Abstract
Background:
Cardiac allograft vasculopathy (CAV) causes impaired blood flow in both epicardial coronary arteries and the microvasculature. A leading cause of post-transplant mortality, CAV impacts 50% of heart transplant (HT) recipients within 10 years of HT.
Objectives:
This analysis examined the outcomes of HT recipients with reduced myocardial blood flow reserve (MBFR) and microvascular CAV detected by 13N-ammonia positron emission tomography myocardial perfusion imaging (PET).
Methods:
181 HT recipients who underwent PET to assess for CAV were included with a median follow-up of 4.7 years. Patients were classified into two groups according to the total MBFR: >2.0 and ≤2.0. Microvascular CAV was defined as no epicardial CAV detected by PET and/or coronary angiography, but with an MBFR ≤2.0 by PET.
Results:
71 (39%) patients had an MBFR ≤2.0. Patients with an MBFR ≤2.0 experienced an increased risk for all outcomes: 7-fold increase in death or retransplantation (HR 7.05, 95% CI 3.2–15.6, p<0.0001), 12-fold increase in cardiovascular death (HR 12.0, 95% CI 2.64–54.12, p=0.001), and 10-fold increase in cardiovascular hospitalization (HR 10.1, 95% CI 3.43–29.9, p<0.0001). Five-year mean survival was 302 days less than those with an MBFR >2.0 (95% CI 260.2–345.4 days, p<0.0001). Microvascular CAV (adjusted HR 3.86, 95% CI 1.58–9.40, p=0.003) was independently associated with an increased risk of death or retransplantation.
Conclusions:
Abnormal myocardial blood flow reserve, even in the absence of epicardial CAV, identifies patients at a high risk of death or retransplantation. Measures of myocardial blood flow provide prognostic information in addition to traditional CAV assessment.
Keywords: Heart transplant, cardiac allograft vasculopathy, microvascular dysfunction, prognosis, positron emission tomography
Condensed Abstract:
Cardiac allograft vasculopathy (CAV) causes impaired blood flow in both epicardial coronary arteries and the microvasculature and is a leading cause of post-transplant morbidity and mortality. Non-invasive assessment of CAV with PET myocardial perfusion imaging provides measurements of myocardial blood flow. HT recipients with a reduced (≤2.0) myocardial blood flow reserve had a 7-fold increase in death or retransplantation, 12-fold increase in CV death, and 10-fold increase in CV hospitalization. Patients with isolated microvascular CAV had a nearly four times the risk of death or retransplantation. Measures of myocardial blood flow provide prognostic information in addition to traditional CAV assessment.
Introduction:
Cardiac allograft vasculopathy (CAV) is unique to heart transplant recipients, impacting both epicardial coronary arteries and the microvasculature. It is marked by intimal thickening and fibrosis, tapering of epicardial vessels, and decreased myocardial blood flow resulting in restrictive physiology. Unfortunately, CAV is common amongst heart transplant recipients with 30–45% of patients having some degree of epicardial CAV by 5 years post-transplant and 50–65% at 10 years.1,2 In addition to being a prevalent condition, it carries a significant risk of mortality. In the International Society for Heart and Lung Transplantation (ISHLT) registry, CAV is one of the most common causes of death, and when graft failure is included (listed as a distinct mode of death, though often due to CAV) it is the most common.1
Evaluation for CAV historically has been centered on invasive coronary angiography and stenosis severity, and the current ISHLT definition of and grading criteria for CAV does not extend beyond epicardial angiography.3 The microvasculature, where abnormalities have been linked with adverse events independent of epicardial CAV,4 is not comprehensively assessed with angiography. Physiologic assessment of CAV, both invasive and non-invasive, has recently been demonstrated to provide greater discrimination. Invasively measured fractional flow reserve and markers of microvascular dysfunction (coronary flow reserve and index of microcirculatory resistance) have both been demonstrated to predict death or cardiac retransplantation.5,6 Non-invasive assessment of myocardial flow reserve with rubidium-82 positron emission tomography (PET) myocardial perfusion imaging also have demonstrated reduced survival with reductions in myocardial blood flow reserve.7,8 The goal of this analysis was to assess the prognostic ability of total myocardial blood flow reserve (MBFR) and isolated microvascular CAV measured by 13N-ammonia PET on post-transplant outcomes.
Methods:
Study Population
In 2016 our center changed the CAV screening protocol from biannual coronary angiography with intravascular ultrasound (IVUS) alternating with Dobutamine Stress Echocardiography (DSE), replacing DSE with 13N-ammonia PET due to our and others contemporary experience with DSE (e.g. limited sensitivity and frequency of non-diagnostic studies).9,10 Patients with an abnormal PET were referred for coronary angiography with IVUS if renal function was acceptable. This observational retrospective cohort study included all adult heart transplant recipients who underwent 13N-ammonia PET myocardial perfusion imaging from June 2016 through September 2017, targeting 5 years of follow-up for clinical outcomes. Final study follow-up date was December 31st, 2021. Demographic and clinical data were collected from the electronic medical record. Cardiac allograft vasculopathy was defined and graded according to the ISHLT angiographic criteria.11 Right heart catheterization was performed at the same time as the angiogram. Angiograms were graded by one of six board certified interventional cardiologists who were not aware of the PET results. Angiograms were included if they were performed within three months prior to the PET or in the subsequent 12 months to maximize the number of patients with contemporary angiographic data (median time between studies: 322 days).
Study Definitions:
Patients were classified into two groups according to the total MBFR: >2.0 and ≤2.0. A MBFR value of 2.0 was chosen as the cut-off based on the definition of microvascular dysfunction according to standardized COVADIS (Coronary Vasomotion Disorders International Study Group) diagnostic criteria 12, prior data demonstrating the prognostic significance of a PET derived MBFR below 2.0 in atherosclerosis 13,14, and a receiver operator characteristic curve analysis demonstrating an optimal cut-off of 1.96 (Supplemental Figure 1). Patients were secondarily classified, and epicardial CAV was defined by PET derived ischemia (summed difference score [SDS] ≥2) or ISHLT CAV 1 or greater angiographically, and an MBFR >2.0. Microvascular CAV was defined as an MBFR ≤2.0 with no evidence of epicardial CAV (PET SDS <2 or ISHLT CAV Grade 0 angiographically). Patients with mixed CAV had both ischemia (SDS ≥2 or ISHLT CAV 1 or greater) and an MBFR ≤2.0.
PET Protocol
PET myocardial perfusion imaging was conducted using a Siemens PET-CT mCT 64-slice scanner. For rest images, 8 to 12 mCi of 13N-ammonia was injected intravenously before acquisition of cardiac perfusion images. For the stress portion of the examination, patients underwent pharmacologic stress with dipyridamole (0.56 mg/kg), adenosine (140 mcg/kg/min), or regadenoson (0.4 mg), at which time they received another 8 to 12 mCi of 13N-ammonia after a delay of 50 minutes (5 half-lives) from rest imaging. All patients were closely monitored for transient conduction system abnormalities. After all images were obtained, the reconstructed perfusion images were analyzed using Invia software (4DM; Ann Arbor, MI) according to standard-of-care methods. Myocardial ischemia or infarction was assessed using described semi-quantitative assessment of a 17-segment model by board-certified nuclear cardiologists.15 Summed rest, summed stress, and summed difference (stress-rest) scores were calculated. Stress and rest left ventricular ejection fraction (LVEF) was measured from gated images. Myocardial blood flow (in milliliters per minute per gram of myocardial tissue) was calculated at rest and stress using validated 2-compartment models. MBFR was calculated as the ratio of stress to rest myocardial blood flow and adjusted for rate pressure product using a reference value of 9,000. Low-dose computed tomography was used for attenuation correction and allowed for visual estimation of coronary artery calcium (VECAC), which has previously been validated.16 Average radiation effective dose for each PET study was 2–3 mSv, less than the annual background radiation in the United States.17
Study Outcomes
The primary study endpoint was death or retransplantation, ascertained from medical records. Major secondary endpoints included cardiovascular death or retransplantation and cardiovascular hospitalization, which involved heart failure hospitalization, acute coronary syndrome, percutaneous coronary intervention, and acute rejection.
Statistical Analysis:
Demographic and clinical variables were expressed as mean (± standard deviation) or median with interquartile range (IQR) for continuous variables depending on normality and count (with percentage) for categorical variables. Group comparisons were made with X2 test, Fisher’s exact test, and the Mann Whitney U Test where appropriate. Kaplan-Meier survival analysis and Cox proportional-hazards regression were performed to compare outcomes between the groups. Cardiovascular mortality was assessed using a Fine and Gray subdistribution hazard model to account for the competing risk of non-CV mortality. A multivariable Cox model was generated to assess for significant predictors and confounders of death or retransplantation. Variables considered included those listed in Table 3. The adjusted model was generated using Akaike Information Criterion model selection to find the best-fit model. A secondary model was generated with backward selection, including all variables and removing the least significant variables until all included variables had a p-value <0.10. The difference in post-PET survival between groups was determined using restricted mean survival time (RMST) (20). RMST is analogous to the area under the survival curve and, in this analysis, represents the mean event-free survival from PET scan to the end of study follow-up. A two-tailed p-value of less than 0.05 was considered significant. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina). The study was approved by the Columbia University Irving Medical Center Institutional Review Board.
Table 3.
Univariable and multivariable predictors of death or retransplantation.
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
|---|---|---|---|---|
|
| ||||
| MBFR≤2 | 7.05 (3.2–15.5) | <0.0001 | 4.04 (1.72–9.46) | 0.001 |
| Donor Age | 0.99 (0.97–1.03) | 0.82 | ||
| Age | 1.008 (0.99–1.03) | 0.50 | ||
| Non-white | 0.93 (0.47–1.81) | 0.83 | ||
| Time Since Transplant | 1.04 (0.99–1.10) | 0.15 | ||
| Female | 0.30 (0.11–0.86) | 0.02 | ||
| Rest EF | 0.95 (0.92–0.98) | 0.004 | 0.95 (0.92–1.00) | 0.04 |
| ISHLT CAV 2 or 3 | 4.56 (1.99–10.46) | 0.0003 | 2.68 (1.07–8.46) | 0.04 |
| PET Assessed Ischemia (SDS>2) | 6.14 (2.80–13.16) | <0.0001 | 3.70 (1.62–8.46) | 0.002 |
| Ischemic Cardiomyopathy | 1.49 (0.73–3.04) | 0.27 | ||
| PSI | 0.47 (0.18–1.21) | 0.12 | ||
| Statin | 0.52 (0.23–1.14) | 0.10 | 0.34 (0.14–0.80) | 0.01 |
| Aspirin | 1.15 (0.35–3.77) | 0.81 | ||
| Pre-Transplant Cigarette Use | 1.25 (0.63–2.49) | 0.42 | ||
| Diabetes Mellitus | 3.51 (1.77–6.97) | 0.0003 | 3.67 (1.71–7.88) | 0.001 |
| Prior Stroke | 2.41 (0.74–7.88) | 0.15 | ||
| BMI | 0.99 (0.93–1.04) | 0.59 | ||
| Stage 3+ CKD (GFR<60 ml/min/1.73 m2) | 0.66 (0.73–3.81) | 0.23 | ||
| DSA | 1.77 (0.91–3.47) | 0.09 | ||
| Prior ACR | 1.55 (0.78–3.08) | 0.21 | ||
| Prior AMR | 2.66 (1.10–6.41) | 0.03 | ||
ACR=Acute cellular rejection, AMR=Antibody mediated rejection, BMI=Body mass index, CAV=Cardiac allograft vasculopathy, CKD=Chronic kidney disease, DSA=Donor specific antibodies, EF=Ejection fraction, MBFR=Myocardial blood flow reserve, PSI=Proliferation signal inhibitor, SDS=Summed difference score
Results:
Baseline Characteristics
Two-hundred and six consecutive heart transplant recipients who underwent a PET scan were assessed, of which 181 patients comprised the study cohort (17 were excluded for high resting myocardial blood flow [>1.1 mL/minute/g], 5 had technical difficulties, and 3 were repeat studies for patients previously included, Figure 1). Baseline characteristics of the study cohort are presented in Table 1. Overall, 181 patients were enrolled, of whom 110 had an MBFR >2.0 and 71 had an MBFR ≤2.0. Median follow-up was 4.7 years (IQR 4.0–5.2). The median age of individuals with reduced MBFR was greater at the time of the PET scan (65 years [IQR 56–69] vs. 59 years [IQR 47–67], p=0.02), and there were trends towards a greater median donor age (34 years [IQR 25–48.5] vs. 29 years [IQR 21–44], p=0.07) and a greater median time since transplant (9 years [5–12] vs. 7 years [IQR 4–10], p=0.11). Patients with an MBFR ≤2.0 were significantly more likely to have had an ischemic cardiomyopathy as indication for heart transplant, have had a prior stroke, had smoked prior to transplantation, and had diabetes mellitus; they were less likely to be taking aspirin. They also had significantly more prior acute cellular rejection, antibody mediated rejection, and chronic kidney disease (Table 1). Among patients who had rejection, the time from AMR to PET was less among those with an MBFR ≤2.0, while time from ACR to PET was greater. No patient fulfilled the ISHLT criteria for restrictive physiology and right atrial pressure, pulmonary capillary wedge pressure, and cardiac index were similar between groups. Individual hemodynamic abnormalities were associated with the risk of death or retransplantation (Supplemental Table 1).
Figure 1: Study flow diagram outlining the reasons for patient exclusion.
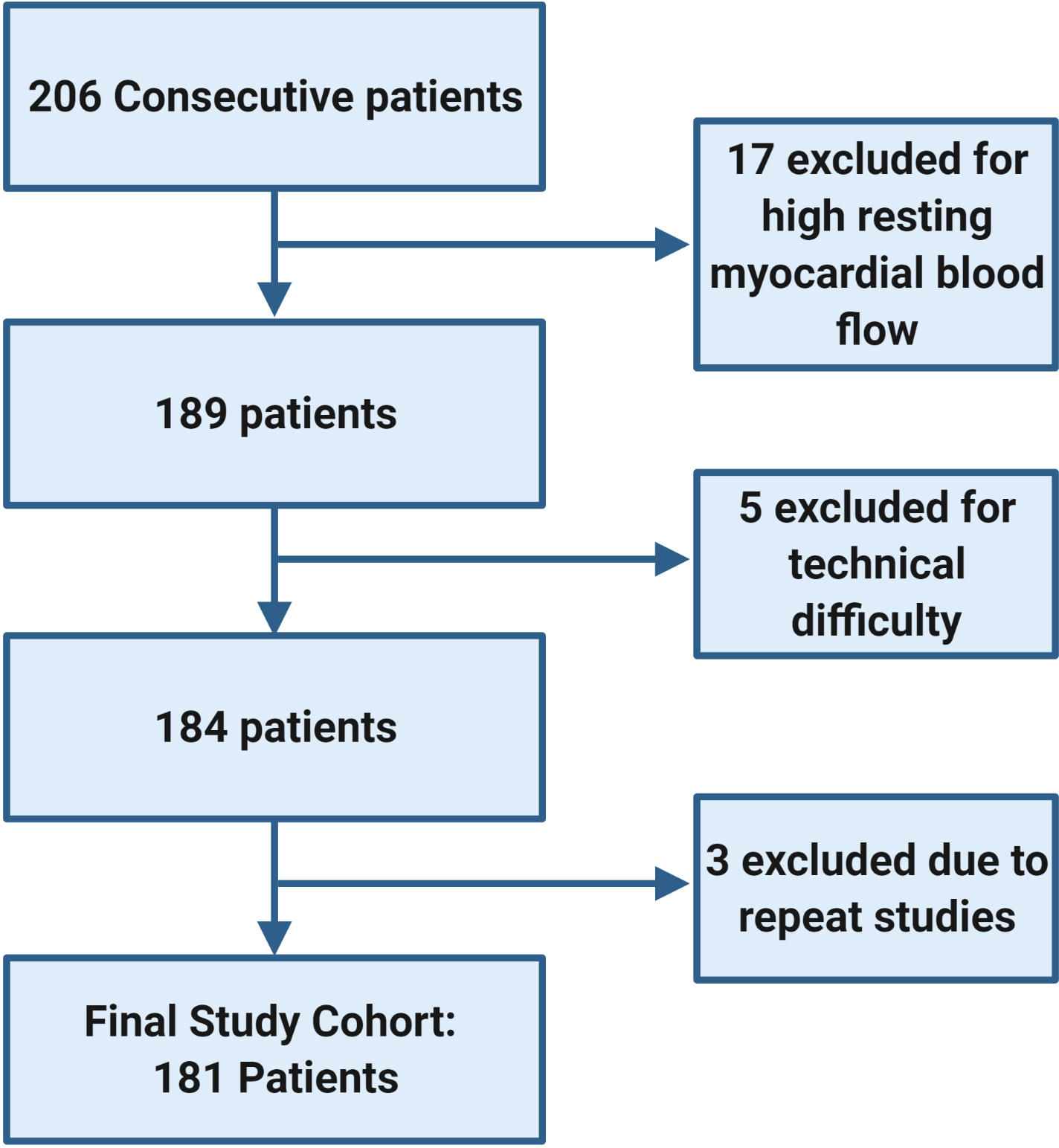
The study included all adult heart transplant recipients who underwent 13N-ammonia PET myocardial perfusion imaging from June 2016 through September 2017, targeting five years of follow-up. Patients were excluded for high resting myocardial blood flow, technical difficulties, or patients with repeat studies during the study period.
Table 1.
Baseline Characteristics
| All Patients | MBFR>2.0 | MBFR≤2.0 | p-value | |
|---|---|---|---|---|
|
| ||||
| n | 181 | 110 | 71 | |
| Male (%) | 133 (73.5) | 76 (69.1) | 57 (80.3) | 0.10 |
| Age at PET Scan | 62.0 (49–68) | 59 (47–67) | 65 (56–69) | 0.02 |
| Age at Transplant | 54.4 (41–62) | 51.5 (37–61) | 56 (46–63) | 0.06 |
| Time Since Heart Transplant | 7 (4–11) | 7.0 (4.0–10.0) | 9.0 (5.0–12.0) | 0.11 |
| Donor Age | 29 (21–44) | 34 (25–48.5) | 0.07 | |
| Ethnicity (%) | 0.49 | |||
| White | 102 (56.4) | 59 (53.6) | 43 (60.5) | |
| Black | 38 (21.0) | 25 (22.7) | 13 (18.3) | |
| Hispanic | 31 (17.1) | 18 (16.4) | 13 (18.3) | |
| Other | 10 (5.5) | 8 (7.3) | 2 (2.8) | |
| HF Etiology (%) | 0.02 | |||
| Ischemic | 41 (22.6) | 18 (16.4) | 23 (32.4) | |
| Non-ischemic | 122 (67.4) | 84 (76.4) | 38 (53.5) | |
| Restrictive/Infiltrative | 4 (2.2) | 2 (1.8) | 2 (2.8) | |
| Retransplant | 7 (3.9) | 2 (1.8) | 5 (7.1) | |
| Congenital | 7 (3.9) | 4 (3.6) | 3 (4.2) | |
| Immunosuppression | ||||
| CNI | 177 (97.8) | 108 (98.2) | 69 (97.2) | 0.66 |
| Tacrolimus | 132 (72.9) | 86 (78.2) | 46 (64.8) | |
| Cyclosporine | 55 (30.4) | 22 (20.0) | 23 (32.4) | |
| Proliferation Signal Inhibitor | 48 (26.5) | 31 (28.2) | 37 (24.3) | 0.47 |
| Everolimus | 36 (19.9) | 25 (22.7) | 11 (15.7) | |
| Sirolimus | 12 (6.6) | 6 (5.5) | 6 (8.6) | |
| Anti-metabolite | 112 (61.9) | 66 (60.0) | 46 (64.8) | 0.43 |
| Mycophenolate Mofetil | 108 (59.7) | 63 (57.3) | 45 (63.4) | |
| Azathioprine | 4 (2.2) | 3 (2.7) | 1 (1.4) | |
| Medication | ||||
| ACEi/ARB/ARNI | 62 (34.3) | 38 (34.6) | 24 (33.8) | 0.92 |
| Calcium Channel Blocker | 72 (40.0) | 40 (36.4) | 32 (45.1) | 0.24 |
| Statin | 156 (86.2) | 96 (87.3) | 60 (84.5) | 0.60 |
| Other lipid lowering agent | 42 (23.8) | 29 (36.4) | 14 (19.7) | 0.10 |
| Aspirin | 164 (90.6) | 105 (95.5) | 59 (83.1) | 0.005 |
| BMI | 26.7 (23.7–29.2) | 26.6 (23.7–28.8) | 26.7 (24.0–30.8) | 0.42 |
| HTN | 127 (70.2) | 76 (69.1) | 51 (71.8) | 0.69 |
| Tobacco Use Pre-transplant | 48 (26.5) | 29 (26.1) | 29 (40.3) | 0.04 |
| Insulin Dependent Diabetes Mellitus (%) | 50 (27.6) | 20 (18.2) | 30 (42.3) | 0.0004 |
| Diabetes Mellitus (%) | 65 (35.9) | 31 (28.2) | 34 (47.9) | 0.007 |
| Prior Stroke (%) | 8 (4.4) | 2 (1.8) | 6 (8.5) | 0.03 |
| CKD (%) | 0.0002 | |||
| GFR>60 mL/min | 51 (28.2) | 39 (35.5) | 12 (16.9) | |
| GFR 30–60 mL/min | 85 (47.0) | 56 (50.9) | 29 (40.9) | |
| GFR<30 mL/min | 33 (18.2) | 12 (10.9) | 21 (29.5) | |
| ESRD | 12 (6.6) | 3 (2.7) | 9 (12.7) | |
| LDL (mg/dL) | 83 (68–101) | 85.4 ±23.7 | 82.8±25.6 | 0.39 |
| Prior ACR | 54 (29.8) | 26 (23.6) | 28 (39.4) | 0.02 |
| Prior ACR≥2R | 23 (12.7) | 11 (10.0) | 12 (16.9) | 0.17 |
| Time Since ACR | 8.2 (2.7–12.2) | 6.6 (2.4–9.5) | 9.3 (3.3–15.2) | 0.05 |
| Prior AMR | 14 (7.7) | 4 (3.6) | 10 (14.1) | 0.01 |
| Time Since AMR | 3.1 (2.5–4.5) | 4.0 (3.1–7.7) | 2.8 (2.1–3.7) | 0.12 |
| Post-Transplant DSA | 56 (30.9) | 30 (27.3) | 26 (36.6) | 0.19 |
| Cardiac Allograft Vasculopathy (n=174) | 0.005 | |||
| CAV 0 | 92 (50.8) | 64 (61) | 28 (40.6) | |
| CAV 1 | 69 (38.1) | 37 (35.2) | 32 (46.4) | |
| CAV 2 | 5 (2.7) | 0 (0) | 5 (5.8) | |
| CAV 3 | 8 (4.4) | 4 (3.8) | 4 (5.8) | |
| Right Heart Catheterization | ||||
| RA | 5 (3–8) | 5 (3–8) | 6 (3–8) | 0.24 |
| PA Systolic | 29 (26–35) | 28 (25–31.5) | 32 (28–40) | 0.006 |
| PA Diastolic | 11 (8–14) | 11 (8–13.5) | 12 (9–17) | 0.02 |
| PCWP | 10 (8–13) | 10 (8–13) | 11 (8–15) | 0.25 |
| Cardiac Index | 2.71 (2.35–3.24) | 2.73 (2.34–3.26) | 2.70 (2.38–3.20) | 0.87 |
ACEi=Angiotensin converting enzyme inhibitor, ARB=Angiotensin II receptor blocker, ARNI=Angiotensin Receptor-Neprilysin Inhibitor, ACR=Acute cellular rejection, AMR=Antibody mediated rejection, CAV=Cardiac allograft vasculopathy, CKD=Chronic kidney disease, DSA=Donor specific antibodies, EF=Ejection fraction, ESRD=End stage renal disease, GFR=Glomerular filtration rate, LDL=Low density lipoprotein, MBFR=Myocardial blood flow reserve, mmHg=Millimeters of mercury, PA=Pulmonary Artery, PCWP=Pulmonary capillary wedge pressure, PSI=Proliferation signal inhibitor. St Dev=Standard Deviation. Continuous data presented as median with interquartile range unless otherwise specified.
PET Characteristics
Dipyridamole was the predominant coronary vasodilator and was utilized for 95% of the studies with no difference between groups (Table 2). There were no significant conduction disturbances following vasodilator administration in this cohort. At rest, total myocardial blood flow was similar between the two groups (0.94 mL/min/g [IQR 0.85–1.03] vs 0.90 mL/min/g [IQR 0.82–1.0], p=0.08), whereas there was a significant difference in total stress myocardial blood flow (2.31 mL/min/g [2.03–2.62] vs. 1.47 mL/min/g [1.22–1.69], p<0.0001), which drove the difference in the MBFR (2.60 [IQR 2.20–3.0] vs. 1.62 [1.35–1.80], p<0.0001). Rest and stress left ventricular ejection fraction was lower in the MBFR ≤2.0 group, however both remained within the normal range (Table 2). Ischemia was more common among those with an MBFR ≤2.0, with significant ischemia (>5% of myocardium) accounting for most of the difference. Coronary calcium was more prevalent in the MBFR ≤2.0 group (37.5% vs 19.8%, p=0.01), however visually estimated coronary artery calcium score categories did not significantly differ (Table 2).
Table 2.
PET Scan characteristics
| MBFR>2.0 | MBFR≤2.0 | p-value | |
|---|---|---|---|
|
| |||
| Stress Agent | 0.21 | ||
| Adenosine | 3 (2.8) | 2 (2.8) | |
| Dipyridamole | 105 (97.2) | 67 (94.4) | |
| Regadenoson | 0 (0) | 2 (2.8) | |
| Myocardial Blood Flow | |||
| Total Stress Myocardial Blood Flow | 2.32 (2.03–2.62) | 1.47 (1.22–1.69) | <0.0001 |
| Total Rest Myocardial Blood Flow | 0.94 (0.85–1.03) | 0.90 (0.82–1.0) | 0.08 |
| Total Myocardial Flow Reserve | 2.60 (2.20–3.0) | 1.63 (1.35–1.80) | <0.0001 |
| Resting Ejection Fraction | 60.1±7.4 | 57.3±10.4 | 0.06 |
| Stress Ejection Fraction | 63.0±7.0 | 59.4±10.6 | 0.03 |
| Any Ischemia | 6 (5.5) | 12 (16.9) | 0.02 |
| <5% of myocardium | 4 (3.6) | 2 (2.8) | |
| 5–10% of myocardium | 1 (0.9) | 5 (7.0) | |
| >10% of myocardium | 1 (0.9) | 5 (7.0) | |
| Transient ischemic dilation | 0 (0) | 1 (1.4) | 0.21 |
| Coronary Calcium | 0.01 | ||
| No Coronary Calcium | 85 (80.2) | 40 (62.5) | |
| Any Coronary Calcium | 21 (19.8) | 24 (37.5) | |
| VECAC | |||
| 0 | 85 (80.2) | 40 (62.5) | 0.11 |
| 1–99 | 5 (4.7) | 8 (12.5) | |
| 10–99 | 7 (6.6) | 7 (10.9) | |
| 100–399 | 7 (6.6) | 6 (9.4) | |
| 400–999 | 2 (1.9) | 3 (4.7) | |
Data presented as count (%) and median (interquartile range). VECAC=Visually estimated coronary artery calcium
Clinical Outcomes
Patients with an MBFR ≤2.0 experienced more than a 7-fold increased risk of death or retransplantation (HR 7.05, 95% CI 3.2–15.6, p<0.0001, Central Illustration). The survival difference manifested soon after the PET (2-year absolute decrease 9.7%, 95% CI 4.1–14.5%, p<0.0001) and expanded with time (5-year absolute decrease 33.8%, 95% CI 26.5–39.9%, p<0.0001, Figure 2). This translated into a five-year restricted mean survival that was 302.8 days less (95% CI 260.2–345.4 days, p<0.0001, Figure 3). Peak stress myocardial blood flow was equally prognostic (Peak MBF<1.8: HR 7.12, 95% 3.11–16.34, p<0.0001). The risk of cardiovascular hospitalization was ten times greater among those with MBFR ≤2.0 (HR 10.1, 95% CI 3.43–29.9, p<0.0001). Similarly, the risk of cardiovascular death was twelve-fold greater for those with an MBFR ≤2.0 (HR 12.0, 95% CI 2.64–54.12, p=0.001). While a binary MBFR cut-off of 2.0 was thoughtfully selected, the association between MBFR and an increased risk of death or retransplantation demonstrated a gradient of effect. We separated the cohort into tertiles (Figure 4), and when compared with individuals with an MBFR >2.0 patients with an MBFR less than 1.5 had a risk of death or retransplantation that was more than eight times greater (HR 8.45, 95% CI 3.49–20.45, p<0.0001), while individuals with an MBFR between 1.5 and 2.0 had a six-fold increased risk (HR 6.10, 95% CI 2.56–14.59, p<0.0001). When treated as a continuous variable, the risk of death or retransplantation increased 19% (95% CI 1.11–1.27, p<0.0001) for each 0.1 decrease in MBFR.
Central Illustration: Five-year outcomes according to myocardial blood flow reserve.
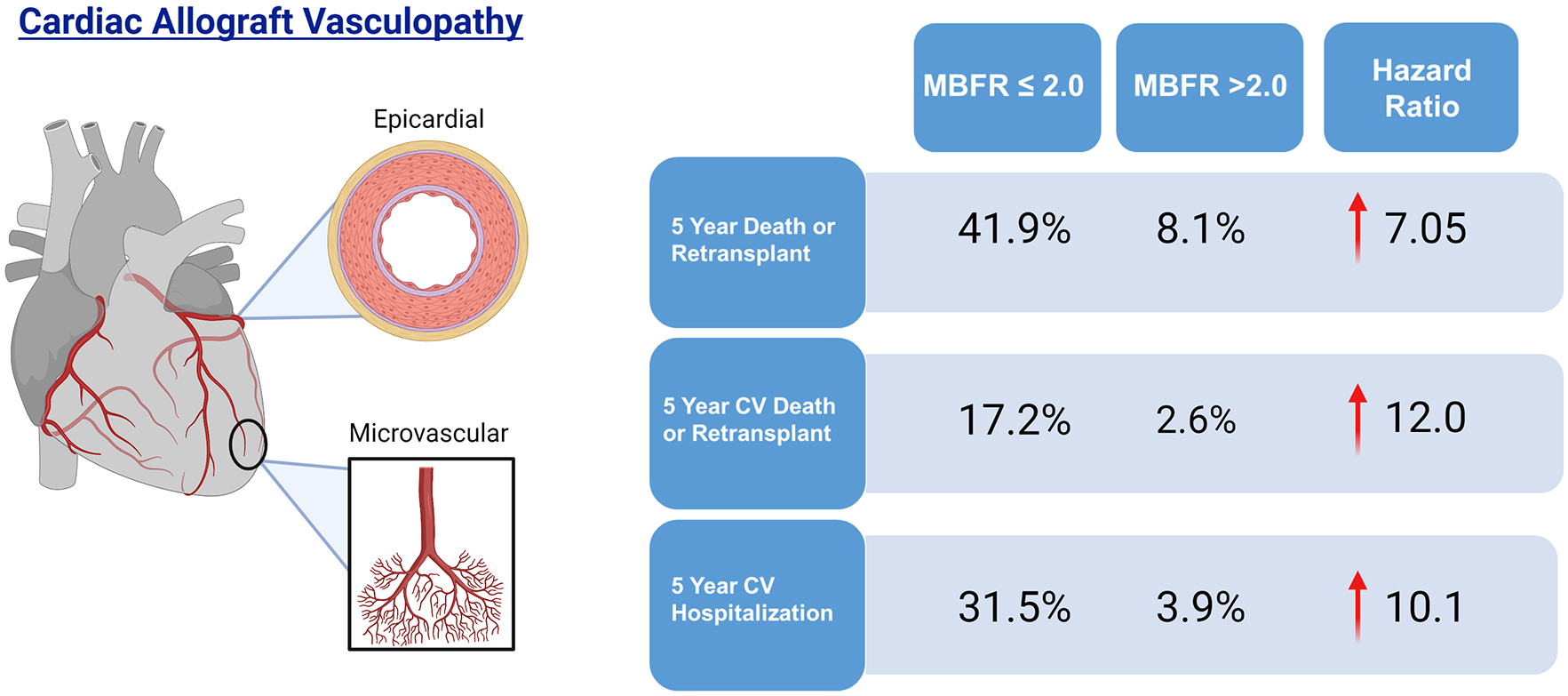
Patients with a myocardial blood flow reserve ≤2.0 have a 7-fold increased 5-year risk of death or retransplantation, 12-fold increased risk of cardiovascular death or retransplantation, and 10-fold increased risk of cardiovascular hospitalization.
Figure 2. Primary Outcome.
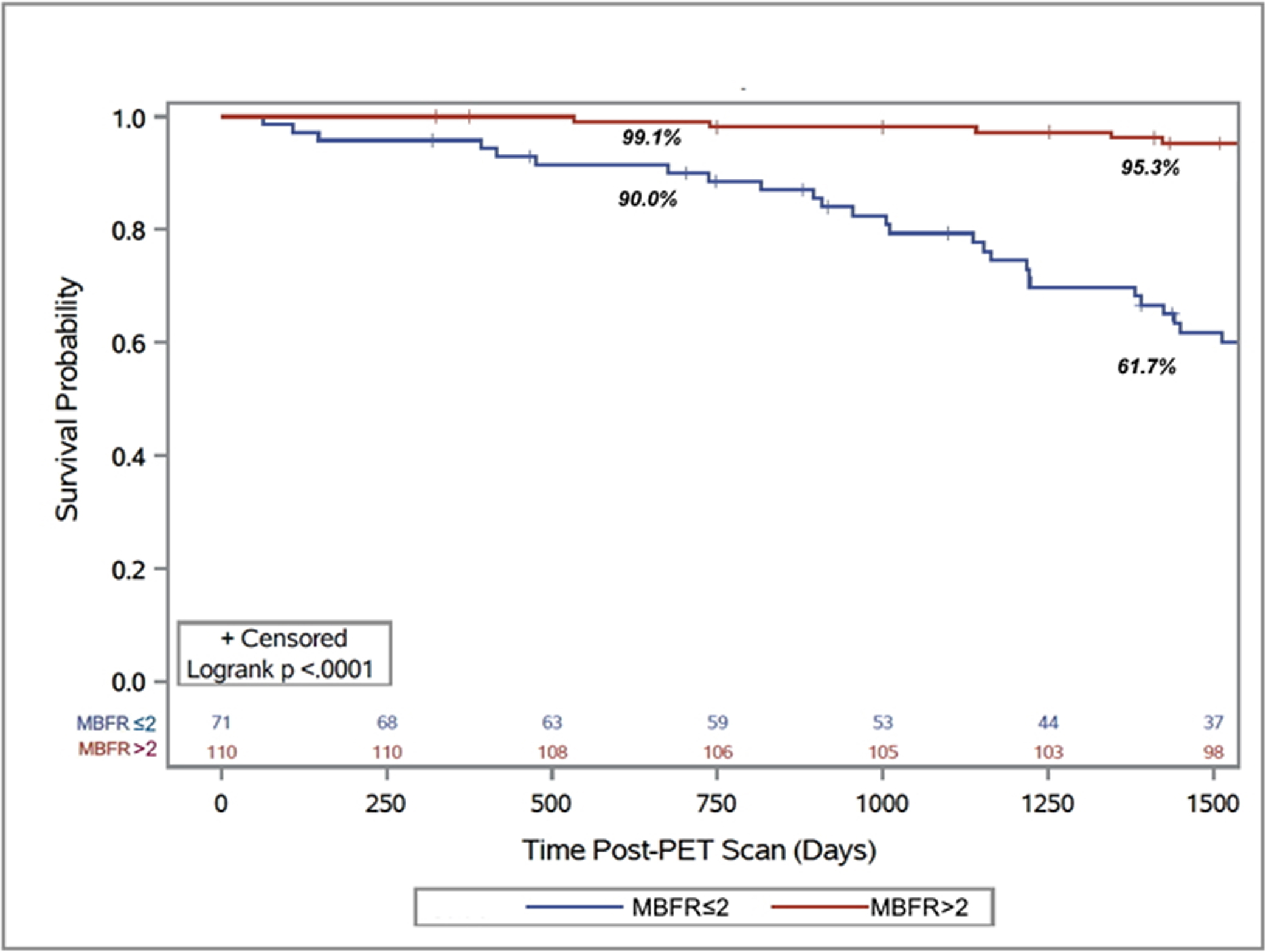
Kaplan-Meier survival curve for post-PET scan freedom from death or retransplantation demonstrating an early decrease in retransplant free survival (2 year: 90.0% vs. 99.1%) and the magnitude of that decrease continued to increase as time post-PET scan progressed (4 year: 61.7% vs. 95.3%).
Figure 3. Differences in restricted mean survival time:
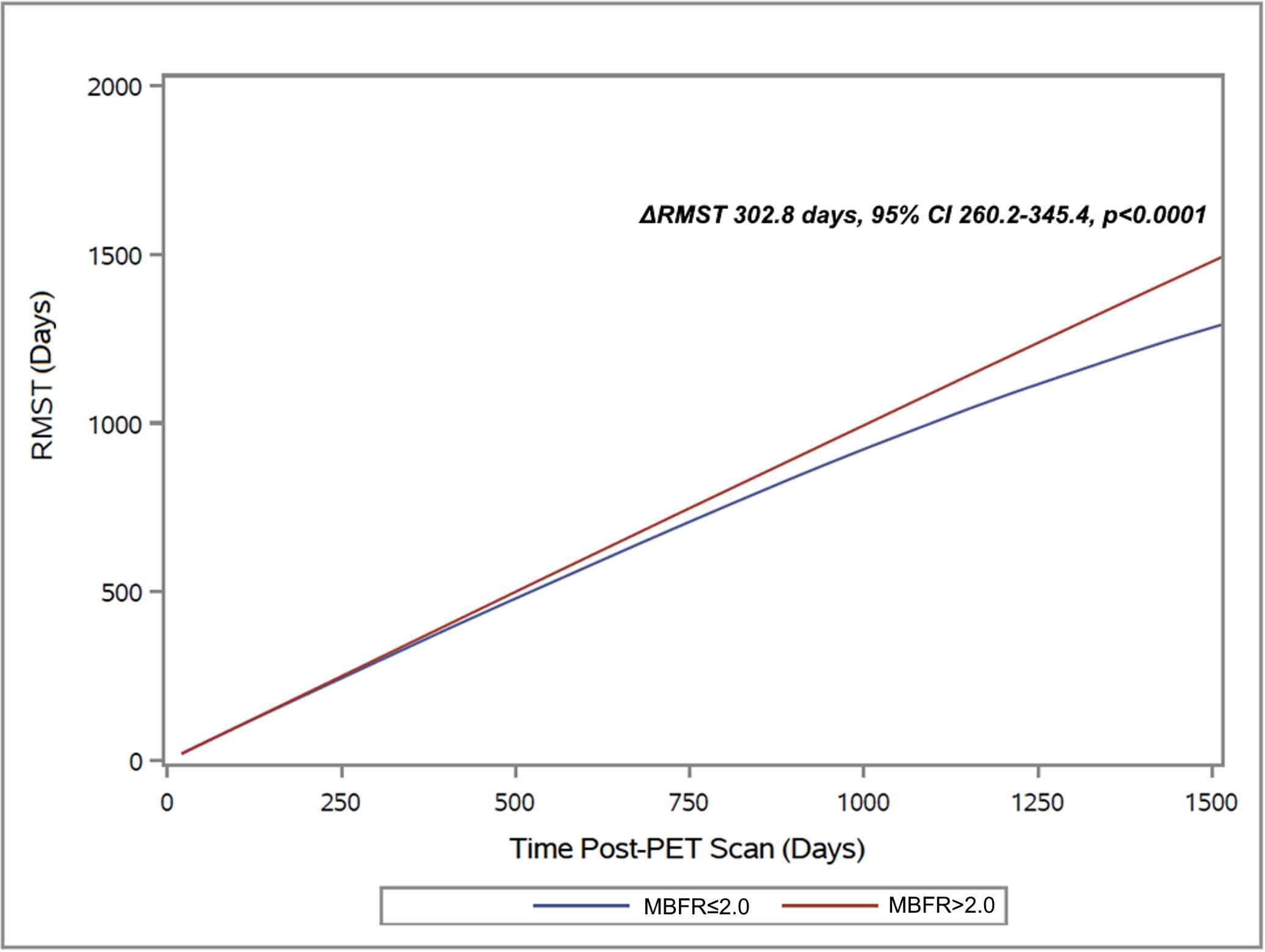
The difference in restricted mean survival time between patients with a MBFR of >2.0 and ≤2.0 began to manifest early and after five-years post-PET scan those with MBFR ≤2.0 survived 302.8 days less.
Figure 4. Prevalence of Death or Retransplantation for Various MBFR Ranges.
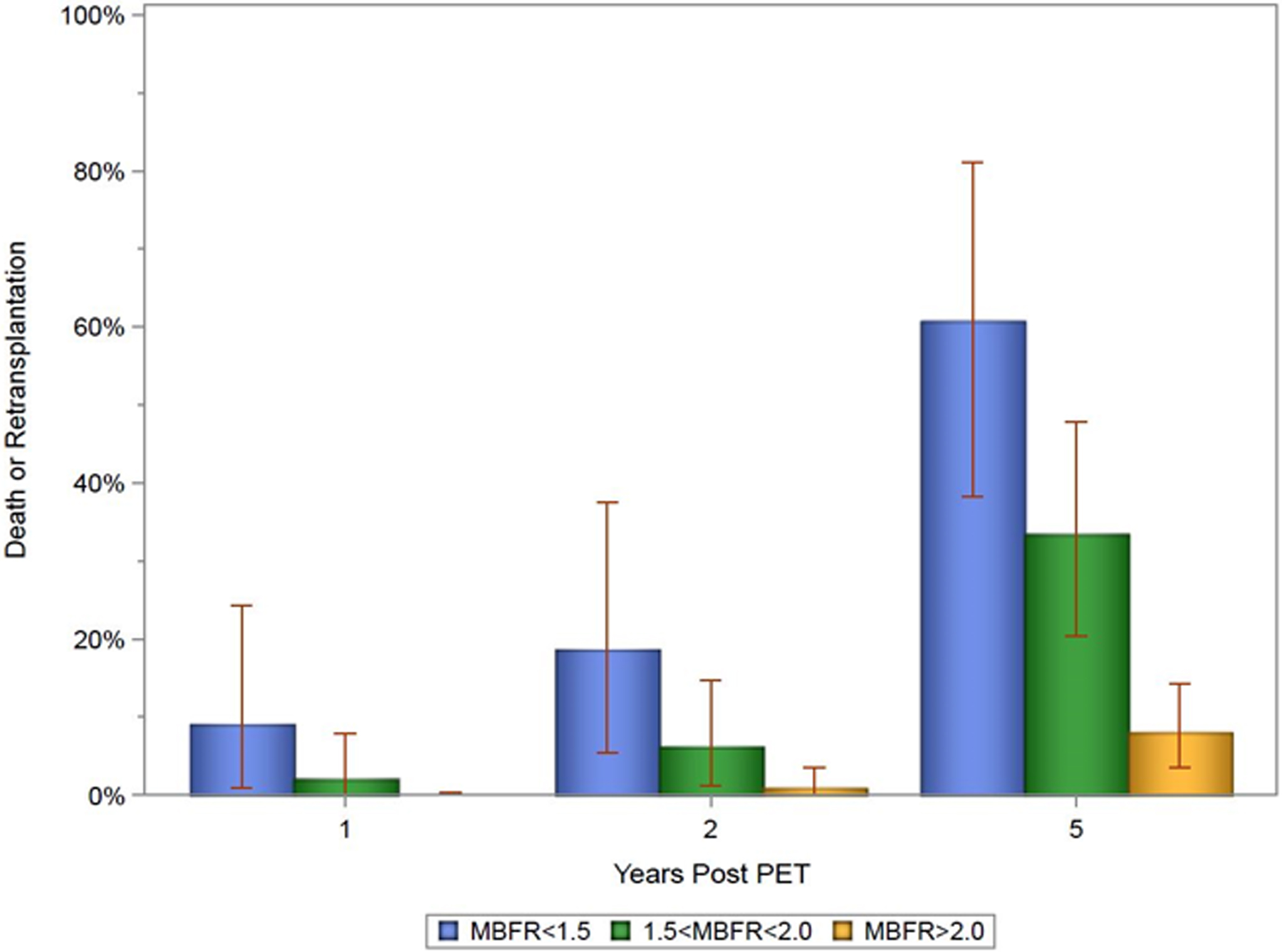
Bar chart exhibiting the prevalence of death or retransplantation, comparing patients with MBFR >2.0, 1.5<MBFR≤2.0, and MBFR≤1.5. This demonstrates a gradient effect as post-PET death or retransplantation increased as MBFR decreased.
There was an interaction between MBFR and time post-transplant (interaction p-value 0.04) therefore we analyzed outcomes by time post-transplant. As an independent variable, time post-transplant was not predictive of death or retransplantation (HR 1.003 per month post-transplant, 95% CI 0.998–1.007, p=0.28). Comparing the early (1- <5 years post-transplant), mid-term (5–10 years post-transplant), and late (10+ years post-transplant) periods post-transplant, an MBFR ≤2.0 portended a poor prognosis at all times (Supplemental Figure 2). Early post-transplant patients (n=53) in the low MBFR group had a mean survival that was 6 months less in the subsequent five years (191.1 days, 95% CI 82.0–300.1, p=0.07), and mid-term patients (n=66) had similar outcomes (187.1 days, 95% CI 72.5–301.8, p=0.03). Among individuals with an MBFR ≤2.0 more than 10 years after transplantation (n=62), the impact was substantial as patients had a mean survival that was 470 days less (95% CI 298.9–640.7, p<0.0001) during the following five years.
Using a multivariable model to adjust for between group differences, MBFR ≤2.0 remained a significant risk factor for death or retransplantation (adjusted HR 4.04, 95% CI 1.72–9.46, p=0.001, Table 3). Similar results were obtained when a multivariable model was generated by backward selection (aHR 3.64, 95% CI 1.52–8.68, p=0.004, Supplemental Table 2). When the outcome was limited to CV death, the magnitude of risk was even greater for those with an MBFR ≤2.0 (aHR 7.45, 95% CI 1.44–38.5, p=0.02) and MBFR ≤2.0 and PET diagnosed ischemia were the only significant predictors after adjustment (Supplemental Table 3).
Outcomes by Sub-Type of CAV
Patients were grouped into four groups: epicardial CAV, microvascular CAV, mixed CAV, and no CAV as described in the Methods section. When PET-only criteria were utilized, individuals with Mixed CAV had the worst outcomes (5-year survival 25.4%, 95% CI 4.2%-56.3%; Time lost 691.0 days, 95% CI 356.9–1,025.0) followed by microvascular CAV (5-year survival 64.4%, 95% CI 51.1%-76.7%; Time lost 282.0 days, 95% CI 163.5–400.5, Figure 5A). Patients with epicardial CAV with an MBFR >2 had outcomes (5-year survival 83.3%, 95% CI 46.5%-99.9%; Time lost 38.5 days, 95% CI −30.4–107.4) that were similar to patients without CAV (5-year survival 92.5%, 95% CI 86.4%-97.0%; Time lost 43.1 days, 95% CI 18.7–36.7). The findings were replicated using angiography to classify epicardial CAV (Figure 5B). Patients with mixed CAV (5-year survival 54.9%, 95% CI 38.9%-70.5%; Time lost 387.7 days, 95% CI 221.4–554.1) and microvascular CAV (5-year survival 62.8%, 95% CI 43.9%-79.9%; Time lost 297.5 days, 95% CI 132.7–462.4) continued to have poor outcomes, while individuals with isolated epicardial CAV (5 year survival 92.2%, 95% CI 81.8%-98.5%; Time lost 35.7 days, 95% CI −6.0–77.3) and patients without CAV (5-year survival 91.6%, 95% CI 83.1%-97.2%; Time lost 47.5 days, 95% CI −2.1–97.1) continued to have similar outcomes.
Figure 5. Freedom from Death or Retransplantation by CAV Subtype:
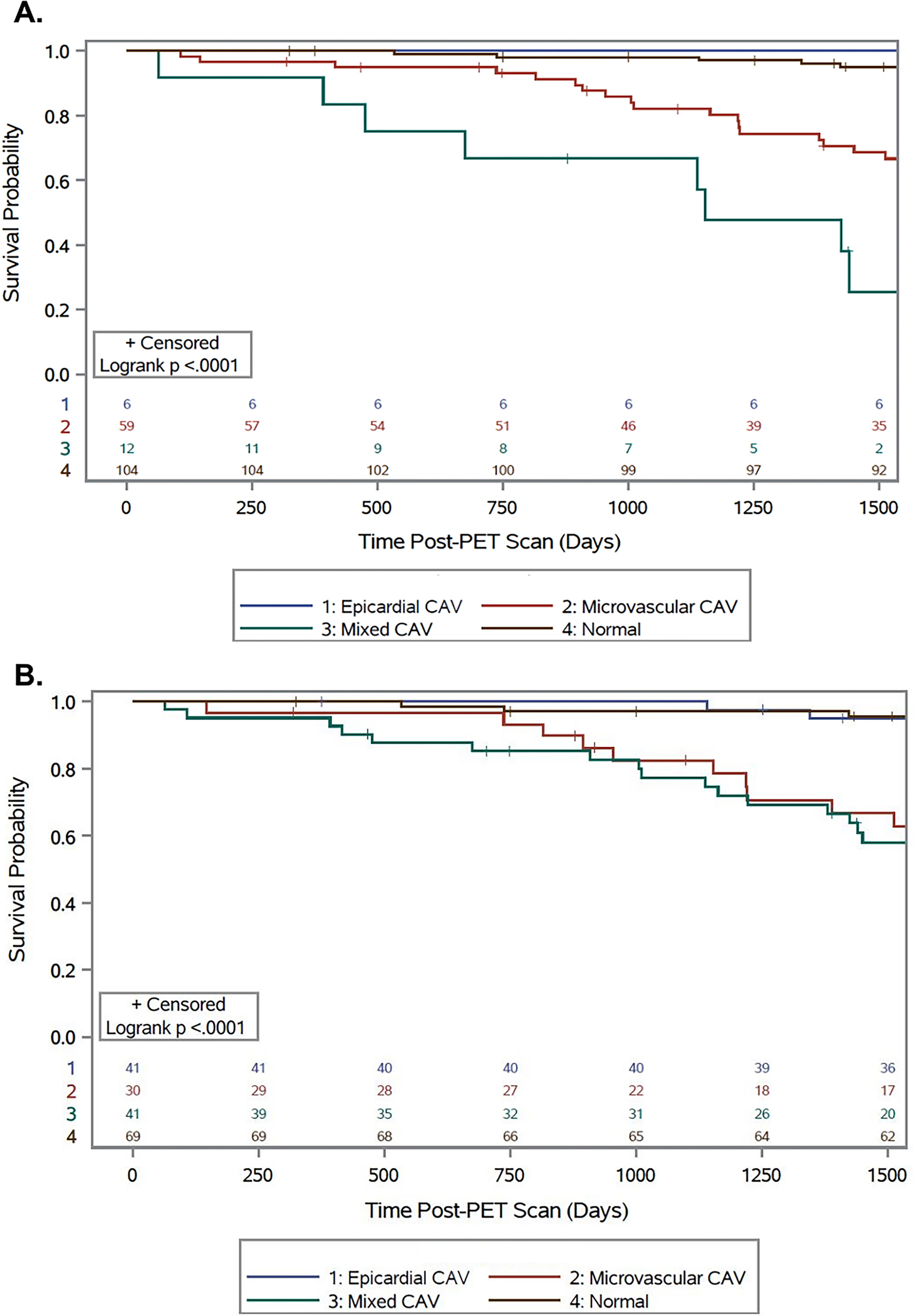
Patient outcomes classified by type of CAV as defined by (A) PET only or (B) PET and coronary angiography. Outcomes were similar by both methods and patients with mixed CAV had the lowest post-PET survival and patients with microvascular CAV also having poor outcomes.
We next assessed if mixed CAV and microvascular CAV remained associated with an increased risk of death or retransplantation in regression with multivariable adjustment. Mixed CAV (HR 7.37, 95% CI 3.31–15.41, p<0.0001) and microvascular CAV (HR 2.96, 95% CI 1.52–5.76, p=0.001) replaced MBFR in a multivariable model that included all covariates listed in Table 3. In this model both microvascular CAV (aHR 3.86, 95% CI 1.58–9.40, p=0.003) and mixed CAV (aHR 11.19, 95% CI 3.57–35.01, p<0.0001) remained substantial risk factors for death or retransplantation.
Cause of Primary Endpoint
The primary study endpoint of death or retransplantation occurred in 10 (9.1%) of those with an MBFR >2.0 and 25 (35.2%) of those with MBFR ≤2.0 (p<0.0001). The causes are listed in Table 4. Cardiovascular causes of death were more common (11 vs. 2, p=0.0007) in the MBFR ≤2.0 group. Among the group with an MBFR >2.0, 80% of the deaths were due to infection or malignancy.
Table 4.
Cause of the primary endpoint
| MBFR>2.0 | MBFR≤2.0 | p-value | |
|---|---|---|---|
|
| |||
| Death or Retransplant (%) | 10 (9.1) | 25 (35.2) | <0.0001 |
| All Cause Death (%) | 10 (9.1) | 22 (31.0) | 0.0001 |
| Cardiovascular Death (%) | 2 (1.8) | 11 (15.5) | 0.0004 |
| Etiology (% of Events) | 0.06 | ||
| Cardiac arrest | 1 (10.0) | 9 (36.0) | |
| Infection | 3 (30.0) | 4 (16.0) | |
| COVID-19 | 1 (10) | 2 (8.0) | |
| MSOF | 0 (0) | 3 (12.0) | |
| Heart Failure | 1 (10) | 2 (8.0) | |
| Malignancy | 5 (50.0) | 2 (8.0) | |
COVID-19=Coronavirus disease 2019, MSOF=Multi-system organ failure
Discussion
Cardiac allograft vasculopathy remains a cause of significant morbidity and mortality following heart transplantation and requires assiduous screening. Anatomic assessment of CAV has long been the standard, however this study assessed the addition of non-invasive physiologic measures and demonstrated: 1) Patients with an MBFR ≤2 had a 7-fold increased risk of death or retransplantation over the subsequent five years, 2) Microvascular CAV was independently associated with death or retransplantation, 3) Reduced MBFR was associated with an increased risk of death or retransplantation at all times post-transplant, but especially after 10 years, 4) Patients with mixed CAV had the greatest risk of death or retransplantation, and 5) Patients with MBFR >2.0 had excellent survival (one year 100%, five year 92%).
Coronary angiography has been performed since the 1960s and was adopted as the method to screen for CAV after it was appreciated to be a nefarious post-transplant complication as early as 1969.18,19 The introduction of intravascular ultrasound in 199220 furthered the understanding of the underlying pathophysiology of CAV and appreciation that CAV may develop early following heart transplantation. Much like the diagnosis of coronary atherosclerosis, which evolved from angiography and stenosis severity to include intravascular imaging and now physiologic measurements (e.g. fractional flow reserve (FFR), resting full-cycle ratio, instantaneous wave-free ratio), physiology is now being explored for the diagnosis of CAV. Invasive pressure-temperature sensor guidewire assessment of intracoronary physiology has demonstrated increased mortality associated with a reduced FFR or evidence of microvascular disease.5 More recently, evidence of epicardial CAV (FFR<0.80 in LAD) and microvascular disease (coronary flow reserve (CFR) ≤2.0 or index of microcirculatory resistance (IMR) ≥25) at one year post-transplant has been shown be associated a 3-fold and 2-fold increased risk of death or retransplantation after 10 years. Non-invasive measurements of MBFR (the non-invasive counterpart of CFR) have been associated with poor outcomes. In a study of patients who were a median of 9 years post-transplant, McArdle et al. demonstrated using rubidium-82 PET that individuals at a median of 8.2 years since transplantation with a reduced MBFR (<1.75) had a 441% increased risk of MACE (14 MACE events) during 1.5 years of follow-up. A smaller study of 89 patients who were, on average, 7 years from transplant found that an MBFR<1.5 was associated with a 3-fold increased risk of death (40 deaths).8 Here we report the results of our study, in which we used a different MBFR cut-off (≤2.0) and had a longer follow-up time of nearly five years. Our results support the previously published data and highlight the continuous hazard of reduced MBFR post-transplant. In our study, when MBFR was considered as a continuous variable there was a consistent decrement in survival (19% increased risk of death or retransplant for each 0.1 decrease in MBFR) and patients with an MBFR of <1.5 had a more than 8-fold increased risk of death or re-transplantation. Additionally technical differences between the extraction fraction of 82Rb (used in the two other studies) and 13N-ammonia (this study) and vasodilators (one utilized regadenoson, which has reduced hyperemia compared with dipyridamole) may have also contributed.21,22 Nevertheless, the findings are consistent across a range of reduced MBFR cut-offs and irrespective of the radiotracer used, highlighting the generalizability of these findings and this imaging modality.
CAV is known to be a pan-arterial disease with involvement of both the epicardial arteries and the microvasculature. The modalities recommended in the ISHLT guidelines to diagnose CAV (invasive: coronary angiography, intravascular imaging may be considered; non-invasive: dobutamine stress echocardiography, coronary computed tomography angiography) do not allow for careful assessment of the microvasculature. Pathologic studies have demonstrated that stenotic microvascular disease was associated with nearly 3-year reduction in post-transplant survival, independent of epicardial CAV.4 Invasively measured microvascular CAV or dysfunction has been shown to portend a poor short-term prognosis when elevated at one year after transplantation.23 A more recent multicenter study including 254 patients found that invasively measured microvascular disease at 1 year post-transplant, defined as a CFR ≤2.0 or IMR of ≥25 in the absence of significant epicardial disease, resulted in a 233% increased risk of death or retransplantation after 10 years of follow-up. These findings, in a population different from this study, in some respects parallel the findings of our study where isolated microvascular CAV was associated with nearly a 4-fold increased risk of death or retransplantation after 5 years. This further highlights the importance of assessing the microvasculature as microvascular CAV, whether detected on pathology, via invasive pressure-temperature sensor guidewire assessment, or PET, has been consistently associated with an increased risk of death or retransplantation, independent of epicardial CAV.6
The prevalence of CAV increases with time post-transplantation (30–45% at 5 years, 50–65% at 10 years) and it also becomes a leading cause of death as time progresses.1 In this study, patients ranged from 1 year to 28 years post-transplant (75% within the first 11 years) and MBFR was prognostic at each time point. Even early reductions in MBFR (<5 years since transplant) carry significant consequence, as patients with a MBFR ≤2.0 had three times the risk and a 21% absolute increase in the risk of death or retransplantation in the next five years. Put a different way, these patients had 6 less months alive during the five years follow-up than those with a MBFR >2.0. An alternative and more optimistic way of looking at these data is that a MBFR>2.0 portends an excellent prognosis: one-year survival of 100%, two-year survival of 99.1% and 92% five-year survival.
There are several limitations to our study that are important to acknowledge. The first is that this is a single center, non-randomized cohort study of consecutive patients who underwent a PET scan, and while the findings are consistent with other external cohorts, the findings may differ among different populations. Next is that while the study included 181 heart transplant recipients and is the largest to evaluate PET derived MBFR, the sample size remains modest. As such, multivariable models carried the risk of overfitting, however consistent results across multiple model building techniques helped to assuage those concerns. Third, there is no codified definition of microvascular CAV. We utilized a definition that was consistent with other studies 6,12 and the findings were similar whether angiography or PET was used to define epicardial CAV, however this definition requires further validation. Furthermore, intravascular ultrasound (IVUS) was not performed on each patient limiting the comparison of IVUS with MBFR, however recent data have shown the importance of physiology even when accounting for IVUS.6 Since angiography and PET scans were not contemporaneous, the inclusion of angiograms that were performed up to one year after the PET scan may have inflated the influence of angiographic measures given the progressive nature of CAV. Lastly, the role of the immunosuppression protocol on MBFR can’t be fully assess in this study and will need to be evaluate in a prospective randomized study.
Conclusion
Reduced myocardial blood flow reserve on 13N-ammonia PET was associated with an increased risk of death or retransplantation, irrespective of the time since transplantation. Patients with epicardial CAV and reduced MBFR had the greatest risk of death or retransplantation. Microvascular CAV was associated with an increased risk of death or retransplantation, independent of epicardial CAV, and warrants screening.
Supplementary Material
Supplemental Figure 1 Receiver operator characteristic curve: Receiver operator characteristic curve to identify the ideal myocardial blood flow reserve cut-off of 1.96 to predict death or retransplantation.
Supplemental Figure 2. Restricted mean survival time differentiated by time post-transplant: Restricted mean survival differences between patients with a MFR of >2.0 and ≤2.0 stratified by time post-transplant demonstrating that patients with a MBFR ≤2.0 had reduced survival when (A) 1-5 years post-transplant, (B) 5-10 years post-transplant, and (C) 10+ years post-transplant.
Supplemental Table 1. Univariate and multivariable models (including MBFR≤2.0 and the individual univariate predictor) for individual hemodynamic parameters for the association with death or retransplantation.
Supplemental Table 2. Multivariable model for death or retransplantation using backward selection.
Supplemental Table 3. Multivariable model for cardiovascular mortality
Clinical Perspectives.
Competency in Patient Care:
In patients who have undergone heart transplantation, non-invasive identification of isolated microvascular allograft vasculopathy is associated with a poor prognosis.
Translational Outlook:
Future research will explore interventions targeted at improving myocardial blood flow in patients with cardiac allograft vasculopathy.
Acknowledgements:
The authors would like to thank Dr. Sabahat Bokhari for his contribution to this project.
Funding:
KJC has been supported by NIH K23 HL148528.
Abbreviations:
- CAV
Cardiac allograft vasculopathy
- CFR
Coronary flow reserve
- HT
Heart transplant
- ISHLT
International Society for Heart and Lung Transplantation
- IVUS
Intravascular ultrasound
- FFR
Fractional flow reserve
- MBFR
Myocardial blood flow reserve
- PET
Positron emission tomography
Footnotes
Disclosures: VKT has been supported by NIH K08 HL146964. AJE received speaker’s fee from Ionetix, consulting fees from W. L. Gore & Associates; institution has grants/grants pending from Canon Medical Systems, GE Healthcare, Roche Medical Systems, and W. L. Gore & Associates. PCC has received consulting fees from Abbott. The remaining authors have nothing to disclose.
References:
- 1.Khush KK, Hsich E, Potena L, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult heart transplantation report 2021; Focus on recipient characteristics. J Heart Lung Transplant 2021;40(10):1035–1049. DOI: 10.1016/j.healun.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal S, Parashar A, Kapadia SR, et al. Long-term mortality after cardiac allograft vasculopathy: implications of percutaneous intervention. JACC Heart Fail 2014;2(3):281–8. DOI: 10.1016/j.jchf.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Costanzo MR, Costanzo MR, Dipchand A, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant 2010;29(8):914–956. DOI: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 4.Hiemann NE, Wellnhofer E, Knosalla C, et al. Prognostic impact of microvasculopathy on survival after heart transplantation: evidence from 9713 endomyocardial biopsies. Circulation 2007;116(11):1274–82. DOI: 10.1161/circulationaha.106.647149. [DOI] [PubMed] [Google Scholar]
- 5.Yang HM, Khush K, Luikart H, et al. Invasive Assessment of Coronary Physiology Predicts Late Mortality After Heart Transplantation. Circulation 2016;133(20):1945–50. (In eng). DOI: 10.1161/circulationaha.115.018741. [DOI] [PubMed] [Google Scholar]
- 6.Ahn J-M, Zimmermann FM, Arora S, et al. Prognostic value of comprehensive intracoronary physiology assessment early after heart transplantation. Eur Heart J 2021;42(48):4918–4929. DOI: 10.1093/eurheartj/ehab568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ardle BAM, Davies RA, Chen L, et al. Prognostic Value of Rubidium-82 Positron Emission Tomography in Patients After Heart Transplant. Circ Cardiovasc Imaging 2014;7(6):930–937. DOI: doi: 10.1161/CIRCIMAGING.114.002184. [DOI] [PubMed] [Google Scholar]
- 8.Feher A, Srivastava A, Quail MA, et al. Serial Assessment of Coronary Flow Reserve by Rubidium-82 Positron Emission Tomography Predicts Mortality in Heart Transplant Recipients. JACC Cardiovasc Imaging 2020;13:109–120. DOI: doi: 10.1016/j.jcmg.2018.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chirakarnjanakorn S, Starling RC, Popović ZB, Griffin BP, Desai MY. Dobutamine stress echocardiography during follow-up surveillance in heart transplant patients: Diagnostic accuracy and predictors of outcomes. J Heart Lung Transplant 2015;34(5):710–717. DOI: 10.1016/j.healun.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Clerkin KJ, Farr MA, Restaino SW, Ali ZA, Mancini DM. Dobutamine stress echocardiography is inadequate to detect early cardiac allograft vasculopathy. J Heart Lung Transplant 2016;35(8):1040–1041. DOI: 10.1016/j.healun.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehra MR, Crespo-Leiro MG, Dipchand A, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant 2010;29(7):717–27. DOI: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 2018;250:16–20. DOI: 10.1016/j.ijcard.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 13.Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014;129(24):2518–27. DOI: 10.1161/circulationaha.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziadi MC, Dekemp RA, Williams KA, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58(7):740–8. DOI: 10.1016/j.jacc.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 15.Paz Y, Morgenstern R, Weinberg R, et al. Relation of Coronary Flow Reserve to Other Findings on Positron Emission Tomography Myocardial Perfusion Imaging and Left Heart Catheterization in Patients With End-stage Renal Disease Being Evaluated for Kidney Transplant. Am J Cardiol 2017;120(11):1909–1912. DOI: 10.1016/j.amjcard.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Einstein AJ, Johnson LL, Bokhari S, et al. Agreement of visual estimation of coronary artery calcium from low-dose CT attenuation correction scans in hybrid PET/CT and SPECT/CT with standard Agatston score. J Am Coll Cardiol 2010;56(23):1914–21. (In eng). DOI: 10.1016/j.jacc.2010.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirshfeld John W, Ferrari Victor A, Bengel Frank M, et al. 2018 ACC/HRS/NASCI/SCAI/SCCT Expert Consensus Document on Optimal Use of Ionizing Radiation in Cardiovascular Imaging: Best Practices for Safety and Effectiveness. J Am Coll Cardiol 2018;71(24):e283–e351. DOI: 10.1016/j.jacc.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Bieber CP, Stinson EB, Shumway NE, Payne R, Kosek J. Cardiac transplantation in man. VII. Cardiac allograft pathology. Circulation 1970;41(5):753–72. DOI: 10.1161/01.cir.41.5.753. [DOI] [PubMed] [Google Scholar]
- 19.Thomson JG. PRODUCTION OF SEVERE ATHEROMA IN A TRANSPLANTED HUMAN HEART. The Lancet 1969;294(7630):1088–1092. DOI: 10.1016/S0140-6736(69)90700-4. [DOI] [PubMed] [Google Scholar]
- 20.Goar FGS, Pinto FJ, Alderman EL, et al. Intracoronary ultrasound in cardiac transplant recipients. In vivo evidence of “angiographically silent” intimal thickening. Circulation 1992;85(3):979–987. DOI: doi: 10.1161/01.CIR.85.3.979. [DOI] [PubMed] [Google Scholar]
- 21.Murthy VL, Bateman TM, Beanlands RS, et al. Clinical Quantification of Myocardial Blood Flow Using PET: Joint Position Paper of the SNMMI Cardiovascular Council and the ASNC. J Nucl Med 2018;59(2):273–293. DOI: 10.2967/jnumed.117.201368. [DOI] [PubMed] [Google Scholar]
- 22.Johnson NP, Gould KL. Regadenoson versus dipyridamole hyperemia for cardiac PET imaging. JACC Cardiovasc Imaging 2015;8(4):438–447. DOI: 10.1016/j.jcmg.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Yang H-M, Khush K, Luikart H, et al. Invasive Assessment of Coronary Physiology Predicts Late Mortality After Heart Transplantation. Circulation 2016;133(20):1945–1950. DOI: doi: 10.1161/CIRCULATIONAHA.115.018741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Receiver operator characteristic curve: Receiver operator characteristic curve to identify the ideal myocardial blood flow reserve cut-off of 1.96 to predict death or retransplantation.
Supplemental Figure 2. Restricted mean survival time differentiated by time post-transplant: Restricted mean survival differences between patients with a MFR of >2.0 and ≤2.0 stratified by time post-transplant demonstrating that patients with a MBFR ≤2.0 had reduced survival when (A) 1-5 years post-transplant, (B) 5-10 years post-transplant, and (C) 10+ years post-transplant.
Supplemental Table 1. Univariate and multivariable models (including MBFR≤2.0 and the individual univariate predictor) for individual hemodynamic parameters for the association with death or retransplantation.
Supplemental Table 2. Multivariable model for death or retransplantation using backward selection.
Supplemental Table 3. Multivariable model for cardiovascular mortality


