Abstract
Background
Periprosthetic joint infection (PJI) following total joint arthroplasty (TJA) is a serious complication for patients. Some joint surgeons have tried to use vancomycin powder (VP) in total knee and total hip arthroplasty to prevent postoperative PJI, but its effect is still not clear. At present, there is no meta-analysis that specifically analyses the effect of different doses of vancomycin powder on the incidence of PJI.
Methods
We carried out a search based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and identified the studies we needed. Review Manager (RevMan) 5.3 software was employed for statistical analysis.
Results
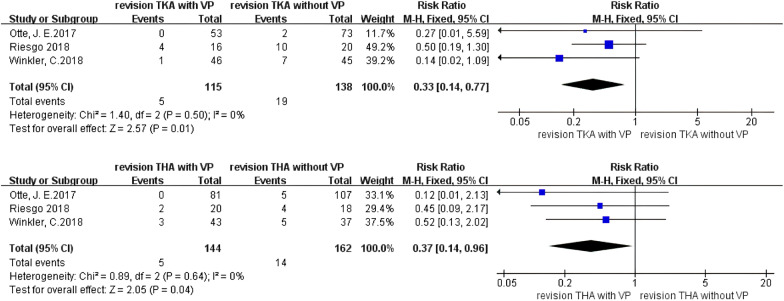
The analysis of primary TKA (PTKA) showed that using 1 g (RR 0.38, 95% CI 0.22–0.67 [P = 0.0008]) and 2 g (RR 0.48, 95% CI 0.31–0.74 [P = 0.0008]) of vancomycin powder in primary TKA (PTKA) could all significantly prevent PJI. The analysis of primary THA (PTHA) showed that using 1 g (RR 0.37, 95% CI 0.17–0.80 [P = 0.01]) of vancomycin powder effectively decreased the incidence of PJI, while using 2 g (RR 1.02, 95% CI 0.53–1.97 [P = 0.94]) of vancomycin powder had no significant effect on preventing PJI. Because the data were abnormal, we believed the conclusion that using 2 g of vancomycin powder in primary THA had no effect on preventing PJI was doubtful. Using vancomycin powder in revision TKA (RTKA) significantly reduced the PJI rate (RR 0.33, 95% CI 0.14–0.77 [P = 0.01]), similar to revision THA (RTHA) (RR 0.37, 95% CI 0.14–0.96 [P = 0.04]).
Conclusions
In primary TKA, both 1 g and 2 g of vancomycin powder can effectively prevent PJI. In primary THA, using 1 g of vancomycin powder is a better choice, while the effect of using 2 g of vancomycin powder is not clear, and a more prospective randomized controlled trial should be done to verify it. In revision TKA and revision THA, vancomycin powder is a good choice to prevent PJI.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13018-022-03445-2.
Keywords: Periprosthetic joint infection, THA, TKA, TJA, Vancomycin
Introduction
Periprosthetic joint infection (PJI) following TJA is a serious complication for patients. Patients who experience PJI must undergo debridement, antibiotics and implant retention (DAIR) or revision surgery, and they bear tremendous psychological and economic burdens. Surveys have shown that patients undergoing TJA due to PJI have a poorer functional prognosis and less satisfaction with the surgery than patients undergoing revision for other reasons, which leads to more lasting negative consequences [1]. Endogenous or exogenous bacteria that infect the joint area following surgery may induce PJI [2]. The most common pathogen is coagulase-negative staphylococci [3], but infection caused by fungi should not be ignored as extending antibiotic prophylaxis may cause a shift in causative organisms. Surgeons have adopted various methods for preventing infection after surgery [4], but the postoperative PJI rate after THA is still between 0.86% and 1.03% [5] and the postoperative PJI rate after TKA is between 1.41% and 2.01% [6, 7].
To further reduce the infection rate, some surgeons have tried to use vancomycin powder in wounds. Vancomycin is a glycopeptide antibiotic that achieves a bactericidal effect by inhibiting the synthesis of the cell wall of gram-positive bacteria [8]. Vancomycin powder has already been used by spinal surgeons to prevent deep infections after surgery and has a good effect [9, 10]. However, there is no consensus on the effect of vancomycin powder in joint surgery. Some authors think that local vancomycin powder can prevent PJI [11–18], but some authors think that vancomycin powder is not effective in preventing postoperative infections [19–24]. At present, there are few meta-analyses of whether vancomycin powder can reduce the postoperative PJI rate, and there is much controversy.
In the published meta-analyses, the authors mainly compared the PJI rate of total joint arthroplasty without considering the dose of vancomycin powder, and they did not report whether there was any difference in the effects of vancomycin powder at different doses. In addition, the effects of vancomycin powder on the PJI rate of primary arthroplasty and revision arthroplasty may also be different. Therefore, we conducted this study to answer the following questions:
Can vancomycin powder effectively prevent PJI after primary TKA? Whether different doses of vancomycin powder can all prevent PJI after primary TKA or not?
Can vancomycin powder effectively prevent PJI after primary THA? Whether different doses of vancomycin powder can all prevent PJI after primary THA or not?
Can vancomycin powder used in revision TKA and revision THA prevent PJI?
Data sources and methods
We registered our study in the PROSPERO database on October 24, 2021 (registration number: CRD42021287003) [25], and was performed based on the PRISMA guidelines [26].
Literature screening
Five researchers searched four major databases (PubMed, Embase, Cochrane Library and WOS) for documents up until 2021–11–10. The criteria for inclusion articles were as follows: (1) studies that reported on the use of intrawound vancomycin powder during primary and revision THA and TKA; (2) follow-up time ≥ 3 months; (3) retrospective and prospective studies; and (4) the language of the study is English. Studies with less than 3-month follow-up, those without complete infection data, non-English studies, and without full-text availability were excluded. The search terms included vancomycin (MeSH); arthroplasty, replacement, knee (MeSH); arthroplasty, replacement, hip (MeSH); total hip replacement (MeSH); and total knee arthroplasty (MeSH) (for the detailed search strategy, see Additional file 1: Table S1).
Data extraction
After the screening, qualified articles were assessed entirely based on the same inclusion and exclusion criteria and the characteristics extracted from databases included the first author’s name, the year of publication, evidence level, study types, vancomycin powder dosage, different types of surgery, sample size of experimental and control groups, follow-up time, and number of PJI occurrences in the vancomycin-treated group and control group. We used the Newcastle–Ottawa Scale (NOS) [27] to evaluate the quality of studies, with 7–9 points indicating high quality.
Publication bias
The publication bias is evaluated by a flow diagram, where the x-axis represents the effect size (RR). The smaller the value is, the closer it is to the left end. The vertical line in the middle is the ideal effect value. Ideally, the studies should be evenly distributed on both sides of the vertical line. The y-axis represents the standard error, which is the sample size. The larger the sample size is, the higher the distribution and the smaller the standard error.
Statistical analysis
The PJI rate of each study was utilized to calculate the relative risk (RR) and confidence intervals (CIs). The chi-square test was used to measure study heterogeneity. If I2 was between 25 and 50%, the heterogeneity was considered small; if I2 was between 50 and 75%, the heterogeneity was considered moderate; if I2 > 75%, the heterogeneity was significant [28, 29]. When I2 is more than 50%, the random-effects model is employed for the evaluation; alternatively, the fixed-effects model is applied. RevMan 5.3 software was employed to perform all statistical analyses.
Results
Search results
The full text of eligible articles was filtered according to inclusion and exclusion criteria, and different articles were reviewed by another author. We excluded duplicate studies and screened studies individually, and then we evaluated the full text of the included studies to exclude studies that did not fulfil the requirements. The screening process is shown in Fig. 1. We searched the articles we needed in the PubMed, Web of Science, Embase and Cochrane Library databases. A total of 625 articles were screened out in the first screening, and 14 original articles were ultimately included. The funnel plot (Fig. 2) showed no indication of publication bias among the 14 studies.
Fig. 1.
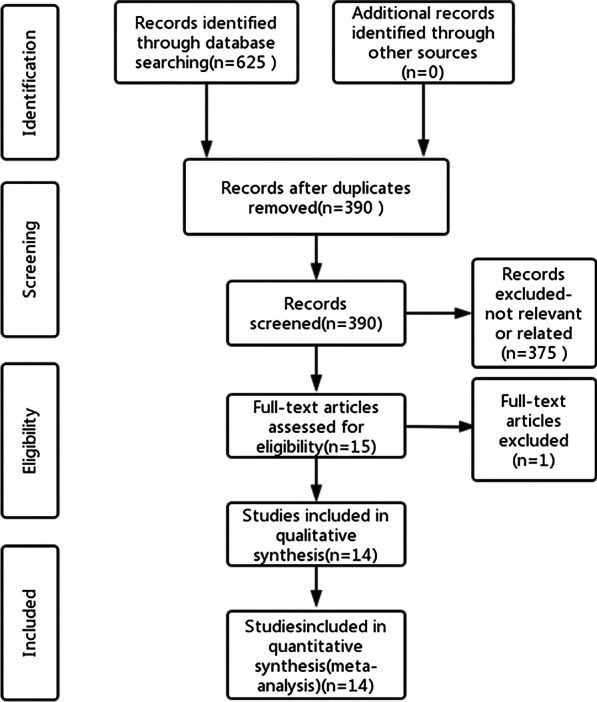
PRISMA flow diagram of the search strategy
Fig. 2.
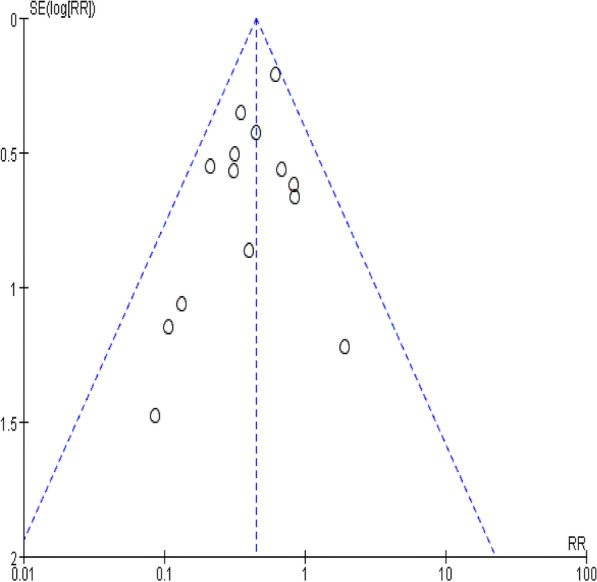
Funnel plot of included studies
Research nature
This analysis comprised 14 studies (Additional file 2: Table S2), 12 of which were retrospective cohort studies and 2 of which were prospective cohort studies. The study comprised 35,418 participants, including 20,368 treated with vancomycin powder and 15,050 not treated with vancomycin powder. Based on the NOS (Table 1), all studies scored at least 7 points and indicated that they were all high-quality studies.
Table 1.
Quality assessment according to the NOS of each cohort study
| Study | Selection | Comparability | Outcome | Total score |
|---|---|---|---|---|
| Buchalter, D. B | 3 | 2 | 3 | 8 |
| Cohen, E. M | 4 | 2 | 3 | 9 |
| Dial, B. L | 3 | 2 | 3 | 8 |
| Khatri, K | 3 | 2 | 3 | 8 |
| Koutalos, A. A | 4 | 2 | 3 | 9 |
| Otte, J. E | 3 | 2 | 2 | 7 |
| Patel, N. N | 3 | 2 | 3 | 8 |
| Tahmasebi, M. N | 3 | 2 | 3 | 8 |
| Winkler, C | 3 | 2 | 2 | 7 |
| Xu, X | 3 | 2 | 3 | 8 |
| Yavuz, I. A | 3 | 2 | 3 | 8 |
| Riesgo | 3 | 2 | 3 | 8 |
| Hanada | 3 | 2 | 3 | 8 |
| Matziolis,G | 3 | 2 | 3 | 8 |
Statistical results
Can vancomycin powder effectively prevent PJI after primary TKA? Whether different doses of vancomycin powder can all prevent PJI after primary TKA or not?
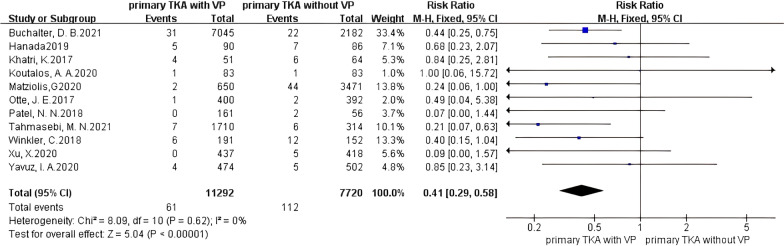
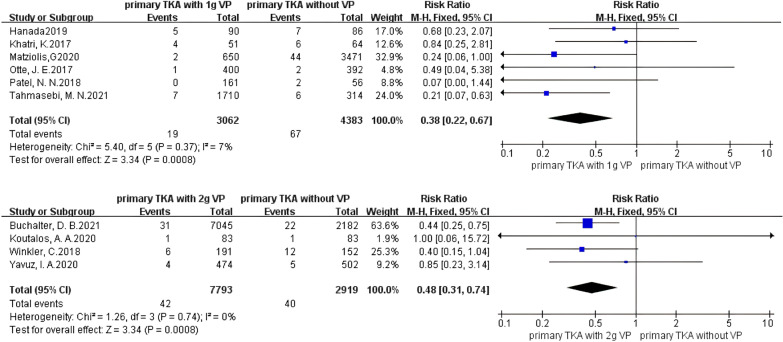
The analysis of the primary TKA (PTKA) showed that the incidence of PJI was significantly reduced when using vancomycin powder (Fig. 3), with no heterogeneity existing in the studies (I2 = 0%, P = 0.62), and PJI was significantly prevented in patients treated with vancomycin powder (RR 0.41, 95% CI 0.29–0.58 [P < 0.00001]). For 1 g of vancomycin powder (Fig. 4), no heterogeneity existed in the studies (I2 = 7%, P = 0.37), and the effect on preventing PJI in the vancomycin-treated group was significantly different from that in the control group (RR 0.38, 95% CI 0.22–0.67 [P = 0.0008]). For 2 g of vancomycin powder (Fig. 4), no heterogeneity existed in the studies (I2 = 0%, P = 0.74), and the effect on preventing PJI in the vancomycin-treated group was significantly different from that in the control group (RR 0.48, 95% CI 0.31–0.74 [P = 0.0008]). It is suggested that both the use of 1 g and 2 g of vancomycin powder in primary TKA can significantly reduce the PJI rate.
Fig. 3.
Forest plot of the risk of PJI after PTKA with VP
Fig. 4.
Forest plot of the risk of PJI after PTKA with different doses of VP
Can vancomycin powder effectively prevent PJI after primary THA? Whether different doses of vancomycin powder can all prevent PJI after primary THA or not?
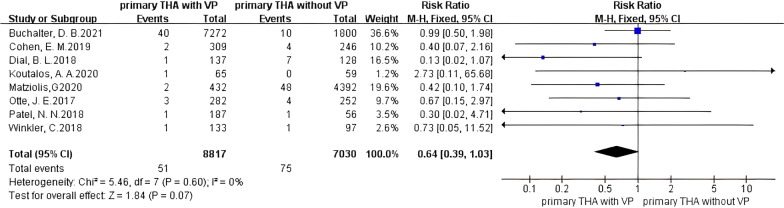
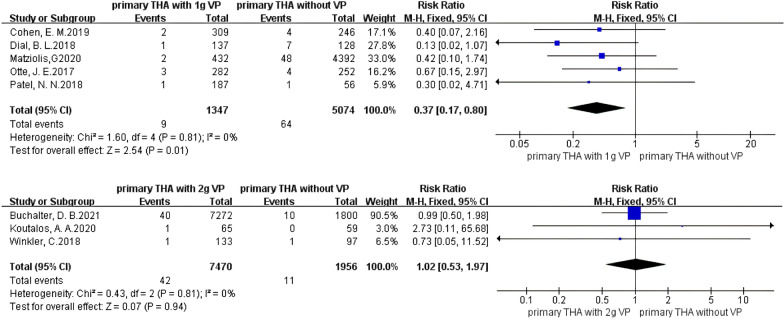
An analysis of primary THA (PTHA) showed that the incidence of PJI was not significantly reduced when using vancomycin powder (Fig. 5), and no heterogeneity existed in the studies (I2 = 0%, P = 0.60). Vancomycin powder had no obvious preventive effect on PJI (RR 0.64, 95% CI 0.39–1.03 [P = 0.07]), but the relative risk ratio was 0.64, indicating that the incidence of PJI in the vancomycin-treated group had a decreasing trend. For 1 g of vancomycin powder (Fig. 6), no heterogeneity existed in the studies (I2 = 0%, P = 0.81), and the PJI rate was significantly decreased in patients treated with vancomycin powder (RR 0.37, 95% CI 0.17–0.80 [P = 0.01]). For 2 g of vancomycin powder (Fig. 6), no heterogeneity existed in the studies (I2 = 0%, P = 0.81), and PJI was not effectively prevented in the vancomycin-treated group (RR 1.02, 95% CI 0.53–1.97 [P = 0.94]). It is suggested that in primary THA, using 1 g of vancomycin powder had a significant effect on reducing the PJI rate, while the effect was not obvious when 2 g of vancomycin powder was used.
Fig. 5.
Forest plot of the risk of PJI after PTHA with VP
Fig. 6.
Forest plot of the risk of PJI after PTHA with different doses of VP
Can vancomycin powder used in revision TKA and revision THA prevent PJI?
A separate analysis of revision TKA (RTKA) (Fig. 7) showed that the incidence of PJI was significantly decreased in the vancomycin-treated group (RR 0.33, 95% CI 0.14–0.77 [P = 0.01]), and no heterogeneity existed in the studies (I2 = 0%, P = 0.50). This suggests that using vancomycin powder in revision TKA can significantly decrease the incidence of PJI.
Fig. 7.
Forest plot of the risk of PJI after RTKA and RTHA with VP
A separate analysis of revision THA (RTHA) (Fig. 7) showed that the incidence of PJI was significantly decreased in the vancomycin-treated group (RR 0.37, 95% CI 0.14–0.96 [P = 0.04]), and no heterogeneity existed in the studies (I2 = 0%, P = 0.64). This suggests that using vancomycin powder in revision THA can significantly decrease the incidence of PJI.
Discussion
Vancomycin powder was first used in spinal surgery to prevent deep postoperative infections. Infection of the surgical site after spinal surgery is a serious complication [30]. According to the guidelines of the North American Spine Association [31], cefazolin is usually used in spinal surgery to prevent infections caused by Staphylococcus aureus. However, the number of cases of infections with methicillin-resistant Staphylococcus aureus (MRSA) is increasing [32–35]. Based on data from the USA and the UK [32–35], approximately 50% of infected patients in the ICU have MRSA infections. Therefore, vancomycin powder began to be used clinically. In the literature on spinal surgery, the infection rate significantly decreased after using vancomycin powder in lumbar spine surgery [36], cervical spine surgery [37], and some deformity surgeries [38].
Vancomycin powder in joint arthroplasty surgery has been used only in recent years, and the effect of reducing the postoperative PJI rate is still unclear. According to the results of some previously published meta-analyses [39–41], the use of vancomycin powder has a significant effect on reducing the postoperative PJI rate. However, we believe that the published meta-analyses are not comprehensive and have not considered the effects of different surgical sites, different surgery types, and different vancomycin powder doses on the PJI rate.
Therefore, we performed this meta-analysis to analyse the effect of different doses of vancomycin powder in preventing PJI in joint arthroplasty. In the analysis of primary TKA in this study, we found that 1 g or 2 g of vancomycin powder could all prevent PJI. In the analysis of primary THA, we found that 1 g of vancomycin powder reduced the incidence of PJI. However, no significant change in the incidence of PJI was found in the experimental group when 2 g of vancomycin powder was used in primary THA. This result might be contrary to clinical experience. Overall, in the study of using 1 g of vancomycin powder, the PJI rates of the experimental group and control group were 0.67% and 1.26%, respectively; in the study of using 2 g of vancomycin powder, the PJI rates of the experimental group and control group were all 0.56%. We analysed the data and found that the PJI rate of experimental group treated with 2 g of vancomycin powder (0.56%) was slightly lower than that treated with 1 g of vancomycin powder (0.67%), and this result was logical. However, the PJI rate of the control group in the study of using 2 g of vancomycin powder (0.56%) was much lower than that in the study of using 1 g of vancomycin powder (1.26%). These data were obviously abnormal and there might be a potential bias that was not detected by the classical tools of systematic reviews. In addition, there were only 1,956 patients included in the control group of the study of using 2 g of vancomycin powder (compared to the study of using 1 g of vancomycin powder, 5074 patients were included in the control group). The small number of patients enrolled might also affect the accuracy of the results. So we believed the conclusion that using 2 g of vancomycin powder in primary THA had no effect on preventing PJI was doubtful, and more prospective randomized controlled trial studies were needed to verify it. Furthermore, the failure to include more studies on using 2 g of vancomycin powder in primary THA was also one of the disadvantages of this paper.
Part of the studies included in this paper had a follow-up period of only 3 months. However, a large proportion of PJI occurred more than 1 year after surgery. If the follow-up time is too short, some PJI may not be detected so that affecting the accuracy of the results. However, we believe that the PJI that occurred more than 1 year after surgery is more related to host factors, and less related to the surgical technique of the surgeon and the treatment regimen of vancomycin powder. Therefore, we think that the follow-up period of 3 months has a limited influence on the accuracy of the results.
The advantages of this study are as follows: (1) Most of the previously published meta-analyses on whether vancomycin powder can prevent PJI following joint arthroplasty did not separate the comparisons for TKA and THA or those for primary arthroplasty and revision arthroplasty. (2) To the best of our knowledge, there is no specific meta-analysis that analyses the effect of different doses of vancomycin powder. This study not only analysed the different surgical sites and different surgical types but also studied the effect of vancomycin powder at different doses on PJI, making the research more comprehensive. The disadvantages of this study are as follows: (1) There are few literature reports on the effect of vancomycin powder on the PJI rate in revision joint arthroplasty, and there is no specific analysis of the effect of different doses of vancomycin powder. (2) In view of the result that the use of 2 g of vancomycin powder in THA has no obvious effect on the prevention of PJI, we made a corresponding conjecture, but there is no specific experiment to support this. (3) The failure to include more studies on using 2 g of vancomycin powder in primary THA might influence the accuracy of the final results.
Conclusion
These results suggest that using vancomycin powder in primary TKA has a rather clear effect on preventing PJI. Both 1 g and 2 g of vancomycin powder can be used for surgery. For primary THA, it is better to use 1 g of vancomycin powder for treatment. The effect of 2 g of vancomycin powder on preventing PJI is not clear, and more prospective randomized controlled trial studies are needed to verify it. Using vancomycin powder in revision TKA and revision THA to reduce the incidence of PJI is relatively effective, but the preventive effect of different doses of vancomycin powder on PJI in revision TKA and THA needs to be verified by more studies.
Supplementary Information
Additional file 1. Table S1. Search strategy.
Additional file 2. Table S2. Characteristics of patients extracted from all included studies.
Acknowledgements
We would like to thank the researchers and study participants for their contributions.
Abbreviations
- PJI
Periprosthetic joint infection
- TJA
Total joint arthroplasty
- TKA
Total knee arthroplasty
- THA
Total hip arthroplasty
- PTKA
Primary total knee arthroplasty
- PTHA
Primary total hip arthroplasty
- RTKA
Revision total knee arthroplasty
- RTHA
Revision total hip arthroplasty
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- VP
Vancomycin powder
- NOS
Newcastle–Ottawa Scale
Author contributions
SL and JL contributed to conceptualization and methodology. SL, ZY, XL, and JC contributed to data curation and writing—original draft preparation. SL and ZY contributed to writing—reviewing and editing. All authors made substantial contributions to this manuscript and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
This is a meta-analysis and therefore no ethics committee and informed consents are required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shiyu Liao, Email: 1787646223@qq.com.
Zhize Yang, Email: 1830598183@qq.com.
Xiao Li, Email: 465919387@qq.com.
Jintian Chen, Email: 511710700@qq.com.
Jian-guo Liu, Email: liujg6@126.com.
References
- 1.Kunutsor SK, Beswick AD, Peters TJ, et al. Health care needs and support for patients undergoing treatment for prosthetic joint infection following hip or knee arthroplasty: a systematic review. PLoS ONE. 2017;12(1):e0169068. doi: 10.1371/journal.pone.0169068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387(10016):386–394. doi: 10.1016/S0140-6736(14)61798-0. [DOI] [PubMed] [Google Scholar]
- 3.Parvizi J, Heller S, Berend KR, Della Valle CJ, Springer BD. Periprosthetic joint infection: the algorithmic approach and emerging evidence. Instr Course Lect. 2015;64:51–60. [PubMed] [Google Scholar]
- 4.Shahi A, Parvizi J. Prevention of periprosthetic joint infection. Arch Bone Jt Surg. 2015;3(2):72–81. [PMC free article] [PubMed] [Google Scholar]
- 5.Gundtoft PH, Overgaard S, Schønheyder HC, Møller JK, Kjærsgaard-Andersen P, Pedersen AB. The "true" incidence of surgically treated deep prosthetic joint infection after 32,896 primary total hip arthroplasties: a prospective cohort study. Acta Orthop. 2015;86(3):326–334. doi: 10.3109/17453674.2015.1011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huotari K, Peltola M, Jämsen E. The incidence of late prosthetic joint infections: a registry-based study of 112,708 primary hip and knee replacements. Acta Orthop. 2015;86(3):321–325. doi: 10.3109/17453674.2015.1035173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52–56. doi: 10.1007/s11999-009-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine DPJCid. Vancomycin: a history. 2006;42(Supplement_1):S5-S12. [DOI] [PubMed]
- 9.Xiong L, Pan Q, Jin G, Xu Y, Hirche C. Topical intrawound application of vancomycin powder in addition to intravenous administration of antibiotics: a meta-analysis on the deep infection after spinal surgeries. Orthop Traumatol Surg Res. 2014;100(7):785–789. doi: 10.1016/j.otsr.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Bakhsheshian J, Dahdaleh NS, Lam SK, Savage JW, Smith ZA. The use of vancomycin powder in modern spine surgery: systematic review and meta-analysis of the clinical evidence. World Neurosurg. 2015;83(5):816–823. doi: 10.1016/j.wneu.2014.12.033. [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Zhang X, Zhang Y, Chen C, Yu H, Xue E. Role of intra-wound powdered vancomycin in primary total knee arthroplasty. Orthop Traumatol Surg Res. 2020;106(3):417–420. doi: 10.1016/j.otsr.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Otte JE, Politi JR, Chambers B, Smith CA. Intrawound vancomycin powder reduces early prosthetic joint infections in revision hip and knee arthroplasty. Surg Technol Int. 2017;30:284–289. [PubMed] [Google Scholar]
- 13.Patel NN, Guild GN, 3rd, Kumar AR. Intrawound vancomycin in primary hip and knee arthroplasty: a safe and cost-effective means to decrease early periprosthetic joint infection. Arthroplast Today. 2018;4(4):479–483. doi: 10.1016/j.artd.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tahmasebi MN, Vaziri AS, Vosoughi F, Tahami M, Khalilizad M, Rabie H. Low post-arthroplasty infection rate is possible in developing countries: long-term experience of local vancomycin use in Iran. J Orthop Surg Res. 2021;16(1):199. doi: 10.1186/s13018-021-02344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matziolis G, Brodt S, Böhle S, Kirschberg J, Jacob B, Röhner E. Intraarticular vancomycin powder is effective in preventing infections following total hip and knee arthroplasty. Sci Rep. 2020;10(1):13053. doi: 10.1038/s41598-020-69958-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkler C, Dennison J, Wooldridge A, et al. Do local antibiotics reduce periprosthetic joint infections? A retrospective review of 744 cases. J Clin Orthop Trauma. 2018;9(Suppl 1):S34–S39. doi: 10.1016/j.jcot.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchalter DB, Teo GM, Kirby DJ, Schwarzkopf R, Aggarwal VK, Long WJ. Does the organism profile of periprosthetic joint infections change with a topical vancomycin powder and dilute povidone-iodine lavage protocol? J Arthroplasty. 2021;36(7s):S314–S319. doi: 10.1016/j.arth.2020.12.036. [DOI] [PubMed] [Google Scholar]
- 18.Riesgo AM, Park BK, Herrero CP, Yu S, Schwarzkopf R, Iorio R. Vancomycin povidone-iodine protocol improves survivorship of periprosthetic joint infection treated with irrigation and debridement. J Arthroplasty. 2018;33(3):847–850. doi: 10.1016/j.arth.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 19.Dial BL, Lampley AJ, Green CL, Hallows R. Intrawound vancomycin powder in primary total hip arthroplasty increases rate of sterile wound complications. Hip Pelvis. 2018;30(1):37–44. doi: 10.5371/hp.2018.30.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen EM, Marcaccio S, Goodman AD, Lemme NJ, Limbird R. Efficacy and cost-effectiveness of topical vancomycin powder in primary cementless total hip arthroplasty. Orthopedics. 2019;42(5):e430–e436. doi: 10.3928/01477447-20190321-05. [DOI] [PubMed] [Google Scholar]
- 21.Khatri K, Bansal D, Singla R, Sri S. Prophylactic intrawound application of vancomycin in total knee arthroplasty. J Arthros Joint Surg. 2017;4(2):61–64. doi: 10.1016/j.jajs.2017.08.001. [DOI] [Google Scholar]
- 22.Hanada M, Nishikino S, Hotta K, Furuhashi H, Hoshino H, Matsuyama Y. Intrawound vancomycin powder increases post-operative wound complications and does not decrease periprosthetic joint infection in primary total and unicompartmental knee arthroplasties. Knee Surg Sports Traumatol Arthrosc. 2019;27(7):2322–2327. doi: 10.1007/s00167-019-05498-z. [DOI] [PubMed] [Google Scholar]
- 23.Yavuz IA, Oken OF, Yildirim AO, Inci F, Ceyhan E, Gurhan U. No effect of vancomycin powder to prevent infection in primary total knee arthroplasty: a retrospective review of 976 cases. Knee Surg Sports Traumatol Arthrosc. 2020;28(9):3055–3060. doi: 10.1007/s00167-019-05778-8. [DOI] [PubMed] [Google Scholar]
- 24.Koutalos AA, Drakos A, Fyllos A, Doxariotis N, Varitimidis S, Malizos KN. Does intra-wound vancomycin powder affect the action of intra-articular tranexamic acid in total joint replacement? Microorganisms. 2020;8(5):671. doi: 10.3390/microorganisms8050671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiyu L, Zhize Y, Liu J. The effect of different dosage of intrawound vancomycin powder on Periprosthetic joint infection (PJI) in total knee and total hip arthroplasty: a systematic review and meta-analysis. 2021.
- 26.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.GA W. Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2008(5).
- 28.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi H, Iida Y, Yokoyama Y, et al. Use of intrawound vancomycin powder against postoperative infection after spine surgery. Spine Surg Relat Res. 2018;2(1):18–22. doi: 10.22603/ssrr.2016-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaffer WO, Baisden JL, Fernand R, Matz PG. An evidence-based clinical guideline for antibiotic prophylaxis in spine surgery. Spine J. 2013;13(10):1387–1392. doi: 10.1016/j.spinee.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 32.Khan NR, Thompson CJ, DeCuypere M, et al. A meta-analysis of spinal surgical site infection and vancomycin powder. J Neurosurg Spine. 2014;21(6):974–983. doi: 10.3171/2014.8.SPINE1445. [DOI] [PubMed] [Google Scholar]
- 33.Melzer M, Eykyn SJ, Gransden WR, Chinn S. Is methicillin-resistant Staphylococcus aureus more virulent than methicillin-susceptible S. aureus? A comparative cohort study of British patients with nosocomial infection and bacteremia. Clin Infect Dis. 2003;37(11):1453–1460. doi: 10.1086/379321. [DOI] [PubMed] [Google Scholar]
- 34.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 35.Noskin GA, Rubin RJ, Schentag JJ, et al. National trends in Staphylococcus aureus infection rates: impact on economic burden and mortality over a 6-year period (1998–2003) Clin Infect Dis. 2007;45(9):1132–1140. doi: 10.1086/522186. [DOI] [PubMed] [Google Scholar]
- 36.Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: efficacy, drug levels, and patient outcomes. Spine (Phila Pa 1976) 2011;36(24):2084–2088. doi: 10.1097/BRS.0b013e3181ff2cb1. [DOI] [PubMed] [Google Scholar]
- 37.Pahys JM, Pahys JR, Cho SK, et al. Methods to decrease postoperative infections following posterior cervical spine surgery. J Bone Joint Surg Am. 2013;95(6):549–554. doi: 10.2106/JBJS.K.00756. [DOI] [PubMed] [Google Scholar]
- 38.Theologis AA, Demirkiran G, Callahan M, Pekmezci M, Ames C, Deviren V. Local intrawound vancomycin powder decreases the risk of surgical site infections in complex adult deformity reconstruction: a cost analysis. Spine (Phila Pa 1976) 2014;39(22):1875–1880. doi: 10.1097/BRS.0000000000000533. [DOI] [PubMed] [Google Scholar]
- 39.Heckmann ND, Mayfield CK, Culvern CN, Oakes DA, Lieberman JR, Della Valle CJ. Systematic review and meta-analysis of intrawound vancomycin in total hip and total knee arthroplasty: a call for a prospective randomized trial. J Arthroplasty. 2019;34(8):1815–1822. doi: 10.1016/j.arth.2019.03.071. [DOI] [PubMed] [Google Scholar]
- 40.Peng Z, Lin X, Kuang X, Teng Z, Lu S. The application of topical vancomycin powder for the prevention of surgical site infections in primary total hip and knee arthroplasty: A meta-analysis. Orthop Traumatol Surg Res. 2021;107(4):102741. doi: 10.1016/j.otsr.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Xu H, Yang J, Xie J, et al. Efficacy and safety of intrawound vancomycin in primary hip and knee arthroplasty. Bone Joint Res. 2020;9(11):778–788. doi: 10.1302/2046-3758.911.BJR-2020-0190.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. Search strategy.
Additional file 2. Table S2. Characteristics of patients extracted from all included studies.
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].