Abstract
Although dogs have been proposed as carriers of extraintestinal pathogenic Escherichia coli (ExPEC) with infectious potential for humans, presumed host species-specific differences between canine and human ExPEC strains have cast doubt on this hypothesis. The recent discovery that allele III of papG (the P fimbrial adhesin gene) predominates among human cystitis isolates and confers an adherence phenotype resembling that of canine ExPEC prompted the present reevaluation of the canine-human ExPEC connection. Sixteen paired pap-positive urine and rectal E. coli isolates from dogs with urinary tract infection were studied. papG (adhesin) and papA (pilin) allele type, agglutination phenotypes, virulence factor genotypes, and randomly amplified polymorphic DNA and pulsed-field gel electrophoresis fingerprints were analyzed and compared with those of human ExPEC controls. The 16 canine strains contained predominantly papG allele III. Agglutination phenotypes segregated strictly according to papG allele status and were homogeneous among strains with the same papG allele profile irrespective of their human versus canine origin. Canine and human PapG variant III peptide sequences were highly homologous, without host species-specific differences. The most prevalent canine papA allele was F48, a novel variant recently identified among human urosepsis isolates. In addition to pap, human ExPEC-associated virulence genes detected among the canine strains included sfa/focDE, sfaS, fyuA, hlyA, cnf1, cdtB, kpsMT-II and -III, rfc, traT, ompT, and a marker for a pathogenicity-associated island from archetypal human ExPEC strain CFT073. Molecular fingerprinting confirmed the fecal origin of all but one canine urine isolate and showed one pair of O6 canine urine and fecal isolates to be extremely similar to an O6 human urosepsis isolate with which they shared all other genotypic and phenotypic characteristics analyzed. These data demonstrate that canine ExPEC strains are similar to, and in some instances essentially indistinguishable from, human ExPEC strains, which implicates dogs and their feces as potential reservoirs of E. coli with infectious potential for humans.
Urinary tract infection (UTI) is a significant health problem for dogs as well as humans (25, 38, 39, 69, 75). Whether the extraintestinal pathogenic Escherichia coli (ExPEC) strains that cause UTIs and other extraintestinal infections in dogs are also capable of infecting humans is an important but unresolved question. Surveys of canine UTI isolates done approximately 10 years ago led to divergent conclusions regarding the relationship between canine and human ExPEC strains (5, 7, 41, 57, 71, 74). Westerlund et al. found canine ExPEC strains to exhibit a high prevalence of virulence-associated traits typical of human ExPEC strains, including digalactoside-binding P fimbriae (encoded by pap, for “pilus associated with pyelonephritis”), hemolysin (encoded by hly), and the O4 and O6 somatic antigens. Thus, these investigators proposed that canine ExPEC strains might pose an infectious threat to humans (71).
In contrast, subsequent investigators of canine ExPEC strains documented adherence patterns distinct from what at the time was expected of P-fimbriated human ExPEC strains. For example, unlike human pyelonephritis isolates, canine ExPEC isolates typically did not agglutinate digalactoside-coated latex beads (41, 57), ceramide trihexoside (CTH)-coated equine erythrocytes (74), or sometimes even human erythrocytes (particularly after neuraminidase treatment) (5, 41, 57), yet they did agglutinate canine and sheep erythrocytes, which the human ExPEC strain used as controls did not (5, 62).
Low et al. suggested that the atypical agglutination phenotype of most pap-positive canine ExPEC strains might be due to expression of P fimbriae containing a different adhesin molecule than that present in the P fimbriae of human ExPEC strains (41). Garcia et al. further proposed that this putative dog-associated P adhesin variant might be the novel adhesin described by Lund et al. as a second P fimbrial type in archetypal human pyelonephritis isolate J96 (5, 43). Stromberg et al. subsequently showed that this cloned prs (for “pap-related substance”) operon from strain J96 indeed conferred atypical adherence phenotypes similar to those exhibited by most pap-positive canine ExPEC strains but different from those of pyelonephritogenic human ExPEC. The prs-associated phenotype included agglutination of canine but not human erythrocytes, adherence to canine but not human epithelial cells, and binding to extended digalactoside-containing glycolipids, such as Forssman glycolipid (which is present in dogs but not humans), but not to CTH (which is present in humans but not dogs) (62). The further demonstration by Marklund et al. that archetypal canine ExPEC strain 1442 contains a prs-like operon seemed to establish prs as a distinctively dog-associated P fimbrial variant and to confirm canine ExPEC strains as pathogenetically irrelevant to humans (46).
Subsequent developments have necessitated a reappraisal of the importance for humans of the (prs-encoded) Prs adhesin variant, which is also known as Pap-2, Class III G adhesin, or PapG variant III (28, 31, 46). First, recent molecular epidemiological studies have shown that allele III of papG is usually the predominant papG variant among E. coli isolates from women and children with cystitis (12, 19, 23). (This syndrome received comparatively little attention during the first decade following the discovery of P fimbriae in E. coli strains from patients with pyelonephritis, in which context papG allele II usually predominates [12, 51, 59].) Second, PapG variant III is now known to bind preferentially not only to (canine-associated) Forssman glycolipid but also to several extended digalactoside-containing glycolipids which are present in the urinary tract of some humans (e.g., globo-A) (36, 37, 56, 57) or of nearly the entire human population (e.g., sialosyl-galactosyl-globoside) (32, 60, 64).
These discoveries, together with the recent finding that canine ExPEC cells commonly contain cnf (cytotoxic necrotizing factor) and sfa (S fimbriae) (77), plus the provocative early observation by Low et al. of extensive similarities between certain pap-positive E. coli strains from dogs with UTI versus those from women with cystitis (41), prompted the present reassessment of canine ExPEC strains as potential human pathogens. Specifically, in the present study we sought to confirm genetically in more than the single previously tested strain (strain 1442) (46, 62) that canine urine isolates commonly contain papG allele III. We also sought to determine whether PapG variant III as it occurs in canine ExPEC strains is functionally or structurally different from PapG variant III from human ExPEC, using as human-source controls more than just the previously studied prs clone from strain J96 (36, 37). In addition, we investigated the extent to which canine ExPEC strains exhibit non-pap virulence traits characteristic of human ExPEC strains and searched for evidence of E. coli clones capable of causing UTI in both dogs and humans. Finally, because of the potential significance for humans if the canine fecal flora is a reservoir for ExPEC, we sought to confirm using contemporary molecular techniques the early observation by Low et al. that the canine host's own gut is the proximate source of canine UTI isolates (41).
(This work was presented in part at the 99th General Meeting of the American Society for Microbiology, abstr. D/B-104, Chicago, Ill., 30 May to 3 June 1999).
MATERIALS AND METHODS
Strains.
The 16 canine E. coli strains studied were paired urine and rectal isolates collected from eight dogs with UTI, as previously described (41). These represented eight of the nine pairs of urine and rectal isolates (out of the 20 pairs initially screened by Low et al.) in which both members were pap positive (41). The agglutination phenotypes, restriction fragment length polymorphism (RFLP) patterns for pap, hly, and IS5, outer membrane protein (OMP) profiles, pilin sizes, plasmid profiles, and O antigens of these strains have been previously reported (41). Strains that were nontypeable for the O antigen were designated “Ont,” and those that were O-antigen negative were designated “O−.”
As agglutination controls, wild-type E. coli isolates from humans and recombinant derivatives thereof were used. These included IA2 and HB101/pDC1 (papG allele II; provided by Steve Clegg) (3, 16); BOS035, BOS060, BOS080, and BOS117 (papG allele III; provided by Joel Maslow) (17, 48); P678-54/pJFK102 (papG allele III; provided by Sheila Hull) (16, 31); HU968-298 (S fimbriae; provided by Sheila Hull); and C1845 (Dr family [F1845] adhesins; provided by Sima Bilge and Steve Moseley) (2, 49). Seventy-five well-characterized blood isolates of E. coli from adult humans with urosepsis which had been collected in the mid-1980s (14, 18, 20, 21) were screened for similarities to the canine strains. Human bacteremia isolate CP9 was used as an alternative source for papG allele III (24). Strains were stored at −70°C until ready for use and were grown with an appropriate antibiotic selection as needed.
Agglutination assays.
Digalactoside-coated latex beads were from Chembiomed (Edmonton, Alberta, Canada; now defunct). Human A1P1 and OP1 erythrocytes were from two of us (J.R.J. and T.T.O., respectively). Human Pk2 and p phenotype erythrocytes were provided by Jane Swanson (15, 28). Washed erythrocytes were stored at 4°C as a 50% (vol/vol) stock suspension in Alsever's solution (Gamma Biologicals, Houston, Tex.), which on the day of use was diluted 1:10 in phosphate-buffered saline (PBS), pH 7.4, plus 5% (wt/vol) α-methyl-d-mannoside (PBS-mannose). In selected experiments, human A1P1 or OP1 erythrocytes were incubated with neuraminidase (1:10; Type III from Vibrio cholerae; Sigma Chemical, St. Louis, Mo.), 0.02 U per 100 μl of 5% erythrocytes, for 1 h at 37°C and then washed and resuspended to 5% (vol/vol) in PBS-mannose (15, 28, 41).
Fetuin (10% [wt/vol] in PBS [35, 41]) (Sigma), pigeon egg white (15, 22, 29), and bovine serum albumin (BSA) (10% [wt/vol] in PBS) (Sigma) were used as specific inhibitors of S fimbriae and P fimbriae and a negative control, respectively. Bacteria harvested from agar plates after overnight growth at 37°C were suspended in PBS-mannose at concentrations of approximately 1011 CFU/ml and used in slide agglutination assays, which were done at 4°C and interpreted microscopically, as previously described (15, 28). Agglutination intensity was graded on a semiquantitative 0 to 4+ scale. Inhibition was defined as a decrease in agglutination intensity by ≥2 intensity levels in the presence of an inhibitor. A similar decrement in agglutination intensity after neuraminidase treatment of human erythrocytes was defined as neuraminidase-sensitive (NS) agglutination (41).
Detection of papG alleles, papA alleles, and other virulence-associated genes.
Previously described multiplex PCR assays were used to detect the three alleles of papG (Fig. 1); the 12 alleles of papA corresponding with the 11 serologically defined F types of P fimbriae (F7-1 through F16), plus a newly identified papA variant (F48) (Fig. 1); papAH, papC, papEF, and papG (Fig. 1); and 22 non-pap putative virulence gene regions of ExPEC (16, 26, 27). PCR products were resolved by size in ethidium bromide-stained agarose gels. ompT, which encodes the outer membrane endopeptidase OmpT and is epidemiologically associated with extraintestinal virulence (4, 44, 61), was detected by dot-blot hybridization. Hybridization was done under stringent conditions as previously described (23), using a probe that was generated and digoxigenin-labeled by PCR using primers ompT-f (5′-ATCTAGCCGAAGAAGGAGGC-3′) and ompT-r (5′-CCCGGGTCATAGTGTTCATC-3′), which are based on the published ompT sequence (GenBank accession no. X06903). All genotyping assays were done at least in duplicate using template DNA boiled lysates prepared independently from separate colonies of each strain.
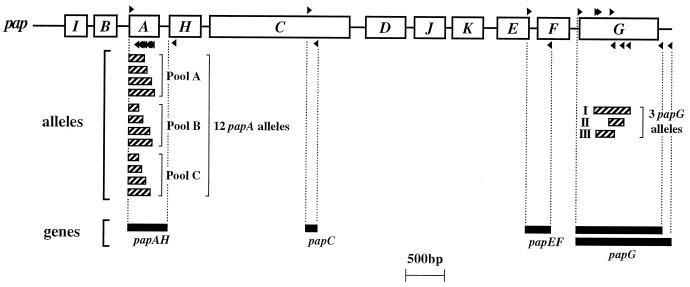
FIG. 1.
Map positions of pap primers and corresponding PCR products. Primers are indicated by small black triangles above (forward primers) or below (reverse primers) their respective recognition sites on the pap operon map. Corresponding PCR products are shown as horizontal bars below the pap operon map, in two groups: (i) alleles of papA and papG and (ii) individual pap genes (papAH, papC, papEF, and papG). Vertical dashed lines connect primers with corresponding PCR products. The two bars shown for full-length papG PCR products correspond with papG allele I (longer bar) and papG alleles II and III (shorter bars).
Genomic fingerprinting and cluster analysis of fingerprints.
Since randomly amplified polymorphic DNA (RAPD) fingerprinting using multiple primers provides a reasonable proxy for multilocus enzyme electrophoresis (MLEE) for defining phylogenetic relationships among pathogenic E. coli (68), RAPD fingerprints were generated for selected strains with each of five decamer oligonucleotide primers (i.e., primers 1247, 1254, 1281, 1283, and 1290) as previously described (68). Each strain's fingerprints from the five primers were digitally combined head-to-toe by using the application Molecular Analyst (Bio-Rad, Hercules, Calif.) to yield a (virtual) composite fingerprint for each strain. Composite fingerprints were compared between strains as operator-independent analog densitometric scans, without assignment of band positions, using Pearson's correlation coefficient. Dendrograms were inferred from the resulting similarity matrices using the unpaired group method with averaging (UPGMA) (58). Clonal groups were defined at the 70% similarity level.
To resolve similarities and differences between closely related strains (1), pulsed-field gel electrophoresis (PFGE) of XbaI-digested total DNA was done as previously described (52). PFGE fingerprints were interpreted by using the criteria of Tenover et al., according to which epidemiologically related isolates that differ by two or three bands are considered to be probably clonally related (65).
DNA sequencing, sequence alignments, and cluster analysis of sequences.
papAH amplicons were generated by using forward primer PapAf (5′-ATGGCAGTGGTGTCTTTTGGTG-3′), which has a recognition site in the consensus signal sequence region of papA, and reverse primer PapAr (5′-CGTCCCACCATACGTGCTCTTC-3′), which has a recognition site within the 5′ end of papH, just downstream from papA (26). Full-length papG amplicons were generated by using forward flanking primer pGf (5′-CTGTAATTACGGAAGTGATTTCTG-3′) and reverse flanking primer pG1"r* (5′-TCCAGAAATAGCTCATGTAACCCG-3′; specific for allele I) or pGr (5′-ACTATCCGGCTCCGGATAAACCAT-3′; specific for alleles II and III) (26). After column purification of papAH and papG amplicons, both strands were directly sequenced as previously described (27). DNA sequences were translated into peptides and aligned by using CLUSTAL-W (66). Dendrograms were inferred according to the neighbor-joining (NJ) method (54) using the application MEGA (34).
For comparison with predicted PapG peptide sequences from the present study, published PapG sequences for variant I from strain J96 (human source), for variant II from strains AD110, DS17, GR12, and IA2 (all human sources), and for variant III from strains J96 (human source), 83972 (human source), 1442 (canine source), and 4787 (porcine source, F165 fimbriae) were included in the PapG dendrogram (6, 11, 33, 42, 45, 46, 62, 67). GenBank accession numbers for these sequences were X61239 (J96, allele I), M20182 (AD110), M94076 (GR12), X61237 (DS17), M20181 (IA2), X62158 (1442), X61238 (J96, allele III), AF097355 (83972), and L07092 (4787).
Comparison of previous versus present analyses of canine ExPEC.
In a previous study (27, 41), the 16 canine strains were tested for hemagglutination (HA) of human erythrocytes from a single donor (with and without neuraminidase digestion and in the presence and absence of inhibitors only of S fimbriae), and for agglutination of P beads. The present study included, in addition, erythrocytes representing multiple human blood phenotypes, control strains for the three papG alleles and for non-P-mannose-resistant (NPMR) adhesins, and specific inhibitors of both P and S fimbriae. Previously, pap status was analyzed as a dichotomous variable and hly was the only virulence factor studied other than pap (27, 41). In the present study, pap status was assessed by specific detection of four subregions of the pap operon, the three papG alleles, and the 12 papA alleles and by sequence analysis of papA and papG from selected strains. In addition, 23 non-pap virulence-associated genes were analyzed. Previously, phylogenetic relationships were inferred from O antigens, RFLP patterns for pap, hly, and IS5 and OMP profiles (27, 41). In the present study, cluster analysis of composite RAPD fingerprints was used to define the overall population structure and PFGE was used to assess close clonal relationships between strains.
Statistical methods.
Comparisons of the prevalence of different virulence genes within the population were tested by using McNemar's test, with a P value of <0.05 as the criterion for statistical significance.
Nucleotide sequence accession numbers.
DNA sequences from the present study have been deposited in GenBank under accession numbers AF234626 (F48 papA control from strain U7), AF234627 (F10 papA control from strain V29), AF237472 through AF237475 (papG allele III from strains U7, CP9, 1U, and 4R, respectively), and AF237478 through AF237482 (papA from strains 2U, 4U, 5U, 6R, and 8U, respectively).
RESULTS
RAPD fingerprinting.
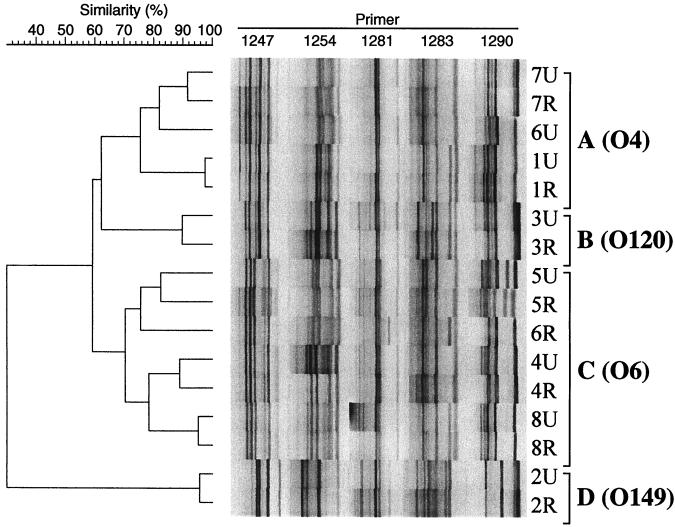
Analysis of the 16 paired urine and rectal E. coli by composite RAPD fingerprinting indicated that the corresponding rectal strain was most similar to the same host's urine strain for seven of eight strain pairs (Fig. 2). These findings were concordant with previous typing results derived by other methods (41).
FIG. 2.
Dendrogram of RAPD fingerprints from 16 canine urine and rectal isolates of E. coli. Composite fingerprints were constructed by combining single-primer fingerprints for each strain, as generated with decamer primers 1247, 1254, 1281, 1283, and 1290. Strain names (dog number, urine versus rectal source), clonal groups (brackets), and O antigens correspond with those shown in Table 1. Similarities were assessed by Pearson's correlation coefficient. Cluster analysis was by UPGMA. Except for paired isolates 6U and 6R, observed differences between paired urine-rectal isolates from the same host are within the reproducibility limits of the assay (not shown).
The RAPD dendrogram, which was used to delineate the underlying phylogenetic relationships between the strains (68), revealed two major clusters (Fig. 2). The first major cluster (designated clonal group A) comprised all five O4 strains. The second major cluster (clonal group C) comprised the four O6 strains plus the three Ont or O− strains that were paired with an O6 strain (Fig. 2; Table 1). Two pairs of urine and rectal strains (pairs 3U-3R and 2U-2R) were not included in either of these major clusters, but instead constituted independent clonal groups (B and D, respectively). Previously determined OMP profiles conformed to this phylogenetic distribution (Fig. 2; Table 1).
TABLE 1.
Virulence traits of 16 rectal-urine E. coli strains from eight dogs with UTIa
| Strain name | O antigenb | OMP pattern, clonal groupb | Agglutination patternb | No. of pap RFLP bandsb | Pilin size (kDa)b | F type (papA allele) | papc | papG allele(s)d | Presence of:
|
PAI marker | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sfa-foc | sfaS | fyuA | hlyA | cnf1 | cdtB | kpsMT (II) | kpsMT (III) | rfc | traT | ||||||||||
| 7U | O4 | A | 6 | F10 | + | II, IIIe | + | − | + | + | + | − | + | − | + | − | + | ||
| 7R | O4 | A | NS | 6 | 19.7 | F10 | + | II, IIIe | + | − | + | + | + | − | + | − | + | − | + |
| 6U | O4 | A | NS | 5 | 19.7 | F7-2,10 | + | III | + | − | + | + | + | − | + | − | + | − | + |
| 1U | O4 | A | NS | 2 | 19.7 | F10 | + | III | + | − | + | + | + | − | − | + | + | + | + |
| 1R | O4 | A | NS | 2 | 19.7 | F10 | + | III | + | − | + | + | + | − | − | + | + | + | + |
| 3U | O120 | B | NS | 6 | 19.5 | F12,13 | + | III | + | − | + | + | + | + | − | − | − | + | + |
| 3R | O120 | B | NS | 6 | 19.5 | F12,13 | + | III | + | − | + | + | + | + | − | − | − | + | + |
| 5U | Ont | C | NS | 2 | 18.7 | F48 | + | III | + | + | + | + | + | − | + | − | − | + | + |
| 5R | O6 | C | NS | 2 | 18.7 | F48 | + | III | + | + | + | + | + | − | + | − | − | + | + |
| 6R | O6 | C | NS | 3 | 18.7 | F48 | + | III | + | + | + | + | + | − | + | − | − | + | + |
| 4U | Ont | C | NS | 4 | 18.7 | F48 | + | III | + | + | + | + | + | − | + | − | − | − | + |
| 4R | O6 | C | NS | 3 | 18.7 | F48 | + | III | + | + | + | + | + | − | + | − | − | − | + |
| 8U | O6 | C | NS | 3 | 18.7 | F48 | + | III | + | + | + | + | + | − | + | − | − | + | + |
| 8R | O− | C | NS | 3 | 18.7 | F48 | + | III | + | + | + | + | + | − | + | − | − | + | + |
| 2U | O149 | D | P | 2 | 18.5, 19.7 | F48 | + | II | − | − | − | − | − | − | − | − | − | + | − |
| 2R | O− | D | P | 2 | 18.5, 19.7 | F48 | + | II | − | − | − | − | − | − | − | − | − | + | − |
All strains were positive for fimH and ompT; all were negative for focG (F1C fimbrial adhesin), afaBC, bmaE (M fimbriae), gafD (G fimbriae), nfaE (nonfimbrial adhesins), iutA (aerobactin uptake), kpsMT-K1 (K1 capsule synthesis), ibeA (invasion of brain endothelium), and cvaC (colicin V). Strain name consists of dog number and source (urine [U] or rectal [R]).
As previously reported (41). Ont, O non-typeable; O−, O-antigen negative; No. of pap RFLP bands, no. of bands in Southern hybridization of pap probe with HindIII-digested genomic DNA. Clonal groups were defined by RAPD fingerprinting (Fig. 1).
All strains were positive for papAH, papC, and papEF. All strains were positive with allele II-III consensus flanking primers. Strains 7U and 7R were positive also with allele I-flanking primers. (This finding will be reported in detail separately).
papG allele was as determined by PCR using allele-specific primers internal to papG (16).
Strains 7U and 7R were subsequently found also to contain a variant of papG allele I which was not detected by published allele I-specific primers. (This finding will be reported in detail separately.)
papG alleles.
All 13 canine E. coli isolates which previously had exhibited the NS agglutination phenotype were found to contain papG allele III, as was the previously agglutination-negative urine strain (7U) which was paired with an NS phenotype rectal strain (7R) (Table 1). (The latter two strains also contained papG allele II in addition to allele III [Table 1].) In contrast, the two strains which previously had exhibited P-pattern agglutination (2U and 2R) were found to contain only papG allele II (Table 1). Thus, papG allele III was significantly more prevalent in the population than was papG allele II (P < 0.05, McNemar's test), whether analyzed among all 16 isolates or only among the nine unique genotypes. Moreover, papG allele content corresponded with historical agglutination phenotypes.
Agglutination phenotypes.
To determine whether agglutination phenotypes correspond most closely with host species or with papG allele content, agglutination phenotypes were reassessed for the 16 canine strains by using P latex beads, diverse erythrocyte types (with or without neuraminidase pretreatment), and various inhibitors of agglutination, in comparison with a panel of human-derived control strains containing papG alleles II or III, S fimbriae, or Dr family adhesins. As previously observed (41), only two of the canine strains (2U and 2R) agglutinated P beads; these were the two strains containing only papG allele II. Similar results were obtained with the control strains, of which only the papG allele II controls were P-bead positive (Table 2).
TABLE 2.
Agglutination patterns of human and canine pap-positive strains for erythrocytes and P latex beads
| Source | Adhesin (no. of strains)b | MRHA of human RBCsa
|
Agglutination of P beadsc | |||||
|---|---|---|---|---|---|---|---|---|
| Inhibitor of A1P1 and/or OP1 RBCs
|
Neuraminidase treatment
|
Pk or p RBCs | ||||||
| PBS or BSA | Pigeon egg white | Fetuin | A1P1 RBCs | OP1 RBCs | ||||
| Human | papG allele II (2) | + | − | + | + | + | (+) | + |
| papG allele III (5) | + | − | + | + | − | − | − | |
| Sfa (1) | + | + | − | − | − | + | − | |
| Dr (1) | + | + | + | + | + | + | − | |
| Canine | papG allele II (2) | + | − | + | + | + | (+) | + |
| papG allele III (13) | + | − | + | + | − | − | − | |
For MRHA of A1P1 and OP1 RBCs, symbols are as follows: +, MRHA was as intense as positive control; −, MRHA was ≥2 intensity levels weaker than positive control (including absent MRHA). For MRHA of Pk or p RBCs, symbols are as follows: +, MRHA was as intense as positive control; (+), MRHA was 0 to 2 intensity levels weaker than positive control; −, absent MRHA. RBCs, red blood cells.
Human-source control strains included IA2 and HB101/pDC1 (papG allele II); BOS035, BOS060, BOS080, BOS117, and HB101/pJFK102 (papG allele III); HU968-298 (Sfa); and C1845 (Dr adhesins). Agglutination-positive canine strains (from present study) included 2U and 2R (papG allele II only); 1U, 1R, 3U-6R, 8U, and 8R (papG allele III only); and 7R (papG alleles II plus III, plus allele I variant). Strain 7U (papG alleles II + III, plus allele I variant) was agglutination negative.
For agglutination of P beads, presence (+) and absence (−) of agglutination is indicated.
Irrespective of papG allele content, all of the canine strains (other than the previously HA-negative strain) and all of the control strains exhibited mannose-resistant HA (MRHA) of both A1P1 and OP1 human erythrocytes (Table 2). Pigeon egg white inhibited MRHA for all the HA-positive canine strains and for the papG allele II- and III-containing control strains but not for the Sfa and Dr control strains, evidence that MRHA as expressed by canine papG allele III-containing strains is digalactoside specific, i.e., is due to P fimbriae rather than to non-P MR adhesins. Fetuin inhibited MRHA for the Sfa control strain but for none of the canine or other control strains, evidence against S fimbriae as mediators of MRHA by the pap-positive canine and control strains.
In experiments done to further characterize the NS agglutination phenotype, for strains containing papG allele III (irrespective of their human versus canine origin), neuraminidase pretreatment of the erythrocytes yielded reduced MRHA intensity with OP1 but not A1P1 human erythrocytes (Table 2). In contrast, for the Sfa control strain, neuraminidase pretreatment reduced MRHA intensity with both OP1 and A1P1 erythrocytes, whereas for the strains containing papG allele II only and for the Dr control strain, neuraminidase pretreatment had no effect on MRHA intensity with either erythrocyte type (Table 2). These findings confirmed an association of the NS agglutination phenotype with papG allele III independent of host species and demonstrated that a specificity for OP1 erythrocytes differentiated the NS phenotype associated with papG allele III from that associated with Sfa.
Further phenotypic differentiation of strains containing papG allele III was obtained by using Pk2 and p phenotype human erythrocytes, which lack the extended digalactoside-containing receptors preferred by PapG variant III. With these rare phenotype erythrocytes, strains containing papG allele III (whether from dogs or humans) yielded trace or absent MRHA. In contrast, strains containing papG allele II (whether from dogs or humans) exhibited MRHA that was robust (Pk2 cells) or of only moderately reduced intensity (p cells) (Table 2). The Sfa and Dr control strains agglutinated both Pk and p erythrocytes equivalently to A1P1 and OP1 cells (Table 2). Taken together, the agglutination results indicated that the human and canine strains containing papG allele III exhibited indistinguishable digalactoside-specific agglutination phenotypes that collectively were distinct from those exhibited by canine and human strains containing papG allele II and from those conferred by non-P MR adhesins.
Canine versus human ExPEC: PapG variant III sequence comparisons.
Despite the similar agglutination phenotypes exhibited by human-derived strains versus canine-derived strains containing papG allele III, it was hypothesized that these two groups still might differ with respect to the peptide sequences of their respective allele III PapG variants and hence might exhibit subtle host-specific differences in the precise receptor specificities of their P fimbriae. To test this hypothesis, the nucleotide sequence was determined for full-length papG amplicons from two allele III-containing canine urine isolates from the present study, i.e., strains 1R (serogroup O4) and 4U (serogroup O6), and from two papG allele III-containing human bacteremia isolates, i.e., strains CP9 (serogroup O4) (24, 53) and U7 (serogroup O6) (26). Predicted PapG peptides corresponding with these papG sequences were compared with published sequences for PapG variant III from human ExPEC strain J96 (46, 70), canine ExPEC strain 1442 (5, 46), human asymptomatic bacteriuria isolate 83972 (11), and porcine septicemia isolate 4787 (10), yielding a total of four human, three canine, and one porcine representatives of the PapG variant III peptide.
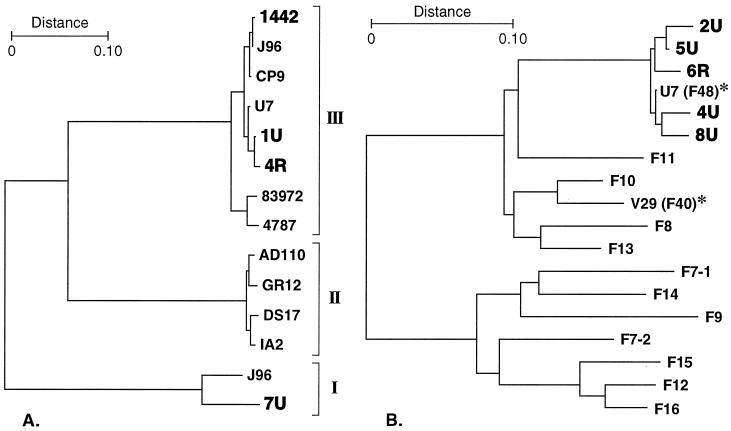
The eight predicted PapG variant III peptides were highly homologous overall, exhibiting 91.7% identity and 5.1% strong similarity (Fig. 3A). At every position in the aligned peptides at which a polymorphism was detected there was sequence identity between at least one human and one canine PapG variant III peptide (Fig. 3A). In a similarity dendrogram, the eight PapG variant III peptides were grouped as three closely related clusters, each of which contained representatives from two different host species (Fig. 4A). Human and canine variants were intermixed in two of the three clusters (Fig. 4A). The aggregate PapG variant III cluster was well removed from the clusters containing, respectively, the variant I and variant II PapG peptides (Fig. 4A). Taken together, these data indicate that versions of PapG variant III from dogs versus from humans do not segregate according to host species even at the peptide level.
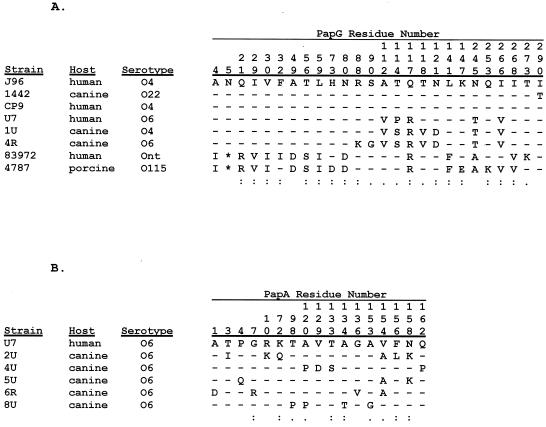
FIG. 3.
Peptide polymorphisms for versions of PapG variant III (A) and the F48 PapA variant (B) from animal and human E. coli isolates. Comparisons included only predicted mature peptides. Alignments were by CLUSTAL-W (66). Only polymorphic positions are shown. Dashes indicate identity, and asterisks indicate gaps. Numbers above sequences indicate residue number (counting from the first residue of the mature peptide). Symbols: colons, highly similar amino acid substitutions; periods, slightly similar substitutions; blanks, differences. The single-letter amino acid code used is as follows: A, Ala; C, Cys; D, Asp; E, Glu; F, Phe; G, Gly; H, His; I, Ile; K, Lys; L, Leu; M, Met; N, Asn; P, Pro; Q, Gln; R, Arg; S, Ser; T, Thr; V, Val; W, Trp; Y, Tyr. (A) For PapG variant III, the version from strain J96 was used as the comparator for versions from three canine isolates, three human isolates, and one porcine isolate. (B) For the F48 PapA variant, the version from index strain U7 (a human urosepsis isolate) was used as the comparator for versions from each of the five unique F48-positive canine isolates from the present study (strains 2U, 4U, 5U, 6R, and 8U).
FIG. 4.
Dendrogram of PapG (A) and PapA (B) peptide sequences. Cluster analysis of predicted mature peptides was by the NJ method (54). (A) Representatives of PapG variants I, II, and III are compared. The allele III variants include three from dogs (1442, 1U, and 4R), four from humans (J96, CP9, U7, and 83972), and one from porcine septicemia isolate 4787. A novel allele I variant identified in canine strain 7U (also shown in bold) will be reported separately. Sequences for CP9, U7, 1U, and 4R (all variant III) and 7U (variant I) were newly determined in the present study; sequences for the other PapG variants were as previously published. (B) Five canine F48 PapA variants (from canine strains 2U, 4U, 5U, 6R, and 8U) are compared with a representative of each of the 11 serologically defined F types of P fimbriae, plus recently discovered PapA variants F40 and F48 (asterisks) (27).
Other pap elements.
Each of the canine strains appeared to contain a complete copy of pap, as evidenced by PCR detection in each strain of all four pap regions, i.e., papAH, papC, papEF, and papG. The single MRHA-negative strain had no detectable abnormality of its pap genotype to account for its altered phenotype (Table 2).
papA alleles.
Although all 16 canine strains were PCR positive for papAH, only seven had a PCR-detectable papA allele corresponding with 1 of the 11 serologically defined F types of P fimbriae, i.e., F7-1, F7-2, and F8-F16 (Table 1). The strains were next tested by using a primer specific for a novel papA allele (F48) that was recently discovered in human urosepsis isolate U7 (27). With the F48-specific primer, all nine of the canine strains that were F PCR negative in the initial testing now yielded an amplicon of the size expected for the F48 PCR product, evidence that they contained the F48 papA variant (Table 1). This was confirmed by full-length DNA sequencing of the papAH amplicon from one representative of each of the F48-positive pairs of urine and rectal strains (i.e., strains 2U, 4U, 5U, and 8U), plus from the sole unpaired F48-positive strain (strain 6R). The corresponding predicted PapA peptide sequences were highly homologous with one another and with the original F48 PapA sequence from (human) source strain U7 (Fig. 3B) but were distant from the other 11 PapA alleles (Fig. 4B). At each of the 17 positions in the F48 PapA peptide at which a polymorphism was detected among the six variants analyzed, the human-derived F48 variant exhibited identity with two or more of the five canine variants, and at only one position (residue 154) was the human-derived variant not identical to the majority of the canine variants (Fig. 3B). The F48 peptide from human isolate U7 was actually more similar overall to two of the canine F48 peptides than were the other three canine F48 variants (Fig. 4B).
Comparison of newly determined papA alleles with previously determined pilin sizes revealed close associations between the F48 papA allele and an 18.7-kDa pilin and between the F10 papA allele and a 19.7 kDa pilin (Table 1). The F48 papA variant accounted for all strains in the O6 cluster (clonal group C), but also occurred in the two outlier strains containing papG allele II only (clonal group D). In contrast, the F10 papA variant was limited to the O4 cluster (clonal group A) and occurred in all O4 strains, one of which also had the F7-2 papA allele (Table 1). These data provided phenotypic validation of the genetic differences detected by the F PCR assay, demonstrated the presence among canine ExPEC strains of papA alleles that are also prevalent among human ExPEC strains (50), and suggested both a phylogenetic distribution of papA alleles and horizontal mobility of the F48 papA variant.
Non-pap virulence factor (VF) genes.
In addition to pap, the canine strains contained multiple other virulence-associated genes characteristic of human ExPEC strains (13). These included sfa/focDE (common to S fimbriae and F1C fimbriae), sfaS (S fimbrial adhesin), fyuA (yersiniabactin), cnf1, hlyA, cdtB (encoding cytolethal distending toxin), kpsMT-II and -III (group II and group III polysaccharide capsule synthesis), rfc (O4 lipopolysaccharide synthesis), traT (serum resistance), ompT, and a marker for a pathogenicity-associated island (PAI) from human ExPEC strain CFT073 (13). Several of these genes exhibited a clear-cut clonal distribution (Fig. 2; Table 1), as has been demonstrated also among human urosepsis isolates (26).
Commonality between canine and human ExPEC.
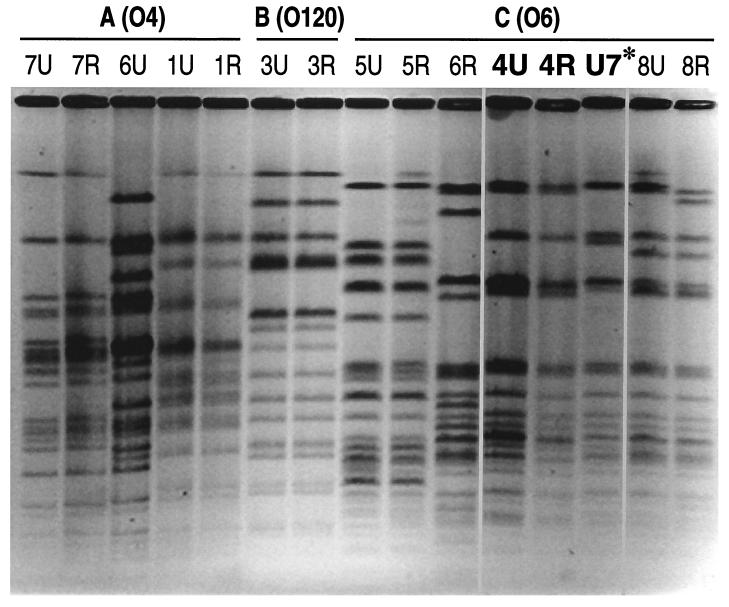
To determine whether any overlap could be identified at the individual clone level between canine and human ExPEC strains, the 16 canine strains from the present study were compared with 75 previously described human urosepsis isolates with respect to O antigen, papG alleles, papA alleles, and genotype for multiple non-pap VFs (as shown in Table 1). One perfect match was found: human blood isolate U7 (O6:K?:HN), the source strain for the F48 papA allele (13, 27), matched canine strains 4U and 4R with respect to all of the characteristics analyzed. PFGE analysis showed human isolate U7 to be almost indistinguishable from these paired canine isolates, meeting criteria for “probably clonally related” according to Tenover et al. (65). Human isolate U7 actually resembled canine strains 4U and 4R more closely than did any of the other canine isolates, including even members of the same O6 clonal group (Fig. 5). Strains 4U, 4R, and U7 were subsequently found to be indistinguishable from one another by RAPD fingerprinting (not shown).
FIG. 5.
SmaI PFGE fingerprints from 14 canine urine and rectal isolates, plus one human urosepsis isolate, of E. coli. For the canine isolates, strain names (dog number, urine versus rectal source) correspond with those shown in Table 1. Highly similar strains 4U (canine), 4R (canine), and U7 (human) are shown in bold. (Canine isolates 2U and 2R [not shown] were indistinguishable from one another and different from all other strains.)
DISCUSSION
In the present study we have analyzed genotypic and phenotypic properties of ExPEC strains isolated from dogs and humans. Our results indicate that papG allele III, which predominates among human cystitis isolates, also predominates among pap-positive canine UTI isolates. Our data also show that (i) PapG variant III sequences from dogs and humans are largely indistinguishable, (ii) canine ExPEC strains exhibit many non-pap virulence genes characteristic of human ExPEC strains, and (iii) even with highly sensitive typing methods, certain canine UTI isolates are essentially indistinguishable from certain human ExPEC strains, providing evidence that there are papG allele III-containing “crossover clones” of E. coli whose members are capable of causing UTI in both canine and human hosts.
Previous investigations of the MR adhesins of canine ExPEC strains led to conflicting conclusions regarding the relationship between canine and human ExPEC strains. It has been reported that canine ExPEC strains typically do not express P fimbriae and thus are fundamentally different from human ExPEC strains (74), that they express P fimbriae and hence are similar to human ExPEC strains (71), and that they express variant forms of P fimbriae that are dissimilar to the P fimbriae of most human ExPEC strains (5, 7, 36, 37, 41, 56, 57), but may be present in some human isolates (36, 37, 41, 56, 57). These investigations were done before assays for the three papG alleles were generally available (12, 16), before the epidemiological association of papG allele III with human cystitis had been demonstrated (12, 23, 59), and before it was known that receptors for PapG variant III are not confined to nonhuman hosts (5, 7, 46, 62, 63) or to humans of the A blood phenotype (36, 37, 56) but are present generally in humans, including within the human urogenital tract (e.g., as sialosyl-galactosyl-globoside) (31, 32, 60, 64). These more recent developments, together with the results of the present study, clarify several points. They provide an explanation for the characteristic NS phenotype of pap-positive canine ExPEC strains (41), which is probably due to desialation by neuraminidase of sialosyl-galactosyl-globoside, the major receptor on human erythrocytes for the variant III PapG adhesin (32, 56, 60, 64). They account for the seemingly discrepant MRHA results reported for canine ExPEC strains by various investigators (5, 7, 56, 57, 62, 71, 74), since the observed phenotypic variability is consistent with conflicting published data regarding PapG variant III (15, 28, 32, 36, 62). They explain the nonreactivity of canine ExPEC strains with digalactoside-coated latex beads (41, 71) and with CTH-coated equine erythrocytes (74), which lack the extended structures preferred as receptors by PapG variant III (36, 60, 62, 64). Finally, they contradict the assumption that canine ExPEC strains have an adhesin distinct from that of human ExPEC strains, one which recognizes a receptor not present in humans (5, 7, 46, 62) or present in only a minority of humans (36, 37, 56).
The results presented here confirm previous reports regarding the high prevalence of pap, hly, sfa/foc, and cnf among canine ExPEC strains (5, 57, 71, 74, 77) and add sfaS, fyuA, cdtB, kpsMT II and -III, rfc, traT, ompT, and the PAI marker from CFT073 to the list of virulence-associated genes present in both canine and human ExPEC strains (9, 26, 55). It is of interest that whereas the aerobactin system was not present in any of the canine strains, which is consistent with the low prevalence reported by others (71, 77), the yersiniabactin siderophore system (fyuA), which has recently been associated with human septicemic E. coli (26, 55), was present in all but two of the strains. In addition, the presence of the PAI marker in most of the canine strains suggests that these strains have inherited a block of urovirulence genes similar to that present in archetypal human ExPEC strain CFT073 (9, 26, 30) and hence likely share with CFT073 and other human ExPEC strains many additional as-yet-unrecognized virulence genes.
Our demonstration of the near-identity of two canine strains (paired urine and rectal isolates from one canine host) and a blood isolate from an adult human with urosepsis confirms the existence of crossover canine-human ExPEC clones. These three strains shared the O6 antigen and had papG allele III, the F48 allele of papA, a uniform extended virulence genotype, and genomic fingerprints that were nearly (PFGE) or completely (RAPD) indistinguishable. Although previous studies have demonstrated overlap between canine and human ExPEC strains with respect to the O serogroup (5, 41, 71, 72, 74, 76), O:K serotype (57), allozyme type (72), or pap, hly, and IS5 RFLP patterns (41), PFGE is more discriminating than any of these typing methods (1, 40, 47, 48). Consequently, the present study provides the best evidence to date of commonality at the individual clone level between canine and human ExPEC strains, albeit with only a single example.
That only one of the 75 human isolates screened could be matched precisely to a canine strain does not necessarily mean that commonality between canine and human ExPEC strains is rare. The human strain collection screened in the present study, which was selected because its members had already been extensively characterized (14, 18, 20, 21, 26), consisted of blood isolates from patients with urosepsis and included only five papG allele-III containing strains. It seems probable that human strains that match canine ExPEC strains would be found more frequently within collections of human cystitis isolates, which exhibit a higher prevalence of papG allele III than do bacteremia isolates (12, 14, 17, 19, 23, 51). Consistent with this prediction, we have recently discovered that the (papG allele III-containing) O6;F48 clonal group that accounted for most of the canine isolates in the present study is one of the most prevalent clonal groups also among urine isolates from women with acute cystitis (unpublished data). These findings indicate that future studies need not investigate whether overlap exists between human and canine ExPEC strains, but instead should determine how extensive such overlap is, to what degree dogs and humans actually exchange ExPEC strains between species, and to what extent cross-species transmission of uropathogens contributes to UTI in humans.
Seven of the eight canine urine isolates from the present study were essentially indistinguishable from the corresponding rectal isolate from the same host, but were distinct from urine and rectal isolates from other canine hosts, with respect to RAPD and PFGE fingerprints and to profiles for multiple virulence-associated genes. This provides strong added support for the hypothesis that canine ExPEC strains (41), like human ExPEC strains (8), typically originate from within the host's own fecal flora and are the predominant fecal E. coli strains at the time of UTI. Other data suggest that even healthy dogs may commonly be fecally colonized with ExPEC strains (73, 77). It is likely that through contact with dogs or their excreta many humans are routinely exposed (on a macroscopic or microscopic level) to elements of the canine fecal flora. Further study of the health implications for humans of such exposures is needed. If pets and pet feces are found to represent significant sources of pathogens or antibiotic resistance elements, interventions that could directly block transmission of fecal bacteria from animals to humans or that could reduce the prevalence and intensity of colonization of pets with pathogenic and/or antimicrobial-resistant E. coli strains conceivably could offer important new measures to prevent human disease.
Summary.
In the present study, papG allele III was found to be the predominant papG allele among E. coli isolates from dogs with UTI, to account for the atypical agglutination pattern of these canine ExPEC isolates and to be genetically indistinguishable among canine versus human ExPEC strains. Multiple virulence genes associated with human ExPEC isolates were identified in pap-positive canine E. coli isolates. One pair of canine urine and rectal isolates was nearly indistinguishable from a human urosepsis isolate with respect to all characteristics studied, including PFGE and RAPD fingerprints, hence presumably represented essentially the same clone. The rectal-urine hypothesis of canine UTI pathogenesis was confirmed by RAPD and PFGE fingerprinting. These findings implicate dogs, and specifically canine feces, as potential reservoirs of E. coli strains with extraintestinal pathogenic capability for humans.
ACKNOWLEDGMENTS
Grant support was from VA Merit Review (J.R.J. and T.A.R.) and National Institutes of Health grants DK-47504 (J.R.J.), AI-23348 (D.A.L.), and AI-42059 (T.A.R.).
Jane Swanson provided Pk2 and p phenotype human erythrocytes. Dave Prentiss helped prepare the figures. Diana Owensby and Ann Emery assisted with manuscript preparation.
REFERENCES
- 1.Arbeit R D, Arthur M, Dunn R, Kim C, Selander R K. Resolution of recent evolutionary divergence among Escherichia coli from related lineages: the application of pulsed field electrophoresis to molecular epidemiology. J Infect Dis. 1990;161:230–235. doi: 10.1093/infdis/161.2.230. [DOI] [PubMed] [Google Scholar]
- 2.Bilge S S, Clausen C R, Lau W, Moseley S L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence to diarrhea-associated Escherichia coli. J Bacteriol. 1989;171:4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clegg S. Cloning of genes determining the production of mannose-resistant fimbriae in a uropathogenic strain of Escherichia coli belonging to serogroup O6. Infect Immun. 1982;38:739–744. doi: 10.1128/iai.38.2.739-744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foxman B, Zhang L, Palin K, Tallman P, Marrs C F. Bacterial virulence characteristics of Escherichia coli isolates from first-time urinary tract infection. J Infect Dis. 1995;171:1514–1521. doi: 10.1093/infdis/171.6.1514. [DOI] [PubMed] [Google Scholar]
- 5.Garcia E, Bergmans H E N, van den Bosch J F, Orskov I, van der Zeijst B A M, Gaastra W. Isolation and characterization of dog uropathogenic Escherichia coli strains and their fimbriae. Antonie Leeuwenhoek. 1988;54:149–163. doi: 10.1007/BF00419202. [DOI] [PubMed] [Google Scholar]
- 6.Garcia E, Bergmans H E N, van der Zeijst B A M, Gaastra W. Nucleotide sequences of the major subunits of F9 and F12 fimbriae of uropathogenic Escherichia coli. Microb Pathog. 1992;13:161–166. doi: 10.1016/0882-4010(92)90076-z. [DOI] [PubMed] [Google Scholar]
- 7.Garcia E, Hamers A M, Bergmans H E N, van der Jeijst B A M, Gaastra W. Adhesion of canine and human uropathogenic Escherichia coli and Proteus mirabilis strains to canine and human epithelial cells. Curr Microbiol. 1988;17:333–337. [Google Scholar]
- 8.Grüneberg R N. Relationship of infecting urinary organism to the faecal flora in patients with symptomatic urinary infection. Lancet. 1969;i:766–768. doi: 10.1016/s0140-6736(69)90478-4. [DOI] [PubMed] [Google Scholar]
- 9.Guyer D M, Kao J-S, Mobley H L T. Genomic analysis of a pathogenecity island in uropathogenic Escherichia coli CFT073: distribution of homologous sequences among isolates from patients with pyelonephritis, cystitis, and catheter-associated bacteriuria and from fecal samples. Infect Immun. 1998;66:4411–4417. doi: 10.1128/iai.66.9.4411-4417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harel J, Forget C, Saint-Amand J, Daigle F, Dubreil D, Jacques M, Fairbrother J M. Molecular cloning of a determinant coding for fimbrial antigen F165, a Prs-like fimbrial antigen for porcine septicaemic Escherichia coli. J Gen Microbiol. 1992;138:1495–1502. doi: 10.1099/00221287-138-7-1495. [DOI] [PubMed] [Google Scholar]
- 11.Hull R A, Rudy D C, Donovan W H, Wieser I E, Stewart C, Darouiche R O. Virulence properties of Escherichia coli 83972, a prototype strain associated with asymptomatic bacteriuria. Infect Immun. 1999;67:429–432. doi: 10.1128/iai.67.1.429-432.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johanson I-M, Plos K, Marklund B-I, Svanborg C. pap, papG and prsG DNA sequences in Escherichia coli from the fecal flora and the urinary tract. Microb Pathog. 1993;15:121–129. doi: 10.1006/mpat.1993.1062. [DOI] [PubMed] [Google Scholar]
- 13.Johnson J R. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson J R. papG alleles among Escherichia coli strains causing urosepsis: associations with other bacterial characteristics and host compromise. Infect Immun. 1998;66:4568–4571. doi: 10.1128/iai.66.9.4568-4571.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson J R, Ahmed P, Brown J J. Diversity of hemagglutination phenotypes among P fimbriated wild-type strains of Escherichia coli according to papG repertoire. Clin Diagn Lab Immunol. 1998;5:160–170. doi: 10.1128/cdli.5.2.160-170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson J R, Brown J J. A novel multiply-primed polymerase chain reaction assay for identification of variant papG genes encoding the Gal(α1-4)Gal-binding PapG adhesins of Escherichia coli. J Infect Dis. 1996;173:920–926. doi: 10.1093/infdis/173.4.920. [DOI] [PubMed] [Google Scholar]
- 17.Johnson J R, Brown J J, Maslow J N. Clonal distribution of the three alleles of the Gal(α1-4)Gal-specific adhesin gene papG among Escherichia coli strains from patients with bacteremia. J Infect Dis. 1998;177:651–661. doi: 10.1086/514230. [DOI] [PubMed] [Google Scholar]
- 18.Johnson J R, Goullet P H, Picard B, Moseley S L, Roberts P L, Stamm W E. Association of carboxylesterase B electrophoretic pattern with presence and expression of urovirulence factor determinants and antimicrobial resistance among strains of Escherichia coli causing urosepsis. Infect Immun. 1991;59:2311–2315. doi: 10.1128/iai.59.7.2311-2315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson J R, Johnson C E, Maslow J N. Clinical and bacteriologic correlates of the papG alleles among Escherichia coli strains from children with acute cystitis. Pediatr Infect Dis J. 1999;18:446–451. doi: 10.1097/00006454-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Johnson J R, Moseley S, Roberts P, Stamm W E. Aerobactin and other virulence factor genes among strains of Escherichia coli causing urosepsis: association with patient characteristics. Infect Immun. 1988;56:405–412. doi: 10.1128/iai.56.2.405-412.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson J R, Orskov I, Orskov F, Goullet P, Picard B, Moseley S L, Roberts P L, Stamm W E. O, K, and H antigens predict virulence factors, carboxylesterase B pattern, antimicrobial resistance, and host compromise among Escherichia coli strains causing urosepsis. J Infect Dis. 1994;169:119–126. doi: 10.1093/infdis/169.1.119. [DOI] [PubMed] [Google Scholar]
- 22.Johnson J R, Ross A E. P1-antigen-containing avian egg whites as inhibitors of P adhesins among wild-type Escherichia coli strains from patients with urosepsis. Infect Immun. 1993;61:4902–4905. doi: 10.1128/iai.61.11.4902-4905.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson J R, Russo T A, Brown J J, Stapleton A. papG alleles of Escherichia coli strains causing first episode or recurrent acute cystitis in adult women. J Infect Dis. 1998;177:97–101. doi: 10.1086/513824. [DOI] [PubMed] [Google Scholar]
- 24.Johnson J R, Russo T A, Scheutz F, Brown J J, Zhang L, Palin K, Rode C, Bloch C, Marrs C F, Foxman B. Discovery of disseminated J96-like strains of uropathogenic Escherichia coli O4:H5 containing genes for both PapGJ96 (“Class I”) and PrsGJ96 (“Class III”) Gal(α1-4)Gal-binding adhesins. J Infect Dis. 1997;175:983–988. doi: 10.1086/514006. [DOI] [PubMed] [Google Scholar]
- 25.Johnson J R, Stamm W E. Urinary tract infections in women: diagnosis and treatment. Ann Intern Med. 1989;111:906–917. doi: 10.7326/0003-4819-111-11-906. [DOI] [PubMed] [Google Scholar]
- 26.Johnson J R, Stell A L. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J Infect Dis. 2000;181:261–272. doi: 10.1086/315217. [DOI] [PubMed] [Google Scholar]
- 27.Johnson J R, Stell A L, Scheutz F, O'Bran T T, Russo T A, Carlino U B, Fasching C C, Kavle J, van Dijk L, Gaastra W. Analysis of F antigen-specific papA alleles of extraintestinal pathogenic Escherichia coli using a novel multiplex polymerase chain reactions-based assay. Infect Immun. 2000;68:1587–1599. doi: 10.1128/iai.68.3.1587-1599.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson J R, Swanson J L, Barela T J, Brown J J. Receptor specificities of variant Gal(α1-4)Gal-binding PapG adhesins of uropathogenic Escherichia coli as assessed by hemagglutination phenotypes. J Infect Dis. 1997;175:373–381. doi: 10.1093/infdis/175.2.373. [DOI] [PubMed] [Google Scholar]
- 29.Johnson J R, Swanson J L, Neill M A. Avian P1 antigens inhibit agglutination mediated by P fimbriae of uropathogenic Escherichia coli. Infect Immun. 1992;60:578–583. doi: 10.1128/iai.60.2.578-583.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kao J, Stucker D M, Warren J W, Mobley H L T. Pathogenicity island sequences of pyelonephritogenic Escherichia coli CFT073 are associated with virulent uropathogenic strains. Infect Immun. 1997;65:2812–2820. doi: 10.1128/iai.65.7.2812-2820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karr J F, Nowicki B, Truong L D, Hull R A, Hull S I. Purified P fimbriae from two cloned gene clusters of a single pyelonephritogenic strain adhere to unique structures in the human kidney. Infect Immun. 1989;57:3594–3600. doi: 10.1128/iai.57.11.3594-3600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karr J F, Nowicki B J, Truong L D, Hull R A, Moulds J J, Hull S I. Pap-2-encoded fimbriae adhere to the P blood group-related glycosphinglolipid stage-specific embryonic antigen 4 in the human kidney. Infect Immun. 1990;58:4055–4062. doi: 10.1128/iai.58.12.4055-4062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klann A G, Hull R A, Hull S I. Sequences of the genes encoding the minor tip components of Pap-3 pili of Escherichia coli. Gene. 1992;119:95–100. doi: 10.1016/0378-1119(92)90071-v. [DOI] [PubMed] [Google Scholar]
- 34.Kumar S, Tamura K, Nei M. MEGA: molecular evolutionary genetics analysis, version 1.01. University Park: The Pennsylvania State University; 1993. [Google Scholar]
- 35.Kunin C M, Hua T, Krishman C, Van Arsdale White L, Hacker J. Isolation of a nicotinamide-requiring clone of Escherichia coli O18:K1:H7 from women with acute cystitis: resemblance to strains found in neonatal meningitis. Clin Infect Dis. 1993;16:412–416. doi: 10.1093/clind/16.3.412. [DOI] [PubMed] [Google Scholar]
- 36.Lindstedt R, Baker N, Falk P, Hull R, Hull S, Karr J, Leffler H, Svanborg Eden C, Larson G. Binding specificities of wild-type and cloned Escherichia coli strains that recognize Globo-A. Infect Immun. 1989;57:3389–3394. doi: 10.1128/iai.57.11.3389-3394.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindstedt R, Larson G, Falk P, Jodal U, Leffler H, Svanborg Eden C. The receptor repertoire defines the host range for attaching Escherichia coli strains that recognize Globo-A. Infect Immun. 1991;59:1086–1092. doi: 10.1128/iai.59.3.1086-1092.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ling G V. Urinary tract infections. In: Ling G V, editor. Lower urinary tract diseases of dogs and cats. St. Louis, Mo: Mosby; 1995. pp. 116–128. [Google Scholar]
- 39.Ling G V, Bibestein E L, Hirsh D C. Bacterial pathogens associated with urinary tract infections. Vet Clin N Am Small Anim Pract. 1979;9:617–630. doi: 10.1016/s0195-5616(79)50077-1. [DOI] [PubMed] [Google Scholar]
- 40.LiPuma J J, Stull T L, Dasen S E, Pidcock K A, Kaye D, Korzeniowski O M. DNA polymorphisms among Escherichia coli isolated from bacteriuric women. J Infect Dis. 1989;159:526–532. doi: 10.1093/infdis/159.3.526. [DOI] [PubMed] [Google Scholar]
- 41.Low D A, Braaten B A, Ling G V, Johnson D L, Ruby A L. Isolation and comparison of Escherichia coli strains from canine and human patients with urinary tract infections. Infect Immun. 1988;56:2601–2609. doi: 10.1128/iai.56.10.2601-2609.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lund B, Lindberg F, Normark S. Structure and antigenic properties of the tip-located P pilus proteins of uropathogenic Escherichia coli. J Bacteriol. 1988;170:1887–1894. doi: 10.1128/jb.170.4.1887-1894.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lund B, Marklund B, Strömberg N, Lindberg F, Karlsson K, Normark S. Uropathogenic Escherichia coli can express serologically identical pili of different receptor binding specificities. Mol Microbiol. 1988;2:255–263. doi: 10.1111/j.1365-2958.1988.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 44.Lundrigan M D, Webb R M. Prevalence of ompT among Escherichia coli isolates of human origin. FEMS Microbiol Lett. 1991;97:51–56. doi: 10.1016/0378-1097(92)90362-r. [DOI] [PubMed] [Google Scholar]
- 45.Maiti S N, DesGroseillers L, Fairbrother J M, Harel J. Analysis of genes coding for the major and minor fimbrial subunits of the Prs-like fimbriae F165(1) of porcine septicemic Escherichia coli strain 4787. Microb Pathog. 1994;16:15–25. doi: 10.1006/mpat.1994.1002. [DOI] [PubMed] [Google Scholar]
- 46.Marklund B I, Tennent J M, Garcia E, Hamers A, Baga M, Lindberg F, Gaastra W, Normark S. Horizontal gene transfer of the Escherichia coli pap and prs pili operons as a mechanism for the development of tissue-specific adhesive properties. Mol Microbiol. 1992;6:2225–2242. doi: 10.1111/j.1365-2958.1992.tb01399.x. [DOI] [PubMed] [Google Scholar]
- 47.Maslow J N, Slutsky A M, Arbeit R D. The application of pulsed field gel electrophoresis to molecular epidemiology. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology: principles and applications. Washington, D.C.: American Society for Microbiology; 1993. pp. 563–572. [Google Scholar]
- 48.Maslow J N, Whittam T S, Gilks C F, Wilson R A, Mulligan M E, Adams K S, Arbeit R D. Clonal relationships among bloodstream isolates of Escherichia coli. Infect Immun. 1995;63:2409–2417. doi: 10.1128/iai.63.7.2409-2417.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nowicki B, Labigne A, Moseley S, Hull R, Hull S, Moulds J. The Dr hemagglutinin, afimbrial adhesins AFA-I and AFA-III, and F1845 fimbriae of uropathogenic and diarrhea-associated Escherichia coli belong to a family of hemagglutinins with Dr receptor recognition. Infect Immun. 1990;58:279–281. doi: 10.1128/iai.58.1.279-281.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orskov I, Orskov F. Serologic classification of fimbriae. Curr Top Microbiol Immunol. 1990;151:71–90. [PubMed] [Google Scholar]
- 51.Otto G, Sandberg T, Marklund B I, Ullery P, Svanborg Eden C. Virulence factors and pap genotype in Escherichia coli isolates from women with acute pyelonephritis, with or without bacteremia. Clin Infect Dis. 1993;17:448–456. doi: 10.1093/clinids/17.3.448. [DOI] [PubMed] [Google Scholar]
- 52.Russo T, Stapleton A, Wenderoth S, Hooton T M, Stamm W E. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli causing recurrent urinary tract infections in young women. J Infect Dis. 1995;172:440–445. doi: 10.1093/infdis/172.2.440. [DOI] [PubMed] [Google Scholar]
- 53.Russo T A, Carlino U B, Mong A, Jodush S T. Identification of genes in an extraintestinal isolate of Escherichia coli with increased expression after exposure to human urine. Infect Immun. 1999;67:5306–5614. doi: 10.1128/iai.67.10.5306-5314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 55.Schubert S, Rakin A, Karch H, Carniel E, Heesemann J. Prevalence of the “high-pathogenicity island” of Yersinia species among Escherichia coli strains that are pathogenic to humans. Infect Immun. 1998;66:480–485. doi: 10.1128/iai.66.2.480-485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Senior D, Baker N, Cedergren B, Falk P, Larson G, Lindstedt R, C. S E. Globo-A—a new receptor specificity for attaching Escherichia coli. FEMS Lett. 1988;237:123–127. doi: 10.1016/0014-5793(88)80184-4. [DOI] [PubMed] [Google Scholar]
- 57.Senior D F, deMan P, Svanborg C. Serotype, hemolysin production, and adherence characteristics of strains of Escherichia coli causing urinary tract infection in dogs. Am J Vet Res. 1992;53:494–498. [PubMed] [Google Scholar]
- 58.Sokal R R, Sneath P H A. Principles of numerical taxonomy. W. H. San Francisco, Calif: Freeman; 1963. [Google Scholar]
- 59.Stapleton A E, Fennell C L, Wobbe C, Denton A, Phin P, Johnson J R, Andreu A, Stamm W E. Associations of Escherichia coli papG adhesin classes with urinary tract infection. Clin Infect Dis. 1997;25:440. [Google Scholar]
- 60.Stapleton A E, Stroud M R, Hakomori S I, Stamm W E. The globoseries glycosphingolipid sialosyl galactosyl globoside is found in urinary tract tissues and is a preferred binding receptor in vitro for uropathogenic Escherichia coli expressing pap-encoded adhesins. Infect Immun. 1998;66:3856–3861. doi: 10.1128/iai.66.8.3856-3861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stathopoulos C. Structural features, physiological roles, and biotechnological applications of the membrane proteases of the OmpT bacterial endopeptidase family: a micro-review. Membr Cell Biol. 1998;12:1–8. [PubMed] [Google Scholar]
- 62.Strömberg M, Marklund B I, Lund B, Ilver D, Hamers A, Gaastra W, Karlsson K A, Normark S. Host-specificity of uropathogenic Escherichia coli depends on differences in binding specificity to Galα1-4Gal-containing isoreceptors. EMBO J. 1990;9:2001–2010. doi: 10.1002/j.1460-2075.1990.tb08328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strömberg N, Nyholm P G, Pascher I, Normark S. Saccharide orientation at the cell surface affects glycolipid receptor function. Proc Natl Acad Sci USA. 1991;88:9340–9344. doi: 10.1073/pnas.88.20.9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stroud M R, Stapleton A E, Levery S B. The P histo-blood group-related glycosphingolipid sialosyl galactosyl globoside as a preferred binding receptor for uropathogenic Escherichia coli: isolation and structural characterization from human kidney. Biochemistry. 1998;37:17420–17428. doi: 10.1021/bi9814639. [DOI] [PubMed] [Google Scholar]
- 65.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Muray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Die I, van den Hondel C, Hamstra H J, Hoekstra W, Bergmans H. Studies on the fimbriae of an Escherichia coli O6:K2:H1:F7 strain: molecular cloning of a DNA fragment encoding a fimbrial antigen responsible for mannose-resistant hemagglutination of human erythrocytes. FEMS Microbiol Lett. 1983;19:77–82. [Google Scholar]
- 68.Wang G, Whittam T S, Berg C M, Berg D E. RAPD (arbitrary primer) PCR is more sensitive than multilocus enzyme electrophoresis for distinguishing related bacterial strains. Nucleic Acids Res. 1993;21:5930–5933. doi: 10.1093/nar/21.25.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Warren J W. Clinical presentations and epidemiology of urinary tract infections. In: Mobley H L T, Warren J W, editors. Urinary tract infections. Molecular pathogenesis and clinical management. Washington, D.C.: ASM Press; 1996. pp. 3–27. [Google Scholar]
- 70.Welch R A, Dellinger E P, Minsheu B, Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981;294:665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- 71.Westerlund B, Pere A, Korhonen T K, Järvinen A-K, Siitonen A, Williams P H. Characterisation of Escherichia coli strains associated with canine urinary tract infections. Res Vet Sci. 1987;42:404–406. [PubMed] [Google Scholar]
- 72.Whittam T S, Wolfe M L, Wilson R A. Genetic relationships among Escherichia coli isolates causing urinary tract infections in humans and animals. Epidemiol Infect. 1988;102:37–46. doi: 10.1017/s0950268800029666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilkinson G T. O-groups of E. coli in the vagina and alimentary tract of the dog. Vet Rec. 1974;94:105. doi: 10.1136/vr.94.6.105-a. [DOI] [PubMed] [Google Scholar]
- 74.Wilson R A, Keefe T J, Davis M A, Browning M T, Ondrusek K. Strains of Escherichia coli associated with urogenital disease in dogs and cats. Am J Vet Res. 1988;49:743–746. [PubMed] [Google Scholar]
- 75.Wooley R E, Blue J L. Quantitative and bacteriological studies of urine specimens from canine and feline urinary tract infections. J Clin Microbiol. 1976;4:326–329. doi: 10.1128/jcm.4.4.326-329.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuri K, Nakata K, Katae H, Tsukamoto T, Hasegawa A. Serotypes and virulence factors of Escherichia coli strains isolated from dogs and cats. J Vet Med Sci. 1998;61:37–40. doi: 10.1292/jvms.61.37. [DOI] [PubMed] [Google Scholar]
- 77.Yuri K, Nakata K, Katae H, Yamamoto S, Hasegawa A. Distribution of uropathogenic virulence factors among Escherichia coli strains isolated from dogs and cats. J Vet Med Sci. 1998;60:287–290. doi: 10.1292/jvms.60.287. [DOI] [PubMed] [Google Scholar]