Abstract
Objectives
In this study, we explored how adiponectin mediated urotensin II (UII)-induced tumor necrosis factor-α (TNF-α) and α-smooth muscle actin (α-SMA) expression and ensuing intracellular signaling pathways in adventitial fibroblasts (AFs).
Methods
Growth-arrested AFs and rat tunica adventitia of vessels were incubated with UII and inhibitors of signal transduction pathways for 1‒24 h. The cells were then harvested for TNF-α receptor (TNF-α-R) messenger RNA (mRNA) and TNF-α protein expression determination by reverse transcription-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA), respectively. Adiponectin and adiponectin receptor (adipoR) expression was measured by RT-PCR, quantitative real-time PCR (qPCR), immunohistochemical analysis, and cell counting kit-8 (CCK-8) cell proliferation experiments. We then quantified TNF-α and α-SMA mRNA and protein expression levels by qPCR and immunofluorescence (IF) staining. RNA interference (RNAi) was used to explore the function of the adipoR genes. To investigate the signaling pathway, we applied western blotting (WB) to examine phosphorylation of adenosine 5'-monophosphate (AMP)-activated protein kinase (AMPK). In vivo, an adiponectin (APN)-knockout (APN-KO) mouse model mimicking adventitial inflammation was generated to measure TNF-α and α-SMA expression by application of qPCR and IF, with the goal of gaining a comprehensive atlas of adiponectin in vascular remodeling.
Results
In both cells and tissues, UII promoted TNF-α protein and TNF-α-R secretion in a dose- and time-dependent manner via Rho/protein kinase C (PKC) pathway. We detected marked expression of adipoR1, T-cadherin, and calreticulin as well as a moderate presence of adipoR2 in AFs, while no adiponectin was observed. Globular adiponectin (gAd) fostered the growth of AFs, and acted in concert with UII to induce α-SMA and TNF-α through the adipoR1/T-cadherin/calreticulin/AMPK pathway. In AFs, gAd and UII synergistically induced AMPK phosphorylation. In the adventitial inflammation model, APN deficiency up-regulated the expression of α-SMA, UII receptor (UT), and UII while inhibiting TNF-α expression.
Conclusions
From the results of our study, we can speculate that UII induces TNF-α protein and TNF-α-R secretion in AFs and rat tunica adventitia of vessels via the Rho and PKC signal transduction pathways. Thus, it is plausible that adiponectin is a major player in adventitial progression and could serve as a novel therapeutic target for cardiovascular disease administration.
Keywords: Urotensin II, Adiponectin, Signal transduction, Adventitial fibroblast, RNA interference (RNAi), Adiponectin-knockout (APN-KO)
1. Introduction
Cardiovascular diseases are essentially a chronic, persistent, low-degree inflammation. During inflammation, the vascular adventitia functions as a participant and mediator (Li and Glass, 2002; Tinajero and Gotlieb, 2020). Adventitial fibroblasts (AFs) represent the most pivotal cell clusters in adventitia. After intrinsic injury or vasoactive peptide stimulation, quiescent AFs are activated and further differentiate into myofibroblasts which are able to produce a wide array of cytokines and molecules including interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1), transforming growth factor-β1 (TGF-β1), and leukotriene C4 (LTC4), which are involved in inflammation and vascular remodeling (Zhang et al., 2008a, 2008b; Enzerink and Vaheri, 2011; Dong et al., 2013; Lu et al., 2019). The physiological and pathophysiological roles of AFs in adventitial inflammation, and thus their involvement in cardiovascular disease, remain elusive.
Urotensin II (UII) is a vasoactive peptide that causes vasoconstriction. UII and its receptor (UT) are primarily expressed in cardiovascular tissues such as AFs, cardiomyocytes, vascular smooth muscle cells (VSMCs), and endothelial cells (Ames et al., 1999). In addition to its vasoconstrictor activity, UII exerts multiple effects on cell proliferation, migration, hypertrophy, apoptosis, and fibrosis, and triggers inflammatory responses in various cell types (Avagimyan et al., 2022). Research has evidenced the upregulation of UII and UT in cardiovascular disease and metabolic syndrome (Pereira-Castro et al., 2019). In diabetic apolipoprotein-E knockout (Apo-E KO) mice, UT antagonism significantly attenuated diabetes-associated atherosclerosis (Watson et al., 2013). Aside from its role in cardiovascular disease, UII is critical in various disease settings (Ames et al., 1999). Hassan et al. (2005) found that increased expression of UII in coronary atherosclerosis was associated with subendothelial inflammation. Furthermore, UII was able to induce IL-1β and IL-6 expression in human umbilical vein endothelial cells and monocytes (Park et al., 2013).
Predominantly secreted by adipose tissues (Sowka and Dobrzyn, 2021), adiponectin is an adipokine which accounts for 0.01% of human plasma proteins (Lau et al., 2017). The globular function domain, recognized as the globular adiponectin (gAd), derived from full-length adiponectin (fAd) by proteolytic cleavage, has displayed independent and enhanced biological activities in relation to fAd (Bobbert et al., 2008; Zhang et al., 2013). Adiponectin is widely expressed in different organs and tissues, and by interacting with specific cell-surface receptors and adiponectin receptors (adipoRs), it is able to carry out pleotropic functions (Yamauchi et al., 2003). Recently, studies have shown that adiponectin is crucial in regulating immunity and inflammation (Rami et al., 2022), and is associated with the development of atherosclerosis, hypertension, and coronary heart disease (Liu et al., 2022). Though the adventitial expression and underlying effects of adiponectin and its receptors remain elusive, we could conclude from current bodies of work that adiponectin is involved in the pathogenesis of vascular inflammation.
In this study, we examined tumor necrosis factor-α (TNF-α) and TNF-α receptor (TNF-α-R) messenger RNA (mRNA) expression in AFs as well as tunica adventitia of vessels in rats, and sought to identify the underlying molecular mechanisms in UII-initiated inflammatory response. We also compared the expression levels of adiponectin and its receptors in different rat tissues. To investigate the association between adiponectin and UII, we explored the proliferation, cytokine production, and phenotypic conversion of in vitro cultured AFs. Lastly, in an adventitial inflammation model of an adiponectin (APN)-knockout (APN-KO) mouse, we investigated whether adiponectin deficiency directly affected TNF-α and α-smooth muscle actin (α-SMA) expression.
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats weighing 180–200 g and male C57BL/6 mice (seven weeks) were provided by the Animal Center of the Health Science Center at Peking University (Beijing, China). The Shanghai Nanfang Laboratory for Model Organisms (Shanghai, China) generated APN-KO mice (whole-body knockout).
2.2. Adventitial inflammation model
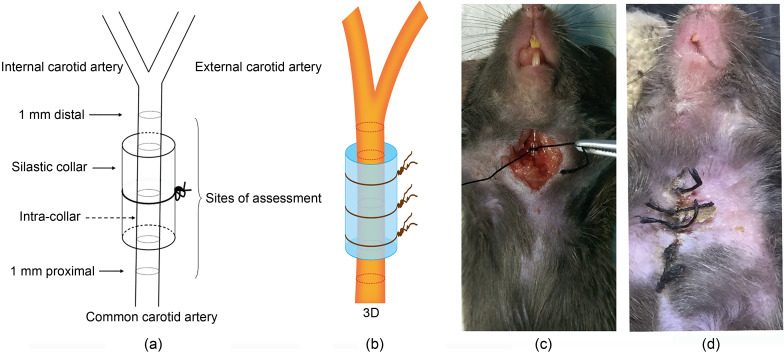
We engineered an adventitial inflammation model according to an established method. The model was modified by utilizing perivascular constrictive silica collars (length 3.0 mm, inside diameter 0.3 mm) placed on the right common carotid arteries of the mice (von der Thüsen et al., 2001). A total of 24 mice were divided into four groups (n=6) as follows: normal mice group, normal mice surgery group, APN-KO mice group, and APN-KO mice surgery group. All mice were fed with a normal diet and housed at a constant temperature (24 ℃) under a 12-h dark/light cycle. After 6 d, mice were sacrificed; the silica collars were carefully removed from the right common carotid arteries, and carotid arteries were excised for further research (Fig. 1). Eye blood was obtained from each mouse, and used to measure peripheral cytokine secretion.
Fig. 1. Schematic representation of adventitial inflammation model and assessment sites. (a) Schematic diagram of model; (b) Three dimensional (3D) display; (c) The procedure: incise the skin of the mouse's neck, and bluntly separate the subcutaneous tissue, expose the carotid artery, trap a silicone tube over the carotid artery and then use surgical stitches to fix it further, and then suture the skin layer by layer; (d) The appearance of the skin after successful molding.
2.3. Reagents
Rat UII and adiponectin were purchased from Phoenix Pharmaceuticals Inc. (Belmont, CA, USA). Antibodies against adipoR1, adipoR2, T-cadherin, calreticulin, and α-SMA were purchased from Abcam (Cambridge, UK), and anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH) was purchased from Kangcheng (Shanghai, China). Antibodies against adenosine 5'-monophosphate-activated protein kinase (AMPK) and phospho-AMPK (p-AMPK) were obtained from Cell Signaling Technology (Beverly, MA, USA) and compound C was purchased from Calbiochem (Merck Kgaa, Darmstadt, Germany). Cyclosporin A (CSA) and nicardipine were obtained from Sigma (St. Louis, MO, USA).
Signal transduction inhibitors PD98059, Y-27632, and H-7 were acquired from Calbiochem (Darmstadt, Germany); High-Capacity complementary DNA (cDNA) Reverse Transcription Kits and SYBR Select Master Mix were purchased from Applied Biosystems (Foster City, CA, USA); polymerase chain reaction (PCR) primers were designed and synthesized by Sangon Biotech (Shanghai, China), and the sequences are shown in Tables S1 and S2. All other chemicals and reagents were of analytical grade.
2.4. Tissue incubation
Rats were first anesthetized with pentobarbital and then rapidly decapitated. The full length of the thoraco-abdominal aorta was excised under aseptic conditions and quenched in 4 ℃ pre-cold Krebs-Henseleit (KH). The longitudinally-sectioned aorta was removed from collateral vessels. After gently rubbing the lumen with the blunt side of dissecting scissors, endothelial cells were successfully removed. By peeling off the medial layer using two forceps, the adventitia was acquired and then cut into small sections. After careful weighing (approximately 100 mg per tube), adventitia pieces were placed into 1.5-mL Eppendorf tubes and kept in different intervention conditions at 37 ℃ in a cell incubator.
According to the study design, tissues were handled in three ways: (1) control groups, tissues incubated in serum-free Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 (DMEM/F12)-only; (2) UII groups, serum-free medium containing 1×10-10–1×10-6 mol/L UII; (3) UII+inhibitor groups, tissues pretreated with different signal transduction blockers (at a concentration of 1×10-5 mol/L), including nicardipine, PD98059, H7, CSA, and Y-27632, in serum-free medium in 0.5 h prior to additional supplementation with UII. The samples were collected after a 24-h-UII incubation for further research.
2.5. Cell culture
The AFs were cultured according to the methods described by Zhang et al. (2008b). To determine the TNF-α protein and TNF-α-R mRNA expression, AFs were treated with different concentrations of UII (1×10-10–1×10-7 mol/L) for 1‒24 h to test the molecular preference. AFs were preincubated with the various signal transduction pathway blockers for 30 min with UII. Group intervention remained the same as indicated above.
2.6. RNA interference for adipoRs
Due to low expression of adipoR2, we used RNA interference (RNAi) to down-regulate the expression of adipoRs. AdipoR1/T-cadherin/calreticulin and negative control used in small interference RNA (siRNA) were designed and synthesized by the Genepharma Biotechnology Company (Shanghai, China). The sequences are listed in Table S3.
2.7. RT-PCR and qPCR analyses
We extracted total RNA from AFs or tissues using TRIzol (Thermo Fisher Scientific, Waltham, MA, USA), and quantified RNA concentration using a spectrophotometer (NanoDrop2000, thermo, USA). cDNA was synthesized using High-Capacity cDNA Reverse Transcription Kits. PCR primer sequences are listed in Table S2.
2.8. Western blot analysis
Western blotting was carried out according to established methods (Zhang et al., 2008b). We used primary antibodies at 1:1000 (volume ratio, the same below) dilution: anti-adipoR1, anti-adipoR2, anti-T-cadherin, anti-calreticulin, anti-α-SMA, anti-AMPK, and anti-p-AMPK. The control plot was detected with an anti-GAPDH antibody (1:2000).
2.9. Immunofluorescence staining
Immunofluorescence (IF) staining was done according to the instructions in the kit. First, we incubated the fibroblasts with the following primary antibodies: adipoR1 (1:250), adipoR2 (1:250), T-cadherin (1:250), and calreticulin (1:250). We then processed the images with fluorescence microscopy (Olympus, Tokyo, Japan) and measured the integrated optical density (IOD) of immunostaining in at least five randomly picked high-magnification fields using Image-Pro Plus 5.0 (Media Cybernetics, Maryland, USA).
2.10. Immunohistochemistry and hematoxylin-eosin staining
We followed the usual procedure to process the samples, using primary antibodies against adipoR1 (1:50), adipoR2 (1:400), T-cadherin (1:500), and calreticulin (1:2000).
2.11. Cell proliferation assay
Cell proliferation was detected using a cell counting kit-8 (CCK-8; Beyotime, Shanghai, China) according to the manufacturer’s instructions.
2.12. ELISA
A TNF-α enzyme-linked immunosorbent assay (ELISA) kit was purchased from Sangon Biotech and TNF-α concentration was measured according to the manufacturer’s instructions.
2.13. Statistical analysis
Data are expressed as mean±standard error of the mean (SEM). Statistical differences were analyzed by one-way analysis of variance (ANOVA), Dunnett’s tests, or t-test, as indicated. All data were obtained using GraphPad Prism 5.0 statistical software (San Diego, CA, USA). P<0.05 was considered statistically significant.
3. Results
3.1. Regulation of TNF-α-R mRNA expression by AF and UII
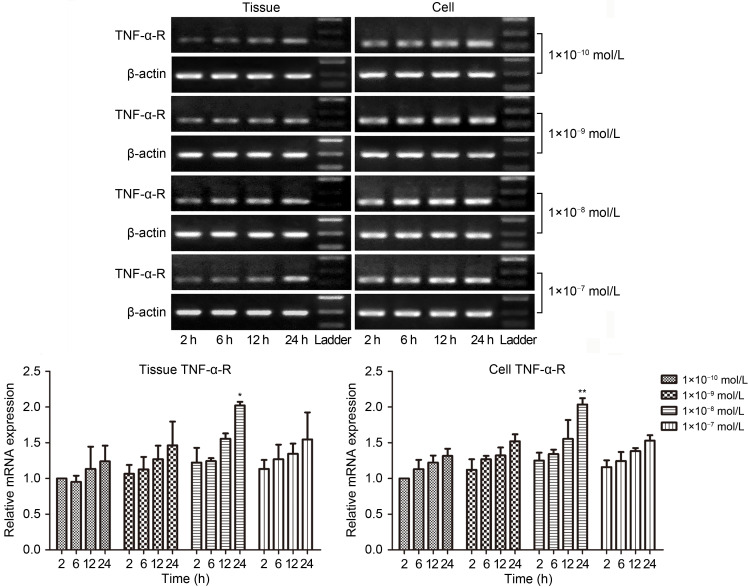
TNF-α-Rs are constitutively expressed across multiple tissues and cells. In the present study, we explored TNF-α-R distribution on the tunica adventitia of vessels and AFs. To determine the role of UII in regulating TNF-α-R mRNA, we incubated tunica adventitia tissues and AFs with a supplement of UII (1×10-10 to 1×10-7 mol/L) at indicated time points. As illustrated in Fig. 2, TNF-α-R mRNA expression was upregulated in a time- and dose-dependent manner. We found a marked elevation after a 24-h intervention with 1×10-8 mol/L UII (tissue P<0.05, cell P<0.01), which was in alignment with our previous findings (Zhang et al., 2008b). All subsequent experiments were performed at the same time point and concentration.
Fig. 2. Time and concentration courses of UII treatment on TNF-α-R mRNA expression from tunica adventitia of vessels and AFs. After rat tunica adventitia of vessels and AFs were treated with UII (1×10 - 10 to 1×10 - 7 mol/L) for the indicated time, tissues and AFs were collected. Total RNA was extracted and reverse transcription into cDNA for RT-PCR and qPCR was carried out. The data are presented as mean±standard error of the mean (SEM), n=3. * P<0.05, ** P<0.01 vs. 2 h at the same concentration. UII: urotensin II; TNF-α-R: tumor necrosis factor-α (TNF-α) receptor; mRNA: messenger RNA; AFs: adventitial fibroblasts; cDNA: complementary DNA; RT-PCR: reverse transcription-polymerase chain reaction (PCR); qPCR: quantitative real-time PCR.
3.2. Stimulation of TNF-α protein secretion and TNF-α-R mRNA expression by UII in the tunica adventitia of vessels and AFs via the Rho/PKC pathway
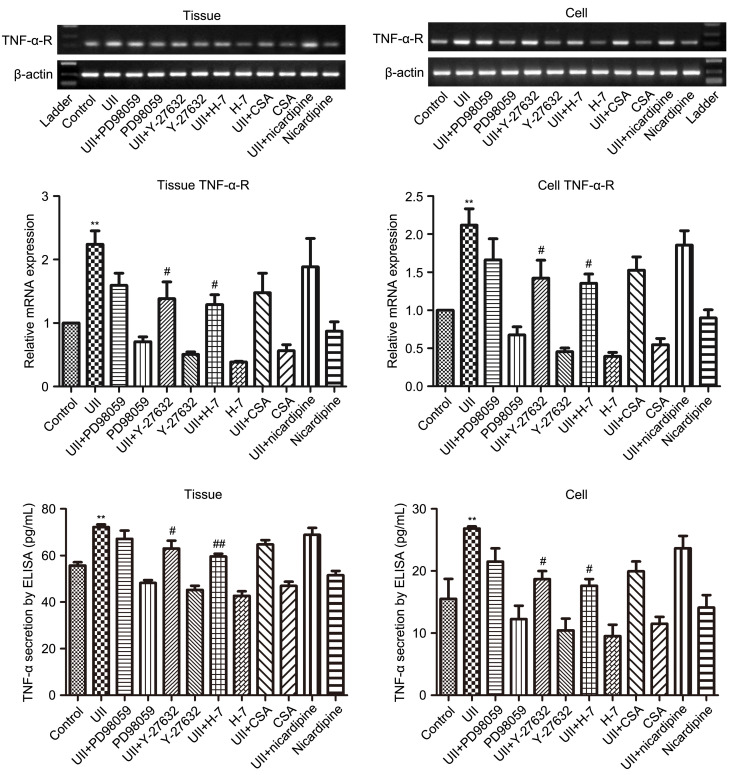
The PCR and quantitative real-time PCR (qPCR) results revealed that UII (1×10-8 mol/L) intervention for 24 h dramatically promoted the expression of TNF-α-R mRNA in vessel tunica adventitia and AFs (tissue P<0.01, cell P<0.01). UII-induced elevation of TNF-α-R mRNA expression was partly abolished when AFs were pre-treated with different inhibitors (Fig. 3) including PD98059, Y-27632, H-7, CSA, and nicardipine, with statistical significance observed in the UII+Y-27632 and UII+H-7 groups. The results suggested involvement of Rho/protein kinase C (PKC) signaling other than the MAPK/calcineurin/Ca2+ pathway in UII-elicited TNF-α-R mRNA expression. ELISA detection of TNF-α secretion coincided with the PCR and qPCR results. Taking these findings together, we could conclude that UII-associated TNF-α and its receptor upregulation acted in both a paracrine and autocrine manner in vessel tunica adventitia and AFs.
Fig. 3. Effects of different inhibitors on UII-induced TNF-α-R mRNA and TNF-α expression in tunica adventitia of vessels and AFs. After preincubation with PD98059 (1×10 - 5 mol/L), Y-27632 (1×10 - 5 mol/L), H-7 (1×10 - 5 mol/L), CSA (1×10 - 5 mol/L), or nicardipine (1×10 - 5 mol/L) for 30 min, the rat tunica adventitias of vessels and AFs were incubated with UII (1×10 - 8 mol/L) and the inhibitors for 24 h. Tissues and AFs were collected, total RNA was extracted, and reverse transcription into cDNA for RT-PCR and qPCR were carried out. Medium was collected for ELISA. The data are presented as mean±standard error of the mean (SEM), n=3. ** P<0.01 vs. control; # P<0.05, ## P<0.01 vs. UII group. UII: urotensin II; TNF-α-R: tumor necrosis factor-α (TNF-α) receptor; mRNA: messenger RNA; AFs: adventitial fibroblasts; CSA: cyclosporin A; cDNA: complementary DNA; RT-PCR: reverse transcription-polymerase chain reaction (PCR); qPCR: quantitative real-time PCR; ELISA: enzyme-linked immunosorbent assay.
3.3. Expression of adiponectin and adipoRs in different organs, vessels, and AFs
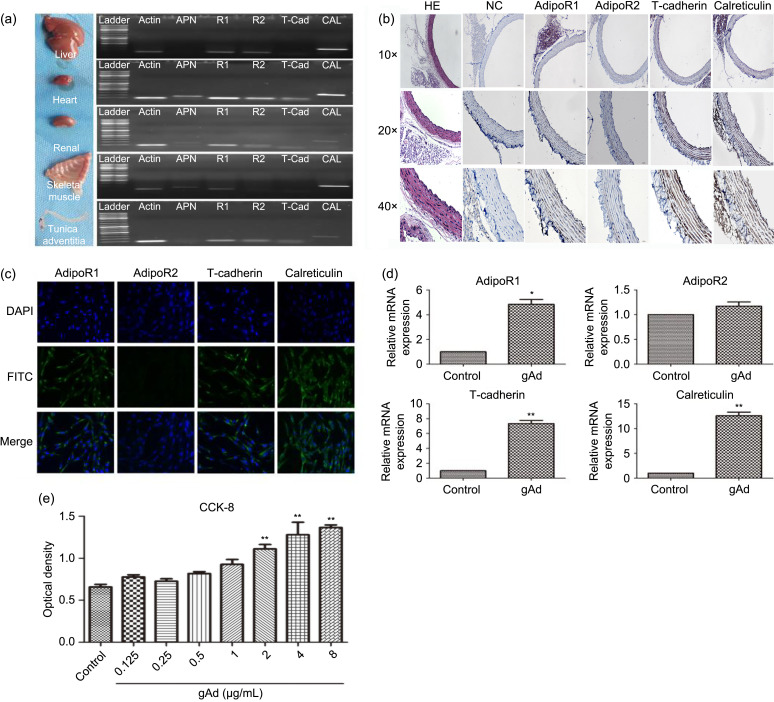
We set out to examine the expression profiles of adiponectin and the corresponding adipoRs in AFs and tissues. The heart, kidney, and muscle were adiponectin reservoirs but we failed to detect its expression in the liver organ or adventitia. AdipoR1 and calreticulin mRNAs were highly expressed in all tissues. We found a marked hepatic, cardiac, and renal accumulation of adipoR2 and relatively low expression in muscle and adventitia. T-cadherin levels were elevated in the heart, kidney, and adventitia, while it was minimally expressed in liver and muscle (Figs. 4a and 4b). Temperature-gradient reverse transcription-PCR (RT-PCR) was used to evaluate adipoR expression levels. In AFs, expression levels of adipoR1 and calreticulin mRNAs were the highest, followed by adipoR2 and T-cadherin (Fig. 4c). To validate our findings, we treated AFs with gAd for 24 h (Fig. 4d) and then analyzed the expression discrepancy of adipoRs. High levels of adipoR1 (P<0.05), T-cadherin (P<0.01), and calreticulin (P<0.01) were detected in the gAd treatment group, in contrast to unchanged levels of adipoR2. We believe that the activation of gAd in AFs required adipoR1, T-cadherin, and calreticulin participation. To explore the sources of adipoRs, IF and IHC analyses were performed on AFs and rat thoracic aorta (Figs. 4b and 4c). The results were similar, as AFs predominantly expressed adipoR1, T-cadherin, and calreticulin proteins in cellular membranes and the cytoplasm. The entire blood vessel was positively stained for adipoR1, T-cadherin, and a strong presence of calreticulin. The expression of adventitial calreticulin was lower compared to that in the tunica intima and tunica media. However, adipoR2 could be detected in the tunica intima and tunica media, but not in the adventitia. We then tested the effects of gAd on AF proliferation (Fig. 4e). By performing a CCK-8 assay, we found that gAd promoted the growth of AFs in a dose-dependent manner (P<0.01). Briefly, we assessed the organ distribution of adiponectin and adipoRs, and found a proliferative effect for gAd.
Fig. 4. Expression of adiponectin and its receptors in different organs, vessels, and AFs. (a) Adiponectin, adipoR1, adipoR2, T-cadherin, and calreticulin mRNA expression in liver, heart, renal, skeletal muscle, and tunica adventitia of vessels. Representative data from three independent experiments are shown. (b) Distribution of adipoRs in rat thoracic aorta. (c) Immunofluorescence staining in AFs revealed that the fibroblast cell membrane was the main location of adipoRs. Plasmalemma localization was revealed using FITC, and nuclear localization was revealed with DAPI. Magnification 200×. (d) The mRNA expression levels of the adipoR1, adipoR2, T-cadherin, and calreticulin proteins in AFs were investigated by qPCR. (e) AFs were grown into a 96-hole cell culture plate. After AFs were stimulated with different concentrations of gAd (0, 0.125, 0.25, 0.5, 1, 2, 4, and 8 μg/mL), proliferation of AFs was evaluated by CCK-8 using a 450-nm wavelength for optical density measurements. A concentration-dependent effect of gAd on AF proliferation was observed. The results are shown as mean±standard error of the mean (SEM), n=3. * P<0.05, ** P<0.01 vs. control. AFs: adventitial fibroblasts; AdipoR: adiponectin receptor; mRNA: messenger RNA; FITC: fluorescein isothiocyanate; DAPI: 4',6-diamidino-2-phenylindole; qPCR: quantitative real-time polymerase chain reaction; CCK-8: cell counting kit-8; gAd: globular adiponectin; HE: hematoxylin-eosin; NC: negative control.
3.4. Effects of adiponectin synergy with UII on AF synthesis of α-SMA and TNF-α via the adipoR1/T-cadherin/calreticulin/AMPK pathway
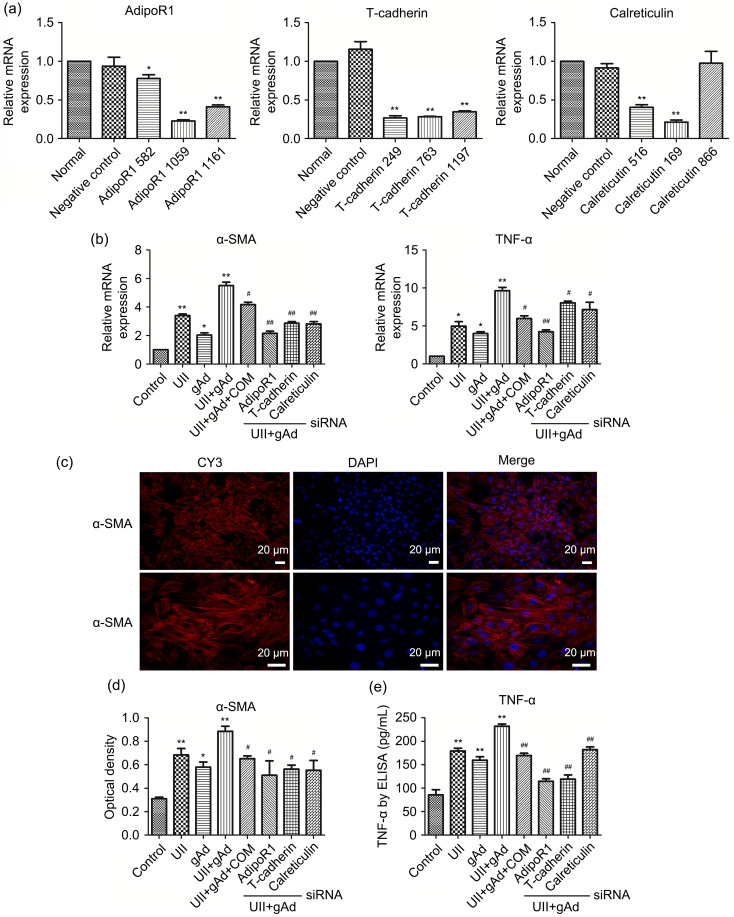
First, we transfected candidate siRNA fragments into AFs and successfully screened adipoR1 (serial number: 1059), T-cadherin (serial number: 249), and calreticulin (serial number: 169) (>75%, P<0.01) siRNAs as specific adipoR gene-knockdown siRNAs (Fig. 5a). qPCR demonstrated that UII or gAd alone was capable of eliciting expression of α-SMA and TNF-α mRNAs in AFs, and the expression level reached its zenith with a combination of UII and gAd (Fig. 5b). This was further validated by ELISA and IF, as evidenced by increased expression of cytoplasm α-SMA and TNF-α secretion (Figs. 5c–5e). With regard to the cellular pathway of gAd-activated AFs, we found that the synergistic effect was abolished in the UII+gAd+compound C (AMPK inhibitor) group. In the presence of UII+gAd treatment, when the adipoRs were silenced, the same phenomenon was observed. It could be inferred that adiponectin-associated activation, together with UII, synergically fostered α-SMA and TNF-α syntheses via the AMPK/adipoR1/T-cadherin/calreticulin signaling pathway in AFs.
Fig. 5. AdipoR1, T-cadherin, calreticulin, and AMPK were involved in UII-induced AF syntheses of α-SMA and TNF-α. (a) Screening of RNAi fragments. AFs were transfected with control siRNA or siRNA-targeting adipoR1, T-cadherin, and calreticulin for 24 h (except for adipoR2, due to its low expression in AFs). The mRNA expression of these siRNA adipoRs in AFs was examined by qPCR analysis. (b) AFs were stimulated with gAd, UII, compound C (COM), and adipoR RNAi fragments for 24 h. The mRNA expression of α-SMA and TNF-α in AFs was examined by qPCR analysis. (c) α-SMA protein expression in AFs was examined by cell immunofluorescence, cytoplasm protein localization was assessed using CY3, and nuclear localization was assessed with DAPI. Scale bar=20 μm. (d) Optical density was measured for analysis. (e) TNF-α protein expression in AFs was examined by ELISA. Data are expressed as mean±standard error (SE), n=3. * P<0.05, ** P<0.01 vs. control; # P<0.05, ## P<0.01 vs. UII+gAd. AdipoR: adiponectin receptor; AMPK: adenosine 5'-monophosphate (AMP)-activated protein kinase; UII: urotensin II; AF: adventitial fibroblast; α-SMA: α-smooth muscle actin; TNF-α: tumor necrosis factor-α; RNAi: RNA interference; siRNA: small interference RNA; mRNA: messenger RNA; qPCR: quantitative real-time polymerase chain reaction; gAd: globular adiponectin; CY3: cyanine 3; DAPI: 4',6-diamidino-2-phenylindole; ELISA: enzyme-linked immunosorbent assay.
3.5. Effect of adiponectin synergy with UII on phosphorylation of AMPK in AFs
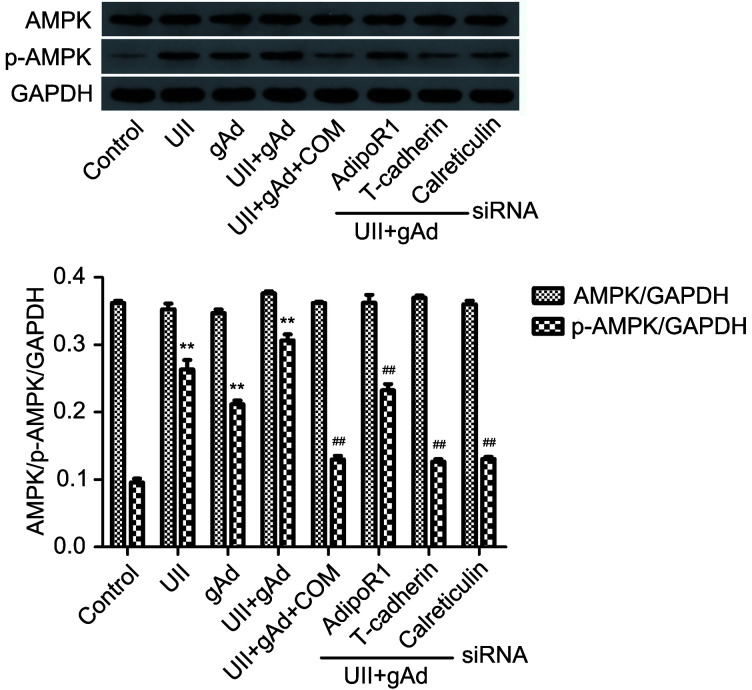
Having identified the adiponectin-mediated AMPK signaling pathway in cell activation, we also confirmed phosphorylation of AMPK in AFs. The western blot analysis shown in Fig. 6 revealed that both UII and gAd increased expression levels of p-AMPK (P<0.01), the maximum level was measured when there was synergic use of UII and gAd (P<0.01). Mixture of UII and gAd, either supplemented with AMPK inhibitor compound C or adipoR RNAi, mitigated AMPK phosphorylation (P<0.01). Therefore, we showed that gAd acted in concert with UII, along with an adipoR1/T-cadherin/calreticulin-triggered AMPK signaling cascade, to promote inflammation in AFs.
Fig. 6. Effects of APN on UII-induced phosphorylation of AMPK in AFs. AFs were stimulated with APN, UII, compound C (COM), and adipoR RNAi fragments for 24 h. The AFs were analyzed for phosphorylation of AMPK by western blotting. Data are expressed as mean±standard error (SE), n=3. ** P<0.01 vs. control; ## P<0.01 vs. UII+gAd. APN: adiponectin; UII: urotensin II; AF: adventitial fibroblast; AMPK: adenosine 5'-monophosphate (AMP)-activated protein kinase; p-AMPK: phospho-AMPK; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; gAd: globular adiponectin; AdipoR: adiponectin receptor; RNAi: RNA interference; siRNA: small interference RNA.
3.6. Effects of APN deficiency on the expression of α-SMA, UT, UII, and TNF-α
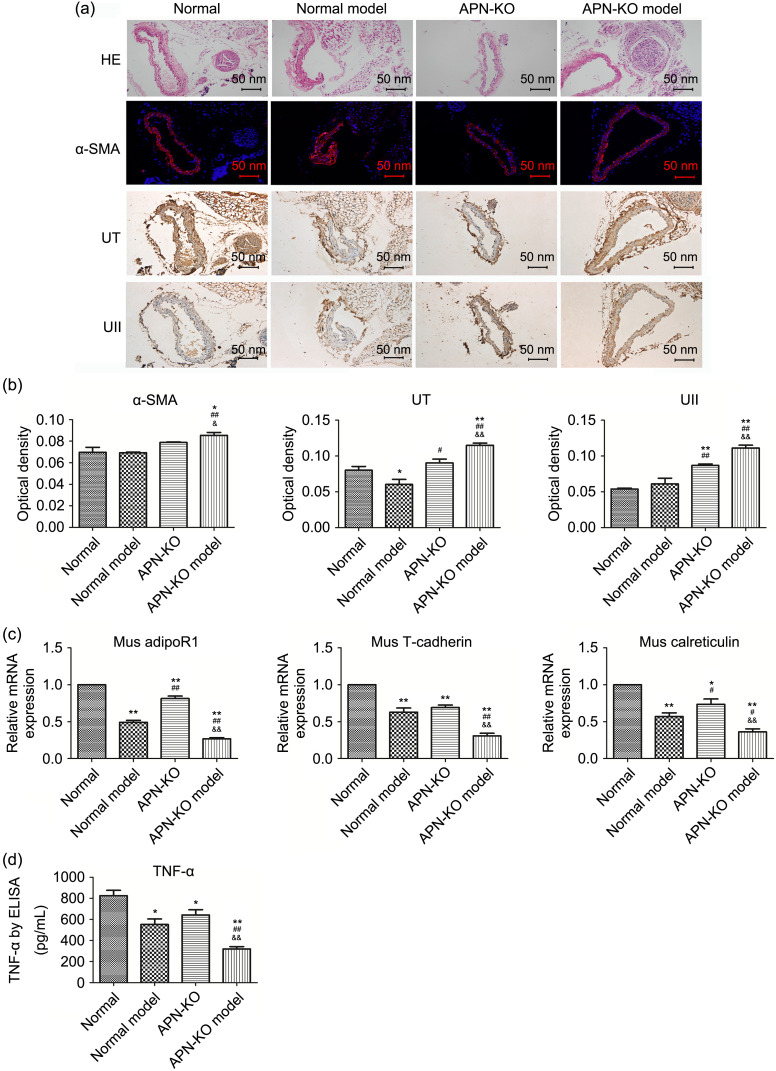
Based on our in vitro cell findings, we examined how inflammatory signals were influenced in APN-deficient settings. As is evident from Figs. 7a and 7b, the APN-KO model group expressed higher α-SMA (P<0.01) in the adventitial inflammation model, while no difference was observed in the normal group, normal model group, or APN-KO group. UT expression in the normal model group was reduced after surgery. Also, we found a stepwise increase in the APN-KO (P<0.05) and APN-KO model (P<0.01) groups. With regard to UII expression, our results indicated that APN-KO (P<0.01) and extra adventitial injury (APN-KO model group, P<0.01) significantly elevated protein production, but this was not the case in the normal model group (P>0.05).
Fig. 7. Changes of α-SMA and TNF-α in the adventitial inflammation model. (a) HE staining showed the structure of each layer of the common carotid artery in the inflammation model. α-SMA expression in common carotid adventitia was examined by immunofluorescence, while UT and UII expression was examined by immunohistochemistry. Scale bar=50 nm. (b) Optical density of the target proteins in the inflammation model (IPP6.0 software was used to analyze the optical density of immunofluorescence and immunohistochemistry sections). (c) Vascular tissue was taken from the modeled part and RNA was extracted. The mRNA expression of adipoR1, T-cadherin, and calreticulin was examined by qPCR analysis. (d) TNF-α changes in mouse plasma in the inflammation model were tested by ELISA. Data are expressed as mean±standard error (SE), n=3. * P<0.05, ** P<0.01 vs. normal group; # P<0.05, ## P<0.01 vs. normal model group; & P<0.05, && P<0.01 vs. APN-KO group. α-SMA: α-smooth muscle actin; TNF-α: tumor necrosis factor-α; HE: hematoxylin-eosin; UII: urotensin II; UT: UII receptor; mRNA: messenger RNA; AdipoR: adiponectin receptor; qPCR: quantitative real-time polymerase chain reaction; ELISA: enzyme-linked immunosorbent assay; APN-KO: adiponectin-knockout; Mus: mouse.
We also discovered moderate changes in adipoRs (Fig. 7c). All groups showed a significant decrease in adipoR1 (P<0.01) compared with the normal group. A marked downregulation was found in the APN-KO model group (P<0.01) compared with the APN-KO group. This result was mirrored in the calreticulin expression level of the four groups, with the most obvious reduction found in the APN-KO model group (P<0.01).
In addition, ELISA was used to assess TNF-α protein concentration. As shown in Fig. 7d, secretion of cytokine TNF-α was markedly attenuated in the APN-KO model group compared with the normal group (P<0.01) and APN-KO group (P<0.01). The data supported our belief that genetic APN deficiency up-regulates expression of α-SMA, UT, and UII in vivo, as well as inhibits TNF-α expression.
4. Discussion
Vascular remodeling is critical in cardiovascular physiology and holds great potential for translation from basic research to clinical applications (Zhao et al., 2020, 2021; Evans et al., 2022). As the most sophisticated and heterogeneous layer of the vessel wall, the adventitia is the initiation site of vascular remodeling (Stenmark et al., 2013). AFs, functioning as cytoskeletal cells, could be activated and differentiate into myofibroblasts after injury and stimulation, demonstrating their contractile properties. In this regard, vascular remodeling is an inflammatory response, but the underlying mechanisms in this process are still poorly understood.
The vascular inflammatory response entails the involvement of TNF-α as it favors the expression of several adhesion molecules, cytokines, and chemokines as well as growth factors which foster inflammatory cell recruitment and adhesion. This triggers the initiation and progression of cardiovascular disease, including hypertension, atherosclerosis, and restenosis. However, so far there is limited acknowledgement of TNF-α and TNF-α-R expression in AFs.
Our group previously reported abundant expression of UII in the adventitia (Zhang et al., 2008a), and found that it prompted phenotypic conversion, cell proliferation, and collagen synthesis (Zhang et al., 2008b). UII-stimulated AFs secreted elevated levels of inflammatory signals such as TGF-β1 (Zhang et al., 2010), LTC4 (Dong et al., 2013), IL-6, and MCP-1 (Zhang et al., 2014), all of which are crucial mediators in vascular remodeling. In the present study, we found that TNF-α-R was expressed in adventitia and AFs. The secretion of TNF-α protein by AFs could act on itself and adventitia in an autocrine/paracrine manner. Furthermore, UII promoted TNF-α secretion and TNF-α-R expression in adventitia and AFs in a time- and dose-dependent manner. It should be noted that these effects could be eliminated by blockage of Rho kinase and PKC inhibitors. Although the findings differ slightly from our former observations (Zhang et al., 2008b), we concluded that UII-induced TNF-α and TNF-α-R expression activates intracellular Rho/PKC signaling pathway.
Because they are either surrounded or embedded in perivascular adipose tissue (PVAT), blood vessels are not only cradles of vasculature that offer primarily mechanistic support but they also regulate vascular/endothelial function by synthesizing adipocytokines, such as leptin, resistin, and adiponectin (Nosalski and Guzik, 2017). Growing evidence has exemplified the multifaceted effects of adiponectin in regulating insulin sensitivity, energy balance, and cellular metabolism. It targets the liver, heart, kidney, pancreatic cells, skeletal muscle, and many other tissues via a receptor-dependent mechanism (Ruan and Dong, 2016; Wang and Scherer, 2016; Zhou et al., 2020). Previous reports have delineated the anti-inflammatory and anti-atherosclerotic function of adiponectin as being reliant on expression levels of adiponectin, as well as adipoRs. In light of these intriguing observations, we speculated that adiponectin is endowed with anti-inflammatory traits; but whether it could possibly modulate UII in AFs is unclear.
In this study, we found that adipoRs are expressed in AFs. In particular, there is enhanced expression of adipoR1, T-cadherin, and calreticulin in contrast to a relative low expression of adipoR2, and no detectable presence of adiponectin. This finding suggests that adiponectin affects the adventitia and AFs in a paracrine rather than autocrine manner. We also examined the effects of UII and gAd on syntheses of α-SMA and TNF-α mRNAs in AFs, and showed that UII or gAd alone facilitated AF syntheses of α-SMA and TNF-α and UII and gAd synergistically promoted their production. When applying siRNAs for adipoRs, the results showed down-regulation of α-SMA and TNF-α expression, indicating gAd, coupled with UII, induced AF syntheses of α-SMA and TNF-α via the adipoR1/T-cadherin/calreticulin pathway. AMPK inhibitor compound C suppressed target protein expression, highlighting the indispensable role of AMPK transduction in the synergetic effect of gAd and UII. In brief, UII, together with gAd, promotes AMPK phosphorylation, accompanied by involvement of adipoR1/T-cadherin/calreticulin.
In vivo, we successfully constructed an inflammation model in APN-KO mice. Compared with the normal group (wild type), the other three groups showed lower expression of adipoRs and detectable changes in α-SMA and TNF-α. Yet, we failed to understand the opposite changes in UII and UT expression and why in vivo and in vitro experiments yielded different results. We speculated that the possible reasons are: first, the exquisite balance is a three-dimensional (3D) regulation network, while the interference factor is a single variant in vitro but rather complex in vivo; second, dose effect and feedback regulation; and finally, though we knocked out the adiponectin gene in the mouse model, the application of gAd treatment in vitro did not serve as an ideal alternative because the circulating adiponectin was in fact a mixture of fAd and gAd.
In summary, our work examined the presentation of TNF-α-R in the adventitia and AFs. Also, we found that AFs are TNF-α protein-secreting cells. UII is capable of inducing TNF-α secretion and TNF-α-R expression in the adventitia and AFs in a time- and dose-dependent manner. These effects could be blocked by inhibitors targeting Rho kinase and PKC. AdipoRs expressed in AFs and gAd synergy but not inhabited with UII promote α-SMA and TNF-α syntheses via the adipoR1/T-cadherin/calreticulin/AMPK pathway. The behavior of APN is a little different in vivo vs. in vitro.
5. Conclusions
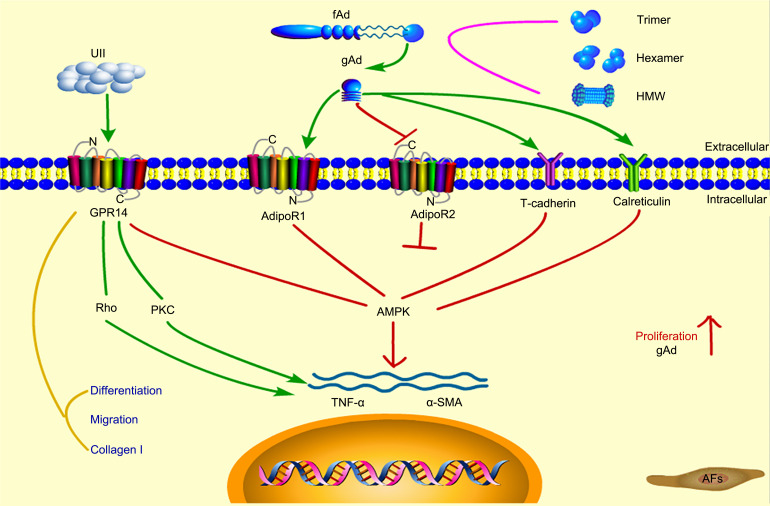
Overall, our study provides a profound understanding of the mechanisms contributing to progression of cardiovascular disease in association with UII and gAd. Moreover, these results broaden our understanding of novel effects of UII and gAd and may shed new light on the mechanisms involved in adventitial inflammation. We hope that this will permit better depiction of vascular pathologies in the early stages and development of new therapeutic strategies targeting UII and gAd (Fig. 8).
Fig. 8. Mechanism of action of adiponectin and UII. UII: urotensin II; GPR14: G protein-coupled receptor 14; PKC: protein kinase C; TNF-α: tumor necrosis factor-α; α-SMA: α-smooth muscle actin; AMPK: adenosine 5'-monophosphate (AMP)-activated protein kinase; AdipoR: adiponectin receptor; fAd: full-length adiponectin; gAd: globular adiponectin; AFs: adventitial fibroblasts; HMW: high molecular weight.
Supplementary information
Acknowledgments
This work was supported by the the National Natural Science Foundation of China (No. 82003372), the Natural Science Foundation of Hubei Province (Nos. 2018CFB747 and 2018CFB537), and the Educational Commission of Hubei Province (Nos. B2017112 and B20181130), China. We are grateful to Yongfeng QI, Liling WU, and Chaoshu TANG (School of Basic Medical Sciences, Peking University, Beijing, China), Dingfang BU, Dan LU, Fen PEN, and Yongyan HU (Peking University First Hospital, Beijing, China) for their helpful suggestions.
Author contributions
Jun LI and Limin LUO completed all the experimental operations and drafted the article. Xiao DONG was responsible for the culture of cells and the feeding of animals. Yonggang ZHANG conducted statistics and analysis of experimental data. Shuyi DANG and Xiaogang GUO took part in drafting the article or revising it critically for important intellectual content. Wenhui DING contributed to conception and design. All authors have read and approved the final manuscript, and therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines
Jun LI, Limin LUO, Yonggang ZHANG, Xiao DONG, Shuyi DANG, Xiaogang GUO, and Wenhui DING declare that they have no conflict of interest.
The study design was carried out under the approval of the Committee on the Ethics of Animal Experiments of Peking University First Hospital (No. J201166). All animal surgeries were performed under sodium pentobarbital anesthesia and efforts were made to alleviate suffering. All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- Ames RS, Sarau HM, Chambers JK, et al. , 1999. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature, 401(6750): 282-286. 10.1038/45809 [DOI] [PubMed] [Google Scholar]
- Avagimyan A, Kajaia A, Gabunia L, et al. , 2022. Urotensin-II as a promising key-point of cardiovascular disturbances sequel. Curr Probl Cardiol, 47(11): 101074. 10.1016/j.cpcardiol.2021.101074 [DOI] [PubMed] [Google Scholar]
- Bobbert P, Antoniak S, Schultheiss HP, et al. , 2008. Globular adiponectin but not full-length adiponectin induces increased procoagulability in human endothelial cells. J Mol Cell Cardiol, 44(2): 388-394. 10.1016/j.yjmcc.2007.10.018 [DOI] [PubMed] [Google Scholar]
- Dong X, Ye XJ, Song NN, et al. , 2013. Urotensin II promotes the production of LTC4 in rat aortic adventitial fibroblasts through NF-κB-5-LO pathway by p38 MAPK and ERK activations. Heart Vessels, 28(4): 514-523. 10.1007/s00380-012-0291-0 [DOI] [PubMed] [Google Scholar]
- Enzerink A, Vaheri A, 2011. Fibroblast activation in vascular inflammation. J Thromb Haemost, 9(4): 619-626. 10.1111/j.1538-7836.2011.04209.x [DOI] [PubMed] [Google Scholar]
- Evans BR, Yerly A, van der Vorst EPC, et al. , 2022. Inflammatory mediators in atherosclerotic vascular remodeling. Front Cardiovasc Med, 9: 868934. 10.3389/fcvm.2022.868934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan GS, Douglas SA, Ohlstein EH, et al. , 2005. Expression of urotensin-II in human coronary atherosclerosis. Peptides, 26(12): 2464-2472. 10.1016/j.peptides.2005.05.028 [DOI] [PubMed] [Google Scholar]
- Lau WB, Ohashi K, Wang YJ, et al. , 2017. Role of adipokines in cardiovascular disease. Circ J, 81(7): 920-928. 10.1253/circj.CJ-17-0458 [DOI] [PubMed] [Google Scholar]
- Li AC, Glass CK, 2002. The macrophage foam cell as a target for therapeutic intervention. Nat Med, 8(11): 1235-1242. 10.1038/nm1102-1235 [DOI] [PubMed] [Google Scholar]
- Liu LH, Shi ZH, Ji XH, et al. , 2022. Adipokines, adiposity, and atherosclerosis. Cell Mol Life Sci, 79(5): 272. 10.1007/s00018-022-04286-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Peng F, Li J, et al. , 2019. Urotensin II promotes secretion of LTB4 through 5-lipoxygenase via the UT-ROS-Akt pathway in RAW 264. 7 macrophages. Arch Med Sci, 15(4): 1065-1072. 10.5114/aoms.2019.85197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosalski R, Guzik TJ, 2017. Perivascular adipose tissue inflammation in vascular disease. Br J Pharmacol, 174(20): 3496-3513. 10.1111/bph.13705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SL, Lee BK, Kim YA, et al. , 2013. Inhibitory effect of an urotensin II receptor antagonist on proinflammatory activation induced by urotensin II in human vascular endothelial cells. Biomol Ther (Seoul), 21(4): 277-283. 10.4062/biomolther.2013.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Castro J, Brás-Silva C, Fontes-Sousa AP, 2019. Novel insights into the role of urotensin II in cardiovascular disease. Drug Discov Today, 24(11): 2170-2180. 10.1016/j.drudis.2019.08.005 [DOI] [PubMed] [Google Scholar]
- Rami AZA, Hamid AA, Anuar NNM, et al. , 2022. Exploring the relationship of perivascular adipose tissue inflammation and the development of vascular pathologies. Mediators Inflamm, 2022: 2734321. 10.1155/2022/2734321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H, Dong LQ, 2016. Adiponectin signaling and function in insulin target tissues. J Mol Cell Biol, 8(2): 101-109. 10.1093/jmcb/mjw014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowka A, Dobrzyn P, 2021. Role of perivascular adipose tissue-derived adiponectin in vascular homeostasis. Cells, 10(6): 1485. 10.3390/cells10061485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark KR, Yeager ME, el Kasmi KC, et al. , 2013. The adventitia: essential regulator of vascular wall structure and function. Annu Rev Physiol, 75: 23-47. 10.1146/annurev-physiol-030212-183802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinajero MG, Gotlieb AI, 2020. Recent developments in vascular adventitial pathobiology: the dynamic adventitia as a complex regulator of vascular disease. Am J Pathol, 190(3): 520-534. 10.1016/j.ajpath.2019.10.021 [DOI] [PubMed] [Google Scholar]
- von der Thüsen JH, van Berkel TJC, Biessen EAL, 2001. Induction of rapid atherogenesis by perivascular carotid collar placement in apolipoprotein E-deficient and low-density lipoprotein receptor-deficient mice. Circulation, 103(8): 1164-1170. 10.1161/01.CIR.103.8.1164 [DOI] [PubMed] [Google Scholar]
- Wang ZV, Scherer PE, 2016. Adiponectin, the past two decades. J Mol Cell Biol, 8(2): 93-100. 10.1093/jmcb/mjw011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AMD, Olukman M, Koulis C, et al. , 2013. Urotensin II receptor antagonism confers vasoprotective effects in diabetes associated atherosclerosis: studies in humans and in a mouse model of diabetes. Diabetologia, 56(5): 1155-1165. 10.1007/s00125-013-2837-9 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, et al. , 2003. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature, 423(6941): 762-769. 10.1038/nature01705 [DOI] [PubMed] [Google Scholar]
- Zhang R, Wu J, Liu D, et al. , 2013. Anti-inflammatory effect of full-length adiponectin and proinflammatory effect of globular adiponectin in esophageal adenocarcinoma cells. Oncol Res, 21(1): 15-21. 10.3727/096504013X13786659070235 [DOI] [PubMed] [Google Scholar]
- Zhang YG, Li YG, Wei RH, et al. , 2008a. Urotensin II is an autocrine/paracrine growth factor for aortic adventitia of rat. Regul Pept, 151(1-3): 88-94. 10.1016/j.regpep.2008.10.004 [DOI] [PubMed] [Google Scholar]
- Zhang YG, Li J, Li YG, et al. , 2008b. Urotensin II induces phenotypic differentiation, migration, and collagen synthesis of adventitial fibroblasts from rat aorta. J Hypertens, 26(6): 1119-1126. 10.1097/HJH.0b013e3282fa1412 [DOI] [PubMed] [Google Scholar]
- Zhang YG, Hu YC, Mao YY, et al. , 2010. Transforming growth factor-β1 involved in urotensin II-induced phenotypic differentiation of adventitial fibroblasts from rat aorta. Chin Med J (Engl), 123(24): 3634-3639. 10.3760/cma.j.issn.0366-6999.2010.24.023 [DOI] [PubMed] [Google Scholar]
- Zhang YG, Bao SL, Kuang ZJ, et al. , 2014. Urotensin II promotes monocyte chemoattractant protein-1 expression in aortic adventitial fibroblasts of rat. Chin Med J (Engl), 127(10): 1907-1912. 10.3760/cma.j.issn.0366-6999.20132795 [DOI] [PubMed] [Google Scholar]
- Zhao K, Yang CX, Li P, et al. , 2020. Epigenetic role of N 6-methyladenosine (m6A) RNA methylation in the cardiovascular system. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 21(7): 509-523. 10.1631/jzus.B1900680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K, Zhang J, Xu TH, et al. , 2021. Low-intensity pulsed ultrasound ameliorates angiotensin II-induced cardiac fibrosis by alleviating inflammation via a caveolin-1-dependent pathway. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 22(10): 818-838. 10.1631/jzus.B2100130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Li JQ, Wei LJ, et al. , 2020. Silencing of DsbA-L gene impairs the PPARγ agonist function of improving insulin resistance in a high-glucose cell model. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 21(12): 990-998. 10.1631/jzus.B2000432 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.