Abstract
Context
The mechanisms leading to increased cardiovascular disease in patients with nonalcoholic fatty liver disease (NAFLD) and advanced liver fibrosis remain incompletely understood.
Objective
This study assessed HDL-bound proteins in patients with NAFLD with or without advanced fibrosis.
Methods
This cross-sectional study at a university hospital included 185 patients with or without type 2 diabetes (T2D). Patients underwent liver proton magnetic resonance spectroscopy to measure intrahepatic triglyceride accumulation and those with NAFLD underwent a percutaneous liver biopsy. Advanced lipid testing with lipoprotein subfraction measurements and targeted proteomics of HDL-bound proteins was performed.
Results
Patients with and without advanced fibrosis had similar clinical characteristics, except for lower HDL-C (34 ± 8 vs 38 ± 9 mg/dL, P = 0.024) and higher prevalence of T2D in advanced fibrosis. Patients with advanced fibrosis had lower HDL particle number. A panel of 28 HDL-bound proteins were targeted and quantified by multiple reaction monitoring liquid chromatography–tandem mass spectrometry. Five proteins were found to be decreased in patients with advanced fibrosis (ApoC-I [P < 0.001], ApoC-IV [P = 0.012], ApoM [P = 0.008], LCAT [P = 0.014], and SAA4 [P = 0.016]). No differences were observed in these proteins in patients with vs without NAFLD or steatohepatitis. The pCAD index, associated with coronary artery disease and cardiovascular mortality, was significantly higher in patients with advanced fibrosis (97 ± 5 vs 86 ± 25, P = 0.04).
Conclusion
Patients with NAFLD with advanced fibrosis showed significant differences in HDL-bound protein levels; this translated into increased cardiovascular risk based on pCAD index. Different lipoprotein composition and function may explain the link between liver disease and increased cardiovascular mortality in these patients.
Keywords: NAFLD, NASH, cirrhosis, cholesterol, dyslipidemia
As a result of the obesity epidemic, nonalcoholic fatty liver disease (NAFLD) has become the most common chronic liver condition worldwide (1, 2). This condition ranges from isolated steatosis (hepatic fat accumulation in the absence of significant inflammation and necrosis) to more severe forms of liver disease, such as nonalcoholic steatohepatitis (NASH) and NAFLD-related cirrhosis. In addition to liver-related morbidity and mortality, NAFLD is also associated with a number of metabolic derangements (3, 4) and increased cardiovascular disease (5). However, it has been extremely difficult to establish whether NAFLD is directly associated with cardiovascular disease, or if this relationship is just the result of shared metabolic risk factors (ie, insulin resistance, hyperglycemia, hypertension, dyslipidemia). If indeed there is a direct association between the two, the actual mechanisms have remained elusive. It is also unclear whether the histological severity of liver disease impacts this potential association.
As the liver plays a key role in the synthesis of several lipoprotein-bound proteins, there has been interest in assessing if differences in the degree of liver disease could affect the lipoprotein profile. Low-density lipoprotein (LDL)-migration index, an indicator of small, dense LDL, was found to be elevated in patients with NASH vs isolated steatosis in a study with 156 patients (6). However, in a study with 81 patients with liver biopsy, Siddiqui et al (7) found no significant differences in lipoprotein profile between NASH and isolated steatosis. In one of the largest studies to date on advanced lipid testing in patients with liver biopsies, we showed that differences in LDL particle size, as well as differences in apolipoprotein (Apo) B and A-I levels, were directly associated with the amount of intrahepatic triglyceride content and insulin resistance, but not to the severity of liver histology (3).
In recent years, several studies have suggested that the stage of liver fibrosis is the most important predictor of mortality in patients with NAFLD (8, 9). While this may be mainly driven by an increase in liver-related mortality, there is also evidence that cardiovascular mortality may be increased in the presence of liver fibrosis (10, 11). However, paradoxically, there is also evidence that the development of cirrhosis is associated with relative improvement in the lipoprotein profile, with reduction of circulating triglycerides, lower very low-density lipoprotein (VLDL) particle size and number, and a reduction in LDL (as well as small, dense LDL) particle number (7, 12). High-density lipoprotein cholesterol (HDL-C) is also reduced in patients with decompensated cirrhosis and plasma HDL-C predicts survival in these patients (13). However, there is limited information regarding HDL particle function and lipoprotein composition at earlier stages of liver disease in NAFLD. Therefore, the aim of this study was to assess if worse liver histology in NAFLD is associated with changes in HDL protein composition and if these changes are associated with increased cardiovascular risk.
Methods
Research Subjects
Subjects included in this study were formerly recruited for the purpose of previously reported studies (4, 14–17). Patients with available serum samples, as well as complete data from liver proton magnetic resonance spectroscopy (1H-MRS) and percutaneous liver biopsy if they had a diagnosis of NAFLD, were included in the study. Briefly, they were originally recruited at the University of Florida in Gainesville, FL and the University of Texas Health Science Center at San Antonio (UTHSCSA) in San Antonio, TX from the general population and from hepatology and endocrinology clinics. Patients (ages 21-70 years old) were included, as long as they had no evidence of a secondary cause of liver steatosis (autoimmune hepatitis, hemochromatosis, viral hepatitis, alcohol consumption ≥30 g/day for males or ≥20 g/day for females, etc.) or use of medications that can affect intrahepatic triglyceride content (ie, vitamin E, pioglitazone, sodium-glucose cotransporter 2 inhibitors, glucagon like peptide 1 receptor agonists or other weight loss medications, amiodarone, glucocorticoids, methotrexate, olanzapine, protease inhibitors). The only glucose-lowering medications allowed were metformin, sulfonylureas, and insulin. The study was approved by the institutional review boards at the University of Florida and University of Texas Health Science Center at San Antonio (UTHSCSA), and a written informed consent was obtained from each patient prior to their participation.
Study Design
In this cross-sectional study, patients underwent a 2-step screening for liver disease based on initial liver 1H-MRS to establish or rule out the presence of NAFLD, and a percutaneous liver biopsy (if they had a diagnosis of NAFLD) to assess for the severity of liver disease. Comprehensive demographic, anthropometric, and biochemical data were also obtained. Finally, a serum sample was blindly provided to Quest Diagnostics to perform advanced lipid testing by ion mobility as well as measurement of HDL-bound proteins as described below.
HDL-Bound Proteomic Signature
Twenty eight HDL-associated proteins were isolated from human serum as previously described with modifications (18). Briefly, target acquisition parameters were obtained via in silico proteolytic digestion using Skyline MS to screen target peptides for each protein of interest. Selection of 1-2 peptides per protein and 2 collision energy optimized multiple reaction monitoring (MRM) transitions for each peptide was determined empirically using digests of each pure protein and corresponding internal standard.
Serum samples were incubated with 15N-labeled, His6-tagged ApoA-I and purified using Nickel-based IMAC columns (PhyNexus) in 96-well tip format. The extract was denatured at 85 °C for 5 minutes followed by digestion with endoproteinase LysC. After digestion, a mixture of stable isotope labeled internal standard peptides (Vivitide) with well-defined concentrations were added. Samples were injected onto a Phenomenex Kinetex C18 50 × 2.1 mm, 2.6 um directly coupled to an Agilent 6495A triple quadrupole mass spectrometer for analysis. Protein quantitation was achieved by single-point internal calibration, using the internal standard as reference, and normalized to the quantity of recovered 15N-His6-ApoA-I in each sample to account for variations in the enrichment process. As such, the reported values for each quantified HDL-associated protein are expressed as a molar ratio.
pCAD Index
Of the 28 targeted HDL-associated proteins using the proteomic measurement platform, 5 proteins that correlated with cell-based assessment of cholesterol efflux capacity (Apolipoproteins A-I, C-I, C-II, C-III, and C-IV) were optimized for stratification of patient cohorts having coronary artery disease and healthy controls by multivariable logistic regression as previously described (19). The resulting pCAD Index exists on a probabilistic continuum from 0 to 100, where higher values represent increased risk of coronary artery disease, and in patients with known diagnosis of coronary artery disease is associated with a higher incidence of cardiovascular mortality (20).
Intrahepatic Triglyceride Content and Liver Histology
Liver 1H-MRS was performed following standard methods previously reported (21). Briefly, intrahepatic triglyceride content was estimated from 3 different liver areas of 3 × 3 × 3 cm. A single experienced observer (F.B.) analyzed all spectra using commercial software (NUTS, Acorn NMR, Inc. Livermore, CA). Intrahepatic triglyceride was expressed as: (area under the curve [AUC] fat peak/[AUC fat peak + water peak]) × 100. Measurements were corrected for T1 and T2 relaxation using methods previously described (22). A liver fat content of > 5.56% was considered diagnostic of NAFLD (23, 24). In patients with a diagnosis of NAFLD, a percutaneous, ultrasound-guided biopsy was performed. Standard histological criteria were used to define presence of definite NASH and classify fibrosis stages (25, 26).
Statistical Analysis
Continuous data have been presented as mean ± SD in the text and tables and mean ± SEM in figures. Categorical variables have been summarized as percentages. Comparisons between 2 groups were performed by t test or Kruskal–Wallis for continuous measures (depending on distribution) and by chi-square or Fisher exact test for categorical measures. Comparisons among more than 2 groups were performed by one-way ANOVA. Multiple linear regression was used to independently assess the effect of NAFLD, type 2 diabetes (T2D), and obesity on lipoprotein subfractions. Differences in HDL-bound proteins (28 proteins measured) were adjusted for multiple comparisons, controlling for a false discovery rate (FDR) of 0.10 by Benjamini–Hochberg method (P < 0.017 were considered statistically significant). For other secondary outcomes, a two-tailed value of P < 0.05 was considered to indicate statistical significance. Analyses were performed with Stata 11.1 (StataCorp LP, College Station, TX) and graphs with Prism 6.0 (GraphPad Software, Inc., La Jolla, CA).
Results
Patient Characteristics
A total of 185 patients were recruited for this study over a period of 5 years. Of these, 45 (24%) were found not to have NAFLD based on liver 1H-MRS and were used as controls. The other 140 patients underwent a percutaneous liver biopsy and were categorized based on the presence or absence of advanced fibrosis (defined as fibrosis stages 3 or 4). Table 1 summarizes patient characteristics comparing patients with vs without advanced fibrosis. Characteristics of controls were also included in this table. As expected, both groups with NAFLD showed an overall worse metabolic profile compared with controls. As can be observed, patients with NAFLD had higher body mass index (BMI), higher prevalence of diabetes, worse dyslipidemia, and more elevated plasma aminotransferase levels.
Table 1.
Patients’ characteristics based on the presence of NAFLD and presence of advanced liver fibrosis
| NAFLD with F0-F2 (n = 114) |
NAFLD with advanced fibrosis (n = 26) |
P between NAFLD groups | Controls without NAFLD (n = 45) |
|
|---|---|---|---|---|
| Age, years | 52 ± 11 | 54 ± 9 | 0.58 | 55 ± 11 |
| Gender, male/female, % | 76%/24% | 77%/23% | 0.95 | 67%/33% |
| Ethnicity | 0.58 | |||
| Caucasian, % | 33% | 46% | 47% | |
| Hispanic, % | 58% | 50% | 27% | |
| African American, % | 8% | 4% | 22% | |
| Other, % | 1% | 0% | 4% | |
| Weight, kg | 101 ± 16 | 101 ± 17 | 0.99 | 91 ± 18 |
| Body mass index, kg/m2 | 34.5 ± 4.6 | 34.2 ± 4.3 | 0.76 | 30.8 ± 5.1 |
| Presence of | 0.33 | |||
| Overweight, % | 18% | 27% | 33% | |
| Obese, % | 82% | 73% | 58% | |
| Presence of diabetes, % | 68% | 85% | 0.084 | 60% |
| Diabetes medications | ||||
| Metformin, % | 46% | 73% | 0.013 | 38% |
| Sulfonylureas, % | 28% | 42% | 0.164 | 21% |
| Insulin, % | 13% | 31% | 0.038 | 12% |
| Statin use, % | 63% | 58% | 0.60 | 57% |
| Total cholesterol, mg/dL | 172 ± 43 | 188 ± 50 | 0.105 | 160 ± 32 |
| LDL-C, mg/dL | 101 ± 38 | 112 ± 39 | 0.21 | 94 ± 27 |
| HDL-C, mg/dL | 38 ± 9 | 34 ± 8 | 0.024 | 45 ± 13 |
| Triglyceride, mg/dL | 151 (105-194) | 162 (122-257) | 0.168 | 101 (79-123) |
| A1c, % | 6.5 ± 1.0 | 7.3 ± 1.3 | <0.001 | 6.4 ± 1.3 |
| Fasting plasma glucose, mg/mL | 127 ± 35 | 139 ± 39 | 0.131 | 127 ± 43 |
| Intrahepatic triglyceride content, % | 16 ± 9 | 15 ± 8 | 0.706 | 3 ± 1 |
| Aspartate aminotransferase, U/L | 39 ± 20 | 56 ± 27 | <0.001 | 25 ± 14 |
| Alanine aminotransferase, U/L | 56 ± 35 | 73 ± 38 | 0.033 | 27 ± 21 |
Abbreviations: A1c, hemoglobin A1c; F0, fibrosis stage 0; F2, fibrosis stage 2; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NAFLD, nonalcoholic fatty liver disease.
Patients with NAFLD, with or without advanced fibrosis, were well-matched for most clinical characteristics. They had similar age, sex and ethnic distribution, weight, and BMI. Prevalence of diabetes was also similar between these 2 groups, although patients with advanced fibrosis had higher hemoglobin A1c levels, despite greater use of metformin and insulin. Results of routine lipid panel were not significantly different between these 2 groups, except for lower plasma HDL-C level in patients with advanced fibrosis. Quantification of intrahepatic triglyceride content was similar in both groups, but patients with advanced fibrosis had higher plasma aspartate aminotransferase and alanine aminotransferase levels.
Lipoprotein Profile Based on Severity of Liver Histology
Table 2 compares the results from advanced lipoprotein profiling by ion mobility between patients with vs without advanced fibrosis. No differences were observed in LDL particle number, subtypes, or particle size. There were only mild differences in the overall HDL particle number between the groups, that was mainly driven by lower levels of small HDL particles. We also observed an increase in the number of small intermediate-density lipoprotein (IDL) particles in patients with advanced fibrosis. In Table 3, we divided patients with NAFLD based on the presence or absence of definite NASH (ie, coexistence of steatosis, inflammation, and hepatocyte ballooning). A relatively small difference was observed in HDL particle number, with lower particle numbers in patients with NASH. This was related to a reduction in both small and large HDL particles. No other significant difference was observed in any of the other lipoprotein subfractions between patients with or without NASH.
Table 2.
Characteristics of lipoprotein particles among patients with NAFLD based on the presence of advanced fibrosis
| NAFLD with F0-F2 (n = 114) |
NAFLD with advanced fibrosis (n = 26) |
P value | |
|---|---|---|---|
| HDL Particle number, nmol/L | 24 050 ± 5296 | 21 761 ± 5083 | 0.048 |
| HDL, Small, nmol/L | 19 057 ± 4052 | 17 269 ± 3705 | 0.042 |
| HDL, Large, nmol/L | 4993 ± 1348 | 4491 ± 1512 | 0.098 |
| MIDZONE, nmol/L | 599 ± 156 | 569 ± 167 | 0.39 |
| LDL Peak size, Angstrom | 214 ± 6 | 214 ± 7 | 0.89 |
| Total LDL, nmol/L | 1008 ± 310 | 1008 ± 300 | 0.99 |
| Non-HDL Particle Number, nmol/L | 1290 ± 363 | 1320 ± 363 | 0.71 |
| LDL, Very Small-d, nmol/L | 75 ± 23 | 70 ± 24 | 0.38 |
| LDL, Very Small-c, nmol/L | 92 ± 39 | 88 ± 47 | 0.68 |
| LDL, Very Small-b, nmol/L | 115 ± 78 | 119 ± 85 | 0.81 |
| LDL, Very Small-a, nmol/L | 105 ± 67 | 110 ± 66 | 0.70 |
| LDL Small, nmol/L | 200 ± 93 | 208 ± 102 | 0.72 |
| LDL Medium, nmol/L | 192 ± 78 | 186 ± 75 | 0.72 |
| LDL, Medium & Small, nmol/L | 392 ± 153 | 394 ± 165 | 0.97 |
| LDL, Large-b, nmol/L | 105 ± 48 | 99 ± 37 | 0.56 |
| LDL, Large-a, nmol/L | 124 ± 52 | 127 ± 58 | 0.78 |
| IDL, Small, nmol/L | 80 ± 24 | 92 ± 35 | 0.049 |
| IDL, Large, nmol/L | 114 ± 34 | 123 ± 42 | 0.21 |
| VLDL, Small, nmol/L | 43 ± 13 | 48 ± 18 | 0.151 |
| VLDL, Medium, nmol/L | 35 ± 12 | 38 ± 17 | 0.28 |
| VLDL, Large, nmol/L | 10 ± 5 | 11 ± 6 | 0.43 |
Abbreviations: F0, fibrosis stage 0; F2, fibrosis stage 2; HDL, high-density lipoprotein; IDL, intermediate-density lipoprotein; LDL, low-density lipoprotein; NAFLD, nonalcoholic fatty liver disease; VLDL, very low-density lipoprotein.
Table 3.
Characteristics of lipoprotein particles among patients with NAFLD based on the presence of steatohepatitis (NASH)
| NAFLD without NASH (n = 70) |
NAFLD with NASH (n = 70) |
P value | |
|---|---|---|---|
| HDL Particle Number, nmol/L | 24 689 ± 5785 | 22 557 ± 4607 | 0.018 |
| HDL, Small, nmol/L | 19 532 ± 4440 | 17 915 ± 3443 | 0.019 |
| HDL, Large, nmol/L | 5158 ± 1446 | 4641 ± 1290 | 0.029 |
| MIDZONE, nmol/L | 615 ± 171 | 572 ± 142 | 0.110 |
| LDL Peak Size, Angstrom | 215 ± 6 | 214 ± 6 | 0.61 |
| Total LDL, nmol/L | 1021 ± 334 | 996 ± 280 | 0.64 |
| Non-HDL Particle Number, nmol/L | 1310 ± 396 | 1282 ± 327 | 0.65 |
| LDL, Very Small-d, nmol/L | 74 ± 20 | 73 ± 25 | 0.83 |
| LDL, Very Small-c, nmol/L | 92 ± 38 | 90 ± 43 | 0.78 |
| LDL, Very Small-b, nmol/L | 115 ± 75 | 117 ± 82 | 0.87 |
| LDL, Very Small-a, nmol/L | 105 ± 66 | 107 ± 68 | 0.85 |
| LDL Small, nmol/L | 203 ± 96 | 201 ± 93 | 0.90 |
| LDL Medium, nmol/L | 197 ± 90 | 184 ± 62 | 0.31 |
| LDL, Medium & Small, nmol/L | 400 ± 170 | 385 ± 140 | 0.56 |
| LDL, Large-b, nmol/L | 108 ± 55 | 101 ± 34 | 0.37 |
| LDL, Large-a, nmol/L | 127 ± 58 | 123 ± 48 | 0.68 |
| IDL, Small, nmol/L | 82 ± 24 | 83 ± 30 | 0.83 |
| IDL, Large, nmol/L | 117 ± 38 | 114 ± 33 | 0.67 |
| VLDL, Small, nmol/L | 45 ± 14 | 44 ± 15 | 0.75 |
| VLDL, Medium, nmol/L | 36 ± 12 | 35 ± 14 | 0.62 |
| VLDL, Large, nmol/L | 10 ± 5 | 10 ± 5 | 0.89 |
Abbreviations: HDL, high-density lipoprotein; IDL, intermediate-density lipoprotein; LDL, low-density lipoprotein; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; VLDL, very low-density lipoprotein.
The role of intrahepatic triglyceride accumulation in the lipoprotein profile was also assessed, comparing patients with vs without NAFLD, after adjustment for the presence of T2D and obesity in a multiple regression analysis. Table 4 summarizes the β coefficient for those lipoprotein subfractions that were independently associated with NAFLD, T2D or obesity (no adjustments for multiple comparisons were performed for this analysis). As can be observed, presence of NAFLD was associated with lower LDL size, more small and medium LDL particles, more VLDL particles of any size, and larger IDL particles. No significant associations were found between NAFLD and HDL particle numbers. After adjustment for NAFLD and T2D, obesity was not associated with any lipoprotein subfraction, except for a mild association with large HDL particles (β = 508, P = 0.042). Type 2 diabetes was independently associated with lower LDL size, and more small LDL particles. No significant increase in the overall VLDL particle number was observed with T2D after adjusting for NAFLD. However, there was a significant decrease in the small VLDL particles with T2D.
Table 4.
Effect of NAFLD, presence of T2D, and obesity on lipoprotein subfractions in a multiple regression analysis
| Β coefficient (CI 95%) P value |
NAFLD | T2D | Obesity |
|---|---|---|---|
| HDL Particle Number, nmol/L | 1077 (−765 to 2919) 0.25 |
19 (−1653 to 1690) 0.98 |
1560 (−253 to 3372) 0.091 |
| HDL, Small, nmol/L | 1060 (−330 to 2450) 0.134 |
80 (−1182 to 1341) 0.90 |
1051 (−317 to 2419) 0.131 |
| HDL, Large, nmol/L | 17 (−480 to 515) 0.94 |
−61 (−512 to 390) 0.79 |
508 (19 to 998)
0.042 |
| MIDZONE, nmol/L | 50 (−7 to 107) 0.086 |
16 (−36 to 68) 0.55 |
33 (−23 to 89) 0.25 |
| LDL Peak Size, Angstrom |
−3.2 (−5.4 to −1.0)
0.005 |
−4.3 (−6.3 to −2.3)
<0.001 |
−1.0 (−3.2 to 1.1) 0.34 |
| Total LDL, nmol/L |
155 (49 to 261)
0.004 |
103 (6 to 199)
0.037 |
26 (−79 to 130) 0.63 |
| Non-HDL Particle Number, nmol/L |
173 (47 to 299)
0.007 |
77 (−37 to 191) 0.184 |
21 (−103 to 144) 0.74 |
| LDL, Very Small-d, nmol/L | 7 (−1 to 15) 0.072 |
4 (−3 to 11) 0.31 |
0 (−8 to 7) 0.96 |
| LDL, Very Small-c, nmol/L | 13 (0 to 26) 0.057 |
12 (0 to 25)
0.046 |
2 (−12 to 15) 0.80 |
| LDL, Very Small-b, nmol/L |
30 (4 to 56)
0.024 |
29 (5 to 52)
0.017 |
4 (−22 to 29) 0.76 |
| LDL, Very Small-a, nmol/L |
31 (9 to 52)
0.006 |
28 (8 to 47)
0.006 |
1 (−20 to 23) 0.91 |
| LDL Small, nmol/L |
48 (17 to 79)
0.003 |
41 (13 to 69)
0.005 |
9 (−22 to 40) 0.56 |
| LDL Medium, nmol/L |
28 (2 to 55)
0.038 |
15 (−9 to 39) 0.21 |
10 (−16 to 36) 0.44 |
| LDL, Medium & Small, nmol/L |
76 (23 to 129)
0.005 |
56 (9 to 104)
0.021 |
19 (−33 to 71) 0.46 |
| LDL, Large-b, nmol/L | 6 (−10 to 21) 0.47 |
−6 (−20 to 8) 0.41 |
2 (−14 to 17) 0.82 |
| LDL, Large-a, nmol/L | −7 (−25 to 11) 0.44 |
−20 (−36 to −4)
0.016 |
−2 (−20 to 16) 0.81 |
| IDL, Small, nmol/L |
−13 (−24 to −2)
0.022 |
−11 (−21 to −1)
0.028 |
−3 (−13 to 8) 0.65 |
| IDL, Large, nmol/L |
18 (5 to 30)
0.005 |
−10 (−21 to 1) 0.080 |
2 (−10 to 14) 0.74 |
| VLDL, Small, nmol/L |
5 (0 to 11)
0.037 |
−5 (−10 to 0)
0.032 |
−1 (−6 to 4) 0.75 |
| VLDL, Medium, nmol/L |
6 (1 to 10)
0.015 |
0 (−5 to 4) 0.87 |
−3 (−7 to 2) 0.24 |
| VLDL, Large, nmol/L |
2 (0 to 4)
0.016 |
1 (0 to 3) 0.106 |
−1 (−3 to 1) 0.25 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; IDL, intermediate-density lipoprotein; NAFLD, nonalcoholic fatty liver disease; T2D, type 2 diabetes mellitus; VLDL, very low-density lipoprotein.
Apolipoproteomic Composition of HDL Particles
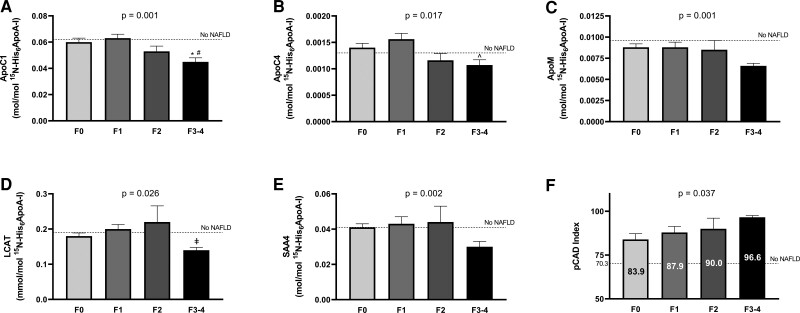
In Table 5, we have summarized the results of the apolipoproteomic approach implemented in isolated ApoA-I-containing particles dividing patients based on the presence or absence of advanced fibrosis. A total of 28 proteins were identified and measured. Of these, 5 were found to be significantly different between the groups after adjustment for multiple testing as described in the statistical section. As can be observed in Table 5, ApoC-I, ApoC-IV, ApoM, LCAT, and SAA4 were significantly reduced in patients with advanced fibrosis. Moreover, these results were unchanged after adjustment for the presence of diabetes (ApoC-I [P = 0.001], ApoC-IV [P = 0.010], ApoM [P = 0.015], LCAT [P = 0.014], and SAA4 [P = 0.015]). No association was found between any of the HDL-bound proteins and hemoglobin A1c. In Fig. 1 (panels A to E) we have plotted these protein levels among patients with NAFLD based on their liver fibrosis stage. Levels in patients without NAFLD by 1H-MRS (n = 45) can also be observed in the figure as a dotted line. As can be observed, ApoC-I and ApoC-IV showed a reduction even with moderate fibrosis (stage 2). In contrast, ApoM, LCAT, and SAA4 showed an abrupt reduction once advanced fibrosis was reached, but no stepwise reduction with moderate fibrosis.
Table 5.
Characteristics of Apo-A1 particles based on presence of NAFLD and/or advanced fibrosis
| [mol/mol 15N-His6ApoA-I] | NAFLD with F0-F2 (n = 114) |
NAFLD with advanced fibrosis (n = 26) |
P between NAFLD groups |
|---|---|---|---|
| ApoA1 | 0.27 ± 0.09 | 0.23 ± 0.06 | 0.036 |
| ApoC1 | 0.060 ± 0.020 | 0.045 ± 0.015 | <0.001 |
| ApoC2 | 0.023 ± 0.012 | 0.019 ± 0.008 | 0.087 |
| ApoC3 | 0.055 ± 0.029 | 0.045 ± 0.023 | 0.098 |
| ApoC4 | 0.0014 ± 0.0007 | 0.0011 ± 0.0005 | 0.012 |
| A1AT | 0.019 ± 0.021 | 0.017 ± 0.009 | 0.65 |
| ANGT | 0.0007 ± 0.0004 | 0.0006 ± 0.0002 | 0.35 |
| ApoA2 | 1.13 ± 0.64 | 0.91 ± 0.26 | 0.083 |
| ApoA4 | 0.0008 ± 0.0006 | 0.0008 ± 0.0005 | 0.53 |
| ApoD | 0.0091 ± 0.0052 | 0.0069 ± 0.0031 | 0.038 |
| ApoE | 0.006 ± 0.005 | 0.004 ± 0.002 | 0.085 |
| ApoL1 | 0.0022 ± 0.0015 | 0.0018 ± 0.0005 | 0.132 |
| ApoM | 0.0086 ± 0.0038 | 0.0066 ± 0.0017 | 0.008 |
| CTEP | 0.0008 ± 0.0008 | 0.0009 ± 0.0007 | 0.65 |
| CLUS | 0.004 ± 0.004 | 0.003 ± 0.001 | 0.36 |
| CO3 | 0.005 ± 0.006 | 0.004 ± 0.002 | 0.50 |
| HPT | 0.024 ± 0.037 | 0.019 ± 0.030 | 0.53 |
| KAIN | 0.0013 ± 0.0012 | 0.0011 ± 0.0006 | 0.40 |
| LCAT [×103] | 0.19 ± 0.10 | 0.14 ± 0.04 | 0.014 |
| LpPLA2 [×103] | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.64 |
| PLTP [×103] | 0.16 ± 0.08 | 0.15 ± 0.06 | 0.38 |
| PON1 | 0.0028 ± 0.0018 | 0.0022 ± 0.0008 | 0.103 |
| PON3 [×103] | 0.048 ± 0.025 | 0.037 ± 0.014 | 0.043 |
| RET4 | 0.0004 ± 0.0003 | 0.0003 ± 0.0001 | 0.101 |
| SAA1/2 | 0.0060 ± 0.0064 | 0.0043 ± 0.0031 | 0.185 |
| SAA4 | 0.042 ± 0.024 | 0.030 ± 0.014 | 0.016 |
| TTHY | 0.043 ± 0.052 | 0.033 ± 0.019 | 0.35 |
| VTNC | 0.0037 ± 0.0025 | 0.0032 ± 0.0010 | 0.28 |
Abbreviations: Apo, apolipoprotein; A1AT, alpha-1-antitrypsin; ANGT, angiotensinogen; CTEP, cholesteryl ester transfer protein; CLUS, clusterin/Apo J; CO3, complement C3; HPT, haptoglobin; KAIN, kallistatin; LCAT, lecithin cholesterol acyl-transferase; LpPLA2, lipoprotein-associated phospholipase A2; NAFLD, nonalcoholic fatty liver disease; PLTP, phospholipid transfer protein; PON, paraoxonase; RET4, retinol binding protein 4; SAA, serum amyloid A; TTHY, transthyretin; VTNC, vitronectin.
Figure 1.
Protein levels (panels A-E) and pCAD index (panel F) across the different fibrosis stages. Dotted line represents levels in patients without NAFLD by 1H-MRS (n = 45). P values in the graphs represent trend across all fibrosis stages. Bonferroni-adjusted pairwise comparisons: *P = 0.009 vs F0; #P = 0.001 vs F1; ʌP = 0.013 vs F1; ‡P = 0.037 vs F2.
When patients were divided based on the presence or absence of biopsy-proven NASH, no differences were observed in any of the HDL-bound proteins (Table 6). When comparing patients with vs without NAFLD, we observed that 2 proteins were significantly different between the groups after adjusting for multiple comparison and for the presence of diabetes. These proteins were ApoA-I (0.32 ± 0.02 vs 0.26 ± 0.01 mol/mol 15N-His6ApoA-I, P = 0.001) and PON3 (0.066 ± 0.006 vs 0.046 ± 0.002 mmol/mol 15N-His6ApoA-I, P < 0.001). Of note, these 2 proteins were also similarly (and independently) associated with the presence of diabetes ApoA-I (P < 0.001) and PON3 (P < 0.001).
Table 6.
Characteristics of Apo-A1 particles based on presence of NASH
| [mol/mol 15N-His6ApoA-I] | NAFLD without NASH (n = 70) |
NAFLD with NASH (n = 70) |
P value |
|---|---|---|---|
| ApoA1 | 0.27 ± 0.09 | 0.26 ± 0.09 | 0.49 |
| ApoC1 | 0.059 ± 0.020 | 0.056 ± 0.020 | 0.41 |
| ApoC2 | 0.023 ± 0.012 | 0.021 ± 0.010 | 0.25 |
| ApoC3 | 0.057 ± 0.032 | 0.050 ± 0.023 | 0.185 |
| ApoC4 | 0.0014 ± 0.0007 | 0.0013 ± 0.0006 | 0.25 |
| A1AT | 0.018 ± 0.014 | 0.020 ± 0.023 | 0.66 |
| ANGT | 0.0007 ± 0.0004 | 0.0007 ± 0.0004 | 0.99 |
| ApoA2 | 1.11 ± 0.45 | 1.07 ± 0.71 | 0.70 |
| ApoA4 | 0.0009 ± 0.0007 | 0.0008 ± 0.0005 | 0.38 |
| ApoD | 0.0091 ± 0.0036 | 0.0082 ± 0.0060 | 0.28 |
| ApoE | 0.006 ± 0.005 | 0.005 ± 0.005 | 0.59 |
| ApoL1 | 0.0021 ± 0.0011 | 0.0022 ± 0.0017 | 0.76 |
| ApoM | 0.0087 ± 0.0037 | 0.0079 ± 0.0035 | 0.179 |
| CTEP | 0.0009 ± 0.0009 | 0.0008 ± 0.0006 | 0.39 |
| CLUS | 0.003 ± 0.003 | 0.004 ± 0.005 | 0.64 |
| CO3 | 0.005 ± 0.004 | 0.005 ± 0.006 | 0.67 |
| HPT | 0.020 ± 0.017 | 0.026 ± 0.048 | 0.31 |
| KAIN | 0.0012 ± 0.0009 | 0.0012 ± 0.0013 | 0.99 |
| LCAT [×103] | 0.19 ± 0.00009 | 0.18 ± 0.09 | 0.41 |
| LpPLA2 [×103] | 0.02 ± 0.00001 | 0.02 ± 0.01 | 0.76 |
| PLTP [×103] | 0.16 ± 0.00006 | 0.16 ± 0.08 | 0.85 |
| PON1 | 0.0029 ± 0.0017 | 0.0025 ± 0.0017 | 0.24 |
| PON3 [×103] | 0.047 ± 0.025 | 0.045 ± 0.022 | 0.60 |
| RET4 | 0.0004 ± 0.0002 | 0.0004 ± 0.0003 | 0.90 |
| SAA1/2 | 0.0065 ± 0.0075 | 0.0049 ± 0.0037 | 0.108 |
| SAA4 | 0.043 ± 0.024 | 0.038 ± 0.021 | 0.21 |
| TTHY | 0.039 ± 0.029 | 0.043 ± 0.061 | 0.70 |
| VTNC | 0.0036 ± 0.0018 | 0.0037 ± 0.0028 | 0.78 |
Abbreviations: Apo, apolipoprotein; A1AT, alpha-1-antitrypsin; ANGT, angiotensinogen; CTEP, cholesteryl ester transfer protein; CLUS, clusterin/Apo J; CO3, complement C3; HPT, haptoglobin; KAIN, kallistatin; LCAT, lecithin cholesterol acyl-transferase; LpPLA2, lipoprotein-associated phospholipase A2; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PLTP, phospholipid transfer protein; PON, paraoxonase; RET4, retinol binding protein 4; SAA, serum amyloid A; TTHY, transthyretin; VTNC, vitronectin.
Sensitivity Analyses
Because we observed a lower HDL particle number in patients with NAFLD and advanced fibrosis compared to those without advanced fibrosis (Table 2), we also calculated differences in HDL-bound protein levels after adjustment for HDL particle number. With this approach, we observed an overall similar trend, but with attenuated significance. Only ApoC-I levels remained significantly different between patients with and without advanced fibrosis (ApoC-I [P = 0.020], ApoC-IV [P = 0.066], ApoM [P = 0.128], LCAT [P = 0.130], and SAA4 [P = 0.071]). When considering a more stringent false discovery rate (FDR) of only 0.025, we also observed that differences in ApoC-I levels remained statistically significant. Finally, because patients with advanced liver fibrosis had higher use of metformin, sulfonylureas, and insulin, we also repeated the analyses after adjusting for their use. Sulfonylurea or insulin use had no effect on HDL-bound protein composition, and all proteins remained significantly different between patients with or without advanced liver fibrosis (ApoC-I [P < 0.001], ApoC-IV [P = 0.005], ApoM [P = 0.010], LCAT [P = 0.011], and SAA4 [P = 0.014]). However, when adjusting for metformin use, ApoM (P = 0.040), LCAT (P = 0.034), and SAA4 (P = 0.044) showed a small reduction in the strength of the association, suggesting some interaction with metformin use.
Cardiovascular Risk in Patients With NAFLD Based on Histological Severity
Based on measurements of 5 of the above apolipoproteins (ApoA-I and ApoC-I to C-IV), the pCAD index was calculated (19). Patients with NAFLD with advanced fibrosis had significantly higher pCAD index compared to patients with NAFLD without advanced fibrosis (97 ± 5 vs 86 ± 25, P = 0.04). Figure 1 (panel F) shows pCAD index for each fibrosis stage. As can be observed, there was a stepwise increase in pCAD index with every fibrosis stage (P for trend 0.037). Of note, pCAD index was significantly increased in all patients with NAFLD compared to controls without NAFLD (represented as dotted line in Fig. 1D). No difference was observed in pCAD index between patients with or without NASH (89 ± 23 vs 87 ± 23, P = 0.67).
Discussion
There is growing evidence that the presence of NAFLD is associated with an increased risk of cardiovascular mortality (27). Due to the typically abnormal metabolic profile of patients with NAFLD, it would be reasonable to think that this is the result of common metabolic abnormalities. However, liver fibrosis stages have been shown to predict cardiovascular and overall mortality in these patients, independently of other well-known cardiovascular risk factors (10, 11). Despite prior efforts to elucidate the mediators of this liver–heart crosstalk, these have basically remained elusive. In this work, we have identified a potential mechanism to explain increased cardiovascular mortality in patients with NAFLD with advanced liver fibrosis. Specifically, we observed that the apolipoproteinic composition of HDL particles may be affected in these patients (ie, decreased ApoC-I, ApoC-IV, ApoM, LCAT, and SAA4). Moreover, these changes translated into a higher pCAD index (19), a score that is associated with coronary artery disease and cardiovascular mortality, independently of circulating levels of ApoA-I and ApoB (20).
The liver is the main source of production of many of the apolipoproteins that participate in the formation of VLDL, as well as HDL particles. Therefore, it is reasonable to think that increasing severity of liver fibrosis could progressively affect their synthesis. In support of this, patients with cirrhosis, especially those with decompensation, have significantly lower levels of total cholesterol, HDL-C, and LDL-C (28). Moreover, HDL-C levels have been shown to predict mortality among patients with cirrhosis (13). Of note, in prior studies, HDL-C levels were only minimally decreased in patients with stable cirrhosis, but markedly reduced with acute decompensation or acute on chronic liver failure (13). Our cohort of patients included 26 patients with advanced fibrosis (21 had fibrosis stage 3 and only 5 had non-decompensated [stable] cirrhosis or fibrosis stage 4). Thus, our study included patients with significantly less severe liver disease compared to studies focusing on cirrhotic patients. Therefore, it is not surprising that we did not observe the changes previously described for patients with decompensated liver cirrhosis. It is also reasonable to consider that gradual increments of liver fibrosis can be associated with more subtle changes in lipoproteins, like the ones described in this work.
Specifically, our work identified 5 HDL-bound apolipoproteins that were significantly decreased in patients with NAFLD and advanced fibrosis: ApoC-I, ApoC-IV, ApoM, LCAT, and SAA4. In line with this, prior studies in patients with chronic liver disease identified similar differences in some of these proteins. Trieb et al (29) described reduced serum LCAT activity in patients with compensated cirrhosis, as well as a reduction in ApoC-I in isolated HDL from patients with cirrhosis (only statistically significant for those with decompensation). Similar to our study, they did not find differences in ApoA-I levels in isolated HDL particles in patients with stable cirrhosis (29). Niu et al (30) also described a significant reduction in ApoC-I and Apo-M levels in serum of patients with cirrhosis. Moreover, these authors also reported a reduction in serum ApoM levels in patients with NAFLD and T2D compared with healthy controls. ApoM is the major carrier of sphingosine-1-phosphate (SP1) and resides primarily in HDL particles (31). Several studies suggest that ApoM maintains and regulates SP1 levels, which have been shown to be associated with liver fibrosis in several studies (31). In a recent study assessing untargeted lipidomics in patients with cirrhosis, SP1 was found to be significantly reduced in patients with cirrhosis (32). However, the exact role of SP1 in liver fibrosis is unclear, as studies have shown both protective as well as harmful effects (31). In addition, the ApoM/SP1 axis is associated with proliferator-activated receptor-γ (PPAR-γ), the specific target of thiazolidinediones, a group of drugs with known beneficial effects in NAFLD. Indeed, rosiglitazone increases ApoM expression in rat livers (33). We found no prior reports on SAA4 or ApoC-IV levels in liver diseases.
In addition to NAFLD-related fibrosis, many of these proteins have also been found to be decreased in patients with advanced liver fibrosis from other etiologies. As they are highly expressed in the liver in normal conditions, this may imply that advanced fibrosis can be associated with some degree of impairment in their synthesis. Therefore, it is possible that the findings reported in our manuscript may apply to patients with advanced fibrosis from other etiologies. For example, ApoC-I was identified as one of the serum proteins that allowed to correctly differentiate patients with hepatitis C virus (HCV)-related cirrhosis compared with lower stages of fibrosis (F1 and F2) (34). Decreased LCAT activity was reported more than 50 years ago in patients with cirrhosis of any cause (35). In support of the hypothesis that advanced fibrosis may affect hepatic synthesis of these HDL-related proteins, we observed no significant differences in HDL-bound proteins when comparing patients with vs without NASH. Moreover, the comparison of patients with NAFLD vs no NAFLD only found differences in HDL-associated ApoA-I and PON3, 2 HDL-associated proteins with well-described reductions in insulin-resistant states and hyperglycemia (36, 37). Of note, these 2 HDL-associated proteins were also significantly (and independently) reduced in patients with T2D.
Prior studies by our (3) and other groups (7, 38), have shown that patients with NAFLD are characterized by smaller LDL particles and more ApoB particles. Yet, these differences dissipated when patients with NASH were compared to those with isolated steatosis (ie, NAFLD without NASH) or when patients with advanced fibrosis/cirrhosis were compared to patients with less severe liver disease (3, 7). This finding conflicts with data showing higher cardiovascular disease and mortality with worse liver histology (ie, increasing fibrosis stage) (27), and suggests that additional mechanisms (different from LDL or ApoB particles) may be participating. Nevertheless, prior studies focusing on HDL subfractions failed to show marked differences in patients with severe liver disease (7). Similar to prior studies, in the current work, we did not observe profound differences in lipoprotein subfractions or LDL particle size with more severe liver histology. We did observe a lower number of HDL particles in patients with NASH and in those with advanced fibrosis. However, due to the relatively small magnitude of this difference, it is unlikely to fully explain the reported increased cardiovascular risk of patients with advanced fibrosis. The identification of significant changes in some HDL-bound protein levels may suggest that HDL function (rather than HDL levels) may be impaired in these patients. In support of this, similar trends persisted after adjusting for HDL particle numbers, suggesting that these changes may reflect a difference in HDL composition. Most importantly, the observed differences in HDL-bound proteins translated into increased cardiovascular risk with worsening liver fibrosis based on the pCAD score. This score has previously been validated and found to be independently associated with presence of coronary artery disease, as well as cardiovascular mortality (19, 20). This suggests that the observed changes in HDL-bound proteins may at least partially explain the increased cardiovascular risk in patients with NAFLD with advanced fibrosis. Specifically, pCAD index calculation includes ApoA-I and ApoC-I to C-IV levels. Therefore, based on significant differences in ApoC-I and ApoC-IV in our cohort, it is possible that these 2 proteins may be playing a larger role in the increased cardiovascular risk. However, although they did not reach statistical significance ApoA-I, ApoC-II, and ApoC-III were also numerically lower in patients with advanced fibrosis. Therefore, larger studies are needed to assess whether these proteins also play a role in the cardiovascular risk of these patients. Prior reports have reported both pro- (39) and anti-atherogenic (40) properties of Apo-CI levels, further complicating the interpretation of the results.
Patients with NAFLD are characterized by a cluster of metabolic abnormalities. Hence, it is sometimes necessary to assess whether differences are due to liver disease or any of the other metabolic abnormalities. Patients with vs without advanced fibrosis in our cohort were well-matched for most of the important clinical and demographic characteristics, thus minimizing the risk of confounding factors. However, an important limitation of the study is that patients with advanced fibrosis had higher presence of diabetes, higher A1c, and higher use of diabetes medications, which could have confounded the results. In order to minimize the potential effect of diabetes, all analyses were repeated after adjustment for presence of diabetes, without any significant differences. In addition, due to their higher cardiovascular risk, our cohort had a significant use of statins, which could have also affected results. However, statin use was similar in patients with or without advanced fibrosis, so this was unlikely to have an important impact on results. Finally, our advanced fibrosis group was relatively small (n = 26), so results may need to be confirmed in larger studies.
In summary, we have shown that patients with NAFLD and advanced fibrosis have different levels of HDL-bound proteins. Moreover, these changes translated into a worse cardiovascular risk score (pCAD index). If confirmed in larger studies, these findings may explain why patients with NAFLD and advanced fibrosis have increased cardiovascular mortality, independently of other cardiovascular risk factors. It is possible that these changes are related to hepatic synthetic defects secondary to fibrosis deposition. Furthermore, these changes may not be specific to patients with NAFLD and advanced fibrosis, and they may extrapolate to other liver conditions. While more research is needed to understand the true implications of the changes observed in our study, it appears that HDL particles may play a key role in the crosstalk between liver and heart.
Abbreviations
- Apo
apolipoprotein
- BMI
body mass index
- HDL
high-density lipoprotein
- HDL-C
high-density lipoprotein cholesterol
- 1H-MRS
proton magnetic resonance spectroscopy
- IDL
intermediate-density lipoprotein
- LDL
low-density lipoprotein
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- SP1
sphingosine-1-phosphate
- T2D
type 2 diabetes
- VLDL
very low-density lipoprotein
Contributor Information
Fernando Bril, Endocrinology, Diabetes and Metabolism, Department of Medicine, University of Alabama at Birmingham, Birmingham, AL 35233, USA; Division of Endocrinology, Diabetes and Metabolism, Birmingham VA Medical Center, Birmingham, AL 35233, USA.
Ryan W Pearce, Quest Diagnostics Cardiometabolic Center of Excellence, Cleveland HeartLab, Cleveland, OH 44103, USA.
Timothy S Collier, Quest Diagnostics Cardiometabolic Center of Excellence, Cleveland HeartLab, Cleveland, OH 44103, USA.
Michael J McPhaul, Division of Endocrinology, Diabetes & Metabolism, Quest Diagnostics Nichols Institute, San Juan Capistrano, CA 92675, USA.
Funding
Early-Career Research Grant from The Obesity Society (F.B.); University of Florida Clinical and Translational Science Institute Pilot Project Award (F.B.). Research reported in this publication was supported by the University of Florida Clinical and Translational Science Institute, which is supported in part by the National Institutes of Health (NIH) National Center for Advancing Translational Sciences under award number UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author Contributions
F.B.: study design, funding, patient recruitment and follow-up, data acquisition and interpretation, statistical analysis, and writing, editing, and final revision of the manuscript. R.W.P.: development of experimental methods, data interpretation, and critical revision of the manuscript. T.S.C.: development of experimental methods, data interpretation, and critical revision of the manuscript. M.J.M.: study design, data interpretation, and critical revision of the manuscript. F.B. is the guarantor of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
F.B.: nothing to disclose in relation to this manuscript; R.W.P.: Employed by Quest Diagnostics; T.S.C.: Employed by and owns stock in Quest Diagnostics; M.J.M.: Employed by and owns stock in Quest Diagnostics.
Data Availability
Data available upon request to corresponding author.
References
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. [DOI] [PubMed] [Google Scholar]
- 2. Godinez-Leiva E, Bril F. Nonalcoholic Fatty Liver Disease (NAFLD) for primary care providers: beyond the liver. Curr Hypertens Rev. 2021;17(2):94–111. [DOI] [PubMed] [Google Scholar]
- 3. Bril F, Sninsky JJ, Baca AM, et al. . Hepatic steatosis and insulin resistance, but not steatohepatitis, promote atherogenic dyslipidemia in NAFLD. J Clin Endocrinol Metab. 2016;101(2):644–652. [DOI] [PubMed] [Google Scholar]
- 4. Bril F, Barb D, Portillo-Sanchez P, et al. . Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017;65(4):1132–1144. [DOI] [PubMed] [Google Scholar]
- 5. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589–600. [DOI] [PubMed] [Google Scholar]
- 6. Imajo K, Hyogo H, Yoneda M, et al. . LDL-Migration Index (LDL-MI), an indicator of small dense low-density lipoprotein (sdLDL), is higher in non-alcoholic steatohepatitis than in non-alcoholic fatty liver: a multicenter cross-sectional study. PLoS One. 2014;9(12):e115403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Siddiqui MS, Fuchs M, Idowu MO, et al. . Severity of nonalcoholic fatty liver disease and progression to cirrhosis are associated with atherogenic lipoprotein profile. Clin Gastroenterol Hepatol. 2015;13(5):1000–1008.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Angulo P, Kleiner DE, Dam-Larsen S, et al. . Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389–397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dulai PS, Singh S, Patel J, et al. . Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65(5):1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Henson JB, Simon TG, Kaplan A, Osganian S, Masia R, Corey KE. Advanced fibrosis is associated with incident cardiovascular disease in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2020;51(7):728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ekstedt M, Hagström H, Nasr P, et al. . Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–1554. [DOI] [PubMed] [Google Scholar]
- 12. Patel S, Siddiqui MB, Chandrakumaran A, et al. . Progression to cirrhosis leads to improvement in atherogenic milieu. Dig Dis Sci. 2021;66(1):263–272. [DOI] [PubMed] [Google Scholar]
- 13. Trieb M, Rainer F, Stadlbauer V, et al. . HDL-related biomarkers are robust predictors of survival in patients with chronic liver failure. J Hepatol. 2020;73(1):113–120. [DOI] [PubMed] [Google Scholar]
- 14. Bril F, Kadiyala S, Portillo Sanchez P, et al. . Plasma thyroid hormone concentration is associated with hepatic triglyceride content in patients with type 2 diabetes. J Investig Med. 2016;64(1):63–68. [DOI] [PubMed] [Google Scholar]
- 15. Bril F, Maximos M, Portillo-Sanchez P, et al. . Relationship of vitamin D with insulin resistance and disease severity in non-alcoholic steatohepatitis. J Hepatol. 2015;62(2):405–411. [DOI] [PubMed] [Google Scholar]
- 16. Bril F, Portillo-Sanchez P, Liu IC, Kalavalapalli S, Dayton K, Cusi K. Clinical and histologic characterization of nonalcoholic steatohepatitis in African American patients. Diabetes Care. 2018;41(1):187–192. [DOI] [PubMed] [Google Scholar]
- 17. Cusi K, Orsak B, Bril F, et al. . Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305–315. [DOI] [PubMed] [Google Scholar]
- 18. Collier TS, Jin Z, Topbas C, Bystrom C. Rapid affinity enrichment of human apolipoprotein A-I associated lipoproteins for proteome analysis. J Proteome Res. 2018;17(3):1183–1193. [DOI] [PubMed] [Google Scholar]
- 19. Jin Z, Collier TS, Dai DLY, et al. . Development and validation of apolipoprotein AI-associated lipoprotein proteome panel for the prediction of cholesterol efflux capacity and coronary artery disease. Clin Chem. 2019;65(2):282–290. [DOI] [PubMed] [Google Scholar]
- 20. Natarajan P, Collier TS, Jin Z, et al. . Association of an HDL apolipoproteomic score with coronary atherosclerosis and cardiovascular death. J Am Coll Cardiol. 2019;73(17):2135–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bril F, Barb D, Lomonaco R, Lai J, Cusi K. Change in hepatic fat content measured by MRI does not predict treatment-induced histological improvement of steatohepatitis. J Hepatol. 2020;72(3):401–410. [DOI] [PubMed] [Google Scholar]
- 22. Bril F, Ortiz-Lopez C, Lomonaco R, et al. . Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int. 2015;35(9):2139–2146. [DOI] [PubMed] [Google Scholar]
- 23. Chalasani N, Younossi Z, Lavine JE, et al. . The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. [DOI] [PubMed] [Google Scholar]
- 24. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) . EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59(6):1121–1140. [DOI] [PubMed] [Google Scholar]
- 25. Kleiner DE, Brunt EM, Van Natta M, et al. . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. [DOI] [PubMed] [Google Scholar]
- 26. Bedossa P, Poitou C, Veyrie N, et al. . Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56(5):1751–1759. [DOI] [PubMed] [Google Scholar]
- 27. Mantovani A, Csermely A, Petracca G, et al. . Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(11):903–913. [DOI] [PubMed] [Google Scholar]
- 28. Cicognani C, Malavolti M, Morselli-Labate AM, Zamboni L, Sama C, Barbara L. Serum lipid and lipoprotein patterns in patients with liver cirrhosis and chronic active hepatitis. Arch Intern Med. 1997;157(7):792–796. [PubMed] [Google Scholar]
- 29. Trieb M, Horvath A, Birner-Gruenberger R, et al. . Liver disease alters high-density lipoprotein composition, metabolism and function. Biochim Biophys Acta. 2016;1861(7):630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Niu L, Geyer PE, Wewer Albrechtsen NJ, et al. . Plasma proteome profiling discovers novel proteins associated with non-alcoholic fatty liver disease. Mol Syst Biol. 2019;15(3):e8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen Z, Hu M. The apoM-S1P axis in hepatic diseases. Clin Chim Acta. 2020;511:235–242. [DOI] [PubMed] [Google Scholar]
- 32. Clària J, Curto A, Moreau R, et al. . Untargeted lipidomics uncovers lipid signatures that distinguish severe from moderate forms of acutely decompensated cirrhosis. J Hepatol. 2021;75(5):1116–1127. [DOI] [PubMed] [Google Scholar]
- 33. Luo G, Feng Y, Zhang J, et al. . Rosiglitazone enhances apolipoprotein M (Apom) expression in rat's Liver. Int J Med Sci. 2014;11(10):1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Göbel T, Vorderwulbecke S, Hauck K, Fey H, Haussinger D, Erhardt A. New multi protein patterns differentiate liver fibrosis stages and hepatocellular carcinoma in chronic hepatitis C serum samples. World J Gastroenterol. 2006;12(47):7604–7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Simon JB, Scheig R. Serum cholesterol esterification in liver disease. N Engl J Med. 1970;283(16):841–846. [DOI] [PubMed] [Google Scholar]
- 36. Shen Y, Ding FH, Sun JT, et al. . Association of elevated apoA-I glycation and reduced HDL-associated paraoxonase1, 3 activity, and their interaction with angiographic severity of coronary artery disease in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2015;14(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shao B, Zelnick LR, Wimberger J, et al. . Albuminuria, the high-density lipoprotein proteome, and coronary artery calcification in type 1 diabetes Mellitus. Arterioscler Thromb Vasc Biol. 2019;39(7):1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sonmez A, Nikolic D, Dogru T, et al. . Low- and high-density lipoprotein subclasses in subjects with nonalcoholic fatty liver disease. J Clin Lipidol. 2015;9(4):576–582. [DOI] [PubMed] [Google Scholar]
- 39. Hansen JB, Fernández JA, Notø AT, Deguchi H, Bjorkegren J, Mathiesen EB. The apolipoprotein C-I content of very-low-density lipoproteins is associated with fasting triglycerides, postprandial lipemia, and carotid atherosclerosis. J Lipids. 2011;2011:271062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yan LR, Wang DX, Liu H, et al. . A pro-atherogenic HDL profile in coronary heart disease patients: an iTRAQ labelling-based proteomic approach. PLoS One. 2014;9(5):e98368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available upon request to corresponding author.