Abstract
Context
Clinical hypothyroidism (CH) and subclinical hypothyroidism (SCH) have been linked to various metabolic comorbidities but the underlying metabolic alterations remain unclear. Metabolomics may provide metabolic insights into the pathophysiology of hypothyroidism.
Objective
We explored metabolic alterations in SCH and CH and identify potential metabolite biomarkers for the discrimination of SCH and CH from euthyroid individuals.
Methods
Plasma samples from a cohort of 126 human subjects, including 45 patients with CH, 41 patients with SCH, and 40 euthyroid controls, were analyzed by high-resolution mass spectrometry–based metabolomics. Data were processed by multivariate principal components analysis and orthogonal partial least squares discriminant analysis. Correlation analysis was performed by a Multivariate Linear Regression analysis. Unbiased Variable selection in R algorithm and 3 machine learning models were utilized to develop prediction models based on potential metabolite biomarkers.
Results
The plasma metabolomic patterns in SCH and CH groups were significantly different from those of control groups, while metabolite alterations between SCH and CH groups were dramatically similar. Pathway enrichment analysis found that SCH and CH had a significant impact on primary bile acid biosynthesis, steroid hormone biosynthesis, lysine degradation, tryptophan metabolism, and purine metabolism. Significant associations for 65 metabolites were found with levels of thyrotropin, free thyroxine, thyroid peroxidase antibody, or thyroglobulin antibody. We successfully selected and validated 17 metabolic biomarkers to differentiate 3 groups.
Conclusion
SCH and CH have significantly altered metabolic patterns associated with hypothyroidism, and metabolomics coupled with machine learning algorithms can be used to develop diagnostic models based on selected metabolites.
Keywords: subclinical hypothyroidism, clinical hypothyroidism, metabolomics, thyrotropin, free thyroxine, biomarkers
As the common endocrine system disease, clinical hypothyroidism (CH) affects 4% to 10% of the population, and the prevalence of subclinical hypothyroidism (SCH) is as high as 10% (1). CH is diagnosed when thyroid-stimulating hormone (TSH) concentrations are increased above the reference range and free thyroxine (FT4) concentrations fall below the reference range. SCH, which is commonly regarded as the sign of early thyroid failure, is diagnosed when TSH levels are detected above the upper limit of the reference range with FT4 within the normal range. The annual risk of SCH progression to CH is from 1% to 5%, depending on the presence of other risk factors (2).
The definition of hypothyroidism is primarily determined by biochemical parameters because clinical manifestations vary widely and often lack specific symptoms. However, in recent years, it has been a matter of debate whether the existing reference ranges for TSH and thyroid hormones (THs) should be applied to the diagnosis of thyroid dysfunction, as symptoms or the risk of adverse disease are not considered (2). A series of studies have confirmed an increased risk of stroke, sudden cardiac death, hyperlipidemia, and Alzheimer disease with variations in thyroid function even within their reference ranges (1–6). Thus, a reliable prediction of hypothyroidism consist of metabolic changes and biochemical testing is helpful to improve our understanding of the normal physiology and the pathophysiology and is of clinical importance in determining clinical treatment.
Metabolomics is a rapidly developing field of life science that uses advanced analytical techniques, such as mass spectrometry (MS) or nuclear magnetic resonance (NMR), and sophisticated statistical methods to comprehensively characterize the metabolome, a collection of all the small molecular metabolites and metabolic pathways for a given biological system (7). Metabolomics has increasingly been used to uncover novel metabolite biomarkers and metabolic pathways under various metabolic disease conditions (8). Furthermore, metabolomics is yielding important new insights into how metabolites influence organ function, immune modulate, and gut physiology (9). The capacity to detect a large number of widely varying metabolites makes metabolomics particularly attractive for the study and diagnosis of metabolic disorders (10–13). As a result, a number of important concepts indicating the role of “metabotoxins” in disease has been proposed, such as “diabetogens”, which refer to compounds that lead to diabetes and insulin resistance at chronically high concentrations (7).
Genome-wide association studies have explained only a small proportion of thyroid function variability in patients with hypothyroidism (14,15). In other words, it indicates that many other factors can also regulate TSH and TH production, including demographic factors (age and sex (2,16,17)), intrinsic factors (microbiota (18), stress (19)), and environmental factors (20). “Metabotoxins”, including endogenous compounds and xenobiotic molecules in serum or organs probably play a role in regulating TSH and TH levels. On the other hand, TH regulates metabolic processes essential for normal growth and development and plays an important role in regulating metabolism in the adults (21). TSH increases hepatic gluconeogenesis (22), controls lipid homeostasis (23), and alters monoaminergic function (24). Thus, a systemic profiling of metabolic alterations associated with SCH and CH by metabolomics may provide novel insights into the underlying mechanisms of hypothyroidism-associated complications.
In this study, we applied an ultrahigh performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS)-based plasma metabolomic approach to characterize the global metabolic perturbations of SCH and CH. The aim of the study is to provide new insights into the pathogenesis of hypothyroidism at the metabolic level and to develop prediction models based on potential metabolic biomarkers for the discrimination of euthyroid controls, individuals with SCH, and patients with CH. Such an approach may have a tremendous impact not only on understanding the molecular basis of SCH and CH, but also on improving current clinical practice in SCH and CH.
Methods
Study Participants
We recruited 126 participants (32 males and 94 females) from the Endocrine Outpatient Clinics of Gansu Provincial Hospital, Gansu, China, between October 2019 and November 2021, including 40 healthy adults and 86 newly diagnosed and untreated patients with hypothyroidism (45 patients with SCH and 41 individuals with CH).
The following exclusion criteria were utilized: age younger than 18 or older than 60 years; pregnancy or breastfeeding; obesity (body mass index [BMI] ≥28 kg/m2); a history of or current illness, especially known metabolic diseases, such as diabetes or prediabetes, hyperuricemia or gout, hypertension, heart disease, renal or hepatic impairment, cancer, autoimmune diseases, or blood diseases; and current medication use, such as antithyroid drugs or levothyroxine, heparin, lipid medications, or corticosteroids. CH was defined as an elevation of serum TSH levels above the reference range (0.35-4.94 mIU/L) and reduced serum FT4 levels below the reference value (9.01-19.05 pmol/L) and SCH was diagnosed with TSH levels above the upper limit of the reference range and FT4 within the normal range. Subjects in the control groups exhibited normal thyroid function and no clinical or biochemical signs of any disease. The study was approved by the Ethics Committee of the Gansu Provincial Hospital (Registration Number: 2019-196). Informed consent was obtained from all participants.
Clinical and Laboratory Measurements
Data were collected by an experienced physicians using a structured questionnaire with the following: demographic information, smoking and drinking habits, history of diseases, current medication use, blood pressure, body weight, and height.
Baseline serum and plasma samples were obtained from all participants and analyzed immediately or stored at −80 °C. Laboratory measurements of serum total bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin (IBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (CREA), fasting blood glucose (FBG), uric acid (UA), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were measured by standard methods (Ci 1620 Automatic biochemical immune analysis system, Abbott Laboratories, USA). TSH, thyroxine (T4), triiodothyronine (T3), FT4, free triiodothyronine (FT3), thyroid peroxidase antibody (TPOAb) and thyroglobulin antibody (TgAb) were determined on the IMMULITE 2000 system (Abbott Laboratories, USA) using Chemiluminesent Immunoassay detection methods.
Plasma Sample Processing
Around 50 μL plasma was treated with 200 μL cold methanol with internal standard (fluorouracil). After centrifugation at 14 000 rpm for 10 minutes at 4 °C, the supernatant was collected for LC-MS analysis. The quality control (QC) sample was prepared by pooling all the serum samples aliquots of 10 μL.
Untargeted Metabolomics Analysis
Metabolomics analysis was performed based on a previously published protocol (13). In brief, UHPLC (Nexera UHPLC LC-30A, SHIMADZU Technologies, Japan) coupled to a quadruple time-of-flight (QTOF) mass spectrometer (AB 6600 TripleTOF, SCIEX, CANADA) was utilized to acquire data in a data-dependent acquisition (DDA) mode in both positive and negative ionization modes. LC separation was performed using Waters ACQUITY UPLC BEH Amide column (100 mm × 2.1 mm, 1.7 μm).
Metabolomics Data Processing
The raw data (.wiff files) were converted to mzML format using ProteoWizard software (version 3.0.21020.0, https://proteowizard.sourceforge.io/). R package XCMS (25) (version 3.14.1) was used to extract metabolites features. In brief, peaks were detected within a tolerance of 20 ppm and peak width of 5 to 30 seconds, a subset-based method was applied to adjust retention time drifts based on QC injected in regular intervals, then peaks presenting in 80% of QC or samples were corresponded to one metabolite feature. Missing values were imputed with half of minimum intensity of each feature. To remove batch effects, a support vector machine (SVM) based normalization (26) was performed, and feature of internal standard was pick up to correct. After excluding features with coefficient of variation in QC > 30%, 10 000 features were included in the following analysis.
Metabolites identification was performed by matching exact mass, retention time, and MS/MS spectrum with an integrated database. The database contained our in-house chemical standards and public databases, including HMDB (27), MassBank (28), GNPS (29), and KEGG (30). R package Spectra (version 1.2.2) were employed to calculate MS/MS spectrum similarity through a dot-product algorithm, which considered both fragments and intensities. According to Metabolomics Standards Initiative (MSI) (31), metabolites identified by in-house database were considered as level 1, which matched in both exact mass, retention time and MS/MS spectrum, and others identified by public database as level 2. In addition, unannotated features were analyzed by a metabolic reaction network based recursive algorithm (32) (MetDNA) to reveal novel metabolites, which given a MSI level 4 identification.
Statistical Analysis
The study design and data analysis workflow are illustrated in Fig. 1A. Clinical and laboratory measurements of the control, SCH, and CH groups were expressed by mean ± SD for normally distributed data. Variables with a skewed distribution, were given as median and upper and lower quartiles. The differences among the 3 groups were analyzed by one-way analysis of variance (ANOVA) or Kruskal-Wallis H tests. The proportions were analyzed using chi-squared tests. All the data analysis was performed by R (version 4.1.0). Features with coefficient of variation in QC < 30% were utilized in principal component analysis by R package mixOmics (version 6.13.3). Annotated compounds data was log transformed before ANOVA. After Benjamini & Hochberg adjusted, metabolites with false discovery rate (FDR) <0.05 were z-score normalized and showed in heatmap by R package ComplexHeatmap (version 2.8.0). To discovery differential metabolites, Wilcoxon test was used to ascertain the significance of metabolites alterations and the threshold of FDR and fold-change (FC) were set to 0.05 and 4/3 or 3/4. We utilized a quantitative enrichment analysis–based method Metabolite Set Enrichment Analysis (MSEA) (33) to perform pathway analysis. Using KEGG pathway database as metabolites sets, the top 20 disturbed pathway were shown. Then, we calculated the Pearson correlation between thyroid function parameters and metabolites playing a role in known metabolism pathway (in KEGG pathway). A correlation network drawn by R package igraph (version 1.2.11) showed metabolites significantly correlated with thyroid function parameters (P < 0.05). We applied Least Absolute Shrinkage and Selection Operator (LASSO) regression, random forest (RF), and support vector regression (SVR) to build models to predict CH and SCH using R package caret (version 6.0.90). To optimize predictive performance, a multi-variables selection algorithm unbiased variable selection in R (MUVR) (34) was used to screen metabolites as biomarkers. In brief, variables were selected by performing recursive variable elimination in 200 repeated double cross-validation. The top 7 robust predictive variables (metabolites) or the most optimized subset of variables (features) were selected as biomarker to construct prediction models. Receiver operating characteristic (ROC) curve were using to evaluate the performance of each model (R package pROC, version 1.18.0)
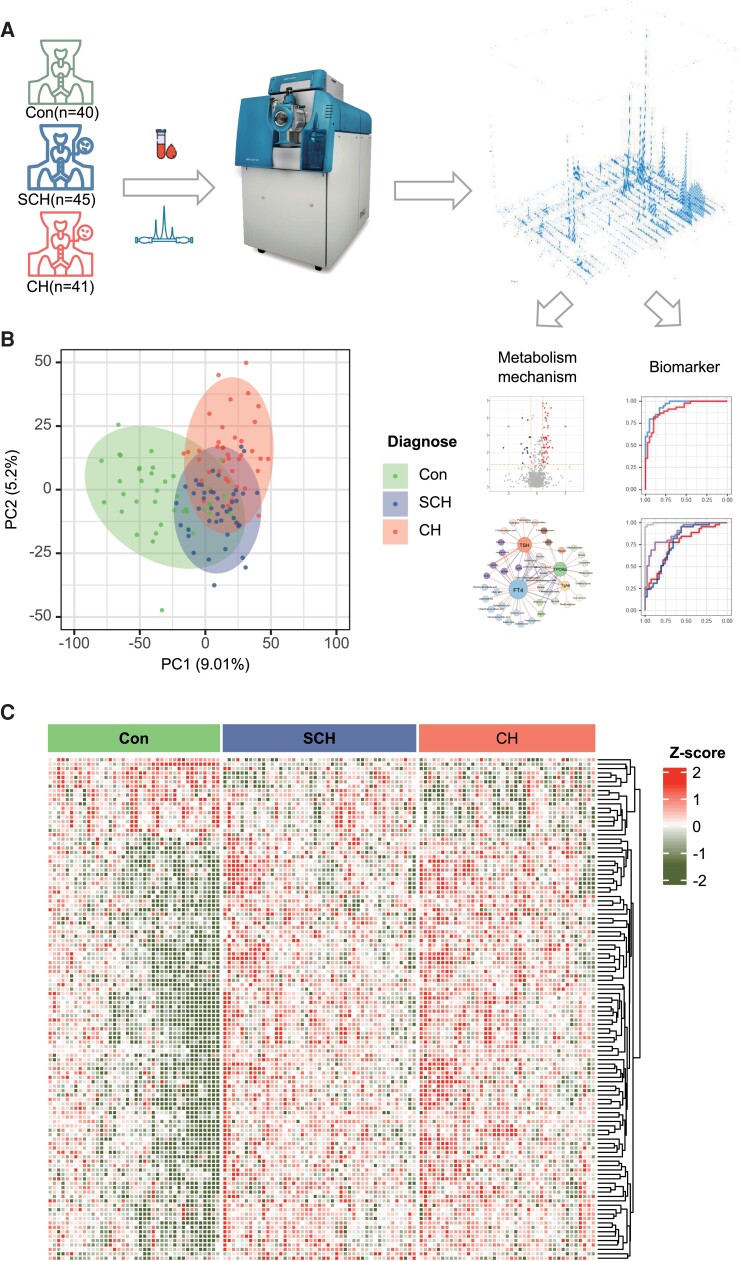
Figure 1.
Untargeted metabolomic profile of participants serum. A, Scheme of design: 40 healthy control (Con), 41 patients diagnosed as subclinical hypothyroidism (SCH), and 45 patients with clinical hypothyroidism were included. B, Principal component analysis (PCA) based on all features, top 2 PCs explaining the largest variation were shown. C, Heatmap of metabolites with false discovery rate (FDR) <0.05 in ANOVA.
Results
Clinical and Biochemical Characteristics of the Enrolled Participants
In this study, 41 patients with CH, 45 individuals with SCH, and 40 healthy participants were included. The clinical characteristics of these subjects are shown in Table 1. The median age of participants was 36 years (21-59), and there were no significant differences in age and sex among the 3 groups. CH and SCH groups had significantly higher serum TSH levels, while markedly lower levels of T4 and FT4 than the euthyroid control group. Lower serum T3 and FT3 levels were only observed in subjects with CH. Compared with the control group, patients with SCH or CH had dramatically higher TPOAb and TgAb levels. Because strict exclusion criteria were established to exclude the interference of comorbidities on metabolism, almost all participants were of normotension and of normal weight to slightly overweight. Additionally, there were no significant differences in serum TBIL, DBIL, IBIL, ALT, AST, CREA, FBG, UA, and TG levels, while TC and LDL-C levels were found to be significantly higher in the CH group than in the other 2 groups.
Table 1.
Baseline characteristics of the enrolled participants
| Parameters | Control (n = 40) | SCH (n = 45) | CH (n = 41) | P |
|---|---|---|---|---|
| Age, year | 35.7 (10.7) | 39.0 (10.2) | 39.0 (9.2) | 0.232 |
| Female, n (%) | 30 (75%) | 32 (76.2%) | 32 (72.7%) | 0.932 |
| Smoking, n (%) | 6 (15%) | 4 (9.5%) | 10 (22.7%) | 0.242 |
| Drinking, n (%) | 15 (37.5%) | 18 (42.9%) | 21 (47.7%) | 0.639 |
| BMI, kg/m2 | 22.66 (3.13) | 21.54 (2.72) | 22.81 (3.06) | 0.114 |
| SBP, mmHg | 117 (14) | 114 (14) | 115 (17) | 0.717 |
| DBP, mmHg | 74 (8) | 70 (13) | 71 (12) | 0.395 |
| TSH, mIU/L | 2.06 (0.54–4.38) | 12.32 (5.58–100)a | 50.6 (9.65–100)a,b | <0.001 |
| T3, nmol/L | 1.57 (0.96–2.04) | 1.65 (0.98–2.05) | 1.09 (0.31–2.03)a,b | <0.001 |
| T4, nmol/L | 98.19 (64.14–126.32) | 82.38 (50.97–151.12)a | 41.99 (11.71–76.77)a,b | <0.001 |
| FT3, pmol/L | 4.38 (3.00–7.08) | 4.66 (1.99–6.24) | 3.02 (1.42–15.79)a,b | <0.001 |
| FT4, pmol/L | 13.29 (10.36–17.10) | 10.95 (9.17–14.93)a | 6.50 (2.41–10.83)a,b | <0.001 |
| TgAb, IU/mL | 1.05 (0.10–336.28) | 70.72 (0–1000)a | 125.41 (0.56–1000)a | <0.001 |
| TPOAb, IU/mL | 0.39 (0–110.84) | 379.76 (0–1000)a | 702.41 (0–1000)a | <0.001 |
| TBIL, umol/L | 17.97 (9.73–55.06) | 20.30 (3.70–113.00) | 17.60 (8.80–42.23) | 0.677 |
| DBIL, μmol/L | 4.56 (2.50–7.55) | 4.75 (2.30–14.60) | 4.20 (2.50–8.40) | 0.132 |
| IBIL, μmol/L | 14.2 (8.5–24.4) | 12.8 (0.5–31.6) | 13.0 (6.0–31.4) | 0.317 |
| ALT, U/L | 17 (8–35) | 17 (4–54) | 20 (8–47) | 0.290 |
| AST, U/L | 19 (12–32) | 20 (11–54) | 20 (12–47) | 0.144 |
| CREA, μmol/L | 58.08 (41.50–78.00) | 56.55 (40.52–85.70) | 65.30 (40.30–96.67) | 0.136 |
| UA, μmol/L | 272.47 (86.22) | 282.95 (77.12) | 298.31 (90.94) | 0.427 |
| FBG, mmol/L | 4.84 (0.65) | 4.85 (0.61) | 4.96 (0.52) | 0.611 |
| TC, mmol/L | 3.78 (2.19–14.53) | 4.16 (2.96–6.79) | 4.83 (3.82–8.12)a,b | <0.001 |
| TG, mmol/L | 1.03 (0.43–5.44) | 1.18 (0.41–3.27) | 1.16 (0.54–5.49) | 0.299 |
| LDL-C, mmol/L | 2.40 (0.62) | 2.55 (0.69) | 3.20 (0.75)a,b | <0.001 |
| HDL-C, mmol/L | 1.21 (0.23) | 1.25 (0.21) | 1.34 (0.28)a | 0.061 |
Data are expressed as medians and upper and lower quartiles, unless indicated otherwise. Age, BMI, SBP, DBP, UA, FBG, LDL-C, and HDL-C are shown by mean ± SD. Smoking: current or had a history of smoking; drinking: current or had a history of drinking.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CREA, creatinine; DBP, diastolic blood pressure; DBIL, direct bilirubin; FBG, fasting blood glucose; FT3, free triiodothyronine; FT4, free thyroxine; HDL-C, high-density lipoprotein cholesterol; IBIL, indirect bilirubin; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; T3, triiodothyronine; T4, thyroxine; TBIL, total bilirubin; TC, total cholesterol; TG, triglyceride; TgAb, thyroglobulin antibody; TPOAb, thyroid peroxidase antibody; TSH, thyrotropin; UA, uric acid.
Compared to control group, P < 0.05.
Compared to SCH group, P < 0.05.
Plasma Metabolomic Profiles of the Euthyroid Controls, SCH, and CH
An untargeted metabolomic analysis using high-resolution MS was performed in this study, and 11 376 peaks in positive and negative ionization modes were detected (Fig. 1A). After the exclusion of natural isotope peaks, 1222 and 1112 metabolites were identified in positive and negative modes, respectively. Among the annotated metabolites, 22 compounds were identified by our in-house database (MSI level 1), 561 metabolites with MS/MS spectra matched to a public database (MSI level 2).
To examine the intrinsic metabolic variations and data quality of the metabolomic features data, we performed an unsupervised principal component analysis (PCA) in the statistical evaluation. Metabolites in the SCH and CH groups demonstrated a better separation from healthy control, whereas individuals with SCH and CH showed less obvious separation (Fig. 1B).
Furthermore, an ANOVA was performed to identify differential metabolites of the 3 groups and these significantly different metabolites were used for clustering analysis in a heatmap (Fig. 1C). The heatmap visually suggested that metabolites in the CH and SCH group had obvious differences compared to the control, whereas metabolic alterations showed similar patterns between the SCH and CH groups.
Differential Metabolites and Dysregulated Metabolic Pathways of the Euthyroid Controls, SCH, and CH
To exclude possible confounding variables and to maximize the variations between groups in metabolomics analysis, and detect metabolites that significantly contribute to the variation, a supervised orthogonal partial least squares discriminant analysis (OPLS-DA) was applied (Supplementary Fig. 1A–1F (35)). Compared with the PCA, the OPLS-DA analysis showed a better separation of metabolites in the hypothyroid (SCH group and CH group) and euthyroid control groups without overfitting. Notably, even the SCH and CH were clearly separated (Supplementary Fig. 1E and 1F (35)).
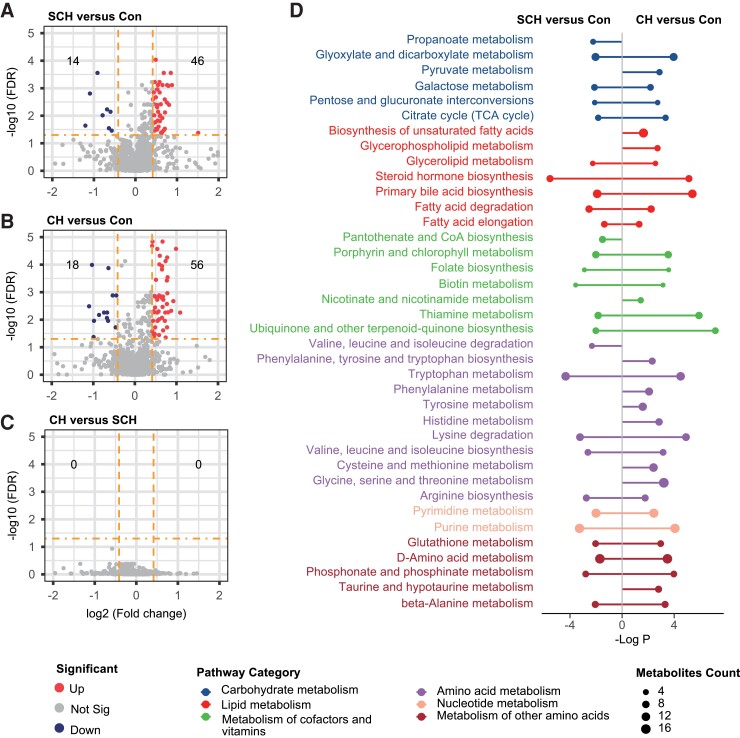
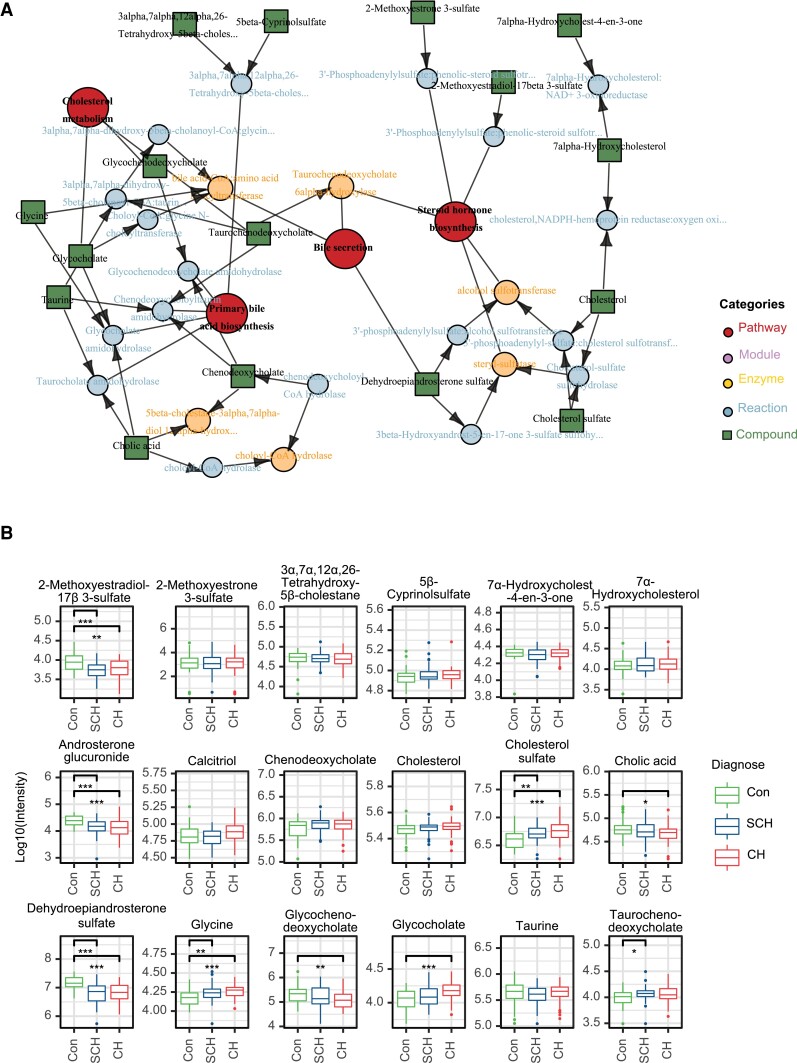
To identify those metabolites that are changed significantly between any 2 of the 3 groups, we performed univariate nonparametric Wilcoxon’s analysis based on the fold-change and FDR of metabolites. Metabolites with an FDR of < 0.05 and fold-change of > 4/3 or < 3/4 were considered as differential metabolites. As shown in the volcano plot, among the 110 metabolites with an FDR of < 0.05, 46 and 14 metabolites were significantly upregulated and downregulated, respectively, in the SCH group compared with the euthyroid control group (Fig. 2A, Supplementary Table 1 (35)). In Fig. 2B, the volcano plot showed that 141 metabolites had an FDR < 0.05, among which 56 metabolites were significantly upregulated, whereas 18 metabolites were significantly downregulated when comparing the CH group to the euthyroid control group (Supplementary Table 2 (35)). These significantly regulated metabolites fell into diverse categories of structural identities, including lipids, bile acids, steroid hormones, amino acids, nucleotides, and purine metabolites. For example, compared with the control group, SCH and CH patients showed significantly higher plasma levels of glycerophospholipids, including phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), phosphatidylserine (PS), lysophosphatidylcholine (LPC), lysophosphatidylethanolamine (LPE), and lysobisphosphatidic acid (LPA). Notably, there were no significantly changed metabolites between the CH group and the SCH group (Fig. 2C). Next, we examined global distribution on KEGG pathways by MSEA. As a result, 53 of 194 mapped pathways showed significant changes (FDR < 0.05, global test). The pathway analysis showed a broad range of pathway categories and the detailed impacts of hypothyroidism-related alterations in metabolic networks (Fig. 2D). We found that, in both SCH and CH groups, the enriched pathways mainly involved in primary bile acid (BA) biosynthesis, steroid hormone biosynthesis, thiamine metabolism, lysine degradation, tryptophan metabolism, purine metabolism, and phosphonate and phosphinate metabolism. Metabolites and metabolic pathways closely associated with hypothyroidism were shown in Fig. 3 and Supplementary Figs. 2 and 3 (35). We found that cholesterol seems to be a critical metabolite, which acts as a bridge linking cholesterol metabolism, primary BA biosynthesis, bile secretion, and steroid hormone biosynthesis (Fig. 3A). As shown in Fig. 3B, levels of cholesterol sulfate were significantly elevated from control to SCH and CH, while the levels of cholesterol showed similar trends without statistical significance. The levels of 2-methoxyestradiol-17β-3-sulfate, androsterone glucuronide, and dehydroepiandrosterone sulfate (DHEA-S) were significantly decreased in SCH and CH, whereas glycine and glycocholate showed opposite trends.
Figure 2.
Differential metabolites and disturbed pathway. A, Volcano plot shows altered metabolites between CH and Con, which increase (red, FDR<0.05 and fold-change >4/3) or decrease (blue, FDR <0.05 and fold-change <3/4) in CH. B, Volcano plot shows altered metabolites between SCH and Con, which increase (red, FDR<0.05 and fold-change >4/3) or decrease (blue, FDR<0.05 and fold-change <3/4) in SCH. C, Volcano plot shows altered metabolites between CH and SCH, which increase (red, FDR<0.05 and fold-change >4/3) or decrease (blue, FDR<0.05 and fold-change <3/4) in CH. D, Pathway analysis by Metabolite Set Enrichment Analysis (MSEA), pathway involved in metabolism were included for MSEA, significantly disturbed pathway between SCH and Con (left), CH and Con (right) are shown.
Figure 3.
Metabolites involved in steroid and bile acid metabolism. A, Network of detected metabolites and deductive enzymes involved in steroid and bile acid metabolism. B, Relative intensity of metabolites in the above network.
As shown in supplemental Fig. 2A (35), amino acid metabolism was notably altered in CH patients compared to the control, with significantly reduced levels of L-tyrosine, L-lysine, and L-histidine, with increased levels of glycine. Interestingly, L-arginine was significantly increased while glycine was decreased in subjects with SCH and CH compared to control. Notably, the levels of 5-hydroxytryptamine, tryptophan metabolism, and lysine degradation metabolic pathways were markedly altered in CH and SCH. Interestingly, however, levels of D-aspartate, indole-3-acetaldehyde, and indole-3-ethanol, were only significantly elevated in SCH but not in CH (Supplementary Fig. 2B (35)).
Although the serum urate levels were not significantly different among the 3 groups, purine and nucleotide metabolism pathways were enriched as the significantly altered pathways in SCH and CH compared to control (Supplementary Fig. 3A and 3B (35)). Hypothyroid patients showed increased abundances of several purine metabolites, such as adenine, hypoxanthine, xanthine, and UA degradation metabolites, including 5-hydroxyisourate, 2-oxo-4-hydroxy-4-carboxy-5-ureidoimidazole, and allantoic acid. These data appear to suggest that hypothyroid patients increase purine metabolism and UA breakdown to similar extent without significantly affecting serum urate levels.
Correlations of Thyroid Function Parameters and Plasma Metabolites
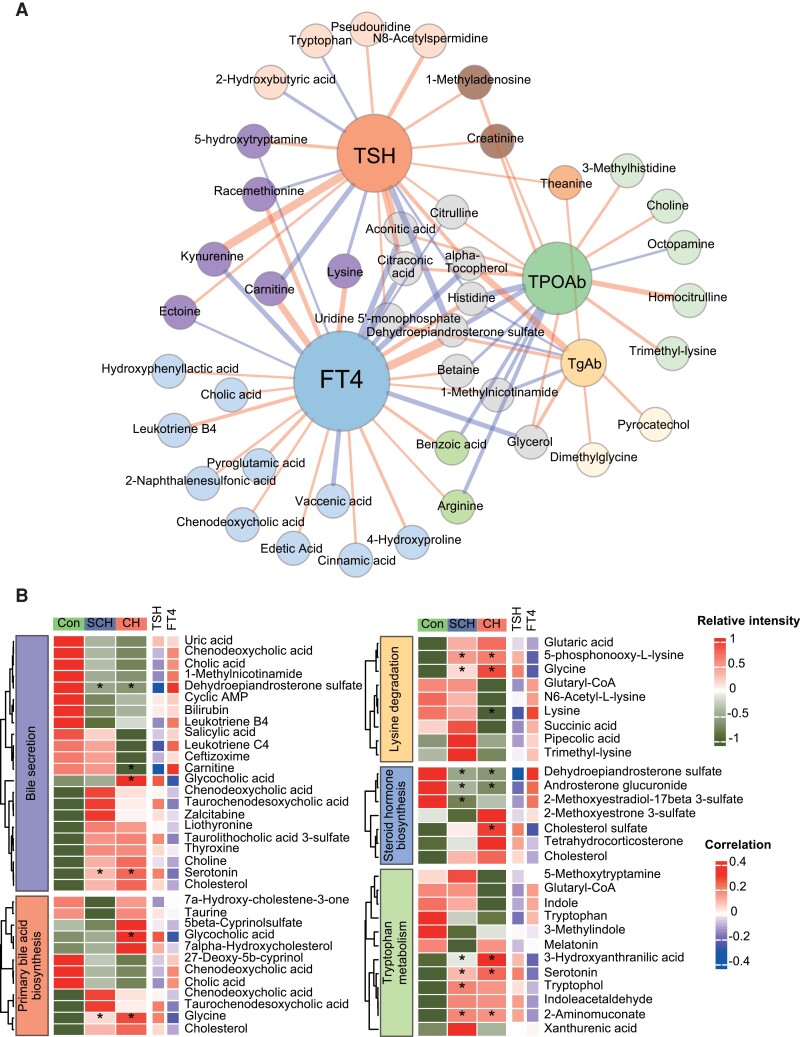
Circulating levels of TSH, FT4, TPOAb, and TgAb are routinely used to diagnose SCH and CH in clinical settings. To further investigate whether these hormones are associated with metabolic alterations identified by the metabolomics, we calculated the Pearson correlations between these serum thyroid function parameters and metabolites and metabolic pathways (in the KEGG pathway). A correlation network was constructed based on statistically significant metabolites with hormone levels (P < 0.05), among which 65 metabolites and 5 primary metabolism pathways were significantly associated with serum TSH, FT4, TPOAb, and TgAb, respectively (Fig. 4A and B). Among the 5 metabolic pathways, DHEA-S in steroid hormone biosynthesis emerges as a key node connecting these 4 thyroid function parameters. Furthermore, levels of DHEA-S and androsterone glucuronide were significantly elevated in the SCH and CH group, and positively correlated with FT4 levels but negatively correlated with TSH. On the other hand, cholesterol sulfate decreased only in the CH group, with a positive correlation with TSH but inverse correlation with FT4 (Fig. 4). Moreover, the other 2 cholesterol-related metabolic pathways, bile secretion and primary BA biosynthesis, were also found to be critically correlated with TSH, FT4, and TPOAb. Consistent with analysis in previous section, metabolites in lysine degradation and tryptophan metabolism were also significantly correlated: 5-phosphonoxy-L-lysine and glycine were elevated in SCH and CH, and positively correlated with TSH but negatively correlated with FT4. Similar trends were observed for metabolites in tryptophan metabolism, such as 3-hydroxyanthranilic acid, 5-hydroxytryptamine, and 2-aminomuconate.
Figure 4.
Metabolite disturbances related to thyroid function parameters. A, Correlation network of TSH, FT4, TgAb, TPOAb, and metabolites (gender, age, and BMI as the concomitant variable), edges connecting 2 nodes represent positive (red) or negative (blue) correlation and their width represent correlation coefficients. B, Heatmap of metabolites and their enriched pathways associated with TSH and FT4.
Development of Diagnostic Models Based on Metabolites Using Machine Learning Algorithms
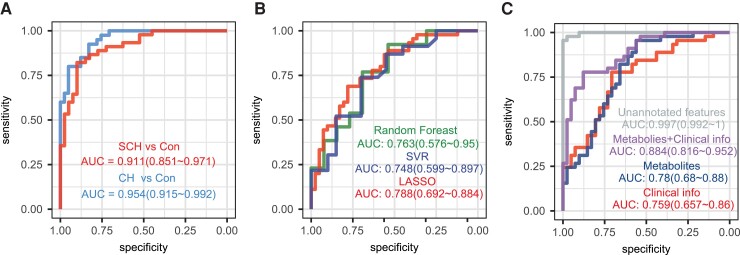
After systematic profiling of metabolomic alteration in plasma of SCH and CH compared to control, we set out to develop prediction models by selecting metabolites to differentiate individuals with SCH and patients with CH from euthyroid controls. We randomly selected two-thirds of the samples in each group as the training set and the rest as the validation set. A MUVR algorithm was used to identify potential metabolites, and the top metabolites in 200 repeated 10-fold cross-validation were selected as potential biomarkers to increase the reliability and clinical suitability of the prediction models. A machine learning model of LASSO was used to establish the prediction models. Optimal variables number and lambda was automatically selected in the 10-fold cross-validation. Only metabolites with MS/MS spectrum matched (dot product >0.3) were selected as potential biomarkers. Although the optimal variables to construct the best predictive models were 67 (CH and Con), 58 (SCH and Con) and 42 (CH and SCH), respectively (Supplementary Fig. 4 (35)), we selected the top 7 important variables in our prediction models for practical purpose. A total of 17 metabolites were selected and further validated the structural identities (Supplementary Figs. 5–7 (35)), and the box plots showed the abundance of these metabolomic biomarkers in the prediction models (Supplementary Fig. 8 (35)). A total of 11 metabolites were identified to distinguish SCH and CH groups from euthyroid control, and LASSO regression was performed to establish the prediction models. In the prediction model to differentiate SCH from control, the 7 selected metabolites: DHEA-S, PC 40:5, 1-palmitoylphosphatidylcholine, tryptophol, LPC (15:0/0:0), PE 38:5, and LPE (16:0/0:0), yielded an area under the curve (AUC) of 0.911 (95% CI, 0.851-0.971) in the validation set. LPC (18:0/0:0), LPC (P-18:0/0:0), LPC (20:0/0:0), 1-palmitoylphosphatidylcholine, DHEA-S, erythro-isoleucine, and LPC (15:0/0:0) were identified as potential biomarkers for the prediction of CH. The AUC in the training set was 0.954, ranging from 0.915 to 0.992, consistent with a large metabolomic difference between the 2 groups (Fig. 5A).
Figure 5.
Prediction models based on machine learning algorithm. A, Receiver operating characteristic (ROC) curve of least absolute shrinkage and selection operator (LASSO) models to discriminate Con and CH (or SCH) based on 7 selected metabolites. B, ROC curves of 3 different models to discriminate SCH and CH based on 7 selected metabolites. C, ROC curve of LASSO model to discriminate CH and SCH based on unannotated features (MS peaks currently not matched in databases), 7 selected metabolites, clinical information (age, TPOAb, TgAb, BMI, SBP, DBP, ALT, AST, CREA, UA, FBG, TC, TG, HDL-C, LDL-C) and combination of metabolites and clinical information.
Due to the limited metabolic alterations between SCH and CH patients, a prediction model based on metabolites alone to dissect these two is more challenging than differentiating SCH and CH from control. Nonetheless, 7 metabolites, carnitine, erythro-isoleucine, racemethionine, histidine, tryptophan, L-lysine, and p-octopamin, were selected to differentiate CH from SCH. To increase the reliability of this prediction model, we validated this model by using 3 machine learning algorithms, RF, SVM, and LASSO regression, which yielded good AUCs (0.748–0.788) although LASSO regression appeared slightly better (Fig. 5B). To improve the performance of the prediction model to differentiate SCH from CH, we added some routine clinical parameters, such as age, TPOAb, TgAb, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), ALT, AST, CREA, UA, FBG, TC, TG, HDL-C, and LDL-C, in addition to the 7 metabolites, which significantly improved this model with an increase of AUC from 0.780 to 0.884. Notably, our combined prediction model has drastically outperformed the current clinical diagnosis based on clinical information (Fig. 5C). Interestingly, we attempted to build a prediction model with unannotated peak features in our metabolomic analysis and achieved a complete distinguishing of SCH from CH (AUC of 0.997). However, the obvious drawback for this model is difficult to perform quantitative analysis due to the lack of standards.
Discussion
Serum TH levels and TSH levels are the major determinants of the current clinical diagnosis of hypothyroidism, including SCH and CH. However, the metabolic alterations associated with SCH and CH compared to healthy control remain poorly defined (36). In this nontargeted UHPLC-MS metabolomics study, strict criteria were established to highlight the influence of hypothyroidism on metabolism and to minimize metabolic interference from other metabolic comorbidities. We included 126 plasma samples of patients with SCH or CH, and euthyroid controls, and systematically defined the metabolic profiles and related pathways in the 3 groups. The plasma metabolomic patterns in the SCH and CH groups were significantly different from those of the control group but the metabolic patterns were surprisingly similar between SCH and CH. Pathway enrichment analysis found diverse significantly dysregulated pathways in individuals with SCH and patients with CH compared to euthyroid controls, including primary BA biosynthesis, steroid hormone biosynthesis, lysine degradation, tryptophan metabolism, and purine metabolism. Furthermore, 65 metabolites and 5 primary metabolism pathways were significantly associated with serum levels of TSH, FT4, TPOAb, and TgAb. We further employed 3 machine learning algorithms to build prediction models based on 17 metabolic biomarkers to differentiate the 3 groups, especially SCH from CH.
This study, for the first time, has presented a comprehensive metabolomic evaluation and comparison of SCH, CH, and healthy controls using nontargeted UHPLC-MS and has developed prediction models for the diagnosis of SCH and CH. To minimize confounding factors from other metabolic conditions, our cohort included participants with an age range from 18 to 60 years and excluded those with a history or current illness, especially the known metabolic diseases. Thus, the metabolomic profiles of our study might be more reflective of metabolism underlying hypothyroidism. Most previous metabolomic studies on CH were performed in animals (37–41), while a few reports have focused only on CH in humans (42–45).
In present study, we observed significantly higher levels of plasma profile of glycerophospholipids, including PC, PE, PG, PI, PS, LPC, LPE, and LPA in the SCH and CH groups than those in the control group (Supplementary Table 1 and 2 (35)). Interestingly, some of the lipid species, such as LPC (18:0/0:0), LPC (P-18:0/0:0), LPC (20:0/0:0) and LPC (15:0/0:0), were identified as potential biomarkers for the prediction of CH, while PC 40:5, LPC (15:0/0:0), PE 38:5, and LPE (16:0/0:0) were potential biomarkers for the prediction of SCH (Fig. 4). Glycerophospholipids have been associated with other diseases, such as atherosclerosis, diabetes, insulin resistance, and metabolic syndrome (46–48). In hypothyroid patients, a previous study had demonstrated an increase of PS, LPC, PE, and sphingomyelin and emphasized the significance of studying phospholipids in the diagnosis of hypothyroidism (49).
Furthermore, metabolic pathways of primary BA biosynthesis and steroid hormone biosynthesis were obviously disturbed in subjects with CH and SCH. Primary BAs, including cholic acid, taurocholic acid, and taurodeoxycholic acid, were decreased, while cholesterol sulfate was increased in CH and SCH compared with euthyroid controls, despite no significant differences in SCH (Fig. 3A). Altered levels of TC and LDL-C are frequently noted in hypothyroidism, which plays an important role in the disrupted function of heart and vascular physiology (1). The conversion of cholesterol to BAs is a required cholesterol clearance pathway to maintain cholesterol homeostasis and altered BA metabolism has been associated with many diseases, such as cardiomyopathy, ulcerative colitis, Crohn disease, and Alzheimer disease (50–53). However, a few studies suggested a possible association between hypothyroidism and bile composition and excretion in patients with SCH or CH (54,55). Thyroid hormone can stimulate hepatic BA synthesis and biliary secretion of lipids, particularly those of cholesterol and phospholipids, while TSH has been reported to promote cholesterol synthesis and inhibit BA synthesis (56,57), although it remains controversial whether the effects are due to increased TSH or decreased FT4 levels. Furthermore, a recent study suggested that the BAs act as signaling molecules regulating the thyroid pituitary axis (58). Interestingly, a previous metabolomic study also identified altered BA profiles in clinical hyperthyroidism and hypothyroidism, in which increased cholic acid and decreased glycodeoxycholic acid levels were detected (43). Cholesterol sulfate is the hydrophilic excretion form of cholesterol. It regulates cholesterol homeostasis, directly or indirectly (59). The levels of plasma cholesterol sulfate have been found strikingly elevated in hypothyroid patients (60).
Our study identified that metabolic pathways associated with steroid hormones are also significantly perturbed in SCH and CH patients: DHEA-S, androsterone glucuronide, pregnanediol, and 2-methoxyestradiol-17beta-3-sulfate decreased both in SCH and CH group. In 1980, Bassi et al discovered lower DHEA-S concentrations in CH patients (61). More recently, Gonulalan et al observed lower levels of DHEA-S in SCH (62), while Ravaglia et al confirmed a positive association between serum DHEA-S levels and serum FT4 levels in women and a negative association between serum DHEA-S levels and serum TSH levels in men (63). Similar trends were found in our study. Surprisingly, in our correlation analysis, DHEA-S was negatively correlated with serum TPOAb. DHEA-S has some protective effects against cardiovascular disease, hypercholesterolemia, and immune-modulated diseases, and decreased serum DHEA-S concentrations have been reported in some autoimmune diseases (64). DHEA-S normalized the defects of a subpopulation of natural killer immune cells in Graves disease and Hashimoto thyroiditis (65). Therefore, future studies are warranted to investigate the roles of DHEA-S in autoimmune hypothyroidism and related complications.
In the metabolic profiles, the amino acid metabolism was notably altered in CH, with reduced levels of L-tyrosine, L-lysine, and L-histidine, and increased levels of glycine, just the inverse of previous reports in hyperthyroid patients (43,66). Notably, the levels of 5-hydroxytryptamine, tryptophan metabolism, and lysine degradation metabolic pathways were markedly altered in CH and SCH compared to control. Tryptophan plays a crucial role in protein synthesis and a precursor for the biosynthesis of 5-hydroxytryptamine, melatonin, kynurenic acid, tryptamine, and also coenzymes important for electron transfer reaction. The levels of tryptophan can be used for diagnosing various metabolic disorders and the symptoms associated with those diseases (67). Furthermore, reduced secretion of tryptophan and kynurenine to tryptophan ratio were associated with Alzheimer disease, which might contribute to hypothyroidism-related cognitive impairment. More importantly, we found that 5-hydroxytryptamine and kynurenine were positively correlated with TSH in the correlation analysis. The pipecolate pathway is the predominant lysine degradative pathway in adult brain, in which μ-crystallin/ketimine reductase (CRYM/KR) is a crucial enzyme. Hallen et al proposed that T3 strongly regulates CRYM/KR activity, suggesting that at least some of the biological effects of T3 are most likely due to the interaction of T3 with CRYM/KR (68). Interestingly, our study also found that FT4 had a positive correlation with L-lysine. Together, our findings suggest that the interplays between amino acid metabolism and thyroid functions are certainly far more complex than we expected, which warrants future studies.
Furthermore, purine metabolism pathway was identified as significantly disturbed in SCH and CH group. Serum UA levels reflect a balance between the metabolic breakdown of purine nucleotides and UA excretion. It remains poorly understood how serum UA affects thyroid function. We observed hypothyroid patients with increased abundances of several purine metabolites (adenine, hypoxanthine, xanthine) and UA degradation metabolites (5-hydroxyisourate, 2-oxo-4-hydroxy-4-carboxy-5-ureidoimidazole, allantoic acid), whereas UA remains similar to the control. In CH, hyperuricemia was previously thought to be associated with reduced renal plasma flow and impaired glomerular filtration (69). On the other hand, TSH and T4 are found not to be related to UA levels (70), especially for the association between SCH and serum UA levels (71).
Metabolomics and machine learning algorithms have been increasingly recognized as enabling techniques for developing diagnostic models for various human diseases (13,72,73). After systematically profiling the metabolic alterations in SCH and CH, we successfully selected potential metabolites to distinguish the 3 groups using machine learning algorithms. In our study, 7 metabolites were identified for discrimination of the SCH group from the euthyroid control group, with an AUC of 0.911 (95% CI, 0.851-0.971), and 7 metabolites were identified to discriminate the CH group from the euthyroid control group, with an AUC of 0.954 (95% CI, 0.915-0.992) in the validation set. Although the metabolic alterations in SCH and CH were quite similar, we successfully identified 7 metabolic biomarkers using 3 machine learning algorithms to differentiate between SCH and CH. Thyroid autoantibodies and demographic factors, including age and sex, were important clinical factors regulating thyroid function status (2). It is worth mentioning that these metabolic biomarkers showed a slightly higher AUC than current clinical factors, and the prediction model combining clinical and metabolomics traits achieved a median AUC of 0.884. Furthermore, unannotated MS features achieved an AUC close to 1 in the validation set.
There are several limitations of the current study. First, to minimize interference of comorbidities on metabolomic differences, we included only a “pure” population of patients with SCH or CH in this cross-sectional study. However, some comorbidities of hypothyroidism are commonly seen in clinics, which may limit generalizability to the general hypothyroid population. Second, due to the strict exclusion criteria, the study had a relatively small sample size, our findings need to be further validated in larger scale studies. Third, it remains to be studied whether the 7 metabolites identified to differentiate SCH from CH or any metabolomics features can be used to predict the progression of SCH to CH in future longitudinal cohorts.
In summary, our untargeted metabolomic analysis has revealed novel metabolomic patterns in CH and SCH group compared with control group. And the strikingly similar metabolomic profiles in plasma of patients with SCH and CH suggest that similar clinical therapeutic strategies may be effective for the early treatment of thyroid dysfunction. A panel of differential metabolites were successfully identified as potential biomarkers to discriminate SCH, CH, and control subjects. In addition to providing new insights into the pathogenesis of hypothyroidism at the metabolic level, our diagnostic models have the potential to improve current clinical assessment and management of SCH and CH, once validated in future studies.
Acknowledgments
The authors thank the research volunteers for their participation in this study.
Abbreviations
- ALT
alanine aminotransferase
- ANOVA
analysis of variance
- AST
aspartate aminotransferase
- AUC
area under the curve
- BA
bile acid
- BMI
body mass index
- CH
clinical hypothyroidism
- DBIL
direct bilirubin
- DBP
diastolic blood pressure
- DHEA-S
dehydroepiandrosterone sulfate
- FBG
fasting blood glucose
- FC
fold-change
- FDR
false discovery rate
- FT3
free triiodothyronine
- FT4
free thyroxine
- HDL-C
high-density lipoprotein cholesterol
- IBIL
indirect bilirubin
- LASSO
Least Absolute Shrinkage and Selection Operator
- LDL-C
low-density lipoprotein cholesterol
- LPC
lysophosphatidylcholine
- LPE
lysophosphatidylethanolamine
- MS
mass spectrometry
- MSEA
metabolite set enrichment analysis
- MSI
Metabolomics Standards Initiative
- OPLS-DA
orthogonal partial least squares discriminant analysis
- PC
phosphatidylcholine
- PCA
principal component analysis
- PE
phosphatidylethanolamine
- QC
quality control
- RF
random forest
- ROC
receiver operating characteristic
- SBP
systolic blood pressure
- SCH
subclinical hypothyroidism
- SVR
support vector regression
- T3
triiodothyronine
- T4
thyroxine
- TBIL
total bilirubin
- TC
total cholesterol
- TG
triglyceride
- TgAb
thyroglobulin antibodies
- TH
thyroid hormone
- TPOAb
thyroid peroxidase antibodies
- TSH
thyrotropin (thyroid-stimulating hormone)
- UA
uric acid
- UHPLC
ultrahigh performance liquid chromatography
Contributor Information
Feifei Shao, The First School of Clinical Medicine, Lanzhou University, Lanzhou, Gansu 730099, China; Department of Endocrinology (Cadre Ward 3), Gansu Provincial Hospital, Lanzhou, Gansu 730099, China; Clinical Research Center for Metabolic Disease, Gansu Province. 204 Donggang West Road, Lanzhou, Gansu 730099, China.
Rui Li, CAS Key Laboratory of Nutrition, Metabolism, and Food Safety, Shanghai Institute of Nutrition and Health, Innovation Center for Intervention of Chronic Disease and Promotion of Health, Chinese Academy of Sciences (CAS), Shanghai, 200031, China; School of Life Science and Technology, ShanghaiTech University, 201210, Shanghai, China.
Qian Guo, Department of Endocrinology (Cadre Ward 3), Gansu Provincial Hospital, Lanzhou, Gansu 730099, China; Clinical Research Center for Metabolic Disease, Gansu Province. 204 Donggang West Road, Lanzhou, Gansu 730099, China.
Rui Qin, Clinical Research Center for Metabolic Disease, Gansu Province. 204 Donggang West Road, Lanzhou, Gansu 730099, China.
Wenxiu Su, Clinical Research Center for Metabolic Disease, Gansu Province. 204 Donggang West Road, Lanzhou, Gansu 730099, China.
Huiyong Yin, CAS Key Laboratory of Nutrition, Metabolism, and Food Safety, Shanghai Institute of Nutrition and Health, Innovation Center for Intervention of Chronic Disease and Promotion of Health, Chinese Academy of Sciences (CAS), Shanghai, 200031, China; School of Life Science and Technology, ShanghaiTech University, 201210, Shanghai, China.
Limin Tian, The First School of Clinical Medicine, Lanzhou University, Lanzhou, Gansu 730099, China; Gansu Provincial Hospital, Lanzhou, Gansu 730099, China; Clinical Research Center for Metabolic Disease, Gansu Province. 204 Donggang West Road, Lanzhou, Gansu 730099, China.
Funding Support
This study was supported by the National Natural Science Foundation of China (No. 82060152, 32030053, 32150710522), Clinical Research Center for Metabolic Diseases, Gansu Province (No. 18JR2FA006) and Research Fund project of Gansu Provincial Hospital (No. 21GSSYB-17).
Disclosures
The authors have nothing to disclose.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Udovcic M, Pena RH, Patham B, et al. . Hypothyroidism and the heart. Methodist Debakey Cardiovasc J. 2017;13(2):55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chaker L, Bianco AC, Jonklaas J, et al. . Hypothyroidism. Lancet. 2017;390(10101):1550–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Tienhoven-Wind LJ, Dullaart RP. Low-normal thyroid function and novel cardiometabolic biomarkers. Nutrients. 2015;7(2):1352–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chaker L, Baumgartner C, den Elzen WP, et al. . Thyroid function within the reference range and the risk of stroke: an individual participant data analysis. J Clin Endocrinol Metab. 2016;101(11):4270–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chaker L, van den Berg ME, Niemeijer MN, et al. . Thyroid function and sudden cardiac death: a prospective population-based cohort study. Circulation. 2016;134(10):713–722. [DOI] [PubMed] [Google Scholar]
- 6. Li GH, Cheung CL, Cheung EY, et al. . Genetically determined TSH level within reference range is inversely associated with Alzheimer’s Disease. J Clin Endocrinol Metab. 2021;106(12):e5064–e5074. [DOI] [PubMed] [Google Scholar]
- 7. Wishart DS. Metabolomics for investigating physiological and pathophysiological processes. Physiol Rev. 2019;99(4):1819–1875. [DOI] [PubMed] [Google Scholar]
- 8. Cui L, Lu H, Lee YH. Challenges and emergent solutions for LC-MS/MS based untargeted metabolomics in diseases. Mass Spectrom Rev. 2018;37(6):772–792. [DOI] [PubMed] [Google Scholar]
- 9. Rinschen MM, Ivanisevic J, Giera M, et al. . Identification of bioactive metabolites using activity metabolomics. Nat Rev Mol Cell Biol. 2019;20(6):353–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang A, Sun H, Wang X. Power of metabolomics in biomarker discovery and mining mechanisms of obesity. Obes Rev. 2013;14(4):344–349. [DOI] [PubMed] [Google Scholar]
- 11. Xuan Q, Ouyang Y, Wang Y, et al. . Multiplatform metabolomics reveals novel serum metabolite biomarkers in diabetic retinopathy subjects. Adv Sci (Weinh). 2020;7(22):2001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Masoodi M, Gastaldelli A, Hyotylainen T, et al. . Metabolomics and lipidomics in NAFLD: biomarkers and non-invasive diagnostic tests. Nat Rev Gastroenterol Hepatol. 2021;18(12):835–856. [DOI] [PubMed] [Google Scholar]
- 13. Shen X, Wang C, Liang N, et al. . Serum metabolomics identifies dysregulated pathways and potential metabolic biomarkers for hyperuricemia and gout. Arthritis Rheumatol. 2021;73(9):1738–1748. [DOI] [PubMed] [Google Scholar]
- 14. Denny JC, Crawford DC, Ritchie MD, et al. . Variants near FOXE1 are associated with hypothyroidism and other thyroid conditions: using electronic medical records for genome- and phenome-wide studies. Am J Hum Genet. 2011;89(4):529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eriksson N, Tung JY, Kiefer AK, et al. . Novel associations for hypothyroidism include known autoimmune risk loci. PLoS One. 2012;7(4):e34442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chaker L, Korevaar TI, Medici M, et al. . Thyroid function characteristics and determinants: the rotterdam study. Thyroid. 2016;26(9):1195–1204. [DOI] [PubMed] [Google Scholar]
- 17. Song Q, Chen X, Su Y, et al. . Age and gender specific thyroid hormones and their relationships with body mass index in a large Chinese population. Int J Endocrinol Metab. 2019;17(1):e66450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knezevic J, Starchl C, Tmava Berisha A, et al. . Thyroid-gut-axis: how does the microbiota influence thyroid function? Nutrients. 2020;12(6):1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Helmreich DL, Parfitt DB, Lu XY, et al. . Relation between the hypothalamic-pituitary-thyroid (HPT) axis and the hypothalamic-pituitary-adrenal (HPA) axis during repeated stress. Neuroendocrinology. 2005;81(3):183–192. [DOI] [PubMed] [Google Scholar]
- 20. Babic Leko M, Gunjaca I, Pleic N, et al. . Environmental factors affecting thyroid-stimulating hormone and thyroid hormone levels. Int J Mol Sci. 2021;22(12):6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mullur R, Liu Y-Y, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Y, Wang L, Zhou L, et al. . Thyroid stimulating hormone increases hepatic gluconeogenesis via CRTC2. Mol Cell Endocrinol. 2017;446:70–80. [DOI] [PubMed] [Google Scholar]
- 23. Sun X, Sun Y, Li WC, et al. . Association of thyroid-stimulating hormone and cardiovascular risk factors. Intern Med. 2015;54(20):2537–2544. [DOI] [PubMed] [Google Scholar]
- 24. Mouri A, Hoshino Y, Narusawa S, et al. . Thyrotoropin receptor knockout changes monoaminergic neuronal system and produces methylphenidate-sensitive emotional and cognitive dysfunction. Psychoneuroendocrinology. 2014;48:147–161. [DOI] [PubMed] [Google Scholar]
- 25. Smith CA, Want EJ, O’Maille G, et al. . XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem. 2006;78(3):779–787. [DOI] [PubMed] [Google Scholar]
- 26. Shen X, Gong X, Cai Y, et al. . Normalization and integration of large-scale metabolomics data using support vector regression. Metabolomics. 2016;12(5):89. [Google Scholar]
- 27. Wishart DS, Feunang YD, Marcu A, et al. . HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018;46(D1):D608–D617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Horai H, Arita M, Kanaya S, et al. . MassBank: a public repository for sharing mass spectral data for life sciences. J Mass Spectrom. 2010;45(7):703–714. [DOI] [PubMed] [Google Scholar]
- 29. Chen L, Lu W, Wang L, et al. . Metabolite discovery through global annotation of untargeted metabolomics data. Nat Methods. 2021;18(11):1377–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kanehisa M, Furumichi M, Tanabe M, et al. . KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45(D1):D353–D361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sumner LW, Amberg A, Barrett D, et al. . Proposed minimum reporting standards for chemical analysis. Metabolomics. 2007;3(3):211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shen X, Wang R, Xiong X, et al. . Metabolic reaction network-based recursive metabolite annotation for untargeted metabolomics. Nat Commun. 2019;10(1):1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xia J, Wishart DS. MSEA: a web-based tool to identify biologically meaningful patterns in quantitative metabolomic data. Nucleic Acids Res. 2010;38(Web Server):W71–W77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shi L, Westerhuis JA, Rosén J, et al. . Variable selection and validation in multivariate modelling. Bioinformatics. 2019;35(6):972–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shao F, Li R, Guo Q, et al. . Plasma Metabolomics Reveals Systemic Metabolic Alterations of Subclinical and Clinical Hypothyroidism. Dataset. Zenodo. Posted August 29,2022. 10.5281/zenodo.7022060 [DOI]
- 36. Yavuz S, Salgado Nunez Del Prado S, Celi FS. Thyroid hormone action and energy expenditure. J Endocr Soc. 2019;3(7):1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Constantinou C, Chrysanthopoulos PK, Margarity M, et al. . GC-MS metabolomic analysis reveals significant alterations in cerebellar metabolic physiology in a mouse model of adult onset hypothyroidism. J Proteome Res. 2011;10(2):869–879. [DOI] [PubMed] [Google Scholar]
- 38. Muller AP, Longoni A, Farina M, et al. . Propylthiouracil-induced hypothyroidism during lactation alters leucine and mannose metabolism in rat cerebellar slices. Exp Biol Med (Maywood). 2013;238(1):31–36. [DOI] [PubMed] [Google Scholar]
- 39. Wu S, Tan G, Dong X, et al. . Metabolic profiling provides a system understanding of hypothyroidism in rats and its application. PLoS One. 2013;8(2):e55599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Han X, Xiao H, Chen J, et al. . The substance basis of Poria ameliorates hypothyroidism other than hyperthyroidism based on proteomics and metabolomics. FASEB J. 2020;34(9):11970–11982. [DOI] [PubMed] [Google Scholar]
- 41. Muñoz-Prieto A, González-Arostegui LG, Rubic´ I, et al. . Untargeted metabolomic profiling of serum in dogs with hypothyroidism. Res Vet Sci. 2021;136:6–10. [DOI] [PubMed] [Google Scholar]
- 42. Song J, Shan Z, Mao J, et al. . Serum polyamine metabolic profile in autoimmune thyroid disease patients. Clin Endocrinol (Oxf). 2019;90(5):727–736. [DOI] [PubMed] [Google Scholar]
- 43. Liu J, Fu J, Jia Y, et al. . Serum metabolomic patterns in patients with autoimmune thyroid disease. Endocr Pract. 2020;26(1):82–96. [DOI] [PubMed] [Google Scholar]
- 44. Zhao C, Ge J, Jiao R, et al. . 1H-NMR Based metabolomic profiling of cord blood in gestational hypothyroidism. Ann Transl Med. 2020;8(6):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Piras C, Pibiri M, Leoni VP, et al. . Analysis of metabolomics profile in hypothyroid patients before and after thyroid hormone replacement. J Endocrinol Invest. 2021;44(6):1309–1319. [DOI] [PubMed] [Google Scholar]
- 46. Ramakrishanan N, Denna T, Devaraj S, et al. . Exploratory lipidomics in patients with nascent metabolic syndrome. J Diabetes Complications. 2018;32(8):791–794. [DOI] [PubMed] [Google Scholar]
- 47. Lu J, Lam SM, Wan Q, et al. . High-coverage targeted lipidomics reveals novel serum lipid predictors and lipid pathway dysregulation antecedent to type 2 diabetes onset in normoglycemic Chinese adults. Diabetes Care. 2019;42(11):2117–2126. [DOI] [PubMed] [Google Scholar]
- 48. Liu P, Zhu W, Chen C, et al. . The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 2020;247:117443. [DOI] [PubMed] [Google Scholar]
- 49. Zelinskaia NB. The significance of studying phospholipids in the diagnosis of hypothyroidism. Vrach Delo. 1989;5:79–81. [PubMed] [Google Scholar]
- 50. Desai MS, Mathur B, Eblimit Z, et al. . Bile acid excess induces cardiomyopathy and metabolic dysfunctions in the heart. Hepatology. 2017;65(1):189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Uchiyama K, Kishi H, Komatsu W, et al. . Lipid and bile acid dysmetabolism in Crohn’s Disease. J Immunol Res. 2018;2018:7270486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sinha SR, Haileselassie Y, Nguyen LP, et al. . Dysbiosis-induced secondary bile acid deficiency promotes intestinal inflammation. Cell Host Microbe. 2020;27(4):659–670.e655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mahmoudiandehkordi S, Arnold M, Nho K. , et al. Altered bile acid profile associates with cognitive impairment in Alzheimer's disease-An emerging role for gut microbiome. Alzheimers Dement. 2019;15(1):76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Laukkarinen J, Sand J, Saaristo R, et al. . Is bile flow reduced in patients with hypothyroidism? Surgery. 2003;133(3):288–293. [DOI] [PubMed] [Google Scholar]
- 55. Song Y, Zhao M, Zhang H, et al. . Thyroid-stimulating hormone levels are inversely associated with serum total bile acid levels: a cross-sectional study. Endocr Pract. 2016;22(4):420–426. [DOI] [PubMed] [Google Scholar]
- 56. Song Y, Xu C, Shao S, et al. . Thyroid-stimulating hormone regulates hepatic bile acid homeostasis via SREBP-2/HNF-4alpha/CYP7A1 axis. J Hepatol. 2015;62(5):1171–1179. [DOI] [PubMed] [Google Scholar]
- 57. Dawson PA, Parini P. Hepatic thyroid hormone receptor beta1 agonism: good for lipids, good for bile? J Lipid Res. 2018;59(9):1551–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ockenga J, Valentini L, Schuetz T, et al. . Plasma bile acids are associated with energy expenditure and thyroid function in humans. J Clin Endocrinol Metab. 2012;97(2):535–542. [DOI] [PubMed] [Google Scholar]
- 59. Sanchez LD, Pontini L, Marinozzi M, et al. . Cholesterol and oxysterol sulfates: pathophysiological roles and analytical challenges. Br J Pharmacol. 2021;178(16):3327–3341. [DOI] [PubMed] [Google Scholar]
- 60. van Doormaal JJ, Muskiet FA, Jansen G, et al. . Increase of plasma and red cell cholesterol sulfate levels in induced hypothyroidism in man. Clin Chim Acta. 1986;155(3):195–200. [DOI] [PubMed] [Google Scholar]
- 61. Bassi F, Pupi A, Giannotti P, et al. . Plasma dehydroepiandrosterone sulphate in hypothyroid premenopausal women. Clin Endocrinol (Oxf). 1980;13(1):111–113. [DOI] [PubMed] [Google Scholar]
- 62. Gonulalan G, Tanrıkulu Y. Relationship of dehydroepiandrosterone sulfate levels with atherosclerosis in patients with subclinical hypothyroidism. Wien Klin Wochenschr. 2022;134(1-2):45–50. [DOI] [PubMed] [Google Scholar]
- 63. Ravaglia G, Forti P, Maioli F, et al. . Dehydroepiandrosterone-sulfate serum levels and common age-related diseases: results from a cross-sectional Italian study of a general elderly population. Exp Gerontol. 2002;37(5):701–712. [DOI] [PubMed] [Google Scholar]
- 64. Tagawa N, Tamanaka J, Fujinami A, et al. . Serum dehydroepiandrosterone, dehydroepiandrosterone sulfate, and pregnenolone sulfate concentrations in patients with hyperthyroidism and hypothyroidism. Clin Chem. 2000;46(4):523–528. [PubMed] [Google Scholar]
- 65. Solerte SB, Precerutti S, Gazzaruso C, et al. . Defect of a subpopulation of natural killer immune cells in Graves’ disease and Hashimoto’s Thyroiditis: normalizing effect of dehydroepiandrosterone sulfate. Eur J Endocrinol. 2005;152(5):703–712. [DOI] [PubMed] [Google Scholar]
- 66. Chng CL, Lim AY, Tan HC, et al. . Physiological and metabolic changes during the transition from hyperthyroidism to euthyroidism in Graves’ disease. Thyroid. 2016;26(10):1422–1430. [DOI] [PubMed] [Google Scholar]
- 67. Kaluzna-Czaplinska J, Gatarek P, Chirumbolo S, et al. . How important is tryptophan in human health? Crit Rev Food Sci Nutr. 2019;59(1):72–88. [DOI] [PubMed] [Google Scholar]
- 68. Hallen A, Jamie JF, Cooper AJ. Lysine metabolism in mammalian brain: an update on the importance of recent discoveries. Amino Acids. 2013;45(6):1249–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Giordano N, Santacroce C, Mattii G, et al. . Hyperuricemia and gout in thyroid endocrine disorders. Clin Exp Rheumatol. 2001;19(6):661–665. [PubMed] [Google Scholar]
- 70. Raber W, Vukovich T, Vierhapper H. Serum uric acid concentration and thyroid-stimulating-hormone (TSH): results of screening for hyperuricaemia in 2359 consecutive patients with various degrees of thyroid dysfunction. Wien Klin Wochenschr. 1999;111(8):326–328. [PubMed] [Google Scholar]
- 71. Xing Y, Yang L, Liu J, et al. . The association with subclinical thyroid dysfunction and uric acid. Int J Endocrinol. 2021;2021:9720618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lappas M, Mundra PA, Wong G, et al. . The prediction of type 2 diabetes in women with previous gestational diabetes mellitus using lipidomics. Diabetologia. 2015;58(7):1436–1442. [DOI] [PubMed] [Google Scholar]
- 73. Yang QJ, Zhao JR, Hao J, et al. . Serum and urine metabolomics study reveals a distinct diagnostic model for cancer cachexia. J Cachexia Sarcopenia Muscle. 2018;9(1):71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.