Abstract
The early warning and tracking of COVID-19 prevalence in the community provided by wastewater surveillance has highlighted its potential for much broader viral disease surveillance. In this proof-of-concept study, 46 wastewater samples from four wastewater treatment plants (WWTPs) in Queensland, Australia, were analyzed for the presence and abundance of 13 respiratory viruses, and the results were compared with reported clinical cases. The viruses were concentrated using the adsorption-extraction (AE) method, and extracted nucleic acids were analyzed using qPCR and RT-qPCR. Among the viruses tested, bocavirus (BoV), parechovirus (PeV), rhinovirus A (RhV A) and rhinovirus B (RhV B) were detected in all wastewater samples. All the tested viruses except influenza B virus (IBV) were detected in wastewater sample from at least one WWTP. BoV was detected with the greatest concentration (4.96–7.22 log10 GC/L), followed by Epstein-Barr virus (EBV) (4.08–6.46 log10 GC/L), RhV A (3.95–5.63 log10 GC/L), RhV B (3.74–5.61 log10 GC/L), and PeV (3.17–5.32 log10 GC/L). Influenza viruses and respiratory syncytial virus (RSV) are notifiable conditions in Queensland, allowing the gene copy (GC) concentrations to be compared with reported clinical cases. Significant correlations (ρ = 0.60, p < 0.01 for IAV and ρ = 0.53, p < 0.01 for RSV) were observed when pooled wastewater influenza A virus (IAV) and RSV log10 GC/L concentrations were compared to log10 clinical cases among the four WWTP catchments. The positive predictive value for the presence of IAV and RSV in wastewater was 97 % for both IAV and RSV clinical cases within the four WWTP catchments. The overall accuracy of wastewater analysis for predicting clinical cases of IAV and RSV was 97 and 90 %, respectively. This paper lends credibility to the application of wastewater surveillance to monitor respiratory viruses of various genomic characteristics, with potential uses for increased surveillance capabilities and as a tool in understanding the dynamics of disease circulation in the communities.
Keywords: Wastewater, Surveillance, Respiratory viruses, qPCR, RT-qPCR, Public health
Graphical abstract
1. Introduction
Wastewater surveillance was first applied to assess the circulation of enteric pathogens such as Salmonella typhi, Vibrio cholerae, and most notably, poliovirus (Wilson, 1993; Paul et al., 1940; Moore, 1951; Barrett et al., 1980; Manor et al., 1999). Since these pathogens are primarily transmitted via the fecal-oral route, it follows logically that wastewater containing human feces could provide insight into their circulation within a community. It was not until 2009 that wastewater was monitored for a respiratory virus, influenza A virus (IAV), primarily transmitted via a non-fecal-oral route (Heijnen and Medema, 2011). Even then, the study was framed to investigate the potential for fecal-oral transmission of pandemic influenza A (H1N1) virus from wastewater. However, the widespread success of wastewater surveillance for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus characterized by respiratory transmission and fecal shedding, suggests that wastewater is most likely not a vehicle of transmission for SARS-CoV-2 but is an excellent matrix for environmental surveillance of COVID-19 (Ahmed et al., 2021a). More recently, wastewater surveillance was used to detect outbreaks of IAV on two college campuses in the USA (Wolfe et al., 2022), and measurements of respiratory syncytial virus (RSV) RNA in wastewater were observed to reflect trends in traditional clinical surveillance of RSV (Hughes et al., 2022). Together, these observations strongly suggest the potential to use wastewater for monitoring the circulation of a wide range of respiratory pathogens in communities. However, the presence of such viruses and their genetic materials in wastewater is not well documented.
Herein, we report the surveillance of 13 respiratory viruses in untreated wastewater from four wastewater treatment plants (WWTPs) in Queensland, Australia. These viruses include – bocavirus (BoV), cytomegalovirus (CmV), Epstein-Barr virus (EBV), IAV, influenza B virus (IBV), parechovirus (PeV), RSV subtype A (RSV A) and B (RSV B), parainfluenza virus type 1 (PiV 1), 2 (PiV 2), and 3 (PiV 3), and rhinovirus subtype A (RhV A) and B (RhV B). The characteristics of these respiratory viruses are presented in Table 1 . Many of these viruses are associated with completely asymptomatic respiratory infections among the immunocompetent individuals, but they can also cause a wide range of illnesses, including gastroenteritis (BoV, IAV, PeV) (Guido et al., 2016; Hutchinson, 2018; Britton et al., 2018), flu (IAV, IBV) (Hutchinson, 2018), febrile illness (CmV) (Dioverti and Razonable, 2016), flu-like illness (PeV) (Britton et al., 2018), common cold (RhV A and RhV B) (Turner and Lee, 2009), and cold-like illness (RSV) (Pandya et al., 2019). Among the immunocompromised, infants and children, and the elderly, infection with some of these respiratory viruses can cause serious illnesses, hospitalization, and even death (Henrickson, 2003; Britton et al., 2018; Pandya et al., 2019). CmV demonstrates latency in various human cells and can cause a wide range of syndromes including a mononucleosis-like illness (Dioverti and Razonable, 2016). EBV infection has been implicated for longer term sequalae including tumor formation, cancer, and multiple sclerosis (Farrell, 2019; Bjornevik et al., 2022). The non-specific or non-existent symptomology associated with many respiratory virus infections likely hampers traditional and syndromic disease surveillance (Henning, 2004). Yet the severity of disease among vulnerable subsegments of the population makes surveillance of these respiratory viruses a vital interest to public health. In this case, wastewater surveillance, which combines the specificity and sensitivity of PCR-based analysis with pooled biological materials collected without care-seeking required (i.e., prodromal surveillance), could be advantageous in observing and managing the circulation of respiratory viruses in the communities.
Table 1.
Characteristics of respiratory viruses analyzed in this study.
| Virus | Family | Genus | Morphology dimensions | Enveloped | Genome type |
|---|---|---|---|---|---|
| Bocavirus (BoV) | Parvoviridae | Bocaparvovirus | Icosahedral 18–26 nm | N | ssDNA |
| Cytomegalovirus (CmV) | Herpesviridae | Cytomegalovirus | Pleomorphic 150–200 nm | Y | dsDNA |
| Epstein-Barr virus (EBV) | Herpesviridae | Lymphocryptovirus | Pleomorphic 120–220 nm | Y | dsDNA |
| Influenza A virus (IAV) | Orthomyxoviridae | Alphainfluenzavirus | Pleomorphic 80–120 nm | Y | ssRNA |
| Influenza B virus (IBV) | Orthomyxoviridae | Betainfluenzavirus | Pleomorphic 80–120 nm | Y | ssRNA |
| Parechovirus (PeV) | Picornaviridae | Parechovirus | Icosahedral 30 nm | N | ssRNA |
| Respiratory syncytial virus (RSV A and RSV B) | Pneumoviridae | Orthopneumovirus | Pleomorphic 150–250 nm | Y | ssRNA |
| Parainfluenza virus type 1 and 3 (PiV 1 and PiV 3) | Paramyxoviridae | Respirovirus | Pleomorphic 150–250 nm | Y | ssRNA |
| Parainfluenza virus type 2 (PiV 2) | Paramyxoviridae | Rubulavirus | Pleomorphic 150–250 nm | Y | ssRNA |
| Rhinovirus (RhV A and RhV B) | Picornaviridae | Enterovirus | Dodecahedral 30 nm | N | ssRNA |
2. Materials and methods
2.1. Sources of wastewater samples
Archived wastewater samples were used in this study from the Queensland Health Wastewater Surveillance program (https://www.data.qld.gov.au/dataset/queensland-wastewater-surveillance-for-sars-cov-2). We selected a total of 46 wastewater samples that had been collected between January 4 and June 2, 2022 from four WWTPs in Queensland, Australia. WWTPs that were selected in this study use activated sludge processes and treat domestic water from ~35,500 to ~93,000 people. These WWTPs are hereafter referred as WWTP A, WWTP B, WWTP C and WWTP D. At each WWTP, untreated wastewater samples ranging from 500 mL to 1 L in volume were collected as time-based composites using an automated sampler operating in time-proportional mode (taking subsamples every 15 min for 24 h) (Ahmed et al., 2021b). These composite wastewater samples had been previously screened by RT-qPCR for SARS-CoV-2 RNA in conjunction with the surveillance program and confirmed to be positive using both the US CDC N1 and N2 assays (https://www.data.qld.gov.au/dataset/queensland-wastewater-surveillance-for-sars-cov-2).
2.2. Virus concentration
Archived wastewater samples (−20 °C) were thawed at 4 °C and viruses were concentrated using the adsorption-extraction (AE) method (Ahmed et al., 2020a). This method is routinely used in our laboratory for the concentration of microorganisms from environmental water and wastewater samples. The high recovery efficiency of this method for DNA and RNA viruses have been reported previously (Ahmed et al., 2015; Ahmed et al., 2020a). A 50 mL subsample from each thawed wastewater sample was supplemented with MgCl2 to achieve a final concentration of 25 mM. After amendment with MgCl2, 50 mL wastewater samples were immediately filtered through a 0.45-μm pore-size, 47-mm diameter electronegative HA membrane (Cat. No. HAWP04700) (Merck Millipore Ltd., KGaA, Darmstadt, Germany) via a magnetic filter funnel (Pall Corporation, New York, USA) and filter flask (Merck Millipore Ltd.) (Ahmed et al., 2020a).
2.3. Nucleic acid extraction
Following filtration, using aseptic technique, the membrane was immediately removed, rolled, and inserted into a 5 mL bead-beating tube of the RNeasy PowerWater Kit (Cat. No. 14700-50-NF) (Qiagen, Hilden, Germany) to directly extract nucleic acid from the membrane. Briefly, 990 μL of buffer PM1 and 10 μL of β-mercaptoethanol (Cat. No. M6250-10 mL) (Sigma-Aldrich, St. Louis, Missouri, USA) were added into each bead-beating tube, which was then homogenized using a Precellys 24 tissue homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France) set for 3 × 15 s at 10,000 rpm at a 10 s interval. After homogenization, the tubes were centrifuged at 4000 g for 5 min to pellet the filter debris and beads. Nucleic acid extraction was carried out with the RNeasy PowerWater Kit (Qiagen) according to the manufacturer's protocol with two modifications: (i) the use of DNase I solution was omitted from the protocol to isolate nucleic acid (i.e., both RNA and DNA) as respiratory panel included both RNA and DNA viruses; (ii) nucleic acid was eluted with 200 μL of DNase and RNase free water. Sample lysate supernatant ranging from 600 to 800 μL in volume was then used to extract nucleic acid following the manufacturer's protocol. Nucleic acid purity was verified by measuring A260/280 ratio using a DeNovix Spectrophotometer & Fluorometer (DeNovix, Wilmington, DE, USA).
2.4. PCR inhibition assessment
After homogenization and before completing the rest of the nucleic acid extraction, known quantity (2.1 × 104 GC) of murine hepatitis virus (MHV) was seeded into each lysate and pellet as an inhibition process control. The same quantity of MHV suspension was also added to a distilled water extraction control (same volume of lysate) and subjected to extraction. The presence of PCR inhibition in nucleic acid samples extracted from wastewater was assessed using an MHV RT-PCR assay (Besselsen et al., 2002). The reference PCR quantification cycle (Cq) values obtained for MHV seeded into distilled water for all methods were compared with the Cq values of the MHV seeded into wastewater lysate to obtain information on potential RT-PCR inhibition. If the Cq value resulting from the sample was >2 Cq difference from the reference Cq value for the distilled water control, the sample was considered inhibited (Ahmed et al., 2022). In addition to the negative extraction control, all samples were analyzed alongside three PCR negative controls.
2.5. RT-PCR, qPCR and RT-qPCR analysis
Previously published RT-PCR assays were used for MHV detection (Besselsen et al., 2002). For the MHV assay, positive control material in the form of gBlocks gene fragments was purchased from Integrated DNA Technologies (Integrated DNA Technology Coralville, IA, USA). For the respiratory viruses, custom TaqMan® Assays were used (Thermofisher Scientific, Waltham, MA, USA). These assays include BoV (assay ID Vi9999003_po), CmV (assay ID Vi06439643_s1), EBV (assay ID Vi06439675_s1), IAV (assay ID Vi99990011_po), IBV (assay ID Vi99990012_po), PeV (assay ID Vi99990006_po), PiV 1 (assay ID Vi06439642_s1), PiV 2 (assay ID Vi06439672_s1), PiV 3 (assay ID Vi06439670_s1), RhV A (assay ID Vi99990016_po), RhV B (Vi99990017_po), RSV A (assay ID Vi99990014_po) and RSV B (assay ID Vi99990015_po). These TaqMan® Assays were rigorously designed by the vendor (Thermofisher Scientific) to ensure maximum strain coverage while minimizing the potential for off-target cross-reactivity. The specificities of these assays were also determined by the vendor (Thermofisher Scientific) against the available subset of respiratory pathogen genomes demonstrating no cross-reactivity of the respiratory pathogen assays with closely related species and other respiratory microbes. The standard curves prepared from linearized plasmid DNA exhibited a linear dynamic range of quantification from 105 to 1 GC/μL. The amplification efficiencies were close to 100 %. The correlation coefficients (r 2) were >0.99. The assay limit of detection (ALOD) was determined to be 1–10 GC/reaction for all assays (TrueMark™ Respiratory Panel 2.0, TaqMan™ Array card (thermofisher.com)). For the respiratory virus qPCR and RT-qPCR assays, a linearized multi-target plasmid pool (Cat. No. A50382) containing all target sequences was used (Applied Biosystems, Waltham, MA, USA). This plasmid pool was used as qPCR/RT-qPCR standards.
qPCR and RT-qPCR analyses were performed in 20 μL reaction mixtures using QuantiNova Probe PCR Kit (Qiagen) and TaqMan™ Fast Virus 1-Step Master Mix (Applied Biosystems), respectively. MHV RT-qPCR mixture contained 5 μL of Supermix, 300 nM of forward primer, 300 nM of reverse primer, 400 nM of probe, and 5 μL of template RNA. Respiratory virus qPCR and RT-qPCR mixtures were prepared that contained 10 μL Supermix (BoV, CmV and EBV) or 5 μL Supermix (all other respiratory viruses), optimized target specific primers and probes and 5 μL of nucleic acid sample. Molecular-grade water was used to amend all PCR reaction mixtures to 20 μL of total volume. For each qPCR and RT-qPCR experimental run, a series of standard (3 × 105 to 3 GC/reaction of plasmid DNA control) and no template controls (n = 3) were included. The qPCR and RT-qPCR assays were performed on a Bio-Rad CFX96 thermal cycler (Bio-Rad Laboratories, Hercules, California, USA) using manual settings for threshold and baseline. All qPCR and RT-qPCR reactions were performed in duplicate reactions.
2.6. qPCR and RT-qPCR assay limit of detection
qPCR and RT-qPCR ALODs were determined using plasmid DNA control (Applied Biosystems) containing all target and control sequences. Plasmid DNA control was diluted (3 × 102 to 0.3 GC/reaction) and analyzed using qPCR and RT-qPCR. At each dilution, 12 replicates were analyzed. The 95 % ALOD was defined by fitting an exponential survival model to the proportion of PCR replicates positive at each step along the gradient (Verbyla et al., 2016).
2.7. Quality control
To minimize qPCR and RT-qPCR contamination, nucleic acid extraction and qPCR and RT-qPCR set up were performed in separate laboratories. A sample negative control was included during the concentration process. An extraction negative control was also included during nucleic acid extraction to account for any contamination during extraction. All sample and extraction negative controls were PCR negative for the analyzed targets.
2.8. Sources of clinical influenza and RSV data
Not all the viruses analyzed in this study are notifiable in Queensland. Among all the viruses analyzed, only influenza and RSV are notifiable. Clinical case numbers were obtained from notifiable conditions reports (https://www.health.qld.gov.au/clinical-practice/guidelines-procedures/diseases-infection/surveillance/reports/notifiable). Notifiable conditions reports provide data on the weekly and annual totals of communicable diseases at different levels across Queensland and can be further broken down by Public Health Units (PHUs) and/or Hospital and Health Services (HHS).
2.9. Data analysis
For qPCR and RT-qPCR, the ALOD is defined as the minimum GC number with a 95 % probability of detection and determined as previously described (Verbyla et al., 2016). For qPCR and RT-qPCR, samples were considered positive (virus detected) if amplification was observed in at least one of the two replicates within 45 cycles. Samples were considered quantifiable if amplification was observed in both replicates with concentrations above the ALOD values. Weekly influenza and RSV case numbers in all four WWTP catchments were compared with IAV and combined RSV A and RSV B wastewater RNA concentration (GC/L) using Spearman's rank correlation (ρ). In the event where there were two wastewater RNA measurements during the period, the computed geometric mean concentration was used. The association between RNA detection in wastewater and the presence of cases was assessed using Fisher's exact test. Since the individual WWTP contingency tables contained zeros, the association between RNA detection in wastewater and the presence of IAV or RSV cases was assessed using the data pooled from all WWTP catchments. The positive and negative predictive values (PPV, NPV) and accuracy of wastewater for IAV or RSV cases was also assessed using the pooled datasets.
3. Results
3.1. qPCR and RT-qPCR assay performance and relevant QA/QC
The A260/280 absorbance ratio of purified RNA from all wastewater samples was >1.80 and considered acceptable RNA quality (Sambrook et al., 1989). The qPCR and RT-qPCR standard curves prepared from linearized plasmid DNA exhibited a linear dynamic range of quantification from 3 × 105 to 3 GC/reaction (1 × 105 to 1 GC/μL). The slopes of the standard curves ranged between −3.57 (PiV 1) and − 3.10 (both RhV A and RhV B) (Table 2 ). The range of amplification efficiencies (91.0 to 110 %) and y-intercepts (35.75 to 40.68) were within the prescribed range of the Minimum Information for Publication of Quantitative Real-Time PCR experiments (MIQE) guidelines (Bustin et al., 2009). The correlation coefficients (r 2) across the standard curves for all assays ranged from 0.97 to 1.00. The ALODs for the RT-qPCR assays were between 3 and 30 GC/reaction (Table 2). All positive controls or standard curve samples were amplified in each PCR run. PCR inhibition was not identified in any nucleic acid samples based on the seeded GC of MHV (all within 2 Cq values of the reference Cq value).
Table 2.
qPCR and RT-qPCR performance characteristics and assay limit of detection (ALOD).
| Assays | qPCR/RT-qPCR performance characteristicsa |
ALOD |
||||
|---|---|---|---|---|---|---|
| Efficiency (%) | Correlation coefficient (r2) | Slope | Y-intercept | qPCR/RT-qPCR GC/reaction | GC/50 mL sample | |
| BoV | 97 | 0.99 | −3.41 | 38.38 | 7.30 | 292 |
| CmV | 106 | 1.00 | −3.18 | 37.54 | 14.2 | 568 |
| EBV | 105 | 1.00 | −3.22 | 37.54 | 5.60 | 224 |
| IAV | 95 | 1.00 | −3.45 | 38.68 | 8.40 | 336 |
| IBV | 97 | 0.97 | −3.39 | 39.21 | 7.32 | 293 |
| PeV | 99 | 0.99 | −3.33 | 38.37 | 8.10 | 324 |
| PiV 1 | 91 | 1.00 | −3.57 | 39.64 | 21.0 | 840 |
| PiV 2 | 102 | 1.00 | −3.28 | 38.01 | 17.1 | 684 |
| PiV 3 | 97 | 1.00 | −3.40 | 38.49 | 17.4 | 696 |
| RhV A | 101 | 1.00 | −3.10 | 36.13 | 9.30 | 372 |
| RhV B | 110 | 1.00 | −3.10 | 35.75 | 7.41 | 296 |
| RSV A | 103 | 0.99 | −3.26 | 37.16 | 7.32 | 293 |
| RSV B | 91 | 0.98 | −3.56 | 40.68 | 6.80 | 272 |
BoV: bocavirus; PiV 1: parainfluenza virus 1; PiV 2: parainfluenza virus 2; PiV 3: parainfluenza virus 3; PeV: parechovirus; RSV A: respiratory syncytial virus A; RSV B: respiratory syncytial virus B; RhV A: rhinovirus A; RhV B: rhinovirus B; EBV: Epstein-Barr Virus; CmV: cytomegalovirus; IAV: influenza A virus; IBV: influenza B virus.
Average values are presented.
3.2. Prevalence and concentrations of respiratory viruses in wastewater
pt?>Among the 13 respiratory viruses, BoV, PeV, RhV A and RhV B were detected in all wastewater samples (Table 3 ). EBV was also highly prevalent, detected in all wastewater samples from WWTP A (100 %), followed by WWTP C (91.7 %), WWTP D (90.9 %) and WWTP B (72.7%). RSV B was more prevalent than RSV A in wastewater samples from WWTP B (72.7 % vs. 45.5 %), WWTP C (41.7 % vs. 16.7 %) and WWTP D (90.9 % vs. 9.09 %). However, RSV A (91.7 %) was more prevalent than RSV B (50 %) in WWTP A. IAV was more frequently detected in wastewater samples from WWTP B (81.8 %) followed by WWTP A (76.9 %), WWTP D (72.7 %) and WWTP C (50 %). IBV could not be detected in any wastewater samples collected during the study. CmV was detected in all four WWTPs with the detection frequency ranging from 9.09 % (WWTPs B and D) to 33.3 % (WWTP A). PiV 1, PiV 2 and PiV 3 were sporadically detected in wastewater samples, with the highest prevalence observed for PiV 3 compared to PiV 1 and PiV 2.
Table 3.
Prevalence of respiratory viruses in wastewater samples collected from four WWTPs in Queensland, Australia.
| Viruses | No. of samples PCR positive/no. of samples tested (%) |
||||
|---|---|---|---|---|---|
| WWTP A | WWTP B | WWTP C | WWTP D | WWTP Pooled | |
| BoV | 12/12 (100) | 11/11 (100) | 12/12 (100) | 11/11 (100) | 46/46 (100) |
| CmV | 4/12 (33.3) | 1/11 (9.09) | 3/12 (25) | 1/11 (9.09) | 9/46 (19.6) |
| EBV | 12/12 (100) | 8/11 (72.7) | 11/12 (91.7) | 10/11 (90.9) | 41/46 (89.1) |
| IAV | 10/13 (76.9) | 9/11 (81.8) | 6/12 (50) | 8/11 (72.7) | 33/46 (71.7) |
| IBV | 0/12 (0) | 0/11 (0) | 0/12 (0) | 0/11 (0) | 0/46 (0) |
| PeV | 12/12 (100) | 11/11 (100) | 12/12 (100) | 11/11 (100) | 46/46 (100) |
| PiV 1 | 0/12 (0) | 2/11 (18.2) | 0/12 (0) | 2/11 (18.2) | 4/46 (8.69) |
| PiV 2 | 0/12 (0) | 2/11 (18.2) | 2/12 (16.7) | 1/11 (9.09) | 5/46 (10.7) |
| PiV 3 | 5/12 (41.7) | 2/11 (18.2) | 0/12 (0) | 2/11 (18.2) | 9/46 (19.6) |
| RhV A | 12/12 (100) | 11/11 (100) | 12/12 (100) | 11/11 (100) | 46/46 (100) |
| RhV B | 12/12 (100) | 11/11 (100) | 12/12 (100) | 11/11 (100) | 46/46 (100) |
| RSV A | 11/12 (91.7) | 5/11 (45.5) | 2/12 (16.7) | 1/11 (9.09) | 19/46 (41.3) |
| RSV B | 6/12 (50) | 8/11 (72.7) | 5/12 (41.7) | 10/11 (90.9) | 29/46 (63.0) |
BoV: bocavirus; PiV 1: parainfluenza virus 1; PiV 2: parainfluenza virus 2; PiV 3: parainfluenza virus 3; PeV: parechovirus; RSV A: respiratory syncytial virus A; RSV B: respiratory syncytial virus B; RhV A: rhinovirus A; RhV B: rhinovirus B; EBV: Epstein-Barr Virus; CmV: cytomegalovirus; IAV: influenza A virus; IBV: influenza B virus.
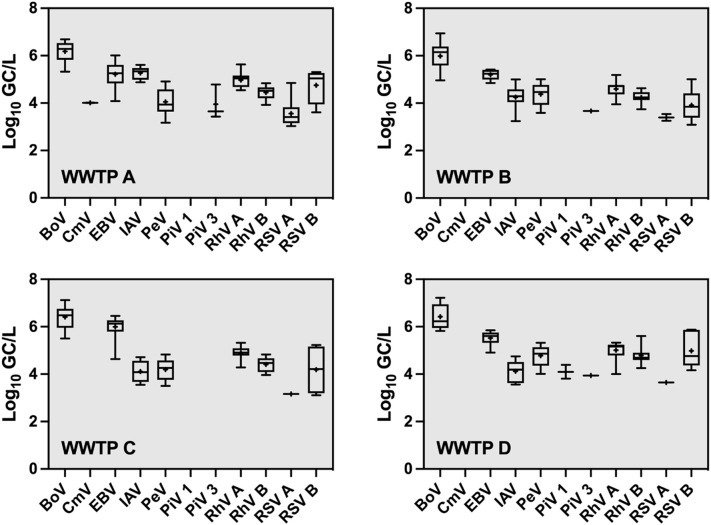
Not all PCR-detected viruses were present at quantifiable concentrations. The number of qPCR and RT-qPCR quantifiable samples, minimum, maximum, mean, and standard deviation were determined for each virus in each WWTP (Supplementary Table ST1). The concentrations of BoV were the highest (4.96–7.22 log10 GC/L) with a mean concentration as high as 6.42 log10 GC/L (WWTP D). EBV ranging from 4.08 to 6.46 log10 GC/L was detected with the greatest mean concentration (6.00 log10 GC/L) at WWTP C. The concentrations of RhV A (3.95–5.63 log10 GC/L), RhV B (3.74–5.61 log10 GC/L), and PeV (3.17–5.32 log10 GC/L) were in the mid-range of the observed concentrations across all the viruses. The highest concentrations of these viruses were found in wastewater samples from WWTP D which is located in a highly urbanized area. The concentrations of IAV, RSV A and RSV B were in the low- to mid-range of 3.24–5.61, 3.03–4.85 and 3.09–5.88 log10 GC/L, respectively. The greatest concentrations of IAV were observed in wastewater samples from WWTP A, while the highest concentrations of RSV A and RSV B were found in WWTP D. CmV was quantifiable in only two samples from WWTP A with a mean concentration of 4.01 log10 GC/L. PiV 1 and PiV 3 were also infrequently quantifiable in a range of 3.81–4.39 and 3.43–4.78 log10 GC/L, respectively. The concentrations of each respiratory viruses in wastewater samples at each WWTP are shown in Fig. 1 . The mean concentrations of viruses from qPCR/RT-qPCR duplicates from each individual wastewater sample at each WWTP are shown in Supplementary Fig. SF1.
Fig. 1.
Box-and-whisker plots of the concentrations (log10 GC/L) of respiratory viruses in wastewater samples collected from four wastewater treatment plants (WWTPs). The lower and upper boxes denote 25th and 75th percentiles. The lower and upper bars represent the 5th and 95th percentiles. + denotes mean.
3.3. Correlation between clinical cases of IAV and RSV with wastewater RNA (GC/L)
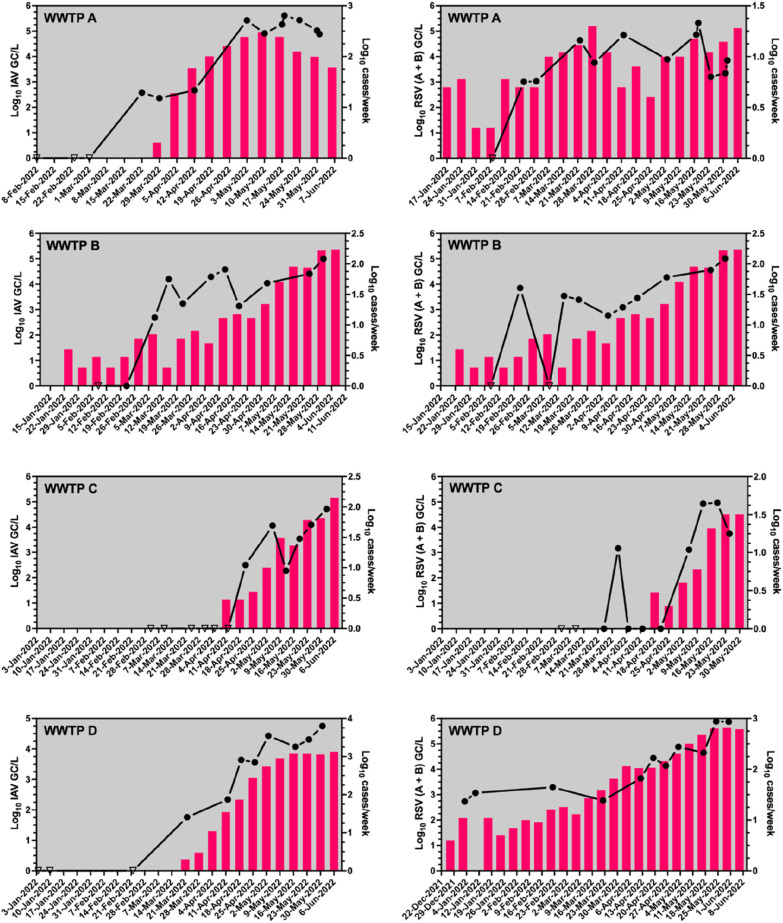
Over the period of the study, the number of clinical cases and wastewater RNA concentration (GC/L) pairs for IAV and RSV was less than or equal to 10 at each WWTP (Table 4 ). Across each WWTP, the Spearman's rank correlation between influenza cases and IAV RNA concentration in wastewater ranged from weak to moderate (0.37 to 0.76), but the small sample size at each WWTP meant the correlation was statistically significant for only one catchment (WWTP D). However, when the observations across all catchments were pooled, IAV RNA concentrations were moderately correlated with cases of influenza (ρ = 0.60, p < 0.01). The Spearman's rank correlations between RSV GC/L (A and B combined) and clinical RSV cases displayed a greater range from weak to strong (ρ = 0.39 to 0.95) correlations within individual WWTP catchments. However, when data were pooled across all WWTPs, RSV GC concentrations in wastewater were also moderately correlated with RSV clinical cases (ρ = 0.53, p < 0.01). Lead indication of virus signals in wastewater to clinical cases was evident for IAV in WWTP A (12 days) and for RSV (A + B) in WWTP C (17 days) catchments, while lead/lag trends for viruses in wastewater compared to clinical cases were not obvious in the rest of the WWTP catchments (Fig. 2 ).
Table 4.
Spearman's rank correlation (ρ) between IAV and RSV RNA (GC/L) in wastewater and number of cases per week in the corresponding WWTP catchment.
| WWTP | IAV | RSV |
|---|---|---|
| WWTP A | n = 7 | n = 9 |
| ρ = 0.61 | ρ = 0.39 | |
| p = 0.17 | p = 0.30 | |
| WWTP B | n = 9 | n = 9 |
| ρ = 0.37 | ρ = 0.58 | |
| p = 0.34 | p = 0.11 | |
| WWTP C | n = 6 | n = 4 |
| ρ = 0.60 | ρ = 0.40 | |
| p = 0.24 | p = 0.75 | |
| WWTP D | n = 8 | n = 10 |
| ρ = 0.76 | ρ = 0.95 | |
| p = 0.03 | p < 0.01 | |
| All WWTPs | n = 30 | n = 32 |
| ρ = 0.60 | ρ = 0.53 | |
| p < 0.01 | p < 0.01 |
Fig. 2.
Correlations between concentrations (log10 GC/L) of IAV and RSV (A + B) with weekly influenza and RSV cases in four WWTP catchments. Triangle symbols represent no detection. Columns are representing weekly reported cases. In some cases, the log10 cases/week values are too small to illustrate.
The relationship between RNA detection in wastewater and the presence or absence of influenza or RSV cases in the corresponding catchment was assessed by means of Fisher's exact test, PPV and NPV. The small sample size prevented an assessment for each individual WWTP catchment as the contingency tables often contained zeros in one or more quadrants. However, for the pooled data, the association between the presence of RNA in wastewater and cases of RSV or IAV was statistically significant (p < 0.01). Overall, the PPV of RNA presence in wastewater was 97 % for cases of influenza and RSV within the catchment. The NPV, on the other hand, was much lower for cases of influenza and RSV, 71 and 67 % respectively, because there were non-detect wastewater results despite the known presence of clinical cases. The overall accuracy of wastewater for predicting influenza cases was 97 % and greater than the accuracy for RSV, which was only 90 %.
4. Discussion
In this proof-of-concept study, we examined the presence and abundance of genetic material from 13 respiratory viruses in wastewater from four WWTPs in Queensland, Australia. Critically, the clinical data needed to assess the usefulness of wastewater for monitoring the circulation of these viruses within communities were only available for two notifiable diseases – influenza and RSV. While the notifiable case number data were limited, each of the 13 viruses we selected to study is known or strongly suspected to be present in the community by clinical experience or known seasonal patterns for respiratory illnesses. However, little was previously known regarding the presence and abundance of these viruses, or their genetic materials, in domestic wastewater. Recently, monitoring of respiratory viruses RSV and IAV in wastewater has been conducted in the USA and Canada and demonstrated reasonable correlations with reported clinical cases and outbreaks (Hughes et al., 2022; Wolfe et al., 2022; Mercier et al., 2022). The wastewater data for the other respiratory viruses included in the current study could also be used to inform public health response to seasonal respiratory pathogens for example messaging concerning cold season and symptoms, masking or other prophylactic measures among vulnerable populations, informing diagnostic triage by clinicians, or the deployment of resources such as hand sanitizer.
Simultaneous monitoring of a comprehensive range of respiratory viruses could provide a useful understanding of the dynamics of respiratory disease circulation in communities, especially when infectious agents are associated with non-specific symptoms or asymptomatic patterns (Guido et al., 2016; Britton et al., 2018; Pandya et al., 2019). A large proportion of respiratory infections do not lead to hospitalization, and therefore, more likely to be undetected and/or unreported. BoV, PeV, RhV A, and RhV B were highly prevalent in all four WWTP catchments suggesting persistent and prevalent infections in the community. BoV contains a subtype that primarily causes respiratory infection and is frequently found in respiratory specimens, and other subtypes targeting the gastrointestinal system (Guido et al., 2016). Moderate clinical prevalence rates were reported in Australia during 2005–2016, with 13 % for respiratory and 8.5 % for gastrointestinal infections, when compared to the global prevalence of 1.0 to 56.8 % and 1.3 to 63.0 % for BoV respiratory and gastrointestinal infections, respectively (Guido et al., 2016). Moreover, seasonal distribution of BoV in environmental water appeared to be regionally specific, as highest detection rates were reported in summer months in Thailand (Kumthip et al., 2021), while they were detected in all seasons but summertime in Germany (Hamza et al., 2009). Due to worldwide occurrence and observed regional-specific temporal patterns, BoV monitoring in local wastewater could provide a better understanding of its circulating dynamics that complement the traditional clinical surveillance.
High prevalence of PeV, another respiratory and gastrointestinal virus, in wastewater in this study was consistent with the frequent outbreaks reported in Australia (Britton et al., 2018; McNeale et al., 2018). PeV is also of global concern as it was found in fecal and wastewater samples from several countries, e.g., Thailand (Chieochansin et al., 2011), Japan (Thongprachum et al., 2018), USA (McCall et al., 2020), and New Zealand (Moore et al., 2015). Slightly higher concentrations than observed in the current study were reported in wastewater in Sweden at 5.89–6.45 log10 GC/L (Wang et al., 2018). RhV is the most common respiratory virus in Australia (Adam et al., 2021) and worldwide (Jafarinejad et al., 2017). A global epidemiology review of chronic obstructive pulmonary diseases during 2000 to 2017 showed that RhV had the highest prevalence, when compared to RSV, IAV, IBV, and PiV (Jafarinejad et al., 2017). Moreover, RhV was the most common etiological agent causing respiratory infections of all age ranges during the first year of COVID-19 pandemic in Turkey, while infection rates from other respiratory agents (e.g., IAV, IBV, PiV, BoV, RSV, etc.) declined (Agca et al., 2021). RhV was also quantified at the greatest concentrations in wastewater solids compared to PiV, RSV A, RSV B, IAV, and IBV (Boehm et al., 2022).
EBV was frequently detected in wastewater samples in this study. This type of herpesvirus is transmitted mainly through saliva and most infected patients are asymptomatic (Idesawa et al., 2004). In contrast, another herpesvirus CmV was detected sporadically in wastewater in the present study, which could be due to low prevalence in the studied communities. CmV was found mostly in South America, Africa, and Asia, with lower prevalence in Western Europe and the USA (Cannon et al., 2010). For a common group of respiratory viruses, IAV was identified as the highest clinical incidence rates in Brisbane, Australia, during 2010–2015, which was higher than RSV, PiV, and IBV (Lam et al., 2019). The clinical incidence pattern corresponded well with the prevalence trend in wastewater in the present study. IAV was detected in German wastewater from 3.0 to 5.5 log10 GC/L in two wastewater treatment plants (Dumke et al., 2022), comparable to the detected concentrations of 3.2–5.6 log10 GC/L in the current study. The detected concentrations of RSV and IAV in wastewater settled solids in the USA ranged up to 4.8 and 4.4 log10 GC/g dry weight, respectively (Hughes et al., 2022; Wolfe et al., 2022). Low prevalence of IBV in certain years (Lam et al., 2019) implied that no IBV detection in wastewater during this study may be due to low infection rates in the study communities. This also corroborates with Australian Influenza Surveillance Report where IBV could not be detected in clinical specimens between Jan to Oct 2022 (https://www.health.gov.au/sites/default/files/documents/2022/10/aisr-fortnightly-report-no-14-26-september-to-9-october-2022.pdf). Global incidence of these respiratory viruses disclosed that other geographical regions could be of high concerns for the viral outbreaks and that wastewater surveillance could provide important data, which could allow a better management of respiratory diseases in the community.
Since this was a proof-of-concept study, we conveniently selected commercial qPCR and RT-qPCR TaqMan® Assays for wastewater analysis. The vendor certifies the assay specificities against a panel of relevant microorganisms and provided ALODs (1–10 GC/μL) for each assay which are comparable with the ALODs empirically determined in the current study (TrueMark™ Respiratory Panel 2.0, TaqMan™ Array card (thermofisher.com)). Due to ongoing demands for rapid analysis in both diagnostic and research applications, customized PCR reagents, including primers, probes, and reaction mixes targeting emerging and re-emerging pathogens recently are commercially available and have been used in wastewater surveillance research for emerging pathogens, for example monkeypox virus (Wurtzer et al., 2022). The primary limitation of these commercially designed qPCR and RT-qPCR TaqMan® Assays is that the primer and probe sequences are not known, and therefore, cannot be characterized in silico independently of the vendor's claims. Furthermore, these assays and standard materials are expensive, and delivery can require a considerable amount of time. With the proof-of-concept demonstrated by the present study, in the future these issues can be alleviated by selecting existing assays from the literature or by designing new assays, which can be thoroughly validated for key performance metrics such as sensitivity and specificity.
For our study, archived wastewater samples were used from the Queensland Health Wastewater Surveillance program (https://www.data.qld.gov.au/dataset/queensland-wastewater-surveillance-for-sars-cov-2). All wastewater samples were archived at −20 °C. Although viral genome degradation could occur when water samples were stored at −20 °C, we were able to quantify multiple viruses in archived samples which could facilitate the retrospective clinical disease surveillance. Our results agree with recent findings that storage at freezing temperatures does not seem to cause loss of the SARS-CoV-2 RNA signal within 58 days at low and ultralow temperatures (Hokajärvi et al., 2021). A recent study also reported no significant decay of the OC43 RNA signal at −80 °C over three weeks following multiple freeze-thaw cycles (McMinn et al., 2021). In contrast, ~90 % loss of SARS-CoV-2 RNA signal following storage at −80 °C for one week has been reported (Weidhaas et al., 2021). Nonetheless, we acknowledge that some degree of target loss is still possible following freeze-thawing and will require further investigation.
For concentrating the viral nucleic acids, we used an AE method which has been successfully used to concentrate enveloped and non-enveloped viruses from water and wastewater (Ahmed et al., 2015; Ahmed et al., 2020a; Ahmed et al., 2021c). This method demonstrated its ability to concentrate a wide range of respiratory viruses from wastewater. However, the recovery efficiencies for the respiratory viruses were not determined in the present study due to a lack of readily available control materials. When gamma-irradiated or heat-treated viruses are available, additional studies should be undertaken to determine the recovery efficiencies. We acknowledge that recovery efficiency of viruses analyzed in this study could vary from virus to virus and sample to sample (Ahmed et al., 2022; Haramoto et al., 2018).
In this study, we leveraged wastewater analysis to provide quantitative data on 13 respiratory viruses very likely circulating in four different WWTP catchments in Queensland, Australia. A recent study analyzed wastewater samples in Belgium for two years and reported the presence of 20 different respiratory viruses which is in accordance with the findings of this study (preprint of Rector et al., 2022). Several other studies also reported the presence of RSV, influenza viruses, PiV and RhV in wastewater besides SARS-CoV-2 (Hughes et al., 2022; Wolfe et al., 2022; Dumke et al., 2022; Mercier et al., 2022; Boehm et al., 2022). We observed significant correlations between the reported clinical cases and respective GC/L among pooled wastewater data from four WWTPs for two notifiable cases (influenza and RSV). This underlines the usefulness of wastewater surveillance tools to understand community disease and lends credibility to the potential usefulness of wastewater for surveillance of other respiratory viruses (Ahmed et al., 2020b; Sangsanont et al., 2022). Our experience is consistent with observations for IAV and RSV in other communities (Hughes et al., 2022; Wolfe et al., 2022; Mercier et al., 2022; Boehm et al., 2022). Moreover, a 17-day lead time of PMMoV-normalized IAV concentrations in primary sludge to clinical detection was observed in Ottawa city (Mercier et al., 2022). In two instances during the present study, wastewater data provided an early warning for the presence of IAV in WWTP A (12 days) and RSV (A + B) in WWTP C (17 days) catchment. However, for some WWTP catchments we did not observe any early signal. This could be because not all households in a sewer catchment are serviced by WWTP, i.e., a portion of the population is serviced by on-site sewer systems or septic tanks. If these population are infected, it is likely that individuals or households will be tested positive by clinical testing (if clinical testings are conducted), but catchment wastewater analysis from WWTPs may remain negative.
Despite the limitations, this study demonstrates the capability to quantify multiple respiratory viruses of varying genomic (i.e., ssDNA, dsDNA, and ssRNA) and phenotypic (i.e., enveloped or nonenveloped virus, morphology, and size) characteristics in wastewater. By integrating a wide range of viral target monitoring, respiratory and enteric viruses could be simultaneously monitored to provide comprehensive perspective of community disease circulation dynamics. Together, these observations justify investment in more intensive wastewater sampling and clinical observation to further characterize the predictive capability of wastewater surveillance. Future research is also needed to better understand the intrinsic characteristics of respiratory virus shedding into wastewater, as well as factors that could affect the environmental degradation and detectability of these viruses in wastewater.
CRediT authorship contribution statement
Warish Ahmed: Methodology, Formal analysis, Investigation, Writing – original draft. Aaron Bivins: Formal analysis, Writing – original draft. Mikayla Stephens: Methodology. Suzanne Metcalfe: Methodology. Wendy J.M. Smith: Methodology. Kwanrawee Sirikanchana: Formal analysis, Writing – original draft. Masaaki Kitajima: Formal analysis, Writing – original draft. Stuart L. Simpson: Investigation, Funding acquisition, Writing – original draft.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank CSIRO Environment for strategic funding to complete this research project. We thank Urban Utilities and University of Queensland for collecting and providing untreated wastewater samples. Thanks to Queensland Health for funding SARS-CoV-2 wastewater surveillance project in Queensland, Australia.
Editor: Damia Barcelo
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2022.161023.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- Adam D.C., Chen X., Scotch M., MacIntyre C.R., Dwyer D., Kok J. The molecular epidemiology and clinical phylogenetics of rhinoviruses among paediatric cases in Sydney,Australia. Int. J. Infect. Dis. 2021;110:69–74. doi: 10.1016/j.ijid.2021.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agca H., Akalin H., Saglik I., Hacimustafaoglu M., Celebi S., Ener B. Changing epidemiology of influenza and other respiratory viruses in the first year of COVID-19 pandemic. J. Infect. Public Health. 2021;14:1186–1190. doi: 10.1016/j.jiph.2021.08.004. [DOI] [PubMed] [Google Scholar]
- Ahmed W., Harwood V.J., Gyawali P., Sidhu J.P., Toze S. Comparison of concentration methods for quantitative detection of sewage-associated viral markers in environmental waters. Appl. Environ. Microbiol. 2015;81:2042–2049. doi: 10.1128/AEM.03851-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R., Mueller J.F., Simpson S.L., Smith W., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bibby K., D’Aoust P.M., Delatolla R., Gerba C.P., Haas C.N., Hamilton K.A., Hewitt J., Julian T.R., Kaya D., Monis P., Moulin L., Naughton C., Noble R.T., Shrestha A., Tiwari A., Simpson S.L., Wurtzer S., Bivins A. Differentiating between the possibility and probability of SARS-CoV-2 transmission associated with wastewater: empirical evidence is needed to substantiate risk. FEMS Microbes. 2021;2 doi: 10.1093/femsmc/xtab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Tscharke B., Bertsch P.M., Bibby K., Bivins A., Choi P., Clarke L., Dwyer J., Edson J., Nguyen T., O'Brien J.W., Simpson S.L., Sherman P., Thomas K.V., Verhagen R., Zaugg J., Mueller J.F. SARS-CoV-2 RNA monitoring in wastewater as a potential early warning system for COVID-19 transmission in the community: a temporal case study. Sci. Total Environ. 2021;761 doi: 10.1016/j.scitotenv.2020.144216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bivins A., Simpson S.L., Smith W.J.M., Metcalfe S., McMinn B., Symonds E.M., Korajkic A. Comparative analysis of rapid concentration methods for the recovery of SARS-CoV-2 and quantification of human enteric viruses and a sewage-associated marker gene in untreated wastewater. Sci. Total Environ. 2021;799 doi: 10.1016/j.scitotenv.2021.149386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bivins A., Metcalfe S., Smith W.J.M., Verbyla M.E., Symonds E.M., Simpson S.L. Evaluation of process limit of detection and quantification variation of SARS-CoV-2 RT-qPCR and RT-dPCR assays for wastewater surveillance. Water Res. 2022;213 doi: 10.1016/j.watres.2022.118132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T.J., Blake P.A., Morris G.K., Puhr N.D., Bradford H.N., Wells J.G. Use of Moore swabs for isolating Vibrio cholerae from sewage. J. Clin. Microbiol. 1980;11:385–388. doi: 10.1128/jcm.11.4.385-388.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besselsen D.G., Wagner A.M., Loganbill J.K. Detection of rodent coronaviruses by use of fluorogenic reverse transcriptase-polymerase chain reaction analysis. Comp. Med. 2002;52(2):111–116. [PubMed] [Google Scholar]
- Bjornevik K., Cortese M., Healy B.C., Kuhle J., Mina M.J., Leng Y., Elledge S.J., Niebuhr D.W., Scher A.I., Munger K.L., Ascherio A. Longitudinal analysis reveals high prevalence of epstein-barr virus associated with multiple sclerosis. Science. 2022;375:296–301. doi: 10.1126/science.abj8222. [DOI] [PubMed] [Google Scholar]
- Boehm A.B., Hughes B., Doung D., Chan-Herur V., Buchman A., Wolfe M.K., White B.J. 2022. Wastewater Surveillance of Human Influenza, Metapneumovirus, Parainfluenza, Respiratory Syncytial Virus (RSV), Rhinovirus, and Seasonal Coronaviruses During the COVID-19 Pandemic. medRxiv, 2022.09.22.22280218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton P.N., Jones C.A., Macartney K., Cheng A.C. Parechovirus: an important emerging infection in young infants. Med. J. Aust. 2018;208:365–369. doi: 10.5694/mja18.00149. [DOI] [PubMed] [Google Scholar]
- Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., Vandesompele J., Wittwer C.T. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Cannon M.J., Schmid D.S., Hyde T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 2010;20:202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- Chieochansin T., Vichiwattana P., Korkong S., Theamboonlers A., Poovorawan Y. Molecular epidemiology, genome characterization, and recombination event of human parechovirus. Virology. 2011;421:159–166. doi: 10.1016/j.virol.2011.09.021. [DOI] [PubMed] [Google Scholar]
- Dioverti M.V., Razonable R.R. Cytomegalovirus. Microbiol. Spectr. 2016;4(4) doi: 10.1128/microbiolspec.DMIH2-0022-2015. [DOI] [PubMed] [Google Scholar]
- Dumke R., Geissler M., Skupin A., Helm B., Mayer R., Schubert S., Oertel R., Renner B., Dalpke A.H. Simultaneous detection of SARS-CoV-2 and influenza virus in wastewater of two cities in Southeastern Germany, January to May 2022. Int. J. Environ. Health Res. Public Health. 2022;19:13374. doi: 10.3390/ijerph192013374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P.J. Epstein-Barr virus and cancer. Annu. Rev. Pathol. 2019;14:29–53. doi: 10.1146/annurev-pathmechdis-012418-013023. [DOI] [PubMed] [Google Scholar]
- Guido M., Tumolo M.R., Verri T., Romano A., Serio F., Giorgi M.D., Donno A.D., Bagordo F., Zizza A. Human bocavirus: current knowledge and future challenges. World J. Gastroenterol. 2016;22:8684–8697. doi: 10.3748/wjg.v22.i39.8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza I.A., Jurzik L., Stang A., Sure K., Überla K., Wilhelm M. Detection of human viruses in rivers of a densly-populated area in Germany using a virus adsorption elution method optimized for PCR analyses. Water Res. 2009;43:2657–2668. doi: 10.1016/j.watres.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Haramoto E., Kitajima M., Hata A., Torrey J.R., Masago Y., Sano D., Katayama H. A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 2018;135:168–186. doi: 10.1016/j.watres.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Heijnen L., Medema G. Surveillance of influenza A and the pandemic influenza A (H1N1) 2009 in sewage and surface water in the Netherlands. J. Water Health. 2011;9:434–442. doi: 10.2166/wh.2011.019. [DOI] [PubMed] [Google Scholar]
- Henning K.J. Overview of syndromic surveillance: what is syndromic surveillance? MMWR. 2004;53:5–11. [PubMed] [Google Scholar]
- Henrickson K.J. Parainfluenza viruses. Clin. Microbiol. Rev. 2003;16:242–264. doi: 10.1128/CMR.16.2.242-264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokajärvi A.M., Rytkönen A., Tiwari A., Kauppinen A., Oikarinen S., Lehto K.M., Kankaanpää A., Gunnar T., Al-Hello H., Blomqvist S., Miettinen I.T., Savolainen-Kopra C., Pitkänen T. The detection and stability of the SARS-CoV-2 RNA biomarkers in wastewater influent in Helsinki,Finland. Sci. Total Environ. 2021;770 doi: 10.1016/j.scitotenv.2021.145274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes B., Duong D., White B.J., Wigginton K.R., Chan E.M.G., Wolfe M.K., Boehm A.B. Respiratory syncytial virus (RSV) RNA in wastewater settled solids reflects RSV clinical positivity rates. Environ. Sci. Technol. Lett. 2022;9:173–178. [Google Scholar]
- Hutchinson E.C. Influenza virus. Trends Microbiol. 2018;26:809–810. doi: 10.1016/j.tim.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Idesawa M., Sugano N., Ikeda K., Oshikawa M., Takane M., Seki K., Ito K. Detection of epstein-barr virus in saliva by real-time PCR. Oral Microbiol. Immunol. 2004;19:230–232. doi: 10.1111/j.1399-302X.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- Jafarinejad H., Moghoofei M., Mostafaei S., Salimian J., Azimzadeh Jamalkandi S., Ahmadi A. Worldwide prevalence of viral infection in AECOPD patients: a meta-analysis. Microb. Pathog. 2017;113:190–196. doi: 10.1016/j.micpath.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumthip K., Khamrin P., Ushijima H., Maneekarn N. Contamination of human Bocavirus genotypes 1, 2, 3, and 4 in environmental waters in Thailand. Microbiol. Spectr. 2021;9 doi: 10.1128/spectrum.02178-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T.T., Tang J.W., Lai F.Y., Zaraket H., Dbaibo G., Bialasiewicz S., Tozer S., Heraud J.M., Drews S.J., Hachette T., Chan P.K., Koay E.S., Lee H.K., Tee K.K., Liu Y., Fraaij P.L.A., Jennings L., Waris M., Krajden M., Corriveau A., Jalal H., Nishimura H., Nymadawa P., Badarch D., Watanabe A., Kabanda A., Sloots T., Kok J., Dwyer D.E., Koopmans M. Comparative global epidemiology of influenza, respiratory syncytial and parainfluenza viruses, 2010–2015. J. Infect. 2019;79:373–382. doi: 10.1016/j.jinf.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor Y., Handsher R., Halmut T., Neuman M., Bobrov A., Rudich H., Vonsover A., Shulman L., Kew O., Mendelson E. Detection of poliovirus circulation by environmental surveillance in the absence of clinical cases in Israel and the Palestinian authority. J. Clin. Microbiol. 1999;37:1670–1675. doi: 10.1128/jcm.37.6.1670-1675.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall C., Wu H., Miyani B., Xagoraraki I. Identification of multiple potential viral diseases in a large urban center using wastewater surveillance. Water Res. 2020;184 doi: 10.1016/j.watres.2020.116160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMinn B.R., Korajkic A., Kelleher J., Herrmann M.P., Pemberton A.C., Ahmed W., Villegas E.N., Oshima K. Development of a large volume concentration method for recovery of coronavirus from wastewater. Sci. Total Environ. 2021;774 doi: 10.1016/j.scitotenv.2021.145727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeale D., Wang C.Y.T., Arden K.E., Mackay I.M. HPeV-3 predominated among parechovirus a positive infants during an outbreak in 2013–2014 in Queensland,Australia. J. Clin. Virol. 2018;98:28–32. doi: 10.1016/j.jcv.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Mercier E., D’Aoust P.M., Thakali O., Hegazy N., Jia J.-J., Zhang Z., Eid W., Plaza-Diaz J., Kabir M.P., Fang W., Cowan A., Stephenson S.E., Pisharody L., MacKenzie A.E., Graber T.E., Wan S., Delatolla R. Municipal and neighbourhood level wastewater surveillance and subtyping of an influenza virus outbreak. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-20076-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B. The detection of enteric carriers in towns by means of sewage examination. J. R. Sanit. Inst. 1951;71:57–60. doi: 10.1177/146642405107100109. [DOI] [PubMed] [Google Scholar]
- Moore N.E., Wang J., Hewitt J., Croucher D., Williamson D.A., Paine S., Yen S., Greening G.E., Hall R.J. Metagenomic analysis of viruses in feces from unsolved outbreaks of gastroenteritis in humans. J. Clin. Microbiol. 2015;53:15–21. doi: 10.1128/JCM.02029-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya M.C., Callahan S.M., Savchenko K.G., Stobart C.C. A contemporary view of respiratory syncytial virus (RSV) biology and strain-specific differences. Pathogens. 2019;8:67. doi: 10.3390/pathogens8020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J.R., Trask J.D., Gard S. II. Poliomyelitic virus in urban sewage. J. Exp. Med. 1940;71(6):765–777. doi: 10.1084/jem.71.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queensland Health; 2022. Queensland wastewater surveillance for SARS-CoV-2.https://www.data.qld.gov.au/dataset/queensland-wastewater-surveillance-for-sars-cov-2 [Google Scholar]
- Rector A., Bloemen M., Thijssen M., Pussig B., Beuselinck K., Ranst V., Wollants E. 2022. Epidemiological Surveillance of Respiratory Pathogens in Wastewater in Belgium. medRxiv. [DOI] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. NY Cold Spring Harbor Laboratory Press; COLD Spring Harbor: 1989. Molecular Cloning: A Laboratory Manual. (1989) [Google Scholar]
- Sangsanont R.S., Kongprajug A., Chyerochana N., Sresung M., Sriporatana N., Wanlapakorn N., Poovorawan Y., Mongkolsuk S., Sirikanchana K. SARS-CoV-2 RNA surveillance in large to small centralized wastewater treatment plants preceding the third COVID-19 resurgence in Bangkok,Thailand. Sci. Total Environ. 2022;809:151169. doi: 10.1016/j.scitotenv.2021.151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongprachum A., Fujimoto T., Takanashi S., Saito H., Okitsu S., Shimizu H., Khamrin P., Maneekarn N., Hayakawa S., Ushijima H. Detection of nineteen enteric viruses in raw sewage in Japan. Infect. Genet. Evol. 2018;63:17–23. doi: 10.1016/j.meegid.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Turner R.B., Lee W.-M. In: Clinical Virology. Richman D.D., Whitley R.J., Hayden F.G., editors. ASM Press; Washington: 2009. Rhinovirus; pp. 1063–1082. [Google Scholar]
- Verbyla M.E., Symonds E.M., Kafle R.C., Cairns M.R., Iriarte M., Mercado Guzmán A., Coronado O., Breitbart M., Ledo C., Mihelcic J.R. Managing microbial risks from indirect wastewater reuse for irrigation in urbanizing watersheds. Environ. Sci. Technol. 2016;50:6803–6813. doi: 10.1021/acs.est.5b05398. [DOI] [PubMed] [Google Scholar]
- Wang H., Sikora P., Rutgersson C., Lindh M., Brodin T., Björlenius B., Larsson D.G.J., Norder H. Differential removal of human pathogenic viruses from sewage by conventional and ozone treatments. Int. J. Hyg. Environ. Health. 2018;221:479–488. doi: 10.1016/j.ijheh.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidhaas J., Aanderud Z.T., Roper D.K., VanDerslice J., Gaddis E.B., Ostermiller J., Hoffman K., Jamal R., Heck P., Zhang Y., Torgersen K., Laan J.V., LaCross N. Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. Sci. Total Environ. 2021;775 doi: 10.1016/j.scitotenv.2021.145790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W.J. Isolation of enteric Bacilli from sewage and waters and its bearing on epidemiology. Br. Med. J. 1993;2(3794):560–562. doi: 10.1136/bmj.2.3794.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe M.K., Duong D., Bakker K.M., Ammerman M., Mortenson L., Hughes B., Arts P., Lauring A.S., Fitzsimmons W.J., Bendall E., Hwang C.E., Martin E.T., White B.J., Boehm A.B., Wigginton K.R. Wastewater-based detection of two influenza outbreaks. Environ. Sci. Technol. Lett. 2022;9:687–692. [Google Scholar]
- Wurtzer S., Levert M., Dhenain E., Boni M., Tournier J.N., Londinsky N., Lefranc A., Sig O., Ferraris O., Moulin L. First detection of Monkeypox virus genome in sewersheds in France. Environ. Sci. Technol. Lett. 2022;9:991–996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.