Abstract
Background
Cardiovascular disease (CVD) remains the number one cause of death and disability worldwide and public health interventions focus on modifiable risk factors, such as diet. Coenzyme Q10 (CoQ10) is an antioxidant that is naturally synthesised by the body and can also be taken as a dietary supplement. Studies have shown that a CoQ10 deficiency is associated with cardiovascular disease.
Objectives
To determine the effects of coenzyme Q10 supplementation as a single ingredient for the primary prevention of CVD.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2013, Issue 11); MEDLINE (Ovid, 1946 to November week 3 2013); EMBASE (Ovid, 1947 to 27 November 2013) and other relevant resources on 2 December 2013. We applied no language restrictions.
Selection criteria
Randomised controlled trials (RCTs) lasting at least three months involving healthy adults or those at high risk of CVD but without a diagnosis of CVD. Trials investigated the supplementation of CoQ10 alone as a single supplement. The comparison group was no intervention or placebo. The outcomes of interest were CVD clinical events and major CVD risk factors, adverse effects and costs. We excluded any trials involving multifactorial lifestyle interventions to avoid confounding.
Data collection and analysis
Two authors independently selected trials for inclusion, abstracted data and assessed the risk of bias.We contacted authors for additional information where necessary.
Main results
We identified six RCTs with a total of 218 participants randomised, one trial awaiting classification and five ongoing trials. All trials were conducted in participants at high risk of CVD, two trials examined CoQ10 supplementation alone and four examined CoQ10 supplementation in patients on statin therapy; we analysed these separately. All six trials were small‐scale, recruiting between 20 and 52 participants; one trial was at high risk of bias for incomplete outcome data and one for selective reporting; all studies were unclear in the method of allocation and therefore for selection bias. The dose of CoQ10 varied between 100 mg/day and 200 mg/day and the duration of the interventions was similar at around three months.
No studies reported mortality or non‐fatal cardiovascular events. None of the included studies provided data on adverse events.
Two trials examined the effect of CoQ10 on blood pressure. For systolic blood pressure we did not perform a meta‐analysis due to significant heterogeneity. In one trial CoQ10 supplementation had no effect on systolic blood pressure (mean difference (MD) ‐1.90 mmHg, 95% confidence interval (CI) ‐13.17 to 9.37, 51 patients randomised). In the other trial there was a statistically significant reduction in systolic blood pressure (MD ‐15.00 mmHg, 95% CI ‐19.06 to ‐10.94, 20 patients randomised). For diastolic blood pressure we performed a random‐effects meta‐analysis, which showed no evidence of effect of CoQ10 supplementation when these two small trials were pooled (MD ‐1.62 mmHg, 95% CI ‐5.2 to 1.96).
One trial (51 patients randomised) looked at the effect of CoQ10 on lipid levels. The trial showed no evidence of effect of CoQ10 supplementation on total cholesterol (MD 0.30 mmol/L, 95% CI ‐0.10 to 0.70), high‐density lipoprotein (HDL)‐cholesterol (MD 0.02 mmol/L, 95% CI ‐0.13 to 0.17) or triglycerides (MD 0.05 mmol/L, 95% CI ‐0.42 to 0.52).
Of the four trials that investigated CoQ10 supplementation in patients on statin therapy, three of them showed that simultaneous administration of CoQ10 did not significantly influence lipid levels or systolic blood pressure levels between the two groups. The fourth trial showed a significant increase in the change in total and low‐density lipoprotein (LDL)‐cholesterol at three months across the four arms of the trial (α‐tocopherol, CoQ10, CoQ10 + α‐tocopherol and placebo), however the way in which the data were presented meant that we were unable to determine if there was any significant difference between the CoQ10 only and placebo arms. In contrast, there was no significant difference in the change in HDL‐cholesterol and triglycerides after three months between the four arms of the trial.
Authors' conclusions
There are very few studies to date examining CoQ10 for the primary prevention of CVD. The results from the ongoing studies will add to the evidence base. Due to the small number of underpowered trials contributing to the analyses, the results presented should be treated with caution and further high quality trials with longer‐term follow‐up are needed to determine the effects on cardiovascular events.
Keywords: Humans, Antioxidants, Antioxidants/administration & dosage, Cardiovascular Diseases, Cardiovascular Diseases/blood, Cardiovascular Diseases/prevention & control, Cholesterol, Cholesterol/blood, Hydroxymethylglutaryl-CoA Reductase Inhibitors, Hydroxymethylglutaryl-CoA Reductase Inhibitors/administration & dosage, Lipids, Lipids/blood, Primary Prevention, Primary Prevention/methods, Randomized Controlled Trials as Topic, Ubiquinone, Ubiquinone/administration & dosage, Ubiquinone/analogs & derivatives
Plain language summary
Co‐enzyme Q10 supplementation for the primary prevention of cardiovascular disease
Cardiovascular disease (CVD) is the result of complications of the heart and blood vessels and is a worldwide healthcare burden. However, it is thought that CVD risk can be lowered by changing a number of modifiable risk factors, such as diet. Dietary supplements have received a great deal of attention for the prevention of CVD. One such supplement is coenzyme Q10 (CoQ10), which is an antioxidant. Deficiencies in CoQ10 have been associated with CVD. This review therefore assessed the effectiveness of CoQ10 supplementation for CVD prevention. We included trials administering CoQ10 as a single supplement in healthy adults or those at high risk of CVD (but without a diagnosis of CVD) and measuring cardiovascular events or major CVD risk factors, such as blood pressure and lipid levels. We found six completed randomised controlled trials with a total of 218 participants randomised. All were conducted in participants at high risk of CVD. Two examined CoQ10 supplementation alone and four examined CoQ10 supplementation in patients on statin therapy. The trials were small and short‐term, none measured cardiovascular events or adverse events, and we regarded two of the six trials as being at high risk of bias. Very few small trials contributed to the analyses and no conclusions can be drawn at this time. We also identified five ongoing trials and the results from these will add to the evidence base in due course. More longer‐term trials are needed to determine the effect of CoQ10 on cardiovascular events.
Background
Description of the condition
Cardiovascular disease (CVD) remains the number one cause of death and disability worldwide (WHO 2012). The burden of disease will increase with an aging population and increasing levels of obesity and sedentary lifestyles. CVDs are the result of complications in the heart and blood vessels (WHO 2012), and include cerebrovascular disease, coronary heart disease (CHD) and peripheral arterial disease (PAD). Around 29.6% of total global deaths can be attributed to CVD (WHO 2003), and it is estimated that 17 million deaths per year are caused by CVD (Mackay 2004).
One of the main mechanisms thought to cause CVD is atherosclerosis, where the arteries become clogged by plaques or atheromas (NHS 2010). Atherosclerosis can cause CVD when the arteries are completely blocked by a blood clot or when blood flow is restricted by a narrowed artery limiting the amount of blood and oxygen that can be delivered to organs or tissue (BHF 2012). Whilst arteries may naturally become harder and narrower with age, this process may be accelerated by factors such as smoking, high cholesterol, high blood pressure, obesity, a sedentary lifestyle and ethnicity (NHS 2010).
Prevention of CVD by targeting modifiable factors remains a key public health priority. Diet plays a major role in the aetiology of many chronic diseases including CVD, thereby contributing to a significant geographical variability in morbidity and mortality rates across different countries and populations worldwide (WHO/FAO 2002). A number of dietary factors have been found to be associated with CVD risk, such as a low consumption of fruit and vegetables (Begg 2007), a high intake of saturated fat and a high consumption of salt (He 2011; Siri‐Tarino 2010). These factors are important since they can be modified in order to lower CVD risk making them a prime target for interventions aimed at primary prevention and management of CVD.
Description of the intervention
The intervention examined in this review is co‐enzyme Q10 (CoQ10) supplementation as a single ingredient. CoQ10 or ubiquinone is an important intracellular antioxidant naturally synthesised in the body. It is also available as a non‐prescription nutritional dietary supplement. CoQ10 plays a vital role in energy (ATP) production in the body by acting as an electron carrier in mitochondria and as a co‐enzyme for mitochondrial enzymes (Langsjoen 1985; Niklowitz 2007).
Studies have shown that a CoQ10 deficiency is associated with cardiovascular disease (Kumar 2009; Niklowitz 2007); however, it is uncertain whether a CoQ10 deficiency is the cause or the effect of disease, especially in observational studies (Niklowitz 2007). Patients with ischaemic heart disease and dilated cardiomyopathy have been found to have significantly lower levels of CoQ10 compared to healthy controls (Langsjoen 1990). In addition, the concentrations of CoQ10 in blood and myocardial tissue decline with increasing severity of heart disease (Littarru 1972). CoQ10 deficiency has also been observed in patients with hypertension; enzymatic deficiency of CoQ10 has been reported in 39% of hypertensive patients compared with only 6% of healthy controls (Kumar 2009).
Much of the research on CoQ10 is related to the secondary prevention of CVD and results of clinical trials support the use of CoQ10 in the treatment of congestive heart failure (Hofman‐Bang 1995; Morisco 1993). In other trials, CoQ10 has been used in combination with other micronutrients (Alehagen 2013). In primary prevention, a meta‐analysis of observational studies and clinical trials (12 studies, total of 362 patients) has shown that CoQ10 supplementation reduces blood pressure (Rosenfeldt 2007). The 12 studies consisted of three randomised controlled trials (RCTs), one cross‐over study and eight open‐label observational studies. Dose of CoQ10 ranged between 34 and 225 mg/day and duration of individual studies from one to 56 weeks. CoQ10 reduced systolic blood pressure by between 11 to 17 mmHg and reduced diastolic blood pressure by between 8 to 10 mmHg. This meta‐analysis also concluded that CoQ10 appears to be generally safe with no significant adverse effects. A recent Cochrane systematic review of RCTs in participants with primary hypertension confirmed these findings (Ho 2009). Dose of CoQ10 ranged from 100 to 120 mg/day. The meta‐analysis found that systolic blood pressure was reduced by 11 mmHg and the diastolic blood pressure was lowered by 7 mmHg compared to placebo, however, due to the possible unreliability of some of the included studies, the authors concluded that it is still uncertain if CoQ10 could be a useful in the treatment of hypertension (Ho 2009).
A CoQ10 deficiency occurs with age; however, certain drugs can cause depletion of CoQ10 levels, particularly hydroxy‐methylglutaryl‐coenzyme A (HMG‐CoA) reductase inhibitors, or statins. Statins are prescribed to reduce cholesterol levels and work by inhibiting HMG‐CoA reductase and the mevalonate metabolic pathway (Folkers 1990). Mevalonate is used to synthesise cholesterol as well as CoQ10 (Schaars 2008), therefore, when statin drugs lower cholesterol levels they simultaneously lower CoQ10 levels. Statins are known to block CoQ10 biosynthesis and reduce serum concentrations of CoQ10 by up to 40% (Kumar 2009). Furthermore, statin use is often associated with a variety of muscle‐related symptoms or myopathies. Research has suggested that CoQ10 supplementation may decrease muscle pain associated with statin treatment (Caso 2007).
How the intervention might work
Considering the key role of CoQ10 in cellular energy production, and the high energy requirements of cardiac cells, CoQ10 has a potential role in the prevention and treatment of heart ailments by improving cardiac bioenergetics (Kumar 2009).
Dietary supplementation with CoQ10 results in increased levels of ubiquinol‐10 (the reduced form of CoQ10) within circulating lipoproteins. In its reduced form the CoQ10 molecule acts as a powerful intracellular antioxidant due to its ability to hold electrons rather loosely, and will quite easily give up one or both electrons. The antioxidant and free radical scavenger effects of CoQ10 can therefore help to prevent lipid peroxidation and thus the progression of atherosclerosis (Kumar 2009; Mohr 1992). Furthermore, CoQ10 has also been found to modulate the amount of ß‐integrin levels on the surface of blood monocytes, strongly suggesting that the anti‐atherogenic effects of CoQ10 are mediated by other mechanisms beside its antioxidant properties (Turunen 2002).
There are also a number of ways that CoQ10 could act favourably to reduce blood pressure. CoQ10 could act directly on vascular endothelium and decrease total peripheral resistance by acting as an antagonist of vascular superoxide, by either scavenging it, or suppressing its synthesis (McCarty 1999). Further to this, a recent meta‐analysis has associated CoQ10 supplementation with a significant improvement in arterial endothelial function in patients with and without CVD (Gao 2012). CoQ10's antioxidant properties may also result in the quenching of free radicals that cause inactivation of endothelium‐derived relaxing factor or fibrosis of arteriolar smooth muscle, or both (Ignarro 1989). In addition, CoQ10 has been found to decrease blood viscosity and improve blood flow to cardiac muscle in patients with ischaemic heart disease; therefore it may reduce blood pressure (Kato 1990).
Why it is important to do this review
Most systematic reviews and meta‐analyses have looked at CoQ10 for the secondary prevention of cardiovascular diseases (Kendler 2006; Sander 2006; Shekelle 2003; Soja 1997), and those that focus on primary prevention have only looked at the effect of CoQ10 on hypertension (Ho 2009; Rosenfeldt 2003; Rosenfeldt 2007). Furthermore, a recent Cochrane review has looked at the efficacy of a number of cardioprotective agents (including CoQ10) in preventing heart damage in cancer patients treated with anthracyclines (van Dalen 2011). The outcomes assessed in that review include clinical and subclinical heart failure, quality of life and adverse effects, however, only one trial was identified using CoQ10 and it does not meet the selection criteria outlined in the current review.
This review is important because it looks at a broader range of outcomes including mortality, CVD events, changes in blood pressure and lipid levels, type 2 diabetes as a major risk factor for CVD, adverse effects, quality of life and costs. This review summarises the best available current evidence from RCTs of the effectiveness of CoQ10 supplementation for the primary prevention of CVD.
Objectives
To determine the effects of coenzyme Q10 supplementation as a single ingredient for the primary prevention of CVD.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs).
Types of participants
Adults, aged 18 and over from the general population, and adults at high risk of CVD. This review focused on the effects of CoQ10 supplementation on participants in primary prevention trials. We excluded those in secondary prevention trials, i.e. those who have experienced a previous myocardial infarction (MI), stroke, revascularisation procedure (coronary artery bypass grafting (CABG) or percutaneous transluminal coronary angioplasty (PTCA)) and those with angina or with angiographically defined CHD.
Types of interventions
The intervention was CoQ10 supplementation alone. We placed no limit on the amount of CoQ10 taken. We have stratified the results by those also taking statins. We only considered trials where the comparison group was placebo or no intervention. We intended to stratify by dose of CoQ10, however, there was an insufficient number of trials so this was not possible.
Types of outcome measures
Primary outcomes
Cardiovascular mortality
All‐cause mortality
Non‐fatal endpoints such as MI, CABG, PTCA, angina or angiographically defined CHD, stroke, carotid endarterectomy, PAD
Secondary outcomes
Changes in blood pressure (systolic and diastolic blood pressure) and blood lipids (total cholesterol, high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol, triglycerides)
Occurrence of type 2 diabetes as a major CVD risk factor
Quality of life
Adverse effects
Costs
We intended to focus on follow‐up periods of six months or more as these are most relevant for public health interventions. However, we also considered trials with a minimum follow‐up of three months where these were lacking.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases, without language restrictions, on 2 December 2013:
CENTRAL (2013, Issue 11);
MEDLINE (OVID, 1946 to November week 3 2013);
EMBASE Classic + EMBASE (OVID, 1947 to 27 November 2013);
Web of Science (Thomson Reuters, 1970 to 2 December 2013);
Health Technology Assessment (HTA) Database (Issue 4 of 4, 2013) on The Cochrane Library;
Database of Abstracts of Reviews of Effects (DARE) (Issue 4 of 4, 2013) on The Cochrane Library;
NHS Economic Evaluation Database (NEED) (Issue 4 of 4, 2013) on The Cochrane Library.
We used medical subject headings (MeSH) or equivalent and text word terms. We used the Cochrane sensitivity‐maximising RCT filter (Lefebvre 2011). We designed searches in accordance with the Cochrane Heart Group methods and guidance. The search strategies are shown in Appendix 1.
Searching other resources
We also checked reference lists of reviews and retrieved articles for additional studies.
We searched the metaRegister of controlled trials (mRCT) (www.controlled‐trials.com/mrct), ClinicalTrials.gov (www.clinicaltrials.gov/) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/) for ongoing trials (search date 22 July 2013) and Google Scholar for additional studies. We also searched OpenGrey to identify any relevant grey literature (www.opengrey.eu/). The search strategies are shown in Appendix 2.
We contacted authors where necessary for any additional information.
Data collection and analysis
Selection of studies
From the searches, two authors (NF, DT) reviewed the title and abstract of each paper and retrieved potentially relevant references. Two authors (NF, DT or LH) then independently selected studies to be included in the review by using predetermined inclusion criteria. In all cases authors resolved disagreements about study inclusion by consensus and consulted a third author (KR/LH) if disagreements persisted.
Data extraction and management
Two authors (NF, LH) independently extracted data using a pre‐standardised data extraction form. We contacted chief investigators to provide additional relevant information where necessary. We extracted details of the study design, participant characteristics, study setting, intervention (including duration and dose) and outcome data (including details of outcome assessment, adverse effects and methodological quality (randomisation, blinding and attrition)) from each included study. Authors resolved any disagreements about extracted data by consensus and consulted a third author (KR) if disagreements persisted.
Assessment of risk of bias in included studies
We assessed risk of bias by examining the quality of the random sequence generation and allocation concealment, description of drop‐outs and withdrawals (including analysis by intention‐to‐treat), blinding (participants, personnel and outcome assessment) and selective outcome reporting (Higgins 2011). We categorised risk of bias as 'low', 'unclear' or 'high'. Two authors (NF, LH) independently assessed the risk of bias in included studies.
Measures of treatment effect
We processed data in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For continuous outcomes we compared net changes (i.e. intervention group minus control group differences) and calculated a mean difference (MD) and 95% CI for each study.
Unit of analysis issues
Studies with multiple intervention groups
We included studies reporting multiple comparison groups in this review. Where this was the case, we used the data for the control group for each intervention group comparison and reduced the weight assigned to the control group by dividing the control group N by the number of intervention groups. However, we found no studies with more than one intervention group in this current review.
Cluster‐randomised trials
We included cluster‐randomised trials in this review by using the unit of randomisation (cluster) as the number of observations. Where necessary, we utilised individual level means and standard deviations adjusted for clustering together with the number of clusters in the denominator, in order to weight the trials appropriately. However, we found no cluster‐randomised trials in this current review.
Cross‐over trials
We included cross‐over trials in this review by using data only from the first half as a parallel‐group design. We only considered risk factor changes (i.e. blood pressure, lipid levels) before patients crossed over to the other therapy and where the duration was a minimum of three months before cross‐over occurred. However, we found no cross‐over randomised controlled trials in this current review.
Dealing with missing data
We contacted the authors of studies that met our inclusion criteria if missing data were unclear or if data had not been fully reported. We captured missing data in the data extraction form and reported this in the 'Risk of bias' tables. If a trial collected an outcome measure at more than one time point, we used the longest period of follow‐up where there was also 20% or less loss to follow‐up.
Assessment of heterogeneity
For each outcome, we conducted tests of heterogeneity using the Chi2 test of heterogeneity and the I2 statistic. Where there was no heterogeneity we performed a fixed‐effect meta‐analysis. If substantial heterogeneity was detected (50% or greater), we looked for possible explanations for this (e.g. participants and intervention). If the source of heterogeneity could not be explained, we considered the following options: provide a narrative overview and not aggregate the studies at all or use a random‐effects model with appropriate cautious interpretation.
Assessment of reporting biases
There were insufficient trials included in the review to date to examine the effects of publication bias using funnel plots (Sterne 2011).
Data synthesis
We carried out statistical analysis using The Cochrane Collaboration's statistical software, Review Manager 2012. We entered continuous data as the change in means and standard deviations from baseline to follow‐up measurements. We were only able to combine the results of two trials statistically.
Subgroup analysis and investigation of heterogeneity
We stratified results by those taking statins. It was our intention to stratify studies by 'dose' of CoQ10 intake and duration of the intervention but there were insufficient trials that met the inclusion criteria to do this.
Sensitivity analysis
There were insufficient trials to carry out sensitivity analyses excluding studies at high risk of bias.
Results
Description of studies
Results of the search
The searches generated 1348 hits after duplicates were removed. Screening of titles and abstracts identified 96 papers to go forward for formal inclusion and exclusion. Of these, six RCTs met the inclusion criteria. We also identified five ongoing trials from trial registers and there is one trial (five papers) awaiting classification. Details of the flow of studies through the review are shown in the PRISMA flow diagram in Figure 1.
1.
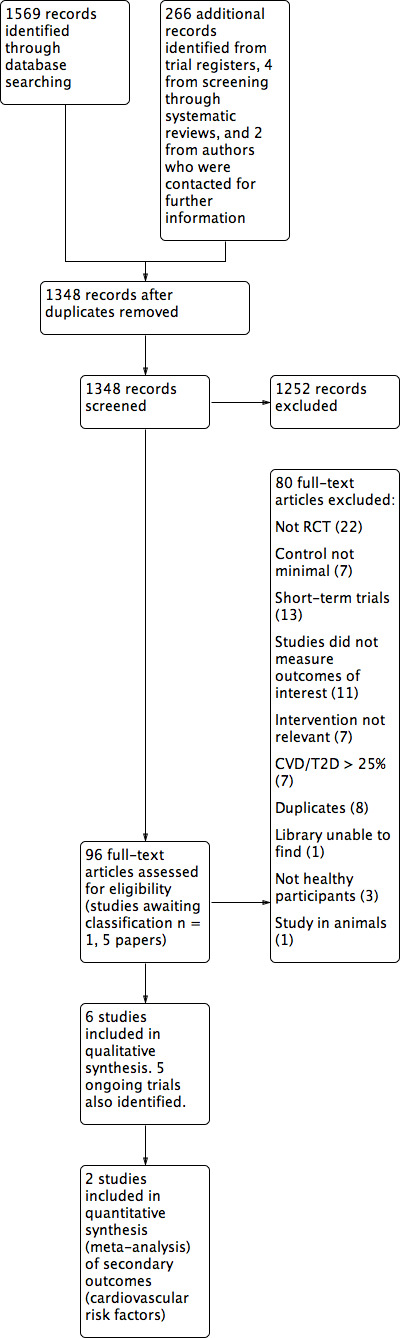
Study flow diagram.
Included studies
Details of the methods, participants, intervention, comparison group and outcome measures for each of the studies included in the review are shown in the Characteristics of included studies. We included six trials, with 218 participants randomised. Five trials recruited male and female participants (184 randomised). The remaining trial did not specify the gender of participants (Bargossi 1994). Two trials (69 participants randomised) were conducted in Japan (Mabuchi 2007; Yamagami 1986). The remaining studies were conducted in Seoul, Republic of Korea (Lee 2011), Finland (Kaikkonen 2000), Italy (Bargossi 1994), and New Zealand (Young 2007).
The duration of the intervention and follow‐up periods were similar for all trials. More specifically, for three of the trials, intervention and follow‐up period was defined as 12 weeks (Lee 2011; Yamagami 1986; Young 2007); three months (Kaikkonen 2000); and 90 days (Bargossi 1994). For the remaining trial, the intervention period was 12 weeks but follow‐up was at 16 weeks (four weeks after withdrawal of CoQ10 intervention) (Mabuchi 2007).
The dose of CoQ10 supplementation that was used varied from 100 to 200 mg/day and for four of the trials, participants were also taking statin therapy (Bargossi 1994; Kaikkonen 2000; Mabuchi 2007; Young 2007).
Baseline CoQ10 status varied by country, being lowest in Seoul, Republic of Korea (630 to 650 µg/L) and highest in Italy (10,800 µg/L to 12,000 µg/L). The country of recruitment, baseline plasma/serum CoQ10 level, dose of CoQ10 supplementation and duration of the intervention for each study are shown in Table 1.
1. Country, baseline CoQ10 levels and dose of CoQ10 supplementation.
| Study | Country | Baseline CoQ10 level | Dose of CoQ10 level studied (mg/day) | Statin therapy | Duration of CoQ10 intervention |
| Bargossi 1994 | Italy | Plasma 1.20 mg/dL equivalent to 12,000 µg/L (intervention group) 1.08 mg/dL equivalent to 10,800 µg/L (control group) |
100 | Yes | 90 days |
| Kaikkonen 2000 | Finland | Plasma 0.83 ± 0.04 µmol/L equivalent to 716.57 ± 34.53 µg/L (intervention group) 1.07 ± 0.10 µmol/L equivalent to 923.77 ± 86.33 µg/L (control group) |
200 | Yes | 3 months |
| Lee 2011 | Seoul, Republic of Korea | Serum 0.63 ± 0.25 µg/ml equivalent to 630 ± 250 µg/L (intervention group) 0.65 ± 0.27 µg/ml equivalent to 650 ± 270 µg/L (control group) |
200 | No | 12 weeks |
| Mabuchi 2007 | Japan | Plasma 1.113 ± 0.444 µmol/L equivalent to 960.90 ± 383.32 µg/L (intervention group) 1.180 ± 0.282 µmol/L equivalent to 1018.74 ± 243.46 µg/L (control group) |
100 | Yes | 12 weeks |
| Yamagami 1986 | Japan | Serum 0.704 ± 0.04 µg/ml equivalent to 704 ± 40µg/L (intervention group) 0.626 ± 0.05 µg/ml equivalent to 626 ± 50 µg/L (control group) |
100 | No | 12 weeks |
| Young 2007 | New Zealand | Plasma 1.3 (1.0 to 1.4) µmol/L equivalent to 1122.34 ± (863.34 to 1208.68) µg/L (intervention group) 1.4 (1.1 to 1.8) µmol/L equivalent to 1208.68 ± (949.67 to 1554.01) µg/L (control group) |
200 | Yes | 12 weeks |
Conversions: Mass (g) = No moles(n) x molecular mass (gmol‐1)
Molecular mass CoQ10 is 863.34 g
All six trials recruited participants that were at high risk of CVD. For example, four of the trials recruited hypercholesteraemic participants (Bargossi 1994; Kaikkonen 2000; Mabuchi 2007; Young 2007), one trial recruited participants with hypertension (Yamagami 1986), and the remaining trial recruited obese participants (body mass index (BMI) ≥ 25 kg/m2) (Lee 2011).
Details of the trial awaiting assessment are presented in the Characteristics of studies awaiting classification table. We are awaiting a response for requests for missing data from the author.
We identified five ongoing trials from trial registers. Details of these trials are provided in the Characteristics of ongoing studies table. Two of these trials have been completed but are not yet published. The first of these looked at CoQ10 supplementation (100 mg orally, once daily for three months) or placebo (100 mg, once daily for three months) in participants with hypertension and the outcome was blood pressure (Mozaffari 2011). The second investigated the effect of dietary supplementation with coenzyme Q10 (100 mg taken orally, twice daily for three months) on endothelial function (Young 2008). Participants were males with the metabolic syndrome who are concurrently on statin treatment. Outcomes were markers of cardiovascular risk, systolic and diastolic blood pressure and quality of life. The manuscript is currently being written for publication.
Of the remaining three trials, one of them examined the effects of CoQ10 supplementation (100 mg three times a day, or 300 mg three times a day) or matching placebo for 3.5‐month periods (Golomb 2009). The participants were Gulf War Veterans with chronic health problems and the outcome measured was quality of life (subjective health; syndrome defining symptoms (fatigue, muscle pain, muscle strength; and cognition)). No anticipated end date was provided for this trial. We contacted the author for further information but no response was received.
Another ongoing trial is examining CoQ10 supplementation (100 mg soft gel capsule twice a day for three months) or placebo tablets (containing 100 mg of soy oil twice a day for three months) in participants with the metabolic syndrome (Usefzadeh 2012). Outcomes include total cholesterol, HDL‐cholesterol, LDL‐cholesterol, blood pressure and triglycerides. The recruitment status is complete but no anticipated end date was provided for this trial. We contacted the author for further information but no response was received.
The final ongoing trial investigates CoQ10 (100 mg twice daily) or placebo (twice daily) for 12 weeks in inadequately treated hypertensive patients with the metabolic syndrome and the outcomes are systolic and diastolic blood pressure (Molyneux 2008). The recruitment status is closed and follow‐up is complete. We contacted the author for further information but no response was received.
Excluded studies
Details and reasons for exclusion for the studies that most closely missed the inclusion criteria are presented in the Characteristics of excluded studies. The reason for exclusion for the majority of studies was their short duration (less than three months). Other reasons for exclusion were that no outcomes of interest were measured, participants were not healthy, the control was not no intervention/placebo, or use of CoQ10 supplementation in combination with other micronutrients.
Short‐term studies
As stated above, the reason for exclusion for the majority of studies was that they were short‐term (less than three months follow‐up). We focused on six months or more follow‐up (or three months where these were lacking) as we were interested in the sustained effects of CoQ10, which are most relevant for public health interventions.
Risk of bias in included studies
Details are provided for each of the included studies in the 'Risk of bias' tables in Characteristics of included studies and summaries are provided in Figure 2 and Figure 3.
2.
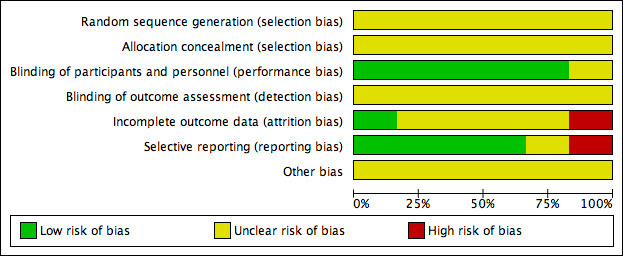
'Risk of bias' graph: authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
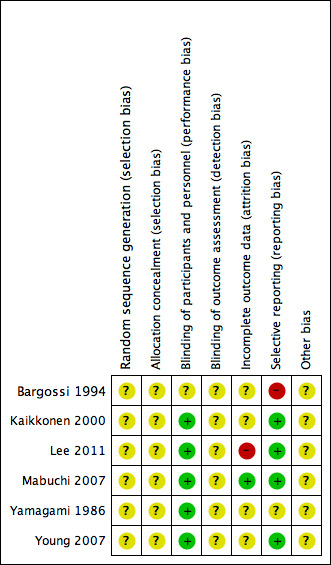
'Risk of bias' summary: authors' judgements about each risk of bias item for each included study.
Allocation
In all six trials, the methods of random sequence generation and allocation concealment were not stated and so we regarded them as being at unclear risk of bias.
Blinding
Five of the six trials stated they were 'double‐blind' (participants and personnel were blinded to treatment allocation) and we regarded them as low risk of bias, while one trial did not state if it had used blinding (Bargossi 1994). In all six trials no details were provided as to whether outcome assessors were blinded and so we regarded them as being at unclear risk of bias.
Incomplete outcome data
We judged one of the trials as high risk as 30% of participants were lost to follow‐up overall and no intention‐to‐treat analysis was carried out (Lee 2011). We judged one study as low risk as all participants completed the trial (Mabuchi 2007). We judged all the remaining four trials as unclear risk of bias as insufficient information was provided; three of these trials provided no information on loss to follow‐up (Kaikkonen 2000; Yamagami 1986; Young 2007), and the remaining study provided some loss to follow‐up information but it was unclear which arm these participants were from (Bargossi 1994).
Selective reporting
We judged one of the trials as high risk as outcome data for LDL‐cholesterol, HDL‐cholesterol and triglycerides were not reported fully (Bargossi 1994). We judged one study as unclear as there was insufficient information to judge selective reporting (Yamagami 1986). We judged all the remaining four trials at low risk of bias as all pre‐specified outcomes were reported (Kaikkonen 2000; Lee 2011; Mabuchi 2007; Young 2007).
Other potential sources of bias
For all six trials, there was insufficient information to judge the risk of bias from other potential sources and so we regarded them as being at unclear risk of bias
Effects of interventions
Primary outcomes
Cardiovascular and all‐cause mortality and non‐fatal cardiovascular events
None of the included studies provided data for any of our primary outcomes, including cardiovascular and all‐cause mortality and non‐fatal cardiovascular events.
Secondary outcomes
Cardiovascular risk factors
CoQ10 only intervention without concurrent use of statins
Two trials examined the effect of CoQ10 on blood pressure (Lee 2011; Yamagami 1986). For systolic blood pressure (SBP), we found significant heterogeneity (I2 = 78%) between the trials and so we did not perform a meta‐analysis (Analysis 1.1). In one of the trials (Lee 2011), CoQ10 supplementation had no effect on systolic blood pressure (mean difference (MD) ‐1.90 mmHg, 95% confidence interval (CI) ‐13.17 to 9.37). In the other trial (Yamagami 1986), there was a statistically significant reduction in systolic blood pressure (MD ‐15.00 mmHg, 95% CI ‐19.06 to ‐10.94).
1.1. Analysis.
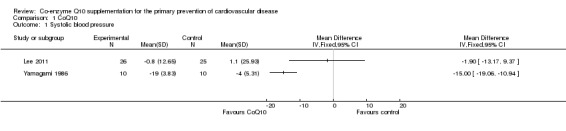
Comparison 1 CoQ10, Outcome 1 Systolic blood pressure.
For diastolic blood pressure (DBP) we observed moderate heterogeneity between the studies (I2 = 35%) so we performed a random‐effects meta‐analysis. There was no evidence of effect of CoQ10 supplementation in the pooled analysis of these two trials (71 participants randomised, MD ‐1.62 mmHg, 95% CI ‐5.20 to 1.96) (Analysis 1.2).
1.2. Analysis.
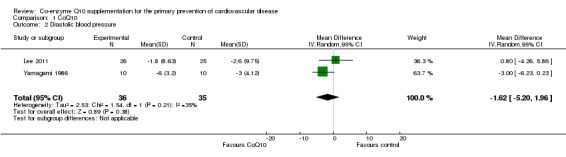
Comparison 1 CoQ10, Outcome 2 Diastolic blood pressure.
Only one trial looked at the effect of CoQ10 on lipids (total cholesterol, HDL‐cholesterol and triglycerides) (Lee 2011). The trial showed no evidence of effect of CoQ10 supplementation on total cholesterol (MD 0.30 mmol/L, 95% CI ‐0.10 to 0.70), HDL‐cholesterol (MD 0.02 mmol/L, 95% CI ‐0.13 to 0.17) or triglycerides (MD 0.05 mmol/L, 95% CI ‐0.42 to 0.52).
None of the trials reported type 2 diabetes as an outcome as a major risk factor for cardiovascular disease (CVD).
CoQ10 supplementation in patients on statin therapy
Four trials looked at the effects of CoQ10 supplementation in patients on statin therapy and we have analysed these separately. These studies met our inclusion criteria as the outcomes were lipid levels (total cholesterol, LDL‐cholesterol, HDL‐cholesterol and triglycerides), but we have not meta‐analysed them and have described them narratively below for completeness as these trials are not contributory to the question posed of the effects of CoQ10 on lipid levels, as statin therapy is designed to reduce lipid levels. In addition, for three of the trials the main focus was on statin tolerance and statin‐associated myalgia (Young 2007), to evaluate whether statins modify blood levels of CoQ10 (Bargossi 1994), to assess whether there is an interaction between CoQ10 and vitamin E (in a combined supplementation) in the antioxidative efficiency and in the change of plasma concentrations, and whether CoQ10 would increase the antioxidative resistance of atherogenic plasma lipoproteins (Kaikkonen 2000).
In one of these trials (Young 2007), there was no significant difference in the change in lipid levels between the groups of patients treated with simvastatin alone (increasing dose from 10 to 40 mg/day), or simvastatin (increasing dose from 10 to 40 mg/day) plus CoQ10 (200 mg/day) for 12 weeks. Total cholesterol: (CoQ10 + simvastatin (median, interquartile range): ‐1.5 mmol/L (‐2.4 to ‐0.7); simvastatin alone (median, interquartile range): ‐1.6 mmol/L (‐2.4 to ‐0.9); P value = 0.57)). LDL‐cholesterol: (CoQ10 + simvastatin (median, interquartile range): ‐1.7 mmol/L (‐2.4 to ‐1.0); simvastatin alone (median, interquartile range): ‐1.3 mmol/L (‐2.1 to ‐0.9); P value = 0.53)). HDL‐cholesterol: (CoQ10 + simvastatin (median, interquartile range): 0.01 mmol/L (‐0.08 to 0.15); simvastatin alone (median, interquartile range): ‐0.02 mmol/L (‐0.09 to 0.15); P value = 0.65). Triglycerides (CoQ10 + simvastatin (median, interquartile range): ‐0.4 mmol/L (‐0.6 to 0.3); simvastatin alone (median, interquartile range): ‐0.3 mmol/L (‐0.6 to 0.03); P value = 0.90).
A similar trial looked at groups of patients treated with simvastatin alone (20 mg/day), or simvastatin (20 mg/day) plus CoQ10 (100 mg/day) for three months (Bargossi 1994). The complete outcome data were not available, however, the author stated that simultaneous administration of CoQ10 did not significantly influence total cholesterol, LDL‐cholesterol, HDL‐cholesterol, triglycerides or systolic blood pressure levels between the two groups.
One trial looked at groups of patients treated with atorvastatin (10 mg/day), or atorvastatin (10 mg/day) plus CoQ10 (100 mg/day) for 12 weeks. Treatment with atorvastatin plus CoQ10 decreased mean (± standard deviation (SD)) total cholesterol by 30% (repeated measurement ANOVA, P value < 0.0001), while in the atorvastatin only group by 36% (P value < 0.0001) at study week 12, and there were no significant differences between the two groups. LDL‐cholesterol levels in the atorvastatin plus CoQ10 group and the atorvastatin only groups decreased by 43% and by 49% at study week 12, respectively (P value = 0.0001 in both groups), and there were no differences between the groups. The HDL‐cholesterol levels increased in the atorvastatin plus CoQ10 group but not in the atorvastatin only groups (repeated measurement ANOVA, P value = 0.0033 and P value = 0.0583, respectively), but there were no significant differences between the groups. The serum triglyceride levels decreased significantly in both groups, but there were no significant differences between the groups (Mabuchi 2007).
The final study was a four‐arm trial (α‐tocopherol, CoQ10, CoQ10 + α‐tocopherol, and placebo) with patients on statin therapy (statin and dose not stated) (Kaikkonen 2000). We were interested in the CoQ10 and placebo arms only. After three months of CoQ10 supplementation (200 mg/day), mean changes (± standard error (SE)) in lipid levels were as follows: total cholesterol (CoQ10: 0.33 mmol/L ± 0.14; placebo: ‐0.43 mmol/L ± 0.19); LDL‐cholesterol (CoQ10: 0.12 mmol/L ± 0.12; placebo: ‐0.45 mmol/L ± 0.15); HDL‐cholesterol (CoQ10: 0.21 mmol/L ± 0.04; placebo: 0.09 mmol/L ± 0.06) and triglycerides (CoQ10: ‐0.30 mmol/L ± 0.14; placebo: ‐0.54 mmol/L ± 0.22). The trial showed a significant increase in total and LDL‐cholesterol at three months across the four arms of the trial, however, the way in which the data were presented meant that we were unable to determine if there was any significant difference between the CoQ10 and placebo arms only. In contrast, there was no significant difference in the change in HDL‐cholesterol and triglycerides after three months between the four arms of the trial.
Quality of life
None of the included studies provided data on quality of life.
Adverse events
None of the included studies provided data on adverse events.
Costs
None of the included studies provided data on costs.
Discussion
Summary of main results
We identified six trials that randomised 218 participants in studies of three or more months duration from the 1348 papers screened. Four of these studies included patients on statin therapy and we analysed these separately.
None of the trials measured clinical events or mortality as they were small, short‐term and conducted in relatively healthy participants.
Only two trials where patients were not on statin therapy contributed to the main analysis. There was a reduction in systolic blood pressure in one of the two trials, with no evidence of effect in the other, and no evidence of effect on diastolic blood pressure in the pooled analysis of both trials. Only one trial examined the effects of coenzyme Q10 (CoQ10) on lipid levels and found no evidence of effect of CoQ10 supplementation on total cholesterol, high‐density lipoprotein (HDL)‐cholesterol or triglycerides.
Of the four trials that investigated CoQ10 supplementation in patients on statin therapy, three of them showed that simultaneous administration of CoQ10 did not significantly influence lipid levels or systolic blood pressure levels between the two groups. The fourth trial showed a significant increase in the change in total and low‐density lipoprotein (LDL)‐cholesterol at three months across the four arms of the trial, however, the way in which the data were presented meant that we were unable to determine if there was any significant difference between the CoQ10 and placebo arms only. In contrast, there was no significant difference in the change in HDL‐cholesterol and triglycerides after three months between the four arms of this trial.
None of the studies investigated our other secondary outcomes, adverse effects, quality of life or costs.
Overall completeness and applicability of evidence
This review included adult participants who were at varying levels of cardiovascular disease (CVD) risk and included both free‐living men and women. None of the six included studies examined our primary outcomes as trials were small, relatively short‐term and participants were predominantly healthy. We were also not able to examine the effects of 'dose' or duration of CoQ10 supplementation due to the limited number of included trials.
The effectiveness of CoQ10 could not be rigorously assessed as only two small trials including patients without statin therapy (71 participants) examined cardiovascular risk factors at three months (Lee 2011; Yamagami 1986).
The remaining four trials included patients on statin therapy. Whilst these trials met our inclusion criteria, their outcomes were lipid levels, which are affected by statin therapy alone. We analysed these trials separately and they did not contribute to our main findings.
The five ongoing trials will add to the evidence base.
Quality of the evidence
The studies included in this review were at some risk of bias and, as such, the results should be treated with caution. In all six of the included trials the methods of random sequence generation and allocation concealment were not stated. Five of the six studies stated that they were double‐blind and in one trial, blinding of participants and personnel was not stated. Risk of bias related to incomplete outcome data was unclear in four studies, low in one study and high in one study. We considered bias due to selective outcome reporting to be low in four studies, high in one study and unclear in one study. For all studies there was insufficient information to judge the risk of other biases.
In addition, small study bias is a risk in this review as most trials were very small and there were also inconsistencies between trials. We were unable to examine the effects of publication bias in funnel plots due to the limited number of included studies. However, small studies are often less methodologically robust, more likely to be conducted in selected populations and have been shown to report larger beneficial effects than larger trials (Nüesch 2010; Sterne 2000; Sterne 2001). The results of the review need to be interpreted with this in mind.
Potential biases in the review process
We conducted a comprehensive search across major databases for interventions involving CoQ10. We also screened systematic review reference lists and contacted authors when necessary. Two authors independently carried out all screening, inclusion and exclusion and data abstraction.
Our decision to restrict this review to interventions only investigating CoQ10 as a single supplement avoided the potential confounding effects of other nutritional and behavioural interventions on our outcomes, e.g. those involving additional micronutrient supplements, different dietary interventions or interventions that focused on weight loss or exercise. However, this limited the number of trials eligible for inclusion. In addition, the small number of trials on which this review is based, limitations in reporting methodological quality, an unclear risk of bias in most trials and few or no data for primary or secondary outcomes mean that caution should be used when interpreting the results of this review.
Agreements and disagreements with other studies or reviews
There are three systematic reviews and meta‐analyses that focus on the effect of CoQ10 on hypertension (Ho 2009; Rosenfeldt 2003; Rosenfeldt 2007). One review concluded that CoQ10 has the potential in hypertensive patients to lower systolic blood pressure by up to 17 mmHg and diastolic blood pressure by up to 10 mmHg (Rosenfeldt 2007). The most recent meta‐analysis concluded it was uncertain whether or not CoQ10 reduces blood pressure in the long‐term management of primary hypertension due to the possible unreliability of some of the included studies (Ho 2009). Our review produced few data with which to compare to previous studies and no conclusions can be drawn at present. Finally, a significant reduction in cardiovascular mortality has been observed in a recent trial using a combined CoQ10‐selenium supplementation, over a five‐year follow‐up, in a group of elderly Swedish individuals (Alehagen 2013). However, the effects seen could be due to either CoQ10, selenium or a combination of the two micronutrients.
Authors' conclusions
Implications for practice.
Six trials met the inclusion criteria for our review and none reported our primary outcomes. Furthermore, only two trials were included with patients not on statin therapy, and just one of these small trials found a significant reduction in systolic blood pressure. The review found no evidence of effect of coenzyme Q10 (CoQ10) supplementation on reducing lipid levels in the one small trial reporting this. Due to the small number of trials included, with a small number of participants randomised, short follow‐up and trials at some risk of bias, the results should be treated with caution. High quality trials are needed to examine the effects of CoQ10 supplementation on cardiovascular risk factors and events over a longer period of time. Given the very limited evidence to date, this review does not make any recommendations about changing practice.
Implications for research.
There is a lack of randomised controlled trials looking at the effects of CoQ10 supplementation for the primary prevention of cardiovascular disease. The completion of the five ongoing trials will add to the evidence base. We found no trials reporting our primary outcome, cardiovascular disease events, or the secondary outcomes adverse effects, health‐related quality of life or costs.
Acknowledgements
We are grateful to Nicole Martin for conducting the searches for this review. We would like to acknowledge Dr Jo Young and Dr Hassan Mozaffari‐Khosravi for providing additional data and/or information on their trials (Mozaffari 2011; Young 2008; Young 2007). We would also like to acknowledge Dr Noriko Cable, Dr Mariana Dyakova, Dr Thomas Hamborg and Dr William Tigbe for their help with translating articles.
Appendices
Appendix 1. Search strategies November 2013
CENTRAL, DARE, HTA, NEED on The Cochrane Library
#1 MeSH descriptor: [Cardiovascular Diseases] explode all trees #2 cardio* #3 cardia* #4 heart* #5 coronary* #6 angina* #7 ventric* #8 myocard* #9 pericard* #10 isch?em* #11 emboli* #12 arrhythmi* #13 thrombo* #14 atrial next fibrillat* #15 tachycardi* #16 endocardi* #17 (sick next sinus) #18 MeSH descriptor: [Stroke] explode all trees #19 (stroke or stokes) #20 cerebrovasc* #21 cerebral next vascular #22 apoplexy #23 (brain near/2 accident*) #24 ((brain* or cerebral or lacunar) near/2 infarct*) #25 MeSH descriptor: [Hypertension] explode all trees #26 hypertensi* #27 (peripheral next arter* next disease*) #28 ((high or increased or elevated) near/2 blood pressure) #29 MeSH descriptor: [Hyperlipidemias] explode all trees #30 hyperlipid* #31 hyperlip?emia* #32 hypercholesterol* #33 hypercholester?emia* #34 hyperlipoprotein?emia* #35 hypertriglycerid?emia* #36 MeSH descriptor: [Arteriosclerosis] explode all trees #37 MeSH descriptor: [Cholesterol] explode all trees #38 cholesterol #39 "coronary risk factor*" #40 MeSH descriptor: [Blood Pressure] this term only #41 "blood pressure" #42 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 #43 MeSH descriptor: [Ubiquinone] this term only #44 ubiquinone #45 ubiquinol‐10 #46 ubidecarenone #47 "Coenzyme Q" #48 "coenzyme Q10" #49 CoQ10 #50 caomet #51 decorenone #52 mitocor #53 neuquinone #54 "quinone q 10" #55 ubimaior #56 ubiten #57 #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 #58 #42 and #57
MEDLINE OVID
1. exp Cardiovascular Diseases/ 2. cardio*.tw. 3. cardia*.tw. 4. heart*.tw. 5. coronary*.tw. 6. angina*.tw. 7. ventric*.tw. 8. myocard*.tw. 9. pericard*.tw. 10. isch?em*.tw. 11. emboli*.tw. 12. arrhythmi*.tw. 13. thrombo*.tw. 14. atrial fibrillat*.tw. 15. tachycardi*.tw. 16. endocardi*.tw. 17. (sick adj sinus).tw. 18. exp Stroke/ 19. (stroke or stokes).tw. 20. cerebrovasc*.tw. 21. cerebral vascular.tw. 22. apoplexy.tw. 23. (brain adj2 accident*).tw. 24. ((brain* or cerebral or lacunar) adj2 infarct*).tw. 25. exp Hypertension/ 26. hypertensi*.tw. 27. peripheral arter* disease*.tw. 28. ((high or increased or elevated) adj2 blood pressure).tw. 29. exp Hyperlipidemias/ 30. hyperlipid*.tw. 31. hyperlip?emia*.tw. 32. hypercholesterol*.tw. 33. hypercholester?emia*.tw. 34. hyperlipoprotein?emia*.tw. 35. hypertriglycerid?emia*.tw. 36. exp Arteriosclerosis/ 37. exp Cholesterol/ 38. cholesterol.tw. 39. "coronary risk factor* ".tw. 40. Blood Pressure/ 41. blood pressure.tw. 42. or/1‐41 43. coenzyme Q10.tw. 44. Ubiquinone/ 45. Ubiquinone.tw. 46. Ubiquinol‐10.tw. 47. Ubidecarenone.tw. 48. Coenzyme Q.tw. 49. CoQ10.tw. 50. caomet.tw. 51. decorenone.tw. 52. mitocor.tw. 53. neuquinone.tw. 54. quinone q 10.tw. 55. ubimaior.tw. 56. ubiten.tw. 57. or/43‐56 58. 42 and 57 59. randomized controlled trial.pt. 60. controlled clinical trial.pt. 61. randomized.ab. 62. placebo.ab. 63. drug therapy.fs. 64. randomly.ab. 65. trial.ab. 66. groups.ab. 67. 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 68. exp animals/ not humans.sh. 69. 67 not 68 70. 58 and 69
EMBASE OVID
1. exp cardiovascular disease/ 2. cardio*.tw. 3. cardia*.tw. 4. heart*.tw. 5. coronary*.tw. 6. angina*.tw. 7. ventric*.tw. 8. myocard*.tw. 9. pericard*.tw. 10. isch?em*.tw. 11. emboli*.tw. 12. arrhythmi*.tw. 13. thrombo*.tw. 14. atrial fibrillat*.tw. 15. tachycardi*.tw. 16. endocardi*.tw. 17. (sick adj sinus).tw. 18. exp cerebrovascular disease/ 19. (stroke or stokes).tw. 20. cerebrovasc*.tw. 21. cerebral vascular.tw. 22. apoplexy.tw. 23. (brain adj2 accident*).tw. 24. ((brain* or cerebral or lacunar) adj2 infarct*).tw. 25. exp hypertension/ 26. hypertensi*.tw. 27. peripheral arter* disease*.tw. 28. ((high or increased or elevated) adj2 blood pressure).tw. 29. exp hyperlipidemia/ 30. hyperlipid*.tw. 31. hyperlip?emia*.tw. 32. hypercholesterol*.tw. 33. hypercholester?emia*.tw. 34. hyperlipoprotein?emia*.tw. 35. hypertriglycerid?emia*.tw. 36. exp Arteriosclerosis/ 37. exp Cholesterol/ 38. cholesterol.tw. 39. "coronary risk factor*".tw. 40. Blood Pressure/ 41. blood pressure.tw. 42. or/1‐41 43. ubidecarenone/ 44. coenzyme Q10.tw. 45. ubiquinone.tw. 46. ubiquinol‐10.tw. 47. ubidecarenone.tw. 48. Coenzyme Q.tw. 49. CoQ10.tw. 50. caomet.tw. 51. decorenone.tw. 52. mitocor.tw. 53. neuquinone.tw. 54. quinone q 10.tw. 55. ubimaior.tw. 56. ubiten.tw. 57. or/43‐56 58. 42 and 57 59. random$.tw. 60. factorial$.tw. 61. crossover$.tw. 62. cross over$.tw. 63. cross‐over$.tw. 64. placebo$.tw. 65. (doubl$ adj blind$).tw. 66. (singl$ adj blind$).tw. 67. assign$.tw. 68. allocat$.tw. 69. volunteer$.tw. 70. crossover procedure/ 71. double blind procedure/ 72. randomized controlled trial/ 73. single blind procedure/ 74. 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 75. (animal/ or nonhuman/) not human/ 76. 74 not 75 77. 58 and 76 78. limit 77 to embase
Web of Science
#15 #14 AND #13 #14 TS=((random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*)) #13 #12 AND #9 #12 #11 OR #10 #11 TS=(caomet or decorenone or mitocor or neuquinone or (quinone NEXT q NEXT 10) or ubimaior or ubiten) #10 TS=((coenzyme NEXT Q10) or Ubiquinone or Ubiquinol‐10 or Ubidecarenone or (Coenzyme NEXT Q) or CoQ10) #9 #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 #8 TS=(arteriosclerosis or cholesterol or "coronary risk factor*" or "blood pressure") #7 TS=(hyperlipid* or hyperlip?emia* or hypercholesterol* or hypercholester?emia* or hyperlipoprotein?emia* or hypertriglycerid?emia*) #6 TS=(high near/2 "blood pressure" or increased near/2 "blood pressure" or elevated near/2 "blood pressure") #5 TS=(brain* near/2 infarct or cerebral near/2 infarct or lacunar near/2 infarct* or hypertensi* or "peripheral arter* disease*") #4 TS=("cerebral vascular" or apoplexy or brain near/2 accident*) #3 TS=("atrial fibrillat*" or tachycardi* or endocardi* or "sick sinus" or stroke or stokes or cerebrovasc*) #2 TS=(myocard* or pericard* or isch?em* or emboli* or arrhythmi* or thrombo*) #1 TS=(cardio* or cardia* or heart* or coronary* or angina* or ventric*)
Appendix 2. Search strategies for trial registers
metaRegister of controlled trials (mRCT), Clinical trials.gov, the WHO International Clinical Trials Registry platform (ICTRP)
1. Coenzyme Q10
Data and analyses
Comparison 1. CoQ10.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systolic blood pressure | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Diastolic blood pressure | 2 | 71 | Mean Difference (IV, Random, 95% CI) | ‐1.62 [‐5.20, 1.96] |
| 3 Total cholesterol | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.10, 0.70] |
| 4 HDL‐cholesterol | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.13, 0.17] |
| 5 Triglycerides | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.42, 0.52] |
1.3. Analysis.
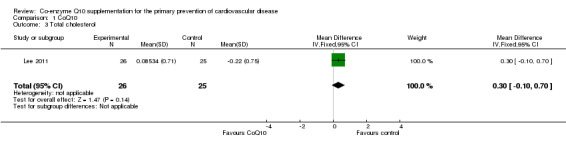
Comparison 1 CoQ10, Outcome 3 Total cholesterol.
1.4. Analysis.
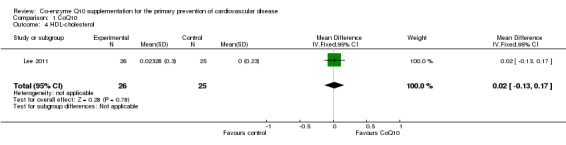
Comparison 1 CoQ10, Outcome 4 HDL‐cholesterol.
1.5. Analysis.
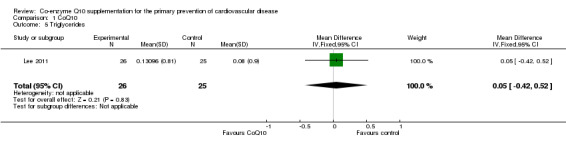
Comparison 1 CoQ10, Outcome 5 Triglycerides.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bargossi 1994.
| Methods | RCT cross‐over design (analysed as parallel group, using data only from the first 3 months of intervention before patients crossed over to the other therapy) | |
| Participants | 34 outpatients with primary hypercholesterolaemia (LDL‐cholesterol > 190 mg/dl, triglycerides < 200 mg/dl, 5 ° percentile < HDL‐cholesterol < 95 ° percentile of a reference population recruited in Italy | |
| Interventions | After a drug‐free controlled diet period participants were randomly allocated to 2 treatment groups: Intervention (statin + CoQ10): simvastatin (20 mg/day) plus CoQ10 (100 mg/day) for 3 months Control (statin): simvastatin (20 mg/day) for 3 months After this first 3‐month (90‐day) phase the 2 groups were crossed over |
|
| Outcomes | Blood pressure, total cholesterol, HDL‐cholesterol, LDL‐cholesterol, triglycerides | |
| Notes | Age, sex and ethnicity of participants were not specified. Number of participants randomised to each arm was not specified. Outcome data were not fully reported. We could not find any contact details for the authors of this paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to judge |
| Selective reporting (reporting bias) | High risk | Outcomes for LDL‐cholesterol, HDL‐cholesterol and triglycerides are not reported |
| Other bias | Unclear risk | Insufficient information to judge |
Kaikkonen 2000.
| Methods | RCT of parallel‐group design | |
| Participants | 40 participants (11 men and 29 postmenopausal women, 60.7 ± 5.7 years) with mild hypercholesterolaemia (5.90 ± 0.96 mmol/L) taking a regular HMG‐CoA reductase inhibitor treatment were recruited from newspaper advertisements from Eastern Finland in Spring 1997. BMI of participants was 26.9 ± 3.6 kg/m2 Exclusion criteria: regular intake of antioxidants, any drug with antioxidative properties, acetyl‐salicylic acid or other investigational products within the last month, malabsorption, treatment with oral oestrogen, use of anticoagulants, manifest insulin diabetes, cancer or other severe diseases that could cause difficulties in the participation Participants were recruited to 4 arms: α‐tocopherol, CoQ10, CoQ10 + α‐tocopherol and placebo |
|
| Interventions | Intervention (CoQ10): 10 participants were randomised. Oil‐based CoQ10 (2 x 100 mg daily) for 3 months. All capsules contained soybean oil. Participants were advised to take capsules in the morning and evening with meals (2 capsules in the morning and 2 in the evening) and to maintain their statin treatment, smoking and normal exercise and dietary habits during the study Control (placebo): 10 participants were randomised. Placebo capsules contained soybean oil. Participants were advised to take placebo capsules in the morning and evening with meals (2 capsules in the morning and 2 in the evening) and to maintain their statin treatment, smoking and normal exercise and dietary habits during the study |
|
| Outcomes | Total cholesterol, LDL‐cholesterol, HDL‐cholesterol, triglycerides | |
| Notes | 4‐arm trial (α‐tocopherol, CoQ10, CoQ10 + α‐tocopherol and placebo). We used the CoQ10 and placebo arms only. Age and sex were not specified in each arm of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to judge |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Insufficient information to judge |
Lee 2011.
| Methods | RCT of parallel‐group design | |
| Participants | 50 obese adults of either sex, greater than 20 years of age, and whose BMI was greater than 25 kg/m2 were recruited by advertisement in Seoul, Republic of Korea Exclusion criteria: taking vitamins or antioxidants, taking lipid‐lowering drugs such as statins or fenofibrates, uncontrolled hypertension (SBP ≥ 160 mmHg or DBP ≥ 100 mmHg), uncontrolled diabetes mellitus with a fasting blood sugar ≥ 150 mg/dL, hyperlipidaemia (triglycerides ≥ 400mg/dL or total cholesterol ≥ 250 mg/dL), or a history of cardiovascular disease |
|
| Interventions | Intervention (CoQ10): 26 participants (11 men and 15 women, mean age 42.7 ± 11.3 years) were given a 200 mg coenzyme Q10 pill once a day for 12 weeks Control (placebo): 25 participants (10 men and 15 women, mean age 42.5 ± 11.2 years) were given a placebo pill once a day for 12 weeks |
|
| Outcomes | Systolic blood pressure, diastolic blood pressure, total cholesterol, HDL‐cholesterol, triglycerides | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 30% lost to follow‐up overall |
| Selective reporting (reporting bias) | Low risk | All outcomes stated were reported |
| Other bias | Unclear risk | Report states "the strict exclusion criteria were meant to reduce confounding factors but may have resulted in a relatively healthy study population that attenuated the favourable effects of CoQ10." Also states, "the small sample size may have resulted in a Type II error" |
Mabuchi 2007.
| Methods | RCT of parallel‐group design | |
| Participants | 49 Japanese, hypercholesteraemic (above 220 mg/dL) patients of either sex Exclusion criteria: pregnant or lactating women; women of childbearing potential; patients with familial hypercholesterolaemia; patients taking other lipid‐lowering drugs such as fibrates or bile acid‐binding resins and other drugs known to affect statin metabolism, such as fibrates, cyclosporine, tamoxifen, corticosteroids, macrolide antibiotics and others; patients taking antioxidants such as ascorbic acid and tocopherol |
|
| Interventions | After a 4‐week dietary lead‐in period (less than 300 mg/day of low cholesterol diet), patients were randomised as follows: Intervention (statin + CoQ10): 24 participants (6 men and 18 women, mean age 61 ± 8 years) took atorvastatin (10 mg/day) for 16 weeks plus CoQ10 (100 mg/day) for 12 weeks Control (statin + placebo): 25 participants (8 men and 17 women, mean age 60 ± 8 years) took atorvastatin (10 mg/day) for 16 weeks plus placebo for 12 weeks Patients were instructed not to change their dietary and smoking habits throughout the study |
|
| Outcomes | Serum total cholesterol, LDL‐cholesterol, HDL‐cholesterol, triglycerides | |
| Notes | Nobody could discriminate the soft capsule of placebo containing only safflower oil from the CoQ10 capsule by appearance, odour and taste | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the trial |
| Selective reporting (reporting bias) | Low risk | All outcomes stated were reported |
| Other bias | Unclear risk | Plasma triglyceride levels in the placebo arm were slightly higher than those in the CoQ10 arm at baseline |
Yamagami 1986.
| Methods | RCT of parallel‐group design | |
| Participants | 52 patients of either sex, with essential hypertension whose blood pressure was higher than 150/90 mmHg were selected at random from the outpatient clinic of The Center for Adult Diseases, Osaka, Japan. 20 patients (8 men, 12 women, mean age 60 years) with low CoQ level and low SDH‐Q reductase activity were randomised Patients receiving conventional therapy for hypertension such as thiazide, beta‐blocker or vasodilators were allowed to enter the study. In the CoQ10 arm, 2 participants were taking thiazide, 3 were taking beta‐blockers, 2 were taking a combination of both of these medications and 3 had no therapy. In the control (placebo) group, 2 participants were taking thiazide, 4 were taking beta‐blockers, 1 was taking a combination of both of these medications, 1 was taking alpha‐blockers and 2 had no therapy. Therapy was continued during the trial period without any change Exclusion criteria: serious complications such as angina pectoris, myocardial infarction or cerebral vascular diseases were not included |
|
| Interventions | Each patient entered a lead‐in period of at least 4 weeks, during which their symptoms and blood pressure were stable. The intervention group (4 men, 6 women; mean age 59.5 ± 2.6 years) received 3 capsules daily. Each capsule contained 33.3 mg CoQ10. The control group (4 men, 6 women; mean age 61.3 ± 3.3 years) received 3 capsules daily of inactive placebo | |
| Outcomes | Systolic blood pressure, diastolic blood pressure | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to judge |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Insufficient information to judge |
Young 2007.
| Methods | RCT of parallel‐group design | |
| Participants | 44 patients (22 male, 22 female) with previous statin‐related myalgia, recruited from New Zealand Exclusion criteria: acute myocardial infarction or cerebral vascular accident within 3 months, alanine aminotransferase or aspartate aminotransferase > 3 times the upper level of normal, calculated glomerular filtration rate 45 ml/min, decompensated heart failure, warfarin treatment and antioxidant vitamin supplementation |
|
| Interventions | Before randomisation, patients underwent a 2‐week washout of coenzyme Q10 supplements and lipid‐modifying therapies, except for ezetimibe (n = 4) The intervention group (12 men, 10 women; mean age 59 ± 2 years) received CoQ10 capsules (200 mg/day) for 12 weeks in combination with upward dose titration of simvastatin from 10 mg/day, doubling every 4 weeks if tolerated to a maximum of 40 mg/day. The control group (10 men, 12 women; mean age 59 ± 2 years) received placebo for 12 weeks in combination with upward dose titration of simvastatin from 10 mg/day, doubling every 4 weeks if tolerated to a maximum of 40 mg/day |
|
| Outcomes | Total cholesterol, LDL‐cholesterol, HDL‐cholesterol, triglycerides | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to judge |
| Selective reporting (reporting bias) | Low risk | All outcomes stated were reported |
| Other bias | Unclear risk | Insufficient information to judge |
BMI: body mass index DBP: diastolic blood pressure HMG‐CoA: hydroxy‐methylglutaryl‐coenzyme A RCT: randomised controlled trial SBP: systolic blood pressure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bonetti 2000 | No outcomes of interest measured |
| Bookstaver 2012 | Participants not healthy (29% had CHD and 45% had diabetes) |
| Burke 2001 | Not a suitable intervention or control (both include vitamin E) |
| Caso 2007 | Not a suitable control group (vitamin E supplementation) |
| Cooke 2008 | Short‐term trial (follow‐up period was 2 weeks) |
| Delgado‐Casado 2011 | Not a suitable intervention (CoQ10 was combined with a Mediterranean diet) |
| Digiesi 1990 | Short‐term trial (follow‐up period was 10 weeks) |
| Gokbel 2010 | No outcomes of interest measured |
| Gul 2011 | No outcomes of interest measured |
| Kaikkonen 1997 | Short‐term trial (follow‐up period was 2 months) |
| Kelly 2005 | Not a suitable control group (vitamin E supplementation). Short‐term trial (follow‐up period was 30 days) |
| Kuettner 2005 | Participants not healthy (56% with CAD) |
| Malm 1997 | No outcomes of interest measured |
| Nuku 2007 | Short‐term trial (follow‐up period was 4 weeks) |
| Nukui 2008 | Short‐term trial (follow‐up period was 4 weeks) |
| Ostman 2012 | No outcomes of interest measured |
| Raitakari 2000 | Short‐term trial (follow‐up period was 4 weeks) |
| Shah 2007 | Short‐term trial (follow‐up period was 8 hours) |
| Shojaei 2011 | Participants not healthy (haemodialysis patients) |
| Svensson 1995 | Short‐term trial (follow‐up period was 20 days) |
| Weston 1997 | Short‐term trial (follow‐up period was 4 weeks) |
| Ylikoski 1997 | No outcomes of interest measured |
| Young 2012 | Participants not healthy (53% with diabetes and 30% with CVD) |
| Zheng 2008 | No outcomes of interest measured |
| Zita 2003 | No outcomes of interest measured |
CHD: coronary heart disease CoQ10: co‐enzyme Q10 CVD: cardiovascular disease
Characteristics of studies awaiting assessment [ordered by study ID]
Fedacko 2013.
| Methods | RCT of parallel‐group design |
| Participants | 60 patients with statin‐associated myopathy |
| Interventions | Randomisation 1: 200 mg CoQ10 daily or the corresponding placebo
Randomisation 2: 200 µg selenium daily or the corresponding placebo 4 subgroups were studied: (1) Group Q10Se, 200 mg of CoQ10 (active) + 200 µg of selenium (active) (daily) (2) Group Q10SePla, 200 mg CoQ10 (active) + selenium placebo (daily) (3) Group Q10PlaSe, CoQ10 placebo + 200 µg selenium (active) (daily) (4) Group Q10PlaSePla, CoQ10 placebo and selenium placebo |
| Outcomes | Total cholesterol, LDL‐cholesterol, HDL‐cholesterol, triglycerides, systolic blood pressure, diastolic blood pressure |
| Notes | Contacted author and received response but still waiting for specific data |
CAD: coronary artery disease
CoQ10: co‐enzyme Q10
Characteristics of ongoing studies [ordered by study ID]
Golomb 2009.
| Trial name or title | Q10 for Gulf War Veterans |
| Methods | Randomised, double‐blind (participant, investigator, outcomes assessor), placebo‐controlled, cross‐over study |
| Participants | 46 Gulf War veterans with chronic health problems (Gulf War Illness). Minimum age: 18 years. maximum age: n/a. Gender: both Inclusion criteria: ‐ Deployed to the Middle East for any period between August 1990 and July 1991 ‐ Adherence to CDC criteria for Gulf War illness: chronic symptoms, for at least 6 months, first arising after Gulf deployment, in at least 2 of the 3 areas of fatigue, musculoskeletal and mood/cognition ‐ Adherence to Kansas criteria for Gulf War illness. To aid specificity, these criteria are more involved than CDC criteria. Veterans are asked about symptoms in several general categories (e.g. respiratory, gastrointestinal, neuropsychological, sleep disturbances, pain), as well as symptoms (e.g. fatigue, headache) for which no single category is apparent. Gulf War illness criteria symptoms must have persisted or recurred in the year prior to interview and first have been a problem for respondents in 1990 or later. ‐ Willing to agree to defer initiating other over the counter medications until after completion of study participation ‐ Willing to defer participation in other clinical trials until after completion of study participation ‐ If female of childbearing potential, willing to be on 2 forms of birth control during study participation Exclusion criteria: ‐ Any factor that might compromise participation for the full duration of the study ‐ Known active cancer (except non‐melanoma skin cancer), neurodegenerative disease, or HIV ‐ Active medical problems distinct from Gulf War symptomatology that confer a significant probability of hospitalisation, medication change or change in clinical state during the course of participation ‐ Use of coumadin ‐ Use of Q10‐containing products, including lotions, toothpastes or supplements in the prior 2 months ‐ Current use of drugs known to be mitochondrial toxins: amiodarone, protease inhibitors, fluoroquinoline ("floxin") antibiotics ‐ Nursing or pregnant women |
| Interventions | Coenzyme Q10 at 100 mg 3 times a day or 300 mg 3 times a day or matching placebo for 3.5‐month periods |
| Outcomes | Quality of life (subjective health; syndrome defining symptoms (fatigue, muscle pain, muscle strength; and cognition)) |
| Starting date | Date of first enrolment: July 2008 |
| Contact information | Beatrice A Golomb, MD, PhD, Principal Investigator University of California San Diego La Jolla California 92093 |
| Notes | This study is ongoing, but not recruiting participants Emailed to find out further information (http://gulfstudy.ucsd.edu/Contact_Us.htm) but no response received |
Molyneux 2008.
| Trial name or title | A double‐blind, randomised, placebo‐controlled, 12‐week, cross‐over study to assess the effect of coenzyme Q10 treatment on 24‐hour mean ambulatory systolic and diastolic blood pressure in inadequately treated hypertensive patients with the metabolic syndrome |
| Methods | Randomised, controlled, cross‐over trial |
| Participants | Age minimum: 25 years
Age maximum: 75 years
Gender: both males and females Inclusion criteria: hypertension (average sitting systolic BP of > 139 mmHg or > 129/80 if patient has type 2 diabetes) and stabilised on antihypertensives for at least 1 month. The metabolic syndrome Exclusion criteria: uncontrolled hypertension. Cerebrovascular accident within 12 months prior. Taking warfarin treatment or antioxidant vitamin supplements |
| Interventions | Coenzyme Q10 (100 mg twice daily) or placebo (twice daily) for 12 weeks via oral capsule, followed by a 4‐week washout period, then 12 weeks of the alternate 'treatment' |
| Outcomes | Systolic and diastolic blood pressure |
| Starting date | Date of first enrolment: December 2008 |
| Contact information | Name: Sarah Molyneux Address: Canterbury Health Laboratories Biochemistry Unit, P.O. Box 151, Christchurch, 8140, New Zealand Telephone: +64 3 3641594 Email: sarah.molyneux@cdhb.govt.nz |
| Notes | Recruitment status: Closed. Follow‐up complete Contacted Sarah Molyneux for information but no response received |
Mozaffari 2011.
| Trial name or title | The study of coenzyme Q10 supplementation on blood pressure, inflammation factors and adiponectin in hypertensive patients |
| Methods | Randomisation: randomised. Blinding: double‐blind. Placebo: used. Assignment: parallel |
| Participants | Age minimum: 35
Age maximum: 55
Gender: both male and female Inclusion criteria: willingness to co‐operate in the project and complete a written informed consent, diagnosis of hypertensive patients based on diagnostic criteria and clinical examination by a physician, no pregnant or breastfeeding women, the absence of any autoimmune disorders, cardiovascular or renal diseases, lack of nutritional supplements during the past 6 months Exclusion criteria: diabetes mellitus type 1 or type 2, the history of disease such as myocardial infarction, cardiac dysfunction, cardiac arrhythmia, angina and kidney disease, autoimmune diseases like MS, rheumatism, etc., BMI > 40, factors causing secondary hypertension, supplementation of vitamin, mineral or other nutritional supplements, alcohol or drug use, incidence of severe side effects, non‐compliance with study protocol |
| Interventions | Coenzyme Q10, 100 mg orally, once daily for 3 months Placebo, 100 mg, once daily for 3 months |
| Outcomes | Hypertension. Time point: at baseline, and 6 and 12 weeks after baseline assessment. Method of measurement: Measured by standard mercury blood pressure measuring device sitting |
| Starting date | Date of first enrolment: March 2011 |
| Contact information | Name: Dr Hassan Mozaffari Address: 3rd floor, Central Building of Shahid Sadughi University of Medical Sciences and Health Services, Shahid Bahonar Sq. Yazd, Islamic Republic of Iran Telephone: +00 98 3517249333 Email: mozaffari.kh@gmail.com Affiliation: Shahid Sadughi University of Medical Sciences and Health Services Name: Seied Mohammad Mohammadi Zarchi Address: Shahid Sadughi University of Medical Sciences and Health Services,Yazd Diabetes Research Center Yazd, Islamic Republic of Iran Telephone: 00989123468307 Email: drc@ssu.ac.ir Affiliation: Shahid Sadughi University of Medical Sciences and Health Services |
| Notes | Target sample size: 72. Recruitment status: complete Contacted Dr Hassan Mozaffari and Seied Mohammad Mohammadi Zarchi. Response was received from Dr Hassan Mozaffari to say the trial was done but they are unable to write the article. Dr Hassan Mozaffari was contacted again to find out further information on who is responsible for writing the article/reasons why they are unable to write the article |
Usefzadeh 2012.
| Trial name or title | Effect of Q10 in the treatment of metabolic syndrome |
| Methods | Randomisation: randomised. Blinding: double‐blind. Placebo: used. Assignment: parallel |
| Participants | Inclusion criteria: 1. Having the metabolic syndrome according to the NCEP ATP III definition: Having at least 3 of the following conditions: a) Abdominal obesity (waist circumference = 102 cm in men and = 88 cm in women) b) High blood pressure (systolic blood pressure = 130 mmHg and/or diastolic blood pressure = 85 mmHg) c) Low HDL‐cholesterol (< 40 mg/dl in men and < 50 mg/dl in women) d) TG = 150 mg/dl e) FBS = 100 mg/ dl 2. Being the age limit from 35 to 55 years 3. Patients with hypertension treated with antihypertensive drugs in the past 3 months (which has not changed and changes in drug therapy not occurring during the study period) 4. Patients with diabetes whose treatment in the past 6 weeks has not changed (changes in drug therapy not occurring during the study period) 5. Consent to participate in the study Exclusion criteria: 1. Statin use in the last month 2. History of cardiovascular disease in the past 3 months 3. Having an active infection 4. Risk of renal failure 5. Liver disease (viral hepatitis, treatments for liver disease) 6. Alcohol consumption 7. Pregnancy or taking contraceptive drugs 8. History of hospitalisation in the past 2 months 9. Consumption of nutritional supplements such as vitamins, trace elements, antioxidants Exclusion criteria: Age minimum: 35 Age maximum: 55 Gender: both male and female |
| Interventions | Intervention group: Q10, 100 mg soft gel capsule twice a day for 3 months Placebo group: placebo tablets containing 100 mg of soy oil twice a day for 3 months |
| Outcomes | Primary: HDL‐cholesterol, blood pressure, TG (at the beginning of the study and 3 months after intervention) Secondary: LDL‐cholesterol, total cholesterol (at the beginning of the study and 3 months after intervention) |
| Starting date | Date of first enrolment: October 2012 |
| Contact information | Name: Dr Gholamreza Usefzadeh Address: Shariati Ave, Kerman, Islamic Republic of Iran Telephone: +00 98 3412459003 Email: uousefzadeh@kmu.ac.ir Affiliation: Kerman Physiology Research Center Name: Dr Azadeh Najarzadeh Address: Bahonar Sq Yazd, Islamic Republic of Iran Telephone: +00 98 3516238505 Email: azmm1383@yahoo.com Affiliation: Shahid Sadoghi University of Medical Sciences |
| Notes | Recruitment status complete Contacted Dr Gholamreza Usefzadeh and Dr Azadeh Najarzadeh for further information but no response was received |
Young 2008.
| Trial name or title | Randomised, double‐blind, placebo‐controlled, cross‐over study to investigate the effect of dietary supplementation with coenzyme Q10 on endothelial function in males with the metabolic syndrome who are concurrently on statin treatment |
| Methods | Randomised, controlled, cross‐over trial |
| Participants | Age minimum: 35 years
Age maximum: 65 years
Gender: males Inclusion criteria: Waist circumference >= 94 cm Triglycerides >= 1.7 mmol/L or specific therapy for triglycerides Systolic blood pressure >= 130 mmHg or diastolic blood pressure >= 85 mmHg or treatment for hypertension Statin naive or treatment with <= 40mg/day simvastatin or equivalent Exclusion criteria: Pre‐treatment low‐density lipoprotein (LDL)‐cholesterol < 1.5 mmol/L or > 6.5 mmol/L Pre‐treatment triglycerides > 5 mmol/L History of cardiovascular event or intervention within the 6 months prior to screening Type 1 diabetes mellitus Type 2 diabetes mellitus requiring oral antidiabetic agents or insulin Current smoker Treatment with fibrates or other lipid‐modifying agents other than statin therapy |
| Interventions | Coenzyme Q10 dietary supplement is a tablet (100 mg) to be taken orally, twice daily for 3 months. There is a 1‐month washout period between intervention and control or vice versa depending on treatment allocation |
| Outcomes | Markers of cardiovascular risk measured from blood tests Quality of life Systolic and diastolic blood pressure |
| Starting date | Date of first enrolment: October 2008 |
| Contact information | Name: Jo Young Address: Lipid and Diabetes Research Group First Floor, Hagley Hostel Christchurch Hospital, Private Bag 4710, Christchurch 8140, New Zealand Telephone: +64 3 364 1186 Email: joanna.young@cdhb.govt.nz |
| Notes | Contacted Jo Young for further information. Received response to say the trial was completed and they are currently writing the manuscript for publication |
NCEP ATP III: National Cholesterol Education Program Adult Treatment Panel III
BMI: body mass index BP: blood pressure CDC: Centers for Disease Control and Prevention MS: multiple sclerosis
TG: Triglycerides
Differences between protocol and review
It was our intention to perform stratified analysis to examine the effects of 'dose' and duration of CoQ10 supplementation but the review included an insufficient number of trials to do this. We also intended to create funnel plots to assess publication bias. We will address these in future updates of this review when more evidence is available. We also defined what we considered to be significant heterogeneity in terms of the I2 statistic (50% or greater) after the protocol was published.
Contributions of authors
All authors contributed to protocol development. NF and DT screened the titles and abstracts, and NF, DT and LH assessed the studies for formal inclusion and exclusion. NF and LH assessed methodological rigour and abstracted data. NF and KR conducted the analysis and wrote the review. All authors contributed to later drafts.
Sources of support
Internal sources
Warwick Medical School, University of Warwick, UK.
External sources
NIHR Cochrane Programme Grant, UK.
Declarations of interest
None known.
New
References
References to studies included in this review
Bargossi 1994 {published data only}
- Bargossi AM, Grossi G, Fiorella PL, Gaddi A, Giuliol R, Battino M. Exogenous CoQ10 supplementation prevents plasma ubiquinone reduction induced by HMG‐CoA reductase inhibitors. Molecular Aspects of Medicine 1994;15 Suppl:s187‐93. [DOI] [PubMed] [Google Scholar]
Kaikkonen 2000 {published data only}
- Kaikkonen J, Nyyssonen K, Tomasi A, Iannone A, Tuomainen TP, Porkkala‐Sarataho E, et al. Antioxidative efficacy of parallel and combined supplementation with coenzyme Q10 and d‐α‐tocopherol in mildly hypercholesterolemic subjects: a randomised placebo‐controlled clinical study. Free Radical Research 2000;33:329‐40. [DOI] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee YJ, Cho WJ, Kim JK, Lee DC. Effects of coenzyme Q10 on arterial stiffness, metabolic parameters, and fatigue in obese subjects: a double‐blind randomised controlled study. Journal of Medicinal Food 2011;14(4):386‐90. [DOI] [PubMed] [Google Scholar]
Mabuchi 2007 {published data only}
- Mabuchi H, Nohara A, Kobayashi J, Kawashiri M, Katsuda S, Inazu A, et al. Effects of CoQ10 supplementation on plasma lipoprotein lipid, CoQ10 and liver and muscle enzyme levels in hypercholesterolemic patients treated with atorvastatin: a randomized double‐blind study. Atherosclerosis 2007;195:e182–9. [DOI] [PubMed] [Google Scholar]
Yamagami 1986 {published data only}
- Yamagami T, Takagi M, Akagami H, Kubo H, Toyama S, Okamoto T, et al. Effect of coenzyme Q10 on essential hypertension, a double blind controlled study. Biomedical and Clinical Aspects of Coenzyme Q10: Proceedings of the Fifth International Symposium on the Biomedical and Clinical Aspects of Coenzyme Q10. 1986; Vol. 5:337‐43.
Young 2007 {published data only}
- Young JM, Florkowski CM, Molyneux SL, McEwan RG, Frampton CM, Georgeb PM, et al. Effect of coenzyme Q10 supplementation on simvastatin‐induced myalgia. American Journal of Cardiology 2007;100:1400–3. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bonetti 2000 {published data only}
- Bonetti A, Solito F, Carmosino G, Bargossi AM, Fiorella PL. Effect of ubidecarenone oral treatment on aerobic power in middle‐aged trained subjects. Journal of Sports Medicine & Physical Fitness 2000;40(1):51‐7. [PubMed] [Google Scholar]
Bookstaver 2012 {published data only}
- Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin‐induced myalgias. American Journal of Cardiology 2012;110(4):526‐9. [DOI] [PubMed] [Google Scholar]
Burke 2001 {published data only}
- Burke BE, Neuenschwander R, Olson RD. Randomized, double‐blind, placebo‐controlled trial of coenzyme Q10 in isolated systolic hypertension. Southern Medical Journal 2001;94(11):1112‐7. [DOI] [PubMed] [Google Scholar]
Caso 2007 {published data only}
- Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. American Journal of Cardiology 2007;99(10):1409‐12. [DOI] [PubMed] [Google Scholar]
Cooke 2008 {published data only}
- Cooke M, Iosia M, Buford T, Shelmadine B, Hudson G, Kerksick C, et al. Effects of acute and 14‐day coenzyme Q10 supplementation on exercise performance in both trained and untrained individuals. Journal of the International Society of Sports Nutrition 2008;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Delgado‐Casado 2011 {published data only}
- Delgado‐Casado N, Yubero‐Serrano EM, Perez‐Martinez P, Tasset‐Cuevas I, Santos‐Gonzalez M, Delgado‐Lista J, et al. The supplementation of coenzyme Q10 to a Mediterranean diet improves antioxidant systems and reduces cellular oxidation in elderly subjects. Atherosclerosis Supplements 2010;11(2):91. [Google Scholar]
Digiesi 1990 {published data only}
- Digiesi V, Cantini F, Brodbeck B. Effect of coenzyme Q10 on essential arterial hypertension. Current Therapeutic Research ‐ Clinical and Experimental 1990;47(5):841‐5. [Google Scholar]
Gokbel 2010 {published data only}
- Gokbel H, Gergerlioglu HS, Okudan N, Gul I, Buyukbas S, Belviranli M. Effects of coenzyme Q10 supplementation on plasma adiponectin, interleukin‐6, and tumor necrosis factor‐alpha levels in men. Journal of Medicinal Food 2010;13(1):216‐8. [DOI] [PubMed] [Google Scholar]
Gul 2011 {published data only}
- Gul I, Gokbel H, Belviranli M, Okudan N, Buyukbas S, Basarali K. Oxidative stress and antioxidant defense in plasma after repeated bouts of supramaximal exercise: the effect of coenzyme Q10. Journal of Sports Medicine and Physical Fitness 2011;51(2):305‐12. [PubMed] [Google Scholar]
Kaikkonen 1997 {published data only}
- Kaikkonen J, Nyyssonen K, Porkkala‐Sarataho E, Poulsen HE, Metsa‐Ketela T, Hayn M, et al. Effect of oral coenzyme q10 supplementation on the oxidation resistance of human VLDL+LDL fraction: absorption and antioxidative properties of oil and granule‐based preparations. Free Radical Biology and Medicine 1997;22(7):1195‐202. [DOI] [PubMed] [Google Scholar]
Kelly 2005 {published data only}
- Kelly P, Vasu S, Getato M, McNurlan M, Lawson WE. Coenzyme Q10 improves myopathic pain in statin treated patients (abstract). Journal of the American College of Cardiology 2005;45:3A. [Google Scholar]
Kuettner 2005 {published data only}
- Kuettner A, Pieper A, Koch J, Enzmann F, Schroeder S. Influence of coenzyme Q10 and cerivastatin on the flow‐mediated vasodilation of the brachial artery: results of the ENDOTACT study. International Journal of Cardiology 2005;98(3):413‐9. [DOI] [PubMed] [Google Scholar]
Malm 1997 {published data only}
- Malm C, Svensson M, Ekblom B, Sjodin B. Effects of ubiquinone‐10 supplementation and high intensity training on physical performance in humans. Acta Physiologica Scandinavica 1997;161(3):379‐84. [DOI] [PubMed] [Google Scholar]
Nuku 2007 {published data only}
- Nuku K, Matsuoka Y, Yamagishi T, Miyawaki H, Sato K. Safety assessment of PureSorb‐Q40 in healthy subjects and serum coenzyme Q10 level in excessive dosing. Journal of Nutritional Science & Vitaminology 2007;53(3):198‐206. [DOI] [PubMed] [Google Scholar]
Nukui 2008 {published data only}
- Nukui K, Yamagishi T, Miyawaki H, Kettawan A, Okamoto T, Belardinelli R, et al. Blood CoQ10 levels and safety profile after single‐dose or chronic administration of PureSorb‐Q40: animal and human studies. BioFactors 2008;32(1‐4):209‐19. [DOI] [PubMed] [Google Scholar]
Ostman 2012 {published data only}
- Ostman B, Sjodin A, Michaelsson K, Byberg L. Coenzyme Q10 supplementation and exercise‐induced oxidative stress in humans. Nutrition 2012;28(4):403‐17. [DOI] [PubMed] [Google Scholar]
Raitakari 2000 {published data only}
- Raitakari OT, McCredie RJ, Witting P, Griffiths KA, Letters J, Sullivan D, et al. Coenzyme Q improves LDL resistance to ex vivo oxidation but does not enhance endothelial function in hypercholesterolemic young adults. Free Radical Biology & Medicine 2000;28(7):1100‐5. [DOI] [PubMed] [Google Scholar]
Shah 2007 {published data only}
- Shah SA, Sander S, Cios D, Lipeika J, Kluger J, White CM. Electrocardiographic and hemodynamic effects of coenzyme Q10 in healthy individuals: a double‐blind, randomized controlled trial. Annals of Pharmacotherapy 2007;41(3):420‐5. [DOI] [PubMed] [Google Scholar]
Shojaei 2011 {published data only}
- Shojaei M, Djalali M, Khatami M, Siassi F, Eshraghian M. Effects of carnitine and coenzyme Q10 on lipid profile and serum levels of lipoprotein(a) in maintenance hemodialysis patients on statin therapy. Iranian Journal of Kidney Diseases 2011;5(2):114‐8. [PubMed] [Google Scholar]
Svensson 1995 {published data only}
- Svensson M, Malm C, Tonkonogi M, Ekblom B, Sjodin B, Sahlin K. Effect of Q10 supplementation on tissue Q10 levels and adenine nucleotide catabolism during high‐intensity exercise. International Journal of Sport Nutrition 1999;9(2):166‐80. [DOI] [PubMed] [Google Scholar]
Weston 1997 {published data only}
- Weston SB, Zhou S, Weatherby RP, Robson SJ. Does exogenous coenzyme Q10 affect aerobic capacity in endurance athletes?. International Journal of Sport Nutrition 1997;7(3):197‐206. [DOI] [PubMed] [Google Scholar]
Ylikoski 1997 {published data only}
- Ylikoski T, Piirainen J, Hanninen O, Penttinen J. The effect of coenzyme Q10 on the exercise performance of cross‐country skiers. Molecular Aspects of Medicine 1997;18 Suppl:s283‐90. [DOI] [PubMed] [Google Scholar]
Young 2012 {published data only}
- Young JM, Florkowski CM, Molyneux SL, McEwan RG, Frampton CM, Nicholls MG, et al. A randomized, double‐blind, placebo‐controlled crossover study of coenzyme Q10 therapy in hypertensive patients with the metabolic syndrome. American Journal of Hypertension 2012;25(2):261‐70. [DOI] [PubMed] [Google Scholar]
Zheng 2008 {published data only}
- Zheng A, Moritani T. Influence of CoQ10 on autonomic nervous activity and energy metabolism during exercise in healthy subjects. Journal of Nutritional Science & Vitaminology 2008;54(4):286‐90. [DOI] [PubMed] [Google Scholar]
Zita 2003 {published data only}
- Zita C, Overvad K, Mortensen SA, Sindberg CD, Moesgaard S, Hunter DA. Serum coenzyme Q(10) concentrations in healthy men supplemented with 30 mg or 100 mg coenzyme Q(10) for two months in a randomised controlled study. BioFactors 2003;18(1‐4):185‐93. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Fedacko 2013 {published data only}
- Fedacko J, Pella D, Fedackova P, Hänninen O, Tuomainen P, Jarcuska P, et al. Coenzyme Q10 and selenium in statin‐associated myopathy treatment. Canadian Journal of Physiology and Pharmacology 2013;91:165‐70. [DOI] [PubMed] [Google Scholar]
- Fedacko J, Pella D, Rybar R, Fedackova P, Dudova D. Coenzyme Q10 and selenium in statin‐associated myopathy treatment. Results of randomized double‐blind clinical study. European Heart Journal 2009;30:234. [Google Scholar]
- Fedacko J, Pella D, Rybar R, Fedackova P, Jarcuska P, Vargova V. Influence of Coenzyme Q10 on left ventricular diastolic function in statin treated patients. 14th World Congress on Clinical Nutrition and 5th International Congress on Cardiovascular Diseases. 40128 Bologna: Medimond S R L, 2009. [Google Scholar]
- Fedacko J, Pella D, Rybar R, Fedackova P, Mechirova V. Coenzyme Q10 and selenium supplementation in patients with statin‐associated myopathy. Atherosclerosis Supplements 2009;10:2. [Google Scholar]
- Fedacko J, Pella D, Rybar R, Lopuchovsky T, Tuomainen P, Merkovska L, et al. The role of selenium supplementation on top of coenzyme Q10 in statins treated patients with possible diastolic dysfunction of left ventricular. European Heart Journal 2012;33:613. [Google Scholar]
References to ongoing studies
Golomb 2009 {unpublished data only}
- Golomb BA. Q10 for Gulf War Veterans (GULF). NCT01011348: http://clinicaltrials.gov/show/NCT01011348(accessed 22 July 2013).
Molyneux 2008 {unpublished data only}
- Molyneux S. A double‐blind, randomised, placebo controlled, 12‐week cross‐over study to assess the effect of Coenzyme Q10 treatment on 24hr mean ambulatory systolic and diastolic blood pressure in inadequately treated hypertensive patients with the metabolic syndrome. ACTRN12611000241932: http://apps.who.int/trialsearch/trial.aspx?trialid=ACTRN12611000241932 (accessed 22 July 2013).
Mozaffari 2011 {unpublished data only}
- Mozaffari H, Mohammadi Zarchi SM. The study of coenzyme Q10 supplementation on blood pressure, inflammation factors and adiponectin in hypertensive patients. IRCT201103086002N1: http://apps.who.int/trialsearch/trial.aspx?trialid=IRCT201103086002N1 (accessed 22 July 2013).
Usefzadeh 2012 {unpublished data only}
- Usefzadeh G, Najarzadeh A. Effect of coenzyme Q10 Supplementation on Metabolic Syndrome Parameters, lipid peroxidation, hs‐CRP and homocysteine in patients with metabolic syndrome. IRCT2012101311092N2: http://apps.who.int/trialsearch/trial.aspx?trialid=IRCT2012101311092N2 (accessed 22 July 2013).
Young 2008 {unpublished data only}
- Young J. Randomised, double blind, placebo controlled, cross over study to investigate the effect of dietary supplementation with coenzyme Q10 on endothelial function in males with the metabolic syndrome who are concurrently on statin treatment. ACTRN12609000232235: http://apps.who.int/trialsearch/trial.aspx?trialid=ACTRN12609000232235 (accessed 22 July 2013).
Additional references
Alehagen 2013
- Alehagen U, Johansson P, Björnstedt M, Rosén A, Dahlström U. Cardiovascular mortality and N‐terminal‐proBNP reduced after combined selenium and coenzyme Q10 supplementation: a 5‐year prospective randomized double‐blind placebo‐controlled trial among elderly Swedish citizens. International Journal of Cardiology 2013;167:1860‐6. [DOI] [PubMed] [Google Scholar]
Begg 2007
- Begg S, Vos T, Barker B, Stevenson C, Stanley L, Lopez A. The Burden of Disease and Injury in Australia 2003. Canberra: Australian Institute of Health and Welfare, 2007. [Cat. no. PHE 82] [Google Scholar]
BHF 2012
- British Heart Foundation. Cardiovascular Disease. 2012. http://www.bhf.org.uk/heart‐health/conditions/cardiovascular‐disease.aspx (accessed April 2012).
Caso 2007
- Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. American Journal of Cardiology 2007;99(10):1409‐12. [DOI] [PubMed] [Google Scholar]
Folkers 1990
- Folkers K, Langsjoen P, Willis R, Richardson P, Xia LJ, Ye CQ, et al. Lovastatin decreases coenzyme Q levels in humans. Proceedings of the National Academy of Sciences of the United States of America 1990;87:8931‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gao 2012
- Gao L, Mao Q, Cao J, Wang Y, Zhou X, Fan L. Effects of coenzyme Q10 on vascular endothelial function in humans: a meta‐analysis of randomized controlled trials. Atherosclerosis 2012;221(2):311‐6. [DOI] [PubMed] [Google Scholar]
He 2011
- He F, Burnier M, MacGregor G. Nutrition in cardiovascular disease: salt in hypertension and heart failure. European Heart Journal 2011;32:3073‐80. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ho 2009
- Ho MJ, Bellusci A, Wright JM. Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007435.pub2] [DOI] [PubMed] [Google Scholar]
Hofman‐Bang 1995
- Hofman‐Bang C, Rehnquist N, Swedberg K, Wiklund I, Astrom H. Coenzyme Q10 as an adjunctive in the treatment of chronic congestive heart failure. The Q10 study group. Journal of Cardiac Failure 1995;1:101‐7. [DOI] [PubMed] [Google Scholar]
Ignarro 1989
- Ignarro LJ. Biological actions and properties of endothelium‐derived nitric oxide formed and released from artery and vein. Circulation Research 1989;65:1‐21. [DOI] [PubMed] [Google Scholar]
Kato 1990
- Kato T, Yoneda S. Reduction in blood viscosity by treatment with coenzyme Q10 in patients with ischemic heart disease. International Journal of Clinical Pharmacology, Therapy and Toxicology 1990;28(3):123‐6. [PubMed] [Google Scholar]
Kendler 2006
- Kendler BS. Supplemental conditionally essential nutrients in cardiovascular disease therapy. Journal of Cardiovascular Nursing 2006;21(1):9‐16. [DOI] [PubMed] [Google Scholar]
Kumar 2009
- Kumar A, Kaur H, Devi P, Mohan V. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere‐like syndrome. Pharmacology and Therapeutics 2009;124(3):259‐68. [DOI] [PubMed] [Google Scholar]
Langsjoen 1985
- Langsjoen PH, Vadhanavikit S, Folkers K. Response of patients in classes III and IV of cardiomyopathy to therapy in a blind and crossover trial with coenzyme Q10. Proceedings of the National Academy of Sciences of the United States of America 1985;82:4240‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langsjoen 1990
- Langsjoen PH. A six‐year clinical study of therapy of cardiomyopathy with coenzyme Q10. International Journal of Tissue Reactions 1990;12(3):169‐71. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011], The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Littarru 1972
- Littarru GP, Ho L, Folkers K. Deficiency of coenzyme Q10 in human heart disease. Part I. International Journal for Vitamin and Nutrition Research 1972;42:291‐305. [PubMed] [Google Scholar]
Mackay 2004
- Mackay J, Manesh GA. The Atlas of Heart Disease and Stroke. Geneva: World Health Organization, 2004. [Google Scholar]
McCarty 1999
- McCarty MF. Coenzyme Q versus hypertension: does CoQ decrease endothelial superoxide generation?. Medical Hypotheses 1999;53(4):300‐4. [DOI] [PubMed] [Google Scholar]
Mohr 1992
- Mohr D, Bowry VW, Stocker R. Dietary supplementation with coenzyme Q10 results in increased levels of ubiquinol‐10 within circulating lipoproteins and increased resistance of human low‐density lipoprotein to the initiation of lipid peroxidation. Biochimica et Biophysica Acta 1992;1126(3):247‐54. [DOI] [PubMed] [Google Scholar]
Morisco 1993
- Morisco C, Trimarco B, Condorelli M. Effect of coenzyme Q10 therapy in patients with congestive heart failure: a long‐term, multicentre randomised study. The Clinical Investigator 1993;71(8 Suppl):S134‐6. [DOI] [PubMed] [Google Scholar]
NHS 2010
- National Health Service. Atherosclerosis. 2010. http://www.nhs.uk/conditions/Atherosclerosis/Pages/Introduction.aspx#commentCountLink (accessed April 2012).
Niklowitz 2007
- Niklowitz P, Sonnenschein A, Janetzky B, Andler W, Menke T. Enrichment of coenzyme Q10 in plasma and blood cells: defense against oxidative damage. International Journal of Biological Sciences 2007;3(4):257‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rosenfeldt 2003
- Rosenfeldt F, Hilton D, Pepe S, Krum H. Systematic review of effect of coenzyme Q10 in physical exercise, hypertension and heart failure. Biofactors 2003;18(1‐4):91‐100. [DOI] [PubMed] [Google Scholar]
Rosenfeldt 2007
- Rosenfeldt FL, Haas SJ, Krum H, Hadj A, Ng K, Leong J‐Y, et al. Coenzyme Q10 in the treatment of hypertension: a meta‐analysis of the clinical trials. Journal of Human Hypertension 2007;21:297‐306. [DOI] [PubMed] [Google Scholar]
Sander 2006
- Sander S, Coleman CI, Patel AA, Kluger J, White CM. The impact of coenzyme Q10 on systolic function in patients with chronic heart failure. Journal of Cardiac Failure 2006;12(6):464‐72. [DOI] [PubMed] [Google Scholar]
Schaars 2008
- Schaars CF, Stalenhoef AF. Effects of ubiquinone (coenzyme Q10) on myopathy in statin users. Current Opinion in Lipidology 2008;19:553‐7. [DOI] [PubMed] [Google Scholar]
Shekelle 2003
- Shekelle P, Morton S, Hardy ML. Effect of supplemental antioxidants vitamin C, vitamin E, and coenzyme Q10 for the prevention and treatment of cardiovascular disease. Evidence Report/Technology Assessment (Summary) 2003;83:1‐3. [PMC free article] [PubMed] [Google Scholar]
Siri‐Tarino 2010
- Siri‐Tarino P, Sun Q, Hu F, Krauss R. Saturated fat, carbohydrate, and cardiovascular disease. American Journal of Clinical Nutrition 2010;91:502‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soja 1997
- Soja AM, Mortensen SA. Treatment of congestive heart failure with coenzyme Q10 illuminated by meta‐analyses of clinical trials. Molecular Aspects of Medicine 1997;18 Suppl:S159‐68. [DOI] [PubMed] [Google Scholar]
Sterne 2000
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta‐analysis: power of statistical tests and prevalence in the literature. Journal of Clinical Epidemiology 2000;53:1119‐29. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta analysis. BMJ 2001;323:101‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Turunen 2002
- Turunen M, Wehlin L, Sjoberg M, Lundahl J, Dallner G, Brismar K, et al. Beta2‐Integrin and lipid modifications indicate a non‐antioxidant mechanism for the anti‐atherogenic effect of dietary coenzyme Q10. Biochemical and Biophysical Research Communications 2002;296:255‐60. [DOI] [PubMed] [Google Scholar]
van Dalen 2011
- Dalen EC, Caron HN, Dickinson HO, Kremer LCM. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD003917.pub4] [DOI] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization. The World Health Report 2003: Shaping the Future. http://www.who.int/whr/2003/en/ (accessed April 2012).
WHO 2012
- World Health Organization. Cardiovascular diseases (CVDs). Fact Sheet Number 317. September 2012. http://www.who.int/mediacentre/factsheets/fs317/en/index.html (accessed April 2012).
WHO/FAO 2002
- Joint WHO/FAO Expert Consultation. Diet, nutrition and the prevention of chronic diseases: report of a joint WHO/FAO expert consultation. 2002. WHO technical report series; 916. http://www.who.int/dietphysicalactivity/publications/trs916/en/ (accessed April 2012).


