Abstract
Purpose
Timely and moderate luteinizing hormone (LH) secretion plays critical roles in follicle development and maturation. However, the role of LH supplementation in in vitro fertilization/intracytoplasmic sperm injection and embryo transfer (IVF/ICSI-ET) cycles remains unclear. Can LH supplementation improve the clinical outcomes of patients who receive long-acting gonadotropin-releasing hormone agonist (GnRHa) pituitary downregulation in IVF/ICSI-ET cycles?
Patients and Methods
This is a retrospective cohort study of 2600 long-acting GnRHa down-regulated IVF/ICSI cycles from 2017 to 2020 in our reproductive medicine centre of Nanjing Drum Tower Hospital. Total cycles were divided into two groups according to LH supplementation or not. In addition, we conducted a secondary analysis that used propensity-score matching to reduce the effects of confounding.
Results
Exogenous LH addition was not significantly correlated with the clinical pregnancy rate (OR=0.910, 95% CI: 0.623–1.311, p=0.61) in logistic regression analysis. After propensity-score matching, we also found no significant association between LH supplementation and the clinical pregnancy rate (OR=0.792, 95% CI: 0.527–1.191, p=0.26).
Conclusion
There is no obvious effect of exogenous LH supplementation on the clinical pregnancy rate in non-selective patients receiving long-acting GnRHa IVF/ICSI-ET cycles, which suggests that exogenous LH addition is not always needed, which can help us avoid drug overuse to a certain extent.
Keywords: LH supplementation, long-acting GnRHa, clinical pregnancy rate, logistic regression analysis, propensity-score matching
Introduction
Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) play important roles in folliculogenesis at different stages of follicle development.1 FSH is essential for the recruitment and development of follicles as well as the induction of many enzymes and hormones, which are subsequently controlled by LH and are necessary for the continuous maturation of follicles.2 LH plays a variety of roles in follicle development, including inducing ovulation, completing meiosis I, early luteinization and progesterone synthesis.3 In the process of follicular development, LH plays a synergistic role with FSH in follicles of 9–10 mm in size. LH binding to its receptors promotes theca cells to synthesize androgen.4 In the mid-follicular phase, an increase in LH levels stimulates oestrogen secretion by granulosa cells, which can induce an increase in LH, leading to final follicular maturation.5 However, premature LH elevation may result in earlier luteinization and follicular atresia.6 Therefore, timely and moderate LH secretion plays critical roles in follicle development and maturation.
However, the role of LH supplementation in assisted reproductive technology (ART) cycles remains unclear. The importance of LH supplementation in the controlled ovarian hyperstimulation (COH) process has been masked by conflicting data. For hypogonadotropic hypogonadism patients with low endogenous LH levels, poor pregnancy outcomes have been detected with FSH stimulation alone.7 Therefore, FSH and LH supplementation seems to be essential for follicular development, embryo implantation, and persistent pregnancy.8 In addition, studies have shown that increased LH concentration is closely related to abnormal fertilization, embryo implantation failure and adverse pregnancy outcomes.9
Most of the clinical studies on LH supplementation have focused on gonadotropin-releasing hormone (GnRH) antagonist cycles, and there is no consensus. In addition, there is a lack of research on LH supplementation in GnRH agonist (GnRHa) cycles. Studies have shown that slowed follicular development, poor-quality follicles, abnormal fertilization rates, reduced clinical pregnancy rates and increased spontaneous abortion rates may be detected in GnRHa cycles with FSH alone. Exogenous LH supplementation can improve follicle and embryo quality, which leads to better pregnancy outcomes.10,11 Prospective studies also suggest that with exogenous LH supplementation, the dosage and days of gonadotropin use can be decreased and the number of normal fertilized embryos increases.12 Similarly, other studies have indicated that LH addition in GnRHa cycles does not benefit all patients and may only have a positive effect on elderly patients or patients with a low ovarian response.3,13
In this study, we attempted to clarify the roles of exogenous LH supplementation in clinical outcomes in COH cycles pre-treated with long-acting GnRHa.
Materials and Methods
Basic Information of Study Population
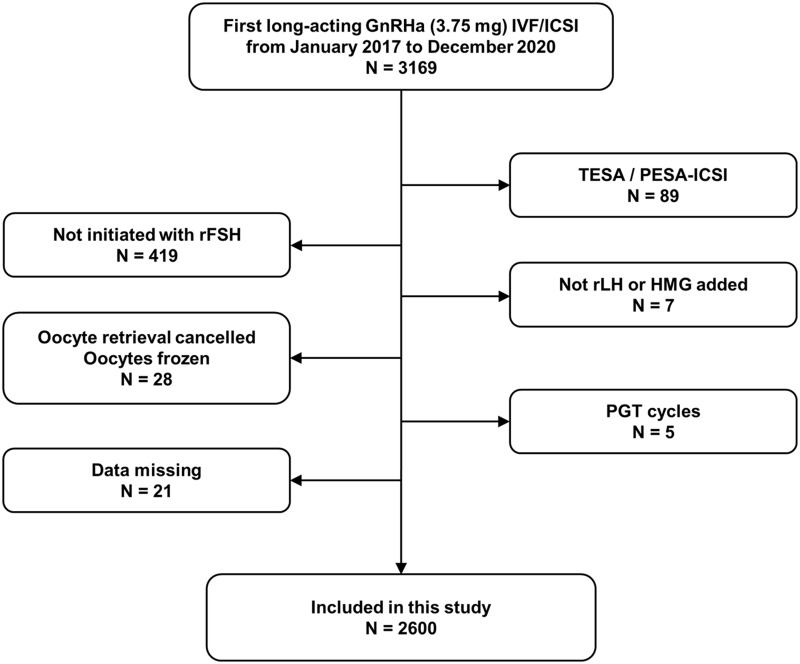
This retrospective analysis was conducted on women who were undergoing the first long-acting GnRHa in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) cycles at the Reproductive Medicine Centre of Nanjing Drum Tower Hospital from January 2017 to December 2020. Each couple was informed of the possibility of using their data during IVF/ICSI therapy and signed an informed consent form. In addition, we obtained approval from the ethics committee of Drum Tower Hospital affiliated with Nanjing University Medical School. All the patients received GnRHa (Decapeptyl, triptorelin acetate, 3.75 mg, Ferring GmbH, Germany) in the early follicular phase, followed by recombinant FSH (rFSH) alone or combined with recombinant LH (rLH, Merck Sereno, Switzerland)/human menopausal gonadotropin (HMG, Livzon Pharm, China). The exclusion criteria were as follows: (1) intracytoplasmic sperm injection (ICSI) cycles in which sperm collected from testicular sperm aspiration (TESA) or percutaneous epididymal sperm aspiration (PESA) was used; (2) cycles initiated with special type of FSH; (3) cycles in which other kinds of exogenous LH were added; (4) cycles in which oocyte retrieval was cancelled or oocytes were frozen; and (5) preimplantation genetic testing (PGT) cycles. There were 2600 cycles included in our study (Figure 1).
Figure 1.
A flow chart of the inclusion and exclusion of patients.
Controlled Ovarian Stimulation Protocol
Long-acting GnRHa (Decapeptyl, triptorelin acetate, 3.75 mg, Ferring GmbH, Germany) was given in the early follicular or mid-luteal phase. Oestradiol (E2), FSH, LH and progesterone (P) were measured 35–40 days later. The follicle diameter was monitored by vaginal ultrasound. The standard of downregulation included FSH < 5 mIU/mL, LH < 5 mIU/mL, E2 < 30 pg/mL and follicle diameter 4.5–5 mm.14,15 After meeting the above criteria, 75–300 IU rFSH (Gonal-F, Merck Sereno, Switzerland) with or without 75–150 IU rLH (Luveris, Merck Sereno, Switzerland) or 75–300 IU HMG (Menotropins for Injection, Livzon Pharm, China) was injected daily. The starting dose of gonadotropin (Gn) depended on the patients’ age, body mass index (BMI) and anti-Mullerian hormone (AMH) level. The dosage was adjusted according to the follicle size and serum hormone levels (FSH, LH, E2 and P). A total of 10,000 IU of human chorionic gonadotropin (hCG) (Chorionic Gonadotropin for Injection, Livzon Pharm, China) was injected when the diameter of 1–2 dominant follicles reached 18 mm to trigger oocyte maturation. When the number of follicles on the trigger day was more than 15 and the E2 level was more than 5000 pg/mL, 250 μg recombinant hCG (rhCG, Merck Sereno, Switzerland) or 5000 IU hCG was used to avoid ovarian hyperstimulation syndrome (OHSS). The oocytes were collected 36–38 hours after triggering. Mature oocytes (metaphase II, MII) were cultured to cleavage stage embryos or blastocysts after fertilization.
Embryo Transfer and Pregnancy Detection
Except for the patients who gave up transfer for various reasons (prevention of OHSS, abnormal progesterone increase, intrauterine effusion, occupation disease in uterine cavity, no transplantable embryo, etc.), fresh embryos were transferred under the guidance of abdominal ultrasound on the 3rd or 5th day after retrieving oocytes. After pregnancy, luteal support was continued until two months after embryo transfer. Biochemical pregnancy was defined as a positive serum β-hCG concentration (more than 200 mIU/mL) measured 12–14 days after embryo transfer. Ultrasound examination was performed 30 days after embryo transfer. Clinical pregnancy was defined as the presence of a gestational sac. The embryo implantation rate was defined as the ratio of the gestational sac number to the number of transferred embryos.
Patient and Public Involvement
The Ethics Committee of Drum Tower Hospital affiliated to Nanjing University Medical School agreed to waive the informed consent step of patients because this is a retrospective study which does not involve patient privacy and does not affect the clinical treatment. Our study strictly complies with the Declaration of Helsinki and keeps patient data confidential.
Statistical Analysis
The patients were divided into groups A and B according to whether they were supplemented with exogenous LH. In fresh embryo transfer (fET) cycles, a relationship between LH supplementation and the clinical pregnancy rate was observed. An initial multivariable logistic-regression model included baseline factors and clinical factors (identified by the univariate analysis) was conducted. In addition, to help account for the nonrandomized treatment administration of LH, we used propensity-score methods to reduce the effects of confounding. Matching was performed with the use of a 1:2 matching protocol without replacement, with a caliper width equal to 0.01 of the standard deviation of the logit of the propensity score. The individual propensities for receipt of LH treatment were estimated with the use of a multivariable logistic-regression model that included the similar covariates (identified by the univariate analysis) as the previous regression model. All analyses were performed with R (http://www.R-project.org) and EmpowerStats software (www.empowerstats.com, X&Y solutions, Inc. Boston MA). P < 0.05 was considered statistically significant.
Results
Characteristics of the Patients, COH and Embryos
In all, 2600 cycles were involved in this study. In 409 cycles (group A), the women did not receive LH supplementation, while in 2191 cycles (group B), the women received exogenous LH. The patients in these two groups were similar in baseline FSH levels. Patients’ age, body mass index (BMI) and infertility duration in group A was lower than those in group B. However, the antral follicle count (AFC) and basal LH level (9.23±5.99 mIU/mL vs 7.66±5.14 mIU/mL, p<0.01) in group A was higher than that in the other group. The initial dose of group A was higher, while the days of Gn stimulation and total Gn dosage of group A were lower. The levels of LH and P in group A were similar to those in group B on the human chorionic gonadotropin (hCG) trigger day, while the E2 level of group A was markedly higher (3772.97±2031.47 pg/mL vs 3167.78±1762.77 pg/mL, p<0.01). The endometrial thickness on hCG day of group A was lower. The number of oocytes greater than 14 mm, retrieved oocytes, mature oocytes (MII), normal fertilized oocytes and high-quality embryos (classified as grade II or above on the third day) were significantly greater in group A than in group B. However, no significant difference was observed in the MII oocyte rate, normal fertilization rate or high-quality embryo rate in the two groups. Compared to that in group B, the proportion of cancelling fresh embryo transfer was much higher (49.4% vs 33.5%, p<0.01) in group A. In all cases, 209 patients had concurrent ovarian hyperstimulation syndrome (OHSS), including 45 and 164 patients in groups A and B, respectively. The OHSS incidence in group A was markedly higher than that in group B (Table 1).
Table 1.
Recombinant Follicle Stimulating Hormone (rFSH) Alone versus Combined rFSH and LH Supplementation During Long-Acting GnRH Agonist Cycles
| Variable | Group A (n=409) | Group B (n=2191) | p value |
|---|---|---|---|
| Baseline characteristics | |||
| Age (y) | 28.0±3.2 | 29.1±3.4 | <0.01 |
| BMI (kg/m2) | 23.0±3.3 | 24.1±3.6 | <0.01 |
| Duration of infertility (y) | 3.1±2.0 | 3.3±2.1 | 0.03 |
| AFC (n) | 23.4±3.3 | 23.1±4.0 | <0.01 |
| FSH levels (mIU/mL) | 6.77±1.63 | 6.87±1.70 | 0.28 |
| LH levels (mIU/mL) | 9.23±5.99 | 7.66±5.14 | <0.01 |
| Medication during stimulation | |||
| Starting doses of Gn (IU) | 111.7±23.0 | 116.5±24.6 | <0.01 |
| Total doses of Gn (IU) | 1535.4±608.8 | 2090.6±796.3 | <0.01 |
| Gn duration (d) | 12.4±3.4 | 13.2±3.1 | <0.01 |
| Hormone variation on trigger day | |||
| LH (mIU/mL) | 1.13±0.67 | 1.19±0.72 | 0.07 |
| E2 (pg/mL) | 3772.97±2031.47 | 3167.78±1762.77 | <0.01 |
| P (ng/mL) | 0.68±0.48 | 0.65±0.48 | 0.18 |
| Em (mm) | 11.6±2.3 | 12.1±2.6 | <0.01 |
| Over 14 mm oocyte count (n) | 9.3±3.7 | 8.6±3.3 | <0.01 |
| Retrieved oocyte count (n) | 16.5±6.4 | 14.0±5.5 | <0.01 |
| MII oocyte count (n) | 15.3±6.3 | 12.9±5.3 | <0.01 |
| Normal fertilized oocyte count (n) | 11.9±5.4 | 10.2±4.6 | <0.01 |
| Good-quality embryos count (n) | 6.1±3.9 | 5.0±3.2 | <0.01 |
| MII oocyte rate (%) | 92.5±10.3 | 91.7±7.3 | 0.11 |
| Normal fertilization rate (%) | 89.8±11.0 | 90.8±11.3 | 0.13 |
| Good-quality embryos rate (%) | 46.0±21.9 | 44.3±22.5 | 0.22 |
| fET cancle rate (%) | 49.4 (202/409) | 33.5 (734/2191) | <0.01 |
| Incidence of OHSS (%) | 11.0 (45/409) | 7.5 (164/2191) | <0.01 |
Abbreviations: BMI, Body mass index; AFC, Antral follicle count; Em, Endometrial thickness; fET, fresh embryo transfer.
Outcomes of Fresh Embryo Transfer (fET)
The clinical results of fET are listed in Table 2. Among all the cycles, 1664 were conducted with fET: 207 and 1457 cycles in groups A and B, respectively. The endometrial thickness on the trigger day of group A was lower than that of group B, while the number of transferred embryos were higher in group A. The biochemical pregnancy rate, clinical pregnancy rate and embryo implantation rate of group A were slightly higher than those of group B, but the difference was not statistically significant.
Table 2.
Pregnancy Outcomes of Fresh Embryos Transfer
| Variable | Group A (n=207) | Group B (n=1457) | p value |
|---|---|---|---|
| Em (mm) | 11.6±2.4 | 12.1±2.6 | 0.01 |
| No. of transfer embryos | 1.71±0.45 | 1.62±0.49 | 0.01 |
| Biochemical pregnancy rate | 78.7% (163/207) | 73.7% (1074/1457) | 0.12 |
| Clinical pregnancy rate | 75.4% (156/207) | 69.4% (1011/1457) | 0.08 |
| Implantation rate | 62.1% (220/354) | 57.0% (1344/2356) | 0.07 |
Abbreviation: Em, Endometrial thickness.
To identify the association between LH supplementation and clinical outcomes based on different ages, we divided the data in different subgroups by patients’ age (Table S1). In the subgroup of women aged 35 and over, the retrieved oocyte number, high-quality embryo number and rate of clinical pregnancy were similar in the two groups. The number of retrieved eggs (16.6±6.3 vs 14.0±5.5, p<0.01) and high-quality embryos (6.2±4.0 vs 5.1±3.3, p<0.01) of patients without LH supplementation in the subgroup that was younger than 35 years old were obviously higher than those in the subgroup of patients supplemented with LH. Additionally, it was shown that the rate of clinical pregnancy was slightly lower in cycles with added LH (not statistically significant). In all cycles of group B, there were two main kinds of exogenous LH for supplementation: rLH and HMG. To explore the effect of different kinds of exogenous LH on clinical outcomes, we divided all data of group B into two subgroups (Table S2, B1 for rLH and B2 for HMG). The number of retrieved oocytes in cycles with rLH supplementation were significantly higher and the proportion of mature follicles (MII) was lower in rLH group than those in the HMG group. In addition, no significant difference in high-quality embryo proportion was detected in these two groups and the clinical pregnancy rate in the B2 group was slightly increased compared to those in the B1 group (no significant difference).
Outcomes Based on Multivariate Regression Analysis
To control confounding variables, a multivariable logistic-regression model was employed. Preliminary univariate analysis was used to identify confounding factors that might affect clinical pregnancy outcomes (Table S3). Patients’ baseline factors (Age; AFC; Baseline LH levels) and treatment factors (Starting doses of Gn; P levels on hCG day; Retrieved oocyte count; MII oocyte count; Normal fertilized oocyte count; Good-quality embryos count; No. of transfer embryos) were selected as adjustment variables for multivariate regression analysis. LH supplementation was not significantly correlated with the clinical pregnancy rate (Table 3, adjusted OR=0.910, 95% CI: 0.623–1.311, p=0.61).
Table 3.
Multivariate Analysis for LH Supplementation Involved in the Clinical Pregnancy Rate
| Variable | Adjust I | Adjust II | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p value | Odds Ratio | 95% CI | p value | |
| LH supplementation | 0.827 | 0.587–1.164 | 0.28 | 0.910 | 0.623–1.311 | 0.61 |
Notes: Adjust I model adjust for: Age; AFC; Baseline LH levels. Adjust II model adjust for: Starting doses of Gn; P levels on hCG day; Retrieved oocyte count; MII oocyte count; Normal fertilized oocyte count; Good-quality embryos count; No. of transfer embryos.
Comparisons After the Propensity-Score Matching
Given the differences in the baseline characteristics between the two groups (Table 1), Propensity-score matching was used to identify a cohort of patients with similar baseline characteristics. As shown in Table 4, the baseline characteristics of patients with fET cycles were similar between the two groups after the propensity-score matching. The endometrial thickness on the trigger day of group A was lower than that of group B, while the number of transferred embryos were higher in group A (Table 5). The biochemical pregnancy rate, clinical pregnancy rate and embryo implantation rate of group A were slightly higher than those of group B without significant difference (Table 5). In addition, univariate analysis was also used to identify confounding factors that might affect clinical pregnancy outcomes (Table S4). Patients’ baseline factors (Age; AFC) and treatment factors (Starting doses of Gn; Retrieved oocyte count; MII oocyte count; Normal fertilized oocyte count; MII oocyte rate; No. of transfer embryos) were selected as adjustment variables for multivariate regression analysis. LH supplementation was not significantly correlated with the clinical pregnancy rate (Table 6, adjusted OR=0.792, 95% CI: 0.527–1.191, p=0.26).
Table 4.
Baseline Characteristics Before and After Propensity-Score Matching
| Variable | Before Matching | Before Matching | ||||
|---|---|---|---|---|---|---|
| Group A (n=207) | Group B (n=1457) | p value | Group A (n=203) | Group B (n=406) | p value | |
| Age (y) | 28.2±3.2 | 29.2±3.5 | <0.01 | 28.2±3.2 | 28.0±3.2 | 0.63 |
| BMI (kg/m2) | 23.1±3.4 | 24.1±3.5 | <0.01 | 23.0±3.3 | 24.1±3.6 | 0.31 |
| Infertility Duration (y) | 2.9±2.0 | 3.3±2.2 | <0.01 | 3.1±2.0 | 3.3±2.1 | 0.67 |
| AFC (n) | 23.0±3.6 | 22.7±3.9 | 0.12 | 23.4±3.3 | 23.1±4.0 | 0.90 |
| FSH levels (mIU/mL) | 6.87±1.62 | 7.01±1.75 | 0.39 | 6.77±1.63 | 6.87±1.70 | 0.44 |
| LH levels (mIU/mL) | 9.05±6.24 | 7.52±5.06 | <0.01 | 9.23±5.99 | 7.66±5.14 | 0.91 |
Abbreviations: BMI, Body mass index; AFC, Antral follicle count.
Table 5.
Pregnancy Outcomes of Fresh Embryos Transfer After Propensity-Score Matching
| Variable | Group A (n=203) | Group B (n=406) | p value |
|---|---|---|---|
| Em (mm) | 11.5±2.3 | 12.0±2.5 | 0.04 |
| No. of transfer embryos | 1.71±0.46 | 1.61±0.49 | 0.02 |
| Biochemical pregnancy rate | 78.3% (159/203) | 75.1% (305/406) | 0.38 |
| Clinical pregnancy rate | 75.4% (153/203) | 69.5% (282/406) | 0.13 |
| Implantation rate | 62.0% (215/347) | 57.0% (373/654) | 0.08 |
Abbreviation: Em, Endometrial thickness.
Table 6.
Multivariate Analysis for LH Supplementation Involved in the Clinical Pregnancy Rate After Propensity-Score Matching
| Variable | Adjust I | Adjust II | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p value | Odds Ratio | 95% CI | p value | |
| LH supplementation | 0.728 | 0.495–1.071 | 0.11 | 0.792 | 0.527–1.191 | 0.26 |
Notes: Adjust I model adjust for: Age; AFC. Adjust II model adjust for: Starting doses of Gn; Retrieved oocyte count; MII oocyte count; MII oocyte rate; Normal fertilized oocyte count; No. of transfer embryos.
Discussion
FSH and LH secreted by the pituitary gland play a key role in the development of follicles in a natural cycle.16 To prevent premature ovulation, GnRHa is used to inhibit LH levels during controlled ovulation induction. However, the effect of reduced LH levels on follicular maturation and pregnancy outcomes has been controversial.
Some studies suggest that exogenous LH supplementation can improve the quality of follicles and embryos and improve pregnancy outcomes, which is inconsistent with our conclusion. Our retrospective study showed that LH addition had no significant effect on non-selective patients receiving COH with long-acting GnRHa. Some previous studies also considered that endogenous LH could meet the needs of follicular development in most patients with a normal ovarian response, and the addition of exogenous LH is not necessary.10,11,17,18 Chappel et al19 suggested that a few LH receptors (less than 1%) in most patients were enough to maintain normal steroid production and follicular development. When GnRH agonists or antagonists decrease the level of endogenous LH, the “resting state” of the LH concentration may be sufficient.20 Some authors have reported that when the LH level is less than 0.5–0.7 mIU/mL, clinical outcomes of FSH-induced COH cycles are impaired (ie, increased early spontaneous abortion, reduced normal fertilization rate, etc.).21,22 Other studies have indicated that the lower limit of the LH threshold ranges between 0.5 and 1.2 mIU/mL.21,23,24 In our study, the levels of LH were similar between the two groups on the hCG day (approximately 1.2 mIU/mL), which was higher than the reported LH level (0.5–0.7 mIU/mL). Therefore, it may be that the endogenous LH level in the GnRHa-downregulated ovulation cycle is enough to support the growth and development of follicles and that the addition of exogenous LH has no significant effect on the clinical results.
Furthermore, there are few studies exploring the effects of basal LH levels on exogenous LH supplementation in COH. Previous studies have suggested that exogenous LH supplementation when endogenous LH is greater than a certain value during COH leads to a higher rate of embryo implantation and clinical pregnancy.25 The authors speculated that this phenomenon may occur according to desensitization of the ovarian LH receptor induced by high concentrations of endogenous LH.26 Therefore, the collected data were stratified again in our study according to the basal LH levels. Regardless of whether the basal LH level was greater or less than 10 mIU/mL, the rates of clinical pregnancy and embryo implantation were slightly higher in the unsupplemented LH cycles than in the LH-supplemented cycles. However, when analysing only the LH-supplemented group, the clinical pregnancy rate and embryo implantation rate were slightly lower in patients with a basal LH<10 mIU/mL than in those with a basal LH ≥ 10 mIU/mL (data not shown). This result suggests that exogenous LH supplementation in COH may be more beneficial in patients with a higher basal LH level, which needs further study.
Studies have shown that the biological activity of LH and the number of LH receptors in follicular granulosa cells gradually decrease in women over 35 years old. Adding LH may benefit older women.13,27,28 Some randomized controlled trials also suggest that exogenous rLH may be beneficial to patients over 35 years old. Matorras et al18 found that adding rLH to 35- to 39-year-old patients led to higher implantation and live birth rates. Adding rLH can increase the clinical pregnancy rate of FSH-induced COH patients with poor ovarian response (POR) by approximately 30%.3 Therefore, we divided the patients into subgroups according to their age. The number of high-quality embryos and the rate of clinical pregnancy of elderly patients (≥ 35 years old) with LH supplementation were lower than those of patients without LH supplementation, but there was no significant difference. In this study, only 14 cycles of elderly women did not receive LH supplementation. In clinical work, we may be inclined to treat elderly women with exogenous LH, which leads to a certain result bias. Moreover, the morphology scoring used in the study may not accurately reflect the potential of embryo implantation, which might have a few effects on the embryo implantation rate in the two groups. In contrast, LH supplementation seems to reduce the clinical pregnancy rate. However, some other studies have similar results to our study.29 Some scholars contend that a higher LH concentration in elderly women may affect oocyte maturation, meiosis and zygotic mitosis.30 Therefore, more studies should be carried out to clarify the benefits of LH supplementation for elderly women.
Different types of LH supplementation have different effects on the clinical outcomes of ART cycles. Some studies have suggested that the number of oocytes obtained in FSH combined with rLH COH is higher than that obtained in HMG COH, but there is no significant difference in the rate of clinical pregnancy and live birth.31 There are two main kinds of exogenous LH (rLH and HMG) used in COH cycles at our reproductive medical centre. In this study, the subgroup analysis of different kinds of LH supplementation showed that more oocytes could be obtained in the rLH-addition subgroup than in the HMG-addition subgroup, while the proportion of mature follicles (MII) in the HMG addition subgroup was slightly higher. The clinical pregnancy rate were slightly higher than that in the rLH addition subgroup. However, the above data are not statistically significant. Therefore, expanding the sample size for retrospective analysis or clinical randomized controlled trials is necessary for further evaluation.
Meta-analysis suggests that rLH supplementation may contribute to pregnancy3,13,32 in women with POR. None of these meta-analyses included a separate assessment of normal ovarian responsive women who responded relatively well to FSH stimulation alone after GnRHa downregulation. Clinical evidence suggests that a small number of women with a normal ovarian response do not respond well to FSH and may develop LH deficiency after GnRHa use. These women account for approximately 10–14%10,33,34 of young women with a normal ovarian response receiving ART treatment. These POR patients can obtain more oocytes and improve the clinical pregnancy rate with LH supplementation.3 However, at our reproductive medical centre, the long-acting GnRHa program is used mainly for patients with normal ovarian response or high ovarian response. Therefore, this study did not include POR patients, and a few POR patients were not analysed separately, which is worthy of further attention.
Previous studies have mostly explored the role of LH addition in GnRH antagonist IVF/ICSI cycles. However, several scholars previously believed that exogenous LH addition seemed beneficial due to the deep inhibition of long-acting GnRHa on the LH level. However, our study suggests that LH supplementation can not improve the clinical pregnancy rate of non-selective patients with long-acting GnRHa IVF/ICSI cycles, which has certain innovation. At the same time, our study has a retrospective design, which is the main drawback. In addition, the administration time of LH and LH levels on the LH supplementation day were not included in this study. To further clarify the roles of LH supplementation in clinical outcomes, higher-quality and large randomized controlled trials are needed.
Conclusion
In conclusion, the results of this retrospective study showed that the clinical pregnancy rate of non-selective women who received long-acting GnRHa therapy were not associated with exogenous LH supplementation.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81801530) and Reproductions Research Program of Young and Middle-aged Physicians and China Health Promotion Foundation (BJHPA-2022-SHZHYXZHQNYJ-LCH-002). There is no conflict of interests.
Data Sharing Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Ethics Approval
We obtained approval from the ethics committee of Drum Tower Hospital affiliated with Nanjing University Medical School. We did not need patient consent, and permission to participate was also not appropriate, because our review was a retrospective study of data reuse, and the message of the patients was anonymous.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Chenyang Huang, Qingqing Shi, and Yuan Yan should be regarded as joint First Authors.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Alviggi C, Clarizia R, Mollo A, Ranieri A, De Placido G. Who needs LH in ovarian stimulation? Reprod Biomed Online. 2011;22(Suppl 1):S33–41. doi: 10.1016/S1472-6483(11)60007-2 [DOI] [PubMed] [Google Scholar]
- 2.Hillier SG. Gonadotropic control of ovarian follicular growth and development. Mol Cell Endocrinol. 2001;179(1–2):39–46. doi: 10.1016/S0303-7207(01)00469-5 [DOI] [PubMed] [Google Scholar]
- 3.Lehert P, Kolibianakis EM, Venetis CA, et al. Recombinant human follicle-stimulating hormone (r-hFSH) plus recombinant luteinizing hormone versus r-hFSH alone for ovarian stimulation during assisted reproductive technology: systematic review and meta-analysis. Reprod Biol Endocrinol. 2014;12:17. doi: 10.1186/1477-7827-12-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hugues JN. Impact of ‘LH activity’ supplementation on serum progesterone levels during controlled ovarian stimulation: a systematic review. Hum Reprod. 2012;27(1):232–243. doi: 10.1093/humrep/der380 [DOI] [PubMed] [Google Scholar]
- 5.Revelli A, Chiado A, Guidetti D, Bongioanni F, Rovei V, Gennarelli G. Outcome of in vitro fertilization in patients with proven poor ovarian responsiveness after early vs. mid-follicular LH exposure: a prospective, randomized, controlled study. J Assist Reprod Genet. 2012;29(9):869–875. doi: 10.1007/s10815-012-9804-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diedrich K, Diedrich C, Santos E, et al. Suppression of the endogenous luteinizing hormone surge by the gonadotrophin-releasing hormone antagonist Cetrorelix during ovarian stimulation. Hum Reprod. 1994;9(5):788–791. doi: 10.1093/oxfordjournals.humrep.a138597 [DOI] [PubMed] [Google Scholar]
- 7.Orvieto R, Meltzer S, Rabinson J, Gemer O, Anteby EY, Nahum R. Does day 3 luteinizing-hormone level predict IVF success in patients undergoing controlled ovarian stimulation with GnRH analogues? Fertil Steril. 2008;90(4):1297–1300. doi: 10.1016/j.fertnstert.2007.10.058 [DOI] [PubMed] [Google Scholar]
- 8.Shoham Z, Smith H, Yeko T, O’Brien F, Hemsey G, O’Dea L. Recombinant LH (lutropin alfa) for the treatment of hypogonadotrophic women with profound LH deficiency: a randomized, double-blind, placebo-controlled, proof-of-efficacy study. Clin Endocrinol. 2008;69(3):471–478. doi: 10.1111/j.1365-2265.2008.03299.x [DOI] [PubMed] [Google Scholar]
- 9.Shoham Z. The clinical therapeutic window for luteinizing hormone in controlled ovarian stimulation. Fertil Steril. 2002;77(6):1170–1177. doi: 10.1016/S0015-0282(02)03157-6 [DOI] [PubMed] [Google Scholar]
- 10.Hill MJ, Levy G, Levens ED, Hill MJ, Levy G, Levens ED. Does exogenous LH in ovarian stimulation improve assisted reproduction success? An appraisal of the literature. Reprod Biomed Online. 2012;24(3):261–271. doi: 10.1016/j.rbmo.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 11.Bleau N, Agdi M, Son W, Tan S, Dahan MH. A comparison of outcomes from in vitro fertilization cycles stimulated with follicle stimulating hormone plus either recombinant luteinizing hormone or human menopausal gonadotropins in subjects treated with long gonadotropin releasing hormone agonist protocols. Int J Fertil Steril. 2017;11(2):79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durnerin CI, Erb K, Fleming R, et al. Effects of recombinant LH treatment on folliculogenesis and responsiveness to FSH stimulation. Hum Reprod. 2008;23(2):421–426. doi: 10.1093/humrep/dem388 [DOI] [PubMed] [Google Scholar]
- 13.Hill MJ, Levens ED, Levy G, et al. The use of recombinant luteinizing hormone in patients undergoing assisted reproductive techniques with advanced reproductive age: a systematic review and meta-analysis. Fertil Steril. 2012;97(5):1108–14 e1. doi: 10.1016/j.fertnstert.2012.01.130 [DOI] [PubMed] [Google Scholar]
- 14.Runxiang LL, Yue L. Effect of long-term down-regulation time of early follicular phase on IVF-ET pregnancy outcome. Contemp Med. 2021;27(621):3. [Google Scholar]
- 15.Sun H. Effect of pituitary down-regulation on the synchronization of follicular development. J Reprod Med. 2010;19:3. [Google Scholar]
- 16.Rinaldi L, Selman H. Profile of follitropin alpha/lutropin alpha combination for the stimulation of follicular development in women with severe luteinizing hormone and follicle-stimulating hormone deficiency. Int J Womens Health. 2016;8:169–179. doi: 10.2147/IJWH.S88904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maia MC, Approbato MS, da Silva TM, Fleury EA, Sanchez EG, Sasaki RS. Use of recombinant luteinizing hormone for controlled ovarian hyperstimulation in infertile patients. JBRA Assist Reprod. 2016;20(2):78–81. doi: 10.5935/1518-0557.20160018 [DOI] [PubMed] [Google Scholar]
- 18.Matorras R, Prieto B, Exposito A, et al. Mid-follicular LH supplementation in women aged 35–39 years undergoing ICSI cycles: a randomized controlled study. Reprod Biomed Online. 2011;22(Suppl 1):S43–51. doi: 10.1016/S1472-6483(11)60008-4 [DOI] [PubMed] [Google Scholar]
- 19.Chappel SC, Howles C. Reevaluation of the roles of luteinizing hormone and follicle-stimulating hormone in the ovulatory process. Hum Reprod. 1991;6(9):1206–1212. doi: 10.1093/oxfordjournals.humrep.a137513 [DOI] [PubMed] [Google Scholar]
- 20.Humaidan P. Are endogenous LH levels during ovarian stimulation for IVF using GnRH analogues associated with the probability of ongoing pregnancy? A systematic review. Hum Reprod Update. 2006;12(3):325. doi: 10.1093/humupd/dml005 [DOI] [PubMed] [Google Scholar]
- 21.Humaidan P, Bungum L, Bungum M, Andersen CY. Ovarian response and pregnancy outcome related to mid-follicular LH levels in women undergoing assisted reproduction with GnRH agonist down-regulation and recombinant FSH stimulation. Hum Reprod. 2002;17(8):2016–2021. doi: 10.1093/humrep/17.8.2016 [DOI] [PubMed] [Google Scholar]
- 22.Westergaard LG, Laursen SB, Andersen CY. Increased risk of early pregnancy loss by profound suppression of luteinizing hormone during ovarian stimulation in normogonadotrophic women undergoing assisted reproduction. Hum Reprod. 2000;15(5):1003–1008. doi: 10.1093/humrep/15.5.1003 [DOI] [PubMed] [Google Scholar]
- 23.Westergaard LG, Erb K, Laursen SB, Rex S, Rasmussen PE. Human menopausal gonadotropin versus recombinant follicle-stimulating hormone in normogonadotropic women down-regulated with a gonadotropin-releasing hormone agonist who were undergoing in vitro fertilization and intracytoplasmic sperm injection: a prospective randomized study. Fertil Steril. 2001;76(3):543–549. doi: 10.1016/s0015-0282(01)01973-2 [DOI] [PubMed] [Google Scholar]
- 24.Lahoud R, Al-Jefout M, Tyler J, Ryan J, Driscoll G. A relative reduction in mid-follicular LH concentrations during GnRH agonist IVF/ICSI cycles leads to lower live birth rates. Hum Reprod. 2006;21(10):2645–2649. doi: 10.1093/humrep/del219 [DOI] [PubMed] [Google Scholar]
- 25.Humaidan P, Bungum M, Bungum L, Yding Andersen C. Effects of recombinant LH supplementation in women undergoing assisted reproduction with GnRH agonist down-regulation and stimulation with recombinant FSH: an opening study. Reprod Biomed Online. 2004;8(6):635–643. doi: 10.1016/S1472-6483(10)61643-4 [DOI] [PubMed] [Google Scholar]
- 26.Amsterdam A, Tajima K, Frajese V, Seger R. Analysis of signal transduction stimulated by gonadotropins in granulosa cells. Mol Cell Endocrinol. 2003;202(1–2):77–80. doi: 10.1016/S0303-7207(03)00066-2 [DOI] [PubMed] [Google Scholar]
- 27.Ramachandran A, Jamdade K, Kumar P, Adiga SK, Bhat RG, Ferrao SR. Is there a need for luteinizing hormone (LH) estimation in patients undergoing ovarian stimulation with gonadotropin-releasing hormone (GnRH) antagonists and recombinant follicle-stimulating hormone (rFSH)? J Clin Diagn Res. 2014;8(1):90–92. doi: 10.7860/JCDR/2014/5728.3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosch E, Labarta E, Crespo J, Simon C, Remohi J, Pellicer A. Impact of luteinizing hormone administration on gonadotropin-releasing hormone antagonist cycles: an age-adjusted analysis. Fertil Steril. 2011;95(3):1031–1036. doi: 10.1016/j.fertnstert.2010.10.021 [DOI] [PubMed] [Google Scholar]
- 29.Konig TE, van der Houwen LE, Overbeek A, et al. Recombinant LH supplementation to a standard GnRH antagonist protocol in women of 35 years or older undergoing IVF/ICSI: a randomized controlled multicentre study. Hum Reprod. 2013;28(10):2804–2812. doi: 10.1093/humrep/det266 [DOI] [PubMed] [Google Scholar]
- 30.Weghofer A, Munne S, Brannath W, et al. The impact of LH-containing gonadotropin stimulation on euploidy rates in preimplantation embryos: antagonist cycles. Fertil Steril. 2009;92(3):937–942. doi: 10.1016/j.fertnstert.2008.07.1735 [DOI] [PubMed] [Google Scholar]
- 31.Orvieto R. HMG versus recombinant FSH plus recombinant LH in ovarian stimulation for IVF: does the source of LH preparation matter? Reprod Biomed Online. 2019;39(6):1001–1006. doi: 10.1016/j.rbmo.2019.08.010 [DOI] [PubMed] [Google Scholar]
- 32.Mochtar MH, Van V, Ziech M, van Wely M. Recombinant Luteinizing Hormone (rLH) for controlled ovarian hyperstimulation in assisted reproductive cycles. Cochrane Database Syst Rev. 2007;1(2):CD005070. [DOI] [PubMed] [Google Scholar]
- 33.Ferraretti AP, Gianaroli L, Magli MC, D’Angelo A, Farfalli V, Montanaro N. Exogenous luteinizing hormone in controlled ovarian hyperstimulation for assisted reproduction techniques. Fertil Steril. 2004;82(6):1521–1526. doi: 10.1016/j.fertnstert.2004.06.041 [DOI] [PubMed] [Google Scholar]
- 34.De Placido G, Alviggi C, Perino A, et al. Recombinant human LH supplementation versus recombinant human FSH (rFSH) step-up protocol during controlled ovarian stimulation in normogonadotrophic women with initial inadequate ovarian response to rFSH. A multicentre, prospective, randomized controlled trial. Hum Reprod. 2005;20(2):390–396. doi: 10.1093/humrep/deh625 [DOI] [PubMed] [Google Scholar]