Abstract
Hypophosphatemic rickets typically presents in infancy or early childhood with skeletal deformities and growth plate abnormalities. The most common causes are genetic (such as X-linked hypophosphatemia), and these typically will result in lifelong hypophosphatemia and osteomalacia. Knowledge of phosphate metabolism, including the effects of fibroblast growth factor 23 (FGF23) (an osteocyte produced hormone that downregulates renal phosphate reabsorption and 1,25-dihydroxyvitamin-D (1,25(OH)2D) production), is critical to determining the underlying genetic or acquired causes of hypophosphatemia and to facilitate appropriate treatment. Serum phosphorus should be measured in any child or adult with musculoskeletal complaints suggesting rickets or osteomalacia. Clinical evaluation incudes thorough history, physical examination, laboratory investigations, genetic analysis (especially in the absence of a guiding family history), and imaging to establish etiology and to monitor severity and treatment course. The treatment depends on the underlying cause, but often includes active forms of vitamin D combined with phosphate salts, or anti-FGF23 antibody treatment (burosumab) for X-linked hypophosphatemia. The purpose of this article is to explore the approach to evaluating hypophosphatemic rickets and its treatment options.
Keywords: hypophosphatemia, rickets, fibroblast growth factor 23, X-linked hypophosphatemia, burosumab
Background
Clinical Presentation of Hypophosphatemic Rickets
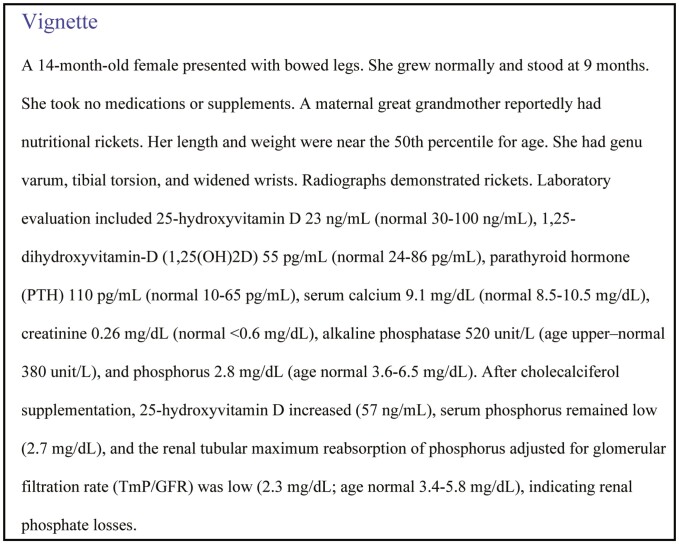
Rickets is a skeletal abnormality that results from impaired apoptosis of hypertrophic chondrocytes and delayed mineralization of growth plate cartilage (1). Most rickets remains nutritional in origin (about 85%); however, hypophosphatemic disorders are important to recognize (2). Children with hypophosphatemic rickets present similarly to nutritional rickets with poor growth, deformities of weight-bearing limbs such as genu varum or valgus, a “rachitic rosary” involving the costochondral junctions, or enlargement of wrist, knees, or ankles. Children may have hypotonia, delayed motor development, myopathy, or bone pain. Gait may waddling with in-toeing. Children with rickets, especially hypophosphatemic rickets, often have short stature with disproportionately short lower extremities, typically after age 1 year (3). Frontal bossing is common, while some may develop craniosynostosis (4). Dental abscesses are also common in X-linked hypophosphatemia (XLH). Family history may lead to detection of genetic forms prior to demonstration of rickets.
In adults, chronic hypophosphatemia causes osteomalacia with musculoskeletal pain and weakness, especially involving the lower extremities and impaired mobility (1). Bone pain may accompany acute or chronic insufficiency fractures or pseudofractures. Adults with hypophosphatemic osteomalacia may also have fatigue, enthesopathy, osteoarthritis, and dental disease, and are often short with persistence of childhood skeletal deformities (5-7).
Phosphorus Metabolism
Hydroxyapatite incorporates about 85% of the body’s inorganic phosphate; thus phosphate is critical to skeletal growth and function (8). Insufficient phosphate causes failure of hypertrophic chondrocytes to apoptose, disorganization of the growth plate, and delayed mineralization of the growth plate and osteoid (9). The intracellular compartment contains most remaining phosphate, with roles in DNA, RNA, energy metabolism, cellular structural components, and intracellular signaling processes. Serum phosphorus measurements detect production of a phosphomolybdate complex from inorganic phosphate (10). Hypophosphatemia impairs several cellular processes, causing neuromuscular dysfunction, especially when acute or severe. However, chronic hypophosphatemia induces rickets and osteomalacia (8).
Phosphate is abundant in most foods; therefore, nutritional phosphate deficiency is uncommon. Paracellular sodium–independent phosphate absorption is passive and concentration dependent (11). Active intestinal phosphate absorption is upregulated by 1,25(OH)2D through expression of sodium phosphate transporter IIb and type III sodium–dependent phosphate transporters.
Fibroblast growth factor 23 (FGF23) is a protein hormone produced primarily in osteocytes (12). FGF23 gene expression is increased by 1,25(OH)2D, high dietary phosphate intake, or hyperphosphatemia, though the phosphate-sensing mechanism has been elusive. High phosphate diet induces tyrosine phosphorylation of FGFR1c in the absence of FGF ligands, activating extracellular signal–regulated kinase signaling and inducing expression of GALNT3 to regulate post-translational modification of FGF23 protein (13). O-glycosylation of intact FGF23 via N-acetyl-galactosaminyltransferase 3 (encoded by GALNT3) prevents proteolytic cleavage of FGF23 into inactive fragments (14). Increasing GALNT3 expression contributes to increases in serum intact FGF23 (13). FGF23 gene expression is also stimulated by FGFR1 liganded signaling, PTH, leptin, catecholamines, mineralocorticoids, and iron deficiency (15, 16). Hypocalcemia appears to impede FGF23 production (17). PHEX and DMP1 are expressed in osteoblast/osteocytes and dentinoblasts (18, 19). Deficiency of PHEX or DMP1 results in increased FGF23 gene expression and circulating FGF23, though it is not clear that PHEX has a role in normal FGF23 regulation (15, 20, 21). FGF23 binds to alpha klotho as a cofactor to form a ternary subunit which stimulates FGFR1 (12).
Phosphorus balance is regulated at the kidney. Phosphorus is filtered at the glomerulus; then mostly reabsorbed in the proximal renal tubule. There is no paracellular transport of phosphorus in the kidney due to tight junction proteins. Active phosphorus reabsorption is downregulated by PTH and FGF23; both decrease surface expression of sodium-dependent phosphate cotransport proteins (NaPi2a and NaPi2c) in the proximal renal tubules, resulting in phosphaturia (1). Excesses of either PTH or FGF23 cause hypophosphatemia, while hyperphosphatemia develops during deficiency of either PTH or FGF23, indicating that neither hormone can fully compensate for the absence of the other (22).
FGF23 and PTH have opposing effects on vitamin D metabolism. FGF23 inhibits vitamin D activation via renal 1-alpha-hydroxylase and stimulates vitamin D degradation via 24-hydroxylase. In contrast, PTH stimulates vitamin D activation and inhibits its degradation. During dietary phosphate deficiency, FGF23 declines and 1,25(OH)2D increases, together resulting in higher intestinal absorption and renal reabsorption (11). High-dose 1,25(OH)2D partially antagonizes the phosphaturic effect of FGF23 (23).
Differential Diagnosis of Hypophosphatemia
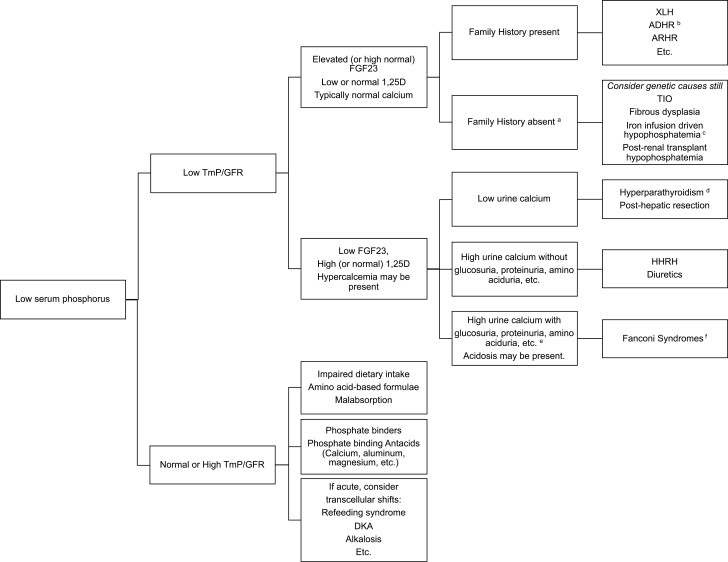
While hypophosphatemia may occur in settings where calcium-related abnormalities are the primary feature, such as severe vitamin D deficiency, genetic primary vitamin D metabolism defects (24-27), or primary or secondary hyperparathyroidism, in this article we focus on conditions with hypophosphatemia as a primary manifestation and mechanism. Hypophosphatemia occurs by 3 mechanisms: transcellular shifts, impaired gastrointestinal intake or absorption, or renal losses. Chronic hypophosphatemia from gastrointestinal or renal mechanisms causes rickets (8). Figure 1 illustrates the differential diagnosis of chronic hypophosphatemia.
Figure 1.
Algorithm for assessing causes of hypophosphatemic rickets. Note that before applying this algorithm, the calciopenic causes of rickets (such as vitamin D deficiency, etc., must first be excluded). The calciopenic forms are often marked by hypocalcemia along with marked elevation of PTH. Once calciopenic forms are excluded, evaluation for causes of hypophosphatemia is pursued beginning with TmP/GFR. aConsider genetic causes even without family history. bIron deficiency itself triggers hypophosphatemia in ADHR. cIron infusions especially iron carboxymaltose or polymaltose can also trigger hypophosphatemia, likely through impaired cleavage of FGF23. dFGF23 may be high in hyperparathyroidism. eHypercalciuria may not always be present in tubulopathies. fNon-FGF23–mediated causes of hypophosphatemia may still result in high FGF23 concentrations if moderate to severe chronic kidney disease develops.
Shifts from extracellular to intracellular compartments instigates acute hypophosphatemia during refeeding syndrome or treatment of diabetic ketoacidosis with insulin, when phosphate moves intracellularly for glucose utilization (28). Transient cellular phosphate uptake also occurs due to respiratory alkalosis during salicylate poisoning or rapid mechanical ventilation. The resulting hypophosphatemia can be severe and have neuromuscular consequences but is too brief to cause rickets.
Dietary phosphate deficiencies typically occur in combination with vitamin D and other nutritional deficiencies (eg, alcoholism, anorexia, starvation, or malabsorption). Premature infants have higher phosphate demands than older infants, and those with impaired nutritional intake or absorption, or during parenteral nutrition are at risk for hypophosphatemia (8). Infants and children receiving certain amino acid–based nutritional formulae may develop hypophosphatemic rickets and osteomalacia due to impaired phosphate bioavailability (29, 30). Antacids and phosphate binders impair absorption of phosphate (28).
Hypophosphatemia due to renal losses can be FGF23 dependent or FGF23 independent. FGF23-mediated conditions typically involve isolated renal phosphate losses. Non-FGF23–mediated hypophosphatemia is often accompanied by hypercalciuria.
FGF23-mediated Hypophosphatemia
X-linked hypophosphatemia
XLH, caused by X-linked dominant variants in PHEX, is the most common inherited rickets. Over 870 variants in PHEX have been described (including missense, nonsense, frameshifts, insertions, deletions, duplications, and splice variants) (31). Sporadic PHEX variants occur commonly. Clinical severity generally appears unrelated to PHEX genotype or sex (male hemizygous vs female heterozygous). Individual kindreds demonstrate wide clinical variability (32). PHEX inactivation increases FGF23 expression (20), with consequent low TmP/GFR, hypophosphatemia, and low/normal 1,25(OH)2D.
Children with XLH are born without rickets and signs of rickets develop over several months, presenting with bowing of lower limbs and short stature (33). Patients with XLH also have frequent dental abscesses, wide dental canals, and open apices, which may require root canal treatments or tooth extractions (33-35). Sensorineural hearing loss is common in adults and some children with XLH, and may include cochlear malformation (36). Chiari malformation and craniosynostosis also may occur in XLH (37, 38). Importantly, XLH causes lifelong hypophosphatemia, and adults may have muscle weakness, bone pain from osteomalacia, gait abnormalities from bone deformities, osteoarthritis, and enthesopathy (39). Bone density is typically not low in patients with XLH (40). Past studies that used self-reports have indicated that fractures were not more common in persons with XLH than among familial controls (41). However, osteomalacic pseudofractures are common, especially in adults with XLH. Patients with XLH often are unaware of their existing or past pseudofractures or of the difference between fractures, pseudofractures, or stress fractures, and more recent articles have tended to report these grouped together without distinguishing them, indicating high rates of “fractures/pseudofractures” in XLH, based on skeletal surveys (42) or on survey self-reporting (39, 43).
Autosomal recessive hypophosphatemic rickets
Inactivating variants in multiple genes cause autosomal recessive hypophosphatemic rickets (ARHR) including dentin matrix protein 1 (DMP1), ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) and family with sequence similarity 20, member C (FAM20C). DMP1 deficiency causes defective osteocyte maturation, and increases FGF23 expression (19). Children with DMP1 variants present similarly to XLH, with rickets and a significant dental phenotype (19).
FAM20C variants cause Raine syndrome, along with hypophosphatemia (44, 45), cerebral calcifications, osteosclerosis of long bones, facial and acral dysmorphism, and dental decay. FAM20C enhances DMP1 expression (46) and phosphorylates FGF23, causing impairment of GALNT3-mediated O-glycosylation and making FGF23 prone to cleavage (20). FAM20C deficiency both increases FGF23 gene expression and decreases FGF23 cleavage.
ENPP1 deficiency causes generalized arterial calcification of infancy (47, 48), which is lethal in over half of affected infants but bisphosphonates are thought to improve survival. Hypophosphatemia develops in survivors causing rickets and osteomalacia (49). ENPP1 cleaves adenosine triphosphate to produce pyrophosphate, which impairs mineralization. In ENPP1 deficiency, insufficient pyrophosphate leads to vascular calcification, but not concurrent increased bone mineralization. Instead, FGF23 increases, and hypophosphatemia causes impaired skeletal mineralization. Osteomalacia is evident in most patients by age 14 years, and heterozygous adults develop osteoporosis (50, 51).
Autosomal dominant hypophosphatemic rickets
Autosomal dominant hypophosphatemic rickets (ADHR) is caused by FGF23 variants affecting a cleavage site, leading to impaired cleavage and high levels of intact FGF23 (52-54). ADHR may present at any age as these variants have incomplete and variable timing of penetrance. While some develop hypophosphatemic rickets in early childhood, others have normal serum phosphorus without rickets during childhood and demonstrate delayed onset of hypophosphatemia with osteomalacia in adolescence or adulthood (53, 55). Spontaneous remission, with normalized FGF23 and phosphate, and recurrence of hypophosphatemia both occur.
Human and mouse studies demonstrated that high intact FGF23 and hypophosphatemia only develop in ADHR during iron deficiency (56, 57). FGF23 expression and levels are normal during iron sufficiency. FGF23 expression increases during iron deficiency, and when combined with the impaired FGF23 cleavage in ADHR leads to high intact FGF23 concentrations and hypophosphatemia. In ADHR patients oral iron replacement normalized FGF23 and serum phosphate (58).
Iron Infusion–induced Hypophosphatemia
Since iron deficiency increases FGF23 gene expression, this may be relevant to general iron deficiency. Multiple studies demonstrate an inverse relationship between ferritin or serum iron with C-terminal FGF23 (these assays detect inactive fragments plus intact FGF23) (57, 59). However, without FGF23 mutations, intact FGF23 is typically normal during iron deficiency. For uncertain reasons, iron infusions, especially with iron carboxymaltose or iron polymaltose, appear to acutely impair FGF23 cleavage, triggering transient intact FGF23 elevations and hypophosphatemia in over 70% of patients during prospective studies (60, 61). Serum phosphorus should be monitored in these patients, as several reports describe severe hypophosphatemic osteomalacia following iron infusions, and the symptoms can be nonspecific.
Tumor-induced osteomalacia
Tumor-induced osteomalacia (TIO) is an acquired renal phosphate wasting disorder that has an indolent presentation, usually in adulthood, caused by typically small benign mesenchymal tumors secreting excess FGF23 (62, 63). The resulting renal phosphate losses, hypophosphatemia, and low 1,25(OH)2D cause myopathy, bone pain, and insufficiency fractures (64). Some causative tumors have an FN1-FGFR1 (fibronectin and fibroblast growth factor receptor 1) transcriptional fusion (62). The tumors can be difficult to detect despite advanced imaging.
Fibrous Dysplasia
Fibrous dysplasia (FD) of bone can be monostotic or polyostotic and is often associated with café au lait macules, precocious puberty, or other endocrine hyperfunction in McCune–Albright syndrome due to GNAS mutations (8). FGF23 is expressed in FD lesions and C-terminal FGF23 levels correlate with the total body FD quantity (65). Intact FGF23 is often not high, which is postulated to be due to less FGF23 glycosylation and altered cleavage (66). However, patients with FD may develop high intact FGF23, hypophosphatemia, and osteomalacia or rickets.
Lesions similar to FD occur in osteoglophonic dysplasia and cutaneous skeletal hypophosphatemia syndrome (67-69). Osteoglophonic dysplasia, caused by dominant activating variants in FGFR1, presents as a skeletal dysplasia with rhizomelic short stature, craniofacial bone anomalies, and focal nonossifying skeletal lesions (67, 68). Cutaneous skeletal hypophosphatemia syndrome is a neuroectodermal disorder with elevated levels of immunoglobin E, PTH, and FGF23, presenting with seizures, developmental defects, skeletal and cutaneous lesions, and hypophosphatemia (70, 71), caused by somatic activating mutations in HRAS, KRAS, and NRAS (72).
Kidney Transplant and Hepatic Resection
Patients with end-stage kidney disease have severe elevations of FGF23 (73). Following kidney transplant, patients commonly develop prolonged hypophosphatemia secondary to persistence of elevated FGF23 (18). Transient hypophosphatemia after hepatic resection lasts from 1 to several days (74-76). While previously reported to be independent of FGF23, it is noted that FGF23 gene expression in liver macrophages is stimulated during acute liver injury (77) and in 2 infants with biliary atresia, FGF23-mediated hypophosphatemia normalized after liver transplantation (78).
Non-FGF23–mediated Renal Hypophosphatemia
In several conditions, the renal tubule contains the primary defect mediating hypophosphatemia, and FGF23 is appropriately suppressed. These are often accompanied by hypercalciuria and include the Fanconi syndromes.
Hereditary hypophosphatemic rickets with hypercalciuria (HHRH) is caused by recessive SLC34A3 variants leading to insufficient renal sodium-dependent phosphate cotransporter 2c, impaired phosphate reabsorption, and often severe rickets (79, 80). In HHRH, high 1,25(OH)2D production causes gastrointestinal hyperabsorption of calcium and hypercalciuria with nephrolithiasis and nephrocalcinosis. Heterozygotes are also prone to nephrolithiasis.
Several genetic renal tubulopathies (Fanconi syndromes) cause hypophosphatemia, including mutations in chloride channel 5 (Dent disease), sodium phosphate cotransporter 2a, cystinosin (CTNS, causing nephropathic cystinosis), and hereditary tyrosinemia (81-86). In addition to renal phosphate wasting, these cause various renal losses of amino acids, glucose, bicarbonate, and other solutes. Some NaPi2a (SLC34A1) variants result in abnormal processing and intracellular transport of the protein and tubular damage, causing a generalized tubulopathy (83). Other NaPi2a (SLC34A1) variants cause infantile hypercalcemia due to secondary 1,25(OH)2D production or cause nephrolithiasis in adults (84, 85). Dent disease is an X-linked recessive disorder, due to chloride channel 5 or OCRL1 variants impairing lysosomal trafficking and causing tubular damage (81). In addition to hypophosphatemic rickets, patients have significant proteinuria, high 1,25(OH)2D, hypercalciuria, nephrocalcinosis, and develop chronic kidney disease. Nephropathic cystinosis causes tissue damage due to cystine accumulation in many organs including the kidneys, causing generalized renal tubule substrate losses and chronic kidney disease.
Fanconi syndromes with hypophosphatemia may be caused by drug or toxins such as heavy metals antibiotics, and antiretroviral and anticancer medications (8, 87). For example, the nucleotide reverse transcriptase inhibitor tenofovir causes mitochondrional DNA depletion and ultrastructural mitochondrial abnormalities in renal proximal tubules, and a reversible Fanconi syndrome can develop (88-90).
Evaluation
History
Detailed history should consider the age at apparent onset of hypophosphatemia or of symptoms or signs. Symptoms related to gait, skeletal deformities, and neurologic symptoms should be sought. Short stature is common to many of the causes of hypophosphatemic rickets and does not differentiate them. Dietary intake, pediatric formula feeding, and supplements should be recorded. A history of malabsorptive conditions, iron deficiency, kidney stones, or other systemic symptoms (such as precocious puberty or endocrine hyperfunction in McCune–Albright syndrome) may help identify etiologies. A history of polyuria, polydipsia, and failure to thrive may indicate Fanconi syndromes, such as cystinosis. Similarly, a history or evidence of chronic kidney disease may be a clue to diagnosis of Dent disease or hereditary hyperphosphatemic rickets with hypercalciuria. Vision problems might indicate possible Lowe syndrome or cystinosis, or optic nerve compression from craniofacial fibrous dysplasia. Medication history should note phosphate binders, medications causing tubular damage, and iron infusions.
Family history and inheritance pattern is helpful if positive. However, a negative family history does not rule out genetic causes. ADHR can have incomplete penetrance or delayed onset of hypophosphatemia. XLH is often sporadic and mild XLH may go undetected until adulthood. ARHR may appear to be a spontaneous disorder without known family history. A family history of neonatal death (especially in a sibling) from heart failure or arterial calcifications raises the possibility of autosomal recessive hypophosphatemic rickets due to mutations in the ENPP1 gene.
Physical Examination
Physical examination identifies the consequences of hypophosphatemia and features guiding the differential diagnosis. Children may have bowing of limbs, waddling gait, in-toeing, enlargement of the wrist, knees, or ankles, frontal bossing, fusion of skull sutures, expansion of the costochondral junctions, or a horizontal groove along the thoracic border. Bone pain evident on palpation, movement, or weight bearing may be secondary to osteomalacia alone, but should prompt consideration of fracture or pseudofracture. Dental abscesses are common in XLH (33). Muscle weakness and abnormal gait may be present. Enthesopathy often manifests as decreased range of motion at the spine or large joints due to calcification of entheses in adults with XLH (62). If TIO is considered, a mass may be palpated in any part of body (8, 91). Skin examination may detect café au lait macules (FD and McCune–Albright syndrome) or abnormal nevi with a variety of morphologies (pink/orange hairless plaques or brown verrucous lesions that are exophytic and friable; nevi on mucous membranes, mouth, or eyes, which could represent linear sebaceous nevi) (70). FD may result in deformities of long bones or facial asymmetry due involved craniofacial bones.
Laboratory
We recommend collecting samples before initiating treatment to avoid confounding the results. If a patient is already receiving treatment, stopping phosphate and calcitriol for a week before testing can clarify the diagnosis. Initial evaluation includes measuring serum calcium, phosphorus, PTH, alkaline phosphatase, and 25-hydroxyvitamin D. While secondary hyperparathyroidism often accompanies rickets, rickets is not usually caused by primary hyperparathyroidism. Hypocalcemia would suggest abnormal vitamin D stores or vitamin D metabolism or a tubulopathy. Alkaline phosphatase levels are typically elevated in rickets but may be only mildly high (8). Normal serum phosphorus and alkaline phosphatase values are higher in infants and young children and decrease with age gradually to adulthood; therefore, one must be certain to compare these to age-appropriate normal ranges to ensure appropriate diagnosis. Using an adult normal range for serum phosphorus could result in missing the diagnosis of hypophosphatemia.
TmP/GFR is determined using either a fasting second morning void or a 2-hour morning urine collection, with concurrent measures of serum and urine phosphorus and creatinine. TmP/GFR is also higher in infants and children than in adults; in general, the normal TmP/GFR ranges at any age are similar to the normal serum phosphorus ranges for age. While a nomogram (92) or an algorithm (93, 94) may be used to determine TmP/GFR, these are less accurate in children, and the nomogram does not incorporate the full pediatric normal range. We suggest using the following equation for TmP/GFR in children (95, 96): TmP/GFR = serum phosphate – [(urine phosphate × serum creatinine)/urine creatinine]. This equation has the advantage of performing similarly in fasting and nonfasting conditions, which is useful in young children and in those with afternoon clinical appointments. A high TmP/GFR indicates appropriate renal phosphate conservation (thus a gastrointestinal or dietary cause of hypophosphatemia), while low TmP/GFR indicates inappropriate renal phosphate wasting.
High or high–normal FGF23 levels support an FGF23-mediated cause (97). However, the diagnosis is often clear without FGF23 measurements. In XLH and other FGF23-mediated hypophosphatemia, 1,25(OH)2D is often low or low–normal but is often high in non-FGF23–mediated hypophosphatemia. Hypercalciuria in the untreated patient often indicates a non-FGF23–mediated cause such as HHRH or Fanconi syndrome. However, treatment with vitamin D or calcitriol also can cause hypercalciuria. Unless the family history indicates an FGF23-mediated cause, it is critical to evaluate for generalized proximal renal tubulopathies (including Fanconi syndromes, Dent disease, and others) by performing urinalysis (such as urine dipstick) and additional urine studies paying key attention to elevated urine pH (suggesting bicarbonaturia), glucosuria, albuminuria or general proteinuria, amino aciduria or loss of other substrates, in addition to urine calcium, and urine phosphorus. High urinary albumin and low molecular weight protein excretion (eg, alpha-1 microglobulin, beta-2-microglobulin, retinol-binding protein) may suggest Dent disease or other tubulopathies.
Acidosis may be present, and serum bicarbonate may also be useful in this regard. Fanconi syndrome due to cystinosis although not FGF23 mediated may cause chronic kidney disease, which can then lead to elevated FGF23 concentrations, but by that point the patient is likely no longer hypophosphatemic. Serum ferritin and iron evidence for iron deficiency may direct diagnostic considerations. We recommend that serum phosphorus be measured routinely during treatment with intravenous iron, especially if requiring multiple doses.
Regardless of family history or age of presentation, consider genetic testing where available. Family history can target gene testing but multigene hypophosphatemia panels are available. Alternatively, family members can be screened using age-appropriate serum phosphorus and alkaline phosphatase ranges, acknowledging false negatives may occur especially in the first 3 months of age, which may delay initiation of treatment.
Imaging
Radiographs should be obtained of the wrists and lower limbs (and other areas of deformity or pain) to detect signs and severity of rickets, fractures, or pseudofractures (98, 99). Radiographs of growth plates demonstrate metaphyseal widening, cupping, flaring, or lucency (100). XLH clinical trials applied a 10-point Rickets Severity Scale to wrist and knee radiographs (99, 101, 102). Radiographs may reveal coxa varum, genu varum or valgum, femoral or tibial bowing, tibial torsion, or other deformities. Ribs may have expanded costochondral junctions. Treatment effects are monitored with serial radiographs. With new or worsening bone pain, targeted radiographs may detect fracture or pseudofracture. Skull radiographs may indicate skull shape abnormalities, while computed tomography (CT) scans can evaluate concerns for craniosynostosis or Chiari malformations.
FD can be detected by nuclear medicine techniques. Magnetic resonance imaging and CT scans can evaluate for craniofacial or spinal neurovascular impingement. Plain radiographs or other focused imaging are useful to evaluate changes in FD lesions, or new or worsening symptoms.
Whole body imaging in TIO may include magnetic resonance imaging, CT, or positron emission tomography/CT to detect a causative tumor. All imaging modalities have low sensitivity, but functional whole body imagining with 68Ga-DOTATATE positron emission tomography/CT is useful when available (103-105). Selective venous sampling for FGF23 is described (105, 106). If the causative tumors are not identifiable, periodic repeat imaging is needed.
Management
The treatment goals for hypophosphatemic rickets in children are to improve growth, rickets, skeletal deformity, bone pain, muscle strength, and ambulation. Treatment during growth can improve height and lower limb deformities. However, many children still require corrective surgeries for lower limb deformities. In adults, treatment goals are to decrease bone pain, heal or prevent fractures, and improve mobility. Treatment must be balanced against the complications of therapy.
Phosphate Salts and Active Vitamin D
Conventional therapy for XLH (and other FGF23-mediated hypophosphatemia) has involved high doses of active vitamin D (calcitriol, alfacalcidol, etc.) and oral phosphate. Oral phosphate doses typically range from 20 to 60 mg/kg per day of elemental phosphorus divided into 3 to 5 doses (33, 100). However, treating XLH with phosphate salts alone (without active vitamin D) is inappropriate and ineffective, and can cause severe hyperparathyroidism (107). Calcitriol (or other forms of active vitamin D) stimulate intestinal phosphate absorption, and is necessary to heal rickets and osteomalacia (108, 109). Calcitriol dosing is typically 20 to 30 ng/kg per day divided into 2 to 3 doses, while alfacalcidol dosing is 40 to 60 ng/kg per day (33, 100, 110). Some patients require higher (or lower) doses of phosphate or active vitamin D.
Dosage is titrated based on laboratory testing, improvement in rickets and skeletal deformity on radiographs, and minimization of gastrointestinal and other side effects. Conventional therapy does not correct renal phosphate wasting, so, during treatment with calcitriol and phosphate, we do not target normal serum phosphorus due to safety concerns (nephrocalcinosis and hyperparathyroidism). Oral phosphate and calcitriol are administered multiple times a day due to short half-life and ongoing renal losses, limiting compliance.
Since the goal is for alkaline phosphatase and rickets to improve with treatment, it may be reasonable to increase doses of calcitriol or phosphate carefully to help reach this goal, unless the other safety laboratory assessments (normal to high serum phosphorus, high calcium, high PTH, or high urine calcium excretion) or other safety issues would prohibit that change. Recognize also that improvements in alkaline phosphatase takes time, and often alkaline phosphatase increases transiently on initiation of therapy for hypophosphatemic rickets or osteomalacia. Once stable dosing (of calcitriol and phosphate, or of burosumab) is established, monitoring is recommended about every 3 to 4 months for serum phosphorus, calcium, creatinine, and alkaline phosphatase, as well as urine calcium and creatinine. Importantly if the fasting serum phosphorus is low on treatment, that is not itself an indication to adjust phosphate salts or calcitriol.
Treating non-FGF23–mediated hypophosphatemia is more complex; hypercalciuria is often present. HHRH is treated with phosphate salts as monotherapy because of inherently elevated 1,25(OH)2D and hypercalciuria. When treating HHRH with phosphate salts, high 1,25(OH)2D may persist, though hypercalciuria can improve (79, 80), while phosphaturia persists and hyperparathyroidism remains a risk. Similarly, patients with renal tubulopathies are at risk for hypercalciuria, limiting treatment with active vitamin D and requiring careful monitoring for hypercalciuria and progressive kidney disease. Targeted therapy for cystinosis is available as cysteamine.
Burosumab
Burosumab is a monoclonal antibody that binds FGF23 to inhibit its activity (111). It is FDA approved as monotherapy for children and adults with XLH or TIO. Further studies are needed regarding other FGF23-mediated disorders (112-114). Burosumab is mechanistically inappropriate when FGF23 concentrations are low and is contraindicated in moderate to severe kidney disease.
Clinical trials of burosumab in children and adults with XLH demonstrate increases in TmP/GFR, serum phosphorus, and 1,25(OH)2D (102, 111, 115). The half-life of subcutaneously burosumab is about 13 to 19 days, and dosing is every 4 weeks in adults (115, 116), and every 2 weeks in children (111). The peak increase in TmP/GFR occurs about 7 days after injection, and the peak serum phosphorus and 1,25(OH)2D are between 3 and 7 days after injection (116). The peak serum 1,25(OH)2D rises into the supraphysiologic range for at least the first several injections (111, 116). However, hypercalcemia and hypercalciuria did not develop in the clinical trials. With repeated dosing, the magnitude of the post-dose 1,25(OH)2D surge lessens to more physiologic levels.
Calcitriol and phosphate treatment should be stopped 1 week prior to starting burosumab. Children start burosumab at 0.8 mg/kg every 2 weeks, which can be titrated up to 2 mg/kg every 2 weeks targeting a low to mid-normal serum phosphorus concentration, while avoiding hyperphosphatemia. The standard dose in adults is 1 mg/kg every 4 weeks. The maximum weight-based dose is 90 mg. Hyperphosphatemia occasionally requires dose reduction. Thus, periodic measurement of serum phosphorus at peak and trough time points is important to monitor burosumab.
In a randomized controlled XLH trial, children aged 1-12 years having active rickets (after prior conventional therapy for a mean of over 3 years) were then randomized to either continue conventional therapy or start burosumab for a 64-week controlled trial period (102). Burosumab improved fasting serum phosphorus and TmP/GFR, while conventional therapy did not. Both treatment groups had improvements in rickets severity and alkaline phosphatase, with greater improvements demonstrated with burosumab. Similar improvements in rickets were seen among the children <5 years and those 5-12 years old at enrollment (117). Modest growth improvements were seen with burosumab, but the effect on final adult height is not known. Additionally, the influence of burosumab on the need for corrective skeletal surgery is not known.
The clinical severity of XLH varies widely, as does the response to conventional therapy. It is reasonable to start all children on conventional therapy to monitor initial response and to avoid delays in starting treatment (and for cost and insurance reasons many may require a period of treatment with conventional therapy before being approved for burosumab). However, for those with more severe rickets, more severe deformities, and short stature, starting burosumab earlier (or as initial therapy) is likely to be beneficial. Among children who are tolerating conventional therapy with good results in terms of growth and healing of rickets, normalization of alkaline phosphatase, and lack of deformity, continuing on conventional therapy is reasonable. These children should be monitored though for complications of conventional therapy such as hyperparathyroidism, which may be a reason to consider switching to burosumab. For children who are having persistent rickets during conventional therapy, bone pain, failure to correct alkaline phosphatase, or worsening growth deficit, a switch to burosumab should be considered. Compliance is difficult with conventional therapy, requiring multiple daily dosing, and may be a further reason for limited response to conventional therapy. In this situation, children may also benefit from the switch to injections of burosumab every 2 weeks for a more consistent therapeutic effect.
There are no clinical trials or other studies of burosumab addressing the transition period from adolescence to adulthood to provide evidence-based treatment guidance in this regard. It has been our practice to continue to treat with the every 2 week pediatric dose regimen until the patient has normal alkaline phosphatase for age and has clearly finished growing based on either bone age or lack of further growth, and then to transition to every 4 week dosing of burosumab in accordance with adult dosing. When switching from every 2 week to every 4 week dosing, laboratory monitoring should be followed to ensure the phosphorus remains in the target range and to guide dose adjustment.
Adults with XLH also have a substantial burden of disease, related to ongoing effects of hypophosphatemia, osteomalacia, enthesopathy, osteoarthritis, bone pain, and fractures (39). A 24-week, randomized, double-blind, placebo-controlled trial in adults with XLH and chronic pain demonstrated improvements in serum phosphorus, while bone turnover markers increased, with improvements in pain, stiffness, physical function, and mobility (42, 118). At baseline, half the patients had nephrocalcinosis, 99% had radiographic enthesopathy, and approximately half of the patients in each group had active fractures or pseudofractures. After burosumab, 43.1% of the active fractures/pseudofractures had completely healed by week 24 (compared with 7.7% of the placebo group), increasing to 63% during the 48-week extension trial, demonstrating a positive effect, and illustrating the difficulty healing these chronic lesions in XLH.
Monitoring
Monitoring is similar for conventional therapy or for burosumab. Initial monthly laboratory monitoring can extend to every 3 to 4 months once stable dosing is established for serum phosphorus, calcium, creatinine, alkaline phosphatase, and urine calcium and creatinine. Importantly, hypophosphatemia during conventional therapy is not an indication to adjust phosphate salts or calcitriol. In contrast, burosumab treatment targets low–normal trough phosphorus requiring periodic measurements at peak and trough time points. If serum phosphorus is elevated, regardless of treatment modality, the doses should decrease. Worsening kidney function or nephrocalcinosis also prompt dose decreases.
Hypercalcemia is an indication to decrease calcitriol dosing. We would also consider decreasing burosumab doses for hypercalcemia or hypercalciuria. Some authors have recommended monitoring 1,25(OH)2D during treatment (33). In fact, the mean peak values of 1,25(OH)2D were supraphysiologic during the registration clinical trials (42, 102, 111, 115, 116), but the trials did not adjust doses based on 1,25(OH)2D. Decreasing the dose based on 1,25(OH)2D levels may limit the effectiveness of burosumab by allowing persistent hypophosphatemia. Thus, monitoring 1,25(OH)2D adds cost without clear benefit, and we have not recommended monitoring 1,25(OH)2D during treatment. There is greater rationale for monitoring urine calcium excretion and serum calcium as the likely signals of true adverse 1,25(OH)2D effects. PTH should be assessed every 6 months and during hypercalcemia. If PTH is elevated, either the dose of calcitriol should be increased (unless there is hypercalcemia), or the dose of phosphate decreased. Patients may require supplementation to maintain normal 25-hydroxyvitamin D concentrations. Alkaline phosphatase often increases transiently on initiation of therapy, but over time normalization is a marker of therapeutic effect. Burosumab dosing is titrated based on serum phosphorus rather than alkaline phosphatase. Monitor growth and radiographs annually in children.
While enthesopathy and osteoarthritis are often debilitating features in adults with XLH, there are no data that conventional therapy or burosumab prevents or improves these in humans or mouse models (39, 119-121). The burosumab trials could not evaluate changes in structural abnormalities of osteophytes and enthesopathy, since enthesopathy and osteoarthritis develop over many years. Adults with XLH require symptom monitoring, directed imaging, and consideration of the impact of enthesopathy, osteoarthritis, or spinal stenosis on mobility and quality of life. Joint replacements and spinal surgery are common.
Dental abscesses and periodontal disease remain common and sometimes severe with either conventional therapy or burosumab. Consequently, regular dental care is necessary. Some data suggest dental complications are less severe among adults receiving conventional therapy than in those not receiving ongoing treatment (121, 122). To date, burosumab studies have not indicated improvements in dental health, but in the relatively short controlled trials (24-64 weeks), there were more dental events during burosumab than during conventional therapy. In the hyp mouse, both anti-FGF23 antibody and calcitriol improved dentoalveolar mineralization, and calcitriol increased dentin volume and thickness over anti-FGF23 antibody, but neither treatment normalized dental features (123).
TIO Treatment
The first-line treatment for TIO is curative complete surgical resection (63, 124), but the tumor may be inoperable or not locatable. Generally patients have been treated for osteomalacia similarly to XLH with calcitriol and phosphate, though adjunctive therapy with cinacalcet may be beneficial (125). Burosumab also improves phosphate, mobility, fracture healing, and osteomalacia in TIO (126). After resection, medications can be tapered if cured (127).
Complications of Medical Therapy
Important side effects complicate treatment of hypophosphatemic rickets. Phosphate salts cause gastrointestinal symptoms, including dyspepsia and a laxative effect. Decreasing the dose and gradual upward titration may improve tolerability.
Secondary hyperparathyroidism is commonly present prior to starting treatment in XLH, but is also worsened by phosphate salts. Secondary hyperparathyroidism affects over 80% of patients with XLH (128), and up to 25% to 30% of adults with XLH develop hypercalcemic tertiary hyperparathyroidism during conventional therapy, with lower rates in childhood (128, 129). Tertiary hyperparathyroidism tends to occur with higher phosphate doses and longer durations of treatment (130, 131). A calcimimetic (ie, cinacalcet) may be attempted; however, insurance approval may be a barrier, and nausea is a common side effect. Regarding surgical intervention, resection of multiple parathyroid glands is typically required due to multigland hyperplasia. Risks include persistent hypercalcemia after incomplete resection, recurrence of hypercalcemia years later, or postsurgical hypoparathyroidism.
We recommend titrating phosphate salts and calcitriol to try to normalize the PTH. Limited data suggest that burosumab may improve though not necessarily normalize secondary hyperparathyroidism (118). However, once parathyroid autonomy is established, burosumab likely will not normalize hyperparathyroidism. Patients who are already hypercalcemic or hypercalciuric may be at risk for worsening serum or urine calcium during post-burosumab spikes in 1,25(OH)2D. If considering burosumab in patients with tertiary hyperparathyroidism, clinicians should address the hyperparathyroidism first and monitor calcium closely.
Nephrocalcinosis affects over 50% of persons with XLH as a complication of conventional therapy. Nephrocalcinosis is associated with renal tubular acidosis and hypercalciuria in XLH (132). In the setting of nephrocalcinosis or hypercalciuria, patients may benefit from decreasing their doses of calcitriol, or possibly adding a thiazide diuretic. Chronic kidney disease is reported in up to 17.6% of XLH patients with nephrocalcinosis (128), and, though usually mild, there are rare occurrences of end-stage kidney disease. Other ectopic calcifications are also possible, and 1 study reported intracranial calcifications in 32% of adults with XLH over age 40 years (119). Nephrocalcinosis is still a potential risk of burosumab as hyperphosphatemia may occur and 1,25(OH)2D concentrations increase (118). However, an observational study reported burosumab’s TmP/GFR improvements were accompanied by decreases in the urine calcium/creatinine ratio (133). In burosumab clinical trials, a difference in risk could not be determined. Renal ultrasounds should be used to screen and monitor nephrocalcinosis at least every 1 to 2 years (100).
In the pediatric trials, fever, myalgia, and transient injection site reactions were common (102, 111). Although hypersensitivity reactions were commonly reported in burosumab-treated children, none of the children withdrew from the trial. Other reported side effects were headache, cough, pain in extremities, and nasopharyngitis (102, 111). Some report early increases in bone pain during initiation of burosumab that improves over time.
Restless legs syndrome in adults with XLH was first reported during the uncontrolled open-label burosumab phase 2 clinical trial (116). The placebo-controlled trial in adults with XLH confirmed an 11.8% incidence of restless legs syndrome with burosumab, while the placebo group had a 7.6% incidence of restless legs syndrome (42). In our experience, restless legs syndrome is frequent among untreated adults with XLH, but a substantial proportion develop new or worsening restless legs syndrome after starting burosumab. Some require medication for restless legs syndrome. However, restless legs syndrome may sometimes subsequently improve or resolve during ongoing burosumab.
Pregnancy
Little is known about the risks and benefits of treating XLH during pregnancy.
In pregnancy, the high calcium and phosphate demands of the fetus might prompt medical treatment with calcitriol and phosphate (5, 134). Breast milk from humans with XLH has low phosphorus content, while that of the hyp mouse is normal (135, 136). However, maternal risks of treatment with calcitriol and phosphate include worsening kidney function or nephrocalcinosis. Mouse model data indicate that although the hyp mouse mother is hypophosphatemic, both the hyp fetus and the normal fetus of this mother are normophosphatemic in utero (134). As a monoclonal antibody, burosumab crosses the placenta and could have potential effects, but the risk for complications of burosumab during pregnancy or lactation is not known. Thus, in the absence of clear data on benefit or harm during pregnancy, treatment should be approached cautiously and with a thorough discussion regarding known and unknown risks from either conventional therapy or burosumab. A decision to treat would be based on maternal skeletal related symptoms rather than on an expected benefit for the fetus. Regardless of therapeutic approach, we strongly recommend that if the mother is treated, she should be monitored at least monthly with laboratory tests for safety.
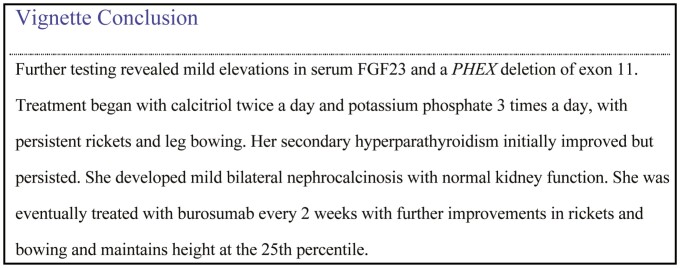
Conclusion
Hypophosphatemic rickets/osteomalacia represent a set of rare disorders with many genetic and acquired causes and potential in long-term complications for children and adults, and diminishing physical function and quality of life. Therapy for hypophosphatemic rickets is directed by the underlying cause and management is complex requiring careful monitoring. Treatment for the most common cause of hypophosphatemic rickets, XLH, has expanded to include the option of anti-FGF23 therapy with burosumab. Many questions remain regarding the long-term effects of burosumab on several outcomes of interest to patients.
Abbreviations
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- ADHR
autosomal dominant hypophosphatemic rickets
- ARHR
autosomal recessive hypophosphatemic rickets
- CT
computed tomography
- FD
fibrous dysplasia
- FGF23
Fibroblast growth factor 23
- HHRH
hereditary hypophosphatemic rickets with hypercalciuria
- PTH
parathyroid hormone
- TIO
tumor-induced osteomalacia
- TmP/GFR
tubular maximum reabsorption of phosphorus adjusted for glomerular filtration rate
- XLH
X-linked hypophosphatemia
Contributor Information
Sarah A Ackah, Department of Medicine, Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN 46202, USA.
Erik A Imel, Department of Medicine, Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN 46202, USA.
Funding
This work was supported in part by funding from the NIH by NIAMS (P30AR072581). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
E.A.I. receives research funding and fees for consulting for Ultragenyx Pharmaceuticals and Kyowa Hakko Kirin, Pharmaceuticals. S.A.A. has nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Mughal MZ. Rickets. Curr Osteoporos Rep. 2011;9(4):291–299. [DOI] [PubMed] [Google Scholar]
- 2. Beck-Nielsen SS, Jensen TK, Gram J, Brixen K, Brock-Jacobsen B. Nutritional rickets in Denmark: a retrospective review of children’s medical records from 1985 to 2005. Eur J Pediatr. 2009;168(8):941–949. [DOI] [PubMed] [Google Scholar]
- 3. Mao M, Carpenter TO, Whyte MP, et al. Growth curves for children with X-linked hypophosphatemia. J Clin Endocrinol Metab. 2020;105(10):3243–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arenas MA, Jaimovich S, Perez Garrido N, et al. Hereditary hypophosphatemic rickets and craniosynostosis. J Pediatr Endocrinol Metab. 2021;34(9):1105–1113. [DOI] [PubMed] [Google Scholar]
- 5. Lecoq AL, Brandi ML, Linglart A, Kamenický P. Management of X-linked hypophosphatemia in adults. Metab Clin Exp. 2020;103(s):154049. [DOI] [PubMed] [Google Scholar]
- 6. Nehgme R, Fahey JT, Smith C, Carpenter TO. Cardiovascular abnormalities in patients with X-linked hypophosphatemia. J Clin Endocrinol Metab. 1997;82(8):2450–2454. [DOI] [PubMed] [Google Scholar]
- 7. Lin X, Li S, Zhang Z, Yue H. Clinical and genetic characteristics of 153 Chinese patients with X-linked hypophosphatemia. Front Cell Dev Biol. 2021;9:617738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Imel EA, Econs MJ. Approach to the hypophosphatemic patient. J Clin Endocrinol Metab. 2012;97(3):696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sabbagh Y, Carpenter TO, Demay MB. Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc Natl Acad Sci USA. 2005;102(27):9637–9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dubourg LD, Aurelle M, Chardon L, Flammier S, Lemoine S, Bacchetta J. TmP/GFR reference values from childhood to adulthood in the era of IDMS-standardized creatinine values. Nephrol Dial Transplant. 2021. doi: 10.1093/ndt/gfab331. Online ahead of print. [DOI] [Google Scholar]
- 11. Capuano P, Radanovic T, Wagner CA, et al. Intestinal and renal adaptation to a low-Pi diet of type II NaPi cotransporters in vitamin D receptor- and 1alphaOHase-deficient mice. Am J Physiol Cell Physiol. 2005;288(2):C429–C434. [DOI] [PubMed] [Google Scholar]
- 12. Suzuki Y, Kuzina E, An SJ, et al. FGF23 contains two distinct high-affinity binding sites enabling bivalent interactions with alpha-Klotho. Proc Natl Acad Sci USA. 2020;117(50):31800–31807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takashi Y, Kosako H, Sawatsubashi S, et al. Activation of unliganded FGF receptor by extracellular phosphate potentiates proteolytic protection of FGF23 by its O-glycosylation. Proc Natl Acad Sci USA. 2019;116(23):11418–11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frishberg Y, Ito N, Rinat C, et al. Hyperostosis-hyperphosphatemia syndrome: a congenital disorder of O-glycosylation associated with augmented processing of fibroblast growth factor 23. J Bone Miner Res. 2007;22(2):235–242. [DOI] [PubMed] [Google Scholar]
- 15. Lang F, Leibrock C, Pandyra AA, Stournaras C, Wagner CA, Föller M. Phosphate homeostasis, inflammation and the regulation of FGF-23. Kidney Blood Press Res. 2018;43(6):1742–1748. [DOI] [PubMed] [Google Scholar]
- 16. Onal M, Carlson AH, Thostenson JD, et al. A novel distal enhancer mediates inflammation-, PTH-, and early onset murine kidney disease-induced expression of the mouse Fgf23 gene. JBMR Plus. 2018;2(1):31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. David V, Dai B, Martin A, Huang J, Han X, Quarles LD. Calcium regulates FGF-23 expression in bone. Endocrinology. 2013;154(12):4469–4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beck-Nielsen SS, Mughal Z, Haffner D, et al. FGF23 and its role in X-linked hypophosphatemia-related morbidity. Orphanet J Rare Dis. 2019;14(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feng JQ, Ward LM, Liu S, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38(11):1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clinkenbeard EL, White KE. Heritable and acquired disorders of phosphate metabolism: Etiologies involving FGF23 and current therapeutics. Bone. 2017;102:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin A, Liu S, David V, et al. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 2011;25(8):2551–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ovejero D, Hartley IR, de Castro Diaz LF, et al. PTH and FGF23 exert interdependent effects on renal phosphate handling: evidence from patients with hypoparathyroidism and hyperphosphatemic familial tumoral calcinosis treated with synthetic human PTH 1-34. J Bone Miner Res. 2022;37(2):179–184. [DOI] [PubMed] [Google Scholar]
- 23. Martins JS, Liu ES, Sneddon WB, Friedman PA, Demay MB. 1,25-Dihydroxyvitamin D maintains brush border membrane NaPi2a and attenuates phosphaturia in Hyp mice. Endocrinology. 2019;160(10):2204–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jimenez M, Ivanovic-Zuvic D, Loureiro C, et al. Clinical and molecular characterization of Chilean patients with X-linked hypophosphatemia. Osteoporos Int. 2021;32(9):1825–1836. [DOI] [PubMed] [Google Scholar]
- 25. Dhull RS, Jain R, Deepthi B, et al. Vitamin D-dependent rickets (VDDR) type 1: case series of two siblings with a CYP27B1 mutation and review of the literature. J Bras Nefrol. 2020;42(4):494–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thacher TD, Levine MA. CYP2R1 mutations causing vitamin D-deficiency rickets. J Steroid Biochem Mol Biol. 2017;173:333–336. [DOI] [PubMed] [Google Scholar]
- 27. Chavez MB, Kramer K, Chu EY, Thumbigere-Math V, Foster BL. Insights into dental mineralization from three heritable mineralization disorders. J Struct Biol. 2020;212(1):107597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liamis G, Milionis HJ, Elisaf M. Medication-induced hypophosphatemia: a review. QJM. 2010;103(7):449–459. [DOI] [PubMed] [Google Scholar]
- 29. Gonzalez Ballesteros LF, Ma NS, Gordon RJ, et al. Unexpected widespread hypophosphatemia and bone disease associated with elemental formula use in infants and children. Bone. 2017;97:287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Creo AL, Epp LM, Buchholtz JA, Tebben PJ. Prevalence of metabolic bone disease in tube-fed children receiving elemental formula. Horm Res Paediatr. 2018;90(5):291–298. [DOI] [PubMed] [Google Scholar]
- 31. Sarafrazi S, Daugherty SC, Miller N, et al. Novel PHEX gene locus-specific database: Comprehensive characterization of vast number of variants associated with X-linked hypophosphatemia (XLH). Hum Mutat. 2022;43(2):143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng B, Wang C, Chen Q, et al. Functional characterization of PHEX gene variants in children with X-linked hypophosphatemic rickets shows no evidence of genotype-phenotype correlation. J Bone Miner Res. 2020;35(9):1718–1725. [DOI] [PubMed] [Google Scholar]
- 33. Haffner D, Emma F, Eastwood DM, et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol. 2019;15(7):435–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bradley H, Dutta A, Philpott R. Presentation and non-surgical endodontic treatment of two patients with X-linked hypophosphatemia: a case report. Int Endod J. 2021;54(8):1403–1414. [DOI] [PubMed] [Google Scholar]
- 35. Clayton D, Chavez MB, Tan MH, et al. Mineralization defects in the primary dentition associated with X-linked hypophosphatemic rickets. JBMR Plus. 2021;5(4):e10463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gagnon LH, Bauschatz JD, Davisson MT, et al. New intragenic deletions in the Phex gene clarify X-linked hypophosphatemia-related abnormalities in mice. Mamm Genome. 2004;15(3):151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Willis FR, Beattie TJ. Craniosynostosis in X-linked hypophosphataemic rickets. J Paediatr Child Health. 1997;33(1):78–79. [DOI] [PubMed] [Google Scholar]
- 38. Vega RA, Opalak C, Harshbarger RJ, et al. Hypophosphatemic rickets and craniosynostosis: a multicenter case series. J Neurosurg Pediatr. 2016;17(6):694–700. [DOI] [PubMed] [Google Scholar]
- 39. Javaid MK, Ward L, Pinedo-Villanueva R, et al. Musculoskeletal features in adults with X-linked hypophosphatemia: an analysis of clinical trial and survey data. J Clin Endocrinol Metab. 2022;107(3):e1249–e1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reid IR, Murphy WA, Hardy DC, Teitelbaum SL, Bergfeld MA, Whyte MP. X-linked hypophosphatemia: skeletal mass in adults assessed by histomorphometry, computed tomography, and absorptiometry. Am J Med. 1991;90(1):63–69. [DOI] [PubMed] [Google Scholar]
- 41. Econs MJ, Samsa GP, Monger M, Drezner MK, Feussner JR. X-Linked hypophosphatemic rickets: a disease often unknown to affected patients. Bone Miner. 1994;24(1):17–24. [DOI] [PubMed] [Google Scholar]
- 42. Insogna KL, Briot K, Imel EA, et al. A randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with X-linked hypophosphatemia: week 24 primary analysis. J Bone Miner Res. 2018;33(8):1383–1393. [DOI] [PubMed] [Google Scholar]
- 43. Skrinar A, Dvorak-Ewell M, Evins A, et al. The lifelong impact of X-linked hypophosphatemia: results from a burden of disease survey. J Endocr Soc. 2019;3(7):1321–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hirst L, Abou-Ameira G, Critchlow S. Hypophosphataemic rickets secondary to Raine syndrome: a review of the literature and case reports of three paediatric patients’ dental management. Case Rep Pediatr. 2021;2021:6637180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Elalaoui SC, Al-Sheqaih N, Ratbi I, et al. Non lethal Raine syndrome and differential diagnosis. Eur J Med Genet. 2016;59(11):577–583. [DOI] [PubMed] [Google Scholar]
- 46. Kinoshita Y, Hori M, Taguchi M, Fukumoto S. Functional analysis of mutant FAM20C in Raine syndrome with FGF23-related hypophosphatemia. Bone. 2014;67:145–151. [DOI] [PubMed] [Google Scholar]
- 47. Brunod I, Tosello B, Hassid S, Gire C, Thomachot L, Panuel M. Generalized arterial calcification of infancy with a novel ENPP1 mutation: a case report. BMC Pediatr. 2018;18(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ferreira CR, Kavanagh D, Oheim R, et al. Response of the ENPP1-deficient skeletal phenotype to oral phosphate supplementation and/or enzyme replacement therapy: comparative studies in humans and mice. J Bone Miner Res. 2021;36(5):942–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rutsch F, Böyer P, Nitschke Y, et al. Hypophosphatemia, hyperphosphaturia, and bisphosphonate treatment are associated with survival beyond infancy in generalized arterial calcification of infancy. Circ Cardiovasc Genet. 2008;1(2):133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oheim R, Zimmerman K, Maulding ND, et al. Human heterozygous ENPP1 deficiency is associated with early onset osteoporosis, a phenotype recapitulated in a mouse model of ENPP1 deficiency. J Bone Miner Res. 2020;35(3):528–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ferreira CR, Hackbarth ME, Ziegler SG, et al. Prospective phenotyping of long-term survivors of generalized arterial calcification of infancy (GACI). Genet Med. 2021;23(2):396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60(6):2079–2086. [DOI] [PubMed] [Google Scholar]
- 53. Imel EA, Hui SL, Econs MJ. FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J Bone Miner Res. 2007;22(4):520–526. [DOI] [PubMed] [Google Scholar]
- 54. ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26(3):345–348. [DOI] [PubMed] [Google Scholar]
- 55. Econs MJ, McEnery PT. Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J Clin Endocrinol Metab. 1997;82(2):674–681. [DOI] [PubMed] [Google Scholar]
- 56. Farrow EG, Yu X, Summers LJ, et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci USA. 2011;108(46):E1146–E1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ. Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab. 2011;96(11):3541–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Imel EA, Liu Z, Coffman M, Acton D, Mehta R, Econs MJ. Oral iron replacement normalizes fibroblast growth factor 23 in iron-deficient patients with autosomal dominant hypophosphatemic rickets. J Bone Miner Res. 2020;35(2):231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Braithwaite V, Prentice AM, Doherty C, Prentice A. FGF23 is correlated with iron status but not with inflammation and decreases after iron supplementation: a supplementation study. Int J Pediatr Endocrinol 2012;2012(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wolf M, Rubin J, Achebe M, et al. Effects of iron isomaltoside vs ferric carboxymaltose on hypophosphatemia in iron-deficiency anemia: two randomized clinical trials. JAMA. 2020;323(5):432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;28(8):1793–1803. [DOI] [PubMed] [Google Scholar]
- 62. Colazo JM, DeCorte JA, Gillaspie EA, Folpe AL, Dahir KM. Hiding in plain sight: Gene panel and genetic markers reveal 26-year undiagnosed tumor-induced osteomalacia of the rib concurrently misdiagnosed as X-linked hypophosphatemia. Bone Rep. 2021;14:100744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Folpe AL, Fanburg-Smith JC, Billings SD, et al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004;28(1):1–30. [DOI] [PubMed] [Google Scholar]
- 64. Zanchetta MB, Jerkovich F, Nunez S, et al. Impaired bone microarchitecture and strength in patients with tumor-induced osteomalacia. J Bone Miner Res. 2021;36(8):1502–1509. [DOI] [PubMed] [Google Scholar]
- 65. Riminucci M, Collins MT, Fedarko NS, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112(5):683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bhattacharyya N, Wiench M, Dumitrescu C, et al. Mechanism of FGF23 processing in fibrous dysplasia. J Bone Miner Res. 2012;27(5):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kuthiroly S, Yesodharan D, Ghosh A, White KE, Nampoothiri S. Osteoglophonic dysplasia: phenotypic and radiological clues. J Pediatr Genet. 2017;6(4):247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. White KE, Cabral JM, Davis SI, et al. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am J Hum Genet. 2005;76(2):361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ovejero D, Lim YH, Boyce AM, et al. Cutaneous skeletal hypophosphatemia syndrome: clinical spectrum, natural history, and treatment. Osteoporos Int. 2016;27(12):3615–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hoffman WH, Jueppner HW, Deyoung BR, O’Dorisio MS, Given KS. Elevated fibroblast growth factor-23 in hypophosphatemic linear nevus sebaceous syndrome. Am J Med Genet. 2005;134(3):233–236. [DOI] [PubMed] [Google Scholar]
- 71. Narazaki R, Ihara K, Namba N, Matsuzaki H, Ozono K, Hara T. Linear nevus sebaceous syndrome with hypophosphatemic rickets with elevated FGF-23. Pediatr Nephrol. 2012;27(5):861–863. [DOI] [PubMed] [Google Scholar]
- 72. Lim YH, Ovejero D, Sugarman JS, et al. Multilineage somatic activating mutations in HRAS and NRAS cause mosaic cutaneous and skeletal lesions, elevated FGF23 and hypophosphatemia. Hum Mol Genet. 2014;23(2):397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64(6):2272–2279. [DOI] [PubMed] [Google Scholar]
- 74. Nafidi O, Lapointe RW, Lepage R, Kumar R, D’Amour P. Mechanisms of renal phosphate loss in liver resection-associated hypophosphatemia. Ann Surg. 2009;249(5):824–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hallet J, Karanicolas PJ, Zih FSW, et al. Hypophosphatemia and recovery of post-hepatectomy liver insufficiency. Hepatobiliary Surg Nutr. 2016;5(3):217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Squires MH, Dann GC, Lad NL, et al. Hypophosphataemia after major hepatectomy and the risk of post-operative hepatic insufficiency and mortality: an analysis of 719 patients. HPB. 2014;16(10):884–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kumar P, Liu Y, Shen Y, Maher JJ, Cingolani F, Czaja MJ. Mouse liver injury induces hepatic macrophage FGF23 production. PLoS One. 2022;17(3):e0264743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wasserman H, Ikomi C, Hafberg ET, Miethke AG, Bove KE, Backeljauw PF. Two case reports of FGF23-induced hypophosphatemia in childhood biliary atresia. Pediatrics. 2016;138(2):e20154453. [DOI] [PubMed] [Google Scholar]
- 79. Yu Y, Sanderson SR, Reyes M, et al. Novel NaPi IIc mutations causing HHRH and idiopathic hypercalciuria in several unrelated families: long-term follow-up in one kindred. Bone. 2012;50(5):1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bergwitz C, Miyamoto KI. Hereditary hypophosphatemic rickets with hypercalciuria: pathophysiology, clinical presentation, diagnosis and therapy. Pflug Arch Eur J Phy. 2019;471(1):149–163. [DOI] [PubMed] [Google Scholar]
- 81. Devuyst O, Thakker RV. Dent’s disease. Orphanet J Rare Dis. 2010;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nasir S, Raza M, Siddiqui SI, Saleem A, Abbas A. Hereditary tyrosinemia compounded with hyperinsulinemic hypoglycemia: challenging diagnosis of a rare case. Cureus. 2020;12(11):e11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Magen D, Berger L, Coady MJ, et al. A loss-of-function mutation in NaPi-IIa and renal Fanconi’s syndrome. N Engl J Med. 2010;362(12):1102–1109. [DOI] [PubMed] [Google Scholar]
- 84. Schlingmann KP, Ruminska J, Kaufmann M, et al. Autosomal-recessive mutations in SLC34A1 encoding sodium-phosphate cotransporter 2A cause idiopathic infantile hypercalcemia. J Am Soc Nephrol. 2016;27(2):604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Arcidiacono T, Mingione A, Macrina L, Pivari F, Soldati L, Vezzoli G. Idiopathic calcium nephrolithiasis: a review of pathogenic mechanisms in the light of genetic studies. Am J Nephrol. 2014;40(6):499–506. [DOI] [PubMed] [Google Scholar]
- 86. Hohenfellner K, Rauch F, Ariceta G, et al. Management of bone disease in cystinosis: Statement from an international conference. J Inherit Metab Dis. Sep 2019;42(5):1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Duck L, Devogelaer JP, Persu A, et al. Osteomalacia due to chemotherapy-induced Fanconi syndrome in an adult patient. Gynecol Oncol. 2005;98(2):329–331. [DOI] [PubMed] [Google Scholar]
- 88. Lebrecht D, Venhoff AC, Kirschner J, Wiech T, Venhoff N, Walker UA. Mitochondrial tubulopathy in tenofovir disoproxil fumarate-treated rats. J Acquir Immune Defic Syndr. 2009;51(3):258–263. [DOI] [PubMed] [Google Scholar]
- 89. Papaleo A, Warszawski J, Salomon R, et al. Increased β-2 microglobulinuria in human immunodeficiency virus-1-infected children and adolescents treated with tenofovir. Pediatr Infect Dis J. 2007;26(10):949–951. [DOI] [PubMed] [Google Scholar]
- 90. Mateo L, Holgado S, Mariñoso ML, et al. Hypophosphatemic osteomalacia induced by tenofovir in HIV-infected patients. Clin Rheumatol. 2016;35(5):1271–1279. [DOI] [PubMed] [Google Scholar]
- 91. Abramson M, Glezerman IG, Srinivasan M, Ross R, Flombaum C, Gutgarts V. Hypophosphatemia and FGF23 tumor-induced osteomalacia in two cases of metastatic breast cancer. Clin Nephrol. 2021;95(2):104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Walton RJ, Bijvoet OL. Nomogram for derivation of renal threshold phosphate concentration. Lancet. 1975;2(7929):309–310. [DOI] [PubMed] [Google Scholar]
- 93. Payne RB. Renal tubular reabsorption of phosphate (TmP/GFR): indications and interpretation. Ann Clin Biochem. 1998;35(Pt 2):201–206. [DOI] [PubMed] [Google Scholar]
- 94. Barth JH, Jones RG, Payne RB. Calculation of renal tubular reabsorption of phosphate: the algorithm performs better than the nomogram. Ann Clin Biochem. 2000;37(Pt 1):79–81. [DOI] [PubMed] [Google Scholar]
- 95. Stark H, Eisenstein B, Tieder M, Rachmel A, Alpert G. Direct measurement of TP/GFR: a simple and reliable parameter of renal phosphate handling. Nephron. 1986;44(2):125–128. [DOI] [PubMed] [Google Scholar]
- 96. Brodehl J, Krause A, Hoyer PF. Assessment of maximal tubular phosphate reabsorption: comparison of direct measurement with the nomogram of Bijvoet. Pediatr Nephrol. 1988;2(2):183–189. [DOI] [PubMed] [Google Scholar]
- 97. Endo I, Fukumoto S, Ozono K, et al. Clinical usefulness of measurement of fibroblast growth factor 23 (FGF23) in hypophosphatemic patients: proposal of diagnostic criteria using FGF23 measurement. Bone. 2008;42(6):1235–1239. [DOI] [PubMed] [Google Scholar]
- 98. Hardy DC, Murphy WA, Siegel BA, Reid IR, Whyte MP. X-linked hypophosphatemia in adults: prevalence of skeletal radiographic and scintigraphic features. Radiology. 1989;171(2):403–414. [DOI] [PubMed] [Google Scholar]
- 99. Thacher TD, Pettifor JM, Tebben PJ, et al. Rickets severity predicts clinical outcomes in children with X-linked hypophosphatemia: utility of the radiographic Rickets Severity Score. Bone. 2019;122:76–81. [DOI] [PubMed] [Google Scholar]
- 100. Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL. A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res. 2011;26(7):1381–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Thacher TD, Fischer PR, Pettifor JM, Lawson JO, Manaster BJ, Reading JC. Radiographic scoring method for the assessment of the severity of nutritional rickets. J Trop Pediatr. 2000;46(3):132–139. [DOI] [PubMed] [Google Scholar]
- 102. Imel EA, Glorieux FH, Whyte MP, et al. Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open-label, phase 3 trial. Lancet. 2019;393(10189):2416–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yang M, Doshi KB, Roarke MC, Nguyen BD. Molecular imaging in diagnosis of tumor-induced osteomalacia. Curr Probl Diagn Radiol. 2019;48(4):379–386. [DOI] [PubMed] [Google Scholar]
- 104. Zhang J, Zhu Z, Zhong D, et al. 68Ga DOTATATE PET/CT is an accurate imaging modality in the detection of culprit tumors causing osteomalacia. Clin Nucl Med. 2015;40(8):642–646. [DOI] [PubMed] [Google Scholar]
- 105. Kawai S, Ariyasu H, Furukawa Y, et al. Effective localization in tumor-induced osteomalacia using 68Ga-DOTATOC-PET/CT, venous sampling and 3T-MRI. Endocrinol Diabetes Metab Case Rep. 2017;2017. doi: 10.1530/EDM-17-0005.eCollection [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Florenzano P, Gafni RI, Collins MT. Tumor-induced osteomalacia. Bone Rep. 2017;7:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Stickler GB, Hayles AB, Rosevear JW. Familial hypophosphatemic vitamin D resistant rickets: effect of increased oral calcium and phosphorus intake without high doses of vitamin D. Am J Dis Child. 1965;110(6):664–667. [DOI] [PubMed] [Google Scholar]
- 108. Drezner MK, Lyles KW, Haussler MR, Harrelson JM. Evaluation of a role for 1,25-dihydroxyvitamin D3 in the pathogenesis and treatment of X-linked hypophosphatemic rickets and osteomalacia. J Clin Invest. 1980;66(5):1020–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Glorieux FH, Marie PJ, Pettifor JM, Delvin EE. Bone response to phosphate salts, ergocalciferol, and calcitriol in hypophosphatemic vitamin D-resistant rickets. N Engl J Med. 1980;303(18):1023–1031. [DOI] [PubMed] [Google Scholar]
- 110. Linglart A, Biosse-Duplan M, Briot K, et al. Therapeutic management of hypophosphatemic rickets from infancy to adulthood. Endocr Connect. 2014;3(1):R13–R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Carpenter TO, Whyte MP, Imel EA, et al. Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med. 2018;378(21):1987–1998. [DOI] [PubMed] [Google Scholar]
- 112. Imanishi Y, Ito N, Rhee Y, et al. Interim analysis of a phase 2 open-label trial assessing burosumab efficacy and safety in patients with tumor-induced osteomalacia. J Bone Miner Res. 2021;36(2):262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Amarnani R, Travis S, Javaid MK. Novel use of burosumab in refractory iron-induced FGF23-mediated hypophosphataemic osteomalacia. Rheumatology (Oxford). 2020;59(8):2166–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gladding A, Szymczuk V, Auble BA, Boyce AM. Burosumab treatment for fibrous dysplasia. Bone. 2021;150:116004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Carpenter TO, Imel EA, Ruppe MD, et al. Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J Clin Invest. 2014;124(4):1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Imel EA, Zhang X, Ruppe MD, et al. Prolonged correction of serum phosphorus in adults with x-linked hypophosphatemia using monthly doses of KRN23. J Clin Endocrinol Metab. 2015;100(7):2565–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ward LM, Glorieux FH, Whyte MP, et al. Effect of burosumab compared with conventional therapy on younger vs older children with X-linked hypophosphatemia. J Clin Endocrinol Metab. 2022;107(8):e3241–e3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Portale AA, Carpenter TO, Brandi ML, et al. Continued beneficial effects of burosumab in adults with X-linked hypophosphatemia: results from a 24-week treatment continuation period after a 24-week double-blind placebo-controlled period. Calcif Tissue Int. 2019;105(3):271–284. [DOI] [PubMed] [Google Scholar]
- 119. Gjorup H, Kjaer I, Beck-Nielsen SS, Poulsen MR, Haubek D. A radiological study on intra- and extra-cranial calcifications in adults with X-linked hypophosphatemia and associations with other mineralizing enthesopathies and childhood medical treatment. Orthod Craniofac Res. 2016;19(2):114–125. [DOI] [PubMed] [Google Scholar]
- 120. Cauliez A, Zhukouskaya VV, Hilliquin S, et al. Impact of early conventional treatment on adult bone and joints in a murine model of X-linked hypophosphatemia. Front Cell Dev Biol. 2020;8:591417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Connor J, Olear EA, Insogna KL, et al. Conventional therapy in adults with X-linked hypophosphatemia: effects on enthesopathy and dental disease. J Clin Endocrinol Metab. 2015;100(10):3625–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Biosse Duplan M, Coyac BR, Bardet C, et al. Phosphate and vitamin D prevent periodontitis in X-linked hypophosphatemia. J Dent Res. 2017;96(4):388–395. [DOI] [PubMed] [Google Scholar]
- 123. Lira Dos Santos EJ, Chavez MB, Tan MH, et al. Effects of active vitamin D or FGF23 antibody on hyp mice dentoalveolar tissues. J Dent Res. 2021;100(13):1482–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sun ZJ, Jin J, Qiu GX, Gao P, Liu Y. Surgical treatment of tumor-induced osteomalacia: a retrospective review of 40 cases with extremity tumors. BMC Musculoskelet Disord. 2015;16(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Geller JL, Khosravi A, Kelly MH, Riminucci M, Adams JS, Collins MT. Cinacalcet in the management of tumor-induced osteomalacia. J Bone Miner Res. 2007;22(6):931–937. [DOI] [PubMed] [Google Scholar]
- 126. Jan de Beur SM, Miller PD, Weber TJ, et al. Burosumab for the treatment of tumor-induced osteomalacia. J Bone Miner Res. 2021;36(4):627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chong WH, Andreopoulou P, Chen CC, et al. Tumor localization and biochemical response to cure in tumor-induced osteomalacia. J Bone Miner Res. 2013;28(6):1386–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. DeLacey S, Liu Z, Broyles A, et al. Hyperparathyroidism and parathyroidectomy in X-linked hypophosphatemia patients. Bone. 2019;127:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lecoq AL, Chaumet-Riffaud P, Blanchard A, et al. Hyperparathyroidism in patients with X-linked hypophosphatemia. J Bone Miner Res. 2020;35(7):1263–1273. [DOI] [PubMed] [Google Scholar]
- 130. Makitie O, Kooh SW, Sochett E. Prolonged high-dose phosphate treatment: a risk factor for tertiary hyperparathyroidism in X-linked hypophosphatemic rickets. Clin Endocrinol (Oxf). 2003;58(2):163–168. [DOI] [PubMed] [Google Scholar]
- 131. Kato H, Koga M, Kinoshita Y, et al. Incidence of complications in 25 adult patients with X-linked hypophosphatemia. J Clin Endocrinol Metab. 2021;106(9):e3682–e3692. [DOI] [PubMed] [Google Scholar]
- 132. Seikaly M, Browne R, Baum M. Nephrocalcinosis is associated with renal tubular acidosis in children with X-linked hypophosphatemia. Pediatrics. 1996;97(1):91–93. [PubMed] [Google Scholar]
- 133. Harada D, Ueyama K, Oriyama K, et al. Switching from conventional therapy to burosumab injection has the potential to prevent nephrocalcinosis in patients with X-linked hypophosphatemic rickets. J Pediatr Endocrinol Metab. 2021;34(6):791–798. [DOI] [PubMed] [Google Scholar]
- 134. Ohata Y, Yamazaki M, Kawai M, et al. Elevated fibroblast growth factor 23 exerts its effects on placenta and regulates vitamin D metabolism in pregnancy of Hyp mice. J Bone Miner Res. 2014;29(7):1627–1638. [DOI] [PubMed] [Google Scholar]
- 135. Delzer PR, Meyer RA Jr. Normal milk composition in lactating X-linked hypophosphatemic mice despite continued hypophosphatemia. Calcif Tissue Int. 1983;35(6):750–754. [DOI] [PubMed] [Google Scholar]
- 136. Jonas AJ, Dominguez B. Low breast milk phosphorus concentration in familial hypophosphatemia. J Pediatr Gastroenterol Nutr. 1989;8(4):541–543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.