Abstract
Label-free technologies for isolating rare circulating cells in breast cancer patients are widely available; however, they are mostly validated on metastatic patient blood samples. Given the need to use blood-based biomarkers to inform on disease progression and treatment decisions, it is important to validate these technologies in non-metastatic patient blood samples. In this study, we specifically focus on a recently established label-free microfluidic technology Labyrinth and assess its capabilities to phenotype a variety of rare circulating tumor cells indicative of epithelial-to-mesenchymal transition as well as cancer-associated macrophage-like (CAML) cells. We specifically chose a patient cohort that is non-metastatic and selected to undergo neoadjuvant chemotherapy to assess the performance of the Labyrinth technology. We enrolled 21 treatment naïve non-metastatic breast cancer patients of various disease stages. Our results indicate that (i) Labyrinth microfluidic technology is successfully able to isolate different phenotypes of CTCs despite the counts being low. (ii) Invasive phenotypes of CTCs such as transitioning CTCs and mesenchymal CTCs were found to be present in high numbers in stage III patients as compared to stage II patients. (iii) As the total load of CTCs increased, the mesenchymal CTCs were found to be increasing. (iv) Labyrinth was able to isolate CAMLs with the counts being higher in stage III patients as compared to stage II patients. Our study demonstrates the ability of the Labyrinth microfluidic technology to isolate rare cancer-associated cells from the blood of treatment naïve non-metastatic breast cancer patients, laying the foundation for tracking oncogenic spread and immune response in patients undergoing neoadjuvant chemotherapy.
I. INTRODUCTION
Neoadjuvant chemotherapy (NAC) is used in breast cancer to reduce the need for mastectomies by shrinking the primary tumor.1,2 However, resistance to treatment can cause disease progression and promote distant organ metastasis.3,4 Therefore, longitudinal monitoring of NAC response is needed to identify resistant tumors early so that second-line therapies can be appropriately instituted. Currently, the response to chemotherapy is clinically determined by calculating the residual tumor burden at the time of surgery after completion of proposed chemotherapy. Clearly, identifying tumor resistance before completing the entire regimen of NAC would avoid unnecessary doses of toxic treatment, and predicting a complete pathological response may preclude the need for surgery. However, longitudinal monitoring using serial tissue biopsies is impractical. In contrast, routine blood draws are a standard practice in the clinic, making liquid biopsy an attractive approach to address these dilemmas. Thus, identifying blood-based biomarkers in breast cancer patients that can predict NAC response is essential for personalized cancer treatment.
Liquid biopsy is an effective approach to track circulating tumor cells (CTCs) in metastatic breast cancer patients.5–7 CTCs have been identified as critical determinants of the onset and advancement of metastasis.8–10 CTCs are the primary tumor cells that exit the breast parenchyma, enter the bloodstream, and survive systemic circulation. CTCs further rewire themselves and grow in distant organs, causing the spread of cancer. Since mortality in breast cancer patients undergoing neoadjuvant chemotherapy results from disease progression caused by metastasis,11,12 technologies evaluating CTCs in the blood of patients undergoing NAC are needed.
Breast cancer also initiates a significant host immune response, invoking inflammatory cells such as natural killer cells, T-cells, macrophages, etc.13,14 Intriguingly, recent studies show cancer-associated macrophage-like cells (CAMLs) in the blood of breast, prostate, pancreatic, esophageal, lung, and renal cell cancer patients.15–20 These reports suggest that CAMLs are either involved in engulfing tumor cells or aiding the transport of primary tumor cells during circulation.15–21 As CAMLs and CTCs are both present in the blood of cancer patients15–22 and represent the delicate balance between the oncogenic spread and host immune response, it is desirable to develop methods that enable simultaneous investigation of these cells. Such techniques will enable comprehensive profiling of rare circulating cells indicative of metastatic potential (CTCs) and host immunity (CAMLs), which can be a compelling means to predict NAC response in patients using routine blood draws.
Prior approaches to isolate CTCs from the blood of breast cancer patients undergoing NAC have used antibody-based markers.23–26 As shown in Table I, these affinity-based technologies use epithelial (E+) markers such as EpCAM (epithelial cellular adhesion molecule) and cytokeratin to isolate specific groups of CTCs. Given that tumor progression involves epithelial to mesenchymal transition and host immune response, capture and enumeration of only E+ CTCs remain a significant limitation of affinity-based techniques27–29 as shown in Table I. Indeed, studies show that CTCs also exhibit mesenchymal (M+ CTCs) markers such as vimentin, N-cadherin, and fibronectin and can stain positive for both E+ and M+ markers (E+M+ CTCs).30,31 It is crucial to enumerate the mesenchymal-type CTCs since they are known to be responsible for chemoresistance.32,33 Thus, marker-based isolation technologies do not allow comprehensive profiling of CTCs and CAMLs in the blood of NAC patients.
TABLE I.
CTC studies in breast cancer patients undergoing neoadjuvant chemotherapy and the technologies used for isolating rare cells in their blood.
| Study | Technology | Rare cells enumerated | |
|---|---|---|---|
| Marker-based Rare Cell Isolation technologies | Serrano et al.25 | Cytokeratin immunomagnetic cell separation | E+ CTCs |
| Hall et al.23 | Cell search | E+ CTCs | |
| Onstenk et al.20 | Cell search | E+ CTCs | |
| Bauer et al.26 | AdnaTest | Isolated cells were not enumerated | |
| Pierga et al.40 | Cell search | E+ CTCs | |
| Riethdorf et al.41 | Cell search | E+ CTCs | |
| Marker-free Rare Cell Isolation technologies | Gwark et al., 202034 | Smart biopsy system isolation kit | E+ CTCs |
| Ni et al.7–35 | CanPatrol | Isolated cells were not enumerated | |
| Jakabova et al.36 | MetaCell | Isolated cells were not enumerated | |
| This study | Labyrinth | E+ CTCs, M+ CTCs, E+M+ CTCs and CAMLs |
Addressing the limitation of marker-based technologies, label-free or antibody-independent technologies have been used to isolate CTCs in NAC patients.34–36 For example, Gwark et al.34 used the Smart Biopsy System Isolation kit37 to isolate CTCs from the blood of patients undergoing NAC. However, the study did not enumerate the mesenchymal phenotypes of the CTCs and CAMLs. Ni et al.35 used CanPatrol™38 technology and ribonucleic acid-in situ hybridization (RNA-ISH) to identify the expression of epithelial and mesenchymal genes in isolated cells, which enabled the classification of patients as CTC-positive and CTC-negative. Similarly, Jakabova et al.36 used the MetaCell39 size-based filtration technique to isolate CTCs and classify patients as CTC-positive and CTC-negative based on the quantitative polymerase chain reaction (qPCR) analysis. In summary, current studies have not interrogated the ability of marker-free technologies to comprehensively enumerate the various rare cells that could be found at different stages in patients selected for NAC.
The focus of our investigation is to determine whether marker-free technology Labyrinth can be used to isolate and enumerate CTC phenotypes (E+, M+, and E+M+ CTCs) and CAMLs in patients selected for NAC. Labyrinth technology uses inertial focusing and isolates CTCs based on size and deformability.42 The long spiral channels and sharp turns of the Labyrinth microfluidic device help in the distinctive and efficient focusing of CTCs and blood cells. The basic mechanism for particle separation in curved channels involves the inertial lift force that stabilizes particle position (i.e., particle focusing), while the Dean drag force aids in lateral migration due to cross-sectional circulation (i.e., particle separation).42–45 In the Labyrinth device, the turns help to have long channels in a small footprint as well as tight curvatures, both of which increase the opportunity to focus smaller particles. Previously, the Labyrinth technology was shown to isolate heterogeneous CTCs and CTC clusters in metastatic breast, lung, pancreatic, and prostate cancer patients.42–47 Still, it is unclear whether this technology has the capability to enumerate the low CTC counts typically associated with treatment-naïve non-metastatic breast cancer patients. In addition, the ability of Labyrinth to identify and enumerate CAMLs remains to be explored. This evaluation is necessary to inform on whether the baseline counts or the real-time change in those counts can be used to predict the treatment response to the NAC.
II. MATERIALS AND METHODS
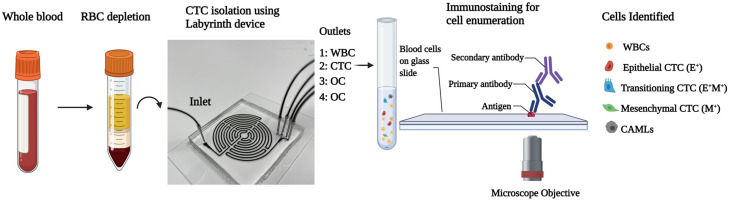
Our workflow for isolating rare cells from the blood of patients selected for NAC is shown in Fig. 1. The Labyrinth device is used to isolate specific cell populations based on size and deformability using the principle of inertial focusing.42 The total channel length of 637 mm is compressed in 11 long loops. The height of the channel is 100 μm and the width is 500 μm. The device has four outlets, where WBCs are collected in the first outlet, CTCs are collected in the second outlet, and other components (OC) of blood are collected in the third and fourth outlets. Immunostaining is subsequently performed to identify the different cell types. Below, we describe the detailed methodology associated with this workflow.
FIG. 1.
Schematic of the workflow from blood collection to cell identification in treatment naïve non-metastatic breast cancer patients. For CTC isolation, the outlet 2 was used. Outlet 1 separated white blood cells (WBCs). Outlets 3 and 4 separated other components (OC) from blood.
A. Blood collection
To study the baseline, we recruited treatment naïve non-metastatic breast cancer patients who consented to receive NAC at the UMC Cancer Center, Texas Tech University Health Sciences Center (TTUHSC). This study was approved by the Institutional Review Board (IRB, Protocol No. L19-043) of TTUHSC, Lubbock. Blood samples were collected from 21 non-metastatic treatment naïve patients undergoing NAC after obtaining written informed consent. Blood samples were collected in BD Vacutainer blood collection tubes (Franklin Lakes, NJ).
B. Depletion of red blood cells
Depletion of red blood cells (RBCs) from 5 ml of whole blood was done using Ficoll-Paque™ Plus (Cytiva Life Sciences, Marlborough, MA) density gradient.48 Ficoll-Paque™ was layered in 15 ml conical centrifuge tubes with 1:1 diluted blood in phosphate buffer saline (PBS, Gibco, Gaithersburg, MD), as per the manufacturer's protocol. After centrifugation, the buffy layer containing peripheral blood mononuclear cells was collected and diluted 5× with PBS to make a total volume of 25 ml for further processing through the Labyrinth chip.
C. CTC isolation
Labyrinth microfluidic chip was primed with 1% pluronic solution (Sigma Aldrich, St. Louis, MO) by flowing 1 ml of the solution at a flow rate of 100 μl/min, followed by 10 min of incubation to prevent cell adhesion to channel walls. After the pluronic treatment, the 5× diluted blood was run through the Labyrinth chip at 2.5 ml/min. The flow was allowed to stabilize for a minute, after which the product from the CTC outlet was collected (see Fig. 1). Images showing separation of cancer cells in the CTC outlet are shown in Fig. S1 in the supplementary material.
D. Immunostaining for cell enumeration
Isolated CTCs underwent the slide centrifugation process using Cytospin™ (Epredia, Kalamazoo, MI). The product collected from the CTC outlet was loaded into EZ Megafunnel™ (Epredia, Kalamazoo, MI), after which Cytospin™ coated cells in a single layer on poly-lysine-coated glass slides. The Cytospin was run at 800 rpm for 10 min. Furthermore, the cells were fixed using 4% paraformaldehyde (PFA, Thermo Fisher Scientific, Waltham, MA) for 10 min and then permeabilizations by 0.2% Triton X-100 (Sigma Aldrich, St. Louis, MO) for 3 min. After the permeabilization step, the slide was washed 3× with PBS for 5 min each. Blocking was done at room temperature using 10% normal goat serum (Thermo Fisher Scientific, Waltham, MA) for 30 min. A cocktail of primary antibodies was added to the slides made using mouse anti-human PanCK IgG1 (Bio-Rad, Hercules, CA), rabbit anti-human Vimentin (Abcam, Waltham, MA), and mouse anti-human CD45 IgG2 (Bio-Rad, Hercules, CA). The slides were incubated overnight with the antibody cocktail at 4 °C in a humidified chamber. After incubation, the slides were washed 3× with PBS, followed by secondary antibody cocktail incubation for 90 min. The secondary antibody cocktail consisted of goat anti-mouse IgG1 AF 546, goat anti-rabbit AF 647, and goat anti-mouse IgG2 AF 488. All the secondary antibodies were procured from Thermo Fisher Scientific. After secondary antibody incubation, the slides were washed 3× with PBS and mounted with Prolong Gold Antifade Mountant with DAPI (Thermo Fisher Scientific, Waltham, MA).
E. Imaging
After immunostaining, an Olympus IX81 microscope (Waltham, MA) and a Hamamatsu digital camera (ImagEM X2 EM-CCD, Bridgewater, NJ) were used for imaging. The microscope was equipped with a Thorlabs automated stage (Newton, NJ) and was controlled by software Slidebook 6.1 (3i Intelligent Imaging Innovations Inc., Denver, CO). The images were acquired at 20× magnification under DAPI, FITC, TRITC, and Cy5 fluorescent filters with exposure times between 20 and 100 ms. The imaging resolution was 0.8 micrometers per pixel. Images were analyzed using a Slidebook Reader.
F. Cell identification
Approximately 2500 images were acquired under each fluorescent filter for every patient sample. Images were analyzed manually using a Slidebook 6 Reader (3i Intelligent Imaging Innovations Inc., Denver, CO) to determine the cell counts. The images were analyzed by simultaneously switching between DAPI, FITC (CD45), TRITC (Cytokeratin), and CY5 (Vimentin) signals across each image for identification of CTC phenotypes and CAMLs.
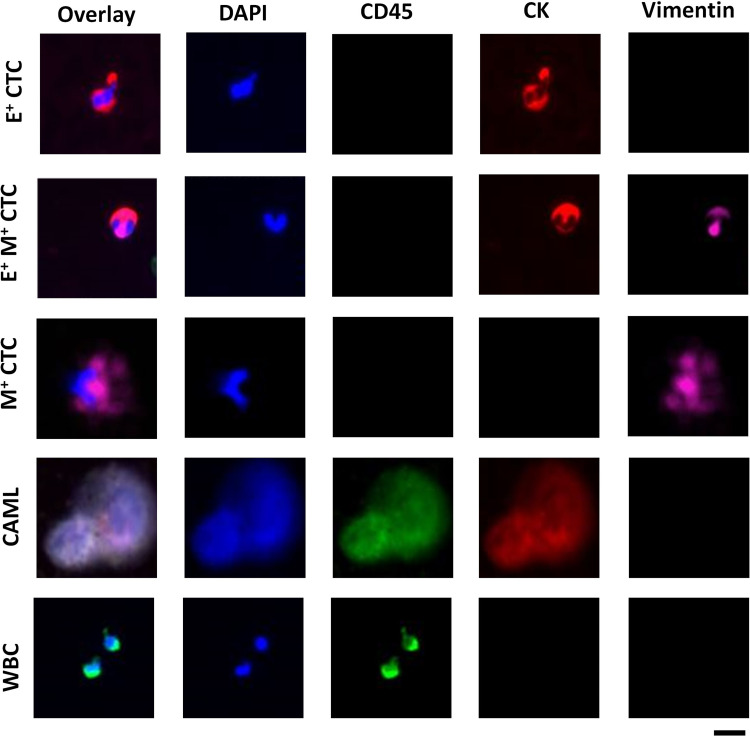
Figure 2 shows the cellular profiling in the blood of treatment naïve breast cancer patients. CTCs were identified as epithelial CTCs (E+ CTCs) if they were positive for nucleus (DAPI) and cytokeratin (TRITC).46–52 Transitioning CTCs (E+M+ CTCs) were positive for nucleus (DAPI), cytokeratin (FITC), and vimentin (Cy5) markers.50–53 Similarly, CTCs were classified as mesenchymal if they were positive for nucleus (DAPI) and Vimentin (Cy5).49–55 Manual scoring of different cell types did not present ambiguity except for some epithelial CTCs, in which case it was classified as an epithelial CTC only if the cytokeratin expression was 50% higher than a reference WBC.
FIG. 2.
Comprehensive cellular profiling in treatment naïve breast cancer patients using Labyrinth technology. E+ denotes epithelial CTCs (DAPI+/CD45-/CK+/Vim-), E+ M+ denote EMT transition CTCs (DAPI+/CD45−/CK+/Vim+), M+ denotes mesenchymal CTCs (DAPI+/CD45−/CK−/Vim+), CAML are DAPI+/CD45+/CK+/Vim−, and WBC are DAPI+/CD45+/CK+/Vim−. Scale bar is 20 μm.
To identify cells as CAMLs, we used the same criteria as that of Adams et al. (for pancreatic, prostate, and breast cancers),15–20 Gironda et al. (for esophageal cancer),17 Zhu et al. (renal cell carcinoma),18 and Augustyn et al. (for lung cancer).20 In these studies, and our work, CAMLs were identified as cells exhibiting nucleus (DAPI), CD45 (FITC), and cytokeratin (TRITC).15–20 We also measured the size of CAMLs in representative samples and found it to range from 14 to 150 μm, which was congruent with the size range of 14–300 μm reported by Adams et al.15
G. Performance of labyrinth device
The performance metrics of the Labyrinth device with respect to purity and separation has been previously established with both cell lines and metastatic cancer patient samples.42 To validate the performance, we conducted several complementary studies. To determine the recovery of CTCs in different outlets, we spiked the breast cancer MCF-7 cell line in whole blood (1000 cells/ml) and found ∼90% recovery from CTC outlet consistently across 10 trials (see Fig. S2 in the supplementary material). We performed a live/dead assay before and after recovery on MCF-7 cells, and we consistently found ∼99.5% cells alive across five trials (Fig. S3 in the supplementary material). Additionally, we did experiments where we spiked the breast cancer cell line (MCF-7) in very low numbers such as 30, 100, and 300 cells/ml, and the recovery was found to be 80%–90% (see Fig. S4 in the supplementary material). These results indicate that the separation efficiency of the labyrinth device is high. In the CTC outlet, we find 3000–6000 WBCs/ml of whole blood. Thus, the purity is rather high since there is about two to three log-fold depletion in WBCs.
III. RESULTS
A. Characterization of CTC phenotypes in staged cohorts of treatment naïve breast cancer patients
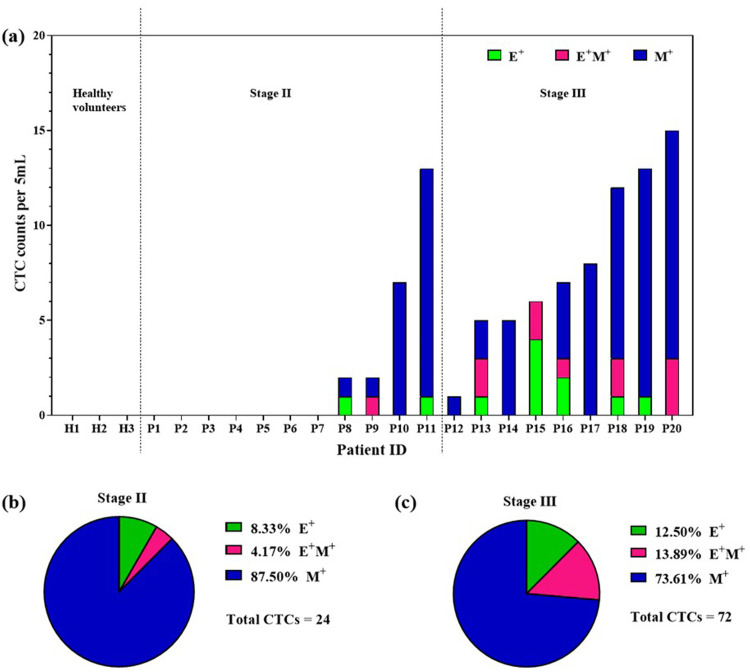
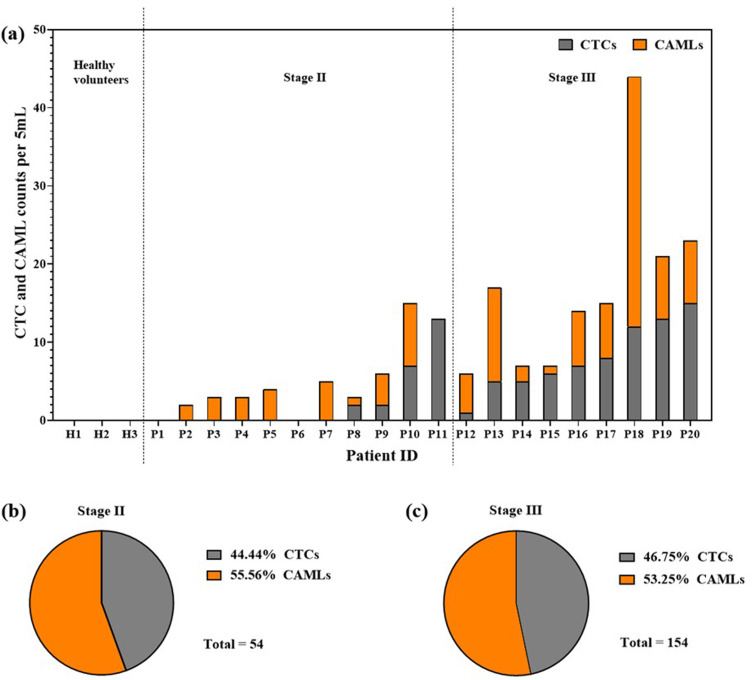
There have not been systematic studies enumerating different phenotypes of CTCs in patients selected for NAC. We phenotyped different CTCs using label-free microfluidic technology Labyrinth in 21 treatment naïve non-metastatic breast cancer patients. Out of 21 patients, one had stage I, 11 had stage II, and nine had stage III disease. Additionally, we had three healthy volunteers in our control cohort. All cell counts were reported per 5 ml of whole blood. Healthy volunteers did not show any CTCs [Fig. 3(a)]. With CTC phenotypes absent in all healthy volunteers, these counts validate the Labyrinth technique of CTC isolation and characterization.
FIG. 3.
CTC profiling in neoadjuvant breast cancer patients prior to treatment across stages II and III. (a) Overview of CTC phenotypes observed in healthy volunteers and stage II and III patients. (b) and (c) Profiling stage CTC sub-phenotypes in stage II and III treatment naïve breast cancer patients.
Figure 3(a) gives an overview of the presence of epithelial (E+), transitioning (E+M+), and mesenchymal (M+) phenotypes of CTCs in the blood of treatment naïve breast cancer patients. Only 4 (36%) of the stage II patients showed 1 CTCs/5 ml, while 7 (64%) had no CTCs. E+ CTCs and E+M+ CTCs were found in 2 (18%) and 1 (9%) of stage II patients. All stage II patients with 1 CTC/5 ml showed the presence of mesenchymal CTCs. All stage III patients had atleast 1 CTCs/5 ml. Five (55%) of the stage III patients showed E+ CTCs and E+M+ transitioning CTCs, while 8 (89%) of stage III patients had M+ CTCs. Overall, these results indicate that stage III disease is significantly associated with a higher CTC count than stage II.
It is interesting to note that patients P10, P12, P14, and P17 only show mesenchymal CTCs and no other rare circulating cells. Both P14 and P17 patients are human epidermal growth positive (HER2+) patients, and HER2+ tumors tend to be more aggressive cancers.56 Since mesenchymal cells are correlated with that of more aggressive tumors, this may explain why we observe only mesenchymal cells in these two patients.50 Additionally, we also find only mesenchymal CTCs in P10 and P12, and both patients were triple negative. Triple negative cancers are known to be more aggressive as no targeted therapies can be used toward mitigating the growth of tumors.57,58 This may explain why we only observe mesenchymal CTCs in more aggressive cancers.
Next, we investigated among the different CTC phenotypes found in stage II and stage III patients, which phenotypes were dominant by pooling enumerated cell counts from patients within a cohort. As shown in Fig. 3(b), the total CTC count across all stage II patients was 24, of which 21 (87.5%) were of mesenchymal phenotype. The epithelial and transitioning phenotypes were not as dominant as the mesenchymal phenotype. Similarly, pooling the counts from stage III patients, as shown in Fig. 3(c), a total of 72 CTCs were found in circulation. 53 (73.61%) of the total CTCs were of the mesenchymal phenotype, followed by 9 (12.5%) epithelial and 10 (13.89%) CTCs of transitioning phenotypes. In both stage II and stage III patients, the mesenchymal phenotype was dominant in treatment naïve patients. More than 50% of CTCs were found to belong to the mesenchymal category in stage II and stage III patients. This highlights the advantage of Labyrinth technology compared to affinity-based isolation techniques that may fail to capture these types of CTCs. Thus, the Labyrinth technology increases CTC phenotyping depth, potentially enabling tracking of NAC response in breast cancer patients.
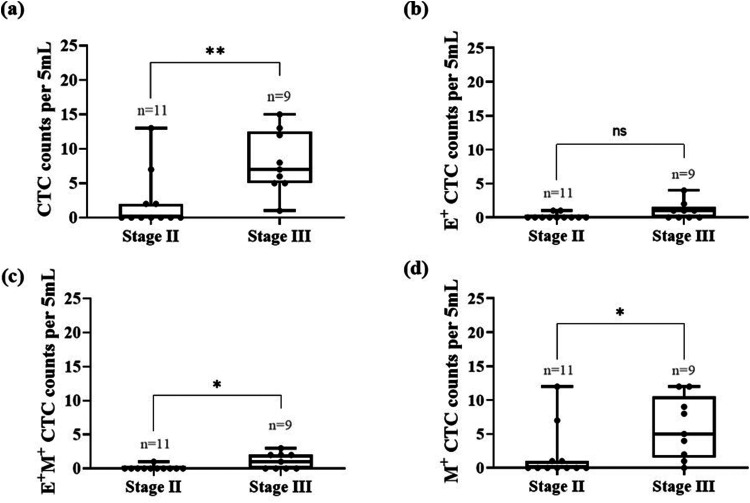
Next, we determined the effect of disease stage on the CTC phenotypes to understand whether specific phenotypes are more prevalent in stage II vs stage III patients. We computed the median of a specific CTC phenotype and statistically compared the two cohorts. As shown in Fig. 4(a), the median number of CTCs found in stage III patients was 7 CTCs/5 ml, as compared to 0 CTCs/5 ml in stage II patients (p = 0.0041), indicating the presence of significantly higher number of CTCs in stage III patients. With regard to E+ CTC, as shown in Fig. 4(b), stage II patients had a median of 0 CTCs/5 ml and patients with stage III showed 1 CTC/5 ml, which was not statistically significant (p = 0.0975). The median number of transitioning CTCs (E+M+) in stage II patients was 0 CTCs/5 ml, and for stage III patients, it was 1 CTCs/5 ml, which was statistically significant (p = 0.026). As shown in Fig. 4(d), the median counts of mesenchymal CTCs (M+) in stage II and stage III patients was 0 and 5 CTCs/5 ml, respectively (p = 0.0169), indicating the invasive cells are present in higher number in stage III patients.
FIG. 4.
Profiling the counts of CTC phenotypes as a function of stage for breast cancer patients prior to treatment. The box plots represent the CTC counts in stage II and stage III patients. The line inside the box represents the median (unpaired Mann–Whitney t-test, *p < 0.05, **p < 0.01, ns = not significant).
B. Characterization of CAMLs vis-à-vis CTCs staged cohorts of treatment naïve breast cancer patients
Our study focused on identifying CAMLs in addition to CTC phenotypes as a distinct biomarker for tracking NAC response. This section discusses the CAML counts vis-à-vis CTC counts for stage II and stage III patients. As shown in Fig. 5(a), the three healthy volunteers did not show any CAMLs or CTCs. In stage II and III patients, we observed CAMLs in 8 out of 11 patients and in 9 out of 9 patients, respectively. Although five of the stage II patients did not show CTCs but showed CAMLs, one patient did not show CAMLs but showed CTCs. In stage III patients, all nine showed both CTCs and CAMLs in varying proportions.
FIG. 5.
CTC profiling in neoadjuvant breast cancer patients prior to treatment. (a) Overview of CTCs and CAMLs observed in healthy volunteers and Stage I, II, III patients. (b) and (c) Profiling stage II and III treatment naïve breast cancer patients based on CTCs and CAMLs.
Upon pooling the cell counts across all the patients in stage II, as shown in Fig. 5(b), out of a total of 54 cells, 24 (44.44%) cells were CTCs and 30 (56.56%) cells were CAMLs. Similarly, as shown in Fig. 5(c), in stage III patients, out of 154 cells identified, 72 (46.75%) were CTCs and 82 (53.25%) were CAMLs. The percentage of CAMLs was higher than the percentage of CTCs in both stage II and III treatment naïve breast cancer patients.
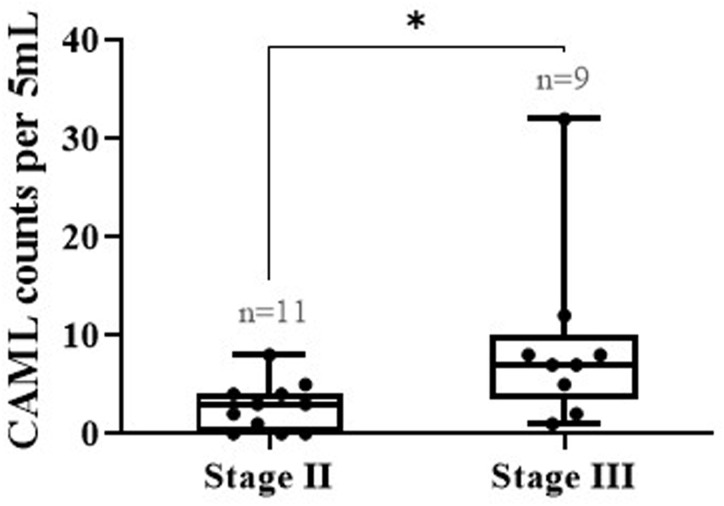
We further wanted to investigate the effect of the disease stage on CAMLs by identifying the median number of CAMLs across different stages. As shown in Fig. 6, the median count found in stage II and III patients was 3 CAMLs/5 ml and 7 CAMLs/5 ml, respectively (p = 0.0175), indicating that stage III patients had more CAMLs compared to stage II patients.
FIG. 6.
Comparing the counts of CAMLs as a function of stage for neoadjuvant breast cancer patients prior to treatment (unpaired Mann–Whitney t-test, *p < 0.05).
C. Relationship between CTC phenotypes and CAML counts
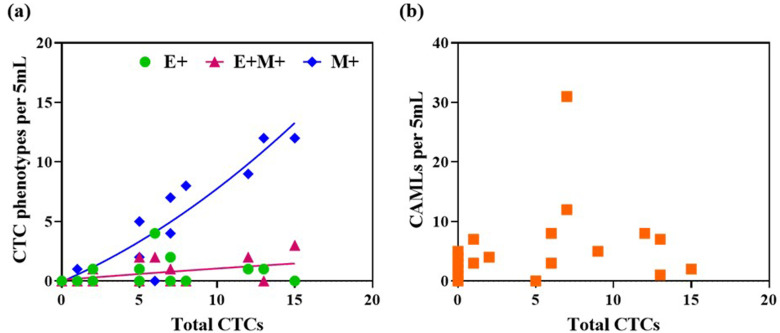
Given that the Labyrinth technology provides label-free isolation of CTCs and CAMLs, we sought to investigate whether there is a relationship between the counts of different cell types isolated from this technology. Figure 7(a) shows the variation in individual CTC phenotypes with the total number of CTCs by pooling data from all patients irrespective of the disease stage. We find that as the total number of CTCs increases, the mesenchymal phenotype also significantly increases. Furthermore, it is also seen that there is a slight increase in the transitioning phenotype as the number of CTCs increases; however, no change is noted in the epithelial CTC counts. This suggests that if the CTC load is high, it is more likely that a significant fraction of them are the mesenchymal or the invasive phenotype. Concerning the variation of CAML counts with CTC counts [Fig. 5(b)], we did not find any discernable relationship. A high load of CTCs did not necessarily translate into strong presence of CAMLs in the blood of treatment naïve non-metastatic breast cancer patients.
FIG. 7.
Relationship between cellular counts identified from Labyrinth technology irrespective of the disease stage in treatment naïve breast cancer patients. (a) CTC phenotypes and total number of CTCs; (b) CAMLs and total CTCs.
IV. DISCUSSION
A. Labyrinth microfluidic technology isolates CTCs and CAMLs in treatment naïve non-metastatic breast cancer patients
Affinity-based technologies for isolating CTCs do not comprehensively profile rare cells present in the blood of cancer patients since only a specific group of cells with targeted markers are isolated.23–26 Marker-free isolation technologies that address this gap have not been widely evaluated for their capability to phenotype the potentially wide variety of rare circulating cancer-associated cells. Increasing the depth of phenotyping provides a broader set of readouts that could be useful for tracking the treatment response in NAC patients.
Labyrinth microfluidic technology, a marker-free approach, has been previously used to isolate cancer cells from the blood of metastatic breast, prostate, and lung cancer patients.42 Specifically, Labyrinth isolated >50 CTCs per 7.5 ml of blood in metastatic breast cancer patients.42 Isolation of CTCs from the blood of metastatic breast cancer patients is relatively more efficient, as they are present in such high numbers. However, the suitability of Labyrinth to isolate CTCs in earlier stages of breast cancer, where CTCs are present in relatively low numbers, remains to be determined. In this study, we show that Labyrinth microfluidic technology is successfully able to isolate low numbers of CTCs (0–15 CTCs/5 ml of blood) and CAMLs (0–35 CAMLs/5 ml of blood). Thus, Labyrinth microfluidic technology can isolate CTCs and CAMLs in treatment naïve non-metastatic breast cancer patients as well as setting the stage for evaluation of its use to track treatment response in NAC patients.
B. Epithelial to mesenchymal transition status in stage II and stage III patients
Epithelial to mesenchymal transition (EMT), the precursor of metastasis, is a phenomenon during which the epithelial tumor cells become invasive by losing their intercellular connections and gaining motility.30 EMT promotes the primary tumor cells to enter the bloodstream and metastasize at distant organs.59 EMT in primary tumor cells or epithelial CTCs (E+ CTCs) is activated by transcription factors such as ZEB, TWIST, and SNAIL,33 along with rearrangement in the intermediate filaments to promote cell motility.60 A significant increase in markers such as Vimentin, N-cadherin, and Fibronectin is seen in mesenchymal CTCs (M + CTCs) and CTCs undergoing epithelial-mesenchymal transition (E+M+ CTCs).31 These mesenchymal-type CTCs are also known to be responsible for chemoresistance.32,33 Thus, tracking EMT status using liquid biopsy is a powerful means to monitor disease progression.
EMT status has been evaluated using liquid biopsy in breast cancer patients. In a study by Kallergi et al.,54 CTCs were isolated from the blood of 25 early-stage and 25 metastatic breast cancer patients by WBC depletion using Dynal CELLection beads.61 The cells were identified as CTC positive using anti-cytokeratin antibody. These cytokeratin-positive CTCs were further tested for markers such as Vimentin and Twist to identify CTCs as EMT CTCs (E+M+ CTCs). The results indicated that ∼70% of early-stage breast cancer patients had transitioning CTCs and 100% of the metastatic patients had transitioning CTCs, indicating EMT prevalence in the blood of metastatic patients.54
In another study by Yu et al.,62 CTCs were isolated from the blood of 41 metastatic breast cancer patients using the label-based microfluidic herringbone chip coated with antibodies against EpCAM, human epidermal growth factor receptor 2 (HER2), and epidermal growth factor receptor (EGFR). The results showed that 17 out of 41 patients expressed EMT features. Among the 17 patients expressing EMT features, they found that the patients with lobular cancer (ER+/PR+) had more epithelial CTCs, whereas the patients with ductal cancers (triple negative, HER2+) had more mesenchymal CTCs.
We did not find any studies evaluating EMT status in treatment naïve non-metastatic breast cancer patients. Our results demonstrate that Labyrinth could not only isolate CTCs in low numbers of 0–3 CTCs/ml of whole blood of patients with non-metastatic breast cancer disease, but it can also capture different CTC phenotypes present across the spectrum of EMT. We also found that 9% and 36% of stage II patients showed transitioning and mesenchymal CTCs, respectively, whereas 55% and 88% of stage III patients showed transitioning and mesenchymal CTCs, respectively. Additionally, we also noticed that both the transitioning and mesenchymal CTCs were present in significantly high numbers in stage III patients compared to stage II patients. Thus, our study indicates that EMT status could be tracked in response to NAC.
C. CAMLs as an important biomarker for liquid biopsy
CAMLs are inflammatory immune cells of myeloid lineage known to be found in the blood of cancer patients.13 Adams et al.15 isolated CAMLs from the blood of early to late-stage breast, cancer patients using microfilter technology CellSieve.63 CAMLs were found in 97% (28/29) of breast cancer patients. Out of the 29 breast cancer patients, 5 patients (stage unknown) were treatment naïve. The average number of CAMLs in these treatment naïve patients was found to be 3 CAMLs/7.5 ml, whereas the mean CAML count was much higher (29 CAMLs/7.5 ml) in breast cancer patients undergoing chemotherapy, highlighting the differences between innate immune response and chemotherapy-induced immune response. Furthermore, they found that 2/3 patients with stage I/II, 8/8 patients with stage III, and 17/18 patients with stage IV had CAMLs with majority of the data collected from subjects undergoing hormone therapy or chemotherapy.
In contrast to the work of Adams et al.,15 we pursued a systematic study with treatment naïve non-metastatic patients in the context of NAC. Using Labyrinth microfluidic technology, we also found CAMLs with mean counts of 3 and 9 CAMLs/5 ml in stage II and stage III patients. CAMLs were found in all (9/9) stage III patients but only 8/11 (72%) of stage II patients. Interestingly, some of the stage II patients that did not have any CTCs had CAMLs in circulation, whereas patient P11, who had the highest number of CTCs, had no CAMLs. This could indicate that CAMLs may play a role in expunging CTCs from circulation. We also found that CAMLs and CTCs are predominantly found in the blood of stage II and III treatment naïve breast cancer patients, indicating that these cells can provide meaningful insights into the role of cancer-associated cells and their interactions with the immune system while in circulation.
V. CONCLUSIONS
In this study, we have presented the capability of Labyrinth microfluidic technology to isolate different cancer-associated rare cells in low numbers from the blood of treatment naïve non-metastatic breast cancer patients selected for NAC. A systematic study was conducted where blood from 21 patients (one stage I, 11 stage II, and nine stage III) and three healthy volunteers was collected. The blood was processed through the label-free Labyrinth microfluidic chip, and cells were immunostained to identify E+ CTCs, M+ CTCs, E+M+ CTCs, and CAMLs.
The performance of Labyrinth for NAC application was established by (i) none of the healthy volunteers' blood that showed cancer-associated circulating cells and (ii) the ability to enumerate CTCs and CAMLs despite the counts being low in the selected patients. In contrast to previous technologies (listed in Table I), the Labyrinth technology is able to isolate rare circulating cells with not only sufficient sensitivity but enables phenotyping CTCs and enumeration of CAMLs.
This study also provided important results on how CTC phenotypes vary with disease stage. We found that the total number of CTCs was significantly high in stage III patients as compared to stage II patients. We also found that the transitioning and mesenchymal phenotypes, also known as the invasive phenotypes, were present at significantly higher counts in stage III patients as compared to stage II patients. However, epithelial CTCs alone showed no significant difference across stages II and III. Interestingly, the counts of mesenchymal CTCs increases with CTC load.
Given the importance of host immune response on disease progression in breast cancer, we enumerated CAMLs as biomarkers of host immunity. We found that the Labyrinth technology was able to successfully isolate CAMLs with counts varying from 0 to 32 CAMLs/5 ml of blood in non-metastatic breast cancer patients. We also found that CAMLs were present in significantly higher numbers in stage III patients as compared to stage II patients. Interestingly, no obvious relationship was found between total CTC and CAML counts in our small dataset, suggesting that more studies are needed to uncover the relationship between the host immune response and tumor burden in circulation.
In summary, our work sets the stage for the use of Labyrinth microfluidic technology to track CTC phenotypes and CAMLs since we showed that robust baseline data can be achieved in stage II and stage III patients prior to treatment. Currently, the response to chemotherapy is clinically determined by calculating the residual tumor burden at the time of surgery after completion of proposed chemotherapy. In the future, it would be interesting to design a NAC study, where blood draws are done during treatment and after treatment completion to determine how the CTC and CAML counts change between pre-therapy and post-therapy blood draws. If the CTC and/or CAML counts are able to predict pathological complete response (i.e., no residual tumor burden), then it would avoid unnecessary doses of toxic treatment and may preclude the need for surgery. This study represents the first step in achieving this predictive capability.
SUPPLEMENTARY MATERIAL
See the supplementary material for testing data obtained from Labyrinth microfluidic technology using MCF-7 breast cancer cells.
ACKNOWLEDGMENTS
We sincerely appreciate the patients and healthy volunteers participating in this study. We also thank the Clinical Research Institute of TTUHSC and the nursing staff at UMC Cancer Center, Lubbock, for helping with patient recruitment, blood draws, and data collection. We acknowledge the Cancer Prevention and Research Institute of Texas (Grant No. RP190658) for funding this work.
AUTHOR DECLARATIONS
Conflict of Interest
The authors have no conflicts to disclose.
Ethics Approval
The study was approved by the Institutional Review Board (Protocol No. L19-043) of Texas Tech University Health Sciences, Lubbock. Written and informed consent was obtained from all patients before enrolling them in the study.
Author Contributions
Adity A. Pore: Formal analysis (equal); Methodology (equal); Validation (equal); Visualization (equal); Writing – original draft (equal); Writing – review & editing (equal). Swastika S. Bithi: Formal analysis (equal); Methodology (equal); Writing – review & editing (equal). Mina Zeinali: Formal analysis (supporting); Methodology (supporting); Writing – review & editing (supporting). Hunaiz Bin Navaid: Resources (supporting); Writing – review & editing (supporting). Sunitha Nagrath: Resources (equal); Writing – review & editing (supporting). Rakhshanda Layeequr Rahman: Conceptualization (equal); Project administration (equal); Resources (equal); Supervision (equal); Writing – original draft (equal); Writing – review & editing (equal). Siva A. Vanapalli: Conceptualization (equal); Funding acquisition (equal); Project administration (lead); Resources (equal); Supervision (equal); Visualization (equal); Writing – original draft (equal); Writing – review & editing (equal).
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Thompson A. M. and Moulder-Thompson S. L., “Neoadjuvant treatment of breast cancer,” Ann. Oncol. 23(Suppl 10), x231–x236 (2012). 10.1093/annonc/mds324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masood S., “Neoadjuvant chemotherapy in breast cancers,” Womens Health (London) 12, 480–491 (2016). 10.1177/1745505716677139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikeda T., Jinno H., Matsui A., Masamura S., and Kitajima M., “The role of neoadjuvant chemotherapy for breast cancer treatment,” Breast Cancer 9, 8–14 (2002). 10.1007/BF02967540 [DOI] [PubMed] [Google Scholar]
- 4.Hanna A., Birla R., Iosif C., Boeriu M., and Constantinoiu S., “Benefits and disadvantages of neoadjuvant radiochemotherapy (RCT) in the multimodal therapy of squamous esophageal cancer (ESC),” Chirurgia (Bucur) 111, 12–25 (2016). [PubMed] [Google Scholar]
- 5.Moussavi-Harami S. F., Wisinski K. B., and Beebe D. J., “Circulating tumor cells in metastatic breast cancer: A prognostic and predictive marker,” J. Patient Cent. Res. Rev. 1, 85–92 (2014). 10.17294/2330-0698.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giordano A. and Cristofanilli M., “CTCs in metastatic breast cancer,” Recent Results Cancer Res. 195, 193–201 (2012). 10.1007/978-3-642-28160-0_18 [DOI] [PubMed] [Google Scholar]
- 7.Arya S. K., Lim B., and Rahman A. R. A., “Enrichment, detection and clinical significance of circulating tumor cells,” Lab Chip 13, 1995–2027 (2013). 10.1039/c3lc00009e [DOI] [PubMed] [Google Scholar]
- 8.Rodrigues P. and Vanharanta S., “Circulating tumor cells: Come together, right Now, over metastasis,” Cancer Discov. 9, 22–24 (2019). 10.1158/2159-8290.CD-18-1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Micalizzi D. S., Maheswaran S., and Haber D. A., “A conduit to metastasis: Circulating tumor cell biology,” Genes Dev. 31, 1827–1840 (2017). 10.1101/gad.305805.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alix-Panabières C. and Pantel K., “Technologies for detection of circulating tumor cells: Facts and vision,” Lab Chip 14, 57–62 (2014). 10.1039/C3LC50644D [DOI] [PubMed] [Google Scholar]
- 11.Redig A. J. and McAllister S. S., “Breast cancer as a systemic disease: A view of metastasis,” J. Intern. Med. 274, 113–126 (2013). 10.1111/joim.12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selli C. and Sims A. H., “Neoadjuvant therapy for breast cancer as a model for translational research,” Breast Cancer: Basic Clin. Res. 13, 117822341982907–1178223419829072 (2019). 10.1177/1178223419829072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez H., Hagerling C., and Werb Z., “Roles of the immune system in cancer: From tumor initiation to metastatic progression,” Genes Dev. 32, 1267–1284 (2018). 10.1101/gad.314617.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Standish L. J. et al. , “Breast cancer and the immune system,” J. Soc. Integr. Oncol. 6, 158–168 (2008). [PMC free article] [PubMed] [Google Scholar]
- 15.Adams D. L. et al. , “Circulating giant macrophages as a potential biomarker of solid tumors,” Proc. Natl. Acad. Sci. U.S.A. 111, 3514–3519 (2014). 10.1073/pnas.1320198111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams D. L. et al. , “Circulating cancer-associated macrophage-like cells differentiate malignant breast cancer and benign breast conditions,” Cancer Epidemiol. Biomarkers Prev. 25, 1037–1042 (2016). 10.1158/1055-9965.EPI-15-1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gironda D. J. et al. , “Cancer associated macrophage-like cells and prognosis of esophageal cancer after chemoradiation therapy,” J. Transl. Med. 18, 413 (2020). 10.1186/s12967-020-02563-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu P. et al. , “Detection of tumor-associated cells in cryopreserved peripheral blood mononuclear cell samples for retrospective analysis,” J. Transl. Med. 14, 198 (2016). 10.1186/s12967-016-0953-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mu Z. et al. , “Prognostic values of cancer associated macrophage-like cells (CAML) enumeration in metastatic breast cancer,” Breast Cancer Res. Treat. 165, 733–741 (2017). 10.1007/s10549-017-4372-8 [DOI] [PubMed] [Google Scholar]
- 20.Augustyn A. et al. , “Giant circulating cancer-associated macrophage-like cells are associated with disease recurrence and survival in non-small-cell lung cancer treated with chemoradiation and atezolizumab,” Clin. Lung Cancer 22, e451–e465 (2021). 10.1016/j.cllc.2020.06.016 [DOI] [PubMed] [Google Scholar]
- 21.Quinn M. T. and Schepetkin I. A., “Role of NADPH oxidase in formation and function of multinucleated giant cells,” J. Innate Immun. 1, 509–526 (2009). 10.1159/000228158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krebs M. G., Hou J.-M., Ward T. H., Blackhall F. H., and Dive C., “Circulating tumour cells: Their utility in cancer management and predicting outcomes,” Ther. Adv. Med. Oncol. 2, 351–365 (2010). 10.1177/1758834010378414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall C. et al. , “Circulating tumor cells after neoadjuvant chemotherapy in stage I–III triple-negative breast cancer,” Ann. Surg. Oncol. 22, 552–558 (2015). 10.1245/s10434-015-4600-6 [DOI] [PubMed] [Google Scholar]
- 24.Onstenk W. et al. , “Improved circulating tumor cell detection by a combined EpCAM and MCAM CellSearch enrichment approach in patients with breast cancer undergoing neoadjuvant chemotherapy,” Mol. Cancer Ther. 14, 821 (2015). 10.1158/1535-7163.MCT-14-0653 [DOI] [PubMed] [Google Scholar]
- 25.Serrano M. J. et al. , “Dynamics of circulating tumor cells in early breast cancer under neoadjuvant therapy,” Exp. Ther. Med. 4, 43–48 (2012). 10.3892/etm.2012.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasimir-Bauer S. et al. , “Does primary neoadjuvant systemic therapy eradicate minimal residual disease? Analysis of disseminated and circulating tumor cells before and after therapy,” Breast Cancer Res. 18, 20 (2016). 10.1186/s13058-016-0679-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowalik A., Kowalewska M., and Góźdź S., “Current approaches for avoiding the limitations of circulating tumor cells detection methods—Implications for diagnosis and treatment of patients with solid tumors,” Transl. Res. 185, 58–84.e15 (2017). 10.1016/j.trsl.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 28.Pantel K. and Speicher M. R., “The biology of circulating tumor cells,” Oncogene 35, 1216–1224 (2016). 10.1038/onc.2015.192 [DOI] [PubMed] [Google Scholar]
- 29.Shen Z., Wu A., and Chen X., “Current detection technologies for circulating tumor cells,” Chem. Soc. Rev. 46, 2038–2056 (2017). 10.1039/C6CS00803H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalluri R. and Weinberg R. A., “The basics of epithelial-mesenchymal transition,” J. Clin. Invest. 119, 1420–1428 (2009). 10.1172/JCI39104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamouille S., Xu J., and Derynck R., “Molecular mechanisms of epithelial-mesenchymal transition,” Nat. Rev. Mol. Cell Biol. 15, 178–196 (2014). 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jie X.-X., Zhang X.-Y., and Xu C.-J., “Epithelial-to-mesenchymal transition, circulating tumor cells and cancer metastasis: Mechanisms and clinical applications,” Oncotarget 8, 81558–81571 (2017). 10.18632/oncotarget.18277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brabletz T., Kalluri R., Nieto M. A., and Weinberg R. A., “EMT in cancer,” Nat. Rev. Cancer 18, 128–134 (2018). 10.1038/nrc.2017.118 [DOI] [PubMed] [Google Scholar]
- 34.Gwark S. et al. , “Analysis of the serial circulating tumor cell count during neoadjuvant chemotherapy in breast cancer patients,” Sci. Rep. 10, 17466 (2020). 10.1038/s41598-020-74577-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ni C. et al. , “Prospective study of the relevance of circulating tumor cell status and neoadjuvant chemotherapy effectiveness in early breast cancer,” Cancer Med. 9, 2290–2298 (2020). 10.1002/cam4.2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jakabova A. et al. , “Characterization of circulating tumor cells in early breast cancer patients receiving neoadjuvant chemotherapy,” Ther. Adv. Med. Oncol. 13, 175883592110284 (2021). 10.1177/17588359211028492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee S. J. et al. , “Evaluation of a novel approach to circulating tumor cell isolation for cancer gene panel analysis in patients with breast cancer,” Oncol. Lett. 13, 3025–3031 (2017). 10.3892/ol.2017.5807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu S. et al. , “Classification of circulating tumor cells by epithelial-mesenchymal transition markers,” PLos One 10, e0123976 (2015). 10.1371/journal.pone.0123976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolostova K., Zhang Y., Hoffman R. M., and Bobek V., “In vitro culture and characterization of human lung cancer circulating tumor cells isolated by size exclusion from an orthotopic nude-mouse model expressing fluorescent protein,” J. Fluoresc. 24, 1531–1536 (2014). 10.1007/s10895-014-1439-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pierga J. Y. et al. , “Circulating tumour cells and pathological complete response: Independent prognostic factors in inflammatory breast cancer in a pooled analysis of two multicentre phase II trials (BEVERLY-1 and -2) of neoadjuvant chemotherapy combined with bevacizumab,” Ann. Oncol. 28, 103–109 (2017). 10.1093/annonc/mdw535 [DOI] [PubMed] [Google Scholar]
- 41.Riethdorf S. et al. , “Prognostic impact of circulating tumor cells for breast cancer patients treated in the neoadjuvant ‘geparquattro’ trial,” Clin. Cancer Res. 23, 5384–5393 (2017). 10.1158/1078-0432.CCR-17-0255 [DOI] [PubMed] [Google Scholar]
- 42.Lin E. et al. , “High-throughput microfluidic labyrinth for the label-free isolation of circulating tumor cells,” Cell Syst. 5, 295–304.e4 (2017). 10.1016/j.cels.2017.08.012 [DOI] [PubMed] [Google Scholar]
- 43.Gossett D. R. and Carlo D. D., “Particle focusing mechanisms in curving confined flows,” Anal. Chem. 81, 8459–8465 (2009). 10.1021/ac901306y [DOI] [PubMed] [Google Scholar]
- 44.Di Carlo D., Irimia D., Tompkins R. G., and Toner M., “Continuous inertial focusing, ordering, and separation of particles in microchannels,” Proc. Natl. Acad. Sci. U.S.A. 104, 18892–18897 (2007). 10.1073/pnas.0704958104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gangadhar A. and Vanapalli S. A., “Inertial focusing of particles and cells in the microfluidic labyrinth device: Role of sharp turns,” Biomicrofluidics 16, 044114 (2022). 10.1063/5.0101582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeinali M. et al. , “High-throughput label-free isolation of heterogeneous circulating tumor cells and CTC clusters from non-small-cell lung cancer patients,” Cancers 12, 127 (2020). 10.3390/cancers12010127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rivera-Báez L. et al. , “Expansion of circulating tumor cells from patients with locally advanced pancreatic cancer enable patient derived xenografts and functional studies for personalized medicine,” Cancers 12, 1011 (2020). 10.3390/cancers12041011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fuss I. J., Kanof M. E., Smith P. D., and Zola H., “Isolation of whole mononuclear cells from peripheral blood and cord blood,” Curr. Protoc. Immunol. 85, 7.1.1–7.1.8 (2009). 10.1002/0471142735.im0701s85 [DOI] [PubMed] [Google Scholar]
- 49.Horimoto Y. et al. , “Analysis of circulating tumour cell and the epithelial mesenchymal transition (EMT) status during eribulin-based treatment in 22 patients with metastatic breast cancer: A pilot study,” J. Transl. Med. 16, 287 (2018). 10.1186/s12967-018-1663-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang S. et al. , “Mesenchymal phenotype of circulating tumor cells is associated with distant metastasis in breast cancer patients,” Cancer Manag. Res. 9, 691–700 (2017). 10.2147/CMAR.S149801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z. et al. , “Perioperative circulating tumor cells (CTCs), MCTCs, and CTC-white blood cells detected by a size-based platform predict prognosis in renal cell carcinoma,” Dis. Markers 2021, 9956142. 10.1155/2021/9956142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stott S. L. et al. , “Isolation of circulating tumor cells using a microvortex-generating herringbone-chip,” Proc. Natl. Acad. Sci. U.S.A. 107, 18392 (2010). 10.1073/pnas.1012539107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao W. et al. , “Tumor antigen-independent and cell size variation-inclusive enrichment of viable circulating tumor cells,” Lab Chip 19, 1860–1876 (2019). 10.1039/C9LC00210C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kallergi G. et al. , “Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients,” Breast Cancer Res. 13, R59 (2011). 10.1186/bcr2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manjunath Y. et al. , “PD-L1 expression with epithelial mesenchymal transition of circulating tumor cells is associated with poor survival in curatively resected non-small cell lung cancer,” Cancers (Basel) 11, 806 (2019). 10.3390/cancers11060806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pupa S. M. et al. , “HER2 signaling and breast cancer stem cells: The bridge behind HER2-positive breast cancer aggressiveness and therapy refractoriness,” Cancers (Basel) 13, 4778 (2021). 10.3390/cancers13194778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asaoka M. et al. , “Clinical and pathological predictors of recurrence in breast cancer patients achieving pathological complete response to neoadjuvant chemotherapy,” Eur. J. Surg. Oncol. 45, 2289–2294 (2019). 10.1016/j.ejso.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 58.Liedtke C. et al. , “Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer,” J. Clin. Oncol. 26, 1275–1281 (2008). 10.1200/JCO.2007.14.4147 [DOI] [PubMed] [Google Scholar]
- 59.Heerboth S. et al. , “EMT and tumor metastasis,” Clin. Transl. Med. 4, e6 (2015). 10.1186/s40169-015-0048-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun B. O., Fang Y., Li Z., Chen Z., and Xiang J., “Role of cellular cytoskeleton in epithelial-mesenchymal transition process during cancer progression,” Biomed. Rep. 3, 603–610 (2015). 10.3892/br.2015.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naume B. et al. , “Increased sensitivity for detection of micrometastases in bone-marrow/peripheral-blood stem-cell products from breast-cancer patients by negative immunomagnetic separation,” Int. J. Cancer 78, 556–560 (1998). [DOI] [PubMed] [Google Scholar]
- 62.Yu M. et al. , “Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition,” Science (New York, N.Y.) 339, 580–584 (2013). 10.1126/science.1228522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adams D. L. et al. , “The systematic study of circulating tumor cell isolation using lithographic microfilters,” RSC Adv. 9, 4334–4342 (2014). 10.1039/C3RA46839A [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See the supplementary material for testing data obtained from Labyrinth microfluidic technology using MCF-7 breast cancer cells.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.