Abstract
Background
Assessing medication adherence in rheumatoid arthritis (RA) is clinically significant as low adherence is associated with high disease activity. Self-reported medication adherence surveys have been shown to have problems with overestimation of adherence due to social desirability bias. However, no MTX adherence studies adjusted for social desirability have been conducted to date. This study aimed to evaluate adherence to MTX and perform an investigatory search for factors associated with MTX adherence including social desirability.
Methods
This cross-sectional multicenter study was conducted among adult RA patients consuming oral MTX for ≥ 3 months. We examined the distribution of MTX adherence, according to the eight-item Morisky Medication Adherence Scale (MMAS-8). Social desirability was using the Social Desirability Scale (SDS). Furthermore, an exploratory factor analysis involving social desirability was examined to identify factors associated with MTX adherence using linear regression analysis. To deal with missing values, we used multiple imputations with chained equations methods.
Results
A total of 165 RA patients were enrolled. The median age was 64 years, and 86.1% were women. Based on the MMAS-8, low, medium, and high adherences were noted in 12.1%, 60.0%, and 27.9% of participants, respectively. High social desirability (coefficient, 0.14; 95% confidence interval [CI], 0.05–0.23; p < 0.05) and high age (coefficient per 10 years, 0.16; 95% CI, 0.01–0.03; p < 0.05) were associated with high MTX adherence, whereas full-time work was negatively associated with high MTX adherence (coefficient, -0.50; 95% CI, -0.95–-0.05; p < 0.05).
Conclusions
A large proportion of patients with RA do not take MTX as prescribed. High social desirability, high educational level, and non-full-time work may be associated with high MTX adherence. Physicians should confirm MTX adherence before switching or adding disease-modifying anti-rheumatic drugs in cases of uncontrolled disease activity.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41927-022-00305-8.
Keywords: Rheumatoid arthritis, Methotrexate, Drug monitoring
Background
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by chronic inflammation. RA affects the joints and leads to functional disability in the absence of appropriate treatment [1]. The 2015 American College of Rheumatology (ACR) and the 2019 European League against Rheumatism recommendations suggested the early use of disease-modifying antirheumatic-drugs (DMARDs), usually methotrexate (MTX), in most active RA patients [2, 3]. Several studies have reported that low medication adherence is associated with high RA disease activity [4, 5]. Hence, MTX adherence in RA patients is crucial.
Non-adherence can be categorized as non-initiation, poor execution (accidental or intentional) or non-persistence [6]. As a chronic disease, RA is important for drug continuation, and attention should be focused on non-persistence non-adherence. Several studies on adherence to MTX have been conducted. However, MTX adherence differed among the studies because of the various definitions of adherence, different follow-up durations, and heterogeneity of patient populations. A previous study reported that MTX adherence is associated with age, sex, race, RA disease activity, patient beliefs about the medication, disease duration, mental health, and socio-economic status (SES) [4, 7–11]. In a systematic review, beliefs on the necessity and efficacy of MTX, high mood, mild disease, and MTX monotherapy were identified as the most reliable variables related to MTX adherence [12].
The common propensity for people to show themselves in the most favorable light in relation to society values and standards is known as social desirability [13]. Clinically, social desirability may result in underreporting of socially and culturally "undesirable" habits and behaviors, which may eventually result in lost chances for intervention and unrecognized pharmacological contraindications [14, 15]. It is well known to influence the use of self-reported questionnaires in research. A previous study reported that social desirability is the tendency to respond to self-reported items in a way that reflects better on the respondent rather than acting accurately and truthfully [16]. Self-reported medication adherence surveys have been shown to have problems with overestimation of adherence due to social desirability bias [17, 18]. A previous MTX adherence study has reported the need to control this bias [19]; however, no studies have investigated the MTX adherence survey by controlling for social desirability. Thus, this study aimed to evaluate the effect of already known variables plus social desirability.
Methods
Study design
This cross-sectional study was conducted in four hospitals: Showa University Hospital, Showa University Northern Yokohama Hospital, Showa University Fujigaoka Hospital, and Kanto Rosai Hospital. Data collection was conducted only once during the survey.
Patient population
Participants were outpatients who met the 2010 ACR criteria for RA [20]. The inclusion criteria were age ≥ 20 years and consumption of oral MTX for ≥ 3 months. The exclusion criteria were patients with dementia, restlessness, and severe psychiatric disorders. Consecutive sampling was employed. Patients were recruited between August 2013 and October 2014.
Outcome
The primary outcome was the distribution of MTX adherence according to MMAS-8 at the time of the survey based on the patient’s description. MMAS-8 consists of an eight-item questionnaire [21–23]. MMAS-8 scores range from 0 to 8 and are classified as follows: low, < 6; medium, > 6 and < 8; and high, 8. Originally, MMAS-8 was developed for daily administration of oral medicine. MTX was administered once a week; thus, we developed the Japanese questionnaire for MTX accordingly after obtaining the original developer’s permission. First, a physician (N.Y.), a pharmacist (T.K.) with experience in scale development translated the scale into Japanese. Second, it was back-translated into English by two professional translators. N.Y., T.K. and two professional translators compared the items with the original items, and discussed the questionnaire and achieved a consensus, and revised the translated and back-translated versions. Finally, we were sent to the original author to confirm the semantic content, and some minor improvements were made. The final version was approved by the original author. Then, five Japanese RA participants took part in a pilot test to see if they could understand the questions and respond to them clearly, if the language was clear, if there were any technical or strange words that the subjects couldn't understand, and if the questions were appropriate for Japanese culture.
Data collection
Social desirability is typically assessed using the Social Desirability Scale (SDS) developed by Crowne and Marlowe in 1960, with 33 items [24]. By "the urge to seek acceptance by acting in a culturally relevant and acceptable way," this scale defines social desirability. Furthermore, a 13-item SDS was developed with confirmed validity and reliability [25–27]. In this study, we used the 13-item SDS. The responses are recorded on “True” or “False. Add 1 point to the score for each “True” response to statements 5, 7, 9, 10, and 13. Add 0 points to the score for each “False” response to these statements. Add 1 point to the score for each “False” response to statements 1, 2, 3, 4, 6, 8, 11, and 12. Add 0 points to the score for each “True” response to these statements. The score by domains ranges from 0 to 13. Higher scores indicated higher social desirability.
MTX dose, MTX dosing frequency, duration of MTX treatment were collected. RA disease activity was assessed using the Disease Activity Score in 28 joints (DAS28-ESR). [28]. Activities of daily living (ADL) was assessed using the modified Health Assessment Questionnaire (mHAQ), which is a self-reported questionnaire that measures function, including ADL performance. Depression state was defined using the Centre for Epidemiological Studies-Depression (CES-D) scale[29]. The patient’s perceptions towards medications were assessed using the Belief about Medicines Questionnaire-Specific (BMQ-specific), which includes two domains, BMQ-Specific necessity (5 items) and BMQ-Specific concern (5 items). We used the BMQ necessity-concern differential (“BMQ-Specific necessity” minus “BMQ-Specific concern”) predicted adherence most strongly in studies affecting adherence [30]. Pain severity was assessed using the Brief Pain Inventory (BPI).We used the average numerical rating scale (NRS) pain score other clinical studies have applied in the multivariable analysis. Short Form-8 (SF-8) is a health-related quality of life (QoL) questionnaire [31, 32] We used two summary scores: mental component summary (MCS; showing mental status) and physical component summary (PCS; showing physical status). The Japanese versions of mHAQ, CES-D, BMQ, BPI, SDS, and SF-8 were validated [33, 34].
Additionally, we obtained data on SES, including marital status, educational level, employment status, and living status. Data on patient characteristics, medication, and SES were further collected using questionnaires. Other data were obtained from medical chart records. After consulting the doctor, the participants answered the Morisky Medication Adherence Scale (MMAS-8), mHAQ, CES-D, BMQ, BPI, SDS, SF-8, and SES questionnaires; they returned the completed questionnaires to the data center by mail.
Statistical analyses
MMAS-8, sex, CES-D, and SES were used as categorical variables, whereas age, MTX dose, MTX dosing frequency, duration of MTX treatment, disease duration, DAS28-ESR, mHAQ, BMQ, BPI, SDS, and SF-8 were used as continuous variables. Summary statistics were presented as median with interquartile range (IQR) and numbers with proportion (%). First, we evaluated the distribution of MTX adherence according to MMAS-8. Subsequently, we compared MMAS-8 and pre-described factors using one-way analysis of variance (ANOVA), Kruskal–Wallis test, and chi-squared test. For significant factors, we conducted a trend analysis. Finally, multiple linear regression analysis was performed to exploratively assess factors associated with MTX adherence The co-variables selected were as follows: age, sex, disease duration, RA disease activity (DAS28-ESR), depression state (CES-D), reliability of the medication (BMQ necessity-concern differential), social desirability (SDS), educational level (more than, equal to college-level, or not), and employment status (full-time work or not). These factors were further selected based on previous studies and clinical importance [4, 7–11, 35–37]. To compare the mean DAS-28 scores among the three groups using ANOVA, the effect size (small, 0.1; medium, 0.25; and large: 0.4) customarily proposed by Cohen [38] was set at medium (0.25), the significance level was set at 5% on both sides, and the power was set at 80%. The total number of cases was calculated to be 159. The target number of cases was 176, assuming a dropout rate of 10%.
To deal with missing values, we used multivariable multiple imputations with chained equations methods to increase power and minimize selection bias since we considered missing data to be an assumption of missing at random. We included age, sex, disease duration, RA disease activity, depression state, reliability of the medication, social desirability, educational level, and employment status for each imputation model. We generated 10 imputed datasets and combined coefficient estimates using Rubin’s rules for each imputation.
A two-sided p-value < 0.05 was considered statistically significant. All statistical analyses were conducted using STATA 14.2 (StataCorp LP, College Station, TX) software.
Ethical considerations
The ethics committee of Showa University Hospital (approval number 1446) and Showa University Toyosu Hospital, Showa University Northern Yokohama Hospital, Kanto Rosai Hospital approved this study, and informed consent was obtained from all participants before study enrolment. All study procedures were performed in accordance with the Declaration of Helsinki. Patient information was anonymized and de-identified before analysis.
Results
Patient flow chart
Figure 1 shows the patient flow chart. Initially, 181 RA patients were invited. Of 169 patients eligible for the study, four were excluded because of missing MTX adherence data (n = 2) and clinical data (n = 2). A total of 165 RA patients were included in the final analysis.
Fig. 1.
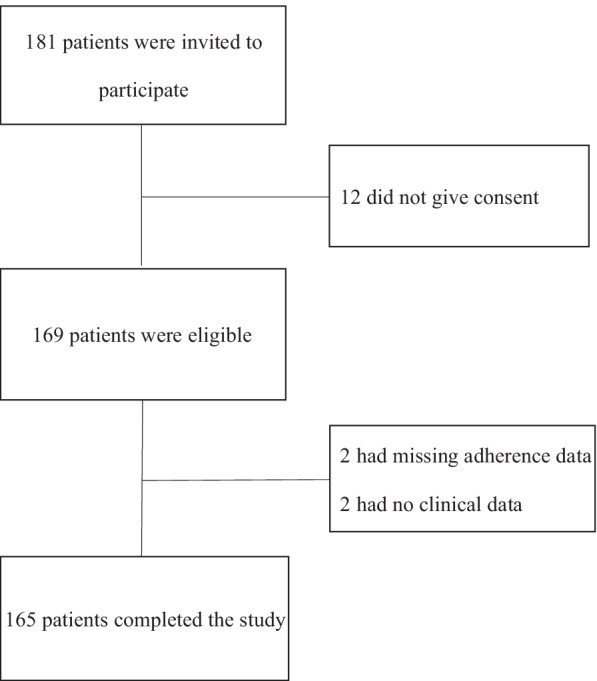
Patient flow chart
Baseline characteristics
The characteristics of the participants are shown in Table 1. The median age of the patients was 64 years (IQR, 54–72), and 86.1% were women. The median dose of MTX was 8 mg per week (IQR, 6–12), median MTX dosing frequency was three times per week (IQR, 2–3), and median duration of MTX treatment was 36 months (IQR, 17–75). The proportion of missing data varied from 0 to 15.2% (Additional file 1: Table S1).
Table 1.
Patient characteristics by grade of MTX adherence divided by MMAS-8 (n = 165)
| All (n = 165) |
MMAS-8 | p value | |||
|---|---|---|---|---|---|
| Low (n = 20) |
Medium (n = 99) |
High (n = 46) |
|||
| Age, median (IQR) |
64 (54–72) |
58 (44–62) |
65 (53–73) |
65.5 (59–74) |
0.01 |
| Female, n (%) | 142 (86.1) | 18 (90.0) | 84 (84.6) | 38 (82.6) | 0.74 |
| MTX dose (mg/week), median (IQR) | 8 (6–12) | 10 (8–12) | 8 (6–10) | 10 (8–12) | 0.17 |
| MTX dosing frequency (times/week), median (IQR) | 3 (2–3) | 3 (2–3) | 2 (2–3) | 2 (2–3) | 0.54 |
|
Duration of MTX treatment (month), median (IQR) |
36 (17–75) | 32 (16–79) | 35 (18–80) | 41 (13–74) | 0.15 |
|
Disease duration (month), median (IQR) |
53.5 (22.5–123.5) |
35 (22–120) |
47 (22–111) |
60.5 (24–158) |
0.95 |
| Concomitant medication | |||||
| NSAIDS, n (%) | 59 (35.8) | 5 (25.0) | 36 (36.4) | 8 (39.1) | 0.53 |
| Corticosteroid, n (%) | 27 (16.4) | 2 (10.0) | 17 (17.2) | 8 (17.4) | 0.71 |
| Biologics, n (%) | 48 (29.1) | 7 (35.0) | 28 (28.3) | 13 (28.3) | 0.83 |
| DAS28-ESR, median (IQR) |
2.30 (1.68–3.00) |
2.01 (1.37–2.23) |
2.32 (1.68–3.02) |
2.40 (2.01–3.15) |
0.08 |
| mHAQ, median (IQR) |
0 (0–0.13) |
0 (0–0.25) |
0 (0–0) |
0.13 (0–0.38) |
0.02 |
| BMQ necessity-concern differential, median (IQR) | 1 (0–3) | 2 (0–3) | 1 (0–3) | 2 (0–3) | 0.81 |
| CES-D (total score ≥ 16), n (%) | 37 (26.4) | 8 (47.1) | 21 (8.3) | 8 (20.0) | 0.10 |
| BPI (average NRS pain score), median (IQR) | 2 (1–3) | 1 (1–4) | 1 (1–3) | 1 (3–4) | 0.13 |
| SDS, median (IQR) | 6 (4–7) | 4 (3–6) | 6 (4–6) | 6.5 (5–8) | 0.00 |
| SF-8 (PCS), median (IQR) |
48.1 (42.4–52.1) |
47.4 (45.8–52.1) |
49.8 (43.5–52.1) |
45.6 (40.2–50.2) |
0.04 |
| SF-8 (MCS), median (IQR) |
50.2 (45.6–54.1) |
47.7 (43.0–53.2) |
50.3 (46.3–53.9) |
50.0 (46.1–55.0) |
0.36 |
| Marital status (married), n (%) | 95 (57.9) | 11 (55.0) | 61 (62.2) | 23 (50.0) | 0.37 |
| Educational level (greater than or equal to college level), n (%) | 36 (21.8) | 5 (25.0) | 17 (17.2) | 14 (30.4) | 0.19 |
| Employment status (full-time), n (%) | 38 (23.0) | 12 (60.0) | 22 (22.2) | 4 (8.7) | 0.00 |
| Living status (living alone), n (%) | 43 (26.4) | 7 (35.0) | 24 (24.5) | 12 (26.7) | 0.62 |
IQR interquartile range; DAS28-ESR Disease Activity Score (28 joint count)- erythrocyte sedimentation rate; mHAQ modified Health Assessment Questionnaire; BMQ Beliefs about Medicines Questionnaire; CES-D Center for Epidemiological Studies Depression; BPI Brief Pain Inventory; NRS Numerical Rating Scale; SDS Social Desirability Scale; SF-8 8-item Short-Form Health Survey; PCS physical component summary; MCS mental component summary; MTX methotrexate; MMAS-8 Morisky Medication Adherence Scale
Distribution of MTX adherence
Based on MMAS-8, low, medium, and high adherence rates were noted in 12% (20/165), 60% (99/165), and 28% (46/165) of cases, respectively. The median MMAS-8 score was 7 (IQR, 6.5–8).
Patient’s characteristics by grade of MTX adherence
Patient’s characteristics by grade of MTX adherence divided by MMAS-8 are shown in Table 1. A significant correlation between levels of MTX adherence and age, mHAQ, SDS, SF-8 (PCS), and employment status was found. Based on trend analysis, older participants had significantly high adherence, and the higher the social desirability, the higher the adherence. However, a significant correlation between MTX adherence and mHAQ and SF-8 (PCS) was not observed in the trend analysis.
Factors associated with high MTX adherence considering social desirability
In the multiple linear regression analysis adjusted for age, sex, disease duration, RA disease activity (DAS28-ESR), depression state (CES-D), reliability of the medication (BMQ necessity-concern differential), social desirability (SDS), educational level (more than or equal to college-level or not), and employment status (full-time work or not), higher social desirability (coefficient, 0.14; 95% confidence interval [CI], 0.05–0.23; p < 0.05) and higher age (coefficient per 10 years, 0.16; 95% CI, 0.01–0.03; p < 0.05) were associated with high MTX adherence, whereas full-time work was negatively related to high MTX adherence (coefficient, −0.50; 95% CI, −0.95 to −0.05; p < 0.05) (Table 2).
Table 2.
Factors associated with MTX adherence using linear regression analysis (n = 165)
| Coef | 95% CI | p value | |
|---|---|---|---|
| Age | 0.02 | 0.00–0.03 | 0.03 |
| Female | − 0.09 | − 0.58 to 0.40 | 0.73 |
| Disease duration | 0.00 | − 0.00 to 0.00 | 0.41 |
| RA activity (DAS28-ESR) | 0.16 | − 0.03 to 0.35 | 0.09 |
| Depression state (CES-D) | − 0.26 | − 0.69 to 0.18 | 0.25 |
| Belief about medicine (BMQ necessity-concern differential) | 0.01 | − 0.07 to 0.09 | 0.86 |
| Social desirability (SDS) | 0.14 | 0.05–0.23 | 0.00 |
| Educational level (more than or equal to college level or not) | 0.36 | − 0.10 to 0.82 | 0.13 |
| Employment status (full-time work or not) | − 0.50 | − 0.95 to 0.05 | 0.03 |
Linear regression analysis was used to detect factors associated with MTX adherence
MTX methotrexate; RA rheumatoid arthritis; DAS28-ESR Disease Activity Score (28 joint count)-erythrocyte sedimentation rate; BMQ Beliefs about Medicines Questionnaire; CES-D Center for Epidemiological Studies Depression; SDS Social Desirability Scale; Coef regression coefficients; CI confidence interval
Discussion
Elderly participants and high social desirability were significantly associated with high adherence based on trend analysis. Multiple linear regression analysis showed that high MTX adherence in RA patients is associated with high social desirability, high educational level, and non-full-time work. In our study, only 27.9% of the participants showed high medication adherence. Several studies reported that adherence to DMARDS was 40%–107% [10, 39, 40]. There are several potential reasons for the observed low adherence. First, the method of adherence measurement in each study was different. There were interviews, questionnaires, tablet count, drug concentration, and doctor’s judgment as measurements. A previous study showed that the adherence to interview, tablet count, drug concentration, and doctor’s judgment were 96%, 77%, 58%, and 42%, respectively, which showed vast differences [41]. Since each measurement has advantages and disadvantages, the gold standard is not determined. We chose a self-reported questionnaire in this study since this scale is not expensive or invasive and can be possibly validated. Second, the time point of adherence measurement was different between the studies. We measured adherence during the maintenance period (mean disease duration, 53 months). A previous report suggested that the adherence of DMARDs in the early period was low (58%) [5]. Another study reported the adherence to salazosulfapyridine as 87% at three years from the start of medication [36]. In contrast, the adherence to DMARDs in the maintenance period varied from 84%,4 70% [42], to 95.4% [43]. Previous reports have suggested that the adherence to DMARDs was not different between the early and maintenance periods. It is crucial to note that the time point of measuring medication adherence is the maintenance period because RA is a chronic disease that requires DMARDs for a long duration. The third is the difference in participant characteristics. Living status and marital status affected adherence [7, 11]. Our study could not adjust the living status and marital status because we could not include more factors based on our sample size. In addition, the age of the participants may have had an effect. In the original MMAS-8 article [21], the mean age was 52 years; however, in our study, the median age was 64 years, which means that the participants in our study were older, and this may have influenced the results. Furthermore, older people may not have revealed the truth. Thus, when referring to drug adherence studies, the method of measuring adherence, whether the target patient is in the disease’s early or maintenance phase, and the patient’s characteristics should be confirmed before adaptation.
Our study detected high social desirability, high educational level, and non-full-time work as factors affecting MTX adherence. A previous study reporting MTX adherence found that adherence is associated with age, sex, race, RA disease activity, patient beliefs about the medication, disease duration, mental health, and SES [4, 7–11, 35–37]. The discrepancy between our findings and those of other studies could be attributed to three reasons. First, we measured and adjusted social desirability, which possibly affected the answers in the questionnaire. Although a recent MTX adherence research mentioned the necessity to account for this bias [19], no studies have examined how MTX adherence survey accounts for social desirability. The results may have differed since we conducted a factor search that included social desirability. Second, racial differences may have led to different outcomes. Our study was based on a Japanese population; however, previous studies were mainly on white people, black people, and Hispanic people. Canon et al. reported that the adherence to MTX was associated with the Caucasian race [4]. Therefore, adherence may be influenced by race. Third, the definition of medication adherence varies between studies. We used MMAS-8 in our study. Conversely, previous studies used other methods to assess medication adherence, such as physicians’ estimation, different self-reported scales, drug concentration, tablet count electronic monitors, and other questionnaires. A prior study reported that medication adherence differs because the measurement method employed seems to vary [12]. Therefore, we need to practice caution when using research results because the results may differ depending on the method of measuring adherence and the variables incorporated, including social desirability.
Our study had three strengths. First, we assessed social desirability, leading to social desirability bias. To our knowledge, no clinical studies adjusting social desirability have investigated the association between MTX adherence and various factors. Second, to our knowledge, this is the first research to examine MTX adherence in an Asian population. Third, the response rate to the questionnaires was high (93.4%), thereby strengthening internal validity.
This study has significant implications for the clinician. Our findings may prevent unnecessary DMARDs changes since physicians tend to overestimate patients' medication adherence [44]. They should confirm MTX adherence before switching or adding DMARDS. Accordingly, this provides an opportunity to reduce the healthcare cost and adverse events of medication.
However, this study had several limitations. First, in the multivariable analysis, some immeasurable essential factors (such as characteristics of each participant, joint deformities, number of medications, and MTX dosing frequency) that could affect MTX adherence possibly exist. We did not determine patient personality due to the difficulty in quantifying it. In addition, we did not collect radiographs even if they are necessary for determining joint deformity because they are burdensome for patients. However, we adjusted for joint deformity using the mHAQ to reduce its possible influence on the findings. In addition, although the number of MTX doses was collected, it was not possible to incorporate variables due to sample size limitations. Second, the generalizability of our findings is limited. Our study setting was primarily university hospitals in Japan; thus, the results of this study cannot be applied to the clinical setting, in small- and medium-sized hospitals, or in settings including other races. Furthermore, the highest dose of MTX in Japan in the study was 8 mg per week. This dose is less than the standard dose of 20 mg worldwide, which limits the indications. Third, we used a self-reported questionnaire of adherence. Hence, the completed questionnaire possibly influenced social desirability. Although we could not adjust this bias completely, social desirability was added as a factor for analysis. Furthermore, MMAS-8 is "self-perceived adherence" and not true drug adherence. Therefore, even a factorial search including social desirability may not reveal the truth. Forth, The Marlowe-Crowne Social Desirability scale may have inadequate validity and reliability [45]. The validity of this scale has not been examined in the target population of RA patients. There are also negative studies on the reliability of the SDS scale. As a future study, we would like to conduct a validity study of this scale in RA patients.
Conclusions
Our results demonstrated that sixty percent of the RA patients had moderate adherence to MTX. Moreover, high MTX adherence in RA patients is associated with high social desirability, high educational level, and non-full-time work.
Supplementary Information
Additional file 1. Table S1. Missing data of patient characteristics.
Acknowledgements
We thank Noboru Murata (Kikuna Memorial Hospital) and Osamu Namiki for critically reviewing the manuscript.
Abbreviations
- ADL
Activities of daily living
- BMQ
Beliefs about Medicines Questionnaire
- BPI
Brief Pain Inventory
- CES-D
Center for Epidemiological Studies Depression
- DAS28-ESR
Disease Activity Score (28 joint count)-erythrocyte sedimentation rate
- DMARDs
Disease-modifying antirheumatic-drugs
- MCS
Mental component summary
- mHAQ
Modified Health Assessment Questionnaire
- MMAS-8
Morisky Medication Adherence Scale
- MTX
Methotrexate
- NRS
Numerical rating scale
- PCS
Physical component summary
- RA
Rheumatoid arthritis
- SDS
Social desirability scale
- SF-8
8-Item Short-Form Health Survey
Author contributions
NY: Methodology, Project administration, Writing—original draft, Writing—Review and Editing. TK: Supervision, Conceptualization, Project Administration, Writing—original draft, Writing—Review and Editing. RT: Data curation, Writing—original draft, Writing—Review and Editing. HN: Data curation, Writing—original draft, Writing—Review and Editing. YT: Data curation, Writing—original draft, Writing—Review and Editing. KO: Data curation, Writing—original draft, Writing—Review and Editing. TO: Data curation, Writing—original draft, Writing—Review and Editing. TK: Data curation, Writing—original draft, Writing—Review and Editing. DEM: Supervision, Writing—original draft, Writing—Review and Editing. TY: Formal Analysis, Supervision, Writing—original draft, Writing—Review and Editing. TK: Supervision, Writing—original draft, Writing—Review and Editing. All authors read and approved the final manuscript.
Funding
The authors have not received funding to conduct this study.
Availability of data and materials
The dataset analyzed in this paper is available from the corresponding author on reasonable request. About MMAS8: The use of the MMAS diagnostic adherence assessment instrument is protected by US copyrighted and trademarked laws. Permission for use is required. A license is available from MORISKY MEDICATION ADHERENCE RESEARCH, LLC., Donald E. Morisky, ScD, ScM, MSPH, MMAR, LLC, 294 Lindura Ct., Las Vegas, NV 89138; dmorisky@gmail.com.
Declarations
Ethics approval and consent to participate
The ethics committee of Showa University Hospital (Approval number 1446) and other each institution approved this study, and informed consent was obtained from all participants before study enrolment. All study procedures were performed in accordance with the Declaration of Helsinki. Patient information was anonymized and de-identified before analysis.
Consent for publication
Not applicable.
Competing interests
Donald E. Morisky holds a copyright for the Morisky Medication Adherence Scale-8, is one of its authors, and collects fees in exchange for licenses to use the scale in research. This does not alter the authors’ commitment to objectivity in research or adherence to data sharing policies. He was not involved in any data analysis. For the remaining authors, none were declared.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.van der Heijde DM, van Leeuwen MA, van Riel PL, Koster AM, vant Hof MA, van Rijswijk MH, et al. Biannual radiographic assessments of hands and feet in a three-year prospective followup of patients with early rheumatoid arthritis. Arthritis Rheum. 1992;35:26–34. doi: 10.1002/art.1780350105. [DOI] [PubMed] [Google Scholar]
- 2.Singh JA, Saag KG, Bridges SL, Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68:1–25. doi: 10.1002/acr.22783. [DOI] [PubMed] [Google Scholar]
- 3.Smolen JS, Landewe RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:685–699. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 4.Cannon GW, Mikuls TR, Hayden CL, Ying J, Curtis JR, Reimold AM, et al. Merging Veterans Affairs rheumatoid arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res. 2011;63:1680–1690. doi: 10.1002/acr.20629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Contreras-Yanez I, Ponce De Leon S, Cabiedes J, Rull-Gabayet M, Pascual-Ramos V. Inadequate therapy behavior is associated to disease flares in patients with rheumatoid arthritis who have achieved remission with disease-modifying antirheumatic drugs. Am J Med Sci. 2010;340:282–90. doi: 10.1097/MAJ.0b013e3181e8bcb0. [DOI] [PubMed] [Google Scholar]
- 6.Brodtkorb E, Samsonsen C, Sund JK, Bråthen G, Helde G, Reimers A. Treatment non-adherence in pseudo-refractory epilepsy. Epilepsy Res. 2016;122:1–6. doi: 10.1016/j.eplepsyres.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 7.De Cuyper E, De Gucht V, Maes S, Van Camp Y, De Clerck LS. Determinants of methotrexate adherence in rheumatoid arthritis patients. Clin Rheumatol. 2016;35:1335–1339. doi: 10.1007/s10067-016-3182-4. [DOI] [PubMed] [Google Scholar]
- 8.DiBenedetti DB, Zhou X, Reynolds M, Ogale S, Best JH. Assessing methotrexate adherence in rheumatoid arthritis: a cross-sectional survey. Rheumatol Ther. 2015;2:73–84. doi: 10.1007/s40744-015-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salt E, Frazier SK. Adherence to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: a narrative review of the literature. Orthop Nurs. 2010;29:260–275. doi: 10.1097/NOR.0b013e3181e5c2c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheiman-Elazary A, Duan L, Shourt C, Shourt C, Agrawal H, Ellashof D, et al. The rate of adherence to antiarthritis medications and associated factors among patients with rheumatoid arthritis: a systematic literature review and metaanalysis. J Rheumatol. 2016;43:512–523. doi: 10.3899/jrheum.141371. [DOI] [PubMed] [Google Scholar]
- 11.Waimann CA, Marengo MF, de Achaval S, Cox VL, Garcia-Gonzalez A, Reveille JD, et al. Electronic monitoring of oral therapies in ethnically diverse and economically disadvantaged patients with rheumatoid arthritis: consequences of low adherence. Arthritis Rheum. 2013;65:1421–1429. doi: 10.1002/art.37917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hope HF, Bluett J, Barton A, Hyrich KL, Cordingley L, Verstappen SM. Psychological factors predict adherence to methotrexate in rheumatoid arthritis; findings from a systematic review of rates, predictors and associations with patient-reported and clinical outcomes. RMD Open. 2016;2:e000171. doi: 10.1136/rmdopen-2015-000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tracey TJG. A note on socially desirable responding. J Counsel Psychol. 2016;63:224–32. doi: 10.1037/cou0000135. [DOI] [PubMed] [Google Scholar]
- 14.Copeland K, Checkoway H, McMichael A, Holbrook R. Bias due to the misclassification in the estimation of relative risk. Am J Epidemiol. 1977;105:488–495. doi: 10.1093/oxfordjournals.aje.a112408. [DOI] [PubMed] [Google Scholar]
- 15.Richman WL, Weisband S, Kiesler S, Drasgow FA. meta-analytic study of social desirability distortion in computer-administered questionnaires, traditional questionnaires, and interviews. J Appl Psychol. 1999;84:754–775. doi: 10.1037/0021-9010.84.5.754. [DOI] [Google Scholar]
- 16.Holtgraves T. Social desirability and self-reports: testing models of socially desirable responding. Pers Soc Psychol Bull. 2004;30:161–172. doi: 10.1177/0146167203259930. [DOI] [PubMed] [Google Scholar]
- 17.Hebel S, Kahn-Woods E, Malone-Thomas S, McNeese M, Thornton L, Sukhija-Cohen A, et al. Brief report: discrepancies between self-reported adherence and a biomarker of adherence in real-world settings. J Acquir Immune Defic Syndr. 2020;85:454–457. doi: 10.1097/QAI.0000000000002486. [DOI] [PubMed] [Google Scholar]
- 18.van der Straten A, Montgomery ET, Hartmann M, Minnis A. Methodological lessons from clinical trials and the future of microbicide research. Curr HIV/AIDS Rep. 2013;10:89–102. doi: 10.1007/s11904-012-0141-9. [DOI] [PubMed] [Google Scholar]
- 19.Aaltonen KJ, Turunen JH, Sokka T, Puolakka K, Valleala H. A survey on the medication adherence to methotrexate among rheumatoid arthritis patients treated with self-administered biologic drugs. Clin Exp Rheumatol. 2016;34:694–697. [PubMed] [Google Scholar]
- 20.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 21.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Berlowitz DR, Foy CG, Kazis LE, Bolin LP, Conroy MB, Fitzpatrick P, et al. Effect of intensive blood-pressure treatment on patient-reported outcomes. N Engl J Med. 2017;377:733–744. doi: 10.1056/NEJMoa1611179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bress AP, Bellows BK, King JB, Hess R, Beddhu S, Zhang Z, et al. Cost-effectiveness of intensive versus standard blood pressure control. N Engl J Med. 2017;377:745–755. doi: 10.1056/NEJMsa1616035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crowne DP, Marlowe D. A new scale of social desirability independent of psychopathology. J Consult Psychol. 1960;24:349–354. doi: 10.1037/h0047358. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds WM. Development of reliable and valid short forms of the Marlowe-Crowne social desirability scale. J Clin Psych. 1982;38:119–125. doi: 10.1002/1097-4679(198201)38:1<119::AID-JCLP2270380118>3.0.CO;2-I. [DOI] [Google Scholar]
- 26.Barger SD. The Marlowe-Crowne affair: Short forms, psychometric structure, and social desirability. J Pers Assess. 2003;79:286–305. doi: 10.1207/S15327752JPA7902_11. [DOI] [PubMed] [Google Scholar]
- 27.Nederhof AJ. Methods of coping with social desirability bias: A review. Eur J Soc Psychol. 1985;15:263–280. doi: 10.1002/ejsp.2420150303. [DOI] [Google Scholar]
- 28.Prevoo ML, vant Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 29.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 30.Horne R, Weinman J. Patients' beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555–567. doi: 10.1016/S0022-3999(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 31.Ware JE, GlaxoSmithKline. How to score and interpret single-item health status measures: a manual for users of the of the SF-8 health survey: (with a supplement on the SF-8 health survey). Lincoln, RI; QualityMetric, Inc.; Boston, MA: Health Assessment Lab. 2001.
- 32.Birrell FN, Hassell AB, Jones PW, Dawes PT. How does the short form 36 health questionnaire (SF-36) in rheumatoid arthritis (RA) relate to RA outcome measures and SF-36 population values? A cross-sectional study. Clin Rheumatol. 2000;19:195–199. doi: 10.1007/s100670050155. [DOI] [PubMed] [Google Scholar]
- 33.Iihara N, Suzuki K, Kurosaki Y, Morita S, Hori K. Factorial invariance of a questionnaire assessing medication beliefs in Japanese non-adherent groups. Pharm World Sci. 2010;32:432–439. doi: 10.1007/s11096-010-9388-7. [DOI] [PubMed] [Google Scholar]
- 34.Uki J, Mendoza T, Cleeland CS, Nakamura Y, Takeda F. A brief cancer pain assessment tool in Japanese: the utility of the Japanese Brief Pain Inventory–BPI-J. J Pain Symptom Manage. 1998;16:364–373. doi: 10.1016/S0885-3924(98)00098-0. [DOI] [PubMed] [Google Scholar]
- 35.Lorish CD, Richards B, Brown S. Missed medication doses in rheumatic arthritis patients: intentional and unintentional reasons. Arthritis Care Res. 1989;2:3–9. doi: 10.1002/anr.1790020103. [DOI] [PubMed] [Google Scholar]
- 36.Brus HL, van de Laar MA, Taal E, Rasker JJ, Wiegman O. Effects of patient education on compliance with basic treatment regimens and health in recent onset active rheumatoid arthritis. Ann Rheum Dis. 1998;57:146–151. doi: 10.1136/ard.57.3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viller F, Guillemin F, Briancon S, Moum T, Suurmeijer T, van den Heuvel W. Compliance with drug therapy in rheumatoid arthritis A longitudinal European study. Joint Bone Spine. 2000;67:178–82. [PubMed] [Google Scholar]
- 38.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 39.van den Bemt BJ, Zwikker HE, van den Ende CH. Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert Rev Clin Immunol. 2012;8:337–351. doi: 10.1586/eci.12.23. [DOI] [PubMed] [Google Scholar]
- 40.Curtis JR, Bykerk VP, Aassi M, Schiff M. Adherence and persistence with methotrexate in rheumatoid arthritis: a systematic review. J Rheumatol. 2016;43:1997–2009. doi: 10.3899/jrheum.151212. [DOI] [PubMed] [Google Scholar]
- 41.Pullar T, Peaker S, Martin MF, Bird HA, Feely MP. The use of a pharmacological indicator to investigate compliance in patients with a poor response to antirheumatic therapy. Br J Rheumatol. 1988;27:381–384. doi: 10.1093/rheumatology/27.5.381. [DOI] [PubMed] [Google Scholar]
- 42.van den Bemt BJ, den Broeder AA, van den Hoogen FH, Benraad B, Hekster YA, van Riel PL, et al. Making the rheumatologist aware of patients' non-adherence does not improve medication adherence in patients with rheumatoid arthritis. Scand J Rheumatol. 2011;40:192–196. doi: 10.3109/03009742.2010.517214. [DOI] [PubMed] [Google Scholar]
- 43.Park DC, Hertzog C, Leventhal H, Morrell RW, Leventhal E, Birchmore D, et al. Medication adherence in rheumatoid arthritis patients: older is wiser. J Am Geriatr Soc. 1999;47:172–183. doi: 10.1111/j.1532-5415.1999.tb04575.x. [DOI] [PubMed] [Google Scholar]
- 44.Gossec L, Molto A, Romand X, Puyraimond-Zemmour D, Lavielle M, Beauvais C, et al. Recommendations for the assessment and optimization of adherence to disease-modifying drugs in chronic inflammatory rheumatic diseases: a process based on literature reviews and expert consensus. Joint Bone Spine. 2019;86:13–19. doi: 10.1016/j.jbspin.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Nurumov K, Hernández-Torrano D, Ait Si Mhamed A, Ospanova U. Measuring social desirability in collectivist countries: a psychometric study in a representative sample from Kazakhstan. Front Psychol. 2022;13:822931. doi: 10.3389/fpsyg.2022.822931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. Missing data of patient characteristics.
Data Availability Statement
The dataset analyzed in this paper is available from the corresponding author on reasonable request. About MMAS8: The use of the MMAS diagnostic adherence assessment instrument is protected by US copyrighted and trademarked laws. Permission for use is required. A license is available from MORISKY MEDICATION ADHERENCE RESEARCH, LLC., Donald E. Morisky, ScD, ScM, MSPH, MMAR, LLC, 294 Lindura Ct., Las Vegas, NV 89138; dmorisky@gmail.com.


