Abstract
Background
Osteoporosis (OP) has become a major public health issue, threatening the bone health of middle-aged and elderly people from all around the world. Changes in the gut microbiota (GM) are correlated with the maintenance of bone mass and bone quality. However, research results in this field remain highly controversial, and no systematic review or meta-analysis of the relationship between GM and OP has been conducted. This paper addresses this shortcoming, focusing on the difference in the GM abundance between OP patients and healthy controls based on previous 16S ribosomal RNA (rRNA) gene sequencing results, in order to provide new clinical reference information for future customized prevention and treatment options of OP.
Methods
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), we comprehensively searched the databases of PubMed, Web of Science, Embase, Cochrane Library, and China National Knowledge Infrastructure (CNKI). In addition, we applied the R programming language version 4.0.3 and Stata 15.1 software for data analysis. We also implemented the Newcastle-Ottawa Scale (NOS), funnel plot analysis, sensitivity analysis, Egger’s test, and Begg’s test to assess the risk of bias.
Results
This research ultimately considered 12 studies, which included the fecal GM data of 2033 people (604 with OP and 1429 healthy controls). In the included research papers, it was observed that the relative abundance of Lactobacillus and Ruminococcus increased in the OP group, while the relative abundance for Bacteroides of Bacteroidetes increased (except for Ireland). Meanwhile, Firmicutes, Blautia, Alistipes, Megamonas, and Anaerostipes showed reduced relative abundance in Chinese studies. In the linear discriminant analysis Effect Size (LEfSe) analysis, certain bacteria showed statistically significant results consistently across different studies.
Conclusions
This observational meta-analysis revealed that changes in the GM were correlated with OP, and variations in some advantageous GM might involve regional differences.
Keywords: Osteoporosis, Microbiome, Intestinal, 16S ribosomal RNA (rRNA) sequencing
1. Introduction
As the global issue of population aging is becoming increasingly prominent, the comprehensive prevalence of osteoporosis (OP) has grown to 21.7%. In particular, its value has reached 24.3% among the Asian population. As a result, OP can be considered a major public health crisis that is severely threatening the bone health of middle-aged and elderly people worldwide (Anonymous, 1993; Salari et al., 2021). Moreover, osteoporotic fractures cause patients to suffer from high treatment costs, severe lifelong disability, and a heightened risk of death. Therefore, the prevention and treatment of OP has become of particular concern and must be urgently addressed.
Tens of thousands of gut microbiota (GM) evolve along with their host organism, creating a mutually beneficial symbiotic relationship (Danne et al., 2017; Wei et al., 2019). GM comprise a complicated and large microbial metabolic system that participates in regulating the stability of the internal and external environment, and also has a key role in healthy human physiological processes and disease development (Juárez-Fernández et al., 2020; Shi et al., 2020). If the homeostasis between the human body and GM is damaged, it could lead to many types of human chronic diseases such as metabolic, mental, neurodegenerative, cardiovascular, and cancer diseases (Baker et al., 2011; Li et al., 2017; Hou et al., 2021). In recent years, researchers have established that changes in the GM are also related to the maintenance of bone mass and quality, while GM impact the dynamic balance between bone formation maintained by osteoblasts and bone resorption maintained by osteoclasts, which relies on the gut-bone axis (Liang et al., 2018). Sjögren et al. (2012) found that the bone mass of germ-free mice was abnormally high. When implanting GM into these mice, they were able to induce and activate cluster of differentiation-positive (CD+) T cells. GM triggered the excretion of several cytokines in the bone to promote osteoclast activation and initiate the bone resorption mechanism, and normalized the bone mass of ovariectomized (OVX) mice. Since 2017, five clinical randomized controlled trials have shown that replenishing probiotics can prevent and treat OP as well as improve bone quality in OP, and fecal microbiota transplantation (FMT) could become a new therapeutic standard for OP (Jafarnejad et al., 2017; Lambert et al., 2017; Nilsson et al., 2018; Takimoto et al., 2018; Jansson et al., 2019). However, as different populations have unique GM abundance characteristics, applying beneficial and customized GM-mixed preparations may be a safer and more effective choice (Tyagi et al., 2018). As a result, identifying advantageous bacterial strains or metabolites with positive bone effects, as well as constructing customized and precise GM therapies based on different OP, may provide new, safe, and effective OP treatment options.
The 16S ribosomal RNA (rRNA) genes are present in the genome of all bacteria. Consequently, 16S rRNA gene sequencing has become widely applied in the research on microbial phylogeny and accurate species classifications. In addition, it has significant value when explaining the relationship between GM and disease. Several clinical studies based on populations have used GM 16S rRNA gene sequencing to analyze the abundance of GM in patients (Rettedal et al., 2021; Wang ZX et al., 2021; Xu et al., 2021). These results verified that changes occurred in the relative abundance of several species of microorganisms, which may be related to GM impacting the bone metabolism. However, these 16S rRNA gene sequencing results were dispersed and inconsistent, lacked systematic reviews and summaries, and were unable to provide new reference values for current and future large-scale clinical trials. Prompted by this deficiency, in this paper, we conducted a systematic review and meta-analysis of the differences in GM abundance between OP and healthy control (HC) from previous 16S rRNA gene sequencing results, to more effectively explain the role of GM in OP, and to provide new clinical reference information for customized OP prevention and treatment.
2. Results
2.1. Search flow and overview of studies
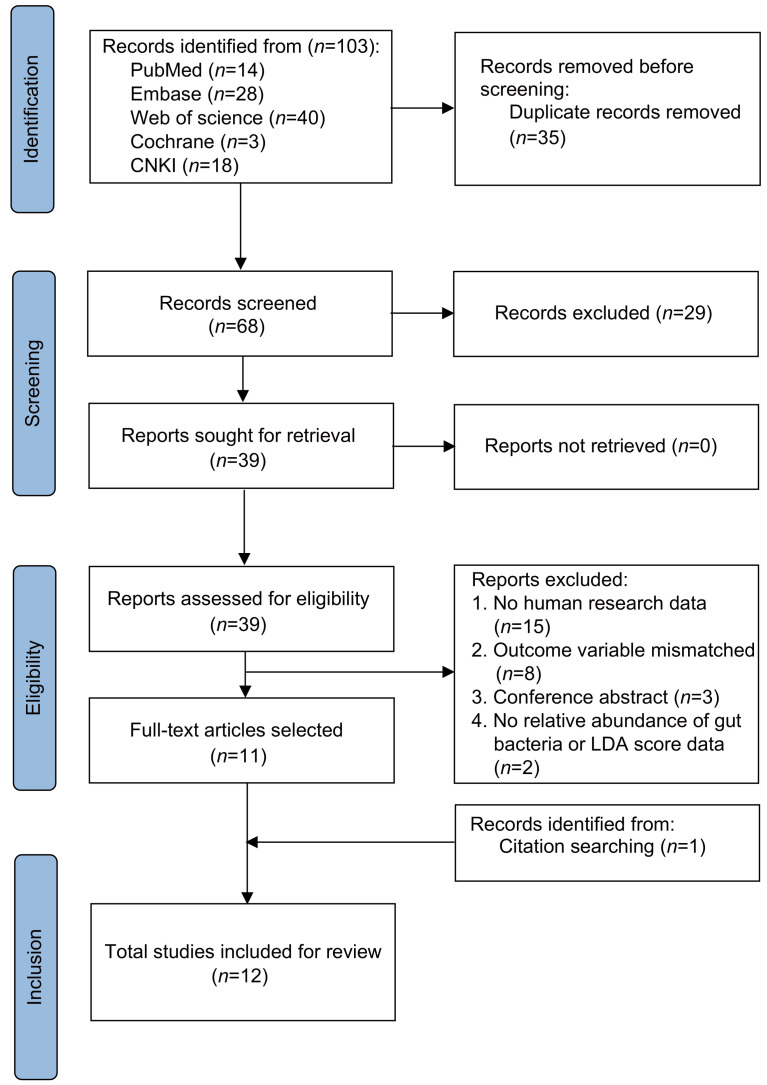
A total of 103 items of data were selected through database retrieval. Among them, 35 repetitive studies were excluded, 29 studies with inconsistent data were excluded, and the remaining 39 articles were evaluated. After carefully reading the titles and abstracts, 26 articles were excluded because they did not meet the inclusion criteria. Two articles were excluded as they did not provide relevant data on the relative abundance of GM, including one with a sample size fewer than ten patients. Two articles were excluded for not providing sufficient data on analyses of alpha diversity. One study on Bacteroides was excluded as it had high heterogeneity. One article was included by the combination of manual retrieval and literature tracking. After reading the full texts carefully and comparing the selection criteria, 12 studies were finally included (Fig. 1) (Das et al., 2019; Li C et al., 2019; Li LS, 2019; He et al., 2020; Palacios-González et al., 2020; Xu et al., 2020; Ling et al., 2021; Lyu et al., 2021a, 2021b; Rettedal et al., 2021; Wang ZX et al., 2021; Wei et al., 2021). The basic data and sequencing methods of the population are shown in Table S1. The meta-analysis included studies covering a period from 2019 to 2021, including 604 OP patients and 1429 HC. Among them, five studies included all female participants (He et al., 2020; Palacios-González et al., 2020; Lyu et al., 2021b; Rettedal et al., 2021; Wang ZX et al., 2021), while all participants in another were exclusively male (Lyu et al., 2021a). The total proportion of female participants was 67.8%, except that Li et al. (2019) did not adjust for gender-related confounding factors. The results of other included studies showed significant differences in the intestinal flora among different groups after adjusting for sex-related confounding factors. As for population areas, one study was conducted in Ireland (Das et al., 2019), one in Mexico (Palacios-González et al., 2020), one in New Zealand (Rettedal et al., 2021), and nine in China (Li C et al., 2019; Li LS, 2019; He et al., 2020; Xu et al., 2020; Ling et al., 2021; Lyu et al., 2021a, 2021b; Wang ZX et al., 2021; Wei et al., 2021). All studies measured bone mineral density (BMD) using dual-energy X-ray (DXA), and all of them used sequencing schemes, including 16S rRNA sequencing, to evaluate intestinal flora samples. One study utilized 16S rRNA to measure the V3-V5 regions (Das et al., 2019), two studies measured the V4 region (Li, 2019; Palacios-González et al., 2020), and seven studies measured the V3 and V4 regions (Li et al., 2019; He et al., 2020; Xu et al., 2020; Ling et al., 2021; Lyu et al., 2021a, 2021b; Wei et al., 2021). One study did not specify the sequencing region (Rettedal et al., 2021). Two studies defined patients as people with low BMD (Li et al., 2019; Palacios-González et al., 2020). Nine studies reported data on the relative abundance of microflora (Das et al., 2019; Li, 2019; He et al., 2020; Palacios-González et al., 2020; Xu et al., 2020; Lyu et al., 2021a, 2021b; Wang ZX et al., 2021; Wei et al., 2021), and ten studies reported data on the linear discriminant analysis (LDA) scores (Li C et al., 2019; Li LS, 2019; He et al., 2020; Palacios-González et al., 2020; Xu et al., 2020; Ling et al., 2021; Lyu et al., 2021a, 2021b; Rettedal et al., 2021; Wang ZX et al., 2021). Seven studies reported the alpha diversity data (Das et al., 2019; Li, 2019; Palacios-González et al., 2020; Xu et al., 2020; Ling et al., 2021; Rettedal et al., 2021; Wei et al., 2021). Two studies reported the specific value of Firmicutes and Bacteroidetes (F/B) (Li et al., 2019; Palacios-González et al., 2020). Three studies used Spearman’s correlation analysis to evaluate the correlation between GM at the phylum and genus levels and the BMD measurements (Li et al., 2019; Xu et al., 2020; Wei et al., 2021). The average overall quality score of these studies was eight stars (Table S2).
Fig. 1. Search and selection procedures of the literature for the systematic review, described in detail by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart. CNKI: China National Knowledge Infrastructure; LDA: linear discriminant analysis.
2.2. Alpha diversity and Firmicutes/Bacteroidetes (F/B) ratio
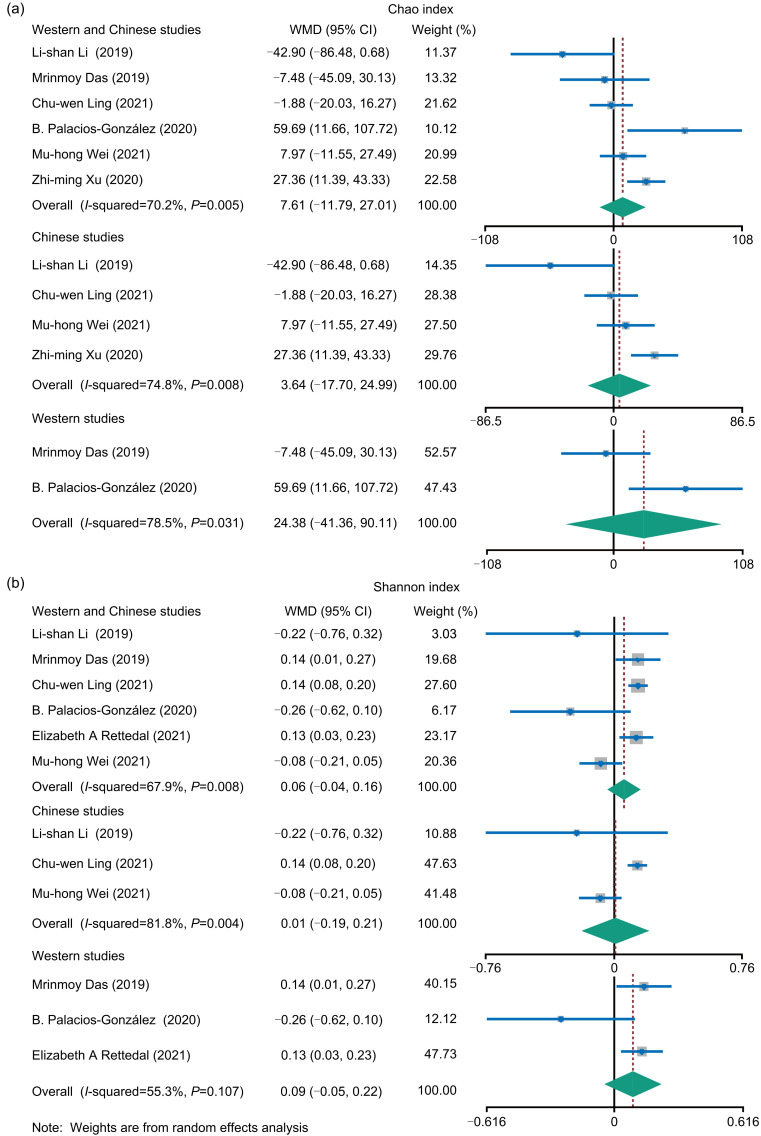
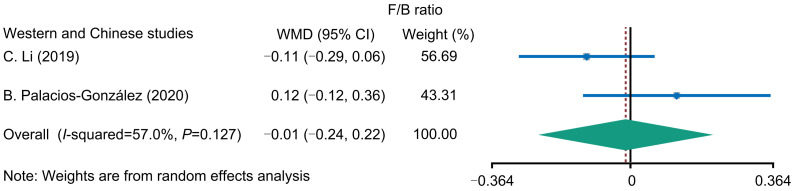
Alpha diversity can reflect the richness and diversity of microbial communities in samples. We analyzed the alpha diversity indices (Chao and Shannon) between OP and HC, and found that the two indices did not significantly differ between the Western and the Chinese populations, within the Chinese population, or within the Western population (Fig. 2). The contents of F/B play a dominant role in intestinal microbial composition, and the F/B ratio was associated with the dietary intake, energy metabolism, and GM imbalance (Rinaldi et al., 2019; Zhao et al., 2019). Of the 12 included studies, two studies reported the F/B ratios, and our analysis showed no significant difference in F/B ratios between OP and HC (Fig. 3).
Fig. 2. Forest map of alpha diversity differences by Chao index (a) and Shannon index (b). WMD: weight mean difference; CI: confidence interval.
Fig. 3. Forest plot of Firmicutes/Bacteroidetes (F/B) ratio. WMD: weight mean difference; CI: confidence interval.
2.3. Bacterial phyla
As shown in Table 1, the relative abundance of Firmicutes in the OP group was decreased in five groups (Li et al., 2019; Xu et al., 2020; Lyu et al., 2021a, 2021b; Wei et al., 2021). It should be noted that in the Irish and Mexico results, the relative abundance of Firmicutes increased in the OP group. Meanwhile, the relative abundance of Bacteroidetes increased in the OP group in China and Mexico (Li, 2019; Palacios-González et al., 2020; Xu et al., 2020; Lyu et al., 2021a, 2021b; Wei et al., 2021). We found that the relative abundance of Bacteroidetes decreased only in the OP group in Ireland, but not in China or Mexico.
Table 1.
Changes at the taxonomic level in phyla associated with intestinal flora
| Phylum | Higher in osteoporosis | Higher in healthy control | ||
|---|---|---|---|---|
| Reference | Ratio (%)# | Reference | Ratio (%)# | |
| Firmicutes | Das et al., 2019 | 79.13 | Wei et al., 2021 | 54.51 |
| Palacios-González et al., 2020& | 38.01 | Xu et al., 2020 | 84.14 | |
| Lyu et al., 2021b | 75.70 | |||
| Li, 2019 | 61.90 | |||
| Lyu et al., 2021a | 67.86 | |||
| Bacteroidetes | Li, 2019 | 29.57 | Das et al., 2019 | 15.32 |
| Wei et al., 2021 | 38.93 | |||
| Xu et al., 2020* | 12.03 | |||
| Palacios-González et al., 2020& | 52.79 | |||
| Lyu et al., 2021b | 12.81 | |||
| Lyu et al., 2021a | 10.36 | |||
# Approximation proportion of relative abundance histogram. & Patients characterized by low bone mineral density (BMD). * The difference was statistically significant.
2.4. Bacterial genera
A total of eight studies reported the relative abundance data at the genus level, as shown in Table 2 (Das et al., 2019; Li, 2019; He et al., 2020; Xu et al., 2020; Lyu et al., 2021a, 2021b; Wang ZX et al., 2021; Wei et al., 2021). Blautia were decreased in five Chinese OP groups (Li, 2019; He et al., 2020; Xu et al., 2020; Lyu et al., 2021b; Wei et al., 2021); research by He et al. (2020) also indicated that Blautia was directly correlated with lumbar BMD in postmenopausal osteopenia patients, and was negatively correlated with metabolite L-lysine. The relative abundance of Alistipes was decreased in four Chinese OP groups (Li, 2019; He et al., 2020; Wang ZX et al., 2021; Wei et al., 2021), and increased in an Irish OP group (Das et al., 2019). The relative abundance of Megamonas was decreased in two OP groups in China (Li, 2019; Xu et al., 2020). The relative abundance of Anaerostipes was increased in one Irish OP group (Das et al., 2019) and decreased in two Chinese OP groups (Li, 2019; Wei et al., 2021). The relative abundance of Lactobacillus and Ruminococcus was increased in the OP groups (Das et al., 2019; Li, 2019; Lyu et al., 2021a, 2021b; Wei et al., 2021). Li (2019) found that Lactobacillus was positively correlated with BMD, while Bacteroides was negatively correlated with BMD (Fig. S1). The relative abundance of Bacteroides was increased in seven OP groups (Li et al., 2019; He et al., 2020; Xu et al., 2020; Lyu et al., 2021b; Rettedal et al., 2021; Wang ZX et al., 2021; Wei et al., 2021), and decreased in an Irish OP group (Das et al., 2019).
Table 2.
Differences in the relative abundance of gut bacteria at the genus level between osteoporosis patients and healthy control
| Genus | Higher in osteoporosis | Higher in healthy control | ||
|---|---|---|---|---|
| Reference | Ratio (%)# | Reference | Ratio (%)# | |
| Blautia | Wei et al., 2021 | 7.63 | ||
| Lyu et al., 2021b | 9.36 | |||
| Li, 2019 | 14.46 | |||
| Xu et al., 2020* | 4.21 | |||
| He et al., 2020** | ||||
| Alistipes | Das et al., 2019 | 1.23 | Wang ZX et al., 2021 | 2.71 |
| Wei et al., 2021 | 2.05 | |||
| He et al., 2020 | 0.95 | |||
| Li, 2019 | 1.65 | |||
| Megamonas | Xu et al., 2020 | 5.14 | ||
| Li, 2019 | 3.10 | |||
| Anaerostipes | Das et al., 2019 | 2.21 | Li, 2019 | 2.69 |
| Wei et al., 2021 | 1.63 | |||
| Lactobacillus | Das et al., 2019 | 8.60 | ||
| Li, 2019 | 1.65 | |||
| Lyu et al., 2021a | 8.75 | |||
| Lyu et al., 2021b | 5.94 | |||
| Ruminococcus | Wei et al., 2021 | 2.86 | ||
| Das et al., 2019 | 3.68 | |||
| Bacteroides | He et al., 2020* | 46.74 | Das et al., 2019 | 16.42 |
| Wei et al., 2021 | 29.63 | |||
| Xu et al., 2020 | 6.36 | |||
| Lyu et al., 2021b | 7.76 | |||
| Wang ZX et al., 2021 | 48.12 | |||
| Li et al., 2019& | ||||
| Rettedal et al., 2021 | ||||
# Approximation proportion of relative abundance histogram. * The difference was statistically significant. ** The difference was significant in postmenopausal osteopenia. & Patients characterized by low bone mineral density (BMD).
2.5. Controversial results
For certain bacteria, contradictory results were found in studies from the same or different countries; however, these bacteria were still associated with bone health (Table 3). For example, Xu et al. (2020) indicated that the OP changes were related to Faecalibacterium and found a negative correlation between Bifidobacterium and BMD, while Li et al. (2019) found a positive correlation between Bifidobacterium and Roseburia and BMD (Fig. S1). A Chinese study by Wang ZX et al. (2021) revealed that Prevotella was less abundant in postmenopausal women with OP.
Table 3.
Contradictory results of differences in the relative abundance of gut bacteria at the genus level between osteoporosis patients and healthy control
| Genus | Higher in osteoporosis | Higher in healthy control | ||
|---|---|---|---|---|
| Reference | Ratio (%)# | Reference | Ratio (%)# | |
| Bifidobacterium | Lyu et al., 2021b | 27.40 | Xu et al., 2020 | 4.66 |
| Wei et al., 2021 | 3.41 | Li, 2019 | 4.96 | |
| Lyu et al., 2021a | 13.51 | |||
| Das et al., 2019 | 2.45 | |||
| Faecalibacterium | Lyu et al., 2021b | 16.90 | Li, 2019 | 4.55 |
| Xu et al., 2020* | 32.71 | Lyu et al., 2021a | 19.29 | |
| He et al., 2020 | 1.87 | |||
| Wei et al., 2021 | 6.81 | |||
| Das et al., 2019 | 19.12 | |||
| Wang ZX et al., 2021 | 3.14 | |||
| Roseburia | Wei et al., 2021 | 1.50 | He et al., 2020 | 1.08 |
| Wang ZX et al., 2021 | 1.92 | Das et al., 2019 | 12.75 | |
| Prevotella | Das et al., 2019 | 4.41 | Wang ZX et al., 2021 | 26.81 |
| Wei et al., 2021 | 3.41 | |||
# Approximation proportion of relative abundance histogram. * The difference was statistically significant.
2.6. Linear discriminant analysis (LDA)
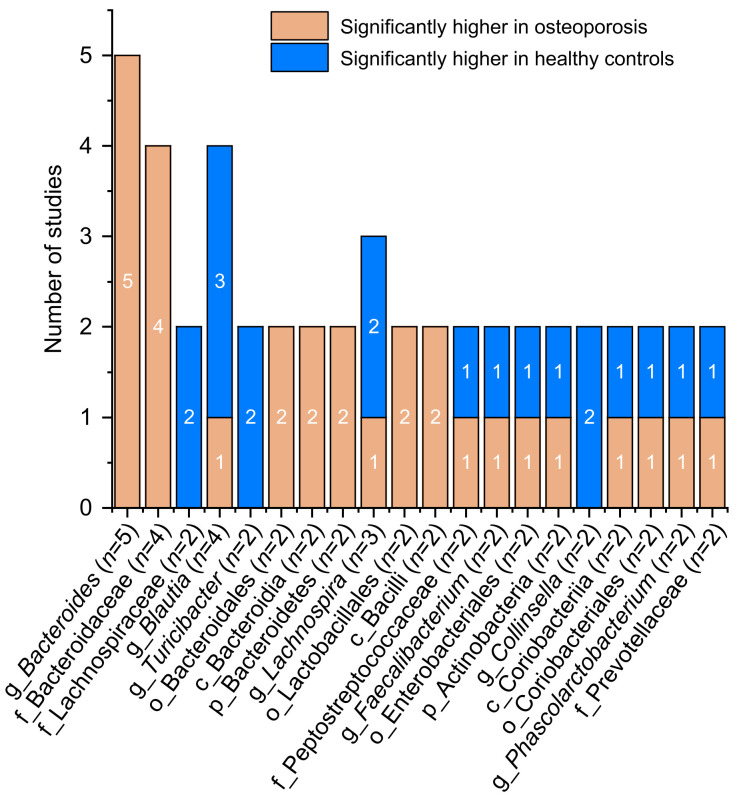
LDA Effect Size (LEfSe) analysis can be used to compare the statistical differences between the OP and HC groups, to find the microflora with significant differences between groups. From the 12 studies included, ten reported the LDA scores of the OP and HC groups (Li C et al., 2019; Li LS, 2019; He et al., 2020; Palacios-González et al., 2020; Xu et al., 2020; Ling et al., 2021; Lyu et al., 2021a, 2021b; Rettedal et al., 2021; Wang ZX et al., 2021). Here, we describe the strains that have been reported more than twice. In the OP group, g_Bacteroides, f_Bacteroidaceae, o_Bacteroidales, c_Bacteroidia, p_Bacteroidetes, o_Lactobacillales, and c_Bacilli were significantly different microflora, whereas in the HC group, f_Lachnospiraceae, g_Turicibacter, and g_Collinsella were the significantly different microflora. Contradictory results were reported for the remaining strains (Fig. 4). Among them, g_Lachnospira was considered as significantly different microflora in low-BMD postmenopausal women in Mexico and significantly different microflora in the HC group in China (He et al., 2020; Palacios-González et al., 2020; Wang ZX et al., 2021). f_Peptostreptococcaceae was considered as significantly different microflora in the OP group in China and significantly different microflora in the HC group in New Zealand (Lyu et al., 2021b; Rettedal et al., 2021).
Fig. 4. Number of studies with statistically differences in the key microflora of the osteoporosis group or healthy control group.
2.7. Publication bias
We assessed the risk of bias for each of the included articles, and Begg’s and Egger’s regression tests indicated that there was no significant bias in the results of these meta-analyses (Fig. S2).
3. Discussion
Changes in the GM have an important impact on the physiological processes and disease prognosis of many organisms, and these changes differ between developed and developing countries (Vangay et al., 2018). As a result, assessing the correlation between GM and OP has a definite reference value for discovering the potential targets of GM regulating OP. Currently, research on the relationship between GM and bone health has become extremely popular. Based on our understanding, our work is the first meta-analysis to identify the correlation between GM and OP, including the population data of a total of 2033 individuals. Our results showed that, in the Chinese studies, the relative abundance of Firmicutes in the intestine of patients with OP was lower than that of healthy people. At the same time, we found that the relative abundance of Blautia, Alistipes, Megamonas, and Anaerostipes decreased in the intestine of patients with OP. Blautia is an anaerobic bacterium with probiotic properties that can promote the secretion of short-chain fatty acids (SCFAs) from intestinal dietary fiber. It can also impact the immune response of regulatory T cells (Tregs). Tregs can stimulate the bone marrow to generate CD8+ T cells, upregulate the expression of the protein Wnt family member 10b (Wnt10b), secrete interleukin-10 (IL-10), and maintain the homeostasis of the host’s immune system, which can in turn increase bone mass (Rey et al., 2010; Juanola et al., 2018; Tyagi et al., 2018; Liu XM et al., 2021). Alistipes, which belongs to the Bacteroidetes phylum, can produce SCFAs and reduce intestinal inflammation. Moreover, the decrease of its abundance can reduce the amount of SCFAs, impacting intestinal homeostasis (Wang BK et al., 2021). Both of our included studies with data on Megamonas, which were two original studies, came from China. A report indicated that Megamonas was difficult to find among European and North American populations, but could be detected among Chinese populations, and thus may be endemic to Asian populations (Yachida et al., 2019). Therefore, further research is needed to confirm whether Megamonas is a potential marker of GM among Chinese or Asian OP populations. The most recent study reported that, under certain conditions, a portion of Anaerostipes strains could generate propionate, butyrate, and SCFAs (Bui et al., 2021).
In all the studies we included, we found that the relative abundance of the Firmicutes, Ruminococcus and Lactobacillus, increased in the case group. Ruminococcus is a key bacterial genus for the degradation of resistant starch (RS) (Suzuki and Ley, 2020). RS can promote equol production, and research has shown that a diet supplemented with RS can reduce bone loss in OVX mice (Tousen et al., 2019). Other studies found that Lactobacillus reuteri can inhibit the base level of tumor neurosis factor-α (TNF-α) messenger RNA (mRNA) in the intestine, increase the bone mass of healthy male mice (Mccabe et al., 2013), and guard against bone loss in OVX mice (Britton et al., 2014). One study found that Lactobacillus salivarius, Leuconostoc lactis, and Lactobacillus paracasei were probiotics that inhibited gut pathogenic bacteria (Liu ZJ et al., 2021). More in-depth research is needed to explain the relationship between the bone protection mechanism of Lactobacillus and its increased relative abundance in OP.
We found that the relative abundance of Bacteroidetes increased in the OP group in China and Mexico. Bacteroidetes is composed of various kinds of Gram-negative bacteria found in the gastrointestinal tract, and its two most commonly seen types are Bacteroides and Prevotella. Bacteroides is a vitamin K-synthesizing strain (Mandatori et al., 2021). Vitamin K2 can impact bone metabolism through the γ-carboxylation of osteocalcin (OC) (Atkins et al., 2009). An animal model using a combination of vitamin K2 and teriparatide found enhanced osteoblast function and increased serum levels of carboxylated osteocalcin (Gla-OC), a specific marker of bone formation, in OVX rats (Nagura et al., 2015; Akbari and Rasouli-Ghahroudi, 2018). Prevotella includes more than 50 different species (Tett et al., 2021), such as Prevotella histicola. Wang ZX et al. (2021) found that this species inhibited osteoclast activity, changed the expression of IL-1β, TNF-α, and other osteoclastic cytokines, and reduced bone loss in OVX mice.
We found that different studies had contradictory results for Bifidobacterium, Faecalibacterium, and Roseburia. Bifidobacterium belongs to Actinobacteria, which can mediate lipopolysaccharide (LPS)-induced inflammatory responses (Khokhlova et al., 2012). One study found that, when a low concentration of LPS induces bone mesenchymal stem cells (BMSCs), they could differentiate into osteoblasts, but a high concentration of LPS would reduce their differentiation ability (Ding, 2017). As a result, further research is needed to confirm how Bifidobacterium impacts bone quality. Faecalibacterium and Roseburia can generate butyrate bacteria (Crespo-Piazuelo et al., 2021). Butyrate can upregulate the expression of Wnt10b and enhance the osteogenic differentiation ability of BMSCs.
Ethnic or regional differences may be important factors affecting the composition and structure of GM. In this meta-analysis, the relative abundance of Firmicutes, Bacteroidetes, Alistipes, Anaerostipes, and Bacteroides in the gut of Irish OP patients showed inconsistent alterations compared with other regions. In the future, more population studies and meta-analyses are needed to explain this regional difference. Although the overall quality of the included original studies was good, we should not overlook the important fact that drugs can also influence GM composition and structure. It is well known that depression is one of the high-risk factors of OP. Wan et al. (2022) found that the new antidepressant drug R-ketamine can alleviate bone loss in OVX mice through the anti-inflammatory effect of GM, which reveals the potential therapeutic targets of OP based on the pathogenesis of GM. Thus, individualized treatment for specific patients can be achieved, which is expected to be beneficial for the treatment of OP. In addition, it should be noted that, although we were unable to incorporate GM metabolites into this systematic review and meta-analysis, clinical studies exploring the relationship between specific gut microbial-derived metabolites and OP may constitute valuable future research directions.
Currently, the exact relationship between GM and OP is complicated and remains unclear. The numerous changes in GM abundance may be unable to directly explain the impact of GM on bone health. However, we look forward to the application of this meta-analysis to provide reference information for future research designs. Applying GM as a target to improve bone health involves an enormous amount of potential, and research focusing on specific advantageous strains may be a direction worth exploring for improved diagnostic and treatment options for OP.
4. Limitations
In this work, we relied on established inclusion standards to comprehensively search for all of the studies that matched our requirements, and strictly implemented a systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guide (Moher et al., 2009). However, some limitations of this study could not be avoided. First, most of the included studies had a high level of heterogeneity. Different from the meta-analysis of randomized controlled trials, heterogeneity is a widely known and unavoidable problem of observational studies (Stroup et al., 2000). The included studies had a considerably large clinical and methodological heterogeneity, while specific situations among different individuals also exhibited relatively large differences. For instance, differences between factors such as the participants’ sample size, ethnicity, place of residence, season, diet, medications, age and gender, physical exercise, and stress tend to impact the composition and structure of their GM (Gupta et al., 2017). The researchers’ DNA extraction methods used to targe the 16S rRNA gene area for sequencing, the sequencing platform, and sequencing depth were all reasons that could cause inconsistent results (Duvallet, 2018). We could not guarantee that the meta-analysis results display all of the strains correlated with OP. Furthermore, we were only able to identify the correlation between changes in GM and OP. However, neither does this definitively indicate that the two are causally related, nor can it conclusively verify that a particular advantageous bacterium can protect bones. At the same time, changes in relative abundance cannot always accurately react variations in absolute abundance (Smets et al., 2016). Furthermore, although 16S rRNA analysis is a powerful technique, it cannot provide reliable in-depth identification (Ravi et al., 2018).
5. Conclusions
Our meta-analysis results indicated no significant difference in alpha diversity (Chao and Shannon) indices or the F/B ratio between OP and HC. In the included research papers, it was observed that, compared with the HC group, the relative abundance of Lactobacillus and Ruminococcus increased, while the relative abundance for Bacteroides of Bacteroidetes increased (except for Ireland) in the OP group. Meanwhile, Firmicutes, Blautia, Alistipes, Megamonas, and Anaerostipes had reduced relative abundance in the included Chinese studies. In the LEfSe analysis, certain bacteria showed statistically significant results consistently across different studies. In addition, there may have been regional differences in the changes in some intestinal flora. The pathogenicity of GM is still controversial, while more basic and clinical research is needed to explain the role of microbes in bone microbiology, to help explore new strategies for using GM to prevent and treat OP.
Materials and methods
Detailed methods are provided in the electronic supplementary materials of this paper.
Supplementary information
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81860391), the Guangxi Medical High-level Backbone Talents Training “139” Program Training Project (No. [2020]15), and the Guangxi Hundred Thousand Talents Project (No. [2019]32), China.
Author contributions
Conceptualization: Gaofeng ZENG and Shaohui ZONG; Methodology: Jieqiong HUANG; Formal analysis: Rui HUANG and Huihua LI; Resources: Yeping SU; Writing ‒ original draft preparation: Rui HUANG and Pan LIU; Writing ‒ review and editing: Rui HUANG, Ruixin MA, and Quan ZHOU; Visualization: Rui HUANG and Yiguang BAI; Project administration: Rui PAN; Funding acquisition: Gaofeng ZENG and Shaohui ZONG. All authors have read and approved the final manuscript, and therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines
Rui HUANG, Pan LIU, Yiguang BAI, Jieqiong HUANG, Rui PAN, Huihua LI, Yeping SU, Quan ZHOU, Ruixin MA, Shaohui ZONG, and Gaofeng ZENG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Akbari S, Rasouli-Ghahroudi AA, 2018. Vitamin K and bone metabolism: a review of the latest evidence in preclinical studies. Biomed Res Int, 2018: 4629383. 10.1155/2018/4629383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous , 1993. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med, 94(6): 646-650. 10.1016/0002-9343(93)90218-e [DOI] [PubMed] [Google Scholar]
- Atkins GJ, Welldon KJ, Wijenayaka AR, et al. , 2009. Vitamin K promotes mineralization, osteoblast-to-osteocyte transition, and an anticatabolic phenotype by γ-carboxylation-dependent and -independent mechanisms. Am J Physiol Cell Physiol, 297(6): C1358-C1367. 10.1152/ajpcell.00216.2009 [DOI] [PubMed] [Google Scholar]
- Baker RG, Hayden MS, Ghosh S, 2011. NF-κB, inflammation, and metabolic disease. Cell Metab, 13 (1): 11 -22. 10.1016/j.cmet.2010.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton RA, Irwin R, Quach D, et al. , 2014. Probiotic L . reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol, 229(11): 1822-1830. 10.1002/jcp.24636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui TPN, Mannerås-Holm L, Puschmann R, et al. , 2021. Conversion of dietary inositol into propionate and acetate by commensal Anaerostipes associates with host health. Nat Commun, 12: 4798. 10.1038/s41467-021-25081-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Piazuelo D, Lawlor PG, Ranjitkar S, et al. , 2021. Intestinal microbiota modulation and improved growth in pigs with post-weaning antibiotic and ZnO supplementation but only subtle microbiota effects with Bacillus altitudinis . Sci Rep, 11: 23304. 10.1038/s41598-021-01826-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danne C, Ryzhakov G, Martínez-López M, et al. , 2017. A large polysaccharide produced by Helicobacter hepaticus induces an anti-inflammatory gene signature in macrophages. Cell Host Microbe, 22(6): 733-745.e5. 10.1016/j.chom.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Cronin O, Keohane DM, et al. , 2019. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology (Oxford), 58(12): 2295-2304. 10.1093/rheumatology/kez302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, 2017. The Effects of LPS on the Proliferation and Osteogenic Differentiation of Human Bone Marrow Mesen chymal Stem Cells and the Study on the Related Mechanisms. MS Thesis, Naval Medical University, Shanghai, China: (in Chinese). [Google Scholar]
- Duvallet C, 2018. Meta-analysis generates and prioritizes hypotheses for translational microbiome research. Microb Biotechnol, 11(2): 273-276. 10.1111/1751-7915.13047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta VK, Paul S, Dutta C, 2017. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front Microbiol, 8: 1162. 10.3389/fmicb.2017.01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JQ, Xu SB, Zhang BZ, et al. , 2020. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging (Albany NY), 12(9): 8583-8604. 10.18632/aging.103168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou M, Xu GL, Ran MS, et al. , 2021. APOE-ε4 carrier status and gut microbiota dysbiosis in patients with alzheimer disease. Front Neurosci, 15: 619051. 10.3389/fnins.2021.619051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarnejad S, Djafarian K, Fazeli MR, et al. , 2017. Effects of a multispecies probiotic supplement on bone health in osteopenic postmenopausal women: a randomized, double-blind, controlled trial. J Am Coll Nutr, 36(7): 497-506. 10.1080/07315724.2017.1318724 [DOI] [PubMed] [Google Scholar]
- Jansson PA, Curiac D, Lazou Ahrén I, et al. , 2019. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol, 1(3): e154-e162. 10.1016/S2665-9913(19)30068-2 [DOI] [PubMed] [Google Scholar]
- Juanola O, Piñero P, Gómez-Hurtado I, et al. , 2018. Regulatory T cells restrict permeability to bacterial antigen translocation and preserve short-chain fatty acids in experimental cirrhosis. Hepatol Commun, 2(12): 1610-1623. 10.1002/hep4.1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez-Fernández M, Porras D, García-Mediavilla MV, et al. , 2020. Aging, gut microbiota and metabolic diseases: management through physical exercise and nutritional interventions. Nutrients, 13(1): 16. 10.3390/nu13010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhlova EV, Smeianov VV, Efimov BA, et al. , 2012. Anti-inflammatory properties of intestinal Bifidobacterium strains isolated from healthy infants. Microbiol Immunol, 56(1): 27-39. 10.1111/j.1348-0421.2011.00398.x [DOI] [PubMed] [Google Scholar]
- Lambert MNT, Thybo CB, Lykkeboe S, et al. , 2017. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: a randomized controlled trial. Am J Clin Nutr, 106(3): 909-920. 10.3945/ajcn.117.153353 [DOI] [PubMed] [Google Scholar]
- Li C, Huang Q, Yang R, et al. , 2019. Gut microbiota composition and bone mineral loss-epidemiologic evidence from individuals in Wuhan, China. Osteoporos Int, 30(5): 1003-1013. 10.1007/s00198-019-04855-5 [DOI] [PubMed] [Google Scholar]
- Li LS, 2019. Study of Correlation Between Structural Characteristics of Gut Microbiota and TH17/Treg Ratio in Osteoporosis. MS Thesis, Southern Medical University, Guangzhou, China(in Chinese). [Google Scholar]
- Li SY, Wang ZL, Yang Y, et al. , 2017. Lachnospiraceae shift in the microbial community of mice faecal sample effects on water immersion restraint stress. AMB Express, 7: 82. 10.1186/s13568-017-0383-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Peng SL, Li J, et al. , 2018. Inhibition of osteoblastic Smurf1 promotes bone formation in mouse models of distinctive age-related osteoporosis. Nat Commun, 9: 3428. 10.1038/s41467-018-05974-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling CW, Miao ZL, Xiao ML, et al. , 2021. The association of gut microbiota with osteoporosis is mediated by amino acid metabolism: multiomics in a large cohort. J Clin Endocrinol Metab, 106(10): e3852-e3864. 10.1210/clinem/dgab492 [DOI] [PubMed] [Google Scholar]
- Liu XM, Mao BY, Gu JY, et al. , 2021. Blautia—a new functional genus with potential probiotic properties? Gut Microbes, 13(1): 1875796. 10.1080/19490976.2021.1875796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZJ, Xu C, Tian R, et al. , 2021. Screening beneficial bacteriostatic lactic acid bacteria in the intestine and studies of bacteriostatic substances. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 22(7): 533-547. 10.1631/jzus.B2000602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu J, Zhao HP, Yu Y, et al. , 2021a. Composition and gene function of intestinal microbiota in male osteoporotic patients. Chin J Osteoporosis Bone Miner Res, 14(5): 457-469 (in Chinese). 10.3969/j.issn.1674-2591.2021.05.003. [DOI] [Google Scholar]
- Lyu J, Zhao HP, Yu Y, et al. , 2021b. Profile and gene functional analysis of gut microbiota in women with postmenopausal osteoporosis. Chin J Microbiol Immunol, 41(11): 867-874 (in Chinese). 10.3760/cma.j.cn112309-20210425-00134 [DOI] [Google Scholar]
- Mandatori D, Pelusi L, Schiavone V, et al. , 2021. The dual role of vitamin K2 in “bone-vascular crosstalk”: opposite effects on bone loss and vascular calcification. Nutrients, 13(4): 1222. 10.3390/nu13041222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccabe LR, Irwin R, Schaefer L, et al. , 2013. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol, 228(8): 1793-1798. 10.1002/jcp.24340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, et al. , 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ, 339: b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagura N, Komatsu J, Iwase H, et al. , 2015. Effects of the combination of vitamin K and teriparatide on the bone metabolism in ovariectomized rats. Biomed Rep, 3(3): 295-300. 10.3892/br.2015.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson AG, Sundh D, Bäckhed F, et al. , 2018. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med, 284(3): 307-317. 10.1111/joim.12805 [DOI] [PubMed] [Google Scholar]
- Palacios-González B, Ramírez-Salazar EG, Rivera-Paredez B, et al. , 2020. A multi-omic analysis for low bone mineral density in postmenopausal women suggests a relationship between diet, metabolites, and microbiota. Microorganisms, 8(11): 1630. 10.3390/microorganisms8111630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi A, Avershina E, Angell IL, et al. , 2018. Comparison of reduced metagenome and 16S rRNA gene sequencing for determination of genetic diversity and mother-child overlap of the gut associated microbiota. J Microbiol Methods, 149: 44-52. 10.1016/j.mimet.2018.02.016 [DOI] [PubMed] [Google Scholar]
- Rettedal EA, Ilesanmi-Oyelere BL, Roy NC, et al. , 2021. The gut microbiome is altered in postmenopausal women with osteoporosis and osteopenia. JBMR Plus, 5(3): e10452. 10.1002/jbm4.10452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey FE, Faith JJ, Bain J, et al. , 2010. Dissecting the in vivo metabolic potential of two human gut acetogens. J Biol Chem, 285(29): 22082-22090. 10.1074/jbc.M110.117713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi E, Consonni A, Cordiglieri C, et al. , 2019. Therapeutic effect of bifidobacterium administration on experimental autoimmune myasthenia gravis in Lewis rats. Front Immunol, 10: 2949. 10.3389/fimmu.2019.02949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salari N, Ghasemi H, Mohammadi L, et al. , 2021. The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J Orthop Surg Res, 16: 609. 10.1186/s13018-021-02772-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HL, Yu YH, Lin DH, et al. , 2020. β-Glucan attenuates cognitive impairment via the gut-brain axis in diet-induced obese mice. Microbiome, 8: 143. 10.1186/s40168-020-00920-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren K, Engdahl C, Henning P, et al. , 2012. The gut microbiota regulates bone mass in mice. J Bone Miner Res, 27(6): 1357-1367. 10.1002/jbmr.1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets W, Leff JW, Bradford MA, et al. , 2016. A method for simultaneous measurement of soil bacterial abundances and community composition via 16S rRNA gene sequencing. Soil Biol Biochem, 96: 145-151. 10.1016/j.soilbio.2016.02.003 [DOI] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, et al. , 2000. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA, 283(15): 2008-2012. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- Suzuki TA, Ley RE, 2020. The role of the microbiota in human genetic adaptation. Science, 370(6521): eaaz6827. 10.1126/science.aaz6827 [DOI] [PubMed] [Google Scholar]
- Takimoto T, Hatanaka M, Hoshino T, et al. , 2018. Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: a randomized, placebo-controlled, double-blind clinical trial. Biosci Microbiota Food Health, 37(4): 87-96. 10.12938/bmfh.18-006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tett A, Pasolli E, Masetti G, et al. , 2021. Prevotella diversity, niches and interactions with the human host. Nat Rev Microbiol, 19(9): 585-599. 10.1038/s41579-021-00559-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousen Y, Matsumoto Y, Nagahata Y, et al. , 2019. Resistant starch attenuates bone loss in ovariectomised mice by regulating the intestinal microbiota and bone-marrow inflammation. Nutrients, 11(2): 297. 10.3390/nu11020297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi AM, Yu MC, Darby TM, et al. , 2018. The microbial metabolite butyrate stimulates bone formation via T regulatory cell-mediated regulation of WNT10B expression. Immunity, 49(6): 1116-1131.e7. 10.1016/j.immuni.2018.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangay P, Johnson AJ, Ward TL, et al. , 2018. US immigration westernizes the human gut microbiome. Cell, 175(4): 962-972.e10. 10.1016/j.cell.2018.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan XY, Eguchi A, Fujita Y, et al. , 2022. Effects of (R)-ketamine on reduced bone mineral density in ovariectomized mice: a role of gut microbiota. Neuropharmacology, 213: 109139. 10.1016/j.neuropharm.2022.109139 [DOI] [PubMed] [Google Scholar]
- Wang BK, Zhou YH, Mao YL, et al. , 2021. Dietary supplementation with Lactobacillus plantarum ameliorates compromise of growth performance by modulating short-chain fatty acids and intestinal dysbiosis in broilers under Clostridium perfringens challenge. Front Nutr, 8: 706148. 10.3389/fnut.2021.706148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZX, Chen K, Wu CC, et al. , 2021. An emerging role of Prevotella histicola on estrogen deficiency-induced bone loss through the gut microbiota-bone axis in postmenopausal women and in ovariectomized mice. Am J Clin Nutr, 114(4): 1304-1313. 10.1093/ajcn/nqab194 [DOI] [PubMed] [Google Scholar]
- Wei MH, Li C, Dai Y, et al. , 2021. High-throughput absolute quantification sequencing revealed osteoporosis-related gut microbiota alterations in Han Chinese elderly. Front Cell Infect Microbiol, 11: 630372. 10.3389/fcimb.2021.630372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei MY, Shi S, Liang C, et al. , 2019. The microbiota and microbiome in pancreatic cancer: more influential than expected. Mol Cancer, 18: 97. 10.1186/s12943-019-1008-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Wu ZF, Wang Y, et al. , 2021. High-throughput sequencing identifies salivary microbiota in Chinese caries-free preschool children with primary dentition. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 22(4): 285-294. 10.1631/jzus.B2000554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZM, Xie Z, Sun JG, et al. , 2020. Gut microbiome reveals specific dysbiosis in primary osteoporosis. Front Cell Infect Microbiol, 10: 160. 10.3389/fcimb.2020.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachida S, Mizutani S, Shiroma H, et al. , 2019. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat Med, 25(6): 968-976. 10.1038/s41591-019-0458-7 [DOI] [PubMed] [Google Scholar]
- Zhao ZH, Shi AM, Wang Q, et al. , 2019. High oleic acid peanut oil and extra virgin olive oil supplementation attenuate metabolic syndrome in rats by modulating the gut microbiota. Nutrients, 11(12): 3005. 10.3390/nu11123005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.