Abstract
Health care–acquired viral respiratory infections are common and cause increased patient morbidity and mortality. Although the threat of viral respiratory infection has been underscored by the coronavirus disease 2019 (COVID-19) pandemic, respiratory viruses have a significant impact in health care settings even under normal circumstances. Studies report decreased nosocomial transmission when aggressive infection control measures are implemented, with more success noted when using a multicomponent approach. Influenza vaccination of health care personnel furthers decrease rates of transmission; thus, mandatory vaccination is becoming more common. This article discusses the epidemiology, transmission, and control of health care–associated respiratory viral infections.
Keywords: Influenza, SARS-CoV-2, COVID-19, Coronavirus, Respiratory virus, Infection prevention, Nosocomial infection, Health care–acquired infection
Key points
-
•
Health care–acquired viral respiratory infections are common, with increased patient morbidity and mortality.
-
•
Multicomponent infection control measures consisting of education, hand washing, isolation, consistent use of personal protective equipment, cohorting patients, and cohort nursing reduces transmission of respiratory infections.
-
•
Health care worker influenza vaccination is recommended, with mandatory vaccination policies becoming more common.
Introduction
Although the threat of viral respiratory infection has been underscored by the current coronavirus disease 2019 (COVID-19) pandemic, respiratory viruses have a significant impact in health care settings even under normal circumstances. It is estimated that approximately 19,000 nosocomial respiratory virus infections occur in US hospitals each year, resulting in increased patient morbidity, mortality, and health care costs.1 Approximately 20% of patients with health care–associated pneumonia have viral respiratory infections, with an incidence that typically reflects the level of virus activity within the community.2 , 3 Respiratory viruses can be transmitted through multiple pathways, including contact, droplet, and airborne routes, with the relative contributions of each route depending on the viral species and environmental factors. Controlled laboratory studies to determine which transmission routes are possible and epidemiologic studies to determine which routes most contribute to real-world transmission are both needed to inform prevention and control. This article describes the current understanding of the epidemiology, transmission, and control of health care–associated respiratory viral infections.
Severe acute respiratory syndrome coronavirus-2 and other human coronaviruses
Epidemiology
Among the human coronaviruses (HCoVs), there are 4 seasonal viruses (229E, OC43, NL63, and HKU1) that cause annual epidemics of primarily mild respiratory infections. In addition, several novel coronaviruses have emerged from zoonotic reservoirs in recent decades causing severe lower respiratory disease, most notably the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus responsible for the recent COVID-19 pandemic.
As of March 1, 2021, the COVID-19 pandemic caused by SARS-CoV-2 resulted in 113,467,303 confirmed cases and 2,520,550 deaths worldwide, with the United States accounting for approximately 25% of the cases and 20% of the deaths.4 , 5 A large study performed in the United States and United Kingdom found that health care personnel (HCP) were more than 3 times more likely to be infected with SARS-CoV-2 compared with the general community after adjusting for likelihood of testing.6 These infections strained health care capacity and put patient safety at risk. Characterizations of nosocomial outbreaks early in the pandemic noted that mortality among patients with health care–acquired COVID-19 was much higher than in the general population, potentially reflecting older age and poorer health.7, 8, 9 Residents of long-term care facilities have accounted for more than a quarter of all US COVID-19 deaths as of mid-February 2021.10
Although the COVID-19 pandemic is the first documented pandemic caused by an HCoV, there are other recent examples of novel HCoV emergence causing epidemics. In 2002 to 2003, the novel SARS-CoV caused an epidemic of severe respiratory illness (coined severe acute respiratory syndrome [SARS]) resulting in 8096 cases globally and 774 deaths (9.6% case fatality ratio) with notable nosocomial outbreaks in Singapore and Toronto.11 , 12 Middle East Respiratory Syndrome Coronavirus (MERS-CoV) was identified in the Arabian Peninsula in 2012.13 As of January 2021, 2566 cases in 27 countries have been reported, with 882 deaths (34.4% case fatality ratio).14 , 15 Although direct contact with dromedary camels has remained a major risk factor for MERS, there have been multiple MERS nosocomial outbreaks and 13% to 70% of nosocomial MERS infections were among HCP.15, 16, 17, 18, 19
Nosocomial infection with seasonal coronaviruses has also been described,1 , 20 , 21 including large outbreaks among patients and HCP in pediatric and neonatal intensive care units.22
Transmission
The median incubation period of SARS-CoV-2 is 4 to 5 days, with symptoms developing within 12 days in 95% of symptomatic infections.23 , 24 Between 1 in 3 and 1 in 6 SARS-CoV-2 infections remain asymptomatic.25 , 26 Asymptomatic patients may have viral loads higher or comparable with symptomatic patients, but shorter durations of shedding.27 , 28 Among symptomatic individuals, viral shedding peaks on or just before the day of symptom onset.28 In uncomplicated infections, shedding of virus capable of replication lasts less than 10 days,28 but individuals with severe COVID-1929 or who are immunocompromised30 may be infectious for much longer. Taken together, the long incubation period, high proportion of asymptomatic infections, presymptomatic shedding, and long duration of shedding contribute to a high proportion of unobserved transmission.
SARS-CoV-2 is predominantly transmitted through close contact via the large droplet and direct contact routes.31 , 32 However, there is evidence that SARS-CoV-2 may be transmitted over longer distances and periods of time via smaller droplets or aerosols in certain circumstances.32, 33, 34 Aerosol transmission may be of particular concern in health care settings where procedures are performed that can generate infectious respiratory droplets and aerosols (aerosol-generating procedures) such as endotracheal intubation and extubation, nebulizer administration, and airway suctioning.35 Studies have also shown frequent contamination of surfaces, with prolonged infectivity up to 96 hours on nonporous surfaces and up to 72 hours on cardboard.34 , 36 However, instances of indirect fomite transmission have not been conclusively documented.
In contrast with SARS-CoV-2, MERS-CoV has low human-to-human transmission potential. However, inadequate or inconsistent infection control measures have been cited as factors in MERS-CoV transmission during nosocomial outbreaks.16 , 17 MERS-CoV may transmit by large droplets, contact, and aerosols following aerosol-generating procedures.37
The seasonal HCoVs, unlike their higher-severity counterparts, seem to be largely spread by droplet transmission.38 Performing aerosol-generating procedures and contact with pediatric patients have been identified as risk factors for HCP infection with seasonal HCoV.39
Prevention and Control
Patients with suspected or confirmed SARS-CoV-2 infection, or those who would otherwise be required to quarantine, should be placed in single rooms with a closed door and dedicated bathroom.40 Patients undergoing aerosol-generating procedures should be placed in airborne infection isolation rooms if possible. Limiting patients with SARS-CoV-2 to specific units should be considered, and their movement should be limited. In addition to standard precautions, HCP entering the rooms of patients with SARS-CoV-2 should use an N-95-equivalent or higher-level respirator, eye protection, gloves, and a gown. These best practices have not always been feasible given limited personal protective equipment (PPE) resources, and the US Centers for Disease Control and Prevention (CDC) has outlined contingencies and strategies to preserve PPE.41 The World Health Organization (WHO) and several US state health departments have issued more lenient guidance permitting use of medical masks rather than respirators in the absence of aerosol-generating procedures.42 Although the SARS-CoV-2 research base has developed in an extraordinarily short time, these differing recommendations highlight remaining gaps in the knowledge of SARS-CoV-2 epidemiology, transmission, and infection control best practices.43
CDC has also recommended general measures to reduce health care–associated transmission.40 These measures have been extremely effective for reducing risk of health care–associated SARS-CoV-2 infection and could be considered for use during nonpandemic respiratory virus seasons.44 , 45
-
•
Telehealth strategies should be used to reduce in-person medical visits where possible.
-
•
Visitors to the health care facility should be limited, especially during times of high community transmission.
-
•
All patients, visitors, and HCP should be screened for symptoms of COVID-19 or contact with a suspected or confirmed case in the past 14 days.
-
•
Patients, visitors, and HCP should wear a well-fitting cloth mask,46 surgical mask, or respirator at all times as a method of universal source control.
-
•
Physical distancing should be encouraged to maintain at least 2 m (6 feet) between individuals when possible.
Both the Infectious Diseases Society of America (IDSA) and CDC recommend nucleic acid amplification testing for all individuals, hospitalized or in the community, with either symptoms of COVID-19 or who have been exposed to a suspected or confirmed patient with SARS-CoV-2 infection.47 , 48 Negative results in symptomatic hospitalized patients should be treated with suspicion with repeated testing, including specimens from the lower respiratory tract if possible. Testing of asymptomatic patients on hospital admission is also recommended by IDSA and CDC during periods of high community transmission.40 , 48 Periodic testing of asymptomatic HCP without known exposure is recommended for those working in long-term care facilities, and may be considered in hospital settings if resources are available.49
As of March 2021, 3 vaccines for the prevention of COVID-19 have received emergency use authorizations from the US Food and Drug Administration (FDA), and all have shown substantial efficacy, particularly against severe disease.50, 51, 52 Frontline HCP are among those designated as highest priority for vaccination by the CDC’s Advisory Committee for Immunization Practices (ACIP).53 , 54 High HCP vaccination coverage is critical to protecting both HCP and patients. It remains to be seen whether requirements for HCP COVID vaccination will emerge as has occurred with influenza vaccination. As the experience and comfort with these new vaccines increases and full FDA approval is issued, such requirements may be justified, especially if an impact on transmission is confirmed.
Hospitalized patients with MERS-CoV should be placed in contact and airborne precautions with the use of eye protection.55
Although seasonal HCoV are not specifically mentioned in CDC infection prevention guidelines, droplet and contact precautions may be considered based on likely routes of transmission.38
Seasonal influenza
Epidemiology
Influenza infects approximately 2% to 10% of the US population annually, resulting in 140,000 to 810,000 hospitalizations and 12,000 to 61,000 deaths.56 , 57 Transmission of influenza has been reported in a variety of health care settings, and HCP are often implicated as index cases for nosocomial outbreaks.58 A recent study found that nearly 90% of influenza-infected HCP were asymptomatic or mildly symptomatic with no fever or cough.59 Although asymptomatic and mildly symptomatic individuals shed less virus for shorter duration than those who are symptomatic,60 there is still potential for transmitting infection to patients or other HCP. Even when symptomatic, HCP often work while acutely ill for a variety of reasons.61 , 62 High levels of patient and HCP infection can also disrupt hospital operations during seasonal influenza epidemics.63 , 64
Transmission
The average incubation period for influenza is approximately 3 days, but can range from 1 to 4 days.65 Viral shedding begins before the appearance of symptoms and within the first 24 hours following infection, peaks on the second day following infection, and usually declines rapidly thereafter.65 , 66 Virus is typically no longer detectable after 6 to 10 days after inoculation. Prolonged viral shedding has been documented in children,67 those hospitalized for severe influenza,68 and immunocompromised adults.69
The possibility for influenza transmission to occur through direct contact,70 , 71 indirect contact with fomites,72 , 73 large droplet,74 , 75 and aerosol76 , 77 routes has been shown in both laboratory and field studies. However, in both the community and health care settings, large droplet and direct contact transmission predominates.78 Although possible, indirect fomite transmission and aerosol transmission across long distances and time periods have not been conclusively shown and are unlikely. Consistent with this, several randomized controlled trials have found that no significant advantage of N95 respirators compared with surgical masks has been shown for standard clinical care.79, 80, 81 The exception may be for aerosol-generating procedures, which can increase risk of transmission to health care workers involved in or in close proximity to the procedure.82 , 83
Prevention and Control
In addition to standard precautions, the CDC recommends implementation of droplet precautions to prevent health care–associated influenza (Tables 1 and 2 ).3 , 84 , 85 Droplet precautions should continue for 7 days after illness onset or until 24 hours after the resolution of fever and respiratory symptoms, whichever is longer, but may be extended for immunocompromised or other patients who may have prolonged viral shedding. HCP should wear respiratory protection equivalent to a fitted N95 filtering respirator mask if patients with influenza undergo aerosol-generating procedures.85
Table 1.
Precautions for preventing transmission of respiratory infections
| Precautions | Component | Recommendation |
|---|---|---|
| Standard | Hand hygiene |
|
| Respiratory hygiene |
|
|
| Gloves | Wear when contact with respiratory secretions could occur | |
| Gowns | Wear during procedures and activities when contact of clothing or exposed skin with respiratory secretions is anticipated | |
| Masks and eye protection | Wear during procedures and activities likely to generate splashes or sprays of respiratory secretions | |
| Contacta | Patient placement | Place patient in a single-patient room, if possible, or cohort with other patients infected with the same organism Limit patient movement to medically necessary purposes |
| Gloves and gowns | Wear on room entry whenever contact is likely with the patient, patient’s respiratory secretions, or potentially contaminated items in the patient’s vicinity, including equipment and environmental surfaces | |
| Masks and eye protection | As per standard precautions | |
| Dropleta | Patient placement | Place patient in a single-patient room, if possible, or cohort with other patients infected with the same organism Limit patient movement to medically necessary purposes, and patients should wear a mask and follow respiratory hygiene during transport |
| Gloves, gowns, and eye protection | As per standard precautions | |
| Masks | Wear a surgical mask on room entry if close contact (eg, <1 m [3 feet]) with the patient is anticipated | |
| Airbornea | Patient placement | Place infected patients in a single-patient airborne infection isolation roomb Limit patient movement to medically necessary purposes, and patients should wear a mask and follow respiratory hygiene during transport |
| Gloves, gowns, and eye protection | As per standard precautions | |
| Masks | Wear a fit-tested N95 respirator before room entry |
Contact, droplet, and airborne precautions include hand hygiene and respiratory hygiene as per standard precautions.
Airborne infection isolation room consists of negative pressure relative to the surrounding area, 6 to 12 air changes per hour, and air is exhausted directly to the outside or recirculated through high-efficiency particulate air (HEPA) filtration before return.
From Siegel JD, Rhinehart E, Jackson M, et al; Health Care Infection Control Practices Advisory C. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 2007;35:S65–164; with permission.
Table 2.
Infection prevention recommendations for viral respiratory pathogens
| Common Measures for Reducing Transmission in the Health Care Setting |
|---|
| Hand hygiene |
| Respiratory hygiene/cough etiquette |
| Standard precautions |
| Restrict ill visitorsa |
| Restrict ill personnel (prevent so-called presenteeism) |
| Cohort nursing |
| Prompt diagnosis of respiratory infections among patients by diagnostic testsb |
| Restrict elective admissions of patients during outbreaks in the community and/or facility |
| Surveillance for an increase in activity of viral infections within the community |
| Universal source control with well-fitting masks |
| Measures for Reducing Transmission of Specific Pathogens in the Health Care Setting | |||||||
|---|---|---|---|---|---|---|---|
| Influenza |
Novel Coronavirus | ||||||
| Intervention | RSV | Adenovirus | Parainfluenza Virus/HMPV | Rhinovirus | Seasonal | Novel | |
| Precautions | — | — | — | — | — | — | — |
| Contact | ● | ● | ● | — | — | ● | ● |
| Droplet | — | ● | — | ● | ● | ● | |
| Airborne | — | — | — | — | ●c | ● | ○d |
| Eye protection | — | — | — | — | — | ● | ● |
| Vaccination of personnel | — | — | — | — | ● | — | — |
| Chemoprophylaxis | ○e | — | — | — | ○f | ○ | — |
Closed circles (●) denote recommended measures. Open circles (○) denote measures recommended in certain circumstances.
Abbreviations: HMPV, human metapneumovirus; RSV, respiratory syncytial virus.
Institutions may restrict only young children and/or screen all visitors for illness by using a trained health care worker to assess for signs and symptoms or by using an educational patient information list to advise ill visitors.
To control outbreaks, institutions may perform preadmission screening of patients for infection.
The Centers for Disease Control and Prevention recommends an N95 respirator for HCP performing aerosol-generating procedures.
The Centers for Disease Control and Prevention recommends use of a fit-tested N95 respirator. The WHO and some US state health departments recommend medical masks.
In addition to other infection control measures, palivizumab prophylaxis of high-risk infants has been used to control outbreaks in the neonatal intensive care unit.
During a facility outbreak of influenza, administer antiviral chemoprophylaxis to all patients in the involved unit, regardless of vaccination status, and to unvaccinated HCP working in the involved unit. If feasible, administer facility-wide chemoprophylaxis for all residents in long-term care facilities. Chemoprophylaxis may also be administered to personnel when the outbreak strain is not well matched by the vaccine.
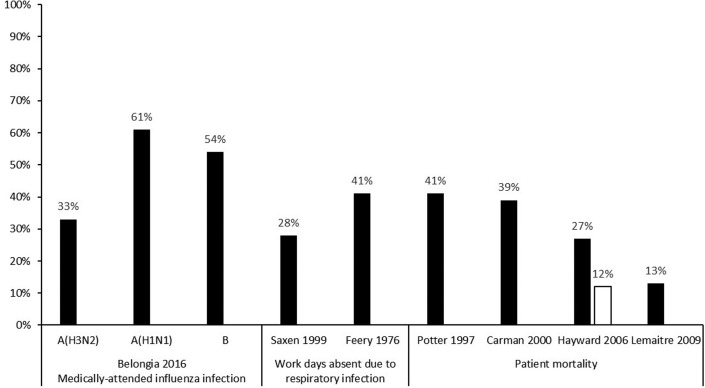
Annual influenza vaccination is recommended for HCP by the ACIP.86 Although influenza vaccine effectiveness can vary annually, it is the best available tool for the prevention of influenza infection (Fig. 1 ).87 Vaccinated HCP are less likely to miss work because of respiratory illness and miss fewer days when they are ill.88 , 89 Improved HCP vaccination coverage has also been linked with decreased health care–associated influenza among patients and personnel,90 , 91 and reduced mortality among nursing home patients.92, 93, 94, 95, 96 Since 2013, multiple professional societies and safety advocacy organizations have endorsed policies for mandatory influenza vaccination for HCP (Box 1 ). As a result, the proportion of hospitals with mandatory influenza vaccination programs increased from 37% in 2013 to 61% in 2017.97 During the 2019 to 2020 influenza season, HCP vaccination coverage was 81% overall; 52% in settings where vaccines were not required, promoted, or offered on site; and 94% where vaccination was required.98
Fig. 1.
Percentage reduction in noted outcomes in HCP receiving influenza vaccination.
Box 1. Professional societies and safety advocacy organisms that recommend mandatory health care personnel influenza immunization.
American Academy of Family Physicians (AAFP)
American Academy of Pediatrics (AAP)
American College of Physicians (ACP)
American Hospital Association (AHA)
American Medical Directors Association (AMDA)
American Nurses Association (ANA)
American Pharmacists Association
American Public Health Association (APHA)
Association for Professionals in Infection Control and Epidemiology (APIC)
IDSA
National Association of County and City Health Officials (NACCHO)
National Business Group on Health
National Patient Safety Foundation (NPSF)
Society for Healthcare Epidemiology of America (SHEA)
Veterans Health Administration (VHA) Department of Veterans Affairs
Data from observational studies and controlled trials support recommendations to provide antiviral chemoprophylaxis to residents in long-term care facilities, regardless of vaccination status, during an institutional influenza outbreak.99 , 100 In this setting, chemoprophylaxis should be continued for 14 days or for at least 7 days after the onset of symptoms in the last person infected, whichever is longer.99 , 100 Effectiveness of employee chemoprophylaxis in the acute care setting is unclear, but may be considered for unvaccinated or high-risk HCP during an institutional outbreak.99 , 100
In addition to vaccination and antiviral chemoprophylaxis, interventions to prevent health care–associated influenza include source control (ie, wearing a mask), cohort nursing, exclusion of ill HCP and visitors through comprehensive sick leave policies and entry screening, and early diagnostic testing of symptomatic patients.85 , 99 Influenza testing is recommended on admission for all hospitalized patients with acute respiratory illness during the influenza season.99
Novel pandemic influenza
Over the past century, there have been 4 major influenza pandemics caused by novel viruses resulting from reassortment of human influenza genes with those of avian or swine strains: 1918 to 1919 A (H1N1), 1957 to 1958 A (H2N2), 1968 to 1969 A (H3N2), and 2009 A (H1N1). Several zoonotic influenza viruses are currently being monitored for pandemic potential, including avian H5 and H7 viruses.
Highly pathogenic avian influenza A (H5N1) virus was first reported to cause human infections in 1997 in China; however, no other human infections were reported until the virus reemerged in Hong Kong in 2003.101 Since that time, it has caused 862 infections worldwide, of whom 455 patients have died (case fatality ratio: 53%), although only 1 case was identified in 2020.102 Human infection with avian influenza A (H7N9) was first reported 2013 in China, subsequently causing seasonal outbreaks of severe respiratory illness accounting for a total of 616 deaths from a total of 1568 cases (case fatality ratio: 39%).103 However, only 4 human cases have been identified following mass immunization of Chinese poultry with a bivalent influenza A (H7N9) and A (H5N1) vaccine beginning in September 2017.103 , 104
The CDC has issued interim guidelines for infection control for patients with suspected novel influenza A infection associated with severe disease, including H5N1, H7N9, and other emerging strains. Avian influenza should be suspected in patients presenting with a severe respiratory illness with recent contact with potentially infected birds or with travel to a country with avian influenza activity in the past 10 days.105 Contact and airborne precautions should be used for patients with suspected novel influenza (see Tables 1 and 2). In addition, all HCP should wear eye protection when entering the patient’s room. Transmission-based precautions for novel influenza should be continued throughout the duration of the patient’s stay. Antiviral chemoprophylaxis for 5 days should also be considered for HCP with unprotected exposures if the exposure was not ongoing, or for 10 days if the exposure was ongoing.106
Respiratory syncytial virus
Epidemiology
Respiratory syncytial virus (RSV) is the most common cause of pneumonia and bronchiolitis in infants107 and a common cause of hospitalization in older and high-risk adults.108 Rates of respiratory hospitalizations secondary to RSV infection are highest among infants, although older patients (≥75 years) have similar rates to children aged 1 to 4 years.109 Outbreaks of RSV have occurred in a variety of health care settings.110, 111, 112, 113 Secondary infection risks between 19% and 45% among patients, and between 34% and 56% among HCP, on infant wards have been reported when limited or no infection control measures are implemented.110 , 114 , 115 Most infected HCP are symptomatic, but asymptomatic shedding of RSV occurs in 15% to 20%.116 As with other viruses, symptomatic HCP who work while ill are a concern, and this is reported to occur with a high frequency (51%–75%) in some populations.117 , 118
Transmission
Transmission of RSV occurs primarily through direct contact or by self-inoculation after touching contaminated fomites119 , 120; inoculation is most efficient via the eyes and nose.121 RSV has been recovered on countertops for up to 6 hours, on rubber gloves for up to 2 hours, and on cloth gowns and hands for 15 to 60 minutes after contamination with infected nasal secretions.122 The duration of viral shedding among hospitalized infants averages 6.7 days but can be as long as 21 days123; in a large Kenyan household cohort, shedding averaged 11 days, with approximately 13% shedding for more than 21 days.124 Similar durations of viral shedding have also been shown for older adults.125 Younger children, infants with lower respiratory disease, and those with a compromised immune status have more prolonged shedding and shed greater quantities of virus.123 , 124
Prevention and Control
Although several RSV vaccines are in development,126 there is no licensed vaccine or specific treatment readily available for RSV. Therefore, effective infection control measures are paramount for minimizing transmission. In addition to standard precautions, the CDC recommends contact precautions to prevent health care–associated RSV (see Tables 1 and 2).84 These precautions should continue for the duration of illness but may be extended for immunocompromised patients because of prolonged viral shedding. Some studies have suggested that eye protection may further reduce transmission given the importance of the eye as a portal of entry.127 , 128
Palivizumab is a humanized mouse immunoglobulin G monoclonal antibody that is effective in preventing hospitalizations caused by RSV infections,129 and the use of palivizumab as prophylaxis for susceptible infants to control outbreaks in neonatal intensive care units has been described.111 , 130 The American Academy of Pediatrics recommends that palivizumab be administered to the following groups of patients131 , 132:
-
•
Infants younger than 12 months of age and born before 29 weeks’ gestation at the beginning of RSV season
-
•
Preterm infants with chronic lung disease of prematurity who are born before 32 weeks’ gestation during the first year of life
-
•
Infants younger than 12 months of age with hemodynamically significant heart disease
Other respiratory viruses
Adenovirus
Health care–associated outbreaks of adenovirus have been reported from various settings with high secondary infection risks among patients (15%–56%).133, 134, 135 Adenoviruses can result in severe or fatal disseminated disease among severely immunocompromised patients.136 Adenoviruses are transmitted through large respiratory droplets but are also notable for their ability to survive on nonporous surfaces for up to 49 days.137 As a result, transmission also occurs via self-inoculation after contact with contaminated fomites. In addition to standard precautions, the CDC recommends contact and droplet precautions to prevent health care–associated adenovirus infection (see Tables 1 and 2).3 , 84
Parainfluenza Virus
Transmission of parainfluenza has been documented in pediatric wards,138 neonatal nurseries,139 and adult transplant units.140 Transmission of parainfluenza primarily occurs by direct person-to-person contact. Parainfluenza can survive up to 4 hours on porous surfaces and up to 10 hours on nonporous surfaces.141 However, viral recovery from hands declines rapidly, with only 5% detected after 10 minutes.142 The CDC recommends contact precautions for the prevention of health care–associated parainfluenza infection (see Tables 1 and 2).3 , 84
Rhinovirus
Among studies with broad molecular respiratory virus identification, rhinoviruses are typically the most common health care–associated respiratory viral infection among both children and adults.1 , 20 , 21 Nosocomial rhinovirus infections have been linked with increased need for respiratory support and longer lengths of stay in high-risk neonates143 and deaths in long-term care facilities.144 However, the clinical significance of rhinovirus infections is debated because this virus is frequently detected in asymptomatic children.145 The CDC recommends droplet precautions for the prevention of health care–associated rhinovirus infection (see Tables 1 and 2).84
Human Metapneumovirus
Nosocomial outbreaks of human metapneumovirus have been described in various settings with secondary infection risks ranging from 36% to 56%.146, 147, 148, 149 Human metapneumovirus survival on nonporous surfaces has been shown for up to 8 hours, but for much shorter durations on porous surfaces.150 The CDC recommends contact precautions for the prevention of health care–associated human metapneumovirus infection (see Tables 1 and 2).151
Empiric clinical guidance for patients with suspected respiratory viral infections
For suspected respiratory virus infections, CDC recommends empiric contact plus droplet precautions until viruses specifically requiring droplet precautions can be ruled out.3 , 84 Empiric treatment with influenza antiviral medications is also recommended for any patient with suspected or confirmed influenza infection who is hospitalized, has severe illness, or is at high risk of complications.100
Diagnostic Testing
In many cases, diagnosing a respiratory virus infection can inform clinical management, improve infection prevention,152 , 153 and may improve antibiotic stewardship.154 In addition to virus-specific guidance previously discussed for SARS-CoV-2 and influenza, IDSA recommends respiratory virus panel testing of immunocompromised patients who are hospitalized with respiratory symptoms, and advises that panel testing can be used more broadly in hospitalized patients if it might influence clinical care or infection prevention.99 The diagnostic landscape continues to rapidly evolve and more research is needed regarding best practices, clinical interpretation, and cost-effectiveness of respiratory virus testing.155
Summary
Transmission of respiratory viruses occurs in a variety of health care settings, resulting in increased patient morbidity and health care costs. Different viruses have different modes of transmission, and prevention of transmission requires early recognition of symptomatic patients and prompt institution of appropriate transmission-based precautions, in addition to adherence to basic infection control practices such as hand hygiene. In addition to virus-specific infection control measures, vaccination of HCP is a priority for prevention of health care–associated SARS-CoV-2 and influenza infection.
Clinics care points
-
•
Vaccination of health care personnel is a priority for the prevention of healthcare acquired influenza and SARS-CoV-2 infection.
-
•
Recommended precautions preventing healthcare acquired viral respiratory diseases vary by viral species.
-
•
droplet precautions until viruses specifically requiring droplet precautions can be ruled out.
-
•
In many cases, diagnosing a respiratory virus infection can inform clinical management, improve infection prevention, and may improve antibiotic stewardship.
Acknowledgments
Disclosure
J.G. Petrie and T.R. Talbot have no disclosures.
Footnotes
This work was funded in whole or in part with federal funds by the National Institute of Allergy and Infectious Diseases (Grant No. K01 AI141579 to J.G.P.).
References
- 1.Chow E.J., Mermel L.A. Hospital-Acquired Respiratory Viral Infections: Incidence, Morbidity, and Mortality in Pediatric and Adult Patients. Open Forum Infect Dis. 2017;4(1) doi: 10.1093/ofid/ofx006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall C.B. Nosocomial viral respiratory infections: Perennial weeds on pediatric wards. Am J Med. 1981;70(3):670–676. doi: 10.1016/0002-9343(81)90594-5. [DOI] [PubMed] [Google Scholar]
- 3.Tablan O.C., Anderson L.J., Besser R., et al. Guidelines for preventing health-care--associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep Cent Dis Control. 2004;53(RR-3):1–36. [PubMed] [Google Scholar]
- 4.Weekly operational update on COVID-19 - 1 March 2021. https://www.who.int/publications/m/item/weekly-operational-update-on-covid-19---1-march-2021 Available at: Accessed March 1, 2021.
- 5.Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; 2021. COVID Data Tracker weekly review.https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html Available at: Accessed March 1, 2021. [Google Scholar]
- 6.Nguyen L.H., Drew D.A., Graham M.S., et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5(9):e475–e483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rickman H.M., Rampling T., Shaw K., et al. Nosocomial Transmission of Coronavirus Disease 2019: A Retrospective Study of 66 Hospital-acquired Cases in a London Teaching Hospital. Clin Infect Dis. 2021;72(4):690–693. doi: 10.1093/cid/ciaa816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Praet J.T., Claeys B., Coene A.-S., et al. Prevention of nosocomial COVID-19: Another challenge of the pandemic. Infect Control Hosp Epidemiol. 2020;41(11):1355–1356. doi: 10.1017/ice.2020.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMichael T.M., Currie D.W., Clark S., et al. Epidemiology of Covid-19 in a Long-Term Care Facility in King County, Washington. N Engl J Med. 2020;382(21):2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Healthcare Safety Network (NHSN) COVID-19 Nursing Home Data. 2021. https://data.cms.gov/stories/s/COVID-19-Nursing-Home-Data/bkwz-xpvg/ Available at: Accessed March 2, 2021.
- 11.Goh K.-T., Cutter J., Heng B.-H., et al. Epidemiology and control of SARS in Singapore. Ann Acad Med Singap. 2006;35(5):301–316. [PubMed] [Google Scholar]
- 12.Varia M., Wilson S., Sarwal S., et al. Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. CMAJ Can Med Assoc J. 2003;169(4):285–292. [PMC free article] [PubMed] [Google Scholar]
- 13.Zaki A.M., van Boheemen S., Bestebroer T.M., et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 14.WHO EMRO | MERS outbreaks | MERS-CoV | Health topics. http://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html Available at: Accessed February 23, 2021.
- 15.Hui D.S., Azhar E.I., Kim Y.-J., et al. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018;18(8):e217–e227. doi: 10.1016/S1473-3099(18)30127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Assiri A., McGeer A., Perl T.M., et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balkhy H.H., Alenazi T.H., Alshamrani M.M., et al. Notes from the Field: Nosocomial Outbreak of Middle East Respiratory Syndrome in a Large Tertiary Care Hospital--Riyadh, Saudi Arabia, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(6):163–164. doi: 10.15585/mmwr.mm6506a5. [DOI] [PubMed] [Google Scholar]
- 18.Hunter J.C., Nguyen D., Aden B., et al. Transmission of Middle East Respiratory Syndrome Coronavirus Infections in Healthcare Settings, Abu Dhabi. Emerg Infect Dis. 2016;22(4):647–656. doi: 10.3201/eid2204.151615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chowell G., Abdirizak F., Lee S., et al. Transmission characteristics of MERS and SARS in the healthcare setting: a comparative study. BMC Med. 2015;13:210. doi: 10.1186/s12916-015-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi H.-S., Kim M.-N., Sung H., et al. Laboratory-based surveillance of hospital-acquired respiratory virus infection in a tertiary care hospital. Am J Infect Control. 2017;45(5):e45–e47. doi: 10.1016/j.ajic.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrie J.G., Lauring A.S., Martin E.T., et al. Hospital Associated Respiratory Virus Infection in Children and Adults: It Does Not Just Occur During Cold and Flu Season. Open Forum Infect Dis. 2020;7(6) doi: 10.1093/ofid/ofaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gagneur A., Vallet S., Talbot P.J., et al. Outbreaks of human coronavirus in a pediatric and neonatal intensive care unit. Eur J Pediatr. 2008;167(12):1427–1434. doi: 10.1007/s00431-008-0687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Q., Guan X., Wu P., et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauer S.A., Grantz K.H., Bi Q., et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med. 2020 doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oran D.P., Topol E.J. The Proportion of SARS-CoV-2 Infections That Are Asymptomatic. Ann Intern Med. 2021 doi: 10.7326/M20-6976. [DOI] [PubMed] [Google Scholar]
- 26.Buitrago-Garcia D., Egli-Gany D., Counotte M.J., et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLOS Med. 2020;17(9):e1003346. doi: 10.1371/journal.pmed.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasanoglu I., Korukluoglu G., Asilturk D., et al. Higher viral loads in asymptomatic COVID-19 patients might be the invisible part of the iceberg. Infection. 2020:1–10. doi: 10.1007/s15010-020-01548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cevik M., Tate M., Lloyd O., et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2(1):e13–e22. doi: 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Kampen JJA, van de Vijver D.A.M.C., Fraaij P.L.A., et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19) Nat Commun. 2021;12(1):267. doi: 10.1038/s41467-020-20568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarhini H., Recoing A., Bridier-Nahmias A., et al. Long term SARS-CoV-2 infectiousness among three immunocompromised patients: from prolonged viral shedding to SARS-CoV-2 superinfection. J Infect Dis. 2021 doi: 10.1093/infdis/jiab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization (WHO) Transmission of SARS-CoV-2: implications for infection prevention precautions. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions Available at: Accessed March 22, 2021.
- 32.Centers for Disease Control and Prevention Science Brief: SARS-CoV-2 and Potential Airborne Transmission. 2020. https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-sars-cov-2.html Available at: Accessed March 22, 2021.
- 33.Morawska L., Milton D.K. It Is Time to Address Airborne Transmission of Coronavirus Disease 2019 (COVID-19) Clin Infect Dis. 2020;71(9):2311–2313. doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasnick S., Carlos W.G., Dela Cruz C.S., et al. SARS-CoV-2 Transmission and the Risk of Aerosol-Generating Procedures. Am J Respir Crit Care Med. 2020;202(4):P13–P14. doi: 10.1164/rccm.2024P13. [DOI] [PubMed] [Google Scholar]
- 36.Chia P.Y., Coleman K.K., Tan Y.K., et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat Commun. 2020;11(1):2800. doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otter J.A., Donskey C., Yezli S., et al. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92(3):235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Committee on Infectious Diseases; American Academy of Pediatrics. Kimberlin D.W., Brady M.T., Jackson M.A., et al. 2018. Red Book® 2018 | red Book online.https://redbook.solutions.aap.org/book.aspx?bookid=2205 Available at: Accessed March 25, 2021. [Google Scholar]
- 39.Cummings D.A.T., Radonovich L.J., Gorse G.J., et al. Risk Factors for Healthcare Personnel Infection with Endemic Coronaviruses (HKU1, OC43, NL63, 229E): Results from the Respiratory Protection Effectiveness Clinical Trial (ResPECT) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention (CDC) 2020. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic.https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html Available at: Accessed March 2, 2021. [Google Scholar]
- 41.Centers for Disease Control and Prevention Strategies for Optimizing the Supply of Facemasks. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/face-masks.html Available at: Accessed March 2, 2021.
- 42.World Health Organization (WHO) Advice on the use of masks in the community, during home care and in healthcare settings in the context of the novel coronavirus (COVID-19) outbreak. https://www.who.int/publications/i/item/advice-on-the-use-of-masks-in-the-community-during-home-care-and-in-healthcare-settings-in-the-context-of-the-novel-coronavirus-(2019-ncov)-outbreak Available at: Accessed March 17, 2021.
- 43.Mody L, Akinboyo IC, Babcock HM, et al. COVID-19 Research Agenda for Healthcare Epidemiology. Infect Control Hosp Epidemiol. 2021:1-81. doi:10.1017/ice.2021.25 [DOI] [PMC free article] [PubMed]
- 44.Richterman A., Meyerowitz E.A., Cevik M. Hospital-Acquired SARS-CoV-2 Infection: Lessons for Public Health. JAMA. 2020 doi: 10.1001/jama.2020.21399. [DOI] [PubMed] [Google Scholar]
- 45.Wang X., Ferro E.G., Zhou G., et al. Association Between Universal Masking in a Health Care System and SARS-CoV-2 Positivity Among Health Care Workers. JAMA. 2020 doi: 10.1001/jama.2020.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention Your Guide to Masks. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/about-face-coverings.html Available at: Accessed March 17, 2021.
- 47.Centers for Disease Control and Prevention Overview of Testing for SARS-CoV-2 (COVID-19) 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html Available at: Accessed March 5, 2021.
- 48.Hanson K.E., Caliendo A.M., Arias C.A., et al. The Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19: Molecular Diagnostic Testing. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab048. ciab048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention . 2020. Interim guidance on testing healthcare personnel for SARS-CoV-2.https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-healthcare-personnel.html Available at: Accessed March 5, 2021. [Google Scholar]
- 50.Food and Drug Administration FACT SHEET FOR HEALTHCARE PROVIDERS ADMINISTERING VACCINE (VACCINATION PROVIDERS) EMERGENCY USE AUTHORIZATION (EUA) OF THE PFIZER-BIONTECH COVID-19 VACCINE TO PREVENT CORONAVIRUS DISEASE 2019 (COVID-19) 2021. https://www.fda.gov/media/144413/download Available at: Accessed March 5, 2021.
- 51.Food and Drug Administration FACT SHEET FOR HEALTHCARE PROVIDERS ADMINISTERING VACCINE (VACCINATION PROVIDERS) EMERGENCY USE AUTHORIZATION (EUA) OF THE MODERNA COVID-19 VACCINE TO PREVENT CORONAVIRUS DISEASE 2019 (COVID-19) 2020. https://www.fda.gov/media/144637/download Available at: Accessed March 5, 2021.
- 52.Food and Drug Administration FACT SHEET FOR HEALTHCARE PROVIDERS ADMINISTERING VACCINE (VACCINATION PROVIDERS) EMERGENCY USE AUTHORIZATION (EUA) OF THE JANSSEN COVID-19 VACCINE TO PREVENT CORONAVIRUS DISEASE 2019 (COVID-19) 2021. https://www.fda.gov/media/144413/download Available at: Accessed March 5, 2021.
- 53.Dooling K. The Advisory Committee on Immunization Practices’ Updated Interim Recommendation for Allocation of COVID-19 Vaccine — United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69 doi: 10.15585/mmwr.mm695152e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oliver S.E. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Janssen COVID-19 Vaccine — United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70 doi: 10.15585/mmwr.mm7009e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention MERS-CoV: Prevention and Control for Hospitalized Patients. https://www.cdc.gov/coronavirus/mers/infection-prevention-control.html Available at: Accessed March 22, 2021.
- 56.Rolfes M.A., Foppa I.M., Garg S., et al. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018;12(1):132–137. doi: 10.1111/irv.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention . 2020. Past seasons estimated influenza disease Burden.https://www.cdc.gov/flu/about/burden/past-seasons.html Available at: Accessed March 8, 2021. [Google Scholar]
- 58.Talbot T.R., Bradley S.F., Cosgrove S.E., et al. Influenza Vaccination of Healthcare Workers and Vaccine Allocation for Healthcare Workers During Vaccine Shortages. Infect Control Hosp Epidemiol. 2005;26(11):882–890. doi: 10.1086/502512. [DOI] [PubMed] [Google Scholar]
- 59.Bénet T., Amour S., Valette M., et al. Incidence of asymptomatic and symptomatic influenza among healthcare workers: a multicenter prospective cohort study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1109. [DOI] [PubMed] [Google Scholar]
- 60.Ip D.K.M., Lau L.L.H., Leung N.H.L., et al. Viral Shedding and Transmission Potential of Asymptomatic and Paucisymptomatic Influenza Virus Infections in the Community. Clin Infect Dis. 2017;64(6):736–742. doi: 10.1093/cid/ciw841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chow E.J., Mermel L.A. More Than a Cold: Hospital-Acquired Respiratory Viral Infections, Sick Leave Policy, and A Need for Culture Change. Infect Control Hosp Epidemiol. 2018;39(7):861–862. doi: 10.1017/ice.2018.94. [DOI] [PubMed] [Google Scholar]
- 62.Jiang L., McGeer A., McNeil S., et al. Which healthcare workers work with acute respiratory illness? Evidence from Canadian acute-care hospitals during 4 influenza seasons: 2010-2011 to 2013-2014. Infect Control Hosp Epidemiol. 2019;40(8):889–896. doi: 10.1017/ice.2019.141. [DOI] [PubMed] [Google Scholar]
- 63.Shearer M.P., Meyer D., Divya Hosangadi M., et al. Operational stresses on New York City Health+Hospitals Health System frontline hospitals during the 2017-18 influenza season. Am J Disaster Med. 2020;15(2):99–111. doi: 10.5055/ajdm.2020.0360. [DOI] [PubMed] [Google Scholar]
- 64.Poland G.A., Tosh P., Jacobson R.M. Requiring influenza vaccination for health care workers: seven truths we must accept. Vaccine. 2005;23(17):2251–2255. doi: 10.1016/j.vaccine.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 65.Ip D.K.M., Lau L.L.H., Chan K.-H., et al. The Dynamic Relationship Between Clinical Symptomatology and Viral Shedding in Naturally Acquired Seasonal and Pandemic Influenza Virus Infections. Clin Infect Dis. 2016;62(4):431–437. doi: 10.1093/cid/civ909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murphy B.R., Chalhub E.G., Nusinoff S.R., et al. Temperature-Sensitive Mutants of Influenza Virus. III. Further Characterization of the ts-1[E] Influenza A Recombinant (H3N2) Virus in Man. J Infect Dis. 1973;128(4):479–487. doi: 10.1093/infdis/128.4.479. [DOI] [PubMed] [Google Scholar]
- 67.Hall C.B., Douglas R.G. Nosocomial Influenza Infection as a Cause of Intercurrent Fevers in Infants. Pediatrics. 1975;55(5):673–677. [PubMed] [Google Scholar]
- 68.Lee N., Chan P.K.S., Hui D.S.C., et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492–500. doi: 10.1086/600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Englund J.A., Champlin R.E., Wyde P.R., et al. Common emergence of amantadine- and rimantadine-resistant influenza A viruses in symptomatic immunocompromised adults. Clin Infect Dis. 1998;26(6):1418–1424. doi: 10.1086/516358. [DOI] [PubMed] [Google Scholar]
- 70.Grayson M.L., Melvani S., Druce J., et al. Efficacy of soap and water and alcohol-based hand-rub preparations against live H1N1 influenza virus on the hands of human volunteers. Clin Infect Dis. 2009;48(3):285–291. doi: 10.1086/595845. [DOI] [PubMed] [Google Scholar]
- 71.Thomas Y., Boquete-Suter P., Koch D., et al. Survival of influenza virus on human fingers. Clin Microbiol Infect. 2014;20(1):O58–O64. doi: 10.1111/1469-0691.12324. [DOI] [PubMed] [Google Scholar]
- 72.Bean B., Moore B.M., Sterner B., et al. Survival of influenza viruses on environmental surfaces. J Infect Dis. 1982;146(1):47–51. doi: 10.1093/infdis/146.1.47. [DOI] [PubMed] [Google Scholar]
- 73.Boone S.A., Gerba C.P. The occurrence of influenza A virus on household and day care center fomites. J Infect. 2005;51(2):103–109. doi: 10.1016/j.jinf.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 74.Klontz K.C., Hynes N.A., Gunn R.A., et al. An outbreak of influenza A/Taiwan/1/86 (H1N1) infections at a naval base and its association with airplane travel. Am J Epidemiol. 1989;129(2):341–348. doi: 10.1093/oxfordjournals.aje.a115137. [DOI] [PubMed] [Google Scholar]
- 75.Morens D.M., Rash V.M. Lessons from a nursing home outbreak of influenza A. Infect Control Hosp Epidemiol. 1995;16(5):275–280. doi: 10.1086/647107. [DOI] [PubMed] [Google Scholar]
- 76.Moser M.R., Bender T.R., Margolis H.S., et al. An outbreak of influenza aboard a commercial airliner. Am J Epidemiol. 1979;110(1):1–6. doi: 10.1093/oxfordjournals.aje.a112781. [DOI] [PubMed] [Google Scholar]
- 77.Milton D.K., Fabian M.P., Cowling B.J., et al. Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog. 2013;9(3):e1003205. doi: 10.1371/journal.ppat.1003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brankston G., Gitterman L., Hirji Z., et al. Transmission of influenza A in human beings. Lancet Infect Dis. 2007;7(4):257–265. doi: 10.1016/S1473-3099(07)70029-4. [DOI] [PubMed] [Google Scholar]
- 79.Radonovich L.J., Simberkoff M.S., Bessesen M.T., et al. N95 Respirators vs Medical Masks for Preventing Influenza Among Health Care Personnel: A Randomized Clinical Trial. JAMA. 2019;322(9):824–833. doi: 10.1001/jama.2019.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Loeb M., Dafoe N., Mahony J., et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302(17):1865–1871. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- 81.MacIntyre C.R., Wang Q., Cauchemez S., et al. A cluster randomized clinical trial comparing fit-tested and non-fit-tested N95 respirators to medical masks to prevent respiratory virus infection in health care workers. Influenza Other Respir Viruses. 2011;5(3):170–179. doi: 10.1111/j.1750-2659.2011.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuster S.P., Coleman B.L., Raboud J., et al. Risk factors for influenza among health care workers during 2009 pandemic, Toronto, Ontario, Canada. Emerg Infect Dis. 2013;19(4):606–615. doi: 10.3201/eid1904.111812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tellier R. Aerosol transmission of influenza A virus: a review of new studies. J R Soc Interf. 2009;6(suppl_6):S783–S790. doi: 10.1098/rsif.2009.0302.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siegel J.D., Rhinehart E., Jackson M., et al. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am J Infect Control. 2007;35(10, Supplement 2):S65–S164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Centers for Disease Control and Prevention (CDC) Prevention Strategies for Seasonal Influenza in Healthcare Settings. https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm Available at: Accessed March 15, 2021.
- 86.Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC) Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep. 2011;60(RR-7):1–45. [PubMed] [Google Scholar]
- 87.Belongia E.A., Simpson M.D., King J.P., et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16(8):942–951. doi: 10.1016/S1473-3099(16)00129-8. [DOI] [PubMed] [Google Scholar]
- 88.Murti M., Otterstatter M., Orth A., et al. Measuring the impact of influenza vaccination on healthcare worker absenteeism in the context of a province-wide mandatory vaccinate-or-mask policy. Vaccine. 2019;37(30):4001–4007. doi: 10.1016/j.vaccine.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 89.Frederick J., Brown A.C., Cummings D.A., et al. Protecting Healthcare Personnel in Outpatient Settings: The Influence of Mandatory Versus Nonmandatory Influenza Vaccination Policies on Workplace Absenteeism During Multiple Respiratory Virus Seasons. Infect Control Hosp Epidemiol. 2018;39(4):452–461. doi: 10.1017/ice.2018.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saxén H., Virtanen M. Randomized, placebo-controlled double blind study on the efficacy of influenza immunization on absenteeism of health care workers. Pediatr Infect Dis J. 1999;18(9):779–783. doi: 10.1097/00006454-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 91.Feery B.J., Evered M.G., Morrison E.I. Different Protection Rates in Various Groups of Volunteers Given Subunit Influenza Virus Vaccine in 1976. J Infect Dis. 1979;139(2):237–241. doi: 10.1093/infdis/139.2.237. [DOI] [PubMed] [Google Scholar]
- 92.Carman W.F., Elder A.G., Wallace L.A., et al. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: a randomised controlled trial. Lancet. 2000;355(9198):93–97. doi: 10.1016/S0140-6736(99)05190-9. [DOI] [PubMed] [Google Scholar]
- 93.Potter J., Stott D.J., Roberts M.A., et al. Influenza Vaccination of Health Care Workers in Long-Term-Care Hospitals Reduces the Mortality of Elderly Patients. J Infect Dis. 1997;175(1):1–6. doi: 10.1093/infdis/175.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hayward A.C., Harling R., Wetten S., et al. Effectiveness of an influenza vaccine programme for care home staff to prevent death, morbidity, and health service use among residents: cluster randomised controlled trial. BMJ. 2006;333(7581):1241. doi: 10.1136/bmj.39010.581354.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lemaitre M., Meret T., Rothan-Tondeur M., et al. Effect of Influenza Vaccination of Nursing Home Staff on Mortality of Residents: A Cluster-Randomized Trial. J Am Geriatr Soc. 2009;57(9):1580–1586. doi: 10.1111/j.1532-5415.2009.02402.x. [DOI] [PubMed] [Google Scholar]
- 96.Ahmed F., Lindley M.C., Allred N., et al. Effect of influenza vaccination of healthcare personnel on morbidity and mortality among patients: systematic review and grading of evidence. Clin Infect Dis. 2014;58(1):50–57. doi: 10.1093/cid/cit580. [DOI] [PubMed] [Google Scholar]
- 97.Greene M.T., Fowler K.E., Ratz D., et al. Changes in Influenza Vaccination Requirements for Health Care Personnel in US Hospitals. JAMA Netw Open. 2018;1(2):e180143. doi: 10.1001/jamanetworkopen.2018.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Centers for Disease Control and Prevention Influenza Vaccination Information for Health Care Workers. https://www.cdc.gov/flu/professionals/healthcareworkers.htm Available at: Accessed March 16, 2021.
- 99.Uyeki T.M., Bernstein H.H., Bradley J.S., et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza. Clin Infect Dis. 2019;68(6):e1–e47. doi: 10.1093/cid/ciy866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Centers for Disease Control and Prevention Influenza Antiviral Medications: Clinician Summary. 2021. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm Available at: Accessed March 16, 2021.
- 101.World Health Organization (WHO) H5N1 highly pathogenic avian influenza: Timeline of major events. 2014. https://www.who.int/influenza/human_animal_interface/H5N1_avian_influenza_update20140317.pdf Available at: Accessed March 17, 2021.
- 102.World Health Organization (WHO) Cumulative number of confirmed human cases of avian influenza A(H5N1) reported to WHO. http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/ Available at: Accessed March 17, 2021.
- 103.Food and Agriculture Organization of the United Nations (FAO) H7N9 situation update. 2021. http://www.fao.org/ag/againfo/programmes/en/empres/H7N9/situation_update.html Available at: Accessed March 17, 2021.
- 104.Wu J., Ke C., Lau E.H.Y., et al. Influenza H5/H7 Virus Vaccination in Poultry and Reduction of Zoonotic Infections, Guangdong Province, China, 2017–18. Emerg Infect Dis. 2019;25(1) doi: 10.3201/eid2501.181259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Centers for Disease Control and Prevention Interim Guidance on Case Definitions for Investigations of Human Infection with Highly Pathogenic Avian Influenza A H5 Viruses in the United States. https://www.cdc.gov/flu/avianflu/hpai/case-definitions.htm Available at: Accessed March 17, 2021.
- 106.Centers for Disease Control and Prevention Interim Guidance on Follow-up of Close Contacts of Persons Infected with Novel Influenza A Viruses Associated with Severe Human Disease and on the Use of Antiviral Medications for Chemoprophylaxis. https://www.cdc.gov/flu/avianflu/novel-av-chemoprophylaxis-guidance.htm Available at: Accessed March 17, 2021.
- 107.Shay D.K., Holman R.C., Newman R.D., et al. Bronchiolitis-associated hospitalizations among US children, 1980-1996. J Am Med Assoc. 1999;282(15):1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 108.Falsey A.R., Hennessey P.A., Formica M.A., et al. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 109.Goldstein E., Greene S.K., Olson D.R., et al. Estimating the hospitalization burden associated with influenza and respiratory syncytial virus in New York City, 2003–2011. Influenza Other Respir Viruses. 2015;9(5):225–233. doi: 10.1111/irv.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hall C.B., Douglas R.G., Geiman J.M., et al. Nosocomial Respiratory Syncytial Virus Infections. N Engl J Med. 1975;293(26):1343–1346. doi: 10.1056/NEJM197512252932604. [DOI] [PubMed] [Google Scholar]
- 111.Halasa N.B., Williams J.V., Wilson G.J., et al. Medical and Economic Impact of a Respiratory Syncytial Virus Outbreak in a Neonatal Intensive Care Unit. Pediatr Infect Dis J. 2005;24(12):1040–1044. doi: 10.1097/01.inf.0000190027.59795.ac. [DOI] [PubMed] [Google Scholar]
- 112.Englund J.A., Anderson L.J., Rhame F.S. Nosocomial transmission of respiratory syncytial virus in immunocompromised adults. J Clin Microbiol. 1991;29(1):115–119. doi: 10.1128/jcm.29.1.115-119.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mathur U., Bentley D.W., Hall C.B. Concurrent Respiratory Syncytial Virus and Influenza A Infections in the Institutionalized Elderly and Chronically III. Ann Intern Med. 1980;93(1_Part_1):49–52. doi: 10.7326/0003-4819-93-1-49. [DOI] [PubMed] [Google Scholar]
- 114.Hall B.C., Geiman J.M., Douglas R.G., Jr., et al. Control of nosocomial respiratory syncytial viral infections. Pediatrics. 1978;62(5):728–732. [PubMed] [Google Scholar]
- 115.Hall C.B., Kopelman A.E., Douglas R.G., Jr., et al. Neonatal Respiratory Syncytial Virus Infection. N Engl J Med. 1979;300(8):393–396. doi: 10.1056/NEJM197902223000803. [DOI] [PubMed] [Google Scholar]
- 116.Weinstein R.A., Hall C.B. Nosocomial Respiratory Syncytial Virus Infections: The “Cold War” Has Not Ended. Clin Infect Dis. 2000;31(2):590–596. doi: 10.1086/313960. [DOI] [PubMed] [Google Scholar]
- 117.Jena A.B., Baldwin D.C., Daugherty S.R., et al. Presenteeism among resident physicians. JAMA. 2010;304(11):1166–1168. doi: 10.1001/jama.2010.1315. [DOI] [PubMed] [Google Scholar]
- 118.Jena A.B., Meltzer D.O., Press V.G., et al. Why physicians work when sick. Arch Intern Med. 2012;172(14):1107–1108. doi: 10.1001/archinternmed.2012.1998. [DOI] [PubMed] [Google Scholar]
- 119.Hall C.B., Douglas R.G. Modes of transmission of respiratory syncytial virus. J Pediatr. 1981;99(1):100–103. doi: 10.1016/s0022-3476(81)80969-9. [DOI] [PubMed] [Google Scholar]
- 120.Leclair J.M., Freeman J., Sullivan B.F., et al. Prevention of nosocomial respiratory syncytial virus infections through compliance with glove and gown isolation precautions. N Engl J Med. 1987;317(6):329–334. doi: 10.1056/NEJM198708063170601. [DOI] [PubMed] [Google Scholar]
- 121.Hall C.B., Douglas R.G., Schnabel K.C., et al. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect Immun. 1981;33(3):779–783. doi: 10.1128/iai.33.3.779-783.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hall C.B., Douglas R.G., Jr., Geiman J.M. Possible Transmission by Fomites of Respiratory Syncytial Virus. J Infect Dis. 1980;141(1):98–102. doi: 10.1093/infdis/141.1.98. [DOI] [PubMed] [Google Scholar]
- 123.Hall C.B., Douglas R.G., Geiman J.M. Respiratory syncytial virus infections in infants: Quantitation and duration of shedding. J Pediatr. 1976;89(1):11–15. doi: 10.1016/s0022-3476(76)80918-3. [DOI] [PubMed] [Google Scholar]
- 124.Munywoki P.K., Koech D.C., Agoti C.N., et al. Influence of age, severity of infection, and co-infection on the duration of respiratory syncytial virus (RSV) shedding. Epidemiol Infect. 2015;143(4):804–812. doi: 10.1017/S0950268814001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Walsh E.E., Peterson D.R., Kalkanoglu A.E., et al. Viral Shedding and Immune Responses to Respiratory Syncytial Virus Infection in Older Adults. J Infect Dis. 2013;207(9):1424–1432. doi: 10.1093/infdis/jit038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.PATH. RSV Vaccine and mAb Snapshot. https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/ Available at: Accessed March 18, 2021.
- 127.Gala C.L., Hall C.B., Schnabel K.C., et al. The use of eye-nose goggles to control nosocomial respiratory syncytial virus infection. JAMA. 1986;256(19):2706–2708. [PubMed] [Google Scholar]
- 128.Agah R., Cherry J.D., Garakian A.J., et al. Respiratory syncytial virus (RSV) infection rate in personnel caring for children with RSV infections. Routine isolation procedure vs routine procedure supplemented by use of masks and goggles. Am J Dis Child. 1987;141(6):695–697. doi: 10.1001/archpedi.1987.04460060111049. [DOI] [PubMed] [Google Scholar]
- 129.Feltes T.F., Cabalka A.K., Meissner H.C., et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143(4):532–540. doi: 10.1067/s0022-3476(03)00454-2. [DOI] [PubMed] [Google Scholar]
- 130.Kurz H., Herbich K., Janata O., et al. Experience with the use of palivizumab together with infection control measures to prevent respiratory syncytial virus outbreaks in neonatal intensive care units. J Hosp Infect. 2008;70(3):246–252. doi: 10.1016/j.jhin.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 131.American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee Updated Guidance for Palivizumab Prophylaxis Among Infants and Young Children at Increased Risk of Hospitalization for Respiratory Syncytial Virus Infection. Pediatrics. 2014;134(2):e620–e638. doi: 10.1542/peds.2014-1666. [DOI] [PubMed] [Google Scholar]
- 132.Pediatrics AA of AAP Publications Reaffirmed. Pediatrics. 2019;144(2) doi: 10.1542/peds.2019-1767. [DOI] [PubMed] [Google Scholar]
- 133.Brummitt C.F., Cherrington J.M., Katzenstein D.A., et al. Nosocomial Adenovirus Infections: Molecular Epidemiology of an Outbreak Due to Adenovirus 3a. J Infect Dis. 1988;158(2):423–432. doi: 10.1093/infdis/158.2.423. [DOI] [PubMed] [Google Scholar]
- 134.James L., Vernon M.O., Jones R.C., et al. Outbreak of Human Adenovirus Type 3 Infection in a Pediatric Long-Term Care Facility—Illinois, 2005. Clin Infect Dis. 2007;45(4):416–420. doi: 10.1086/519938. [DOI] [PubMed] [Google Scholar]
- 135.Lessa F.C., Gould P.L., Pascoe N., et al. Health Care Transmission of a Newly Emergent Adenovirus Serotype in Health Care Personnel at a Military Hospital in Texas, 2007. J Infect Dis. 2009;200(11):1759–1765. doi: 10.1086/647987. [DOI] [PubMed] [Google Scholar]
- 136.Ison M.G. Adenovirus Infections in Transplant Recipients. Clin Infect Dis. 2006;43(3):331–339. doi: 10.1086/505498. [DOI] [PubMed] [Google Scholar]
- 137.Gordon Y.J., Gordon R.Y., Romanowski E., et al. Prolonged recovery of desiccated adenoviral serotypes 5, 8, and 19 from plastic and metal surfaces in vitro. Ophthalmology. 1993;100(12):1835–1839. doi: 10.1016/s0161-6420(93)31389-8. [discussion: 1839–40] [DOI] [PubMed] [Google Scholar]
- 138.Karron R.A., O’Brien K.L., Froehlich J.L., et al. Molecular epidemiology of a parainfluenza type 3 virus outbreak on a pediatric ward. J Infect Dis. 1993;167(6):1441–1445. doi: 10.1093/infdis/167.6.1441. [DOI] [PubMed] [Google Scholar]
- 139.Meissner H.C., Murray S.A., Kiernan M.A., et al. A simultaneous outbreak of respiratory syncytial virus and parainfluenza virus type 3 in a newborn nursery. J Pediatr. 1984;104(5):680–684. doi: 10.1016/s0022-3476(84)80943-9. [DOI] [PubMed] [Google Scholar]
- 140.Zambon M., Bull T., Sadler C.J., et al. Molecular Epidemiology of Two Consecutive Outbreaks of Parainfluenza 3 in a Bone Marrow Transplant Unit. J Clin Microbiol. 1998;36(8):2289–2293. doi: 10.1128/jcm.36.8.2289-2293.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brady M.T., Evans J., Cuartas J. Survival and disinfection of parainfluenza viruses on environmental surfaces. Am J Infect Control. 1990;18(1):18–23. doi: 10.1016/0196-6553(90)90206-8. [DOI] [PubMed] [Google Scholar]
- 142.Ansari S.A., Springthorpe V.S., Sattar S.A., et al. Potential role of hands in the spread of respiratory viral infections: studies with human parainfluenza virus 3 and rhinovirus 14. J Clin Microbiol. 1991;29(10):2115–2119. doi: 10.1128/jcm.29.10.2115-2119.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zinna S., Lakshmanan A., Tan S., et al. Outcomes of Nosocomial Viral Respiratory Infections in High-Risk Neonates. Pediatrics. 2016;138(5):e20161675. doi: 10.1542/peds.2016-1675. [DOI] [PubMed] [Google Scholar]
- 144.Louie J.K., Yagi S., Nelson F.A., et al. Rhinovirus outbreak in a long term care facility for elderly persons associated with unusually high mortality. Clin Infect Dis. 2005;41(2):262–265. doi: 10.1086/430915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rhedin S., Lindstrand A., Rotzén-Östlund M., et al. Clinical utility of PCR for common viruses in acute respiratory illness. Pediatrics. 2014;133(3):e538–e545. doi: 10.1542/peds.2013-3042. [DOI] [PubMed] [Google Scholar]
- 146.Liao R.S., Appelgate D.M., Pelz R.K. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility for the elderly in Oregon. J Clin Virol. 2012;53(2):171–173. doi: 10.1016/j.jcv.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 147.Lee N., Chan P.K.S., Yu I.T., et al. Co-circulation of human metapneumovirus and SARS-associated coronavirus during a major nosocomial SARS outbreak in Hong Kong. J Clin Virol. 2007;40(4):333–337. doi: 10.1016/j.jcv.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cheng V.C.C., Wu A.K.L., Cheung C.H.Y., et al. Outbreak of human metapneumovirus infection in psychiatric inpatients: implications for directly observed use of alcohol hand rub in prevention of nosocomial outbreaks. J Hosp Infect. 2007;67(4):336–343. doi: 10.1016/j.jhin.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 149.Kim S., Sung H., Im H.J., et al. Molecular epidemiological investigation of a nosocomial outbreak of human metapneumovirus infection in a pediatric hemato-oncology patient population. J Clin Microbiol. 2009;47(4):1221–1224. doi: 10.1128/JCM.01959-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kutter J.S., Spronken M.I., Fraaij P.L., et al. Transmission routes of respiratory viruses among humans. Curr Opin Virol. 2018;28:142–151. doi: 10.1016/j.coviro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tollefson S.J., Cox R.G., Williams J.V. Studies of culture conditions and environmental stability of human metapneumovirus. Virus Res. 2010;151(1):54–59. doi: 10.1016/j.virusres.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van Rijn A.L., Nijhuis R.H.T., Bekker V., et al. Clinical implications of rapid ePlex® Respiratory Pathogen Panel testing compared to laboratory-developed real-time PCR. Eur J Clin Microbiol Infect Dis. 2018;37(3):571–577. doi: 10.1007/s10096-017-3151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Youngs J., Marshall B., Farragher M., et al. Implementation of influenza point-of-care testing and patient cohorting during a high-incidence season: a retrospective analysis of impact on infection prevention and control and clinical outcomes. J Hosp Infect. 2019;101(3):276–284. doi: 10.1016/j.jhin.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 154.Barlam T.F., Cosgrove S.E., Abbo L.M., et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–e77. doi: 10.1093/cid/ciw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hanson K.E. America (IDSA) for the DC of the IDS of, Azar MM, et al. Molecular Testing for Acute Respiratory Tract Infections: Clinical and Diagnostic Recommendations From the IDSA’s Diagnostics Committee. Clin Infect Dis. 2020;71(10):2744–2751. doi: 10.1093/cid/ciaa508. [DOI] [PMC free article] [PubMed] [Google Scholar]