Abstract
Increases in plastic-related pollution and their weathering can be a serious threat to environmental sustainability and human health, especially during the present COVID-19 (SARS-CoV-2 coronavirus) pandemic. Planetary risks of plastic waste disposed from diverse sources are exacerbated by the weathering-driven alterations in their physical-chemical attributes and presence of hazardous pollutants mediated through adsorption. Besides, plastic polymers act as vectors of toxic chemical contaminants and pathogenic microbes through sorption onto the ‘plastisphere’ (i.e., plastic-microbe/biofilm-environment interface). In this review, the effects of weathering-driven alterations on the plastisphere are addressed in relation to the fate/cycling of environmental contaminants along with the sorption/desorption dynamics of micro-/nano-scale plastic (MPs/NPs) polymers for emerging contaminants (e.g., endocrine-disrupting chemicals (EDCs), polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), pharmaceuticals and personal care products (PPCPs), and certain heavy metals). The weathering processes, pathways, and mechanisms governing the adsorption of specific environmental pollutants on MPs/NPs surface are thus evaluated in relation to the physicochemical alterations based on several kinetic and isotherm studies. Consequently, the detailed evaluation on the role of the complex associations between weathering and physicochemical properties of plastics should help us gain a better knowledge with respect to the transport, behavior, fate, and toxicological chemistry of plastics along with the proper tactics for their sustainable remediation.
Keywords: Nanoscale plastic, Environmental fate, Microplastic weathering, Emerging contaminants, Sorption kinetics, Coronavirus pandemic
Graphical Abstract
1. Introduction
Global-scale contamination by plastic polymers is occurring at an alarming pace, especially during present novel coronavirus (SARS-CoV-2) outbreak (Patrício Silva et al., 2021). Based on global regulatory institutions like the United Nations Environment Program (UNEP), plastic contamination and associated weathering mechanisms are an emergent environmental and human health concern (UNEP, 2014, Klingelhöfer et al., 2020). This is because of the weathering driven high surface reactivity of microplastics (MPs) and nanoplastics (NPs) that allow adsorption of hazardous chemical contaminants on ‘plastisphere’ (i.e., novel human-made ecosystem with close interface of plastic litter and microbiome) (Rochman et al., 2013; Rocha-Santos and Duarte, 2015; Jakubowska et al., 2020; Cortés-Arriagada, 2021). Further, the weathering induced physical-chemical changes are inextricably linked with the adsorption of hazardous pollutants on MPs and NPs to pose human health risks (Duan et al., 2021, Fu et al., 2021, Luo et al., 2022).
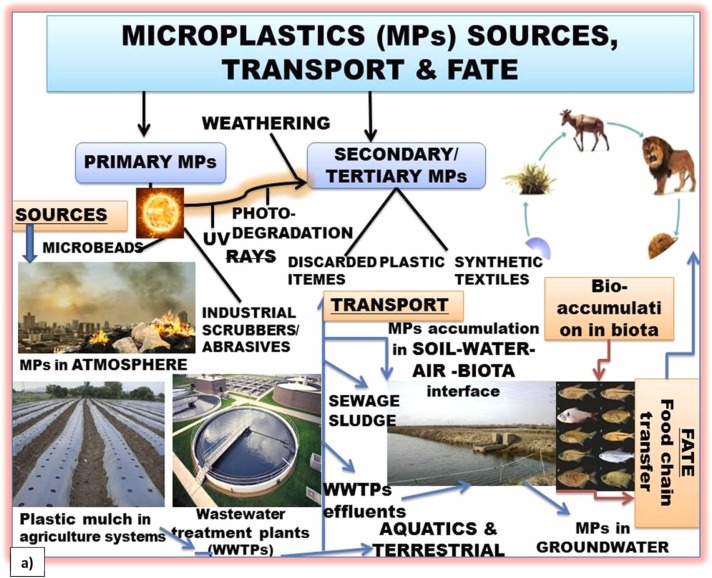
Among diverse environmental matrices, the pronounced weathering processes in marine ecosystems can serve as main depositories and reservoirs of MPs (with expected storage potential of 250 Mt (metric tons)) by 2025 (Sun et al., 2020). In the marine environment, increased weathering induced adsorption of inorganic, organic, and emerging contaminants on plastisphere and their subsequent desorption at biotic interface threatens the build-up of a ‘Blue Economy’ (i.e., ocean resources underpinning the economy and human well-being) (da Costa, 2018, Peng and Fu, 2020). Nonetheless, the terrestrial ecosystem is also not free from such a problem as it also represents a large fraction (14%) of global plastic pollution, which is further exacerbated through weathering processes and adsorption of environmental contaminants (Horton et al., 2017a, Horton et al., 2017b, Wanner, 2020, Vieira et al., 2021).
Plastic weathering processes can increase the environmental abundance of MPs/NPs to perturb the environmental sustainability and planetary public health due to their role as effective adsorbents/vectors of toxic inorganic contaminants such as heavy metals (Bradney et al., 2019), organic (Agboola and Benson, 2021, Fu et al., 2021), emerging pollutants (e.g., bisphenol A: BPA) (Cortés-Arriagada, 2021), and pathogenic microbes (Wu et al., 2019a, Wu et al., 2019b, Zettler et al., 2013; Zhang et al., 2020a, Zhang et al., 2020b; Luo et al., 2022). It is well known that the weathering of plastic particles can allow microbial adhesion or colonization (Sun et al., 2020). Therefore, the plastisphere can act as a potential vector of pathogenic microbes such as Pseudomonas and Vibrio to pose human health risks (Koelmans et al., 2019). Also, weathered plastic surface associated with biofilm or microbial substrata can adsorb more pollutants relative to virgin MPs (Wang et al., 2021). In addition to the weathering induced hazardous effects, uncontrolled incineration of medical plastic waste as management option may contribute to the release of greenhouse gases (GHG) and other potentially dangerous pollutants such as polychlorinated biphenyls (PCBs), polyaromatic hydrocarbons (PAHs), dioxins, furans, and heavy metals (Patrício Silva et al., 2021). The recent outburst of the SARS-CoV-2 pandemic has substantially raised the consumption and disposal rates of plastic products by the general public and para-medical/medical personals due to the increased global demand for PPE products (e.g., single-use face mask, medical gloves, gowns, etc.), packaged meals, plastic cups, and home-delivered groceries (Adyel, 2020, Prata et al., 2020, Patrício Silva et al., 2021). Therefore, the weathering of disposed plastic products (e.g., especially during COVID-19) can further enhance the chemical adsorption on plastisphere to exacerbate their hazardous effects.
The weathering linked physical-chemical factors (e.g., pH, temperature, solar irradiation, and the presence of salts such as NaCl and CaCl2) and biotic factors (e.g., microbial diversity) influence the sorption-desorption mechanism of chemicals and pathogens on MPs/NPs surface (Zhang et al., 2019a). Therefore, adsorption of pollutants on plastic polymers and consequential physical-chemical processes are also controlled by weathering (Bradney et al., 2019, Rodrigues et al., 2019). Plastic weathering results in the formation of MPs/NPs in specific particle size ranges (e.g., MPs less than 5 mm in diameter and NPs with less than 100 nm) with salient physical-chemical and adsorptive attributes (Rocha-Santos and Duarte, 2015; da Costa et al., 2019; Hoang and Kim, 2020; Harraq and Bharti, 2021; Luo et al., 2022).
Our basic knowledge of the sorption chemistry of environmental contaminants on the plastisphere has been upgraded by enormous efforts in this research field (Yu et al., 2019, Fred-Ahmadu et al., 2020, Seeley et al., 2020, Rai et al., 2021a, Rai et al., 2021b, Rai et al., 2021c). Nevertheless, useful information is yet scanty to elucidate the influence of plastic weathering on adsorptive attributes. In this respect, a survey on the ‘Science Direct’ search engine also reveals the rapid increase in the number of articles (research, reviews, book chapters, and encyclopedia articles) on MP and NP pollution in the recent decade (2011 to on-going 2021) ( Fig. 1a). Notably, there is a considerable increase in the research concerning the sorption chemistry of MPs/NPs for various emergent chemicals over the past five years (Fig. 1(a,b)). However, the number of research articles related to sorption of chemicals on the plastic surface is relatively low in proportion to the total number of publications covering the diverse aspects of MPs and NPs (Fig. 1b). Further, only 11 reviews are found to be made over the last five years that cover the sorption of contaminants on MPs and NPs surfaces to a large degree (e.g., Ribeiro et al., 2019; Yu et al., 2019; Mammo et al., 2020; Fred-Ahmadu et al., 2020; Wang et al., 2021). Such efforts were confined by their quest on plastisphere adsorption of organic/emerging pollutants (Agboola and Benson, 2021, Fu et al., 2021, Vieira et al., 2021) or the interactive role of plastic-microbes as vector of pollutants (Wang et al., 2021, Luo et al., 2022). As such, the past research efforts were inadequate to empirically address the effects of weathering on the adsorptive attributes of MPs and NPs.
Fig. 1.
Trends in the research publications related to MPs/NPs emphasizing lesser studies on pollutants adsorption on plastisphere, especially in terms of empirical or critical reviews during the last decade. (a) The number of experimental researches, review, book chapters, and encyclopedia publications pertaining to wide health/analytical or multiple prospects of MPs and NPs (duration 2011-ongoing 2021; Source-Science direct using the keywords: “Microplastics” and “Nanoplastics”; values in cyan color represent reviews in multiple aspects of MPs/NPs). (b) The number of research articles related to chemicals sorption on MPs/NPs in proportion to the total number and lack of a holistic review on sorption of diverse contaminants on plastics (keyword: ‘chemical sorption on plastics); values in cyan color represent reviews which partially mentioned sorption of pollutants on MPs/NPs.
The lack of our knowledge in the weathering-physical-chemical interface operating in tandem is yet significant not to gain an integrated overview as previous adsorption studies on MPs/NPs did not encompass a broad range of environmental pollutants. Therefore, our knowledge horizon on the influence of weathering on adsorptive and physical-chemical attributes is still narrow despite the noticeable progress made in the recent past.
To the best of our knowledge, the explicit holistic reviews on the sorption of diverse environmental contaminants on plastisphere are scanty (Fig. 1b). Besides, there is a knowledge gap in elucidating the role of weathering and subsequent alterations in physical-chemical/environmental processes on the sorption kinetics/isotherms of hazardous pollutants over MPs/NPs surfaces. Henceforth, the present review aims to fill the knowledge voids in weathering-plastisphere sorption interrelationship under different physical-chemical conditions. The recent increase in the global plastic pollution under the event of COVID-19 pandemic further exacerbated the investigation of their sorption processes and related mechanisms in relation to their interrelationship with weathering and physical-chemical properties (particle size and surface chemistry). A detailed analysis of these interactive factors (e.g., pollutant adsorption, sorption kinetics, environmental behavior, and ecotoxicity of MPs/NPs) is also made to allow the comparative evaluation of pollutant adsorption on plastic particles and non-plastic environmental substrata (e.g., biochar and nano-adsorbents). This review is thus expected to offer a better knowledge on the plastic weathering and their influence on adsorption mechanism of diverse hazardous chemicals.
2. Influence of weathering on adsorption chemistry of plastic particles
2.1. Weathering pathway/mechanisms and influence on plastisphere adsorption
Weathering is one of the most critical processes affecting the physical-chemical attributes of plastic particles (MPs/NPs) and their sorption affinity for contaminants in the surrounding environment (Lambert et al., 2017, Duan et al., 2021, Vieira et al., 2021, Luo et al., 2022) ( Fig. 2a and b). Thus, the weathering and physical-chemical attributes operate in tandem to influence the pollutant adsorption on MPs/NPs surface. Although both weathering and alterations in physical-chemical attributes act in concert, each of them is discussed in separate sections to explicitly elucidate their influence on pollutant adsorption by MPs/NPs. There are several weathering, physical-chemical, and environmental stress processes that exert controls on plastisphere adsorption in several respects: (i) physical factors (via abrasion by sand particles), (ii) chemical factors (e.g., pH, ionic strength, and oxidizing agents), (iii) photo/thermal-oxidative factors (e.g., the climate in terms of light and heat), and (iv) biological factors by microbial degradation (Fig. 2b). Notably, the effect of weathering on plastic physico-chemical properties is a more complicated process in the real environment due to the simultaneous action of environmental attributes (in terms of climate, regional conditions, and biological factors) (Luo et al., 2022). Hence, the individualistic effects of a single environmental stress factor on plastic weathering initiated the physical-chemical attributes and their influence on pollutant adsorption are commonly investigated under controllable laboratory simulations (Sun et al., 2020). In this scenario, the effects of physical and chemical environmental stress factors on plastic weathering and sorption capability are frequently elucidated in laboratory research. For instance, MPs released into real field conditions are expected to undergo physical abrasion and chemical reaction accelerated by increasing temperature, in line with the Arrhenius relationship (Geburtig et al., 2019). In addition to abrasion, thermal oxidation and photo-oxidation are projected to trigger polymeric yellowing when exposed to near-UV and visible radiation via various free-radical reaction mechanisms that proceed according to the following steps (Gardette et al., 2013).
-
A.Chain initiation
(1) -
B.Propagation (chain growth and branch formation)
(2) (3) (4) (5)
-
C.
Termination of the chain
| (6) |
| (7) |
| (8) |
Fig. 2.
Influence of weathering on adsorption and environmental fate of MPs (a) sources and the fate of MPs under the event of weathering and (b) classification of MP weathering caused by multiple abiotic/biotic environmental stressors and their influence on chemical sorption, environmental fate, and eco-toxicity (Modified 2b after Klein et al., 2018).
Photo-oxidative processes could also lead to significant alterations in the MPs surface chemistry, mainly by forming new functional groups such as -C = O; -C O, and –OH in the presence of O/N oxides, OH radicals, and other photo-generated radicals (Chandra and Rustgi, 1998, Bandow et al., 2017, Duan et al., 2021). Ageing under photo-oxidative reactions may also induce microscopic surface cracks and polymeric plastic backbone fragmentation induced by light and oxygen radical reactions with their internal layers (Luo et al., 2020, Luo et al., 2020a, Luo et al., 2020). MPs aged under these weathering conditions showed increased surface area (Lambert et al., 2017). For instance, the surface area of polyethylene (PE) MPs increased from 3.4 ± 0.1–5.8 ± 0.3 m2g-1 as a result of cracking and fragmentation after exposure to Xenon light- (ZH-XD-150 Xenon-lamp aging test chamber) for 8 weeks (Luo et al., 2020, Luo et al., 2020a, Luo et al., 2020). In this case, the environmental factors were tightly regulated at 60 °C, 50% humidity, and light irradiance 1200 Wm−2 to demonstrate the influence of photo-oxidative ageing on PE MP surface area (Luo et al., 2020, Luo et al., 2020a, Luo et al., 2020). Similarly, in another study, the specific surface area of the naturally weathered plastic debris (polystyrene (PS) MPs) collected from the coastal beaches of North China was increased from 2.0 ± 0.1–7.9 ± 0.2 m2 g-1 due to fragmentation upon oxidative weathering under solar irradiation when compared with virgin PS MPs foams (Zhang et al., 2018a)
However, it was noted that weathered PS MPs had a smaller average pore diameter (5.1 ± 0.2 nm) relative to virgin PS MPs with an average pore size of 39.3 ± 0.5 nm due to the pore deformation under solar weathering effects (Zhang et al., 2018a). In this context, the aged or weathered MP pellets offer a larger surface area for the sorption of multiple toxic organic contaminants (Wang and Wong, 2018, Wang et al., 2018a). Concomitantly, aged MP pellets allow better diffusion and transfer mechanisms for hazardous chemicals to penetrate deep inside their internal structure (Fries and Zarfl, 2012, Fries et al., 2013, Mendoza and L.M., Jones, 2015). In marine environments, it was observed that the sorption affinity of MP for organic chemicals (e.g., PCBs, PAHs, and DDT) decreased in the order PE > PP (polypropylene)> PS due to the weathering effects (Wang and Wong, 2018, Wang et al., 2018a). Moreover, the weathering of aged PS pellets also leads to the leaching of toxic chemicals such as hexabromocyclododecanes (HBCDDs) as the result of the photooxidation process (Liu et al., 2019, Liu et al., 2020a, Liu et al., 2020b, Liu et al., 2020c, Liu et al., 2019d).
As the weathering proceeds actively, the changes in physical-chemical characteristics of plastic particles take place to influence their potential for chemical adsorption (Luo et al., 2020, Luo et al., 2020a, Luo et al., 2020; Agboola and Benson, 2021; Fu et al., 2021). For example, exposure to UV radiation reduced the average molecular weight of PVC MPs while increasing their surface roughness and size to impact the adsorption behavior of triclosan (Ma et al., 2019). In contrast to photo-oxidation, biological weathering by Chlorella pyrenoidosa could improve plastic stability through biological secretions of extracellular polymeric substances (EPS) through the formation of a plastic-microbe biofilm (Mao et al., 2020). The formed plastic-microbe biofilm should reduce the aggregation of MP and NP particles in the surrounding environment, thereby improving their chemical stability (Mao et al., 2020). A study on the aggregation kinetics using Derjaguin–Landau–Verwey–Overbeek (DLVO) calculations revealed that the energy barrier of PS NPs (in water) was dependent on electrostatic repulsion, van der Waals forces, and the pH of the surrounding media (Mao et al., 2020). Additionally, chemical kinetic models indicated that a number of factors (e.g., DLVO/xDLVO ratio, ionic strength, and the PS NPs/chemical additives ratio) could impact PS NPs mobility and stability in the environment due to a charge shielding effect (Hu et al., 2020). Therefore, in addition to weathering, aggregation of MPs is a vital attribute governing their life cycle in real environmental conditions. This may be estimated using a set of chemical interactions.
| (9) |
| (10) |
| (11) |
Here, V T(d) represents the total interaction energy, V vdw(d) denotes van der Waals interaction energy, and V edl (d) is the electric double layer interaction energy in Eq. (9). In Eq. (10), A is the Hamaker constant for the NP dispersion system, a is the radius of NPs, and d is the separation distance between NPs. In Eq. (11), ε denotes the dielectric constant of the aqueous phase, ε0 reflects the dielectric constant of vacuum, k is the Boltzmann constant, T is the absolute temperature, qe is the electron charge, z denotes the charge number, ψ represents the surface potential of NPs, and ҡ denotes the Debye length.
The above equations of DLVO theory are vital in explaining the role of environmental factors such as inorganic ions, pH, organic matter, ionic strength, and natural minerals in controlling the aggregation of NPs (Mao et al., 2020, Wang et al., 2021). For example, a positive charge on NPs under low solution pH (acidic conditions) can reduce ψ value and stimulate the aggregation process. Conversely, a negative charge on NPs at a higher pH (alkaline medium) with negative ε –potentials can cause an increase in ψ value, which results in increased stability of PS NPs (Mao et al., 2020). Furthermore, an increased ionic strength could reduce the V edl (d) factor, thereby facilitating the aggregation process of NPs (Wu et al., 2020a, Wu et al., 2020b, Wu et al., 2020c, Wu et al., 2020d, Wang et al., 2021). In this respect, natural minerals also facilitate hetero-aggregate formation by increasing the electrostatic interactions between the particles of NPs (Singh et al., 2019). The hydrophobic MPs with net surface zero charges increased the capability of MPs to attract and adsorb various substances (nutrients, biomolecules, organic matter, environmental contaminants, and pathogenic microbes) from the surrounding environment (Galloway et al., 2017). The layer of organic matter formed on the MPs surface can lead to eco-corona formation that enhances NPs stability while the aggregation process decreased with an increase in the ψ value (Saavedra et al., 2019, Wang et al., 2021). Accordingly, a MP surface covered with bio-molecules (eco-corona) potentially acts as a vehicle for transferring pathogens and persistent organic pollutants (POPs) through the environmental media as mediated through the adsorption process (Galloway et al., 2017). Similarly, NPs can also interact with biomolecules like protein, leading to the generation of eco-corona/“coronal protein rings”, which had a remarkable impact on endocytosis in biota (Huang et al., 2018). Therefore, the formation of an eco-corona can influence the adsorption, fate, and eco-toxicity of MPs/NPs.
Fig. 2b shows the effects of weathering on the environmental fate and ecotoxicity of MPs. For example, solar radiation can enhance photo-oxidation, physicochemical abrasion, and microbial colonization of plastic polymers (Sun et al., 2020). These weathering attributes remarkably influence their environmental stability, transport/migration processes, sorption behavior, and toxicological chemistry. In some cases, long-term weathering could also increase the leaching rate of some chemical additives added during MP manufacturing (e.g., antioxidants or brominated flame retardants) and/or other adsorbed contaminants on plastic polymers (e.g., HBCDD) into the surrounding environment (Brandon et al., 2016). Note that antioxidant plastic additives are frequently added to increase plastic stability upon long-term aging through capturing the generated photo-oxidative free radicals. However, brominated flame retardants (categorized as POP) have been of particular interest given their potential to cause environmental damage and human health effects (Rai et al., 2021a). For instance, leaching of HBCDD from PS MPs increased in open seawater (42.9 mg g-1; 61%) under photo-oxidative weathering compared with dark exposure (25.6 mg g-1; 37%) after five days of aging (Rani et al., 2017). Furthermore, according to the simulation of weathering processes in several plastic polymers, photo-oxidative ageing promoted the leaching of many contaminants (e.g., Cu, Zn, Ca, Cl, and total organic carbon), compared to thermal oxidation (Bandow et al., 2017). In this context, the photo-oxidative ageing of MPs was also observed by introducing new adsorption bonds (e.g., C-O, C O, and OH), unlike thermally induced oxidation (Bandow et al., 2017).
The sorption affinity of aged MPs for chemical contaminants is complex and depends on various factors such as contaminant types, chemical structure of plastic polymers, weathering conditions, and sorption mechanism. In principle, the weathering factors could decrease or increase MP adsorption affinity for target contaminants by altering the physicochemical attributes of MPs along with their textural and surface properties by the action of reactive oxygen species, as described in (1), (2), (3), (4), (6), (7), (8) (Wang et al., 2021). For instance, beach-weathered MPs were found to have more oxygen-mediated functional groups than pristine MPs (Turner and Holmes, 2015; Huffer et al., 2018; Tiwari et al., 2019; Agboola and Benson, 2021). Furthermore, during the initial stages of photooxidative weathering, the crystallinity of aged MPs can increase due to plastic degradation (amorphous polymers) associated with oxidative chain scission reactions and subsequent rearrangement into shorter segments (Carrasco et al., 2001, Lv et al., 2015, Rouillon et al., 2016, Fu et al., 2021). Unlike photo-oxidation, thermal oxidation of pre-degraded PE MPs in the marine environment did not significantly affect crystallinity (Karlsson et al., 2018). In contrast, thermal oxidation was found to increase the extent of biofouling and density of pre-degraded PE MPs in a marine environment. This indicates that the variations in the weathering factors (e.g., photo or thermal-oxidation processes) can have different influences on the physicochemical features of plastics in the real environment.
The sorption chemistry of environmental contaminants is inextricably linked with multiple weathering and ageing factors controlling the alterations in physical-chemical process (e.g., particle size). For instance, the sorption capacity of 2, 2 ’, 4, 4’-tetrabromodiphenyl ether (BDE-47) on virgin PS MPs (6.16 ng g-1) was reduced to 4.96, 3.75, and 3.53 ng g-1 upon ageing PS plastics for 90 days under seawater, seawater with UV irradiation, and UV irradiation alone, respectively (Wu et al., 2020a, Wu et al., 2020b, Wu et al., 2020c, Wu et al., 2020d). The low sorption of BDE-47 on PS aged in seawater was due to surface biofilm formation, which reduces the MPs hydrophobicity and the number of available sorption sites. However, the sorption of BDE-47 on PS MPs was further reduced in seawater irradiated with UV due to the increased number of oxygen-containing functional groups (Wu et al., 2020a, Wu et al., 2020b, Wu et al., 2020c, Wu et al., 2020d). These oxygen-mediated functional groups formed hydrogen bonds between the MP surface and the surrounding water molecules, increasing surface hydrophilicity and reducing hydrophobic BDE-47 sorption (Wu et al., 2020a, Wu et al., 2020b, Wu et al., 2020c, Wu et al., 2020d). In several other studies, an enhancement in the adsorption of chemical contaminants on MPs surface was observed with the progress of plastic weathering (Liu et al., 2019c, Liu et al., 2019d, Liu et al., 2019). Herein, the increased adsorption was ascribed to increased surface area and formation of more favorable active sorption sites on the plastisphere. For example, the laboratory simulated weathering of PS and polyvinylchloride (PVC) plastic wastes enhanced the adsorption of hydrophilic antibiotics contaminants (e.g., ciprofloxacin) by 123.3% and 20.4%, respectively (Liu et al., 2019c, Liu et al., 2019d, Liu et al., 2019). The enhanced adsorption performance of the antibiotics was ascribed to the increased negative surface zeta potential that enhances electrostatic interaction and hydrogen bonding between ciprofloxacin and the weathered plastic surface (Liu et al., 2019c, Liu et al., 2019d, Liu et al., 2019). The physical abrasion (cryo-milled) and chemically induced (acid-treated) weathering factors also enhanced the sorption of difenoconazole and metformin pollutants on aged polyamide (PA), polypropylene (PP), and PS MPs (Goedecke et al., 2017). Furthermore, surface modifications of MPs through mechanical weathering (etching) also facilitated the sorption of PAHs (logK 3.85–5.18) more than UV-induced ageing (logK 3.71–4.98) when compared with pristine MPs (logK 3.80–4.95) (Li et al., 2020a, Li et al., 2020b). Herein, the etching process accelerated intraparticle diffusion, π-π interactions, and hydrogen bonding mechanisms between MP and PAH aromatic rings.
In addition to organic chemical adsorption, the weathered PVC MPs were demonstrated to have a high adsorption capability against inorganic contaminants such as silver and copper ions when aged over 502 days in marine ecosystems (Kedzierski et al., 2018). Notably, the adsorption of metallic contaminants onto aged PVC MPs was induced after the desorption of estrogenic compounds from PVC plastics to the aquatic environment. The sorption of inorganic contaminants (e.g., Cr, Ni, Zn, Cd, Pb, Mn, and Al) also increased upon aging PVC, PP, PE, and PET MPs over one year in the natural aquatic environment of San Diego Bay (Rochman et al., 2014). Other inorganic contaminants (like Cd, Pb, and Br) were also associated with MPs during field and lab-based weathering studies at two different beaches of southwest England (Massos and Turner, 2017). Inorganic contaminants (e.g., Ag, Cd, Co, Cr, Cu, Hg, Ni, Pb, and Zn) were strongly adsorbed on weathered or beached PE MPs relative to the virgin plastic particles of suspended river sediments. (Turner and Holmes, 2015). In this study, the higher adsorption of metallic contaminants on beached PE MPs than virgin pellets was attributed to surface modification by photooxidation (i.e., improved electrostatic interactions with increasing oxygenated surface functionalities) (Turner and Holmes, 2015, Turner et al., 2020). Further, these authors observed an adsorption isotherm of virgin PE against Hg and Pb with a maximum distribution coefficient of 6.0 mL g-1. Conversely, the weathered or beached plastic pellets adsorbed with Pb and Ag (instead of Hg) recorded comparatively higher distribution coefficients on the order of 1.0 × 102 mL g-1 (Turner and Holmes, 2015). Interestingly, an increase in photo-oxidative weathering duration (from 200 to 500 h UV exposure) promoted the adsorption of metallic contaminants on PE MPs like Cu2+ (from 55 to 60–160–175 µg g-1) and Zn2+ (from 45 to 50–75–80 µg g-1) (Turner and Holmes, 2015, Wang et al., 2020a, Wang et al., 2020b).
In a laboratory simulation, it was observed that the addition of external chemical stimuli such as Fenton’s reagent (a combination of H2O2 and ferrous ions with catalytic potential) could efficiently accelerate the aging process of MPs to subsequently alter the sorption potential for various chemical contaminants (Lang et al., 2020). In this context, Fenton’s reagent can induce a more substantial aging effect on PS MP surfaces than H2O2, while the aging of PS MPs via a photo-mediated Fenton’s reagent reaction was reported to decrease the adsorption efficiency for atorvastatin (Liu et al., 2020a, Liu et al., 2020b, Liu et al., 2020c). Conversely, oxygen-mediated functional groups such as carbonyl or carboxyl released during the aging of PS MPs increased the adsorption of amlodipine due to the high surface charge, hydrophilicity, and electrostatic/hydrogen bonding interactions on the MPs surface (Liu et al., 2019c, Liu et al., 2019d, Liu et al., 2019). Compared with aged PS MPs, the adsorption of pharmaceuticals onto pristine PS MPs is mainly controlled by hydrophobic and π-π interaction mechanisms. In another report, the sorption potential of BDE-47 on aged PS MPs was reduced relative to pristine MPs, irrespective of changes in pH and DOM concentration (Wu et al., 2020a, Wu et al., 2020b, Wu et al., 2020c, Wu et al., 2020d). The decreased BDE-47 sorption was attributed to the reduced number of hydrophobic sorption sites on the aged PS MPs surface due to the increased oxygen-mediated surface functionalities during aging. Zhang et al., 2020a, Zhang et al., 2020b, Zhang et al., 2020c, Zhang et al., 2020a, Zhang et al., 2020b noted a decline in sorption potential of all the MPs after their UV-induced photooxidative weathering as new oxygenated functional groups occupied the available sorption sites. As discussed in this section, the weathering of MPs is of paramount importance in driving the changes in physical-chemical processes and plastic properties/attributes which remarkably influence the pollutants adsorption on their surface. Therefore, an explicit evaluation on the effects of weathering on the alterations in physical-chemical attributes or properties of plastics can better elucidate the adsorption chemistry of pollutant, as described below.
2.2. Physical-chemical changes on plastisphere and their influence on pollutant adsorption
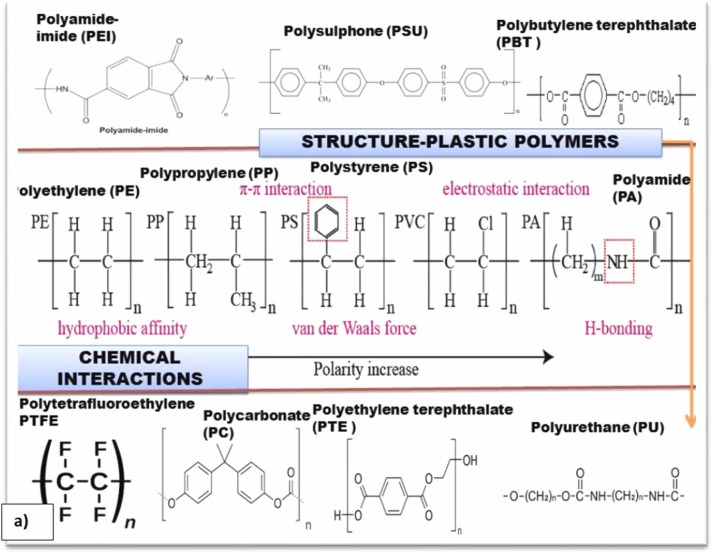
It is well-known that the chemical interactions of emerging contaminants with plastic surfaces are affected by both intrinsic (plastic characteristics) and extrinsic (environmental/biotic) attributes ( Fig. 3a,b). These intrinsic/extrinsic attributes regulate the sorption affinity of plastic polymers for contaminants and their toxicological chemistry (Fig. 3c). In this respect, the physical-chemical characteristics of MPs/NPs play a vital role in determining their sorption chemistry (Ma et al., 2020).
Fig. 3.
Chemical interactions within plastic particles and sorption chemistry of inorganic, organic, and emerging environmental contaminants on plastisphere (a) Chemical interactions among MP plastic polymers, structure, and trends in polarity, (b) explanation of plastic-chemical interactions considering POP as a model example in elucidating adsorption mechanisms, and (c) weathering driven changes in physical-chemical factors affecting pollutant adsorption on plastic (MPs and NPs) surface which further influence their environmental fate, behavior, and eco-toxicity.
2.2.1. Physical attributes
2.2.1.1. Effect of size and shape
It is well-known that the sorption affinity and ecotoxicity of plastics are dependent on their size and shape (Wright et al., 2013, Lambert et al., 2017, Ma et al., 2020, Fu et al., 2021). Weathering factors could cause fragmentation of larger MPs to smaller (nano-scale) particles and alter their surface functionalities (i.e., active sorption sites) and affinity for target adsorbates in the surrounding environmental matrices (Sun et al., 2020, Duan et al., 2021, Luo et al., 2022). For example, the downsizing of virgin PP MPs (of size 3–5 mm) by cryo-milling to 0.2–0.6 mm increased the plastic surface area (about 5–14 times) and its sorption capacity for difenoconazole by approx. 4 times (Goedecke et al., 2017). Furthermore, the sorption rates of PCBs on PS NPs were 1–2 fold higher than PE MPs, demonstrating the synergistic role of size and surface area of plastic polymers on the adsorption process (Velzeboer et al., 2014). The distribution coefficient of phenanthrene on NPs (log Kd: 5.82 ± 5.23) in freshwater was also higher than MPs (log Kd: 4.23 ± 3.04) (Ma et al., 2016). Accordingly, the toxic effects of NPs on biota (Daphnia magna and feathered minnow) might be greater than MPs (Ma et al., 2016, Hoang and Kim, 2020), thereby emphasizing the effect of size on ecotoxicity. It was also noted that MPs could be manufactured in different shapes (e.g., fibers, fragments, film, foam, and pellets) (Bond et al., 2018, Zhang et al., 2019a, Zhang et al., 2019b). In addition, the plastic shape can also influence the adsorption and eco-toxicity of chemical pollutants (Koelmans et al., 2019). Among these shapes, fiber-shaped PP MPs are more toxic to Hyalella azteca (an amphipod) than PP beads (Au et al., 2015). Plastic particles of irregular and needle shapes bind to biotic surfaces more readily and have a greater toxic potential, which is ascribed to increased specific surface area (Lambert et al., 2017).
2.2.1.2. Effect of plastic color
The visual appearance of MPs may be linked with pseudo-ingestion by biota and may influence the sorption and toxicological chemistry of several organic contaminants (Ma et al., 2020). The color could also be altered by the extent of weathering of plastic polymers. In this respect, lighter color beached plastic pellets exhibit a higher affinity to absorb lower-molecular-weight PAHs, while darker plastics tend to preferentially adsorb higher-molecular-weight PAHs (Fisner et al., 2013). Black beached MPs adsorbed more organic chemical contaminants (e.g., PCBs and PAHs) compared to white MPs (Frias et al., 2013; Ma et al., 2020). Greater adsorption on black MPs was ascribed to the enhanced-photo-oxidative weathering under solar light irradiation (relative to aged white MPs). As such, the surface area of weathered black MPs increased for organics or chemical additive adsorption. Furthermore, white-colored MPs demonstrated differential growth inhibition for green algae (Scenedesmus obliquus) due to the presence of ethanol residue (toxic agent for microbial pathogen) in white-colored MPs (Chen et al., 2020). Therefore, the color of plastic particles can also impact the pollutants' adsorption and biotic eco-toxicity.
2.2.1.3. Effect of plastic density and polymer type
The MP density plays a key role in controlling the sorption dynamics of plastics for various organic and inorganic contaminants (Cole et al., 2013, Song et al., 2014, Desforges et al., 2015, Rummel et al., 2017, Carbery et al., 2018). For instance, lower-density PE (LDPE) MPs were seen to exhibit higher adsorption affinity for PCBs, phenanthrene, and PAHs than the higher-density PE (HDPE) MPs (Mato et al., 2001; Karapanagioti and Klontza, 2008; Fries and Zarfl, 2012). Similarly, the LDPE MPs displayed a higher diffusion coefficient for PAHs when compared to the higher-density MPs such as PVC and PP (Chen et al., 2018, Lee et al., 2018, Seidensticker et al., 2017, Wang and Wong, 2018, Wang et al., 2018a). The lower sorption of PP and PVC MPs for PAHs can be ascribed to their glassy attributes (with a glass transition temperature of > 80 °C) and the increased surface hydrophilicity (e.g., lower hydrophobic and π-π interaction with PAHs). In addition, LDPE MPs have a large free volume between the molecules (relative to high-density MPs (PVC/PP)) with fewer void spaces of 0.2–10% (George and Thomas, 2001, Mammo et al., 2020). Such textural properties increase the free volume and available adsorption sites onto LDPE MPs, accelerating the PAH sorption process by a diffusion mechanism. Accordingly, PE MPs recorded a higher partition coefficient for PAHs (log Kd: 4.58 ± 3.75) than PP (log Kd: 3.34 ± 2.23) and PVC MPs (log Kd: 3.22 ± 2.30) (Wang and Wong, 2018, Wang et al., 2018a). The types of plastic polymer affect the adsorption kinetics of Cd(II) ions on PE, PP, PVC, and PS MPs in conjunction with other factors such as pH, humic acid concentration, and ionic strength of the solution (Guo et al., 2020). In this respect, the adsorption capacity of Cd(II) ions was ranked as follows: PVC (151.4 mg Kg-1) > PS (134.1 mg Kg-1) > PP (123.6 mg Kg-1) > PE (113.5 mg Kg-1).
2.2.2. Chemical attributes
2.2.2.1. Effect of plastic surface chemistry
The surface chemistry of plastics profoundly controls their sorption and eco-toxicity mechanisms in the relevant environmental matrix. This is due to the effect of plastic surface chemistry on the interaction between MPs and chemical contaminants as well as bioaccumulation/bioavailability processes. The adsorption patterns of emerging pharmaceutical contaminants (e.g., sulfadiazine, amoxicillin, tetracycline, ciprofloxacin, and trimethoprim) on five MPs (i.e., PE, PP, PS, PVC, and polyamide [PA]) were studied as a function of their surface functionalities (Li et al., 2018). Accordingly, PA was found to have the highest adsorption capacity for all pharmaceutical contaminants due to H-bond formation between the amide groups of PA and the pharmaceuticals. Likewise, the adsorption affinity of 18 perfluoroalkyl substances (PFASs) on MPs decreased in the order of PS > PS-COOH > PE (Llorca et al., 2018). This resulted from the extent of the hydrophobic interactions between MPs and PFASs. Naphthalene (NAP) MPs with charged functional groups such as -NH2, -OH, and -COOH also demonstrated lower adsorption of PAHs (e.g., Kd: 4.5–6.3 L/g) than uncharged naphthalene derivatives (with -CH3; Kd: 11.6–12.0 L/g) (Yu et al., 2020a, Yu et al., 2020b). The lower sorption of PAHs on charged naphthalene derivatives was ascribed to the higher NAP MPs surface polarity (i.e., lower hydrophobicity in terms of Log KOW). The charged functional groups tend to attenuate the π- π interactions and enhance the repulsion between NAP/NAP derivative molecules and MP particles, leading to reduced sorption capacity of PAHs on charged NAP MP derivatives (Yu et al., 2020a, Yu et al., 2020b).
PS-MPs with carboxylated functional groups (PS-COOH) increased the toxicity of inorganic chemical contaminants (e.g., Ni) in D. magna relative to unmodified PS due to the contribution of PS-COOH for concentrating Ni pollutants on its surface via a sorption process (Kim et al., 2017). Similarly, the exposure of mysid shrimp to PS-COOH produced a more toxic outcome than exposure to fresh PS due to their bio-accumulation in the stomachs of mysid shrimp (Wang et al., 2020a, Wang et al., 2020b). Upon exposing sea-urchin embryos to two functional derivatives of 40-nm PS (PS-COOH and PS-NH2), only PS-NH2 caused potential oxidative stress and apoptosis (Vega and Epel, 2004). Similarly, another eco-toxicity study in sea urchins also noted higher toxicity of positively charged 40-nm PS-NH2 NPs when compared with negatively charged PS-COOH NPs (Della et al., 2014). It is suggested that PS-NH2 disrupts the lysosomal membrane stability and induces oxy-radical production, causing cellular apoptosis and forming a PS-NH2 protein corona. In contrast, PS-COOH NPs tend to micro-aggregate in ambient media, resulting in lower bioavailability and ecotoxicity (Canesi et al., 2016).
2.2.2.2. Effect of plastic microstructure
The crystalline structure and molecular composition of plastic polymers are crucial to understanding the sorption-desorption mechanism of various chemical contaminants (Teuten et al., 2007, Wang et al., 2015). This is due to the effects of molecular composition and structural crystallinity (relates to crosslinking, glass transition temperature, and packing) on the density and permeability of plastic polymers (Lambert et al., 2017). In general, more ordered and firmer structures of plastic polymers have a higher degree of crystallinity (Rodrigues et al., 2019). On this basis, it was noted that amorphous PE and PP polymers with a low degree of packing and greater free volume demonstrated a stronger propensity for chemical sorption of many organics, such as PCBs (Cornelissen et al., 2005, Pascall et al., 2005), PBDEs (Rochman et al., 2014a), PAHs, hexachlorocyclohexanes, and chlorinated benzenes (Lee et al., 2014) in comparison to crystalline PS polymers. Based on glass transition temperature, amorphous polymeric regions are subdivided into glassy and rubbery. Glassy PE/PS polymers have lower diffusivity and more accessible adsorption sites for hydrophobic organic contaminants (e.g., phenanthrene, lindane, and naphthalene) when compared to rubbery polymers (Guo rt al, 2012; Rodrigues et al., 2019). Highly crystalline plastic polymers do not facilitate the sorption of pharmaceuticals (such as ciprofloxacin) because of the requirement for significant energies to stabilize their highly ordered polymer chain regions (Liu et al., 2019c, Liu et al., 2019d, Liu et al., 2019). It has also been suggested that more crystalline PE MPs usually exhibit smoother, cleaner surfaces with a reduced number of adsorption sites for chemical contaminants (e.g., they demonstrate lower sorption for phenanthrene, lindane, and naphthalene pollutants) (Guo et al., 2012). Herein, Guo et al. (2012) noted that the adsorption of phenanthrene, lindane, and naphthalene was increased when the crystallinity of PE MPs decreased from 59% to 26%. In this context, the PS MPs with high crystallinity showed higher adsorption for ciprofloxacin than PVC MPs with lower crystallinity (Liu et al., 2019c, Liu et al., 2019d, Liu et al., 2019). Further, glassy polymers with a high degree of cross-linking (e.g., PVC and PS) exhibit nano-holes, which serve as strong adsorption sites for organic chemical contaminants (Hüffer and Hofmann, 2016, Hartmann et al., 2017).
2.2.2.3. Effect of environmental parameters (water pH and ionic strength)
The external environment (biotic and abiotic) also plays a vital role in controlling the adsorption behavior of plastics and their eco-toxicity. In general, the sorption capacity and ecotoxicity of chemical contaminants sorbed onto MPs are remarkably influenced by variations in water pH and ionic strength. For instance, at alkaline pH (> 8), the adsorption of sulfamethoxazole (SMX) on PE MPs greatly declined due to the electrostatic repulsion between the negatively charged PE surface and the anionic SMX (Xu et al., 2018a, Xu et al., 2018b). The adsorption efficiency of Triclosan on PS MPs was significantly decreased from 80% to 10% as solution pH increased from 4 to 10 (Seidensticker et al., 2018). The reduced adsorption at higher pH was ascribed to alterations in Triclosan ionic charge that induced electrostatic repulsion with PS MP. The chemical adsorption of positively charged sertraline and propranolol on negatively charged PE MPs also increased at neutral pH (6.85) due to the accelerated electrostatic attraction mechanism during the adsorption process (Razanajatovo et al., 2018).
The effects of salinity on chemical adsorption onto the plastisphere were demonstrated by the difference in values of partitioning coefficients in seawater (log KMP-SW) and water (log KMP-W) (Wang and Wong, 2018, Wang et al., 2018a). Nevertheless, salinity does not demonstrate a consistent pattern in relation to its effect on chemical adsorption (i.e., a consistent increase or decrease) (Mammo et al., 2020). For example, the PAH partition coefficient in seawater (log KMP-SW) is higher than for water (log KMP-W) due to the ‘salting-out effect’ (Wang and Wong, 2018, Wang et al., 2018a ). This explains the decreased solubility of organic chemicals following an increase in salinity, leading to enhanced chemical availability for sorption on MPs surfaces. Similarly, the KMP-SW values of phenanthrene and pyrene were 7% and 5% greater than their KMP-W (Bakir et al., 2014). In contrast, hexachlorobenzene's partition coefficient in seawater was lower than its partition coefficient in pure water. A higher water salinity also significantly increased the sorption of Triclosan on PE MPs. However, no effect was observed on the sorption of other emerging chemical contaminants (e.g., carbamazepine, 4-methylbenzylidene camphor, and 17α-ethinyl estradiol) (Wu et al., 2016). Under a saline effect of NaCl and CaCl2 addition, the sorption of PFOS on PE/PS MPs increased due to its existence in anionic form (Wang et al., 2015). Accordingly, the insignificant effects of salinity on chemical sorption of organic compounds on MP surfaces can be attributed to the predominant physisorption mechanism via hydrophobic and van der Waals interactions, which are not key factors in solution pH or salinity.
Accordingly, the sorption of hazardous inorganic/organic chemicals on MPs is also dependent on weather factors, NOM concentration, salinity, pH, and biofilms due to their influence on MP crystallinity, oxygen groups, surface area, and physical-chemical attributes (Liu et al., 2020a, Liu et al., 2020b, Liu et al., 2020c). The chemical stability of MPs and NPs in the aquatic environment also depends on surrounding conditions such as pH, aging, metal cations, and EPS (Mao et al., 2020). The interactions of MPs/NPs with environmental particulates and organic matter also influence their sorption affinity for chemical contaminants, aggregation, and fate behavior. Shiu et al. (2020) observed that the hydrophobic interactions of 25 nm PS NPs with DOM in freshwater led to microgel formation, accelerating the DOM-particulate organic matter (POM) transition. These interactions result in the development of large organic particles. Thus, the settling of NPs can alter the dynamics of aquatic ecosystems. In a real terrestrial environment, it was also noted that the identification/separation of plastics is complicated due to their similar or comparable physical-chemical attributes to that of soil organic matter (SOM) (Hidalgo-Ruz et al., 2012, Dekiff et al., 2014, Lima et al., 2014). The plastic-microbe interactions (‘biofilms’) release EPS, which influences the chemical adsorption and biodegradation (Rai et al., 2021b). Further, microbial exposure to MPs sorbed with environmental contaminants (heavy metals/ antibiotics) significantly alters their antibiotic resistance profile (Mammo et al., 2020). In the absence of biofilms, microbes on MPs can pave the way to degrade plastic polymers into metabolites of the tricarboxylic acid (TCA) cycle (Ghosh et al., 2019, Mammo et al., 2020). As such, the changes in physical-chemical properties of MPs/NPs during the weathering process are found to remarkably influence the potential of pollutant adsorption. Unraveling the interactive role of ‘weathering-physicochemical processes’ and ‘pollutant adsorption’ in an interrelated framework can remarkably help expand our knowledge horizon of MPs/NPs sorption chemistry.
3. Adsorption chemistry of pollutants on the plastic surface and eco-toxic effects
3.1. Sorption of environmental contaminants onto plastics and effects on biota
A good number of organic chemical additives (e.g., BPA, parabens, benzophenones, organotins, phthalates, polybrominated flame retardants) are used to manufacture plastic polymers with improved quality and higher chemical stability under various environmental conditions (i.e., making plastics more resistant to radiative and microbial degradation). However, it was noted that some of these additives might bind/absorb to MPs and NPs surfaces during their manufacturing stage and be released to biota under harsh weathering conditions (Lithner et al., 2009, Lithner et al., 2011, Sofia et al., 2015, Luo et al., 2020; Duan et al., 2021). Most of these released organic additives act as harmful chemicals which are categorized as endocrine-disrupting chemicals (EDCs), which are hazardous to the environment and human health (Gallo et al., 2018, Rai et al., 2018a, Rai et al., 2018b, Rai et al., 2019, Rai et al., 2020, Amereh et al., 2020, Cortés-Arriagada, 2021). This fact was verified by a 48 h microcosm study on the movement of Daphnia exposed to 32 plastic leachates (EC50: 5–80 g plastic material L-1) (Lithner et al., 2009). The results showed no impact on Daphnia mobility even at high concentrations up to 70–100 g L-1. In contrast, the dramatic accumulation of phenanthrene in Arenicola marina (lugworm) was seen when exposed to ambient concentrations of only 1 µg PE/g sediment (Teuten et al., 2007). Similar findings were observed in Nitocra spinipes (marine copepod), where 38% of 21 plastic samples showed negative effects (Sofia et al., 2015). Further, it was noted that di (2-ethylhexyl) phthalate (DEHP) and other phthalates are EDCs with potential renal, reproductive, cardio, and neuro-toxic effects on biota (Rowdhwal and Chen, 2018). In Bohai and the Yellow Sea of China, phthalate concentrations of 6.09 ng/g were recorded for MPs (PE, PP, and PS) in beach samples, of which 64% consisted of DEHP (Zhang et al., 2018a).
BPA is also a chemical additive that constitutes 65% of polycarbonate (PC) and 30% of epoxy resin plastics as monomeric units with potential estrogenic properties (Fred-Ahmadu et al., 2020). The maximum BPA adsorption on PVC MPs was 0.19 ± 0.02 mg g-1 at the initial plastic concentration of 1.5 g L-1 (Wu et al., 2019a, Wu et al., 2019b). Likewise, flame retardants (such as polybrominated diphenyl ethers (PBDE)) have been demonstrated to be carcinogenic, mutagenic, and teratogenic with potential reproductive, liver, and neurotoxicity in mice (USEPA, 2017, Fred-Ahmadu et al., 2020). In light of these toxic effects, adsorption studies of PBDE on MPs were emphasized in biota such as a marine amphipod Allorchestes compressa (Chua et al., 2014). Accordingly, MP adsorption was greater in higher PBDE congeners (BDE-154/-153) than in lower brominated congeners such as BDE-28/-47. Likewise, a significant correlation was found between the density of MPs and the absorbed concentration of PBDE (BDEs 183–209) in the biomass of marine myctophid fish (Rochman et al., 2014). Similarly, the sorption of PBDEs (congeners BDE 47, 99, and 153) was demonstrated to occur on several MPs (PE, PP, PS, and PE) under different conditions (e.g., experimental pHs, temperatures, salinities, and organic matter concentrations) (Xu et al., 2019). In field conditions in Swiss lakes, several plastic additives (phthalates, BPA, PBDEs, and NP) were measured in collected MPs samples at concentrations above detection limits (Faure et al., 2015). These NPs are often considered as other examples of toxic EDCs that leach from MPs (e.g., PET, high-density PE, and PVC bottles), yielding concentrations ranging from 180 ng/L (from HDPE) to 300 ng/L (from PVC) (Loyo-Rosales et al., 2004). The hazardous impact of NP leaching from plastics inhibits mitochondrial complex 1, perturbing normal respiratory chain functioning (Belaiche et al., 2009). Plastic polymers can also act as potential vectors or sources of organic/inorganic/emerging contaminants in biota, which can pose serious threats to biota (Kaposi et al., 2014, Rochman et al., 2015, Avio et al., 2015, Agboola and Benson, 2021, Fu et al., 2021, Vieira et al., 2021). Table 1 summarizes a list of the emerging contaminants adsorbed on plastic particles under the event of weathering and methods used for their measurement. Further, the list of organic and inorganic contaminants adsorbed on the weathered plastic particles and their associated eco-toxicity is compiled in Table 2. Thus, the chemical additives associated with plastic particles can be released into environmental matrices under the event of weathering. The release or leaching of additives can potentially act as hazardous pollutants with adverse effects on biota.
Table 1.
Sorbed chemical contaminants on several weathered plastic particles (MP and NP) investigated in microcosm (M)/lab and field (F) conditions with the instrumental techniques used.
| S.No. | MPs/NPs polymer types in the environment/ Organism | MPs-size | Organic sorbed chemicals | Detection techniques | Study type [Microcosm (M)/Field (F)] | Reference |
|---|---|---|---|---|---|---|
| 1. | PE & PS M. galloprovincialis |
< 100 µm | Pyrene | HPLC-Fluorimetric detection |
M | Avio et al. (2015); Rodrigues et al. (2019) |
| 2. | PS | 5 µm | Dibutyl phthalate (DBP) | SEM & Flow cytometer | M | Li et al., 2020a, Li et al., 2020b |
| 3. | PA, PE, PVC, PS | < 250 µm | n-Hexane, Cyclohexane Benzene Toluene Chlorobenzene Ethylbenzoate Naphthalene |
GC-MS | M | Hüffer and Hofmann (2016) |
| 4. | PET, PE-HD, PP, PVC PE-LD |
Pellets (<5 mm) | Acenaphthalene, Acenaphthene Fluorene, Phenanthrene Anthracene, Fluoranthene Pyrene, Benz(a)anthracene Chrysene, Benzo(b)fluoranthene Benzo(k)fluoranthene Benzo(a)pyrene, Indeno(123-cd)pyrene, Dibenzo(ah)anthracene Benzo(ghi)perylene, CB congeners: 8, 18, 28, 52, 44, 60, 101, 81, 77, 123, 118, 114, 153, 105, 138, 126, 187, 128, 167, 156, 180, 169, 170, 189, 196, 206, 209 |
GC-MS | F | Rochman et al. (2013); Rodrigues et al. (2019) |
| 5. | PP | 0.45–0.85 mm | Tonalide, Musk xylene, Musk ketone | GC-MS | M | Zhang et al. (2017) |
| 6. | PE | 10–180 µm | PCB 118 PCB 126 |
GC-MS | M | Velzeboer et al. (2014); Rodrigues et al. (2019) |
| 7. | PS | 70 nm (nano) | Phenanthrene, Anthracene, Fluoranthene, Pyrene Benzo(a)anthracene, Chrysene Benzo(b)fluoranthene Benzo(k)fluoranthene Benzo(a)pyrene Benzo[g,h,i]perylene |
HPLC-fluorescence Detector |
M | Liu et al. (2016) |
| 8. | PE | 10–20 µm Zebrafish (Danio rerio) |
Benzo(a)pyrene | Fluorescence spectroscopy |
M | Batel et al. (2018) |
| 9. | PVC PE |
200–250 µm A.marina, Fish, Seabird (Fulmarus glacialis) |
Dichlorodiphenyltrichloroethane Phenanthrene Bis(2-ethylhexyl)phthalate |
LSC | M | Bakir et al., (2016) |
| 10. | PE-LD | 20–25 µm M. galloprovincialis |
Benzo(a)pyrene | GC-MS | M | Pittura et al. (2018) |
| 11. | PE | 212–250 µm Bacteria |
Phenanthrene, Anthracene | GC-MS | M | Kleinteich et al. (2018) |
| 12. | PE | Pellets (<5 mm) | PCBs (38 PCB congeners) | GC-MS | M | Rodrigues et al. (2019) |
| 13. | PE, PP, PS | < 250 µm | Phenanthrene, Fluoranthene Anthracene, Pentachlorobenzene Chrysene, Benzo(a)pyrene, Dibenz(a,h)anthracene, Benzo(ghi)perylene, Pyrene αß-HCH, ߥ-HCH, ¥--HCH, £ -HCH Hexachlorobenzene |
GC-ECD (HCHs, PCBs) HPLC- Fluorescence detector (PAHs) |
M | Lee et al. (2014); Rodrigues et al. (2019) |
Table 2.
Chemical contaminants (organic/inorganic) adsorbed on MPs under the event of weathering and their eco-toxic impacts.
| S.No. | CHEMICAL CONTAMINANTS | MPs | Model organism/ species | Eco-toxicity (cumulative-MPs+ adsorbed chemical) | Reference |
|---|---|---|---|---|---|
| ORGANIC CHEMICAL CONTAMINANTS | |||||
| 1. | PAHs/PCBs/PBDEs | PE; PS; PSNPs and humic acid-PS-matrix | Japanese medaka (Oryzias latipes); Mussel (Mytilus galloprovincialis); Daphnia magna; Gammarus roeseli |
Adsorption of PAHs led to genotoxicity, neurotoxicity, oxidative stress, and endocrine disruption | Rochman et al. (2014); Avio et al. (2015); Ma et al. (2020); Lin et al. (2020); Bartonitz et al. (2020) |
| 2. | Procainamide and doxycycline (pharmaceuticals) |
Red fluorescent polymer microspheres |
Tetraselmis chuii (marine microalga) |
Adsorbed pharmaceutical chemicals enhanced reduction in chlorophyll | Prata (2018) |
| 3. | Triclosan (TCS) | PE;PS;PVC; PVC800 |
Skeletonema costatum (microalga) |
The adsorption of TCS led to reduced growth | Zhu et al. (2019) |
| 4. | 14C Phenanthrene | PS MPs | Daphnia magna | Enhanced the eco-toxicity | Ma et al. (2020) |
| 5. | Perfluorinated compounds (PFCs), PCBs, methyl-mercury | LDPE | Zebrafish | Adsorbed LDPE caused acute liver toxicity in fish | Rainieri et al. (2018) |
| 6. | Organic pyrethroid insecticide (deltamethrin) | PE MPs | Daphnia magna | Enhanced eco-toxic responses | Felten et al. (2020) |
| 7. | Tetrabromobisphenol a (TBBPA) | Cosmetic-derived MP (PE microbeads) | Zebrafish | Enhanced oxidative stress | Yu et al., 2020a, Yu et al., 2020b |
| 8. | Decabromodiphenyl ether (BDE-209) | PS MPs | Marine scallop (Chlamys farreri) |
Adverse impacts on hemocyte phagocytosis and ultrastructural changes in gills and digestive gland | Xia et al. (2020) |
| 9. | Polybrominated diphenyl ethers (BDE-47) | PS MPs | Marine mussel (Mytilus coruscus) | Elevated respiration rate and antioxidant enzyme levels | Gu et al. (2020) |
| INORGANIC CHEMICAL CONTAMINANTS | |||||
| 10. | Hg | Fluorescence red polymer microspheres |
European seabass (Dicentrarchus labrax) |
Hg adsorption on polymer enhanced the eco-toxic impact in the form of oxidative stress and neuro-toxicity | Antao Barboza et al. (2018); Ma et al. (2020) |
| 11. | Cu | PS | Zebrafish | MPs associated with natural organic matter (NOM) elevated the toxicity of Cu, manifested through increased levels of malonaldehyde and metallothionein while reducing super-oxide dismutase (SOD) | Qiao et al. (2019) |
| 12. | Ni | PS; PS-COOH | Daphnia magna | Adsorption of Ni on PS demonstrated antagonistic effect on eco-toxicity, whereas PS-COOH had a synergistic effect with Ni |
Kim et al. (2017) |
| 13. | Pb & Cr | Powdered MPs | Microcystis aeruginosa | Concentrations (>10 µg L−1) led to inhibition of photosynthesis | Luo et al., 2020a, Luo et al., 2020, Luo et al., 2020 |
| 14. | Pb | polyolefins & PVC | Avian physiologically-based extraction test (PBET) | Bioaccessible concentrations of added Pb (as chemical additive) were significantly higher than environmentally adsorbed | Turner et al. (2020) |
| 15. | Ag | PE microbeads from cosmetics | L. minor and Daphnia magna | Ag on aged microbeads exhibited eco-toxicological impacts | Kalčíková et al. (2020) |
The weathering driven degradation of plastics and consequential leaching of fluorescent additives (such as 3,3′-diaminobenzidine and similar substances) from PUMPs (of 1.6 g L−1 concentration) was also reported to inhibit microalgae (Chlorella vulgaris) photosynthesis (Luo et al., 2019). Note that the leaching or desorption of these chemical/additive contaminants depends on the MPs' physicochemical and textural attributes and other environmental factors during the process of weathering (Fred-Ahmadu et al., 2020). Recently, Gu et al. (2020) also found that the presence of polybrominated diphenyl ethers (BDE-47) on the PS MPs surface should cause anti-oxidative stress in marine mussels (Table 2). Further, the common herbicide glyphosate, combined with PS-NP-NH2, exhibited increased toxicity in M. aeruginosa relative to glyphosate alone. In contrast, PS-NP-NH2 particles alone showed no toxicity (Zhang et al., 2018a, Zhang et al., 2018b, Zhang et al., 2018c). Further, Yu et al., 2020a, Yu et al., 2020b investigated the sorption of flame retardant tetrabromobisphenol A [TBBPA] on cosmetic-MPs (PE µBs) and noted an elevated production of harmful free radicals to put oxidative stress on zebrafish.
The association of MPs with other environmental contaminants (e.g., natural organic matter (NOM), minerals and nano-scale particles) can enhance their ecotoxicity (Oriekhova and Stoll, 2018, Qiao et al., 2019). In addition to the surface charge on the plastic polymeric surface, the charges on NOM and nanoscale particles are mainly responsible for increases in toxicity. For instance, neutral or positively charged MPs exacerbated the toxic impact of synthetic nanoparticles (e.g., TiO2) on marine algae Chlorella sp. (Thiagarajan et al., 2019). Conversely, negatively charged PS-COOH MPs decreased TiO2 NP toxicity which was ascribed to hetero-aggregation between PS MPs and synthetic nanoparticles, unlike positively charged MPs. It was also found that the oxidative stress trend was dependent on the surface charge of PS (PS-NH2/TiO2 > PS-COOH/TiO2), signifying its significant effect on the adsorption potential of environmental contaminants on the plastisphere (Thiagarajan et al., 2019).
As heavy metals also adsorb on plastic particles, their subsequent release can take place to increase algae toxicity (Luo et al., 2020, Luo et al., 2020a, Luo et al., 2020, Capolupo and Sørensen, 2020). In this regard, high concentrations of Pb and Cr (e.g., greater than 10 µg·L-1) inhibited photosynthesis in blue-green algae (Luo et al., 2020, Luo et al., 2020a, Luo et al., 2020). In addition to contaminants, microbiomes (such as bacteria and algae) could also be adsorbed onto MPs/NPs. Subsequent ingestion of these microbiomes may exertharmful effects on higher trophic level biota (Xu et al., 2018a, Xu et al., 2018b, Shen et al., 2019). The majority of the studies describing the eco-toxic effects of plastic weathering and subsequent leaching of pollutants were regulated under controlled environment at laboratory scale. However, the effects of weathered plastic particles on the trophic level/food chain can be pragmatically assessed with prioritization of eco-toxic studies in field environment. In light of these hazardous biological impacts, the influence of weathering on the association between MPs and environmental contaminants through several adsorptive attributes should be investigated more thoroughly (Luo et al., 2022, Zhang et al., 2019b),especially based on the scenario of the coronavirus (COVID-19) pandemic.
3.2. Sorption chemistry of environmental contaminants onto plastics
The sorption chemistry of environmental contaminants on MPs involves both absorption and adsorption processes. The adsorptive attributes of plastic particles and associated chemistry are tightly regulated by weathering processes. Each of those processes influences the transport, fate, and toxicity of MPs and other environmental contaminants ( Fig. 4) (Duan et al., 2021). In absorption, the chemical contaminant penetrates through MPs/NPs solid molecules by diffusion or osmosis processes and becomes associated within the solid phase matrix (Endo and Koelmans, 2016, Fu et al., 2021). However, in the case of adsorption, the chemical contaminant adheres to the MP and NP solid surfaces through physical attachment. Further, the adsorbate interacts with a sorbent at the sorbent-sorbate interface. In contrast, the sorbate (e.g., liquid) interacts internally with the sorbent of different medium (usually a solid matrix) in absorption. Hence, the partitioning of the chemical contaminants takes place due to the chemical forces within MPs (Fotopoulou and Karapanagioti, 2012, Endo and Koelmans, 2016, Agboola and Benson, 2021). Therefore, in the case of MPs, the chemical partitioning is usually synonymous with absorption. In contrast, a wide array of physical (reversible bonds) and chemical (irreversible covalent bonds) interactions are involved in the case of the adsorption process (Fig. 3a). However, it should be noted that the sorption mechanism of chemical contaminants on plastic particles is dependent on various physicochemical and environmental abiotic/biotic factors (Fig. 3b), such as the type and characteristics of pollutants, free energy relationships, and the textural and surface chemistry of the MP/NP solid phase (Fred-Ahmadu et al., 2020).
Fig. 4.
Weathering mediated transformation of macroplastics to microplastics (MPs) and eventually nanoplastics (NPs) and their interactions with different chemical contaminants through sorption processes (adsorption and/or absorption) (i.e., effects on biota and the food chain).
The variance in polarity and hydrophobicity of organic chemicals is also an important factor that influences adsorption chemistry on MPs/NPs (Wang et al., 2021). Specifically, hydrophobic organic chemicals tend to adsorb on NPs with Kpw > 1 to reflect their greater enrichment on plastispheres than in the aqueous phase (Liu et al., 2018). Furthermore, the sorption of polar chemical contaminants frequently occurs on the surfaces of aged NPs according to the polarity (i.e., surface interaction mechanisms) (Liu et al., 2018). Conversely, non-polar or less polar chemicals are adsorbed within the inner matrices of plastic polymers (i.e., pore diffusion mechanism) (Liu et al., 2018, Wang et al., 2021). Such differences in adsorption chemistry of weathered plastics, as driven by the polarity/hydrophobicity of chemicals, can also lead to changes in their mobility and bioavailability.
The sorption affinity of MPs/NPs for organic contaminants is influenced strongly by weathering effects (such as UV- photo-oxidation and microbial degradation processes) through which alterations are induced in their surface chemistry, texture, and crystallinity (Fotopoulou and Karapanagioti, 2012, Endo and Koelmans, 2016, Yu et al., 2019; Harraq and Bharti, 2021). In this context, the PVC MP exhibited a high efficiency for adsorbing five BPAs via physical adsorption mechanisms such as hydrophobic interactions, electrostatic force, and non-covalent bonds (Wu et al., 2019a, Wu et al., 2019b). It has also been recognized that UV-induced weathering increased the surface area of PVC (1.85 times) and PLA MPs (2.66 times) when compared with the virgin PVC/PLA pellets (Fan et al., 2021). Herein, this increase in surface area of weathered PVC/PLA MPs enhanced the adsorption potential of antibiotics (ciprofloxacin and tetracycline) due to the increased number of oxygen-containing surface functionalities (e.g., carbonyl groups) (Fan et al., 2021). This is supported by the decrease in the zeta potential of PLA (from −7.79 to −13.51 mV) and PVC MPs (from −4.96 to −8.34 mV) when compared with the virgin PVC/PLA pellets due to UV-weathering effects (Fan et al., 2021). Accordingly, weathering can also cause alterations in the chemical interactions between MPs/NPs and chemical contaminants (Reichel et al., 2021). In this sense, the π-π-interactions were the primary adsorption mechanism of organic chemicals on virgin PS NPs. In contrast, the sorption mechanisms for aged PS NPs were altered to electrostatic interactions and hydrogen bonding (Liu et al., 2020a, Liu et al., 2020b, Liu et al., 2020c). Such alteration in the adsorption mechanism was attributed to the change of MPs/NPs surface chemistry under weathering conditions (i.e., increased oxygen functional groups on aged PS NPs) (Liu et al., 2020a, Liu et al., 2020b, Liu et al., 2020c). The nucleophilic nature of the outer surface of PET NPs can remarkably facilitate the mass transfer and intraparticle diffusion of emerging contaminants (e.g., BPA) into their inner matrices to form stable complexes with a maximum adsorption energy of 19 kcal/mol (Cortés-Arriagada, 2021). The adsorption energy of BPA onto PET NP complexes was comparable or even higher than those of synthetic or engineered nano-scale adsorbents like graphene, carbon nanotubes, and activated carbon. Further, higher adsorption of BPA-PET NP complexes was mainly attributed to dispersion (38–49%) and electrostatic energies (43–50%) with the lowest role of π-π-interactions (Cortés-Arriagada, 2021). Notably, the BPA adsorption on PET NPs can increase the potential of their leaching and transport into aquatic ecosystems, thereby posing a severe threat to the environment, biota, and human health (Cortés-Arriagada, 2021).
Plastic debris can act as a reservoir for several organic contaminants like PAHs (e.g., fluoranthene, chrysene, pyrene, and phenanthrene) and PCBs (e.g., congeners: 18, 31, 138, and 187) in aquatic ecosystems (Zhang et al., 2019a). Many types of environmentally hazardous organic chemicals such as perfluorinated compounds, PCBs, PAHs, methyl-mercury, pharmaceutical antibiotics, and DDT adsorb on the surfaces of plastic particles (Rainieri et al., 2018; Sun, 2018; Zhou et al., 2018; Yu et al., 2020a, Yu et al., 2020b). In this context, the sorption strength of phenanthrene on plastic decreases in the order of PE > PP > PVC (Alimi et al., 2018). Such an observation may affect the transport of hydrophobic chemical contaminants in biota and their bioaccumulation and eco-toxicity (Koelmans et al., 2016). A total of 16 PAHs were also detected in plastic debris (within 0.33–5 mm sized fractions) in marine ecosystems in China (Mai et al., 2018). The subsequent desorption of PAHs is considered hazardous due to their carcinogenic, mutagenic, and teratogenic nature (Yu et al., 2020a, Yu et al., 2020b). Likewise, polymeric materials such as PS MPs can adsorb diethylhexyl phthalate, diisobutyl phthalate, dibutyl phthalate, 2,4-di-tert-butyl phenol, and dimethyl phthalate benzaldehyde in ocean basins (Zhang et al., 2019a). In addition, a good number of chemical components in plastic debris (e.g., PCB congeners, PAH isomers, dichlorodiphenyltrichloroethane (DDT) metabolites, and hexachlorocyclohexane (HCH) isomers) have also been reported to interact with MPs and NPs (Wang and Wong, 2018, Wang et al., 2018a). In another study, three types of MPs (PE, PS, and PVC) were tested for the sorption of tetracycline (Yu et al., 2020a, Yu et al., 2020b). Among them, PE was superior for tetracycline adsorption relative to PS and PVC, in view of its non-planar structure and van der Waals interactions (Yu et al., 2020a, Yu et al., 2020b). Unlike non-planar PE MPs, PS MPs obtained higher planarity with an affinity for hydrophobic and π–π interactions with tetracycline. Moreover, as the adsorption of tetracycline on PE MPs depends on the coexisting metallic contents in the solutions, their sorption capacity can be reduced by competitive sorption on the available sorption sites (Yu et al., 2019). Such a decrease in the sorption capacity was also attributed to the effect of biofilms on the adsorption process of tetracycline on PE MPs surface. Also, the adsorption of tetracycline on PE MPs was impacted by alterations in salinity and pH, which influenced sorption conditions by changing the chemical state of adsorbate and adsorbent (Yu et al., 2019). In another study, dissolved organic matter (DOM) was found to negatively impact the adsorption process of tetracycline on MPs surfaces (Xu et al., 2018a, Xu et al., 2018b). In this context, the sorption of tetracycline on MPs (PE, PP, and PS) decreased in the presence of DOM (as 90% fulvic acid at 20 mg L-1) (Xu et al., 2018a, Xu et al., 2018b). This is due to the greater affinity of DOM for taking up fulvic acid (rather than being adsorbed on the MPs surfaces). Thus, the MPs played a minor role in determining the environmental fate and transport of tetracycline in the presence of DOM (Xu et al., 2018a, Xu et al., 2018b). On this basis, it can be concluded that the sorption process of chemical contaminants on MPs surface is highly dependent on the weathering driven alterations in physicochemical properties of the environment (in terms of coexisting metallic ions, pH, DOM, etc.).
The polar MPs (e.g., polybutylene succinate (PBS), polycaprolactone (PCL), and polyurethane (PU)) also exhibited a higher affinity to absorb polar organics (e.g., PAH derivatives, phenanthrene, and pyrene) (Zhao et al., 2020). In this context, the sorption of polar phenanthrene and pyrene derivatives on PU MPs (capacity of 1.06 × 104 and 5.87 × 103 mg/kg, respectively) fit well with the Langmuir model to support the interactions between π (n) - π electron donor-acceptors (Zhao et al., 2020). Further, a high adsorption capacity for pharmaceutical (nonsteroidal anti-inflammatory drugs (NSAIDs)) contaminants was noted on the PE/PP MP surface in relevant field conditions (Elizalde-Velázquez et al., 2020). Herein, NSAID adsorption on MPs was ranked in the order of diclofenac (DCF) (Kd-4.51 L kg−1) > ibuprofen (IBU) (Kd-3.97 L kg−1) > naproxen (NPX) (Kd-3.18 L kg−1) ( Fig. 5 and Table 3). In this respect, the Kd values of PE/PS/PP MPs for these NSAIDs were in the order DCF(Kd:10.9) > NPX(Kd:6.5) > IBU(Kd:3.4). Similarly, in soil samples irrigated with wastewater, the sorptive potential of NPX (Kd:2.39) on plastic particles was noted to be higher than IBU (Kd:1,05–2.26), as shown in Fig. 5. A similar sorption trend on MPs (DCF (Kd:4.66) > IBU (Kd:1.69)) was noted in a natural aquifer and sandy sediments (Fig. 5) (Elizalde-Velázquez et al., 2020). Elizalde-Velázquez et al. (2020) attributed the varying extent of NSAID sorption on various MPs to the roles of pH and hydrophobic interactions. In other studies, the chemical configuration of hydrophobic contaminants also influenced the sorption efficiency of plastic particles. For example, planar PAHs (e.g., phenanthrene and heterocyclic atrazine) had higher sorption coefficients on nonpolar PS MPs than their non-planar counterparts (e.g., 1-nitronapthalene and 1- napthylamine) (Velzeboer et al., 2014). In addition, the Kd (Lkg-1) values of the PAHs and their derivatives (e.g., phenanthrene, pyrene, 1-nitronapthalene, 1-napthylamine, and atrazine) ranged from 29.6 to 1.42 × 105 on diverse MP plastic polymers (e.g., PS, PU, PCL, and PBS MPs) (Zhao et al., 2020). It was also noted that the adsorption affinities of PS, PU, PCL, and PBS MPs toward different organic aromatic contaminants decreased in the order of PAHs > derivates of PAHs > atrazine (Zhao et al., 2020). This adsorption trend was attributed to variable polarity, chemical kinetics, hydrophobic interaction, and hydrogen bonding. In this respect, the calculated log KOC/log KOW values of PAHs and its derivatives on polar MPs (e.g., PU, PCL, and PBS) were higher than unity, indicating the vital role of hydrogen bonding during the adsorption process (Zhao et al., 2020). The sorption coefficient of PAHs (10-5 to 1 µg L-1) was also observed to be independent of the aggregation state of PS NPs (70 nm) due to π–π interactions between planar PAHs and the aromatic PS polymeric surface (Liu et al., 2016). In this case, the higher hydrophobicity of aromatic chemicals facilitates the adsorption process onto non-polar MPs via a hydrophobic interaction mechanism.
Fig. 5.
Sorption coefficients, Kd (L kg−1) of nonsteroidal anti-inflammatory drugs (NSAIDs) [diclofenac (DCF), ibuprofen (IBU), and naproxen (NPX)] in different environmental matrices.
Source ref. Durán–Álvarez et al., 2014, Durán-Álvarez et al., 2012, Elizalde-Velázquez et al., 2020, González-Naranjo et al., 2013, Scheytt et al., 2005, Yamamoto et al., 2009, Zhang et al., 2017.
Table 3.
The specific adsorption and mechanisms of inorganic, organic, and emerging environmental contaminants on weathered plastic surfaces.
| S.No. | MP/NP polymeric type | Adsorbed contaminant | Adsorption capacity & chemistry/kinetics | Main environmental /chemical factor (s) | Reference |
|---|---|---|---|---|---|
| 1. | Natural NP at North Atlantic Gyre | Pb (II) | 78.5–97% Pb adsorption on NP; Pseudo-first-order; Freundlich model | pH 7 showed maximum adsorption | Davranche et al. (2019) |
| 2. | PE beads (dose-2–14 g L-1) |
Cr(VI) | Adsorption capacity of Cr (VI)− 0.39 to 1.36 mg g-1; |
pH and SDBS concentrations |
Zhang et al., 2020a, Zhang et al., 2020b, Zhang et al., 2020c, Zhang et al., 2020a, Zhang et al., 2020b |
| 3. | PE, PP, PVC and PS MPs |
Cd (II) | –Adsorption capacity on PVC MPs (151.4 mgKg-1) > PS (134.1 mg Kg-1) > PP (123.6 mg Kg-1) > PE (113.5 mg Kg-1); Freundlich isotherm non-linear model better suited than Langmuir model |
pH, humic acid, polymeric types and ionic strength of solution |
Guo et al. (2020) |
| 4. | PE, PP and PS MPs | 9-Nitroanthracene (9-NAnt) | 9-NAnt adsorption was maximized on PE MPs (734 μg g−1); adsorption mechanism better described with linear isothermal model | Hydrophobic and electrostatic processes | J. Zhang et al. (2020) |
| 5. | PE MPs and PP MPs | Pharmaceuticals (three Nonsteroidal anti-inflammatory drugs i.e., ibuprofen, naproxen, diclofenac) | Highest adsorption capacity noted for Diclofenac | Highest sorption noted at lower pH (2) | Elizalde-Velázquez et al. (2020) |
| 6. | PS NPs and caboxylated (PS-COOH) NPs | Fluoroquinolones | Langmuir model was the best fit model for adsorption isotherms | pH (sorption of fluoroquinolones on PS NPs were higher at pH 5 while pH 6 facilitated their sorption PS-COOH NPs) | H. Zhang et al. (2020) |
| 7. | PS NPs | PAHs (conc. 10–5 µg L−1 to 1 µg L−1) |
log Kf varied from 5.7 to 6.0, adsorption isotherms-nonlinear with π–π interactions; distribution coefficients at lower end of isotherm- 109 L kg− 1 | Pre-extraction with C18 polydimethyl-siloxane reduced the sorption of PAHs | Liu et al. (2016) |
The PS-COOH NPs also showed higher adsorption of fluoroquinolone (norfloxacin and levofloxacin) chemicals than virgin PS NPs (Zhang et al., 2020a, Zhang et al., 2020b, Zhang et al., 2020c, Zhang et al., 2020a, Zhang et al., 2020b). This was as a result of improved polar/ electrostatic interactions and hydrogen bonding adsorption mechanisms between fluoroquinolones and PS-COOH NPs. Such chemical changes alter the surface charge, significantly influencing their adsorption behavior, fate, transport, and aggregation processes in the relevant environment. For instance, positively charged NPs exhibited more harmful effects on biota health than negatively charged NPs, given their greater efficiency to penetrate the cell membrane (Shen et al., 2019). It was reported that 17 PCBs on PS NP (70 nm) surfaces recorded a Kpw (denotes sorption coefficient of the chemical from water to plastic) value of 109 L kg-1 (Velzeboer et al., 2014). Further, the Kpw observed for PCBs adsorption on PS NP was higher than those of PS MPs, nanoscale fullerenes, and multiwalled carbon nanotubes. This observation was attributed to the increased surface contact area of PS NPs and the enhanced chemical interactions (hydrophobic and π-π), as the adsorption of PCBs onto PS NPs surfaces can be promoted by such factors (Velzeboer et al., 2014). Despite the progress in NP chemistry research, there is still a knowledge gap in the contaminant release or desorption mechanism and influence of weathering (Shen et al., 2019). Knowing that weathering and physical-chemical attributes in tandem affect the sorption of chemicals on NPs, the significance of those factors needs to be evaluated further through in-depth holistic analyses.
3.3. Kinetic and isotherm modeling of plastic sorption mechanism
Adsorption and desorption are known to be weathering and time dependent processes in which two basic phenomena should be described: equilibria and kinetics. With regards to sorption kinetics, the phenomenological coefficients characterizing the transfer of substances from a mobile phase (liquid or gaseous) to a solid phase can be determined. As these phenomenological coefficients are sometimes strongly kinetically controlled, sorption isotherms must be specified to describe the how the sorption mechanism depends on the equilibrium concentration of sorbate and the change of sorbent surface chemistry through time. Accordingly, the kinetic and isotherm coefficient constants can provide information to understand the final state of the sorption system and the changes in the chemical properties of both adsorbates and adsorbents based on adsorption/desorption mechanisms. Various kinetic and isotherm models have been recommended to determine the rate-governing adsorption of chemical contaminants on the plastic adsorbents and their sorption mechanisms under various environmental conditions (Razanajatovo et al., 2018, Mammo et al., 2020, Yu et al., 2020a, Yu et al., 2020b, Vieira et al., 2021).
Among kinetic models ( Fig. 6), pseudo-first-order (PFO) and pseudo-second-order (PSO) adsorption models are commonly used to determine the rate-governing adsorption process of chemical contaminants on MP/NP plastic surfaces (Yu et al., 2020a, Yu et al., 2020b). Further, many isotherm models can be used to analyze the equilibria of retention/release processes of a solute on solid plastic particles under various environmental conditions including the Langmuir, Freundlich, and Dubinin-Radushkevich isotherm models (Fig. 6). These isotherm models have also been frequently applied to predict and simulate the sorption mechanism of diverse contaminants on various plastic polymers. The suitability of the Langmuir model was confirmed in the prediction of the sorption of different chemical contaminants onto localized MP/NP sorption sites (Chen, 2015, Tran et al., 2017). This indicates that many chemical contaminants followed a linear, reversible sorption/desorption process onto MPs and NPs surfaces. In some cases, kinetic models can also be used to investigate the effect of weathering and ageing conditions on adsorption capability and mechanism of plastic waste (Liu et al., 2019c, Liu et al., 2019d, Liu et al., 2019).
Fig. 6.
Sorption models (kinetic and isotherm) used to study the interactions of chemical contaminants on plastic surfaces. Here, qm and qe are the amount of adsorbate at equilibrium or adsorption capacity (mg/g), qt is the adsorbed amount at time t, Kid and Ci are intra-particle diffusion constants, Bt is the Boyd constant, Kd is the partition coefficient between the sorbent and the solution at equilibrium, Ce is the sorbate concentration at equilibrium, Qmax is the maximum adsorption capacity, KL is the Langmuir constant (mg/g), Kf is the sorption affinity coefficient (L/mg), and 1/n is adsorption intensity (Freundlich constants).
For instance, the adsorption of ciprofloxacin on pristine PVC/PS MPs was well-fitted by PFO rather than PSO kinetics (Liu et al., 2019c, Liu et al., 2019d, Liu et al., 2019). Such fitting adequacy was however reversed when tested against aged PVC and PS MPs as adsorbents. Likewise, adsorption of phenanthrene on PE and poly-butylene adipate co-terephthalate (PBAT) MPs can be suitably explained using the Freundlich isotherm and PSO kinetics models (Liu et al., 2016; Zuo et al., 2019). In this context, the linear PFO equation is only appropriate to simulate the initial 20–30 min of the adsorption process rather than the whole sorption range (Tran et al., 2017). Also, kinetic (or isotherm) models on a linear basis are less accurate with several errors and thus are not ideal for elucidating weathering mechanisms (Zhang et al., 2020a, Zhang et al., 2020b, Zhang et al., 2020c, Zhang et al., 2020a, Zhang et al., 2020b). As such, a non-linear PSO model was used effectively to address the effect of weathering on chemical adsorption (e.g., pristine and weathered MPs) (Liu et al., 2019c, Liu et al., 2019d, Liu et al., 2019). Herein, adsorption on pristine and weathered MPs occurred mainly via complex physicochemical adsorption mechanisms. In addition, other studies on chemical sorption also demonstrated that the non-linear isotherms should establish a better link between experimental and simulated data (Tran et al., 2017). Henceforth, it should be noted that in studying the adsorption kinetics of chemical contaminants on the sorbent (plastic) surface, a nonlinear kinetic (or isotherm) model should be used (Tran et al., 2017, Liu et al., 2019; Seidensticker et al., 2019; Lima et al., 2020; Zhang et al., 2020a, Zhang et al., 2020b, Zhang et al., 2020c, Zhang et al., 2020a, Zhang et al., 2020b). Furthermore, Zhang et al., 2020a, Zhang et al., 2020b, Zhang et al., 2020c, Zhang et al., 2020a, Zhang et al., 2020b also used a non-linear Langmuir model to estimate the adsorption potential of norfloxacin and levofloxacin on PS NPs ( Table 4). Likewise, Liu et al. (2016) used a non-linear adsorption isotherm to study the sorption of PAHs on the PS NPs. In this case, adsorption of PAHs on PS NP surfaces was controlled by π–π interactions between planar PAHs and the aromatic PS polymeric surface. Similarly, the interactions of PS MPs (0.047 µm) with five soil types was best explained by the non-linear PFO model (R2 = 0.998) (Luo et al., 2020, Luo et al., 2020a, Luo et al., 2020). Here, the sorption efficiency of PS MPs on soil types decreased with increased pH. Notably, the sorption coefficient (Kd) of PS MPs within the soil matrix was positively correlated with soil organic carbon content (R2 = 0.999) and Fe2O3 content (R2 = 0.967). On the other hand, the clay content showed an inverse correlation (R2 = 0.952) with the Kd of PS MPs (Luo et al., 2020, Luo et al., 2020a, Luo et al., 2020). This observation indicates that the sorption mechanisms of MPs within soils are mainly guided by electrostatic and hydrophobic interactions (Luo et al., 2020, Luo et al., 2020a, Luo et al., 2020).
Table 4.
Concentrations of organic, inorganic, and emerging contaminants (sorbate) on plastic polymers (sorbent) and their eco-toxic cellular responses on exposure to biota.
| S.No. | Adsorbed Chemical contaminants (Sorbent) | Microplastic (MP) polymeric types (Sorbate) |
Adsorption capacity (ng g-1) | Eco-toxic responses | Reference |
|---|---|---|---|---|---|
| ORGANIC CONTAMINANTS | |||||
| 1. | PAH derivatives | PE, PP | 24,400–164,900 | Through covalent bonding to DNA, RNA, proteins & other biomolecules have cytotoxic, mutagenic, teratogenic & carcinogenic effects on biota | Mammo et al. (2020) |
| 2. | PCBs (18 congeners) | PE, PP | 5–18,700 | Endocrine disrupting chemicals (EDCs) perturbing gap junctional intercellular communication | Endo et al. (2005) |
| 3. | DDTs | PE, PP | 1.69–276 | Cytotoxic effects & DNA damage | Ogata et al. (2009) |
| INORGANIC CONTAMINANTS | |||||
| 4. | Cd | PE, PP, & PS | 38–1980 | Perturbs cellular proliferation & differentiation leading to cell death or apoptosis | Massos and Turner (2017) |
| 5. | Halogens (Bromine) | PE, PP, & PS | 4–13,300 | Perturbs hepatic metabolism & gene expression | Massos and Turner (2017) |
| 6. | Pb | PE, PP, & PS | 12–4820 | Binds calmodulin by replacing calcium and disturbs protein confirmation & enzyme regulation | Massos and Turner (2017) |
| 7. | Metalloid (As) |
PE, PP, cellophane, & polyester | 0.35–2.89 | Enzyme inhibition by binding through sulfhydryl (-SH) groups and adversely influencing the cellular respiration | Mohsen et al. (2019) |
| 8. | Cu | PE, PP | 80.9–500.60 | Inducts oxygen species (ROS) by binding to the thiol group | Wang et al. (2017) |
| 9. | Cr | PE | 44–430 | Perturbs cellular energy metabolism | Holmes et al. (2012) |
| EMERGING CONTAMINANTS (microcosm/lab based observation on contaminant’s concentrations) | |||||
| 10. | Sertraline; Propranolol | PE | 0–80,000 Sertraline; 0–60,000 Propranolol |
Antioxidative & cytotoxic responses, DNA damage | Mamitiana et al. (2018) |
| 11. | DEP (Diethyl phthalate) | PE, PS, PVC | 10,000–30,000 | Binds & forms DEP-haemoglobin complexes thereby perturbing the normal physiology | P. Liu et al. (2019) |
| 12. | Sulfamethoxazole | PE, PP, PS, PET, PVC. | 660–96,400 | Perturbs cell development by blocking nucleic acids and proteins synthesis | Guo et al. (2019) |
| 13. | Triclosan | PS | 720,000–1,000,000 | Hazardous chemical inhibiting lipid synthesis & cell membrane disruption leading to apoptosis | Li et al. (2019) |
| 14. | 17b-Estradiol (E2) |
PA, PE, PP, PS, PC, PVC | 2500–20,000 | E2 binds with DNA and forms complex that can lead to mutation by inhibiting other biomolecules | G. Liu et al. (2019) |
| 15. | DBP (Dibutyl phthalate) | PS, PE, PVC | 1,100,000–2,400,000 | Like DEP, forms DBP- haemoglobin complexes adversely impacting normal physiology | P. Liu et al. (2019); Mammo et al. (2020) |
Besides kinetic and isotherm modeling, many partition coefficient terms are utilized to assess the sorption potential of emergent chemicals onto plastic polymers and their sorption mechanisms in different environmental streams (air, water, and soil). In this regard, Kpw metric is a vital parameter that defines the ratio of Cp (mg kg-1 plastic adsorbed fractions of chemicals in plastic) and Cw (mg L-1 water free residual chemical in water) at equilibrium. The Kpw metric can be estimated based on another partition coefficient (octanol-water coefficient (Kow)) term, which is often applied to investigate the equilibrium ratio of target contaminant between two liquid phases (organic solvent (octanol) and water) (Mammo et al., 2020). Using the logarithmic equation (Eq. 12), the Kpw can easily be estimated based on Kow values. The Kpw value is mainly dependent on the types of MPs and the pollutants (Endo and Koelmans, 2016).
| (12) |
Here, the α and β are the coefficient terms calculated using experimental data for Kpw and Kow values.
Notably, if the ratio Cp/Cw < Kpw, the sorption process of chemical contaminants only occurs via the mass transfer/diffusion from water to the plastisphere. In contrast, the desorption process is possible when Cp/Cw > Kpw (Endo and Koelmans, 2016). Under the conditions of Cp/Cw ≈ Kpw, sorption equilibrium is reached (e.g., neither adsorption nor desorption). It has been shown that an usually low concentration of sorbate generally encourages the adsorption process, while the high concentration of the sorbate facilitates the absorption process due to the increase in volume (Hartmann et al., 2017, Fred-Ahmadu et al., 2020). Additionally, linear sorption is more favorable if Kpw is constant and independent of the type of environmental contaminants such as heavy metals, PCBs, and nano-materials (Endo and Koelmans, 2016). However, if the Kpw is dependent on the concentration of contaminants, non-linear sorption could occur in a more preferable mechanism.
Another partition coefficient (Kd) based on a linear sorption model (Eq. 13) can elucidate the sorption mechanism occurring on the MPs surfaces because the Kd defines the relative movement of target contaminants from one environmental system to MPs/NPs solid particle (Wang and Wong, 2018, Wang et al., 2018a).
| (13) |
In Eq. 13, Kd denotes partition coefficient (L kg-1), qe is the mass of chemicals adsorbed on the plastic surface at equilibrium (μg chemical. kg-1 of plastic), and Ce (μg L-1) represents the concentration of a chemical in the liquid phase at the equilibrium stage (Wang and Wong, 2018, Wang et al., 2018a).
The Kf term is another partition coefficient that denotes the sorption capacity, and it can be computed using the Freundlich model shown in Eq. 14.
| (14) |
Here, 1/nf represents the Freundlich exponent for modeling the energy distribution and sorbent heterogeneity at adsorption sites (Mammo et al., 2020). Kf represents the non-linearity of how low hydrophobic chemical contaminants behave. Generally, the values of 1/nf range between 0.7 and 1 reflect the relative decrease in adsorption with an increase in sorbate concentrations. It is also linked with the availability of adsorption sites (Endo and Koelmans, 2016, Fred-Ahmadu et al., 2020).
For example, PE MPs were found to have a higher partition coefficient for DDT (log Kow: 6.79 and log Kf: 5.9) than that of phenol (log Kow: 4.5 and log Kf: 4.9), DEHP (log Kf 2.4), and perfluorooctanesulfonic acid (PFOS; log Kf 1.25) adsorption (Teuten et al., 2007, Velzeboer et al., 2014). In this respect, the units of log Kow and log Kf are variable and may be denoted as Lchemical/Lplastic or Lchemical/Kgplastic, depending on the concentrations/medium of sorbate and sorbent (Endo and Koelmans, 2016). The decreasing trend in values of sorption coefficients (DDT > phenol> DEHP > PFOS) can be attributed to a decrease in planarity of a molecule (Velzeboer et al., 2014). In this respect, the high planarity of a molecule (e.g., DDT and phenol) encourages the proximity and movement of chemicals close to the plastic particle surface, thereby facilitating the adsorption process.
In terms of partition coefficient terms, the one-parameter linear free energy relationship (OP-LFERs) is one of the less frequently used models to describe the sorption of chemical contaminants on MPs/NPs in various environments. This model is inadequate to study chemical-MP complexes associated with multiple chemical interactions, such as H-bonding, hydrophobic, and electrostatic interactions (Nguyen et al., 2005, Endo and Koelmans, 2016). Instead, poly-parameter, linear free-energy relationships (PP-LFERs) have proven to be useful for predicting and characterizing the equilibrium partitioning of most chemical contaminants on MPs/NPs in various environments and for predicting their respective partition coefficients (Stenzel et al., 2013, Fred-Ahmadu et al., 2020). In this context, the Abraham Linear Solvation Energy Relationships (LSERs) for Kpw may be used to assess partition coefficients of a wide range of chemical contaminants on plastics, as per Eq. (15):
| (15) |
Here, E denotes excess molar fraction, S is the solute dipolarity/polarizability, A is the solute H-bond donor property (acidity), B represents the solute H-bond acceptor property (basicity), and V is the molar volume. Solute descriptors (denoted by E, S, A, B, and V), and the regression coefficients (represented by c, e, s, a, b and v) can be studied using Eq. (15).
Therefore, in the LSERs model, both the potential of chemical contaminants to undergo molecular interactions (as defined by solute descriptors) and the variances in interface/phase attributes between MPs and aqueous media (as defined by regression coefficients) are taken into consideration (Endo and Koelmans, 2016, Hüffer et al., 2018, Fred-Ahmadu et al., 2020). The PP-LFERs model (with R2 > 0.84; RMSE (root mean square error) −0.27) performed better when compared with the Freundlich isotherm with R2 > 0.5 and a RMSE of 0.27 (Uber et al., 2019). Likewise, adsorption studies of hydrophobic chemical contaminants on MPs using Abraham LSERs further revealed that the non-linear Freundlich isotherm (R2 > 0.75) was even more efficient than the linear isotherm form (with R2 > 0.5), suggesting the preferable use of nonlinear models in sorption studies.
Information on the adsorption kinetics of various chemical contaminants on plastic polymers is summarized under different physical, chemical, and environmental factors (Table 3 and Fig. 5). Table 3, Table 4 also list the measured concentrations of organic, inorganic, and emerging contaminants on plastic polymers in the literature and their eco-toxic cellular responses upon exposure to biota. Although hydrophobic surfaces of MPs and NPs are favorable to facilitate the adsorption of most organic chemical contaminants (Gu et al., 2020), the adsorption of 9-nitroanthrene on PE, PP, and PS MPs was controlled by both hydrophobic and electrostatic interactions (J. Zhang et al., 2020). Among those MPs, PE was superior for 9-nitroanthrene (734 μg g−1) adsorption, and its performance was independent of pH and ionic strength due to the role of hydrophobic interactions as the main adsorption mechanism. In another study, a non-linear Langmuir model was useful to estimate the adsorption mechanism of fluoroquinolone pharmaceuticals (such as norfloxacin and levofloxacin) on PS NPs and PS NP-COOH surfaces (Zhang et al., 2020a, Zhang et al., 2020b, Zhang et al., 2020c, Zhang et al., 2020a, Zhang et al., 2020b). The data revealed that the adsorption potential of PSNP-COOH for those pharmaceuticals is larger than that of PS NPs due to the polar surface, which facilitated electrostatic interactions and H-bonding. On the other hand, hydrophobic interactions provoked high sorption of these pharmaceutical drugs on PS/PE NPs under acidic (at pH 2) conditions, although adsorption was significantly suppressed under environmentally realistic chemical conditions (Elizalde-Velázquez et al., 2020).
The eco-toxic impacts of chemically adsorbed PAHs onto PS NPs and humic acid-PS MPs were also investigated on Daphnia magna using a biodynamic kinetic model (Lin et al., 2020). The reported data confirmed that the PS NPs delayed the intestinal bioaccumulation of less hydrophobic PAHs in D. magna, while the humic acid complexed PS NPs facilitated the allocation of PAHs to the gut. Fisner et al. (2013) emphasized that the sorption interrelationship of MPs with hazardous organic chemicals can vary in vertical aquatic sediment strata. Therefore, characterization at each sediment level is required for explicit elucidation of MP-chemical interactions.
Different organochlorine pesticides (e.g., DDT (dichlorodiphenyltrichloroethane) and HCHs (hexachlorocyclohexane) isomers) can also be adsorbed on MP and NP surfaces in various environments (Rai et al., 2021a). In addition to organic chemicals, inorganic contaminants such as heavy metals (e.g., Cd, Pb, and Cr) and metalloids (e.g., As) can also be adsorbed to the surface of MPs (Holmes et al., 2012, Mohsen et al., 2019). In this case, it was found that the adsorption of Cr (VI) on PE MPs was promoted by the addition of sodium dodecylbenzene sulfonate (SDBS) at pH < 6 (i.e., capacity increased from 0.39 to 1.36 mg g-1), while the capacity decreased at pH > 6 (Zhang et al., 2020a, Zhang et al., 2020b, Zhang et al., 2020c, Zhang et al., 2020a, Zhang et al., 2020b). This is due to the higher inhibitory competition between SDBS and CrO2- 4 for binding at the PE adsorption sites in an alkali pH solution. During photo-oxidative weathering, the increased availability of oxygenated binding sites greatly improved the Pb adsorption on NPs (150–450 nm) surfaces via chemical electrostatic interactions (PSO model fit) as the main rate-limiting adsorption step (Davranche et al., 2019). The adsorption of Cu ion on modified or fractured plastic pellets in conjunction with DOM was 180.64 ng g-1, which was greater than other heavy metals (Zn (178.03 ng g-1), Ni (12.69 ng g-1) and Pb (1.17 ng g-1)) (Wijesekara et al., 2018). This is attributed to the enhanced surface complexation resulting from oxygen-mediated functional groups introduced after the interaction with DOM. Hence, the sorptive potential of chemical contaminants (e.g., heavy metals, pharmaceuticals, hydrocarbons, and organic pesticides) on plastic surfaces can be varied in multiple environmental matrices. Therefore, multiple hazardous contaminants (organic, inorganic, and emerging) can be concentrated on MPs/NPs via the absorption or adsorption process to be subsequently released into environmental matrices (via desorption). Such release from plastisphere can threaten the biota and human-well-being. Plastic particles therefore act as both sources and a sink of environmental contaminants (i.e., increase their toxicity levels) which should be explicitly unveiled in future studies toward pollutant adsorption on MPs/NPs surface.
3.4. Adsorption chemistry of pollutants between plastics and non-plastic environmental matrices
The recent advances in comparative adsorption chemistry of pollutants and ENPs on plastisphere or weathered plastic particles and other environmental matrices have helped us expand our understanding of plastic polymer science (Roes et al., 2012, Lambert et al., 2017). These chemically synthesized ENPs display colloidal attributes that are similar to NPs that are omnipresent in diverse environmental settings in terms of their shape, surface charge, and highly poly-disperse attributes (Praetorius et al., 2014, Besseling et al., 2017, Rai et al., 2018a, Rai et al., 2018b, Hadri et al., 2020). Under weathering effects, ENPs (e.g., AgO/CuO ENPs and TiO2/CeO2 ENPs) usually undergo surface-based dissolution reactions depending on their chemical composition (Kaegi et al., 2013, Conway et al., 2015, Vencalek et al., 2016, OECD, 2016). Nonetheless, non-engineered MPs/NPs do not undergo dissolution; instead, they can undergo fragmentation to be subject to weathering effects (Hüffer et al., 2017). Therefore, synthetic or engineered nano-scale particles display colloidal behavior while their adsorption mechanisms are also different from MPs (Hadri et al., 2020).
Adsorption science on weathered plastic particles is different from other solid waste pollutants and biochar in terms of their small sized colloidal attributes and physical-chemical attributes (Zhou et al., 2007, Vikrant and Kim, 2020). Their chemical interactions with adsorbates (both biological and chemical) can thus be distinguished during adsorption on plastisphere (Fu et al., 2021). Hydrophobic interaction is the main mechanism of adsorption of organic pollutants on MPs surface along with other ones like hydrogen bonds, π–π interactions, and halogen bonds (Agboola and Benson, 2021, Fu et al., 2021).
Like emerging contaminants, certain ENPs can act as adsorbates to be adsorbed to surfaces of weathered plastic particles (Li et al., 2020a, Li et al., 2020b). For instance, Li et al., 2020a, Li et al., 2020b recognized that Ag-ENPs could be adsorbed on PS MP surfaces via a monolayer adsorption mechanism (Langmuir fit), depending on the mass ratio of NPs to ENPs and π-π interactions. However, it should be noted that the adsorbed ENPs can also act as adsorbents to chemical contaminants in line with plastic particles. For example, the sorption of 17 PCB congeners on multi-walled carbon nanotubes (MWCNT), fullerene-C60, and PS NPs adsorbents was reported to be influenced greatly by the presence of organic matter (Velzeboer et al., 2014). Notably, the adsorption affinity of MWCNTs and C60 was 3–4 times higher for PCBs than that of PS MPs (Velzeboer et al., 2014). The encapsulation of ENPs (TiO2-ENPs) to plastic polymers (PS MPs) also enhanced their transport in quartz sand hetero-aggregates to influence their environmental fate and ecotoxicity (Cai et al., 2019). Therefore, ENPs can either act as the adsorbate on MPs/NPs or as adsorbents (like plastic particles) to emerging chemical contaminants. Also, ENPs can be additives to plastic particles and influence their environmental transport and fate pathways (Cai et al., 2019).
The studies on colloidal and interfacial attributes of MPs/NPs can remarkably facilitate in decoupling of their weathering and physico-chemical interrelationship while concomitantly help couple their potential effects and remediation technologies (Harraq and Bharti, 2021). Adsorption of certain emerging (pharmaceutical) contaminants such as tetracyclin on organic montmorillonite fitted well with non-linear Freundlich model, in accord with sorption chemistry on plastic surface (Liu et al., 2012; Yu et al., 2020a, Yu et al., 2020b). Further, the supplementation of plastics in several carbon based nano-adsorbents was reported to augment the efficiency of environmental remediation (Zhou et al., 2007). In this respect, further studies are warranted to explicitly differentiate the adsorption mechanisms of pollutant between MPs/NPs surface and other non-plastic environmental matrices (e.g., ENPs, biochar, and other engineered adsorbents). Interestingly, such comparative studies between plastic and non-plastic surfaces can help broaden the factors and processes governing the sorption chemistry of pollutants to implement the sustainable environmental remediation.
4. COVID-19 pandemic: challenge and opportunity for plastic weathering driven adsorption studies
The recent upsurge of COVID-19 pandemic has significantly disrupted the planetary health, environmental quality, and sustainability (Rai, 2021). The emergence of the COVID-19 pandemic has remarkably increased the consumption and disposal rates of plastic products to strengthen their environmental footprint (Silva et al., 2020, Patrício Silva et al., 2021). An increase in plastic footprint which may reflect global environmental burdens of plastic products can be exemplified through the projection of the growing global need for personal protective equipment (PPE) such as 129 billion face masks (e.g., N95) and 65 billion gloves during this pandemic along with an upsurge in other commodities (e.g., packaged meals, plastic cups, and home-delivered groceries) (Adyel, 2020, Patrício Silva et al., 2021). Note that the poor management on PPE plastic waste can be a serious issue because of its subsequent deleterious implications on natural ecosystems and public health. This is because most PPE waste products are composed of polyethylene terephthalate (PET), polypropylene (PP), and nonwoven materials that are weathered into smaller MPs/NPs to enhance the adsorption propensity of hazardous chemicals on plastisphere.
The weathered plastic waste, in addition to chemicals adsorption, can indeed act as a medium for coronavirus contamination, as the COVID-19 virus was demonstrated to survive on polypropylene plastic for two to three days (van Doremalen et al., 2020; Wu et al., 2020a, Wu et al., 2020b, Wu et al., 2020c, Wu et al., 2020d; Wang et al., 2021). Accordingly, the adsorption-desorption of hazardous chemicals or pathogens (like SARS-CoV-2) within these ‘plastispheres’ can create multiple ecological challenges (Lehner et al., 2019, Rubio et al., 2020). In this sense, plastic-microbe interactions are also found to influence the fate of pathogens by inducing genomic changes such as horizontal gene transfer and promotion of antibiotic-resistant genes (Cole, 2016, McCormick et al., 2016, Rai et al., 2021b). Besides adsorption, the weathered plastics can also influence the fate of specific hazardous chemicals (like PAHs, nonylphenol (NP), PCBs, and heavy metals) through their involvement in metabolomics, biodegradative metabolic pathways, pathogenicity determinants, and evolution of microbiomes (Oliveira et al., 2019, Rogers et al., 2020, Rai et al., 2021b, Luo et al., 2022).
The enormous increase in quantity of disposed plastic products during coronavirus outbreak further exacerbated the challenges to elucidate the influence of weathering on plastisphere adsorptive attributes. In addition, the increased use and disposal of plastics during COVID-19 pandemic strained the sustainable management prospects. Nevertheless, the coronavirus pandemic can be considered as an opportunity to better unveil the interactive roles between weathering and physico-chemical adsorption on plastisphere. Concomitantly, the pragmatic future studies on plastic waste management can explicitly help us minimize the plastic waste footprint and to effectively sustain the planetary public and environmental health.
5. Conclusions
As the adsorption of pollutants on plastic particles is remarkably influenced by weathering, the mechanisms governing such interaction is important enough to govern the potential risks associated with plastic pollution. The use of plastic products and their release into environmental systems increased drastically during the COVID-19 pandemic. Micro- and nano-scale plastic polymers (MPs/ NPs) can serve as the potential vectors of inorganic, organic, and emerging environmental contaminants. These hazardous contaminants can be associated with MPs/NPs via the absorption or adsorption process to be subsequently released into environmental matrices. Weathering of plastic polymers under the effects of both abiotic and biotic environmental stressors can induce significant alterations in the physical-chemical processes and properties of plastics to affect the pollutant adsorption on plastisphere significantly. Concomitantly, adsorption dynamics for different contaminants can also vary due to their interactions with diverse factors (e.g., surface chemistry, size, shape, and crystallinity of MPs particles) during weathering process. Furthermore, the factors governing the changes in physicochemical attributes can be evaluated to learn more about the adsorption/desorption mechanisms in relation to the fate, behavior, transport, and eco-toxicity of MPs/NPs. The adsorption kinetics and isotherm models of environmental pollutants on the plastisphere can also be used to expand our knowledge toward the most effective remediation technologies for target pollutants. In this sense, an assessment of interactive relationship between pollutant adsorption on plastic/other non-plastic substrata (e.g., ENPs and biochar) and weathering induced alterations in physical-chemical/environmental factors may help us better understand the detailed mechanisms of plastisphere sorption so as to achieve an optimized remediation/sustainable management approach to effectively resolve plastic pollution.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
PKR is grateful to the Department of Science and Technology (DST)-Nexus Project on Water Technology Initiative (WTI; no. DST/TMD/EWO/WTI/2K19/EWFH/2019 (C)) for financial assistance. This work was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ITC (MSIT) of the Korean government (Grant No: 2021R1A3B1068304).
Editor: Dr. H. Artuto
References
- Adyel T.M. Accumulation of plastic waste during COVID-19. Science. 2020;369(6509):1314–1315. doi: 10.1126/science.abd9925. [DOI] [PubMed] [Google Scholar]
- Agboola O.D., Benson N.U. Physisorption and chemisorption mechanisms influencing micro (nano) plastics-organic chemical contaminants interactions: a review. Front. Environ. Sci. 2021;9 [Google Scholar]
- Alimi O.S., et al. Microplastics and nanoplastics in aquatic environments: aggregation, deposition, and enhanced contaminant transport. Environ. Sci. Technol. 2018;52(4):1704–1724. doi: 10.1021/acs.est.7b05559. [DOI] [PubMed] [Google Scholar]
- Antao Barboza L.G.A., Vieira L.R., V. Branco N., et al. Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758) Aquat. Toxicol. 2018;19:49–57. doi: 10.1016/j.aquatox.2017.12.008. [DOI] [PubMed] [Google Scholar]
- Amereh F., et al. The emerging risk of exposure to nano(micro)plastics on endocrine disturbance and reproductive toxicity: from a hypothetical scenario to a global public health challenge. Environ. Pollut. 2020;261 doi: 10.1016/j.envpol.2020.114158. [DOI] [PubMed] [Google Scholar]
- Au S.Y., Bruce T.F., Bridges W.C., Klaine S.J. Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ. Toxicol. Chem. 2015;34(11):2564–2572. doi: 10.1002/etc.3093. [DOI] [PubMed] [Google Scholar]
- Avio C.G., et al. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015;198:211–222. doi: 10.1016/j.envpol.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Bakir A., Rowland S.J., Thompson R.C. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 2014;185:16–23. doi: 10.1016/j.envpol.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Bakir A., O'Connor I.A., Rowland S.J., Hendriks A.J., Thompson R.C. Thompson, Relative importance of microplastics as a pathway for the transfer of hydrophobic organic chemicals to marine life. Environ. Pollut. 2016;219:56–65. doi: 10.1016/j.envpol.2016.09.046. [DOI] [PubMed] [Google Scholar]
- Bandow N., Will V., Wachtendorf V., Simon F.G. Contaminant release from aged microplastic. Environ. Chem. 2017;14:394–405. [Google Scholar]
- Bartonitz A., et al. Modulation of PAH toxicity on the freshwater organism G. roeseli by microparticles. Environ. Pollut. 2020;260 doi: 10.1016/j.envpol.2020.113999. [DOI] [PubMed] [Google Scholar]
- Batel A., Borchert F., Reinwald H., Erdinger, T L. Braunbeck, Microplastic accumulation patterns and transfer of benzo[a]pyrene to adult zebrafish (Danio rerio) gills and zebrafish embryos. Environ. Pollut. 2018;235:918–930. doi: 10.1016/j.envpol.2018.01.028. [DOI] [PubMed] [Google Scholar]
- Belaiche C., Holt A., Saada A. Nonylphenol ethoxylate plastic additives inhibit mitochondrial respiratory chain complex I. Clin. Chem. 2009;55:1883–1884. doi: 10.1373/clinchem.2009.130054. [DOI] [PubMed] [Google Scholar]
- Besseling E., et al. Fate of nano- and microplastic in freshwater systems: a modeling study. Environ. Pollut. 2017;220(Part A):540–548. doi: 10.1016/j.envpol.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Bond T., Ferrandiz-Mas V., Felipe-Sotelo M., van Sebille E. The occurrence and degradation of aquatic plastic litter based on polymer physicochemical properties: a review. Crit. Rev. Environ. Sci. Technol. 2018:1–38. [Google Scholar]
- Bradney L., et al. Particulate plastics as a vector for toxic trace-element uptake by aquatic and terrestrial organisms and human health risk. Environ. Int. 2019;131 doi: 10.1016/j.envint.2019.104937. [DOI] [PubMed] [Google Scholar]
- Brandon J., Goldstein M., Ohman M.D. Long-term aging and degradation of microplastic particles: comparing in situ oceanic and experimental weathering patterns. Mar. Pollut. Bull. 2016;110:299–308. doi: 10.1016/j.marpolbul.2016.06.048. [DOI] [PubMed] [Google Scholar]
- Cai Li, et al. Influence of titanium dioxide nanoparticles on the transport and deposition of microplastics in quartz sand. Environ. Pollut. 2019;253:351–357. doi: 10.1016/j.envpol.2019.07.006. [DOI] [PubMed] [Google Scholar]
- Canesi L., Ciacci C., Fabbri R., et al. Interactions of cationic polystyrene nanoparticles with marine bivalve hemocytes in a physiological environment: role of soluble hemolymph proteins. Environ. Res. 2016;150:73–81. doi: 10.1016/j.envres.2016.05.045. [DOI] [PubMed] [Google Scholar]
- Capolupo M., Sørensen L., et al. Chemical composition and ecotoxicity of plastic and car tire rubber leachates to aquatic organisms. Water Res. 2020;169 doi: 10.1016/j.watres.2019.115270. [DOI] [PubMed] [Google Scholar]
- Carbery M., et al. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018;115:400–409. doi: 10.1016/j.envint.2018.03.007. [DOI] [PubMed] [Google Scholar]
- Carrasco F., Pages P., Pascual S., Colom X. Artificial aging of high-density polyethylene by ultraviolet irradiation. Eur. Polym. J. 2001;37:1457–1464. [Google Scholar]
- Chandra R., Rustgi R. Biodegradable polymers. Prog. Polym. Sci. 1998;23(7):1273–1335. [Google Scholar]
- Chen Q., et al. Is color a matter of concern during microplastic exposure to Scenedesmus obliquus and Daphnia magna? J. Hazard. Mater. 2020;383 doi: 10.1016/j.jhazmat.2019.121224. [DOI] [PubMed] [Google Scholar]
- Chen Q., Reisser J., Cunsolo S., et al. Pollutants in plastics within the North pacific subtropical gyre. Environ. Sci. Technol. 2018;52(2):446–456. doi: 10.1021/acs.est.7b04682. [DOI] [PubMed] [Google Scholar]
- Chen X. Modeling of experimental adsorption isotherm data. Information. 2015;6:14–22. [Google Scholar]
- Chua E.M., et al. Assimilation of polybrominated diphenyl ethers from microplastics by the marine amphipod, Allorchestes compressa. Environ. Sci. Technol. 2014;48(14):8127–8134. doi: 10.1021/es405717z. [DOI] [PubMed] [Google Scholar]
- Cole M., Lindeque P., Fileman E., et al. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013:47. doi: 10.1021/es400663f. [DOI] [PubMed] [Google Scholar]
- Cole M.A. Novel method for preparing microplastic fibers. Sci. Rep. 2016;6:34519. doi: 10.1038/srep34519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway J.R., et al. Aggregation, dissolution, and transformation of copper nanoparticles in natural waters. Environ. Sci. Technol. 2015;49(5):2749–2756. doi: 10.1021/es504918q. [DOI] [PubMed] [Google Scholar]
- Cornelissen G., Gustafsson O., Bucheli T.D., M.T.O, et al. Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ. Sci. Technol. 2005;39:6881–6895. doi: 10.1021/es050191b. [DOI] [PubMed] [Google Scholar]
- Cortés-Arriagada D. Elucidating the co-transport of bisphenol A with polyethylene terephthalate (PET) nanoplastics: a theoretical study of the adsorption mechanism. Environ. Pollut. 2021;270 doi: 10.1016/j.envpol.2020.116192. [DOI] [PubMed] [Google Scholar]
- da Costa J.P. Micro- and nanoplastics in the environment: Research and policymaking. Curr. Opin. Environ. Sci. Health. 2018;1:12–16. [Google Scholar]
- da Costa J.P., Paco A., Santos P.S.M., et al. Microplastics in soils: assessment, analytics and risks. Environ. Chem. 2019;16:18–30. [Google Scholar]
- Davranche M., Veclin C., et al. Are nanoplastics able to bind significant amount of metals? The lead example. Environ. Pollut. 2019;249:940–948. doi: 10.1016/j.envpol.2019.03.087. [DOI] [PubMed] [Google Scholar]
- Dekiff J.H., et al. Occurrence and spatial distribution of microplastics in sediments from Norderney. Environ. Pollut. 2014;186:248–256. doi: 10.1016/j.envpol.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Della T.C., Bergami E., Salvati A., et al. Accumulation and embryotoxicity of polystyrene nanoparticles at early stage of development of sea urchin embryos Paracentrotus lividus. Environ. Sci. Technol. 2014;48:12302–12311. doi: 10.1021/es502569w. [DOI] [PubMed] [Google Scholar]
- Desforges J.P., Galbraith M., Ross P.S. Ingestion of microplastics by zooplankton in the Northeast Pacific Ocean. Arch. Environ. Contam. Toxicol. 2015:69. doi: 10.1007/s00244-015-0172-5. [DOI] [PubMed] [Google Scholar]
- Duan J., et al. Weathering of microplastics and interaction with other coexisting constituents in terrestrial and aquatic environments. Water Res. 2021;196 doi: 10.1016/j.watres.2021.117011. [DOI] [PubMed] [Google Scholar]
- Durán–Álvarez J., Prado Ferroud, Juayerk Jiménez-Cisneros. Sorption, desorption and displacement of ibuprofen, estrone, and 17β estradiol in wastewater irrigated and rainfed agricultural soils. Sci. Total Environ. 2014;473–474:189–198. doi: 10.1016/j.scitotenv.2013.12.018. [DOI] [PubMed] [Google Scholar]
- Durán-Álvarez J., Prado-Pano, Jiménez-Cisneros Sorption and desorption of carbamazepine, naproxen and triclosan in a soil irrigated with raw wastewater: estimation of the sorption parameters by considering the initial mass of the compounds in the soil. Chemosphere. 2012;88(1):84–90. doi: 10.1016/j.chemosphere.2012.02.067. [DOI] [PubMed] [Google Scholar]
- Elizalde-Velázquez A., et al. Sorption of three common nonsteroidal anti-inflammatory drugs (NSAIDs) to microplastics. Sci. Total Environ. 2020;715 doi: 10.1016/j.scitotenv.2020.136974. [DOI] [PubMed] [Google Scholar]
- Endo S., Koelmans A.A. Sorption of hydrophobic organic compounds in marine environments: equilibrium. Hazard. Chem. Assoc. Plast. Mar. Environ. 2016:1–20. [Google Scholar]
- Endo S., Takizawa R., Okuda K., Takada H., Chiba K., Kanehiro H., Ogi H., Yamashita R., Date T. Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: variability among individual particles and regional differences. Mar. Pollut. Bull. 2005;50:1103–1114. doi: 10.1016/j.marpolbul.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Fan X., et al. Investigation on the adsorption and desorption behaviors of antibiotics by degradable MPs with or without UV ageing process. J. Hazard. Mater. 2021;401 doi: 10.1016/j.jhazmat.2020.123363. [DOI] [PubMed] [Google Scholar]
- Faure F., Demars C., Wieser O., Kunz M., de Alencastro L.F. Plastic pollution in Swiss surface waters: nature and concentrations, interaction with pollutants. Environ. Chem. 2015;12:582. [Google Scholar]
- Felten V., et al. Microplastics enhance Daphnia magna sensitivity to the pyrethroid insecticide deltamethrin: effects on life history traits. Sci. Total Environ. 2020;714 doi: 10.1016/j.scitotenv.2020.136567. [DOI] [PubMed] [Google Scholar]
- Fisner M., et al. Polycyclic aromatic hydrocarbons (PAHs) in plastic pellets: variability in the concentration and composition at different sediment depths in a sandy beach. Mar. Pollut. Bull. 2013;70(1):219–226. doi: 10.1016/j.marpolbul.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Fotopoulou K.N., Karapanagioti H.K. Surface properties of beached plastic pellets. Mar. Environ. Res. 2012;81:70–77. doi: 10.1016/j.marenvres.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Fred-Ahmadu O.H., et al. Interaction of chemical contaminants with microplastics: principles and perspectives. Sci. Total Environ. 2020;706 doi: 10.1016/j.scitotenv.2019.135978. [DOI] [PubMed] [Google Scholar]
- Fries E., et al. Identification of polymer types and additives in marine microplastic particles using pyrolysis- GC/MS and marine scanning electron microscopy. Environ. Sci. Process. Impacts. 2013;15:1949–1956. doi: 10.1039/c3em00214d. [DOI] [PubMed] [Google Scholar]
- Fries E., Zarfl C. Sorption of polycyclic aromatic hydrocarbons (PAHs) to low and high density polyethylene (PE) Environ. Sci. Pollut. Res. 2012;19(4):1296–1304. doi: 10.1007/s11356-011-0655-5. [DOI] [PubMed] [Google Scholar]
- Fu L., et al. Adsorption behavior of organic pollutants on microplastics. Ecotoxicol. Environ. Saf. 2021;217 doi: 10.1016/j.ecoenv.2021.112207. [DOI] [PubMed] [Google Scholar]
- Gallo F., Fossi C., Weber R., et al. Marine litter plastics and microplastics and their toxic chemicals components: the need for urgent preventive measures. Environ. Sci. Eur. 2018;30(13):1–14. doi: 10.1186/s12302-018-0139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway T.S., Cole M., Lewis C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017;1(5):0116. doi: 10.1038/s41559-017-0116. [DOI] [PubMed] [Google Scholar]
- Gardette M., et al. Photo- and thermal-oxidation of polyethylene: comparison of mechanisms and influence of unsaturation content. Polym. Degrad. Stab. 2013;98:2383–2390. [Google Scholar]
- Geburtig A., Wachtendorf V., Trubiroha P. Exposure response function for a quantitative prediction of weathering caused aging of polyethylene. Materialpruefung. 2019;61:517–526. [Google Scholar]
- George S.C., Thomas S. Transport phenomena through polymeric systems. Prog. Polym. Sci. 2001;26(6):985–1017. [Google Scholar]
- Ghosh S., Qureshi A., Purohit H.J. In: Contaminants in Agriculture and Environment: Health Risks and Remediation. 1. Kumar V., Kumar R., Singh J., Kumar P., editors. Agro Environ Media; Haridwar, India: 2019. Microbial degradation of plastics: biofilms and degradation pathways; pp. 184–198. [Google Scholar]
- Goedecke C., Mülow-stollin U., Hering S., et al. A first pilot study on the sorption of environmental pollutants on various microplastic materials. J. Environ. Anal. Chem. 2017;4:1–8. [Google Scholar]
- González-Naranjo V., Boltes K., Biel M. Mobility of ibuprofen, a persistent active drug, in soils irrigated with reclaimed water. Plant Soil Environ. 2013;59(2):68–73. [Google Scholar]
- Gu H., et al. Microplastics aggravate the adverse effects of BDE-47 on physiological and defense performance in mussels. J. Hazard. Mater. 2020;398 doi: 10.1016/j.jhazmat.2020.122909. [DOI] [PubMed] [Google Scholar]
- Guo X., et al. Sorption properties of cadmium on microplastics: the common practice experiment and a two-dimensional correlation spectroscopic study. Ecotoxicol. Environ. Saf. 2020;190 doi: 10.1016/j.ecoenv.2019.110118. [DOI] [PubMed] [Google Scholar]
- Guo X., et al. Sorption of four hydrophobic organic compounds by three chemically distinct polymers: role of chemical and physical composition. Environ. Sci. Technol. 2012;46(13):7252–7259. doi: 10.1021/es301386z. [DOI] [PubMed] [Google Scholar]
- Guo X., Chen C., Wang J. Sorption of sulfamethoxazole onto six types of microplastics. Chemosphere. 2019;238:300–308. doi: 10.1016/j.chemosphere.2019.04.155. [DOI] [PubMed] [Google Scholar]
- Hadri H.E., et al. Nanoplastic from mechanically degraded primary and secondary microplastics for environmental assessments. NanoImpact. 2020;17 [Google Scholar]
- Harraq A.F., Bharti B. Microplastics through the Lens of Colloid Science. ACS Environ. Au. 2021 doi: 10.1021/acsenvironau.1c00016. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann N.B., et al. Microplastics as vectors for environmental contaminants: exploring sorption, desorption, and transfer to biota. Integr. Environ. Assess. Manag. 2017;13:488–493. doi: 10.1002/ieam.1904. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Ruz V., et al. Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ. Sci. Technol. 2012;46:3060–3075. doi: 10.1021/es2031505. [DOI] [PubMed] [Google Scholar]
- Hoang T.C., Kim M.F. Microplastic consumption and excretion by fathead minnows (Pimephales promelas): influence of particles size and body shape of fish. Sci. Total Environ. 2020;704 doi: 10.1016/j.scitotenv.2019.135433. [DOI] [PubMed] [Google Scholar]
- Holmes L.A., Turner A., Thompson R.C. Adsorbtion of trace metals to plastic resin pellets in the marine environment. Environ. Pollut. 2012;160:42–48. doi: 10.1016/j.envpol.2011.08.052. [DOI] [PubMed] [Google Scholar]
- Horton A.A., et al. Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017;586:127–141. doi: 10.1016/j.scitotenv.2017.01.190. [DOI] [PubMed] [Google Scholar]
- Horton A.A., et al. Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017;586:127–141. doi: 10.1016/j.scitotenv.2017.01.190. [DOI] [PubMed] [Google Scholar]
- Hu E., et al. Cotransport of naphthalene with polystyrene nanoplastics (PSNP) in saturated porous media: effects of PSNP/naphthalene ratio and ionic strength. Chemosphere. 2020;245 doi: 10.1016/j.chemosphere.2019.125602. [DOI] [PubMed] [Google Scholar]
- Huang Z., He K., Song Z., Zeng G., et al. Antioxidative response of Phanerochaete chrysosporium against silver nanoparticle-induced toxicity and its potential mechanism. Chemosphere. 2018;211:573–583. doi: 10.1016/j.chemosphere.2018.07.192. [DOI] [PubMed] [Google Scholar]
- Hüffer T., Hofmann T. Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution. Environ. Pollut. 2016;214:194–201. doi: 10.1016/j.envpol.2016.04.018. [DOI] [PubMed] [Google Scholar]
- Hüffer T., Weniger A.-K., Hofmann T. Sorption of organic compounds by aged polystyrene microplastic particles. Environ. Pollut. 2018;236:218–225. doi: 10.1016/j.envpol.2018.01.022. [DOI] [PubMed] [Google Scholar]
- Hüffer T., Praetorius A., Wagner S., et al. Microplastic exposure assessment in aquatic environments: learning from similarities and differences to engineered nanoparticles. Environ. Sci. Technol. 2017;51:2499–2507. doi: 10.1021/acs.est.6b04054. [DOI] [PubMed] [Google Scholar]
- Jakubowska M., et al. Effects of chronic exposure to microplastics of different polymer types on early life stages of sea trout Salmo trutta. Sci. Total Environ. 2020;740 doi: 10.1016/j.scitotenv.2020.139922. [DOI] [PubMed] [Google Scholar]
- Karapanagioti H.K., Klontza I. Testing phenanthrene distribution properties of virgin plastic pellets and plastic eroded pellets found on Lesvos island beaches (Greece) Mar. Environ. Res. 2008;65(4):283–290. doi: 10.1016/j.marenvres.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Kaegi R., et al. Fate and transformation of silver nanoparticles in urban wastewater systems. Water Res. 2013;47(12):3866–3877. doi: 10.1016/j.watres.2012.11.060. [DOI] [PubMed] [Google Scholar]
- Kalčíková G., et al. An environmental concentration of aged microplastics with adsorbed silver significantly affects aquatic organisms. Water Res. 2020;175 doi: 10.1016/j.watres.2020.115644. [DOI] [PubMed] [Google Scholar]
- Kaposi K.L., et al. Ingestion of microplastic has limited impact on a Marine Larva. Environ. Sci. Technol. 2014;48(3):1638–1645. doi: 10.1021/es404295e. [DOI] [PubMed] [Google Scholar]
- Karlsson T.M., Hassellov M., Jakubowicz I. Influence of thermooxidative degradation on the in situ fate of polyethylene in temperate coastal waters. Mar. Pollut. Bull. 2018;135:187–194. doi: 10.1016/j.marpolbul.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Kedzierski M., D’Almeida M., Magueresse A., Le Grand A., Duval H., César G., Sire O., Bruzaud S., Le Tilly V. Threat of plastic ageing inmarine environment. Adsorption/ desorption of micropollutants. Mar. Pollut. Bull. 2018;127:684–694. doi: 10.1016/j.marpolbul.2017.12.059. [DOI] [PubMed] [Google Scholar]
- Kim D., Chae Y., An Y.-J. Mixture toxicity of nickel and microplastics with different functional groups on Daphnia magna. Environ. Sci. Technol. 2017;51(21):12852–12858. doi: 10.1021/acs.est.7b03732. [DOI] [PubMed] [Google Scholar]
- Kleinteich J., Seidensticker S., Marggrander N., Zarf C. Microplastics reduce short-term effects of environmental contaminants. part II: polyethylene particles decrease the effect of polycyclic aromatic hydrocarbons on microorganisms. Int. J. Environ. Res. Public Health. 2018:15. doi: 10.3390/ijerph15020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S., Dimzon I.K., Eubeler J., Knepper T.P. In: Wagner M., Lambert S., editors. Vol. 58. Springer; Cham.: 2018. Analysis, occurrence, and degradation of microplastics in the aqueous environment. (Freshwater Microplastics. The Handbook of Environmental Chemistry). [DOI] [Google Scholar]
- Klingelhöfer D., et al. Research landscape of a global environmental challenge: microplastics. Water Res. 2020;170 doi: 10.1016/j.watres.2019.115358. [DOI] [PubMed] [Google Scholar]
- Koelmans A., et al. Microplastic as a vector for chemicals in the aquatic environment: critical review and model-supported re-interpretation of empirical studies. Environ. Sci. Technol. 2016;50(7):3315–3326. doi: 10.1021/acs.est.5b06069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelmans A.A., et al. Microplastics in freshwaters and drinking water: critical review and assessment of data quality. Water Res. 2019;155:410–422. doi: 10.1016/j.watres.2019.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S., Scherer C., Wagner M. Ecotoxicity testing of microplastics: considering the heterogeneity of physicochemical properties. Integr. Environ. Assess. Manag. 2017:470–475. doi: 10.1002/ieam.1901. [DOI] [PubMed] [Google Scholar]
- Lang M., et al. Fenton aging significantly affects the heavy metal adsorption capacity of polystyrene microplastics. Sci. Total Environ. 2020;722 doi: 10.1016/j.scitotenv.2020.137762. [DOI] [PubMed] [Google Scholar]
- Lee H., Byun D.E., Kim J.M., Kwon J.H. Desorption modeling of hydrophobic organic chemicals from plastic sheets using experimentally determined diffusion coefficients in plastics. Mar. Pollut. Bull. 2018;126:312–317. doi: 10.1016/j.marpolbul.2017.11.032. [DOI] [PubMed] [Google Scholar]
- Lee H., Shim W.J., Kwon J.-H. Sorption capacity of plastic debris for hydrophobic organic chemicals. Sci. Total Environ. 2014;470–471:1545–1552. doi: 10.1016/j.scitotenv.2013.08.023. [DOI] [PubMed] [Google Scholar]
- Lehner R., et al. Emergence of nanoplastic in the environment and possible impact on human health. Environ. Sci. Technol. 2019;53(4):1748–1765. doi: 10.1021/acs.est.8b05512. [DOI] [PubMed] [Google Scholar]
- Li J., Zhang K., Zhang H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018;237:460–467. doi: 10.1016/j.envpol.2018.02.050. [DOI] [PubMed] [Google Scholar]
- Li P., et al. A preliminary study of the interactions between microplastics and citrate-coated silver nanoparticles in aquatic environments. J. Hazard. Mater. 2020;385 doi: 10.1016/j.jhazmat.2019.121601. [DOI] [PubMed] [Google Scholar]
- Li Y., Li M., Li Z., Yang L., Liu X. Chemosphere effects of particle size and solution chemistry on Triclosan sorption on polystyrene microplastic. Chemosphere. 2019;231:308–314. doi: 10.1016/j.chemosphere.2019.05.116. [DOI] [PubMed] [Google Scholar]
- Li Z., et al. Evaluating the effect of different modified microplastics on the availability of polycyclic aromatic hydrocarbons. Water Res. 2020;170 doi: 10.1016/j.watres.2019.115290. [DOI] [PubMed] [Google Scholar]
- Lima A., Costa M., Barletta M. Distribution patterns of microplastics within the plankton of a tropical estuary. Environ. Res. 2014;2(13):146–155. doi: 10.1016/j.envres.2014.03.031. [DOI] [PubMed] [Google Scholar]
- Lima E.C., et al. Is one performing the treatment data of adsorption kinetics correctly? J. Environ. Chem. Eng. 2020 [Google Scholar]
- Lin W., et al. Joint effect of nanoplastics and humic acid on the uptake of PAHs for Daphnia magna: a model study. J. Hazard. Mater. 2020;391 doi: 10.1016/j.jhazmat.2020.122195. [DOI] [PubMed] [Google Scholar]
- Lithner D., et al. Leachates from plastic consumer products screening for toxicity with Daphnia magna. Chemosphere. 2009;74(9):1195–1200. doi: 10.1016/j.chemosphere.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Lithner D., Larsson A., Dave G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011;409(18):3309–3324. doi: 10.1016/j.scitotenv.2011.04.038. [DOI] [PubMed] [Google Scholar]
- Liu G., Zhu Z., Yang Y., Sun Y., Yu F., Ma J. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ. Pollut. 2019;246:26–33. doi: 10.1016/j.envpol.2018.11.100. [DOI] [PubMed] [Google Scholar]
- Liu J., Ma Y., Zhu D., et al. Polystyrene nanoplastics-enhanced contaminant transport: role of irreversible adsorption in glassy polymeric domain. Environ. Sci. Technol. 2018;52:2677–2685. doi: 10.1021/acs.est.7b05211. [DOI] [PubMed] [Google Scholar]
- Liu L., Fokkink R., Koelmans A.A. Sorption of polycyclic aromatic hydrocarbons to polystyrene nanoplastic. Environ. Toxicol. Chem. 2016;35:1650–1655. doi: 10.1002/etc.3311. [DOI] [PubMed] [Google Scholar]
- Liu P., et al. New insights into aging behavior of microplastics accelerated by advanced oxidation processes. Environ. Sci. Technol. 2019;53(7):3579–3588. doi: 10.1021/acs.est.9b00493. [DOI] [PubMed] [Google Scholar]
- Liu P., et al. Effect of aging on adsorption behavior of polystyrene microplastics for pharmaceuticals: adsorption mechanism and role of aging intermediates. J. Hazard. Mater. 2020;384 doi: 10.1016/j.jhazmat.2019.121193. [DOI] [PubMed] [Google Scholar]
- Liu P., et al. Effect of aging on adsorption behavior of polystyrene microplastics for pharmaceuticals: adsorption mechanism and role of aging intermediates. J. Hazard. Mater. 2020;384 doi: 10.1016/j.jhazmat.2019.121193. [DOI] [PubMed] [Google Scholar]
- Liu P., et al. Effect of weathering on environmental behavior of microplastics: properties, sorption and potential risks. Chemosphere. 2020;242 doi: 10.1016/j.chemosphere.2019.125193. [DOI] [PubMed] [Google Scholar]
- Liu N., et al. Sorption of tetracycline on organo-montmorillonites. J. Hazard Mater. 2012;28(35):225–226. doi: 10.1016/j.jhazmat.2012.04.060. [DOI] [PubMed] [Google Scholar]
- Liu Z., Yu P., Cai M., Wu D., Zhang M., Huang Y., Zhao Y. Polystyrene nanoplastic exposure induces immobilization, reproduction, and stress defense in the freshwater cladoceran Daphnia pulex. Chemosphere. 2019;215:74–81. doi: 10.1016/j.chemosphere.2018.09.176. [DOI] [PubMed] [Google Scholar]
- Llorca M., et al. Adsorption of perfluoroalkyl substances on microplastics under environmental conditions. Environ. Pollut. 2018;235:680–691. doi: 10.1016/j.envpol.2017.12.075. [DOI] [PubMed] [Google Scholar]
- Loyo-Rosales, J.E., Rosales-Rivera, G.C., Lynch, A.M., Rice, C.P., Torrents, A., 2004. Migration of nonylphenol from plastic containers to water and a milk surrogate. doi: 10.1021/JF0345696. [DOI] [PubMed]
- Luo H., et al. Environmental behaviors of microplastics in aquatic systems: a systematic review on degradation, adsorption, toxicity and biofilm under aging conditions. J. Hazard. Mater. 2022;423(A) doi: 10.1016/j.jhazmat.2021.126915. [DOI] [PubMed] [Google Scholar]
- Luo H., et al. Effects of accelerated aging on characteristics, leaching, and toxicity of commercial lead chromate pigmented microplastics. Environ. Pollut. 2020;257 doi: 10.1016/j.envpol.2019.113475. [DOI] [PubMed] [Google Scholar]
- Luo H., Xiang Y., He D., Li Y., Zhao Y., Wang S., Pan X. Leaching behavior of fluorescent additives from microplastics and the toxicity of leachate to Chlorella vulgaris. Sci. Total Environ. 2019;678:1–9. doi: 10.1016/j.scitotenv.2019.04.401. [DOI] [PubMed] [Google Scholar]
- Luo H., Zhao Y., Li Y., Xiang Y., He D., Pan X. Aging of microplastics affects their surface properties, thermal decomposition, additives leaching and interactions in simulated fluids. Sci. Total Environ. 2020;714 doi: 10.1016/j.scitotenv.2020.136862. [DOI] [PubMed] [Google Scholar]
- Luo Y., et al. Distribution characteristics and mechanism of microplastics mediated by soil physicochemical properties. Sci. Total Environ. 2020;726 doi: 10.1016/j.scitotenv.2020.138389. [DOI] [PubMed] [Google Scholar]
- Lv Y.D., Huang Y.J., Yang J.L., Kong M.Q., Yang H., Zhao J.C., Li G.X. Outdoor and accelerated laboratory weathering of polypropylene: a comparison and correlation study. Polym. Degrad. Stab. 2015;112:145–159. [Google Scholar]
- Ma H., Pu S., Liu S., et al. Microplastics in aquatic environments: Toxicity to trigger ecological consequences. Environ. Pollut. 2020;261 doi: 10.1016/j.envpol.2020.114089. [DOI] [PubMed] [Google Scholar]
- Ma J., Zhao J.H., Zhu Z.L., Li L.Q., Yu F. Effect of microplastic size on the adsorption behavior and mechanism of triclosan on polyvinyl chloride. Environ. Pollut. 2019:254. doi: 10.1016/j.envpol.2019.113104. [DOI] [PubMed] [Google Scholar]
- Ma Y., Huang A., Cao S., Sun F., Wang L., Guo H., Ji R. Effects of nanoplastics and microplastics on toxicity, bioaccumulation, and environmental fate of phenanthrene in fresh water. Environ. Pollut. 2016;219:166–173. doi: 10.1016/j.envpol.2016.10.061. [DOI] [PubMed] [Google Scholar]
- Mamitiana R., Ding J., Zhang S., Jiang H., Zou H. Sorption and desorption of selected pharmaceuticals by polyethylene microplastics. Mar. Pollut. Bull. 2018;136:516–523. doi: 10.1016/j.marpolbul.2018.09.048. [DOI] [PubMed] [Google Scholar]
- Mammo F.K., et al. Microplastics in the environment: Interactions with microbes and chemical contaminants. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140518. [DOI] [PubMed] [Google Scholar]
- Mao Y., et al. Nanoplastics display strong stability in aqueous environments: Insights from aggregation behaviour and theoretical calculations. Environ. Pollut. 2020;258 doi: 10.1016/j.envpol.2019.113760. [DOI] [PubMed] [Google Scholar]
- Massos A., Turner A. Cadmium, lead and bromine in beached microplastics. Environ. Pollut. 2017;227:139–145. doi: 10.1016/j.envpol.2017.04.034. [DOI] [PubMed] [Google Scholar]
- Mato Y., Isobe T., Takada H., Kanehiro H., Ohtake C., Kaminuma T. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 2001:35. doi: 10.1021/es0010498. [DOI] [PubMed] [Google Scholar]
- McCormick A., et al. Microplastic in surface waters of urban rivers: concentration, sources, and associated bacterial assemblages. Ecosphere. 2016;7:01556. [Google Scholar]
- Mendoza R., L.M., Jones P.R. Characterisation of microplastics and toxic chemicals extracted from microplastic samples from the North Pacific Gyre. Environ. Chem. 2015;12(5):611–617. [Google Scholar]
- Mohsen M., Wang Q., Zhang L., Sun L., Lin C., Yang H. Heavy metals in sediment, microplastic and sea cucumber Apostichopus japonicus from farms in China. Mar. Pollut. Bull. 2019;143:42–49. doi: 10.1016/j.marpolbul.2019.04.025. [DOI] [PubMed] [Google Scholar]
- Nguyen, T.N., Goss, K.-U., Ball,W.P., 2005. Polyparameter linear free energy relationships for estimating the equilibrium partition of organic compounds between water and the natural organic matter in soils and sediments. doi: 10.1021/ES048839S. [DOI] [PubMed]
- OECD, 2016. Nanomaterials in waste streams: current knowledge on risks and impacts, Paris.
- Ogata Y., et al. International Pellet Watch: global monitoring of persistent organic pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs, DDTs, and HCHs. Mar. Pollut. Bull. 2009;58(10):1437–1446. doi: 10.1016/j.marpolbul.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Oliveira M., Almeida M., Miguel I. A micro(nano)plastic boomerang tale: a never ending story? Trends Anal. Chem. 2019;112:196–200. [Google Scholar]
- Oriekhova O., Stoll S. Heteroaggregation of nanoplastic particles in the presence of inorganic colloids and natural organic matter. Environ. Sci.: Nano. 2018;5(3):792–799. [Google Scholar]
- Pascall M.A., Zabik M.E., Zabik, R.J M.J. Hernandez, Uptake of polychlorinated biphenyls (PCBs) from an aqueous medium by polyethylene, polyvinyl chloride, and polystyrene films. J. Agric. Food Chem. 2005;53:164–169. doi: 10.1021/jf048978t. [DOI] [PubMed] [Google Scholar]
- Patrício Silva A.L., Prata J.C., Walker T.R., et al. Increased plastic pollution due to COVID-19 pandemic: challenges and recommendations. Chem. Eng. J. 2021;405 doi: 10.1016/j.cej.2020.126683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Fu D., et al. Micro- and nano-plastics in marine environment: source, distribution and threats—a review. Sci. Total Environ. 2020;698 doi: 10.1016/j.scitotenv.2019.134254. [DOI] [PubMed] [Google Scholar]
- Pittura L., Avio C.G., Giuliani, G M.E., et al. Microplastics as vehicles of environmental PAHs to marine organisms: combined chemical and physical hazards to the Mediterranean mussels, Mytilus galloprovincialis. Front. Mar. Sci. 2018:5. [Google Scholar]
- Praetorius A., et al. The road to nowhere: equilibrium partition coefficients for nanoparticles. Environ. Sci.: Nano. 2014;1(4):317–323. [Google Scholar]
- Prata J.C. Airborne microplastics: consequences to human health? Environ. Pollut. 2018;234:115–126. doi: 10.1016/j.envpol.2017.11.043. [DOI] [PubMed] [Google Scholar]
- Prata J.C., Silva A., Walker T.R., Duarte A.C., Rocha-Santos T.A.P. COVID-19 pandemic repercussions on the use and management of plastics. Environ. Sci. Technol. 2020;54(2020):1–6. doi: 10.1021/acs.est.0c02178. [DOI] [PubMed] [Google Scholar]
- Qiao R., et al. Combined effects of polystyrene microplastics and natural organic matter on the accumulation and toxicity of copper in zebrafish. Sci. Total Environ. 2019;682:128–137. doi: 10.1016/j.scitotenv.2019.05.163. [DOI] [PubMed] [Google Scholar]
- Rai, P.K., 2021. Environmental degradation by invasive alien plants in the anthropocene: challenges and prospects for sustainable restoration. ANPS, In Press; doi: 10.1007/s44177-021-00004-y.
- Rai P.K., et al. Progress, prospects, and challenges in sampling and standardization of the methods for micro- and nano-plastics analysis in the environment. J. Clean. Prod. 2021;325 [Google Scholar]
- Rai P.K., Kim K.H., Lee S.S., Lee J.-H. Molecular mechanisms in phytoremediation of environmental contaminants and prospects of engineered transgenic plants/microbes. Sci. Total Environ. 2020;705 doi: 10.1016/j.scitotenv.2019.135858. [DOI] [PubMed] [Google Scholar]
- Rai P.K., Kumar V., Lee S.S., Naddem R., Ok Y.S., Kim K.H., Tsang D.S.W. Nanoparticle-plant interaction: implications in energy, the environment, and agriculture. Environ. Int. 2018;119:1–19. doi: 10.1016/j.envint.2018.06.012. [DOI] [PubMed] [Google Scholar]
- Rai P.K., Lee J., Kailasa S.K., Kwon E.E., Tsang Y.F., Ok Y.S., Kim K.H. A critical review of ferrate(VI)-based remediation of soil and groundwater. Environ. Res. 2018;160:420–428. doi: 10.1016/j.envres.2017.10.016. [DOI] [PubMed] [Google Scholar]
- Rai P.K., Lee S., Brown J., Kim K.H. Environmental fate, ecotoxicity biomarkers, and potential health effects of micro- and nano-scale plastic contamination. J. Hazard. Mater. 2021;403 doi: 10.1016/j.jhazmat.2020.123910. [DOI] [PubMed] [Google Scholar]
- Rai P.K., Lee S., Brown J., Kim K.H. Micro- and nanoplastic pollution: behavior, microbial ecology, and remediation technologies. J. Clean. Prod. 2021;291 [Google Scholar]
- Rai P.K., Lee S.S., Zhang M.M., Tsang Y.F., Kim K.H. Heavy metals in food crops: health risks, fate, mechanisms, and management. Environ. Int. 2019;125:365–385. doi: 10.1016/j.envint.2019.01.067. [DOI] [PubMed] [Google Scholar]
- Rainieri S., Conlledo N., Larsen B.K., Granby K., Barranco A. Combined effects of microplastics and chemical contaminants on the organ toxicity of zebrafish (Danio rerio) Environ. Res. 2018;162:135–143. doi: 10.1016/j.envres.2017.12.019. [DOI] [PubMed] [Google Scholar]
- Rani M., Shim W.J., Jang M., Han G.M., Hong S.H. Releasing of hexabromocyclododecanes from expanded polystyrenes in seawater -field and laboratory experiments. Chemosphere. 2017;185:798–805. doi: 10.1016/j.chemosphere.2017.07.042. [DOI] [PubMed] [Google Scholar]
- Razanajatovo R.M., Ding J., Zhang S., Jiang H., Zou H. Sorption and desorption of selected pharmaceuticals by polyethylene microplastics. Mar. Pollut. Bull. 2018;136:516–523. doi: 10.1016/j.marpolbul.2018.09.048. [DOI] [PubMed] [Google Scholar]
- Reichel J., et al. Organic contaminants and interactions with micro-and nano-plastics in the aqueous environment: review of analytical methods. Molecules. 2021;26(4):1164. doi: 10.3390/molecules26041164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro F., O’Brien J.W., et al. Accumulation and fate of nano- and micro-plastics and associated contaminants in organisms. Trends Anal. Chem. 2019;111:139–147. [Google Scholar]
- Rocha-Santos T., Duarte A.C. A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. Trends Analyt. Chem. 2015;65:47–53. [Google Scholar]
- Rochman C.M., Browne M.A., et al. Policy: classify plastic waste as hazardous. Nature. 2013;494:169–171. doi: 10.1038/494169a. [DOI] [PubMed] [Google Scholar]
- Rochman C.M., et al. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ. 2014;493:656–661. doi: 10.1016/j.scitotenv.2014.06.051. [DOI] [PubMed] [Google Scholar]
- Rochman C.M., et al. Anthropogenic debris in seafood: plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci. Rep. 2015;5(14):340. doi: 10.1038/srep14340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman C.M., Lewison R.L., Eriksen M., Allen H., Cook A.M., Teh S.J. Polybrominated diphenyl ethers (PBDEs) in fish tissue may be an indicator of plastic contamination in marine habitats. Sci. Total Environ. 2014;476–477:622–633. doi: 10.1016/j.scitotenv.2014.01.058. [DOI] [PubMed] [Google Scholar]
- Rodrigues J.P., et al. Significance of interactions between microplastics and POPs in the marine environment: a critical overview. Trends Anal. Chem. 2019;111:252–260. [Google Scholar]
- Roes L., Patel M.K., Worrell E., Ludwig C. Preliminary evaluation of risks related to waste incineration of polymer nanocomposites. Sci. Total Environ. 2012;417:76–86. doi: 10.1016/j.scitotenv.2011.12.030. [DOI] [PubMed] [Google Scholar]
- Rogers K.L., Carreres-Calabuig J.A., Gorokhova E., Posth N.R. Micro-by-micro interactions: How microorganisms influence the fate of marine microplastics. Limnol. Oceanogr. Lett. 2020;5:18–36. [Google Scholar]
- Rouillon C., Bussiere P.O., Desnoux E., Collin S., Vial C., Therias S., Gardette J.L. Is carbonyl index a quantitative probe to monitor polypropylene photodegradation? Polym. Degrad. Stab. 2016;128:200–208. [Google Scholar]
- Rowdhwal S.S.S., Chen J. Toxic effects of di-2-ethylhexyl phthalate: an overview. Biomed. Res. Int. 2018;2018:1–10. doi: 10.1155/2018/1750368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio L., et al. Potential adverse health effects of ingested micro and nanoplastics on humans. Lessons learned from in vivo and in vitro mammalian models. J. Toxicol. Environ. Health, Part B. 2020;23(2):51–68. doi: 10.1080/10937404.2019.1700598. [DOI] [PubMed] [Google Scholar]
- Rummel C.D., Jahnke A., Gorokhova E., Kühnel D., Schmitt-Jansen M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017;4(7):258–267. [Google Scholar]
- Saavedra J., Stoll S., Slaveykova V.I. Influence of nanoplastic surface charge on eco-corona formation, aggregation and toxicity to freshwater zooplankton. Environ. Pollut. 2019;252:715–722. doi: 10.1016/j.envpol.2019.05.135. [DOI] [PubMed] [Google Scholar]
- Scheytt T., Mersmann P., Lindstädt R., Heberer T. Determination of sorption coefficients of pharmaceutically active substances carbamazepine, diclofenac, and ibuprofen, in sandy sediments. Chemosphere. 2005;60(2):245–253. doi: 10.1016/j.chemosphere.2004.12.042. [DOI] [PubMed] [Google Scholar]
- Seeley M.E., et al. Microplastics affect sedimentary microbial communities and nitrogen cycling. Nat. Commun. 2020;11:2372. doi: 10.1038/s41467-020-16235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidensticker S., et al. Microplastic–contaminant interactions: influence of nonlinearity and coupled mass transfer. Environ. Toxicol. Chem. 2019;38(8):1635–1644. doi: 10.1002/etc.4447. [DOI] [PubMed] [Google Scholar]
- Seidensticker S., Grathwohl P., Lamprecht J., Zarfl C. A combined experimental and modeling study to evaluate pH-dependent sorption of polar and non-polar compounds to polyethylene and polystyrene microplastics. Environ. Sci. Eur. 2018;30(1):3. doi: 10.1186/s12302-018-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidensticker S., Zarfl C., Cirpka O.A., Fellenberg G., Grathwohl P. Shift in mass transfer of wastewater contaminants from microplastics in the presence of dissolved substances. Environ. Sci. Technol. 2017;51(21):12254–12263. doi: 10.1021/acs.est.7b02664. [DOI] [PubMed] [Google Scholar]
- Shen S., Zhang Y., Zhu Y., et al. Recent advances in toxicological research of nanoplastics in the environment: a review. Environ. Pollut. 2019;252:511–521. doi: 10.1016/j.envpol.2019.05.102. [DOI] [PubMed] [Google Scholar]
- Shiu R.F., et al. Nano-plastics induce aquatic particulate organic matter (microgels) formation. Sci. Total Environ. 2020;706 doi: 10.1016/j.scitotenv.2019.135681. [DOI] [PubMed] [Google Scholar]
- Singh N., Tiwari E., Khandelwal N., Darbha G.K. Understanding the stability of nanoplastics in aqueous environments: effect of ionic strength, temperature, dissolved organic matter, clay, and heavy metals. Environ. Sci. Nano. 2019;6:2968–2976. [Google Scholar]
- Silva A.P.L., Prata J.C., Walker T.R., et al. Rethinking and optimising plastic waste management under COVID-19 pandemic: policy solutions based on redesign and reduction of single-use plastics and personal protective equipment. Sci. Total Environ. 2020;742 doi: 10.1016/j.scitotenv.2020.140565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia B., Matthew M.L., Christian B., Magnus B. Toxicity of leachate from weathering plastics: an exploratory screening study with Nitocra spinipes. Chemosphere. 2015;132:114–119. doi: 10.1016/j.chemosphere.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Song Y.K., et al. Large accumulation of micro-sized synthetic polymer particles in the sea surface microlayer. Environ. Sci. Technol. 2014;48(16):90140–99021. doi: 10.1021/es501757s. [DOI] [PubMed] [Google Scholar]
- Stenzel A., Goss K.-U., Endo S. Determination of polyparameter linear free energy relationship (pp-LFER) substance descriptors for established and alternative flame retardants. Environ. Sci. Technol. 2013 doi: 10.1021/es304780a. 130125075250002. [DOI] [PubMed] [Google Scholar]
- Sun M., et al. Changes in tetracycline partitioning and bacteria/ phage-comediated ARGs in microplastic-contaminated greenhouse soil facilitated by sophorolipid. J. Hazard. Mat. 2018;345:131–139. doi: 10.1016/j.jhazmat.2017.11.036. [DOI] [PubMed] [Google Scholar]
- Sun Y., et al. Laboratory simulation of microplastics weathering and its adsorption behaviors in an aqueous environment: a systematic review. Environ. Pollut. 2020;265 doi: 10.1016/j.envpol.2020.114864. [DOI] [PubMed] [Google Scholar]
- Teuten E.L., Rowland S.J., Galloway T.S., Thompson R.C. Potential for plastics to transport hydrophobic contaminants. Environ. Sci. Technol. 2007;41(22):7759–7764. doi: 10.1021/es071737s. [DOI] [PubMed] [Google Scholar]
- Thiagarajan V., et al. Influence of differently functionalized polystyrene microplastics on the toxic effects of P25 TiO2 NPs towards marine algae Chlorella sp. Aquat. Toxicol. 2019;207:208–216. doi: 10.1016/j.aquatox.2018.12.014. [DOI] [PubMed] [Google Scholar]
- Tiwari M., Rathod T.D., Ajmal P.Y., Bhangare R.C., Sahu S.K. Distribution and characterization of microplastics in beach sand from three different Indian coastal environments. Mar. Pollut. Bull. 2019;140:262–273. doi: 10.1016/j.marpolbul.2019.01.055. [DOI] [PubMed] [Google Scholar]
- Tran H.N., et al. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Res. 2017;120:88–116. doi: 10.1016/j.watres.2017.04.014. [DOI] [PubMed] [Google Scholar]
- Turner A., et al. Metals and marine microplastics: adsorption from the environment versus addition during manufacture, exemplified with lead. Water Res. 2020;173 doi: 10.1016/j.watres.2020.115577. [DOI] [PubMed] [Google Scholar]
- Turner A., Holmes L.A. Adsorption of trace metals by microplastic pellets in fresh water. Environ. Chem. 2015;12:600–610. [Google Scholar]
- Uber T.H., Hüffer T., Planitz S., Schmidt T.C. Characterization of sorption properties of high-density polyethylene using the poly-parameter linearfree-energy relationships. Environ. Pollut. 2019;248:312–319. doi: 10.1016/j.envpol.2019.02.024. [DOI] [PubMed] [Google Scholar]
- UNEP, 2014. Valuing plastics: the business case for measuring, managing and disclosing plastic use in the consumer goods industry. United Nations Environment Program, Nairobi.
- USEPA, 2017. Technical fact sheet – Polybrominated Diphenyl ethers (PBDEs). U.S. EPA Fed. Facil. Restor. Reuse off, pp. 1–5.
- van Doremalen N., Bushmaker T., Morris, et al. Aerosol and surface stability of SARSCoV- 2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega R.L., Epel D. Stress-induced apoptosis in sea urchin embryogenesis. Mar. Environ. Res. 2004;58(2–5):799–802. doi: 10.1016/j.marenvres.2004.03.096. [DOI] [PubMed] [Google Scholar]
- Velzeboer I., et al. Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ. Sci. Technol. 2014;48(9):4869–4876. doi: 10.1021/es405721v. [DOI] [PubMed] [Google Scholar]
- Vencalek B.E., et al. In situ measurement of CuO and Cu(OH)2 nanoparticle dissolution rates in quiescent freshwater mesocosms. Environ. Sci. Technol. Lett. 2016;3(10):375–380. [Google Scholar]
- Vieira Y., et al. Microplastics physicochemical properties, specific adsorption modelling and their interaction with pharmaceuticals and other emerging contaminants. Sci. Total Environ. 2021;753 doi: 10.1016/j.scitotenv.2020.141981. [DOI] [PubMed] [Google Scholar]
- Vikrant K., Kim K.H., et al. Adsorption performance of standard biochar materials against volatile organic compounds in air: a case study using benzene and methyl ethyl ketone. Chem. Eng. J. 2020;387 [Google Scholar]
- Wang F., Shih K.M., Li X.Y. The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics. Chemosphere. 2015;119:841–847. doi: 10.1016/j.chemosphere.2014.08.047. [DOI] [PubMed] [Google Scholar]
- Wang F., Wong C.S., et al. Interaction of toxic chemicals with microplastics: a critical review. Water Res. 2018;139:208–219. doi: 10.1016/j.watres.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Wang J., Guo X., Xue J. Biofilm-developed microplastics as vectors of pollutants in aquatic environments. Environ. Sci. Technol. 2021;55(19):12780–12790. doi: 10.1021/acs.est.1c04466. [DOI] [PubMed] [Google Scholar]
- Wang J., Peng J., Tan Z., Gao Y., Zhan Z., Chen Q., Cai L. Microplastics in the surface sediments from the Beijiang River littoral zone: composition, abundance, surface textures and interaction with heavy metals. Chemosphere. 2017;171:248–258. doi: 10.1016/j.chemosphere.2016.12.074. [DOI] [PubMed] [Google Scholar]
- Wang Q., Zhang Y., Wangjin X., Wang Y., Meng G., Chen Y. The adsorption behavior of metals in aqueous solution by microplastics effected by UV radiation. J. Environ. Sci. 2020;87:272–280. doi: 10.1016/j.jes.2019.07.006. [DOI] [PubMed] [Google Scholar]
- Wang T., et al. Adsorption behavior and mechanism of five pesticides on microplastics from agricultural polyethylene films. Chemosphere. 2020;244 doi: 10.1016/j.chemosphere.2019.125491. [DOI] [PubMed] [Google Scholar]
- Wang Z., Chen M., Zhang L., Wang K., Yu X., Zheng Z., Zheng R. Sorption behaviors of phenanthrene on the microplastics identified in a mariculture farm in Xiangshan Bay, southeastern China. Sci. Total Environ. 2018;628–629:1617–1626. doi: 10.1016/j.scitotenv.2018.02.146. [DOI] [PubMed] [Google Scholar]
- Wanner P. Plastic in agricultural soils – a global risk for groundwater systems and drinking water supplies? – a review. Chemosphere. 2020 doi: 10.1016/j.chemosphere.2020.128453. [DOI] [PubMed] [Google Scholar]
- Wijesekara H., Bolan N.S., Bradney L., Obadamudalige N., Seshadri B., Kunhikrishnan A., Dharmarajan R., Ok Y.S., Rinklebe J., Kirkham M.B., Vithanage M. Trace element dynamics of biosolids-derived microbeads. Chemosphere. 2018;199:331–339. doi: 10.1016/j.chemosphere.2018.01.166. [DOI] [PubMed] [Google Scholar]
- Wright S.L., Thompson R.C., Galloway T.S. The physical impacts of microplastics on marine organisms: a review. Environ. Pollut. 2013;178:483–492. doi: 10.1016/j.envpol.2013.02.031. [DOI] [PubMed] [Google Scholar]
- Wu C., Zhang K., Huang X., Liu J. Sorption of pharmaceuticals and personal care products to polyethylene debris. Environ. Sci. Pollut. Res. 2016;23(9):8819–8826. doi: 10.1007/s11356-016-6121-7. [DOI] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., et al. Effects of polymer aging on sorption of 2, 2 ’, 4, 4’-tetrabromodiphenyl ether by polystyrene microplastics. Chemosphere. 2020;253 doi: 10.1016/j.chemosphere.2020.126706. [DOI] [PubMed] [Google Scholar]
- Wu P., et al. Adsorption mechanisms of five bisphenol analogues on PVC microplastics. Sci. Total Environ. 2019;650:671–678. doi: 10.1016/j.scitotenv.2018.09.049. [DOI] [PubMed] [Google Scholar]
- Wu X., et al. Adsorption of triclosan onto different aged polypropylene microplastics: critical effect of cations. Sci. Total Environ. 2020;717 doi: 10.1016/j.scitotenv.2020.137033. [DOI] [PubMed] [Google Scholar]
- Wu X., Lyu X., Li Z., Gao B., Zeng X., Wu J., Sun Y. Transport of polystyrene nanoplastics in natural soils: effect of soil properties, ionic strength and cation type. Sci. Total Environ. 2020;707 doi: 10.1016/j.scitotenv.2019.136065. [DOI] [PubMed] [Google Scholar]
- Wu X., Pan J., Li M., Li Y., Bartlam M., Wang Y. Selective enrichment of bacterial pathogens by microplastic biofilm. Water Res. 2019;165 doi: 10.1016/j.watres.2019.114979. [DOI] [PubMed] [Google Scholar]
- Xia B., et al. Polystyrene microplastics increase uptake, elimination and cytotoxicity of decabromodiphenyl ether (BDE-209) in the marine scallop Chlamys farreri. Environ. Pollut. 2020;258 doi: 10.1016/j.envpol.2019.113657. [DOI] [PubMed] [Google Scholar]
- Xu B., Liu F., Brookes P.C., Xu J. The sorption kinetics and isotherms of sulfamethoxazole with polyethylene microplastics. Mar. Pollut. Bull. 2018;131 doi: 10.1016/j.marpolbul.2018.04.027. 191–19. [DOI] [PubMed] [Google Scholar]
- Xu B., Liu F., Brookes P.C., Xu J. Microplastics play a minor role in tetracycline sorption in the presence of dissolved organic matter. Environ. Pollut. 2018;240:87. doi: 10.1016/j.envpol.2018.04.113. [DOI] [PubMed] [Google Scholar]
- Xu P., Ge W., Chai C., Zhang Y., Jiang T., Xia B. Sorption of polybrominated diphenyl ethers by microplastics. Mar. Pollut. Bull. 2019;145:260–269. doi: 10.1016/j.marpolbul.2019.05.050. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., et al. Persistence and partitioning of eight selected pharmaceuticals in the aquatic environment: laboratory photolysis, biodegradation, and sorption experiments. Water Res. 2009;43(2):351–362. doi: 10.1016/j.watres.2008.10.039. [DOI] [PubMed] [Google Scholar]
- Yu F., Yang C., Zhu Z., Bai X., Ma J. Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment. Sci. Total Environ. 2019 doi: 10.1016/j.scitotenv.2019.133643. [DOI] [PubMed] [Google Scholar]
- Yu H., et al. The effects of functional groups on the sorption of naphthalene on microplastics. Chemosphere. 2020;261 doi: 10.1016/j.chemosphere.2020.127592. [DOI] [PubMed] [Google Scholar]
- Yu Y., et al. Enhanced adsorption of tetrabromobisphenol a (TBBPA) on cosmetic-derived plastic microbeads and combined effects on zebrafish. Chemosphere. 2020;248 doi: 10.1016/j.chemosphere.2020.126067. [DOI] [PubMed] [Google Scholar]
- Zettler E.R., Mincer T.J., Amaral-Zettler L.A. Life in the “plastisphere”: microbial communities on plastic marine debris. Environ. Sci. Technol. 2013;47:7137–7146. doi: 10.1021/es401288x. [DOI] [PubMed] [Google Scholar]
- Zhang H., et al. Sorption of fluoroquinolones to nanoplastics as affected by surface functionalization and solution chemistry. Environ. Pollut. 2020;262 doi: 10.1016/j.envpol.2020.114347. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wang J., Zhou B., Zhou Y., Dai Z., Zhou Q., Chriestie P., Luo Y. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: kinetics, isotherms and influencing factors. Environ. Pollut. 2018;243:1550–1557. doi: 10.1016/j.envpol.2018.09.122. [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhou Q., Xie Z., Zhou Y., Tu C., Fu C., Mi W., Ebinghaus R., Christie P., Luo Y. Occurrences of organophosphorus esters and phthalates in the microplastics from the coastal beaches in north China. Sci. Total Environ. 2018;616–617:1505–1512. doi: 10.1016/j.scitotenv.2017.10.163. [DOI] [PubMed] [Google Scholar]
- Zhang J., et al. Adsorption behavior and mechanism of 9-Nitroanthracene on typical microplastics in aqueous solutions. Chemosphere. 2020;245 doi: 10.1016/j.chemosphere.2019.125628. [DOI] [PubMed] [Google Scholar]
- Zhang Price, Jamieson Burton, Khosravi Sorption and desorption of selected nonsteroidal anti-inflammatory drugs in an agricultural loam-textured soil. Chemosphere. 2017;174:628–637. doi: 10.1016/j.chemosphere.2017.02.027. [DOI] [PubMed] [Google Scholar]
- Zhang Q., et al. The combined toxicity effect of nanoplastics and glyphosate on Microcystis aeruginosa growth. Environ. Pollut. 2018;243:1106–1112. doi: 10.1016/j.envpol.2018.09.073. [DOI] [PubMed] [Google Scholar]
- Zhang S., et al. Microplastics in the environment: a review of analytical methods, distribution, and biological effects. Trends Anal. Chem. 2019;111:62–72. [Google Scholar]
- Zhang S., et al. Interactive effects of polystyrene microplastics and roxithromycin on bioaccumulation and biochemical status in the freshwater fish red tilapia (Oreochromis niloticus) Sci. Total Environ. 2019;648:1431–1439. doi: 10.1016/j.scitotenv.2018.08.266. [DOI] [PubMed] [Google Scholar]
- Zhang W., et al. The mechanism for adsorption of Cr(VI) ions by PE microplastics in ternary system of natural water environment. Environ. Pollut. 2020;257 doi: 10.1016/j.envpol.2019.113440. [DOI] [PubMed] [Google Scholar]
- Zhang Y., et al. Atmospheric microplastics: A review on current status and perspectives. Earth-Sci. Rev. 2020;203 [Google Scholar]
- Zhang Y., et al. Potential risks of microplastics combined with superbugs: enrichment of antibiotic resistant bacteria on the surface of microplastics in mariculture system. Ecotoxicol. Environ. Saf. 2020;187 doi: 10.1016/j.ecoenv.2019.109852. [DOI] [PubMed] [Google Scholar]
- Zhao L., et al. Sorption of five organic compounds by polar and nonpolar microplastics. Chemosphere. 2020;257 doi: 10.1016/j.chemosphere.2020.127206. [DOI] [PubMed] [Google Scholar]
- Zhou, A., Ma, X., Song, C. 2007. Nanoporous carbon adsorbent preparation coupled with hydrogen production by chemical activation of coal, plastics and biomass. United Kingdom.
- Zhou Q., et al. The distribution and morphology of microplastics in coastal soils adjacent to the Bohai Sea and the Yellow Sea. Geoderma. 2018;322:201–208. [Google Scholar]
- Zhu Z.-l, et al. Joint toxicity of microplastics with triclosan to marine microalgae Skeletonema costatum. Environ. Pollut. 2019;246:509–517. doi: 10.1016/j.envpol.2018.12.044. [DOI] [PubMed] [Google Scholar]