Abstract
As the world has been facing several deadly virus crises, including Zika virus disease, Ebola virus disease, severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and Coronavirus disease 2019 (COVID-19), lateral flow assays (LFAs), which require minimal equipment for point-of-care of viral infectious diseases, are garnering much attention. Accordingly, there is an increasing demand to reduce the time and cost required for manufacturing LFAs. The current study introduces an equipment-free method of salt-mediated immobilization of nucleic acids (SAIoNs) for LFAs. Compared to general DNA immobilization methods such as streptavidin–biotin, UV-irradiation, and heat treatment, our method does not require special equipment (e.g., centrifuge, UV-crosslinker, heating device); therefore, it can be applied in a resource-limited environment with reduced production costs. The immobilization process was streamlined and completed within 30 min. Our method improved the color intensity signal approximately 14 times compared to the method without using SAIoNs and exhibited reproducibility with the long-term storage stability. The proposed method can be used to detect practical targets (e.g., SARS-CoV-2) and facilitates highly sensitive and selective detection of target nucleic acids with multiplexing capability and without any cross-reactivity. This novel immobilization strategy provides a basis for easily and inexpensively developing nucleic acid LFAs combined with various types of nucleic acid amplification.
Abbreviations: AuNPs, gold nanoparticles; BSA, bovine serum albumin; LF, lateral flow; LFA, lateral flow assay; LFIA, lateral flow immuno-assay; LOD, limit of detection; LAMP, loop-mediated isothermal amplification; MERS, Middle East respiratory syndrome; NC, nitrocellulose; NTC, no-template control; NALFA, nucleic acid lateral flow assay; POC, point-of-care; RT-qPCR, quantitative reverse transcription-polymerase chain reaction; RPA, recombinase polymerase amplification; SAIoNs, salt-mediated immobilization of nucleic acids; SARS, severe acute respiratory syndrome
Keywords: Metal salt, Immobilization, Lateral flow assay, Nucleic acid lateral flow assay, Equipment-free, Streptavidin, Biotin
Graphical Abstract
1. Introduction
Since the beginning of the 21st century, the world has faced several deadly virus crises, including Zika virus disease, Ebola virus disease, severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and Coronavirus disease 2019 (COVID-19) [1]. The prevalence of viral diseases has been caused by either the evolution of existing viruses or the emergence of new viral species, incurring enormous damage to society [2]. In order to control these threats, one key requirement is new diagnostic tests capable of prompt, accurate identification of viruses at the point-of-care (POC) [3]. The current clinical standard is quantitative reverse transcription-polymerase chain reaction (RT-qPCR) that is only used in centralized hospital laboratories, not in the field, due to its complexity and high cost [4]. In this respect, lateral flow assays (LFAs) have received special attention because they are well suited to facilitate nucleic acid testing at the patient level with minimal instrumentation [5]. Due to their portability, low cost, and simple operation, LFAs have been widely used in various fields, ranging from medical to environmental and safety monitoring [6].
There are two main types of LFA currently in use: a lateral flow immuno-assay (LFIA) using an antigen–antibody interaction, and a nucleic acid lateral flow assay (NALFA) using sequence specific DNA hybridization [7]. LFIA is commonly used for the detection of target proteins such as bacteria or viruses [8]. Several serological kits are already on the market to cope with the emergency detection of SARS-CoV-2 antibodies [9]. LFIA based assay for SARS-CoV-2 provide rapid diagnostic results in 15–30 min, which is a relatively short period of time [10], [11]. However, they either have unsatisfactory detection sensitivity or are unable to detect the early onset of COVID-19, which is evidenced by a poor clinical sensitivity of only 18.4% compared to RT-qPCR [12]. This is possibly because LFIAs are difficult to combine with a method of amplifying the target protein. On the contrary, NALFA methods can combine the power of exponential enzymatic amplification (e.g., polymerase chain reaction, PCR; recombinase polymerase amplification, RPA; loop-mediated isothermal amplification, LAMP) of the target gene sequence together with the sensitivity and ease of use offered by the LFA technique. Several studies have reported the combined use of nucleic acid amplification with LFA detection [13], [14], [15]. The first NALFA was developed by Yu and co-workers for the simultaneous detection of three regions of the SARS-CoV-2 genome (RdRp, ORF3a, and the nucleocapsid [N]-protein gene) combined with LAMP [16]. It facilitates the detection of SARS-CoV-2 with a sufficient limit of detection (LOD) of each gene, showing results comparable to those of RT-qPCR, which is the gold standard [17].
To perform NALFA, capture DNA that specifically recognizes a target must be immobilized on a nitrocellulose (NC) membrane. Biotin-modified capture DNA and streptavidin have been extensively used for this purpose [18]. However, it requires the pre-incubation of the streptavidin and biotin-modified capture DNA and separation of unbound streptavidin molecules to prepare a streptavidin–biotin complex of capture DNA, complicating the preparation of NALFA (Fig. S1A). In addition, since the streptavidin–biotin interaction is used for the immobilization of capture DNA, other molecules (e.g., digoxigenin) and their antibodies (e.g., anti-digoxigenin antibody) are necessary for the generation of colorimetric signals, increasing the overall assay cost. As an alternative, unmodified DNA is directly immobilized on the NC membrane using ultraviolet (UV) irradiation (Fig. S1B) or heat treatment (Fig. S1C) [19], [20]. The direct immobilization of capture DNA is highly advantageous but special equipment, such as UV-crosslinkers or heating devices, are required and capture DNA could be damaged because of these harsh conditions (e.g., UV excitation or heating at 60 °C). Therefore, it is imperative to reduce production costs and time to promote the implementation of NALFAs.
In this study, we aimed to develop a simple and inexpensive strategy for the salt-mediated immobilization of nucleic acids onto an NC membrane, hereinafter referred to as SAIoNs. Our new method streamlines the direct immobilization of capture DNA without the need for a biotin–streptavidin complex or special equipment (e.g., UV-crosslinkers or heating devices) and importantly, it improves the color intensity signal approximately 14 times compared to methods without using SAIoNs ( Fig. 1). To demonstrate its utility in biological applications, the new method was applied to analyze various SARS-CoV-2 and human genes (e.g., Orf1b, N, and RPP). The strategy is universally applicable for various capture DNAs and allow for the highly sensitive and selective detection of target nucleic acids with multiplexing capability and without any cross-reactivity.
Fig. 1.
Schematic illustration of the salt-mediated immobilization of nucleic acids (SAIoNs).
2. Material and methods
2.1. Reagents and materials
The oligonucleotides used in this study (Table S1) were purchased from Integrated DNA Technologies (Skokie, IL, USA) and Bionics (Seoul, Korea); HAuCl₄, Tween 20, and sodium azide from Sigma-Aldrich (St. Louis, MO, USA); sodium carbonate, sucrose, NaCl, MgCl2, KCl, and CaCl2 from Daejung Chemicals & Metals (Gyeonggi-do, Korea); bovine serum albumin (BSA) and PCR premix (RT500S) from Enzynomics (Daejeon, Korea); streptavidin from Biolegend (CA, USA); backing card and absorbent pads from TWOHANDS (Gyeonggi-do, Korea); and NC membrane from Whatman (Maidstone, UK).
2.2. Synthesis of gold nanoparticles (AuNPs)
AuNPs were synthesized by adding 0.04 g of HAuCl₄ to 400 mL of distilled water, followed by addition of 3.2 mL of 1% trisodium citrate. The mixture was boiled for 15 min with stirring, and then cooled to room temperature. Synthesized AuNPs were stored at 4 °C and characterized using the ultraviolet–visible (UV–vis) spectrophotometer (Spectramax iD5 multi-mode microplate reader) from Molecular Devices (CA, San Jose, USA).
2.3. Preparation of streptavidin-coated AuNPs
The AuNPs synthesized in the previous step were centrifuged at 1400 × g for 60 min and prepared to reach an absorbance value at 534 nm (A534) of 5 units. To this solution, sodium carbonate and streptavidin were individually added at a final concentration of 1.28 mM and 40 μg/mL, respectively, and were incubated for 1 h at room temperature. Then, BSA was added at the final concentration of 0.1 mg/mL and incubated for 1 h at room temperature. Finally, to remove the unbound streptavidin and BSA, the mixture was centrifuged at 1400 × g for 30 min and resuspended in 10 mM Tris-HCl (pH 8) to reach an A534 of 5 units. The prepared streptavidin-coated AuNPs were stored at 4 °C until further use.
2.4. Assembly of LF strips
LF strips consist of three parts: a backing card, an NC membrane, and an absorbent pad. The NC membrane and absorbent pad were connected to each other on the backing card to ensure continuous capillary transfer. First, a 22 × 300 mm absorbent pad was placed on the downstream side of the NC membrane attached to the backing card, overlapping with the NC membrane by 2 mm. Next, the cards were cut into pieces that were 3 mm wide and 43 mm long.
2.5. Confirmation of the pre-treatment effect on DNA immobilization
The pre-treatment buffer was 20 mM sodium borate (pH 8.0) containing 0.8 M KCl, 0.2% (v/v) Tween 20. An amount of 0.3 μL of 300 µM bare capture DNA was dispensed and dried at room temperature for 10 min. Before and after dispensing bare capture DNA, NC membranes were fully immersed in pre-treatment buffer and dried for 10 min. For comparison, 300 μM bare capture DNA was mixed with pre-treatment buffer or each component of the pre-treatment buffer, and an amount of 0.3 μL of above mixtures was dispensed and dried at room temperature for 10 min. Finally, all LF strips were exposed to 254 nm UV light using a UV-crosslinker (Korea Ace Science, Seoul, Korea) with a total energy imparted of 90 mJ/cm2 at 15 cm distance, for 5 min
2.6. Various DNA immobilization methods
An amount of 0.3 μL of 300 μM bare capture DNA with or without 0.8 M KCl was spotted on the NC membrane and dried at room temperature for 10 min. After that, the strips were treated by the following conventional methods: for conventional UV-mediated immobilization, prepared strips were exposed to 254 nm UV light using a UV-crosslinker (Korea Ace Science, Seoul, Korea) with a total energy imparted of 90 mJ/cm2 for 5 min; for conventional heat-mediated DNA immobilization, prepared strips were incubated using a heat block (DAIHAN Scientific, Seoul, Korea) at 60 °C for 10 min; and for no-treatment, prepared strips were incubated at room temperature for 30 min.
2.7. LFA
We mixed 0.3 μL of synthetic DNA product (Table S1) at various concentrations (at 10 nM unless otherwise stated), 3 μL of streptavidin-coated AuNPs, and 26.7 μL of running buffer containing 20 mM sodium borate (pH 8.0), 2% (w/v), sucrose, 0.6 M NaCl, 0.2% (v/v) Tween 20%, and 0.1% (w/v) sodium azide. The LF strips were then dipped into the mixtures and incubated for 10 min. Then, the LF strips were dipped into PBS and incubated for 5 min. The color intensity was then analyzed using Image J software, which was normalized by subtracting the background signal of each strip. Unless otherwise indicated, 0.4 μL of 80 μM 5′-C12 NH2 modified capture DNA (Table S1) containing 0.8 M CaCl2 was spotted on the NC membrane, which was dried for 30 min at room temperature.
2.8. Detection of synthetic target gene of SARS-CoV-2
For the selectivity test, 1x PCR premix was mixed with 0.12 μM forward primer and 1.2 μM reverse primer for the RPP gene, or 0.03 μM forward primer and 0.3 μM reverse primer for the Orf1b gene, or 0.15 μM forward primer and 1.5 μM reverse primer for the N gene. Then, 10 pM synthetic cDNA of each gene was mixed for asymmetric PCR. For the sensitivity test, 1x PCR premix, 0.1 μM forward primer and 1 μM reverse primer for each gene, and various concentrations of each synthetic cDNA were mixed. Asymmetric PCR was performed in a CFX Connect (Bio-Rad Laboratories, Inc., USA) by using the following pre-determined conditions: initial denaturation at 94 °C for 15 min, followed by 50 cycles of denaturation at 95 °C for 10 s, annealing at 57 °C for 15 s, and extension at 72 °C for 30 s. We added 0.6 μL of each asymmetric PCR amplicon, 3 μL of streptavidin-coated AuNPs, and running buffer up to 30 μL. The strips were then dipped into the mixtures and incubated for 10 min. The color intensity was then analyzed using Image J software, which was normalized by subtracting the background signal of each strip.
3. Results and discussion
3.1. Comparison between various immobilization conditions
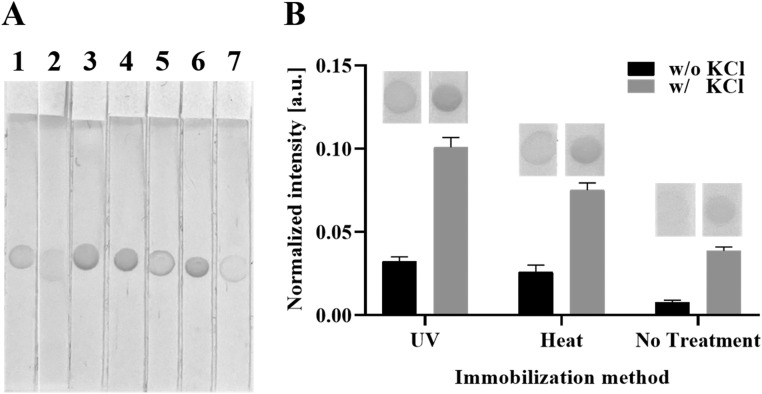
Motivated by a recent study reporting that the pre-treatment of the entire LF strip with the solution can improve the signal intensity in LFA [21], we first investigated the effect of pre-treatment on the colorimetric signal in LFA. We pre-treated NC membrane in various conditions with pre-treatment buffer containing 20 mM sodium borate (pH 8.0), 0.8 M KCl and 0.2% (v/v) Tween 20. As shown in LF strip 3 ( Fig. 2A), the LFA signal increased when the entire NC membrane was pre-treated before the capture DNA was immobilized. As the pre-treatment step of the entire NC membrane increases usage of the pre-treatment buffer and preparation time, it is more desirable to skip this step, and thus we attempted to achieve this goal by preparing capture DNA that contained components of the pre-treatment buffer (LF strips 4, 5, 6, and 7; Fig. 2B). Interestingly, when capture DNA mixed with pre-treatment buffer was immobilized (LF strip 4 in Fig. 2A), the color intensity was comparable to that of the LF strip pre-treated on the entire NC membrane (LF strip 3 in Fig. 2A). Next, we investigated which components of the pre-treatment buffer were the major contributors to the improved colorimetric signal in the LFA. The colorimetric signal increased substantially when 0.8 M KCl was mixed with the bare capture DNA (LF strip 6; Fig. 2A). Based on these results, we assumed that the KCl neutralized the negatively charged phosphate backbone of the capture DNA and reduced the intermolecular electrostatic repulsion of the DNA, which consequently mediated the effective immobilization of capture DNA onto the NC membrane, resulting in a more intense colorimetric signal.
Fig. 2.
Comparison of signal intensity between various immobilization conditions. (A) Effect of pre-treatment buffer or its components on DNA immobilization. 1: without pre-treatment on entire nitrocellulose (NC) membrane, 2: with pre-treatment on entire NC membrane after dispensing bare capture DNA, 3: with pre-treatment on entire NC membrane before dispensing bare capture DNA, 4: Bare capture DNA mixed with pre-treatment buffer, 5: Bare capture DNA mixed with 20 mM borate, 6: Bare capture DNA mixed with 0.8 M KCl, 7: Bare capture DNA mixed with 0.2% (v/v) Tween 20. Conventional UV-mediated immobilization was used to all LF strips. (B) Effect of KCl on DNA immobilization with various immobilization methods. UV: conventional UV-mediated immobilization, Heat: conventional heat-mediated DNA immobilization.
We checked the effect of KCl on the various DNA immobilization methods (Fig. 2B). In all three cases (with UV-irradiation, heat treatment, or without any treatment), the colorimetric signal was increased by more than three times by the presence of KCl in comparison to the absence of KCl (Fig. 2B). Importantly, the colorimetric signal of the non-treated LFA in the presence of KCl was superior to the conventional strategies that rely on UV-irradiation or heat treatment without KCl, paving the way for an equipment-free DNA immobilization method.
3.2. Optimization of SAIoNs
Based on these promising results, we optimized the conditions for SAIoNs ( Fig. 3). We made efforts to achieve a normalized colorimetric intensity of 0.1 in the non-treated conditions because a normalized colorimetric intensity of 0.1 (as evidenced in Fig. 2B) was high enough to clearly detect the results with the naked eye. Because we found that 0.8 M KCl in the pretreatment buffer increases the immobilization efficiency in the previous experiment (Fig. 2), we started the optimization experiment under this condition. First, we investigated the effect of amine (NH2) modification and the concentration of the capture DNA (Fig. 3A and Fig. S2A). When 5′-C6 NH2- modified capture DNA was used, the colorimetric signal was higher than that of bare capture DNA at all concentrations. Thus, NH2-modified capture DNA at a concentration of 80 µM was selected for the subsequent optimization experiments to minimize the amount of capture DNA. Next, we varied the types of NH2 modification in the capture DNA. As shown in Fig. 3B and Fig. S2B, the NH2 modification improved the colorimetric signal in all cases, but the greatest enhancement was observed when using 5′-C12 NH2 capture DNA. Furthermore, we compared the effects of other metal salts (NaCl, MgCl2, and CaCl2) instead of KCl based on our assumption that the neutralization of the phosphate backbone charge of the capture DNA by metal salts would increase the immobilization efficiency (Fig. 3C and Fig. S2C). All tested salts substantially improved the colorimetric signal, with CaCl2 showing the best results and a concentration of 0.8 M CaCl2 showed the clearest signal (Fig. 3D and Fig. S2D). In addition, the mechanism for our SAIoNs technology was proposed in Fig. 3E. It is known that the NC forms dipoles which consist of nitrogen of partial positive charge and oxygen of partial negative charge [22]. Without salt, the negatively charged phosphate group of the DNA backbone is attracted to the nitrogen of the partial positive charge in NC and at the same time is repelled by the oxygen of the partial negative charge in NC. On the other hand, in the presence of salt at optimal concentration, the phosphate group of the DNA backbone and metal ions form a complex by charge-charge interaction [23], which consequently minimizes the repulsive force between DNA and NC. More specifically, the metal ion and phosphate group of DNA-metal complex are arranged to have an attraction with the oxygen of NC and nitrogen of NC, respectively, thereby placing DNA and NC in proximity. However, when a high concentration of salt is present, an excess of metal ions surrounds DNA [24] and oxygen of NC, respectively, which makes it difficult for DNA to approach NC due to the repulsive force, leading to the reduction in the immobilization efficiency. Therefore, we assumed that metal salt at optimal concentration increases the chance of non-covalent and irreversible physical adsorption by placing NCs and DNA in proximity [25], consequently improving the immobilization efficiency. Finally, we confirmed the influence of drying time (Fig. S3A). It was observed that the signal increased over time; 30 min drying time was sufficient since a normalized colorimetric intensity above 0.1 was achieved. Notably, the signal at 0 min was also high enough to detect the color intensity signal with naked eye. We confirmed the influence of drying temperature (Fig. S3B). It was observed that the signal increased with increasing temperature. This result suggests that SAIoNs work well even in resource-limited environments, implying that the developed method would be suitable for POC. In addition, the prepared LF strips were stable for long-term storage, as evidenced by no reduction in signal up to 56 days after storage at room temperature or 4 °C (Fig. S4).
Fig. 3.
Optimization of salt-mediated immobilization of nucleic acids (SAIoNs). (A) Signal intensity with various capture DNA concentrations. (B) Signal intensity with different DNA modifications. No: bare capture DNA; 5′-C6: 5′-C6 NH2 modified capture DNA; 5′-C12: 5′-C12 NH2 modified capture DNA; 3′-C3: 3′-C3 NH2 modified capture DNA; 5′-C6, 3′-C3: 5′ -C6, 3′-C3 NH2 modified capture DNA. All capture DNAs contain 0.8 M KCl. (C) Signal intensity with different metal chlorides. All metal chlorides were used at 0.8 M. (D) Signal intensity at various CaCl2 concentrations. (E) Schematic illustration of the expected mechanism of SAIoNs.
3.3. Comparison of different DNA immobilization methods
Under the optimized conditions, we compared the normalized colorimetric intensity of our method with other DNA immobilization methods. SAIoNs substantially improved the colorimetric signal over non-SAIoNs methods, whether UV or heat was applied or not ( Fig. 4A). The results in Fig. 4B and C show that the colorimetric signal in all cases increased as the concentration of the synthetic DNA product increased. Importantly, the LF strips prepared by our method detected the target DNA at a lower concentration than the method using only UV-irradiation (Fig. 4C). Furthermore, when SAIoNs and UV were used together, the synthetic DNA product was determined at the lowest concentration. The visual limit of detection (LODs) in the three cases, SAIoNs with UV, SAIoNs, and Conventional UV, were approximately 0.5, 1, and 2 nM respectively. Because UV irradiation or heating is known to increase the immobilization efficiency of capture DNA, this enhancement effect was also applied to SAIoNs. These results confirm that the newly developed SAIoNs have good detection performance and can be used for the effective immobilization of capture DNA onto the NC membrane.
Fig. 4.
Comparison of different DNA immobilization methods. (A) Signal intensity with various immobilization methods without (w/o) salt-mediated immobilization of nucleic acids (SAIoNs): 300 μM of bare capture DNA; with (w/) SAIoNs: 80 μM of 5′-C12 NH2 modified capture DNA with 0.8 M CaCl2. (B) Signal intensity with various synthetic DNA product concentrations. (C) Lateral flow strip images of various synthetic DNA product concentrations. SAIoNs + UV: 80 μM of 5′-C12 NH2 modified capture DNA containing 0.8 M CaCl2 with 5 min UV-irradiation; SAIoNs: 80 μM of 5′-C12 NH2 modified capture DNA containing 0.8 M CaCl2 with 30 min incubation at room temperature; Conventional UV: 300 μM of bare capture DNA with UV-irradiation for 5 min. Black arrows indicate visual limits of detection in each case.
3.4. Detection of SARS-CoV-2
The proposed SAIoNs was applied to the detection of SARS-CoV-2 ( Fig. 5). For the specific detection, we designed three different capture DNAs, respectively targeting the Orf1b and N gene of SARS-CoV-2, and the human RPP gene, which is conventionally used as an internal control. These capture DNAs were immobilized onto the separate test zones of one LF strip using SAIoNs. After an asymmetric PCR with specifically designed primers (Table S1), the three kinds of single-stranded amplicons (Orf1b, N, and human RPP genes) were mixed with running buffer. The LF strips were dipped into the mixture. Because the reverse primer was modified with biotin, the single-stranded amplicons were labeled with biotin, which was bound to streptavidin-coated AuNPs, generating the colorimetric signal in each test zone. A high sequence specificity between capture DNA and single-stranded amplicons with no cross-reactivities clearly demonstrate the multiplexing capability ( Fig. 6A). In addition, the detection sensitivities of the Orf1b and N genes of SARS-CoV-2 were evaluated. When confirmed by visual LOD, they were detectable even at concentrations of 300 aM and 500 aM (Fig. 6B and C). These results support the contention that SAIoNs are sufficiently applicable in the real, practical detection of different pathogenic bacteria or viruses [26].
Fig. 5.
Schematic illustration of detecting synthetic target gene of SARS-CoV-2 using LF strips prepared by salt-mediated immobilization of nucleic acids (SAIoNs).
Fig. 6.
Detection of synthetic target gene of SARS-CoV-2 using asymmetric polymerase chain reaction (PCR) with LF strips prepared by salt-mediated immobilization of nucleic acids (SAIoNs) (A) Cross-reactivity test with each capture DNA. (−) and (+) indicate PCR products amplified in the absence and presence of 10 pM of each synthetic cDNA, respectively. (B) Lateral flow strip images and signal intensity with various concentrations of Orf1b synthetic cDNA. (C) Lateral flow strip images and signal intensity with various concentrations of N synthetic cDNA. For the control test, the PCR products amplified in the presence of 10 pM RPP synthetic cDNA was used. Dashed red line in (B) and (C) indicates the cutoff value, which is calculated by the following formula: the normalized intensity of NTC +3 × S.D. of NTC, where NTC, non-template control, S.D., standard deviation.
4. Conclusions
In this paper, we introduce a novel technique for the salt-mediated immobilization of nucleic acids (SAIoNs) onto NC membrane. Compared to conventional DNA immobilization methods such as streptavidin–biotin, UV-irradiation, and heat treatment, our method does not require special equipment (e.g., centrifuge, UV-crosslinker, heating device) and can therefore be applied in a resource-limited environment, with the concomitant advantages of reducing production costs and time ( Table 1). Through the application testing, our newly developed method shows excellent immobilization performance and reproducibility with the long-term storage stability. However, we confirmed only limited kinds of chloride compounds (NaCl, MgCl2, KCl, and CaCl2) and the effect on NH2 modification; therefore, other types of ionic compounds and DNA modification should be studied in order to ensure more versatile applications. Nonetheless, the new method with the improved performance was successfully validated by the detection of practical targets (e.g., SARS-CoV-2) with multiplexing capability, suggesting that it can be a promising molecular diagnostic platform for the simple and rapid detection of various pathogens.
Table 1.
Comparison among UV-mediated immobilization, streptavidin-biotin method, and salt-mediated immobilization of nucleic acids (SAIoNs).
| Conventional method |
SAIoNs |
||||
|---|---|---|---|---|---|
| UV | Heat | Streptavidin (SA)-Biotin | (our method) | ||
| Capture DNA amount | 300 μM | 100 pmole | over 100 μM | 80 μM (32 pmole) | |
| Capture DNA preparation | method | UV irradiation | Baking in 70 °C | Incubation of SA with biotin, and removal of excess SA | No need |
| time | ~15 min | 30 min | 2 h | 30 min | |
| Instrument | UV crosslinker | Oven centrifuge | Heat block and | No need | |
| Cost of Instrument | $2350 | $750 ~ 1170 | $1900 | $0 | |
| Necessary reagents | Bare DNA | Bare DNA | Streptavidin and biotin-labeled DNA | Bare DNA or amine-labeled DNA and salt | |
| Cost per 100 strips | $1.68 | $1.40 | $7.31 | $0.45 ~ 2.21 | |
| Reference | [18], [20], [27], [28] | [29] | [27], [30], [31] | This study | |
CRediT authorship contribution statement
Jung Soo Park: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft. Seokjoon Kim: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft. Jinjoo Han: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft. Jung Ho Kim: Methodology, Formal analysis, Investigation. Ki Soo Park: Writing – review & editing, Funding acquisition, Resources, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by a grant from the National Research Foundation of Korea (NRF), funded by the Korea government (Ministry of Science and ICT) (No. NRF-2020R1C1C1012275), and by a grant (21163MFDS501) from Ministry of Food and Drug Safety in 2021.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Supplementary information
The supporting information is available free of charge as the following files: Schematic illustration of conventional DNA immobilization methods (Fig. S1); Optimization of salt-mediated immobilization of nucleic acids (Fig. S2); Signal intensity at various drying conditions (Fig. S3); Storage durability test (Fig. S4); Oligonucleotide sequences used in this study (Table S1).
Biographies
Jung Soo Park received his BS degree in 2020 from Konkuk University, Republic of Korea. Currently, he is studying for his master degree in the Department of Biological Engineering, Konkuk University, Republic of Korea.
Seokjoon Kim received his BS degree in 2019 from Konkuk University, Republic of Korea. Currently, he is studying for his Ph.D. degree in the Department of Biological Engineering, Konkuk University, Republic of Korea.
Jinjoo Han received her BS degree in 2021 from Konkuk University, Republic of Korea. Currently, she is studying for her master degree in the Department of Biological Engineering, Konkuk University, Republic of Korea.
Jung Ho Kim received his BS degree in 2020 from Konkuk University, Republic of Korea. Currently, she is studying for his master degree in the Department of Biological Engineering, Konkuk University, Republic of Korea.
Ki Soo Park is an associate professor in the Department of Biological Engineering at Konkuk University, Republic of Korea. He received his PhD from the Department of Chemical and Biomolecular Engineering from KAIST, Republic of Korea. He worked as a post‐doctoral researcher in Harvard Medical School/Massachusetts General Hospital. His research interests include exosome analysis platforms, nucleic acid bioengineering, nano‐biotechnology for biomedical sensing, and point‐of‐care diagnostic systems.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.snb.2021.130975.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Pokhrel P., Hu C., Mao H. Detecting the coronavirus (CoVID-19) ACS Sens. 2020;5:2283–2297. doi: 10.1021/ACSSENSORS.0C01153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindhout P., Reniers G. Reflecting on the safety zoo: developing an integrated pandemics barrier model using early lessons from the Covid-19 pandemic. Saf. Sci. 2020;130 doi: 10.1016/j.ssci.2020.104907. (https://doi.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang K., Qin W., Hou Y., Xiao K., Yan W. The application of lateral flow immunoassay in point of care testing: a review. Nano Biomed. Eng. 2016;8:172–183. doi: 10.5101/nbe.v8i3.p172-183. [DOI] [Google Scholar]
- 4.Grant B.D., Anderson C.E., Williford J.R., Alonzo L.F., Glukhova V.A., Boyle D.S., Weigl B.H., Nichols K.P. SARS-CoV-2 coronavirus nucleocapsid antigen-detecting half-strip lateral flow assay toward the development of point of care tests using commercially available reagents. Anal. Chem. 2020;92:11305–11309. doi: 10.1021/acs.analchem.0c01975. (https://doi.org/) [DOI] [PubMed] [Google Scholar]
- 5.Koczula K.M., Gallotta A. Lateral flow assays. Essays Biochem. 2016;60:111–120. doi: 10.1042/EBC20150012. (https://doi.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahadır E.B., Sezgintürk M.K. Lateral flow assays: principles, designs and labels. TrAC - Trends Anal. Chem. 2016;82:286–306. doi: 10.1016/j.trac.2016.06.006. (https://doi.org/) [DOI] [Google Scholar]
- 7.Posthuma-Trumpie G.A., Korf J., Van Amerongen A. Lateral flow (immuno)assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009;393:569–582. doi: 10.1007/s00216-008-2287-2. (https://doi.org/) [DOI] [PubMed] [Google Scholar]
- 8.Andryukov B.G. Six decades of lateral flow immunoassay: from determining metabolic markers to diagnosing covid-19. AIMS Microbiol. 2020;6:280–304. doi: 10.3934/microbiol.2020018. (https://doi.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.West R.M., Kobokovich A., Connell N., Gronvall G.K. Antibody (serology) tests for COVID-19: a case study. MSphere. 2021;6 doi: 10.1128/msphere.00201-21. (https://doi.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant B.D., Anderson C.E., Williford J.R., Alonzo L.F., Glukhova V.A., Boyle D.S., Weigl B.H., Nichols K.P. SARS-CoV-2 coronavirus nucleocapsid antigen-detecting half-strip lateral flow assay toward the development of point of care tests using commercially available reagents. Anal. Chem. 2020;92:11305–11309. doi: 10.1021/acs.analchem.0c01975. (https://doi.org/) [DOI] [PubMed] [Google Scholar]
- 11.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Sun R., Wang Y., Hu B., Chen W., Zhang Y., Wang J., Huang B., Lin Y., Yang J., Cai W., Wang X., Cheng J., Chen Z., Sun K., Pan W., Zhan Z., Chen L., Ye F. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020;92:1518–1524. doi: 10.1002/jmv.25727. (https://doi.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruno R., Mondelli M., Brunetti E., Di Matteo A., Seminari E., Maiocchi L., Zuccaro V., Pagnucco L., Mariani B., Ludovisi S., Lissandrin R., Parisi A., Sacchi P., Patruno S.F.A., Michelone G., Gulminetti R., Zanaboni D., Novati S., Maserati R., Orsolini P., Vecchia M., Sciarra M., Asperges E., Colaneri M., Di Filippo A., Sambo M., Biscarini S., Lupi M., Roda S., Pieri T.C., Gallazzi I., Sachs M., Valsecchi P., Perlini S., Alfano C., Bonzano M., Briganti F., Crescenzi G., Falchi A.G., Guarnone R., Guglielmana B., Maggi E., Martino I., Pettenazza P., Pioli Di Marco S., Quaglia F., Sabena A., Salinaro F., Speciale F., Zunino I., De Lorenzo M., Secco G., Dimitry L., Cappa G., Maisak I., Chiodi B., Sciarrini M., Barcella B., Resta F., Moroni L., Vezzoni G., Scattaglia L., Boscolo E., Zattera C., Tassi M.F., Capozza V., Vignaroli D., Bazzini M., Iotti G., Mojoli F., Belliato M., Perotti L., Mongodi S., Tavazzi G., Marseglia G., Licari A., Brambilla I., Barbarini D., Cambieri P., Campanini G., Comolli G., Corbella M., Daturi R., Furione M., Monzillo E., Paolucci S., Parea M., Percivalle E., Piralla A., Rovida F., Sarasini A., Zavattoni M., Adzasehoun G., Bellotti L., Cabano E., Casali G., Dossena L., Frisco G., Garbagnoli G., Girello A., Landini V., Lucchelli C., Maliardi V., Pezzaia S., Premoli M., Bonetti A., Caneva G., Cassaniti I., Corcione A., Di Martino R., Di Napoli A., Ferrari A., Ferrari G., Fiorina L., Giardina F., Mercato A., Novazzi F., Ratano G., Rossi B., Sciabica I.M., Tallarita M., Nepita E.V., Calvi M., Tizzoni M., Nicora C., Triarico A., Petronella V., Marena C., Muzzi A., Lago P., Baldanti F. Performance of VivaDiag COVID-19 IgM/IgG rapid test is inadequate for diagnosis of COVID-19 in acute patients referring to emergency room department. J. Med. Virol. 2020;92:1724–1727. doi: 10.1002/jmv.25800. (https://doi.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivanov A.V., Safenkova I.V., Zherdev A.V., Dzantiev B.B. Nucleic acid lateral flow assay with recombinase polymerase amplification: solutions for highly sensitive detection of RNA virus. Talanta. 2020;210 doi: 10.1016/j.talanta.2019.120616. (https://doi.org/) [DOI] [PubMed] [Google Scholar]
- 14.Kim J.M., Park J.S., Yoon T.H., Park J., Park K.S. Nucleic acid lateral flow assay for simultaneous detection of hygiene indicator bacteria. Anal. Bioanal. Chem. 2021;413:5003–5011. doi: 10.1007/s00216-021-03462-w. (https://doi.org/) [DOI] [PubMed] [Google Scholar]
- 15.Allgöwer S.M., Hartmann C.A., Holzhauser T. The development of highly specific and sensitive primers for the detection of potentially allergenic soybean (Glycine max) using loop-mediated isothermal amplification combined with lateral flow dipstick (LAMP-LFD) Foods. 2020;9 doi: 10.3390/foods9040423. (https://doi.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu S., Nimse S.B., Kim J., Song K.S., Kim T. Development of a lateral flow strip membrane assay for rapid and sensitive detection of the SARS-CoV-2. Anal. Chem. 2020;92:14139–14144. doi: 10.1021/acs.analchem.0c03202. (https://doi.org/) [DOI] [PubMed] [Google Scholar]
- 17.Antiochia R. Paper-based biosensors: frontiers in point-of-care detection of covid-19 disease. Biosensors. 2021;11 doi: 10.3390/bios11040110. (https://doi.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pongsuchart M., Sereemaspun A., Ruxrungtham K. UV treatment nucleic acid probe without biotin-labeling is sensitive and sufficient for the fabrication of nucleic acid lateral flow (NALF) strip test. J. Life Sci. Technol. 2013:172–175. doi: 10.12720/jolst.1.3.172-175. (https://doi.org/) [DOI] [Google Scholar]
- 19.Javani A., Javadi-Zarnaghi F., Rasaee M.J. A multiplex protein-free lateral flow assay for detection of microRNAs based on unmodified molecular beacons. Anal. Biochem. 2017;537:99–105. doi: 10.1016/j.ab.2017.09.005. (https://doi.org/) [DOI] [PubMed] [Google Scholar]
- 20.Pongsuchart M., Sereemaspun A., Ruxrungtham K. Sensitivity enhancement of nucleic acid detection by lateral flow strip test using UV crosslink method. Asian Biomed. 2012;6:459–463. doi: 10.5372/1905-7415.0603.077. (https://doi.org/) [DOI] [Google Scholar]
- 21.Tang R.H., Li M., Liu L.N., Zhang S.F., Alam N., You M., Ni Y.H., Li Z.D. Chitosan-modified nitrocellulose membrane for paper-based point-of-care testing. Cellulose. 2020;27:3835–3846. doi: 10.1007/s10570-020-03031-x. (https://doi.org/) [DOI] [Google Scholar]
- 22.Costa M.N., Veigas B., Jacob J.M., Santos D.S., Gomes J., Baptista P.V., Martins R., Inácio J., Fortunato E. A low cost, safe, disposable, rapid and self-sustainable paper-based platform for diagnostic testing: lab-on-paper. Nanotechnology. 2014;25 doi: 10.1088/0957-4484/25/9/094006. (https://doi.org/) [DOI] [PubMed] [Google Scholar]
- 23.Kang F., Wang H., Gao Y., Long J., Wang Q. Ca2+ promoted the low transformation efficiency of plasmid DNA exposed to PAH contaminants. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058238. (https://doi.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsarskaia O., Roosen-Runge F., Schreiber F. Multivalent ions and biomolecules: attempting a comprehensive perspective. ChemPhysChem. 2020;21:1742–1767. doi: 10.1002/cphc.202000162. (https://doi.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stillman B.A., Tonkinson J.L. FAST(TM) slides: a novel surface for microarrays. Biotechniques. 2000;29:630–635. doi: 10.2144/00293pf01. (https://doi.org/) [DOI] [PubMed] [Google Scholar]
- 26.Corman V.M., Haage V.C., Bleicker T., Schmidt M.L., Mühlemann B., Zuchowski M., Jo W.K., Tscheak P., Möncke-Buchner E., Müller M.A., Krumbholz A., Drexler J.F., Drosten C. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: a single-centre laboratory evaluation study. Lancet Microbe. 2021;2:e311–e319. doi: 10.1016/S2666-5247(21)00056-2. (https://doi.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javani A., Javadi-Zarnaghi F., Rasaee M.J. Development of a colorimetric nucleic acid-based lateral flow assay with non-biotinylated capture DNA. Appl. Biol. Chem. 2017;60:637–645. doi: 10.1007/s13765-017-0321-9. (https://doi.org/) [DOI] [Google Scholar]
- 28.Bruno J.G. Application of DNA aptamers and quantum dots to lateral flow test strips for detection of foodborne pathogens with improved sensitivity versus colloidal gold. Pathogens. 2014;3:341–355. doi: 10.3390/pathogens3020341. (https://doi.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Javani A., Javadi-Zarnaghi F., Rasaee M.J. A multiplex protein-free lateral flow assay for detection of microRNAs based on unmodified molecular beacons. Anal. Biochem. 2017;537:99–105. doi: 10.1016/j.ab.2017.09.005. (https://doi.org/) [DOI] [PubMed] [Google Scholar]
- 30.Mao X., Ma Y., Zhang A., Zhang L., Zeng L., Liu G. Disposable nucleic acid biosensors based on gold nanoparticle probes and lateral flow strip. Anal. Chem. 2009;81:1660–1668. doi: 10.1021/ac8024653. (https://doi.org/) [DOI] [PubMed] [Google Scholar]
- 31.Gao X., Xu H., Baloda M., Gurung A.S., Xu L.P., Wang T., Zhang X., Liu G. Visual detection of microRNA with lateral flow nucleic acid biosensor. Biosens. Bioelectron. 2014;54:578–584. doi: 10.1016/j.bios.2013.10.055. (https://doi.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material