Abstract
Background
Although Vanins are closely related to neutrophil regulation and response to oxidative stress, and play essential roles in inflammatory diseases with clinical significance, their contribution to periodontitis remains to be determined. This research was designed to assess the expression of Vanins in human gingiva, and to define the relationship between Vanins and periodontitis.
Methods
Forty-eight patients with periodontitis and forty-two periodontal healthy individuals were enrolled for gingival tissue sample collection. Expression levels of VNN1, VNN2 and VNN3 were evaluated by RT-qPCR and validated in datasets GSE10334 and GSE16134. Western blot and immunohistochemistry identified specific proteins within gingiva. The histopathological changes in gingival sections were investigated using HE staining. Correlations between Vanins and clinical parameters, PD and CAL; between Vanins and inflammation, IL1B; and between Vanins and MPO in periodontitis were investigated by Spearman's correlation analysis respectively. Associations between VNN2 and indicators of neutrophil adherence and migration were further validated in two datasets.
Results
Vanins were at higher concentrations in diseased gingival tissues in both RT-qPCR and dataset analysis (p < 0.01). Assessment using western blot and immunohistochemistry presented significant upregulations of VNN1 and VNN2 in periodontitis (p < 0.05). The higher expression levels of Vanins, the larger the observed periodontal parameters PD and CAL (p < 0.05), and IL1B (p < 0.001). Moreover, positive correlations existed between VNN2 and MPO, and between VNN2 and neutrophil-related indicators.
Conclusion
Our study demonstrated upregulation of Vanins in periodontitis and the potential contribution of VNN2 to periodontitis through neutrophils-related pathological processes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-022-02583-7.
Keywords: Vanin, Periodontitis, Gingival tissue, Neutrophil
Background
Periodontitis is a widely prevalent infectious inflammatory disease of the periodontal tissues in response to the invasion of bacterial pathogens [1, 2]. Severe periodontitis affects 10–15% of population, while 40–60% of adults suffer from moderate periodontitis [3]. The imbalance between proinflammatory and anti-inflammatory cytokines and deleterious effects during the host’s response against bacterial infection could lead to irreversible periodontium destruction, including periodontal pockets formation and alveolar bone resorption [4–7].
During the pathological process of periodontitis, neutrophils not only play protective roles, such as helping to maintain periodontal tissue homeostasis, but may also lead to destructive effects, such as affecting inflammatory bone loss [8]. Neutrophils can be recruited quickly to the attacked site in acute inflammation, whereas they are also involved in chronic inflammatory diseases [9]. In chronic periodontitis, a larger number of longer-lived hyperactivated neutrophils promote the lymphocyte-rich periodontium lesion, resulting in aggravating periodontal tissue destruction [9]. In addition, hyperactive neutrophils promote reactive oxygen species production, leading to dysregulated metabolites of lipid peroxidation, damage of DNA and proteins during pathological process of periodontal lesions [10]. Factors regulating neutrophil functions were therefore hypothesized to affect periodontitis pathological process [9, 11].
Vanins (VNNs) are pantetheinase enzymes, including three members VNN1, VNN2, and VNN3, which mediate hydrolyzation of pantetheine in Coenzyme A circulation [12]. Numerous studies have revealed pivotal roles of Vanins in fatty acid metabolism, oxidative stress, cell migration and inflammatory response [13–15]. VNN1 was first identified as a regulator of adhesion of thymocytes and thymus homing, and reported to affect oxidative stress [16]. VNN2 is mainly distributed on human neutrophils and monocytes, with secretory vesicles as the reservoir of intracellular VNN2 [17, 18]. Working as a modulator of Mac-1 (CD11b/CD18), VNN2 was reported to regulate β2 integrin-mediated cell adhesion, cell migration, and motility of neutrophils [16]. Recent studies indicated that expression levels of Vanins were abnormal in diverse diseases, especially inflammatory diseases, including diabetes, sepsis, rheumatoid arthritis and inflammatory bowel disease [19–23]. The dysregulated Vanins played significant roles of biomarkers in disease diagnosis and prognosis [24]. In view of these findings, we would consider the potential role of Vanins in periodontitis.
A recent transcriptomic analysis of circulating lymphocytes and monocytes indicated that the VNN1 gene was upregulated in patients suffering from type II diabetes mellitus, periodontitis, and dyslipidemia simultaneously in contrast with normal individuals [25]. However, the expression profile of Vanins in periodontitis on both the genetic and protein levels has not been reported, and it was unclear how Vanins contribute to periodontitis. Therefore, the study was mainly aimed to analyze the relationship between Vanins and periodontitis. Comparison of the expression of Vanins in human gingival tissues was conducted between patients with periodontitis and periodontal healthy individuals. In addition, the Vanins expression levels were hypothesized to associate with periodontitis on the aspect of clinical parameters, the severity of inflammation and neutrophil response.
Methods
Study design and participants
The study was performed between February 2022 to October 2022 at the Department of Stomatology, Renji Hospital affiliated to Shanghai Jiao Tong University School of Medicine, following the STROBE guidelines (Supplementary File 1). Approval from the Institutional Review Board was obtained before subject enrollment. The study design was developed according to Helsinki Declaration (KY2021-196-B). The research group obtained the written informed consent from all participants enrolled.
Patients diagnosed with periodontitis or impacted mandibular third molars were considered to be potentially eligible. The screening was conducted to confirm eligibility after clinical examination and interviews of demographic information. After the screening study, the subjects were enrolled into the study with informed consent. According to the classification of periodontal conditions updated in 2017, inclusion criteria for the periodontal healthy groups were as follows: (1) probing depth (PD) ≤ 3 mm, bleeding on probing (BOP) < 10% and no clinical attachment loss (CAL), no history of periodontitis and no history of pericoronitis; (2) aged between 18 and 65 [26]. Inclusion criteria for the periodontitis group were as follows: (1) diagnosed with stage II-IV periodontitis, whose maximum PD ≥ 5 mm, CAL ≥ 3 mm at the most severe site, bone loss ≥ 15% in radiographic examinations; (2) needed periodontal flap surgery in the mandibular posterior region; and (3) aged between 18 and 65 [27]. Subjects were excluded with (1) smoking history, (2) pregnant, lactating, or menopausal status, (3) taking any medication which could affect periodontal healthy conditions over the past quarter, or (4) accompanied with systemic diseases relating to periodontal lesions, including but not limited to diabetes, cardiovascular diseases, systemic lupus erythematosus and chronic renal diseases [6]. In total, this study enrolled 48 periodontitis patients and 42 periodontal healthy individuals as the flow diagram presented (Supplementary Fig. 1).
Sample collection
The demographic information was obtained by interviewers, and the clinical characteristics were collected by a specialist of periodontology when screening the eligibility (Table 1). The clinical parameters, including PD and CAL, were performed at six sites (mesial-buccal, mid-buccal, distal-buccal, mesial-lingual, mid-lingual, and distal-lingual) and the deepest site was chosen for clinical parameters collection. Gingival tissue samples were collected from 48 periodontitis patients during periodontal flap surgery, in which the inner margin of the flap was trimmed. For periodontal healthy group, tissue samples were obtained from 42 donors who had their impacted teeth extracted. All attached gingival tissue samples were from mandibular posterior regions, comprising the epithelial and connective tissue. The collected samples were rinsed with 0.9% normal saline and kept at − 80 °C for further experiments, while others were fixed with 4% Paraformaldehyde for further Hematoxylin–eosin (HE) staining and IHC. In total, 48 periodontitis and 42 healthy samples were collected, of which 30 pairs were used for RT-qPCR, six pairs were used for western blot, and the other 12 periodontitis and six healthy samples were used for histological analysis.
Table 1.
Demographic information and clinical characteristics of periodontal healthy individuals and patients with periodontitis enrolled in this study
| Characteristics | Periodontal healthy (n = 42) | Periodontitis (n = 48) |
|---|---|---|
| Age (years) | 30.81 ± 4.70 | 33.25 ± 5.22 |
| Gender (Male/Female) | 21/21 | 24/24 |
| Number of Tooth loss | 0.00 ± 0.00 | 0.40 ± 0.82*** |
| PD (mm) | 2.26 ± 0.54 | 6.75 ± 1.41**** |
| CAL (mm) | 0.00 ± 0.00 | 6.06 ± 2.04**** |
| BOP (%) | 7.21 ± 1.76 | 72.29 ± 14.37**** |
Data are expressed as mean ± SD. Statistical significance is indicated as *** p < 0.001, and **** p < 0.0001
PD Probing depth, CAL Clinical attachment loss, BOP Bleeding on probing
RT-qPCR
Gingival tissue samples of periodontitis (n = 30) and periodontal healthy (n = 30) donors were utilized for RT-qPCR as previously reported [6]. Total RNA was isolated with TRIzol Reagent (Invitrogen, Carlsbad, CA, USA), and was later qualified and quantified. Using PrimeScript RT Master Mix (Takara Bio, Otsu, Shiga, Japan), RNA was reverse-transcribed into cDNA according to manufacturer's protocol. RT-qPCR was performed in usage of FastStart Universal SYBR Green Master Mix (Roche, Nutley, NJ, USA) on the available QuantStudio7 Flex Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The relative mRNA expression levels of detected VNN1, VNN2, VNN3, and IL1B were normalized to the housekeeping gene GAPDH in the 2^-ΔCT method. Experiments designed were all repeated at least in triplicate. The primers from Sangon Biotech (Shanghai, China) were available in Table 2 with details of primer design.
Table 2.
Primer sequences for RT-qPCR validation
| Gene | Forward primer (5’ → 3’) | Reverse primer (5’ → 3’) |
|---|---|---|
| VNN1 | TCTGCAGTGGTGAACTGGAC | GTCAAATGCCCCTAGAGCGT |
| VNN2 | GTGCTACTTACCGAAATTCATC | TTTACCAAACGCCCATCTT |
| VNN3* | GATCATTCTAAGTGGGAGTCA | CGTCCATCTCTTGAAATCTCA |
| IL1B | AGCTACGAATCTCCGACCAC | CGTTATCCCATGTGTCGAAGAA |
| GAPDH | GGAAGATGGTGATGGGATT | GGATTTGGTCGTATTGGG |
*VNN3, VNN3P Vanin 3, pseudogene (Homo sapiens)
Validation on GEO datasets
The periodontitis datasets were searched within the datasets registered in the GEO of the accessible National Center for Biotechnology Information (NCBI) (www.ncbi.nlm.nih.gov/geo/). Two datasets, GSE10334 and GSE16134, which examined gene expression profiles in the diseased and healthy human gingival sites, were obtained. For the GSE10334 dataset, the transcriptomes of 183 diseased and 64 periodontal healthy gingival sites were reported. For the GSE16134 dataset, the gene expression profiling of human periodontitis was presented with a total number of 310 gingival sites (241 diseased and 69 healthy sites). Both GSE10334 and GSE16134 were from the platform GPL570. To validate Vanin expression levels and their correlation with inflammation, relative expression levels of VNN1, VNN2, VNN3 and IL1B were selected for further analysis. The correlation between Vanins and neutrophils, especially in regards to the previously reported β2 integrin-dependent neutrophil adherence and migration, was conducted using relative expression levels of subunits of α/β integrins and ligands of β2 integrins on these two datasets [28, 29]. The average value was calculated if there were multiple probes for the same gene.
Western blot
Six pairs of gingival tissue samples were used in western blot. As reported in the previous article of research group, the total protein was extracted by RIPA Lysis Buffer (Beyotime, Shanghai, China) with 1% PMSF (Beyotime, Shanghai, China) on ice from gingival samples of six periodontitis donors and six normal controls [6]. The Thermo Scientific Pierce BCA Protein Assay Kit was used to measure protein concentrations. Based on the protein concentration, protein solutions were diluted, denatured, and loaded onto sodium dodecyl sulfate–polyacrylamide gels. The polyvinylidene fluoride membrane loaded proteins from gels and were further blocked with 5% bovine serum albumin (BSA). Primary antibodies against VNN1 (ab205912, Abcam), VNN2 (#39964, Signalway Antibody) and GAPDH (5174 s, Cell Signaling Technology) were used for overnight membrane treatment at 4 °C. Next, the 1 × phosphate buffered saline (PBS) with Tween detergent (PBST) was used to wash the membranes, which were later treated with Anti-rabbit IgG, HRP-linked Antibody (Cell Signaling Technology, Danvers, MA, USA). PBST washed the membranes again. The Chemiluminescence Reagents (Millipore, Billerica, MA, USA) helped visualize immunoreactive bands. Quantitative analysis of blots was conducted on ImageJ software.
Sections preparation
The gingival tissues of six periodontal healthy individuals and 12 periodontitis patients were fixed with 4% Paraformaldehyde for more than 24 h at 4 °C. Specimens were embedded in paraffin and cut into 4-μm sections. The section was later dewaxed with routine xylene, followed by dehydration. The sections were prepared for HE staining and IHC assay.
HE staining
Sections of gingival samples were treated with Hematoxylin solution, followed by Hematoxylin differentiation solution treatment as well as Hematoxylin Scott Tap Bluing step. Next, the sections were dehydrated and stained with Eosin dye and sealed with neutral gum. The histopathological changes of human gingival tissues were observed by researchers under an optical microscope to define the periodontal status. The results of HE staining were evaluated and recognized by oral pathologists as well.
Immunohistochemistry assay
To specifically detect target antigens, sections were blocked with 3% BSA after antigen retrieval. After removal of excess liquid, gingival sections were incubated with a rabbit antibody against VNN1 (1:500, ab205912; Abcam), a rabbit antibody against VNN2 (1:500, #39964; Signalway Antibody) and a rabbit antibody against MPO (1:1000, GB11224; Servicebio) overnight according to protocols. Sections, which were washed in PBS for three times, were incubated with biotinylated goat anti-rabbit IgG secondary antibody for 50 min at room temperature. The positive is brownish yellow after DAB chromogenic reaction. After nucleus counterstaining, dehydration and mounting, the sections were scanned under a microscope. The positive area percentage was analyzed as a positive rate in quantitative analysis. Analysis of high-power fields was conducted after random selection to determine percentages of positive areas and the average optical density (AOD).
Statistical analysis
In this study, all data were statistically analyzed with GraphPad Prism 8.0 and reported mean ± standard deviation (SD). Mann–Whitney test was used to compare two groups of independent samples. Correlations between Vanins expression levels and the periodontal clinical parameters, including PD and CAL, were analyzed according to Spearman's correlation analysis. Correlation analysis was further conducted between Vanins and IL1B expression based on RT-qPCR and datasets, between Vanins and MPO based on IHC, and between VNN2 and neutrophil-related indicators based on datasets. * p < 0.05 was considered to be statistically significant. Other forms were expressed as ** p < 0.01, *** p < 0.001 and **** p < 0.0001.
Results
Upregulated expression levels of Vanins in periodontitis gingival tissue samples
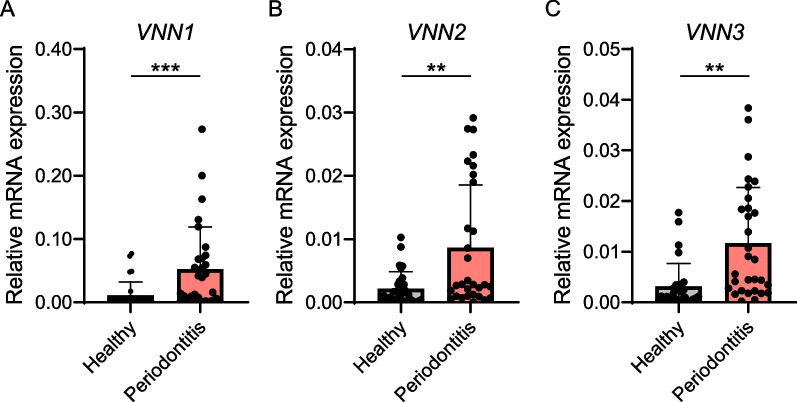
RT-qPCR was used to determine the relative expression levels of VNN1, VNN2 and VNN3. All three were significantly upregulated in diseased gingival tissues from periodontitis (n = 30) in comparison with the periodontal healthy samples (n = 30) (Fig. 1).
Fig. 1.
Upregulation of VNNs in periodontitis in RT-qPCR. Genetic expression levels of VNN1 (A), VNN2 (B), and VNN3 (C), in gingival tissues from periodontal healthy (n = 30) and periodontitis (n = 30) subjects were evaluated. The data were presented in the form of mean ± SD. Significance was defined in the figure as ** p < 0.01 and *** p < 0.001
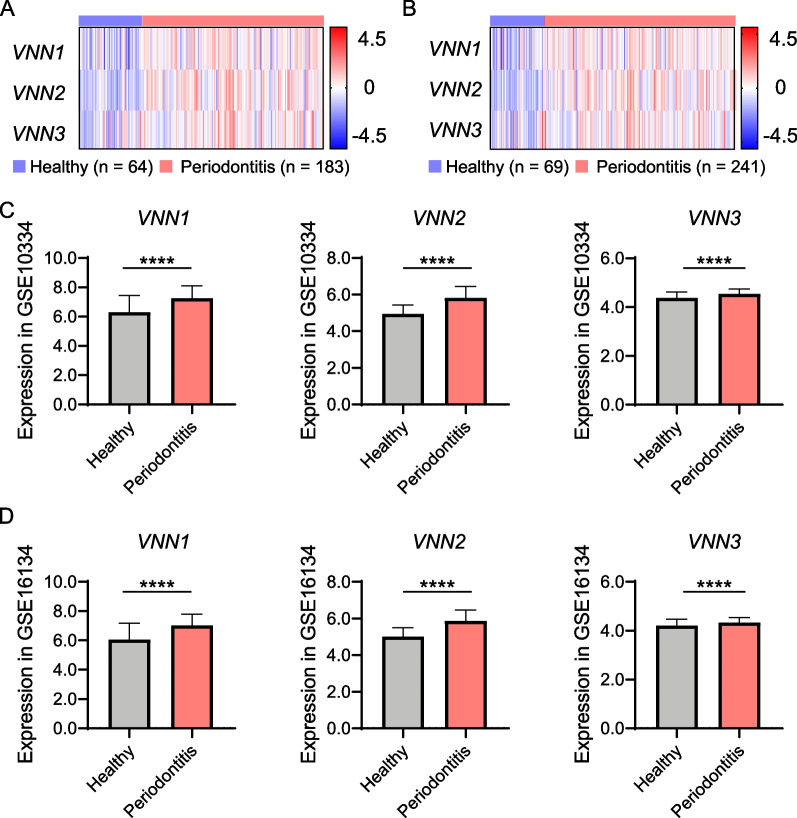
Two datasets, GSE10334 and GSE16134, were studied to further validate Vanins upregulation in human periodontitis. Consistent with the RT -qPCR result from our samples, the expression levels of VNN1, VNN2, and VNN3 in the datasets were significantly higher in diseased gingival tissues (p < 0.0001) (Fig. 2). Generally speaking, expression levels of VNN1, VNN2 and VNN3 were higher in gingival tissues from periodontitis patients at the genetic level in both RT-qPCR and GEO dataset analysis. For VNN3 was previously reported to be a pseudogene for the frame shift owing to the absence of one nucleotide, we decided to only study VNN1 and VNN2 in the proceeding experiments.
Fig. 2.
Validation of gene expression levels of VNNs in datasets. A, C VNNs in healthy (n = 64) and periodontitis (n = 183) sites in dataset GSE10334 were presented. B, D VNNs in healthy (n = 69) and periodontitis (n = 241) sites in dataset GSE16134 were presented. Z-score was analyzed and enrolled in the establishment of heatmaps. Significance was defined in the figure as **** p < 0.0001
As immunoreactive bands and the matched quantitative assessment shown in Fig. 3, VNN1 and VNN2 presented significantly higher concentrations in diseased gingival tissues (n = 6) on the protein level in comparison with periodontal healthy ones (n = 6) (p < 0.01). The upregulation of VNN1 and VNN2 protein levels in western blot was consistent with the results of genetic expression.
Fig. 3.
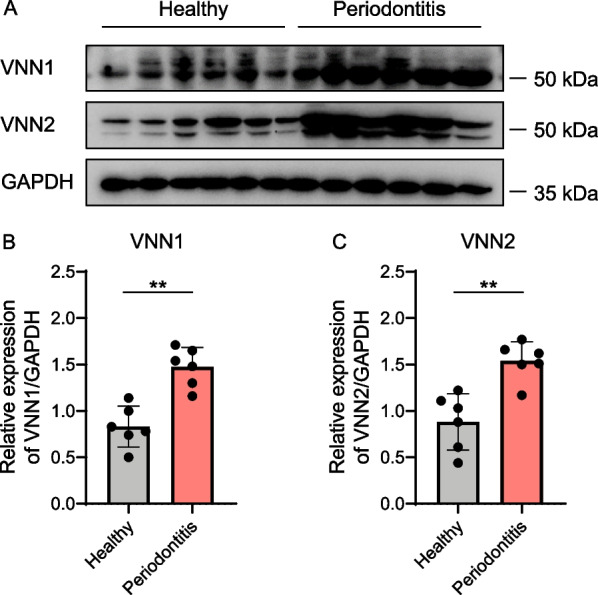
Upregulation of VNN1 and VNN2 in periodontitis in western blot. A Protein expression levels of VNN1 and VNN2 in gingival tissue samples from periodontal healthy (n = 6) and periodontitis (n = 6) subjects were evaluated. B Relative expression level of VNN1 protein when normalized to GAPDH. C Relative expression level of VNN2 protein when normalized to GAPDH. Significance was defined in the figure as ** p < 0.01
Expression and involvement of VNN1 and VNN2 in the histopathological changes of gingival tissues with periodontitis
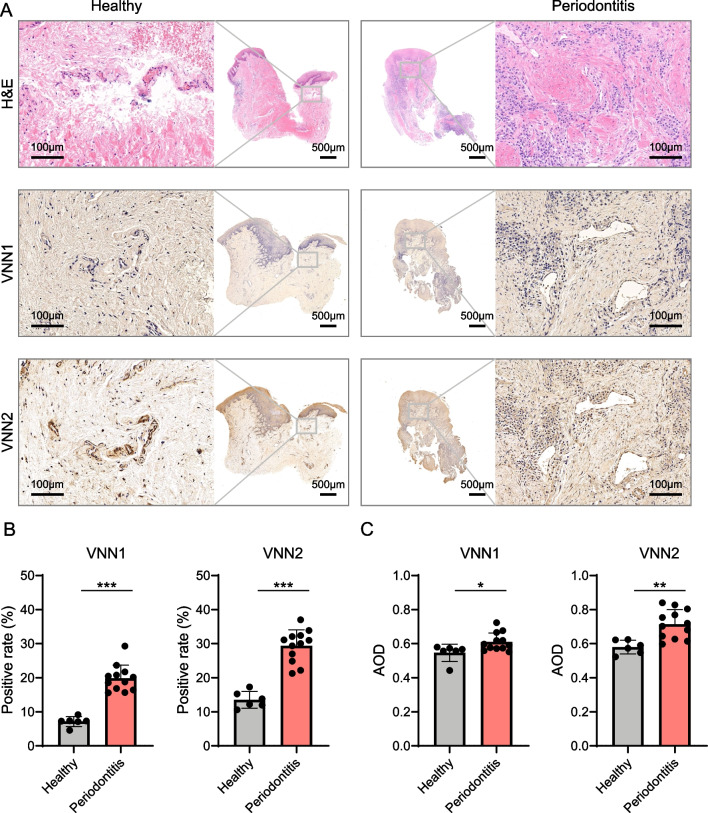
HE staining demonstrated histological characteristics in diseased and periodontal healthy gingival tissues (Fig. 4A). The structure of healthy gingival tissues was normal with clear epithelial and connective tissue layers, and free of inflammation. Whereas in the diseased gingival tissues, a large number of immune cells infiltrated the connective tissues after HE staining. Positive expressions of VNN1 and VNN2 were presented in the connective tissue regions, as indicated by IHC (Fig. 4A). The positive rates of VNN1 and VNN2 were significantly higher in inflamed gingival tissues than in healthy samples (p < 0.001), as shown in Fig. 4B. The average optical density (AOD) of VNN1 and VNN2 was significantly higher in periodontitis gingival tissues than periodontal healthy samples as well (p < 0.05) (Fig. 4C). The results supported that VNN1 and VNN2 were enriched in gingival tissues affected by periodontitis, especially in the part of connective tissues.
Fig. 4.
VNN1 and VNN2 were at higher concentrations and got involved in the histopathological changes of gingival tissues with periodontitis. A HE staining and immunohistochemical staining of VNN1 and VNN2 in gingival tissues of periodontal healthy individuals and patients with periodontitis. Positive expression areas of VNN1 and VNN2 were brownish yellow in the connective tissue. B The positive area percentage of VNN1 and VNN2 in the gingiva from periodontal healthy individuals (n = 6) and patients with periodontitis (n = 12). C The quantitative analysis of AOD of VNN1 and VNN2 in the gingiva from healthy (n = 6) and periodontitis (n = 12) subjects. Significance was defined in the figure as * p < 0.05, ** p < 0.01, and *** p < 0.001. AOD, average optical density
Clinical significance of Vanins in human periodontitis
The association between Vanin family members, VNN1 and VNN2, and periodontal parameters, PD and CAL, were investigated according to Spearman’s correlation analysis (Table 3). The expression level of VNN1 was significantly positively correlated to PD (R = 0.4498, p = 0.0126, moderate) and CAL (R = 0.5867, p = 0.0007, moderate). In addition, the relative expression level of VNN2 was significantly positively related to PD (R = 0.6218, p = 0.0002, moderate) and CAL (R = 0.5912, p = 0.0006, moderate). The positive correlations indicate potential roles Vanins play in periodontitis, and that VNN2 displays a stronger correlation with clinical parameters than VNN1.
Table 3.
Correlation analysis of overexpressed VNNs and periodontal parameters
| PD (mm) | CAL (mm) | |||
|---|---|---|---|---|
| R | p | R | p | |
| VNN1 | 0.4498* | 0.0126 | 0.5867*** | 0.0007 |
| VNN2 | 0.6218*** | 0.0002 | 0.5912*** | 0.0006 |
Statistical significance was indicated as * p < 0.05, and *** p < 0.001
PD Probing depth, CAL Clinical attachment loss
Correlation between upregulated Vanins and inflammation in periodontitis
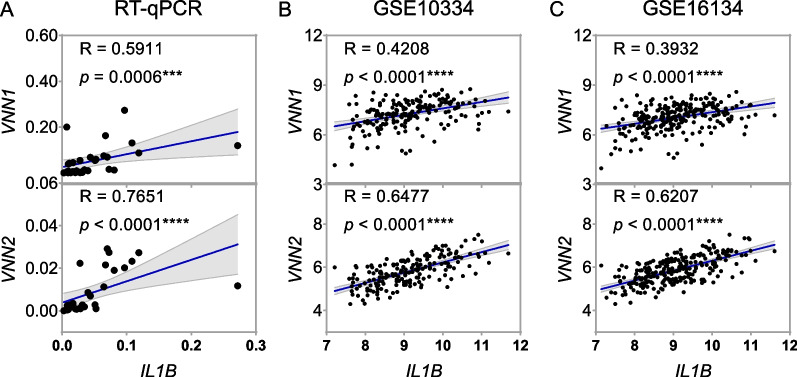
The relationship between VNN1 and VNN2, and IL1B, an indicator of inflammation, was investigated based on datasets and RT-qPCR. As shown in Fig. 5, correlation analysis supported that VNN1 expression was significantly positively correlated with IL1B in inflamed periodontal tissues by RT-qPCR (n = 30, R = 0.5911, p = 0.0006, moderate), and in the datasets GSE10334 (n = 183, R = 0.4208, p < 0.0001, moderate), and GSE16134 (n = 241, R = 0.3932, p < 0.0001, weak). For VNN2, positive correlations were also shown in the tissue samples (n = 30, R = 0.7651, p < 0.0001, strong) and datasets, GSE10334 (n = 183, R = 0.6477, p < 0.0001, moderate), and GSE16134 (n = 241, R = 0.6207, p < 0.0001, moderate). All these results indicated a potential association between Vanin family members and periodontitis.
Fig. 5.
Upregulated VNNs were positively correlated with inflammation. A Correlation between expression levels of VNN1 and IL1B, and between expression levels of VNN2 and IL1B, in gingival tissues of human periodontitis in RT-qPCR (n = 30). B Correlation between VNN1 and IL1B expression levels, and between VNN2 and IL1B expression levels, in periodontitis in GSE10334 (n = 183). C Correlation between VNN1 and IL1B expression levels, and between VNN2 and IL1B expression levels, in periodontitis in GSE16134 (n = 241). Significance was defined in the figure as *** p < 0.001, and **** p < 0.0001
Potential contribution of VNN2 to periodontitis through neutrophils
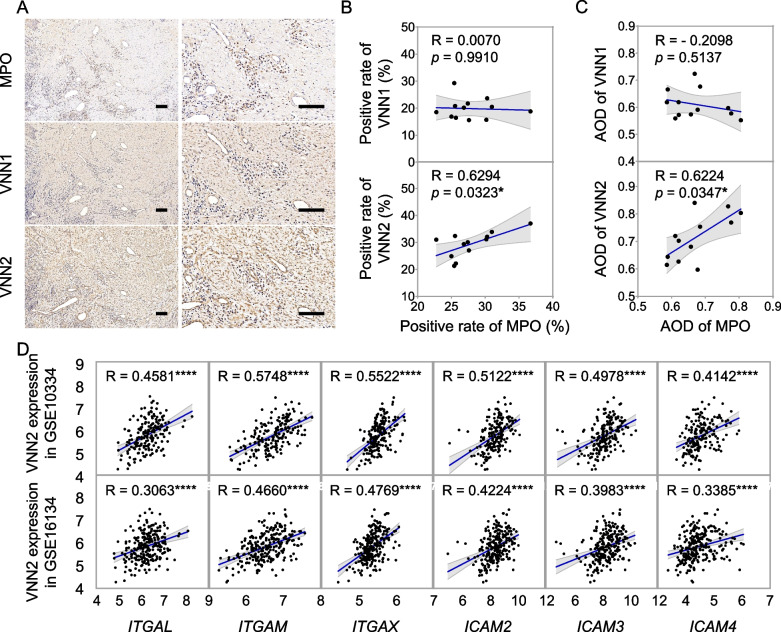
Myeloperoxidase (MPO) expression was measured by IHC (Fig. 6A) in inflamed gingival tissues from periodontitis patients (n = 12). As shown in Fig. 6B, there was a significant and positive correlation between the positive rate of VNN2 and MPO (R = 0.6294, p = 0.0323, moderate). The AOD of VNN2 was also significantly positively correlated to MPO (R = 0.6224, p = 0.0347, moderate) in Fig. 6C. No significant correlation was observed between MPO and VNN1 expression (Fig. 6B, 6C).
Fig. 6.
Upregulated VNN2 was correlated with neutrophil-related immune response in gingival tissues with periodontitis. A Immunohistochemical staining of MPO, VNN1 and VNN2 in gingival tissues with periodontitis. Positive expression areas of VNN1 and VNN2 were brownish yellow in the connective tissue. Positive expression of MPO was nucleus staining. Scale bar = 100 μm. B Scatter plot showed correlation between positive rate of MPO and that of VNN1 and VNN2 in diseased gingival tissues from periodontitis patients (n = 12). C The Scatter plot showed correlation between AOD of MPO and that of VNN1 and VNN2 in inflamed gingival tissues (n = 12). D Scatter plot showed correlation between VNN2 gene expression and indicators of neutrophil adherence and migration in diseased gingival tissues of periodontitis in two datasets, GSE10334 and GSE16134. Significance was defined in the figure as * p < 0.05, and **** p < 0.0001. AOD, average optical density
To further explore the correlation between VNN2 and neutrophils, five genes of integrins, ITGB2/CD18, ITGAL/CD11a, ITGAM/CD11b, ITGAX/CD11c, and ITGAD/CD11d, and 14 genes of 12 ligands, JAM-1, JAM-3, ICAM‑1, ICAM‑2, ICAM‑3, ICAM‑4, ICAM-5, Laminin 8, uPAR, RAGE, Fibrinogen, and VCAM-1 were enrolled as indicators of β2 integrin-dependent neutrophil adherence and migration. As presented in Table 4 and Fig. 6D. The correlation analysis showed the positive association between VNN2 expression level and indicators of neutrophil adherence and migration, including ITGAL, ITGAM, ITGAX, ICAM2, ICAM3 and ICAM4, in periodontitis in both two datasets (R = 0.40–0.69, p < 0.0001, moderate). Taken together, VNN2 was suggested to potentially influence the inflammatory response via regulating β2 integrin-dependent neutrophil adherence and migration in periodontitis.
Table 4.
Correlation between VNN2 and indicators of β2 integrin-dependent neutrophil adherence and migration in periodontitis
| Gene | Type | GSE10334 (n = 183) | GSE16134 (n = 241) | |||
|---|---|---|---|---|---|---|
| R | p | R | p | |||
| ITGB2/CD18 | Integrin | β2 | 0.3993**** | < 0.0001 | 0.2649**** | < 0.0001 |
| ITGAL/CD11a | Integrin | αL | 0.4581**** | < 0.0001 | 0.3063**** | < 0.0001 |
| ITGAM/CD11b | Integrin | αM | 0.5748**** | < 0.0001 | 0.4660**** | < 0.0001 |
| ITGAX/CD11c | Integrin | αX | 0.5522**** | < 0.0001 | 0.4769**** | < 0.0001 |
| ITGAD/CD11d | Integrin | αD | 0.0643 | 0.3874 | 0.0358 | 0.5799 |
| ICAM1 | Ligand | ICAM-1 | 0.0845 | 0.2556 | 0.0371 | 0.5666 |
| ICAM2 | Ligand | ICAM-2 | 0.5122**** | < 0.0001 | 0.4224**** | < 0.0001 |
| ICAM3 | Ligand | ICAM-3 | 0.4978**** | < 0.0001 | 0.3983**** | < 0.0001 |
| ICAM4 | Ligand | ICAM-4 | 0.4142**** | < 0.0001 | 0.3385**** | < 0.0001 |
| ICAM5 | Ligand | ICAM-5 | 0.0623 | 0.4022 | 0.0266 | 0.6813 |
| F11R | Ligand | JAM-1 | -0.3305**** | < 0.0001 | -0.2903**** | < 0.0001 |
| JAM3 | Ligand | JAM-3 | 0.0734 | 0.3233 | 0.0103 | 0.8733 |
| AGER | Ligand | RAGE | -0.0695 | 0.3499 | -0.1317* | 0.0411 |
| FGA | Ligand | Fibrinogen | 0.0694 | 0.3507 | 0.1180 | 0.0674 |
| FGB | Ligand | Fibrinogen | 0.1198 | 0.1063 | 0.0721 | 0.2647 |
| FGG | Ligand | Fibrinogen | -0.2677*** | 0.0002 | -0.2179*** | 0.0007 |
| PLAUR | Ligand | uPAR | 0.2739*** | 0.0002 | 0.2040** | 0.0015 |
| LAMC1 | Ligand | Laminin 8 | 0.3070**** | < 0.0001 | 0.2647**** | < 0.0001 |
| VCAM1 | Ligand | VCAM1 | 0.2536*** | 0.0005 | 0.2056** | 0.0013 |
Statistical significance was indicated as * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001
Discussion
In the current study, we analyzed the expression profile of Vanin family members and their correlation with periodontitis on the aspect of clinical parameters, the severity of inflammation and neutrophil response. Results suggested that the Vanins were upregulated in the gingival tissues from patients with periodontitis when compared with periodontal healthy individuals, among which two members, VNN1 and VNN2 were elevated on both the genetic and protein levels. Correlation analyses further demonstrated that VNN1 and VNN2 were respectively associated with clinical parameters, PD and CAL, and indicator of inflammation, IL-1B. What’s more, the VNN2 might interact with neutrophil during periodontitis pathogenesis, with positive evidence between VNN2 and neutrophil-related biomarkers, MPO and indicators of β2 integrin-dependent neutrophil adherence and migration. This study characterized the expression profile of Vanins family members and their contribution to periodontitis.
For clinical relevance, Vanins have been closely related to clinical indicators in diseased animal models, and other human samples, alone with the conclusion that the abnormal accumulation of Vanins may lead to decreased biological function and prognosis [19–21, 30–33]. For instance, plasma VNN1 is increased in trauma patients and is independently associated with the risk of sepsis [20]. For patients with rheumatoid arthritis, VNN2 presents high concentrations in synovial fluids, indicating its role in inflammatory disease [21]. Vanins are also associated with decreased kidney function on the aspect of worse clinical parameters of renal function in rat model and human beings [31–33]. The clinical significance of Vanins provides clues for further studies about diagnosis and prognosis of diseases. In the current study, we performed comparison of Vanins expression between periodontitis and periodontal healthy gingival tissues, and concluded that higher concentrations of Vanins were accompanied with worse periodontal health conditions. With the primary aim to create accessibility for deep untreated pockets, periodontal surgery should be limited to periodontal pockets deeper than 5 mm to avoid mechanical damage to the periodontium[34]. In this study, gingival tissue samples, from patients with PD = (6.75 ± 1.41) mm and CAL = (6.06 ± 2.04) mm, were collected during periodontal flap surgery. The results may be more valid among the patients with severe periodontitis due to the sample collection methods. In the future, periodontitis of different severity, from mild to severe, could be recruited to better demonstrate the relationship between Vanins and this inflammatory disease, and to improve the generalizability of the findings.
In this study, correlation analysis was conducted between Vanins and IL1B, which is a highly inflammatory cytokine and promotes the recruitment of neutrophils at the inflamed sites through the overexpression of E-selectin and ICAM-1 [35–37]. NLRP3 inflammasome-dependent IL-1β production was reported to promote neutrophil recruitment, and neutrophils were in turn important sources of IL-1β in acute inflammatory disorders [38, 39]. Due to close relationships among IL-1β, neutrophils, and severity of inflammation, the positive correlation between Vanins and IL1B presented the potential functions of affecting the severity of the disease.
The mechanism behind Vanins in inflammation could be closely related to the regulation of neutrophil, including neutrophil activation, neutrophil degranulation, and leukocyte adhesion [40]. Previous studies have suggested that VNN2 regulates leukocyte adherence and migration through physically associating with Mac-1 (CD11b/CD18), an adhesive molecule essential to neutrophil function [41, 42]. In colorectal carcinogenesis, both Vanins and MPO interact with Mac-1 (CD11b/CD18), and regulate tissue destruction by activating inflammatory cells to the inflamed site [43]. In the current study, the positive correlation between VNN2 and MPO in IHC presented the potential regulation of VNN2 during this process. The correlation analysis between VNN2 and indicators of β2 integrin-dependent neutrophil adherence and migration further provided evidence to the contribution of VNN2 through regulating neutrophil migration by associating with Mac-1 (CD11b/CD18) on the human neutrophil surface in periodontitis.
On the other hand, VNN1 was not correlated to MPO in IHC, which led us to think further about the possible mechanism. It is well known that VNN1 could influence oxidative stress response and license the production of inflammatory mediators by antagonizing peroxisome proliferator-activated receptor-γ (PPAR-γ), which works as a negative regulatory factor of NF-κB with anti-inflammatory effects [44]. The absence of VNN1 could reduce inflammation and oxidative stress during infection or injury [45]. In acute pancreatitis, upregulation of S100A9 induces cell injury and inflammatory response through activating NLRP3 via targeting VNN1-mediated ROS release [15]. Considerable evidence has implicated the association of reactive oxygen response with the pathogenesis of periodontitis [10, 46]. The inflammatory response in periodontitis was suggested to be associated with increased local and systemic oxidative stress and impaired antioxidant capacity [4, 10, 47]. The link between periodontitis, oxygen stress and VNN1 may provide clues for further exploration of the pathological process of periodontitis [10]. Therefore, further studies need to be carried out to investigate the deeper mechanism behind Vanins’ regulation in periodontitis.
Conclusion
In summary, this study revealed the upregulation of Vanin family members in periodontitis at both genetic and protein levels. The clinical significance of Vanins and their positive association with inflammation indicated their potential involvement in periodontitis. Furthermore, VNN2 was positively correlated with indicators of neutrophils, indicating the potential contribution of VNN2 to periodontitis via affecting neutrophil adherence and migration during immune response. All our results indicated possible significance of Vanin family members in human periodontitis and might act as potential therapeutic targets to treat periodontitis.
Supplementary Information
Additional file 1: Supplementary File 1. STROBE checklist. The study has followed the STROBE guidelines and added the STROBE checklist.
Additional file 2: Supplementary Figure 1. Flow diagram for the study groups. The enrollment of participants for the periodontitis group (A) and the periodontal healthy group (B) were presented respectively. Supplementary Figure 2. Uncropped images of western blot in Figure 3, including bolts of VNN1 (A), VNN2 (B), and GAPDH (C). P, periodontitis group; H, periodontal healthy group.
Acknowledgements
Not applicable.
Abbreviations
- AOD
Average optical density
- BOP
Bleeding on probing
- CAL
Clinical attachment loss
- GEO
Gene Expression Omnibus
- HE
Hematoxylin–eosin
- IHC
Immunohistochemistry
- MPO
Myeloperoxidase
- NCBI
National Center for Biotechnology Information
- PPAR-γ
Peroxisome proliferator-activated receptor-γ
- PBS
Phosphate buffered saline
- PD
Probing depth
- RT-qPCR
Real-time quantitative polymerase chain reaction
- SD
Standard deviation
- VNN
Vanin
Authors’ contributions
Research design: Weijun Yu, Min Jin, Eryi Lu; Methodology: Shucheng Hu, Lu Lin, Yuting Gu; Project administration: Weijun Yu, Ruhan Yang; Research supervision: Yuting Gu, Bin Jiang, Yuhua Gong, Eryi Lu; Data analysis: Shucheng Hu, Chuanyuan Mao, Yuting Gu, Guanglong Li; Writing-original draft: Weijun Yu, Shucheng Hu; Writing-review & editing: Bin Jiang, Yuhua Gong, Eryi Lu. All authors read and approved the final version of manuscript and agreed to be accountable for all aspects of the work.
Funding
This study was supported by National Natural Science Foundation of China (52171075), Science and Technology Commission of Shanghai Municipality (21DZ2294700, 201409006300), the Opening Project of Shanghai Key Laboratory of Orthopaedic Implant (KFKT2021001) and the Project of Shanghai Key Laboratory for Nucleic Acid Chemistry and Nanomedicine (2021ZYA009).
Availability of data and materials
Data will be available from corresponding authors on the reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Renji Hospital, Shanghai Jiao Tong University School of Medicine (KY2021-196-B). The study design was developed according to Helsinki Declaration. The research group obtained the written informed consent from all participants enrolled.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bin Jiang, Email: 13916963436@qq.com.
Yuhua Gong, Email: rjgongyuhua@163.com.
Eryi Lu, Email: lueryi222@outlook.com.
References
- 1.Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases Nat Rev Dis Primers. 2017;3:17038. doi: 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- 2.Slots J. Periodontitis: facts, fallacies and the future. Periodontol 2000. 2017;75(1):7–23. doi: 10.1111/prd.12221. [DOI] [PubMed] [Google Scholar]
- 3.Frencken JE, Sharma P, Stenhouse L, Green D, Laverty D, Dietrich T. Global epidemiology of dental caries and severe periodontitis - a comprehensive review. J Clin Periodontol. 2017;44(Suppl 18):S94–s105. doi: 10.1111/jcpe.12677. [DOI] [PubMed] [Google Scholar]
- 4.Sczepanik FSC, Grossi ML, Casati M, et al. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontol 2000. 2020;84(1):45–68. doi: 10.1111/prd.12342. [DOI] [PubMed] [Google Scholar]
- 5.Curtis MA, Diaz PI, Van Dyke TE. The role of the microbiota in periodontal disease. Periodontol 2000. 2020;83(1):14–25. doi: 10.1111/prd.12296. [DOI] [PubMed] [Google Scholar]
- 6.Lin L, Yu W, Zhang W, et al. Expression profile of lipoxygenases in gingival tissues of human periodontitis. Oral Dis. 2021;27(3):567–576. doi: 10.1111/odi.13558. [DOI] [PubMed] [Google Scholar]
- 7.Yu W, Gu Q, Wu D, et al. Identification of potentially functional circRNAs and prediction of circRNA-miRNA-mRNA regulatory network in periodontitis: Bridging the gap between bioinformatics and clinical needs. J Periodontal Res. 2022;57(3):594–614. doi: 10.1111/jre.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajishengallis G, Moutsopoulos NM, Hajishengallis E, Chavakis T. Immune and regulatory functions of neutrophils in inflammatory bone loss. Semin Immunol. 2016;28(2):146–158. doi: 10.1016/j.smim.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajishengallis G, Chavakis T, Hajishengallis E, Lambris JD. Neutrophil homeostasis and inflammation: novel paradigms from studying periodontitis. J Leukoc Biol. 2015;98(4):539–548. doi: 10.1189/jlb.3VMR1014-468R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Andrukhov O, Rausch-Fan X. Oxidative Stress and Antioxidant System in Periodontitis. Front Physiol. 2017;8:910. doi: 10.3389/fphys.2017.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajishengallis G. New developments in neutrophil biology and periodontitis. Periodontol 2000. 2020;82(1):78–92. doi: 10.1111/prd.12313. [DOI] [PubMed] [Google Scholar]
- 12.Nawaz MZ, Attique SA, Ain QU, et al. Discovery and characterization of dual inhibitors of human Vanin-1 and Vanin-2 enzymes through molecular docking and dynamic simulation-based approach. Int J Biol Macromol. 2022;213:1088–1097. doi: 10.1016/j.ijbiomac.2022.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Kaskow BJ, Proffitt JM, Blangero J, Moses EK, Abraham LJ. Diverse biological activities of the vascular non-inflammatory molecules - the Vanin pantetheinases. Biochem Biophys Res Commun. 2012;417(2):653–658. doi: 10.1016/j.bbrc.2011.11.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Millet V, Gensollen T, Maltese M, et al. Harnessing the Vnn1 pantetheinase pathway boosts short chain fatty acids production and mucosal protection in colitis. Gut. 2022;gutjnl-2021–325792. 10.1136/gutjnl-2021-325792. Online ahead of print. [DOI] [PubMed]
- 15.Xiang H, Guo F, Tao X, et al. Pancreatic ductal deletion of S100A9 alleviates acute pancreatitis by targeting VNN1-mediated ROS release to inhibit NLRP3 activation. Theranostics. 2021;11(9):4467–4482. doi: 10.7150/thno.54245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nitto T, Onodera K. Linkage between coenzyme a metabolism and inflammation: roles of pantetheinase. J Pharmacol Sci. 2013;123(1):1–8. doi: 10.1254/jphs.13R01CP. [DOI] [PubMed] [Google Scholar]
- 17.Dahlgren C, Karlsson A, Sendo F. Neutrophil secretory vesicles are the intracellular reservoir for GPI-80, a protein with adhesion-regulating potential. J Leukoc Biol. 2001;69(1):57–62. doi: 10.1189/jlb.69.1.57. [DOI] [PubMed] [Google Scholar]
- 18.Soler DC, Kerstetter-Fogle A, Young AB, et al. Healthy myeloid-derived suppressor cells express the surface ectoenzyme Vanin-2 (VNN2) Mol Immunol. 2022;142:1–10. doi: 10.1016/j.molimm.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen S, Zhang W, Tang C, Tang X, Liu L, Liu C. Vanin-1 is a key activator for hepatic gluconeogenesis. Diabetes. 2014;63(6):2073–2085. doi: 10.2337/db13-0788. [DOI] [PubMed] [Google Scholar]
- 20.Lu H, Zhang A, Wen D, et al. Plasma Vanin-1 as a Novel Biomarker of Sepsis for Trauma Patients: A Prospective Multicenter Cohort Study. Infect Dis Ther. 2021;10(2):739–751. doi: 10.1007/s40121-021-00414-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Takeda Y, Watanabe T, Sendo F. A sandwich ELISA for detection of soluble GPI-80, a glycosylphosphatidyl-inositol (GPI)-anchored protein on human leukocytes involved in regulation of neutrophil adherence and migration–its release from activated neutrophils and presence in synovial fluid of rheumatoid arthritis patients. Microbiol Immunol. 2001;45(6):467–471. doi: 10.1111/j.1348-0421.2001.tb02646.x. [DOI] [PubMed] [Google Scholar]
- 22.Gensollen T, Bourges C, Rihet P, et al. Functional polymorphisms in the regulatory regions of the VNN1 gene are associated with susceptibility to inflammatory bowel diseases. Inflamm Bowel Dis. 2013;19(11):2315–2325. doi: 10.1097/MIB.0b013e3182a32b03. [DOI] [PubMed] [Google Scholar]
- 23.Jansen PA, Kamsteeg M, Rodijk-Olthuis D, et al. Expression of the vanin gene family in normal and inflamed human skin: induction by proinflammatory cytokines. J Invest Dermatol. 2009;129(9):2167–2174. doi: 10.1038/jid.2009.67. [DOI] [PubMed] [Google Scholar]
- 24.Bartucci R, Salvati A, Olinga P, Boersma YL. Vanin 1: Its Physiological Function and Role in Diseases. Int J Mol Sci. 2019;20(16):3891. doi: 10.3390/ijms20163891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corbi SCT, de Vasconcellos JF, Bastos AS, et al. Circulating lymphocytes and monocytes transcriptomic analysis of patients with type 2 diabetes mellitus, dyslipidemia and periodontitis. Sci Rep. 2020;10(1):8145. doi: 10.1038/s41598-020-65042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapple ILC, Mealey BL, Van Dyke TE, et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89(Suppl 1):S74–s84. doi: 10.1002/JPER.17-0719. [DOI] [PubMed] [Google Scholar]
- 27.Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89(Suppl 1):S173–S182. doi: 10.1002/JPER.17-0721. [DOI] [PubMed] [Google Scholar]
- 28.Yun J, Duan Q, Wang L, et al. Differential expression of leukocyte β2 integrin signal transduction-associated genes in patients with symptomatic pulmonary embolism. Mol Med Rep. 2014;9(1):285–292. doi: 10.3892/mmr.2013.1780. [DOI] [PubMed] [Google Scholar]
- 29.Schittenhelm L, Hilkens CM, Morrison VL. β(2) Integrins As Regulators of Dendritic Cell, Monocyte, and Macrophage Function. Front Immunol. 2017;8:1866. doi: 10.3389/fimmu.2017.01866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeda Y, Kurota Y, Kato T, et al. GPI-80 Augments NF-κB Activation in Tumor Cells. Int J Mol Sci. 2021;22(21):12027. doi: 10.3390/ijms222112027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosohata K, Jin D, Takai S, Iwanaga K. Involvement of Vanin-1 in Ameliorating Effect of Oxidative Renal Tubular Injury in Dahl-Salt Sensitive Rats. Int J Mol Sci. 2019;20(18):4481. doi: 10.3390/ijms20184481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosohata K, Matsuoka H, Iwanaga K, Kumagai E. Urinary vanin-1 associated with chronic kidney disease in hypertensive patients: A pilot study. J Clin Hypertens (Greenwich) 2020;22(8):1458–1465. doi: 10.1111/jch.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosohata K, Matsuoka H, Kumagai E. Association of urinary vanin-1 with kidney function decline in hypertensive patients. J Clin Hypertens (Greenwich) 2021;23(7):1316–1321. doi: 10.1111/jch.14295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graziani F, Karapetsa D, Alonso B, Herrera D. Nonsurgical and surgical treatment of periodontitis: how many options for one disease. Periodontol 2000. 2017;75(1):152–88. doi: 10.1111/prd.12201. [DOI] [PubMed] [Google Scholar]
- 35.Mitroulis I, Kambas K, Ritis K. Neutrophils, IL-1β, and gout: is there a link? Semin Immunopathol. 2013;35(4):501–512. doi: 10.1007/s00281-013-0361-0. [DOI] [PubMed] [Google Scholar]
- 36.Drummond RA, Swamydas M, Oikonomou V, et al. CARD9(+) microglia promote antifungal immunity via IL-1β- and CXCL1-mediated neutrophil recruitment. Nat Immunol. 2019;20(5):559–570. doi: 10.1038/s41590-019-0377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibuya R, Ishida Y, Hanakawa S, et al. CCL2‒CCR2 Signaling in the Skin Drives Surfactant-Induced Irritant Contact Dermatitis through IL-1β‒Mediated Neutrophil Accumulation. J Invest Dermatol. 2022;142(3 Pt A):571–82.e9. doi: 10.1016/j.jid.2021.07.182. [DOI] [PubMed] [Google Scholar]
- 38.Amaral FA, Costa VV, Tavares LD, et al. NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B(4) in a murine model of gout. Arthritis Rheum. 2012;64(2):474–484. doi: 10.1002/art.33355. [DOI] [PubMed] [Google Scholar]
- 39.Iula L, Keitelman IA, Sabbione F, et al. Autophagy Mediates Interleukin-1β Secretion in Human Neutrophils. Front Immunol. 2018;9:269. doi: 10.3389/fimmu.2018.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nitto T, Takeda Y, Yoshitake H, Sendo F, Araki Y. Structural divergence of GPI-80 in activated human neutrophils. Biochem Biophys Res Commun. 2007;359(2):227–233. doi: 10.1016/j.bbrc.2007.05.087. [DOI] [PubMed] [Google Scholar]
- 41.Takeda Y, Kato T, Ito H, et al. The pattern of GPI-80 expression is a useful marker for unusual myeloid maturation in peripheral blood. Clin Exp Immunol. 2016;186(3):373–386. doi: 10.1111/cei.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inoue T, Kato T, Hikichi Y, et al. Stent-induced neutrophil activation is associated with an oxidative burst in the inflammatory process, leading to neointimal thickening. Thromb Haemost. 2006;95(1):43–48. doi: 10.1160/TH05-08-0591. [DOI] [PubMed] [Google Scholar]
- 43.Mariani F, Roncucci L. Role of the Vanins-Myeloperoxidase Axis in Colorectal Carcinogenesis. Int J Mol Sci. 2017;18(5):918. doi: 10.3390/ijms18050918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang B, Lo C, Shen L, et al. The role of vanin-1 and oxidative stress-related pathways in distinguishing acute and chronic pediatric ITP. Blood. 2011;117(17):4569–4579. doi: 10.1182/blood-2010-09-304931. [DOI] [PubMed] [Google Scholar]
- 45.van Diepen JA, Jansen PA, Ballak DB, et al. Genetic and pharmacological inhibition of vanin-1 activity in animal models of type 2 diabetes. Sci Rep. 2016;6:21906. doi: 10.1038/srep21906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhattarai G, Poudel SB, Kook SH, Lee JC. Resveratrol prevents alveolar bone loss in an experimental rat model of periodontitis. Acta Biomater. 2016;29:398–408. doi: 10.1016/j.actbio.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 47.Chen M, Cai W, Zhao S, et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: A systematic review and meta-analysis. J Clin Periodontol. 2019;46(6):608–622. doi: 10.1111/jcpe.13112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary File 1. STROBE checklist. The study has followed the STROBE guidelines and added the STROBE checklist.
Additional file 2: Supplementary Figure 1. Flow diagram for the study groups. The enrollment of participants for the periodontitis group (A) and the periodontal healthy group (B) were presented respectively. Supplementary Figure 2. Uncropped images of western blot in Figure 3, including bolts of VNN1 (A), VNN2 (B), and GAPDH (C). P, periodontitis group; H, periodontal healthy group.
Data Availability Statement
Data will be available from corresponding authors on the reasonable request.