Abstract
Camels are susceptible to a variety of infectious diseases, such as trypanosomiasis, anthrax, hemorrhagic septicemia, brucellosis, mange, and pox, which can also affect other farm animal species. Camelpox is one of the most infectious skin diseases, which is caused by the Camelpox virus (CPV), a member of the Orthopoxvirus genus in the Poxviridae family. This study mainly aimed to detect and isolate CPV affecting camels in Wasit province, Iraq. Initially, the study focused principally on clinical manifestations of the disease in affected animals. Afterward, 110 skin samples were collected from infected animals under strict aseptic conditions. They were to be subjected to inoculation into the local embryonated chicken eggs to isolate the virus from the chicken embryo fibroblast cell culture. Finally, the isolates were confirmed using the molecular polymerase chain reaction (PCR) assay. Camelpox virus isolates appeared as several necrotic pock lesions on the Chorioallantoic Membrane (CAM) with cell clustering and sloughing or detachment from the monolayer. Molecular testing was conducted using the PCR assay by targeting the ATIP gene at 881bp. The findings demonstrated that all the investigated isolates were poxvirus positive. In addition, it was found that Camelpox disease is significantly endemic in Wasit province, Iraq. The visualization of the characteristic features of the virus also revealed it can easily adapt to replication in the CAM and cell culture.
Keywords: ATIP gene, Chorioallantoic membrane, Embryonated chicken eggs, PCR
1. Introduction
Camels are susceptible to a variety of infectious diseases, such as trypanosomiasis, anthrax, hemorrhagic septicemia, brucellosis, mange, and pox, that affect other farm animal species as well ( 1 , 2 ). Camelpox is one of the most infectious skin diseases that is caused by the Camelpox virus (CPV), a member of the Orthopoxvirus (OPXV) genus in the Poxviridae family ( 3 ). It is considered an enclosed, brick-shaped virus with an average virion size of 224-389 nm and an unevenly organized tubular structure covering the outer membrane ( 4 ). Each infectious virion has an envelope outer membrane with two internal membranes. Serologically, the virus strains of all animal species are strongly related; therefore, an enzootic disease exists in all areas where camels are herding, which causes significant economic loss ( 5 ). In Iraq, CPV was first reported in 1979, and every 2-3 years, outbreaks of Camelpox occur ( 6 ). Worldwide, this disease is observed in countries where camels live ( 7 ). Camelpox can be transmitted either directly from infected to vulnerable animals or indirectly through contaminated environments ( 8 ). It has been suggested that an arthropod vector has a role in spreading this disease and the tick population during the rainy season affects its dissemination ( 9 ). The virus enters the infected animals’ bodies through respiration and skin abrasions causing viremia, during which it can be excreted throughout the milk, saliva, and ocular/nasal discharges ( 10 , 11 ). Clinically, the severe form of this disease usually appears in young camels, and only the mild form can be seen in older animals ( 9 ). However, fever, lymph node enlargement, and skin sores are the main symptoms observed in affected animals. Skin lesions emerge 1-3 days after the onset of fever, beginning as erythematous macules and then, progressing to papules, vesicles, as well as pustules. Crusts form on the burst pustule ( 12 ).
The identification of the classic indicators is commonly carried out through clinical symptoms; however, confirmatory diagnosis is also essential to rule out other diseases, such as contagious ecthyma, papillomavirus, insect bite reaction, as well as other OPXVs and para-poxvirus infections ( 13 ). The Camelpox virus can be propagated in a large variety of cell cultures, such as Vero, MA-104 and MS monkey kidney, baby hamster kidney, Dubai camel skin, as well as the following primary cell cultures: lamb testis, lamb kidney, camel embryonic kidney, calf kidney, and chicken embryo fibroblast ( 14 ). Due to the limitations of traditional approaches, polymerase chain reaction (PCR) has emerged as a preferred method for detecting and distinguishing CPV from other poxviruses. Commonly, ATIP (A-type inclusion protein), hemagglutinin, and ankyrin repeat protein (C18L)-based PCR techniques are preferred for the specific diagnosis of Camelpox ( 15 , 16 ).
Therefore, this study was designed to detect and isolate CPV affecting camels in Wasit province, Iraq.
2. Materials and Methods
2.1. Sampling and Research Area
This research was carried out in different regions of Wasit province, Iraq, such as Al-Numaniyah, Al-Hay, Sheik Saad, and Badra districts from April 2018 to August 2019. Totally, 110 samples of skin crusts and/or scrapings were collected from free-ranging camels that showed various stages of Camelpox-like skin lesions. Case history data showed that the investigated animals had not received any specific vaccination against CPV. All collected samples were kept in sterile vials with buffered glycerol saline, antibiotics, and antifungal until they were tested. They were then processed for inoculums preparation according to El-Kenawy, Abdel-Galil ( 17 ).
2.2. Virus Isolation
2.2.1. Embryonated Chicken Eggs
Local Embryonated chicken eggs (ECE) of 10-13 days old were subjected for inoculation with 0.3 ml of inoculums into the Chorioallantoic membrane (CAM) and then, incubated at 37˚C. Up to the fourth passage, the extracted CAMs were checked for pock lesions. All techniques were conducted at highly aseptic conditions, as mentioned by Robinson and Balassu ( 18 ).
2.2.2. Primary and Secondary Chicken Embryo Fibroblast
The embryo was dissected into small pieces with sterile scissors and forceps, and then, the bones, as well as viscera, were removed. To remove blood, the remaining tissues were rinsed in phosphate buffer saline. The embryo body was chopped into small pieces with sterile scissors. The utilized medium for growing and maintaining cell growth was prepared following a unique formula from (GIBCOR, UK). The monolayer of the ECE was used for cell culture with 10% bovine fetal serum to acclimatize the cells to grow. As described in previous studies ( 19 ), about 1 ml of CAMs suspension was obtained from samples having characteristic pock lesions, and they were then filtered and seeded in the confluent monolayers in a 25 cm2 tissue culture falcon. The falcons were checked for cytopathic effects (CPE) for up to 5 days every day to each passage. The method described by Cunningham ( 20 ) was followed for preparing primary fibroblast cell cultures from 9 days old chicken embryos. After 36-48 h of incubation, homogeneous cell sheets were created.
In the chick embryo fibroblasts (CEF) cell culture, the prepared viral samples were propagated, and approximately 0.3 ml of virus inoculums was introduced to the monolayers in falcon. The excess inoculum was removed from tubes after 2 h of viral adsorption at 37˚C; then, the maintenance medium was added and incubated at 37˚C. The media from five infected falcons were pooled after 5 days of PROPIDIUM IODIDE, as well as three cycles of alternate freezing and thawing. A portion of the pooled material was inoculated at 0.3 ml per falcon into a new passage of two cell culture falcons (control and infected) while a representative amount was kept at 20˚C.
2.3. Polymerase Chain Reaction
The DNAs were extracted from the suspension of cell culture, as well as from skin lesions, following the manufacturer’s instructions (Bioneer, Korea). The concentration and purity of the extracted DNAs were evaluated using the Nanodrop device (UVIS Drop/ACTGene, USA). The ATIP gene was targeted on a set of primers [(F: 5′-AATACAAGGAGGATCT-3′) and (R: 5'-CTTAACTTTTTCTTTCTC-3′)] ( 21 ) to prepare the PCR to master mix at a final volume of 25 l, which was subjected to thermocycler conditions: 1 cycle initial denaturation (94˚C for 5 min), 30 cycles for denaturation (94˚C for 1 min), annealing (45˚C for 1 min), extension (72˚C for 2.5 min), and 1 cycle final extension (72˚C for 10 min). The PCR products were subjected to electrophoresis in gel stained with ethidium bromide at 100 volts, 80mAm for 1 h, and then visualized under UV to detect positive samples at 881bp.
3. Results
3.1. Clinical Findings
Infected camels showed signs of fever, depression, weakness, and anorexic. Due to the restricted suckling and grazing caused by widespread oral sores, most of the animals were seen with a lowered weight. The infected animals showed some degree of hair loss on the affected portions of their skin as a result of rubbing against hard objects. There were papules vesicles, ocular lacrimation, and thick scabs on their lips and nose, as well as other body regions, such as the trunk (Figure 1).
Figure 1.
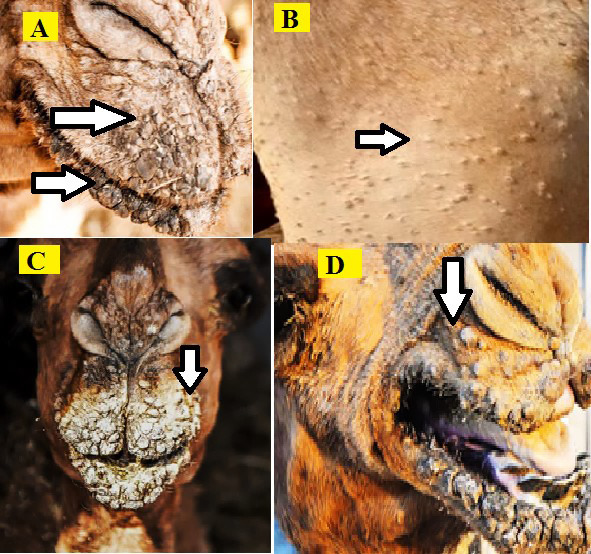
Camelpox clinical findings on lips and the body
3.2. Inoculation of the Virus on Chorioallantoic Membrane
The virus stock was inoculated on the CAM of 9-11 days old ECE for four consecutive passages producing the characteristic pock lesion of CPV (circular, opaque, white swollen, and hemorrhagic patches) (Figure 2).
Figure 2.
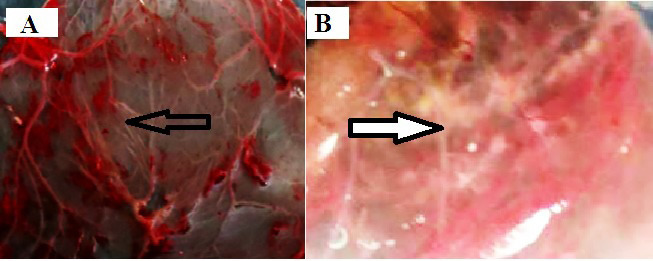
Pock formation by the Camelpox virus at the third (A) and fourth (B) passages
3.3. Tissue Culture Expansion
After four days of inoculation, the virus was reproduced in CEF cells and caused CPE. Rounded and separated cells from the glass were the characteristics of the CPE. There were also a few small syncytia and cell floatings after five passages. Gradually, these CPEs from being mild in the first and second passages became abundant when reaching the fourth and fifth passages (Figure 3).
Figure 3.
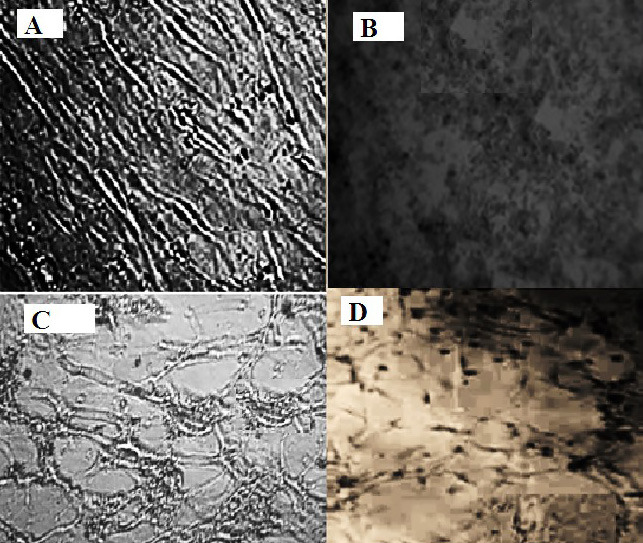
Cytopathic effects on the extracellular fluid cells inoculated with the suspected inoculum after 48 h showed the clustering of cells and their detachment from the glass.
(A): Control not infected, (B): The second passage, (C): The third passage, (D): The fifth passage
3.4. Molecular Assays
The PCR positive bands were detected at a product size of 881bp. All suspected samples revealed a positive amplification targeting the ATIP of the poxvirus (Figure 4).
Figure 4.
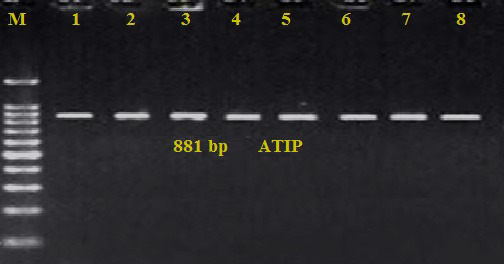
Agarose electrophoresis gel electrophoresis targeting the ATIP gene
Lane (M): Ladder marker (100-1500bp), Lane (1): Positive control, Lanes (2-4): Representative positive samples from cell culture, Lanes (5-6): Representative positive samples from cutaneous lesions
4. Discussion
Worldwide, while the battle to eradicate smallpox is nearly completed, other similar viruses, including Camelpox, Buffalopox, and Monkeypox, remain widespread. It is still unclear how dangerous these viruses are to humans ( 22 , 23 ). It is, however, possible that these viruses will re-evolve into smallpox or a smallpox-like disease in a less immune human population with only minimal antigenic changes between the Camelpox and smallpox virus ( 24 , 25 ). The biological properties of the Iraqi CPV were comparable to those of the Iranian strain, according to the findings presented in a previous study (6). This is understandable given that the Etha 78 virus was discovered along the Iraq-Iran border. Poxviridae is a vast family of viruses that contains ds-DNA, which can infect mammals, birds, and insects. Viruses that infect vertebrates belong to the Chordopoxvirinae subfamily ( 26 ). Pox is the most common infectious viral disease among camels, and thus, the most well-known ( 27 ).
There were typical clinical manifestations of CPV found in this investigation in infected camels, which ranged from acute (camel calves) to mild infection in adults, indicating that the disease could exhibit itself in two or more clinical forms ( 28 ). These findings were consistent with those observed previously where infected camels showed one type with varying degrees of severity, as typical Camelpox infections have four stages ( 3 , 28 ). Samples from potential Camelpox lesions effectively inoculated into the CAM and ECF cell culture resulted in recognizable pock lesions and distinctive cytopathic effects on CAMs and cells, respectively. Pock lesions formed as dense, grayish-white, as well as expanded regions, after the fourth passage, and embryos were alive 5 days after the injection. The findings were consistent with those detected by Al-Zi'abi, Nishikawa ( 8 ). After the second passage, Yousif and Al-Naeem ( 29 ) described the pock lesion as dense and grayish-white with an elevated center. The CPV may be isolated and propagated in numerous cell cultures, including CEF. In the current study, discrete pock lesions and distinctive cytopathic effects were detected on CAMs and CEF cells, respectively. Cell rounding, multinucleated giant cell development, as well as aggregation, and the detachment of cell sheet from the monolayer, followed by plaque formation was observed in the field isolates ( 30 ).
Camelpox virus is still widespread among camel herds in Iraq. Although clinical signs of this disease have been identified, the availability of quick, specific, sensitive, and cost-effective tools might be critical for the confirmation of diagnosis. In addition to higher sensitivity, a PCR-based test is preferred.
Authors' Contribution
Study concept and design: M. A. S. A.
Acquisition of data: H. A. M. A.
Analysis and interpretation of data: H. A. M. A.
Drafting of the manuscript: H. A. M. A.
Critical revision of the manuscript for important intellectual content: M. A. S. A.
Statistical analysis: Z. H. A.
Administrative, technical, and material support: Z. H. A.
Ethics
The research was performed in conformity with the recommendations of the Animal Ethics Committee, as well as local laws and regulations. Camel owners’ agreement was also obtained.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgment
The writers want to express their gratitude to the owners of camel farms and certain vets who helped in collecting samples. They are also grateful to the veterinary hospital staff for their help in viral isolation.
Publisher’s Note
In terms of jurisdictional claims in the published institutional affiliation, Veterinary World maintains a neutral position.
References
- 1.Al-Gharban H, Al-Taee H. Seroclinical diagnosis of Anaplasma marginale bacteria in carrier arabian one-humped camels. Basrah J Vet Res. 2006;15:346–59. [Google Scholar]
- 2.Al-Hassani MKA, Al-Gharban HA, foad Manher L. Application of Three Diagnostic Serologic Techniques to Detect of Dromedary Camel’ s Brucellosis. Al-Qadisiyah J Pure Sci. 2018;23(2):61–74. [Google Scholar]
- 3.Bhanuprakash V, Balamurugan V, Hosamani M, Venkatesan G, Chauhan B, Srinivasan V, et al. Isolation and characterization of Indian isolates of camel pox virus. Trop Anim Health Prod. 2010;42(6):1271–5. doi: 10.1007/s11250-010-9560-z. [DOI] [PubMed] [Google Scholar]
- 4.Haagmans BL, Van Den Brand JM, Raj VS, Volz A, Wohlsein P, Smits SL, et al. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science. 2016;351(6268):77–81. doi: 10.1126/science.aad1283. [DOI] [PubMed] [Google Scholar]
- 5.Essbauer S, Pfeffer M, Meyer H. Zoonotic poxviruses. Vet Microbiol. 2010;140(3-4):229–36. doi: 10.1016/j.vetmic.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Falluji M, Tantawi H, Shony M. Isolation, identification and characterization of camelpox virus in Iraq. Epidemiol Infect. 1979;83(2):267–72. doi: 10.1017/s002217240002605x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhanuprakash V, Prabhu M, Venkatesan G, Balamurugan V, Hosamani M, Pathak KM, et al. Camelpox: epidemiology, diagnosis and control measures. Expert Rev Anti Infect. 2010;8(10):1187–201. doi: 10.1586/eri.10.105. [DOI] [PubMed] [Google Scholar]
- 8.Al-Zi'abi O, Nishikawa H, Meyer H. The first outbreak of camelpox in Syria. J Vet Med Sci. 2007;69(5):541–3. doi: 10.1292/jvms.69.541. [DOI] [PubMed] [Google Scholar]
- 9.Bayisa DA. Review on camel pox: epidemiology, public health and diagnosis. ARC J Animal Vet Sci. 2019;5(4):22–33. [Google Scholar]
- 10.Alehegn E, Chanie M, Mengesha D. A systematic review of serological and clinicopathological features and associated risk factors of avian pox. Br J Poult Sci. 2014;3:78–87. [Google Scholar]
- 11.Gharban HA, Al-Shaeli SJ, Al-Fattli HH, Altaee MN. Molecular and histopathological confirmation of clinically diagnosed lumpy skin disease in cattle, Baghdad Province of Iraq. Vet world. 2019;12(11):1826. doi: 10.14202/vetworld.2019.1826-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mozamel Abdoelmotalab Ibrahim Y. Isolation and Identification of Camelpox Virus in Eastern Sudan. SUST Journal of Agricultural and Veterinary Sciences (SJAVS) 2014; 15 (2):73–81. [Google Scholar]
- 13.Joseph S, Kinne J, Nagy P, Juhász J, Barua R, Patteril NAG, et al. Outbreak of a Systemic Form of Camelpox in a Dromedary Herd (Camelus dromedarius) in the United Arab Emirates. Viruses. 2021;13(10):1940. doi: 10.3390/v13101940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tripathy DN, Reed WM. Pox. Diseases of poultry. :2013, 333–49. [Google Scholar]
- 15.Balamurugan V, Bhanuprakash V, Hosamani M, Jayappa KD, Venkatesan G, Chauhan B, et al. A polymerase chain reaction strategy for the diagnosis of camelpox. Journal of Veterinary Diagnostic Investigation. 2009;21(2):231–7. doi: 10.1177/104063870902100209. [DOI] [PubMed] [Google Scholar]
- 16.Yadav S, Hosamani M, Balamurugan V, Bhanuprakash V, Singh RK. Partial genetic characterization of viruses isolated from pox-like infection in cattle and buffaloes: evidence of buffalo pox virus circulation in Indian cows. Archives of virology. 2010;155(2):255–61. doi: 10.1007/s00705-009-0562-y. [DOI] [PubMed] [Google Scholar]
- 17.El-Kenawy A, Abdel-Galil Y, El-Mekkawi M, Enany M. Studies on camel pox virus isolated from camels in Sharkia govemorate. J Egyptian Vet Med Assoc. 1989;49:389–95. [Google Scholar]
- 18.Robinson A, Balassu T. Contagious pustular dermatitis (orf) Vet Bull. 1981;51(10):771–82. [Google Scholar]
- 19.Kaaden O, Walz A, Czerny C, Wernery U, editors. Progress in the development of a camel pox vaccine. Proc 1st Int Camel Conf: 1992. [Google Scholar]
- 20.Cunningham C. A laboratory guidein virology. 1960. p. 173. [Google Scholar]
- 21.Meyer H, Pfeffer M, Rziha H-J. Sequence alterations within and downstream of the A-type inclusion protein genes allow differentiation of Orthopoxvirus species by polymerase chain reaction. J Gen Virol. 1994;75(8):1975–81. doi: 10.1099/0022-1317-75-8-1975. [DOI] [PubMed] [Google Scholar]
- 22.Goyal T, Varshney A, KSingh R. Poxviruses, Rubella, Coxsackie, and other viruses: Comprehensive Approach to Infections in Dermatology. 2016. p. 260. [Google Scholar]
- 23.Petersen E, Kantele A, Koopmans M, Asogun D, Yinka-Ogunleye A, Ihekweazu C, et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin. 2019;33(4):1027–43. doi: 10.1016/j.idc.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olson VA, Shchelkunov SN. Are we prepared in case of a possible smallpox-like disease emergence? . Viruses. 2017;9(9):242. doi: 10.3390/v9090242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds MG, Carroll DS, Karem KL. Factors affecting the likelihood of monkeypox's emergence and spread in the post-smallpox era. Curr Opin Virol. 2012;2(3):335–43. doi: 10.1016/j.coviro.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter GR, Wise DJ, Flores EF. A concise review of veterinary virology. 2004 [Google Scholar]
- 27.Duraffour S, Meyer H, Andrei G, Snoeck R. Camelpox virus. Antivi Res. 2011;92(2):167–86. doi: 10.1016/j.antiviral.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Khalafalla A, Mohamed ME, Ali B. Camel pox in the Sudan: Part 1. Isolation and identification of the causative virus. J Camel Pract Res. 1998;5(2):229–33. [Google Scholar]
- 29.Yousif A, Al-Naeem A. Molecular characterization of enzootic Camelpox virus in the Eastern Kingdom of Saudi Arabia. Int J Virol. 2011;7(4):135–46. [Google Scholar]
- 30.Yadav S, Dash BB, Kataria JM, Dhama K, Gupta SK, Rahul S. Pathogenicity study of different avipoxviruses in embryonated chicken eggs and cell cultures. Indian J Vet Pathol. 2007;31:17–20. [Google Scholar]


