Abstract
To compare postoperative complications, functional rehabilitation, surgical outcomes of the radial artery forearm free flap (RAFFF) and split thickness skin graft (STSG) reconstruction of postsurgical defect in T2 lesions of cancer oral cavity. Observational Prospective comparative study. Academic tertiary referral centre. In our study of forty four patients, after tumour resection, half underwent reconstruction using RAFFF (Group I) and another half by STSG (Group II). All of the patients were followed postoperatively to determine and compare their functional outcomes related to donor site and recipient site complications, speech, deglutition and mouth opening. The speech intelligibility and deglutition were each assessed using Articulation Handicap Index and Vedio-fluoroscopy using the Functional oral intake scale. Operative time for STSG reconstruction was shorter at 2.2 ± 0.97 SD hours compared to 5.9 ± 1.24 SD hours for RAFFF reconstruction. Hospital stay was 8.3 ± 1.19 SD days for STSG patients and 12.6 ± 1.7 SD days for RAFFF patients. The functional outcomes of speech quality and swallowing were near comparable in both groups but the donor site complications were significant in the RAFFF group. Operative time, hospital stay and donor site complications are both significantly reduced with the STSG as opposed to RAFF. Functional and oncologic results of both methods are near comparable. To conclude, STSG can be used for reconstruction of the post-surgical defects in T2 lesions of the tongue.
Keywords: Oral cancer, Tongue carcinoma, T2 lesion, RAFFF, STSG, Surgical outcome, Speech, Deglutition
Introduction
The oral cavity cancers represent the sixteenth most common cancer in the world. The annual incidence of head and neck cancers worldwide is 377,713 which are approximately 2% of total cancer cases [1]. The most frequent site of oral cancer is the tongue and the most common histological type of oral cavity cancer is squamous cell carcinoma [1]. The treatment of choice for any H&N cancer is surgical resection and reconstruction using vascularised or non-vascularized grafts followed by radiotherapy and/or chemotherapy except in very advanced diseases where the resection is avoided. Small tumours, T1 lesions (less than 2 cm) are excised followed by primary closure or placement of STSG. T2 lesions (2–4 cm) are treated by excision followed by reconstruction using skin graft (STSG, FTSG) or free tissue transfer (radial artery forearm free flap/RAFFF, anterolateral thigh free flap/ALTFF, lateral arm free flap/LAFF, latissimus dorsi myo-cutaneous free flap/LDMCFF). Tumours more than 4 cm i.e. T3 and tumours involving cortical bone, T4a lesions are treated by excision followed by microvascular free tissue transfer or pedicled flap reconstruction [2, 3].
The decision on how to reconstruct a tongue defect depends on several factors: (1) size of the tongue defect, (2) availability of neck donor vessels, (3) floor-of-mouth involvement, or (4) presence of concurrent mandible bony defect and/or concurrent oropharyngeal defect. The treatment algorithm for various tongue defects can be seen in Fig. 1 [4].
Fig. 1.
Treatment algorithm for defects of the tongue, tongue and pharynx, and tongue and mandible [4]
Radial artery forearm free flap (RAFFF) is the most common free flap for oropharyngeal reconstruction, because of its surgical anatomy making it easier for surgeons to harvest it. The technique of this flap was developed at Shenyan Military Hospital of China, hence also called Chinese flap [4]. This flap is based on the radial artery and the accompanying paired venae comitantes. This flap can incorporate a sensory nerve-lateral antebrachial cutaneous nerve of the forearm which can provide a sensate flap for oral reconstruction. This flap can include the bony segment of the radius providing an osteo-cutaneous flap, which is suitable for mandible reconstruction [5–7]. The RAFFF can be harvested proximally or distally. Distal flaps are thinner, hairless, better quality and have a long pedicle. Proximal flaps are thicker and muscle bellies provide an ideal bed for split skin closure of donor site: the long pedicle may be raised distally and used for anastomosis thus providing retrograde flow [4]. Split-thickness skin graft (STSG) is the skin graft of choice, containing the epidermis and a portion of dermis. Since the inception of the split thickness grafting technique by Ollier in 1872, the thickness of the graft has been considered as a critical factor for healing and reconstruction of the recipient site. The thickness of the graft divides it into: thin (0.013–0.033 cm), medium (0.033–0.046 cm) and thick (0.046–0.076) [8].
The purpose and priorities of reconstruction include restoring oral cavity lining, restoring the bulk & volume of a tissue defect, maintaining oral competence i.e. function of speech and swallowing and providing an acceptable aesthetic result. The choice of reconstructive options depends on patients’ co-morbidities, the surgical defect, any future treatments including radiotherapy and donor site morbidity [3]. With the advent of oral reconstructive surgeries and evolving options available, the management of oral cancers has been revolutionised. There is not enough evidence relating to the positive outcome of different reconstructive procedures for head and neck defects and hence, there is not a definite reconstructive procedure for a particular tissue defect. This paper aims to discuss the two options for reconstruction following ablative surgery of T2 lesions of the tongue when primary closure is not feasible, a non-vascularised graft, STSG and a microvascular reconstruction using RAFFF, regarding functional rehabilitation, surgical outcome and complications.
Materials and Methods
This descriptive prospective study was conducted in the department of Otolaryngology and Head & Neck Surgery, SMGSH, Jammu from June 2018 to July 2022. The study population included 44 patients with following criteria:
Inclusion criteria:
T2 lesions of carcinoma of the tongue.
Tumour 2 cm or less.
Depth of invasion more than 5 mm but no more than 10 mm
Patients with negative neck nodes were managed by tumour excisions and defect reconstruction (STSG or RAFFF).
Exclusion criteria:
Patients with T1 lesions (who underwent primary closure),
T2 lesions of carcinoma of the tongue with tumour size more than 2 cm but less than 4 cm,
T3-T4 lesions (who underwent excision followed by mandibular reconstruction),
Proven nodal metastasis,
T2 lesions of other subsites of the oral cavity,
Patients with problems of range of motion or sensation of the hands or forearms prior to operation.
Patients not willing for surgery or unfit for surgery
Informed written consent was taken followed by a detailed history and local examination which included examination of the tumour and donor site. A complete registration of tumour features (including the clinical and histological diagnosis, location of the tumour, prior treatments such as radiation or chemotherapy, and extension of the defect after tumour ablation) was undertaken which led to the decision of the type of reconstruction procedures (type and composition of the vascularised free flap, size of the skin paddle and type of vascular anastomosis). All routine preoperative investigations were done (blood investigations, flexible endoscopic examination of the upper airways; neck ultrasonography; head and neck CT scan; biopsy diagnostic for SCC, chest X-ray). Allen test was performed in all cases to evaluate the contra-lateral flow for the recovery of the superficial palmar arch by the ulnar artery. The patients were randomly divided into two groups to eliminate selection bias. The resection included tumour with a 1.5–2 cm margin, which constituted removal of 1/3–1/4th of the tongue (partial glossectomy), followed by elective neck dissection. In furtherance of repairing the defect, half of the patients underwent reconstruction using RAFFF (Group I) and others by STSG (Group II). There were two surgical teams: one exclusively performed resection of the primary tumour of the patients planned for reconstruction by RAFFF or STSG; similarly, another team undertook the reconstruction of tissue defect, whether planned for RAFFF or STSG.
In group 1, after tumour resection (by partial glossectomy), distally placed RAFFF was harvested preferentially from the non-dominant arm and the soft tissue defect was closed by STSG and dorsal forearm splinting was applied for 1 week post-operatively. After inset and positioning the pedicle, microvascular anastomosis of radial artery was done with the branches of the external carotid artery using 8–0 interrupted sutures. After confirming good venous outflow from the flap, a 3 mm coupler was used for anastomosis of the venae comitantes and cephalic vein to the facial vein. Acland testing confirmed a patent venous anastomosis. The average size of the skin paddle of RAFFF was 5 × 3 cm and 7 cm for vascular pedicle. Considering surgical details, tracheotomy was done in all the cases to assure the patency of the airway after the surgical procedure. At regular intervals post-operative check was done: colour, turgor, viability (scratch test/pin prick test) of the flap/graft (Figs. 2, 3). In group II, after tumour resection, the STSG was taken using Downey’s skin grafting blade after painting and draping the donor site. The graft was meshed, placed on the recipient bed, dermis down, and bolstered with sutures into the defect. A bolster dressing was done to decrease shearing forces on the graft [8].
Fig. 2.
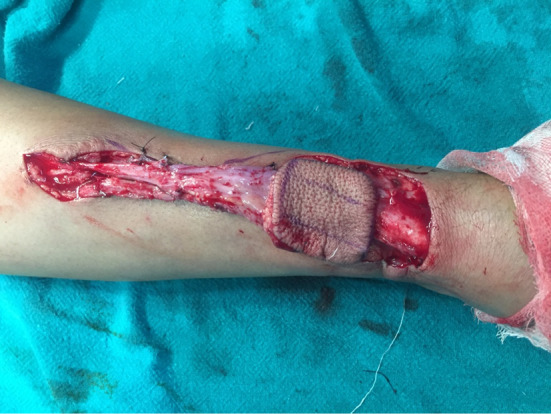
Intra-operative photograph showing harvest of RAFFF
Fig. 3.

Anastomosis between the radial artery and the facial artery
The patients were monitored post operatively, followed up at 4 weeks and 6 months and the two methods of reconstruction were compared using different parameters. The functional outcome was measured by objective tests like VFSS for swallowing assessment and AHI for speech articulation assessment (Figs. 4, 5).
Fig. 4.

Post-operative picture of RAFFF at 6 months follow-up
Fig. 5.

Post-operative picture of donor site of RAFFF at 6 months follow-up
The Articulation Handicap Index (AHI), which has been developed on the basis of the 30 items of the German version of the Voice Handicap Index (VHI), was used for the objective evaluation of speech function in the Speech Therapy Department of our hospital. Three untrained volunteers with normal hearing listened to the tape and transcribed the sounds. The mean AHI score was calculated as the percentage of the words correctly understood by the volunteers. The Grading used was: using a four-category scale with the categories ‘Normal articulation’, ‘Mild impairment’, ‘Moderate impairment’, and ‘Severe impairment’ [9].
| Grade | Description | AHI score |
|---|---|---|
| 1 | Normal articulation | 0–13 |
| 2 | Mild impairment | 14–44 |
| 3 | Moderate impairment | 55–76 |
| 4 | Severe impairment | 77–120 |
VFSS (Vedio-fluoroscopic swallowing study), also called a modified barium swallow or dynamic swallowing study), a video radiographic swallowing study procedure, for oropharyngeal dysphagia, was performed following a standardised VFSS protocol, according to Logemann [10, 11]. The patients were positioned in their usual eating/ drinking position. Test boluses were mixed with radio-opaque material to enable visualization. Test boluses were given in increasing volume to minimize the risk of large amounts of aspiration. A range of different food textures were assessed in a graded fashion, conducted in the lateral position. Images are recorded digitally, for analysis and interpretation [11, 12]. For the purpose of this study focussing on functional outcome, subjective evaluation of deglutition was done by use of the functional oral intake scale (FOIS), a 7-point ordinal scale [12].
| Level | Description |
|---|---|
| 1 | Nothing by mouth |
| 2 | Tube dependent with minimal attempts of food or liquid |
| 3 | Tube dependent with consistent oral intake of food or liquid |
| 4 | Total oral diet of a single consistency |
| 5 | Total oral diet with multiple consistencies but requiring special preparation or compensations |
| 6 | Total oral diet with multiple consistencies without special preparation but with specific food limitations |
| 7 | Total oral diet with no restrictions |
Complications were evaluated and classified as general complications, complications of the flap (such as flap oedema ischemia, partial or total necrosis, fistula formation) and donor site complications (non-healing, dehiscence, scar formation, reduced sensation, reduced range of motion).
Data Analysis
The study cohort was divided according to choice of flap: those reconstructed with RAFFF and those with STSG. The recorded data was compiled and entered in a spreadsheet (Microsoft Excel) and then exported to the data editor of SPSS Version 20.0 (SPSS Inc., Chicago, Illinois, USA). Continuous variables were expressed as Mean ± SD and categorical variables were summarized as frequencies and percentages. Graphically the data was presented by bar diagrams. Student’s independent t-test was employed for comparing continuous variables. Chi-square test or Fisher’s exact test, whichever appropriate, was applied for comparing categorical variables. Intra-group analysis of data was done by applying McNemar chi-square test. A p-value of less than 0.05 was considered statistically significant.
Results
Demographics
In our study, out of 44 patients, 22 underwent reconstruction of tissue defect by RAFFF and 22 by STSG after resection of the tumour. A mean age of 43.4 years was observed (range = 25–60), out of which 42 patients (95.4%) were males and only 2 (4.4%) patients were females (as shown in Table 1 and Fig. 6).
Table 1.
Age distribution, gender distribution, site of the oral tumour, histopathological grade in two groups
| 1: Age distribution of study patients in two groups | |||||
|---|---|---|---|---|---|
| Group | N | Mean | SD | Range | P-value |
| RAFFF | 22 | 42.8 | 1.97 | 25–58 | 0.742 |
| STSG | 22 | 43.1 | 2.41 | 27–60 | |
| 2: Gender distribution | |||||
|---|---|---|---|---|---|
| Gender | RAFFF | STSG | P-value | ||
| No | %age | No | %age | ||
| Male | 21 | 95.45 | 21 | 95.45 | 1.000 |
| Female | 1 | 4.54 | 1 | 4.54 | |
| 3: Site of oral cavity tumour in two groups | |||||
|---|---|---|---|---|---|
| Site of tumour | RAFFF | STSG | P-value | ||
| No | %age | No | %age | ||
| R Lateral border of tongue | 11 | 50.0 | 9 | 40.9 | 0.809 |
| Dorsal surface of tongue | 2 | 9.1 | 3 | 13.7 | |
| L Lateral border of tongue | 9 | 40.9 | 10 | 45.4 | |
| 4: Histopathological grade in two groups | |||||
|---|---|---|---|---|---|
| Histopathological Grade | RAFFF | STSG | p-value | ||
| No | %age | No | %age | ||
| Well differentiated | 10 | 45.4 | 11 | 50.0 | 0.911 |
| Moderately differentiated | 11 | 50.0 | 9 | 40.9 | |
| Poorly differentiated | 1 | 4.5 | 2 | 9.0 | |
The difference is not statistically significant.
Fig. 6.

Distribution of gender in the two groups
Tumour Characteristics
The subsite of tumour was right lateral border of tongue in 20 (45.45%) patients, dorsal surface of tongue in 6 (13.6%) patients and Left lateral border of tongue in 18 patients (40.6%). According to the histopathological grading, 24 (54.16%) patients were well differentiated, 16 (36.3%) patients were moderately differentiated and 4 (9.1%) patients were poorly differentiated. The difference in age distribution, gender distribution, site of tumour distribution and HPE grade distribution between the two cohorts was statistically insignificant (as shown in Table 1).
Operative Time and Hospital Stay
The time taken to harvest RAFFF was more as compared to STSG harvesting, which led to overall variation in the operative time. Hence, after analysis, it was found that Operative time for STSG reconstruction was shorter at 2.2 ± 0.97 SD hours compared to 5.9 ± 1.24 SD hours for RAFFF reconstruction. Hospital stay was 8.3 ± 1.19 SD days for STSG patients and 12.6 ± 1.7 SD days for RAFFF patients. The total operative time and the hospital stay were more in the RAFFF group as compared to the STSG group and the difference between the two groups was statistically significant (p-value = 0.002, p-value < 0.001 respectively) (as shown in Table 2, Figs. 7 and 8).
Table 2.
Comparison based on the operative time (hours) and hospital stay in two groups
| Group | N | Mean | SD | Range | P-value |
|---|---|---|---|---|---|
| Comparison based on operative time (hours) in two groups | |||||
| RAFFF | 22 | 5.9 | 1.24 | 5–7 h | < 0.001* |
| STSG | 22 | 2.2 | 0.77 | 2–3 h | |
| Comparison based on hospital stay (days) in two groups | |||||
| RAFFF | 22 | 12.6 | 1.73 | 11–13 Days | < 0.001* |
| STSG | 22 | 8.3 | 1.19 | 7–9 Days | |
Both are significantly more in group RAFFF
Fig. 7.

Means and range of operative time in the two groups
Fig. 8.

Means and range of hospital stay in the two groups
Complications
All the patients developed one or other immediate post-operative complications like oedema, scab formation, local infection, traction injury or mild dehiscence; which after conservative management, reversed except in one patient where revision vascular anastomosis was required followed by surgical treatment of oro-cutaneous fistula.
Recipient site complications: In group I, 3 patients (13.6%) developed partial necrosis, 2 patients (9.0%) developed hematoma and 1 patient (4.5%) developed oro-cutaneous fistula had flap uptake failure and among these, flap failure occurred in 3 patients (13.6%). In group II, 4 patients (18.17%) developed partial necrosis, 1 patient (4.5%) developed total necrosis, 1 (4.5%) patient had graft contracture and 1 graft (4.5%) dehisced and among these graft uptake failure occurred in 4 patients (18.1%). On statistical analysis, the complications were more in the STSG group, however the difference was not significant (as shown in Table 3 and Fig. 9).
Table 3.
Comparison based on postoperative complications in two groups
| Postoperative complications | RAFFF | STSG | P-value | ||
|---|---|---|---|---|---|
| No | %age | No | %age | ||
| Partial necrosis | 3 | 13.6 | 4 | 18.1 | 0.689 |
| Hematoma | 2 | 9.0 | 0 | 0.0 | 1.000 |
| Total necrosis | 0 | 0.0 | 1 | 4.5 | 1.000 |
| Contracture | 0 | 0 | 1 | 4.5 | 1.000 |
| Dehiscence | 0 | 0.0 | 1 | 4.5 | 1.000 |
| Fistula | 1 | 4.5 | 0 | 0.0 | 1.000 |
Post-operative complications were more in group RAFFF but it was not statistically significant
Fig. 9.
Post-operative recipient site complications
Donor site complications: In group I, donor site scar formation was seen in all 22 (100%) patients, reduced range of motion were seen in 7 patients (36.3%) and, reduced sensation in 9 patients (40.9%). In group II, 10 patients (45.7%) had minimal scar formation and only 2 (9.0%) complained of slightly reduced range of motion. Donor site complications were significantly more in group RAFFF. Range of motion was analysed following reconstruction and demonstrated reduced range of motion in RAFFF, but the significant differences between the two groups were not statistically significant at any time point. Reduced sensation and scar formation were more in the RAFFF group and the difference between the two groups was statistically significant (p-value = 0.37 and 0.005 respectively) (as shown in Table 4 and Fig. 10).
Table 4.
Comparison based on donor site complications in two groups
| Donor site complications | RAFFF | STSG | P-value | ||
|---|---|---|---|---|---|
| No | %age | No | %age | ||
| Reduced range of motion | 7 | 36.3 | 2 | 9.0 | 0.316 |
| Scar formation | 22 | 100 | 10 | 45.7 | 0.005* |
| Reduced sensation | 9 | 40.7 | 0 | 0.0 | 0.037* |
Donor site complications are significantly more in group RAFF
Fig. 10.

Post-operative donor site complications
Speech
Speech articulation test was done preoperatively and after 6 months of post-operative setting. In group I, preoperatively, 4 (18.7%) patients had a normal speech, 7 (31.7%) patients had a mild impairment, 7 (31.7%) patients had a moderate impairment and 4 patients (18.6%) had a severe impairment. In the post-operative setting, 5 patients (22.7%) had normal speech, 11 patients (50.1%) had a mild impairment, 4 patients (18.1%) had a moderate impairment and 2 patients (9.1%) had a severe impairment. In group II, preoperatively, 3 (13.6%) patients had a normal speech, 5 patients (22.7%) had a mild impairment, 9 patients (40.9.1%) had a moderate impairment and 5 patients (22.7%) had a severe impairment. Post-operatively, 7 patients (31.7%) had a normal speech, 9 patients (22.6%) had a mild impairment, 4 patients (33.3%) had a moderate impairment and 2 patients (9.1%) had a severe impairment. Post-operative results of both the groups were compared [as shown in Fig. 11 and Table 5] and on statistical evaluation, the speech intelligibility in both groups was comparable.
Fig. 11.
Bar diagram showing comparison based on speech in two groups showing that the speech intelligibility in both the groups was comparable when compared to each other but it was increased significantly in both groups postoperatively
Table 5.
Comparison based on speech in two groups showing that the speech intelligibility in both the groups was comparable when compared to each other but it was increased significantly in both groups postoperatively
| Grade | RAFFF | STSG | p-value | |||
|---|---|---|---|---|---|---|
| No | %age | No | %age | |||
| Pre-op | Grade 1 | 4 | 18.7 | 3 | 13.6 | 0.31 |
| Grade 2 | 7 | 31.7 | 5 | 22.7 | ||
| Grade 3 | 7 | 31.7 | 9 | 40.9 | ||
| Grade 4 | 4 | 18.6 | 5 | 22.6 | ||
| Post-op | Grade 1 | 5 | 22.7 | 7 | 31.7 | 0.363 |
| Grade 2 | 11 | 50.0 | 9 | 22.6 | ||
| Grade 3 | 4 | 18.1 | 4 | 33.3 | ||
| Grade 4 | 2 | 9.0 | 2 | 9.0 | ||
| P-value (Preop vs Postop) | 0.027* | 0.039* | ||||
Deglutition
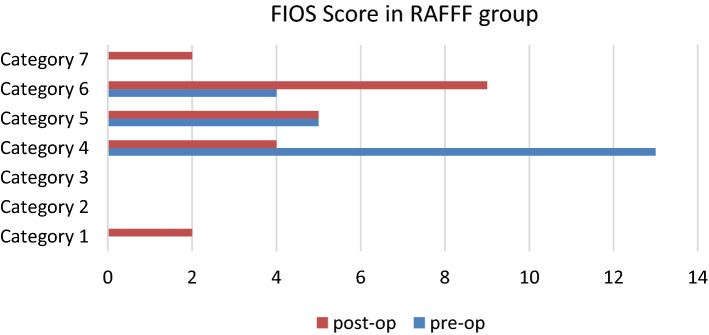
Deglutition function was assessed using VFSS and FIOS scoring was done in patients preoperatively and after a post-operative period of 6 months. In group 1, 2 patients had Nothing by mouth, 4 had a total oral diet of a single consistency, 5 were on a total oral diet with multiple consistencies but requiring special preparation or compensations, 9 were on a total oral diet with multiple consistencies without special preparation but with specific food limitations, 2 were on a total oral diet with no restrictions (as shown in Fig. 12). In group II, 1 patient had Nothing by mouth, 1 patient was tube dependent with consistent oral intake of food or liquid, 3 had a total oral diet of a single consistency, 9 were on a total oral diet with multiple consistencies but requiring special preparation or compensations, 5 were on a total oral diet with multiple consistencies without special preparation but with specific food limitations, 3 patients were on a total oral diet with no restrictions. The post-operative function improved in both groups but the difference was significant in RAFFF. The same function was near comparable between the two groups and the difference was statistically insignificant [as shown in Table 6].
Fig. 12.
Bar diagram showing deglutition in RAFFF group, the quality of Oral intake is significantly improved in group RAFFF post-operatively
Table 6.
Comparison based on deglutition in two groups statistically the post-operative function was comparable in the two groups
| Level | RAFFF | STSG | p-value | |||
|---|---|---|---|---|---|---|
| No | %age | No | %age | |||
| Pre-op | 1 | 0 | 0.0 | 0 | 0.0 | 0.713 |
| 2 | 0 | 0.0 | 0 | 0.0 | ||
| 3 | 0 | 0.0 | 0 | 0.0 | ||
| 4 | 13 | 59.1 | 9 | 40.9 | ||
| 5 | 5 | 22.7 | 7 | 31.8 | ||
| 6 | 4 | 18.1 | 6 | 27.2 | ||
| 7 | 0 | 0.0 | 0 | 0.0 | ||
| Post-op | 1 | 2 | 9.0 | 1 | 4.5 | 0.701 |
| 2 | 0 | 0.0 | 0 | 0.0 | ||
| 3 | 0 | 0.0 | 1 | 4.5 | ||
| 4 | 4 | 18.1 | 3 | 13.6 | ||
| 5 | 5 | 22.7 | 9 | 40.9 | ||
| 6 | 9 | 40.9 | 5 | 22.6 | ||
| 7 | 2 | 9.0 | 3 | 13.6 | ||
| P-value (Pre-op vs Post-op) | 0.043* | 0.179 | ||||
The quality of Oral intake is significantly improved in group RAFFF post-operatively
Discussion
Cancer oral cavity is one of the most common cancers that are encountered by otolaryngologists in India. Because of its site, it is easily detectable, easily diagnosed in early stages and hence if treated early can significantly alter the course of the disease. Our study focuses on T2 tongue lesions and the single-stage tumour resection and reconstruction of the defect by RAFFF and STSG. A Margin of 1.5 to 2 cm is recommended for squamous cell carcinomas; therefore, even T2 lesions of less than 2 cm can lead to relatively large surgical defects which cannot heal by primary closure, hence requiring reconstruction using a skin graft or free tissue transfer [3]. The advantages of RAFFF are consistent surgical anatomy which makes it easier for the surgeon to harvest it compared to other flaps, thin skin and thin subcutaneous tissue and re-innervation potential. The disadvantages include volume of tissue, colour match, and donor site scar [4]. The advantages of STSG are easier to harvest, and less operative time [8]. As described earlier, the goal of reconstruction is to regain swallowing ability, having intelligible speech and later on better QOL. It is debatable whether overall speech and swallow outcomes are improved after reconstruction.
Operative Time and Hospital Stay
Our study indicates that the operative time of group RAFFF was 5.9 ± 1.24 h (range = 5–7 h) and the hospital stay was 12.6 ± 1.73 days (range = 11–13). This was in accordance with a study by Kao H.K. et al., who compared operative time and hospital stay of Medial Sural Artery Perforator Flap with the Radial Forearm Free Flap [13]. It was seen that both operative time and hospital stay were significantly more in the RAFFF group. In a study by Hoefert et al., tumor resection and reconstruction by free microvascular RFF showed the highest LoS (length of stay) of 19.6 days and tumour resection and reconstruction with STSG without tracheotomy with LOS of 10.2 days [14].
Peri-Operative Mortality
There was no peri-operative mortality in our study which is different from the results of previous research. Haughey et al. stated the peri-operative mortality in a series of 241 patients was 2.1% [15]. This was similar to the peri-operative mortality rates reported by other large series of free flap transfers to the head and neck 2% by Souter et al., 7.0% by Shestak et al., 2.0% by McNamara et al., 4.7% by Simpson et al., 6.3% by Jones et al. [7, 15–19]. The discrepancy could be due to the patients in our study that were lost to follow-up.
Post-Operative Complications
The post-operative recipient site complications developed in 4 patients (18.2%) in group RAFFF and 5 patients (22.6%) of subjects in group STSG, however after managing the complications, 2 flaps and 3 grafts were salvaged. The results of postoperative complications of our study corroborate with the results of previous studies. In a retrospective study by Fang et al., out of the 20 radial forearm flaps used to repair the buccal defects, only two flaps suffered venous obstruction and hematoma, respectively which were salvaged, thus, the flap survival rate was 100% [20]. Similarly, the success rate of RAFFF was reported as more than 95% by Kruse et al., 100% by Shibahara et al., and greater than 90% by Song et al. in their respective studies [21–23]. Regarding donor site complications, the results in our study concur favourably with previous findings in the literature. In group I, donor site scar formation was seen in all 22 (100%) patients, reduced range of motion was seen in 7 patients (36.3%), and reduced sensation in 9 patients (40.9%). This was relatable with the study by Kao HK et al., in which 100% of RAFFF patients developed pigmentation of donor area, 26.7% % complained of functional impairment and 80% had numbness at the site [13]. In group II, 10 patients (45.7%) had minimal scar formation and only 2 (9.0%) complained of a slightly reduced range of motion. Donor site complications were significantly more in group RAFFF.
Functional Outcome
The speech outcome in our study was significantly different in pre and post-operative settings. In group RAFFF (73.7%) patients had intelligible speech (grade1,2), which was in line with past studies. In a study by Chien et al., (89%) of 15 patients after RAFFF or ATTFF reconstruction for subtotal or total glossectomy defects developed intelligible speech [24]. Liao et al., stated that 100% of patients after RAFF for partial glossectomy defects retained intelligible speech [25]. Lam et al., reported that patients undergoing anterior tongue resection with free-flap reconstruction had a single word intelligibility of 90% preoperatively, 69.2% at 1 month postoperatively, and improved to 79.6% at 6 months. Sentence intelligibility for patients who underwent anterior tongue resection and reconstruction was 76.9% at 1-month post-surgery and 90.7% at 6 months [26]. In group STSG, 91.7% patients had intelligible speech (grade 1,2). The outcome was satisfactorily comparable between the two groups on follow-up visits.
In our study, 91.67% of the patients in each group have been found to regain deglutition and were free of NG tubes at 4 to 6 months after surgery. The outcome was satisfactorily comparable between the two groups on follow-up visits, however the post-operative deglutition function in group RAFFF was significantly better than group STSG. In contrast to our findings, in a series by Brown et al., 15 patients who underwent resection of the anterior tongue and RAFFF reconstruction, there was no significant difference found in the ability to swallow liquids or tongue mobility at 6 and 12 months after surgery [27]. This variation can be due to resection of > 50% of tongue in this series. Jain et al. reported in their study that in patients who underwent post-surgery rehabilitation therapy had statistically significant deglutition but did not show significant improvement from the preoperative setting [28]. In a study by Gabriele M et al., during FEES, a minor buccal and oral phase deficit was present in 9/14 patients (64%), 5 in the group which underwent microvascular reconstruction of the tongue defect and 4 in the group which underwent closure of the defect by primary healing or secondary intention [29]. Dzioba et al., studied swallow outcome using MDADI and EORTC-H&N35 subscales in 117 undergoing partial glossectomy with reconstruction (using RAFFF most commonly) and found no significant differences between pre-op and 6 months post-surgery function. However, the study cohort included resection of the floor of mouth, resection of the mandible and patients receiving post-operative RT/CT as well [30].
Limitations of Our Study
We are aware that our research has limitations like small sample size, single institution research, patients lost to follow up and it is plausible that these limitations could have influenced the results obtained. We have not compared the effect of radiotherapy on functional outcomes, since radiation causes increased shrinkage of tissue, leading to decreased bulk of the oropharyngeal tongue and thus, poor swallowing function. Similarly, other factors such as the flap type and reinnervation of the flap have also not been focused upon. Due to patients being residents of far-flung localities and the COVID-19 pandemic, patients were lost to follow up and long term survival could not be assessed.
Conclusion
Both the reconstructive techniques can be performed safely with high success rates. Radial artery forearm flap is the most reliable and efficient method for restoring the tissue bulk of post-surgical resection of the tumour site, giving the best functional outcome in terms of deglutition and aesthetics. Even with its numerous advantages, microvascular free flap reconstruction is both time-consuming, requires expertise and is associated with donor-site morbidities.
STSG provides just the adequate bulk to most of the post-surgical tongue defects of early cancers. It may offer advantages of decreased operative time, reduced duration of stay, reduced morbidity, and locally available with minimal deformity to the donor site while maintaining acceptable function as compared to the RAFFF. The evidence of non-inferiority of STSG as established in our study makes it a good option for reconstruction, when the patients have anaesthesia risk for prolonged surgical procedures and in institutions where free flap expertise is not available.
Authors’ Contribution
All authors contributed to the study conception and design. Material preparation, data collection, analysis and the first draft of the manuscript was written by Faizah Deva and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No funding was received for conducting this study. The authors declare that they have no financial interest.
Declarations
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Ethics Approval
Approval was obtained from the ethics committee of our college. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Ragbir M, Brown JS, Mehanna H. Reconstructive considerations in head and neck surgical oncology: United Kingdom national multidisciplinary guidelines. J Laryngol Otol. 2016;130(S2):S191–S197. doi: 10.1017/S0022215116000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent A, Kohlert S, Lee TS, Inman J, Ducic Y. Free-flap reconstruction of the tongue. Semin Plast Surg. 2019;33(1):38–45. doi: 10.1055/s-0039-1677789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soutar DS, Scheker LR, And TNSB, Mcgregor IA. The radial forearm flap: a versatile method for intra-oral reconstruction. Br J Plast Surg. 1983;36:1–8. doi: 10.1016/0007-1226(83)90002-4. [DOI] [PubMed] [Google Scholar]
- 5.Cha YH, Nam W, Cha IH, Kim HJ. Revisiting radial forearm free flap for successful venous drainage. Maxillofac Plastic Reconstr Surg. 2017;39(1):1–4. doi: 10.1186/s40902-017-0110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muhlbauer W, Herndl E, Stock W. The forearm flap. Plast Reconstr Surg. 1982;70(3):336–342. doi: 10.1097/00006534-198209000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Soutar DS, Widdowson WP. Immediate reconstruction of the mandible using a vascularized segment of radius. Head Neck Surg. 1986;8(4):232–246. doi: 10.1002/hed.2890080403. [DOI] [PubMed] [Google Scholar]
- 8.ME Braza, MP Fahrenkopf (2020) Split-thickness skin grafts StatPearls. Treasure Island (FL): StatPearls publishing; 2021 Jan. [PubMed]
- 9.Keilmann A, Konerding U, Oberherr C, et al. Articulation handicap index: an instrument for quantifying psychosocial consequences of impaired articulation. Eur Arch Otorhinolaryngol. 2016;273:4493–4500. doi: 10.1007/s00405-016-4143-x. [DOI] [PubMed] [Google Scholar]
- 10.Royal College of Speech and Language Therapists (RCSLT). Videofluoroscopic evaluation of oropharyngeal swallowing disorders (VFS) in adults: the role of speech and language therapists. RCSLT policy statement. London: RCSLT; 2007.
- 11.Logemann JA, Bytell DE. Swallowing disorders in three types of head and neck surgical patients. Cancer. 1979;44(3):1095–1105. doi: 10.1002/1097-0142(197909)44:3<1095::AID-CNCR2820440344>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.Popa NS, Murith M, Chisholm H, Engmann J. Matching the rheological properties of videofluoroscopic contrast agents and thickened liquid prescriptions. Dysphagia. 2013;28(2):245–252. doi: 10.1007/s00455-012-9441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kao HK, Chang KP, Wei FC, Cheng MH. Comparison of the medial sural artery perforator flap with the radial forearm flap for head and neck reconstructions. Plast Reconstr Surg. 2009;124(4):1125–1132. doi: 10.1097/PRS.0b013e3181b457cf. [DOI] [PubMed] [Google Scholar]
- 14.Hoefert S, Lotter O. Change in reimbursement and costs in German oncological head and neck surgery over the last decade: ablative tongue cancer surgery and reconstruction with split-thickness skin graft vs. microvascular radial forearm flap. Clin Oral Invest. 2018;22:1741–1750. doi: 10.1007/s00784-017-2269-x. [DOI] [PubMed] [Google Scholar]
- 15.Haughey BH, Wilson E, Kluwe L, Piccirillo J, Fredrickson J, Sessions D, Spector G. Free flap reconstruction of the head and neck: analysis of 241 cases. Otolaryngol Head Neck Surg. 2001;125(1):10–17. doi: 10.1067/mhn.2001.116788. [DOI] [PubMed] [Google Scholar]
- 16.Shestak KC, Jones NF. Microsurgical free tissue transfer in the elderly patient. Plast Reconstr Surg. 1991;88:259–263. doi: 10.1097/00006534-199108000-00014. [DOI] [PubMed] [Google Scholar]
- 17.McNamara M, Pope S, Sadler A. Microvascular free flaps in head and neck surgery. J Laryngol Otol. 1994;108:962–968. doi: 10.1017/S0022215100128634. [DOI] [PubMed] [Google Scholar]
- 18.Simpson KH, Murphy PG, Hopkins PM. Prediction of outcomes in 150 patients having microvascular free tissue transfers to the head and neck. Br J Plast Surg. 1996;49:267–273. doi: 10.1016/S0007-1226(96)90154-X. [DOI] [PubMed] [Google Scholar]
- 19.Jones NF, Johnson JT, Shestak KC. Microsurgical reconstruction of the head and neck: interdisciplinary collaboration between head and neck surgeons and plastic surgeons in 305 cases. Ann Plast Surg. 1996;36:37–43. doi: 10.1097/00000637-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Fang QG, Li ZN, Zhang X, Liu FY, Xu ZF, Sun CF. Clinical reliability of radial forearm free flap in repair of buccal defects. World J Surg Oncol. 2013;11:26. doi: 10.1186/1477-7819-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kruse AL, Bredell MG, Lübbers HT, Jacobsen C, Grätz KW, Obwegeser JA. Clinical reliability of radial forearm free-flap procedure in reconstructive head and neck surgery. J Craniofac Surg. 2011;22:822–825. doi: 10.1097/SCS.0b013e31820f36aa. [DOI] [PubMed] [Google Scholar]
- 22.Shibahara T, Mohammed AF, Katakura A, Nomura T. Long-term results of free radial forearm flap used for oral reconstruction: functional and histological evaluation. J Oral Maxillofac Surg. 2006;64:1255–1260. doi: 10.1016/j.joms.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 23.Song M, Chen FJ, Guo ZM, Zhang Q, Yang AK. Application of various flaps to intraoral reconstruction of buccal defects after resection of buccal mucosa carcinoma. Ai Zheng. 2009;28:663–667. [PubMed] [Google Scholar]
- 24.Chien CY, Su CY, Hwang CF, Chuang HC, Jeng SF, Chen YC. Ablation of advanced tongue or base of tongue cancer and reconstruction with free flap: functional outcomes. Eur J Surg Oncol. 2006;32(03):353–357. doi: 10.1016/j.ejso.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Liao G, Su Y, Zhang J, Hou J, Chen Y, Li M. Reconstruction of the tongue with reinnervated rectus abdominis musculoperitoneal flaps after hemiglossectomy. J Laryngol Otol. 2006;120(03):205–213. doi: 10.1017/S002221510600017X. [DOI] [PubMed] [Google Scholar]
- 26.Lam L, Samman N. Speech and swallowing following tongue cancer surgery and free flap reconstruction–a systematic review. Oral Oncol. 2013;49(06):507–524. doi: 10.1016/j.oraloncology.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Brown L, Rieger JM, Harris J, Seikaly H. A longitudinal study of functional outcomes after surgical resection and microvascular reconstruction for oral cancer: tongue mobility and swallowing function. J Oral Maxillofac Surg. 2010;68(11):2690–2700. doi: 10.1016/j.joms.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Jain SK, Raj P, Singh SK, Chugh R, Gupta DK, Goyal S. Post-operative speech and swallowing in partial glossectomy patients: role of effective rehabilitation. Int J Otorhinolaryngol Head Neck Surg. 2018;4:1473–1478. doi: 10.18203/issn.2454-5929.ijohns20184362. [DOI] [Google Scholar]
- 29.Gabriele M, Michael G, Giulia M, et al. Quality of life, swallowing and speech outcomes after oncological treatment for mobile tongue carcinoma. Eur J Plast Surg. 2020;43:247–256. doi: 10.1007/s00238-019-01593-z. [DOI] [Google Scholar]
- 30.Dzioba A, Aalto D, Papadopoulos-Nydam G, Seikaly H, Rieger J, Wolfaardt J, Osswald M, et al. Functional and quality of life outcomes after partial glossectomy: a multi-institutional longitudinal study of the head and neck research network. J Otolaryngol Head Neck Surg. 2017;4(46):1. doi: 10.1186/s40463-017-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]