Abstract
Experiments were performed using the standardized murine model of Helicobacter pylori infection to determine the immunogenicity of H. pylori outer membrane vesicles in immune protection. These vesicles, which are naturally shed from the surface of the bacterium, induce a protective response when administered intragastrically to mice in the presence of cholera holotoxin, despite the absence of the urease enzyme and associated Hsp54 chaperonin. Immunoblotting identified a specific serum immunoglobulin G (IgG) response to an 18-kDa outer membrane protein in a significant number of immunized animals. This commonly expressed, immunodominant protein was subsequently identified as lipoprotein 20 (Lpp20). Hybridoma backpacks secreting an IgG1 subclass monoclonal antibody to Lpp20 were generated in H. pylori-infected mice and were found to significantly reduce bacterial numbers, providing evidence that this surface-exposed antigen is a true vaccine candidate and not merely an antigenic marker for successful, protective immunization.
Helicobacter pylori, a bacterium which is estimated to infect more than half the world's population, is associated with peptic ulcer disease (4) and the development of gastric cancer (32). Immunization against this bacterium represents a cost-effective strategy to reduce global gastric cancer rates (5) and would also have a major impact on H. pylori-related peptic ulcer disease. H. pylori vaccine candidates identified to date include the urease enzyme (20, 40, 51, 55) and the urease enzyme chaperonin heat shock protein A (21). Mice immunized with purified VacA cytotoxin are also protected from challenge with a Tox+ strain of H. pylori (48). A common factor among these three vaccine candidates is their reported association with the outer membrane of H. pylori (1, 16, 17, 27, 36, 52, 57). The potential of catalase as an H. pylori vaccine candidate has also been identified (58). This enzyme, which is found in both the cytosol and the periplasmic space of H. pylori (28), is also thought to be surface exposed (57). More recently, the screening of recombinant H. pylori antigens (30) has identified another five potential H. pylori vaccine candidates. These include Lpp20, a conserved H. pylori lipoprotein that is membrane associated but not surface exposed (38).
In our search for candidate H. pylori vaccine antigens, we have focused on the outer membrane of the bacterium. Like many other gram-negative bacteria (reviewed in reference 25), H. pylori and Helicobacter felis shed part of their outer membrane as vesicles when grown under certain conditions (34). These outer membrane vesicles (OMV) are thought to be formed when the outer membrane of the bacterium expands faster than the underlying peptidoglycan layer, resulting in portions of the membrane blebbing off the surface of growing cells (44). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis reveals that the protein and lipopolysaccharide content of these OMV closely resembles that of a Sarkosyl-insoluble outer membrane preparation of the parent bacterium (J. Keenan, unpublished observation).
We found that 70% of BALB/c mice were protected from infectious challenge with H. felis following intragastric immunization with H. felis OMV and cholera toxin (CT) (Keenan, unpublished). Furthermore, protection from infectious challenge in these animals correlates with marked serum immunoglobulin G (IgG) antibody responsiveness to an 18-kDa antigen present in H. felis OMV (35). H. pylori outer membranes are also immunogenic in mice (14). We found that intragastric immunization with H. pylori OMV in conjunction with CT as an adjuvant elicits a serum IgG response to a similarly sized immunodominant outer membrane antigen (35) which is commonly expressed by H. pylori strains (34).
In this study, we used the recently developed standardized murine model of H. pylori infection (39) and confirmed the immunogenicity of H. pylori OMV in immune protection. As with the H. felis model, antibodies to the 18-kDa outer membrane antigen were a marker for protective immunity in mice. A monoclonal antibody (MAb) to the H. pylori antigen, used to screen an H. pylori genomic expression library, identified this outer membrane antigen as Lpp20. In vivo passive-protection experiments with mice confirmed that Lpp20 is a candidate vaccine antigen and not merely an antigenic marker for successful, protective immunization. In addition, we used immunolabeling studies to show that Lpp20 is surface exposed, not only on H. pylori but also when expressed as a recombinant protein by Escherichia coli.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free, female BALB/c mice were housed according to Health Research Council of New Zealand guidelines and were allowed free access to food and water.
Bacteria.
A well-characterized, Tox+ strain, H. pylori 60190 (41), produced the OMV used to immunize the mice. Mice were subsequently challenged with the SS1 (Sydney) strain of H. pylori (39). Both strains were grown in 2.8% (wt/vol) brucella broth base (Difco, Detroit, Mich.), supplemented with 5% fetal calf serum (Gibco BRL, Auckland, New Zealand). Cultures were incubated at 37°C in a microaerobic environment (10% hydrogen, 10% carbon dioxide, and 80% nitrogen) and were shaken at 120 rpm. E. coli strains were routinely grown in Luria-Bertani (LB) broth or on LB plates (1% [wt/vol] tryptone, 0.5% [wt/vol] yeast extract [Difco], 0.5% [wt/vol] NaCl [pH 7.0]) at 37°C under aerobic conditions with aeration at 200 rpm. Recombinant E. coli organisms were grown in LB medium containing 100 μg of ampicillin/ml as the selectable marker.
H. pylori OMV.
Whole bacteria were harvested from 48- to 72-h broth cultures by two centrifugations (10,000 × g, 15 min, 4°C). The spent-culture supernatants were ultracentrifuged (100,000 × g, 2 h, 4°C), and the resulting pellet of OMV was washed three times with phosphate-buffered saline (PBS) (100,000 × g, 2 h, 4°C) (35). The absence of whole bacteria and flagella in the preparation was confirmed by electron microscopy. The protein concentration of the OMV fraction was assayed (49) prior to storage of the fraction at −20°C until use.
Prophylactic immunization and challenge of mice.
Six- to eight-week-old mice were immunized four times by gastric intubation at weekly intervals. Each dose consisted of 50 μg of H. pylori (60190) OMV protein and 10 μg of CT (Sigma Chemical Co., St. Louis, Mo.) (13). Age-matched control mice were not immunized. Mice were challenged with a single dose of 108 H. pylori (SS1) organisms 7 days after the last immunization.
Assessment of protection.
Twenty-eight days after challenge, the mice were killed by cervical dislocation. The stomach of each animal was removed, bisected longitudinally, and pinned out. Full-thickness tissue (5 by 5 mm) was taken from the antrum-body area of one-half of each stomach and placed in 0.2 ml of urease test medium (29). Urease activity in the samples, identified by a distinctive color change in the medium, was assessed after 24 h of incubation at room temperature (RT). The remainder of the stomach was fixed in 10% buffered formalin and embedded in paraffin. Longitudinal sections, stained with a modified May-Grunwald Giemsa stain, were scanned full length using light microscopy (oil immersion lens). H. pylori cells per longitudinal section were counted and scored as follows: 0 (no bacteria), 1+ (1 to 10 bacteria), 2+ (11 to 50 bacteria), 3+ (51 to 100 bacteria), or 4+ (>100 bacteria). Mice with scores of 0 or 1+ were considered protected (40).
Immunoblot analysis of antibody response to immunization.
Serum antibody specificity was determined by immunoblotting following electrophoretic transfer of SDS-PAGE-separated (12.5% acrylamide) H. pylori OMV to 0.45-μm-pore-size nitrocellulose (NC) membranes. Following a 30-min wash in Tris-saline blotting buffer, antigen-impregnated NC strips (5 μg of protein) were incubated with individual sera for 2 h at RT. After a washing, bound murine antibodies were detected by incubation of the strips in alkaline phosphatase-conjugated goat anti-mouse IgG antibody (Sigma) for 1 h at RT. Secondary antibody binding was detected by reaction with 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium substrate (2).
Production and screening of an anti-H. pylori 18-kDa outer membrane antigen hybridoma.
H. pylori 18-kDa outer membrane antigen-specific MAbs were produced following subcutaneous immunization of a BALB/c mouse, as described previously (35). Briefly, the 18-kDa antigen was identified following its separation from other outer membrane components by preparative SDS-PAGE and immunoblotting to NC. The band was excised and implanted under the dorsal skin of the mouse. Twenty-one days later, the animal was injected intraperitoneally with H. pylori OMV (50 μg of protein). The spleen lymphocytes were fused with FOX-NY mouse myeloma cells at a ratio of 5:1, 7 days after the second immunization (42). Clones were obtained by limiting dilution and were screened by enzyme-linked immunosorbent assay for an antibody response to H. pylori OMV antigens (see above). From these, an IgG1 subclass-producing hybridoma (6A8) was selected for further investigation.
Construction of an H. pylori expression library.
Genomic DNA isolated from an H. pylori clinical strain (53) was partially digested with Sau3A (0.3 U) for 10 min at 37°C, and following electrophoresis, DNA fragments of between 2 and 10 kb were excised from a 1% low-melting-point agarose gel and purified using GELase (Epicentre Technologies, Madison, Wis.). The H. pylori DNA fragments were ligated into the BamHI site of the lambda Zap vector (Stratagene), and the recombinant lambda was packaged by using a MaxPlax packaging extract (Epicentre Technologies) according to the manufacturer's instructions. The packaged phage (1 μl) were used to transfect E. coli XL1-blue (optical density at 600 nm = 0.5) by incubating the cells with the phage at 37°C for 30 min. The phage were plated by pouring the cells onto an LB plate after the addition of 3 ml of melted top agar (containing 10 mM MgSO4, 2.5 mM isopropyl-β-d-thiogalactopyranoside [IPTG], and 4 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside [X-Gal]/ml). A color assay was used to determine the ratio of recombinants to nonrecombinants by plating the library on IPTG–X-Gal.
Antibody screening of the H. pylori expression library.
MAb 6A8, raised against the immunogenic 18-kDa outer membrane antigen (see Fig. 2c), was used to screen the H. pylori expression library. Around 6,000 plaques were plated onto LB plates and incubated at 37°C overnight. IPTG-saturated disks of Hybond-C extra hybridization transfer membrane (Amersham) were placed on the phage to induce expression of the fusion proteins for 6 h at 37°C and then were placed at 4°C overnight. The membranes were removed, blocked in 2% milk powder in PBS–0.1% Tween 20 (PBS-T) for 2 h at RT, and then incubated with MAb 6A8 diluted 1:100 in blocking buffer for 1 h at RT. Following washing with PBS-T, membranes were incubated with a goat anti-mouse IgG-alkaline phosphatase conjugate (Sigma) diluted 1:1,000 in blocking buffer for 1 h at RT. The membranes were washed twice with PBS-T and once with substrate buffer (100 mM Tris, 100 mM NaCl, 10 mM MgCl2). Positive plaques detected by the antibody were visualized by the addition of 50 μg of 5-bromo-4-chloro-3-indolylphosphate (XP)/ml and 0.01% nitroblue tetrazolium as substrates (Sigma). Positive plaques were selected following secondary screening, transferred to sodium-magnesium buffer containing 1/25 volume of chloroform, and vortexed to release the phage particles, which were then stored at 4°C.
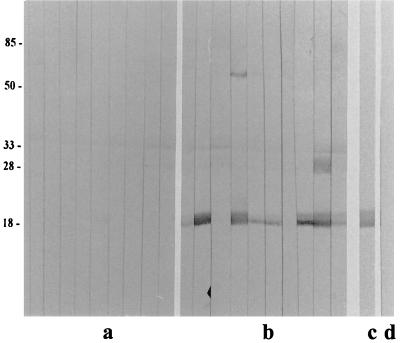
FIG. 2.
Immunoblot analysis of serum IgG reactivity (1:100 dilution) to H. pylori 60190 OMV (5 μg of protein/lane) in individual mice before (a) and after (b) intragastric immunization (H. pylori 60190 OMV and CT) and challenge (H. pylori SS1). Controls were OMV from H. pylori 60190 immunoblotted with MAb 6A8 (c), a MAb specific for the H. pylori 18-kDa outer membrane antigen, and CMRF-82 (d), a MAb specific for tetanus toxoid. Molecular mass markers (in kilodaltons) are indicated (left).
Sequence analysis of phagemid inserts.
The in vivo excision and recircularization of the cloned insert to form a phagemid containing the insert were carried out by coinfecting the phage stock with E. coli XLI-blue and ExAssist helper phage (>106 PFU/ml) according to the manufacturer's instructions. Phagemid DNA was prepared by using a plasmid isolation kit (Bresatec, Adelaide, Australia). The H. pylori DNA inserts in these phagemids were analyzed by automated sequencing (Massey University DAN Analysis Service) across the cloning junctions, using the universal primers T3 and T7. The DNA sequences were compared to the published Institute for Genome Research sequence of H. pylori strain 26695 (63), and the genes contained in each phagemid were identified.
Cloning and expression of H. pylori reading frames.
Oligonucleotide primers were designed to amplify open reading frames (ORFs) HP1456 and HP1457 based on the published genome sequence (63). The primers were designed to amplify the ORF devoid of its signal sequence, with a BamHI site incorporated into the 5′ end and an EcoRI site at the 3′ end as follows (5′ to 3′): for HP 1456, CTTTAGGATCCGTGGGTTGCTGAAG (forward) and TATTTGAATTCAAAACATACGCTTA (reverse); for HP 1457, TCGTAGGATCCAGCCATGCC (forward) and AAGGCGAATTCTTAAAACCCT (reverse). Genomic DNA prepared from H. pylori strain CCUG 17874 was used as the template in the PCR. The PCR products were amplified under standard conditions, using Pwo Taq polymerase (Boehringer Mannheim). The PCR cycle consisted of 30 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 30 s, and an extension step at 72°C for 60 s. Products were visualized on a 1% Tris-acetate-EDTA-agarose gel and purified using a gel purification kit (Qiagen, Clifton Hill, Australia). After digestion with the restriction enzymes BamHI and EcoRI, the purified products were cloned into the compatible sites of the expression vectors pPROex HTb (Life Technologies) and pGEX-6P-3 (Pharmacia Biotech). Recombinant plasmids were purified and transformed into competent E. coli cells by using standard procedures. Recombinant E. coli cells were grown until mid-log phase (optical density at 600 nm = 0.5 to 1.0), and expression of the fusion proteins was induced by the addition of 0.5 mM IPTG. Following induction, the cells were harvested by centrifugation at 12,000 × g and resuspended in 20 mM Tris (pH 8.0)–10 mM MgCl2. Total protein was electrophoresed on SDS-PAGE gels and stained with Coomassie or transferred to a polyvinylidene difluoride (PVDF) membrane for immunoblotting with MAb 6A8 (see above).
In vivo passive-protection experiment.
Eight- to ten-week-old naive BALB/c mice were infected with H. pylori SS1 by intragastric intubation. A total of 108 viable bacteria were given over two consecutive days. Hybridoma cells producing anti-H. pylori Lpp20 (MAb 6A8) or anti-tetanus toxoid (CMRF-82) were grown in RPMI medium, harvested, and washed twice in PBS. CMRF-82, an IgG1 subclass antibody against a tetanus toxoid component, fails to display reactivity against H. pylori OMV antigens (see Fig. 2d) and was used in this experiment as a control. At day 4, 106 hybridoma cells were injected subcutaneously between the scapulae of each mouse to generate IgG1-secreting hybridoma tumors (50, 64). When the experiment was concluded at day 20, every mouse was carrying a large backpack tumor. Immunoblotting of sera from these mice confirmed the presence of circulating MAb. The ability of MAb 6A8 to protect these mice from H. pylori infection was assessed by quantitative culture (22) as well as biopsy urease and histological analyses (see above) of antrum-body tissue samples. To perform quantitative bacterial counts, tissue fragments were homogenized in 500 μl of brucella broth. The homogenates were serially diluted in sterile saline and plated onto selective medium. Bacterial counts were expressed as the mean number of CFU per gram of tissue.
Immunolabeling of whole bacteria with MAb 6A8.
MAb 6A8 was used to immunolabel the surface of H. pylori 60190, as described previously (35). In a similar experiment, the same technique was used to immunolabel E. coli expressing recombinant Lpp20. Briefly, whole bacteria were washed twice with low-salt phosphate, overlaid onto carbon-colloidin-coated mesh grids, and blocked with 0.1% bovine serum albumin (in low-salt phosphate) for 15 min before being incubated in MAb 6A8 (diluted in blocking buffer) for 1 h at RT. Gold (10 nm)-labeled goat anti-mouse IgG was used to detect murine antibody binding. The grids were then negatively stained with 1% aqueous phosphotungstic acid (pH 7.0) prior to examination.
Statistical analysis.
Fisher's exact test was used to evaluate the presence or absence of experimental infection in test and control animals as well as the anti-18-kDa outer membrane antigen response to immunization. P values were determined by the InStat software program (GraphPad, San Diego, Calif.).
RESULTS
Immunization of mice with H. pylori OMV correlates with serum reactivity against an immunodominant 18-kDa major antigen.
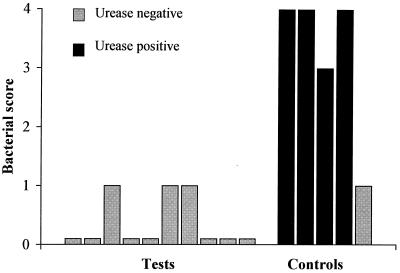
Intragastric immunization with H. pylori (60190) OMV and CT conferred immune protection against H. pylori (SS1) challenge in 10 of 10 mice (100%). In contrast, 4 of 5 (80%) naive control animals were infected with H. pylori (Fig. 1). These differences were statistically significant (P = 0.0013). Protection from infectious challenge correlated with serum antibody reactivity to an H. pylori 60190 OMV antigen with an apparent molecular mass of 18 kDa in 8 of the 10 mice following intragastric immunization (Fig. 2b). Similar reactivity, absent in sera collected from these same animals prior to immunization (Fig. 2a), was seen when these same sera were immunoblotted against OMV from H. pylori SS1 (results not shown). Statistical analysis revealed that this specific antibody response to immunization was significant (P = 0.0007). Sera from mice sham immunized with PBS and CT (35) failed to display similar immune responsiveness (results not shown), indicating that this response is specific to animals immunized with H. pylori OMV.
FIG. 1.
Mice were immunized intragastrically with H. pylori (60190) OMV (50 μg of protein) and 10 μg of CT once weekly for 4 weeks prior to infectious challenge. H. pylori SS1 infection was determined by urease assay and confirmed by enumeration of bacteria in ∼8-mm stained sections of antral-body mucosa. The bacterial scores for immunized animals were found to be significantly lower than scores for unimmunized control mice (Mann-Whitney U test; P = 0.0013).
Immunoscreening of the H. pylori expression library with MAb 6A8.
Immunoscreening of the expression library with MAb 6A8 revealed eight strongly positive plaques. After secondary screening to confirm their reactivity, the H. pylori DNA was excised from each and recircularized to form a phagemid containing the H. pylori insert DNA. Restriction digests of these phagemids revealed that six contained an identical insert of approximately 2 kb, while the remaining two contained inserts of approximately 2.5 kb (data not shown). Sequence analysis of these phagemid inserts revealed that they all mapped to the same region of the H. pylori genome, encompassing the promoter and start codons of three genes (HP 1455, HP 1456, and HP 1457). Each of these three genes contains a putative signal peptide and therefore is a candidate to encode an OMV protein. Two of these genes (HP 1456 and HP 1457), when translated from the putative cleavage point, produce proteins of approximately 19.11 kDa (HP 1456) and 23.31 kDa (HP 1457). Although the predicted size of the gene product indicates that the HP 1456 ORF is likely to encode the 18-kDa antigen detected by the antibody, further experiments were required to confirm this.
Identification of Lpp20 as the immunogenic 18-kDa antigen.
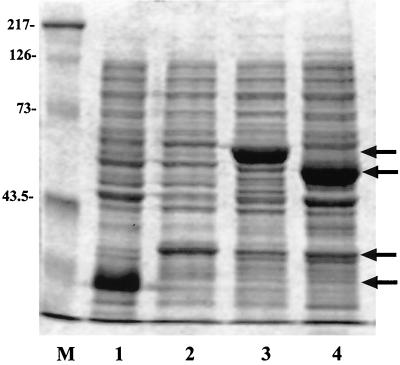
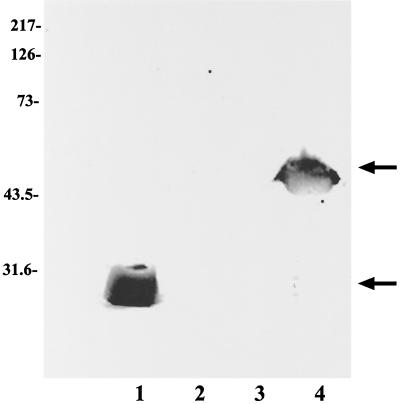
To investigate which ORF (HP 1456 or HP 1457) encoded the 18-kDa antigen, the ORFs of each were cloned and the protein was expressed as a recombinant fusion protein. Oligonucleotide primers were designed to amplify a truncated ORF devoid of the signal sequence. Expression of the recombinant fusion protein was maximally induced in pPROex HTb over 4 h by the addition of 0.5 mM IPTG (results not shown). Expression of HP 1456 and HP 1457 in this vector resulted in the production of fusion proteins of 23.6 and 27.8 kDa, respectively, with the N-terminal signal sequence replaced with a string of six histidines, a spacer region, and an rTEV (a recombinant endopeptidase from the tobacco etch virus) protease cleavage site (Fig. 3, lanes 1 and 2, respectively). Similarly, induction of expression in pGEX-6P-3 resulted in a fusion protein of 42.8 kDa for HP 1456 and 47.0 kDa for HP 1457 (Fig. 3, lanes 4 and 3, respectively), with the N terminus being replaced by glutathione S-transferase (GST) and a recognition site for the protease PreScission. Total protein was prepared from cultures of cells containing each construct, separated on an SDS–12.5% PAGE gel, and transferred to PVDF for Western blotting with MAb 6A8. A positive reaction was seen with both HP 1456 fusion proteins, but no reaction was observed with either HP 1457 protein (Fig. 4). The HP 1456 gene codes for H. pylori Lpp20 (63).
FIG. 3.
SDS-PAGE gel (15% acrylamide) of H. pylori proteins HP 1456 and HP 1457, expressed as either histidine-tagged (lanes 1 and 2) or GST-tagged (lanes 3 and 4) fusion proteins and stained with Coomassie. Lanes: M, Kaleidoscope markers (Bio-Rad); 1, HP 1456-His; 2, HP 1457-His; 3, HP 1457-GST; 4, HP 1456-GST. Molecular mass markers (in kilodaltons) are indicated (left).
FIG. 4.
Identification of HP 1456 with an anti-18-kDa-protein MAb. H. pylori proteins HP 1456 and HP 1457, expressed as either histidine-tagged (lanes 1 and 2) or GST-tagged (lanes 3 and 4) fusion proteins, were analyzed by SDS-PAGE (15% acrylamide), transferred to a PVDF membrane, and screened with MAb 6A8. Lanes: 1, HP 1456-His; 2, HP 1457-His; 3, HP 1457-GST; 4, HP 1456-GST. Molecular mass markers (in kilodaltons) are indicated (left).
Passive protection against H. pylori infection confirms the role of Lpp20 in protective immunity.
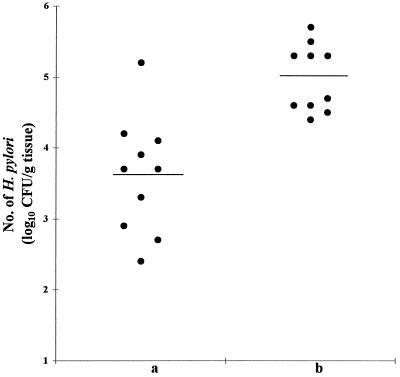
Hybridoma backpacks were generated in mice to investigate whether circulating 6A8, an IgG1 subclass MAb raised against H. pylori Lpp20, could prevent (or reduce) H. pylori colonization in these animals. At sacrifice, each of these mice was shown by immunoblotting to have circulating MAb 6A8. Furthermore, quantitative culturing of gastric tissue revealed these mice to have lower numbers of H. pylori cells, expressed as log10 CFU per gram of stomach, than infected control animals carrying non-H. pylori-reactive hybridoma backpacks (Fig. 5). These differences were highly significant (P = 0.0002). Microscopic grading of gastric tissue confirmed this finding (results not shown).
FIG. 5.
Two groups of female BALB/c mice, each containing 10 animals, were infected with 108 H. pylori SS1 cells. Hydridoma backpack tumors expressing either MAb 6A8 (a) or CMRF-82 IgG1 (b) subclass MAbs were generated in the animals 4 days after infectious challenge. At sacrifice (day 20), the mean numbers of CFU in homogenates of gastric samples were determined from serial counts of each homogenate. Each point represents the log10 CFU per gram of tissue. Horizontal bars represent the geometric means for animals in each group.
MAb 6A8 binds to a surface-exposed epitope on Lpp20.
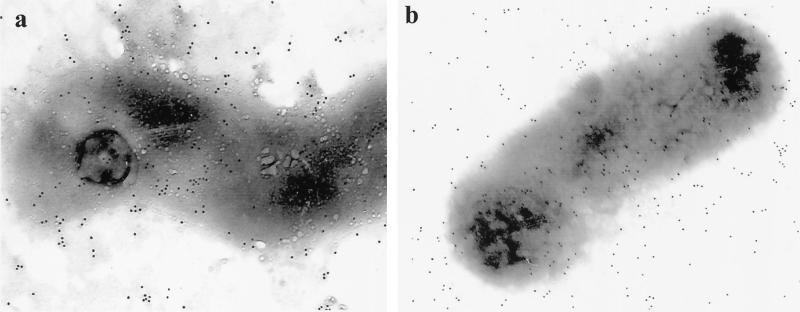
Immunogold electron microscopy with MAb 6A8 showed binding of the MAb to the surface of H. pylori 60190 cells (Fig. 6a). Furthermore, this same technique showed that recombinant Lpp20, produced by E. coli subcloned with an expression vector containing the antigen coding region, was also expressed on the bacterial surface (Fig. 6b).
FIG. 6.
Immunogold electron microscopy of H. pylori 60190 (a) and E. coli expressing recombinant Lpp20 (b) following incubation in 6A8, a MAb specific for Lpp20. A gold-labeled goat anti-mouse IgG antibody detected MAb binding to the outer membranes of both bacteria.
DISCUSSION
The outer membrane is a continuous structure on the surface of gram-negative bacteria and, in bacterial pathogens, has particular significance as a potential target for protective immunity. Outer membrane vaccines have been used with considerable success to induce protection against a number of organisms, including group B Neisseria meningitidis (24), Pasteurella multocida (45), Porphyromonas gingivalis (37), and Moraxella catarrhalis (47). Using OMV shed from the surface of the bacterium during growth in broth culture, we were able to protect 100% of mice from H. pylori SS1 challenge following oral immunization with H. pylori 60190 OMV with CT as a mucosal adjuvant.
Two well-documented vaccine candidates are associated with the surface of Helicobacter (20, 21, 40, 51, 55) and could have contributed to the protective effect seen in these studies. However, both functional and immunological assays used to screen for the presence of the urease enzyme and its associated Hsp54 chaperonin failed to detect either of these antigens in the OMV fraction of H. pylori (36). Our ability to protect mice from infectious challenge in the absence of both urease and associated Hsp suggested the presence of a new vaccine candidate in the outer membrane fraction. Immunoblotting demonstrated specific serum IgG immunoreactivity to an OMV component with an apparent molecular mass of 18 kDa in immunized and protected mice. Using a MAb, we subsequently identified Lpp20 as the potential vaccine candidate.
Lipoproteins are major antigens in a number of bacterial pathogens, including E. coli (33), Haemophilus influenzae (9), Pseudomonas aeruginosa (43), Borrelia burgdorferi (18), and Campylobacter jejuni (7). A number of unrelated studies have now identified Lpp20 as an immunodominant H. pylori antigen (8, 30, 34, 38). Moreover, it is likely that the immunoreactive species-specific 19-kDa H. pylori outer membrane protein described in an earlier study is also Lpp20 (15). H. pylori is noninvasive, and it is likely that outer membrane-associated Lpp20 (38), which is released from the surface of H. pylori during growth in vitro (8), is delivered to the gastric mucosa in the OMV shed from the surface of the bacterium in vivo (36).
Despite the potential problems of qualitative and quantitative expression of outer membrane proteins, the Lpp20 antigen appears to be commonly expressed in all H. pylori strains examined so far (15, 34, 38). Furthermore, no cross-reaction is shown when antibodies (polyclonal and monoclonal) to H. pylori Lpp20 are used to immunoscreen closely related species of Helicobacter (15, 34, 38), Campylobacter (15, 38), or a diverse range of other bacteria (38). This supports a search of data banks which shows the lpp20 gene to be unique to H. pylori (38).
The amino acid sequence of Lpp20 implies outer membrane localization of this protein based on the prediction of Yamaguchi et al. (65). This was confirmed with an anti-Lpp20 MAb. Furthermore, immunolabeling of H. pylori with gold-labeled anti-Lpp20 antibodies confirmed that the protein is expressed on the surface of the bacterium. In contrast, cross-reactivity with H. felis proteins was not demonstrated using MAb 6A8 (35). This supports an earlier observation that mice immunized with either H. pylori or H. felis OMV produce serum antibodies that bind significantly to homologous antigen only (34). Immunolabeling studies of H. pylori by Drouet et al. also find an immunogenic 19-kDa antigen to be surface exposed (15). The failure, therefore, of Kostrzynska et al. to show Lpp20 on the surface of H. pylori may simply reflect conformational differences in the SDS-PAGE-denatured protein used to raise their rabbit polyclonal anti-H. pylori Lpp20 antiserum (38).
Bacterial lipoproteins are well described, not only as vaccine target candidates (23, 61, 62) but also as immunostimulatory molecules (26). We used an in vivo passive-protection model, based on the generation of hybridoma backpack tumors in mice (50, 64), to show that Lpp20 is a true vaccine candidate and not merely an immunogenic marker for protection. Using this model, we demonstrated that an anti-Lpp20-secreting tumor in vivo correlated with a significant decrease in H. pylori colonization of the murine gastric mucosa. A recently published, molecular approach to identifying H. pylori vaccine antigens also identifies Lpp20 as a candidate following the successful immunization of mice with purified recombinant Lpp20 antigen (30).
An isogenic mutant, defective in the production of Lpp20, shows this H. pylori protein to be nonessential for growth in vitro (38). However, in the hybridoma backpack model, the reduction in gastric H. pylori levels correlated with expression of the Lpp20-specific MAb in the circulation of infected mice. This finding strongly suggests a role for antibody-mediated protection against this bacterium, despite recent evidence to the contrary (19, 56). We find that BALB/c mice recognize a similarly sized surface antigen following infection with H. pylori SS1. However, our preliminary evidence suggests that protection is not only related to the specificity but also reliant on the magnitude and subclass of the response (Keenan, unpublished). Whether this surface-exposed immunodominant lipoprotein has a functional role in the outer membrane of the bacterium remains to be elucidated. Some functions ascribed to other bacterial lipoproteins include the stimulation of host cytokine production (46, 60), iron uptake (6, 59), adhesion (54), and the induction of proliferation and immunoglobulin production in mouse B cells (31).
However, a protective antibody response does not have to target a specific bacterial function (3). If protection is mediated (at least in part) by specific antibody action, successful immunization may be the result of sufficient antibody binding to Lpp20 epitopes, thereby cross-linking and agglutinating the bacteria and ultimately enhancing their removal by peristalsis (40). Support for this hypothesis comes from the observation that the most likely H. pylori vaccine candidates identified to date (urease, heat shock protein, and Lpp20) are all surface exposed (1, 16, 17, 27, 35, 52, 57).
Finally, electron microscopy of immunolabeled E. coli transformants expressing recombinant Lpp20 showed the lipoprotein to be surface exposed, an observation which is also noted when the P. aeruginosa lipoprotein gene (oprI) is cloned into E. coli (10). Live carriers are ideal vaccine delivery systems and are being increasingly used to express large amounts of protective recombinant antigens (11, 12, 23, 61, 62). We are currently investigating a role for a recombinant carrier such as Salmonella enterica serovar Typhimurium phoP(Con) (11) to provide a mucosal vaccine vector to deliver Lpp20 to antigen-presenting cells on mucosal surfaces.
ACKNOWLEDGMENTS
We thank Adrian Lee (School of Microbiology & Immunology, University of New South Wales, Kensington, Australia) for providing H. pylori SS1, John Lewis and Peter Elder (Steroid Laboratory, Christchurch Hospital) for advice on MAb production, and Kate Arnold for providing CMRF-82 as a control MAb for this study.
This work was supported by grants from the Canterbury Medical Research Foundation, the University of Otago (J.K.), and the Health Research Council of New Zealand (J.O.).
REFERENCES
- 1.Austin J W, Doig P, Stewart M, Trust T J. Macromolecular structure and aggregation states of Helicobacter pylori urease. J Bacteriol. 1991;173:5663–5667. doi: 10.1128/jb.173.18.5663-5667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blake M S, Johnston K H, Russell-Jones G J, Gotschlich E C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on western blots. Anal Biochem. 1984;136:175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard T G, Czinn S J, Maurer R, Thomas W D, Soman G, Nedrud J G. Urease-specific monoclonal antibodies prevent Helicobacter felis infection in mice. Infect Immun. 1995;63:1394–1399. doi: 10.1128/iai.63.4.1394-1399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser M J. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992;102:720–727. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- 5.Bloom B R. Vaccines for the Third World. Nature. 1989;342:115–120. doi: 10.1038/342115a0. [DOI] [PubMed] [Google Scholar]
- 6.Boulton I C, Gorringe A R, Shergill J K, Jannou C L, Evans R W. A dynamic model of the meningococcal transferrin receptor. J Theor Biol. 1999;198:497–505. doi: 10.1006/jtbi.1999.0928. [DOI] [PubMed] [Google Scholar]
- 7.Burnens A, Stucki U, Nicolet J, Frey J. Identification and characterization of an immunogenic outer membrane protein of Campylobacter jejuni. J Clin Microbiol. 1995;33:2826–2832. doi: 10.1128/jcm.33.11.2826-2832.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao P, McClain M S, Forsyth M H, Cover T L. Extracellular release of antigenic proteins by Helicobacter pylori. Infect Immun. 1998;66:2984–2986. doi: 10.1128/iai.66.6.2984-2986.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chanyangam M, Smith A L, Moseley S L, Kuehn M, Jenny P. Contribution of a 28-kilodalton membrane protein to the virulence of Haemophilus influenzae. Infect Immun. 1991;59:600–608. doi: 10.1128/iai.59.2.600-608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelis P, Sierra J C, Lim A, Jr, Malur A, Tungpradabkul S, Tazka H, Leitao A, Martins C V, di Perna C, Brys L, De Baetselier P, Hamers R. Development of new cloning vectors for the production of immunogenic outer membrane fusion proteins in Escherichia coli. Biotechnology. 1996;14:203–208. doi: 10.1038/nbt0296-203. [DOI] [PubMed] [Google Scholar]
- 11.Corthésy-Theulaz I E, Hopkins S, Bachmann D, Saldinger P F, Porta N, Haas R, Zheng-Xin Y, Meyer T, Bouzourene H, Blum A L, Kraehenbuhl J-P. Mice are protected from Helicobacter pylori infection by nasal immunization with attentuated Salmonella typhimurium phoPc expressing urease A and B subunits. Infect Immun. 1998;66:581–586. doi: 10.1128/iai.66.2.581-586.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cote-Sierra J, Jongert E, Bredan A, Gautam D C, Parkhouse M, Cornelis P, De Baetselier P, Revets H. A new membrane-bound OprI lipoprotein expression vector. High production of heterologous fusion proteins in gram (−) bacteria and the implications for oral vaccination. Gene. 1998;221:25–34. doi: 10.1016/s0378-1119(98)00437-5. [DOI] [PubMed] [Google Scholar]
- 13.Czinn S J, Nedrud J G. Oral immunization against Helicobacter pylori. Infect Immun. 1991;59:2359–2363. doi: 10.1128/iai.59.7.2359-2363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doig P, Trust T J. Identification of surface-exposed outer membrane antigens of Helicobacter pylori. Infect Immun. 1994;62:4526–4533. doi: 10.1128/iai.62.10.4526-4533.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drouet E B, Denoyel G A, Boude M, Wallano E, Andujar M, de Montclos H P. Characterization of an immunoreactive species-specific 19-kilodalton outer membrane protein from Helicobacter pylori by using a monoclonal antibody. J Clin Microbiol. 1991;29:1620–1624. doi: 10.1128/jcm.29.8.1620-1624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunn B E, Campbell G P, Perez-Perez G I, Blaser M J. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990;265:9464–9469. [PubMed] [Google Scholar]
- 17.Dunn B E, Roop R M, II, Sung C-C, Sharma S A, Perez-Perez G I, Blaser M J. Identification and purification of a cpn60 heat shock protein homolog from Helicobacter pylori. Infect Immun. 1992;60:1946–1951. doi: 10.1128/iai.60.5.1946-1951.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erdile L F, Brandt M-A, Warakomski D J, Westrack G J, Sadziene A, Barbour A G, Mays J P. Role of attached lipid in immunogenicity of Borrelia burgdorferi OspA. Infect Immun. 1993;61:81–90. doi: 10.1128/iai.61.1.81-90.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ermak T H, Giannasca P G, Nichols R, Myers G A, Nedrud J, Weltzin R, Lee C K, Kleanthous H, Monath T P. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J Exp Med. 1998;188:2277–2288. doi: 10.1084/jem.188.12.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrero R L, Thiberge J-M, Huerre M, Labigne A. Recombinant antigens prepared from the urease subunits of Helicobacter spp.: evidence of protection in a mouse model of gastric infection. Infect Immun. 1994;62:4981–4989. doi: 10.1128/iai.62.11.4981-4989.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrero R L, Thiberge J M, Kansau I, Wuscher N, Huerre M, Labigne A. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc Natl Acad Sci USA. 1995;92:6499–6503. doi: 10.1073/pnas.92.14.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrero R L, Thiberge J-M, Huerre M, Labigne A. Immune responses of specific-pathogen-free mice to chronic Helicobacter pylori (strain SS1) infection. Infect Immun. 1998;66:1349–1355. doi: 10.1128/iai.66.4.1349-1355.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finke M, Duchêne M, Eckhardt A, Domdey H, von Specht B-U. Protection against experimental Pseudomonas aeruginosa infection by recombinant P. aeruginosa lipoprotein I expressed in Escherichia coli. Infect Immun. 1990;58:2241–2244. doi: 10.1128/iai.58.7.2241-2244.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fredriksen J H, Rosenqvist E, Wedege E, Bryn K, Bjune G, Froholm L O, Lindbak A K, Mogster B, Namork E, Rye U, Stabbetorp G, Winsnes R, Aase B, Closs O. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 1991;14:67–80. [PubMed] [Google Scholar]
- 25.Gamazo C, Moriyón I. Release of outer membrane fragments by exponentially growing Brucella melitensis cells. Infect Immun. 1987;55:609–615. doi: 10.1128/iai.55.3.609-615.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haupl T, Landgraf S, Netusil P, Biller N, Capiau C, Desmons P, Hauser P, Burmester G R. Activation of monocytes by three OspA vaccine candidates: lipoprotein OspA is a potent stimulator of monokines. FEMS Immunol Med Microbiol. 1997;19:15–23. doi: 10.1111/j.1574-695X.1997.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 27.Hawtin P R, Stacey A R, Newell D G. Investigation of the structure and localization of the urease of Helicobacter pylori using monoclonal antibodies. J Gen Microbiol. 1990;136:1995–2000. doi: 10.1099/00221287-136-10-1995. [DOI] [PubMed] [Google Scholar]
- 28.Hazell S, Evans D J, Graham D. Helicobacter pylori catalase. J Gen Microbiol. 1991;137:57–61. doi: 10.1099/00221287-137-1-57. [DOI] [PubMed] [Google Scholar]
- 29.Hazell S L, Borody T J, Gal A, Lee A. Campylobacter pyloridis gastritis. 1. Detection of urease as a marker of bacterial colonization and gastritis. Am J Gastroenterol. 1987;82:292–296. [PubMed] [Google Scholar]
- 30.Hocking D, Webb E, Radcliffe F, Rothel L, Taylor S, Pinczower G, Kapouleas C, Braley H, Lee A, Doidge C. Isolation of recombinant protective Helicobacter pylori antigens. Infect Immun. 1999;67:4713–4719. doi: 10.1128/iai.67.9.4713-4719.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honarvar N, Schaible U E, Galanos C, Wallich R, Simon M M. A 14,000 MW lipoprotein and a glycolipid-like structure of Borrelia burgdorferi induce proliferation and immunoglobulin production in mouse B cells at high frequencies. Immunology. 1994;82:389–396. [PMC free article] [PubMed] [Google Scholar]
- 32.IARC. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:220. [PMC free article] [PubMed] [Google Scholar]
- 33.Ichihara S, Hussain M, Mizushima S. Characterization of new membrane lipoproteins and their precursors of Escherichia coli. J Biol Chem. 1981;256:3125–3129. [PubMed] [Google Scholar]
- 34.Keenan J I, Allardyce R A, Bagshaw P F. Dual silver staining to characterise Helicobacter spp. outer membrane components. J Immunol Methods. 1997;209:17–24. doi: 10.1016/s0022-1759(97)00141-5. [DOI] [PubMed] [Google Scholar]
- 35.Keenan J I, Allardyce R A, Bagshaw P F. Lack of protection following immunisation with H. pylori outer membrane vesicles highlights antigenic differences between H. felis and H. pylori. FEMS Microbiol Lett. 1998;161:21–27. doi: 10.1111/j.1574-6968.1998.tb12924.x. [DOI] [PubMed] [Google Scholar]
- 36.Keenan J I, Allardyce R A, Bagshaw P F. A role for the bacterial outer membrane in the pathogenesis of H. pylori infection. FEMS Microbiol Lett. 2000;182:259–264. doi: 10.1111/j.1574-6968.2000.tb08905.x. [DOI] [PubMed] [Google Scholar]
- 37.Kesavalu L, Ebersole J L, Machen R L, Holt S C. Porphyromonas gingivalis virulence in mice: induction of immunity to bacterial components. Infect Immun. 1992;60:1455–1464. doi: 10.1128/iai.60.4.1455-1464.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kostrzynska M, O'Toole P W, Taylor D E, Trust T J. Molecular characterization of a conserved 20-kilodalton membrane-associated lipoprotein antigen of Helicobacter pylori. J Bacteriol. 1994;176:5938–5948. doi: 10.1128/jb.176.19.5938-5948.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee A, O'Rourke J, Deungria M C, Robertson B, Daskalopoulos G, Dixon M F. A standardized mouse model of Helicobacter pylori infection—introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 40.Lee C K, Weltzin R, Thomas W D, Jr, Kleanthous H, Ermak T H, Soman G, Hill J E, Ackerman S K, Monath T P. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J Infect Dis. 1995;172:161–172. doi: 10.1093/infdis/172.1.161. [DOI] [PubMed] [Google Scholar]
- 41.Leunk R D, Johnson P T, David B C, Kraft W G, Morgan D R. Cytotoxin activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988;26:93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- 42.Lewis J G, Elder P A, Yeo K H J. A monoclonal antibody to prednisone: use of enzyme-linked immunosorbent assay (ELISA) for screening and characterization of antigenic determinants. J Steroid Biochem. 1986;25:659–663. doi: 10.1016/0022-4731(86)90008-7. [DOI] [PubMed] [Google Scholar]
- 43.Lim A, Jr, De Vos D, Brauns M, Mossialos D, Gaballa A, Qing D, Cornelis P. Molecular and immunological characterization of OprL, the 18 kDa outer-membrane peptidoglycan-associated lipoprotein (PAL) of Pseudomonas aeruginosa. Microbiology. 1997;143:1709–1716. doi: 10.1099/00221287-143-5-1709. [DOI] [PubMed] [Google Scholar]
- 44.Loeb M R, Kilner J. Release of a special fraction of the outer membrane from both growing and phage T4-infected Escherichia coli. Biochim Biophys Acta. 1978;514:117–127. doi: 10.1016/0005-2736(78)90081-0. [DOI] [PubMed] [Google Scholar]
- 45.Lu Y-S, Aguila H N, Lai W C, Pakes S P. Antibodies to outer membrane proteins but not to lipopolysaccharide inhibit pulmonary proliferation of Pasteurella multocida in mice. Infect Immun. 1991;59:1470–1475. doi: 10.1128/iai.59.4.1470-1475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma Y, Weis J J. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulating properties. Infect Immun. 1993;61:3843–3853. doi: 10.1128/iai.61.9.3843-3853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maciver I, Unhanand M, McCracken G H, Hansen E J. Effect of immunization on pulmonary clearance of Moraxella catarrhalis in an animal model. J Infect Dis. 1993;168:469–472. doi: 10.1093/infdis/168.2.469. [DOI] [PubMed] [Google Scholar]
- 48.Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 49.Markwell M A K, Haas S M, Bieber L L, Tolbert N E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 50.Michetti P, Mahan M J, Slauch J M, Mekalanos J J, Neutra M R. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992;60:1786–1792. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michetti P, Corthesy-Theulaz I, Davin C, Haas R, Vaney A-C, Heitz M, Bille J, Kraehenbuhl J-P, Saraga E, Blum A L. Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pylori urease. Gastroenterology. 1994;107:1002–1011. doi: 10.1016/0016-5085(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 52.Mobley H L T, Cortesia M J, Rosenthal L E, Jones B D. Characterization of urease from Campylobacter pylori. J Clin Microbiol. 1988;26:831–836. doi: 10.1128/jcm.26.5.831-836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mooney C, Keenan J, Munster D, Wilson I, Allardyce R, Bagshaw P, Chapman B, Chadwick V. Neutrophil activation by Helicobacter pylori. Gut. 1991;32:853–857. doi: 10.1136/gut.32.8.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Odenbreit S, Till M, Hofreuter D, Faller G, Haas R. Genetic and functional characterization of the alpAB gene locus essential for the adhesion of Helicobacter pylori to human gastric tissue. Mol Microbiol. 1999;31:1537–1548. doi: 10.1046/j.1365-2958.1999.01300.x. [DOI] [PubMed] [Google Scholar]
- 55.Pappo J, Thomas W D, Jr, Kabok Z, Taylor N S, Murphy J C, Fox J G. Effect of oral immunization with recombinant urease on murine Helicobacter felis gastritis. Infect Immun. 1995;63:1246–1252. doi: 10.1128/iai.63.4.1246-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pappo J, Torrey D, Castriotta L, Savinainen A, Kabok Z, Ibraghimov A. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infect Immun. 1999;67:337–341. doi: 10.1128/iai.67.1.337-341.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phadnis S H, Parlow M H, Levy M, Ilver D, Caulkins C M, Connors J B, Dunn B E. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect Immun. 1996;64:905–912. doi: 10.1128/iai.64.3.905-912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Radcliff F J, Hazell S L, Kolesnikow T, Doidge C, Lee A. Catalase, a novel antigen for Helicobacter pylori vaccination. Infect Immun. 1997;65:4668–4674. doi: 10.1128/iai.65.11.4668-4674.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reidl J, Mekalanos J J. Lipoprotein e(P4) is essential for hemin uptake by Haemophilus influenzae. J Exp Med. 1996;183:621–629. doi: 10.1084/jem.183.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimizu T, Iwamoto Y, Yanagihara Y, Kurimura M, Achiwa K. Mitogenic activity and the induction of tumor necrosis factor by lipopeptide analogs of the N-terminal part of lipoprotein in the outer membrane of Escherichia coli. Biol Pharm Bull. 1994;17:980–982. doi: 10.1248/bpb.17.980. [DOI] [PubMed] [Google Scholar]
- 61.Sjöstedt A, Sandstrom G, Tärnvik A. Humoral and cell-mediated immunity in mice to a 17-kilodalton lipoprotein of Francisella tularensis expressed by Salmonella typhimurium. Infect Immun. 1992;60:2855–2862. doi: 10.1128/iai.60.7.2855-2862.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steere A C, Sikand V K, Meurice F, Parenti D L, Fikrig E, Schoen R T, Nowakowski J, Schmid C H, Laukamp S, Buscarino C, Krause D S. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 63.Toomb J-F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Watthey L, Wallin E, Hayes W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 64.Winner L, III, Mack J, Weltzin R, Mekalanos J J, Kraehenbuhl J-P, Neutra M R. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect Immun. 1991;59:977–982. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamaguchi K, Yu F, Inouye M. A single amino acid determinant of the membrane localization of lipoproteins in E. coli. Cell. 1988;53:423–432. doi: 10.1016/0092-8674(88)90162-6. [DOI] [PubMed] [Google Scholar]