Abstract
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the cause of coronavirus disease 2019 (COVID-19) which has emerged as a global health crisis. Recently, more than 50 different types of potential COVID-19 vaccines have been developed to elicit a strong immune response against SARS-CoV-2. However, genetic mutations give rise to the new variants of SARS-CoV-2 which is highly associated with the reduced effectiveness of COVID-19 vaccines. There is still no efficient antiviral agent to specifically target the SARS-CoV-2 infection and treatment of COVID-19. Therefore, understanding the molecular mechanisms underlying the pathogenesis of SARS-CoV-2 may contribute to discovering a novel potential therapeutic approach to the management of COVID-19. Recently, extracellular vesicle (EV)-based therapeutic strategies have received great attention on account of their potential benefits in the administration of viral diseases. EVs are extracellular vesicles containing specific biomolecules which play an important role in cell-to-cell communications. It has been revealed that EVs are involved in the pathogenesis of different inflammatory diseases such as cancer and viral infections. EVs are released from virus-infected cells which could mediate the interaction of infected and uninfected host cells. Hence, these extracellular nanoparticles have been considered a novel approach for drug delivery to mediate the treatment of a wide range of diseases including, COVID-19. EVs are considered a cell-free therapeutic strategy that could ameliorate the cytokine storm and its complications in COVID-19 patients. Furthermore, EV-based cargo delivery such as immunomodulatory agents in combination with antiviral drugs may have therapeutic benefits in patients with SARS-CoV-2 infection. In this review, we will highlight the potential of EVs as a therapeutic candidate in the diagnosis and treatment of COVID-19. Also, we will discuss the future perspectives regarding the beneficial effects of Evs in the development of COVID-19 vaccines.
Keywords: Extracellular vesicles, COVID-19, SARS-CoV-2, Therapeutics, Vaccine development
1. Introduction
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been declared a challenging pandemic by the World Health Organization (WHO) on March 11, 2020 [1]. To date, COVID-19 with over 600 million cases has become a serious health crisis all around the world due to the simple transmission through respiratory droplets [2]. COVID-19 is mainly characterized by dysregulation of immune responses, uncontrolled systemic inflammation, cytokine storm, and multi-organ dysfunctions [3]. However, the most frequent complication of COVID-19 is associated with respiratory diseases including, pneumonia, respiratory distress syndromes (RDS), and acute lung injury [4]. SARS-CoV-2 is a member of the coronaviruses family which are spherical RNA viruses enveloped with different viral proteins including, the spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins [5]. The internalization process of SARS-CoV-2 is mediated by the S protein that binds to the receptor-binding domain (RBD) of angiotensin-converting enzyme 2 (ACE2) receptors expressed by host cells. The fusion of the host and the viral membrane is facilitated with the cleavage of S protein into S1 and S2 subunits by the transmembrane serine protease (TMPRSS2) [6]. Besides, CD147 known as EMMPRIN or basigin (BSG), and furin-mediated S protein cleavage are considered the alternative internalization route of the SARS-CoV-2 virus [7]. In addition, integrins are identified as potential alternative receptors for viral entry to facilitate viral transmission and pathogenesis [8]. The blockade of virus entry is a promising therapeutic target in the prevention of COVID-19 [9]. Although the inhibitors of SARS-CoV-2 entry as well as different vaccines have been developed to prevent the SARS-CoV-2 infection and eradicate the COVID-19 pandemic. However, several mutations in the genome of SARS‐CoV‐2 lead to new variants of viruses such as the Alpha, Delta, and Omicron variants which are linked to the altered transmissibility and pathogenicity [10], [11]. For instance, the mutated protein in the Omicron variant results in the enhanced affinity to ACE2 associated with accelerated internalization and fusion process [12]. Therefore, discovering an efficient therapeutic approach for the treatment of SARS-CoV-2 infection and alleviating COVID-19-related symptoms is still of research interest.
Extracellular vesicles (EVs) are lipid-enclosed small particles with an endocytic origin that are produced and secreted by various immune and non-immune cells to the extracellular space in physiological and pathological states [13]. EVs are mainly classified into three subgroups microvesicles (MVs), exosomes, and apoptotic bodies (Abs) based on their characteristics including the biogenesis process, release mechanisms, and physiological functions [13]. EVs are membrane-bound and cell-derived cargos that contain signaling biomolecules such as proteins, microRNAs (miRNA), and metabolites depending on the cell from which they originate. These intercellular signalosomes can be transported through body fluid to convey specific cargo to the target cells. EVs mainly express membrane-bound and cytosolic proteins including, CD9, CD63, and CD81, integrins, heat shock proteins (HSP), actin, and flotillins [14]. Membrane-bound proteins, ligands, receptors, and adhesion molecules expressed on the exosomal membrane mediate cell interaction to exert their effects on target cells [15]. According to the evidence, EVs play a critical role in the pathophysiology of different diseases such as autoimmune disease, cardiovascular disease (CVD), cancer, and viral pathogenicity [16], [17], [18]. Nevertheless, there is controversial evidence regarding the role of EVs in the prevention or promotion of SARS-CoV-2 infectivity. For example, EVs can transfer ACE2 to recipient cells, which supports virus internalization and infection [19]. On the contrary, it has been demonstrated that circulating ACE2-expressing EVs can block different strains of SARS-CoV-2 [20]. In general, EVs implicate a significant modulatory role in immune responses by providing a plethora of signals to activate or suppress the immune system [21]. Furthermore, it has been shown EVs can be a potential tool to reduce the cytokine storm and alleviate the severity of COVID-19 [22], [23]. It is noteworthy that immunomodulatory drugs can be loaded into the EVs which may be an efficient approach for the treatment of COVID-19 infection alongside antiviral drugs. Hence, in this review, we will highlight the important role of EVs in viral infection and the potential application of these exo-transfer mediators in the alleviation of COVID‐19–associated pathologies ( Fig. 1).
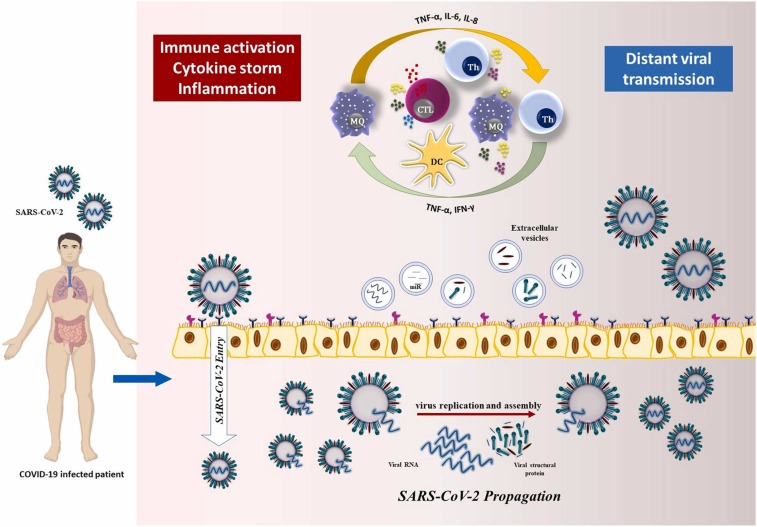
Fig. 1.
The pathogenesis mechanism of SARS-CoV-2 and exososme-related transmission. The internalization process of SARS-CoV-2 is primarily orchestrated by the interaction of S and ACE2 receptors expressed by human cells which are facilitated by the cleavage of S protein priming by TMPRSS2. Then, SARS-CoV-2 starts to replicate inside the cell and is released after assembling into new viruses which are followed by immune activation and cytokine storm. Through this process EVs containing viral particles can infect distant cells through secreted soluble factors, such as hormones and cytokines, to signal propagation.
2. Immunopathogenesis mechanism of COVID-19 and the role of the immune system in response to SARS-CoV-2
The SARS-CoV-2 virus invades the host cell through different receptors and cofactors [24]. The most important mediator of viral invasion is the ACE2 receptor which in turn leads to the entrance of the viral genome [25]. Subsequently, prompt viral replication occurs inside the infected cell and contributed to the dissemination of infection. In general, innate and adaptive immunity are two major arms of the immune system which rapidly respond to potential invaders. Innate immune cells such as monocytes, macrophages, dendritic cells, neutrophils, and natural killer (NK) cells are recognized as the first line of immune defense that plays a fundamental role against viruses, as well as SARS-CoV-2 [26], [27]. Different pattern recognition receptors (PRRs) have been identified as RNA and DNA sensors including, toll-like receptors (TLRs), a retinoic acid-inducible gene I (RIG-I), melanoma differentiation-associated protein 5 (MDA5), absent in melanoma 2 (AIM2), and cGAS [28]. These receptors as the key cellular sensors activated by a diverse range of viral components. Furthermore, SARS-CoV-2 infection may cause inflammatory cell death such as apoptosis, pyroptosis, and necroptosis in infected cells and consequently a release of damage-associated molecular patterns (DAMPs) [29]. Moreover, adaptive immune cells including, T and B cells mediate robust immune responses to viral infection. Following the recognition of viral particles, different series of downstream signaling pathways and various transcriptional mediators including, nuclear transcription factor κB (NF-κB), and interferon regulatory factors (IRFs) activate inside the cell. This process results in the expression of interferon-stimulated genes (ISGs) and type-I interferon (IFN-I) responses as well as the production of cytokines and chemokines such as interleukin-1 beta (IL-1β), IL-2, IL-6, IL-7, tumor necrosis factor-alpha (TNF-α), CCL-2, CCL-3, CXCL8, and CXCL10 [30], [31]. Immune dysregulation and various inflammatory pathways are key underlined mechanisms in the pathogenesis of SARS-CoV-2 [32]. Excessive and generalized cytokine production named cytokine storm or cytokine release syndrome (CRS) occurs as a consequence of unrestricted systemic inflammation and may cause multiple organ damage [33]. Inflammatory cytokine production especially high levels of IL-1β, IL-6, and TNFα significantly related to disease severity [34]. Taken together, immunoinflammatory responses are the important cause of damage to the different organs in COVID-19-infected patients.
3. Immune system and EVs
The SARS-CoV-2 primarily could infect the respiratory tract due to the high expression of ACE2 in type II alveolar epithelial cells. In general, ACE2 is significantly expressed by diverse cell types including, myocardial cells, liver, adipose tissue, small intestine, colon, and bladder indicating the susceptibility of different human tissues to SARS-CoV-2 infection [35]. Although the interaction of the SARS-CoV-2 S protein and ACE2 receptor is the main viral entry mechanism, however, the pathogenesis of COVID-19 is more likely orchestrated through immune dysregulation and impairment of interferon (IFN) immune responses [36]. It has been indicated that the M and N protein of SARS-CoV-2 suppresses the expression of the ISGs which represses IFN-I responses mediated through the RIG-I pathway resulting in immune evasion by SARS-CoV-2 [37], [38]. Moreover, several signaling pathways including, Janus kinase/signal transducer and activator of transcription (JAK/STAT), tyrosine kinases, mitogen-activated protein kinase (MAPK), and mammalian target of rapamycin (mTOR) are the underlying mechanisms associated with COVID-19 pathogenesis [39]. Uncontrolled cytokine production is identified as cytokine storm or cytokine release syndrome (CRS) triggered as a result of unrestricted systemic inflammation which may cause multiple organ damage [40]. Besides, the production of inflammatory cytokines and high levels of IL-1β, IL-6, and TNF-α are remarkably related to the severity of COVID-19 [41], [42]. The cytokine storm significantly occurs in the severe forms of COVID-19 which is characterized by high levels of interferon-gamma (IFN-γ), induced protein 10 (IP10), monocyte chemoattractant protein-1 (MCP-1), granulocyte-colony stimulating factor (G-CSF), TNF-α and IL-1β [43]. Neutrophilia and lymphopenia, hyperactivated T cells, reduced number of regulatory T cells (Tregs), decrease in CD8+ and CD4+ T cells expressing IFN-γ, pro-inflammatory cytokine production including, IL-6, IL-1β, IL-8, and TNF-α are related to the lung injury and severity of the disease [43], [44]. Therefore, immune dysregulation is the key mechanism in the pathogenesis of SARS-CoV-2.
EVs are recognized as novel intercellular communication tools through direct or indirect interactions. Cell-to-cell communication is mediated through gap junctions and protein-protein interactions, while EVs could facilitate distant cells through secreted soluble factors, such as hormones and cytokines, to signal propagation [45]. Moreover, EVs could attach to the surface of target cells through adhesion molecules including, tetraspanins, integrins, extracellular matrix proteins, immunoglobulin superfamily members, proteoglycans, and lectins [46]. It has been demonstrated that EVs are associated with cellular physiological processes including, unwanted protein removal and other genetic products, as well as mRNA and miRNA [47]. EVs are produced by different cell types and play a dual role in immune stimulation or suppression which regulate the immune responses [48]. These extracellular vesicles are involved in antigen presentation independent of the antigen-presenting cells (APCs) or interactions with T cells by providing the MHC-peptide signaling for T cell activation, through direct and indirect mechanisms as well as cross-dressing pathways [49]. Also, EVs enriched with chemokines effectively promote the recruitment of lymphocytes [50]. Li N, et al. have demonstrated that EVs derived from prostate cancer cell lines upregulate the expression of CXCR4 which is mediated by the TLR2/NF-κB pathway. This process results in the recruitment of myeloid-derived suppressor cells (MDSCs) into the tumor microenvironment [51]. In contrast, it has been revealed that EVs play an immunosuppressive role in different types of cancers [52]. For example, EVs bearing mediators of apoptosis such as Fas ligand (FasL) mediates apoptosis of CD8+ T cells [53]. Klibi et al. have demonstrated that EVs containing galectin-9 secreted by Epstein-Barr virus-infected nasopharyngeal carcinoma cells mediate the immunosuppression through apoptosis in EBV-specific CD4+ T cells [54]. Plasma-derived EVs are associated with immunosuppression by impacting immune cells’ function. It has been demonstrated that plasma EVs bearing IL-12 and IL-4 could enhance the Th1/Th2 ratio [50]. Moreover, EVs are bearing different anti-apoptotic mediators as well as microRNAs which mediate the inhibition of apoptosis. For instance, EVs enriched with hsa-miR-7–5p potentially inhibit Bad and activation of the CGMP-PKG pathway which mediates the apoptosis of T cells [55]. Also, plasma-derived EVs containing PD-1 ligands (PDL-1 and PDL-2) synergistically enhance the interaction of EVs and lymphocytes which inhibit the activation of T cells [56]. Similarly, Kim et al. found that plasma EVs carrying MHC II and CD11b suppress antigen-specific immune responses partially through Fas/FasL-dependent pathways [57]. On the other hand, miR-34a, miR-122 and, miR-146a in plasma-derived EVs upregulate the production of pro-inflammatory cytokines including IL-6, IL-1 β, TNF-α and, MIP-2 by macrophages via activation of TLR7-MyD88 pathway. This process induces immune activation by the macrophages and neutrophils migration [50]. Taken together, understanding the molecular mechanisms of EVs could be beneficial in affecting several obstacles toward immunotherapy strategies.
4. The role of EVs in viral infection and COVID-19
EVs are the smallest type of extracellular vesicles that recently received great consideration due to their functional role in the recipient cells. These mediators originate from different cells through endocytic pathways which are actively important in health and disease [58]. EVs can move across the whole body and can be found in different body fluids. It has been demonstrated that the composition of EVs varies in different viral infections and other intracellular pathogens. viral recognition and propagation in host cells are influenced by EVs derived from virus-infected cells [59]. EVs can dynamically influence host cells using different pathways. Cargo delivery is considered the main mechanism to affect recipient cells through the fusion of membranes and the transportation of functional molecules to target cells [60]. The EVs and host cell membrane fusion is mediated by clatherin-dependent, caveolin, and lipid raft as well as phagocytosis and micropinocytosis internalizing mechanisms. This process can be influenced by proteins and glycoproteins expressed by Evs and the recipient cell surface. Besides, EVs could attach to the surface protein such as integrins which may result in the activation of the intracellular signaling pathways [61]. EVs could play a dual role in the inhibition or progression of viral pathogenesis. Based on the evidence, in different viral infections, including, human immunodeficiency virus type-1 (HIV-1), human T cell lymphotropic virus (HTLV), Dengue virus, and hepatitis C virus (HCV), EVs which are derived from viral-infected cells are containing regulatory molecules and modulating the cellular processes [62]. Kaposi’s sarcoma virus (KSV) and Epstein-Barr virus (EBV) are recruiting the exosomal trafficking machinery to secrete microRNAs (miRNA), messenger RNAs (mRNA), and small non-protein-coding RNAs to proteins to modulate special gene expression in target recipient cells. EBV-infected cells release EVs enriched with dUTPaset that can lead to the activation of transcriptional factors such as NF‐κB signal transduction and cytokine production. of macrophage cytokine secretion [63]. Furthermore, EVs derived from HCV- and Zika virus-infected cells mediate the cytokine release, especially INF-α production by macrophages and monocytes, respectively [64].
SARS-CoV-2 is a member of the coronavirus family which shares a great sequential homology with SARS-CoV and MERS-CoV. SARS-CoV-2 viruses can hijack ACE2-positive human type II alveolar cells It has been demonstrated that EVs-mediated mechanisms potentially contribute to the SARS-CoV-2, and SARS-CoV transmission to neighbor cells and tissues, distant organs through systemic circulation and vascular system. EVs membrane tetraspanin facilitates viral entry to the host cells. Several types of viruses use EV machinery to propagate and viral spread in the human body. It has recently been investigated that SARS-CoV-2 infected lung epithelial cells can release EVs bearing viral particles which could facilitate the viral spread into cardiomyocytes and expression of inflammation-related genes [65]. Therefore, it is reasonable to presume that EVs harboring SARS-CoV-2 RNA are an indirect mechanism for viral entry.
5. EVs as a diagnostic tool for COVID-19
EVs are important intercellular transportation systems that are secreted by different cells in health or disease indicating the pathophysiological states. Due to the biochemical characteristics of EVs including simple isolation, high stability, and easy storage, EVs could be served as appropriate diagnostic biomarkers [66]. Recently, it has been proposed that exosomal-miRNA could be considered as a biomarker to monitor patients with hepatitis B virus (HBV) infection. Hu Z, et al. have shown that EVs enriched with miR-142–3p secreted by tumor cells and M1 macrophages in HBV-infected patients with hepatocellular carcinoma [67]. Furthermore, EVs derived from HCV-infected cells are composed of HCV RNA, argonaute-2 (Ago2), HSP90, and miR-122 potentially could enhance the stability of HCV RNA mediating the HCV proliferation [68], [69]. Hence, SARS-CoV-2-infected cells may release EVs with specific proteins that could be considered critical biomarkers for the monitoring of COVID-19. The bulk of research is hinting at the presence of subgenomic SARS-CoV-2 RNAs which may indicate the active COVID-19 infection [70], [71], [72]. Nevertheless, a single study by Soren A, et al. indicated that subgenomic SARS-CoV-2 RNAs could not be considered a reliable biomarker of the active form of SARS-CoV-2 infection [73]. Moreover, another in-vitro study has shown that EVs bearing viral genomes may trigger immune activation through the cGAS/STING and IRF3-dependent pathways [74]. It has been demonstrated that the circulating EVs bearing self- and viral antigens are increased during coronavirus infections which indicates COVID-19 virus-infected cells potentially release EVs containing virus particles [16], [75]. Barberis E, et al. have shown that circulating EVs containing SARS-CoV-2 RNA are strongly contributed to inflammation, coagulation as well as immunomodulation in COVID-19-infected patients [76]. SARS-CoV-2 infected lung epithelial cells could produce EVs enriched with viral particles which facilitate the viral transmission to cardiomyocytes and subsequent inflammation [77]. It has also been indicated in an in-vitro study that circulating EVs enriched with ACE2 could potentially prevent SARS-CoV-2 infection by interaction with the viral S protein [20]. Therefore, it can be perceived that antiviral EV proteins are recognized as an early biomarker for the diagnosis of COVID-19. It has been suggested different exosomal proteins including, CRP, A1AG1, A1AG2, CXCL7, SAMP, and ZA2G proteins could be used as an efficient approach for the diagnosis and management of COVID-19 disease [76]. Therefore, EVs are widely accepted biomarkers for clinical diagnosis in various diseases including, COVID-19.
6. Therapeutic application of EVs in COVID-19
Although different vaccines have been developed for the prevention of COVID-19, however, there are still persistent challenges to finding effective therapeutic solutions for the treatment of infection caused by recent SARS-CoV-2 variants. Recently, mesenchymal stem cells draw a lot of interest in regenerative medicine with plenty of beneficial effects in different pathological states including CVD, cancer, autoimmune disease, and viral infections. For instance, the preliminary results of phase I to III clinical trials have indicated that bone marrow-derived mesenchymal stromal cells have promising efficacy in the treatment of patients with severe COVID-19 [78]. Interestingly, cell-free-based therapies avoid cell toxicity and undesirable side effects, which have become an advantageous strategy in the treatment of a wide range of diseases [79]. EVs are extracellular vehicles containing specific cargo including mRNA, non-coding RNAs, DNA, and proteins from cells of origin. Based on the evidence, EVs transport biomolecules and specific signals to neighboring cells, mediating the reprogramming of recipient cells to control essential cellular functions [80]. These nano-sized vesicles play paradoxical effects on the immune responses in COVID-19, which could either promote SARS-CoV-2 pathogenesis or be beneficial in the treatment of the disease [49]. The potential role of EVs as drug delivery in lung diseases such as asthma chronic obstructive pulmonary disease (COPD) and acute respiratory distress syndrome (ARDS) have been evaluated [81]. Therefore, it would be beneficial to investigate the therapeutic and pharmacological effects of EVs in the treatment and management of COVID-19.
The mesenchymal stem cell-derived EVs are considered an ideal therapeutic strategy for the management of different diseases due to their characteristics including high stability and biocompatibility, low immunogenicity, easy storage, and ability to cross the blood-brain barrier [82]. Basalova N, et al. have reported that mesenchymal stem cell-derived EVs prevent fibroblast-to-myofibroblast transition which might inhibit the development of tissue fibrosis [83]. Also, mesenchymal stem cell-derived EVs mediate lung regeneration in animal models of bronchopulmonary dysplasia by reducing the levels of pro-inflammatory cytokines and macrophage immunomodulation [84], [85], [86]. Furthermore, mesenchymal stem cell-derived EVs containing FGF2 could increase the proliferation of lung epithelial cells [87]. The administration of EVs as immunomodulator agents could decrease the proinflammatory mechanisms and cytokine storm in COVID-19 patients and prevent ARDS pathogenesis [88]. Besides, Worthington EN, et al. have demonstrated the potential of EVs to upregulate the anti-inflammatory cytokine levels which could impact lung injury severity [89]. It has been demonstrated that mesenchymal stem cell-derived EVs enriched miR-146a target specific cells which could prevent the inflammation of cardiomyocytes and facilitate cardiac repair [51]. Also, Li et al. have indicated that transporting of anti-apoptotic miR-21–5p using mesenchymal stem cell-derived EVs potentially has a protective effect against oxidative stress which alleviate ischemia/reperfusion lung injury in the mouse model [90]. EVs containing miR-155 and/or miR-146a which are produced by primary bone marrow-derived dendritic cells play anti-inflammatory effects [91]. Besides, adipose-derived stem cells are frequent types of mesenchymal stem cells characterized by immunomodulatory functions, low immunogenicity, ability to differentiate into various cell types, cell regeneration, and repair of damaged cells. In addition, adipose-derived stem cells can regulate immune responses through EV secretion, providing a microenvironment to modulate inflammation [92]. Zhu et al. have presented the evidence that EVs released from adipose-derived stem cells potentially reduce lung inflammation and injury caused by cigarette smoke through inhibition of alveolar macrophage pyroptosis [93]. Besides, adipose-mesenchymal stem cell-derived EVs represent a capacity to protect against acute lung injury in a mouse model of sepsis [94]. Another study has shown that EVs derived from mesenchymal stem cells are capable to inhibit IL-27 produced by macrophages which potentially alleviates lung injury in sepsis mice model [95]. Therefore, adipose-derived stem cells are considered a potential therapeutic candidate in the treatment of COVID-19 [96]. Hence, mesenchymal stem cell-derived EVs exert anti-inflammatory and immunomodulatory effects, as well as tissue regeneration capacity, indicating the significant therapeutic potential for the management and treatment of SARS-CoV-2.
Based on the evidence, several clinical trials are currently registered to indicate the safety and efficiency of EVs for the treatment of COVID-19 patients ( Table 1) [97]. A phase I randomized clinical trial is evaluating the safety and efficiency of CD24-EVs in 35 patients with COVID-19 infection (NCT04747574). A phase II randomized clinical trial evaluated 155 patients with severe COVID-19 pneumonia who are treated by inhalation of CD24 overexpressed EVs to prevent clinical deterioration (NCT04969172). Another nonrandomized cohort study has indicated the safety of bone marrow mesenchymal stem cell-derived EVs in improving the cytokine storm and alleviation of pneumonia features in COVID-19 patients [98]. In another clinical trial, umbilical cord mesenchymal stem cell-derived EVs were administered intravenously to treat severe COVID-19 patients (ISRCTN33578935). It has been demonstrated that the administration of mesenchymal stem cell-derived EVs to COVID-19 patients with pulmonary fibrosis via inhalation is significantly associated with lung repair in those patients (NCT04276987). Another phase II clinical trial investigated the safety and efficiency of EVs inhalation in 90 individuals with COVID-19 (NCT04602442). Also, the ongoing phase I and II clinical trials are evaluating the safety and efficiency of EVs inhalation in 90 individuals with SARS-CoV-2-associated pneumonia (NCT04491240). Moreover, the phase I clinical trial is investigating the COVID-19-specific T cell-derived EVs in 60 patients with SARS-CoV-2 infection. 120 participants with COVID-19-associated ARDS are treated with extracellular vesicles in phase II clinical trial (NCT04493242).
Table 1.
Clinical trial EVs-based therapeutics for the treatment of COVID-19.
| EVs source | Administration route | Number of participants | Outcome measurement | Reference | Developmental status |
|---|---|---|---|---|---|
| Mesenchymal stem cell | Inhalation | 30 | Adverse reaction, Time to Clinical Recovery, blood oxygen saturation, level of inflammatory markers | NCT04491240 | Phase I and II |
| Mesenchymal stem cell | Inhalation | 24 | Adverse reactions, Time to Clinical Recovery, level of inflammatory markers | NCT04276987 | Phase I |
| Mesenchymal stem cell | Inhalation | 90 | Adverse reaction, Time to Clinical Recovery, blood oxygen saturation, level of inflammatory markers | NCT04602442 | Phase II |
| Bone Marrow Mesenchymal Stem Cell | Intravenous infusion | 120 | Time to Clinical Recovery, Neutrophil count, level of inflammatory markers | NCT04493242 | Phase II |
| Unavailable | inhalation | 155 | blood oxygen saturation, level of inflammatory markers | NCT04969172 | Phase II |
| T-RExTM-293 cells | inhalation | 35 | Adverse reaction, Time to Clinical Recovery, blood oxygen saturation, level of inflammatory markers | NCT04747574 | Phase I |
| T cells | inhalation | 60 | Adverse reaction, Time to Clinical Recovery | NCT04389385 | Phase I |
| Placental Mesenchymal Stem Cell | Intravenous infusion | 64 | Adverse reaction, Time to Clinical Recovery, blood oxygen saturation | ISRCTN33578935 | Phase II |
EVs are identified as a vehicle for drug delivery to infected cells due to their competence to preserve the encapsulated material from degradation and immune response [99]. Also, EVs can cross the blood-brain barrier to transfer the encapsulated cargo to the brain through an endocytic mechanism [100]. EVs loaded with anti-inflammatory drugs could also be considered an effective approach to dealing with COVID-19 disease. A previous study demonstrated that intranasal delivery of two anti-inflammatory drugs encapsulated in EVs could selectively result in the apoptosis of microglial cells and potentially treat various brain inflammation in animal models [101]. The EVs containing immunomodulatory materials in combination with antiviral drugs can be used as a novel tool for treating COVID-19. For instance, EVs containing heparin, which exerts antiviral and anticoagulant features, have served as intrapulmonary drug delivery in the management of COVID-19 patients in a clinical setting [102]. Besides, EVs are the potential to be used as a vehicle for Remdesivir delivery in the treatment of patients with severe COVID-19 [103]. All drugs approved by the food and drug administration for the treatment of COVID-19 infection such as remdesivir can be encapsulated by EVs [104].
On the other hand, EVs can be used as a potential virus-free vaccine by presenting specific viral antigens and induction of a strong immune response. Animal studies and transcriptomics analysis have indicated the off-toxicity of EVs with no hepatotoxicity or a proinflammatory cytokine response [105], [106]. For instance, loading the viral peptides to EVs derived from myeloid cells mediate the activation of CD8+ T cells and the production of IFN-γ [107]. It has also been indicated that EV-based vaccines trigger high antibody-mediate immune responses in comparison with patients with SARS. Based on previous reports, the anti-S protein vaccines potentially stimulate the production of anti-S antibodies as well as increased EVs containing S protein. It has also been demonstrated that a chimeric protein has been developed using S protein and EVs [108]. Despite more than 50 COVID-19 vaccines including live-attenuated and inactivated viruses, the development of different engineered EV-based vaccines by loading full or modified mRNAs/proteins is of paramount importance. It has been demonstrated that EV-based vaccines potentially induce high levels of antibodies in comparison with COVID-19-infected individuals [109]. Currently, EV-based vaccines are produced by Biotechnology companies which are composed of recombinant SARS-CoV-2 S, M, E, and N proteins that trigger prolonged immunity with strong neutralizing antibodies and T cell responses. Although, the development of EV-based COVID-19 vaccines is difficult in terms of scalability and characterization of immune responses. However, these type of vaccines is beneficial in conferring long-lasting immunity with no risk of recurrence of vaccine-induced virulence. Also, EV-based vaccines are considered the best carriers for viral antigens which could present antigens in their native state [110]. Hence, it could be perceived that EVs-based could be administered to patients with COVID-19. Currently, anti-SARS-CoV-2 vaccines are engineered using S protein expressed on EVs surface or carrying mRNA of viral proteins.
7. Conclusion
EVs are nanosized extracellular vesicles produced by different cells that could be engineered for antiviral therapies as well as COVID-19. EVs secreted locally or systematically play a fundamental role in pathological states of different diseases by signal transduction. According to the evidence, EVs may contribute to the propagation and spread of SARS-CoV-2 infection on account of CD9 and ACE2 expression. Also, EVs may have beneficial functions by transferring specific cargo in the treatment of diseases such as COVID-19. EVs are cell-free therapeutic approaches with low toxicity that have been recently recognized as state-of-the-art therapeutic candidates in the treatment of inflammatory diseases including, SARS-CoV-2 infection. It is noteworthy that EVs can control the cytokine storm and alleviate the complication of COVID-19. Besides, the advantages of EVs administration proposed these extracellular vehicles as an ideal candidate to improve the patient's health. Although the production of EVs, purification, and application of these extracellular vesicles are still considered the limitations of EVs-based treatments. Nevertheless, EVs are widely interested as biomarkers, immunomodulators, therapeutics, and vaccines in industries. Currently, ongoing research is implicated to exploit the advantage of EVs in the reduction of cytokine storm and alleviate the complication of SARS-CoV-2 infection. In conclusion, further pre-clinical and clinical analysis is required to evaluate the safety and effectiveness of EVs-based therapy in COVID-19 and other viral infections as well as to clarify the long-term outcomes of these kinds of treatments.
CRediT authorship contribution statement
MI and MA: Conception, design and inviting co-authors to participate. M M and M O: Writing original manuscript draft. HE GG, N JJ, A H, Y B and K M: Review and editing of manuscript critically for important intellectual content and provided comments and feedback for the scientific contents of the manuscript. All authors read, revised and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
None.
References
- 1.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Bio-Med.: Atenei Parm. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khorramdelazad H., Kazemi M.H., Azimi M., Aghamajidi A., Mehrabadi A.Z., Shahba F., Aghamohammadi N., Falak R., Faraji F., Jafari R. Type-I interferons in the immunopathogenesis and treatment of Coronavirus disease 2019. Eur. J. Pharmacol. 2022;927 doi: 10.1016/j.ejphar.2022.175051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front. Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krynytska I., Marushchak M., Birchenko I., Dovgalyuk A., Tokarskyy O. COVID-19-associated acute respiratory distress syndrome versus classical acute respiratory distress syndrome (a narrative review) Iran. J. Microbiol. 2021;13:737–747. doi: 10.18502/ijm.v13i6.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agostinis C., Mangogna A., Balduit A., Aghamajidi A., Ricci G., Kishore U., Bulla R. COVID-19, pre-eclampsia, and complement system. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.775168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson C.B., Farzan M., Chen B., Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022;23:3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rangu R., Wander P.L., Barrow B.M., Zraika S. Going viral in the islet: mediators of SARS-CoV-2 entry beyond ACE2. J. Mol. Endocrinol. 2022;69:R63–R79. doi: 10.1530/JME-21-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sigrist C.J., Bridge A., Le Mercier P. A potential role for integrins in host cell entry by SARS-CoV-2. Antivir. Res. 2020;177 doi: 10.1016/j.antiviral.2020.104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chitsike L., Duerksen-Hughes P. Keep out! SARS-CoV-2 entry inhibitors: their role and utility as COVID-19 therapeutics. Virol. J. 2021;18:154. doi: 10.1186/s12985-021-01624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., Ludden C., Reeve R., Rambaut A., Peacock S.J., Robertson D.L. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. (C.-G.U. Consortium) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorente-González M., Suarez-Ortiz M., Landete P. Evolution and clinical trend of SARS-CoV-2 variants. Open Respir. Arch. 2022;4(2) doi: 10.1016/j.opresp.2022.100169. Epub 2022 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lupala C.S., Ye Y., Chen H., Su X.D., Liu H. Mutations on RBD of SARS-CoV-2 Omicron variant result in stronger binding to human ACE2 receptor. Biochem. Biophys. Res. Commun. 2022;590:34–41. doi: 10.1016/j.bbrc.2021.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doyle L.M., Wang M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8 doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurung S., Perocheau D., Touramanidou L., Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021;19:47. doi: 10.1186/s12964-021-00730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meldolesi J. Exosomes and ectosomes in intercellular communication. Curr. Biol. 2018;28:R435–R444. doi: 10.1016/j.cub.2018.01.059. [DOI] [PubMed] [Google Scholar]
- 16.Gurunathan S., Kang M.H., Kim J.-H. Diverse effects of exosomes on COVID-19: a perspective of progress from transmission to therapeutic developments. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.716407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.K.B. Neves, F.J. Rios, J. Sevilla-Montero, A.C. Montezano, R.M. Touyz, Exosomes, 2022 and the cardiovascular system: role in cardiovascular health and disease. The Journal of Physiology n/a. [DOI] [PMC free article] [PubMed]
- 18.Caobi A., Nair M., Raymond A.D. Extracellular vesicles in the pathogenesis of viral infections in humans. Viruses. 2020;12 doi: 10.3390/v12101200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J., Chen S., Bihl J. Exosome-mediated transfer of ACE2 (Angiotensin-converting enzyme 2) from endothelial progenitor cells promotes survival and function of endothelial cell. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/4213541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Shennawy L., Hoffmann A.D., Dashzeveg N.K., McAndrews K.M., Mehl P.J., Cornish D., Yu Z., Tokars V.L., Nicolaescu V., Tomatsidou A., Mao C., Felicelli C.J., Tsai C.-F., Ostiguin C., Jia Y., Li L., Furlong K., Wysocki J., Luo X., Ruivo C.F., Batlle D., Hope T.J., Shen Y., Chae Y.K., Zhang H., LeBleu V.S., Shi T., Swaminathan S., Luo Y., Missiakas D., Randall G.C., Demonbreun A.R., Ison M.G., Kalluri R., Fang D., Liu H. Circulating ACE2-expressing extracellular vesicles block broad strains of SARS-CoV-2. Nat. Commun. 2022;13:405. doi: 10.1038/s41467-021-27893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussain M.W.A., Jahangir S., Ghosh B., Yesmin F., Anis A., Satil S.N., Anwar F., Rashid M.H. Exosomes for regulation of immune responses and immunotherapy. J. Nanotheranostics. 2022;3:55–85. [Google Scholar]
- 22.Yan Y.Y., Zhou W.M., Wang Y.Q., Guo Q.R., Zhao F.X., Zhu Z.Y., Xing Y.X., Zhang H.Y., Aljofan M., Jarrahi A.M., Makabel B., Zhang J.Y. The potential role of extracellular vesicles in COVID-19 treatment: opportunity and challenge. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.699929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdelgawad M., Bakry N.S., Farghali A.A., Abdel-Latif A., Lotfy A. Mesenchymal stem cell-based therapy and exosomes in COVID-19: current trends and prospects. Stem Cell Res. Ther. 2021;12:469. doi: 10.1186/s13287-021-02542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matusiak M., Schürch C.M. Expression of SARS-CoV-2 entry receptors in the respiratory tract of healthy individuals, smokers and asthmatics. Respir. Res. 2020;21:252. doi: 10.1186/s12931-020-01521-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuo W., Zhao X. Natural killer cells play an important role in virus infection control: antiviral mechanism, subset expansion and clinical application. Clin. Immunol. 2021;227 doi: 10.1016/j.clim.2021.108727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diamond M.S., Kanneganti T.-D. Innate immunity: the first line of defense against SARS-CoV-2. Nat. Immunol. 2022;23:165–176. doi: 10.1038/s41590-021-01091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlee M., Hartmann G. Discriminating self from non-self in nucleic acid sensing. Nat. Rev. Immunol. 2016;16:566–580. doi: 10.1038/nri.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S., Zhang Y., Guan Z., Li H., Ye M., Chen X., Shen J., Zhou Y., Shi Z.-L., Zhou P., Peng K. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct. Target. Ther. 2020;5:235. doi: 10.1038/s41392-020-00334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan Y., Jiang X., Yang L., Chen L., Zeng X., Liu G., Tang Y., Qian C., Wang X., Cheng F., Lin J., Wang X., Li Y. SARS-CoV-2-specific immune response in COVID-19 convalescent individuals. Signal Transduct. Target Ther. 2021;6:256. doi: 10.1038/s41392-021-00686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricci D., Etna M.P., Rizzo F., Sandini S., Severa M., Coccia E.M. Innate immune response to SARS-CoV-2 infection: from cells to soluble mediators. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22137017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y.-y, Li B.-r, Ning B.-t. The comparative immunological characteristics of SARS-CoV, MERS-CoV, and SARS-CoV-2 coronavirus infections. Front. Immunol. 2020;11:2033. doi: 10.3389/fimmu.2020.02033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., Chen Y., Zhang Y. COVID-19: immunopathogenesis and Immunotherapeutics. Signal. Transduct. Target. Ther. 2020;5:128. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong J., Dong H., Xia Q.-S., Huang Z.-Y., Wang D.-K., Zhao Y., Liu W.-H., Tu S.-H., Zhang M.-M., Wang Q. Correlation analysis between disease severity and inflammation-related parameters in patients with COVID-19: a retrospective study. BMC Infect. Dis. 2020;20:1–7. doi: 10.1186/s12879-020-05681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beyerstedt S., Casaro E.B., Rangel É B. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis.: Off. Publ. Eur. Soc. Clin. Microbiol. 2021;40:905–919. doi: 10.1007/s10096-020-04138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ning Q., Wu D., Wang X., Xi D., Chen T., Chen G., Wang H., Lu H., Wang M., Zhu L., Hu J., Liu T., Ma K., Han M., Luo X. The mechanism underlying extrapulmonary complications of the coronavirus disease 2019 and its therapeutic implication. Signal Transduct. Target. Ther. 2022;7:57. doi: 10.1038/s41392-022-00907-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sui L., Zhao Y., Wang W., Wu P., Wang Z., Yu Y., Hou Z., Tan G., Liu Q. SARS-CoV-2 membrane protein inhibits type I interferon production through ubiquitin-mediated degradation of TBK1. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.662989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen K., Xiao F., Hu D., Ge W., Tian M., Wang W., Pan P., Wu K., Wu J. SARS-CoV-2 nucleocapsid protein interacts with RIG-I and represses RIG-mediated IFN-β production. Viruses. 2020;13 doi: 10.3390/v13010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basile M.S., Cavalli E., McCubrey J., Hernández-Bello J., Muñoz-Valle J.F., Fagone P., Nicoletti F. The PI3K/Akt/mTOR pathway: a potential pharmacological target in COVID-19. Drug Discov. Today. 2022;27:848–856. doi: 10.1016/j.drudis.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jamal M., Bangash H.I., Habiba M., Lei Y., Xie T., Sun J., Wei Z., Hong Z., Shao L., Zhang Q. Immune dysregulation and system pathology in COVID-19. Virulence. 2021;12:918–936. doi: 10.1080/21505594.2021.1898790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Del Valle D.M., Kim-Schulze S., Huang H.-H., Beckmann N.D., Nirenberg S., Wang B., Lavin Y., Swartz T.H., Madduri D., Stock A., Marron T.U., Xie H., Patel M., Tuballes K., Van Oekelen O., Rahman A., Kovatch P., Aberg J.A., Schadt E., Jagannath S., Mazumdar M., Charney A.W., Firpo-Betancourt A., Mendu D.R., Jhang J., Reich D., Sigel K., Cordon-Cardo C., Feldmann M., Parekh S., Merad M., Gnjatic S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabaan A.A., Al-Ahmed S.H., Muhammad J., Khan A., Sule A.A., Tirupathi R., Mutair A.A., Alhumaid S., Al-Omari A., Dhawan M., Tiwari R., Sharun K., Mohapatra R.K., Mitra S., Bilal M., Alyami S.A., Emran T.B., Moni M.A., Dhama K. Role of inflammatory cytokines in COVID-19 patients: a review on molecular mechanisms, immune functions, immunopathology and immunomodulatory drugs to counter cytokine storm. Vaccines. 2021;9 doi: 10.3390/vaccines9050436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazini L., Rochette L., Malka G. Exosomes contribution in COVID-19 patients’ treatment. J. Transl. Med. 2021;19:234. doi: 10.1186/s12967-021-02884-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montazersaheb S., Hosseiniyan Khatibi S.M., Hejazi M.S., Tarhriz V., Farjami A., Ghasemian Sorbeni F., Farahzadi R., Ghasemnejad T. COVID-19 infection: an overview on cytokine storm and related interventions. Virol. J. 2022;19:92. doi: 10.1186/s12985-022-01814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367 doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buzás E.I., Tóth E., Sódar B.W., Szabó-Taylor K. Molecular interactions at the surface of extracellular vesicles. Semin. Immunopathol. 2018;40:453–464. doi: 10.1007/s00281-018-0682-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beach A., Zhang H.G., Ratajczak M.Z., Kakar S.S. Exosomes: an overview of biogenesis, composition and role in ovarian cancer. J. Ovarian Res. 2014;7:14. doi: 10.1186/1757-2215-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hazrati A., Soudi S., Malekpour K., Mahmoudi M., Rahimi A., Hashemi S.M., Varma R.S. Immune cells-derived exosomes function as a double-edged sword: role in disease progression and their therapeutic applications. Biomark. Res. 2022;10:30. doi: 10.1186/s40364-022-00374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barros F.M., Carneiro F., Machado J.C., Melo S.A. Exosomes and immune response in cancer: friends or foes? Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao K., Jin J., Huang C., Li J., Luo H., Li L., Huang Y., Jiang Y. Exosomes derived from septic mouse serum modulate immune responses via exosome-associated cytokines. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.01560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu C., Xue J., Xu B., Zhang A., Qin L., Liu J., Yang Y. Exosomes Derived from miR-146a-5p-Enriched Mesenchymal Stem Cells Protect the Cardiomyocytes and Myocardial Tissues in the Polymicrobial Sepsis through Regulating MYBL1. Stem Cell. Int. 2021;2021 doi: 10.1155/2021/1530445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie Q.H., Zheng J.Q., Ding J.Y., Wu Y.F., Liu L., Yu Z.L., Chen G. Exosome-mediated immunosuppression in tumor microenvironments. Cells. 2022;11 doi: 10.3390/cells11121946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abusamra A.J., Zhong Z., Zheng X., Li M., Ichim T.E., Chin J.L., Min W.-P. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells, Mol. Dis. 2005;35:169–173. doi: 10.1016/j.bcmd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Klibi J., Niki T., Riedel A., Pioche-Durieu C., Souquere S., Rubinstein E., Le Moulec S., Guigay J., Hirashima M., Guemira F. Blood diffusion and Th1-suppressive effects of galectin-9–containing exosomes released by Epstein-Barr virus–infected nasopharyngeal carcinoma cells. Blood J. Am. Soc. Hematol. 2009;113:1957–1966. doi: 10.1182/blood-2008-02-142596. [DOI] [PubMed] [Google Scholar]
- 55.Deng J.N., Li Y.Q., Liu Y., Li Q., Hu Y., Xu J.Q., Sun T.Y., Xie L.X. Exosomes derived from plasma of septic patients inhibit apoptosis of T lymphocytes by down-regulating bad via hsa-miR-7-5p. Biochem. Biophys. Res. Commun. 2019;513:958–966. doi: 10.1016/j.bbrc.2019.04.051. [DOI] [PubMed] [Google Scholar]
- 56.Kawamoto E., Masui-Ito A., Eguchi A., Soe Z.Y., Prajuabjinda O., Darkwah S., Park E.J., Imai H., Shimaoka M. Integrin and PD-1 ligand expression on circulating extracellular vesicles in systemic inflammatory response syndrome and sepsis. Shock. 2019;52:13–22. doi: 10.1097/SHK.0000000000001228. [DOI] [PubMed] [Google Scholar]
- 57.Kim S.H., Bianco N.R., Shufesky W.J., Morelli A.E., Robbins P.D. MHC class II+ exosomes in plasma suppress inflammation in an antigen-specific and Fas ligand/Fas-dependent manner. J. Immunol. 1950;179(2007):2235–2241. doi: 10.4049/jimmunol.179.4.2235. [DOI] [PubMed] [Google Scholar]
- 58.Gurung S., Perocheau D., Touramanidou L., Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021;19:47. doi: 10.1186/s12964-021-00730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshikawa F.S.Y., Teixeira F.M.E., Sato M.N., Oliveira L.M.D.S. Delivery of microRNAs by extracellular vesicles in viral infections: could the news be packaged? Cells. 2019;8:611. doi: 10.3390/cells8060611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montecalvo A., Larregina A.T., Shufesky W.J., Stolz D.B., Sullivan M.L., Karlsson J.M., Baty C.J., Gibson G.A., Erdos G., Wang Z., Milosevic J., Tkacheva O.A., Divito S.J., Jordan R., Lyons-Weiler J., Watkins S.C., Morelli A.E. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mulcahy L.A., Pink R.C., Carter D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles. 2014;3:24641. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chahar H.S., Bao X., Casola A. Exosomes and their role in the life cycle and pathogenesis of RNA viruses. Viruses. 2015;7:3204–3225. doi: 10.3390/v7062770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ariza M.E., Rivailler P., Glaser R., Chen M., Williams M.V. Epstein-Barr virus encoded dUTPase containing exosomes modulate innate and adaptive immune responses in human dendritic cells and peripheral blood mononuclear cells. PLOS One. 2013;8 doi: 10.1371/journal.pone.0069827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martínez-Rojas P.P., Quiroz-García E., Monroy-Martínez V., Agredano-Moreno L.T., Jiménez-García L.F., Ruiz-Ordaz B.H. Participation of extracellular vesicles from zika-virus-infected mosquito cells in the modification of naïve cells' behavior by mediating cell-to-cell transmission of viral elements. Cells. 2020;9 doi: 10.3390/cells9010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Y. Kwon, S.B. Nukala, S. Srivastava, H. Miyamoto, N.I. Ismail, J. Rehman, S.-B. Ong, W.H. Lee, S.-G. Ong, Exosomes facilitate transmission of SARS-CoV-2 genome into human induced pluripotent stem cell-derived cardiomyocytes. 2020.
- 66.Zhang Y., Bi J., Huang J., Tang Y., Du S., Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020;15:6917–6934. doi: 10.2147/IJN.S264498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu Z., Yin Y., Jiang J., Yan C., Wang Y., Wang D., Li L. Exosomal miR-142-3p secreted by hepatitis B virus (HBV)-hepatocellular carcinoma (HCC) cells promotes ferroptosis of M1-type macrophages through SLC3A2 and the mechanism of HCC progression. J. Gastrointest. Oncol. 2022;13:754–767. doi: 10.21037/jgo-21-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Conrad K.D., Giering F., Erfurth C., Neumann A., Fehr C., Meister G., Niepmann M. microRNA-122 dependent binding of Ago2 protein to hepatitis C virus RNA is associated with enhanced RNA stability and translation stimulation. PLOS One. 2013;8 doi: 10.1371/journal.pone.0056272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roberts A.P.E., Lewis A.P., Jopling C.L. miR-122 activates hepatitis C virus translation by a specialized mechanism requiring particular RNA components. Nucleic Acids Res. 2011;39:7716–7729. doi: 10.1093/nar/gkr426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parker M.D., Lindsey B.B., Leary S., Gaudieri S., Chopra A., Wyles M., Angyal A., Green L.R., Parsons P., Tucker R.M., Brown R., Groves D., Johnson K., Carrilero L., Heffer J., Partridge D.G., Evans C., Raza M., Keeley A.J., Smith N., Filipe A.D.S., Shepherd J.G., Davis C., Bennett S., Sreenu V.B., Kohl A., Aranday-Cortes E., Tong L., Nichols J., Thomson E.C., Wang D., Mallal S., de Silva T.I. Subgenomic RNA identification in SARS-CoV-2 genomic sequencing data. Genome Res. 2021;31:645–658. doi: 10.1101/gr.268110.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Z., Ng R.W.Y., Lui G., Ling L., Chow C., Yeung A.C.M., Boon S.S., Wang M.H., Chan K.C.C., Chan R.W.Y., Hui D.S.C., Chan P.K.S. Profiling of SARS-CoV-2 subgenomic RNAs in clinical specimens. Microbiol. Spectr. 2022;10 doi: 10.1128/spectrum.00182-22. e00182-00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zou X., Mu S., Wang Y., Guo L., Ren L., Deng X., Li H., Zhao J., Zhang Y., Li H., Lu B., Huang C., Cao B. Characterization of two SARS-CoV-2 subgenomic RNA dynamics in severe COVID-19 patients. Virol. Sin. 2022;37:30–37. doi: 10.1016/j.virs.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alexandersen S., Chamings A., Bhatta T.R. SARS-CoV-2 genomic and subgenomic RNAs in diagnostic samples are not an indicator of active replication. Nat. Commun. 2020;11:6059. doi: 10.1038/s41467-020-19883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Torralba D., Baixauli F., Villarroya-Beltri C., Fernández-Delgado I., Latorre-Pellicer A., Acín-Pérez R., Martín-Cófreces N.B., Jaso-Tamame L., S Á., Iborra I., Jorge G., González-Aseguinolaza J., Garaude M., Vicente-Manzanares J.A., Enríquez M., Mittelbrunn F., Sánchez-Madrid Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat. Commun. 2018;9:2658. doi: 10.1038/s41467-018-05077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosell A., Havervall S., von Meijenfeldt F., Hisada Y., Aguilera K., Grover S.P., Lisman T., Mackman N., Thålin C. Patients with COVID-19 have elevated levels of circulating extracellular vesicle tissue factor activity that is associated with severity and mortality – brief report. Arterioscler., Thromb., Vasc. Biol. 2021;41:878–882. doi: 10.1161/ATVBAHA.120.315547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barberis E., Vanella V.V., Falasca M., Caneapero V., Cappellano G., Raineri D., Ghirimoldi M., De Giorgis V., Puricelli C., Vaschetto R., Sainaghi P.P., Bruno S., Sica A., Dianzani U., Rolla R., Chiocchetti A., Cantaluppi V., Baldanzi G., Marengo E., Manfredi M. Circulating exosomes are strongly involved in SARS-CoV-2 infection. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.632290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwon Y., Nukala S.B., Srivastava S., Miyamoto H., Ismail N.I., Jousma J., Rehman J., Ong S.-B., Lee W.H., Ong S.-G. Detection of viral RNA fragments in human iPSC cardiomyocytes following treatment with extracellular vesicles from SARS-CoV-2 coding sequence overexpressing lung epithelial cells. Stem Cell Res. Ther. 2020;11:514. doi: 10.1186/s13287-020-02033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grégoire C., Layios N., Lambermont B., Lechanteur C., Briquet A., Bettonville V., Baudoux E., Thys M., Dardenne N., Misset B., Beguin Y. Bone marrow-derived mesenchymal stromal cell therapy in severe COVID-19: preliminary results of a phase I/II clinical trial. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.932360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu C., Zhao L., Zhang L., Bao Q., Li L. Mesenchymal stem cell-based cell-free strategies: safe and effective treatments for liver injury. Stem Cell Res. Ther. 2020;11:377. doi: 10.1186/s13287-020-01895-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ginini L., Billan S., Fridman E., Gil Z. Insight into extracellular vesicle-cell communication: from cell recognition to intracellular fate. Cells. 2022;11 doi: 10.3390/cells11091375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Purghè B., Manfredi M., Ragnoli B., Baldanzi G., Malerba M. Exosomes in chronic respiratory diseases. Biomed. Pharmacother. 2021;144 doi: 10.1016/j.biopha.2021.112270. [DOI] [PubMed] [Google Scholar]
- 82.Martinez-Arroyo O., Ortega A., Forner M.J., Cortes R. Mesenchymal stem cell-derived extracellular vesicles as non-coding RNA therapeutic vehicles in autoimmune diseases. Pharmaceutics. 2022;14 doi: 10.3390/pharmaceutics14040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Basalova N., Sagaradze G., Arbatskiy M., Evtushenko E., Kulebyakin K., Grigorieva O., Akopyan Z., Kalinina N., Efimenko A. Secretome of mesenchymal stromal cells prevents myofibroblasts differentiation by transferring fibrosis-associated microRNAs within extracellular vesicles. Cells. 2020;9 doi: 10.3390/cells9051272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chaubey S., Thueson S., Ponnalagu D., Alam M.A., Gheorghe C.P., Aghai Z., Singh H., Bhandari V. Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor TSG-6. Stem Cell Res Ther. 2018;9:173. doi: 10.1186/s13287-018-0903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Willis G.R., Fernandez-Gonzalez A., Anastas J., Vitali S.H., Liu X., Ericsson M., Kwong A., Mitsialis S.A., Kourembanas S. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am. J. Respir. Crit. Care Med. 2018;197:104–116. doi: 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Porzionato A., Zaramella P., Dedja A., Guidolin D., Van Wemmel K., Macchi V., Jurga M., Perilongo G., De Caro R., Baraldi E., Muraca M. Intratracheal administration of clinical-grade mesenchymal stem cell-derived extracellular vesicles reduces lung injury in a rat model of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019;316:L6–l19. doi: 10.1152/ajplung.00109.2018. [DOI] [PubMed] [Google Scholar]
- 87.Kim Y.S., Kim J.Y., Cho R., Shin D.M., Lee S.W., Oh Y.M. Adipose stem cell-derived nanovesicles inhibit emphysema primarily via an FGF2-dependent pathway. Exp. Mol. Med. 2017;49 doi: 10.1038/emm.2016.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abraham A., Krasnodembskaya A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl. Med. 2020;9:28–38. doi: 10.1002/sctm.19-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Worthington E.N., Hagood J.S. Therapeutic use of extracellular vesicles for acute and chronic lung disease. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21072318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Jw, Wei L., Han Z., Chen Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. Eur. J. Pharmacol. 2019;852:68–76. doi: 10.1016/j.ejphar.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 91.Alexander M., Hu R., Runtsch M.C., Kagele D.A., Mosbruger T.L., Tolmachova T., Seabra M.C., Round J.L., Ward D.M., O'Connell R.M. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat. Commun. 2015;6:7321. doi: 10.1038/ncomms8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chang T.H., Wu C.S., Chiou S.H., Chang C.H., Liao H.J. Adipose-derived stem cell exosomes as a novel anti-inflammatory agent and the current therapeutic targets for rheumatoid arthritis. Biomedicines. 2022;10 doi: 10.3390/biomedicines10071725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu Z., Lian X., Su X., Wu W., Zeng Y., Chen X. Exosomes derived from adipose-derived stem cells alleviate cigarette smoke-induced lung inflammation and injury by inhibiting alveolar macrophages pyroptosis. Respir. Res. 2022;23:5. doi: 10.1186/s12931-022-01926-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Deng H., Zhu L., Zhang Y., Zheng L., Hu S., Zhou W., Zhang T., Xu W., Chen Y., Zhou H., Li Q., Wei J., Yang H., Lv X. Differential lung protective capacity of exosomes derived from human adipose tissue, bone marrow, and umbilical cord mesenchymal stem cells in sepsis-induced acute lung injury. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/7837837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang X., Liu D., Zhang X., Yang L., Xia Z., Zhang Q. Exosomes from adipose-derived mesenchymal stem cells alleviate sepsis-induced lung injury in mice by inhibiting the secretion of IL-27 in macrophages. Cell Death Discov. 2022;8:18. doi: 10.1038/s41420-021-00785-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mazini L., Ezzoubi M., Malka G. Overview of current adipose-derived stem cell (ADSCs) processing involved in therapeutic advancements: flow chart and regulation updates before and after COVID-19. Stem Cell Res. Ther. 2021;12:1. doi: 10.1186/s13287-020-02006-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rogers C.J., Harman R.J., Bunnell B.A., Schreiber M.A., Xiang C., Wang F.S., Santidrian A.F., Minev B.R. Rationale for the clinical use of adipose-derived mesenchymal stem cells for COVID-19 patients. J. Transl. Med. 2020;18:203. doi: 10.1186/s12967-020-02380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Elsharkasy O.M., Nordin J.Z., Hagey D.W., de Jong O.G., Schiffelers R.M., Andaloussi S.E.L., Vader P. Extracellular vesicles as drug delivery systems: why and how? Adv. Drug Deliv. Rev. 2020;159:332–343. doi: 10.1016/j.addr.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 100.Chen C.C., Liu L., Ma F., Wong C.W., Guo X.E., Chacko J.V., Farhoodi H.P., Zhang S.X., Zimak J., Ségaliny A., Riazifar M., Pham V., Digman M.A., Pone E.J., Zhao W. Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell. Mol. Bioeng. 2016;9:509–529. doi: 10.1007/s12195-016-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhuang X., Xiang X., Grizzle W., Sun D., Zhang S., Axtell R.C., Ju S., Mu J., Zhang L., Steinman L., Miller D., Zhang H.G. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther.: J. Am. Soc. Gene Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Van Haren F.M., Page C., Laffey J.G., Artigas A., Camprubi-Rimblas M., Nunes Q., Smith R., Shute J., Carroll M., Tree J. Nebulised heparin as a treatment for COVID-19: scientific rationale and a call for randomised evidence. Crit. Care. 2020;24:1–11. doi: 10.1186/s13054-020-03148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., D'Arminio Monforte A., Ismail S., Kato H., Lapadula G., L'Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A.O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S.K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate use of remdesivir for patients with severe covid-19. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mendt M., Kamerkar S., Sugimoto H., McAndrews K.M., Wu C.C., Gagea M., Yang S., Blanko E.V.R., Peng Q., Ma X., Marszalek J.R., Maitra A., Yee C., Rezvani K., Shpall E., LeBleu V.S., Kalluri R. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018;3 doi: 10.1172/jci.insight.99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saleh A.F., Lázaro-Ibáñez E., Forsgard M.A., Shatnyeva O., Osteikoetxea X., Karlsson F., Heath N., Ingelsten M., Rose J., Harris J., Mairesse M., Bates S.M., Clausen M., Etal D., Leonard E., Fellows M.D., Dekker N., Edmunds N. Vol. 11. 2019. Extracellular vesicles induce minimal hepatotoxicity and immunogenicity; pp. 6990–7001. (Nanoscale). [DOI] [PubMed] [Google Scholar]
- 107.Admyre C., Johansson S.M., Paulie S., Gabrielsson S. Direct exosome stimulation of peripheral human T cells detected by ELISPOT. Eur. J. Immunol. 2006;36:1772–1781. doi: 10.1002/eji.200535615. [DOI] [PubMed] [Google Scholar]
- 108.Kuate S., Cinatl J., Doerr H.W., Uberla K. Exosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodies. Virology. 2007;362:26–37. doi: 10.1016/j.virol.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuate S., Cinatl J., Doerr H.W., Überla K. Exosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodies. Virology. 2007;362:26–37. doi: 10.1016/j.virol.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yoo K.H., Thapa N., Kim B.J., Lee J.O., Jang Y.N., Chwae Y.J., Kim J. Possibility of exosome‑based coronavirus disease 2019 vaccine. Mol. Med. Rep. 2022;25:1–9. doi: 10.3892/mmr.2021.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]