Abstract
Introduction
Features of underlying autonomic dysfunction, including sleep disturbances, gastrointestinal problems, and atypical heart rate, have been reported in neurodevelopmental conditions, including autism spectrum disorder (ASD). The current cross‐sectional, between‐groups study aimed to quantify symptoms of autonomic dysfunction in a neurodevelopmental pediatric cohort characterized by clinical diagnoses as well as genetic etiology.
Method
The Pediatric Autonomic Symptom Scales (PASS) questionnaire was used to assess autonomic features across a group of patients with clinical neurodevelopmental diagnoses (NPD; N = 90) and genetic etiologies. Patients were subdivided based on either having a clinical ASD diagnosis (NPD‐ASD; n = 37) or other non‐ASD neurodevelopmental diagnoses, such as intellectual disability without ASD, speech and language disorders, and/or attention deficit hyperactivity disorder (NPD‐OTHER; n = 53). Analyses focused on characterizing differences between the NPD group compared to previously published reference samples, as well as differences between the two NPD subgroups (NPD‐ASD and NPD‐OTHER).
Results
Our results indicate higher PASS scores in our NPD cohort relative to children with and without ASD from a previously published cohort. However, we did not identify significant group differences between our NPD‐ASD and NPD‐OTHER subgroups. Furthermore, we find a significant relationship between quantitative ASD traits and symptoms of autonomic function.
Conclusion
This work demonstrates the utility of capturing quantitative estimates of autonomic trait dimensions that may be significantly linked with psychosocial impairments and other core clinical features of ASD.
Keywords: autism spectrum disorder, autonomic nervous system, developmental brain dysfunction, individual differences
1. BACKGROUND
Although not a part of the core diagnost criteria, symptoms related to underlying dysfunction of the autonomic nervous system (ANS) are commonly observed in patients with autism spectrum disorder (ASD), including sleep disturbances, gastrointestinal problems, and atypical heart rate (Aldinger et al., 2015; Devnani & Hegde, 2015; Kotagal & Broomall, 2012; Kushki et al., 2013; Liu et al., 2006; Mayes & Calhoun, 2009; Neuhaus et al., 2014; Patriquin et al., 2013). The ANS (i.e., the peripheral nervous system) maintains homeostasis in the body via a synchronous balance of sympathetic and parasympathetic activity across functions including heart rate, digestion, respiration, sleep, and sensory processing. Failure of one of the parasympathetic or sympathetic components of the ANS can result in malfunction or disruption of the system. Recent work has described a dysregulated ANS as a potential biological driver of anxiety, sensory disintegration, and psychosocial impairments that are significant clinical features of atypical neurodevelopment, including ASD (Appelhans & Luecken, 2006; Goodwin et al., 2006; Kushki et al., 2013; Kushki et al., 2014; Patriquin et al., 2013; Patriquin et al., 2019; Quintana et al., 2012).
Over the past 15 years, a number of studies have highlighted the role of the ANS in the underlying pathophysiology of brain‐related disorders and assessed autonomic dysregulation and atypical circadian function associated with neurodegenerative disease states (Germain & Kupfer, 2008; Musiek et al., 2015), various forms of psychopathology (Sgoifo et al., 2015; Videnovic & Zee, 2015; Wirz‐Justice et al., 2009; Wulff et al., 2012), and neurodevelopmental conditions such as ASD (Anderson & Colombo, 2009; Bellato et al., 2020; Bharath et al., 2019; Dell'Osso et al., 2022; Rukmani et al., 2016). Studies have reported atypical autonomic responses, including heart rate variability, skin conductance, pupillary response, and neuroendocrine markers (Gabriels et al., 2013; Kushki et al., 2013; Ming et al., 2005; Poquérusse et al., 2018; Song et al., 2016; Tordjman et al., 2014; Wang et al., 2016). These differences in physiological outputs have been interpreted as peripheral biological indicators of discordant autonomic arousal. The observed differences in measures of cardiac output, electrodermal activity, and pupil reactivity have also been theoretically and quantitatively linked to deficits in social cognition and social impairments (Appelhans & Luecken, 2006; Cheshire, 2012; Quintana et al., 2012). More specifically, task‐induced measures of heart rate and sinus arrhythmia have been associated with impaired social reciprocity (Neuhaus et al., 2014; Patriquin et al., 2013), as well as social and emotional perception, cognitive abilities, and restrictive and repetitive behaviors (Billeci et al., 2018; Condy et al., 2017; Kushki et al., 2014; Soker‐Elimaliah et al., 2020). From this lens, differences in physiological outputs associated with ANS dysfunction have been attributed to underlying neurobiological differences and core clinical features of ASD (Dinalankara et al., 2017; Kushki et al., 2014; Ming et al., 2005; Pace et al., 2016; Patriquin et al., 2013). Taken together, this research highlights autonomic function as a possible indicator of dysregulated neurological states that can be linked with atypical neurodevelopment and ASD trait dimensions.
It is standard of care to offer diagnostic genetic testing to patients with ASD and/or other neurodevelopmental disorders to assess for genetic etiologies. Known genetic causes of ASD and other neurodevelopmental disorders are etiologically heterogeneous and include copy number variants (CNVs), sequence‐level variants in single genes (SNVs), and epigenetic alterations (for review, see Rylaarsdam and Guemez Gamboa (2019)). Importantly, many genetic disorders confer high risk for a broad spectrum of neurodevelopmental/psychiatric disorders (NPD), which represent variable expressivity of underlying developmental brain dysfunction (Moreno‐De‐Luca et al., 2013). For example, recurrent pathogenic CNVs (e.g., 22q11.2, 16p11.2) are associated with several psychiatric and neurodevelopmental disorders (Gudmundsson et al., 2019; Martin et al., 2020; Stefansson et al., 2014), including ASD (Hanson et al., 2015; Kates et al., 2007; Niklasson et al., 2009; Shinawi et al., 2010). When examining common variation across the genome, several studies have also identified shared genetic risk across multiple psychiatric and neurodevelopmental disorders, highlighting the role of shared genetic risk factors for clinical diagnoses and phenotypic variability across NPD (Cross‐Disorder Group of the Psychiatric Genomics Consortium, 2013; Lee et al., 2019). Thus, there is increased recognition that genetic influences on psychiatric and neurodevelopmental disorders are pleiotropic and transcend diagnostic boundaries.
Individuals with NPD‐related genetic disorders have been reported to have symptoms such as atypical sleep–wake cycles or disrupted patterns of sleep, gastrointestinal and digestive disturbances, and others that can be interpreted as signs of ANS dysfunction (Brunetti‐Pierri et al., 2008; Cerminara et al., 2021; Kamara et al., 2021; Leader et al., 2021; Shayota & Elsea, 2019). However, there is little known regarding the connection between ANS‐related symptoms and genetic variation associated with atypical neurodevelopment, including ASD (Dell'Osso et al., 2022; Hu et al., 2009; Lorsung et al., 2021). Thus, a more comprehensive understanding of the presence of a common transdiagnostic domain, such as ANS‐related symptoms, in children with neurodevelopmental disorders may lead us closer to the underlying biology that drives behavioral differences in heterogeneous disorders. This transdiagnostic approach aligns with recent shifts in clinical outcomes and behavioral research to focus more on quantitative symptom scales or trait dimensions in a cross‐disorder manner, outside of traditional clinical taxonomy (Dalgleish et al., 2020; Gentes & Ruscio, 2011; Mahoney & McEvoy, 2012). Although it is ideal to capture symptoms via direct measurement, one can also capture variance in a variety of symptom domains using validated self‐ or parental‐report questionnaires. Quantitative measures of autonomic features have been previously utilized in patients with other neurological disorders (Adler et al., 2018; Aziz et al., 2010; Damian et al., 2012), although a majority of previous studies are based on adults (Damian et al., 2012; Sletten et al., 2012) or focused on one symptom domain (Morlino et al., 2019). Studies of ANS function within neurodevelopmental populations have not typically used ANS‐specific surveys, but rather relied on measures of anxiety, sensory processing, or sleep–wake cycles to assess features of arousal that were interpreted to reflect dysregulated ANS function (Keith et al., 2019; Lawson et al., 2020; Wiggs & Stores, 1996; Wiggs & Stores, 2004). One study used an ANS‐specific scale, the Composite Autonomic Symptoms Scales (COMPASS‐31), and noted increased symptoms of autonomic dysfunction in adolescents and young adults with ASD and a relationship between COMPASS‐31 scores and ASD traits (Lawson et al., 2020; Sletten et al., 2012). Another previous study used the Pediatric Autonomic Symptom Scales (PASS; adapted from the CASS and COMPASS‐31) (Sletten et al., 2012; Suarez et al., 1999) to demonstrate that features of autonomic dysfunction are elevated in a small cohort of children with ASD (Ming et al., 2011) (n = 18), for which genetic etiology was unknown.
Here, we capture autonomic symptoms as measured by the PASS (Ming et al., 2011) in a clinically characterized, pediatric cohort with genetic NPD etiologies, a subset of whom have a clinical ASD diagnosis. Although our group analyses focus on clinical diagnoses of ASD versus other non‐ASD NPD, we include comprehensive information on genetic etiology in this genotyped cohort in order to promote future research on such highly heterogeneous, real‐world samples. The overall objective of the current investigation was to quantify symptoms of autonomic dysfunction in a neurodevelopmental pediatric cohort characterized based on both clinical diagnoses as well as clinical genetic etiology. Based on previous studies of autonomic features in neurodevelopmental populations, we hypothesized that individuals with a genetic NPD etiology would demonstrate an increase in atypical symptoms of ANS function as compared to a published reference sample of children with and without ASD (Ming et al., 2011). Additionally, we aimed to assess individual differences in ANS function that may scale with the presence and severity of ASD symptoms across children with and without a clinical diagnosis of ASD and/or other NPDs. Research has demonstrated the link between autonomic dysregulation and psychosocial features, including core diagnostic traits of ASD (Appelhans & Luecken, 2006; Goodwin et al., 2006; Kushki et al., 2013; Kushki et al., 2014; Patriquin et al., 2013; Patriquin et al., 2019; Quintana et al., 2012). As a part of the current study, we characterized the direct relationship between features of autonomic dysfunction and symptom severity across ASD trait dimensions.
2. METHODS
2.1. Participants
The NPD cohort in the current cross‐sectional, between‐groups study was identified based on documentation of a diagnosis of a targeted NPD‐related genetic syndrome and current enrollment in an ongoing research protocol at the authors’ home institution. All eligible participants had consented to an ongoing research protocol, approved by the authors’ home institution's institutional review board (IRB) and ethics committee, that allowed for recontact and participation in an online phenotyping battery, including parent‐report measures (described below). Data were collected between April 2018 and November 2019. Individual genetic syndromes are reported in Table 1, with the most common syndromes including 16p11.2 (BP4‐BP5) deletion (del), n = 14; 17p11.2 del, n = 8; 17q12 del, n = 9; and Trisomy 21, n = 11. Patients at Geisinger's Autism & Developmental Medicine Institute (ADMI) undergo assessment by a multi‐disciplinary team including neurodevelopment pediatricians, clinical psychologists, behavioral specialists, and speech pathologists. Assessment tools and/or structured interviews, such as the ADOS or ADI‐R, may be used but diagnoses are ultimately made by the clinicians using DSM‐5 criteria for ASD after a comprehensive evaluation of the patient. ADOS and ADI‐R scores were not available from the electronic health record for enough patients in the current sample and thus were not used in any analyses.
TABLE 1.
Demographic information and a summary of genetic syndromes (copy number state, coordinates, and pathogenicity) included in the reported NPD sample (N = 90)
| Chromosome region | n, Sex (M/F) | n = ASD | Copy number state | Accepted general coordinates (hg19/GRCh37) | ClinGen pathogenicity |
|
Min | Max | |
|---|---|---|---|---|---|---|---|---|---|
| Copy number losses | |||||||||
| 1q21.1 (BP3‐BP4, wo TAR region) | n = 4, 3 M | 1 | Deletion | chr1:146,577,486‐147,394,506 (hg19) | 3 | 10.25 (6.65) | 2 | 18 | |
| 7q11.23 | n = 1, F | Deletion | chr7:72,744,455‐74,142,510 (hg19) | 3 | 3 | ||||
| 15q11.2 (BP1‐BP2) | n = 1, M | 1 | Deletion | chr15:22,832,519‐23,090‐897 (hg19) | 2 | 13 | |||
| 15q11.2q13.1 | n = 1, M | Deletion | chr15:23,747,996‐28,379,874 (hg19) | 3 | 18 | ||||
| 15q13.3 (BP3‐BP5) | n = 1, F | Deletion | chr15:29,156,959‐32,445,405 (hg19) | 3 | 16 | ||||
| 16p11.2 (BP4‐BP5) | n = 14, 9 M | 6 | Deletion | chr16:29,649,997‐30,199,852 (hg19) | 3 | 11.21 (4.14) | 6 | 17 | |
| 16p11.2 (SH2B1) | n = 2, 1 M | 1 | Deletion | chr16:28,822,635‐29,046,499 (hg19) | 3 | 9.50 (4.95) | 6 | 13 | |
| 16p12.2 (EEF2K) | n = 1, F | 1 | Deletion | chr16:21,948,445‐22,430,804 (hg19) | 2 | 11 | |||
| 16p13.11 | n = 1, F | Deletion | chr16:15,511,711‐16,292,265 (hg19) | 3 | 12 | ||||
| 17p11.2 | n = 8, 5 M | 4 | Deletion | chr17:16,810,028‐20,213,202 | 3 | 10.75 (4.56) | 5 | 18 | |
| 17q12 | n = 9, 7 M | 2 | Deletion | chr17:34,815,072‐36,192,489 (hg19) | 3 | 9.44 (5.00) | 3 | 18 | |
| 17q21.31 | n = 1, F | Deletion | chr17:43,705,166‐44,164,880 (hg19) | 3 | 4 | ||||
| 22q11.2 (3 Mb) | n = 3, 2 M | Deletion | chr22:18,912,231‐21,465,672 (hg19) | 3 | 11.33 (5.51) | 6 | 17 | ||
| Copy number gains | |||||||||
| 1q21.1 (BP2‐BP3, TAR region only) | n = 2, 1 M | 1 | Duplication | chr1:145,386,507‐145,748,064 (hg19) | 1 | 8.50 (0.71) | 8 | 9 | |
| 1q21.1 (BP3‐BP4, wo TAR region) | n = 3, 2 M | 1 | Duplication | chr1:146,577,486‐147,394,506 (hg19) | 3 | 8.00 (5.00) | 3 | 13 | |
| 1q21.1 (coordinates not available) | n = 1, M | 1 | Duplication | n/a | n/a | 13 | |||
| 15q11.1q13.3 (supernumerary isodicentric) | n = 2, 2 F | Triplication | variable | Not evaluated | 8.00 (0.00) | 8 | 8 | ||
| 15q11.2q13.1 | n = 5, 2 M | 3 | Duplication | chr15:23,747,996‐28,379,874 (hg19) | 3 | 14.20 (4.38) | 7 | 17 | |
| 15q11.2q13.1 (interstitial tandem triplication) | n = 1, F | 1 | Triplication | variable | Not evaluated | 3 | |||
| 15q11.2q13.3 (supernumerary isodicentric) | n = 1, F | 1 | Duplication | variable | Not evaluated | 12 | |||
| 15q13.3 (BP3‐BP5) | n = 1, M | 1 | Duplication | chr15:29,156,959‐32,445,405 (hg19) | 1 | 11 | |||
| 16p11.2 (SH2B1) | n = 1, M | 1 | Duplication | chr16:28,822,635‐29,046,499 (hg19) | 1 | 3 | |||
| 16p13.11 | n = 3, 2 M | 2 | Duplication | chr16:15,511,711‐16,292,265 (hg19) | 2 | 2.33 (0.58) | 2 | 3 | |
| 17q12 | n = 7, 6 M | 4 | Duplication | chr17:34,815,072‐36,192,489 (hg19) | 3 | 6.71 (3.09) | 2 | 10 | |
| Trisomy 21, | n = 11, 7 M | 2 | Trisomy | chr21:1‐48,129,895 (hg19) | Not evaluated | 8.91 (3.08) | 4 | 13 | |
| 22q11.2 (3 Mb) | n = 2, 2 M | 1 | Duplication | chr22:18,912,231‐21,465,672 (hg19) | 3 | 10.00 (5.66) | 6 | 17 | |
| Intragenic sequence variant | |||||||||
| 17p11.2 (RAI1 sequence variant) | n = 2, 2 F | Sequence variant | n/a | n/a | 9.00(2.83) | 7 | 11 | ||
| Other copy number variant | |||||||||
| 15q11.2q13.3 | n = 1, M | Not available | variable | Not evaluated | 7 |
Average age () as well as range of age for genetic syndrome is outlined in columns to the right.
All patients with qualifying clinical diagnoses are offered comprehensive clinical genetic testing and result follow‐up with the ADMI genetic counseling team. All clinical diagnoses, including ASD and any comorbidities, and genetic test results are entered into the patient's digital health record. The majority of patients (>85%) also consent/assent to a clinic‐wide research protocol, which allows researchers to access the patient's health record and recontact for additional research. Patients were eligible if they had received a clinical NPD diagnosis with a known genetic etiology and were recontacted to request completion of the parental report measures described below; clinical diagnoses and other parent‐report measures available in individuals’ EHR were also curated for this study. A total of 134 eligible participants completed and returned the PASS. However, due to factors including incomplete responses, age of the proband outside of the targeted age range, and missing clinical, diagnostic, and/or genetic information, data from n = 44 were excluded from analysis, resulting in our reported cohort of N = 90 NPD included in the current study. N = 90 (entire sample) were subdivided based on either having a clinical diagnosis of ASD (NPD‐ASD; n = 37) or another NPD diagnosis (NPD‐OTHER; n = 53). Other non‐ASD NPD diagnoses, present across NPD‐ASD and NPD‐OTHER subgroups, included attention deficit hyperactivity disorder (ADHD), anxiety, speech and language disorder, and mild‐to‐moderate intellectual disability (ID). All of the individuals within the NPD‐ASD subgroup had a diagnosis of ASD and at least one co‐occurring clinical diagnosis of another form of NPD (i.e., intellectual disability, ADHD, anxiety, etc.). To confirm, none of the individuals in the NPD‐OTHER had a clinical diagnosis of ASD; thus, this group comprised only individuals with other, non‐ASD forms on NPD (i.e., not ASD). Table S1 outlines the clinical diagnoses within each of the NPD‐ASD and NPD‐OTHER subgroups.
2.2. Parent‐report measures
All parent‐report measures outlined below were administered via an online phenotyping battery administered and maintained using REDCap. Parents and/or caregivers of consented patients received a personalized link, directing them to complete a comprehensive battery of various symptom questionnaires related to neurodevelopmental traits.
2.2.1. Pediatric autonomic symptom scales (PASS)
The PASS (Ming et al., 2011; Suarez et al., 1999) is a parent/caregiver report questionnaire designed to assess the severity of autonomic dysfunction across multiple systems in children. The questionnaire was adapted for use in pediatric cohorts based on the adult clinical research scale, the Composite Autonomic Symptom Scale (CASS) (Suarez et al., 1999). It includes 80 close‐ended questions from four section subscales grouped by the affected organ or organ systems—(I): Mood, Behavior, and Emotion; (II): Secretomotor/Sensory Integration; (III): Urinary/Gastrointestinal Systems; and (IV): Circulation, Thermoregulation, Sleeping Patterns, and Breathing (see Figure 1). The first type of question is based on the presence or absence of a symptom, such as “Have you noticed that your child seems to have difficulty seeing after coming out of a dark room?” If the answer represented dysfunction, it was scored as “1” whereas absence of the symptom or appropriate function was scored “0.” The second type of question assessed severity or frequency of the symptom, such as ‘‘Does your child urinate frequently, such as more than 10 times daily?’’ or ‘‘Does your child typically skip having a bowel movement for 2 days or more?’’ Item responses are summed, resulting in a Total Autonomic score and four subscale scores for each of the sections outlined above.
FIGURE 1.
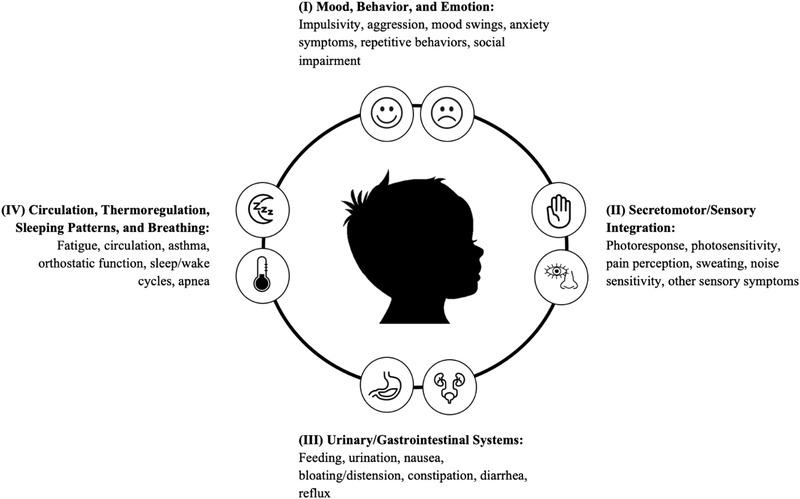
The Pediatric Autonomic Symptoms Scale (PASS). The PASS is an 80‐item parent‐report questionnaire of autonomic symptoms in children. Items are scored based on the presence (Yes = 1) or absence (No = 0) of symptoms. Items are summed resulting in a total score as well as four subscale scores: (I) Mood, Behavior, and Emotion; (II) Secretomotor/Sensory Integration; (III) Urinary/Gastrointestinal Systems; and (IV) Circulation, Thermoregulation, Sleep Patterns, and Breathing.
2.2.2. The social responsiveness scale—2nd edition (SRS‐2)
The SRS‐2 is a 65‐item rating scale widely used to identify social impairment and persons at risk for ASD from 2.5 years to adulthood (Constantino & Frazier, 2013; Constantino & Gruber, 2005; Constantino & Gruber, 2012). The SRS offers the advantage of being sensitive to subclinical ASD behaviors in the general population. Each item, such as “avoids eye contact, or has unusual eye contact” or “would rather be alone than with others” is measured on a 4‐point (0–3) scale: 0 = not true, 1 = sometimes true, 2 = often true, and 3 = almost always true. Group average SRS Total t‐scores, a summary of participants with t‐scores across the mild, moderate, and severe ranges, and total raw and subscale scores with participant demographics are reported in Table 2. We used SRS raw scores in the current study as a proxy of ASD clinical features and to maximize phenotypic variability across our sample.
TABLE 2.
Demographics and SRS scores in N = 90 NPD (55 males) as well as within specific subgroups established from Total NPD sample
| Subgroups defined from Total sample | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total NPD sample N = 90 | NPD‐ASD n = 37 of 90 | NPD‐OTHER n = 53 of 90 | ||||||||||
|
|
Min | Max |
|
Min | Max |
|
Min | Max | ||||
| Age (in years) | 9.71 (4.50) | 2 | 18 | |||||||||
| SRS Total t‐score | 74.51 (13.93) | 40 | 105 | 82.52 (11.48) | 53 | 89 | 67.55 (12.21) | 50 | 72 | |||
| n = Mild range (T‐score 60 −65) | n = 6 | n = 1 | n = 5 | |||||||||
| n = Moderate (T‐score 66 −75) | n = 16 | n = 5 | n = 11 | |||||||||
| n = Severe (T‐score ≥ 76) | n = 23 | n = 16 | n = 7 | |||||||||
| SRS Raw scores (n = 52; Male:33) | ||||||||||||
| Total | 93.65 (33.53) | 11 | 161 | 114.39 (26.86) | 50 | 73 | 77.21 (29.12) | 50 | 64 | |||
| SCI | 75.90 (26.29) | 9 | 128 | 90.96 (21.50) | 50 | 85 | 63.97 (23.71) | 50 | 82 | |||
| RBRI | 17.75 (8.32) | 1 | 33 | 23.43 (6.67) | 50 | 78 | 13.24 (6.61) | 50 | 74 | |||
| Social awareness | 12.39 (4.11) | 3 | 22 | 14.74 (3.26) | 59 | 105 | 10.52 (3.77) | 40 | 89 | |||
| Social cognition | 18.98 (6.71) | 3 | 31 | 22.04 (4.47) | 55 | 88 | 22.04 (4.47) | 31 | 77 | |||
| Social communication | 31.14 (12.18) | 0 | 58 | 37.48 (10.35) | 57 | 83 | 26.10 (11.25) | 48 | 77 | |||
| Social motivation | 13.40 (6.11) | 2 | 27 | 16.69 (6.36) | 50 | 79 | 10.79 (4.49) | 50 | 74 | |||
In addition to clinical diagnoses and parent‐report measures, we curated outcomes of an additional available measure of co‐occurring clinical and behavioral features assessed via the Child Behavior Checklist (CBCL) from the EHR. See Table S2 for additional summary of demographic variables, including parent‐report measures and CBCL scores (school‐age and pre‐school ages). Although the CBCL scores were not part of our main hypotheses, we include them in Supporting Information to provide a more complete characterization of the range of this cohort's emotional and behavioral problems.
2.3. Analysis and statistical methods
Prior to carrying out formal analyses that addressed our primary research questions (outlined below), we assessed the distribution of our measures using a Shapiro–Wilk test of normality. PASS Total and subscale scores deviated from a normal distribution (p’s < .019, NS) with the exception of PASS (I) Mood, Behavior, and Emotion (p > .061, NS). Due to these results, non‐parametric tests were used to assess group comparisons. An alpha‐level of p < .05 was used across all analysis to identify significant results with appropriate correction for multiple comparisons for each stage of our analyses. We also assessed the inter‐correlations among PASS subscales in our reported cohort and noted significant relationships across all PASS subscales. Complete results from this analysis are reported in Table S3.
We analyzed participant data by grouping participants in several ways: (1) For cross‐sectional, between‐groups comparisons, we compared scores from the entire sample to a published reference sample of children with and without ASD from Ming et al. (2011) via Wilcoxon, exact sign procedures in R Studio with continuity correction for comparison between a continuous and discrete population variables. Since the reference sample included children in a 2–5 year age range (Ming et al., 2011) and the current sample included ages 2–18 years, we first confirmed that scores did not differ by age (see complete results from this analysis in Table S4). (2) In additional between‐groups comparisons, we also split the NPD group into two subgroups, those that had a clinical diagnosis of ASD (NPD‐ASD; n = 37) and those that had other, non‐ASD forms of NPDs (NPD‐OTHER; n = 53). Again, non‐parametric sign procedures were used for subgroup comparisons with appropriate corrections for multiple comparisons (i.e., Bonferroni methods were used for comparisons between NPD‐ASD and NPD‐OTHER; continuity correction was used for comparisons between the current cohort and previously published samples). Finally (3), across our larger N = 90 NPD cohort, we dimensionally assessed the relationship between individual differences in symptoms scales of ANS dysfunction and core clinical features of ASD using the SRS via partial correlation procedures controlling for chronological age and sex with correction for multiple comparisons via Bonferroni methods.
3. RESULTS
3.1. Differences in PASS scores in pediatric probands, children with ASD, and healthy controls
We first compared PASS scores collected in the N = 90 NPD group to a published reference sample of children with and without ASD (from Ming et al. (2011)). Children in the NPD group had higher PASS Total scores as compared to children with ASD (p = .003) and healthy controls (p < .0001) from the reference sample. This finding of higher PASS Total scores in the NPD group was also confirmed across all subscales (p’s < .041). See Table 3 for group average PASS scores and results of between group comparisons.
TABLE 3.
Pediatric Autonomic Symptom Scale (PASS) scores on N = 90 NPD identified based on having a genetic syndrome that confers increased risk for ASD or other neurodevelopmental diagnosis
| N = 90 NPD | Ming et al., 2011 | |||||||
|---|---|---|---|---|---|---|---|---|
| n = 18 ASD | n = 24 HC | |||||||
| 7 | Min | Max |
|
|
p‐value | |||
| Age | 9.71 (4.50) | 2 | 18 | |||||
| PASS | ||||||||
| PASS Total | 24.51 (11.14) | 3 | 68 | 20.6 (2.7) | 6.0 (0.8) | |||
|
Section I Mood, Behavior, and Emotion (MBE) |
9.26 (4.50) | 0 | 18 | 10.7 (0.8) | 2.6 (0.3) | |||
|
Section II Secretomotor and Sensory Integration (SS) |
6.37 (3.78) | 0 | 19 | 4.5 (0.8) | 1.5 (0.3) | |||
|
Section III Urinary and Gastrointestinal Systems (UG) |
4.81 (3.37) | 0 | 15 | 3.8 (0.7) | 1.3 (0.2) | |||
|
Section IV Circulation, Thermoregulation, and Sleep (CTS) |
4.08 (3.00) | 0 | 17 | 3.1 (0.7) | 0.9 (0.2) | |||
All p‐values reported below are based on results from Wilcoxon signed test with continuity correction.
Group comparisons between mean PASS scores in NPD in the current research as compared to mean PASS scores for the ASD group reported in Ming et al. (2011).
Group comparisons between mean PASS scores in NPD in the current research as compared to healthy controls reported in Ming et al. (2011).
p < 0.05;.
p < 0.001.
We also explored differences between subgroups of our cohort: those with a clinical diagnosis of ASD (NPD‐ASD, n = 37 of 90) versus those with non‐ASD NPD diagnoses (NPD‐OTHER, n = 53 of 90) (Table 4). NPD‐ASD had higher PASS Total scores as compared to NPD‐OTHER (p = .009) and higher scores relative to the published ASD reference sample (p = .0006). NPD‐ASD demonstrated significantly higher scores on the PASS (I) Mood, Behavior, and Emotion and (II) Secretomotor and Sensory Integration subscales as compared to NPD‐OTHER (p’s < .021), but not the other two PASS subscales (p’s > .516, NS). When compared to the Ming et al. (2011) ASD reference sample, NPD‐ASD also demonstrated significantly higher scores on the PASS (III) Secretomotor and Sensory Integration and (IV) Thermoregulation, Circulation, and Sleep subscales (p’s < .049), but not other subscales (p > .061, NS). Finally, no significant differences in PASS scores were found between NPD‐OTHER as compared to the Ming et al. (2011) ASD reference sample (p’s > .0625, NS), with the exception of the (I) Mood, Behavior, and Emotion subscale (p < .0001). Thus, symptoms of autonomic dysfunction, particularly in the (I) Mood, Behavior, and Emotion and (II) Secretomotor/Sensory Integration domains, are increased in children with a NPD‐related genetic etiology and an ASD diagnosis relative to those without ASD and compared to previously published samples of children with ASD of unknown etiology.
TABLE 4.
PASS scores in NPD probands with ASD (n = 37 NPD‐ASD), probands with other forms of NPD (n = 53 NPD‐OTHER), and ASD subsample reported in Ming et al. (2011)
|
n = 37 of 90 NPD‐ASD |
n = 53 of 90 NPD‐OTHER |
Ming et al. n = 18 ASD |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
Min | Max |
|
Min | Max |
|
p‐value | ||||
| Age | 9.78 (4.91) | 2 | 18 | 9.51 (4.29) | 2 | 18 | |||||
| PASS | |||||||||||
| PASS Total | 28.16 (11.75) | 7 | 68 | 21.96 (10.02) | 3 | 45 | 20.6 (2.7) |
c p = .5353, NS |
|||
|
Section I Mood, Behavior, and Emotion (MBE) |
11.32 (4.31) | 0 | 18 | 7.81 (4.08) | 0 | 18 | 10.7 (0.8) |
b p = .3043, NS |
|||
|
Section II Secretomotor and Sensory Integration (SS) |
7.37 (3.82) | 0 | 18 | 5.66 (3.63) | 1 | 19 | 4.5 (0.8) |
c p = .0625, NS |
|||
|
Section III Urinary and Gastrointestinal Systems (UG) |
5.19 (3.76) | 0 | 15 | 4.55 (3.09) | 0 | 12 | 3.8 (0.7) |
a p = .3945, NS b p = .0611, NS c p = .1434, NS |
|||
|
Section IV Circulation, Thermoregulation, and Sleep (CTS) |
4.27 (2.99) | 0 | 17 | 3.94 (3.03) | 0 | 14 | 3.1 (0.7) |
a p = .6132, NS c p = .2676, NS |
|||
Group comparisons between mean PASS scores in probands with ASD (NPD‐ASD) as compared to mean PASS scores in probands with other forms of neurodevelopmental disorders (NPD‐OTHER). p‐Values reported adjusted via Bonferroni methods.
Group comparisons between mean PASS scores in probands with ASD (NPD‐ASD) as compared to individuals with ASD reported in Ming et al. (2011). p‐Values reported based on Wilcoxon signed test with continuity correction.
Group comparisons between mean PASS scores in probands with other forms of neurodevelopmental disorders (NPD‐OTHER) and individuals with ASD reported in Ming et al. (2011). p‐Values reported based on Wilcoxon signed test with continuity correction.
p < .05;
p < .001.
3.2. Individual differences in autonomic features associated with core clinical ASD features
Next, we assessed the relationship between autonomic features and quantitative traits of ASD across our NPD cohort by characterizing the relationship between the PASS and available SRS scores from n = 52 of 90 NPD (see Table 5) via partial correlation controlling for the combined effects of age and sex. PASS Total scores were significantly associated with SRS Total raw scores (r = 0.723, p < .0001) as well as all subscale scores (p’s < .0001) (see Figure 2). PASS (I) Mood, Behavior, and Emotion and (II) Secretomotor/Sensory Integration subscales were significantly related to Total and subscale SRS raw scores (p’s < .007). PASS (III) Urinary/Gastrointestinal Systems subscale scores were significantly related to SRS RRB and Social Awareness scores (p’s < .038), but no other SRS subscale scores. Finally, PASS (IV) Circulation, Thermoregulation, Sleeping Patterns, and Breathing subscale scores were significantly related to SRS Total raw scores (r = 0.384, p < .006) as well as subscale scores (p’s < .023) with the exception of Social Awareness and Social Motivation scores. See Table 5 for complete results from all correlation analyses between PASS and SRS raw scores. Finally, we also assessed the relationship between PASS and SRS scores separately within each of the NPD‐ASD and NPD‐OTHER subgroups. Complete results from this analysis can be found Tables S5 and S6.
TABLE 5.
Partial correlation between SRS raw scores and PASS (age, sex) in NPD with genetic syndrome; corrected p‐values reported below
| PASS | PASS Section I | PASS Section II | PASS Section III | PASS Section IV | |||
|---|---|---|---|---|---|---|---|
| Total | MBE | SS | UG | CTS | |||
| SRS‐2 (Raw scores) | |||||||
| Total Score | 0.723, p < .0001 ** | 0.751, p < .0001 ** | 0.600, p < .0001 ** | 0.254, p = .076 | 0.384, p = .006 * | ||
| SCI | 0.702, p < .0001 ** | 0.732, p < .0001 ** | 0.586, p = .0001 ** | 0.230, p = .108 | 0.388, p = .005 * | ||
| RBRI | 0.692, p < .0001 ** | 0.713, p < .0001 ** | 0.562, p = .0002 ** | 0.295, p = .038 * | 0.320, p = .023 * | ||
| Social awareness | 0.569, p < .0001 ** | 0.575, p = .0001 ** | 0.499, p = .0002 ** | 0.294, p = .038 * | 0.165, p = .252 | ||
| Social cognition | 0.715, p < .0001 ** | 0.765, p < .0001 ** | 0.582, p = .0001 ** | 0.188, p = .191 | 0.443, p = .001 ** | ||
| Social communication | 0.689, p < .0001 ** | 0.660, p < .0001 ** | 0.587, p = .0001 ** | 0.268, p = .059 | 0.412, p = .003 * | ||
| Social motivation | 0.478, p < .0001 ** | 0.604, p < .0001 ** | 0.374, p = .007 * | 0.052, p = .717 | 0.249, p = .081 | ||
Corrected p‐values reported below.
MBE, Mood, Behavior, and Emotion; SS, Secretomotor/Sensory Integration; UG, Urinary/Gastrointestinal Systems; CTS, Circulation, Thermoregulation, Sleeping Patterns, and Breathing.
p < 0.05;
p < 0.001.
FIGURE 2.
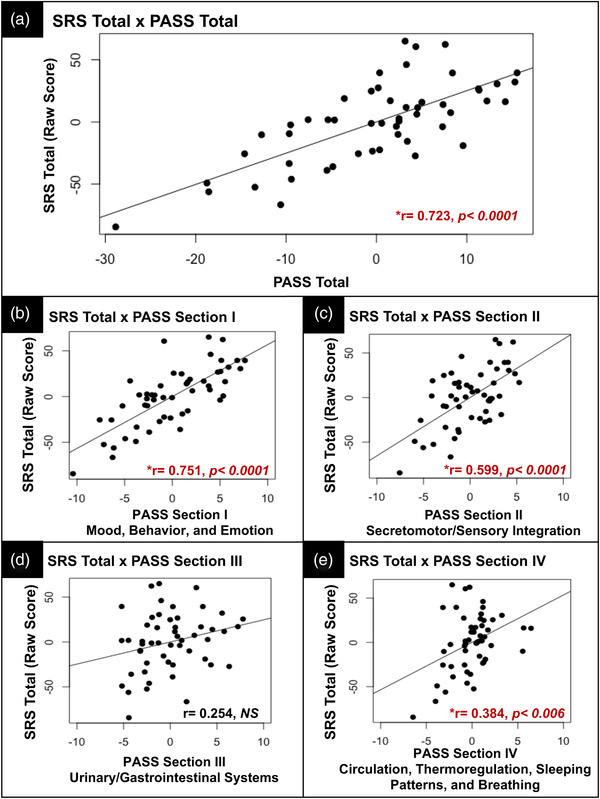
Results from Pearson, pairwise comparisons between SRS total (raw scores) and PASS total (A) and subscale scores (B–E)
4. DISCUSSION
The current investigation aimed to assess features of autonomic dysfunction in children with an NPD‐related genetic etiology. We find elevated symptoms of autonomic dysfunction in this cohort, including those with ASD, as compared to a reference sample of children with ASD of unknown etiology and healthy controls (Ming et al., 2011). Our reported results align with current research on symptoms indicative of autonomic dysfunction such as disrupted sleep patterns as well as gastrointestinal disturbances in NPD‐related genetic disorders, including 16p11.2 del and dup syndrome, Smith Magenis Syndrome, and 15q duplication syndrome, all of which were represented within our current cohort (Brunetti‐Pierri et al., 2008; Cerminara et al., 2021; Kamara et al., 2021; Leader et al., 2021; Shayota & Elsea, 2019). Previous work in ASD either has relied only on clinical diagnoses for inclusion into a study (ignoring genetic etiology) or focused on one‐specific genetic etiology and associated NPD diagnoses (Angkustsiri et al., 2014; Fine et al., 2005; Kumar et al., 2008; Martin et al., 2020; Moreno‐De‐Luca et al., 2013; Moreno‐De‐Luca et al., 2015). Because individual NPD‐related genetic disorders are individually rare, amassing a large cohort with the same genetic etiology is challenging. Scalable methods such as meta‐analyses and curation of existing phenotypic data in larger samples will be necessary to move the field forward toward identifying behavioral domains that may be specifically impacted based on distinct chromosomal anomalies.
While we have ruled out the possible effect of age as a reason for the significant differences in PASS scores within our NPD cohort and a younger reference sample of children with ASD (Ming et al., 2011), there are other possible reasons for our reported results. In addition to being a larger cohort that includes a wider age range, our sample includes a broad spectrum of clinical diagnoses and genetic etiologies. It is also important to highlight the broad scope of ASD traits, assessed via the SRS, in our NPD cohort and across those with and without ASD. SRS raw scores were used in analyses as a measure of ASD traits in order to maximize phenotypic variability observed across our sample. Less than 25% of our NPD group presented with symptoms in the severe range according to SRS T‐scores (see Table 2) and several individuals had co‐occurring diagnoses of mild‐to‐severe intellectual disability (see Table S1). While phenotypic information was not reported as a part of Ming et al. (2011), the current investigation includes individuals from across the diagnostic spectrum, especially those with a more severe clinical presentation (i.e., those that would be clinically described as lower functioning). The wide range of phenotypic traits, ranging from mild to severe, was also critical in analysis regarding the association between SRS and PASS scores. Thus, the heterogeneous and variable phenotypes, including the various genetic etiologies, present within our reported cohort are a major distinction between the current investigation and previous studies using the PASS (Ming et al., 2011). However, it is important to note that comorbidities and genetic diagnoses (with the exception of those with a targeted genetic etiology associated with familial dysautonomia) were not reported in previous published work (i.e., Ming et al., 2011).
Recent research has emphasized the important role of genetics in understanding the variable development and expression of core clinical features of ASD and other NPD. For example, the phenotypic outcomes of high‐penetrance genetic disorders (such as CNVs or single gene disorders) that cause NPD are influenced by other genetic background variance (Finucane et al., 2016; Moreno‐De‐Luca et al., 2015). Recent work on transdiagnostic traits, such as "externalizing" behaviors (Linnér et al., 2019) have proven to be successful at capturing larger amounts of genetic variance than models that strictly rely on clinical diagnostic categories. Thus, there is growing consensus that clinical nosology may need to be set aside in favor of transdiagnostic traits (Dalgleish et al., 2020). Despite this growing realization, research on psychiatric and neurodevelopmental traits has continued to be siloed based on clinically defined diagnostic boundaries. Other work has taken a genetics‐first approach to understanding gene‐behavior relationships (Moreno‐De‐Luca et al., 2015), but these two approaches (diagnosis/phenotype approach vs. genotype‐first approach) can be challenging to marry in real‐world clinical settings. Here, we demonstrate a strategy for sampling and analysis of a clinically heterogeneous population that, if applied to future studies, may help the field overcome limitations of small sample sizes when focusing on homogeneous and rare disease groups. The concurrent use of clinical data, genetic diagnoses, and transdiagnostic trait assessment has the potential to inform correlations between pathogenic variants associated with NPDs and patient phenotypes (Kothari et al., 2018).
The work presented here uses a broad sampling approach that aimed to address whether there is a relationship between ANS dysfunction and ASD traits, while also using a real‐world heterogeneous, genetically characterized cohort. From our perspective, individual differences in autonomic features may represent a measurable, transdiagnostic trait domain that is more closely linked to underlying mechanistic drivers of atypical neurodevelopmental features. Outside of experimental studies of functional autonomic processes (Condy et al., 2017; Kushki et al., 2013; Kushki et al., 2014; Patriquin et al., 2013), features of ANS dysfunction have not been quantitatively assessed across ASD and other NPDs. To measure individual differences in autonomic features, we make use of a parent‐report measure, the PASS. We report a linear relationship between features of autonomic dysfunction (PASS Total and subscale scores) and ASD traits across probands with an identified NPD‐related genetic etiology. In addition to elevated autonomic symptoms across PASS subdomains, individual differences in autonomic features were significantly associated with clinically significant ASD traits. We wish to acknowledge that characterizing the causal relationship between psychosocial impairments and core clinical features of ASD and atypical autonomic processes is beyond the scope of the current study. However, given previous research on autonomic dysfunction in ASD and other studies linking specific autonomic processes such as HRV with psychosocial impairment and emotion regulation (Appelhans & Luecken, 2006; Bunford et al., 2017; Patriquin et al., 2013; Patriquin et al., 2019; Quintana et al., 2012; Thapa et al., 2021), this and the current results provide strong evidence for future work directly assessing the causality of autonomic function as a biological driver for neurodevelopmental traits.
Although this work is the first to capture the PASS scales in patients with a range of NPD, including ASD as well as other neurodevelopmental disorders, other measures have been used to assess symptoms that have been linked with autonomic processes (Bernstein et al., 1997; Woodard et al., 2012). Previous work that has used functional autonomic indices within an experimental context is able to determine whether there is increased or decreased ANS activity in ASD. However, existing results are discordant (Kushki et al., 2014; Wang et al., 2016), with some studies identifying increased sympathetic versus parasympathetic activity in ASD and related NPDs and others finding the opposite relationship (Anderson & Colombo, 2009; Billeci et al., 2018; Condy et al., 2017; Klusek et al., 2015; Neuhaus et al., 2014; Patriquin et al., 2013; Patriquin et al., 2019; Wang et al., 2016). Previous research has also investigated distinct autonomic symptoms (i.e., gastrointestinal problems and sleep disturbances assessed via parent‐report), in conjunction with core features of ASD, as a possible way to stratify clinically meaningful subgroups (Unwin et al., 2013). However, in the current investigation and based on the high inter‐correlations between PASS subscales (see Table S4), it is unclear whether distinct autonomic phenotypes are presented within our reported clinical cohort or if ASD symptoms are more broadly associated with autonomic dysregulation. Furthermore, the PASS does not allow us to specify increased or decreased activity across organ systems or within specific autonomic processes (i.e., sympathetic vs. parasympathetic). Thus, although this work represents the potential for expanded use of the PASS as a quantitative index of autonomic features, additional research in larger samples that links autonomic symptoms, ASD traits, and functional metrics of ANS dysfunction will be an important next step to (i) characterize autonomic function as a biological driver for atypical neurodevelopment and (ii) identify distinct autonomic processes that may differentiate clinical subtypes in neurodevelopmental populations including ASD.
Our sample was characterized by a combination of CNVs and single gene disorders, with a few CNVs with uncertain pathogenicity. Advances in clinical genomic testing have identified numerous pathogenic variants causative for ASD and other complex neurodevelopmental and psychiatric phenotypes; however, characterization of variants of uncertain significance and the discovery of genes and mechanistic pathways connected to neural, cognitive, and biological processes are ongoing (De Rubeis et al., 2014; Iossifov et al., 2014). Genetic variants associated with ASD also confer risk for other neuropsychiatric conditions, medical problems, and other diseases (i.e., ataxias, altered growth patterns, hearing and/or visual impairments) and atypical function in various organ systems regulated by the ANS (Cappuccio et al., 2016; Daghsni et al., 2018; Lecavalier et al., 2019; Robinson et al., 2016; Talkowski et al., 2011; Zhang et al., 2021). The continued study and characterization of symptom dimensions that may be transdiagnostic is an important next step in understanding the underlying biology of known and emerging genetic disorders with complex neurodevelopmental phenotypes (Savatt & Myers, 2021; Srivastava et al., 2019).
This study has several limitations. The current sample included a broad age range that extended beyond previously published results that used the PASS in younger children (Ming et al., 2011). We explored potential age‐related differences in PASS scores across our sample and were unable to identify a main effect of age. Thus, our results represent an expanded approach and increase the generalizability of our findings. While this may be viewed as a relative strength, it is important to acknowledge that the original published use of the PASS was in a much narrower and younger age group (1). In future iterations or as a part of larger studies, the item structure and wording within specific PASS questions should be considered and adapted as needed for a wider age range and to be more developmentally appropriate (i.e., “Does he or she line up toys or other objects…?; Does your child ever tell you he/she feels ‘‘big in the tummy” or bloated?). We also wish to acknowledge that our study design and the included sample were not directly based on a priori power analyses and/or sample size calculations. The current work was supported by an administrative supplement as a part of ongoing NIH R01 (see funding information listed in Acknowledgments) focused on characterizing the impact of CNVs and other genetic variants on neurodevelopmental traits). As stated above, measures of interest (i.e., the PASS) were included in an online phenotyping battery that was administered to already consented parents/caregivers. Thus, we acknowledge that our reported results are based on a convenience sample; however, our included sample sizes are larger than previously published reference samples highlighted in the current investigation (Ming et al., 2011). Additionally, a primary objective of the current study was to quantitatively assess individual differences in autonomic symptoms across multiple organ systems in a heterogeneous cohort, a method and research objective that is in contrast to other studies that have investigated isolated processes of ANS function (i.e., HRV, tonic pupil size) and in smaller samples (Anderson & Colombo, 2009; Condy et al., 2017; Kushki et al., 2014; Ming et al., 2005). Despite this, it will be critical in future studies to perform appropriate sample size calculations and power analyses in order to accurately address research objective and maximize effect sizes. Also due to the nature of the ongoing research funding and the corresponding project, we relied on a combination of parent‐report medical history and clinical data from individuals’ EHR documentation from expert neurodevelopmental diagnosticians at our clinic. This strategy allowed us to eliminate time and participant burden typically required for in‐person testing, but limited comprehensive assessment of other traits, such as cognitive ability. Although this is certainly a limitation, this strategy does allow for inclusion of a more diverse neurodevelopmental population, including some children that may be unable to complete in‐person research testing due to more profound impairment. In contrast to studies that tend to rely on in‐person human subjects testing methods, the strategy used here is amenable to cost‐effective scaling and can be more inclusive to those with more severe cognitive and behavioral impairments that tend to be excluded from traditional in‐person research testing. Furthermore, this approach allows for the capture of meaningful variability in autonomic processes across the full spectrum of NPD. Finally, we wish to acknowledge that the current investigation did not include specific subgroup analyses of genetic variants included in our cohort that have previously been reported to present with atypical autonomic features (Brunetti‐Pierri et al., 2008; Cerminara et al., 2021; Kamara et al., 2021; Leader et al., 2021; Shayota & Elsea, 2019) (e.g., comparing PASS scores and/or assessing PASSxSRS correlations between targeted genetic variants). Smaller samples sizes, even in the more highly represented genetic variants within our cohort (i.e., 16p11.2 del syndrome, n = 14), left us underpowered to draw meaningful group comparisons and identify significant results. While collapsing deletions and duplications within some of our variant subgroups would increase our n’s and power in identifying significant group differences, these alterations are complex with deletions and duplications often leading to highly variable phenotypes (Chawner et al., 2021; Kates et al., 2007; Wenger et al., 2016) and clinical features even within the same variant. Thus, while we see this as an exciting future direction for this work and a critical next step in characterizing the genetic contribution to autonomic dysfunction in neurodevelopmental populations, these analyses were beyond the scope of the current study.
This study quantifies symptoms of autonomic dysfunction in a heterogeneous, neurodevelopmental pediatric cohort characterized based on both clinical diagnoses as well as clinical genetic etiology. Thus, our sampling methods and study findings move toward the characterization of autonomic dysfunction as a transdiagnostic trait within atypical neurodevelopment, including ASD, rather than within a specific diagnostic group (Anderson & Colombo, 2009; Billeci et al., 2018; Condy et al., 2017; Ming et al., 2005; Pace et al., 2016). Results from the current research lay the groundwork for future studies aimed at characterizing individual differences in atypical autonomic processes as important transdiagnostic traits that are associated with clinically significant neurodevelopmental symptoms. Further characterization of the link between ANS functional, neurodevelopmental, and genetic differences is an important next step toward understanding the biological pathways underlying NPD and may lead to the identification of clinical subtypes and objective biomarkers, based on symptomology and genetic etiology that are amenable to novel treatment targets.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
DiCriscio and Troiani had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: DiCriscio and Troiani. Acquisition, analysis, or interpretation of data: DiCriscio, Smith, Beiler, Walsh, Holdren, Wain, and Troiani. Drafting of the manuscript: DiCriscio, Smith, and Troiani. Critical revision of the manuscript for important intellectual content: DiCriscio, Wain, and Troiani. Statistical analysis: DiCriscio. Obtained funding: DiCriscio and Troiani. Administrative, technical, or material support: DiCriscio, Smith, Beiler, Walsh, Holdren, Wain, and Troiani.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.2813
Supporting information
Supplemental Table 1: Clinical diagnosis in NPD‐ASD and NPD‐OTHER subgroups
Supplemental Table 2: Demographics and sample characteristics
Supplemental Table 3: Age based analysis of PASS
Supplemental Table 4: Inter‐correlations between PASS sections
Supplemental Table 5: PASS and SRS correlations, NPD‐ASD
Supplemental Table 6: PASS and SRS correlations, NPD‐OTHER
ACKNOWLEDGMENTS
We would like to extend our gratitude to the patients and families who made this research possible. The authors are grateful to Dr. Christa Martin, PhD, FACMG, Chief Scientific Officer of Geisinger Research, Professor and Director of Geisinger ADMI. This study was funded by an NIH R01 Administrative Supplement (NIMH 3R01 107431‐04S1) awarded to Vanessa Troiani and Antoinette DiCriscio, and Martin as a part of a parent NIMH R01 (PI: Martin; NIMH 3R01 MH107431‐03).
DiCriscio, A. S. , Wain, K. E. , Smith, J. , Beiler, D. , Walsh, L. K. , Holdren, K. , & Troiani, V. (2022). Higher scores on autonomic symptom scales in pediatric patients with neurodevelopmental disorders of known genetic etiology. Brain and Behavior, 12, e2813. 10.1002/brb3.2813
Contributor Information
Antoinette S DiCriscio, Email: asdicriscio@geisinger.edu.
Vanessa Troiani, Email: vtroiani@geisinger.edu.
DATA AVAILABILITY STATEMENT
Data are available on request due to privacy/ethical restrictions
REFERENCES
- Adler, B. L. , Russell, J. W. , Hummers, L. K. , & McMahan, Z. H. (2018). Symptoms of autonomic dysfunction in systemic sclerosis assessed by the COMPASS‐31 questionnaire. Journal of Rheumatology, 45(8), 1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldinger, K. A. , Lane, C. J. , Veenstra‐VanderWeele, J. , & Levitt, P. (2015). Patterns of risk for multiple co‐occurring medical conditions replicate across distinct cohorts of children with autism spectrum disorder. Autism Research, 8(6), 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, C. J. , & Colombo, J. (2009). Larger tonic pupil size in young children with autism spectrum disorder. Developmental Psychobiology, 51(2), 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angkustsiri, K. , Goodlin‐Jones, B. , Deprey, L. , Brahmbhatt, K. , Harris, S. , & Simon, T. J. (2014). Social impairments in chromosome 22q11. 2 deletion syndrome (22q11. 2DS): Autism spectrum disorder or a different endophenotype? Journal of Autism and Developmental Disorders, 44, 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhans, B. M. , & Luecken, L. J. (2006). Heart rate variability as an index of regulated emotional responding. Review of General Psychology, 10(3), 229. [Google Scholar]
- Aziz, N. A. , Anguelova, G. V. , Marinus, J. , Van Dijk, J. G. , & Roos, R. A. C. (2010). Autonomic symptoms in patients and pre‐manifest mutation carriers of Huntington's disease. European Journal of Neurology, 17(8), 1068–1074. [DOI] [PubMed] [Google Scholar]
- Bellato, A. , Arora, I. , Hollis, C. , & Groom, M. J (2020). Is autonomic nervous system function atypical in attention deficit hyperactivity disorder (ADHD)? A systematic review of the evidence. Neuroscience and Biobehavioral Reviews, 108, 182–206. [DOI] [PubMed] [Google Scholar]
- Bernstein, G. A. , Massie, E. D. , Thuras, P. D. , Perwien, A. R. , Borchardt, C. M. , & Crosby, R. D. (1997). Somatic symptoms in anxious‐depressed school refusers. Journal of the American Academy of Child and Adolescent Psychiatry, 36(5), 661–668. [DOI] [PubMed] [Google Scholar]
- Bharath, R. , Moodithaya, S. S. , Bhat, S. U. , Mirajkar, A. M. , & Shetty, S. B. (2019). Comparison of physiological and biochemical autonomic indices in children with and without autism spectrum disorders. Medicina, 55(7), 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeci, L. , Tonacci, A. , Narzisi, A. , Manigrasso, Z. , Varanini, M. , Fulceri, F. , Lattarulo, C. , Calderoni, S. , & Muratori, F. (2018). Heart rate variability during a joint attention task in toddlers with autism spectrum disorders. Frontiers in Physiology, 9, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti‐Pierri, N. , Berg, J. S. , Scaglia, F. , Belmont, J. , Bacino, C. A. , Sahoo, T. , Lalani, S. R. , Graham, B. , Lee, B. , Shinawi, M. , Shen, J. , Kang, S. H. , Pursley, A. , Lotze, T. , Kennedy, G. , Lansky‐Shafer, S. , Weaver, C. , Roeder, E. R. , Grebe, T. A. , … Patel, A. (2008). Recurrent reciprocal 1q21. 1 deletions and duplications associated with microcephaly or macrocephaly and developmental and behavioral abnormalities. Nature Genetics, 40(12), 1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunford, N. , Evans, S. W. , Zoccola, P. M. , Owens, J. S. , Flory, K. , & Spiel, C. F (2017). Correspondence between heart rate variability and emotion dysregulation in children, including children with ADHD. Journal of Abnormal Child Psychology, 45(7), 1325–1337. [DOI] [PubMed] [Google Scholar]
- Cappuccio, G. , Vitiello, F. , Casertano, A. , Fontana, P. , Genesio, R. , Bruzzese, D. , Ginocchio, V. M. , Mormile, A. , Nitsch, L. , Andria, G. , & Melis, D. (2016). New insights in the interpretation of array‐CGH: Autism spectrum disorder and positive family history for intellectual disability predict the detection of pathogenic variants. Italian Journal of Pediatrics, 42(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerminara, M. , Spirito, G. , Pisciotta, L. , Squillario, M. , Servetti, M. , Divizia, M. T. , Lerone, M. , Berloco, B. , Boeri, S. , Nobili, L. , Vozzi, D. , Sanges, R. , Gustincich, S. , & Puliti, A. (2021). Case report: Whole exome sequencing revealed disease‐causing variants in two genes in a patient with autism spectrum disorder, intellectual disability, hyperactivity, sleep and gastrointestinal disturbances. Frontiers in Genetics, 12, 625564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawner, S. J. , Doherty, J. L. , Anney, R. J. , Antshel, K. M. , Bearden, C. E. , Bernier, R. , Chung, W. K. , Clements, C. C. , Curran, S. R. , Cuturilo, G. , Fiksinski, A. M. , Gallagher, L. , Goin‐Kochel, R. P. , Gur, R. E. , Hanson, E. , Jacquemont, S. , Kates, W. R. , Kushan, L. , Maillard, A. M. , … van den Bree, M. B. M. (2021). A genetics‐first approach to dissecting the heterogeneity of autism: Phenotypic comparison of autism risk copy number variants. American Journal of Psychiatry, 178(1), 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshire, W. P. (2012). Highlights in clinical autonomic neuroscience: New insights into autonomic dysfunction in autism. Autonomic Neuroscience, 171(1), 4–7. [DOI] [PubMed] [Google Scholar]
- Condy, E. E. , Scarpa, A. , & Friedman, B. H. (2017). Respiratory sinus arrhythmia predicts restricted repetitive behavior severity. Journal of Autism and Developmental Disorders, 47(9), 2795–2804. [DOI] [PubMed] [Google Scholar]
- Constantino, J. N. , & Frazier, T. W. (2013). Commentary: The observed association between autistic severity measured by the social responsiveness scale (SRS) and general psychopathology – a response to. Journal of Child Psychology and Psychiatry and Allied Disciplines, 54(6), 695–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino, J. N. , & Gruber, C. P. (2005). Social responsive scale (SRS) manual. In Volkmar, F. R. (Ed.) Encyclopedia of autism spectrum disorders. Springer. [Google Scholar]
- Constantino, J. N. , & Gruber, C. P. (2012). Social responsiveness scale: SRS‐2 software kit. Western Psychological Services. [Google Scholar]
- Cross‐Disorder Group of the Psychiatric Genomics Consortium . (2013). Identification of risk loci with shared effects on five major psychiatric disorders: A genome‐wide analysis. Lancet Lond Engl, 381(9875), 1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daghsni, M. , Rima, M. , Fajloun, Z. , Ronjat, M. , Brusés, J. L. , M'rad, R. , & De Waard, M. (2018). Autism throughout genetics: Perusal of the implication of ion channels. Brain and Behavior, 8(8), e00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish, T. , Black, M. , Johnston, D. , & Bevan, A. (2020). Transdiagnostic approaches to mental health problems: Current status and future directions. Journal of Consulting and Clinical Psychology, 88(3), 179–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damian, A. , Adler, C. H. , Hentz, J. G. , Shill, H. A. , Caviness, J. N. , Sabbagh, M. N. , Evidente, V. G. , Beach, T. G. , & Driver‐Dunckley, E. (2012). Autonomic function, as self‐reported on the SCOPA‐autonomic questionnaire, is normal in essential tremor but not in parkinson's disease. Parkinsonism & Related Disorders, 18(10), 1089–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Osso, L. , Massoni, L. , Battaglini, S. , Cremone, I. M. , Carmassi, C. , & Carpita, B. (2022). Biological correlates of altered circadian rhythms, autonomic functions and sleep problems in autism spectrum disorder. Annals of General Psychiatry, 21(1), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rubeis, S. , He, X. , Goldberg, A. P. , Poultney, C. S. , Samocha, K. , Cicek, A. E. , Kou, Y. , Liu, L. , Fromer, M. , Walker, S. , Singh, T. , Klei, L. , Kosmicki, J. , Shih‐Chen, F. , Aleksic, B. , Biscaldi, M. , Bolton, P. F. , Brownfeld, J. M. , Cai, J. , … & Buxbaum, J. D. (2014). Synaptic, transcriptional and chromatin genes disrupted in autism. Nature, 515(7526), 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devnani, P. A. , & Hegde, A. U (2015). Autism and sleep disorders. Journal of Pediatric Neurosciences, 10(4), 304–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinalankara, D. M. , Miles, J. H. , Nicole Takahashi, T. , & Yao, G. (2017). Atypical pupillary light reflex in 2–6‐year‐old children with autism spectrum disorders. Autism Research, 10(5), 829–838. [DOI] [PubMed] [Google Scholar]
- Fine, S. E. , Weissman, A. , Gerdes, M. , Pinto‐Martin, J. , Zackai, E. H. , McDonald‐McGinn, D. M. , & Emanuel, B. S. (2005). Autism spectrum disorders and symptoms in children with molecularly confirmed 22q11.2 deletion syndrome. Journal of Autism and Developmental Disorders, 35(4), 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane, B. , Challman, T. D. , Martin, C. L. , & Ledbetter, D. H. (2016). Shift happens: Family background influences clinical variability in genetic neurodevelopmental disorders. Genetics in Medicine, 18(4), 302–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriels, R. L. , Agnew, J. A. , Pan, Z. , Holt, K. D. , Reynolds, A. , & Laudenslager, M. L (2013). Elevated repetitive behaviors are associated with lower diurnal salivary cortisol levels in autism spectrum disorder. Biological Psychology, 93(2), 262–268. [DOI] [PubMed] [Google Scholar]
- Gentes, E. L. , & Ruscio, A. M. (2011). A meta‐analysis of the relation of intolerance of uncertainty to symptoms of generalized anxiety disorder, major depressive disorder, and obsessive–compulsive disorder. Clinical Psychology Review, 31(6), 923–933. [DOI] [PubMed] [Google Scholar]
- Germain, A. , & Kupfer, D. J. (2008). Circadian rhythm disturbances in depression. Human Psychopharmacology: Clinical and Experimental, 23(7), 571–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin, M. S. , Groden, J. , Velicer, W. F. , Lipsitt, L. P. , Baron, M. G. , Hofmann, S. G. , & Groden, G. (2006). Cardiovascular arousal in individuals with autism. Focus on Autism and Other Developmental Disabilities, 21(2), 100–123. [Google Scholar]
- Gudmundsson, O. O. , Walters, G. B. , Ingason, A. , Johansson, S. , Zayats, T. , Athanasiu, L. , Sonderby, I. E. , Gustafsson, O. , Nawaz, M. S. , Jonsson, G. F. , Jonsson, L. , Knappskog, P. M. , Ingvarsdottir, E. , Davidsdottir, K. , Djurovic, S. , Knudsen, G. P. S. , Askeland, R. B. , Haraldsdottir, G. S. , Baldursson, G. , … Stefansson, K. (2019). Attention‐deficit hyperactivity disorder shares copy number variant risk with schizophrenia and autism spectrum disorder. Translational Psychiatry, 9(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, E. , Bernier, R. , Porche, K. , Jackson, F. I. , Goin‐Kochel, R. P. , Snyder, L. G. , Snow, A. V. , Wallace, A. S. , Campe, K. L. , Zhang, Y. , Chen, Q. , D'Angelo, D. , Moreno‐De‐Luca, A. , Orr, P. T. , Boomer, K. B. , Evans, D. W. , Kanne, S. , Berry, L. , Miller, F. K. , … Chung, W. K. (2015). The cognitive and behavioral phenotype of the 16p11.2 deletion in a clinically ascertained population. Biological Psychiatry, 77(9), 785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, V. W. , Sarachana, T. , Kim, K. S. , Nguyen, A. , Kulkarni, S. , Steinberg, M. E. , Luu, T. , Lai, Y. , & Lee, N. H (2009). Gene expression profiling differentiates autism case–controls and phenotypic variants of autism spectrum disorders: Evidence for circadian rhythm dysfunction in severe autism. Autism Research, 2(2), 78–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov, I. , O'roak, B. J. , Sanders, S. J. , Ronemus, M. , Krumm, N. , Levy, D. , Stessman, H. A. , Witherspoon, K. T. , Vives, L. , Patterson, K. E. , Smith, J. D. , Paeper, B. , Nickerson, D. A. , Dea, J. , Dong, S. , Gonzalez, L. E. , Mandell, J. D. , Mane, S. M. , Murtha, M. T. , … Wigler, M. (2014). The contribution of de novo coding mutations to autism spectrum disorder. Nature, 515(7526), 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamara, D. , De Boeck, P. , Lecavalier, L. , Neuhaus, E. , & Beauchaine, T. P. (2021). Characterizing sleep problems in 16p11. 2 deletion and duplication. Journal of Autism and Developmental Disorders, 1–14. [DOI] [PubMed] [Google Scholar]
- Kates, W. R. , Antshel, K. M. , Fremont, W. P. , Shprintzen, R. J. , Strunge, L. A. , Burnette, C. P. , & Higgins, A. M. (2007). Comparing phenotypes in patients with idiopathic autism to patients with velocardiofacial syndrome (22q11 DS) with and without autism. American Journal of Medical Genetics. Part A, 143(22), 2642–2650. [DOI] [PubMed] [Google Scholar]
- Keith, J. M. , Jamieson, J. P. , & Bennetto, L. (2019). The importance of adolescent self‐report in autism spectrum disorder: Integration of questionnaire and autonomic measures. Journal of Abnormal Child Psychology, 47(4), 741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek, J. , Roberts, J. E. , & Losh, M. (2015). Cardiac autonomic regulation in autism and fragile x syndrome: A review. Psychological Bulletin, 141(1), 141–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotagal, S. , & Broomall, E. (2012). Sleep in children with autism spectrum disorder. Pediatric Neurology, 47(4), 242–251. [DOI] [PubMed] [Google Scholar]
- Kothari, C. , Wack, M. , Hassen‐Khodja, C. , Finan, S. , Savova, G. , O'Boyle, M. , Bliss, G. , Cornell, A. , Horn, E. J. , Davis, R. , Jacobs, J. , Kohane, I. , & Avillach, P. (2018). Phelan‐McDermid syndrome data network: Integrating patient reported outcomes with clinical notes and curated genetic reports. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 177(7), 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, R. A. , KaraMohamed, S. , Sudi, J. , Conrad, D. F. , Brune, C. , Badner, J. A. , Gilliam, T. C. , Nowak, N. J. , Cook, E. H., Jr , Dobyns, W. B. , & Christian, S. L. (2008). Recurrent 16p11.2 microdeletions in autism. Human Molecular Genetics, 17(4), 628–638. [DOI] [PubMed] [Google Scholar]
- Kushki, A. , Brian, J. , Dupuis, A. , & Anagnostou, E. (2014). Functional autonomic nervous system profile in children with autism spectrum disorder. Molecular Autism, 5(1), 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushki, A. , Drumm, E. , Mobarak, M. P. , Tanel, N. , Dupuis, A. , Chau, T. , & Anagnostou, E. (2013). Investigating the autonomic nervous system response to anxiety in children with autism spectrum disorders. PLoS ONE, 8(4), e59730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, L. P. , Richdale, A. L. , Haschek, A. , Flower, R. L. , Vartuli, J. , Arnold, S. R. , & Trollor, J. N. (2020). Cross‐sectional and longitudinal predictors of quality of life in autistic individuals from adolescence to adulthood: The role of mental health and sleep quality. Autism, 24(4), 954–967. [DOI] [PubMed] [Google Scholar]
- Leader, G. , Forde, J. , Naughton, K. , Maher, L. , Arndt, S. , & Mannion, A. (2021). Relationships among gastrointestinal symptoms, sleep problems, challenging behaviour, comorbid psychopathology and autism spectrum disorder symptoms in children and adolescents with 15q duplication syndrome. Jidr, 65(1), 32–46. [DOI] [PubMed] [Google Scholar]
- Lecavalier, L. , McCracken, C. E. , Aman, M. G. , McDougle, C. J. , McCracken, J. T. , Tierney, E. , Smith, T. , Johnson, C. , King, B. , Handen, B. , Swiezy, N. B. , Eugene Arnold, L. , Bearss, K. , Vitiello, B. , & Scahill, L. (2019). An exploration of concomitant psychiatric disorders in children with autism spectrum disorder. Comprehensive Psychiatry, 88, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, P. H. , Anttila, V. , Won, H. , Feng, Y. C. A. , Rosenthal, J. , Zhu, Z. , Tucker‐Drob, E. M. , Nivard, M. G. , Grotzinger, A. D. , Posthuma, D. , Wang, M. M.‐J. , Yu, D. , Walters, R. K. , Anney, R. J. L. , Duncan, L. E. , Ge, T. , Adolfsson, R. , Banaschewski, T. , Belangero, S. , … Weissman, M. M. (2019). Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell, 179(7), 1469–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnér, R. K. , Biroli, P. , Kong, E. , Meddens, S. F. W. , Wedow, R. , Fontana, M. A. , Lebreton, M. , Tino, S. P. , Abdellaoui, A. , Hammerschlag, A. R. , Nivard, M. G. , Okbay, A. , Rietveld, C. A. , Timshel, P. N. , Trzaskowski, M. , Vlaming, R. , Zünd, C. L. , Bao, Y. , Buzdugan, L. , … Beauchamp, J. P. (2019). Genome‐wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nature Genetics, 51(2), 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Hubbard, J. A. , Fabes, R. A. , & Adam, J. B. (2006). Sleep disturbances and correlates of children with autism spectrum disorders. Child Psychiatry and Human Development, 37(2), 179–191. [DOI] [PubMed] [Google Scholar]
- Lorsung, E. , Karthikeyan, R. , & Cao, R. (2021). Biological timing and neurodevelopmental disorders: A role for circadian dysfunction in autism spectrum disorders. Frontiers in Neuroscience, 15, 642745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney, A. E. , & McEvoy, P. M. (2012). A transdiagnostic examination of intolerance of uncertainty across anxiety and depressive disorders. Cognitive Behaviour Therapy, 41(3), 212–222. [DOI] [PubMed] [Google Scholar]
- Martin, C. L. , Wain, K. E. , Oetjens, M. T. , Tolwinski, K. , Palen, E. , Hare‐Harris, A. , Habegger, L. , Maxwell, E. K. , Reid, J. G. , Walsh, L. K. , Myers, S. M. , & Ledbetter, D. H. (2020). Identification of neuropsychiatric copy number variants in a health care system population. JAMA Psychiatry, 77(12), 1276–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes, S. D. , & Calhoun, S. L. (2009). Variables related to sleep problems in children with autism. Research in Autism Spectrum Disorders, 3(4), 931–941. [Google Scholar]
- Ming, X. , Bain, J. M. , Smith, D. , Brimacombe, M. , von‐Simson, G. G. , & Axelrod, F. B. (2011). Assessing autonomic dysfunction symptoms in children: A pilot study. Journal of Child Neurology, 26(4), 420–427. [DOI] [PubMed] [Google Scholar]
- Ming, X. , Julu, P. O. , Brimacombe, M. , Connor, S. , & Daniels, M. L. (2005). Reduced cardiac parasympathetic activity in children with autism. Brain & Development, 27(7), 509–516. [DOI] [PubMed] [Google Scholar]
- Moreno‐De‐Luca, A. , Evans, D. W. , Boomer, K. B. , Hanson, E. , Bernier, R. , Goin‐Kochel, R. P. , Myers, S. M. , Challman, T. D. , Moreno‐De‐Luca, D. , Slane, M. M. , Hare, A. E. , Chung, W. K. , Spiro, J. E. , Faucett, W. A. , Martin, C. L. , & Ledbetter, D. H. (2015). The role of parental cognitive, behavioral, and motor profiles in clinical variability in individuals with chromosome 16p11. 2 deletions. JAMA Psychiatry, 72(2), 119–126. [DOI] [PubMed] [Google Scholar]
- Moreno‐De‐Luca, A. , Myers, S. M. , Challman, T. D. , Moreno‐De‐Luca, D. , Evans, D. W. , & Ledbetter, D. H. (2013). Developmental brain dysfunction: Revival and expansion of old concepts based on new genetic evidence. Lancet Neurology, 12(4), 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlino, S. , Dordoni, C. , Sperduti, I. , Clark, C. J. , Piedimonte, C. , Fontana, A. , Colombi, M. , Grammatico, P. , Copetti, M. , & Castori, M. (2019). Italian validation of the functional difficulties questionnaire (FDQ‐9) and its correlation with major determinants of quality of life in adults with hypermobile Ehlers–Danlos syndrome/hypermobility spectrum disorder. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 180(1), 25–34. [DOI] [PubMed] [Google Scholar]
- Musiek, E. S. , Xiong, D. D. , & Holtzman, D. M. (2015). Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Experimental & Molecular Medicine, 47(3), e148–e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus, E. , Bernier, R. , & Beauchaine, T. P. (2014). Brief report: Social skills, internalizing and externalizing symptoms, and respiratory sinus arrhythmia in autism. Journal of Autism and Developmental Disorders, 44(3), 730–737. [DOI] [PubMed] [Google Scholar]
- Niklasson, L. , Rasmussen, P. , Óskarsdóttir, S. , & Gillberg, C. (2009). Autism, ADHD, mental retardation and behavior problems in 100 individuals with 22q11 deletion syndrome. Research in Developmental Disabilities, 30(4), 763–773. [DOI] [PubMed] [Google Scholar]
- Pace, M. , Dumortier, L. , Favre‐Juvin, A. , Guinot, M. , & Bricout, V. A. (2016). Heart rate variability during sleep in children with autism spectrum disorders. Physiology & Behavior, 167, 309–312. [DOI] [PubMed] [Google Scholar]
- Patriquin, M. A. , Hartwig, E. M. , Friedman, B. H. , Porges, S. W. , & Scarpa, A. (2019). Autonomic response in autism spectrum disorder: Relationship to social and cognitive functioning. Biological Psychology, 145, 185–197. [DOI] [PubMed] [Google Scholar]
- Patriquin, M. A. , Scarpa, A. , Friedman, B. H. , & Porges, S. W. (2013). Respiratory sinus arrhythmia: A marker for positive social functioning and receptive language skills in children with autism spectrum disorders. Developmental Psychobiology, 55(2), 101–112. [DOI] [PubMed] [Google Scholar]
- Poquérusse, J. , Azhari, A. , Setoh, P. , Cainelli, S. , Ripoli, C. , Venuti, P. , & Esposito, G. (2018). Salivary α‐amylase as a marker of stress reduction in individuals with intellectual disability and autism in response to occupational and music therapy. Jidr, 62(2), 156–163. [DOI] [PubMed] [Google Scholar]
- Quintana, D. S. , Guastella, A. J. , Outhred, T. , Hickie, I. B. , & Kemp, A. H. (2012). Heart rate variability is associated with emotion recognition: Direct evidence for a relationship between the autonomic nervous system and social cognition. International Journal of Psychophysiology, 86(2), 168–172. [DOI] [PubMed] [Google Scholar]
- Robinson, E. B. , St Pourcain, B. , Anttila, V. , Kosmicki, J. A. , Bulik‐Sullivan, B. , Grove, J. , Maller, J. , Samocha, K. E. , Sanders, S. J. , Ripke, S. , Martin, J. , Hollegaard, M. V. , Werge, T. , Hougaard, D. M. , Neale, B. M. , Evans, D. M. , Skuse, D. , Mortensen, P. B. , Daly, M. J. , iPSYCH‐SSI‐Broad Autism Group . (2016). Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nature Genetics, 48(5), 552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukmani, M. R. , Seshadri, S. P. , Thennarasu, K. , Raju, T. R. , & Sathyaprabha, T. N. (2016). Heart rate variability in children with attention‐deficit/hyperactivity disorder: A pilot study. Annals of Neurosciences, 23(2), 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylaarsdam, L. E. , & Guemez Gamboa, A. (2019). Genetic causes and modifiers in autism spectrum disorder. Frontiers in Cellular Neuroscience, 13, 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savatt, J. M. , & Myers, S. M. (2021). Genetic testing in neurodevelopmental disorders. Frontiers in Pediatrics, 9, 526779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgoifo, A. , Carnevali, L. , de los Pico Alfonso, M. A. , & Amore, M. (2015). Autonomic dysfunction and heart rate variability in depression. Stress (Amsterdam, Netherlands), 18(3), 343–352. [DOI] [PubMed] [Google Scholar]
- Shayota, B. J. , & Elsea, S. H. (2019). Behavior and sleep disturbance in smith‐magenis syndrome. Current Opinion in Psychiatry, 32(2), 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinawi, M. , Liu, P. , Kang, S. H. L. , Shen, J. , Belmont, J. W. , Scott, D. A. , Probst, F. J. , Craigen, W. J. , Graham, B. H. , Pursley, A. , Clark, G. , Lee, J. , Proud, M. , Stocco, A. , Rodriguez, D. L. , Kozel, B. A. , Sparagana, S. , Roeder, E. R. , McGrew, S. G. , … Lupski, J. R. (2010). Recurrent reciprocal 16p11. 2 rearrangements associated with global developmental delay, behavioural problems, dysmorphism, epilepsy, and abnormal head size. Journal of Medical Genetics, 47(5), 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletten, D. M. , Suarez, G. A. , Low, P. A. , Mandrekar, J. , & Singer, W. (2012). COMPASS 31: A refined and abbreviated composite autonomic symptom score. Mayo Clinic Proceedings, 87(12), 1196–1201. http://www.sciencedirect.com/science/article/pii/S0025619612010385 [DOI] [PMC free article] [PubMed]
- Soker‐Elimaliah, S. , Jennings, C. A. , Hashimi, M. M. , Cassim, T. Z. , Lehrfield, A. , & Wagner, J. B. (2020). Autistic traits moderate relations between cardiac autonomic activity, interoceptive accuracy, and emotion processing in college students. International Journal of Psychophysiology, 155, 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, R. , Liu, J. , & Kong, X. (2016). Autonomic dysfunction and autism: Subtypes and clinical perspectives. North American Journal of Medical Sciences, 9(4), 172–180. [Google Scholar]
- Srivastava, S. , Love‐Nichols, J. A. , Dies, K. A. , Ledbetter, D. H. , Martin, C. L. , Chung, W. K. , Firth, H. V. , Frazier, T. , Hansen, R. L. , Prock, L. , Brunner, H. , Hoang, N. , Scherer, S. W. , Sahin, M. , & Miller, D. T. , NDD Exome Scoping Review Work Group . (2019). Meta‐analysis and multidisciplinary consensus statement: Exome sequencing is a first‐tier clinical diagnostic test for individuals with neurodevelopmental disorders. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 21(11), 2413–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson, H. , Meyer‐Lindenberg, A. , Steinberg, S. , Magnusdottir, B. , Morgen, K. , & Arnarsdottir, S. (2014). CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature, 505, 361–366. [DOI] [PubMed] [Google Scholar]
- Suarez, G. A. , Opfer‐Gehrking, T. L. , Offord, K. P. , Atkinson, E. J. , O'brien, P. C. , & Low, P. A. (1999). The autonomic symptom profile a new instrument to assess autonomic symptoms. Neurology, 52(3), 523–523. [DOI] [PubMed] [Google Scholar]
- Talkowski, M. E. , Mullegama, S. V. , Rosenfeld, J. A. , Van Bon, B. W. , Shen, Y. , Repnikova, E. A. , Gastier‐Foster, J. , Thrush, D. L. , Kathiresan, S. , Ruderfer, D. M. , Chiang, C. , Hanscom, C. , Ernst, C. , Lindgren, A. M. , Morton, C. C. , An, Y. , Astbury, C. , Brueton, L. A. , Lichtenbelt, K. D. , … Elsea, S. H (2011). Assessment of 2q23. 1 microdeletion syndrome implicates MBD5 as a single causal locus of intellectual disability, epilepsy, and autism spectrum disorder. American Journal of Human Genetics, 89(4), 551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa, R. , Pokorski, I. , Ambarchi, Z. , Thomas, E. , Demayo, M. , Boulton, K. , Matthews, S. , Patel, S. , Sedeli, I. , Hickie, I. B. , & Guastella, A. J. (2021). Heart rate variability in children with autism spectrum disorder and associations with medication and symptom severity. Autism Research, 14(1), 75–85. [DOI] [PubMed] [Google Scholar]
- Tordjman, S. , Anderson, G. M. , Kermarrec, S. , Bonnot, O. , Geoffray, M. M. , Brailly‐Tabard, S. , Chaouch, A. , Colliot, I. , Trabado, S. , Bronsard, G. , Coulon, N. , Botbol, M. , Charbuy, H. , Camus, F. , & Touitou, Y. (2014). Altered circadian patterns of salivary cortisol in low‐functioning children and adolescents with autism. Psychoneuroendocrinology, 50, 227–245. [DOI] [PubMed] [Google Scholar]
- Unwin, L. , Maybery, M. , Wray, J. , & Whitehouse, A. (2013). A “Bottom‐Up” approach to aetiological research in autism spectrum disorders. Frontiers in Human Neuroscience, 7. https://www.frontiersin.org/articles/10.3389/fnhum.2013.00606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnovic, A. , & Zee, P. C (2015). Consequences of circadian disruption on neurologic health. Sleep Medicine Clinics, 10(4), 469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Hensley, M. K. , Tasman, A. , Sears, L. , Casanova, M. F. , & Sokhadze, E. M. (2016). Heart rate variability and skin conductance during repetitive TMS course in children with autism. Applied Psychophysiology and Biofeedback, 41(1), 47–60. [DOI] [PubMed] [Google Scholar]
- Wenger, T. L. , Miller, J. S. , DePolo, L. M. , de Marchena, A. B. , Clements, C. C. , Emanuel, B. S. , Zackai, E. H. , McDonald‐McGinn, D. M. , & Schultz, R. T. (2016). 22q11. 2 duplication syndrome: Elevated rate of autism spectrum disorder and need for medical screening. Molecular Autism, 7(1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs, L. , & Stores, G. (1996). Severe sleep disturbance and daytime challenging behaviour in children with severe learning disabilities. Jidr, 40(6), 518–528. [DOI] [PubMed] [Google Scholar]
- Wiggs, L. , & Stores, G. (2004). Sleep patterns and sleep disorders in children with autistic spectrum disorders: Insights using parent report and actigraphy. Developmental Medicine and Child Neurology, 46(6), 372–380. [DOI] [PubMed] [Google Scholar]
- Wirz‐Justice, A. , Bromundt, V. , & Cajochen, C. (2009). Circadian disruption and psychiatric disorders: The importance of entrainment. Sleep Medicine Clinics, 4(2), 273–284. [Google Scholar]
- Woodard, C. R. , Goodwin, M. S. , Zelazo, P. R. , Aube, D. , Scrimgeour, M. , Ostholthoff, T. , & Brickley, M. (2012). A comparison of autonomic, behavioral, and parent‐report measures of sensory sensitivity in young children with autism. Research in Autism Spectrum Disorders, 6(3), 1234–1246. [Google Scholar]
- Wulff, K. , Dijk, D. J. , Middleton, B. , Foster, R. G. , & Joyce, E. M. (2012). Sleep and circadian rhythm disruption in schizophrenia. British Journal of Psychiatry, 200(4), 308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Liu, X. , Gao, H. , He, R. , Chu, G. , & Zhao, Y. (2021). Copy number variations of chromosome 17p11.2 region in children with development delay and in fetuses with abnormal imaging findings. BMC Medical Genomics, 14(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Clinical diagnosis in NPD‐ASD and NPD‐OTHER subgroups
Supplemental Table 2: Demographics and sample characteristics
Supplemental Table 3: Age based analysis of PASS
Supplemental Table 4: Inter‐correlations between PASS sections
Supplemental Table 5: PASS and SRS correlations, NPD‐ASD
Supplemental Table 6: PASS and SRS correlations, NPD‐OTHER
Data Availability Statement
Data are available on request due to privacy/ethical restrictions


