Abstract
Background
Long chain polyunsaturated fatty acids (LCPUFA), especially docosahexaenoic acid (DHA), are the most abundant fatty acids in the brain and are necessary for growth and maturation of an infant's brain and retina. LCPUFAs are named “essential” because they cannot be synthesised efficiently by the human body and come from maternal diet. It remains controversial whether LCPUFA supplementation to breastfeeding mothers is beneficial for the development of their infants.
Objectives
To assess the effectiveness and safety of supplementation with LCPUFA in breastfeeding mothers in the cognitive and physical development of their infants as well as safety for the mother and infant.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (6 August 2014), CENTRAL (Cochrane Library 2014, Issue 8), PubMed (1966 to August 2014), EMBASE (1974 to August 2014), LILACS (1982 to August 2014), Google Scholar (August 2014) and reference lists of published narrative and systematic reviews.
Selection criteria
Randomised controlled trials or cluster‐randomised controlled trials evaluating the effects of LCPUFA supplementation on breastfeeding mothers (including the pregnancy period) and their infants.
Data collection and analysis
Two review authors independently assessed eligibility and trial quality, performed data extraction and evaluated data accuracy.
Main results
We included eight randomised controlled trials involving 1567 women. All the studies were performed in high‐income countries. The longest follow‐up was seven years.
We report the results from the longest follow‐up time point from included studies. Overall, there was moderate quality evidence as assessed using the GRADE approach from these studies for the following outcomes measured beyond 24 months age of children: language development and child weight. There was low‐quality evidence for the outcomes: Intelligence or solving problems ability, psychomotor development, child attention, and child visual acuity.
We found no significant difference in children's neurodevelopment at long‐term follow‐up beyond 24 months: language development (standardised mean difference (SMD) ‐0.27, 95% confidence interval (CI) ‐0.56 to 0.02; two trials, 187 participants); intelligence or problem‐solving ability (three trials, 238 participants; SMD 0.00, 95% CI ‐0.36 to 0.36); psychomotor development (SMD ‐0.11, 95% CI ‐0.48 to 0.26; one trial, 113 participants); motor development (SMD ‐0.23, 95% CI ‐0.60 to 0.14; one trial, 115 participants), or in general movements (risk ratio, RR, 1.12, 95% CI 0.58 to 2.14; one trial, 77 participants; at 12 weeks of life). However, child attention scores were better at five years of age in the group of children whose mothers had received supplementation with fatty acids (mean difference (MD) 4.70, 95% CI 1.30 to 8.10; one study, 110 participants)). In working memory and inhibitory control, we found no significant difference (MD ‐0.02 95% CI ‐0.07 to 0.03 one trial, 63 participants); the neurological optimality score did not present any difference (P value: 0.55).
For child visual acuity, there was no significant difference (SMD 0.33, 95% CI ‐0.04 to 0.71; one trial, 111 participants).
For growth, there were no significant differences in length (MD ‐0.39 cm, 95% CI ‐1.37 to 0.60; four trials, 441 participants), weight (MD 0.13 kg, 95% CI ‐0.49 to 0.74; four trials, 441 participants), and head circumference (MD 0.15 cm, 95% CI ‐0.27 to 0.58; three trials, 298 participants). Child fat mass and fat mass distribution did not differ between the intervention and control group (MD 2.10, 95% CI ‐0.48 to 4.68; one trial, 115 participants, MD ‐0.50, 95% CI ‐1.69 to 0.69; one trial, 165 participants, respectively).
One study (117 infants) reported a significant difference in infant allergy at short‐term follow‐up (risk ratio (RR) 0.13, 95% CI 0.02 to 0.95), but not at medium‐term follow‐up (RR 0.52, 95% CI 0.17 to 1.59).
We found no significant difference in two trials evaluating postpartum depression. Data were not possible to be pooled due to differences in the describing of the outcome. One study (89 women) did not find any significant difference between the LCPUFA supplementation and the control group at four weeks postpartum (MD 1.00, 95%CI ‐1.72 to 3.72).
No adverse effects were reported.
Authors' conclusions
Based on the available evidence, LCPUFA supplementation did not appear to improve children's neurodevelopment, visual acuity or growth. In child attention at five years of age, weak evidence was found (one study) favouring the supplementation. Currently, there is inconclusive evidence to support or refute the practice of giving LCPUFA supplementation to breastfeeding mothers in order to improve neurodevelopment or visual acuity.
Plain language summary
Long chain polyunsaturated fatty acid supplements for mothers who breastfeed
Background
Long chain polyunsaturated fatty acids (LCPUFAs) are abundant in the brain and are necessary for growth and maturation of a young infant’s brain and the retina of the eye. These particular fatty acids include docosahexaenoic acid (DHA) and are said to be ‘essential’ because the human body is not efficient in producing them. This means that infants who are breastfeeding obtain the fatty acids from their mothers’ diet, mainly from fish oil and ocean fish. We reviewed the evidence about the effect of supplementation of LCPUFA on breastfeeding mothers on growth and neurodevelopment of their children.
Study characteristics
We found eight randomised clinical trials. A total of 1567 women from high‐income countries were included in the trials. The quality of evidence was found to be moderate and low.
Main results
This review of trials showed that supplementing a mother’s diet with LCPUFA during the pregnancy and the first four months after birth did not improve the child’s growth or neurodevelopment in terms of problem‐solving ability or intelligence, psychomotor, motor, or language development. In child attention at five years of age, weak evidence was found (one study) favouring the supplementation. The age of the children at the last neurodevelopment assessment was seven years. The children’s visual acuity was not different at five years of age compared with children of the control group of mothers who received supplements of soybean or corn oils.
Conclusions
Currently, there is inconclusive evidence to support or refute the practice of giving LCPUFA supplementation to breastfeeding mothers in order to improve neurodevelopment.
Summary of findings
Summary of findings for the main comparison. Fatty acid supplementation versus placebo compared with improving child growth and development for.
| Fatty acid supplementation versus placebo compared with improving child growth and development | ||||||
| Patient or population: Breastfeeding mothers Settings: Outpatient clinic Intervention: Fatty acid supplementation versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Fatty acid supplementation versus placebo | |||||
| Language development ‐ Beyond 24 months | With no treatment | The mean language development ‐ beyond 24 months in the intervention groups was 0.27 standard deviations lower (0.56 lower to 0.02 higher) | ‐0.27 (‐0.56 to 0.02) | 187 (2 studies) | ⊕⊕⊕⊝ moderate1 | A standard deviation of ‐0.27 represents a small difference between groups. |
| Intelligence or problem‐solving ability ‐ Beyond 24 months | With no treatment | The mean intelligence or problem‐solving ability ‐ beyond 24 months in the intervention groups was 0 standard deviations higher (0.36 lower to 0.36 higher) | 0 (‐0.36 to 0.36) | 238 (3 studies) | ⊕⊕⊝⊝ low2 | A standard deviation of ‐0.36 represents a small difference between groups. |
| Psychomotor development ‐ Beyond 24 months | With no treatment | The mean psychomotor development ‐ beyond 24 months in the intervention groups was 0.11 standard deviations lower (0.48 lower to 0.26 higher) | ‐0.11 (‐0.48 to 0.26) | 113 (1 study) | ⊕⊕⊝⊝ low3 | A standard deviation of ‐0.11 represents a small difference between groups. |
| Child attention ‐ Long term beyond 24 months | With no treatment | The mean child attention ‐ long term beyond 24 months in the intervention groups was 4.70 (1.30 lower to 8.10 higher) | 4.70 (1.30 to 8.10 higher) | 110 (1 study) | ⊕⊕⊝⊝ low3 | A mean difference of 4.70 represents a moderate difference between groups. |
| Child visual acuity ‐ Long term beyond 24 months | With no treatment | The mean child visual acuity ‐ long term beyond 24 months in the intervention groups was 0.33 standard deviations higher (0.04 lower to 0.71 higher) | 0.33 (‐0.04 to 0.71) | 111 (1 study) | ⊕⊕⊝⊝ low3 | A standard deviation of 0.33 represents a small difference between groups. |
| Child weight ‐ Long term beyond 24 months | With no treatment | The mean child weight ‐ long term beyond 24 months in the intervention groups was 0.13 g higher (0.49 lower to 0.74 higher) | 0.13 (‐0.49 to 0.74 higher) | 414 (4 studies) | ⊕⊕⊕⊝ moderate4 | 0.13 g is not a clinically important difference. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The evidence is from two RCTs: Jensen 2005 had "unclear" allocation concealment, blinding of researchers and participants, and losses to follow‐up were not reported; Lauritzen 2004 had important losses to follow‐up. The PICO question is similar in the two studies. There is no evidence of inconsistency between studies. The CIs are narrow. We have downgraded the evidence once for risk of bias. We used Cohen's interpretation of effect sizes to assess the standardised mean difference (Cohen 1988).
2 The evidence comes from three RCTs: Jensen 2005 had "unclear" allocation concealment, blinding of researchers and participants, and losses to follow‐up were not reported; Lauritzen 2004 had important loss of follow‐up; Helland 2001 had important loss of follow‐up. The PICO question is similar in the three studies. There is evidence of inconsistency between studies; the I² = 48%, with the Helland 2001 results opposite to those of the other two trials. The CIs are narrow. We have downgraded once for risk of bias and once for inconsistency. We used Cohen's interpretation of effect sizes to assess the standardised mean difference (Cohen 1988).
3 The evidence comes from one single RCT, Jensen 2005; this trial had "unclear" allocation concealment, blinding of researchers and participants, and losses to follow‐up were not reported. We have downgraded the evidence for risk of bias and for imprecision due to few participants. We used Cohen's interpretation of effect sizes to assess the standardised mean difference (Cohen 1988).
4 The evidence comes from four RCTs: Jensen 2005 had "unclear" allocation concealment, blinding of researchers and participants, and losses to follow‐up were not reported; Lauritzen 2004 had important losses to follow‐up; Helland 2001 had a unclear allocation concealment and attrition bias; Lucia 2007 had unclear allocation concealment and attrition bias. The PICO question was similar in the four studies. There is no evidence of imprecision or inconsistency between studies. No evidence of publication bias. We have downgraded the evidence once for risk of bias. We used Cohen's interpretation of effect sizes to assess the standardised mean difference (Cohen 1988).
Background
Growth of the fetus and small infants, and in particular of the brain, is exceptionally fast during the last trimester of pregnancy and the first year of life and depends on the quality of the environment around them as well as maternal nutrition (Grantham‐McGregor 2007).
The World Health Organization recommends that children should be exclusively breast‐fed until six months of age and emphasises the importance of lactating women's nutrition. A deterioration in their nutritional status can produce a deficit in some nutrients of breast milk and, therefore, in their offspring (Hoddinot 2007; Horta 2007). Breastfeeding has a demonstrated benefit on child neurodevelopment. Breast milk enhances child development through its nutrients, especially through the essential fatty acids (Belfort 2013; Hoddinot 2007; Uauy 2003; Walker 2007).
Special emphasis has been placed on the lipid fraction of breast milk, which represents the main source of energy for children breastfeeding. Some of these lipids, long chain polyunsaturated fatty acids (LCPUFAs), are named “essential” because they can not be synthesised efficiently by the human body and come from the maternal diet. Interest has focused on essential fatty acids because they are involved in the development of nervous system tissue (Campoy 2012; Jensen 2006; Tinoco 2007). Lipids in breast milk are the main energy suppliers (40% to 55% of total energy intake) for appropriate growth. LCPUFAs are the most abundant fatty acids in the brain and are necessary for growth and maturation of the brain and retina (Jensen 2009; Koletzco 2008; Simmer 2011).
Description of the condition
Docosahexaenoic acid (DHA) and arachidonic acid (AA) are not widely distributed in foods and are absent in vegetable oils and fats, but are present in the fat of certain sea fish. These fish are the primary, and almost exclusive, exogenous source. Fatty acids are transferred during pregnancy through the placenta, and postnatally through breast milk. Breast milk provides both AA and DHA. The level of AA is relatively constant, whereas the level of DHA is variable and depends on maternal dietary habits, culture and lifestyle (Agostoni 2005; Innis 2014; Koletzco 2008). The concentration of DHA in maternal serum decreases significantly after childbirth and depends on maternal intake, so that a diet rich in DHA determines higher levels in breast milk (Jensen 2006; Tinoco 2007), in a dose‐dependent relationship (Gibson 1996). DHA supplementation during lactation increases breast milk DHA content and is more effective in raising breast milk DHA content than supplementation limited to pregnancy only (Jensen 2006).
Experimental studies have used LCPUFA‐supplemented formula during the period of lactation in children, and measured results of their development in terms of visual acuity, language and psychomotor development. Two Cochrane reviews concluded that, based on the existing evidence, routine LCPUFA supplementation formula cannot be recommended in term infants (Simmer 2011) and no clear long‐term benefits are evident for preterm infants receiving formula supplemented with LCPUFA (Schulzke 2011). Observational studies have shown that breast‐fed infants have higher concentrations of DHA than those who are fed with formula (Innis 2007), while there were several observations on the improvement of neurodevelopment among infants who have been breast‐fed compared with those who have received formula (Agostoni 2005) containing DHA and AA. It has also been shown that a high concentration of DHA in breast milk is associated with a low prevalence of postpartum depression (Llorente 2003).
How the intervention might work
The most important LCPUFAs are arachidonic acid (20:4 (n‐6) (AA)) and particularly, docosahexaenoic acid (22:6 (n‐3) (DHA)). AA is involved in several pathways of activation through the cell membrane receptors (cell signalling pathway) and is a precursor of eicosanoids, products of remarkable physiological activity, such as prostaglandins and leukotrienes, in several key cellular processes (McCann 2005). DHA is the most abundant fatty acid in the brain and an important component of brain cell membranes and retina. Its known functions are neurogenesis, neurotransmission and protection against oxidative agents in the brain and retina (Fleith 2005; Innis 2007). LCPUFAs are accumulated in the brain tissue mainly during the second half of pregnancy and during the first two years of life (McCann 2005). In terms of safety, the current recommendation is consumption of one to two portions of sea fish per week, including oily fish, which is a rich source of LCPUFA. This intake of oily fish rarely exceeds the tolerable intake of environmental contaminants (Koletzko 2007). An international consensus recommends that the daily intake of DHA in pregnant and lactating women should reach 200 mg (Koletzco 2008).
Why it is important to do this review
Researchers and clinicians have hypothesised that giving LCPUFA supplementation to lactating mothers may affect children's neurodevelopment, visual acuity and growth. Recent experimental studies have assessed the effectiveness of giving LCPUFA supplementation to breastfeeding mothers of exclusively breast‐fed term newborns. However, the evidence provided is not strong and the results are inconsistent since some of these studies included a limited number of individuals and others presented more than 20% losses to follow‐up. These results do not provide strong evidence (Jensen 2005b; Lauritzen 2005; McCann 2005). Therefore, this systematic review aims to establish whether supplementary LCPUFA is beneficial for both lactating mothers and their infants.
This is an update of a Cochrane review first published in 2010 (Delgado‐Noguera 2010).
Objectives
To assess the effectiveness and safety of supplementation with LCPUFA in breastfeeding mothers on the cognitive and physical development of their infants, as well as the safety for the mother and the infant.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised controlled trials or cluster‐randomised controlled trials evaluating the effects of LCPUFA supplementation on breastfeeding mothers and their infants during pregnancy and the postpartum period.
Quasi‐randomised trials were not eligible for inclusion.
Types of participants
Breastfeeding women receiving LCPUFA supplementation in pregnancy and the postpartum period.
Types of interventions
LCPUFA: AA and/or DHA supplementation to pregnant and breastfeeding mothers compared with any control group (placebo, no supplementation).
Both groups should receive the same co‐intervention, if any.
The following trials were not eligible for inclusion.
Trials reporting exclusively biochemical outcomes.
Types of outcome measures
Primary outcomes
Child neurodevelopment outcomes measured by different scales (e.g. Bayley scale, including the Mental Development Index (MDI) and Psychomotor Development Index (PDI); Gesell scale for gross motor development (Infant Planning Test); or as specified in the original trial reports).
Secondary outcomes
Child visual (acuity) development measures (Teller Acuity Card, Visual Evoked Potential (VEP), SWEEP‐VEP visual acuity determination) or as specified in the original trial reports.
Child’s physical growth: weight, length and head circumference in centimetres (child body mass index (BMI), child fat mass, fat mass distribution were added as non pre‐specified outcomes).
Safety of supplementation with LCPUFA to mothers and babies (considered as environmental contamination of supplementation).
Mothers' views about or satisfaction with their diets (as defined by trial authors).
Infant allergy (non pre‐specified outcome).
Postpartum depression (non pre‐specified outcome).
Time to outcome assessment
We included data from the following time points: at birth, at short term (12 months), medium term (12 to 24 months) and long term (beyond 24 months).
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (6 August 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched CENTRAL (Cochrane Library 2014, Issue 8), PubMed (1966 to August 2014), EMBASE (1974 to August 2014) using the search strategies detailed in Appendix 1, Appendix 2, and Appendix 3.
We searched in LILACS (2009 to August 2014) (Appendix 4) and Google Scholar (August 2014) (Appendix 5).
[In the previous version of the review (Delgado‐Noguera 2010), we also carried out an additional search of CINAHL (see Appendix 6 for the search strategy.]
Searching other resources
We searched reference lists of published narrative reviews or related papers. We also contacted authors to provide data.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeDelgado‐Noguera 2010.
For this update, the following methods were used for assessing the eight reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors, Mario Delgado (MD) and Eleni P Kotanidou (EPK) assessed independently for inclusion all the potential studies identified as a results of the search. We resolved any disagreement through discussion or, if required, consulted a referee, Jose Andres Calvache (JAC) or Xavier Bonfill (XB).
Data extraction and management
We designed a standard form to extract data. For eligible studies, two review authors (MD, EPK) extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third person (XB). We entered data into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above is unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (MD, EPK) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a referee (JAC or XB).
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings.
Assessment of the quality of the evidence
For this update, we assessed the quality of the evidence using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following outcomes.
Language development.
Intelligence or problem‐solving ability.
Psychomotor development.
Child attention.
Child visual acuity.
Child weight.
The main comparisons was made for fatty acid supplementation versus placebo for improving child growth and development. These comparisons were made with data from included studies with longer follow‐up, beyond 24 months of age.
We used GRADE profiler (GRADEpro 2014) software to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data (safety of supplementation with long chain polyunsaturated fatty acids to mothers and babies or other results), we presented results as summary risk ratios with 95% confidence intervals.
Continuous data
For continuous data (child neurodevelopment scales, child visual acuity measures, child physical growth, rating of mother postpartum depression), we used the mean difference if outcomes were measured in the same way between trials. We used standardised the mean difference to combine trials measuring the same outcome, but using different methods.
Unit of analysis issues
We did not identify any cluster‐randomised trials for inclusion in this update. If we identify them in future updates, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their standard errors using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Dealing with missing data
We noted the levels of attrition for included studies. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. For all the outcomes, as far as possible, we carried out analysis on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised, minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 50% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Had we identified substantial heterogeneity (above 50%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
Where we suspected reporting bias, we attempted to contact study authors asking them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a Sensitivity analysis.
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using Review Manager software (RevMan 2014). We used fixed‐effect inverse variance meta‐analysis for combining data where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we planned to discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, we presented the results as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We did not carry out planned subgroup analysis in this update. In future updates, If we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, to use random‐effects analysis to produce it.
We will carry out the following subgroup analyses for the primary outcome.
Term/preterm status.
Type of polyunsaturated fatty acids.
Mother's nutritional status at trial entry (adequate/inadequate).
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014) and report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We carried out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this made any difference to the overall result.
Results
Description of studies
Results of the search
Searches in the databases yielded 792 references. The search of the Cochrane Pregnancy and Childbirth Group's Trials Register (PCGBRT, August 2014), CENTRAL (2009, Issue 2), PubMed (2009 to August 2014), EMBASE (2009 to august 2014), LILACS (2009 to August 2014), Google Scholar (August 2014) and reference lists of published narrative and systematic reviews retrieved 21, 114, 220, 75, 78, 0 and 284 reports, respectively.
From the search of the reference lists of trials and reviews, we identified 18 reports for six studies. Eight were new reports for four studies (Furuhjelm 2009; Jensen 2005; Lauritzen 2004; Lucia 2007), included in the first version of this review (Delgado‐Noguera 2010); and 10 reports were identified for the two new included studies (Hauner 2009; van Goor 2010). No new reports were identified for Gibson 1997 or Helland 2001. In summary, for this update (2015), we included eight studies (42 reports).
Included studies
We included eight randomised studies (1567 women), assessing the clinical effects of fatty acid supplementation during both pregnancy and lactation or solely during lactation, on the development and growth of their children. We did not find any studies evaluating outcomes in preterm infants. All the included trials used a randomised clinical trial design and the studies came from high‐income countries. We have provided full details of the eight included studies in the Characteristics of included studies table.
All the studies were funded by governmental and private organisations. Market Biosciences provided the LCPUFA in the Australian and United States studies and BASF in the Danish studies. Peter Møller, Avd. Orkla ASA, and Aktieselskabet Freia Chocoladefabriks Medicinske Fond funded the Norway study. The supplements were donated by Nestle in the German study Lucia 2007. Danone Reserach Center financially supported Hauner 2009. The Swedish study (Furuhjelm 2009) was financed by government funds and GlaxoSmithKline. van Goor 2010 was financially supported by Friesland‐Campina.
Participants
Lauritzen 2004 was conducted in Denmark and women were recruited from the Danish National Birth Cohort (Olsen 2001). Jensen 2005 was conducted in the United States. Helland 2001 was conducted in Norway, and Gibson 1997 and Furuhjelm 2009 were conducted in Australia, and Sweden, respectively. Lucia 2007 and Hauner 2009 were conducted in Germany, and van Goor 2010 was conducted in The Netherlands.
The 1567 women were healthy pregnant and lactating mothers, without pregnancy complications, who were intending to breastfeed their children. Their children were born at term (37 to 43 weeks' gestation) and were healthy and without malformations.
Intervention
The experimental intervention used in the Lauritzen 2004 study consisted of 1.5 g of LCPUFA, 800 mg of docosahexaenoic acid (DHA) + 600 mg of eicosapentaenoic acid (EPA). Furuhjelm 2009 used 1.1 g of DHA +1.6 g EPA. Jensen 2005 and Lucia 2007 each used 200 mg of DHA. Various concentrations of DHA (200 to 1300 g) were used in Gibson 1997. Helland 2001 used 10 mL of cod liver oil. In Hauner 2009, the intervention consisted of 1200 mg n‐3 LCPUFAs (1020 mg DHA and 180 mg EPA) as well as 9 mg vitamin E. van Goor 2010 used 220 mg of DHA and 220 mg of arachidonic acid (AA).
Placebo consisted of olive oil in Lauritzen 2004; soybean oil and corn oil in Jensen 2005; corn oil in Helland 2001; and soy oil in Furuhjelm 2009 and Lucia 2007; and soybean oil in van Goor 2010. The nature of the placebo was not reported in Gibson 1997. In Hauner 2009, the control group received counselling on a healthy balanced diet.
The intervention was initiated in pregnancy in Helland 2001, Lucia 2007, Furuhjelm 2009, Hauner 2009 and van Goor 2010 and continued during the breastfeeding period. In Lauritzen 2004, Jensen 2005 and Gibson 1997, the intervention was initiated postpartum and during the first four months after birth.
We identified no trials of supplementation of other fatty acids (alfa‐linoleic or linolenic) being given to lactating mothers.
Outcomes
The outcomes assessed in the included trials were neurodevelopment and visual acuity; growth related to weight, length, head circumference and body mass index (BMI); fat mass and fat distribution. Infant blood pressure, infant allergy, maternal depression and postpartum blues were also reported.
We considered eight areas of neurodevelopment to assess this primary outcome: language development (Jensen 2005; Lauritzen 2004); intelligence or problem‐solving ability (Helland 2001; Jensen 2005; Lauritzen 2004; van Goor 2010); psychomotor development (Gibson 1997; Jensen 2005; van Goor 2010); motor development (Jensen 2005; Lauritzen 2004); general movements (van Goor 2010); child attention (Jensen 2005); working memory and inhibitory control (Lauritzen 2004); and neurological optimality score (van Goor 2010).
For the visual acuity outcome there were three short‐term studies (Gibson 1997; Jensen 2005; Lauritzen 2004), and one long‐term study (Jensen 2005).
For the child's physical growth outcome, there were four studies (Hauner 2009; Helland 2001; Lauritzen 2004; Lucia 2007) that reported weight, length, head circumference and BMI (short‐, medium‐ and long‐term data).
For child fat mass and fat mass distribution, there were two studies (Hauner 2009; Lucia 2007) reporting short‐term and long‐term data.
Infant allergy was assessed by one study (Furuhjelm 2009) in short‐term and medium‐term context.
Postpartum depression and postpartum blues were reported by two studies (Jensen 2005; van Goor 2010) at short‐term time points.
Outcomes measures
To assess the outcomes, different measurements were used: for language development, Jensen 2005 used CLAMS (Clinical Linguistic and Auditory Milestone Scale) and Vocabulary subset of Weschler Primary and Preschool Scale of Inteligence‐Revised (WPPSI‐R). Lauritzen 2004 used vocabulary comprehension and the CDI scale (MacArthur Communicative Development Inventory‐vocabulary production (number of words)).
For the outcome Intelligence or problems‐solving ability, Helland 2001 used Fegan test for infant intelligence and Mental Processing Composity. Lauritzen 2004 used the Infant Planning Test and a subtest of Woodcock Johnson Test of cognitive abilities III. Jensen 2005 used the Clinical Adaptative Test and the information subset of WPPSI‐R. van Goor 2010 applied the Mental Developmental Index.
For Psychomotor development, Gibson 1997 used the Bayley Scales of Infant Development; Jensen 2005 used the Visual‐Motor Integration Test; van Goor 2010 used the Psychomotor Developmental Index. For motor development, Jensen 2005 used Gesell Gross Motor Scale and Hand movement subtest of Kaufman Assessment Battery (K‐ABC). Lauritzen 2004 used the child's age of sitting without support. van Goor 2010 used the Fluency score. For assessment of general movements, van Goor 2010 used clinical observation of the child. For evaluation of child attention, the Leiter international Performance Scale was used (Jensen 2005). For evaluation of working memory and inhibitory control, Lauritzen 2004 applied a Day/night Stroop task. Finally, van Goor 2010 also applied the Neurological Otimality Score.
Visual acuity outcomes were measured in different ways: Gibson 1997 used the Visual Evoked Potential (VEP) and Sweep VEP. Lauritzen 2004 and Jensen 2005 used Teller Acuity Cards, Sweep VEP and VEP (for a detailed description of visual acuity‐assessment methods see Simmer 2011).
For measurements of growth in weight, length, head circumference and BMI, Helland 2001, Lauritzen 2004, Lucia 2007 and Hauner 2009 used scales in grams and stadiometers in centimetres, respectively.
For fat mass assessment, Hauner 2009 used four‐point skin fold thickness (SFT) and Lucia 2007 three‐point SFT. For fat mass distribution, Hauner 2009 evaluated trunk‐to‐total SFTs.
Regarding safety and tolerance, one study (Hauner 2009) reports no side effects of the supplementation. No other data were found in the other studies.
Jensen 2005 reported maternal depression and Furuhjelm 2009 reported infant allergy. For the outcome postpartum depression, Jensen 2005 used self‐rating measures; van Goor 2010 used the Dutch version of the Edinburgh Postpartum Depression Scale (EPDS). Postpartum blues were evaluated by van Goor 2010 with the Dutch version of the blues questionnaire. For assessment of allergy, Furuhjelm 2009 used the skin prick test, determination of IgE antibodies and clinical examination.
Excluded studies
We excluded 42 trials and created a Characteristics of excluded studies table, giving reasons for exclusion. We excluded trials for the following reasons: the study was not a clinical trial; the intervention was given only during pregnancy; the intervention was not a LCPUFA; or the authors reported only biochemical data.
Characteristics of ongoing studies
Three studies (Campos‐Martinez 2012; Harris 2014; Karlsson 2010), were identified as ongoing studies (seeCharacteristics of ongoing studies).
Characteristics of studies awaiting classification
One study (Nahidi 2012) is awaiting classification. This study investigated an intervention with LCPUFA for maternal depression. However, its original language is Persian and we are awaiting translation (seeCharacteristics of studies awaiting classification).
Risk of bias in included studies
The eight included studies were assessed for risk of bias domains according to the methodology in the Cochrane Handbook for Systematic Reviews of Interventions (chapter 8.7a)(Higgins 2011).
We have included a summary of the 'Risk of bias' assessments for the included trials in the Characteristics of included studies table, in the methodological quality graph (Figure 1) and in the methodological quality summary (Figure 2).
1.
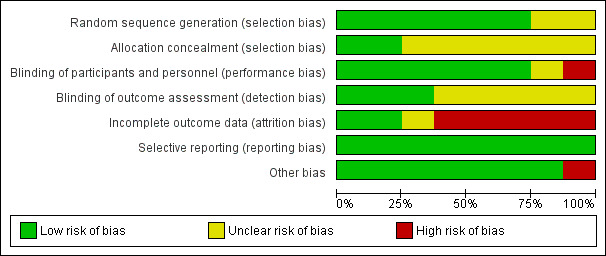
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
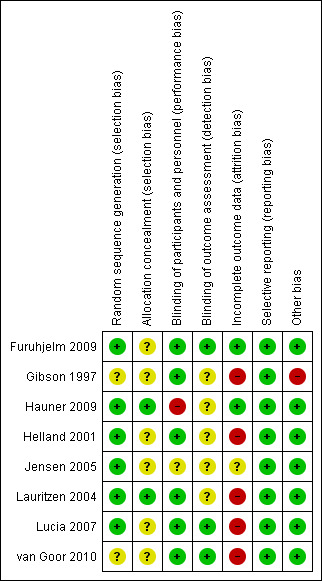
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Six of the eight trials were classified as low risk studies for selection bias: Furuhjelm 2009, Hauner 2009, Helland 2001, Jensen 2005, Lauritzen 2004, and Lucia 2007 performed appropriate random sequence generation. In one study (Gibson 1997), an unclear risk of selection bias was acknowledged since there was insufficient information about the sequence information process. Finally, in the trial of van Goor 2010 an unclear risk of selection bias was identified given that the procedure of allocation was not reported by authors.
Allocation concealment procedures were reported in two studies (Hauner 2009; Lauritzen 2004) and were classified as low risk of bias. The remaining studies were rated as unclear risk of bias.
Blinding
Six of the evaluated studies reported adequate a blinding procedure of the researchers (Furuhjelm 2009; Gibson 1997; Helland 2001; Lauritzen 2004; Lucia 2007; van Goor 2010), and were assessed as low risk for performance bias. In one study (Hauner 2009), a blinding procedure was not performed and thus the trial was assigned as being at high risk of performance bias. Finally, in one trial (Jensen 2005), a blinding procedure was not explicitly reported and the study was classified as unclear risk for the bias.
Assessment of measured outcomes was performed in a blinded procedure in three trials (Furuhjelm 2009; Lucia 2007; van Goor 2010). Five trials were assessed as being at unclear risk for detection bias (Gibson 1997; Hauner 2009; Helland 2001; Jensen 2005, Lauritzen 2004), since no adequate information regarding blinding during the outcome assessment was reported.
Incomplete outcome data
All studies described withdrawals at different stages of follow‐up. Dropout rates during trial performance ranged from 18.3% to 55%. Two studies (Furuhjelm 2009; Hauner 2009), were assessed as low risk trials for attrition bias. In Hauner 2009, 83.65% of the initially stratified study population was assessed at four months follow‐up, (with no discrepancy between intervention and control group losses) and 81.7% of them performed the one‐year follow‐up (all new losses were attributed to the control group only). In the Furuhjelm 2009 study, of the initially stratified population, 82.75% managed to fully receive the intervention of the protocol and thereafter researchers reported only 1.7% losses at three‐month‐follow‐up, 2.5% dropouts at six‐month follow‐up, 4.2% withdrawals at 12‐month assessment, and finally 3.4% loss at 24‐month follow‐up.
One study (Jensen 2005), was classified as of unclear risk for attrition bias since more than 20% dropouts were observed in both intervention and control groups, attributed to similar reasons in each group. The vast majority of missing participants at follow‐up occurred in the short term of the study: from the initially included population 29.5% were lost at four‐month follow‐up, 28.6% were lost at 12‐month assessment, 29.5% were not able to be evaluated at 30‐month assessment, and finally, 47.5% of initially included participants were lost at five‐year follow‐up.
Finally, high risk for attrition bias was identified in the remaining five trials. In Lucia 2007, the differential losses were unclear. At the time of birth, 79.1% of the initially enrolled population were assessed. At one‐month follow‐up, 61.8% were investigated, at three months 60.4%, at 21 months 47.9% of the initially enrolled, but at five‐year follow‐up, 79.8% of the enrolled population were assessed. Data regarding differences in withdrawal rates between study groups were not available. Gibson 1997 was also classified as high risk for attrition bias since at 12‐week follow‐up, 50% of participants were excluded due to practical difficulties in the evaluation procedure of the outcomes. Helland 2001 presented high risk for attrition bias too. At six‐month follow‐up, only 76.8% of initially randomised participants were assessed, at nine months 71.8%, at four years only 24.6% were evaluated, and finally at seven‐year follow‐up, 41.9% of initially randomised participants were assessed. However in all assessments, the dropout rates were equally distributed among intervention and control group; more precisely, at the last follow‐up at the seven years, only 46.8% and 36.7% of the initially planned participants of the intervention and control group were evaluated, respectively.
Lauritzen 2004 also had a high risk of attrition bias. Out of the 122 initially randomised participants, 18% were lost at the nine‐month follow‐up, 27% were lost at one year of age, 41.8% were lost at two years of age, 40.9% were withdrawn at 2.5 years of age, and 19.7% were lost from the final follow‐up at seven years. Justification for missing was data was not reported.
van Goor 2010 was also classified as high attrition bias study due to the fact that only 65% of the initially enrolled mother‐infant pairs managed to completed the intervention and be assessed at one year of life. The comparable proportion of 65.4% of initially enrolled women was assessed for postpartum depression.
Selective reporting
All the trials (Furuhjelm 2009; Gibson 1997; Hauner 2009; Helland 2001; Jensen 2005; Lauritzen 2004; Lucia 2007; van Goor 2010) were classified as low risk for reporting bias, since all the initially planned outcomes were appropriately reported during the follow‐up time points.
Other potential sources of bias
There was no remarkable or apparent source of other potential type of bias identified, in seven out of eight trials studied. Only Gibson 1997 was considered to have a high risk of other bias due to the small number of participants enrolled; it is important to underline that this number was additionally diminished after the excessive dropouts at the intervention completion.
Effects of interventions
See: Table 1
Long chain polyunsaturated fatty acids (LCPUFAs) versus placebo
1. Primary outcome ‐ neurodevelopment
For this outcome, we considered eight areas of neurodevelopment: language development, intelligence or problem‐solving ability, psychomotor development, motor development, general movements, child attention, working memory and inhibitory control, and neurological optimality score. The concept of general movements was described in Hadders‐Algra 2010. For the pooled analysis of these studies, where there was evidence of heterogeneity, we performed a random‐effects model meta‐analysis and expressed the result as standardised mean difference (SMD), because different instruments were used to measure the outcomes among the retrieved studies.
Language development
1.1. Analysis.
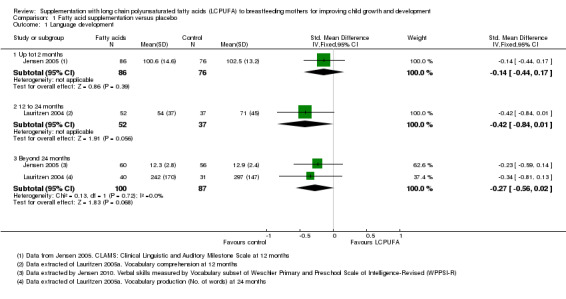
Comparison 1 Fatty acid supplementation versus placebo, Outcome 1 Language development.
Up to 12 months (Subgroup 1.1.1)
Only one study with 162 participants (Jensen 2005), reported data for this outcome. No significant differences were found between LCPUFA supplementation and control groups (SMD ‐0.14, 95% confidence interval (CI) ‐0.44 to 0.17).
12 to 24 months (Subgroup 1.1.2)
One study with 89 participants (Lauritzen 2004), reported this outcome. No significant differences were observed between LCPUFA supplementation and control groups (SMD ‐0.42, 95% CI ‐0.84 to 0.01).
Beyond 24 months (Subgroup 1.1.3)
Two studies providing data from 187 participants (Jensen 2005; Lauritzen 2004), were included in the meta‐analysis. No significant differences were found between LCPUFA supplementation and control groups (SMD ‐0.27, 95% CI ‐0.56 to 0.02). Heterogeneity was not observed between the two studies (Chi² = 0.13, P = 0.72; I² = 0%).
Intelligence or problem‐solving ability
1.2. Analysis.
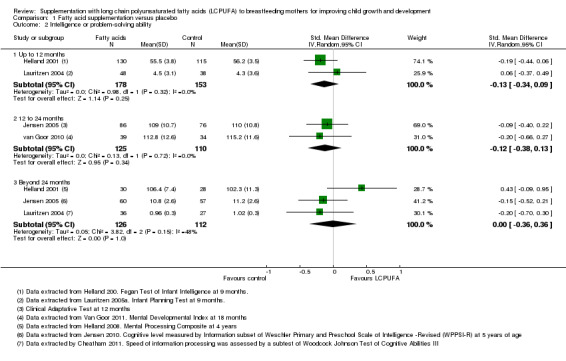
Comparison 1 Fatty acid supplementation versus placebo, Outcome 2 Intelligence or problem‐solving ability.
Up to 12 months (Subgroup 1.2.1)
Two studies with 331 participants (Helland 2001; Lauritzen 2004), reported this outcome and were included in the meta‐analysis. No significant differences were found between LCPUFA supplementation and control groups (SMD ‐0.13, 95% CI ‐0.34 to 0.09). Heterogeneity between studies was not significant (Chi² = 0.98, P = 0.32; I² = 0%).
12 to 24 months (Subgroup 1.2.2)
Two studies with 235 participants (Jensen 2005; van Goor 2010), assessed the outcome and the data were meta‐analysed. No significant difference was found between supplementation and control groups (SMD ‐0.12, 95% CI ‐0.38 to 0.13). Heterogeneity between studies was not significant (Chi² = 0.13, P = 0.72; I² = 0%).
Beyond 24 months (Subgroup 1.2.3)
Three studies with a total of 238 participants (Helland 2001; Jensen 2005; Lauritzen 2004), reported this outcome and were included in meta‐analysis. No significant differences were found between LCPUFA supplementation and control groups (SMD 0.00, 95% CI ‐0.36 to 0.36). Heterogeneity between studies was not significant (Chi² = 3.82, P = 0.15; I² = 48%).
Psychomotor development
1.3. Analysis.
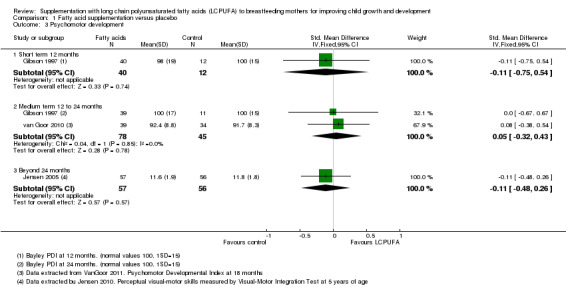
Comparison 1 Fatty acid supplementation versus placebo, Outcome 3 Psychomotor development.
Short term 12 months (Subgroup 1.3.1)
One study with 52 participants (Gibson 1997) reported this outcome. No significant difference was observed between LCPUFA supplementation and control groups (SMD ‐0.11, 95% CI ‐0.75 to 0.54).
Medium term 12 to 24 months (Subgroup 1.3.2)
Two studies (Gibson 1997; van Goor 2010) of 123 participants evaluated this outcome and were included in analysis. No significant difference was found between the LCPUFA supplementation group and the control group (SMD 0.05, 95% CI ‐0.32 to 0.43). Heterogeneity between studies was not significant (Chi² = 0.04, P = 0.85; I² = 0%).
Long term beyond 24 months (Subgroup 1.3.3)
One study of 113 participants (Jensen 2005) reported this outcome. No significant difference was observed between LCPUFA supplementation and control groups (SMD ‐0.11, 95% CI ‐0.48 to 0.26).
Motor development
1.4. Analysis.
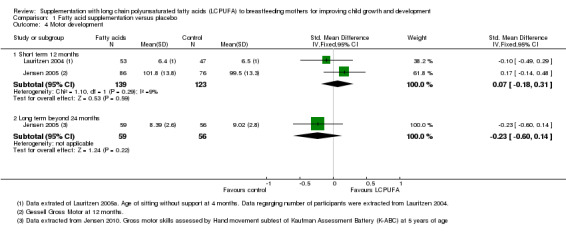
Comparison 1 Fatty acid supplementation versus placebo, Outcome 4 Motor development.
Short term 12 months (Subgroup 1.4.1)
Two studies with a total of 262 participants (Jensen 2005; Lauritzen 2004), measured this outcome. No significant difference was observed between LCPUFA supplementation and control groups (SMD 0.07, 95% CI ‐0.18 to 0.31). Heterogeneity between studies was not significant (Chi² = 1.10, P = 0.29; I² = 9%).
Medium term 12 to 24 months (Subgroup 1.4.2)
One study of 73 participants (van Goor 2010), reported this outcome. No significant difference was found between the LCPUFA group and the control group (median (range):10 (four to 12) versus 10 (six to 12), (P = 0.44)).
Long term beyond 24 months (Subgroup 1.4.3)
One study with 115 participants (Jensen 2005), reported data for this outcome. No significant differences were found between LCPUFA supplementation and control groups (SMD ‐0.23, 95% CI ‐0.60 to 0.14).
General movements
1.5. Analysis.
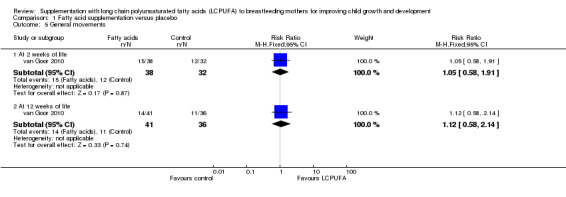
Comparison 1 Fatty acid supplementation versus placebo, Outcome 5 General movements.
At two and 12 weeks of life (Subroup 1.5.1)
One study (van Goor 2010), reported data for this outcome in 70 participants at two weeks of life and in 77 participants at 12 weeks of life, respectively. No significant differences were detected between groups in both times (risk ratio (RR) 1.05, 95% CI 0.58 to 1.91; RR 1.12 95% CI 0.58 to 2.14, respectively).
Child attention
1.6. Analysis.
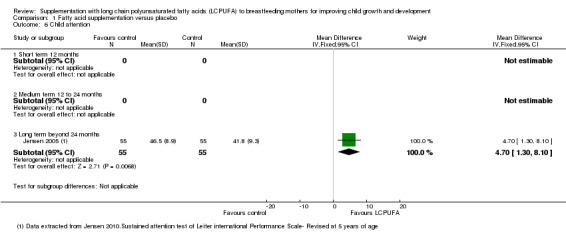
Comparison 1 Fatty acid supplementation versus placebo, Outcome 6 Child attention.
Short term 12 months (Subgroup 1.6.1)
No studies reported data for this outcome.
Medium term 12 to 24 months (Subgroup 1.6.2)
No studies reported data for this outcome.
Long term beyond 24 months (Subgroup 1.6.3)
One study of 110 participants (Jensen 2005), reported data for this outcome. Significant differences were found between LCPUFA supplementation and control groups in favour of the intervention group (mean difference (MD) 4.70, 95% CI 1.30 to 8.10).
Working memory and inhibitory control
1.7. Analysis.
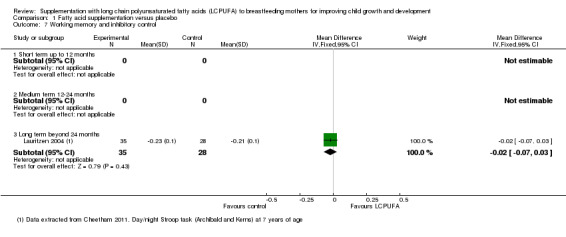
Comparison 1 Fatty acid supplementation versus placebo, Outcome 7 Working memory and inhibitory control.
Short term 12 months (Subgroup 1.7.1)
No studies reported data for this outcome.
Medium term 12 to 24 months (Subgroup 1.7.2)
No studies reported data for this outcome.
Long term beyond 24 months (Subgroup 1.7.3)
One study evaluating 63 participants (Lauritzen 2004), reported data for this outcome. No significant differences were detected between supplementation and control groups (MD ‐0.02 95% CI ‐0.07 to 0.03).
Neurological Optimality Score
1.8. Analysis.
Comparison 1 Fatty acid supplementation versus placebo, Outcome 8 Neurological Optimality Score.
| Neurological Optimality Score | |||||
|---|---|---|---|---|---|
| Study | Study Group | N | Median | Range | p‐value |
| Medium term 12‐24 months | |||||
| van Goor 2010 | LCPUFA | 39 | 48 | 25‐57 | 0.55 |
| van Goor 2010 | Control | 34 | 47.5 | 29‐55 | |
Short term 12 months (Subgroup 1.8.1)
No studies reported data for this outcome.
Medium term 12 to 24 months (Subgroup 1.8.2)
One study (van Goor 2010), reported data for this outcome in a total of 73 participants. No difference was detected between the supplementation and control groups (median (range): 48 (25 to 57) versus 47.5 (29 to 55), (P = 0.55)).
Long term beyond 24 months (Subgroup 1.8.3)
No studies reported data for this outcome.
2. Secondary outcomes
For the pooled analysis of these studies, we performed a random‐effects model meta‐analysis where there was evidence of heterogeneity and expressed the result as SMD, because different instruments were used to measure the outcomes among the retrieved studies
Child visual acuity
1.9. Analysis.
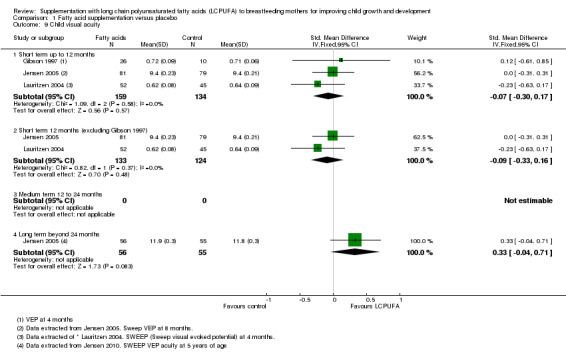
Comparison 1 Fatty acid supplementation versus placebo, Outcome 9 Child visual acuity.
Short term 12 months (Subgroup 1.9.1)
Three studies reported data for this outcome (Gibson 1997; Jensen 2005; Lauritzen 2004). A total of 293 participants were included in meta‐analysis. No significant differences were found (MD ‐0.07, 95% CI ‐0.30 to 0.17). Heterogeneity between studies was not significant (Chi² = 1.09, P = 0.58; I² = 0%).
Medium term 12 to 24 months (Subgroup 1.9.2)
No studies reported data for this outcome.
Long term beyond 24 months (Subgroup 1.9.3)
One study evaluating 111 participants (Jensen 2005), reported data for this outcome. No significant differences were detected between supplementation and control groups (SMD 0.33, 95% CI ‐0.04 to 0.71).
Child weight
For the pooled analysis of outcomes child weight, child length, child head circumference and child BMI of studies, we performed a fixed‐effect model meta‐analysis and expressed the result as MD, because the same instruments were used to measure the outcomes among the retrieved studies.
1.10. Analysis.
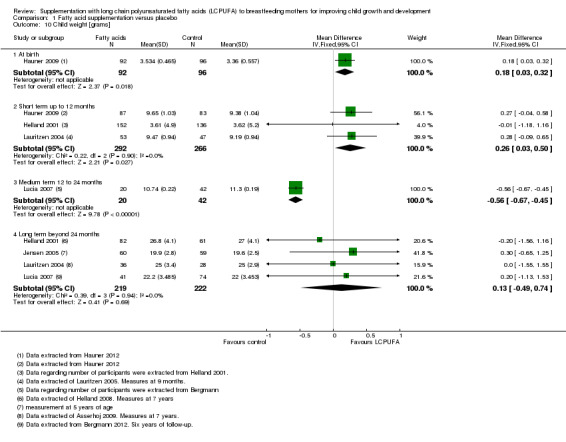
Comparison 1 Fatty acid supplementation versus placebo, Outcome 10 Child weight [grams].
At birth (Subgroup 1.10.1)
One study of 188 participants (Hauner 2009), reported data for this outcome. Significant differences were detected between supplementation and control groups in favour of the LCPUFA intervention group (MD 0.18, 95% CI 0.03 to 0.32).
Short term up to 12 months (Subgroup 1.10.2)
Three studies evaluating a total of 558 participants assessed this outcome (Hauner 2009; Helland 2001; Lauritzen 2004), and were included in meta‐analysis. Significant differences were detected between LCPUFA and control groups in favour of the fatty acid supplementation (MD 0.26, 95% CI 0.03 to 0.50). Heterogeneity between studies was not significant (Chi² = 0.22, P = 0.90; I² = 0%).
Medium term 12 to 24 months (Subgroup 1.10.3)
One study of 62 participants (Lucia 2007), reported this outcome. Significant differences were detected between supplementation and control groups in favour of the control group (MD ‐0.56, 95% CI ‐0.67 to ‐0.45).
Long term beyond 24 months (Subgroup 1.10.4)
Four studies evaluating a total of 441 participants reported this outcome (Helland 2001; Jensen 2005; Lauritzen 2004; Lucia 2007), and were included in the meta‐analysis. No significant differences were detected between LCPUFA and control groups in favour of the fatty acid supplementation group (MD 0.13, 95% CI ‐0.49 to 0.74). Heterogeneity between studies was not significant (Chi² = 0.39, P < 0.69; I² = 0%).
Child length
1.11. Analysis.
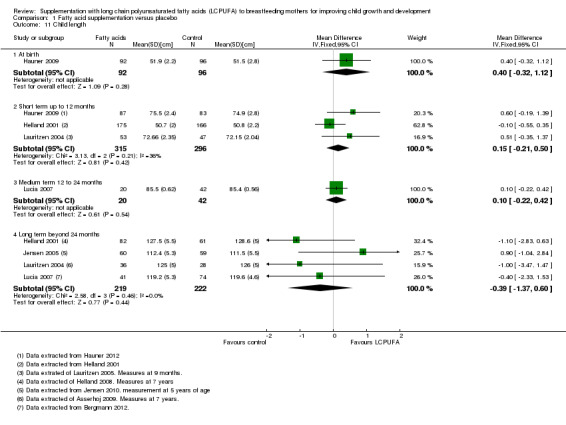
Comparison 1 Fatty acid supplementation versus placebo, Outcome 11 Child length.
At birth (Subgroup 1.11.1)
One study of 188 participants (Hauner 2009), reported data for this outcome. No significant differences were detected between supplementation and control groups (MD 0.40, 95% CI ‐0.32 to 1.12).
Short term up to 12 months (Subgroup 1.11.2)
Three studies evaluating a total of 611 participants assessed this outcome (Hauner 2009; Helland 2001; Lauritzen 2004), and were included in a meta‐analysis. No significant differences were detected between LCPUFA and control groups (MD 0.15, 95% CI ‐0.21 to 0.50). Heterogeneity between studies was not significant (Chi² = 3.13, P = 0.21; I² = 36%).
Medium term 12 to 24 months (Subgroup 1.11.3)
One study of 62 participants (Lucia 2007), reported this outcome. No significant differences were detected between supplementation and control groups (MD 0.10, 95% CI ‐0.22 to 0.42).
Long term beyond 24 months (Subgroup 1.11.4)
Four studies evaluating a total of 441 participants reported this outcome (Helland 2001; Jensen 2005; Lauritzen 2004; Lucia 2007), and were included in a meta‐analysis. No significant differences were detected between LCPUFA and control groups (MD ‐0.39, 95% CI ‐1.37 to 0.60). However, heterogeneity between studies was not significant (Chi² = 2.58, P = 0.46; I² = 0%).
Head circumference
1.12. Analysis.
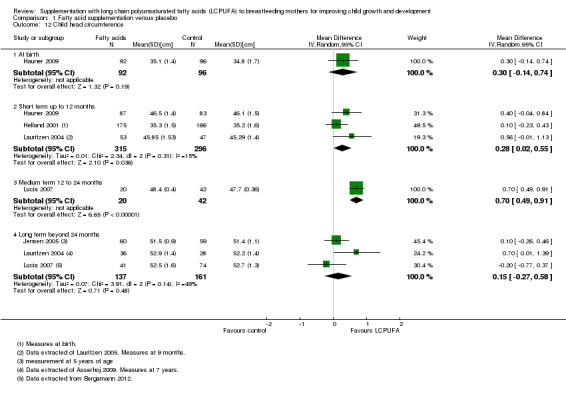
Comparison 1 Fatty acid supplementation versus placebo, Outcome 12 Child head circumference.
At birth (Subgroup 1.12.1)
One study of 188 participants (Hauner 2009), reported data for this outcome. No significant differences were detected between supplementation and control groups (MD 0.30, 95% CI ‐0.14 to 0.74).
Short term up to 12 months (Subgroup 1.12.2)
Three studies evaluating a total of 611 participants assessed this outcome (Hauner 2009; Helland 2001; Lauritzen 2004), and were included in a meta‐analysis. Significant differences were detected between LCPUFA and control groups in favour of the fatty acid supplementation group (MD 0.28, 95% CI 0.02 to 0.55). Heterogeneity between studies was not significant (Chi² = 2.34, P = 0.31; I² = 15%).
Medium term 12 to 24 months (Subgroup 1.12.3)
One study of 62 participants (Lucia 2007), reported this outcome. Significant differences were detected between LCPUFA and control groups in favour of the fatty acid supplementation group (MD 0.70, 95% CI 0.49 to 0.91).
Long term beyond 24 months (Subgroup 1.12.4)
Three studies evaluating a total of 298 participants reported this outcome (Jensen 2005; Lauritzen 2004; Lucia 2007), and were included in a meta‐analysis. No significant differences were detected between LCPUFA and control groups (MD 0.15, 95% CI ‐0.27 to 0.58). No heterogeneity between studies was detected (Chi² = 3.91, P = 0.14; I² = 49%).
Child BMI
1.13. Analysis.
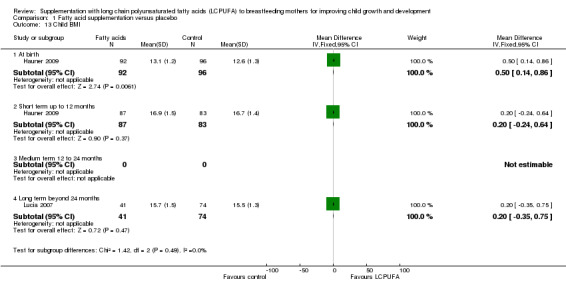
Comparison 1 Fatty acid supplementation versus placebo, Outcome 13 Child BMI.
At birth (Subgroup 1.13.1)
One study evaluated this outcome (Hauner 2009), in a total of 188 participants. Significant differences were detected between the supplementation group and the control group (MD 0.50, 95% CI 0.14 to 0.86).
Short term up to 12 months (Subgroup 1.13.2)
One study of 170 participants reported data for this outcome (Hauner 2009). No significant differences were found between the LCPUFA supplementation group and the control group (MD 0.20, 95% CI ‐0.24 to 0.64).
Medium term 12 to 24 months (Subgroup 1.13.3)
No studies reported data for this outcome.
Long term beyond 24 months (Subgroup 1.13.4)
One study evaluated this outcome (Lucia 2007), in a total of 115 participants. No significant differences were detected between the supplementation group and the control group (MD 0.20, 95% CI ‐0.35 to 0.75).
Child fat mass
1.14. Analysis.
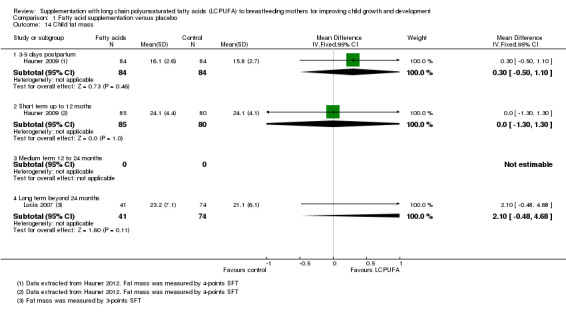
Comparison 1 Fatty acid supplementation versus placebo, Outcome 14 Child fat mass.
Three to five days postpartum (Subgroup 1.14.1)
One study of 168 participants (Hauner 2009), assessed this outcome. No significant differences were found between the LCPUFA supplementation and control groups (MD 0.30, 95% CI ‐0.50 to 1.10).
Short term up to 12 months (Subgroup 1.14.2)
One study of 165 participants (Hauner 2009), reported this outcome. No significant differences were found between the LCPUFA supplementation and control groups (MD 0.00, 95% CI ‐1.30 to 1.30).
Medium term 12 to 24 months (Subgroup 1.14.3)
No studies reported data for this outcome.
Long term beyond 24 months (Subgroup 1.14.4)
One study evaluated this outcome (Lucia 2007), in a total of 115 participants. No significant differences were detected between the supplementation group and the control group (MD 2.10, 95% CI ‐0.48 to 4.68).
Fat mass distribution
1.15. Analysis.
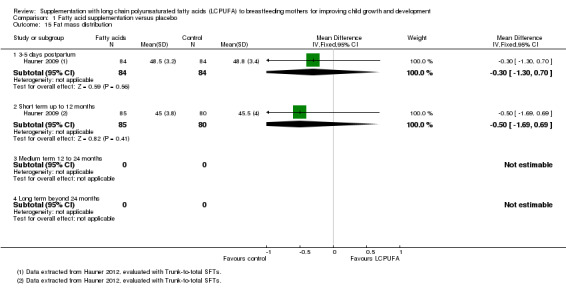
Comparison 1 Fatty acid supplementation versus placebo, Outcome 15 Fat mass distribution.
Three to five days postpartum (Subgroup 1.15.1)
One study of 168 participants (Hauner 2009), assessed this outcome. No significant differences were found between the LCPUFA supplementation and control groups (MD ‐0.30, 95% CI ‐1.30 to 0.70).
Short term up to 12 months (Subgroup 1.14.2)
One study of 165 participants (Hauner 2009), reported this outcome. No significant differences were found between the LCPUFA supplementation and control groups (MD ‐0.50, 95% CI ‐1.69 to 0.69).
Medium term 12 to 24 months (Subgroup 1.14.3)
No studies reported data for this outcome.
Long term beyond 24 months (Subgroup 1.14.4)
No studies reported data for this outcome.
Other non‐prespecified secondary outcomes
Infant allergy
1.16. Analysis.
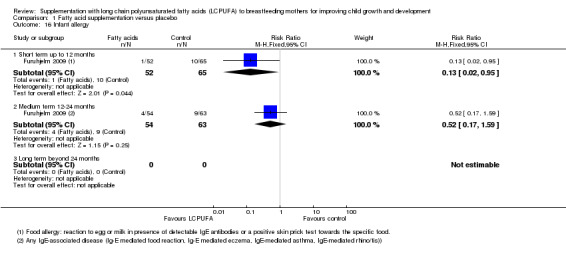
Comparison 1 Fatty acid supplementation versus placebo, Outcome 16 Infant allergy.
Short term up to 12 months (Subgroup 1.16.1)
Infant allergy was evaluated by Furuhjelm 2009 (117 infants). A significant difference was found between LCPUFA supplementation and control groups (RR 0.13, 95% CI 0.02 to 0.95).
Medium term 12 to 24 months (Subgroup 1.16.2)
One study (Furuhjelm 2009, 117 infants), reported this outcome. No difference was detected regarding the risk of allergy between the LCPUFA supplementation (4/54) and control groups (9/63), (RR 0.52, 95% CI 0.17 to 1.59).
Long term beyond 24 months (Subgroup 1.16.3)
No studies reported data for this outcome.
Postpartum depression
1.17. Analysis.
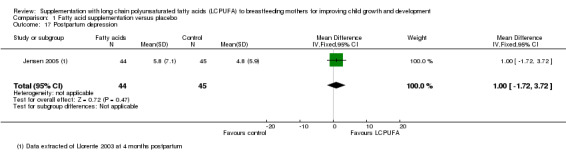
Comparison 1 Fatty acid supplementation versus placebo, Outcome 17 Postpartum depression.
Postpartum depression was evaluated in a study of 89 participants by Jensen 2005, at four months postpartum and also in a study of 77 participants by van Goor 2010 at six weeks postpartum. Data were not possible to be pooled due to differences in the describing of the outcome. The study of Jensen 2005 did not find any significant difference between the LCPUFA supplementation and the control group (MD 1.00, 95% CI ‐1.72 to 3.72). Neither did the study of van Goor 2010 find any significant difference between the LCPUFA supplementation and control groups (median (25th to 75th percentile): 5 (2 to 6) versus 5(2 to 6.5).
Postpartum blues
One study reported this outcome (van Goor 2010). Authors did not provide level of significance nor enough data to calculate mean differences. Median blues scores (25th; 75th percentile) were 8.5 (4.5 to 13.5) for the fatty acid supplementation and 8 (4 to 10) for the control group. No difference was detected between the fatty acid supplementation and the control group. Authors only stated that there we no significant differences, but the level of significance was not stated. Only 60 participants completed the blues questionnaire (initially stratified: 36 participants in the placebo group, 42 participants in the DHA group, 41 participants in the DHA + AA). Sixteen out of 60 (27%) women were considered to suffer from postpartum blues.
Safety of supplementation with LCPUFA to mothers and babies
Hauner 2009 reported this outcome. Safety and blood coagulation variables were similar in both groups.
Mother’s views or satisfaction
We no found trials that reported data on the mother's views or satisfaction.
Subgroup analysis
There were insufficient data to perform the planned subgroup analysis; these will be carried out in subsequent updates of this review, as data become available.
Sensitivity analysis
We performed a sensitivity analysis, removing Gibson 1997 (at high risk of bias) from the analysis of the primary outcome psychomotor development and the secondary outcome, visual acuity. Removing this study from both analyses (Analysis 1.3; Analysis 1.9) made no difference to the results.
Discussion
It has been recognised that long chain polyunsaturated fatty acids (LCPUFAs) are important nutrients, with biologically plausible and potential benefits in development and child growth (McCann 2005).
The human brain exhibits a period of intense growth during the third trimester of pregnancy. This process continues in term infants until the age of two years, while in preterm infants this "brain‐growth‐spurt" may occur postnatally instead of in utero. The "brain‐growth‐spurt" is strongly correlated to the LCPUFA accretion. Since LCPUFAs are not endogenously synthesised, the main source of LCPUFA, particularly docosahexaenoic acid (DHA), during the first six months of life is breast milk (Koletzco 2008).
DHA concentration in breast milk depends on the mother's diet. While the total lipid content of human milk appears generally constant, decreased maternal plasma DHA concentrations have been observed in women after the delivery (Jensen 2006). Otherwise, deficiencies of LCPUFA intake have been associated with impairments in cognitive performance (Campoy 2012). Thus, the fetus and the newborn should receive LCPUFA in amounts sufficient to guarantee visual and cognitive development. The LCPUFA supplementation for mothers during pregnancy and lactation has been proposed from the perspective of preventive nutrition (Koletzco 2008; Larqué 2012).
Taking into consideration the significance of the above‐mentioned biological procedures on the developing human brain and retina, the majority of the commercial available infant formulas are LCPUFA‐enriched. Thus, researchers have hypothesised that for lactating mothers, LCPUFA supplementation might have significant effects on children's neurodevelopment, visual acuity and growth. This systematic review was designed to evaluate the efficacy of LCPUFA supplementation to lactating mothers compared with placebo in the development of their children.
Summary of main results
The current systematic review includes data from eight published trials, including a total of 1567 women. In these trials, LCPUFA supplementation was started either during pregnancy and continued postpartum into breastfeeding, or commenced at the beginning of lactation. Supplementation of LCPUFA exclusively during pregnancy and its effect on neurodevelopmental, visual and growth outcomes of the offspring has been discussed in other reviews (Larqué 2012).
We were able to identify a significant effect of LCPUFA on one neurodevelopmental outcome, but failed to provide evidence of an effect on the majority of other neurodevelopmental outcomes examined. Five different studies tried to elucidate neurodevelopmental status by assessing eight different neurological areas. The longest time point studied was the follow‐up at seven years of age (Lauritzen 2004), and the shortest time point, two weeks of life (van Goor 2010). The supplementation of LCPUFA proved to have no effect on language development, intelligence or problem‐solving ability, psychomotor development, motor development, general movements, working memory and inhibitory control of offspring of supplemented mothers. The only neurodevelopmental outcome that significantly changed as a result of LCPUFA supplementation was child attention at a long‐term follow‐up (beyond 24 months). However, this result was based on only one trial with a relatively small population of children who were assessed at five years of age (Jensen 2005).
Similarly, visual acuity did not appear to be associated with LCPUFA supplementation at any time point studied. Despite the fact that three different studies addressed this outcome at both short‐term and long‐term follow‐up, no association was observed after pooling of data. The longest follow‐up time point studied regarding visual function was five years of age.
Regarding anthropometry, evaluation of participants took place in children from the day of their birth (Hauner 2009), to a follow‐up at the age of seven years of age (Lauritzen 2004), in five different trials. According to the pooled analysis of their data, LCPUFA supplementation resulted in a significantly increased body weight at birth and at short‐term follow‐up, up to 12 months of age, whilst this relationship was not observed at longer‐term follow‐up beyond 24 months. Similarly, head circumference was found to be significantly higher at short‐term and medium‐term time points, but not at longer‐term follow‐up. Body length was not affected by LCPUFA supplementation at any follow‐up, apart from at birth (supplementation led to significantly higher BMI only at the time of birth).
Fat mass was also explored in relation to LCPUFA supplementation in two trials (Hauner 2009; Lucia 2007), at three to five days postpartum, short‐term (12 months) and long‐term follow‐up, with a maximum follow‐up of six years of age. No difference was found between children of supplemented and non‐supplemented mothers at any time point studied. This review did not provide any evidence that fat mass distribution was associated with LCPUFA supplementation. However, this outcome was only assessed after birth (three to five days postpartum) and at short‐term follow‐up, in only one study (Hauner 2009) in a relatively small population.
At short‐term follow‐up (before the age of 12 months), a significant association was seen between LCPUFA supplementation and infant allergy in one trial (Furuhjelm 2009) but not at medium‐term follow‐up (at two years of age).
Finally, postpartum depression and postpartum blues prevalence was also explored in two different trials (Jensen 2005; van Goor 2010) assessing mothers at both six weeks and four months postpartum. No significant relationship between LCPUFA supplementation and depression or blues was identified.
Overall completeness and applicability of evidence
This review did not find sufficient evidence to recommend the supplementation of LCPUFA to lactating mothers.
The results of our review are consistent with the suggestions of McCann 2005 and Cheatham 2006, who concluded that, in order to achieve appropriate neurological and visual development, it is necessary to test different amounts of LCPUFA supplementation, and measurement methods must be homogeneous, sensitive, and specific for the different areas of neurodevelopment (Campoy 2012).
Because maternal DHA status is often diverse and inadequate (Brenna 2007; Torres 2009), and there are different patterns of ocean fish intake, the natural source of LCPUFA (Welch 2002), it seems appropriate to conduct studies in different regions of the world.
Mothers participating in the trials came from high‐income countries. Moreover, they were healthy women with no‐risk pregnancies, so the external validity of this review is limited.
Quality of the evidence
The principal prespecified outcomes were to assess neurodevelopment and visual acuity in relation to LCPUFA supplementation.
We used the GRADE approach to evaluate the body of the evidence and we produced the (Table 1). In general, the quality of the evidence for the most important outcomes was assessed as moderate and low .
For the outcome "language development", the evidence is from two randomised controlled trials (RCTs). The quality was assessed as being moderate and was downgraded due to concerns regarding risk of bias. For the outcome "Intelligence or problem‐solving ability", the evidence is from three RCTs and was assessed as being low quality due to concerns regarding risk of bias and inconsistency. For the outcomes "psychomotor development", "child attention", and "visual acuity", the evidence was assessed as being low quality due to concerns regarding risk of bias and imprecision. For the outcome, child weight, the evidence was evaluated as being moderate quality due to concerns regarding risk of bias.
Infant allergy was assessed in only one study Furuhjelm 2009). Although this study was assessed as being at low risk of bias for all domains, the evidence is restricted to a single study, with a relatively small population that could not be pooled. Finally, Jensen 2005 and van Goor 2010 reported the outcomes postpartum depression and blues. Both outcomes were from studies with moderate risk of bias issues due to concerns regarding attrition bias, blinding and allocation concealment.
However it is important also to note that for the vast majority of outcomes, data were available for more than one study for each time point studied (short term, long term, medium term) and thus pooling of them allowed us to expand the strength of the present review findings.
On the other hand, true adherence of the participants during supplementation period may also be a potential source of bias for the findings of this review. The vast majority of the trials included in this analysis, did not intend or did not manage to address the adherence of the mothers. This may have an impact on the extracted data in terms of true exposure (supplementation with LCPUFAs) or not. Additionally, while the characteristics of mothers participating in the various studies were similar, trials used different doses of DHA and arachidonic acid (AA), varying from the current recommendations for breastfeeding mothers (Koletzco 2008), to an amount five times higher in the Danish studies (Furuhjelm 2009; Lauritzen 2004).
Finally, trials showed high variation in terms of the measurement of outcomes such as neurodevelopment and visual acuity. This wide variation in tools and methods used to assess neurodevelopment resulted in the generation of many different analyses measuring the same outcome (see Analysis 1.1 to Analysis 1.8), and this may make interpretation of the findings difficult, since we were not able to pool much of the retrieved data. On the other hand, measures of growth evaluation were performed in a more standard way and so data could be pooled and were presented in a homogenous way.
Potential biases in the review process
This systematic review addressed prespecified research questions after the application of predefined inclusion criteria of the studies retrieved. Evidence in this review was derived from studies identified in a detailed search process. We acknowledge that not all unpublished trials examining the supplementation of LCPUFAs may have been identified. We attempted to minimise bias in the review process by having two review authors independently assessing studies and extracting data.
No financial, or other type of support was directly or indirectly offered to the authors, in terms of the production of conflict of interest.
Agreements and disagreements with other studies or reviews
This systematic review's findings are in accordance with other reviews (Campoy 2012; Gould 2013). The evidence for LCPUFA supplementation in mothers to improve the neurodevelopment and physical growth of their offspring is inconclusive.
Authors' conclusions
Implications for practice.
Our review did not find strong evidence that supplementation improves child growth or development. The absence of sound evidence regarding LCPUFA supplementation contrasts with the recommendations of a consensus expert (Koletzco 2008) and common practice in many countries.
LCPUFA supplementation is currently being promoted and may be purchased without prescription in different doses and different formulations in various regions all over the world. Supplemenation is widely marketed and promoted (Jensen 2006), and so there is a demand for its use.
Therefore, based on the available evidence, there is little basis for supplementation with LCPUFA in breastfeeding mothers to improve infant growth and development.
Implications for research.
Subgroup analysis based on the timing of the onset of supplementation (pregnancy or postpartum) is needed. These will be carried out in subsequent updates of this review, as data become available.
It is necessary to take into account that variations in measurements make analysis and interpretation of the results difficult. Therefore, it is desirable that future studies ensure standardisation of techniques for measurement of different outcomes of neurodevelopment, so that future studies are able to pool results.
Studies should include populations with mothers and children from different latitudes due to variability in diets, consumption of fish and doses of DHA (Helgi Library). More studies are necessary for the assessment the effectiveness of LCPUFA supplementation in breastfeeding woman in infant allergy and postpartum depression.
What's new
| Date | Event | Description |
|---|---|---|
| 16 December 2014 | New citation required but conclusions have not changed | We added follow‐up data for previously included studies (Furuhjelm 2009; Jensen 2005; Lauritzen 2004; Lucia 2007). In addition, two new studies were added (Hauner 2009; van Goor 2010). |
| 6 August 2014 | New search has been performed | Search updated. A 'Summary of findings' table has been incorporated for this update. |
History
Protocol first published: Issue 3, 2009 Review first published: Issue 12, 2010
| Date | Event | Description |
|---|---|---|
| 29 January 2013 | Amended | Contact details updated. |
Acknowledgements
To Hospital de Sant Creu i Sant Pau‐Iberoamerican Cochrane Centre, Papageorgiuou General Hospital of Thessaloniki, Aristotle University of Thessaloniki, Greece and University of Cauca, Popayán, Colombia for their support.
To Marta Roqué for her methodological and statistical advice. To Dimelza Osorio for her contribution to searching. To Olga Dakou in Greece for their support.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group’s international panel of consumers and the Group’s Statistical Adviser (2010).
Appendices
Appendix 1. CENTRAL search strategy
The Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 8) #1 MeSH descriptor Lactation explode all trees #2 MeSH descriptor breastfeeding explode all trees #3 lactation OR breast‐fe* OR breastfe* OR breast‐milk OR breastmilk OR breastfeed* OR breast‐feed* OR lactating mother* OR lactating woman OR lactating women #4 #1 OR #2 OR #3 OR #4 #5 MeSH descriptor Fatty acids explode all trees #6 fatty acid* #7 omega 3 #8 LCPUFA* OR PUFA* OR LC n‐3 #9 docosahexaenoic acid* OR docosahexanoic acid* OR eicosapentaenoic acid* OR eicosapentanoic acid* #10 MeSH descriptor Fish oils explode all trees #11 fish oil* #12 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR#11 #13 #4 and #12
Appendix 2. PubMed search strategy
(1966 to August 2014)
#1 Lactation[MeSH] #2 lactation[tiab] #3 "breastfeeding"[MesH] #4 breast‐fe*[tiab] OR breastfe*[tiab] #5 breast‐milk[tiab] OR breastmilk[tiab] #6 breastfeed*[tiab] #7 breast‐feed*[tiab] #8 lactating mother*[tiab] #9 lactating woman[tiab] #10 lactating women[tiab] #11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 #12 “Fatty Acids”[MesH] #13 fatty acid*[tw] #14 omega 3[tw] #15 LCPUFA*[tw] #16 LC n‐3 FA*[tw] #17 PUFA*[tw] #18 docosahexaenoic acid*[tiab] OR docosahexanoic acid*[tiab] OR docosahexenoic acid*[tiab] #19 eicosapentaenoic acid*[tiab] OR eicosapentanoic acid*[tiab] OR eicosapentenoic acid*[tiab] #20 “Fish Oils”[MesH] #21 fish oil*[tiab] #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #11 and #22 #24 randomized controlled trial [pt] #25 controlled clinical trial [pt] #26 randomized [tiab] #27 placebo [tiab] #28 drug therapy [sh] #29 randomly [tiab] #30 trial [tiab] #31 groups [tiab] #32 #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 #33 animals [mh] not (humans [mh] and animals [mh]) #34 #23 AND #32 #35 #34 NOT #33
Appendix 3. EMBASE search strategy
(1974 to August 2014, via OVID) 1 exp LACTATION/ 2 lactation.ti,ab. 3 exp breastfeeding/ 4 (lactation OR breast‐fe* OR breastfe* OR breast‐milk OR breastmilk OR breastfeed* OR breast‐feed* OR lactating mother* OR lactating woman OR lactating women).ti,ab. 5 1 or 2 or 3 or 4 6 exp Fatty Acid/ 7 fatty acid*.mp. 8 omega 3.mp. 9 (LCPUFA* or LC n‐3 FA* or PUFA*).mp. 10 (docosahexaenoic acid* or docosahexanoic acid* or docosahexenoic acid*).ti,ab 11 (eicosapentaenoic acid* or eicosapentanoic acid* or eicosapentenoic acid*).ti,ab. 12 exp Fish Oil/ 13 fish oil*.ti,ab. 14 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15 5 and 14 16 Clinical trial/ 17 Randomized controlled trials/ 18 Random Allocation/ 19 Single‐Blind Method/ 20 Double‐Blind Method/ 21 Cross‐Over Studies/ 22 Placebos/ 23 Randomi?ed controlled trial$.tw. 24 RCT.tw. 25 Random allocation.tw. 26 Randomly allocated.tw. 27 Allocated randomly.tw. 28 (allocated adj2 random).tw. 29 Single blind$.tw. 30 Double blind$.tw. 31 ((treble or triple) adj blind$).tw. 32 Placebo$.tw. 33 Prospective Studies/ 34 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 35 Case study/ 36 Case report.tw. 37 Abstract report/ or letter/ 38 35 or 36 or 47 39 34 not 38 40 animal/ not human/ 41 15 and 39 45 41 not 40
Appendix 4. LILACS search strategy
(1982 to August 2014)
First block
((Pt ENSAIO CONTROLADO ALEATORIO OR Pt ENSAIO CLINICO CONTROLADO OR Mh ENSAIOS CONTROLADOS ALEATORIOS OR Mh DISTRIBUICAO ALEATORIA OR Mh MÉTODO DUPLO‐CEGO OR Mh MÉTODO SIMPLES‐CEGO) AND NOT (Ct ANIMALS AND NOT (Ct HUMANO AND Ct ANIMALS)) OR (Pt ENSAIO CLÍNICO OR Ex E05.318.760.535$) OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh PLACEBOS OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR (Mh PROJETOS DE PESQUISA) AND NOT (Ct ANIMALS AND NOT (Ct HUMANO AND Ct ANIMALS)) OR (Ct ESTUDO COMPARATIVO OR Ex E05.337$ OR Mh SEGUIMENTOS OR Mh ESTUDOS PROSPECTIVOS OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct ANIMALS AND NOT (Ct HUMANO AND Ct ANIMALS))) AND NOT Mh ANIMALS
Second block
Mh Eicosapentaenoic Acid OR Mh Docosahexaenoic Acids OR Mh Fatty Acids OR Mh Fish oils OR AB LC‐PUFA OR AB omega‐3 OR AB acido oleico
Third block
Mh breastfeeding OR Mh Lactation OR Mh Milk Human OR AB breast‐fe$ OR breastfe$
Block 1 AND Block 2 AND Block 3
Appendix 5. GOOGLE SCHOLAR search strategy
We use a simple search strategy with the next phrase. We restrict the strategy with the addition of hyphen (‐animals) to search humans only.
Lactation breastfeeding Breast milk Fatty acids Fish oils Docosahexaenoic Acids Eicosapentaenoic Acid Clinical Trial Randomized Controlled Trials ‐animals
Searched August 2014 (With the interval 2009‐2014).
Appendix 6. CINAHL search strategy
(1984 to June 2009, via Ovid)
1 exp LACTATION/ 2 lactation.ti,ab. 3 exp breastfeeding/ 4 (lactation OR breast‐fe* OR breastfe* OR breast‐milk OR breastmilk OR breastfeed* OR breast‐feed* OR lactating mother* OR lactating woman OR lactating women).ti,ab. 5 1 or 2 or 3 or 4 6 exp Fatty Acid/ 7 fatty acid*.mp. 8 omega 3.mp. 9 (LCPUFA* or LC n‐3 FA* or PUFA*).mp. 10 (docosahexaenoic acid* or docosahexanoic acid* or docosahexenoic acid*).ti,ab 11 (eicosapentaenoic acid* or eicosapentanoic acid* or eicosapentenoic acid*).ti,ab. 12 exp Fish Oil/ 13 fish oil*.ti,ab. 14 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15 5 and 14 16 exp prognosis OR exp study design OR random:.mp. 17 15 and 16
Data and analyses
Comparison 1. Fatty acid supplementation versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Language development | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Up to12 months | 1 | 162 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.44, 0.17] |
| 1.2 12 to 24 months | 1 | 89 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐0.84, 0.01] |
| 1.3 Beyond 24 months | 2 | 187 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.56, 0.02] |
| 2 Intelligence or problem‐solving ability | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Up to 12 months | 2 | 331 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.34, 0.09] |
| 2.2 12 to 24 months | 2 | 235 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.38, 0.13] |
| 2.3 Beyond 24 months | 3 | 238 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.36, 0.36] |
| 3 Psychomotor development | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Short term 12 months | 1 | 52 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.75, 0.54] |
| 3.2 Medium term 12 to 24 months | 2 | 123 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.32, 0.43] |
| 3.3 Beyond 24 months | 1 | 113 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.48, 0.26] |
| 4 Motor development | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Short term 12 months | 2 | 262 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.18, 0.31] |
| 4.2 Long term beyond 24 months | 1 | 115 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.60, 0.14] |
| 5 General movements | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 At 2 weeks of life | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.58, 1.91] |
| 5.2 At 12 weeks of life | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.58, 2.14] |
| 6 Child attention | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Short term 12 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Medium term 12 to 24 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Long term beyond 24 months | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 4.70 [1.30, 8.10] |
| 7 Working memory and inhibitory control | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Short term up to 12 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Medium term 12‐24 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Long term beyond 24 months | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.07, 0.03] |
| 8 Neurological Optimality Score | Other data | No numeric data | ||
| 8.1 Short term up to 12 months | Other data | No numeric data | ||
| 8.2 Medium term 12‐24 months | Other data | No numeric data | ||
| 8.3 Long term beyond 24 months | Other data | No numeric data | ||
| 9 Child visual acuity | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Short term up to 12 months | 3 | 293 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.30, 0.17] |
| 9.2 Short term 12 months (excluding Gibson 1997) | 2 | 257 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.33, 0.16] |
| 9.3 Medium term 12 to 24 months | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 Long term beyond 24 months | 1 | 111 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.33 [‐0.04, 0.71] |
| 10 Child weight [grams] | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 At birth | 1 | 188 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [0.03, 0.32] |
| 10.2 Short term up to 12 months | 3 | 558 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [0.03, 0.50] |
| 10.3 Medium term 12 to 24 months | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐0.67, ‐0.45] |
| 10.4 Long term beyond 24 months | 4 | 441 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.49, 0.74] |
| 11 Child length | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 At birth | 1 | 188 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.32, 1.12] |
| 11.2 Short term up to 12 months | 3 | 611 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.21, 0.50] |
| 11.3 Medium term 12 to 24 months | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.22, 0.42] |
| 11.4 Long term beyond 24 months | 4 | 441 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.37, 0.60] |
| 12 Child head circumference | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 At birth | 1 | 188 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.14, 0.74] |
| 12.2 Short term up to 12 months | 3 | 611 | Mean Difference (IV, Random, 95% CI) | 0.28 [0.02, 0.55] |
| 12.3 Medium term 12 to 24 months | 1 | 62 | Mean Difference (IV, Random, 95% CI) | 0.70 [0.49, 0.91] |
| 12.4 Long term beyond 24 months | 3 | 298 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.27, 0.58] |
| 13 Child BMI | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 At birth | 1 | 188 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [0.14, 0.86] |
| 13.2 Short term up to 12 months | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.24, 0.64] |
| 13.3 Medium term 12 to 24 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.4 Long term beyond 24 months | 1 | 115 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.35, 0.75] |
| 14 Child fat mass | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 3‐5 days postpartum | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.50, 1.10] |
| 14.2 Short term up to 12 moths | 1 | 165 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.30, 1.30] |
| 14.3 Medium term 12 to 24 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.4 Long term beyond 24 months | 1 | 115 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [‐0.48, 4.68] |
| 15 Fat mass distribution | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1 3‐5 days postpartum | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.30, 0.70] |
| 15.2 Short term up to 12 months | 1 | 165 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.69, 0.69] |
| 15.3 Medium term 12 to 24 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.4 Long term beyond 24 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Infant allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Short term up to 12 months | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.95] |
| 16.2 Medium term 12‐24 months | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.17, 1.59] |
| 16.3 Long term beyond 24 months | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Postpartum depression | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐1.72, 3.72] |
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Gibson 1997.
| Methods | Randomised double‐blinded trial. | |
| Participants | Adelaide, Australia. 52 "Mothers of term infants (> 37 weeks' gestation) who intended to breast feed for at least 12 weeks". Criteria of eligibility: Quote: "All infants were healthy, appropriate weight for gestation and had Apgar scores greater than 7 at 5 minutes post birth". |
|
| Interventions | Intervention group: DHA‐rich algal oil.The oil contained 43% DHA, 1% n‐6 PUFA, 38% saturates and 18% monounsaturated. 0.2, 0.4, 0.9 or 1.3 g DHA/day. Control group: placebo (not stated). Duration of intervention:12 weeks. Duration of following: 12 months. |
|
| Outcomes | Neurodevelopmental status measured by Bayley MDI and PDI. Visual acuity measured by VEP 12 weeks and 16 weeks. |
|
| Notes | "Financial support was provided by Martek Biosciences, MD, USA and the National Health and Medical Research Council, Australia." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "..These mothers were randomised to receive one of five doses". We evaluated as unclear risk because the information is insufficient about the sequence of generation process. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind placebo control trial...". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Inadequate. For the first outcome, assessment of visual function, 26/52 (50%) were excluded for difficult behaviour at 12 weeks. Quote: "...that large number of exclusions from the VEP acuity determinations reduced the power of the study raising the possibility of a type II error. The number of exclusions was greater than expected at 12 weeks of age which may have been due to the fact that some infants may not have developed binocularity at this age so that some children have had difficulty focusing on the monitor". 50%, rate of lost for assessment of visual function. The greater proportion of missing follow‐up data occurred in the short term. |
| Selective reporting (reporting bias) | Low risk | Not detected. |
| Other bias | High risk | Small number of participants. |
Helland 2001.
| Methods | Randomised trial. Blinded. Helland 2001 is the main study with 3 publications at different time points for the same outcomes. |
|
| Participants | Norway. 590 (intervention group: 301, control group 289). Criteria of eligibility: healthy women with single pregnancy, 19‐35 years of age, with intention to breastfeed. Exclusion criteria of newborns: premature, asphyxia, infections, anomalies that required special attention. |
|
| Interventions | Intervention group: 10 mL of cod liver oil per day. Control group: 10 mL of corn oil. Duration of intervention: 17‐19 weeks of pregnancy to 3 months postpartum. Duration of following: 12 months (Helland 2001); 4 years (Helland 2003); 7 years (Helland 2008). |
|
| Outcomes | 1. Helland 2001 reports cognitive function at 6 and 9 months; weight, length and head circumference at birth and 12 months. 2. Helland 2003 reports measure of intelligence (IQ) at 4 years. 3. Helland 2008 reports measure of intelligence (IQ) and weight, length and head circumference at 7 years. |
|
| Notes | "This study was financed by grants from Peter Møller, Avd. Orkla ASA, and “Aktieselskabet Freia Chocoladefabriks Medicinske Fond." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Done. Quote: "The study was randomised and double‐blinded, and the randomization was performed by a computer program". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was a randomised double‐blinded...". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | In Helland 2001 says: "The participants were assessed by one of the authors (L.S) administering the Fagan Test of Infant Intelligence at 27 and 39 weeks of age". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Inititally randomised number of participants in intervention group was 175. At 6 months the number of participants was 144; at 9 months 130 participants were evaluated; at 4 years the number of participants was 48; and at 7 years participants in the intervention group were 82. The control group was initially comprised of 166 participants; at 6 months of age the number was limited to 118; at 9 months of age there were 115 participants; at 4 years the number of participants were 36; at 7 years of age there were 61 participants. At the last follow‐up study (7 years) only 46.8% and 36.7% of initially planned participants were evaluated, in the intervention and control group, respectively. |
| Selective reporting (reporting bias) | Low risk | Not detected. |
| Other bias | Low risk | No other significant risk of bias found. |
Lauritzen 2004.
| Methods | Randomised trial. Blinded. Lauritzen 2004 is the main study with 8 publications at different times and with different outcomes. |
|
| Participants | Denmark. Participants were selected from cohort of Danish women Olsen 2001. 122 (intervention group:62, control group: 60) exclusive breastfeeding women with a term delivery. Criteria of eligibility: uncomplicated pregnancy, prepregnancy BMI < 30 kg/m², absence of metabolic disorders, intention to breasted for a least 4 months. Newborns healthy, term, singleton, weight adequate for gestational age and Apgar > 7 at 5 minutes. |
|
| Interventions | Intervention group: 4.5 g of fish oil with a content of 1.5 g of n‐3 LCPUFA per day in capsules. Control group: olive oil in capsules. Reference group: 64 mothers with high fish intake. Duration of intervention: 4 months postpartum. Duration of following: 4 months (Lauritzen 2004); 2 years (Lauritzen 2005a); 2.5 years (Ulbak 2004, Lauritzen 2005); 7 years (Asserhoj 2009). |
|
| Outcomes | 1. Lauritzen 2004 reports infant visual acuity at 4 months. 2. Ulbak 2004 reports infant's blood pressure at 2.5 years. 3. Lauritzen 2005 reports infant's growth at 2.5 years. 4. Lauritzen 2005a reports infant's developmental outcomes at 2 years. 5. Asserhoj 2009 reports infant's blood pressure and growth at 7 years. 6. Cheetham 2011 reports infant's neurodevelopment at 7 years of age. 7. Cheetham 2011a is an abstract of Cheetham 2001. |
|
| Notes | "This study was financed by FØTEK —The Danish Research and Development Program for Food and Technology and BASF Aktiengesellschaft". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Done. Quote: "Random block‐wise allocation to the supplement groups was applied in blocks of two in five strata according to mean parental education". |
| Allocation concealment (selection bias) | Low risk | Done. Quote: "After birth, the women with fish intakes below the 50th percentile were randomly assigned to a supplementation group by a randomization schedule prepared by a person uninvolved in the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Owing to the identical appearance for the capsules for the two groups, a person who was not otherwise involved in the project handled the capsules in order to avoid breaking the blinding of the investigators" and "investigators and families were blinded to the randomisation". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No reported blinding of those assessing outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomised: 122 (intervention 62, control 60). At 2.5 years of age N = 65 (55%) fish oil group n = 37 (60%) and control group n = 28 (47%). High number of losses in the follow‐up, no reasons given. |
| Selective reporting (reporting bias) | Low risk | Not detected. |
| Other bias | Low risk | No other bias identified. |
Jensen 2005.
| Methods | Randomised trial, blinded. Jensen (2005) is the main study with 8 publications. |
|
| Participants | USA. 227 (intervention group: 114; control group: 113) exclusive breastfeeding women with a term delivery. Eligibility criteria: healthy mothers: 18 to 40 years, with term infants, 2500 g to 4200 g. |
|
| Interventions | Intervention group: DHA (DHA Algal oil) 200 mg/day for 4 months. Control group: soy and corn oil. Time of intervention: 4 months. Time of following: 30 months (Jensen 2005); 4 months (Llorente 2003); 5 years (Jensen 2004). |
|
| Outcomes | 1. Jensen 2005 reports neurodevelopmental status measure by Bayley Scale at 12, 30 months. Attention measure by Sustained Attention Subtest of the Leíter International Performance Scale (Jensen 2005). 2. Jensen 2004 reports visual acuity measure by Teller Acuity Card and VEP at 4 and 8 months. Attention at 5 years. 3. Llorente 2004 reports postpartum depression measure at 4 months. Jensen 1999a, Jensen 1999b, Jensen 2001, Voigt 1998 and Voigt 2000 are previous abstracts of primary study of Jensen 2005 published in supplements. Jensen 2000 reports fatty acid composition of breast milk lipids and infant plasma phospholipids. 4. Jensen 2010 reports visual function, growth outcomes and neurodevelopment at 5 years of age. |
|
| Notes | Funding by Departament of Agriculture. Martek Biosciences Corp. Mead Johnson. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Women who qualified were assigned randomly in a double‐masked manner with a computer‐generated randomisation scheme". |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "to receive 1 of 2 identical capsules daily for 4 mo". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated clearly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost > 20% in both groups by similar reasons. The greater proportion of missed follow‐up occurred in the short term. |
| Selective reporting (reporting bias) | Low risk | Growth measures (weight, length and head circumference) not provided at Jensen 2005 but in Jensen 2010 these outcomes are appropriately reported. |
| Other bias | Low risk | No other bias identified. |
Lucia 2007.
| Methods | Randomised double‐blinded trial. | |
| Participants | Germany. 144 recruited during pregnancy (at delivery: intervention group 43, control group 74). Criteria of eligibility: exclusion criteria of mothers: lactose intolerance, diabetes, smoking increase risk of premature delivery, multiple pregnancy, allergy to cow milk protein, consumption of alcohol. Exclusion criteria of infants: premature, major malformations, hospitalised for more than 1 week. |
|
| Interventions | Intervention group: "basic supplement with FOS and DHA (200 mg) prepared from fish oil". Control group: "basic supplement containing vitamins and minerals"; second control group: "basic supplement plus the prebiotic, FOS (4.5 g)". Duration of intervention: during pregnancy 21 week‐3 months into lactation. Duration of follow‐up: 21 months. |
|
| Outcomes | 1.Lucia 2007 reports length, weight and head circumference. 2.Bergmann 2006 is an abstract of Lucia 2007. 3.Bergman 2012 reports anthropometrics of children at 6‐years of life. |
|
| Notes | An author belongs to Nestle Nutrition Institute. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Prospective mothers were randomized by a computer program and allocated to one of three groups". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the identity of supplements was blinded to the subjects, support staff and investigators". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The examination was conducted by a pediatrician trained in anthropometric methods (K.E.B.) who was not aware of the original supplementation assignment". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At time of birth, 27/144 "had been lost of follow‐up". For the assessment at 21 months 61/144 were available. No data about differences between the groups in withdrawals. |
| Selective reporting (reporting bias) | Low risk | Not identified. |
| Other bias | Low risk | No other bias identified. |
van Goor 2010.
| Methods | Double‐blind placebo‐controlled randomised trial. | |
| Participants | Netherlands. 183 women between 14th to 20th week of pregnancy without any maternal or neonatal complication. Inclusion criteria: "first or second low‐risk singleton pregnancy". Exclusion criteria: "vegetarian or vegan diets and women with diabetes mellitus". Stop criteria:delivery before 37 or after 42 weeks and any maternal or neonatal complications. |
|
| Interventions | 3 groups: 1st group received 220 mg of DHA, 2nd group received 220 mg DHA + 220 mg AA, 3rd group was the control group receiving soybean oil. Duration of supplementation: from enrolment to 3 months after birth. Duration of follow‐up: 12‐months of life. |
|
| Outcomes | 1. Doornbos 2009 reports a) maternal depression at 6 weeks postpartum measured by Edinburgh Postpartum Depression Scale (EPDS), in week 16 and 36 of pregnancy and 6 weeks postpartum b) postpartum blues assessed with a Dutch version of the blues questionnaire. 2. van Goor 2010 reports general movement quality at 2 weeks i) neurological findings were described firstly by using the clinical classification (normal, mildly abnormal and definitely abnormal neurological condition) and secondly by using the neurological optimality score ii) general movements were assessed spontaneous motility in the supine position was videotaped for at least 5 min; general movement quality was assessed according to Hadders‐Algra 2010 methods] and 12 weeks of life (the infants’ general movements were assessed in the 12th postnatal week‐ spontaneous motility in the supine position was videotaped for at least 5 min; general movement quality was assessed according to Hadders‐Algra 2010 methods. 3. Van Goor 2009 reports the biochemical composition of the breast milk after LCPUFAs supplementation. 4. Van Goor 2009a reports a biochemical outcome of the same study population of the primary study. 5. Van Goor 2011 reports neurodevelopmental status at 18 months of life. |
|
| Notes | Van Goor 2010 was considered as the primary study since this article evaluated our primary outcome. The study was financially supported by Friesland‐Campina. All authors declare that they have no conflict of interest to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Women were randomized to three groups using block randomization." No reporting the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "a double‐blind placebo controlled randomised trial in....". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Two assessors (S. A. v. G. and M. H.‐A.), blinded to the intervention...". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 65% of initially enrolled pairs mother‐infant managed to complete the intervention at 1 year of life for the general movement quality outcome and 65.4% of initially enrolled women were assessed for postpartum depression. |
| Selective reporting (reporting bias) | Low risk | Not identified. |
| Other bias | Low risk | No other bias identified. |
Furuhjelm 2009.
| Methods | Randomised trial. Blinded. | |
| Participants | Sweden. 145 pregnant women with antecedents of allergy (intervention group 70, control group 65). |
|
| Interventions | Intervention group: capsules with 1.1 g of DHA; 1.6 g of EPA. Control group: soy oil capsules. Duration of intervention: 25 week of pregnancy to 3 months postpartum. Duration of follow‐up: 24 months. |
|
| Outcomes | 1. Furuhjelm 2009 reports "risk of allergy sensitization during of first year of life" measures by skin prick test, IgE levels and clinical examinations. 2. Furuhjelm 2011 reports frequency of IgE‐associate diseases at 2 years of life. 3. Furuhjelm 2011a reports biochemical outcomes of the same protocol. |
|
| Notes | "The study was supported financially by Medical Research Council of Southeast Sweden (FORSS), The Otsergotland County Council, The Ekhaga Foundation, Swedish Asthma and Allergy Association, The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS), The Swedish Society of Medicine and Glaxo Smith Kline, Sweden." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The mothers were randomly allocated to dietary supplementation either with omega‐3 fatty acids (x‐3 group) or placebo." "The producer performed the block randomization." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "the research nurses, the pediatricians and the person performing the laboratory analysis were blinded during the intervention and follow‐up." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "the research nurses, the pediatricians and the person performing the laboratory analysis were blinded during the intervention and follow‐up." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 loss at 2 years follow‐up after the complete intervention. |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias. |
| Other bias | Low risk | No other bias identified. |
Hauner 2009.
| Methods | RCT, not‐double blind design. | |
| Participants | Germany. 104 pregnant women in each branch. Inclusion criteria: “Gestational age <15th weeks of gestation; age between 18 and 43 years; BMI at conception between 18 and 30 kg/m²; sufficient German language skills, and written informed consent. Inclusion criteria for follow‐up of the newborns are gestational age at birth between 37th and 42nd weeks; appropriate size for gestational age, and an APGAR score >7 at 5 min PP”. Exclusion criteria: “high‐risk pregnancy (multiple pregnancy, hepatitis B or C infection, or parity >4); hypertension; chronic diseases such as diabetes or gastrointestinal disorders; psychiatric disease; supplementation with n–3 FAs before randomisation; alcohol abuse; hyperemesis gravidarum, and smoking. Exclusion criteria for follow‐up of the newborns are severe malformations or diseases; chromosomal anomaly and inborn metabolic diseases. |
|
| Interventions | "The intervention protocol combined 2 elements to reduce the ratio of n‐6 to n‐3 LCPUFAs from 7:1, which is the mean ratio in the German diet, to a range of 3–3.5:1" Supplementation of n–3 LCPUFAs [1200 mg n‐3 LCPUFAs (1020 mg DHA and 180 mg EPA) as well as 9 mg vitamin E] constituting a low ratio of n–6/n–3 FAs. |
|
| Outcomes | 1. Hauner 2009 reports the rationale of the study. 2. Hauner 2012 reports as primary outcome adipose tissue mass at 3–5 days after birth, 6 weeks, 4 months and 12 months of life, and as secondary outcome anthropometrics at 3–5 days, 6 weeks, 4 months and 12 months after birth. 3. Much 2013a reports anthropometrics and body composition at 1 year of age. 4. Much 2013b reports "th effect of circulating proportions of n‐6 and n‐3 LCPUFAs and their ratio in breast milk on measures of infant growth and body composition at 1 year of life". |
|
| Notes | Included. This document is the rationale of this study (Rationale and Design of the INFAT Study). Danone Reserach Center financially supported the protocol without having any conflict of interest with the study design procedure. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization is conducted by varying block lengths ...using SAS software." |
| Allocation concealment (selection bias) | Low risk | "randomization was performed using a random envelope prepared by Institute of Medical Statistics and Epidemiology..." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "open label randomised control trial." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Fat mass assesses as SFT was initially assessed by unblinded " trained research‐assistants" and secondly "to complement the primary endpoint with another method" fat mass was assessed by blinding personnel: "ultrasound measurements were performed by trained pediatricians". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The 83.65% of eligible participants were assessed at 4 months time point of the study, due to mainly personal reasons in both branches. The 81.7% of the initially eligible participants performed the 1 years follow‐up due to relocation but only in the control group. Intervention group had no dropouts from 4 months to 1 year of age. |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias. |
| Other bias | Low risk | No other bias detected. |
AA: arachidonic acid BMI: body mass index DHA: docosahexaenoic acid EPA: eicosapentaenoic acid FOS: fructooligosaccharide LCPUFA: long chain polyunsaturated fatty acids MDI: Mental Development Index PDI: Psychomotor Development Index RCT: randomised controlled trial SFT: skin fold thickness VEP: visual evoked potential
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anderson 2005 | Excluded because the intervention (trans fatty acids) was not a LCPUFA. |
| Bergmann 2008 | Excluded because this trial reports exclusively biochemical outcomes. |
| Bertschi 2005 | Excluded because the intervention (alpine butter) was not a LCPUFA. |
| Boris 2004 | Excluded because this trial reports exclusively biochemical outcomes. |
| Cant 1991 | Excluded because the intervention (linoleic and gamma‐linolenic acids) was not a LCPUFA. |
| Craig‐Schmidt 1984 | Excluded because the intervention (hydrogenated fat) was not a LCPUFA. |
| Dunstan 2007 | Excluded because the participants were only pregnant women and reporting exclusively biochemical outcomes. |
| Fidler 1999 | Excluded because the trial reports exclusively biochemical outcomes. |
| Fidler 2000 | Excluded because this trial reports exclusively biochemical outcomes. |
| Fung 2010 | Excluded because the intervention and the outcomes are different of the protocol (yogurt). |
| Granot 2011 | Excluded because the trial reports biochemical outcomes. |
| Hasin 2007 | Excluded because the intervention (Isomer of conjugated linoleic acid) was not a LCPUFA. |
| Hawkes 2002 | Excluded because this trial reports exclusively biochemical outcomes. |
| Helland 1998 | Excluded because this trial reports exclusively biochemical outcomes. |
| Helland 2006 | Excluded because this trial reports exclusively biochemical outcomes. |
| Henriksen 2008 | Excluded because the intervention was supplementation of human milk to preterm infants, not to their mothers. |
| Kaapa 1986 | Excluded because this trial reports exclusively biochemical outcomes. |
| Kitz 2006 | Excluded because the interventions of study (gamma‐linolenic acid) was not a LCPUFA. |
| Makrides 1996 | Excluded because this trial reports exclusively biochemical outcomes. |
| Makrides 1997 | Abstract. Excluded because the outcomes are biochemical. |
| Makrides 2002 | Excluded because the intervention (egg yolk) was not a LCPUFA; the participants were infants not breastfeeding mothers. |
| Makrides 2009 | Excluded because de participants were preterm infants with enteral feeds. |
| Martin‐Alvarez 2012 | Abstract. |
| Masters 2002 | Excluded because the intervention (Isomers of octadecadienoic acid) was not a LCPUFA. |
| Mellies 1978 | Excluded because the intervention (varying maternal dietary cholesterol and phytosterol) was not a LCPUFA. |
| Mellies 1979 | Excluded because the intervention (varying maternal dietary fatty acids) was not a LCPUFA. |
| Mosley 2007 | Excluded because the intervention (conjugated linoleic acid) was not a LCPUFA. |
| Palmer 2005 | Excluded because the intervention (cooked and raw egg consumption) was not a LCPUFA. |
| Park 1999 | Excluded because the intervention (high‐fat dairy product) was not a LCPUFA. |
| Potter 1976 | Excluded because the type of study. This study was not a randomised controlled trial. |
| Ritzenthaler 2005 | Excluded because the intervention (conjugated linoleic acid) was not LCPUFA supplementation. |
| Scopesi 2008 | Excluded because the type of study. This study was not a randomised controlled trial. |
| Scopesi 2011 | Abstract. excluded because the outcomes are biochemical. |
| Shahin 2006 | Excluded because the intervention (margarine and butter consumption) was not a LCPUFA. |
| Smithers 2008a | Excluded because the participants were not lactating mothers. |
| Smithers 2008b | Excluded because the participants were not lactating mothers. |
| Smithers 2010 | Excluded because the outcomes were biochemical. |
| Smithers 2011 | Excluded because the supplementation was only in the pregnant period. |
| Tanaka 2009 | This study compared the cognitive function of preterm babies who were breastfeeding with those who were formula‐fed. |
| Valentine 2013 | Excluded because the participants were donors for human milk and the outcome was levels of DHA. |
| Warstedt 2009 | Excluded because the participants were only pregnant women and reporting exclusively biochemical outcomes. |
| Weseler 2008 | Excluded because this trial reports exclusively biochemical outcomes. |
DHA: docosahexaenoic acid LCPUFA: long chain polyunsaturated fatty acids
Characteristics of studies awaiting assessment [ordered by study ID]
Nahidi 2012.
| Methods | Double‐blind clinical trial. |
| Participants | 70 women with postpartum depression. |
| Interventions | Supplementation with 1 g of omega‐3. |
| Outcomes | Maternal depression assessed by Edinburgh’s standard test for diagnosis and Beck’s standard test for depression symptoms severity. |
| Notes | Persian language article, needs translation. |
Characteristics of ongoing studies [ordered by study ID]
Campos‐Martinez 2012.
| Trial name or title | Levels of docosahexaenoic acid in pregnant women and their children after taking a fish oil enriched diet. |
| Methods | Randomised clinical trial. |
| Participants | 149 pregnant women. |
| Interventions | DHA 400 mg/day, up to 4.5 months of lactation. |
| Outcomes | DHA serum levels. |
| Starting date | Unknown. |
| Contact information | Hospital Virgen de las Nieves, Pediatrics, Granada, Spain. |
| Notes | Abstract, ongoing study (planned outcomes: neurodevelopment). |
Harris 2014.
| Trial name or title | |
| Methods | RCT. |
| Participants | 115 pregnant women |
| Interventions | 300 mg DHA or placebo daily from pregnancy to the first 3 months of lactating. |
| Outcomes | BSID at 4 months and 1 year of age, gestation period (gestational length). |
| Starting date | |
| Contact information | |
| Notes | Abstract. |
Karlsson 2010.
| Trial name or title | |
| Methods | Randomised clinical trial. |
| Participants | Pregnant women. |
| Interventions | 1.6 g 25 week of gestation up 3.5 months of lactation. |
| Outcomes | Cognitive development. |
| Starting date | |
| Contact information | |
| Notes | Abstract. |
BSID: Bayley Scale of Infant Development: DHA: docosahexaenoic acid RCT: randomised controlled trial
Differences between protocol and review
Definition of third secondary outcome. Safety was considered as environmental contamination of supplementation.
Non‐prespecified secondary outcomes. We added six new secondary outcomes: child body mass index (BMI), child fat mass, fat mass distribution, postpartum depression, postpartum blues, and infant allergy.
Search methods for identification of studies. We also searched the reference lists of published narrative reviews or related papers and we sent emails to authors who had not reported on some of the data. For the 2015 update, we did not search CINAHL separately as we no longer have access to the database; and the Pregnancy and Childbirth Group's Trials Register now includes a CINAHL search.
Included studies. We changed inclusion criteria to include clinical trials that began the supplementation of LCPUFA during the pregnancy and continued during the lactation period.
Contributions of authors
Mario Delgado conceived, designed and wrote the protocol and the review, and provided a methodological, clinical and policy perspective for the update. Eleni Kotanidou evaluated the studies and wrote the update review. Jose Andres Calvache provided methodological support. Xavier Bonfill and Assimina Galli‐Tsinopoulou commented on the content of the update.
Sources of support
Internal sources
University of Cauca, Popayán, Colombia.
Iberoamerican Cochrane Centre, Spain.
External sources
Erasmus Mundus PUEDES Grant, Other.
Declarations of interest
The authors declare that they have no conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Furuhjelm 2009 {published data only}
- Furuhjelm C, Jenmalm MC, Falth‐Magnusson K, DuchIn K. Th1 and Th2 chemokines, vaccine‐induced immunity, and allergic disease in infants after maternal ‐3 fatty acid supplementation during pregnancy and lactation. Pediatric Research 2011;69(3):259‐64. [DOI] [PubMed] [Google Scholar]
- Furuhjelm C, Warstedt K, Fagerås M, Fälth‐Magnusson K, Larsson J, Fredriksson M, et al. Allergic disease in infants up to 2 years of age in relation to plasma omega‐3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatric Allergy & Immunology 2011;22(5):502‐4. [DOI] [PubMed] [Google Scholar]
- Furuhjelm C, Warstedt K, Larsson J, Fredriksson M, Bottcher MF, Falth‐Magnusson K, et al. Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatrica 2009;98(9):1461‐7. [DOI] [PubMed] [Google Scholar]
Gibson 1997 {published data only}
- Gibson RA, Neumann MA, Makrides M. Effect of increasing breast milk docosahexaenoic acid on plasma and erythrocyte phospholipid fatty acids and neural indices of exclusively breast fed infants. European Journal of Clinical Nutrition 1997;51(9):578‐84. [PUBMED: 9306083] [DOI] [PubMed] [Google Scholar]
Hauner 2009 {published data only}
- Hauner H, Much D, Vollhardt C, Brunner S, Schmid D, Sedlmeier EM, et al. Effect of reducing the n‐6:n‐3 long‐chain PUFA ratio during pregnancy and lactation on infant adipose tissue growth within the first year of life: an open‐label randomized controlled trial. American Journal of Clinical Nutrition 2012;95(2):383‐94. [DOI] [PubMed] [Google Scholar]
- Hauner H, Vollhardt C, Schneider KT, Zimmermann A, Schuster T, Amann‐Gassner U. The impact of nutritional fatty acids during pregnancy and lactation on early human adipose tissue development. Rationale and design of the INFAT study. Annals of Nutrition & Metabolism 2009;54(2):97‐103. [DOI] [PubMed] [Google Scholar]
- Much D, Brunner S, Vollhardt C, Schmid D, Sedlmeier EM, Brüderl M, et al. Breast milk fatty acid profile in relation to infant growth and body composition: Results from the INFAT study. Pediatric Research 2013;74(2):230‐7. [DOI] [PubMed] [Google Scholar]
- Much D, Brunner S, Vollhardt C, Schmid D, Sedlmeier EM, Brüderl M, et al. Effect of dietary intervention to reduce the n‐6/n‐3 fatty acid ratio on maternal and fetal fatty acid profile and its relation to offspring growth and body composition at 1 year of age. European Journal of Clinical Nutrition 2013;67(3):282‐8. [DOI] [PubMed] [Google Scholar]
Helland 2001 {published data only}
- Helland IB, Saugstad OD, Smith L, Saarem K, Solvoll K, Ganes T, et al. Similar effects on infants of n‐3 and n‐6 fatty acids supplementation to pregnant and lactating women. Pediatrics 2001;108(5):E82. [PUBMED: 11694666] [DOI] [PubMed] [Google Scholar]
- Helland IB, Smith L, Blomen B, Saarem K, Saugstad OD, Drevon CA. Effect of supplementing pregnant and lactating mothers with n‐3 very‐long‐chain fatty acids on children's IQ and body mass index at 7 years of age. Pediatrics 2008;122(2):e472‐9. [PUBMED: 18676533] [DOI] [PubMed] [Google Scholar]
- Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very‐long‐chain n‐3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics 2003;111(1):e39‐44. [PUBMED: 12509593] [DOI] [PubMed] [Google Scholar]
Jensen 2005 {published data only}
- Jensen CL, Llorente AM, Voigt RG, Prager TC, Fraley JK, Zou YL, et al. Effects of maternal docosahexaenoic acid (dha) supplementation on visual and neurodevelopmental function of maternal depression and cognitive interference. Pediatric Research 1999;45:284A. [Google Scholar]
- Jensen CL, Maude M, Anderson RE, Heird WC. Effect of docosahexaenoic acid supplementation of lactating women on the fatty acid composition of breast milk lipids and maternal and infant plasma phospholipids. American Journal of Clinical Nutrition 2000;71(1 Suppl):292S‐299S. [PUBMED: 10617985] [DOI] [PubMed] [Google Scholar]
- Jensen CL, Prager TC, Zou Y, Fraley JK, Maude M, Anderson RE, et al. Effects of maternal docosahexaenoic acid supplementation on visual function and growth of breast‐fed term infants. Lipids 1999;34 Suppl:S225. [PUBMED: 10419159] [DOI] [PubMed] [Google Scholar]
- Jensen CL, Voigt RG, Llorente AM, Peters SU, Prager TC, Zou Y, et al. Effect of maternal docosahexaenoic acid (DHA) supplementation on neuropsychological and visual status of former breast‐fed infants at five years of age. Journal of Pediatric Gastroenterology and Nutrition 2004;39(Suppl 1):S10. [Google Scholar]
- Jensen CL, Voigt RG, Llorente AM, Peters SU, Prager TC, Zou YL, et al. Effects of early maternal docosahexaenoic acid intake on neuropsychological status and visual acuity at five years of age of breast‐fed term infants. Journal of Pediatrics 2010;157(6):900‐5. [DOI] [PubMed] [Google Scholar]
- Jensen CL, Voigt RG, Prager TC, Zou YL, Fraley JK, Rozelle JC, et al. Effects of maternal docosahexaenoic acid intake on visual function and neurodevelopment in breastfed term infants. American Journal of Clinical Nutrition 2005;82(1):125‐32. [PUBMED: 16002810] [DOI] [PubMed] [Google Scholar]
- Jensen CL, Voigt RG, Prager TC, Zou YL, Fraley JK, Turcich M, et al. Effect of maternal docosahexaenoic acid (DHA) supplementation on neuropsychological and visual status of former breast‐fed infants at five years of age. Pediatric Research 2001;48:448A. [Google Scholar]
- Llorente AM, Jensen CL, Voigt RG, Fraley JK, Berretta MC, Heird WC. Effect of maternal docosahexaenoic acid supplementation on postpartum depression and information processing. American Journal of Obstetrics and Gynecology 2003;188(5):1348‐53. [DOI] [PubMed] [Google Scholar]
- Voigt RG, Jensen CL, Fraley JK, Brown FR, Heird WC. Maternal docosahexaenoic acid (dha) does not affect neurodevelopmental outcome of breast‐fed term infants at one year of age. Pediatric Research 1998;43(4 Suppl 2):270. [Google Scholar]
- Voigt RG, Jensen CL, Fraley JK, Rozelle J, Turcich M, Heird WC. Effects of maternal docosahexaenoic acid supplementation on neurodevelopmental function of breast‐fed infants at 12 and 30 months of age. Journal of Developmental and Behavioral Pediatrics 2000;21:384‐5. [Google Scholar]
Lauritzen 2004 {published data only}
- Asserhoj M, Nehammer S, Matthiessen J, Michaelsen KF, Lauritzen L. Maternal fish oil supplementation during lactation may adversely affect long‐term blood pressure, energy intake, and physical activity of 7‐year‐old boys. Journal of Nutrition 2009;139(2):298‐304. [PUBMED: 19091800] [DOI] [PubMed] [Google Scholar]
- Cheatham CL, Nerhammer AS, Asserhoj M, Michaelsen KF, Lauritzen L. Fish oil supplementation during lactation: effects on cognition and behavior at 7 years of age. Lipids 2011;46(7):637‐45. [DOI] [PubMed] [Google Scholar]
- Cheatham CL, Nerhammer S, Asserhoj M, Michaelsen KF, Lauritzen L. Fish oil supplementation during lactation: Effects on cognition and behavior at 7 years of age. FASEB Journal 2011;25 Suppl:766.9. [DOI] [PubMed] [Google Scholar]
- Jorgensen MH, Michaelsen KF, Lauritzen L. Long chain polyunsaturated fatty acids and liver biochemistry in breast‐fed infants. Journal of Pediatric Gastroenterology and Nutrition 2005;40(5):631‐2. [Google Scholar]
- Larnkjaer A, Christensen JH, Michaelsen KF, Lauritzen L. Maternal fish oil supplementation during lactation does not affect blood pressure, pulse wave velocity, or heart rate variability in 2.5‐y‐old children. Journal of Nutrition 2006;136(6):1539‐44. [PUBMED: 16702318] [DOI] [PubMed] [Google Scholar]
- Lauritzen L, Halkjaer LB, Mikkelsen TB, Olsen SF, Michaelsen KF, Loland L, et al. Fatty acid composition of human milk in atopic Danish mothers. American Journal of Clinical Nutrition 2006;84(1):190‐6. [PUBMED: 16825695] [DOI] [PubMed] [Google Scholar]
- Lauritzen L, Hoppe C, Straarup EM, Michaelsen KF. Maternal fish oil supplementation in lactation and growth during the first 2.5 years of life. Pediatric Research 2005;58(2):235‐42. [PUBMED: 16006428] [DOI] [PubMed] [Google Scholar]
- Lauritzen L, Jorgensen MH, Mikkelsen TB, Skovgaard M, Straarup EM, Olsen SF, et al. Maternal fish oil supplementation in lactation: effect on visual acuity and n‐3 fatty acid content of infant erythrocytes. Lipids 2004;39(3):195‐206. [PUBMED: 15233397] [DOI] [PubMed] [Google Scholar]
- Lauritzen L, Jorgensen MH, Olsen SF, Straarup EM, Michaelsen KF. Maternal fish oil supplementation in lactation: effect on developmental outcome in breast‐fed infants. Reproduction, Nutrition, Development 2005;45(5):535‐47. [PUBMED: 16188206] [DOI] [PubMed] [Google Scholar]
- Lauritzen L, Kjaer TM, Fruekilde MB, Michaelsen KF, Frokiaer H. Fish oil supplementation of lactating mothers affects cytokine production in 2 1/2‐year‐old children. Lipids 2005;40(7):669‐76. [PUBMED: 16196417] [DOI] [PubMed] [Google Scholar]
- Ulbak J, Lauritzen L, Hansen HS, Michaelsen KF. Diet and blood pressure in 2.5‐y‐old Danish children. American Journal of Clinical Nutrition 2004;79(6):1095‐102. [PUBMED: 15159241] [DOI] [PubMed] [Google Scholar]
Lucia 2007 {published data only}
- Bergmann RL, Bergmann KE, Richter R, Haschke‐Becher E, Henrich W, Dudenhausen JW. Does docosahexaenoic acid (DHA) status in pregnancy have any impact on postnatal growth? Six‐year follow‐up of a prospective randomized double‐blind monocenter study on low‐dose DHA supplements. Journal of Perinatal Medicine 2012;40:677‐84. [DOI] [PubMed] [Google Scholar]
- Bergmann RL, Richter R, Bergmann KE, Dudenhausen JW, Haschke F. 21 months old infants are leaner if their mothers received low dose DHA supplements during pregnancy and lactation. European Journal of Pediatrics 2006;165 Suppl 1:114. [Google Scholar]
- Bergmann RL, Richter R, Bergmann KE, Dudenhausen JW, Haschke F. 21 months old infants are leaner if their mothers received low dose DHA supplements during pregnancy and lactation. European Journal of Pediatrics 2006; Vol. 165, issue 1:114.
- Lucia Bergmann R, Bergmann KE, Haschke‐Becher E, Richter R, Dudenhausen JW, Barclay D, et al. Does maternal docosahexaenoic acid supplementation during pregnancy and lactation lower BMI in late infancy?. Journal of Perinatal Medicine 2007;35(4):295‐300. [PUBMED: 17547539] [DOI] [PubMed] [Google Scholar]
van Goor 2010 {published data only}
- Doornbos B, Goor SA, Dijck‐Brouwer DA, Schaafsma A, Korf J, Muskiet FA. Supplementation of a low dose of DHA or DHA+AA does not prevent peripartum depressive symptoms in a small population based sample. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 2009;33(1):49‐52. [DOI] [PubMed] [Google Scholar]
- Goor SA, Dijck‐Brouwer DA, Doornbos B, Erwich JJ, Schaafsma A, Muskiet FA, et al. Supplementation of DHA but not DHA with arachidonic acid during pregnancy and lactation influences general movement quality in 12‐week‐old term infants. British Journal of Nutrition 2010;103(2):235‐42. [DOI] [PubMed] [Google Scholar]
- Goor SA, Dijck‐Brouwer DA, Hadders‐Algra M, Doornbos B, Erwich JJ, Schaafsma A, et al. Human milk arachidonic acid and docosahexaenoic acid contents increase following supplementation during pregnancy and lactation. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2009;80(1):65‐9. [DOI] [PubMed] [Google Scholar]
- Goor SA, Dijck‐Brouwer DAJ, Erwich JJHM, Schaafsma A, Mijna Hadders‐Algra M. The influence of supplemental docosahexaenoic and arachidonic acids during pregnancy and lactation on neurodevelopment at eighteen months. Prostaglandins Leukotrienes and Essential Fatty Acids 2011;84(5‐6):139‐46. [DOI] [PubMed] [Google Scholar]
- Goor SA, Muskiet FAJ. The effect of a high DHA‐fish oil and arachidonic acid (AA) supplementation during pregnancy and lactation on LCP status of mother and child and on the neurological development of the baby. Netherlands Trial Register (http://www.trialregister.nl) [accessed 1 November 2005] 2005.
- Goor SA, Schaafsma A, Erwich JJ, Dijck‐Brouwer DA, Muskiet FA. Mildly abnormal general movement quality in infants is associated with higher Mead acid and lower arachidonic acid and shows a U‐shaped relation with the DHA/AA ratio. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2010;82(1):15‐20. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anderson 2005 {published data only}
- Anderson NK, Beerman KA, McGuire MA, Dasgupta N, Griinari JM, Williams J, et al. Dietary fat type influences total milk fat content in lean women. Journal of Nutrition 2005;135(3):416‐21. [DOI] [PubMed] [Google Scholar]
Bergmann 2008 {published data only}
- Bergmann RL, Haschke‐Becher E, Klassen‐Wigger P, Bergmann KE, Richter R, Dudenhausen JW, et al. Supplementation with 200 mg/day docosahexaenoic acid from mid‐pregnancy through lactation improves the docosahexaenoic acid status of mothers with a habitually low fish intake and of their infants. Annals of Nutrition & Metabolism 2008;52(2):157‐66. [PUBMED: 18446020] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bertschi 2005 {published data only}
- Bertschi I, Collomb M, Rist L, Eberhard P, Sieber R, Butikofer U, et al. Maternal dietary Alpine butter intake affects human milk: fatty acids and conjugated linoleic acid isomers. Lipids 2005;40(6):581‐7. [DOI] [PubMed] [Google Scholar]
Boris 2004 {published data only}
- Boris J, Jensen B, Salvig JD, Secher NJ, Olsen SF. A randomized controlled trial of the effect of fish oil supplementation in late pregnancy and early lactation on the n‐3 fatty acid content in human breast milk. Lipids 2004;39(12):1191‐6. [PUBMED: 15736915] [DOI] [PubMed] [Google Scholar]
Cant 1991 {published data only}
- Cant A, Shay J, Horrobin DF. The effect of maternal supplementation with linoleic and gamma‐linolenic acids on the fat composition and content of human milk: a placebo‐controlled trial. Journal of Nutritional Science and Vitaminology 1991;37(6):573‐9. [PUBMED: 1668100] [DOI] [PubMed] [Google Scholar]
Craig‐Schmidt 1984 {published data only}
- Craig‐Schmidt MC, Weete JD, Faircloth SA, Wickwire MA, Livant EJ. The effect of hydrogenated fat in the diet of nursing mothers on lipid composition and prostaglandin content of human milk. American Journal of Clinical Nutrition 1984;39(5):778‐86. [DOI] [PubMed] [Google Scholar]
Dunstan 2007 {published data only}
- Dunstan JA, Mitoulas LR, Dixon G, Doherty DA, Hartmann PE, Simmer K, et al. The effects of fish oil supplementation in pregnancy on breast milk fatty acid composition over the course of lactation: a randomized controlled trial. Pediatric Research 2007;62(6):689‐94. [PUBMED: 17957152] [DOI] [PubMed] [Google Scholar]
Fidler 1999 {published data only}
- Fidler N, Sauerwald T, Demmelmair H, Pohl A, Koletzko B. Oxidation of an oil rich in docosahexaenoic acid compared to linoleic acid in lactating women. Annals of Nutrition & Metabolism 1999;43:339‐45. [DOI] [PubMed] [Google Scholar]
Fidler 2000 {published data only}
- Fidler N, Sauerwald T, Pohl A, Demmelmair H, Koletzko B. Docosahexaenoic acid transfer into human milk after dietary supplementation: a randomized clinical trial. Journal of Lipid Research 2000;41(9):1376‐83. [PUBMED: 10974044] [PubMed] [Google Scholar]
Fung 2010 {published data only}
- Fung EB, Ritchie LD, Walker BH, Gildengorin G, Crawford PB. Randomized, controlled trial to examine the impact of providing yogurt to women enrolled in WIC. Journal of Nutrition Education and Behavior 2010;42(3 Suppl):S22‐S29. [DOI] [PubMed] [Google Scholar]
Granot 2011 {published data only}
- Granot E, Jakobovich E, Rabinowitz R, Levy P, Schlesinger M. DHA supplementation during pregnancy and lactation affects infants' cellular but not humoral immune response. Mediators of Inflammation 2011;2011:493925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hasin 2007 {published data only}
- Hasin A, Griinari JM, Williams JE, Shahin AM, McGuire MA, McGuire MK. Consumption of c9,t11‐18:2 or t10,c12‐18:2 enriched dietary supplements does not influence milk macronutrients in healthy, lactating women. Lipids 2007;42(9):835‐43. [DOI] [PubMed] [Google Scholar]
Hawkes 2002 {published data only}
- Hawkes JS, Bryan DL, Makrides M, Neumann MA, Gibson RA. A randomized trial of supplementation with docosahexaenoic acid‐rich tuna oil and its effects on the human milk cytokines interleukin 1 beta, interleukin 6, and tumor necrosis factor alpha. American Journal of Clinical Nutrition 2002;75(4):754‐60. [PUBMED: 11916764] [DOI] [PubMed] [Google Scholar]
Helland 1998 {published data only}
- Helland IB, Saarem K, Saugstad OD, Drevon CA. Fatty acid composition in maternal milk and plasma during supplementation with cod liver oil. European Journal of Clinical Nutrition 1998;52(11):839‐45. [PUBMED: 9846598] [DOI] [PubMed] [Google Scholar]
Helland 2006 {published data only}
- Helland IB, Saugstad OD, Saarem K, Houwelingen AC, Nylander G, Drevon CA. Supplementation of n‐3 fatty acids during pregnancy and lactation reduces maternal plasma lipid levels and provides DHA to the infants. Journal of Maternal‐Fetal and Neonatal Medicine 2006;19(7):397‐406. [PUBMED: 16923694] [DOI] [PubMed] [Google Scholar]
Henriksen 2008 {published data only}
- Henriksen C, Haugholt K, Lindgren M, Aurvag AK, Ronnestad A, Gronn M, et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics 2008;121(6):1137‐45. [PUBMED: 18519483] [DOI] [PubMed] [Google Scholar]
Kaapa 1986 {published data only}
- Kaapa P, Uhari M, Nikkari T, Viinikka L, Ylikorkala O. Dietary fatty acids and platelet thromboxane production in puerperal women and their offspring. American Journal of Obstetrics and Gynecology 1986;155:146‐9. [DOI] [PubMed] [Google Scholar]
Kitz 2006 {published data only}
- Kitz R, Rose MA, Schonborn H, Zielen S, Bohles HJ. Impact of early dietary gamma‐linolenic acid supplementation on atopic eczema in infancy. Pediatric Allergy and Immunology 2006;17(2):112‐7. [DOI] [PubMed] [Google Scholar]
Makrides 1996 {published data only}
- Makrides M, Neumann MA, Gibson RA. Effect of maternal docosahexaenoic acid (DHA) supplementation on breast milk composition. European Journal of Clinical Nutrition 1996;50(6):352‐7. [PUBMED: 8793415] [PubMed] [Google Scholar]
Makrides 1997 {published data only}
- Makrides M, Neumann MA, Gibson RA. Effect of maternal DHA supplementation on breast milk composition and infant fatty acid profiles. Journal of Paediatrics and Child Health 1997;33(Suppl 1):S55. [Google Scholar]
Makrides 2002 {published data only}
- Makrides M, Hawkes JS, Neumann MA, Gibson RA. Nutritional effect of including egg yolk in the weaning diet of breast‐fed and formula‐fed infants: a randomized controlled trial. American Journal of Clinical Nutrition 2002;75(6):1084‐92. [PUBMED: 12036817] [DOI] [PubMed] [Google Scholar]
Makrides 2009 {published data only}
- Makrides M, Gibson RA, McPhee AJ, Collins CT, Davis PG, Doyle LW, et al. Neurodevelopmental outcomes of preterm infants fed high‐dose docosahexaenoic acid: a randomized controlled trial. JAMA 2009;301(2):175‐82. [PUBMED: 19141765] [DOI] [PubMed] [Google Scholar]
Martin‐Alvarez 2012 {published data only}
- Martin‐Alvarez E, Guerrero‐Montenegro B, Romero‐Paniagua MT, Lara‐Villoslada F, Hurtado‐Suazo JA. Maternal docosahexaenoic acid supplementation during pregnancy and lactation can modulate oxidative stress in the term newborn. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(S2):40. [Google Scholar]
Masters 2002 {published data only}
- Masters N, McGuire MA, Beerman KA, Dasgupta N, McGuire MK. Maternal supplementation with CLA decreases milk fat in humans. Lipids 2002;37(2):133‐8. [DOI] [PubMed] [Google Scholar]
Mellies 1978 {published data only}
- Mellies MJ, Ishikawa TT, Gartside P, Burton K, MacGee J, Allen K, et al. Effects of varying maternal dietary cholesterol and phytosterol in lactating women and their infants. American Journal of Clinical Nutrition 1978;31(8):1347‐54. [DOI] [PubMed] [Google Scholar]
Mellies 1979 {published data only}
- Mellies MJ, Ishikawa TT, Gartside PS, Burton K, MacGee J, Allen K, et al. Effects of varying maternal dietary fatty acids in lactating women and their infants. American Journal of Clinical Nutrition 1979;32(2):299‐303. [PUBMED: 420127] [DOI] [PubMed] [Google Scholar]
Mosley 2007 {published data only}
- Mosley SA, Shahin AM, Williams J, McGuire MA, McGuire MK. Supplemental conjugated linoleic acid consumption does not influence milk macronutrient contents in all healthy lactating women. Lipids 2007;42(8):723‐9. [DOI] [PubMed] [Google Scholar]
Palmer 2005 {published data only}
- Palmer DJ, Gold MS, Makrides M. Effect of cooked and raw egg consumption on ovalbumin content of human milk: a randomized, double‐blind, cross‐over trial. Clinical and Experimental Allergy 2005;35(2):173‐8. [DOI] [PubMed] [Google Scholar]
Park 1999 {published data only}
- Park Y, McGuire MK, Behr R, McGuire MA, Evans MA, Shultz TD. High‐fat dairy product consumption increases Delta9c,11t‐18:2 (rumenic acid) and total lipid concentrations of human milk. Lipids 1999;34:543‐9. [DOI] [PubMed] [Google Scholar]
Potter 1976 {published data only}
- Potter JM, Nestel PJ. The effects of dietary fatty acids and cholesterol on the milk lipids of lactating women and the plasma cholesterol of breast‐fed infants. The American Journal of Clinical Nutrition 1976;29(1):54‐60. [PUBMED: 1246976] [DOI] [PubMed] [Google Scholar]
Ritzenthaler 2005 {published data only}
- Ritzenthaler KL, McGuire MK, McGuire MA, Shultz TD, Koepp AE, Luedecke LO, et al. Consumption of conjugated linoleic acid (CLA) from CLA‐enriched cheese does not alter milk fat or immunity in lactating women. Journal of Nutrition 2005;135(3):422‐30. [DOI] [PubMed] [Google Scholar]
Scopesi 2008 {published data only}
- Scopesi F, Traggiai C, Levreri I, Gianotti D, Zucchi C, Calevo MG, et al. Lactating mothers on DHA enriched diet and breast milk composition. Journal of Maternal‐Fetal and Neonatal Medicine 2008;21(Suppl 1):27. [Google Scholar]
Scopesi 2011 {published data only}
- Scopesi F, Calevo MG, Risso FM, Sannia A, Traverso F, Bruschettini M, et al. The impact of a DHA enriched diet on breast milk composition. Early Human Development 2011;87(Suppl):S96. [Google Scholar]
Shahin 2006 {published data only}
- Shahin AM, McGuire MK, Anderson N, Williams J, McGuire MA. Effects of margarine and butter consumption on distribution of trans‐18:1 fatty acid isomers and conjugated linoleic acid in major serum lipid classes in lactating women. Lipids 2006;41(2):141‐7. [DOI] [PubMed] [Google Scholar]
Smithers 2008a {published data only}
- Smithers LG, Gibson RA, McPhee A, Makrides M. Effect of two doses of docosahexaenoic acid (DHA) in the diet of preterm infants on infant fatty acid status: results from the DINO trial. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2008;79(3‐5):141‐6. [PUBMED: 18951004] [DOI] [PubMed] [Google Scholar]
Smithers 2008b {published data only}
- Smithers LG, Gibson RA, McPhee A, Makrides M. Higher dose of docosahexaenoic acid in the neonatal period improves visual acuity of preterm infants: results of a randomized controlled trial. American Journal of Clinical Nutrition 2008;88(4):1049‐56. [DOI] [PubMed] [Google Scholar]
Smithers 2010 {published data only}
- Smithers LG, Markrides M, Gibson RA. Human milk fatty acids from lactating mothers of preterm infants: a study revealing wide intra‐ and inter‐individual variation. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2010;83(1):9‐13. [DOI] [PubMed] [Google Scholar]
Smithers 2011 {published data only}
- Smithers LG, Gibson RA, Makrides M. Maternal supplementation with docosahexaenoic acid during pregnancy does not affect early visual development in the infant: a randomized controlled trial. American Journal of Clinical Nutrition 2011;93(6):1293‐9. [DOI: 10.3945/ajcn.110.009647] [DOI] [PubMed] [Google Scholar]
Tanaka 2009 {published data only}
- Tanaka K, Kon N, Ohkawa N, Yoshikawa N, Shimizu T. Does breastfeeding in the neonatal period influence the cognitive function of very‐low‐birth‐weight infants at 5 years of age?. Brain & Development 2009;31(4):288‐93. [PUBMED: 18640798] [DOI] [PubMed] [Google Scholar]
Valentine 2013 {published data only}
- Valentine CJ, Morrow G, Pennell M, Morrow AL, Hodge A, Haban‐Bartz A, et al. Randomized controlled trial of docosahexaenoic Acid supplementation in midwestern US. Human milk donors. Breastfeeding Medicine 2013;8(1):86‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Warstedt 2009 {published data only}
- Warstedt K, Furuhjelm C, Duchen K, Falth‐Magnusson K, Fageras M. The effects of omega‐3 fatty acid supplementation in pregnancy on maternal eicosanoid, cytokine, and chemokine secretion. Pediatric Research 2009;66(2):212‐7. [DOI] [PubMed] [Google Scholar]
Weseler 2008 {published data only}
- Weseler AR, Dirix CE, Bruins MJ, Hornstra G. Dietary arachidonic acid dose‐dependently increases the arachidonic acid concentration in human milk. Journal of Nutrition 2008;138(11):2190‐7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Nahidi 2012 {published data only}
- Nahidi F, Taghizade S, Sadr S, Majd HA. Effects of omega‐3 Fatty acids on postpartum depression [بزرسی تأثیز اسید بَی چزة امگب‐ 3 بز افسزدگی پس اس سایمبن]. Iranian Journal of Obstetrics, Gynecology and Infertility 2011;14(6):46‐53. [Google Scholar]
References to ongoing studies
Campos‐Martinez 2012 {published data only}
- Campos‐Martinez A, Serrano L, Medina M, Ochoa‐ J, Pena‐Caballero M. Levels of docosahexaenoic acid in pregnant women and their children after taking a fish oil enriched diet. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(S2):92. [Google Scholar]
Harris 2014 {published data only}
- Harris M, Stacy M, Baker S, McGirr K, Davalos D. The omega smart baby project: Effect of maternal DHA on infant development. FASEB Journal 2014;28(1 Suppl):[abstract no: 269.1]. [Google Scholar]
Karlsson 2010 {published data only}
- Karlsson T, Birberg‐Thornberg U, Duchen K, Gustafsson PA. LC‐PUFA supplemented to mothers during pregnancy and breast‐feeding improves cognitive performance in their children four years later ‐ an rct study. 9th Congress of the ISSFAL; 29 May‐2 June 2010; Maastricht, The Netherlands. 2010:113.
Additional references
Agostoni 2005
- Agostoni C, Brunetti I, Marco A. Polyunsaturated fatty acids in human milk and neurological development in breastfed infants. Current Pediatric Reviews 2005;1:25‐30. [Google Scholar]
Belfort 2013
- Belfort MB, Rifas‐Shiman SL, Kleinman KP, Guthrie LB, Bellinger DC, Taveras EM, et al. Infant feeding and childhood cognition at ages 3 and 7 years: Effects of breastfeeding duration and exclusivity. JAMA Pediatrics 2013;167(9):836‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brenna 2007
- Brenna JT, Varamini B, Jensen RG, Diersen‐Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. American Journal of Clinical Nutrition 2007;85(6):1457‐64. [DOI] [PubMed] [Google Scholar]
Campoy 2012
- Campoy C, Escolano‐Margarit MV, Anjos T, Szajewska H, Uauy R. Omega 3 fatty acids on child growth, visual acuity and neurodevelopment. British Journal of Nutrition 2012;107:S85‐S106. [DOI] [PubMed] [Google Scholar]
Cheatham 2006
- Cheatham CL, Colombo J, Carlson SE. N‐3 fatty acids and cognitive and visual acuity development: methodologic and conceptual considerations. American Journal of Clinical Nutrition 2006;83(6 Suppl):1458S‐1466S. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition. Hillsdale, NJ: Lawrence Earlbaum Associates, 1988. [Google Scholar]
Fleith 2005
- Fleith M, Clandinin MT. Dietary PUFA for preterm and term infants: review of clinical studies. Critical Reviews in Food Science and Nutrition 2005;45(3):205‐29. [DOI] [PubMed] [Google Scholar]
Gibson 1996
- Gibson RA, Neumann MA, Makrides M. Effect of dietary docosahexaenoic acid on brain composition and neural function in term infants. Lipids 1996;31:S177‐S181. [DOI] [PubMed] [Google Scholar]
Gould 2013
- Gould JF, Smithers LG, Makrides M. The effect of maternal omega‐3 (n‐3) LCPUFA supplementation during pregnancy on early childhood cognitive and visual development: a systematic review and meta‐analysis of randomized controlled trials. American Journal of Clinical Nutrition 2013;97:351‐44. [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. Version 2014. McMaster University, 2014.
Grantham‐McGregor 2007
- Grantham‐McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B. Developmental potential in the first 5 years for children in developing countries. Lancet 2007;369(9555):60‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hadders‐Algra 2010
- Hadders‐Algra M. Variation and variability: key words in human motor development. Physical Therapy 2010;90(12):1823‐37. [PUBMED: 20966209] [DOI] [PubMed] [Google Scholar]
Helgi Library
- Helgi Library. Fish consumption per capita. http://www.helgilibrary.com/indicators/index/fish‐consumption‐per‐capita accessed at 01.12.2014. [http://www.helgilibrary.com/indicators/index/fish‐consumption‐per‐capita]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoddinot 2007
- Hoddinott P, Tappin D, Wright C. Breast feeding. BMJ 2008;336(7649):881‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horta 2007
- Horta BL, Bahl R, Martines JC, Victora JC. Evidence on the Long‐term Effects of Breastfeeding: Systematic Reviews and Meta‐analyses. Geneva: World Health Organization, 2007. [Google Scholar]
Innis 2007
- Innis SM. Fatty acids and early human development. Early Human Development 2007;83(12):761‐6. [DOI] [PubMed] [Google Scholar]
Innis 2014
- Innis SM. Impact of maternal diet on human milk composition and neurological development of infants. American Journal of Clinical Nutrition 2014;99(3):734S‐41S. [DOI: 10.3945/ajcn.113.072595] [DOI] [PubMed] [Google Scholar]
Jensen 2005b
- Jensen CL, Voigt RG, Prager TC, Zou YL, Fraley JK, Rozelle JC, et al. Effects of maternal docosahexaenoic acid intake on visual function and neurodevelopment in breastfed term infants. American Journal of Clinical Nutrition 2005;82(1):125‐32. [DOI] [PubMed] [Google Scholar]
Jensen 2006
- Jensen CL. Effects of n‐3 fatty acids during pregnancy and lactation. American Journal of Clinical Nutrition 2006;83(6):1452S‐1457S. [DOI] [PubMed] [Google Scholar]
Jensen 2009
- Jensen CL, Lapillonne A. Docosahexaenoic acid and lactation. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2009;81(2‐3):175‐8. [DOI] [PubMed] [Google Scholar]
Koletzco 2008
- Koletzko B, Lien E, Agostoni C, Böhles H, Campoy C, Cetin I, et al. The roles of long‐chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. Journal of Perinatal Medicine 2008;36(1):5‐14. [DOI] [PubMed] [Google Scholar]
Koletzko 2007
- Koletzko B, Cetin I, Brenna T. Dietary fat intakes for pregnant and lactating women. British Journal of Nutrition 2007;98:873–7. [PUBMED: 17688705] [DOI] [PubMed] [Google Scholar]
Larqué 2012
- Larqué E, Gil‐Sánchez A, Prieto‐Sánchez MT, Koletzko B. Omega 3 fatty acids, gestation and pregnancy outcomes. British Journal of Nutrition 2012;107(Suppl 2):S77‐84. [PUBMED: 22591905] [DOI] [PubMed] [Google Scholar]
Lauritzen 2005
- Lauritzen L, Jørgensen MH, Olsen SF, Straarup EM, Michaelsen KF. Maternal fish oil supplementation in lactation: effect on developmental outcome in breast‐fed infants. Reproduction, Nutrition, Development 2005;45(5):535‐47. [DOI] [PubMed] [Google Scholar]
Llorente 2003
- Llorente AM, Jensen CL, Voigt RG, Fraley JK, Berretta MC, Heird WC. Effect of maternal docosahexaenoic acid supplementation on postpartum depression and information processing. American Journal of Obstetrics and Gynecology 2003;188(5):1348‐53. [DOI] [PubMed] [Google Scholar]
McCann 2005
- McCann JC, Ames BN. Is docosahexaenoic acid, an n‐3 long‐chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. American Journal of Clinical Nutrition 2005;82(2):281‐95. [DOI] [PubMed] [Google Scholar]
Olsen 2001
- Olsen J, Melbye M, Olsen SF, Sørensen TI, Aaby P, Andersen AM, et al. The Danish National Birth Cohort‐‐its background, structure and aim. Scandinavian Journal of Public Health 2001;29(4):300‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulzke 2011
- Schulzke SM, Patole SK, Simmer K. Longchain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD000375.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [PUBMED: 19839216] [DOI] [PubMed] [Google Scholar]
Simmer 2011
- Simmer K, Patole SK, Rao SC. Longchain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD000376.pub3] [DOI] [PubMed] [Google Scholar]
Tinoco 2007
- Tinoco SM, Sichieri R, Moura AS, Santos Fda S, Carmo MG. The importance of essential fatty acids and the effect of trans fatty acids in human milk on fetal and neonatal development [Importancia dos acidos graxos essenciais e os efeitos dos acidos graxos trans do leite materno para o desenvolvimento fetal e neonatal]. Cadernos de Saude Publica 2007;23(3):525‐34. [DOI] [PubMed] [Google Scholar]
Torres 2009
- Torres AG, Trugo NM. Evidence of inadequate docosahexaenoic acid status in Brazilian pregnant and lactating women. Revista de Saude Publica 2009;43(2):359‐68. [DOI] [PubMed] [Google Scholar]
Uauy 2003
- Uauy R, Hoffman DR, Mena P, Llanos A, Birch EE. Term infant studies of DHA and ARA supplementation on neurodevelopment: results of randomized controlled trials. Journal of Pediatrics 2003;4:S17‐S25. [DOI] [PubMed] [Google Scholar]
Walker 2007
- Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet 2007;369(9556):145‐57. [DOI] [PubMed] [Google Scholar]
Welch 2002
- Welch AA, Lund E, Amiano P, Dorronsoro M, Brustad M, Kumle M, et al. Variability of fish consumption within the 10 European countries participating in the European Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutrition 2002;6B(5):1273‐85. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Delgado‐Noguera 2010
- Delgado‐Noguera MF, Calvache JA, Bonfill Cosp X. Supplementation with long chain polyunsaturated fatty acids (LCPUFA) to breastfeeding mothers for improving child growth and development. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD007901.pub2] [DOI] [PubMed] [Google Scholar]


