Abstract
Human Coronaviruses (hCoVs) belongs to the enormous and dissimilar family of positive-sense, non-segmented, single-stranded RNA viruses. The RNA viruses are prone to high rates of mutational recombination resulting in emergence of evolutionary variant to alter various features including transmissibility and severity. The evolutionary changes affect the immune escape and reduce effectiveness of diagnostic and therapeutic measures by becoming undetectable by the currently available diagnostics and refractory to therapeutics and vaccines. Whole genome sequencing studies from various countries have adequately reported mosaic recombination between different lineage strain of SARS-CoV-2 whereby RNA dependent RNA polymerase (RdRp) gene reconnects with a homologous RNA strand at diverse position. This all lead to evolutionary emergence of new variant/ lineage as evident with the emergence of XBB in India at the time of writing this review. The continuous periodical genomic surveillance is utmost required for understanding the various lineages involved in recombination to emerge into hybrid variant. This may further help in assessing virus transmission dynamics, virulence and severity factor to help health authorities take appropriate timely action for prevention and control of any future COVID-19 outbreak.
Keywords: COVID-19, SARS-CoV-2, Recombination, Mutation, Evolution
Introduction
COVID-19, a severe respiratory disease, is a continuing global pandemic and has already caused more than 605 million cases and over 6.4 million fatalities worldwide [1]. Many countries have already observed three to four waves of COVID-19 until now and might expect few more in future, with different strains of the virus, which is continuously evolving. Published data revealed that the severity of the disease and differences in symptoms of the COVID-19 properties do vary between all waves, most probably due to the difference in circulating strains. The second wave in several developing nations were unmanageable and uncontrollable as it almost crippled the healthcare system and led to an exponential increase in COVID-19 infection cases and deaths [2]. However, with rapid development and implementation of therapeutics, the severity of COVID-19 was noticed of slowly and gradually decreasing in most countries, especially during third and fourth wave. SARS-CoV-2 in its evolution has emerged to Omicron variant which appears to be a pre warning threat for possible future epidemics/pandemic. The statistics of Omicron cases double in less than two days, suggest high infectivity and transmissibility, but nevertheless low severity than previous SARS-CoV-2 variants, to raise hope of emerging out of COVID-19 pandemic wave soon [3]. The low mortality in the omicron wave, may also be due to mass vaccination to control COVID-19 spread [4]. In addition, the SARS-CoV-2 virus has mutated over time, resulting in many genomic variations in the present circulating SARS-CoV-2 strains. Genetic variants of SARS-CoV-2 arise regularly, and certain mutations in theSARS-CoV-2 strains lead to expressively enhanced or reduced sensitivity in the S-gene target, that is essential for the entry of the virus to the host. In the SARS-CoV-2, most genetic changes have slight to no effect on the virus fitness and infection properties [5]. Conversely, some genetic variations might change the virus’s properties, such as how easily it spreads in communities, the related disease severity, the effect of the vaccine’s activity and/or diagnostic tools, thus having an impact on public health and social measures as well [6].
Many authorities and countries are encouraged to expand whole-genome sequencing capacities to support the epidemiological surveillance and to indicate the extent of transmission of emerging variants found in a particular geographical region and to detect unusual epidemiological events occurring [7]. For the designation and tracking of SARS-CoV-2 new genetic lineages, established nomenclature systems by GISAID, Nextstrain and Pango Lineage are currently used by scientific communities [8, 9]. The emerging SARS-CoV-2 variants are classified into five groups such as variants of concern (VOC), VOC lineages under monitoring (VOC-LUM); variants of interest (VOI); variants under monitoring (VUM) and formerly monitored variants (FMV) by World Health Organization (WHO) [8]. The five variants of concern (VOC) of SARS-CoV-2 namely Alpha B.1.1.7, Beta B.1.351, Gamma P.1, Delta B.1.617.2, and Omicron B.1.1.529 lead to an understanding of the impacts of variants such as transmissibility, risk of reinfection, disease severity and impacts on vaccine and diagnostics performance [10].
Earlier existing corona viruses (SARS-CoV) have their own genomic proofreading mechanisms in RNA-dependent RNA polymerase (RdRp) with 3′–5′ exoribonuclease. SARS-CoV-2 also has exoribonuclease activities by nsp14-ExoN [11, 12]. As Influenza virus appears prone for mutational variation through genetic drift and shift, SARS-CoV-2 has been reported with antigenic shift which might result from viral replication in immune deficient host and inter-species ping-pong transmission between humans and rodents [13–16]. The non-segmented and single-stranded SARS-CoV-2 RNA undergo genomic variation by a hallmark phenomenon of point mutation and recombination [17].
Recombination signifies a major contributor to virus evolution by generating new genotypes with distinct phenotypic appearances which may alter the transmissibility and virulence activity. There is very scarce scientific literature on the role of recombination in the appearance of new variants of SARS-CoV-2 making it imperative to review this important aspect in this article.
Mutation in SARS-CoV-2
Globally, whole-genome sequencing of SARS-CoV-2 facilitated identification of various point mutations in the circulating new genetic variants of SARS-CoV-2. The first D614G point mutation at position 23,403 in spike protein of reference Wuhan SARS-CoV-2 sequence in early March 2020, led to its designation to the “G clade” [13]. The genomic progression of SARS-CoV-2 was initially minimal during the preliminary phase of COVID-19 pandemic, confined to the emergence of a universal mutation called D614G, accompanied by higher transmissibility but without any changes in disease severity [18]. This point mutation was accompanied by another three-point mutation (first C to T change at position 241, second C to T change at position 3,037, and third C toT change at position 14,408 that altered NSP12 (RdRp) P323L) [13]. A single amino acid non-synonymous mutations are important for allowing the virus to bind to the human receptor ACE2, resulting in subsequent higher transmission and infectivity rates.
It is well accepted that host immune pressure and various environmental conditions are the two main triggers for evolution of living things and mutation is central mechanism of this natural process. Viruses are no exemption to this process and they exploit diverse mechanisms for initiation of mutations and genomic variation to ensure their own survival. SARS-CoV-2 emerging variants that are not fit for survival may become extinct due to natural selection. The finding that highly infectious type of SARS-CoV-2 may evolve through genetic modifications in the genome sequence, is an important aspect that should be carefully considered. Some point mutations are able to cause major changes in functional and structural properties of virus and may alter its infectivity, severity of disease and its interactions with host immune system. For instance, the significant E484K point mutation in RBD (receptor binding domain) spike protein in many variants, has been implicated in causing changes in the electrostatic complementarity of antibody binding to the receptor binding domain [19]. Available data indicates L18F mutation present in N-terminal region of Spike protein of the new variants and it also negatively impacts the binding of neutralizing antibodies [20]. Another point mutation P71L occurs in the E protein and this mutation is associated with infection severity and more death rate. This mutation also reduces the binding of SARS-CoV-2 to serum polyclonal neutralizing antibodies helping virus escape immune response [21]. Additionally, several mutations have been demonstrated in virus replication mechanism enzymes, i.e., main protease (Mpro) and RdRp which may act through different mechanisms including reduction of the hydrogen bonding potential or enhancement of the protein flexibility which is important for binding site recognition.
Recombination in SARS-CoV-2
A recombinant SARS-CoV-2 is a variant evolved by the combination of genomes of at- least two different parent lineages. Natural recombination occurs when a minimum of two variant genomes co-infect the same host cell simultaneously and interchange their genetic material[22]. The evolutionary causes to the incidence of recombination, especially RNA viruses, are not as much clear due to huge population sizes and enormous mutation occurrences [23]. An earlier work done on murine coronaviruses, which also has a positive-sense and non-segment RNA, suggested recombination as an important modality contributing to genetic diversity [24]. SARS-CoV-2 also has the positive-sense and non-segment RNA genome, so the appearance of the different variants has drawn the attention of the scientific community to the role of recombination in the emergence of recombinant lineages of the virus[25].
Several studies have scientifically observed the genetic propensity of recombination across different strains of CoVs, and recombination has been directly related with changes in host response, host range, and own virulence activity [26]. Numerous reports have observed the putative importance of recombination in the zoonotic evolution of SARS-CoV-2, as well [27, 28]. A few studies are dedicated to the occurrence of recombination in SARS-CoV-2 throughout the first year of the COVID-19 pandemic. The first evidence for genetic recombination in SARS-CoV-2was presented by Yi et al. 2020, and he found five haplotypes forming loops that typically indicate existing genetic recombination [29]. Another study developed a Recombination Analysis Program (RAPR) lightweight approach and recognized 1,175 putatively recombinant lineages from 537,360 SARS-CoV-2 genomes, implying to recombinants of 0.2% [30]. Pollett et al. highlight the significance of genomic-based investigation to identify recombination events in SARS-CoV-2. They found moderate evidence (depending on the level of confidence of the bioinformatics software used in the study) in eight SARS-CoV-2 recombination events [31]. Accordingly, based on previous studies, a comprehensive representation of recombination in SARS-CoV-2 and its implications on the pandemic is significant for surveillance purposes.
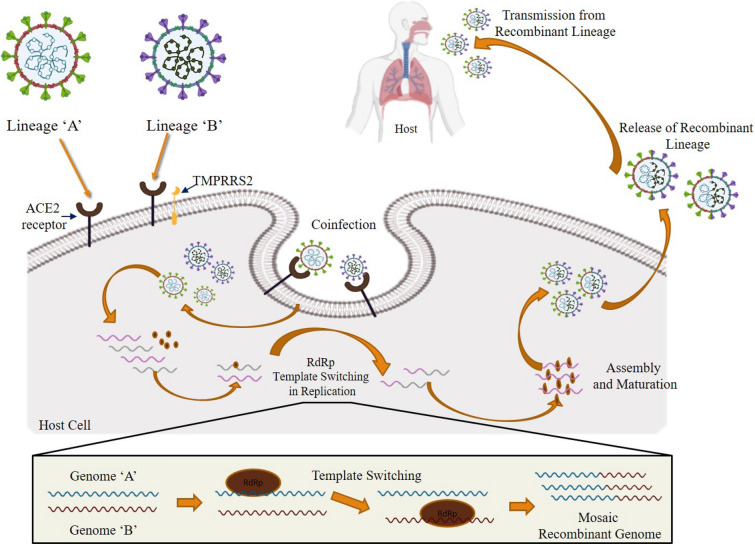
In the homologous recombination mechanism, a fusion/hybrid, or chimera/mosaic RNA genome is produced during the replication process when the RNA-dependent RNA polymerase (RdRp) complex switches from one RNA template (one lineage) to another [32]. The viral genome recombination can be recognized based on the structure of the breakpoint (recombination or crossover) site. Several mosaicism recombination may appear due to breakpoints at several loci on the genome and subsequently their genetic rearrangements. In the direction for RNA mosaicism recombination to be found and then subsequently identified, there must be individuals coinfected by genetically different viruses termed as parental lineage of the recombinant virus [33]. Based on the earlier published data on the molecular mechanism of recombination in RNA viruses, we suggest that the homologous recombination in SARS-CoV-2is possibly through ‘copy-choice replication’ in which the RNA polymerase switches template strands during the replication process between one RNA copy to another in a coinfected host cell (Fig. 1). It is proposed that the RNA polymerase complex (RdRp) disassociates from one RNA genome strand (belonging to lineage 1) to re-associate with another RNA genome strand (belonging to lineage 2), leading to the synthesis of a new recombinant strand. Such switching events may happen one to several times to form a hybrid and thus may lead to mosaicism recombination.
Fig. 1.
Probable homologous recombination mechanism in SARS-CoV-2 genomes and their incidental mosaic genome variance structures
The chances of this mosaicism recombination is understandably very high when the virus prevalence is high, with co-circulation of multiple SARS-CoV-2 lineages within a population providing more opportunity of coinfection of an individual host with virus belonging to multiple lineages. [34]. He et al. recognized a patient who was coinfected with Beta and Delta variants of SARS-CoV-2 and maintained a 1:9 ratio of the relative abundance of concern variants. This study revealed the possible evidence of SARS-CoV-2 recombination in the Orf1ab and Spike genes [35]. List of SARS-CoV-2recombinant lineages detected in various countries across the globe till 31 August 2022 depicted in Table 1.
Table 1.
List of SARS-CoV-2recombinant lineages (As on 20 Oct 2022) [9]
| S. No | Recombinant lineage | Countries (percentage) | First reported date | Number of assigned sequences | Lineage description (parent lineage) |
|---|---|---|---|---|---|
| 1 | XA | UK 100% | 30-01-21 | 44 | B.1.1.7 and B.1.177, UK lineage |
| 2 | XB | USA 79%, Mexico 16%, Guatemala 2%, Honduras 2%, Salvador 1% | 08-07-20 | 3202 | B.1.634 and B.1.631, Central and North America lineage |
| 3 | XC | Japan 100% | 12-08-21 | 25 | AY.29 and B.1.1.7, Japan lineage |
| 4 | XD | France 74%, Denmark 23%, Netherlands 3% | 03-01-22 | 31 | Delta and BA.1, lineage predominantly in France |
| 5 | XE | UK 76%, USA 18%, Israel 3%, Germany 2%, France 1% | 19-01-22 | 2588 | BA.1 and BA.2, UK lineage |
| 6 | XF | UK 100% | 07-01-22 | 33 | Delta and BA.1, UK lineage |
| 7 | XG | Denmark 97%, Germany 1%, UK 1%, Spain 1%, | 11-01-22 | 214 | BA.1 and BA.2, Denmark lineage |
| 8 | XH | Denmark 98%, UK 2% | 30-12-21 | 53 | BA.1 and BA.2, Denmark lineage |
| 9 | XJ | Finland 95%, Sweden 3%, UK 1%, France 1% | 19-01-22 | 69 | BA.1 and BA.2, Finland lineage |
| 10 | XK | Belgium 100% | 10-02-22 | 15 | BA.1 and BA.2, Belgium lineage |
| 11 | XL | UK 88%, USA 12% | 06-02-22 | 64 | BA.1 and BA.2, lineage predominantly in UK |
| 12 | XM | Germany 39%, UK 23%, Denmark 15%, Netherlands 11%, Czech Republic 5% | 14-02-22 | 44 | BA.1.1 and BA.2, European lineage |
| 13 | XN | UK 95%, Denmark 4%, USA 1% | 13-02-22 | 106 | BA.1 and BA.2, lineage predominantly in UK |
| 14 | XP | UK 100% | 22-02-22 | 57 | BA.1.1 and BA.2, UK lineage |
| 15 | XQ | UK 94%, USA 4%, Netherlands 2% | 12-02-22 | 65 | BA.1.1 and BA.2, predominantly in UK lineage |
| 16 | XR | UK 100% | 13-02-22 | 78 | BA.1.1 and BA.2, UK lineage |
| 17 | XS | USA 100% | 19-01-22 | 37 | Delta and BA.1.1, USA lineage |
| 18 | XT | South Africa 100% | 13-12-21 | 12 | BA.1 and BA.2, South Africa lineage |
| 19 | XU | India 71%, Australia 15%, Japan 14% | 20-01-22 | 7 | BA.1 and BA.2, lineage mostly in India |
| 20 | XV | Denmark 81%, Italy 17%, Germany 2% | 31-01-22 | 42 | BA.1 and BA.2, lineage predominantly in Denmark |
| 21 | XW | USA 55%, Germany 27%, UK 12%, Canada 6%, Japan 2% | 13-03-22 | 60 | BA.1 and BA.2, mostly in USA |
| 22 | XY | USA 90%, UK 6%, France 4% | 28-02-22 | 50 | BA.1 and BA.2, mainly in USA |
| 23 | XZ | USA 99%, Virgin Islands 1% | 19-03-22 | 74 | BA.2 and BA.1, USA lineage |
| 24 | XAA | USA 97%, Israel 3% | 12-02-22 | 59 | BA.1 and BA.2, USA lineage |
| 25 | XAB | Germany 84%, Denmark 6%, Italy 4%, Switzerland 2%, Slovakia 2% | 25-02-22 | 81 | BA.1 and BA.2, Germany lineage |
| 26 | XAC | Canada 75%, USA 22%, Singapore 1%, Japan 1%, UK 1% | 11-01-22 | 166 | BA.1 and BA.2, Canada and USA lineage |
| 27 | XAD | Germany 86%, UK 7%, Peru 5%, Slovakia 2% | 15-02-22 | 44 | BA.2 and BA.1, Germany lineage |
| 28 | XAE | USA 54%, Chile 41%, Canada 3%, Netherlands 2% | 26-02-22 | 61 | BA.2 and BA.1, USA and Chile lineage |
| 29 | XAF | USA 48%, Costa Rica 36%, Israel 8%, Chile 4%, Japan 4% | 11-03-22 | 25 | BA.1 and BA.2, Costa Rica lineage |
| 30 | XAG | Brazil 78%, Israel 7%, USA 7%, Denmark 6%, Chile 2% | 10-03-22 | 54 | BA.1 and BA.2, lineage predominantly in Brazil |
| 31 | XAH | Slovenia 67%, Italy 20%, Croatia 5%, Germany 5%, Sweden 3% | 22-02-22 | 73 | BA.2 and BA.1, predominantly in Slovenia |
| 32 | XAJ | UK 79%, USA 12%, South Korea 5%, Israel 2%, Czech Republic 2% | 09-06-22 | 42 | BA.2.12.1 and BA.4, mainly in England |
| 33 | XAK | Germany 94%, Austria 3%, Denmark 2%, Israel 1% | 01-06-22 | 70 | BA.1 and BA.2, Germany lineage |
| 34 | XAL | Germany 74%, Denmark 20%, Slovenia 3%, Portugal 2%, UK 1% | 03-12-21 | 97 | BA.1 and BA.2, Germany lineage |
| 35 | XAM | Panama 54%, USA 35%, Peru 5%, Mexico 3%, Indonesia 3% | 19-03-22 | 348 | BA.1.1 and BA.2.9, lineage occured in Panama and USA |
| 36 | XAN | Denmark 25%, Spain 25%, Canada 12%, Switzerland 10%, Greece 5% | 02-06-22 | 40 | Recombinant lineage of BA.1.1 and BA.2.9 |
| 37 | XAP | USA 87%, Mexico 6%, Canada 1%, Puerto_Rico 1%, Norway 1% | 11-04-22 | 69 | Recombinant lineage of BA.2 and BA.5.1 |
| 38 | XAQ | Canada 92%, United States of America 5%, Hong_Kong 1%, India 1% | 05-03-22 | 76 | Recombinant lineage of BA.2 and BA.1 |
| 39 | XAR | Reunion 88%, France 12% | 11-04-22 | 50 | Recombinant lineage of BA.1 and BA.2 |
| 40 | XAS | USA 65%, Mexico 14%, Canada 13%, Chile 3%, Germany 3% | 31-05-22 | 79 | Recombinant lineage of BA.1 and BA.2 |
| 41 | XAT | Japan 100% | 13-04-22 | 78 | Recombinant lineage of BA.5 and BA.2 |
| 42 | XAU | Spain 36%, UK 18%, USA 10%, France 10%, Germany 4% | 21-03-22 | 77 | Recombinant lineage of BA.2.3.13 and BA.1 |
| 43 | XAV | France 33%, UK 23%, USA 22%, Israel 5%, Sweden 3% | 18-06-22 | 60 | Recombinant lineage of BA.1.1 and BA.2.9 |
| 44 | XAW | Russia 100% | 27-06-22 | 5 | Recombinant lineage of BA.2 and BA.5 |
| 45 | XAY | South_Africa 100% | 28-06-22 | 34 | Recombinant lineage of BA.2 and AY.45 |
| 46 | XAZ | France 18%, Germany 11%, USA 8%, Japan 7%, Denmark 5% | 03-06-22 | 2138 | Recombinant lineage of BA.2.5, BA.5. and BA.2.5 |
| 47 | XBA | "USA 67%, Singapore 33% | 06-09-22 | 4 | Recombinant lineage of perhaps AY.45 and perhaps BA.2 |
| 48 | XBB | Singapore 35%, India 35%, USA 15% others 15% | 06-09-22 | 4466 | BJ.1 and BA.2.75 recombined lineage |
| 49 | XBC | Philippines 50%, Austria 50% | 22-08-22 | 18 | Recombinant lineage of BA.2 and B.1.617.2 |
| 50 | XBD | India 43%, USA 17%, Netherlands 9%, Liechtenstein 9%, Singapore 9% | 21-09-22 | 23 | Recombinant lineage of BA.2.75.2 and BF.5 |
| 51 | XBE | USA 58%, UK 29%, Canada 5%, Denmark 3%, Israel 2%" | 08-08-22 | 112 | Recombinant lineage of BA.5.2 and BE.4.1 |
According to Jackson et al.(2021), sixteen recombinant whole genome sequences from the UK were detected and characterized by subsequent community transmission of recombinant lineage SARS-CoV-2 viruses[33].Recombinant SARS-CoV-2 genomes that share genetic material were tested simultaneously, demonstrating they signify successful onward transmission after the incidence of single ancestral recombination of two lineages. The first instance of lineage recombination, shows the important transmission bunch containing 44 detected cases, which is known as the XA recombinant lineage according to the Pango nomenclature [33]. There is also a possibility that the mosaic recombination may be due to contamination in the laboratory leading to artifacts in the generated sequences [33]
Another study on recombination and phylogenetic investigation revealed the recombinant lineage XB which is previously known as B.1.628 major cluster according to the Pango nomenclature, but XB recombinant lineage is originated through recombination of B.1.631 and B.1.634 lineages [36]. Whenever a new strain of SARS-CoV-2 is detected in a population, a new name is given to it by international health agencies like WHO. However, on further analysis, it is mostly concluded that most of the new strains are a result of genetic recombination of the previously circulating parent lineages.
Another study showed the recombination of 21A (Delta) clade with 20I (Alpha B.1.1.7), mainly between the N genes and ORF6 to suggest the likelihood of multi-lineage infection in a one patient [37].
Notably, Deltacron (Delta + Omicron), XD recombinant SARS-CoV-2 reported from 17 cases in Northwest France by sequencing showed the indication of a prolonged transmission event and circulation of recombinant form, with low disease severity [38]. The evolution of three recombinant lineages of SARS-CoV-2 is mainly XD (Delta and BA.1), XE (BA.1 and BA.2), and XF (Delta and BA.1), which shows probably a high degree of transmissibility that need to be examined by risk assessment investigation [39]. According to WHO, XD (Deltacron) recombinant of SARS-CoV-2 is previously known as VUM and now known as FMV [8] category. The XE recombinant lineage of Omicron BA.1 and BA.2 sub-variants, primarily observed through next generation sequencing in UK. The recombination breakpoint site of this recombinant lineage is occurred in nonstructural protein-6 (NSP6: nucleotide position 11,537) of SARS-CoV-2 [40].
The B.1.617.2 and B.1.529 lineages of SARS-CoV-2 have co-circulated in the US between November 2021 and February 2022, permitting co-infections and likely recombination events. An earlier report recognized eighteen co-infections that exhibited a very few number of Delta-Omicron recombinant lineage in viral population [41]. Researchers also find the 5’-end of the viral genome was from the Delta genome and the 3’-end from Omicron, including the majority of the spike protein gene [41].
Similarly, in January 2022, SARS-CoV-2 XJ recombinant lineages found by genomic surveillance in Finland has a 3’-end of BA.2 and 5’-end of BA.1, along with recombinant breakpoint found in between nucleotide position of 13,296–15, 240 (orf1a and orf1b)[42]. The XJ recombination has been established by nanopore sequencing that generate long-read PCR amplicon spanning the recombination breakpoint, but the origin of the XJ recombinant is still unresolved [42].
First time detected from the India, XBB recombinant variant has been derived from recombination between BJ.1 and BA.2.75. BJ.1 in turn is the result of recombination event between BM.1 and BM.XBB variant and is highly immune evasive strain leading to high chances of replacing the so far dominant BA.2.75 strain as also reported from national agency INSACOG which has confirmed 56% strain in India are XBB and soon going to replace the remaining 44% of BA.2.75 from India [43–45].
These all above discussed studies evidently suggest recombination event occurring between different lineage of SARS-CoV-2 to evolve into new novel variant. However, since the number of confirmed cases of recombinant variants analyzed are very small, the findings cannot be extrapolated for public health measures/significance. Further surveillance is essential for the comprehensive assessment of recombinants’ growth rate and transmissibility.
Conclusion
The natural coinfection generated mosaic recombination events have been found in abundance in the current inter-lineage recombination, which most likely be due to the RdRp template-switching mechanism in SARS-CoV-2 viruses. Various waves of the COVID-19 pandemic, during which there is abundant viral load (belonging to several SARS-CoV-2 lineages) in the community, provide excellent opportunity for recombination. This is also evident by an ever-increasing genetic diversity during subsequent waves, such that it is maximum in 2022, as compared to previous years. Recognizing the recombination incidence is pivotal for characterizing genetic changes occurring in SARS-CoV-2 by applying mathematical model and/or bioinformatics in-silico tools to predict future strains and their transmission rate and virulence. This genetic prediction will help the authorities to initiate appropriate timely action for prevention and control of such epidemics.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Pushpendra Singh, Kuldeep Sharma, Dipika Shaw, Anudita Bhargava and Sanjay Singh Negi. All authors read and approved the final manuscript.
Funding
No funds, grants, or other support was received.
Declarations
Conflict of interest
The authors have no conflict of interest regarding the publication of this article.
Ethics Approval
Not required.
Consent for Publication
Not required.
Consent to Participate
Not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Weekly epidemiological update on COVID-19-14 September 2022 Edition 109. Emerg situational Updat 2022:1–11. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---31-august-2022 (Accessed Sep 18, 2022).
- 2.Singh P, Sharma K, Singh P, Bhargava A, Negi SS, Sharma P, et al. Genomic characterization unravelling the causative role of SARS-CoV-2 Delta variant of lineage B.1.617.2 in 2nd wave of COVID-19 pandemic in Chhattisgarh. India. Microb Pathog. 2022;164:105404. doi: 10.1016/j.micpath.2022.105404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grabowski F, Kochańczyk M, Lipniacki T. The spread of SARS-CoV-2 variant omicron with a doubling time of 2.0–33 days can be explained by immune evasion. Viruses. 2022 doi: 10.3390/v14020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salahshoori I, Mobaraki-Asl N, Seyfaee A, Mirzaei Nasirabad N, Dehghan Z, Faraji M, et al. Overview of COVID-19 disease: virology, epidemiology, prevention diagnosis, treatment, and vaccines. Biol. 2021;1:2–40. doi: 10.3390/biologics1010002. [DOI] [Google Scholar]
- 5.Volz E, Hill V, McCrone JT, Price A, Jorgensen D, O’Toole Á, et al. Evaluating the effects of SARS-CoV-2 spike mutation D614G on transmissibility and pathogenicity. Cell. 2021;184:64–75.e11. doi: 10.1016/j.cell.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiram Guzzi P, Petrizzelli F, Mazza T. Disease spreading modeling and analysis: a survey. Brief Bioinform. 2022 doi: 10.1093/bib/bbac230. [DOI] [PubMed] [Google Scholar]
- 7.Knyazev S, Chhugani K, Sarwal V, Ayyala R, Singh H, Karthikeyan S, et al. Unlocking capacities of genomics for the COVID-19 response and future pandemics. Nat Methods. 2022;19:374–80. doi: 10.1038/s41592-022-01444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Tracking SARS-CoV-2 variants. (2022) https://www.who.int/activities/tracking-SARS-CoV-2-variants (Accessed May 21, 2022).
- 9.Rambaut A, Holmes EC, O’Toole Á, Hill V, McCrone JT, Ruis C, et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. 2020;5:1403–07. doi: 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumache R, Enache A, Macasoi I, Dehelean CA, Dumitrascu V, Mihailescu A, et al. SARS-CoV-2: an overview of the genetic profile and vaccine effectiveness of the five variants of concern. Pathogenus. 2022 doi: 10.3390/pathogens11050516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sevajol M, Subissi L, Decroly E, Canard B, Imbert I. Insights into RNA synthesis, capping, and proofreading mechanisms of SARS-coronavirus. Virus Res. 2014;194:90–9. doi: 10.1016/j.virusres.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gribble J, Stevens LJ, Agostini ML, Anderson-Daniels J, Chappell JD, Lu X, et al. The coronavirus proofreading exoribonuclease mediates extensive viral recombination. PLoS Pathog. 2021;17:e1009226. doi: 10.1371/journal.ppat.1009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812–7.e19. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cameroni E, Bowen JE, Rosen LE, Saliba C, Zepeda SK, Culap K, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2022;602:664–70. doi: 10.1038/s41586-021-04386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yewdell JW. Antigenic drift: understanding COVID-19. Immunity. 2021;54:2681–87. doi: 10.1016/j.immuni.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H, Webster RG, Webby RJ. Influenza virus: dealing with a drifting and shifting pathogen. Viral Immunol. 2018;31:174–83. doi: 10.1089/vim.2017.0141. [DOI] [PubMed] [Google Scholar]
- 17.Pérez-Losada M, Arenas M, Galán JC, Palero F, González-Candelas F. Recombination in viruses: mechanisms, methods of study, and evolutionary consequences. Infect Genet Evol. 2015;30:296–307. doi: 10.1016/j.meegid.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banoun H. Evolution of SARS-CoV-2: review of mutations, role of the host immune system. Nephron. 2021;145:392–403. doi: 10.1159/000515417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jangra S, Ye C, Rathnasinghe R, Stadlbauer D, Alshammary H, Amoako AA, et al. SARS-CoV-2 spike E484K mutation reduces antibody neutralisation. Lancet Microbe. 2021 doi: 10.1016/S2666-5247(21)00068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen RE, Zhang X, Case JB, Winkler ES, Liu Y, VanBlargan LA, et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med. 2021;27:717–26. doi: 10.1038/s41591-021-01294-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammad T, Choudhury A, Habib I, Asrani P, Mathur Y, Umair M, et al. Genomic variations in the structural proteins of SARS-CoV-2 and their deleterious Impact on pathogenesis: a comparative genomics approach. Front Cell Infect Microbiol. 2021 doi: 10.3389/fcimb.2021.765039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu H, Krieter AL, Ponnuraj N, Yung-T Tien Y, Kim T, Jarosinski KW. Coinfection in the host can result in functional complementation between live vaccines and virulent virus. Virulence. 2022;13:980–9. doi: 10.1080/21505594.2022.2082645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao K, Melde RH, Sharp NP. Are mutations usually deleterious? A perspective on the fitness effects of mutation accumulation. Evol Ecol. 2022 doi: 10.1007/s10682-022-10187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai MM, Baric RS, Makino S, Keck JG, Egbert J, Leibowitz JL, et al. Recombination between nonsegmented RNA genomes of murine coronaviruses. J Virol. 1985;56:449–56. doi: 10.1128/JVI.56.2.449-456.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boni MF, Lemey P, Jiang X, Lam TT-Y, Perry BW, Castoe TA, et al. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat Microbiol. 2020;5:1408–17. doi: 10.1038/s41564-020-0771-4. [DOI] [PubMed] [Google Scholar]
- 26.Lange S, Arisan ED, Grant GH, Uysal-Onganer P. MicroRNAs for virus pathogenicity and host responses, identified in SARS-CoV-2 genomes, may play roles in viral-host co-evolution in putative zoonotic host species. Viruses. 2021 doi: 10.3390/v13010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paraskevis D, Kostaki EG, Magiorkinis G, Panayiotakopoulos G, Sourvinos G, Tsiodras S. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect Genet Evol. 2020;79:104212. doi: 10.1016/j.meegid.2020.104212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu A, Niu P, Wang L, Zhou H, Zhao X, Wang W, et al. Mutations, Recombination and Insertion in the Evolution of 2019-nCoV. BioRxiv Preprint Serv Biol 2020:2020.02.29.971101. 10.1101/2020.02.29.971101.
- 29.Yi H. 2019 Novel coronavirus Is undergoing active recombination. Clin Infect Dis. 2020;71:884–7. doi: 10.1093/cid/ciaa219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.VanInsberghe D, Neish AS, Lowen AC, Koelle K (2021) Recombinant SARS-CoV-2 genomes are currently circulating at low levels. BioRxiv Preprint Serv Biol 2021:2020.08.05.238386. 10.1101/2020.08.05.238386.
- 31.Pollett S, Conte MA, Sanborn M, Jarman RG, Lidl GM, Modjarrad K, et al. A comparative recombination analysis of human coronaviruses and implications for the SARS-CoV-2 pandemic. Sci Rep. 2021;11:17365. doi: 10.1038/s41598-021-96626-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chrisman BS, Paskov K, Stockham N, Tabatabaei K, Jung J-Y, Washington P, et al. Indels in SARS-CoV-2 occur at template-switching hotspots. BioData Min. 2021;14:20. doi: 10.1186/s13040-021-00251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson B, Boni MF, Bull MJ, Colleran A, Colquhoun RM, Darby AC, et al. Generation and transmission of interlineage recombinants in the SARS-CoV-2 pandemic. Cell. 2021;184:5179–88.e8. doi: 10.1016/j.cell.2021.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rockett RJ, Draper J, Gall M, Sim EM, Arnott A, Agius JE, et al. Co-infection with SARS-CoV-2 Omicron and Delta variants revealed by genomic surveillance. Nat Commun. 2022;13:2745. doi: 10.1038/s41467-022-30518-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He Y, Ma W, Dang S, Chen L, Zhang R, Mei S, et al. Possible recombination between two variants of concern in a COVID-19 patient. Emerg Microbes Infect. 2022;11:552–5. doi: 10.1080/22221751.2022.2032375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gutierrez B, Castelán Sánchez HG, da Silva Candido D, Jackson B, Fleishon S, Ruis C, et al. Emergence and widespread circulation of a recombinant SARS-CoV-2 lineage in North America. MedRxiv. 2021 doi: 10.1101/2021.11.19.21266601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sekizuka T, Itokawa K, Saito M, Shimatani M, Matsuyama S, Hasegawa H, et al. Genome recombination between delta and alpha variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) MedRxiv. 2021 doi: 10.1101/2021.10.11.21264606. [DOI] [PubMed] [Google Scholar]
- 38.Moisan A, Mastrovito B, De Oliveira F, Martel M, Hedin H, Leoz M, et al. Evidence of transmission and circulation of Deltacron XD recombinant SARS-CoV-2 in Northwest France. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohapatra RK, Kandi V, Tuli HS, Chakraborty C, Dhama K. The recombinant variants of SARS-CoV-2: concerns continues amid COVID-19 pandemic. J Med Virol. 2022 doi: 10.1002/jmv.27780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma K, Chen J. Omicron XE emerges as SARS-CoV-2 keeps evolving. Innov. 2022;3:100248. doi: 10.1016/j.xinn.2022.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolze A, Basler T, White S, Rossi AD, Wyman D, Roychoudhury P, et al. Evidence for SARS-CoV-2 delta and Omicron co-infections and recombination. MedRxiv. 2022 doi: 10.1101/2022.03.09.22272113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindh E, Smura T, Blomqvist S, Liitsola K, Vauhkonen H, Savolainen L, et al. Genomic and epidemiological report of the recombinant XJ lineage SARS-CoV-2 variant, detected in northern Finland, January 2022. Euro Surveill. 2022;27:2200257. doi: 10.2807/1560-7917.ES.2022.27.16.2200257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Focosi D, McConnell S, Casadevall A. The Omicron variant of concern: diversification and convergent evolution in spike protein and escape from anti-Spike monoclonal antibodies. Drug Resist Updat. 2022;65:100882. doi: 10.1016/j.drup.2022.100882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hotez PJ. SARS-CoV-2 variants offer a second chance to fix vaccine inequities. Nat Rev Microbiol. 2022 doi: 10.1038/s41579-022-00824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurhade C, Zou J, Xia H, Liu M, Chang HC, Ren P, et al. Low neutralization of SARS-CoV-2 Omicron BA.2.75.2 BQ.1.1, and XBB.1 by parental mRNA vaccine or a BA.5-bivalent booster. Nat Med. 2022 doi: 10.1038/s41591-022-02162-x. [DOI] [PubMed] [Google Scholar]