Abstract
Purpose:
To evaluate temporal trends, practice variation, and associated outcomes with the use of intravascular ultrasound (US) during deep venous stent placement among Medicare beneficiaries.
Materials and Methods:
All lower extremity deep venous stent placement procedures performed between January 1, 2017, and December 31, 2019 among Medicare beneficiaries were included. Temporal trends in intravascular US use were stratified by procedural setting and physician specialty. The primary outcome was a composite of 12-month all-cause mortality, all-cause hospitalization, or repeat target vessel intervention. The secondary outcome was a composite of 12-month stent thrombosis, embolization, or restenosis.
Results:
Among the 20,984 deep venous interventions performed during the study period, 15,184 (72.4%) utilized intravascular US. Moderate growth in intravascular US use was observed during the study period in all clinical settings. There was a variation in the use of intravascular US among all operators (median, 77.3% of cases; interquartile range, 20.0%–99.2%). In weighted analyses, intravascular US use during deep venous stent placement was associated with a lower risk of both the primary (adjusted hazard ratio, 0.72; 95% confidence interval [CI], 0.69–0.76; P < .001) and secondary (adjusted hazard ratio, 0.32; 95% CI, 0.27–0.39; P < .001) composite end points.
Conclusions:
Intravascular US is frequently used during deep venous stent placement among Medicare beneficiaries, with further increase in use from 2017 to 2019. The utilization of intravascular US as part of a procedural strategy was associated with a lower cumulative incidence of adverse outcomes after the procedure, including venous stent thrombosis and embolization.
Venous thromboembolism (VTE) is common and associated with significant morbidity, as well as health care cost. A study (1) from the Ingenix Impact database involving matched VTE and non-VTE cohorts found that patients in the VTE cohort had significantly higher risks of thrombocytopenia, superficial venous thrombosis, venous ulcer, pulmonary hypertension, stasis dermatitis, and venous insufficiency in addition to higher all-cause health care costs. Another study (2) that aimed to estimate the annual payer burden of venous leg ulcers utilizing data from 2007 to 2011 found that the annual U.S. payer burden was approximately $14.9 billion. Deep venous intervention is used to relieve limb-related edema secondary to extrinsic compression or obstruction predisposing to or related to deep vein thrombosis (DVT) to reduce downstream morbidity and health care costs. One study (3) using Centers for Medicare & Medicaid Services (CMS) 5% research identifiable file data found that catheter-directed thrombectomy and thrombolysis for DVT increased by over 12% between 2007 and 2017. In recent years, there has also been growing utilization of deep venous stent placement to maintain long-term venous patency (4) by an array of providers and in different clinical settings (5). Complications following venous stent placement are not inconsequential and include the risks of migration, embolization, and acute or chronic thrombosis (6).
Intravascular ultrasound (US) is an invasive imaging modality that allows for the examination of the intraluminal vessel and surrounding structures to obtain detailed characteristics that cannot be visualized by standard angiography alone (7). These include, but are not limited to, grading severity of venous compression (8); detection of thrombus; optimization of stent sizing and location of deployment (9); and determining true vessel length and reference measurements. Identification of these characteristics can have important clinical implications. For example, a study (10) that included 106 patients who underwent stent placement for deep venous chronic obstruction found that total occlusion of multiple venous outflow segments was associated with a high rate of reocclusion. Although venous intravascular US utilization has increased in recent years, few studies have examined adoption according to clinical setting and provider specialty. In addition, few studies have evaluated the differences in outcomes following deep venous intervention when using intravascular US, in particular for infrequent but potentially serious events, such as stent embolization.
Leveraging national Medicare insurance claims data, this study aimed to examine nationwide frequency and variation in use as well as outcomes associated with the utilization of intravascular US during lower extremity venous stent placement. Uniquely, this analysis allows the exploration of intravascular US in different procedural settings, including both hospitals and ambulatory surgery centers/office-based laboratories (ASCs/OBLs), as well as among various provider specialties.
MATERIALS AND METHODS
Data Source, Study Cohort, and Exposure
This study was conducted in compliance with the data use agreement in place between the CMS and Beth Israel Deaconess Medical Center (BIDMC) and was approved by the BIDMC Institutional Review Board. The study cohort included all deep venous stent placement procedures performed between January 1, 2017, and December 31, 2019 among fee-for-service Medicare beneficiaries aged ≥66 years with at least 1 year of enrollment before their index procedure. Deep venous stent placement was identified via the Current Procedural Terminology (CPT) and International Classification of Diseases, Tenth Revision, Procedure Coding System claims codes from 100% samples of the Medicare Carrier Fee-for-Service files, Institutional Outpatient files, and MedPAR Inpatient files. The claims codes were also used to identify adjunctive thrombolysis or thrombectomy performed concomitantly with venous stent placement (Table E1, available online on the article’s Supplemental Material page at www.jvir.org.).
Baseline characteristics and comorbidities were ascertained using the CMS Chronic Conditions Warehouse common chronic conditions and other conditions files (11). Sociodemographic information was obtained at the time of the index stent placement using the Master Beneficiary Summary File. Race/ethnicity was classified based on self-reporting using categories specified by Medicare at the time of enrollment. The CPT and International Classification of Diseases, Tenth Revision, Procedure Coding System claims codes used to identify intravascular US exposure during deep venous stent placement are detailed in Table E1 (available online at www.jvir.org). Carrier file data were used to identify procedure location (inpatient, outpatient, or ambulatory surgery center/office-based laboratory [ASC/OBL]) and physician specialty (cardiologist, surgeon, interventional radiologist, or other).
Outcomes
The primary outcome for this analysis was a composite end point of 12-month all-cause mortality, all-cause hospitalization, or repeat target vessel intervention (Table E1, available online at www.jvir.org). In addition, a 30-day blanking period was implemented to account for staged-venous interventions that closely followed the index procedure. Repeat target vessel intervention cannot localize laterality of intervention and could, therefore, encompass treatment of the contralateral limb. The secondary outcome was a composite end point of 12-month hospitalization for venous stent embolization, thrombosis, or restenosis (Table E1, available online at www.jvir.org). Components of the composite end points were also reported. In sensitivity analyses, the rates of events 1, 3, and 6 months after the index procedure were reported.
Statistical Analysis
Categorical variables were reported as counts and percentages, and continuous variables were reported as means and standard deviations. Given the large sample size, standardized mean differences were reported, with a threshold of greater than 10% to define statistical significance (12).
The proportion of deep venous stent placement procedures that used intravascular US were plotted from quarter 1 of 2017 (2017Q1) to quarter 4 of 2019 (2019Q4) to visually display quarterly time trends. Utilization was stratified by practice setting and physician specialty. Each operator’s proportional use of intravascular US during deep venous intervention during the study period was assessed and depicted in a histogram to evaluate for heterogeneity in practice among operators.
The first procedure during the study period for each patient was included for the outcome analysis. Inverse probability weighting was used to correct for potential confounding owing to imbalances in baseline characteristics for patients treated with intravascular US versus venous angiography alone (12). Logistic regression models were used to estimate the propensity score for being treated with intravascular US during deep venous stent placement. All patient, procedural, and hospital characteristics were used in the model (Table 1). Each patient was then weighted based on their propensity score. For outcomes that included death, the traditional Kaplan-Meier methods and Cox regression models were used to estimate the hazard ratios (HRs) comparing intravascular US use during deep venous stent placement versus no intravascular US use. For nondeath outcomes, competing risk analyses were performed based on the Fine-Gray methods, with the cumulative incidence function used to estimate weighted cumulative incidences and Cox regression estimations of the subdistribution HRs (13). All statistical analyses were performed using SAS software (version 9.3; SAS Institute Inc., Cary, North Carolina). A 2-sided P value of <.05 was considered significant.
Table 1.
Preweighting and Postweighting Patient Characteristics
| Subject characteristic | Preweighting |
Postweighting |
||||
|---|---|---|---|---|---|---|
| Non–intravascular US (N = 5,800 subjects) | Intravascular US (N = 15,184 subjects) | Standardized mean difference (%) | Non–intravascular US | Intravascular US | Standardized mean difference (%) | |
| Demographics | ||||||
| Age (y) | ||||||
| Mean ± SD | 74.7 ± 6.6 | 75.4 ± 6.6 | −10.1 | 75.3 ± 6.7 | 75.2 ± 6.6 | 0.9 |
| Male | 45.6% | 41.6% | 8.0 | 42.9% | 43.0% | −0.1 |
| Race | ||||||
| White | 75.7% | 82.9% | −18.0 | 79.8% | 80.2% | −1.2 |
| Black | 15.6% | 10.6% | 14.9 | 13.0% | 12.6% | 1.0 |
| Other | 8.7% | 6.5% | 8.4 | 7.2% | 7.1% | 0.5 |
| Dual enrollment | 20.0% | 17.3% | 7.0 | 18.8% | 18.2% | 1.4 |
| Region | ||||||
| East North Central | 13.7% | 10.8% | 8.9 | 10.6% | 11.5% | −3.0 |
| East South Central | 5.2% | 8.0% | −11.3 | 6.7% | 7.1% | −1.6 |
| West North Central | 6.4% | 4.8% | 6.9 | 4.9% | 5.1% | −1.0 |
| West South Central | 15.3% | 17.9% | −7.1 | 17.7% | 17.5% | 0.6 |
| Middle Atlantic | 11.4% | 11.6% | −0.7 | 13.4% | 11.6% | 5.3 |
| Mountain | 6.8% | 4.6% | 9.4 | 5.5% | 5.3% | 0.9 |
| New England | 3.2% | 2.0% | 7.2 | 2.2% | 2.3% | −0.7 |
| Pacific | 13.4% | 8.0% | 17.6 | 10.2% | 9.7% | 1.8 |
| South Atlantic | 24.6% | 32.2% | −16.9 | 28.9% | 29.9% | −2.4 |
| Comorbidities | ||||||
| Acute myocardial infarction | 7.4% | 6.0% | 5.7 | 6.6% | 6.6% | 0.1 |
| Atrial fibrillation | 23.5% | 24.4% | −2.2 | 24.8% | 24.4% | 0.8 |
| Chronic kidney disease | 61.3% | 52.3% | 18.1 | 54.8% | 55.4% | −1.1 |
| Chronic obstructive pulmonary disease | 39.4% | 37.7% | 3.7 | 38.4% | 38.3% | 0.2 |
| Heart failure | 45.7% | 44.7% | 2.1 | 46.0% | 45.7% | 0.5 |
| Diabetes | 52.1% | 53.8% | −3.3 | 54.5% | 53.9% | 1.3 |
| Ischemic heart disease | 63.6% | 68.6% | −10.6 | 68.8% | 67.8% | 2.1 |
| Stroke/transient ischemic attack | 21.2% | 18.4% | 7.0 | 19.3% | 19.5% | −0.7 |
| Breast cancer | 7.1% | 6.3% | 3.1 | 6.7% | 6.5% | 0.6 |
| Colorectal cancer | 5.1% | 3.3% | 8.7 | 3.5% | 3.7% | −0.8 |
| Prostate cancer | 8.5% | 6.9% | 5.8 | 7.4% | 7.4% | 0.2 |
| Lung cancer | 8.0% | 1.9% | 28.2 | 3.6% | 3.7% | −0.6 |
| Endometrial cancer | 2.9% | 1.8% | 7.1 | 2.1% | 2.1% | 0.0 |
| Anemia | 75.9% | 70.0% | 13.2 | 70.1% | 71.9% | −3.9 |
| Hyperlipidemia | 83.8% | 88.5% | −13.5 | 87.2% | 87.3% | −0.4 |
| Hypertension | 89.6% | 92.0% | −8.2 | 91.7% | 91.5% | 1.0 |
| Tobacco use | 20.1% | 15.3% | 12.8 | 16.8% | 16.9% | −0.1 |
| Acute DVT | 30.9% | 11.0% | 50.5 | 18.7% | 16.0% | 7.2 |
| Chronic DVT | 7.1% | 4.5% | 11.3 | 4.3% | 6.5% | −10.0 |
DVT = deep vein thrombosis; US = ultrasound; SD = standard deviation.
RESULTS
Temporal Trends and Operator Preference for Intravascular US
There were 20,984 deep venous stent placement procedures performed during the study period, of which 15,184 (72.4%) utilized intravascular US. The intravascular US utilization rates by procedure location were the following: (a) 33.6% (1,394/4,149) of inpatient procedures, (b) 74.0% (5,198/7,022) of outpatient procedures, and (c) 87.6% (8,592/9,813) of ASC/OBL procedures. The intravascular US utilization rates by physician specialty were the following: (a) 89.0% (6,607/7,420) of procedures performed by cardiologists, (b) 76.6% (5,175/6,757) of procedures performed by surgeons, (c) 40.8% (2,003/4,905) of procedures performed by interventional radiologists, and (d) 73.6% (1,399/1,902) of procedures performed by other physician specialties. During the study period, the proportion of procedures utilizing intravascular US increased moderately for all procedure locations (Fig 1) and for all physician specialties (Fig 2). Growth over the study period was highest in the inpatient setting (26.0% in 2017Q1 to 39.3% in 2019Q4) and among interventional radiologists (32.3% in 2017Q1 to 54.4% in 2019Q4).
Figure 1.

Temporal trends in intravascular ultrasound (US) use during deep venous stent placement by procedure location. Displayed are rates of intravascular US use from quarter 1 2017 through quarter 4 2019 during lower extremity deep venous stent placement stratified by procedure location. ASC/OBL = ambulatory surgery center/office-based laboratory.
Figure 2.

Temporal trends in intravascular ultrasound (US) use during deep venous stent placement by physician specialty. Displayed are rates of intravascular US use from quarter 1 2017 through quarter 4 2019 during lower extremity deep venous stent placement stratified by physician specialty.
Individual operator proportional use of intravascular US during deep venous stent placement was high but with some variation. The overall median use was 77.3% of cases (interquartile range, 20.0%–99.2%) among all operators, with 33.1% (1,256/3,797) of operators using intravascular US in >90% of their procedures. When restricted to operators who used intravascular US in at least 1 of their procedures, the median use increased to 87.5% (interquartile range, 57.1%–100%), with 64.5% (1,256 of 1,946) of operators using intravascular US in >90% of their procedures (Figs 3, 4).
Figure 3.

Operator variation in intravascular ultrasound (US) use during deep venous stent placement among all operators. Displayed are the proportion of lower extremity deep venous stent placement procedures among individual operators in which intravascular ultrasound was used among all operators.
Figure 4.
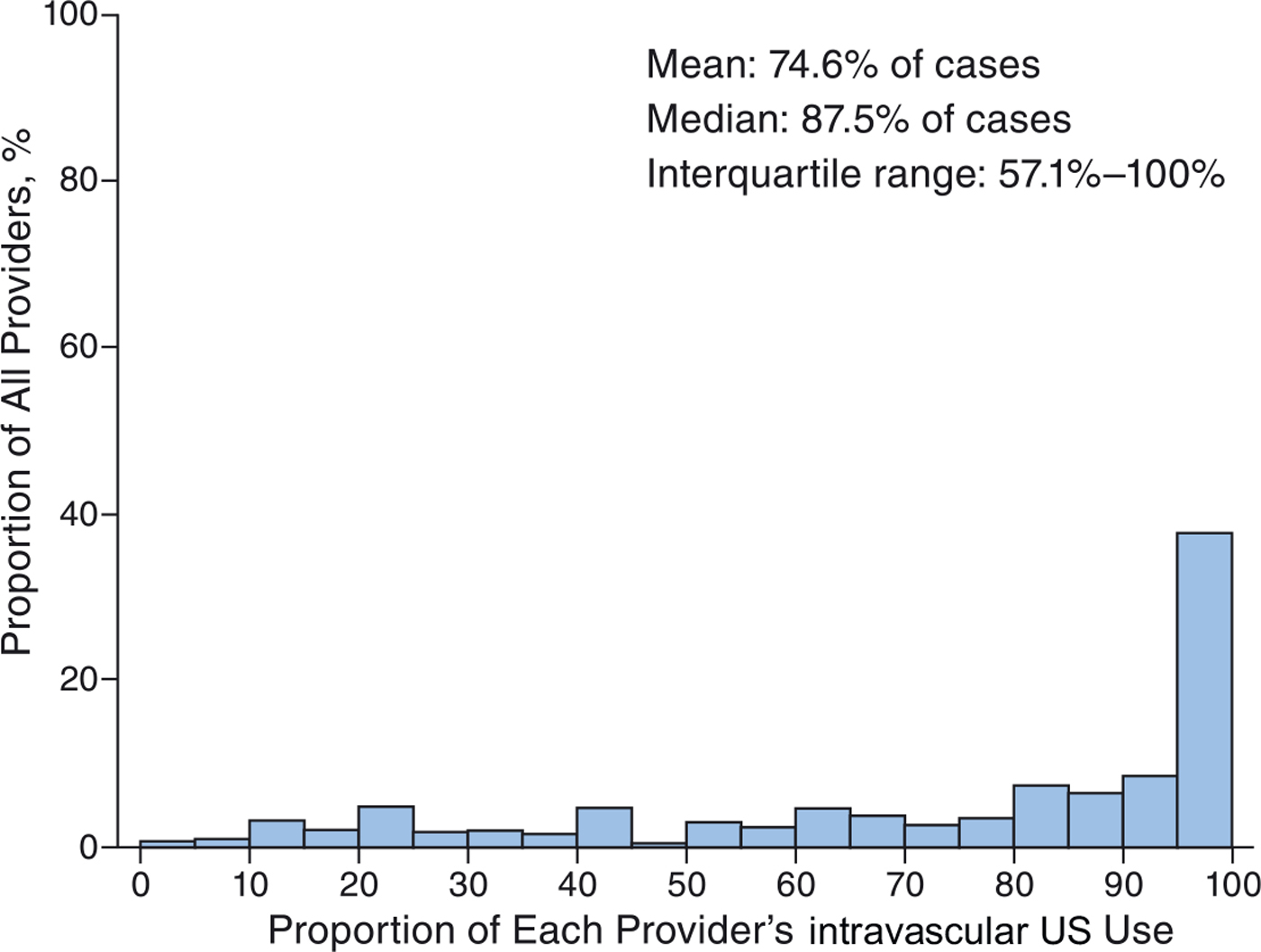
Operator variation in intravascular ultrasound (US) use during deep venous stent placement among all operators who used any intravascular US. Displayed are the proportion of lower extremity deep venous stent placement procedures among individual operators in which intravascular US was used among operators who used any intravascular US.
Patient and Procedural Characteristics by Intravascular US Use
Table 1 displays patient and procedural characteristics between those treated with and without intravascular US. Patients who underwent deep venous stent placement with intravascular US use were older (75.4 years ± 6.6 vs 74.7 years ±6.6), more often White, and residents of East South Central and South Atlantic regions. In addition, patients who underwent intervention with intravascular US had a greater frequency of ischemic heart disease, hyperlipidemia, rheumatoid arthritis, and hypothyroidism. Intravascular US was used less often in procedures involving acute and chronic DVT. During the index procedure, 6.7% of patients underwent adjunctive thrombolysis, and 10.2% underwent adjunctive thrombectomy in addition to stent placement.
Longitudinal Outcomes
Of patients who underwent deep venous stent placement, 34.0% in the intravascular US group and 49.0% in the non–intravascular US group experienced repeat intervention, hospitalization, or death. When intravascular US was included as part of the venous procedure, the unweighted HR of the composite primary outcome was reduced by 42% (95% confidence interval [CI], 0.55–0.61; P < .001) (Table 2). This was driven by a lower risk of death and hospitalization. Conversely, the risk of repeat intervention was marginally greater in the intravascular US group. The association with intravascular US utilization persisted in the weighted analysis, with a 28% reduction in the HR for the primary composite outcome (95% CI, 0.69–0.76; P < .001) (Fig 5). The results were similar in the sensitivity analyses of 1-, 3-, and 6-month outcome data (Table E2, available online at www.jvir.org).
Table 2.
Outcomes (Overall Cohort)
| Outcome | Preweighting |
Postweighting |
||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | Cumulative incidence |
P value | Hazard ratio (95% CI) | Cumulative incidence |
P value | |||
| Intravascular US | Non–intravascular US | Intravascular US | Non–intravascular US | |||||
| Primary outcome | ||||||||
| Composite of 12-month all-cause mortality, all-cause hospitalization, or target vessel repeat intervention | 0.58 (0.55–0.61) | 33.95% | 49.03% | <.001 | 0.72 (0.69–0.76) | 34.91% | 43.40% | <.001 |
| All-cause mortality | 0.21 (0.19–0.23) | 6.18% | 24.95% | <.001 | 0.33 (0.30–0.36) | 7.07% | 19.31% | <.001 |
| All-cause hospitalization | 0.59 (0.56–0.63) | 26.67% | 38.85% | <.001 | 0.75 (0.71–0.80) | 27.85% | 34.07% | <.001 |
| Target vessel repeat intervention* | 1.26 (1.12–1.43) | 9.00% | 7.41% | <.001 | 1.23 (1.09–1.39) | 8.70% | 7.25% | <.001 |
| Secondary outcome | ||||||||
| Composite of 12-month stent thrombosis, embolization, or restenosis | 0.22 (0.18–0.27) | 1.30% | 5.76% | <.001 | 0.32 (0.27–0.39) | 1.55% | 4.86% | <.001 |
| Stent thrombosis | 0.19 (0.15–0.24) | 0.77% | 4.01% | <.001 | 0.28 (0.22–0.36) | 0.90% | 3.22% | <.001 |
| Stent embolization | 0.09 (0.03–0.28) | 0.03% | 0.33% | <.001 | 0.15 (0.06–0.40) | 0.04% | 0.28% | <.001 |
| Stent restenosis | 0.25 (0.19–0.33) | 0.62% | 2.44% | <.001 | 0.33 (0.25–0.43) | 0.75% | 2.32% | <.001 |
CI = confidence interval; US = ultrasound.
Target vessel repeat intervention cannot differentiate between ipsilateral and contralateral interventions. This includes a 30-day blanking period to account for staged procedures.
Figure 5.

Cumulative incidence of the primary composite outcome after deep venous stent placement stratified by use of intravascular ultrasound (US). Displayed are weighted cumulative incidence curves of the primary composite end point (12-month all-cause mortality, all-cause hospitalization, or target vessel repeat intervention [including a 30-day blanking period to account for staged procedures]) stratified by intravascular US utilization during lower extremity deep venous stent placement. CI = confidence interval; HR = hazard ratio.
Stent-related events following venous intervention occurred with frequencies of 1.30% in the intravascular US group and 5.76% in the non–intravascular US group. The risks of individual events included stent thrombosis in 0.77% of the intravascular US group and 4.01% in the non–intravascular US group; stent embolization in 0.03% of the intravascular US group and 0.33% in the non–intravascular US group; and restenosis in 0.62% of the intravascular US group and 2.44% in the non–intravascular US group. When intravascular US was utilized during deep venous stent placement, there was an associated 78% lower unweighted HR of the composite secondary end point of stent thrombosis, embolization, or stenosis (95% CI, 0.18–0.27; P < .001) (Table 2). This association also persisted in the weighted analysis, with a 68% reduction in the HR for the secondary composite outcome (95% CI, 0.27–0.39; P < .001) (Fig 6). Intravascular US was also associated with lower hazards of each component of the composite outcome. The results were again similar in the sensitivity analyses of 1-, 3-, and 6-month outcome data (Table E2, available online at www.jvir.org).
Figure 6.

Cumulative incidence of the secondary composite outcome after deep venous stent placement stratified by use of intravascular ultrasound (US). Displayed are weighted cumulative incidence curves of the secondary composite end point (12-month stent thrombosis, embolization, or stenosis) stratified by intravascular US utilization during deep venous stent placement. CI = confidence interval; HR = hazard ratio.
DISCUSSION
In this large analysis of Medicare beneficiaries undergoing lower extremity deep venous stent placement, the utilization of intravascular US demonstrated moderate growth across procedure settings from 2017 through 2019, yet high utilization was present at the start of the study period. Growth in intravascular US use during the study period was consistent with recent data showing a nearly 12-fold increase in the use of catheter-directed therapies for lower extremity DVTs between 2007 and 2017 among Medicare beneficiaries (3). Among longitudinal outcomes, deep venous stent placement involving intravascular US was associated with reductions in the composite of death, hospitalization, or repeat intervention. This association was driven by lower risks of mortality and hospitalization, whereas the risk of repeat intervention was marginally higher in the intravascular US group. However, claims codes do not allow for the ascertainment of the laterality of target lesion revascularization, and this finding may reflect the greater use of intravascular US among patients with more complex venous diseases that require multiple interventions or intervention to the contralateral limb. Nonetheless, intravascular US use was associated with a decreased risk of stent-related events, including thrombosis and migration.
Intravascular US has become a powerful imaging tool during deep venous intervention owing to the limitations of venography alone and a greater push to clearly define severe lesions that may benefit from revascularization. Indeed, intravascular US is now considered the gold standard by many for the identification of venous obstruction in the iliac and femoral veins (14,15). Several studies have shown that venography alone underestimates percent stenosis (16), has poor sensitivity and a negative predictive value in the detection of significant venous area stenosis (17), and often misses the location of maximal stenosis when compared with venography plus intravascular US (18). The use of intravascular US during peripheral venous stent placement has also been supported by prospective data, such as from the Venogram Versus Intravascular Ultrasound for Diagnosing and Treating Iliofemoral Vein Obstruction (VIDIO) study (19). This study prospectively enrolled 100 patients with symptomatic iliofemoral venous outflow disease and demonstrated that intravascular US use substantially influenced treatment plans. Specifically, intravascular US improved diagnostic accuracy by detecting significant lesions missed on venography and exonerating other lesions that had appeared significant on venography (19).
The use of intravascular US during diagnostic and interventional procedures increases accuracy and, based on the current study, is associated with improved outcomes and reduced complications. Furthermore, intravascular US helps improve the appropriate use of intervention by identifying significant lesions that are ambiguous by angiography and reducing treatment of lesions that are proven insignificant. Additionally, several patients may have undiagnosed and/or underrecognized anatomic obstruction that may be causal (eg, May-Thurner anatomy) (20). Many of these obstructions are not identified by angiography alone because of the often eccentric and web-like nature of associated stenoses. Angiography plus intravascular US is more accurate than angiography alone in the diagnosis of this condition. Furthermore, stent-related complications can result in significant consequences, including migration/embolization that can result in damage to cardiopulmonary structures and the need for endovascular or surgical retrieval (6,21,22). A more accurate assessment of lesions and appropriate sizing of stent implants with the use of intravascular US may be able to reduce the incidence of these complications.
Although intravascular US utilization was overall high in this study, there remained some variability by procedural location and physician specialty. For instance, intravascular US utilization was greatest among procedures performed in the ASC/OBL setting, which, in part, is likely driven by strong outpatient reimbursement. Furthermore, the frequency of intravascular US use was the greatest among procedures performed by cardiologists, which may be a result of greater familiarity with this technology owing to its prevalent use during coronary intervention.
The current study leverages national claims data to evaluate the outcomes associated with intravascular US utilization during deep venous stent placement. The increased risk of repeat intervention in the intravascular US group may not be a true association because the data cannot differentiate between ipsilateral and contralateral interventions. It is also possible that intravascular US may have been used more frequently in patients with complex, and potentially bilateral, lower extremity venous disease requiring multiple attempts at revascularization. Although lacking anatomic characteristics often found in registry and prospective studies, the data from this study allow for the examination of rarer events that may only be detected in case reports and volunteer adverse event databases. This study found an important rate of complications following venous stent placement, with a notable risk of hospitalization for stent embolization. Importantly, intravascular US use during intervention was associated with meaningful reductions in the secondary composite end points. Although causality cannot be proven, these data suggest that intravascular US-guided stent placement has a meaningful role in improving outcomes after deep venous intervention and intravascular US should be considered a central tool during venous revascularization.
Study Limitations
This study has important limitations to consider. First, the CMS data do not include indication for procedure or detailed anatomic or procedural details, including laterality for repeat intervention, or the indication for revascularization (ie, thrombotic disease vs nonthrombotic iliac vein lesions). Accordingly, direct causality relating outcomes with intravascular US is implied but cannot be proven. Second, CPT codes do not identify the specific site of venous location of stent implantation. Nonetheless, most commercially available venous stents are primarily designed for use in the iliofemoral veins. Third, claims codes do not allow for the identification of the timing of intravascular US utilization during the procedure (ie, before or after stent placement). Fourth, well-recognized classification systems, such as the Clinical-Etiology-Anatomy-Pathophysiology classification system (23), and validated venous outcome measures, such as the Villalta scale for postthrombotic syndrome (24) and Venous Clinical Severity Scoring (25), are not available in the CMS dataset. Lastly, unmeasured and residual confounding remain possible because the study was observational in design.
This study examined contemporary nationwide data and found that intravascular US was frequently used during lower extremity deep venous stent placement among Medicare beneficiaries. Intravascular US utilization increased from 2017 to 2019 in all clinical settings and across operator specialties. The utilization of intravascular US during venous stent placement was associated with a lower cumulative incidence of adverse outcomes after the procedure, including venous stent thrombosis and embolization. Current and future efforts should focus on continuing the adoption and standardization of intravascular US as part of lower extremity deep venous intervention.
RESEARCH HIGHLIGHTS.
Among Medicare beneficiaries, there were 20,984 deep venous interventions performed between 2017 and 2019 of which 15,184 (72.4%) utilized intravascular US.
There was variation in use of intravascular US among all operators (median 77.3% of cases, interquartile range (IQR) 20.0%–99.2%).
In weighted analyses, intravascular US use during deep venous stent placement was associated with a lower risk of 12-month reintervention, hospitalization, or death, as well as stent thrombosis, embolization, or restenosis.
STUDY DETAILS.
Study type: Retrospective, observational, cohort study
Level of evidence: 3 (SIR-C)
ACKNOWLEDGMENTS
This work was funded by the Smith Center for Outcomes Research in Cardiology, including a research grant from Philips to the Smith Center.
S.D. is supported by a joint KL2/Catalyst Medical Research Investigator Training (CMeRIT) award from Harvard Catalyst and the Boston Claude D. Pepper Older Americans Independence Center (5P30AG031679-10). M.H.M. is Consultant for Cook Medical for a project using a Delphi consensus panel for venous stenting. M.P.K. is board member at VIVA Physicians, Deputy Editor for the Journal of Vascular and Interventional Radiology, and Global PI of the ELEGANCE registry with Boston Scientific but is not being personally compensated. B.M.H. reports institutional research grants from Behring, Hemostemix, NIH/NHLBI, and Boston Scientific. K.R. is consultant and on scientific advisory board of Angiodynamics, Boston Scientific, Contego, InspireMD, Magneto, Mayo Clinic, Neptune Medical, Philips, Summa Therapeutics, Surmodics, Thrombolex, and Truvic and board member of the National PERT Consortium, a not for profit 501c3 organization dedicated to advancing treatment and improving outcomes in pulmonary embolism and reports grants from the NIH and Boston Scientific and equity from Accolade, Access Vascular, Althea Medical, Contego, Cruzar Systems, Embolitech, Endospan, JanaCare, Magneto, Orchestra, PQ Bypass, Shockwave, Thrombolex, Truvic, and Valcare. S.A.P. is on Advisory Board of Abbott, Boston Scientific, CSI, Janssen, Medtronic, and Philips and reports research grants from Abbott, Boston Scientific (DSMB), Shockwave, Surmodics, TriReme, and Veryan Medical and consulting for Abiomed, Inari, Penumbra, and Terumo. E.A.S. reports research grants from BIDMC—NIH/NHLBI K23HL150290, Food and Drug Administration, Harvard Medical School’s Shore Faculty Development Award, AstraZeneca, BD, Boston Scientific, Cook, CSI, Laminate Medical, Medtronic and Philips—and consulting for Abbott, Bayer, BD, Boston Scientific, Cook, CSI, Endovascular Engineering, Inari, Janssen, Medtronic, Philips, and VentureMed. None of the authors have identified a conflict of interest.
From the 2021 Transcatheter Cardiovascular Therapeutics Meeting (Abstract No. TCT-386, “Utilization of intravascular ultrasound during peripheral venous intervention among medicare beneficiaries”).
ABBREVIATIONS
- ASC/OBL
ambulatory surgery center/office-based laboratory
- CI
confidence interval
- CMS
Centers for Medicare & Medicaid Services
- CPT
Current Procedural Terminology
- DVT
deep vein thrombosis
- HR
hazard ratio
- US
ultrasound
- VTE
venous thromboembolism
Table E1.
Claims Codes Used to Identify Diagnoses, Lower Extremity Deep Venous Stent Placement, Intravascular Ultrasound Exposure during Lower Extremity Deep Venous Stent Placement, and Outcomes
| Diagnosis | CPT | HCPCS | ICD10-PCS | ICD10-CM |
|---|---|---|---|---|
| May-Thurner syndrome | I87.1 | |||
| Acute deep vein thrombosis | I82.220, I82.4, I82.40, I82.401, I82.402, I82.403, I82.409, I82.41, I82.411, I82.412, I82.413, I82.419, I82.42, I82.421, I82.422, I82.423, I82.429, I82.43, I82.431, I82.432, I82.433, I82.439, I82.44, I82.441, I82.442, I82.443, I82.449, I82.45, I82.451, I82.452, I82.453, I82.459, I82.46, I82.461, I82.462, I82.463, I82.469, I82.49, I82.491, I82.492, I82.493, I82.499, I82.4Y, I82.4Y1, I82.4Y2, I82.4Y3, I82.4Y9, I82.4Z, I82.4Z1, I82.4Z2, I82.4Z3, I82.4Z9 | |||
| Chronic deep vein thrombosis | I82.221, I82.5, I82.50, I82.501, I82.502, I82.503, I82.509, I82.51, I82.511, I82.512, I82.513, I82.519, I82.52, I82.521, I82.522, I82.523, I82.529, I82.53, I82.531, I82.532, I82.533, I82.539, I82.54, I82.541, I82.542, I82.543, I82.549, I82.55, I82.551, I82.552, I82.553, I82.559, I82.56, I82.561, I82.562, I82.563, I82.569, I82.59, I82.591, I82.592, I82.593, I82.599, I82.5Y, I82.5Y1, I82.5Y2, I82.5Y3, I82.5Y9, I82.5Z, I82.5Z1, I82.5Z2, I82.5Z3, I82.5Z9 | |||
| Lower extremity venous angioplasty and stent placement | 37238, 37239 | |||
| Lower extremity venous thrombolysis | 37212, 37213, 37214 | |||
| Lower extremity venous thrombectomy | 37187, 37188 | |||
| Lower extremity venous intravascular ultrasound | 37252, 37253 | C1753 | B549ZZ3, B54BZZ3, B54CZZ3, B54DZZ3 | |
| Target vessel repeat procedure* | 37238, 37239, 37212, 37213, 37214, 37187, 37188 | |||
| Lower extremity venous thrombosis | T82.86 | |||
| Lower extremity venous embolism | T82.81 | |||
| Lower extremity venous stenosis | T82.856, T82.857, T82.858 |
CM = Clinical Modification; CPT = Current Procedural Terminology; HCPCS = Health care Common Procedure Coding System; ICD = International Classification of Disease; PCS = Procedure Coding System.
Target vessel repeat intervention cannot differentiate between ipsilateral and contralateral interventions.
Table E2.
Outcomes at Different Time Points after the Index Procedure
| Outcome | Preweighting |
Postweighting |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | Cumulative incidence |
P value | Hazard ratio (95% CI) | Cumulative incidence |
P value | ||||
| Primary outcome | Time point | Intravascular US | Non–intravascular US | Intravascular US | Non–intravascular US | ||||
| Composite of all-cause mortality, all-cause hospitalization, or target vessel repeat intervention (intravascular US vs non–intravascular US) | 1 mo | 0.29 (0.26–0.32) | 3.94% | 12.99% | <.001 | 0.43 (0.39–0.49) | 4.58% | 10.19% | <.001 |
| 3 mo | 0.47 (0.44–0.50) | 12.96% | 25.07% | <.001 | 0.61 (0.57–0.66) | 13.67% | 20.91% | <.001 | |
| 6 mo | 0.52 (0.49–0.55) | 20.65% | 34.93% | <.001 | 0.67 (0.63–0.71) | 21.67% | 29.98% | <.001 | |
| 12 mo | 0.58 (0.55–0.61) | 33.95% | 49.03% | <.001 | 0.72 (0.69–0.76) | 34.91% | 43.40% | <.001 | |
| All-cause mortality | 1 mo | 0.09 (0.07–0.12) | 0.57% | 5.95% | <.001 | 0.16 (0.13–0.20) | 0.70% | 4.28% | <.001 |
| 3 mo | 0.14 (0.12–0.16) | 1.80% | 11.90% | <.001 | 0.24 (0.21–0.28) | 2.17% | 8.60% | <.001 | |
| 6 mo | 0.18 (0.16–0.20) | 3.54% | 17.82% | <.001 | 0.30 (0.27–0.34) | 4.24% | 13.04% | <.001 | |
| 12 mo | 0.21 (0.19–0.23) | 6.18% | 24.95% | <.001 | 0.33 (0.30–0.36) | 7.07% | 19.31% | <.001 | |
| All-cause hospitalization | 1 mo | 0.40 (0.36–0.45) | 3.67% | 8.91% | <.001 | 0.58 (0.51–0.66) | 4.22% | 7.15% | <.001 |
| 3 mo | 0.45 (0.41–0.49) | 8.36% | 17.58% | <.001 | 0.62 (0.57–0.68) | 9.35% | 14.54% | <.001 | |
| 6 mo | 0.51 (0.48–0.55) | 14.55% | 25.92% | <.001 | 0.68 (0.63–0.73) | 15.81% | 21.98% | <.001 | |
| 12 mo | 0.59 (0.56–0.63) | 26.67% | 38.85% | <.001 | 0.75 (0.71–0.80) | 27.85% | 34.07% | <.001 | |
| Target vessel repeat intervention* | 1 mo | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 3 mo | 1.54 (1.29–1.83) | 4.60% | 3.04% | <.001 | 1.38 (1.16–1.64) | 4.30% | 3.15% | <.001 | |
| 6 mo | 1.37 (1.19–1.58) | 6.60% | 4.92% | <.001 | 1.28 (1.11–1.47) | 6.40% | 5.11% | <.001 | |
| 12 mo | 1.26 (1.12–1.43) | 9.00% | 7.41% | <.001 | 1.23 (1.09–1.39) | 8.70% | 7.25% | <.001 | |
| Secondary outcome | |||||||||
| Composite of stent thrombosis, embolization, or restenosis (intravascular US vs non–intravascular US) | 1 mo | 0.23 (0.16–0.33) | 0.34% | 1.46% | <.001 | 0.35 (0.24–0.49) | 0.40% | 1.14% | <.001 |
| 3 mo | 0.23 (0.18–0.30) | 0.67% | 2.82% | <.001 | 0.35 (0.27–0.45) | 0.79% | 2.26% | <.001 | |
| 6 mo | 0.23 (0.18–0.28) | 0.91% | 3.92% | <.001 | 0.36 (0.29–0.45) | 1.14% | 3.06% | <.001 | |
| 12 mo | 0.22 (0.18–0.27) | 1.30% | 5.76% | <.001 | 0.32 (0.27–0.39) | 1.55% | 4.86% | <.001 | |
| Stent thrombosis | 1 mo | 0.19 (0.12–0.30) | 0.20% | 1.04% | <.001 | 0.29 (0.19–0.46) | 0.23% | 0.78% | <.001 |
| 3 mo | 0.20 (0.15–0.27) | 0.42% | 2.05% | <.001 | 0.29 (0.21–0.39) | 0.48% | 1.68% | <.001 | |
| 6 mo | 0.20 (0.15–0.26) | 0.55% | 2.75% | <.001 | 0.31 (0.23–0.40) | 0.68% | 2.19% | <.001 | |
| 12 mo | 0.19 (0.15–0.24) | 0.77% | 4.01% | <.001 | 0.28 (0.22–0.36) | 0.90% | 3.22% | <.001 | |
| Stent embolization | 1 mo | 0.19 (0.04–1.04) | 0.01% | 0.07% | .056 | 0.17 (0.03–0.95) | 0.01% | 0.07% | .044 |
| 3 mo | 0.31 (0.08–1.15) | 0.03% | 0.09% | .079 | 0.44 (0.13–1.48) | 0.04% | 0.09% | .187 | |
| 6 mo | 0.11 (0.04–0.34) | 0.03% | 0.26% | <.001 | 0.18 (0.07–0.48) | 0.04% | 0.23% | <.001 | |
| 12 mo | 0.09 (0.03–0.28) | 0.03% | 0.33% | <.001 | 0.15 (0.06–0.40) | 0.04% | 0.28% | <.001 | |
| Stent restenosis | 1 mo | 0.28 (0.17–0.49) | 0.15% | 0.54% | <.001 | 0.45 (0.26–0.76) | 0.20% | 0.45% | 0.003 |
| 3 mo | 0.26 (0.17–0.38) | 0.28% | 1.08% | <.001 | 0.41 (0.28–0.60) | 0.36% | 0.89% | <.001 | |
| 6 mo | 0.26 (0.19–0.36) | 0.41% | 1.55% | <.001 | 0.40 (0.29–0.56) | 0.52% | 1.28% | <.001 | |
| 12 mo | 0.25 (0.19–0.33) | 0.62% | 2.44% | <.001 | 0.33 (0.25–0.43) | 0.75% | 2.32% | <.001 | |
CI = confidence interval; N/A = not available; US = ultrasound.
Target vessel repeat intervention cannot differentiate between ipsilateral and contralateral interventions. This includes a 30-day blanking period to account for staged procedures.
Contributor Information
Sanjay Divakaran, Division of Cardiovascular Medicine and Cardiovascular Imaging Program, Brigham and Women’s Hospital, Boston, Massachusetts; Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology, Beth Israel Deaconess Medical Center, Boston, Massachusetts; Harvard Medical School, Boston, Massachusetts.
Mark H. Meissner, Department of Surgery, University of Washington School of Medicine, Seattle, Washington.
Maureen P. Kohi, Department of Radiology, University of North Carolina School of Medicine, Chapel Hill, North Carolina.
Siyan Chen, Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology, Beth Israel Deaconess Medical Center, Boston, Massachusetts.
Yang Song, Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology, Beth Israel Deaconess Medical Center, Boston, Massachusetts.
Beau M. Hawkins, Section of Cardiovascular Diseases, University of Oklahoma, Oklahoma City, Oklahoma.
Kenneth Rosenfield, Division of Cardiology, Massachusetts General Hospital, Boston, Massachusetts.
Sahil A. Parikh, Division of Cardiovascular Medicine, Columbia University Medical Center, New York, New York.
Eric A. Secemsky, Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology, Beth Israel Deaconess Medical Center, Boston, Massachusetts; Division of Cardiovascular Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts; Harvard Medical School, Boston, Massachusetts.
REFERENCES
- 1.Lefebvre P, Laliberté F, Nutescu EA, et al. All-cause and potentially disease-related health care costs associated with venous thromboembolism in commercial, Medicare, and Medicaid beneficiaries. J Manag Care Pharm 2012; 18:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons N. Burden of venous leg ulcers in the United States. J Med Econ 2014; 17:347–356. [DOI] [PubMed] [Google Scholar]
- 3.Von Ende E, Gayou EL, Chick JFB, Makary MS. Nationwide trends in catheter-directed therapy utilization for the treatment of lower extremity deep vein thrombosis in Medicare beneficiaries. J Vasc Interv Radiol 2021; 32:1576–1582.e1. [DOI] [PubMed] [Google Scholar]
- 4.Razavi MK, Jaff MR, Miller LE. Safety and effectiveness of stent placement for iliofemoral venous outflow obstruction: systematic review and meta-analysis. Circ Cardiovasc Interv 2015; 8:e002772. [DOI] [PubMed] [Google Scholar]
- 5.Williams ZF, Dillavou ED. A systematic review of venous stents for iliac and venacaval occlusive disease. J Vasc Surg Venous Lymphat Disord 2020; 8:145–153. [DOI] [PubMed] [Google Scholar]
- 6.Sayed MH, Salem M, Desai KR, O’Sullivan GJ, Black SA. A review of the incidence, outcome, and management of venous stent migration. J Vasc Surg Venous Lymphat Disord 2022; 10:482–490. [DOI] [PubMed] [Google Scholar]
- 7.Tardif JC, Pandian NG. Intravascular ultrasound imaging in peripheral arterial and coronary artery disease. Curr Opin Cardiol 1994; 9:627–633. [DOI] [PubMed] [Google Scholar]
- 8.Forauer AR, Gemmete JJ, Dasika NL, Cho KJ, Williams DM. Intravascular ultrasound in the diagnosis and treatment of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol 2002; 13:523–527. [DOI] [PubMed] [Google Scholar]
- 9.Raju S, Buck WJ, Crim W, Jayaraj A. Optimal sizing of iliac vein stents. Phlebology 2018; 33:451–457. [DOI] [PubMed] [Google Scholar]
- 10.Marston WA, Browder SE, Iles K, Griffith A, McGinigle KL. Early thrombosis after iliac stenting for venous outflow occlusion is related to disease severity and type of anticoagulation. J Vasc Surg Venous Lymphat Disord 2021; 9:1399–1407.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chronic Conditions Data Warehouse. CCW Medicare administrative data user guide. 2019, June. Available at: https://www2.ccwdata.org/documents/10280/19002246/ccw-medicare-data-user-guide.pdf. Accessed March 19, 2021.
- 12.Secemsky EA, Shen C, Schermerhorn M, Yeh RW. Longitudinal assessment of safety of femoropopliteal endovascular treatment with paclitaxel-coated devices among Medicare beneficiaries: the SAFE-PAD study. JAMA Intern Med 2021; 181:1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 14.Lau I, Png CYM, Eswarappa M, et al. Defining the utility of anteroposterior venography in the diagnosis of venous iliofemoral obstruction. J Vasc Surg Venous Lymphat Disord 2019; 7:514–521.e4. [DOI] [PubMed] [Google Scholar]
- 15.Radaideh Q, Patel NM, Shammas NW. Iliac vein compression: epidemiology, diagnosis and treatment. Vasc Health Risk Manag 2019; 15:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shammas NW, Shammas GA, Jones-Miller S, et al. Predicting iliac vein compression with computed tomography angiography and venography: correlation with intravascular ultrasound. J Invasive Cardiol 2018; 30:452–455. [PubMed] [Google Scholar]
- 17.Neglén P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg 2002; 35:694–700. [DOI] [PubMed] [Google Scholar]
- 18.Montminy ML, Thomasson JD, Tanaka GJ, Lamanilao LM, Crim W, Raju S. A comparison between intravascular ultrasound and venography in identifying key parameters essential for iliac vein stenting. J Vasc Surg Venous Lymphat Disord 2019; 7:801–807. [DOI] [PubMed] [Google Scholar]
- 19.Gagne PJ, Gasparis A, Black S, et al. Analysis of threshold stenosis by multiplanar venogram and intravascular ultrasound examination for predicting clinical improvement after iliofemoral vein stenting in the VIDIO trial. J Vasc Surg Venous Lymphat Disord 2018; 6:48–56.e1. [DOI] [PubMed] [Google Scholar]
- 20.Harbin MM, Lutsey PL. May-Thurner syndrome: history of understanding and need for defining population prevalence. J Thromb Haemost 2020; 18:534–542. [DOI] [PubMed] [Google Scholar]
- 21.Sharma AK, Ganatra S, Hansen J, et al. A dual-snare percutaneous retrieval of venous stent embolization to the right heart. JACC Cardiovasc Interv 2017; 10:e111–e113. [DOI] [PubMed] [Google Scholar]
- 22.Ogami T, Zimmermann E, Zhu R, Worku B, Avgerinos DV. Embolization of an iliac vein stent to the right atrium. J Card Surg 2018; 33:855–856. [DOI] [PubMed] [Google Scholar]
- 23.Lurie F, Passman M, Meisner M, et al. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord 2020; 8:342–352. [DOI] [PubMed] [Google Scholar]
- 24.Kahn SR. Measurement properties of the Villalta scale to define and classify the severity of the post-thrombotic syndrome. J Thromb Haemost 2009; 7:884–888. [DOI] [PubMed] [Google Scholar]
- 25.Passman MA, McLafferty RB, Lentz MF, et al. Validation of Venous Clinical Severity Score (VCSS) with other venous severity assessment tools from the American Venous Forum, National Venous Screening Program. J Vasc Surg 2011; 54:2S–9S. [DOI] [PubMed] [Google Scholar]


