Abstract
Introduction/Aim
Clinical worsening has been common in people with Parkinson's disease (PD) during the social distancing due to pandemic. It is unclear if telerehabilitation applied during social distancing preserves clinical aspects of people with PD who are frequent exercisers before the pandemic. Thus, we compared the effects of 10 months of supervised, home-based, real-time videoconferencing telerehabilitation (SRTT) and nonexercising control on clinical aspects in people with PD who are frequent exercisers before the pandemic.
Methods
Fifty-seven (SRTT group) and 29 (nonexercising control group) people with PD were retrospectively assessed (Clinical Trials Registry: RBR-54sttfk). Only the SRTT group performed a 60-min online training sessions, 2–3 days per week, for 10 months (April 2020 to January 2021) during social distancing. Quality of life (PD Questionnaire [PDQ-39]), walking (item 28 from the Unified Parkinson’s Disease Rating Scale part III [UPDRS-III]), posture (item 29 from the UPDRS-III), and freezing of gait (New-FOG questionnaire [NFOGQ]) were retrospectively assessed before (February–March 2020) and during social distancing (February–March 2021). The assessments were performed in-person and remotely before and during social distancing, respectively.
Results
There were no between-group differences at baseline (p > 0.05). SRTT preserves PDQ-39 and walking scores but not posture and NFOGQ scores, while nonexercising control worsens scores in all variables. In addition, SRTT is more effective than nonexercising control in preserving PDQ-39 and walking scores.
Conclusion
During social distancing, long-term SRTT preserves the subjective quality of life and walking, but not subjective posture and FOG in people with PD who are frequent exercisers before the pandemic.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13760-022-02160-3.
Keywords: Telerehabilitation, Quality of life, Walking, Pandemic
Introduction
Several countries, including Brazil, announced social distancing policies to contain the spread of the Coronavirus disease 2019 (COVID-19). Social distancing, such as physically distancing from other people, staying at home or avoiding crowded areas decreases social interaction [1] and physical activity levels [2], which negatively affect the quality of life of individuals with chronic diseases [3], such as Parkinson’s disease (PD) [4, 5].
PD is a chronic neurodegenerative disease mainly characterized by motor signs (e.g., gait disturbances). A higher COVID-19 mortality rate has been described in individuals with advanced PD and longer disease duration [4]. People with PD have reported more fear of the COVID-19 pandemic [5, 6], increased self-isolation and social distancing [5], and increased body weight and risk of falls [7]. Social distancing decreased the physical activity levels and the quality of life, as well as worsened clinical aspects (walking, posture, and freezing of gait [FOG]) [5] and balance [7] of people with PD. In Brazil, a country with large socioeconomic differences, the self-reported clinical worsening and the reduction in the physical activity volume in people with PD during social distancing were aggravated by restricted access to telerehabilitation and e-health systems [5], which were already precarious in this country, even before the COVID-19 pandemic [8]. Thus, the implementation of low-cost remote rehabilitation is urgently needed to improve the quality of life of these people during social distancing.
A recent review has reinforced the use of telerehabilitation to improve the remote provision of exercise to people with PD to maintain physical mobility and emotional well-being, mainly during social distancing [9]. Although many recent studies have been published on telerehabilitation in PD [9], which include virtual reality, exergaming, or personal sessions (1:1), there is no evidence of the effect of telerehabilitation, real-time videoconferencing exercise sessions, implemented during social distancing due to COVID-19 pandemic on clinical aspects of PD.
Thus, this retrospective study compared the effects of 10 months of nonexercising control and supervised, home-based, real-time videoconferencing telerehabilitation (SRTT) on clinical aspects (subjective quality of life, walking, posture, and FOG) that worsened during social distancing in people with PD living in Brazil [5]. SRTT included exercise sessions of sitting and standing dance and physiotherapy (lower- and upper-limbs free weight exercises, coordination exercises, and stationary walking with and without dual task), which are known to improve walking, posture, FOG, and quality of life of people with PD [10–12]. Thus, we hypothesized that SRTT but not nonexercising control applied during social distancing would preserve clinical aspects of people with PD who are frequent exercisers before the pandemic.
Materials and methods
Study design and participants
This retrospective study was conducted between February 2020 (before the pandemic) and March 2021 (during the pandemic) during social distancing (e.g., staying at home). The Brazilian Ministry of Health confirmed the first case of COVID-19 on February 25, 2020. As no lockdown was imposed, social distancing was initiated on March 11, 2020.
A convenience sample of people with PD (n = 86) who are frequent exercisers before the pandemic and users from the Brazil Parkinson Association participated in this study. The diagnosis of idiopathic PD was confirmed by a movement disorders specialist from Brazil Parkinson Association in accordance with UK Parkinson’s Disease Society Brain Bank diagnostic criteria [13].
Data of those participants (Hoehn and Yahr stage between 1 and 4 [14]) were included in this study if the participants met all inclusion criteria, as follows: (1) 35 to 90 years of age; (2) social distancing during the COVID-19 pandemic since March 2020; (3) frequent exercises (60-min session, 2–3 days per weeks, for 6 months) before the pandemic and users from Brazil Parkinson Association; (4) absence of dementia and severe depression; (5) absence of severe hearing and visual problems that make it impossible to perform SRTT; and (6) adhered to SRTT or nonexercising control from April 2020 in the Brazil Parkinson Association. Participants were classified as having FOG if they answered affirmatively the first question of the New FOG Questionnaire (NFOGQ) [15] following the presentation of a video showing examples of individuals experiencing FOG.
Individuals gave their written informed consent to participate. The study was approved by University’s Ethical Committee (School of Arts, Sciences and Humanities of the University of Sao Paulo) and registered at the Brazilian Clinical Trials Registry (ReBEC, number: RBR-54sttfk).
Study procedures
Participants were assessed in the clinically defined ‘‘on’’ state (fully medicated) within 1.5 h of taking their morning dose of dopaminergic medication by the same physical therapist before and during social distancing. Although we did not use the previously-suggested criterion for DOPA-responsiveness (difference between on and off scores on the Unified Parkinson’s Disease Rating Scale part III [UPDRS-III] ≥ 5) [16, 17], after participants took the first dosage of medication, they were asked to remain seated for at least 20 min, which has been deemed as an appropriate time window for the medication to improve their motor state [18], as we have previously published [19]. Before social distancing (February to March 2020), the assessments were conducted face-to-face at Brazil Parkinson Association’s facilities. During social distancing (February to March 2021), real-time videoconferencing assessments (post-test) were performed 24 h after the last session of SRTT. Free videoconferencing software programs (e.g., Skype or Google Meet) were used. Participants used a webcam and a computer, a mobile phone or a tablet that they had at home when engaging in the videoconferencing assessments. The participants were instructed to do the assessments in adequate places of their homes (e.g., enough space and access to the quiet and distraction-free area) to avoid communication issues (patient and physical therapist) and limited view of the patients due to the camera angle.
Outcome measures included subjective quality of life, walking, posture, and FOG severity. A physical therapist who did not participate in exercise intervention assessed quality of life using the Parkinson’s Disease Questionnaire (PDQ-39) [20], walking and posture using items 28 and 29 from the UPDRS-III, respectively [21], and FOG severity using the NFOGQ [15]. A previous study demonstrated the feasibility between face-to-face and real-time videoconferencing assessments for items from UPDRS-III including walking and posture [22], which do not require physical interaction between individual with PD and assessors. See Supplementary Material for a detailed description of the assessments.
Interventions
We applied real-time videoconferencing exercise sessions to provide feedback to participants in real-time and to observe if people with PD were performing exercises safely and correctly, as previous studies used assistance via phone calls or email but not real-time videoconferencing have found issues of exercise execution [7, 23, 24].
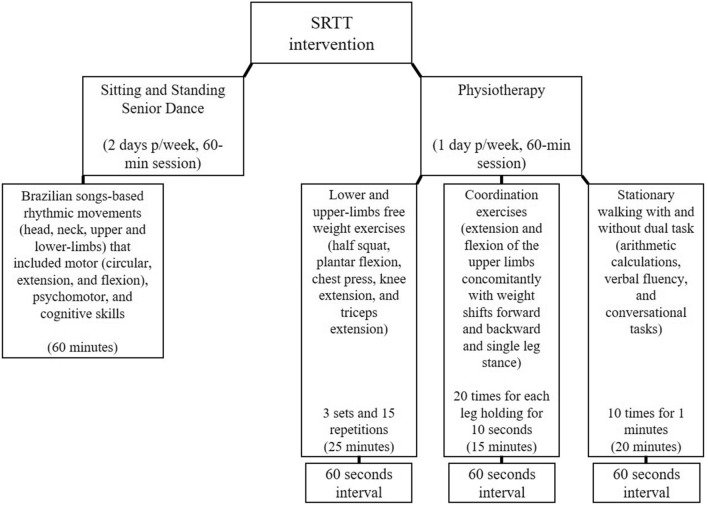
The nonexercising control group did not perform any exercise intervention. Only the SRTT group performed exercise intervention. Participants were trained in the clinically defined ‘‘on’’ state and underwent a 60-min online training session, 2–3 days per week, for 10 months (April 2020 to January 2021). SRTT included two sessions of sitting and standing dance activities and one session of sitting and standing physiotherapy exercises as demonstrated in Fig. 1. During exercise sessions, two physiotherapists supervised up to 12 people per group. One physiotherapist gave instructions on the training protocol and conducted the exercise sessions, while another physiotherapist provided feedback for the people with PD on the correct execution of the movement, which is very important as feedback on performance plays a crucial role in motor rehabilitation [25]. Although telerehabilitation causes some problems related to a limited view of the participant due to camera angle and difficulty obtaining a valid assessment of movement during exercise practice and assessments [24], real-time videoconferencing exercises allow patients to actively interact with the physiotherapist, which increases confidence and feedback on exercise practice [23]. See Supplementary Material for details of SRTT intervention.
Fig. 1.
Schematic representation of the supervised, real-time videoconferencing telerehabilitation (SRTT)
The equipment used to increase the difficulty and progression of the exercises included sticks, weights (plastic bottles or packaged food from 500 g to 2 kg), and elastic bands with different color-coded resistance levels. Training intensity was maintained between 10 and 13 points of the rating of perceived exertion, which are perceived exertion as fairly light and somewhat hard, respectively.
Adherence (how frequently the participants attempted real-time exercise) also was used as a proportion of completed exercises from the total number of exercises to be performed during the total participation online period.
Statistical analyses
Normality and the presence of extreme observations were assessed through the Shapiro–Wilk test and box-plots, respectively. To compare the characteristics between groups at baseline, we used independent t-tests. Chi-square was used to determine whether the proportion of freezers and non-freezers and men and women was different between groups.
Data were analyzed with a magnitude-based inference using effect sizes (ES). The estimated mean and standard deviation (SD) delta changes from each group were used to calculate ES and confidence interval (CI). Thus, to test for the effects of SRTT and nonexercising control on outcomes, ES and CI were calculated for within-group (before vs. during social distancing) and between-groups (changes) comparisons [26]. The 95% CI of the ESs were calculated using a non-central t distribution [27, 28]. Positive and negative CI [i.e., not crossing zero (0)] were considered as significant. The ES has been suggested for group comparisons as it allows the determination of the magnitude of the treatment effects, the interpretation of its practical significance, and it does not give a dichotomic answer (i.e., significant or not significant) [28]. ESs were classified as small (ES 0.20–0.49), medium (ES 0.50–0.79), and large (ES ≥ 0.80) [29].
To compare the levodopa equivalent dose [30], a mixed model for the repeated measure was applied, assuming groups (SRTT and nonexercising control) and times (before and during social distancing) as fixed factors and people with PD as a random factor. The Tukey post hoc was used for multiple comparisons when a significant F value was found.
Results are presented as mean (SD). Statistical procedures were implemented using SAS 9.2® (Institute Inc., Cary, NC, USA) and the level of significance was set at P ≤ 0.05.
Results
Participants’ characteristics
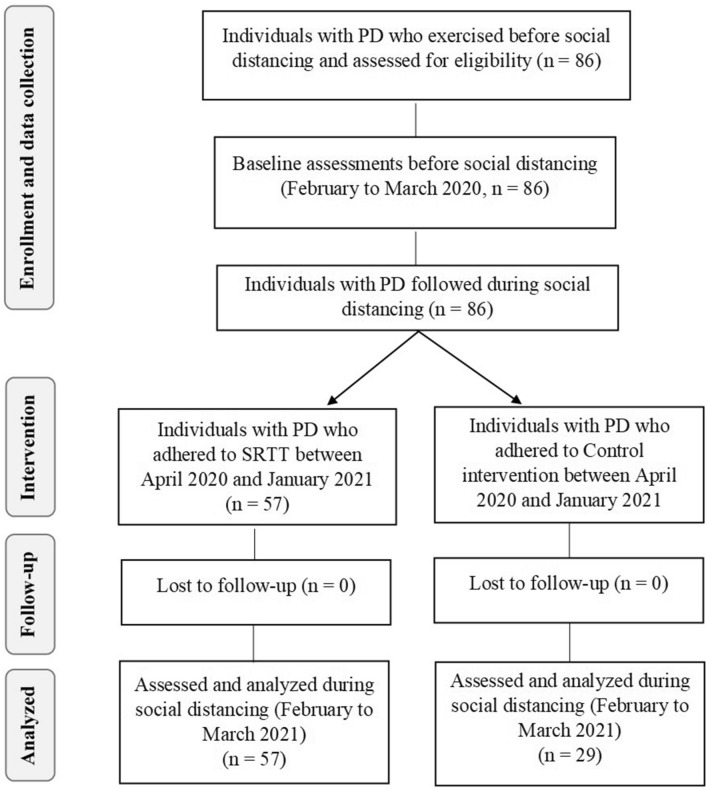
Eighty-six participants from Brazil Parkinson Association who were performing physical exercises before social distancing start in São Paulo city (March 22, 2020) were included in this study. All participants volunteered for the study, signed the written consent and fulfilled inclusion criteria. Eighty-six participants performed baseline testing (before social distancing) and were divided into SRTT (n = 57) and nonexercising control (n = 29) groups, if they adhered (SRTT) or not (nonexercising control) to telerehabilitation during social distancing. No participant dropped out of study (Fig. 2).
Fig. 2.
Consort diagram. SRTT: supervised, real-time videoconferencing telerehabilitation; Control: nonexercising control group
At baseline, there were no between-group differences in demographic, anthropometrical, or clinical outcomes (Table 1), as expected, there was only difference between the number of freezers and non-freezers between groups (P = 0.026).
Table 1.
Characteristics of the participants at baseline, by group. Mean(SD)
| SRTT | Control | p value | |
|---|---|---|---|
| Characteristics | (n = 57) | (n = 29) | |
| Men/women (number) | 30/27 | 15/14 | 0.934 |
| Freezers/Non-freezers (number) | 21/36 | 9/20 | 0.026 |
| Age (years) | 66.9 (9.8) | 65.1 (9.9) | 0.422 |
| Educational level (years) | 11.7 (5.7) | 11.4 (5.6) | 0.795 |
| Body mass (kg) | 70.0 (12.5) | 65.2 (12.1) | 0.110 |
| Height (cm) | 1.6 (0.1) | 1.7 (0.1) | 0.193 |
| Body mass index (kg/m2) | 25.9 (4.6) | 23.9 (5.3) | 0.099 |
| MoCA (score) | 24.5 (2.8) | 24.9 (3.6) | 0.187 |
| Years since diagnosis (years) | 7.6 (5.2) | 8.0 (5.7) | 0.758 |
| Modified Hoehn and Yahr staging scale (a.u) | 2.6 (0.7) | 2.8 (0.6) | 0.359 |
| 1 | 1 | 0 | |
| 1.5 | 1 | 0 | |
| 2 | 20 | 9 | |
| 3 | 31 | 17 | |
| 4 | 4 | 3 | |
| L-Dopa equivalent daily dose (mg/day) | 619.7 (341.3) | 618.1 (451.5) | 0.986 |
| UPDRS-III (score) | 31.8 (17.4) | 33.6 (15.7) | 0.647 |
| Days practicing social distancing (days/week) | 6.4 (1.2) | 6.7 (0.7) | 0.170 |
| Outcomes | |||
| PDQ-39 (%) | 32.3 (13.8) | 33.0 (13.3) | 0.182 |
| Walking from UPDRS-III (score) | 1.3 (0.8) | 1.4 (0.8) | 0.997 |
| Posture from UPDRS-III (score) | 0.9 (1.1) | 1.0 (0.7) | 0.621 |
| NFOGQ (score) | 15.2 (7.1) | 16.5 (7.6) | 0.768 |
SRTT supervised, real-time videoconferencing telerehabilitation, Control nonexercising control group, MoCA Montreal Cognitive Assessment, a.u. arbitrary units, UPDRS-III Unified Parkinson’s Disease Rating Scale part III, PDQ-39 Parkinson´s Disease Questionnaire, NFOGQ New-freezing of gait questionnaire
SRTT preserves the subjective quality of life and walking
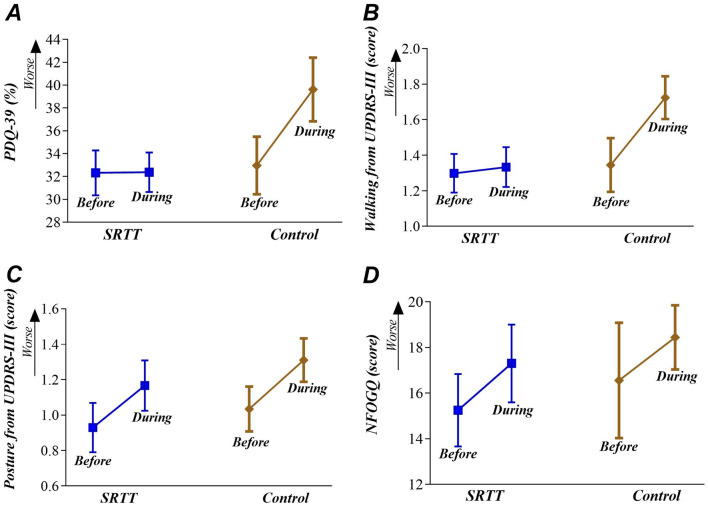
The PDQ-39 and walking scores showed no significant ES after SRTT (ES = − 0.01 and ES = − 0.05, respectively) but showed significant and small ESs after nonexercising control (ES = 0.50 and ES = 0.47, respectively), which demonstrate that only SRTT preserves the subjective quality of life and walking during social distancing in people with PD who are frequent exercisers before the pandemic. When comparing SRTT and nonexercising control groups, SRTT showed a significant and moderate effect on the PDQ-39 scores (ES = − 0.71) and a significant and small effect on the walking scores (ES = − 0.47). Details are given in Table 2 and Fig. 3.
Table 2.
Mean(SD) of the δ, Effect size (ES) and Confidence Interval (CI) for each outcome measure
| Outcomes | SRTT | Control | SRTT vs. Control | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean(SD) | ES | ES 95% CI Low/High | Mean(SD) | ES | ES 95% CI Low/High | Mean(SD) | ES | ES 95% CI Low/High | |
| PDQ-39 (%) | 0.05 (9.23) | − 0.01 | − 0.22/0.21 | 6.65 (14.52) | 0.50 | 0.17/0.82 | 7.70 (14.93) | − 0.71 | − 1.12/− 0.31 |
| Walking (%) | 0.04 (0.73) | − 0.05 | − 0.33/0.24 | 0.38 (0.73) | 0.47 | 0.16/0.77 | 0.55 (0.95) | − 0.47 | − 0.90/− 0.04 |
| Posture (%) | 0.23 (0.60) | 0.23 | 0.05/0.41 | 0.28 (0.84) | 0.41 | 0.01/0.80 | 0.03 (0.82) | 0.08 | − 0.28/0.44 |
| NFOGQ (%) | 2.05 (3.63) | 0.29 | 0.14/0.44 | 1.89 (4.83) | 0.25 | 0.06/0.44 | 0.28 (3.23) | 0.04 | − 0.33/0.42 |
SRTT Supervised, real-time videoconferencing telerehabilitation, Control Nonexercising control group, PDQ-39 Parkinson’s Disease Questionnaire, NFOGQ New-freezing of gait questionnaire
Fig. 3.
Mean (SD) over the two time points (before and during social distancing) in the SRTT group (supervised, real-time videoconferencing telerehabilitation) and Control group (nonexercising) for perception of quality of life (A panel), walking (B panel), posture (C panel), and freezing of gait (D panel). Abbreviations: UPDRS-III: Unified Parkinson’s Disease Rating Scale part III; PDQ-39: Parkinson’s Disease Questionnaire; NFOGQ: New-freezing of gait questionnaire
SRTT does not preserve subjective posture and FOG
The posture and NFOGQ scores showed significant and small ESs after SRTT (ES = 0.23 and ES = 0.29, respectively) and after nonexercising control (ES = 0.41 and ES = 0.25, respectively), which demonstrate that both groups worsen subjective posture and FOG during pandemic. There was no between-groups difference at post-training for posture scores (ES = 0.08) and NFOGQ scores (ES = 0.04). Details are given in Table 2 and Fig. 3.
Adherence and adverse events
Adherence to the SRTT was high. Participants performed 89.2 ± 6.8 sessions (89%). Only two adverse events were reported during SRTT sessions. One participant reported sustained injuries (low-back pain) for three weeks while performing stationary walking with the dual task, but no medical intervention was required. Another participant fell while doing chest-press with an elastic band with a high resistance level, but no medical intervention was required.
Medication dosage through the study
We did not attempt to prevent participants from changing their medication dosage during the study period for ethical reasons. Although at the beginning of the study all participants were taking their usual medication dosage and had stable dopaminergic therapy for at least three months, during the study period some participants modified the dose of their medication. Eleven exercise group participants and 5 control group participants had their levodopa doses increased and 2 control group participants added amantadine to their usual medication dosage. The neurologist decided to adjust the drug treatment based on participant complaints regarding FOG severity during routine appointments. The physical therapist was responsible for registering the changes reported by the patients after the routine appointments with their neurologists. It is important to highlight that we performed additional analyses without these participants (see Supplementary Table 1), which did not significantly influence on the results presented in Table 2. In addition, we did not observe significant changes in medication dose [30] for any group before and during social distancing as demonstrated in Table 3.
Table 3.
Mean(SD) over the two time points (before and during social distancing) in the SRTT group (supervised, real-time videoconferencing telerehabilitation) and control (nonexercising control group) for antiparkinsonian medication dose
| Medications dose | SRTT | Control | ||
|---|---|---|---|---|
| Before | During | Before | During | |
| L-Dopa (mg/day) | 380.7 (343.2) | 394.5 (351.5) | 349.8 (316.6) | 355.7 (337.2) |
| Amantadine (mg/day) | 159.0 (88.1) | 159.0 (88.1) | 125.0 (95.7) | 128.5 (96.1) |
| Entacapone (mg/day) | 62.7 (55.1) | 62.7 (55.1) | 127.7 (44.0) | 127.7 (44.0) |
| MAOB inhibitors (mg/day) | 14.6 (6.4) | 14.6 (6.4) | 13.3 (5.8) | 13.3 (5.8) |
| Dopamine agonists (mg/day) | 2.7 (1.9) | 2.7 (1.9) | 2.3 (2.5) | 2.3 (2.5) |
| L-Dopa-equivalent daily dose (mg/day) | 619.7 (341.3) | 633.5 (363.7) | 618.1 (451.5) | 627.5 (460.3) |
Discussion
To the best of our knowledge, this is the first study to retrospectively compare the effects of 10 months of SRTT and nonexercising control on subjective quality of life, walking, posture, and FOG severity in people with PD who are frequent exercisers before the pandemic. Our findings showed that SRTT preserves the subjective quality of life and walking but not subjective posture and FOG in people with PD who are frequent exercisers before the pandemic. On the other hand, nonexercising control worsens all outcomes during social distancing. Additionally, SRTT was more effective than nonexercising control in preserving PDQ-39 and walking scores at post-training in people with PD who are frequent exercisers before the pandemic.
SRTT preserves the subjective quality of life and walking during social distancing
We have previously demonstrated that reduced quality of life was a common predictor of worse motor (e.g., walking and balance) and non-motor (e.g., sleep problems and fatigue) aspects of daily life experiences during social distancing of people with PD (n = 478) living in Brazil [5]. Decreased quality of life during social distancing was aggravated by the lack of provision and/or access to telerehabilitation programs [5], as physical exercise has an integral impact on the quality of life of individuals with PD [31]. Our study is the first to show that 10 months of SRTT during social distancing can preserve the quality of life compared to before the social distancing. In addition, SRTT is more effective than nonexercising control in preserving the quality of life. Thus, our findings reinforce the need and potential benefits of using telerehabilitation strategies for individuals with PD during social distancing. We used a supervised, group-based real-time videoconferencing telerehabilitation at home and our results are consistent with previous findings in which nine months of individualized, tablet-based physiotherapy program at home improved the quality of life (PDQ-8) of people with PD compared to a nonexercising control group [32]. Thus, home-based telerehabilitation strategies, either individualized [32] or in group (Table 2), can positively impact the quality of life of people with PD who are frequent exercisers before the pandemic.
In addition, high adherence rates to telerehabilitation, such as the one found in our study (89%) seem to be essential for beneficial effects of this intervention in quality of life, as a recent clinical trial showed that high adherence (> 80%) to home-based telerehabilitation is positively correlated to a better quality of life of individuals with PD [33]. Adherence seems to be crucial when exercising at home, since the individual’s motivation to get more involved in his/her own healthcare and to embrace continuity of care are essential for an assertive telerehabilitation practice [34].
Regarding walking, we have previously found that people with PD living in Brazil reported problems with subjective walking (item 2.12 of the MDS-UPDRS part II) during social distancing [5]. Walking is directly related to functional mobility, which is frequently impaired in PD and tends to get worse as the disease progresses [35], even during pharmacological treatment (e.g., levodopa) [35]. Thus, it is expected that people with PD who did not adhere to SRTT (the nonexercising control group) during social distancing had more walking deficits than people who adhered to SRTT. Our findings showed that SRTT was more effective than nonexercising control in preserving walking during social distancing. SRTT involved several exercises, such as standing dance, stationary walking while talking (e.g., cognitive tasks), and lower- and upper-limbs free weight exercises, which are interventions known to positively impact walking in PD [10–12]. Our findings are in accordance with a previous study which showed that seven months of individualized telerehabilitation including motor and cognitive tasks, dual-task activities, and free weight exercises for both upper and lower limbs improved walking (2-m walk test) in people with PD [33]. Taken together, telerehabilitation strategies at home (individualized or group-based) can positively impact the walking of people with PD who are frequent exercisers before the pandemic, although our study is the first to demonstrate the SRTT benefits on walking during social distancing.
SRTT does not preserve subjective posture and FOG during social distancing
We found that subjective posture and FOG got worse after SRTT as much as after nonexercising control. Thus, SRTT is not effective to preserve the posture and FOG of people with PD who are frequent exercisers before the pandemic. In PD, abnormal posture and FOG are the main symptoms linked to falls [36]. Abnormal posture in PD is associated with deficits in flexed posture, trunk flexion and rotation, muscle rigidity, and loss of postural reflexes, which are observed in moderate to severe stages of PD. In fact, in our study, most participants (56%) had moderate stages of PD, which indicate that our participants had abnormal posture. Abnormal posture affects the individual’s ability to speak clearly, moving the neck and upper extremities, postural control, and perception of the body’s position in space. Thus, rehabilitation programs able to improve posture are urgently needed for this population. Schenkman et al. [11] showed that 10 weeks of an individualized exercise-program specifically designed to improve posture (e.g., functional axial rotation) and functional reach improved the spinal flexibility and function of people at moderate stages of PD when compared to nonexercising control (without physical exercise). Our SRTT program did not include specific exercises to improve spinal flexibility, as participants performed most of the exercises in the sitting position, while in the study of Schenkman et al. [11] participants performed exercises moving from a supine to a sitting position. In addition, our participants performed group- and home-based exercises, while in the study of Schenkman et al. [11], participants performed individualized and facility-based training, which could have enabled participants to practice the exercises at their optimal capacity. Therefore, the implementation of exercise interventions designed to target posture is important for people with PD and should be incorporated into telerehabilitation programs in the future.
Our study has also identified that 54.3% and 31% of the people from SRTT and nonexercising control groups, respectively, were characterized as freezers. The NFOGQ scores increased after SRTT and nonexercising control during social distancing compared to before the pandemic, which demonstrates that sitting and standing dance and physiotherapist exercises have no positive effects on subjective FOG severity. A recent meta-analysis showed that interventions aimed directly at the alleviation of subjective FOG severity or FOG-provoking triggers (e.g., external cueing and treadmill training with cues) are beneficial for freezers [10]. Although our SRTT program included motor-cognitive exercises that are beneficial in reducing NFOGQ scores [37], our telerehabilitation protocol was not challenging enough to reduce NFOGQ scores, once triggers for FOG include performing complex activities, such as cognitive challenges while walking and overcoming environmental challenges (e.g., obstacles and turning). Home-based training limits people with PD to perform exercises at their optimal capacity, which result in less improvement in balance and gait when compared to facility-based training [38]. Additionally, people with PD may experience more fear of falling when performing more challenging exercises at home, as fear of falling is a perceived barrier to exercise in this population [39]. Future telerehabilitation programs should be designed to safely target FOG. A possibility is to perform the exercise in the presence of a caregiver. For example, Gandolfi et al. [40] demonstrated that seven weeks of home-based virtual reality balance training with the progressive challenge of postural control and in the presence of a caregiver is a feasible alternative to in-clinic balance training for reducing postural instability in people with PD [40]. Thus, future telerehabilitation studies designed to reduce FOG should consider the presence of a caregiver to monitor the individuals with PD during challenging training sessions, warranting its safety.
Strength and limitations of this study
The strength of this study is that we were able to apply 10 months of SRTT to people with mild-to-severe stages of PD with minimum adverse events and high adherence to treatment during social distancing due to the COVID-19 pandemic, which demonstrate the feasibility of SRTT for this population during social distancing.
Our study also has limitations: i) due to social distancing, post-intervention assessments were all performed by videoconferencing. Although a previous study [22] demonstrated the feasibility between face-to-face and real-time videoconferencing assessments for items from MDS-UPDRS that were used in the present study, there is no study demonstrating the feasibility between face-to-face and real-time videoconferencing assessments for PDQ-39 and NFOGQ, which require future investigation; ii) the most of the participants (57%) had a mild cognitive impairment (score ≤ 25 in the Montreal Cognitive Assessment), which might limit the engagement in remote therapy. However, a previous study found positive effects of home-based telerehabilitation on walking and balance in people with PD with mild cognitive impairment [33], which demonstrate that people with PD with cognitive impairment can perform telerehabilitation; iii) our study did not include follow-up evaluation after the telerehabilitation program. Thus, we do not know if the improvement in subjective quality of life and walking were retained; iv) all participants and investigators were not blinded in this study, which increase the risk of selection and detection bias. However, the same physical therapist who assessed participants before and during social distancing did not participate in the exercise intervention, which decreases the risk of performance bias; v) the present study has an intrinsic limitation because of the retrospective study design itself. Thus, long-term randomized controlled trials are needed to validate the reported benefits of the SRTT on quality of life and walking during social distancing in people with mild-to-severe PD; vi) future studies should compare home-based telerehabilitation vs. in-person rehabilitation in people with PD, as a previous study showed similar gains in arm motor function regardless of type of rehabilitation (in-person or home-based teherehabilitation) in people after Stroke [41]; and vii) although this was a convenience sample size, an exploratory sample size estimate (G*Power v. 3.0.10, Universität Kiel, Germany) suggests that a total sample size of 54 people with PD would be needed to obtain a significant and moderate effect (ES = 0.50, α = 0.05, 1-β = 0.95) on the PDQ-39 scores when comparing SRTT vs. nonexercising control group. Although this indicates that the present study had an appropriate sample size, as we observed a significant and moderate effect (-0.71) on the PDQ-39 scores when comparing SRTT vs. nonexercising control, both groups were unbalance, thus, large prospective and randomized controlled trials are needed to confirm our findings.
Conclusions
SRTT is more effective than nonexercising control in preserving the subjective quality of life and walking in individuals with mild-to-moderate PD who are frequent exercisers before the pandemic, although SRTT does not positively affect the subjective posture and FOG. In addition, SRTT has shown high adherence (89%). Although long-term SRTT can preserve the subjective quality of life and walking during social distancing, future prospective and randomized controlled trials are needed to investigate the effects of the SRTT program on quality of life, walking, posture, and FOG in people with PD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Filipe Oliveira de Almeida who reviewed the manuscript. Participants from the Movement Disorders Clinic from the School of Medicine of the University of São Paulo and the Brazil Parkinson Association for their commitment to study, and CAPES.
Author contributions
The authors’ contributions were as follows: ET, AMN, MVF, EO, FR, ERB, and CSB. Designed research: ET, AMN, MVF, EO, FR, ERB, and CSB; Conducted research: ET, AMN, MVF, EO, FR, ERB, and CSB; Provided essential materials: ET and CSB; Analyzed data/Statistical analysis: ET, AMN, MVF, EO, FR, ERB, and CSB; Wrote paper: ET, AMN, MVF, EO, FR, ERB, and CSB; Primary responsibility for final content: CSB. All authors: read and approved the manuscript.
Funding
This study was supported by Cordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES grant number 88887.464392/2019–00).
Availability of data and materials
All data supporting the results of this study may be made available from the corresponding author on reasonable request.
Declarations
Conflict of interest
ET, AMN, MVF, EO, FR, ERB, and CSB declare that they have no conflicts of interest with the content of this study.
Ethics approval
This study was performed in line with the principles of the 1964 Helsinki Declaration. Approval was obtained from the local Ethical Review Board (No. 46012621.7.0000.5390). Trial registration number (No: RBR-54sttfk) and date of registration (09/14/2021) for retrospective trials. The study was registered at the Brazilian Clinical Trials Registry (ReBEC).
Consent to participate
Each participant provided written consent after being informed of the purpose of the study, experimental procedures, and potential risks.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Calbi M, Langiulli N, Ferroni F, Montalti M, Kolesnikov A, Gallese V, et al. The consequences of COVID-19 on social interactions: an online study on face covering. Sci Rep. 2021;11:2601. doi: 10.1038/s41598-021-81780-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreira-Neto A, Martins B, Miliatto A, Nucci MP, Silva-Batista C. Can remotely supervised exercise positively affect self-reported depressive symptoms and physical activity levels during social distancing? Psychiatry Res. 2021;301:113969. doi: 10.1016/j.psychres.2021.113969. [DOI] [PubMed] [Google Scholar]
- 3.Coupet S, Nicolas G, Louder CN, Meyer M. When public health messages become stressful: Managing chronic disease during COVID-19. Social Sciences & Humanities Open. 2021;4:100150. doi: 10.1016/j.ssaho.2021.100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonini A, Leta V, Teo J, Chaudhuri KR. Outcome of Parkinson's disease patients affected by COVID-19. Movement Disorders: Official Journal of the Movement Disorder Society. 2020 doi: 10.1002/mds.28104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva-Batista C.; Coelho DB.; Junior RCF.; Almeida LR.; Guimaraes A.; Nobrega KCC, et al. (2021) Multidimensional factors can explain the clinical worsening in people with Parkinson's disease during the COVID-19 pandemic: a multicenter cross-sectional trial. Frontiers in Neurology12: 708433. DOI: 10.3389/fneur.2021.708433. [DOI] [PMC free article] [PubMed]
- 6.Prasad S, Holla VV, Neeraja K, Surisetti BK, Kamble N, Yadav R, et al. Parkinson's Disease and COVID-19: Perceptions and Implications in Patients and Caregivers. Movement disorders : official journal of the Movement Disorder Society. 2020 doi: 10.1002/mds.28088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luis-Martinez R, Di Marco R, Weis L, Cianci V, Pistonesi F, Baba A, et al. Impact of social and mobility restrictions in Parkinson's disease during COVID-19 lockdown. BMC Neurol. 2021;21:332. doi: 10.1186/s12883-021-02364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues-de-Paula F.; Lana RC.; Lopes LKR.; Cardoso F.; Lindquist ARR.; Piemonte MEP, et al. Determinants of the use of physiotherapy services among individuals with Parkinson's disease living in Brazil. Arquivos de neuro-psiquiatria. 2018, 76, 592–8. DOI: 10.1590/0004-282X20180087. [DOI] [PubMed]
- 9.Langer A, Gassner L, Flotz A, Hasenauer S, Gruber J, Wizany L, et al. How COVID-19 will boost remote exercise-based treatment in Parkinson's disease: a narrative review. NPJ Parkinson's disease. 2021;7:25. doi: 10.1038/s41531-021-00160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilat M, Ginis P, Zoetewei D, De Vleeschhauwer J, Hulzinga F, D'Cruz N, et al. A systematic review on exercise and training-based interventions for freezing of gait in Parkinson's disease. NPJ Parkinson's disease. 2021;7:81. doi: 10.1038/s41531-021-00224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schenkman M, Cutson TM, Kuchibhatla M, Chandler J, Pieper CF, Ray L, et al. Exercise to improve spinal flexibility and function for people with Parkinson's disease: a randomized, controlled trial. J Am Geriatr Soc. 1998;46:1207–1216. doi: 10.1111/j.1532-5415.1998.tb04535.x. [DOI] [PubMed] [Google Scholar]
- 12.Tomlinson CL.; Patel S.; Meek C.; Herd CP.; Clarke CE.; Stowe R, et al. Physiotherapy versus placebo or no intervention in Parkinson's disease. The Cochrane database of systematic reviews. 2013, CD002817. DOI: 10.1002/14651858.CD002817.pub4. [DOI] [PubMed]
- 13.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Movement disorders : official journal of the Movement Disorder Society. 2004;19:1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 15.Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson's disease and their carers. Gait Posture. 2009;30:459–463. doi: 10.1016/j.gaitpost.2009.07.108. [DOI] [PubMed] [Google Scholar]
- 16.Brown MJ, Almeida QJ. Evaluating dopaminergic system contributions to cued pattern switching during bimanual coordination. Eur J Neurosci. 2011;34:632–640. doi: 10.1111/j.1460-9568.2011.07773.x. [DOI] [PubMed] [Google Scholar]
- 17.Fathipour-Azar Z, Azad A, Akbarfahimi M, Behzadipour S, Taghizadeh G. Symmetric and asymmetric bimanual coordination and freezing of gait in Parkinsonian patients in drug phases. Ann N Y Acad Sci. 2022;1511:244–261. doi: 10.1111/nyas.14759. [DOI] [PubMed] [Google Scholar]
- 18.Colosimo C, Merello M, Hughes AJ, Sieradzan K, Lees AJ. Motor response to acute dopaminergic challenge with apomorphine and levodopa in Parkinson's disease: implications for the pathogenesis of the on-off phenomenon. J Neurol Neurosurg Psychiatry. 1996;60:634–637. doi: 10.1136/jnnp.60.6.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva-Batista C, Mattos EC, Corcos DM, Wilson JM, Heckman CJ, Kanegusuku H, et al. Resistance training with instability is more effective than resistance training in improving spinal inhibitory mechanisms in Parkinson's disease. J Appl Physiol. 1985;2017(122):1–10. doi: 10.1152/japplphysiol.00557.2016. [DOI] [PubMed] [Google Scholar]
- 20.Peto V, Jenkinson C, Fitzpatrick R. PDQ-39: a review of the development, validation and application of a Parkinson's disease quality of life questionnaire and its associated measures. J Neurol. 1998;245(Suppl 1):S10–S14. doi: 10.1007/pl00007730. [DOI] [PubMed] [Google Scholar]
- 21.Fahn S.; Elton RL.; Members. UP. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinson’s disease, Vol 2 Florham Park, NJ: Macmillan Healthcare Information. 1987, 153–63, 293–304. DOI: 959 [pii].
- 22.Stillerova T, Liddle J, Gustafsson L, Lamont R, Silburn P. Remotely Assessing Symptoms of Parkinson's Disease Using Videoconferencing: A Feasibility Study. Neurol Res Int. 2016;2016:4802570. doi: 10.1155/2016/4802570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer R, Bortolini T, Karl JA, Zilberberg M, Robinson K, Rabelo A, et al. Rapid Review and Meta-Meta-Analysis of Self-Guided Interventions to Address Anxiety, Depression, and Stress During COVID-19 Social Distancing. Front Psychol. 2020;11:563876. doi: 10.3389/fpsyg.2020.563876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckingham SA, Sein K, Anil K, Demain S, Gunn H, Jones RB, et al. Telerehabilitation for physical disabilities and movement impairment: A service evaluation in South West England. J Eval Clin Pract. 2022 doi: 10.1111/jep.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim SB, Horslen BC, Davis JR, Allum JH, Carpenter MG. Benefits of multi-session balance and gait training with multi-modal biofeedback in healthy older adults. Gait Posture. 2016;47:10–17. doi: 10.1016/j.gaitpost.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Rhea MR. Determining the magnitude of treatment effects in strength training research through the use of the effect size. J Strength Cond Res. 2004;18:918–920. doi: 10.1519/14403.1. [DOI] [PubMed] [Google Scholar]
- 27.Lixandrao ME, Ugrinowitsch C, Laurentino G, Libardi CA, Aihara AY, Cardoso FN, et al. Effects of exercise intensity and occlusion pressure after 12 weeks of resistance training with blood-flow restriction. Eur J Appl Physiol. 2015;115:2471–2480. doi: 10.1007/s00421-015-3253-2. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82:591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- 29.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale (NJ): L. Erlbaum Associates. Neurology. 1988, 29–35. DOI: 10.4324/9780203771587.
- 30.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 31.Chen K, Tan Y, Lu Y, Wu J, Liu X, Zhao Y. Effect of Exercise on Quality of Life in Parkinson's Disease: A Systematic Review and Meta-Analysis. Parkinson's disease. 2020;2020:3257623. doi: 10.1155/2020/3257623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegert C, Hauptmann B, Jochems N, Schrader A, Deck R. ParkProTrain: an individualized, tablet-based physiotherapy training programme aimed at improving quality of life and participation restrictions in PD patients - a study protocol for a quasi-randomized, longitudinal and sequential multi-method study. BMC Neurol. 2019;19:143. doi: 10.1186/s12883-019-1355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isernia S, Di Tella S, Pagliari C, Jonsdottir J, Castiglioni C, Gindri P, et al. Effects of an Innovative Telerehabilitation Intervention for People With Parkinson's Disease on Quality of Life, Motor, and Non-motor Abilities. Front Neurol. 2020;11:846. doi: 10.3389/fneur.2020.00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connor S, Hanlon P, O'Donnell CA, Garcia S, Glanville J, Mair FS. Understanding factors affecting patient and public engagement and recruitment to digital health interventions: a systematic review of qualitative studies. BMC Med Inform Decis Mak. 2016;16:120. doi: 10.1186/s12911-016-0359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maetzler W, Liepelt I, Berg D. Progression of Parkinson's disease in the clinical phase: potential markers. The Lancet Neurology. 2009;8:1158–1171. doi: 10.1016/S1474-4422(09)70291-1. [DOI] [PubMed] [Google Scholar]
- 36.Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology. 2010;75:116–124. doi: 10.1212/WNL.0b013e3181e7b688. [DOI] [PubMed] [Google Scholar]
- 37.Silva-Batista C, de Lima-Pardini AC, Nucci MP, Coelho DB, Batista A, Piemonte MEP, et al. A Randomized, Controlled Trial of Exercise for Parkinsonian Individuals With Freezing of Gait. Movement disorders : official journal of the Movement Disorder Society. 2020;35:1607–1617. doi: 10.1002/mds.28128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen X, Wong-Yu IS, Mak MK. Effects of Exercise on Falls, Balance, and Gait Ability in Parkinson's Disease: A Meta-analysis. Neurorehabil Neural Repair. 2016;30:512–527. doi: 10.1177/1545968315613447. [DOI] [PubMed] [Google Scholar]
- 39.Ellis T, Boudreau JK, DeAngelis TR, Brown LE, Cavanaugh JT, Earhart GM, et al. Barriers to exercise in people with Parkinson disease. Phys Ther. 2013;93:628–636. doi: 10.2522/ptj.20120279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gandolfi M, Geroin C, Dimitrova E, Boldrini P, Waldner A, Bonadiman S, et al. Virtual Reality Telerehabilitation for Postural Instability in Parkinson's Disease: A Multicenter, Single-Blind, Randomized. Controlled Trial BioMed Research International. 2017;2017:7962826. doi: 10.1155/2017/7962826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cramer SC, Dodakian L, Le V, See J, Augsburger R, McKenzie A, et al. Efficacy of Home-Based Telerehabilitation vs In-Clinic Therapy for Adults After Stroke: A Randomized Clinical Trial. JAMA Neurol. 2019;76:1079–1087. doi: 10.1001/jamaneurol.2019.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the results of this study may be made available from the corresponding author on reasonable request.