Abstract
Breast cancer is one of the most frequent types of malignancies among women and is internationally recognized as the main reason for cancer-caused mortality. Most breast tumors are heterogeneous and genetically complicated due to the involvement of several genes. Therefore, it is clinically important to study genetic variants that increase the risk of breast cancer. It is identified that the presence of polymorphisms in genes encoding regulatory hormones is linked to a higher risk of breast cancer. Additionally, circulating estrogen levels are connected to aromatase (CYP19A1) genes, which is a recognized risk factor for breast cancer progression. In this paper, the authors present a review study on the effect of estrogen and its Single Nucleotide Polymorphisms (SNPs) in the occurrence of breast cancer. This review mainly aimed to find out the connection between CYP19A1 gene variations and the risk of breast cancer, as well as its clinical characteristics and prognosis. Due to the highly special activity of the CYP19A1 enzyme in steroid production, suppression of the targeted CYP19A1 is a focused medication for breast cancer patients, which has only minor adverse effects. Numerous clinical trials over the last decade have shown that Aromatase inhibitors (AIs) not only outperform tamoxifen in terms of effectiveness but also have a lower adverse effect profile. The AI is now widely accepted as a routine therapy option for postmenopausal females with Estrogen receptor-positive (ER+) breast cancer. Furthermore, not only dysregulation of gene expression in different genes related to distinguished pathways, such as estrogen metabolism, is essential in the progression of breast cancer but also particular SNPs can play an essential role in particular genes, such as CYP19A1. Different studies have demonstrated that these SNPs can be located in different sites of these genes, which are collected in this review. In a nutshell, more specific clinical trials are required to demonstrate the precise meditative role of anti-estrogen drugs in the treatment of ER+ breast cancer patients. Furthermore, more genotype analyses are needed to confirm the role of SNPs in the progression of breast cancer.
Keywords: Aromatase genes, Breast cancer, Estrogen, SNPs
1. Context
Breast cancer, the most frequent kind of cancer in females, is responsible for 33% of all diagnosed malignancies. Since this malignancy is the second leading reason for mortality, it is crucial to diagnose it earlier with effective disease management ( 1 ). Breast malignancy is a consequence of various risk factors, including aging, genetic history, early radiotherapy presentations, a history of pregnancy and breastfeeding, as well as hormonal treatment ( 2 ). Just under 1% of breast cancers appear before the age of 25, but beyond 30, the incidence increases dramatically and continues to rise with age ( 3 ). While the average age of breast cancer is between the 60s and 70s, it appears to be high in the global population ( 4 ). The majority of breast cancer incidents are sporadic; however, a considerable percentage is caused by inheriting certain genetic variables, such as gene mutations ( 5 ).
Scientific studies suggested that there is a genetic predisposition in 20% to 30% of breast cancer patients. An additional contributor to breast cancer is long-term estrogen exposure. This hormone is known to contribute to the formation of mammary glands and is also involved in causing and advancing breast malignancies ( 6 ). As a result, one method of determining potential breast cancer genes is to investigate genes involved in the biosynthesis and metabolism of this hormone. Several genes, such as CHEK2, BRIPI, MDM, TGFB, PTEN, TP53, BRCA2, and BRCA1, as well as gene variations in different distinct risk genes, are associated with an increased risk of breast tumor. The estrogen level in blood circulation over life has been proven to be one of the major risk elements for breast malignancy. This explains the reduced frequency of breast malignancy in men due to low amounts of estrogen and breast tissue ( 7 ). In addition, estrogen and progesterone cell surface receptors have a deteriorative function in breast cancer carcinogenesis ( 7 ).
Increased estrogen and progesterone exposure is arguably the most significant risk factor for the occurrence of breast malignancy, according to a study dating back to the early 1970s ( 8 ). On the other hand, obesity has also been recognized as an additional factor considering adipose tissue a rich origin of endogenous hormones, such as estrogens. Moreover, estrogen release in breast tissue has been proven to have a significant function in boosting hormone amounts in this tissue, which in turn stimulates breast cell growth, development, and tumorigenesis ( 9 ). Cytochrome P450 (CYP) family of genes has been demonstrated to function in the production, activity, metabolism, and release of hormones, such as steroids. Among genes in this family, CYP1A1, CYP19, and CYP17 are the most important in the production, metabolism, and maintenance of androgen and estrogen levels. One of the most common kinds of genetic alterations in the human genome is single nucleotide polymorphisms (SNPs). The genetic predisposition to cancer has been connected with genes that affect DNA mismatching, cell cycle control, metabolism, and immunology response ( 10 , 11 ). The identification of the basic procedure of SNPs affecting cancer susceptibility is crucial in determining the molecular pathophysiology of different malignancies. The SNPs can be found in many types of cancers and have therapeutic indications ( 12 ). There are various polymorphisms in breast cancer genes that contribute to the occurrence of breast cancer ( 13 ). There are regulatory regions of the CYP19 gene located in the human genome on chromosome 15q21.2, which has a coding region of 30 kb. This gene encodes aromatase (CYP19A1), an enzyme that catalyzes the transformation of androgens to estrogens. The major source of estrogen in premenopausal women is the ovaries. Meanwhile, in postmenopausal women, estrogen is constructed in other tissues and organs, including the adipose tissue, skin cells, muscles, and the liver, as shown in figure 1 ( 14 ). Genetic variants in CYP19 may be implicated in biological processes, such as Messenger RNA (mRNA) stability, transcription augmentation, or post-translational expression control.
Figure 1.
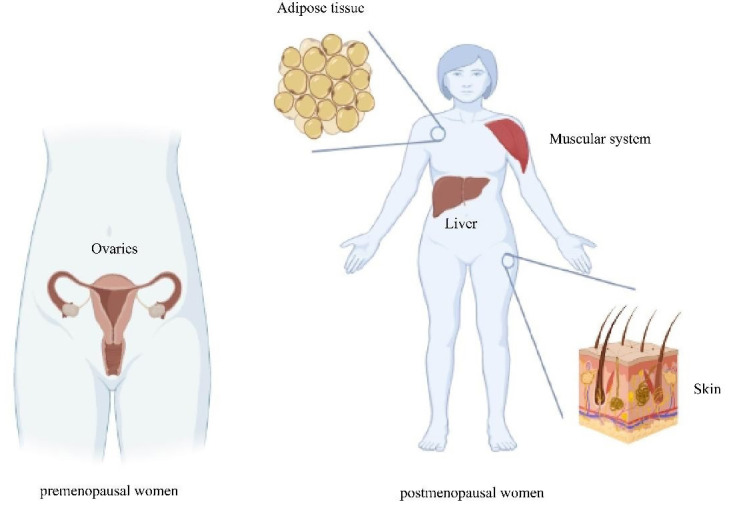
Different sources of estrogen in premenopausal and postmenopausal women
2. Evidence Acquisition
The main aim of this review article was to find out the connection between CYP19A1 gene variations and the risk of breast cancer, as well as its clinical characteristics and prognosis. In this regard, a detailed search was carried out on Google Scholar and PubMed databases to find out relevant research studies. The search process was performed using the following keywords: estrogen metabolism, breast cancer development, CYP, CYP19A1, polymorphisms, and estrogen receptor (ER) blocking drugs.
3. Results
3.1. Estrogen Metabolism
The C27 cholesterol is the source of all steroid hormones, and LDL cholesterol is the primary source of cholesterol used in steroid hormone production (steroidogenesis). Cholesterol is converted to the steroid hormones 21, 19, and 18 carbons. The initial stage in ovarian steroidogenesis is the transport of cholesterol into the mitochondria. The steroidogenic acute regulatory protein gene is responsible for the regulation of this phase. The transformation of cholesterol into pregnenolone is the next step, which is carried out by a collection of mitochondrial lateral chain-cutting enzymes. The 17-hydroxylase /17, 20 lyase enzyme, which is a product of the CYP17A1 gene, metabolizes pregnenolone, which is a steroid hormone precursor, and converts it to progesterone or androstenedione. The conversion of androstenedione to other androgens or estrogens is thus easier. Estrogens are among the few aromatic compounds identified in humans. It is a category of C18 steroids, each of which has a benzene ring, a C3 phenolic hydroxyl group, and a C17 ketone group (17-estradiol). Estradiol (estriol), estrone, and 16 hydroxy estradiol are the most common estrogens found in the human body. Estradiol is one of the main physiologically active estrogens produced predominantly by the ovarian granulosa cells, and estriol is by far the most prevalent estrogen in women’s urine. Follicle-stimulating hormone is responsible for its regulation in the human body. The enzyme 17-hydroxysteroid dehydrogenase converts estrone to estradiol in a reversible manner. However, it functions as a progenitor for estrone and testosterone in the ovaries and peripheral tissue, which is then transformed to estradiol by CYP19A1 ( 15 ).
Ovarian estradiol is the most important estrogen in premenopausal women while the estrone generated in peripheral tissues is dominant in post-menopausal women. The conversion of androgen to estrogen is implemented by a rate-limiting enzyme termed aromatase (CYP19) which is encoded by the CYP19A1 gene ( 16 ). The blockage of CYP19A1 function is a significant therapeutic technique in the management of estrogen-related disorders, such as breast malignancy, endometriosis, and endometrial cancer. Three competitive processes metabolize estradiol and estrone, including irreversible hydroxylation mediated by NADPH-dependent CYP enzymes, such as CYP1A1, CYP1B1, and CYP1A2. Catechol estrogens (2-hydroxyestrone, 4-hydroxyestrone, 2-hydroxyestradiol, and 4-hydroxyestradiol), as well as 16-hydroxyestrone, are formed when estradiol and estrone are hydroxylated at C2, C4, and C16 positions. Estriol is created when estradiol or 16-hydroxyestrone is hydroxylated. Catechol estrogens are additionally transformed (methylated) into methoxy estrogens (2-methoxy estrone, 4-methoxysetron, 2-methoxy estradiol, and 4-methoxycetradiol) by the catechol-O-methyltransferase (COMT) enzyme ( 17 , 18 ).
3.2. Estrogen and Breast Cancer Development
Several epidemiological and clinical studies published in the late 1990s revealed that estrogen has a significant role in the development and progression of many malignancies, including breast, endometrial, prostate, and colorectal cancer. Elevated estrogen levels, which accelerate mitosis, have been linked to the initiation and development of cancer. Exogenous hormones were connected to the growth of these tumors in the beginning, and it was subsequently shown that certain estrogen-sensitive tissues that operate as specific organs release estrogen. Raised hormone levels thus facilitate the proliferation and development of cancer cells ( 33 ). Estrone, estradiol, estriol, and sterol are four primary natural estrogens generated solely during the physiological processes of women and men. Estradiol is a key estrogen that has the highest affinity for ERs and is needed for a variety of physiological processes in both men and women in all stages of life ( 19 ). Aromatase is regarded as one of the major factors in the progression of cancer because of its critical function in the production of different forms of estrogen from androgens, including estradiol from testosterone, estrone from androstenedione, and estriol from 16-dehydroepiandrosterone hydroxylated. While ER is undoubtedly involved in the progression of breast tumors, the mediating actions of the estradiol receptor can also contribute to the development of breast cancers ( 20 ). Estradiol is one of the variables that impact breast cancer. According to epidemiological and experimental research, estrogen therapy promotes and protects breast cancer in a variety of animal models ( 21 ). Breast cancer incidence is reduced by 75% in women who have bilateral ovarian resection before the age of 35.
Premature menstruation, late menopause, extended menopausal estrogen therapy, excessive weight, and high circulating estradiol amounts in premenopausal women are all linked to an increased risk of breast cancer later in life ( 22 ). Results from two valuable studies revealed that postmenopausal women with the top levels of estradiol had 2.5 times higher risks of breast cancer than those with the lowest levels of estradiol over 10 years. Tamoxifen or Raloxifene, which inhibits estrogen action, decreases the risk of breast cancer in high-risk women by 50%-75% ( 23 ). Ultimately, during the adjuvant therapy, blocking estradiol production with aromatase inhibitors (AIs) or decreasing its activity with antiestrogens reduces breast cancer development. Estradiol operates through ER encouraging cell proliferation and causing mutations that lead to mistakes during DNA replication. It then stimulates mutant cell proliferation and accumulation, which eventually lead to cancer.
Apart from the direct role of estrogen in cellular proliferation, human cancers have been associated with increased cell proliferation caused by hormones. Steroid hormones are broken down into quinone metabolites, which directly bind with DNA and create additives, such as genotoxic metabolites of estradiol, which cause DNA mutations ( 24 ). Furthermore, catechol estrogen metabolites contribute to the oxidative oxidation cycle by generating oxygen-free radicals which attack DNA-bound guanine and produce 8-oxoguanine. The findings of Park, Yim ( 25 ) and Ritchie, Hahn ( 26 ) anticipated the "estrogen genotoxic metabolite" theory, which states that women with a combination of mutations in estrogen metabolizing enzymes are more likely to develop breast cancer. A new study reveals that women with breast cancer are considerably more likely to have estrogen-depleting DNA molecules in their urine, in comparison with women at a natural risk of breast cancer, which is in line with the depopulation mechanism ( 27 ).
3.3. Role of Genetic Variants in Estrogen Metabolism
Variations in genes encoding enzymes that are involved in estrogen metabolic pathways and genes encoding ERs are associated with a high risk of breast cancer. Over the last few decades, polymorphisms in genes encoding COMT, CYP1A1, CYP1B1, ER alpha, ER beta, CYP17A1, and CYP19A1 have been extensively studied ( 28 ). The COMT is a phase II enzyme that binds with non-toxic methoxy estrogens to make catechol estrogens inactive. It also blocks the biological transfer of catechol estrogens to other quinone DNA compounds, as well as the generation of reactive oxygen species, which can harm cellular macromolecules, including DNA, lipids, and proteins ( 27 , 29 ).
The CYP, together with peroxidase enzymes, catalyzes the oxidation of catechol estrogens to estrogen quinones and the carcinogenic metabolites of estrogens. In addition, they perform the hydroxylation of major estrogens to catechol estrogens ( 30 ). According to a previous study, polymorphisms in CYP1A1 and CYP1B1 genes are connected to a great risk of breast cancer. In a case-control study, it was observed that African Americans with a polymorphic variation of homozygous Msp1 in CYP1A1 had an odds ratio (OR) of 8.4, compared to the control group ( 31 ). In another case-control study in northern India, Chattopadhyay, Siddiqui ( 32 ) found a substantial link between SNPs in ER (rs2234693), ER (rs2987983), CYP17A1 (rs743572), as well as CYP19A1 (rs700519), and the risk of breast cancer. On the other hand, the results of studies examining distinct SNPs in CYP19A1 and CYP17A1 genes in connection with breast cancer have been inclusive. Talbott, Gammon ( 33 ) discovered that polymorphism alterations in the CYP19 gene with at least one G allele in rs1008805 (A/G) SNP, but not in rs730154 (C/T) SNP, may increase the risk of premenopausal breast cancer.
3.4. Cytochrome P450
The CYP enzymes catalyze the first stage in estrogen metabolism known as hydroxylation. This enzyme is a monooxygenase superfamily that is involved in the oxidative metabolism of a wide range of medicines and compounds, as well as endogenous compounds, such as hormones and steroids. In humans, 57 genes compose the CYP superfamily. They are grouped into 18 families all participating in the production and metabolism of endogenous hormones. There are three families (CYP1, CYP2, and CYP3) that commence oxidative metabolism reactions. Some isoforms of these CYP families are also involved in estrogen metabolism ( 19 ). The CYP enzymes are mainly located in hepatic cells but they are also present in all cells of the body. Many variables in the liver and target tissues influence the expression level of estrogen metabolizing CYP enzymes. As a result, it will not only change the level of estrogen in the cells but also alters physiological consequences on target tissues based on the site or the cell type. Based on the site needed for estrogen, the CYP11A enzyme catalyzes the conversion of cholesterol to pregnenolone. Pregnenolone’s androgen synthesis is catalyzed by CYP17. Androgen and estrogen synthesis is catalyzed by CYP19. Estrone is converted to potent estradiol by 17-beta-hydroxysteroid dehydrogenase. During in vivo and in vitro experiments, CYP isoforms have produced a high variety of hydroxylated estrogen metabolites. Estrogen metabolic pathways have been discovered in both the liver and extrahepatic tissues. Since the liver contains the majority of CYPs, estrogen metabolism takes place predominantly there. Around 80% of estradiol is metabolized to 2-hydroxy estradiol in the liver, whereas 20% is converted to 4-hydroxy estradiol (61). CYP1A1, CYP1A2, and CYP3A4 are frequently found to catalyze the 2-hydroxylation of estradiol rather than the 4-hydroxylation. CYP1B1, on the other hand, has an enzymatic activity for the 4-hydroxylation of estradiol ( 34 ). The activity of CYP3A5 for the 4-hydroxylation of estrogens is also unique (especially estrone). Since CYP1A1 is not present in the liver, CYP1A2 and CYP3A are the major enzymes that catalyze 2-hydroxylation in the liver. The 2-hydroxyestradiol produced by CYP1A1 and CYP3A4 has been found in different tissues, such as the breast, uterus, placenta, brain, and pituitary gland (64–66). As CYP1B1 is highly expressed in the human breast and uterus, 4-hydroxylation is the most common estradiol metabolization process which leads to enhanced cell proliferation ( 35 ). Estrogens, including 6a-, 6b-, 7a-, 12b-, 15a-, 15b-, 16a-, and 16-beta-hydroxy estrogens, are secondary metabolites that exist in addition to the primary quantitative estrogen metabolic processes (2- and 4-hydroxylation). CYP1A1, CYP1A2, CYP1B1, CYP2A6, CYP2C8, CYP3A4, CYP3A5, and CYP3A7 are among the CYP isoforms that contribute to their formation ( 35 ).
3.5. Role of Cytochrome P450 in Cancer
Cytochrome P450 are enzymes that have a substantial role in cancer prevention, genesis, progression, and metastasis. According to an increasing number of findings from epidemiological, diagnostic, and clinical studies, CYPs (1A1, 1A2, 1B1, 2A6, 2A13) (2E1 and 3A4) are connected with the activation of a variety of tumorigenic chemicals in the environment. This includes tobacco-related nitrosamines and polycyclic aromatic hydrocarbons (PAHs) ( 36 ).
The ability of activated compounds to produce reactive derivatives, which lead to the production of covalent DNA adducts, may be investigated through CYP reactions. Cytochrome P450 can effectively activate BAP to DNA binding species, even in the presence of very low amounts of POR in mice. Cytochrome P450s are the most important enzymes involved in the metabolism of PAHs among a vast set of enzymes participating in carcinogen metabolism ( 36 ). In addition, anticancer medication metabolism is predominantly regulated by CYP2A, CYP2B, CYP2C, CYP2D, and CYP3A subfamilies. Drug resistance is among the most serious issues in the treatment of brain malignancies. Increased CYP concentrations may result in drug inactivation inside the cell. Cytochrome P450 up-regulation in tumors may be implicated in the activation or inactivation of chemotherapeutic medicines, thus, the local expression of CYPs in tumors may be required to regulate the carcinogenic process ( 36 ).
As a part of a pleiotropic reaction to cell proliferation, CYPs may be present in tumor cells. They can also slow down the proliferation and metastasis of cancer cells to a certain degree. As a case in point, tumor-specific expression is linked to CYP2W1, CYP1B1, CYP2C9, CYP2C8, CYP2J2, and CYP4A. The share of the CYP family in cancer growth and tumor formation has been identified as a result of these discoveries. Invasion, proliferation, metastasis, and angiogenesis are all influenced by signaling pathways, such as mitogen-activated protein kinase and phosphatidylinositol 3-kinase and protein kinase B (PI3K/Akt) ( 36 ). Recent research has found that 20-HETE- produced from CYP-hydroxylase plays a key role in the activation of PI3K/Akt and extracellular signal‑regulated protein kinase (ERK1/2) in endothelial cells, altering cellular activities like apoptosis and proliferation. The creation of active AIs for breast cancer treatment was the first effective method in cancer therapy to target CYP. Aromatase inhibitors have ushered in a new era in hormone therapy for estrogen-dependent malignancies, paving the door for androgen-dependent prostate cancer to be treated similarly. This discovery resulted in targeting medications for CYPs engaged in the inactivation of vitamin D3 and the anti-cancer metabolites of vitamin A. The discovery of elevated levels of CYPs, such as CYP1B1, in tumor cells has sparked interest in producing pharmacological inhibitors and prodrugs.
3.6. Aromatase (CYP19A1)
Aromatase (EC 1.14.14.1), also called estrogen synthase, is the only member of family 19 of the CYP superfamily and the product of the CYP19A1 gene. This enzyme catalyzes a rate-limiting step in the conversion of three sequential hydroxylated androgens to estrogen aromatization and carbon removal at 19 androgen positions ( 37 ).
Several investigations have demonstrated that many cancers rely on estrogens to survive and spread ( 37 , 38 ). If any transformation of the path is blocked, it may lower the production of estrogen. More specifically, suppression is due to inhibition in the last step limited to the manufacture of estrogen and the inhibition of CYP19A1. Because of the importance of CYP19A1 in estrogen production, there is a surge of attention on possible enzyme inhibitors and their usage as a therapy for endocrine-responsive cancers ( 39 ).
Formerly, it was thought that the ovaries and placenta were the only estrogen-producing organs engaged in female reproductive activities. Nevertheless, further research employing a variety of modern assays and technology has shown that estrogens may be found in the tissues of the male gonads, namely the testis and epididymis, as well as extrauterine tissues, such as the liver and colon. Furthermore, estrogen may be found in the prostate tissue, neural system, adrenal glands, dermal tissues, skeletal system, hair follicles, adipose, and blood vessels. This is owing to the existence of CYP19A1, which in itself is functional in a variety of tissues in both men and women. Therefore, estrogen is generated in both the gonads and tissues outside the gonads ( 15 , 37 - 39 ). CYP19A1s are dimers and two-polypeptide collection; one is a CYP that is a gene CYP19A1 product. Cytochrome reductase is another flavoprotein subunit that is ubiquitous in most cells. Its widespread presence in the human body explains its critical role in a variety of biochemical functions ( 27 ).
3.7. Structure of Aromatase Gene
CYP19A1 is a 123 kb gene that may be located on chromosome 15 in the q21.1 area ( 40 ). The coding region is roughly 34 kb in all tissues and comprises 10 exons and 9 introns whereas the regulatory area is thrice (around 95 kb). This regulatory segment is found in the gene region 5 as a distinct dispersed sequence above the coding sequences. The modulatory region is exceptionally extensive, with at least 10 tissue-specific promoters. In various cell types, these promoters are employed alternatively. The structure of each promoter is controlled by a unique set of DNA modulatory regions and the transcription factors (TFs) that attach to these sites. These TFs attach to the promoter and activate it, causing an untranslated first exon to be truncated at this common junction in the coding region, directly upstream of the translation start point. In diverse tissues, the promoter region comprises the primary transcription elements, notably the TATA box, the CAAT box, and the GC box, as well as additional regulatory elements ( 41 ). The first exon (exon 1), which encodes the CYP19A gene's 5' untranslated region (UTR), is found in a variety of forms and is isolated in a tissue-specific way. Since this gene has several promoters, occasional tissue-specific exon 1 splicing occurs in each tissue. Specific tissue promoters are employed in the gonad, neural system, vascular, skeletal system, adipose tissues, placenta, skin, or fetal liver for the physiologic production of estrogen ( 41 ). Up to the present, 9 distinct exon-1 subtypes, expressed respectively under a particular circumstance, have been identified. Exons 2-10 are gene encoding sequences that are around 34 kb in length. Exon 1 interacts with exon 2 after the intervening regions are spliced. The same exon 2-10 sequence codes for all transcripts.
Breast cancer, endometrial carcinoma, and malignancies of the colon, stomach, liver, lung, ovary, pancreas, as well as prostate, have all been shown to display CYP19A1 gene expression in estrogen-dependent tissues ( 33 ). The existence or lack of CYP19A1 function in different tissues, and the quantity of estrogen present, depend on the specific expression of CYP19A1 in the cell. Since the 1980s, scientists have been studying the control of CYP19A1 transcription and a variety of ideas have been proposed to explain the underlying control of CYP19A1 gene expression in order to better understand the role of CYP19A1 in the genesis and the development of cancer ( 42 ). In addition to these mechanisms, a number of CYP19A1 gene variants are discovered that affect the CYP19A1 activity, which could be utilized as cancer risk predictors ( 40 ).
3.8. Single Nucleotide Polymorphisms
Single Nucleotide Polymorphisms are among the most prevalent forms of genetic changes in humans. The SNPs in genes that govern DNA mismatch control, cell cycle regulation, metabolism, and immunology have been associated with hereditary vulnerability to cancer ( 10 , 11 ). Knowledge of the molecular etiology of diverse cancers requires an understanding of the complex processes of SNP effects that contribute to cancer susceptibility. The SNPs are possible diagnostic and therapeutic biomarkers in a variety of cancers from a clinical standpoint ( 12 ). They may be found in a variety of gene locations, including promoters, exons, introns, as well as the 5' and 3' UTR regions. As a result, depending on where the SNPs are found, alterations in gene expression and their impact on the risk of cancer differ. The SNPs in the promoter impact gene expression by affecting the promoter activity, transcription factor binding, DNA methylation, and histone modifications. Cancer risk is influenced by exon SNPs, which decrease transcription and gene translation. The SNPs in intracellular areas cause shortened transcripts and increase or impair long non- coding RNA (lncRNAs) binding and activity ( 43 ). Those in the 5' UTR region impact translation, while SNPs in the 3' UTR alter microRNA interaction. Through the impacts of long-distance effects, SNPs in locations remote from genes inhibit or enhance gene transcription ( 43 ).
3.9. Role of Polymorphisms in Cancer
3.9.1. SNPs in the Promoter Region and Cancer Susceptibility
Through cis-elements and trans-elements, the promoter region controls the beginning and pace of gene expression. Transcript factor binding is affected by promoter-related polymorphisms that affect promoter activity, gene transcription, mRNA stability, and translation. As a result, these actions change protein levels, which may influence a person’s susceptibility to illnesses, such as cancer. Furthermore, variations in promoter regions influence cancer risk through modifying epigenetic processes, such as DNA methylation and histone changes. TATA box efficiency is impacted by promoter SNPs, and since it is a part of the primary promoter, TATA box polymorphisms decrease promoter activity and restrict genetic transcription. A point mutation (A>C) at position -27 in the EDH17B2 gene’s TATA box, as a case in point, lowers the promoter activation ( 43 ). The influence of SNPs in the promoter region on the interaction of the transcription factor is described as follows. The promoter area includes a wide range of gene transcription sites. Genetic variants in the gene promoter decrease the expression of target genes by blocking transcription factor interaction with the promoter. In the CDH1 promoter, rs16260 (-160C->A) and rs5030625 (-347G->GA) lower transcriptional activity to variable degrees SNS ( 44 ). The rs16260 (-160C>A) inhibits transcription factor interaction with the promoter of the CDH1 gene that results in carcinogenesis, including prostatic cancer, breast malignancy, colon, and pancreatic tumors ( 44 ). CDH1 rs5030625 (-347G->GA) inhibits transcription factor binding and decreases CDH1 expression by tenfold (P=0.001), which is linked to an increased familial vulnerability to the gastric cancer. In contrast, APE1 rs1760944 (-656T>G) is attributed to the enhanced transcription factor binding and an increased risk of cervical cancer (100). The SNPs in promoters impact DNA methylation that occurs largely in the promoter region’s CpG islands. Therefore, SNPs in the promoter region can affect DNA methylation state and significantly impact gene expression. Methylation SNPs are related to DNA methylation at CpG sites ( 45 ). The SNPs in genes with a greater percentage of G and C nucleotides are often mutated in numerous human illnesses. The CpG dinucleotides in the promoter area are modified by SNPs in a CpG island, leading to changes in methylation, histone acetylation, chromatin change, and gene silence. Extensive genome linkage analyses (genome-wide association study) revealed that 38 SNPs in 12 CpG sites were linked to methylation and expression variations in 10 genes termed (IRF6, TSPYL5, CRIM1, CHL1, DDT, PIGC, TMOD1, ZNF266, BDKRB2, and GSTT1) (103). Since it removes methylation sites, CHEK2 rs2236141 (-48G>A) was linked to a lower risk of lung cancer (OR=0.73). Zhang, Gu ( 13 ) also found that it was connected with a lower risk of lung cancer (OR=0.73) (104). As it was methylated and resulted in the decreased EZH2 expression, the EZH2 allele rs6950683C decreases the risk of OSCC relative to the wild-type allele.
3.9.2. Effect of SNPs in the Exon Region on Cancer Occurrence
Non-synonymous and synonymous SNPs (cSNPs) in exons are categorized based on their capacity to substitute encoding amino acids. Exon SNPs increase cancer risk through genetic pathways in most cases ( 12 ). Non-synonymous cSNPs cause amino acid substitutions, which can have an impact on protein function. In most situations, changes in the first two bases of the codon modify the amino acid. Changes in amino acid sequences can impact protein interactions and activities by changing the secondary structure of a protein by increasing or reducing hydrogen bonding and phosphorylation. Due to alterations in the structure and activity of the encoded proteins, non-synonymous SNPs increase cancer occurrence rates. For instance, in the epidermal growth factor (EGFR) gene, a non-synonymous cSNP disables the tyrosine kinase (TKD) domain, which is targeted by the tinny molecule tyrosine kinase (TKI) inhibitors, such as gefitinib and erlotinib. Gefitinib makes two hydrogen bonds with Gly772 in EGFR whereas erlotinib makes a single hydrogen link with Met769. With five polymorphic EGFR proteins, gefitinib has a higher affinity for wild-type EGFR. Polymorphisms in the EGFR-TKD gene cause structural alterations in protein, increasing its activity and susceptibility to TKIs ( 46 ). The synonymous amino acid sequence of the encoded protein is not changed by cSNP. In most circumstances, a nucleotide alteration is followed by a codon base substitution. These proteins were formerly overlooked because their amino acid sequence is similar to that of the wild type. Recent research has found that synonymous cSNPs impact gene function and expression by affecting an adjacent gene’s expression. Protein structure, function, and expression have all been proven to be altered by synonymous mutations in several studies. The expression of a key drug transport protein, p-glycoprotein, is altered by a synonymous SNP in the multidrug resistance gene (MDR1). This has an impact on its expression and activity, as well as drug resistance. In China, Malaysia, and India, SNPs in MDR1, 1236C →T, 3435C →T, and 2677G →T are found in 31% to 49% of the population ( 47 ).
3.10. Effect of Intron SNPs and Sensitivity to Cancer
In particular tissues, introns have a function in gene expression modulation, mRNA transcription, and translation. These gene introns can consist of gene enhancers or other components of cis-elements that cause transcript or participate in elongation procedures. The binding of introns enhances the stability of mRNA in the nucleus. Introns potentially play a role in intermittent splicing and genomic positioning. Operational SNPs in introns are sometimes linked to SNPs in adjacent genes and influence mRNA and lncRNA interactions. This alters the structure and action of mature proteins. Consequently, intron SNPs influence cancer risk through both genetic and epigenetic processes. A genome-wide association study revealed rs2981578 in the fibroblast growth factor 2 receptor (FGFR2) as one of the hazardous alleles in breast cancer initiation. The rs2981578 heterozygotes have significant amounts of the transcription factor Forkhead Box A1 linked to this intron SNP ( 48 ). Three kinds of polymorphisms in the FGFR2 gene (rs2981578, rs35054928, and rs45631563) have been attributed to transcriptional inhibitory elements that diminish FGFR2 expression, enhance estrogen responsiveness, and raise the risk rate of breast cancer occurrence ( 49 ).
3.11. CYP19A1 Gene Polymorphisms
There seem to be multiple alterations in the coding, non-coding, and regulatory sequences of the CYP19A gene. These variations are the consequence of several mutations, including short tandem repetitive (STR) polymorphism and SNP. These modifications may have an impact on CYP19A1 gene expression, enzyme function, vulnerability to cancer progression, and clinical-pathological aspects of cancer. On the other hand, some may serve as cancer resistance and prognosis factors. The number of alleles varies greatly amongst groups, and it has also been demonstrated that discrepancies in plasma amounts of numerous sex steroids, particularly in postmenopausal females, may be attributable to the existence of various alleles. It is thought that genetic variations might explain disparities in cancer risk across various ethnic groups. As a result, the existence of distinct CYP19A1 alleles may result in certain ethnic disparities in the incidence of certain forms of cancer ( 47 ).
3.12. Short Tandem Repetitive Polymorphisms
Short repetition of burst in the gene of the CYP19A1 was detected in the late 1990s. A large incidence of cancer among people carrying various CYP19A1 alleles was found in several studies. The TTTA sequence, a distinct replication from 2 to 13 replications, is one of the most prevalent STRs found in CYP19A2 ( 47 ).
3.13. Insert/Remove TCT in Intron 4
The CYP19A gene’s intron 4 tolerates a TCT insertion/deletion polymorphism (ins/del), with around 50bp upstream (TTTA) n repeat. Numerous investigations have shown the link between breast cancer and TCT deletion of trinucleotides ( 50 , 51 ).
3.14. Single Nucleotide Polymorphisms?
At the beginning of the 1990s, the occurrence of mononucleotide modifications was detected in the CYP19A1 gene’s DNA sequence and 88 SNPs were published in 2005 ( 52 ). Many more SNPs have been found to date and several SNPs have been taken into account due to their vulnerable or protective function in cancer and other disorders. Some of these modifications are uncommon while others are polymorphic with a frequency smaller than 1%.
3.15. Association between CYP19A1 Gene Polymorphisms and Breast Cancer
The multiplication and development of breast cells are affected by estrogens. The risk factors for breast cancer illness in genes involved in biosynthesis and estrogen metabolism were thus taken into account as polymorphisms. Research in the late 1970s has shown that the primary risk factor for breast cancer is prolonged exposure to estrogen and progesterone ( 8 ). The fact that the adipose cells can be the major origin of endogenous estrogens also demonstrated the fact that excessive weight can be a risk factor for breast cancer ( 53 ). Moreover, breast tissue estrogen synthesis was shown to have a key role in boosting hormone levels in brains that drive breast cell proliferation and development, as well as its subsequent development into cancer ( 9 ). In the 1990s, studies on breast cancer focused on the discovery of genetic evidence of susceptibility to the growth of breast cancer, namely, BRCA1 and BRCA2. Substantial studies have led to the gathering of the genetic diversity information of different genes, including the CYP19A1 gene owing to its gene product, CYP19A1. Some CYP19A1 SNP studies highlight that diverse studies of the SNPs’ contribution to breast cancer risk have inadequate reporting, and the influence of SNPs on clinical manifestations is population-specifically differentiated ( 47 ).
3.16. SNPs’ Effect on CYP19A1 Gene in Aromatase Inhibitor Therapy
Novel treatment options for breast cancer have been widely investigated due to the high frequency of this disease worldwide. There are many possibilities for the treatment of various cancers, and there is a typical preference for a combined approach. Clinical therapies rely on the kind of cancer, history of the patient, and tumor properties. Operation, radiation, chemotherapy, and hormone therapy are among the most prevalent treatment choices. Tamoxifen and AIs are the two most prevalent anti-estrogen treatments. The former is utilized in postmenopausal women to treat ER positive (ER+) breast cancer whereas the latter is employed in the treatment of individuals with premenopausal breast cancer ( 47 ). In postmenopausal women, AIs were demonstrated to be more successful in terms of metastasis and adjuvant therapy prognosis than tamoxifen. Nevertheless, there are regular reports of ethnic variances and interpersonal differences due to genetics. The CYP19A1 gene contains a substantial number of SNPs. Some of these SNPs impact the activity of CYP19A1 and thus, the levels of estrogen. Such variations play a key role in the efficacy of treatment approaches and therapeutical impacts. Various studies have assessed the genetic influence on the efficiency of AI in cancer treatment. There have been multiple contradictory studies revealing that SNPs may or may not be linked to AI therapy problems. The SNPs that impact CYP19A1 function and are linked to higher estrogen levels, such as rs6493497 and rs7176005, appear to change the AI impact ( 54 ). However, rs700518, rs10459592, and rs4775936 are substantially related to improved therapeutic efficacy in letrozole therapy. Ferraldeschi, Arnedos ( 55 ) reviewed the AI treatment effects of 56 SNPs, as a prognostic variable, and found that none of them was uniquely related to increasing the AI impact and underlined the significance of additional investigations in genetic markers and their effects on different cancer treatment strategies.
3.17. Breast Cancer Treatment
There are a lot of variables behind breast cancer treatment. Other therapies, such as treatment with medications, hormonal therapy, chemotherapy, and radiation treatments, are also administered after removing a portion of or the full breast tissue. A combination of surgery (lumpectomy), chemotherapy, and radiation therapy is generally employed for treatment based on the concentration of malignant tissue. The prompt diagnosis of this malignancy involves mammography. Many advancements in multiple therapies for breast cancer have been developed in recent decades, which have extended the life span of people. Different therapies depending on the unique problems of each individual are also accessible as a list. This approach is significantly influenced by factors, including tumor stage and characteristics ( 56 ).
3.18. Hormone Therapy in Breast Cancer
Hormones, such as estrogen and progesterone, impact several kinds of breast cancer. Breast cancer cells contain receptors (proteins) that attach to them and assist them to develop. Hormone treatment or endocrine treatment is any therapy to prevent these hormones from binding with their certain receptors. Hormone treatment can reach cancer cells nearly everywhere in the body, not only in the breast; therefore, it is advised for women who have hormone receptor-positive malignancies. This technique doesn’t assist ladies with no hormone receptor in their malignancies, such as triple-negative breast cancer tolerated patients. Hormone therapy is frequently used after surgery (as a supplementary therapy) to lower the chance of cancer recurrence; however, it can also start before surgery (as a novel medication) and is normally taken for at least 5 to 10 years. Hormone therapy would also be used for treating cancer that returns or spreads to other places of the body. Most hormone therapies either reduce estrogen or inhibit estrogens from having an impact on breast cancer cells ( 57 ).
3.19. Estrogen Receptor Blocking Medications
The list of medications that function by preventing estrogen from stimulating the development of breast cancer cells is as follows:
3.19.1. Tamoxifen
Tamoxifen suppresses the activity of ERs in breast malignant cells. It stops estrogen from attaching to cancerous cells and causing them to proliferate. Tamoxifen operates as an antiestrogen in breast cells while in the other tissues, such as the uterus and the bone, it works like estrogen. This is why it is known as a selective estrogen receptor modulator (SERM) which can be utilized to cure females with menopausal or postmenopausal cancer of the breast ( 58 ).
Tamoxifen can be used in several ways. In females with high risk, tamoxifen may be used to minimize the risk of breast cancer. Tamoxifen for five years lowers the probability of ductal carcinoma in situ recurrence in females who have had breast protection surgery with a positive hormone receptor. It also lowers the chance of both breasts developing invasive breast cancer. Tamoxifen can help to minimize the risk of relapse of invasive hormone receptor-positive breast cancer in females who have had surgery and enhance the possibility of survival. It can also lower the chances of acquiring new breast cancer. Tamoxifen is often administered for 5 to 10 years following surgery (adjuvant therapy) or before surgery (neoadjuvant treatment). It is mostly prescribed to women who have early-stage menopausal breast cancer. On the other hand, AIs are typically taken once menopause has passed. Tamoxifen can usually delay and stop the growth of the disease in cancer-tolerated females with hormone-positive breast cancer, and even reduce the size of certain tumors ( 59 ).
3.19.2. Toremifene (Fareston)
Toremifene (Fareston) is another SERM that operates in the same way but is less prevalent and is authorized only in postmenopausal women for treating metastatic breast cancer.
3.19.3. Fulvestrant (Faslodex)
Fulvestrant, a degrader of selective estrogens, is a medicine that inhibits and destroys the receptors of estrogen. It is not a SERM but it works throughout the body as an anti-estrogen. Fulvestrant is presently exclusively licensed for postmenopausal women. It is occasionally used to inactivate the ovaries in premenopausal women, often in conjunction with a luteinizing hormone-releasing hormone agonist. Fulvestrant can be used exclusively for advanced breast cancers not treated with any other hormonal treatment, following the discontinuation of other hormonal medications (such as tamoxifen and typically an AI). It is also used in conjunction with an inhibitor of 6.4 CDK or PI3K to treat metastatic breast cancer as the first hormone treatment or after further testing of the hormone ( 59 ).
3.19.4. Estrogen-lowering Therapies
Certain hormone treatments are used to decrease estrogens. Since the formation of hormonal-receptor-positive breast tumors grows, decreasing estrogens can slow down or prevent cancer from relapse ( 60 ).
3.19.5. Aromatase Inhibitors
The inhibitors of CYP19A1 are medicines that block estrogen production. The majority of estrogen is produced by ovaries before menopause. However, a little estrogen in adipose tissue is still produced by an enzyme termed CYP19A1 for women whose ovaries do not function, either because of menopause or therapy ( 61 ). These medications are beneficial for postmenopausal women, but can also be utilized in postmenopausal women in conjunction with ovarian inhibitors. Inhibitors of CYP19A1 cannot stop ovaries from producing estrogen; therefore, CYP19A1s are mostly utilized for treating postmenopausal women ( 61 ). However, since CYP19A1 inhibitors are far more efficient than tamoxifen in postmenopausal women, the researchers wondered if there was a method to effectively treat people with early-stage hormone receptor-positive with premenopausal CYP19A1 blockers. The findings of the Suppression of Ovarian Function trial, reported in 2015, showed that premenopausal females having hormone receptor-positive breast cancer could be treated with just an AI if their ovarian activity is repressed, as shown in figure 2 ( 62 ).
Figure 2.
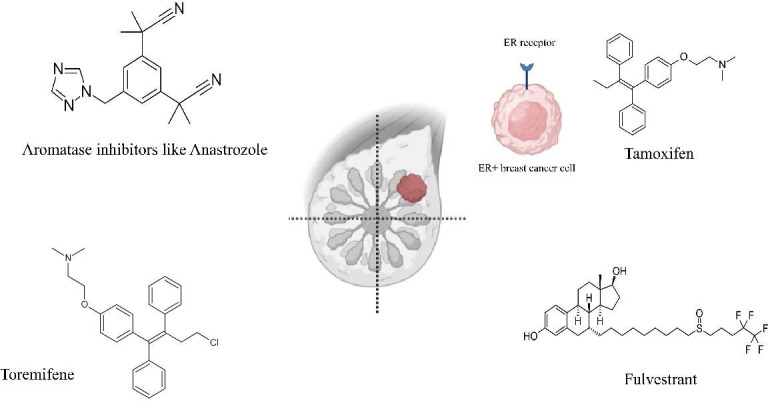
Different medications being used in estrogen-receptor positive breast cancer cells to target estrogen receptor
3.20. White and Dark Sides of Aromatase Inhibitors
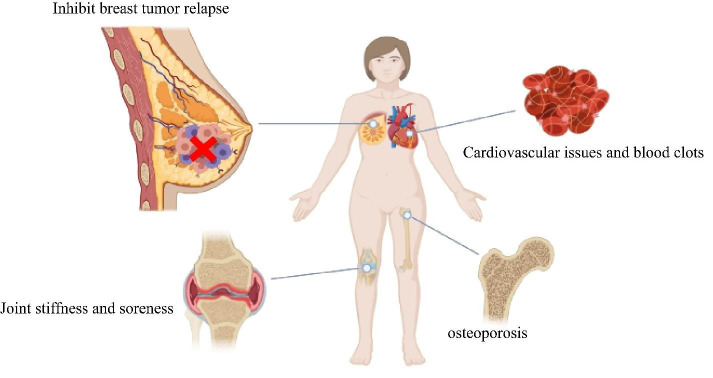
A number of trials have evaluated CYP19A1 inhibitors with tamoxifen in postmenopausal females having early-stage, hormone-receptor-positive breast cancer. According to the findings, the optimum hormonal treatment to start with after the first treatment (surgery, perhaps chemotherapy and radiation therapy) can be an AI. Aromatase inhibitors provide more advantages and less significant adverse effects than tamoxifen for treating early-stage breast cancer with hormone-positive receptors. After using tamoxifen for two to three years (for a total of five years of hormone treatment), using an AI offers greater advantages than using tamoxifen for five years. When compared to not getting therapy after tamoxifen, using an AI for five years following tamoxifen reduces the chance of cancer relapse. The AIs are less likely to produce significant adverse effects such as blood clots, strokes, and endometrial cancer, compared to tamoxifen. However, over the first several years of therapy, AIs can cause greater cardiac issues and osteoporosis than tamoxifen. Joint stiffness or soreness is the most prevalent adverse effect of the Ais, illustrated in figure 3 ( 63 ).
Figure 3.
Advantages and disadvantages of tamoxifen usage in clinics in treating breast cancer patients
4. Conclusion
Due to the highly special activity of the CYP19A1 enzyme in steroid production, targeted CYP19A1 suppression is a focused medication for breast cancer patients, which only has minor adverse effects. Numerous clinical trials over the last decade have shown that AIs not only outperform tamoxifen in terms of effectiveness but also have a lower adverse effect profile. The AI is now widely accepted as a routine therapy option for postmenopausal females with ER+ breast cancer patients. Furthermore, not only dysregulation of gene expression in different genes related to distinguished pathways, such as estrogen metabolism, is essential in the progression of breast cancer but also particular SNPs in particular genes, such as CYP19A1, can play an essential role. Different studies have demonstrated that these SNPs can be located in different sites of these genes, which are collected in this review. In a nutshell, more specific clinical trials are required to demonstrate the precise meditative role of anti-estrogen medications in the treatment of ER+ breast cancer patients. Moreover, another genotype analysis is needed to confirm the role of SNPs in the progression of breast cancer patients.
Authors' Contribution
Study concept and design: A. M. A.
Acquisition of data: J. T. A.
Analysis and interpretation of data: J. T. A.
Drafting of the manuscript: F. A.
Critical revision of the manuscript for important intellectual content: J. T. A.
Administrative, technical, and material support: J. T. A.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.McCullough LE, Santella RM, Cleveland RJ, Millikan RC, Olshan AF, North KE, et al. Polymorphisms in DNA repair genes, recreational physical activity and breast cancer risk. Int J Cancer. 2014;134(3):654–63. doi: 10.1002/ijc.28383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enger SM, Ross RK, Henderson B, Bernstein L. Breastfeeding history, pregnancy experience and risk of breast cancer. Br J Cancer. 1997;76(1):118–23. doi: 10.1038/bjc.1997.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Porter PL. Global trends in breast cancer incidence and mortality. Salud Publica Mex. 2009;51 Suppl 2:s141–6. doi: 10.1590/s0036-36342009000800003. [DOI] [PubMed] [Google Scholar]
- 5.Rheinbay E, Parasuraman P, Grimsby J, Tiao G, Engreitz JM, Kim J, et al. Recurrent and functional regulatory mutations in breast cancer. Nature. 2017;547(7661):55–60. doi: 10.1038/nature22992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Germain D. Estrogen carcinogenesis in breast cancer. Endocrinol Metab Clin North Am. 2011;40(3):473–84, vii. doi: 10.1016/j.ecl.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Wu MH, Chou YC, Yu JC, Yu CP, Wu CC, Chu CM, et al. Hormonal and body-size factors in relation to breast cancer risk: a prospective study of 11,889 women in a low-incidence area. Ann Epidemiol. 2006;16(3):223–9. doi: 10.1016/j.annepidem.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Feigelson HS, Henderson BE. Estrogens and breast cancer. Carcinogenesis. 1996;17(11):2279–84. doi: 10.1093/carcin/17.11.2279. [DOI] [PubMed] [Google Scholar]
- 9.O'Neill JS, Miller WR. Aromatase activity in breast adipose tissue from women with benign and malignant breast diseases. Br J Cancer. 1987;56(5):601–4. doi: 10.1038/bjc.1987.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devilee P, Rookus MA. A tiny step closer to personalized risk prediction for breast cancer. N Engl J Med. 2010;362(11):1043–5. doi: 10.1056/NEJMe0912474. [DOI] [PubMed] [Google Scholar]
- 11.Ulaganathan VK, Sperl B, Rapp UR, Ullrich A. Germline variant FGFR4 p. G388R exposes a membrane-proximal STAT3 binding site. Nature. 2015;528(7583):570–4. doi: 10.1038/nature16449. [DOI] [PubMed] [Google Scholar]
- 12.Deng N, Zhou H, Fan H, Yuan Y. Single nucleotide polymorphisms and cancer susceptibility. Oncotarget. 2017;8(66):110635–49. doi: 10.18632/oncotarget.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Gu L, Qian B, Hao X, Zhang W, Wei Q, et al. Association of genetic polymorphisms of ER-alpha and the estradiol-synthesizing enzyme genes CYP17 and CYP19 with breast cancer risk in Chinese women. Breast Cancer Res Treat. 2009;114(2):327–38. doi: 10.1007/s10549-008-9998-0. [DOI] [PubMed] [Google Scholar]
- 14.Pineda B, Garcia-Perez MA, Cano A, Lluch A, Eroles P. Associations between aromatase CYP19 rs10046 polymorphism and breast cancer risk: from a case-control to a meta-analysis of 20,098 subjects. PLoS One. 2013;8(1): 53902. doi: 10.1371/journal.pone.0053902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, et al. Aromatase--a brief overview. Annu Rev Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- 16.Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005;227(2):115–24. doi: 10.1016/j.canlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Lakhani NJ, Venitz J, Figg WD, Sparreboom A. Pharmacogenetics of estrogen metabolism and transport in relation to cancer. Curr Drug Metab. 2003;4(6):505–13. doi: 10.2174/1389200033489244. [DOI] [PubMed] [Google Scholar]
- 18.Raftogianis R, Creveling C, Weinshilboum R, Weisz J. Estrogen metabolism by conjugation. J Natl Cancer Inst Monogr. 2000;(27):113–24. doi: 10.1093/oxfordjournals.jncimonographs.a024234. [DOI] [PubMed] [Google Scholar]
- 19.Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45(3 Suppl):S116–24. doi: 10.1067/mjd.2001.117432. [DOI] [PubMed] [Google Scholar]
- 20.Yue W, Wang JP, Li Y, Fan P, Liu G, Zhang N, et al. Effects of estrogen on breast cancer development: Role of estrogen receptor independent mechanisms. Int J Cancer. 2010;127(8):1748–57. doi: 10.1002/ijc.25207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zumoff B. Does postmenopausal estrogen administration increase the risk of breast cancer? Contributions of animal, biochemical, and clinical investigative studies to a resolution of the controversy. Proc Soc Exp Biol Med. 1998;217(1):30–7. doi: 10.3181/00379727-217-44202. [DOI] [PubMed] [Google Scholar]
- 22.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344(4):276–85. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 23.Kaaks R, Rinaldi S, Key TJ, Berrino F, Peeters PH, Biessy C, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12(4):1071–82. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 24.Preston-Martin S, Pike MC, Ross RK, Henderson BE. Epidemiologic evidence for the increased cell proliferation model of carcinogenesis. Environ Health Perspect. 1993;101 Suppl 5:137–8. doi: 10.1289/ehp.93101s5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SK, Yim D-s, Yoon K-s, Choi I-m, Choi J-y, Yoo K-y, et al. Combined effect of GSTM1, GSTT1, and COMT genotypes in individual. Breast Cancer Res Treat. 2004;88(1):55–62. doi: 10.1007/s10549-004-0745-x. [DOI] [PubMed] [Google Scholar]
- 26.Ritchie MD, Hahn LW, Roodi N, Bailey LR, Dupont WD, Parl FF, et al. Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am J Hum Genet. 2001;69(1):138–47. doi: 10.1086/321276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaikwad NW, Yang L, Muti P, Meza JL, Pruthi S, Ingle JN, et al. The molecular etiology of breast cancer: evidence from biomarkers of risk. Int J Cancer. 2008;122(9):1949–57. doi: 10.1002/ijc.23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samavat H, Kurzer MS. Estrogen metabolism and breast cancer. Cancer Lett. 2015;356(2 Pt A):231–43. doi: 10.1016/j.canlet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stack DE, Byun J, Gross ML, Rogan EG, Cavalieri EL. Molecular characteristics of catechol estrogen quinones in reactions with deoxyribonucleosides. Chem Res Toxicol. 1996;9(5):851–9. doi: 10.1021/tx960002q. [DOI] [PubMed] [Google Scholar]
- 30.Cavalieri EL, Rogan EG. Unbalanced metabolism of endogenous estrogens in the etiology and prevention of human cancer. J Steroid Biochem Mol Biol. 2011;125(3-5):169–80. doi: 10.1016/j.jsbmb.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taioli E, Bradlow HL, Garbers SV, Sepkovic DW, Osborne MP, Trachman J, et al. Role of estradiol metabolism and CYP1A1 polymorphisms in breast cancer risk. Cancer Detect Prev. 1999;23(3):232–7. doi: 10.1046/j.1525-1500.1999.09912.x. [DOI] [PubMed] [Google Scholar]
- 32.Chattopadhyay S, Siddiqui S, Akhtar MS, Najm MZ, Deo SV, Shukla NK, et al. Genetic polymorphisms of ESR1, ESR2, CYP17A1, and CYP19A1 and the risk of breast cancer: a case control study from North India. Tumour Biol. 2014;35(5):4517–27. doi: 10.1007/s13277-013-1594-1. [DOI] [PubMed] [Google Scholar]
- 33.Talbott KE, Gammon MD, Kibriya MG, Chen Y, Teitelbaum SL, Long CM, et al. A CYP19 (aromatase) polymorphism is associated with increased premenopausal breast cancer risk. Breast Cancer Res Treat. 2008;111(3):481–7. doi: 10.1007/s10549-007-9794-2. [DOI] [PubMed] [Google Scholar]
- 34.Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT. Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology. 2003;144(8):3382–98. doi: 10.1210/en.2003-0192. [DOI] [PubMed] [Google Scholar]
- 35.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Badawi AF, Cavalieri EL, Rogan EG. Role of human cytochrome P450 1A1, 1A2, 1B1, and 3A4 in the 2-, 4-, and 16alpha-hydroxylation of 17beta-estradiol. Metabolism. 2001;50(9):1001–3. doi: 10.1053/meta.2001.25592. [DOI] [PubMed] [Google Scholar]
- 37.Simpson ER, Davis SR. Minireview: aromatase and the regulation of estrogen biosynthesis--some new perspectives. Endocrinology. 2001;142(11):4589–94. doi: 10.1210/endo.142.11.8547. [DOI] [PubMed] [Google Scholar]
- 38.Simpson E, Rubin G, Clyne C, Robertson K, O'Donnell L, Davis S, et al. Local estrogen biosynthesis in males and females. Endocr Relat Cancer. 1999;6(2):131–7. doi: 10.1677/erc.0.0060131. [DOI] [PubMed] [Google Scholar]
- 39.Sasano H, Harada N. Intratumoral aromatase in human breast, endometrial, and ovarian malignancies. Endocr Rev. 1998;19(5):593–607. doi: 10.1210/edrv.19.5.0342. [DOI] [PubMed] [Google Scholar]
- 40.Chen SA, Besman MJ, Sparkes RS, Zollman S, Klisak I, Mohandas T, et al. Human aromatase: cDNA cloning, Southern blot analysis, and assignment of the gene to chromosome 15. DNA. 1988;7(1):27–38. doi: 10.1089/dna.1988.7.27. [DOI] [PubMed] [Google Scholar]
- 41.Shozu M, Zhao Y, Bulun SE, Simpson ER. Multiple splicing events involved in regulation of human aromatase expression by a novel promoter, I.6. Endocrinology. 1998;139(4):1610–7. doi: 10.1210/endo.139.4.5878. [DOI] [PubMed] [Google Scholar]
- 42.McPhaul MJ, Herbst MA, Matsumine H, Young M, Lephart ED. Diverse mechanisms of control of aromatase gene expression. J Steroid Biochem Mol Biol. 1993;44(4):341–6. doi: 10.1016/0960-0760(93)90237-q. [DOI] [PubMed] [Google Scholar]
- 43.Xu Q, Liu JW, He CY, Sun LP, Gong YH, Jing JJ, et al. The interaction effects of pri-let-7a-1 rs10739971 with PGC and ERCC6 gene polymorphisms in gastric cancer and atrophic gastritis. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0089203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin Y, Kim IJ, Kang HC, Park JH, Park HR, Park HW, et al. The E-cadherin -347G->GA promoter polymorphism and its effect on transcriptional regulation. Carcinogenesis. 2004;25(6):895–9. doi: 10.1093/carcin/bgh073. [DOI] [PubMed] [Google Scholar]
- 45.Fan H, Liu D, Qiu X, Qiao F, Wu Q, Su X, et al. A functional polymorphism in the DNA methyltransferase-3A promoter modifies the susceptibility in gastric cancer but not in esophageal carcinoma. BMC Med. 2010;8:12. doi: 10.1186/1741-7015-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raghav D, Sharma V, Agarwal SM. Structural investigation of deleterious non-synonymous SNPs of EGFR gene. Interdiscip Sci. 2013;5(1):60–8. doi: 10.1007/s12539-013-0149-x. [DOI] [PubMed] [Google Scholar]
- 47.Al-Mukaynizi FB, Alanazi M, Al-Daihan S, Parine NR, Almadi M, Aljebreen A, et al. CYP19A1 gene polymorphism and colorectal cancer etiology in Saudi population: case-control study. Onco Targets Ther. 2017;10:4559–67. doi: 10.2147/OTT.S121557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robbez-Masson LJ, Bodor C, Jones JL, Hurst HC, Fitzgibbon J, Hart IR, et al. Functional analysis of a breast cancer-associated FGFR2 single nucleotide polymorphism using zinc finger mediated genome editing. PLoS One. 2013;8(11):e78839. doi: 10.1371/journal.pone.0078839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campbell TM, Castro MAA, de Santiago I, Fletcher MNC, Halim S, Prathalingam R, et al. FGFR2 risk SNPs confer breast cancer risk by augmenting oestrogen responsiveness. Carcinogenesis. 2016;37(8):741–50. doi: 10.1093/carcin/bgw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liotta LA. Cancer cell invasion and metastasis. Sci Am. 1992;266(2):54–9, 62-3. doi: 10.1038/scientificamerican0292-54. [DOI] [PubMed] [Google Scholar]
- 51.Healey CS, Dunning AM, Durocher F, Teare D, Pharoah PD, Luben RN, et al. Polymorphisms in the human aromatase cytochrome P450 gene (CYP19) and breast cancer risk. Carcinogenesis. 2000;21(2):189–93. doi: 10.1093/carcin/21.2.189. [DOI] [PubMed] [Google Scholar]
- 52.Ma CX, Adjei AA, Salavaggione OE, Coronel J, Pelleymounter L, Wang L, et al. Human aromatase: gene resequencing and functional genomics. Cancer Res. 2005;65(23):11071–82. doi: 10.1158/0008-5472.CAN-05-1218. [DOI] [PubMed] [Google Scholar]
- 53.Siiteri PK. Adipose tissue as a source of hormones. Am J Clin Nutr. 1987;45(1 Suppl):277–82. doi: 10.1093/ajcn/45.1.277. [DOI] [PubMed] [Google Scholar]
- 54.Wang M, Chu H, Wang S, Wang M, Wang W, Han S, et al. Genetic variant in APE1 gene promoter contributes to cervical cancer risk. Am J Obstet Gynecol. 2013;209(4):360 e1–7. doi: 10.1016/j.ajog.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 55.Ferraldeschi R, Arnedos M, Hadfield KD, A'Hern R, Drury S, Wardley A, et al. Polymorphisms of CYP19A1 and response to aromatase inhibitors in metastatic breast cancer patients. Breast Cancer Res Treat. 2012;133(3):1191–8. doi: 10.1007/s10549-012-2010-z. [DOI] [PubMed] [Google Scholar]
- 56.Li CI, Malone KE, Porter PL, Weiss NS, Tang MT, Cushing-Haugen KL, et al. Relationship between long durations and different regimens of hormone therapy and risk of breast cancer. J Am Med Assoc. 2003;289(24):3254–63. doi: 10.1001/jama.289.24.3254. [DOI] [PubMed] [Google Scholar]
- 57.von Schoultz E, Rutqvist LE, Stockholm Breast Cancer Study G. Menopausal hormone therapy after breast cancer: the Stockholm randomized trial. J Natl Cancer Inst. 2005;97(7):533–5. doi: 10.1093/jnci/dji071. [DOI] [PubMed] [Google Scholar]
- 58.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339(22):1609–18. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 59.van Leeuwen FE, van den Belt-Dusebout AW, van Leeuwen FE, Benraadt J, Diepenhorst FW, van Tinteren H, et al. Risk of endometrial cancer after tamoxifen treatment of breast cancer. Lancet. 1994;343(8895):448–52. doi: 10.1016/s0140-6736(94)92692-1. [DOI] [PubMed] [Google Scholar]
- 60.Shao W, Brown M. Advances in estrogen receptor biology: prospects for improvements in targeted breast cancer therapy. Breast Cancer Res. 2004;6(1):39–52. doi: 10.1186/bcr742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dutta U, Pant K. Aromatase inhibitors: past, present and future in breast cancer therapy. Med Oncol. 2008;25(2):113–24. doi: 10.1007/s12032-007-9019-x. [DOI] [PubMed] [Google Scholar]
- 62.Gibson L, Lawrence D, Dawson C, Bliss J. Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women. Cochrane Database Syst Rev. 2009;(4):CD003370. doi: 10.1002/14651858.CD003370.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garreau JR, Delamelena T, Walts D, Karamlou K, Johnson N. Side effects of aromatase inhibitors versus tamoxifen: the patients' perspective. Am J Surg. 2006;192(4):496–8. doi: 10.1016/j.amjsurg.2006.06.018. [DOI] [PubMed] [Google Scholar]