Abstract
Immunity to Mycobacterium tuberculosis is dependent upon the generation of a protective gamma interferon (IFN-γ)-producing T-cell response. Recent studies have suggested that interleukin-6 (IL-6) is required for the induction of a protective T-cell response and that IL-4 may suppress the induction of IFN-γ. To evaluate the role of the cytokines IL-6 and IL-4 in the generation of pulmonary immunity to M. tuberculosis, IL-6 and IL-4 knockout mice were infected with M. tuberculosis via aerosol. The absence of IL-6 led to an early increase in bacterial load with a concurrent delay in the induction of IFN-γ. However, mice were able to contain and control bacterial growth and developed a protective memory response to secondary infection. This demonstrates that while IL-6 is involved in stimulating early IFN-γ production, it is not essential for the development of protective immunity against M. tuberculosis. In contrast, while the absence of IL-4 resulted in increased IFN-γ production, this had no significant effect upon bacterial growth.
Interleukin-6 (IL-6) is a pleiotropic cytokine capable of inducing multiple effects upon many target cells. It is involved in the differentiation and proliferation of T and B cells (1, 10, 13) and acts in conjunction with the other proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and IL-1 to initiate the early inflammatory response following infection (10). The specific role of IL-6 in response to a pathological agent varies markedly, depending upon the agent, as is illustrated by the varied consequences of experimental infection in the IL-6 gene-disrupted (knockout [KO]) mouse. Infection by rapidly growing pathogens such as Listeria and Salmonella spp. results in increased susceptibility (9, 14, 28), while little difference has been noted between IL-6 KO and wild-type mice infected with the slow-growing bacterium Leishmania major (21).
The importance of IL-6 in protection from mycobacterial infections is no less complex an issue than for other models. Specifically, Ladel and colleagues reported that while IL-6 was not required to inhibit the growth of Mycobacterium bovis BCG in vivo, IL-6 KO mice were more susceptible than control mice to very high doses of intravenously delivered M. tuberculosis (16). In addition, other studies have reported that IL-6 depletion results in exacerbation of M. avium infection (1) and also reduces the protective effect of culture filtrate protein vaccination against aerogenic M. tuberculosis infection (17). A key observation in the above-mentioned studies was that the absence of IL-6 resulted in reduced production of the protective cytokine gamma interferon (IFN-γ) and, in one study, increased production of IL-4 (16).
Given the above-mentioned data, it would be reasonable to hypothesize that IL-6 plays a protective role in mycobacterial infection. However, the previous studies did not directly address the role of IL-6 in primary infection of the natural target organ, the lung. What they did address is the importance of IL-6 in the differentiation of antigen-exposed Th cells into an IFN-γ-producing phenotype (17) and the importance of IL-6 in responding to an acute bacterial infection of the visceral organs (16). The low-dose aerosol model of M. tuberculosis mimics the natural route of infection and provides a low challenge dose, enabling the mice to develop strong protective immunity and contain the infection over a prolonged period (26). Use of the aerosol model therefore allows the function of IL-6 in the development of early, possibly unique, protective immune responses in the lung to be determined.
In the intravenous challenge model cited above, the increased susceptibility of IL-6 KO mice correlated with increased expression of IL-4 (16). The decrease in IFN-γ seen in IL-6 KO mice may have been a direct result of the lack of IL-6 (as suggested by the work of Leal and colleagues reported in reference 17). It is also possible, however, that the excess IL-4 was responsible for the downregulated IFN-γ production and therefore the increased susceptibility. IL-4 is well known to be able to downregulate protective IFN-γ responses (3, 20), and it is entirely plausible that IL-4 could contribute to the loss of the protective IFN-γ response seen in the murine model of tuberculosis (23). We were therefore also interested in the ability of the IL-4 KO mouse to generate protective T-cell responses in response to aerogenic infection.
To address the issues raised above, both control and KO mice were infected aerogenically with a low-dose aerosol of M. tuberculosis. Both bacterial growth and development of immune parameters were followed over time. We report here that the absence of IL-6 led to a delay in the induction of protective immunity with a subsequent early increase in bacterial load; however, the absence did not affect the induction of normal protective memory responses. The absence of IL-4 did allow more rapid expression of the protective IFN-γ response, but this failed to alter the growth of bacteria within the lung.
MATERIALS AND METHODS
Mice.
Female IL-6 KO or IL-4 KO mice and C57BL/6 wild-type controls were purchased from The Jackson Laboratory, Bar Harbor, Maine. KOs were generated by targeted gene disruption of embryonic stem cells, which were introduced into C57BL/6/129 blastocysts (14, 15). Heterozygous mice (+/−) were then bred to produce both homozygous KOs and wild-type controls.
Bacteria and infection.
M. tuberculosis strains Erdman and CSU 93 were grown from laboratory stocks in Proskauer-Beck liquid medium to mid-log phase, aliquoted, and then frozen at −70°C. Mice were infected using a Middlebrook Airborne Infection apparatus (Middlebrook, Terre Haute, Ind.) such that within each experiment, all groups were exposed simultaneously to the same dose, which results in approximately 100 CFU of M. tuberculosis being deposited in the lungs of each mouse (4, 5, 8, 24, 26, 27). The numbers of viable bacteria in the lung, spleen, and liver were determined at various time points by plating serial dilutions of organ homogenates on nutrient Middlebrook 7H11 agar and counting bacterial colonies after 21 days of incubation at 37°C. The data are expressed as the log10 of the mean number of bacteria recovered per organ (four animals).
In order to examine the ability of previously exposed (memory) mice to respond to challenge, mice were infected intravenously with 105 CFU of M. tuberculosis Erdman via a lateral tail vein. After 1 month, both exposed and naive mice were treated with a combination of rifabutin (Pharmacia, Adina, Dublin, Ohio) at 40 mg/liter) and isoniazid (isonicotinic acid hydroside; Sigma, St. Louis, Mo.) at 200 mg/liter given in the drinking water for 28 days. Mice were then rested for several weeks prior to aerosol infection.
Preparation of lung and spleen cells.
Lungs were aseptically excised and washed free of blood by injection of 10 ml of Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum (FCS), penicillin at 100 U/ml, streptomycin at 100 μg/ml, 10 mM HEPES, 2 mM glutamine, and nonessential amino acids (DMEM–10% FCS) through the heart. The tissue was then finely sliced with sterile razor blades, and tissue from each individual lung was incubated for 60 min in 10 ml of medium containing 0.25% Dispase (Boehringer Mannheim). Undigested tissue was forced through a mesh sieve and centrifuged to pellet cells. The preparations underwent NH4Cl treatment to remove any remaining red blood cells. Cells were then washed, counted, and resuspended in DMEM–10% FCS. Spleen cell suspensions were prepared by forcing the spleen through a mesh sieve, followed by washing, lysis of red blood cells, and resuspension in DMEM–10% FCS.
Culture and assay for cytokine release.
Cultures of 2 × 106 spleen cells, pooled from four mice per group, with or without stimulation by 2 × 106 CFU of M. tuberculosis were incubated in triplicate in a 1-ml volume of DMEM–10% FCS in 24-well plates for 72 h. Cultures of 2 × 105 lung cells, from four individual mice, were incubated in a 200-μl volume of DMEM–10% FCS in 96-well plates for 5 days with antigenic stimuli identical to those used with spleen cells.
Cytokines released into the culture supernatant were detected using a sandwich enzyme-linked immunosorbent assay (ELISA) with appropriate antibody pairs as follows: IFN-γ, R4-6A2 and XMG1.2; IL-6, MP5-20F3 and MP5-32C11; IL-4, BVD4-1D11 and BVD6-24G2 (Pharmingen, San Diego, Calif.). Immunoplate Maxisorb 96-well plates (Nunc, Naperville, Ill.) were used. ELISAs were performed by following the manufacturer's directions.
Isolation of mRNA and analysis of levels of mRNA in infected lung tissue.
Relative amounts of mRNAs for IFN-γ, IL-12 p40 chain, TNF-α, and IL-2 were determined by a quantitative reverse transcriptase PCR protocol as previously described (4). Briefly, tissues were excised, placed in Ultraspec (Cinna/Biotecx, Friendswood, Tex.), and homogenized and RNA was extracted by a phenol-chloroform extraction process. One microgram of total RNA was reverse transcribed, diluted, and subjected to PCR expansion of cytokine-specific cDNA. Fluorescein-tagged cytokine sequence-specific probes were used to determine the amount of cytokine-related product. The fluorescein was detected using an enhanced-chemiluminescence kit (ECL; Amersham, Arlington Heights, Ill.). A limiting number of cycles was used, generating a log-linear relationship between the chemiluminescence signal and the amount of readable cytokine mRNA. The signal derived from four infected mice is divided by the signal derived from four appropriate noninfected control mice, and data are expressed as the mean fold increase in the infected signal over the uninfected signal.
Statistical analysis.
Differences between the means of experimental groups were analyzed using the Student t test. Differences were considered significant at P < 0.05.
RESULTS
Increased bacterial growth in IL-6 KO but not IL-4 KO mice following low-dose M. tuberculosis aerosol infection.
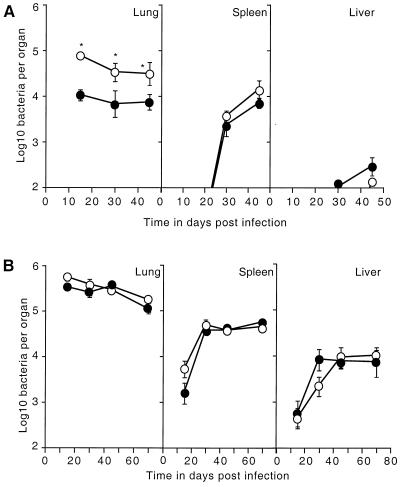
In a first series of experiments, the ability of IL-6 KO mice to control infection with M. tuberculosis was assessed. Control and KO mice were simultaneously infected with 102 CFU of M. tuberculosis Erdman via the aerogenic route, and the number of bacteria was assessed at intervals after infection. Within 14 days postinfection, IL-6 KO mice had approximately 1 log more bacteria in their lungs than did the control mice (Fig. 1A). These mice maintained a higher bacterial load for the duration of the study, although bacterial growth was controlled and dissemination of bacteria into the spleen and liver did not differ significantly between the two mouse strains (Fig. 1A).
FIG. 1.
Course of M. tuberculosis infection in cytokine gene KO mice. (A) Numbers of viable bacteria detected in the lungs, spleens, and livers of both IL-6 KO (open circles) and control (closed circles) mice aerogenically infected with ∼100 CFU of M. tuberculosis Erdman. (B) Numbers of viable bacteria detected in the lungs, spleens, and livers of both IL-4 KO (open circles) and control (closed circles) mice aerogenically infected with ∼100 CFU of M. tuberculosis CSU 93. These data are representative of three similar experiments. The data are the mean log10 number of viable bacteria per organ (four mice) plus the standard deviations. An asterisk indicates a significant difference between the control and IL-6 KO mice (P < 0.005) by the Student t test.
In agreement with previously published data (22), it was found that IL-4 KO mice infected aerogenically with 102 CFU of M. tuberculosis Erdman displayed no significant differences in bacterial growth compared to the wild-type mice (data not shown). To test if the absence of IL-4 affected the growth of a more virulent strain of M. tuberculosis, mice were infected with M. tuberculosis CSU 93 (24). Again, bacterial growth did not differ significantly between control and IL-4 KO mice over a 70-day period (Fig. 1B).
Absence of IL-6 decreases early IFN-γ production following M. tuberculosis infection.
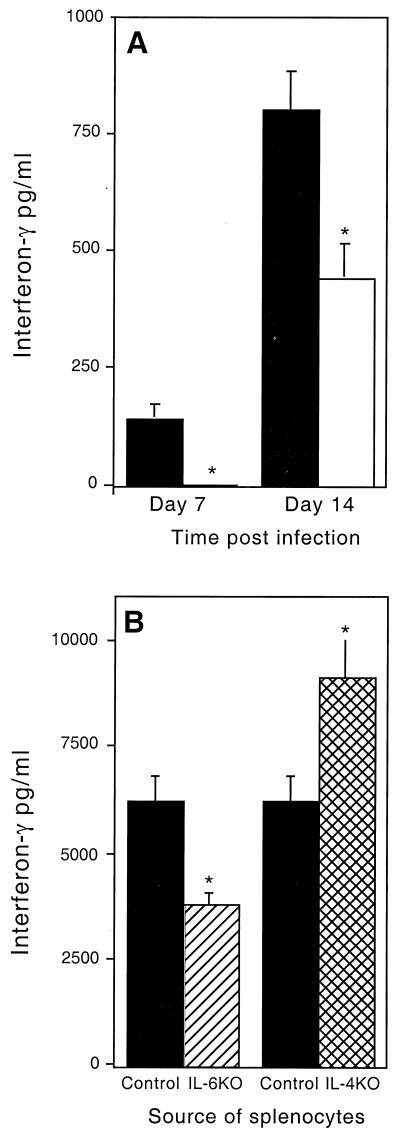
Production of IFN-γ is a critical requirement for protective immunity against M. tuberculosis (6, 11). To assess the role of IL-6 in inducing IFN-γ production, lung cells from M. tuberculosis-infected mice were cultured in vitro with live M. tuberculosis bacteria. IL-6 KO mice produced no detectable IFN-γ at 7 days postinfection and only half of the IFN-γ of wild-type mice at 14 days (Fig. 2A). To confirm that IL-6 was available at this early stage of infection, this cytokine was detected in cultures from control infected, but not uninfected, mice as early as day 7 (data not shown).
FIG. 2.
IFN-γ production by cultures of lung and spleen cells from M. tuberculosis-infected mice. (A) Lung cells from infected control (solid bars) and IL-6 KO (open bars) mice were stimulated for 5 days with live M. tuberculosis organisms, and IFN-γ secretion was analyzed by ELISA. The data are the means and standard deviations of three or four mice per group. (B) Spleen cells from infected control (solid bars), IL-6 KO (hatched bar), or IL-4 KO (cross-hatched bar) mice were stimulated for 72 h with M. tuberculosis organisms. IFN-γ secretion was then analyzed by ELISA. The data are the means and standard deviations of triplicate samples. Spleen and lung cell cultures from uninfected mice produced no detectable IFN-γ during these experiments (data not shown). An asterisk indicates a significant difference between the control and both IL-6 KO and IL-4 KO mice (P < 0.05) by the Student t test.
Spleen cell cultures from both IL-6 and IL-4 KO mice were also stimulated in vitro with live bacteria, and the supernatants were assayed for the production of cytokines. At 14 days postinfection, IL-6 KO mice produced significantly less IFN-γ than did control mice while IL-4 KO mice had significantly increased production of IFN-γ at this same time point (Fig. 2B). No IL-4 was detectable (limit, 75 pg/ml) in the control infected cultures (data not shown).
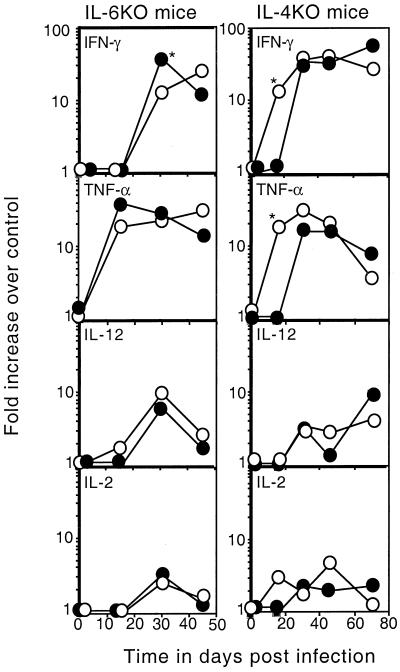
IFN-γ mRNA expression is difficult to detect during the early (days 0 to 15) phase of low-dose aerogenic infection (5, 27). By day 30, however, mRNA is detectable in the lungs of control mice and at this time point the IL-6 KO mice exhibit a decreased ability to express mRNA for IFN-γ (Fig. 3). Interestingly, IL-4 KO mice are able to express IFN-γ mRNA as early as day 14, which is earlier than the control mice. Thus, while it is necessary to restimulate lung cells ex vivo to observe the early defect in IFN-γ production in IL-6 KO mice, the available reverse transcriptase PCR data do support the hypothesis that IL-6 KO mice are less able to express IFN-γ in response to aerogenic infection in vivo.
FIG. 3.
Expression of cytokine mRNA in the lungs of infected mice. Control (closed circles) and KO (open circles; IL-6 KO on the left, IL-4 KO on the right) mice were aerogenically infected with M. tuberculosis as described in the legend to Fig. 1. Lung tissue (four mice) was removed at the times indicated and analyzed for the presence of IFN-γ, TNF-α, IL-2, and IL-12 mRNA by message-specific reverse transcriptase PCR. The data shown are the mean fold increases in the pixel signal of infected animals relative to that of each uninfected control animal (fold increase over control). An asterisk indicates a significant difference between the control and KO mice (P < 0.05) by the Student t test. The standard deviation from the mean was never more than 15%. These data are representative of two separate experiments.
Absence of IL-6 does not affect the generation of a protective memory response against M. tuberculosis.
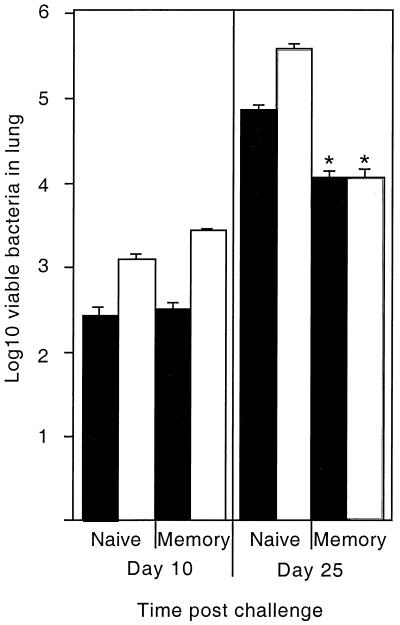
As discussed above, IL-6 appears to play a role in the generation of specific protective immunity, through the differentiation of T lymphocytes to an IFN-γ-producing phenotype (1, 19). Our initial experiments demonstrated the decreased ability of IL-6 KO mice to produce IFN-γ from mixed cell populations derived from both the lung and the spleen. While the IFN-γ may have been derived from antigen-specific T cells, there are many other potential sources of this cytokine in the mixed-population studies reported here. In order to address the role of IL-6 in the induction of protective memory T lymphocytes, the level of protection mediated by prior exposure to M. tuberculosis was compared for both IL-6 KO and control mice. In accordance with previous studies, neither the control nor the IL-6 KO memory mice exhibited increased protection compared to naive mice at day 10 of infection (5). Importantly, however, both the control and IL-6 KO mice exhibited a similar significant decrease in bacterial growth compared to the naive mice by day 25 (Fig. 4).
FIG. 4.
Growth of M. tuberculosis in immune mice. Control (closed bars) and IL-6 KO (open bars) mice were intravenously infected with M. tuberculosis Erdman and after 1 month treated with drugs to clear the infection (memory). Some mice were sham infected and drug treated (naive). Mice were then infected with ∼100 M. tuberculosis Erdman organisms aerogenically, and the numbers of viable bacteria were determined at days 10 and 25 postinfection. The data are mean log10 numbers of viable bacteria per lung (± the standard deviation) of four mice per group. An asterisk indicates a significant difference between the naive and memory control and IL-6 KO mice at day 25 postinfection (P < 0.05). These data are representative of two separate experiments.
DISCUSSION
This study demonstrates that IL-6 is required for the rapid expression of an initial protective IFN-γ response during M. tuberculosis infection. This early response occurs in the lung and is important in the initial containment of mycobacterial growth in this organ. The early lack of containment seen in the absence of IL-6 is compensated for when the acquired immune response is expressed. In addition, IL-6 is not required for the generation of effective memory immunity, as evidenced by the ability of IL-6 KO mice to express strong protective immunity upon rechallenge.
Our study extends an earlier study by Ladel and colleagues (16), who found a protective role for IL-6 in high-dose intravenous infection with M. tuberculosis. In this model, exacerbation of growth was seen in the spleen, liver, and lung, with IL-6 KO mice succumbing to infection beginning 50 days after infection. The fact that IL-6 KO mice did not die in the experiments described here is not contrary to the observations of Ladel (16). In both studies, increased growth of bacteria was seen early in infection; however, when the initial dose was low (and when the high-dose bacteria were less virulent, i.e., BCG [16]), IL-6 KO mice were eventually able to contain the infection.
The precise mechanism by which IL-6 mediates its protective effects in models of infectious disease has not been determined. It is well documented, however, that IL-6 can affect the initiation and development of both the innate and acquired responses in vivo (30). The data presented here strongly suggest that IL-6 has an early, probably innate, protective role which cannot be compensated for by other cytokines. Specifically, the observed kinetics of the increased susceptibility seen in both naive and memory IL-6 KO mice support this hypothesis, as susceptibility precedes the normal expression of protective immunity in this organ (5) and is not improved by the induction of a memory state in the IL-6 KO mouse.
Previous studies with Listeria monocytogenes and Candida albicans have implicated neutrophils as major components of the IL-6 protective response to these agents. In both of these models, the protective effect of IL-6 was abrogated in the absence of neutrophils and one consequence of the absence of IL-6 was defective neutrophilia in response to infection (9, 28). IL-6 has been shown to activate human neutrophils (2), and we have recently identified a protective nonphagocytic role for these cells in tuberculosis infection wherein the depletion of neutrophils resulted in reduced IFN-γ mRNA in the infected organ (25). A hypothesis generated by these observations is that there may be a novel protective mechanism in the M. tuberculosis-infected mouse lung which is dependent upon IL-6 recruitment/activation of neutrophils which then facilitates a rapid IFN-γ response.
Interestingly, both IL-6 KO mice and p47phox KO mice (which lack the ability to generate reactive oxygen intermediates [ROI]) exhibit almost identical phenotypes when aerogenically challenged with M. tuberculosis (8). This similarity may be due to the importance of ROI in the initiation of IL-6 mRNA expression, particularly in the lung (8, 12, 29, 31). The increased susceptibility to early bacterial growth observed in both models may therefore occur as the result of disruption ROI induction of IL-6 (data reported here and in reference 8).
Notwithstanding the strong evidence for an innate role for IL-6 in the control of M. tuberculosis infection, several reports have implicated IL-6 in the initiation and maintenance of acquired antigen-specific cellular immunity. The data reported here show that the susceptibility of IL-6 KO mice occurred prior to the expression of normal cellular immunity (5) and did not affect the expression of protective memory immunity. In addition, both IFN-γ expression and control of bacterial growth in the lung were not compromised once the early stage of infection was over. These observations suggest that in the presence of a viable bacterial infection, IL-6 is not essential for the induction of memory immunity and that protective IFN-γ-producing cells can be induced in the absence of early IL-6-dependent IFN-γ. This is in contrast to the data reported for both M. avium and L. monocytogenes infections (1, 19), wherein IL-6 plays a positive role in the generation of a protective memory response. Thus, while the presence of IL-6 is important for the induction of antigen-specific memory T-cell development during vaccination (17), the presence of a live M. tuberculosis infection abrogates the requirement for this cytokine. It is probable that other cytokines, such as IL-12 (7) and TNF-α (27), that are induced by live M. tuberculosis compensate for the lack of IL-6 in the induction of antigen-specific IFN-γ-producing T cells.
In other models of infection, IL-6 KO mice express increased levels of IFN-γ-downregulating cytokines such as IL-4 (16) and IL-10 (28). It is possible, therefore, that the protective activity of IL-6, as presented here, was mediated by inhibition of IL-4. We were able to examine this question by infecting mice that lack IL-4. Interestingly, the lack of IL-4 did result in earlier and increased expression of IFN-γ, thus supporting the hypothesis that IL-4 did, indeed, inhibit IFN-γ production. It was noticeable, however, that no increased antimycobacterial effect was seen in IL-4 KO mice, suggesting that sufficient IFN-γ was produced in the presence of IL-4 and thus any role of IL-6 in limiting the effects of IL-4 in control mice was essentially moot.
In conclusion, an inability to produce IL-6 in response to infection is not lethal when the dose or virulence of the infective agent is limited. In contrast, a high dose or a rapidly growing pathogen can overwhelm the kind of moderately compromised response seen in IL-6 KO mouse. What we demonstrate here is that IL-6 is required to initiate a very early, possibly innate, IFN-γ response, which then limits bacterial growth until acquired cellular immune responses are expressed. In the normal animal, this early IFN-γ response appears to be optimal, as increasing this response (by removing IL-4, for example) fails to augment bacterial control. The precise nature of this early mechanism mediating control of M. tuberculosis in the lung has not been elucidated; this paper begins to identify some of the components of this mechanism.
ACKNOWLEDGMENTS
We thank the staff of the Laboratory Animal Center, Colorado State University, for animal care and W. Mueller for permission to use the IL-4 KO mice.
This work was supported by National Institutes Health grant AI-40488.
REFERENCES
- 1.Appelberg R, Castro A G, Pedrosa J, Minoprio P. Role of interleukin-6 in the induction of protective T cells during mycobacterial infections in mice. Immunology. 1994;82:361–364. [PMC free article] [PubMed] [Google Scholar]
- 2.Borish L, Rosenbaum R, Albury L, Clark S. Activation of neutrophils by interleukin-6. Cell Immunol. 1989;121:280–289. doi: 10.1016/0008-8749(89)90026-9. [DOI] [PubMed] [Google Scholar]
- 3.Coffman R L, von der Weid T. Multiple pathways for the initiation of T helper 2 (Th2) responses. J Exp Med. 1997;185:373–375. doi: 10.1084/jem.185.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper A M, Callahan J E, Griffin J P, Roberts A D, Orme I M. Old mice are able to control low-dose aerogenic infections with Mycobacterium tuberculosis. Infect Immun. 1995;63:3259–3265. doi: 10.1128/iai.63.9.3259-3265.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper A M, Callahan J E, Keen M, Belisle J T, Orme I M. Expression of memory immunity in the lung following re-exposure to Mycobacterium tuberculosis. Tubercle Lung Dis. 1997;78:67–73. doi: 10.1016/s0962-8479(97)90017-4. [DOI] [PubMed] [Google Scholar]
- 6.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper A M, Magram J, Ferrante J, Orme I M. IL-12 is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–46. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper A M, Segal B H, Frank A A, Holland S M, Orme I M. Transient loss of resistance to pulmonary tuberculosis in p47phox−/− mice. Infect Immun. 2000;68:1231–1234. doi: 10.1128/iai.68.3.1231-1234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalrymple S A, Lucian L A, Slattery R S, McNeil T, Aud D M, Fuchino S, Lee F, Murray R. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect Immun. 1995;63:2262–2268. doi: 10.1128/iai.63.6.2262-2268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinarello C A. Role of interleukin-1 in infectious disease. Immunol Rev. 1992;127:119–146. doi: 10.1111/j.1600-065x.1992.tb01411.x. [DOI] [PubMed] [Google Scholar]
- 11.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franke-Ullman G, Pfortner C, Walter P, Steinmuller C, Lohmann-Matthes M-L, Kobzik L. Characterization of murine lung interstitial macrophages in comparison with alveolar macrophages, in vitro. J Immunol. 1996;157:3097–3104. [PubMed] [Google Scholar]
- 13.Hirano T. Interleukin-6 and its relation to inflammation and disease. Clin Immunol Immunopathol. 1992;62:s60–s65. doi: 10.1016/0090-1229(92)90042-m. [DOI] [PubMed] [Google Scholar]
- 14.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 16.Ladel C H, Blum C, Dreher A, Reifenberg K, Kopf M, Kaufmann S H E. Lethal tuberculosis in interleukin-6-deficient mice. Infect Immun. 1997;65:4843–4849. doi: 10.1128/iai.65.11.4843-4849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leal I S, Smedegard B, Andersen P, Appelberg R. Interleukin-6 and interleukin-12 participate in induction of a type 1 protective T-cell response during vaccination with a tuberculosis subunit vaccine. Infect Immun. 1999;67:5747–5754. doi: 10.1128/iai.67.11.5747-5754.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J R, Koretsky G A. Production of reactive oxygen intermediates following CD40 ligation correlates with c-jun N-terminal kinase activation and IL-6 secretion in murine B lymphocytes. Eur J Immunol. 1998;28:4188–4197. doi: 10.1002/(SICI)1521-4141(199812)28:12<4188::AID-IMMU4188>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Simpson R J, Cheers C. Role of IL-6 in activation of T cells for acquired cellular resistance to Listeria monocytogenes. J Immunol. 1994;152:5375–5380. [PubMed] [Google Scholar]
- 20.Modlin R L, Nutman T B. Type 2 cytokines and negative regulation in human infections. Curr Opin Immunol. 1993;5:511–517. doi: 10.1016/0952-7915(93)90031-m. [DOI] [PubMed] [Google Scholar]
- 21.Moskowitz N H, Brown D, Reiner S L. Efficient immunity against Leishmania major in the absence of interleukin-6. Infect Immun. 1997;65:2448–2450. doi: 10.1128/iai.65.6.2448-2450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.North R J. Mice incapable of making IL-4 or IL-10 display normal resistance to infection with Mycobacterium tuberculosis. Clin Exp Immunol. 1998;113:55–58. doi: 10.1046/j.1365-2249.1998.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orme I M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138:293–298. [PubMed] [Google Scholar]
- 24.Orme I M. Virulence of recent notorious Mycobacterium tuberculosis isolates. Tubercle Lung Dis. 1999;79:379–381. doi: 10.1054/tuld.1999.0221. [DOI] [PubMed] [Google Scholar]
- 25.Pedrosa J, Saunders B M, Appelberg R, Orme I M, Silva M T, Cooper A M. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect Immun. 2000;68:577–583. doi: 10.1128/iai.68.2.577-583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhoades E, Frank A, Orme I. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tubercle Lung Dis. 1997;78:57–66. doi: 10.1016/s0962-8479(97)90016-2. [DOI] [PubMed] [Google Scholar]
- 27.Rhoades E R, Cooper A M, Orme I M. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect Immun. 1995;63:3871–3877. doi: 10.1128/iai.63.10.3871-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romani L, Mencacci A, Cenci E, Spaccapelo R, Toniatti C, Puccetti P, Bistoni F, Poli V. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin-6-deficient mice infected with Candida albicans. J Exp Med. 1996;183:1345–1355. doi: 10.1084/jem.183.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simeonova P P, Toriumi W, Kommineni C, Erkan M, Munson A E, Rom W N, Luster M I. Molecular regulation of IL-6 activation by asbestos in lung epithelial cells: role of reactive oxygen species. J Immunol. 1997;159:3921–3928. [PubMed] [Google Scholar]
- 30.van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida Y, Maruyama M, Fujita T, Arai N, Hayashi R, Araya J, Matsui S, Yamashita N, Sugiyama E, Kobayashi M. Reactive oxygen intermediates stimulate interleukin-6 production in human bronchial epithelial cells. Am J Physiol. 1999;276:900–908. doi: 10.1152/ajplung.1999.276.6.L900. [DOI] [PubMed] [Google Scholar]