Abstract
Background
The increasing demand for healthy eating habits and the emergence of the COVID-19 pandemic, which resulted in a health crisis and global economic slowdown, has led to the consumption of functional and practical foods. Bioactive ingredients can be an alternative for healthy food choices; however, most functional compounds are sensitive to the adverse conditions of processing and digestive tract, impairing its use in food matrices, and industrial-scale applications. Microencapsulation by spray chilling can be a viable alternative to reduce these barriers in food processing.
Scope and approach
This review discusses the use of spray chilling technique for microencapsulation of bioactive food ingredients. Although this technology is known in the pharmaceutical industry, it has been little exploited in the food sector. General aspects of spray chilling, the process parameters, advantages, and disadvantages are addressed. The feasibility and stability of encapsulated bioactive ingredients in food matrices and the bioavailability in vitro of solid lipid microparticles produced by spray chilling are also discussed.
Main findings and conclusions
Research on the microencapsulation of bioactive ingredients by spray chilling for use in foods has shown the effectiveness of this technique to encapsulate bioactive compounds for application in food matrices. Solid microparticles produced by spray chilling can improve the stability and bioavailability of bioactive ingredients. However, further studies are required, including the use of lipid-based encapsulating agents, process parameters, and novel formulations for application in food, beverages, and packaging, as well as in vivo studies to prove the effectiveness of the formulations.
Keywords: Bioactive compounds, Spray congealing, Functional foods, Encapsulation, Natural additives, Solid lipid microparticles
1. Introduction
In March 2020, the Covid-19 pandemic was decreed by the World Health Organization, exposing several challenges for society. Consumer demand will change for years to come due to the pandemic, as longstanding habits, technological acceleration, sanitary and global health protocols, all aimed at providing food safety, health, sustainability, and stability to individuals and countries (Loh, Seah, & Looi, 2021). Some studies have reported that the consumption pattern has changed, with a drop in the consumption of fast food, and an increase in the intake of fresh, healthy products, soups, frozen foods, and dairy products (Chenarides, Grebitus, Lusk, & Printezis, 2021; Eftimov, Popovski, Petković, Seljak, & Kocev, 2020; Galanakis, Aldawoud, Rizou, Rowan, & Ibrahim, 2020).
The Covid −19 pandemic accelerated the trend toward food that supports the immune system, including bioactive ingredients, supplements, and nutraceuticals (Galanakis et al., 2020) By 2030, innovative and sustainable approaches to the supply chain are expected to be taken by food companies. Over the next 10 years, consumers must become more adept at science and technology as they will ensure more affordable, safer, and nutritious food and beverages (Cho, Kim, Kim, Park, & Rhee, 2020).
Bioactive ingredients in foods exhibit a wide range of biological activities, playing key roles in various industries, including food, pharmaceutical, and cosmetics (Ray, Raychaudhuri, & Chakraborty, 2016). However, most of these compounds are very sensitive to processing and adverse environmental conditions such as light, moisture, heat, and oxygen, which represents a great challenge for the use of these compounds on an industrial scale (Figueiredo, MT Lago, et al., 2020). Spray chilling technology also known as spray congealing or spray cooling is one of the microencapsulation techniques that have stood out for decades for solving limitations in the delivery of active pharmaceutical and cosmetic ingredients (Favaro-Trindade et al., 2021, pp. 1–14; Huang, Qian, Sun, & Xia, 2019; Müller, Radtke, & Wissing, 2002b, 2007). In particular, the spray chilling technique is an economical strategy and represents an effective alternative to produce microparticles containing sensitive pigments, flavors, and aromas, antioxidants, and natural preservatives for use in food matrices. Studies have shown that spray chilling encapsulation can maintain the stability of ascorbic acid, vitamin D3, and proanthocyanidins during storage (Carvalho, Oriani, Oliveira, & Hubinger, 2019; Paucar et al., 2016; Tulini et al., 2017).
Microencapsulation by spray chilling consists of the formation of solid lipid microparticles through the atomization of active ingredients that can be of hydrophilic or hydrophobic origin, dissolved or dispersed in molten lipid encapsulating materials (Oriani et al., 2016). It is possible to obtain a solution, emulsion, or dispersion, depending on the solubility of the compounds, which is atomized in a refrigeration chamber, in which the droplets solidify when in contact with cold air, giving rise to solid lipid microparticles with characteristics of a powdered product (Sorita et al., 2021).
Recently, some authors have studied the use of this technology in the food and beverage industry (Carvalho, Oriani, de Oliveira, & Hubinger, 2021; Nahum & Domb, 2021; Queirós, Viriato, Ribeiro, & Gigante, 2020; Tomšik et al., 2019) to overcome challenges related to the stability of sensitive compounds such as vitamins and natural pigments during processing and storage. This technology also allows obtaining fat powders, facilitating the manipulation and the incorporation in different food matrices, besides contributing with lower transport and storage costs due to the smaller volume. In addition, solid lipid microparticles can be added to foods to solve problems related to crystallization events and act as structuring agents (Favaro-Trindade, Okuro, & Matos-Junior, 2016; Landim Neves, de Souza Queirós, Soares Viriato, Badan Ribeiro, & Gigante, 2021). However, further studies are required on the application of this technique in the food sector. According to the Web Of Science database, using the search terms “spray chilling” OR “spray cooling” OR “spray congealing” AND “food encapsulation” AND “solid lipid particles”, a total of 731 articles were found. Review articles and articles that did not fit the spray chilling technology were excluded from the search. Thus, 42 articles were found using this technology for food purposes.
The main objective of this review is to demonstrate the potential of the spray chilling microencapsulation process to improve the applicability, stability, and bioavailability in vitro of bioactive ingredients. The main advantages, challenges, and application potentials of spray chilling will also be addressed.
2. Fundamentals of spray chilling technology
2.1. Process steps
Before understanding the steps of the spray chilling process, it is worth noting that this technology is fully scalable from an industrial point of view (Consoli, Grimaldi, Sartori, Menegalli, & Hubinger, 2016). Concerning the atomization equipment and operations, the spray chiller is closely associated with the spray dryer. Typically, the spray dryer system can be used for spray chilling processes with some adaptations. Although the production of particles from both techniques is based on the generation of droplets by spraying a fluid (solution, dispersion, or emulsion), different mechanisms govern the formation of particles by spray drying and spray chilling (Alvim, Stein, Koury, Dantas, & Cruz, 2016; Jaskulski, Kharaghani, & Tsotsas, 2018).
Briefly, spray drying consists of converting a fluid into a powdered material by evaporating the solvent, usually water, when sprayed in a chamber containing hot air with a temperature higher than the solvent evaporation temperature (Shishir & Chen, 2017).
In turn, spray cooling is based on the solidification of a fluid (containing high melting molten lipids as encapsulating materials) sprayed in a cooling chamber. In detail, spray cooling consists of two steps. The first step requires the encapsulation of the active compound in a lipid matrix, usually a molten lipid or a water-in-oil emulsion (Fig. 1 a) (Bertoni, Albertini, & Passerini, 2019). In the second step, this mixture is sprayed in the form of droplets, usually by a heated atomizing nozzle, to maintain the proper temperature and avoid recrystallization of the lipid compounds (Fig. 1b). When the pulverized material comes into contact with a cooled environment (injection of cold air or liquid nitrogen), with a temperature below the lipid melting point, there is a heat transfer between the lipid and the cold air, leading to solidification of the matrix and resulting in the formation of solid lipid particles. Similar to spray drying, the residence time of the sprayed droplets in the cooling chamber is short. The particles are collected in a container below the cooling chamber, while very fine particles are transported by air to a cyclone, and collected in another container (Consoli et al., 2016).
Fig. 1.
Production of solid lipid particles by spray chilling. (a) Elaboration of a water-in-oil emulsion containing the active ingredient to be atomized in the spray chilling. (b) Atomization of the emulsion in the spray chiller and production of solid lipid particles.
2.2. Structure and composition
Solid lipid particles are an interesting and versatile tool for the delivery of active compounds. The kinetic stability and rigid morphology are the main advantages of lipid particles over vesicular lipid colloidal systems (liposomes, niosomes, etc.).
As previously mentioned, solid lipid particles are often made from emulsion systems of the W/O type or a dispersion (thermodynamically stable systems), at a temperature above the melting point of the oil droplets, followed by cooling to induce droplet crystallization (Müller et al., 2002b; Nahum & Domb, 2021).
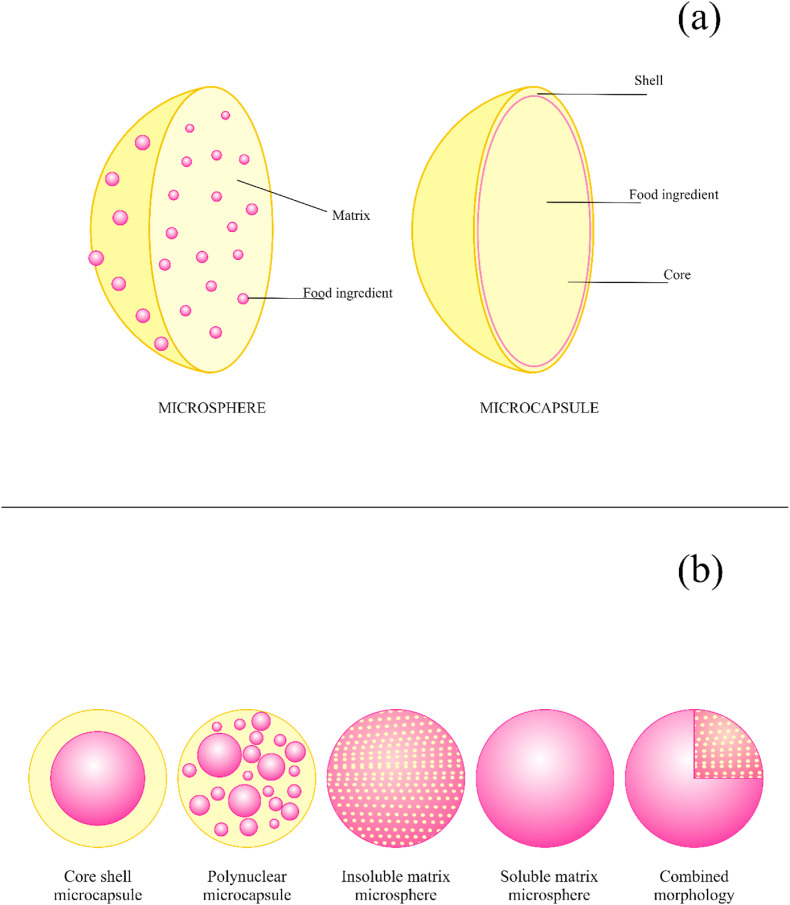
Several microencapsulation techniques are used for different purposes, thus allowing the production of microparticles with distinct morphologies. A wide variety of morphologies can be obtained, depending on the desired characteristics and the processing cost (Arenas-Jal, Suñé-Negre, & García-Montoya, 2020). In general, the microparticles are called microcapsules or microspheres (Fig. 2 a and b).
Fig. 2.
Main morphology of microspheres and microcapsules. (a) Cutout of a microsphere and a microcapsule. (b) Different morphologies of microspheres and microcapsules. It is noteworthy that both morphologies can also exhibit an irregular shape.
The microcapsules have a well-defined inner core (active ingredient) and outer shell (encapsulating material). They can have a single core, multiple cores, or multilayers. Generally, this type of microparticle is obtained by microencapsulation processes such as complex coacervation, polymerization in situ, solvent evaporation, and liposomes (Trojanowska, Nogalska, Valls, Giamberini, & Tylkowski, 2017).
Microspheres are another well-known type of microparticles. They are characterized by having the active ingredient dispersed in the encapsulating matrix. Two types of microsphere morphologies differ from each other by the solubility between the active ingredient and the encapsulating material. The microsphere morphology can be selected according to the characteristics desired for the application. Although microcapsules provide better encapsulation, microspheres provide sufficient protection for some applications and can be achieved by a low-cost technique involving fewer unit operations. The spray drying and spray chilling techniques are widely used in the food, pharmaceutical, and cosmetic industries, and produce typical microsphere structures, in addition to other techniques such as freeze-drying, spray coating (fluid bed), extrusion, and emulsion-based processes. It is also possible to obtain microparticles by combining both microcapsules and microspheres (Arenas-Jal et al., 2020).
Furthermore, microspheres can be classified differently, once their physical properties are affected by the polymorphic transitions of fatty materials. The main types of microspheres obtained according to the type and polymorphic behavior of the lipid material are shown in Fig. 3 (Müller et al., 2002b), which shows the microspheres trapping anthocyanin, which is an active food ingredient.
Fig. 3.
Main types of lipid microparticles produced by spray chilling.
According to the types of lipids used, the microspheres produced by spray chilling can be named as follows (Battaglia & Ugazio, 2019; Fadini et al., 2018; McClements, 2020):
-
•
Solid Lipid Microparticles (SLM);
-
•
Microstructured Lipids Carriers (MLC): type 1, type 2, and type 3;
-
•
Solid Lipid Microcapsules (LMS).
2.2.1. Solid lipid microparticles (SLM)
Solid lipid microparticles are the best-known type of lipid microspheres. SLM is constituted by solid lipids with a diameter between 3 and 800 μm (McClements, 2020). General ingredients include solid lipid(s), surfactant(s), and water. The term lipid is used in a broad sense and includes triglycerides, partial glycerides, fatty acids, steroids, and waxes. All classes of surfactants (concerning the load and molecular weight) have been used to stabilize lipid dispersions; however, polyglycerol polyricinoleate has been efficiently used in W/O type emulsions (Zafimahova-Ratisbonne, Wardhono, Lanoisellé, Saleh, & Clausse, 2014).
SLMs were developed in the early 1990s and have long been considered promising drug carrier systems as they are stable from the physicochemical point of view, and can be easily produced on a large industrial scale, while the raw material and production costs are relatively low. A cross-sectional view of SLM delineates that the active ingredient fraction is embedded in the phospholipid layer (Fig. 4 ). The positive aspect of the solid lipid core is that it reduces drug mobility, thus preventing degradation (Müller, Petersen, Hommoss, & Pardeike, 2007).
Fig. 4.
Production mechanisms (a) and cross-section of SLM (b). Note: in printed version of paper, please do not use color print. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
However, several problems have been associated with these particles such as (i) limited drug-carrying capacity and, (ii) drug release during storage (Müller, Radtke, & Wissing, 2002a; Santos et al., 2019). This behavior is due to the packaging of solid fats, with a predominance of saturated fatty acids, which occurs in a more organized manner (Sato & Ueno, 2011). These limitations encourage researchers to develop a system known as structured micro (>1000 nm) and nano (50–1000 nm) lipid carriers (Battaglia & Ugazio, 2019), and this type of microparticle has been used in spray chilling technology for food ingredients (Carvalho et al., 2019; Consoli et al., 2016; Ribeiro, Arellano, & Grosso, 2012).
2.2.2. Microstructured lipid carriers (MLC)
This system is characterized by the production of solid lipid micro or nanoparticles containing not only solid lipids but also a mixture of solid and liquid lipids, spatially incompatible, leading to special lipid structures, called microstructured lipid carriers (>1000 nm) (Pezeshki et al., 2019).
The formation of a less ordered solid lipid matrix may favor the permanence of the active compound in the lipid matrix. Thus, lipids with different molecules exhibited particles with lipid structure with imperfections, which accommodate differently, favoring the permanence of the active compound inside the particles, which can also be located between the chains, depending on the conformation of the lipid molecules (Müller et al., 2002a).
Micro- or nano-structured lipid microparticles have a higher loading capacity, greater stability during storage, and controlled release of the encapsulated compound (Barroso et al., 2021). These characteristics are achieved due to the disorganized structure of liquid and solid lipids, which can better accommodate the encapsulated material. The mixture of these lipids from different physical states produces a particle with a low crystallinity index and slower polymorphic transition. The presence of a liquid lipid in the core and the spherical structure of the particle contributes to increasing the efficiency of this system. Thus, the micro/nano-structured lipid microparticles, in addition to improving the encapsulation yield and physical stability, have proven to be a valuable alternative to increase the controlled release and bioavailability of functional food ingredients, as reported by several authors (Behbahani, Ghaedi, Abbaspour, Rostamizadeh, & Dashtian, 2019; Carvalho et al., 2019; Gomes et al., 2019; Müller et al., 2007).
There are 3 main types of micro/nano-structured lipid microparticles (Fig. 3): 1 – imperfect type; 2 – amorphous type, and; 3 - multiple type (Müller et al., 2002a). The imperfect type is obtained when the crystallization of lipids is altered by small amounts of oil. In the amorphous type, the lipid matrix is solid, but not crystalline (amorphous state). In the multiple type, the solid lipid matrix contains tiny oil compartments. These microparticles are obtained by mixing a solid lipid with a larger amount of oil, for example blends containing low amounts of stearic acid and high amounts of oleic acid (Battaglia & Ugazio, 2019). The principle is that the microstructure of the lipid matrix increases the charge of the encapsulated material, and prevents the release of compounds during storage (Müller et al., 2002b, 2007).
2.2.3. Solid Lipid Microcapsules (LMS)
Lipid microcapsules have a lipid-core shell microstructure. They are composed of an outer shell, formed by solid lipids and emulsifying agents, and an oily core. Unlike multiple-type MLC, which consist of a solid lipid matrix containing multiple oil compartments, LMS has a single lipid core containing the active ingredient, which is surrounded by a thin solid coating (core coating structure) (Battaglia & Ugazio, 2019).
3. Effect of spray chilling process parameters
3.1. Overview
Knowledge about the effects of the process parameters on the spray chilling technique is important to obtain satisfactory results. Several parameters should be considered, including high process yield, high encapsulation efficiency of the active ingredient, high retention of volatiles, good physical characteristics of the powder, among others (Bertoni, Albertini, & Passerini, 2019).
The main factors that can be optimized in the spray chilling process include (i) the melting temperature of the lipid compounds; (ii) the temperature of the atomizing air; (iii) the temperature of the cooling chamber; (iv) the atomizing air pressure; and (v) the feed flow rate of the molten mixture (Favaro-Trindade et al., 2021, pp. 1–14).
3.1.1. Melting temperature of the lipid compounds
The lipid matrix is the basis for the microencapsulation of active compounds. The matrix melting temperature must be above 45 °C to ensure some characteristics of the solid lipid microparticles, including the solid state over a wide temperature range, low adhesion rate, good fluidity, and high encapsulation efficiency, as well as ensuring the formation, stability, and high process yield (Consoli et al., 2016).
Low melting lipids such as cocoa butter and refined palm oil can cause several problems during and after spray chilling, when used individually (Oriani et al., 2016), with no formation of solid lipid microparticles, as well as high adhesion in the cooling chamber, and low encapsulation efficiency. After the formation of microparticles, a high powder adhesion, agglomeration, and low microparticle fluidity is required (Carvalho et al., 2019; Consoli et al., 2016; Procopio et al., 2018).
On the other hand, lipids with a high melting point (>70 °C) can lead to clogging of the atomizing nozzle, as well as thermal degradation of the active ingredient. In addition, the use of high melting point lipids alone leads to the formation of SLM-type particles, leading to the release of the active ingredient during storage (Müller et al., 2002a). Modifications in the structure to the more perfect crystal form provide less space to accommodate the active ingredient molecules, leading to ingredient release (Bertoni, Albertini, & Passerini, 2019). In contrast, the particles obtained with high melting point lipids have excellent fluidity and stability characteristics (Oriani et al., 2016). Thus, the selection of lipids depends largely on the heat sensitivity of the active ingredient.
3.1.2. Atomizing air temperature
To ensure a continuous and efficient process, the atomizing air temperature must be 10 °C above the highest melting point of the lipids used. If the temperature of the atomizing air is not properly reached, the atomizing nozzle will clog, with no formation of particles (Consoli et al., 2016) since the process must be interrupted to clean the atomizing nozzle, with a subsequent restart of the process.
3.1.3. Cooling chamber temperature
The good quality of the microparticles after the spray cooling process is closely related to the solidification step of the atomized droplets in the cooling chamber (Favaro-Trindade et al., 2021, pp. 1–14), therefore, the lipid materials are largely responsible for the final quality of the product, as also occurring in the encapsulation by spray drying (Fernandes, Borges, & Botrel, 2014). In spray chilling, knowing the solidification behavior of lipids and the various mixtures is fundamental for the success of the technique.
To ensure that all particles are solidified during spraying, it is recommended to use lipid mixtures with a very narrow melting temperature range (Battaglia & Ugazio, 2019; Chambi, Alvim, Barrera-Arellano, & Grosso, 2008). The solidification curve of the molten mixture used as the feed stream must be known. Thus, the material cools down to its solidification temperature when the atomized droplets come into contact with the cooling air. Thereafter, the temperature remains constant during the heat release of the product, with the subsequent formation of stable solid lipid microparticles (Bertoni, Albertini, & Passerini, 2019).
For the complete formation of solid lipid microparticles, the pulverized material is subjected to three cooling stages: (i) cooling of the liquid, (ii) solidification, and (iii) cooling of the solid microparticles (Favaro-Trindade et al., 2021, pp. 1–14).
The solidification of the microparticles happens progressively; first, the temperature of the sprayed droplets decreases as they come into contact with cold air. Then, the droplets reach the solidification temperature and gradually crystallize until complete stability, which will depend on the polymorphic form of the lipid mixture. Therefore, it is recommended to package the solid lipid microparticles at low temperatures after the end of the spray cooling process until they reach stability (Bertoni, Albertini, Ferraro, et al., 2019; Consoli et al., 2016).
In lipids, the stability of the polymorphic forms follows the order α < β' < β. In the case of rapid cooling, the lipid is preferably crystallized in its most unstable form (α). On the other hand, at slow cooling, there is a tendency to the stable form β (Carvalho et al., 2019; Sato, 2001). Therefore, the control and maintenance of the temperature during the solidification of the particles in spray cooling is essential to obtain a high-quality final product.
3.1.4. Atomizing air pressure
The equipment for the production of solid lipid microparticles is a spray dryer adapted for a spray chiller (as shown in Fig. 1), thus the atomizing air pressures between 4 and 6 bar should preferably be used to ensure effective spraying, as recommended by the manufacturer (Labmaq do Brasil, Ribeirão Preto, São Paulo, Brazil).
So far, there are few studies on the effect of spray cooling process variables on the characteristics of the microparticles. Preliminary tests performed by our research team showed that pressures below 4 bar were not sufficient to promote the atomization of the material, regardless of the type of lipid mixture or type of solution (emulsion or dispersion).
Maschke et al. (2007) evaluated the effects of spray cooling atomization pressure on the characteristics of solid lipid microparticles made from glycerol tripalmitate and insulin. The study showed that increasing the atomization pressure from 5 to 6 bar led to a reduction in the particle size, but higher yields were obtained at pressures of 5.5 and 5.9 bar. Thus, the greater the energy supplied, the smaller the droplet size, with lower efficiency, as very small droplets markedly increase the total surface area.
3.1.5. Feed flow rate of the molten mixture
The feed flow rate of the molten mixture can affect both the process yield and the characteristics of the microparticles (Consoli et al., 2016).
The two main factors that affect the flow rate are equipment configurations, for example, with or without a pump system, and the viscosity of the molten mixture, as high viscosities impair atomization, generating larger particles or even clogging of the atomizing nozzle. A high feed rate of the molten mixture increases the particle size while providing greater control of the microparticle size, which can be achieved by controlling the atomizing pressure (Carvalho et al., 2019; Favaro-Trindade et al., 2021, pp. 1–14).
However, further studies are required to investigate the effects of the process variables on the spray chilling technology, mainly taking into account the type of equipment as each equipment has minimum and maximum parameters required for an efficient process.
4. Properties of the microencapsulation process by spray chilling
A precise assessment of the spray chilling properties is necessary, as they have a direct impact on the technological behavior of the lipid microparticles. These properties and the techniques used for analysis will be detailed below.
4.1. Type and characteristics of the lipid encapsulating materials
A careful selection of the encapsulating material must be made for all microencapsulation techniques (Botrel, Fernandes, Borges, & Yoshida, 2014). Encapsulating materials used for the preparation of solid lipid particles are selected for their biocompatibility, availability, and price. For food application purposes, the encapsulating materials are restricted to food grade and must be approved by authorities such as FDA and EFSA or certified as generally recognized as safe (GRAS). Therefore, the alternatives of encapsulating materials are more limited for food products when compared to pharmaceuticals and cosmetics (Furuta & Neoh, 2020). In addition, they must also preserve the bioactive compounds from decomposition during processing and storage conditions, and not chemically interact with other active agents incorporated into the system (Consoli et al., 2016).
In spray chilling, the stability of the microparticles and the encapsulation efficiency are significantly affected by the type and proportion of the encapsulating materials, and food-grade lipids should be used for the development of lipid microparticles for food applications (Favaro-Trindade et al., 2016; Okuro, de Matos, & Favaro-Trindade, 2013).
The selection of the encapsulating material depends on the active ingredient to be encapsulated. For example, for an ingredient sensitive to high temperatures, lipids with a melting point that do not interfere with the properties of the encapsulated material while protecting against the external environment should be used. However, the use of lipid materials with a low melting point may require temperature-controlled storage after the formation of the particles to ensure integrity until application. Lipids with a melting temperature above 45 °C are desirable for the formation and application of lipid microparticles (Consoli et al., 2016). Other requirements for the encapsulating materials include (Oriani et al., 2018; Pelissari et al., 2016; Yin & Cadwallader, 2019):
-
•
Stability in equipment operating conditions;
-
•
Process-friendly rheological properties and ease of handling during encapsulation
-
•
Low reactivity in the medium and no reactivity with the active ingredient;
-
•
Ability to retain the active ingredient within its structure during processing and storage;
-
•
Provide maximum protection for the active ingredient against external environmental conditions (light, heat, humidity) and;
-
•
Specific ability to achieve the release of the active ingredient.
Usually, a single encapsulating material is not able to meet all these requirements, thus blends of these materials are generally used (Oriani et al., 2018). It is also noteworthy that if the active ingredient is not compatible with the matrix material and does not easily settle or separate in the feed tube before spraying, surfactants or dispersing agents can be used.
Various solid and liquid lipids have been widely used in spray chilling technology (Barroso et al., 2021; Battaglia & Ugazio, 2019). They can be divided into several categories, including waxes, vegetable oils, fatty acids, fatty alcohols, and triglycerides. Table 1 shows some examples of materials belonging to each group.
Table 1.
Types of lipids used in microencapsulation by spray chilling.
| Lipids | Chemical composition | Properties | Examples |
|---|---|---|---|
| Fatty acids | Long-chain fatty acids | Melting point = 60–90 °C | Palmitic acid, stearic acid, behenic acid, lauric acid |
| Triglycerides | Monoacid triglycerides | Melting point = 46–73 °C | Glyceryl tripalmitate (Dynasan 116), glyceryl trimyristate, glyceryl trilaurate |
| Waxes | Long-chain fatty acid esters and alcohols | Melting point = 62–86 °C | Carnauba wax, candelilla wax, beeswax, solid paraffin, rice bran wax |
| Hydrogenated and non-hydrogenated vegetable oils | Mixture of triglycerides, free fatty acids, phospholipids | Hydrogenated - melting point = 60–71 °C | Hydrogenated or non-hydrogenated soy and palm oil |
| Fatty alcohol | Mixture of fatty alcohols | Non-hydrogenated - melting point = 13 °C | Cetyl alcohol, lauryl alcohol, stearyl alcohol, oleyl alcohol |
4.2. Determination of the concentration of the active ingredient
The actual or experimental amount of the active compound in the microparticles after the spray chilling is defined as “active ingredient content”, and can be expressed as the percentage by mass (% w/w) (Bertoni, Albertini, & Passerini, 2019).
The content of this ingredient is closely related to the proportion of the active ingredient used in the production of the microparticles. It is affected by the process (operating parameters and equipment) and formulation variables (e.g. hydrophilic or hydrophobic active ingredient and characteristics of the encapsulating material) (Bertoni, Albertini, & Passerini, 2019; Sorita et al., 2021).
In general, the microparticles are treated with solvents or heat to dissolve/melt the encapsulating material for the release/extraction of the encapsulated ingredient. The amount of active compound should be determined using an appropriate analytical method, which may vary according to the size of the microparticles and the theoretical value (Bertoni, Albertini, & Passerini, 2019).
The true concentration of the active ingredient in the microparticles after the spray chilling and the theoretical value is used to estimate the process efficiency, also known as encapsulation efficiency (Jafari, Assadpoor, He, & Bhandari, 2008).
Spray chilling has proven to be a very promising technique for presenting very high encapsulation efficiencies. Some authors studied Gallic acid encapsulated by spray chilling in a matrix of fully hydrogenated soybean oil and fat, at 90:10 (fully hydrogenated soybean oil: soybean oil) and 30:70 (Gallic acid: lipid matrix) ratio, and found an encapsulation efficiency of 100% (Consoli et al., 2016).
In another study using the same technique, ascorbic acid was encapsulated in a matrix containing fully hydrogenated palm oil and fat. The authors produced microparticles with different oil and palm fat ratios and reported encapsulation efficiency ranging from 83 to 96% (Carvalho et al., 2019).
Procopio et al. (2018) evaluated the cinnamaldehyde retention of microencapsulated cinnamon bark oleoresin by spray chilling using fully hydrogenated palm oil and fat. Different concentrations of oleoresin (1 and 2%) and different oil and palm fat ratios were used. The authors reported low retention of cinnamaldehyde, probably due to the use of heating during the atomization process, which may have led to the volatilization of compounds. The maximum percent retention was 69.49% and the minimum 8.28%, with the higher values observed for the formulations containing only fully hydrogenated palm fat, for both concentrations of 1 and 2% oleoresin. However, with the increase in the proportion of liquid oil, higher retention percentages were observed for the concentration of 2% oleoresin when compared to the concentration of 1%. It is worth emphasizing that the microparticles produced only with saturated fat can lead to greater expulsion of the active compound during storage. This behavior is due to the tendency of the lipid crystal chains to reorganize in a more compact structure, thus releasing the active ingredient (Müller et al., 2002a).
Vitamin B12 was encapsulated in a matrix containing vegetable fat with a melting point of approximately 48 °C and soy lecithin. The microparticles obtained by spray chilling showed high encapsulation efficiency (76–100%). Concentrations of 0.10 and 1% of vitamin B12 and 0, 2.5, and 5.0% of soy lecithin were used, and a more effective encapsulation was observed for the formulations containing higher vitamin concentrations (Chalella Mazzocato, Thomazini, & Favaro-Trindade, 2019).
In contrast, Matos-Jr et al. (2015) encapsulated ascorbic acid by spray chilling technique for application of microparticles in an emulsified meat product and reported that the higher the concentration of active material and the smaller the particle diameter, the less effective the encapsulation. Those authors also studied the encapsulation of probiotic microorganisms using the same technique and reported that the microparticles produced by spray chilling were the matrix type, thus the active material was dispersed throughout the volume of the microparticles rather than inside the particles, such as the reservoir type. Thus, the smaller the size of the microparticles, the greater the surface area and the greater the presence of active material on the surface (Okuro et al., 2013).
These examples demonstrate that the performance of the encapsulation by spray chilling is greatly affected by the proportion and nature of the active ingredient and the lipid materials, suggesting that the concentration of the active ingredient and the encapsulating materials should be carefully selected according to the purpose of application of the microparticles.
4.3. Particle size distribution
Particle size is a fundamental property that directly affects the performance of the encapsulated active ingredient. For example, it influences the fluidity and dissolution of microparticles, particle stability, the controlled release, and the stability of the encapsulated compounds (Campelo et al., 2017).
It can be expressed as mean diameter ± standard deviation or mean diameter. The mean diameter can be expressed as d50, d10, and d90, which are so-called percentile values, indicating the size below which 10%, 50%, or 90% of all particles are found. The particle size distribution also provides information on the dispersibility of the samples, most commonly mono- or poly-dispersibility of the microparticle batch (Bertoni, Albertini, & Passerini, 2019).
Studies on spray chilling technique have shown particle diameters ranging from a few microns to hundreds of microns (Carvalho et al., 2019; Consoli et al., 2016; Oriani et al., 2018; Pelissari et al., 2016; Silva et al., 2019). According to those authors, the main factors influencing the particle diameter are the type of atomizing nozzle, atomizing pressure, feed rate, the nature, and proportion of the active compound and matrix, and the viscosity of the molten liquid to be atomized. Increasing the atomizing pressure can lead to a decrease in the particle size while increasing the feed rate can produce a non-uniform distribution of the particles. The low viscosity of the liquid leads to the production of smaller particles, while a higher viscosity allows for the formation of larger particles.
4.4. Morphology of the solid lipid microparticles
The particle morphology, as well as the particle size, exerts influences on technological properties such as fluidity and performance of the active ingredient (Oh, Guo, Wan Sia Heng, & Chan, 2014). Defects such as cracks can facilitate the release and degradation of the ingredient trapped within the microparticle (Botrel et al., 2012), while imperfections or irregular shapes can impair the contact surface area available for transfer of the active compound (Bertoni, Albertini, Ferraro, et al., 2019).
Most studies on solid lipid microparticles obtained by spray chilling in the pharmaceutical, cosmetics, and food sectors have reported that the particles are dense and mostly spherical, and free-flowing (Alemzadeh, Hajiabbas, Pakzad, Sajadi Dehkordi, & Vossoughi, 2020; Okuro et al., 2013; Oriani et al., 2018), which can explain the high encapsulation efficiency found in those studies.
Some factors are directly related to the morphology of the solid lipid microparticles, including solid to liquid fat ratio, the cooling temperature of the microparticles during atomization, and storage conditions (Carvalho et al., 2021; Huang et al., 2019).
Whereas the microparticles produced by spray chilling are based on a lipid matrix, their morphology is closely related to the lipid behavior. Microparticles with high liquid oil content tend to be more agglomerated and undefined in shape. Insufficient cooling for total solidification of the particles during atomization can also potentiate these effects (Oriani et al., 2016). Similar behavior can occur during storage at temperatures above or near the melting temperature of the lipid matrix (Carvalho et al., 2019). The success of the process lies in the correct choice of the lipid materials and process variables, according to the objective of the application of the microparticles.
4.5. Interactions with the active food ingredients
The interactions between the active ingredient and the encapsulating material play a critical role in different aspects and influence the production capacity, physicochemical stability, and performance of the active ingredient. Four types of interactions can occur between the active food ingredient and the encapsulating material, including hydrogen bonding, ionic interaction, dipole-dipole interaction, and van der Waals interaction, and the formation of hydrogen bonds is the most common type of interaction (Bertoni, Albertini, & Passerini, 2019).
In spray chilling processes, the encapsulating material and the active ingredient are intimately mixed before atomization, which favors the interactions between the active ingredient and the molecules of the encapsulating agent in the liquid state. Interactions can also occur during the feeding step or persist after solidification of the solid lipid microparticles. Identifying possible interactions between components while still in the molten state is particularly important in spray chilling, as they can affect important properties of the molten mixture, such as viscosity. Furthermore, the specific interactions between the active ingredient and the encapsulating material play a significant role in the formation of eutectic systems (Van Duong et al., 2018).
Some authors studied the interactions formed between ascorbic acid and refined oil and fully hydrogenated palm oil as encapsulating materials in spray chilling and reported a marked reduction in ascorbic acid crystallinity after the process (Carvalho et al., 2019). The reduction in crystallinity may be due to the interactions between the active compound and the encapsulating agent, or the rapid cooling and solidification of the molten droplets during spray chilling. The rapid solidification of the droplets can prevent the ascorbic acid molecules from reorganizing into their crystalline form. Strong intermolecular bonds between ascorbic acid and the encapsulating materials can provide enough energy to keep the active agent in an “unstable” state, once ascorbic acid remains in an amorphous state or is dispersed at a molecular level within the encapsulating material (Bertoni, Albertini, & Passerini, 2019).
The Fourier Transform Infrared Spectroscopy (FTIR) can be used to investigate the interaction mechanisms at the molecular level. It allows monitoring the vibrations of the functional groups that characterize the molecular structure in solid state reactions. It is also possible to detect changes in the solid state such as hydrogen bonds or π − π interactions that are recognized as peak changes of functional groups (Van Duong & Van den Mooter, 2016).
4.6. Solid state properties of the active ingredient and the encapsulating material
It is worth emphasizing that the active ingredient can be present in three different molecular states, including amorphous, crystalline, or semi-crystalline states, as shown in Fig. 3. These molecular states are dependent on the formulation, and directly affect the dissolution performance of the active ingredient and, consequently, its release and bioavailability. As previously discussed, a large part of the mass of solid lipid microparticles is constituted by the encapsulating materials, thus their characteristics play an important role in their properties and behavior (Bertoni, Albertini, & Passerini, 2019). Therefore, knowledge about the possible polymorphic modifications of lipid materials (for example, from crystalline to semi-crystalline form) during or after the spray chilling process can prevent undesirable reactions in the microparticles.
Furthermore, the mutual effect of the active ingredient and the encapsulating material in its specific solid state should also be considered. Concerning microparticles produced by spray chilling, the following factors contribute to determining the solid state of the active ingredient: (i) active ingredient-encapsulant interaction, as discussed in the previous paragraph; (ii) the nature of the encapsulant, such as its viscosity and tendency to crystallize; (iii) the nature and amount of the active ingredient; (v) the process parameters, mainly the rate and temperature of the cooling phase; and (iv) the storage conditions (Bertoni, Albertini, & Passerini, 2019; Carvalho et al., 2019; Consoli et al., 2016).
Analytical methods are used to detect changes in the solid state of the active ingredient and the encapsulating agent, as well as the microparticles produced by spray chilling. X-ray diffraction (XRD) provides information about the crystal phase of the sample by the presence of diffraction peaks (Santos et al., 2020). The changes in the physical properties of the active ingredient and the encapsulating material can be determined by comparing the diffractograms of each compound separately and the microparticles produced by spray chilling (Müller et al., 2002b). The differential scanning calorimetry (DSC) technique can be used to study the behavior of lipids in phase transitions such as melting and solidification, in addition to the transformations between the various polymorphic forms (Sato, 2001). Nuclear magnetic resonance (NMR) can also predict the overall behavior of lipid and its mixtures, providing solid fat content (SFC) over a wide temperature range. NMR is considered the most accurate technique to identify the absolute content of solid fat content (Oriani et al., 2016; Ribeiro et al., 2012), and SFC curves can be used to predict the type of fat application. For example, the liquid/solid fractions of a lipid at different temperatures can be identified through an iso-solids diagram, which represents the solids fat content of various compositions at constant temperature (Richard, 2009; Timms, 1985). Therefore, this information can be used to understand the behavior of lipids and assist in the development of novel fat-based products.
The physical property of the polymorphic transitions affects the production of solid lipid microparticles, with a low encapsulation efficiency and core release during storage, due to crystal reorganization into a more stable polymorphic form (Ribeiro et al., 2012). When the polymorphic form of the active ingredient and the encapsulating agent is maintained, the peaks are formed at the same diffraction angles, while different polymorphs lead to the formation of different angles, which can also be observed in the case of amorphization, i.e., absence of sharp refraction peaks, called an amorphous halo. Finally, the reduction in crystallinity can be detected by the formation of less sharp and less intense diffraction peaks (Bertoni, Albertini, & Passerini, 2019).
Some authors investigated lycopene dissolved in sunflower oil and encapsulated by spray chilling using fat with a melting point of 51 °C. The microparticles were stored at 4 °C for 90 days and analyzed by X-ray diffractometry. The authors reported that both sunflower oil and lycopene solutions appeared as amorphous materials, and the fat presented a crystalline state. On the other hand, the microparticles exhibited as crystalline materials, governed by the fat behavior. The authors also noticed that increasing the concentration of the lycopene solution in the formulations led to a gradual reduction in the relative intensity of the microparticles diffraction peaks. However, they concluded that the fat crystal was not affected by the process or the incorporation of the lycopene/sunflower oil solution for all concentrations studied. Thus, the diffraction peaks of the diffractograms of both fat and microparticles corresponded to the polymorphic phase β ′ (Pelissari et al., 2016).
5. Applications in foods
The potential increase in consumer interest in healthier and more natural foods has encouraged the food industry to use natural ingredients such as bioactive compounds. However, the sensitivity to processing, storage, and gastrointestinal conditions of these natural compounds has prompted food scientists to develop technologies to overcome these limitations.
Studies have shown that microencapsulation contributes to the development of novel foods, as well as new structures for food packaging. This stimulating potential has led the industry to invest heavily in the development of encapsulation techniques for delivering food ingredients, such as solid lipid nano/microparticles. Microencapsulation plays a huge impact, transforming essential and conventional foods into fortified processed foods for better nutritional, functional, and controlled delivery.
As reported by Bao et al. (2019), it is estimated that more than 40% of the food industries use the microencapsulation process. A study by the market research and consulting journal Markets and Markets, published in March 2020, reported that the global food packaging market was valued at $9.9 billion in 2020 and is projected to exceed $14 billion by 2025 with an annual growth rate (CAGR) of 7.5% during the period. This market is mainly driven by the growing demand for encapsulated flavors and dyes in the food and beverage industry, along with the high consumer demand for fortified and functional foods.
Solid lipid microparticles have been proposed for the release of drugs, enzymes, probiotics, vitamins, minerals, flavors, antioxidants, antimicrobials with several purposes (McClements, 2020), ensuring better bioavailability, stability, and delivery at the desired time and place (Fredes, Osorio, Parada, & Robert, 2018). The microparticles also prevent interactions with the food matrix and allow the conversion of ingredients to easily handled and dispersible powders, in addition to masking undesirable odor and taste, improving the retention of volatile compounds, and promoting controlled release by different factors such as dissolution, temperature, pressure, pH and enzymes, depending on the encapsulating material used (Botrel, Borges, et al., 2014; Campelo-Felix et al., 2017; Carmo et al., 2018; Fernandes, Borges, & Botrel, 2014; Figueiredo, Teixiera, et al., 2020).
The most relevant studies using spray chilling technology in the food area are discussed below and can be seen in Table 2 .
Table 2.
Application of spray chilling technology for the microencapsulation of food ingredients.
| Encapsulating material | Active material | Emulsifier | Reference |
|---|---|---|---|
| Vegetable fat | Vitamin B12 | Soy lecithin | Chalella et al. (2019) |
| Hydrogenated palm oil and fully hydrogenated vegetable oils | Green tea extract | Not used | Cutrim et al. (2019) |
| Palmitic acid, oleic acid, and palm fat | Ginger oil resin | Not used | Oriani et al. (2016) |
| Refined palm oil and fully hydrogenated palm fat | Ascorbic acid | Not used | Carvalho et al. (2019) |
| Vegetable fat and beeswax | Vitamin D3 | Soy lecithin | Paucar et al. (2016) |
| Hydrogenated palm oil | S. boulardii, L. acidophilus, and B. bifdum | Tween 80 | Arslan-Tontul & Erbas (2017) |
| Fully hydrogenated soy fat and refined soy oil | Gallic acid | Polyglycerol polyricinoleate | Consoli et al. (2016) |
| Shortening composed of hydrogenated and interesterified cottonseed, soy and palm oils (mp 51 °C) | Lycopene | Not used | Pelissari et al. (2016) |
| Octacosan paraffin | 2-acetyl-1-pyrroline zinc chloride | Not used | Yin and Cadwallader (2019) |
| Fully hydrogenated palm oil and vegetable oil | Fish oil | Polyglycerol polyricinoleate | Fadini et al. (2018) |
| Vegetable fat | Guarana seed powder extract | Not used | Silva et al. (2019) |
| Stearic acid | Genipap extract | Polyglycerol polyricinoleate | Neri-Numa et al. (2020) |
| Fully hydrogenated palm fat and palm fat | Cinnamon Bark Oil Resin | Not used | Procopio et al. (2018) |
| Lauric acid and oleic acid | Ascorbic acid | Polyglycerol polyricinoleate | Sartori et al. (2015) |
| Interesterified fat from palm oil and corn oil with a melting point of 43 °C | Ascorbic acid | Soy lecithin | de Matos et al. (2017) |
| Stearic acid and hydrogenated fat with melting point 43 °C | Ascorbic acid | Not used | Alvim et al. (2016) |
| Vegetable fat with a melting point of 48 °C | Cinnamon bark extract, proanthocyanidin, and alpha-tocopherol | Not used | Tulini et al. (2017) |
| Vegetable fat with a melting point of 48 °C | Lactobacillus acidophilus LA3 (LA) and Bifdobacterium animalis subsp. Lactis BLC1 (BLC) | Gelatin and gum Arabic | Silva et al. (2019) |
| Palm fat | Capsaicin (66.7%) | Soy lecithin and whey protein isolate | Gunel, Varhan, Koç, Topuz, & Hilal (2021) |
| Vegetable fat with a melting point of 51 °C | Soy Protein Hydrolysate | Polyglycerol polyricinoleate, Tween 80, and soy lecithin | Salvim (2015) |
| Beeswax, carnauba wax, and medium-chain triglycerides (Miglyol 812) | Curcumin (85% purity) | Not used | Sorita et al. (2021) |
| Fully hydrogenated palm fat (mp 63 °C) | Iron, Iodine, and Vitamin A | Soy lecithin | Wegmuller (2006) |
5.1. Vitamins and minerals
Solid lipid microparticles have great potential to efficiently protect vitamins and minerals against degradation, as they are generally sensitive to light and oxygen (Gu et al., 2016; Matos-Junior, Thomazini, & Fávaro-trindade, 2016). Furthermore, they can improve the bioavailability of these compounds (Gomes et al., 2019).
Although ascorbic acid is one of the most studied vitamins by spray chilling to date, other vitamins such as, B12, D3, and E have also been investigated. Different studies have shown the potential of spray chilling in the controlled release of vitamins, and greater protection and stability during storage (Carvalho et al., 2019; Chalella Mazzocato et al., 2019; Gamboa, Gonçalves, & Grosso, 2011; Matos, Comunian, Thomazini, & Favaro-Trindade, 2017; Paucar et al., 2016; Sartori, Consoli, Hubinger, & Menegalli, 2015; Tulini et al., 2017).
Ascorbic acid microparticles were obtained by spray chilling using stearic acid and hydrogenated vegetable shortening as encapsulating materials and applied in short dough biscuits. One of the main objectives of the study was to evaluate the stability of ascorbic acid of these microparticles after baking the cookies. The authors reported that the addition of microparticles (0.3 g/100 g of dough) did not affect the appearance or the manufacturing stages of the cookies. The microparticles adequately protected the ascorbic acid, preserving more than 85% of the ascorbic acid content, when compared to the compound used in free form (28% loss of ascorbic acid). Therefore, the microparticles are potential protection vehicles in the application of sensitive compounds in bakery products (Alvim et al., 2016).
Vitamin D supplementation is increasingly common due to consumer awareness of its important biological roles, whose deficiency causes severe health problems (Maurya, Bashir, & Aggarwal, 2020). Microencapsulation by spray chilling is an excellent alternative to prevent the rapid degradation of this vitamin and improve its absorption. Vitamin D 3 is fat-soluble, thus it requires the use of lipid foods as vehicles, which is not compatible with the consumer demand for low-fat foods. Therefore, the immobilization of vitamin D 3 through fatty encapsulants can overcome this problem. In addition, the release of vitamin D from solid lipid microparticles into the intestine can be regulated by fat digestion, leading to greater bioavailability. Paucar et al. (2016) produced microparticles containing vitamin D3 and vegetable fat and beeswax as encapsulating materials. After a 65-day storage period at 25 °C, a vitamin loss of only 14% was observed for the encapsulated vitamin when compared to a 39% loss of the free vitamin. These results are promising and demonstrate the potential of spray chilling for the production of microparticles containing vitamin D3, encouraging future applications in food.
Gallic acid is considered a potent natural antioxidant and was also microencapsulated by spray chilling in a matrix of soybean oil and fully hydrogenated soybean fat. Although the release kinetics and stability studies have not been performed, the authors found encapsulation efficiency greater than 80% (Consoli et al., 2016), indicating that this technology can be efficient in the microencapsulation of antioxidant compounds.
Some authors evaluated the encapsulation of green tea extract powder (C. sinensis) by spray chilling and ionic gelation. For the production of solid lipid microparticles by spray chilling, the authors used fully hydrogenated palm oil and hydrogenated and interesterified vegetable fat. For the microparticles produced by ionic gelation, amidated low methoxylated pectin, calcium chloride, citric acid, rapeseed oil, and polyglycerol polyricinoleate were used. Satisfactory results were observed and both techniques were able to produce water-insoluble microparticles rich in polyphenols. The spray chilling technology demonstrated greater encapsulation efficiency (approximately 84%) when compared to ionic gelation (73%). Greater antioxidant activity was obtained for solid lipid microparticles (35 IC50 μg/Ml) when compared to the microparticles produced by gelation (33 IC50 μg/Ml). According to the authors, both microparticles were considered suitable as ingredients for the incorporation of polyphenols in foods (Cutrim, Alvim, & Cortez, 2019).
Minerals such as iron and zinc play important roles in the body. Iron plays many critical roles in growth and its deficiency leads to anemia, low immunity, and negatively affects mental development. Although iron fortification of foods can minimize this problem, the incorporation of iron in foods can adversely affect their physicochemical and sensory characteristics. Furthermore, for adequate absorption, iron must be bioavailable after ingestion. Thus, encapsulation can be used to increase the stability and bioavailability of iron-fortified foods, as well as providing greater protection against oxidation and controlled release (Yang, Zhou, Sun, Gao, & Xu, 2015).
5.2. Natural dyes
Little is known about the effects of spray cooling on the production of natural dyes. In a recent study, turmeric was microencapsulated by spray chilling using beeswax, carnauba wax, and medium-chain triglycerides as encapsulating materials. The results showed that it was possible to incorporate curcumin into encapsulating matrices and obtain suitable microparticles. The highest encapsulation efficiency was 85%, which was dependent on the encapsulating materials. However, further studies on particle stability and its application in food matrices are needed to assess the real applicability of these microparticles (Sorita et al., 2021).
Genipap extract represents an emerging source of blue dyes with wide application possibilities. In a recent study, genipap extract was encapsulated using the spray chilling using stearic acid as an encapsulating agent and polyglycerol polyricinoleate as an emulsifying agent. The microparticles showed intermediate protection against degradation of the genipap extract. These microparticles showed desirable effects on the viability of human cancer cells, as well as on the induction of apoptosis in leukemia cells (at a dosage of 25 μg/mL). However, various issues should be assessed, including the toxicological risks of genipap extract, use of different encapsulating materials to produce microparticles containing this extract, the particle release under simulated GI conditions, and application of the microparticles in food matrices and therapeutic use (Neri-Numa et al., 2020).
5.3. Other bioactive compounds
Other types of compounds can be encapsulated by spray chilling for different purposes. Some examples include prebiotics, probiotics, enzymes, microorganisms, peptides, fatty acids (omega 3), essential oils, flavors, fragrances, and antioxidants (quercetin). As discussed above, these compounds, when microencapsulated, can have great potential for the production of fortified foods and beverages (Delshadi, Bahrami, Tafti, Barba, & Williams, 2020; Tavares, Santos, & Zapata Noreña, 2021; Zanetti et al., 2018). Functional and fortified foods are one of the new food trends, especially after the catastrophic consequences on the health of consumers after the Covid-19 pandemic. Furthermore, the sensory characteristics of foods can also be improved using this technology.
6. Controlled release mechanisms and bioavailability in vitro
The controlled release is one of the most desirable properties in encapsulation techniques. It prolongs the biological activities of the encapsulated active ingredients, modifies and/or maintains flavors and odors, such as allowing an explosion of flavors or ensuring long-term maintenance, and increases the bioavailability of the compounds after gastrointestinal digestion (Alemzadeh et al., 2020).
These mechanisms are governed by different physical systems, which aim to modulate the release of the encapsulated active ingredient. In general, this effect is achieved after a specific stimulus, that is, the occurrence of a certain event that allows the particle release. Factors such as the geometry of the active ingredient, the encapsulating material, or the presence of surfactant can determine the particle release mechanism (Boostani & Jafari, 2021; Figueiredo, MT Lago, et al., 2020).
In spray chilling technology, the release of the active ingredient usually occurs by erosion and leaching of the matrix, due to its hydrophobic nature (Favaro-Trindade et al., 2021, pp. 1–14) or by diffusion (Chalella Mazzocato et al., 2019; Paucar et al., 2016). The presence of surfactants, as well as the temperature conditions, can also affect the release of compounds. In these cases, two distinct concepts stand out. The first is related to temperature sensitivity, which is important for materials that expand or shrink when reaching a critical temperature; the second concept is related to the melting of the encapsulating material in response to an increase in temperature, as observed for microparticles consisting of a modified lipid or waxes (Favaro-Trindade et al., 2021, pp. 1–14).
Concerning the vitamins, natural pigments, and essential oils, which are potentially oxidizable compounds, the microencapsulation by spray chilling can, in addition to increasing stability, promote the compound release at the desired location and time, for example, after ingestion of food, that is, in the stomach or intestine, once the lipid matrix can promote the enteric release (Favaro-Trindade et al., 2021, pp. 1–14).
A study evaluated solid lipid microparticles containing vitamin C produced by spray chilling using palm oil and fat, which allowed a slow and controlled vitamin release in an aqueous medium. The authors reported that the different concentrations of the encapsulating materials have also influenced the release of vitamin C. Within 180 min, the microparticles containing different palm oil and fat ratios were able to release 15 to 10% of vitamin C. Therefore, it was it is possible to maintain high vitamin concentration within the microparticles, with a controlled and slower release (Carvalho et al., 2019). Similar results were observed in another study of controlled release in an aqueous medium of vitamin C lipid microparticles (Sartori et al., 2015). All authors reported the effects of the lipid materials on the release of vitamin C.
The limited chemical stability of the bioactive compounds present in vitamins, microorganisms, proteins, and peptides, natural dyes, and essential oils leads to low bioavailability, less transfer across the biological membranes, and low stability in the bloodstream (Battaglia & Ugazio, 2019). Efforts aimed at encapsulation technologies have improved the bioavailability in vitro of these compounds (Gharibzahedi & Smith, 2021; Rodríguez-Roque, Rojas-Graü, Elez-Martínez, & Martín-Belloso, 2013; Zhang et al., 2020).
For effective intestinal absorption, the microparticles must protect the bioactive compounds against acid and enzymatic degradation when exposed to gastric fluid. That is, the wall materials must be tolerant to gastric stimuli, such as extremely low pH and pepsin present in the stomach. In contrast, when microparticles reach the small intestine, they need to efficiently release core materials (Pedroso, Dogenski, Thomazini, Heinemann, & Favaro-Trindade, 2013).
The increase in bioavailability in vitro of the active ingredients by spray chilling depends on two effects: (i) effects independent of the physical state of the active ingredient within the microparticles, and (ii) effects resulting from changes in the original crystalline state of the active ingredient. The second category is strictly dependent on the combination between the active compound and the encapsulating material and should be considered on a case-by-case basis, while the first can be ideally explored to increase the bioavailability of all poorly water-soluble drugs (Favaro-Trindade et al., 2021, pp. 1–14).
Studies on simulated digestion in vitro of the microparticles produced by spray chilling revealed that most microparticles were able to maintain a gradual release in the stomach and especially a greater release in the gut phase, which is an interesting approach since the absorption of bioactive compounds occurs in the intestine. The studies also showed the effect of other variables such as the type of lipid material, use of emulsifiers, and the type of active ingredient in the release and protection of the microparticles in the gastric system (Chalella Mazzocato et al., 2019; Paucar et al., 2016).
A study has shown that solid lipid microparticles produced with cocoa butter protected Lactobacillus acidophilus in gastric fluid and intestine under simulated conditions. However, a 33% loss of microparticles was observed, probably due to the heterogeneous composition and low melting point of cocoa butter. Some triglycerides may have melted at the temperature of the experiment (37 °C), causing a premature release of the probiotics into the gastric fluid. The authors reported that the loss of viability of Bifidobacterium. animalis subsp. lactis and L. acidophilus can be prevented by increasing the initial number of viable cells in the microparticles, aimed to release an appropriate number of cells in the intestine. Another approach suggested by the authors is the production of microparticles using a lipid matrix with a higher melting point (Bampi et al., 2016).
Tulini et al. (2017) reported that the use of soy lecithin as a surfactant in the production of solid lipid microparticles containing vegetable fat and cinnamon extract increased the stability of proanthocyanidins in the gastric fluid, thus increasing the release of this active compound in the intestinal phase.
7. Advantages and disadvantages of spray chilling
The spray chilling technique consumes less time and energy when compared to other methodologies of particle formation, once there is no use of solvents, thus it can be considered an environmentally adequate technique (Bertoni, Albertini, Dolci, & Passerini, 2018). Other advantages include simplicity, formation of free-flowing microparticles, high encapsulation efficiency (90–100%) for a lipid matrix composed of solid and liquid lipids, ease of incorporation into different food matrices, non-toxicity, and the possibility of encapsulating hygroscopic and water-sensitive compounds (Chalella Mazzocato et al., 2019; Oxley, 2012; Tomšik et al., 2019). From an industrial point of view, it can be performed on an industrial scale and adapted for continuous manufacturing. Concerning the atomizing equipment and mechanism procedures, spray cooling is closely linked to spray drying, thus the spray drying apparatus can be used for spray chilling processes with some modifications (Consoli et al., 2016).
As observed for all technological processes, spray chilling also has some disadvantages. The encapsulated material must be stable at the temperature necessary for melting the lipid matrix, and the possibility of physical changes during the process when using lipids such as glycerides and carnauba wax, affecting the stability and dissolution of the encapsulated compound. Clogging of the atomizer when using highly viscous mixtures can also be a disadvantage of the process (Bertoni, Dolci, Albertini, & Passerini, 2018; Chalella Mazzocato et al., 2019; Okuro et al., 2013; Sartori et al., 2015).
8. Current challenges of spray chilling
There are several current challenges of spray chilling technology in the production of food ingredients when compared to other processes. The challenges include the search for encapsulating materials of lipid origin that meet the characteristics of an ideal encapsulating agent, such as the food grade, melting point, oxidative stability, maintenance of the encapsulating structure throughout storage and use, among others. Queirós et al. (2020) developed a fully hydrogenated milk fat to be used as an encapsulating material and reported that fat allowed the formation of solid lipid microparticles in the β’ form. Further studies are needed for the application of solid lipid microparticles in different foods and beverages.
Other challenges include the applicability of the microparticles produced by spray chilling in different food matrices, as well as the bioavailability in vitro and in vivo.
A forthcoming challenge includes process expansion, as well as optimization in equipment and procedures, to improve the performance in the manufacture of solid lipid microparticles.
9. Innovations
The production of enriched, functional, convenient, and tasty beverages is a major challenge for the food industry. In this context, spray chilling technology can provide innovative and satisfactory results to meet this demand. Most foods are made with a lipid base, thus the addition of solid lipid microparticles containing bioactive compounds, aromas, flavors, microorganisms, among other compounds can be incorporated into the common diet of individuals, such as dairy products, ice cream, cereal bars, meat products, and bakery products, thus providing tasty meals with health benefits. Although few studies have addressed the application of this technology in the food sector, it is known that spray chilling is an innovative technology with great potential to enhance the food and beverage sector.
Some recent studies have shown the improvement of microencapsulation by spray chilling in the food sector with the use of co-encapsulation and different encapsulating agents. Co-encapsulation allows the incorporation of two or more ingredients in the same matrix to be subjected to the encapsulation process. For example, the co-encapsulation of proanthocyanidins from cinnamon extract with α-tocopherol in solid lipid microparticles by spray chilling demonstrated an enhanced scavenging ability of α-tocopherol against reactive oxygen and nitrogen species (Tulini et al., 2017). Therefore, the addition of antioxidant compounds such as α-tocopherol to lipid microparticles produced by spray chilling may be a promising approach, as they can present a synergistic interaction and enhance the biological activities of the compounds (Chawda, Shi, Xue, & Young Quek, 2017).
The investigation of different sources of encapsulating materials for use in spray chilling, such as edible butters from the Amazon region, can bring innovations to the food industry. In a recent innovative study, some authors developed lipidic microparticles made with anhydrous milk fat and fully hydrogenated soybean oil and reported the potential of the microparticles for use in foods as crystallization inducers, structuring agents, and as encapsulating material (Landim Neves et al., 2021).
10. Conclusions
Microencapsulation by spray chilling has been relatively little explored in the food sector when compared to spray drying. Although it is not a recent technique, microencapsulation technology has been gaining popularity in the last decade, with a significant increase in studies on the encapsulation of bioactive food ingredients. The increasing demand for healthy foods may have encouraged studies on encapsulated products that allow the incorporation of bioactive ingredients into food matrices. In this context, spray chilling is an expanding technology with many advantages and applications in a wide variety of industries. The technique involves trapping a compound within an encapsulating material of lipid origin, which protects the core material from the surrounding environment. Solid lipid microparticles can enable the production of functional foods and overcome many limitations in the food industry. In addition, the microparticles can increase the stability of aromas, flavors, pigments, and microorganisms, mask undesirable odors and flavors, allow controlled release, and increase the resistance to the GI tract, improving the bioavailability of bioactive compounds, with health benefits for consumers.
Declaration of interest
The authors declare there is no conflict of interest for this research.
Acknowledgments
This study was supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico - Grant number 578 403803/2016-0 (CNPq, Brasília, Brazil), and Fundação de Amparo à Pesquisa do Estado de Minas Gerais - Grant number CAG_APQ_02464_16 (FAPEMIG, Belo Horizonte, Brazil), for financial support.
References
- Alemzadeh I., Hajiabbas M., Pakzad H., Sajadi Dehkordi S., Vossoughi A. Encapsulation of food components and bioactive ingredients and targeted release. International Journal of Engineering, Transactions A: Basics. 2020;33(1):1–11. doi: 10.5829/ije.2020.33.01a.01. [DOI] [Google Scholar]
- Alvim I.D., Stein M.A., Koury I.P., Dantas F.B.H., Cruz C.L.C.V. Comparison between the spray drying and spray chilling microparticles contain ascorbic acid in a baked product application. Lebensmittel-Wissenschaft und -Technologie- Food Science and Technology. 2016;65:689–694. doi: 10.1016/j.lwt.2015.08.049. [DOI] [Google Scholar]
- Arenas-Jal M., Suñé-Negre J.M., García-Montoya E. An overview of microencapsulation in the food industry: Opportunities, challenges, and innovations. European Food Research and Technology. 2020;246(7):1371–1382. doi: 10.1007/s00217-020-03496-x. [DOI] [Google Scholar]
- Arslan-Tontul Sultan., Erbas Mustafa. BİYOAKTİF GIDA BİLEŞENLERİNİN PÜSKÜRTEREK DONDURMA YÖNTEMİ İle MİKROENKAPSÜLASYONU. Gida / the Journal of Food. 2017;43(1):11–20. doi: 10.15237/gida.GD17075. [DOI] [Google Scholar]
- Bampi G.B., Backes G.T., Cansian R.L., de Matos F.E., Ansolin I.M.A., Poleto B.C., et al. Spray chilling microencapsulation of Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis and its use in the preparation of savory probiotic cereal bars. Food and Bioprocess Technology. 2016;9(8):1422–1428. doi: 10.1007/s11947-016-1724-z. [DOI] [Google Scholar]
- Bao C., Jiang P., Chai J., Jiang Y., Li D., Bao W., et al. The delivery of sensitive food bioactive ingredients: Absorption mechanisms, influencing factors, encapsulation techniques and evaluation models. Food Research International. 2019;120(February):130–140. doi: 10.1016/j.foodres.2019.02.024. [DOI] [PubMed] [Google Scholar]
- Barroso L., Viegas C., Vieira J., Ferreira-Pêgo C., Costa J., Fonte P. Lipid-based carriers for food ingredients delivery. Journal of Food Engineering. 2021;295(July 2020):110451. doi: 10.1016/j.jfoodeng.2020.110451. [DOI] [Google Scholar]
- Battaglia L., Ugazio E. Lipid nano- and microparticles: An overview of patent-related research. Journal of Nanomaterials. 2019 doi: 10.1155/2019/2834941. 2019. [DOI] [Google Scholar]
- Behbahani E.S., Ghaedi M., Abbaspour M., Rostamizadeh K., Dashtian K. Curcumin loaded nanostructured lipid carriers: In vitro digestion and release studies. Polyhedron. 2019;164:113–122. doi: 10.1016/j.poly.2019.02.002. [DOI] [Google Scholar]
- Bertoni S., Albertini B., Dolci L.S., Passerini N. Spray congealed lipid microparticles for the local delivery of β-galactosidase to the small intestine. European Journal of Pharmaceutics and Biopharmaceutics. 2018;132(August):1–10. doi: 10.1016/j.ejpb.2018.08.014. [DOI] [PubMed] [Google Scholar]
- Bertoni S., Albertini B., Ferraro L., Beggiato S., Dalpiaz A., Passerini N. Exploring the use of spray congealing to produce solid dispersions with enhanced indomethacin bioavailability: In vitro characterization and in vivo study. European Journal of Pharmaceutics and Biopharmaceutics. 2019;139(December 2018):132–141. doi: 10.1016/j.ejpb.2019.03.020. [DOI] [PubMed] [Google Scholar]
- Bertoni S., Albertini B., Passerini N. Spray congealing: An emerging technology to prepare solid dispersions with enhanced oral bioavailability of poorly water soluble drugs. Molecules. 2019;24(19):3471. doi: 10.3390/molecules24193471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoni S., Dolci L.S., Albertini B., Passerini N. Spray congealing: A versatile technology for advanced drug-delivery systems. Therapeutic Delivery. 2018;9(11):833–845. doi: 10.4155/tde-2018-0049. [DOI] [PubMed] [Google Scholar]
- Boostani S., Jafari S.M. A comprehensive review on the controlled release of encapsulated food ingredients; fundamental concepts to design and applications. Trends in Food Science & Technology. 2021;109(December 2020):303–321. doi: 10.1016/j.tifs.2021.01.040. [DOI] [Google Scholar]
- Botrel D.A., Borges S.V., Barros R. V. De, do Carmo E.L. Optimization of fish oil spray drying using a protein : Inulin system optimization of fish oil spray drying using a protein : Inulin system. Drying Technology. 2014;32(3):279–290. doi: 10.1080/07373937.2013.823621. [DOI] [Google Scholar]
- Botrel D.A., Borges S.V., Fernandes R.V. de B., Viana A.D., Costa J.M.G., Marques G.R. Evaluation of spray drying conditions on properties of microencapsulated oregano essential oil. International Journal of Food Science and Technology. 2012;47(11):2289–2296. doi: 10.1111/j.1365-2621.2012.03100.x. [DOI] [Google Scholar]
- Botrel D.A., Fernandes V.D.B., Borges S.V., Yoshida M.I. In fl uence of wall matrix systems on the properties of spray-dried microparticles containing fi sh oil. FRIN. 2014;62:344–352. doi: 10.1016/j.foodres.2014.02.003. [DOI] [Google Scholar]
- Campelo-Felix P.H., Souza H.J.B., Figueiredo J.D.A., Fernandes R.V. de B., Botrel D.A., de Oliveira C.R., et al. Prebiotic carbohydrates: Effect on reconstitution, storage, release, and antioxidant properties of lime essential oil microparticles. Journal of Agricultural and Food Chemistry. 2017;65(2):445–453. doi: 10.1021/acs.jafc.6b04643. [DOI] [PubMed] [Google Scholar]
- Campelo P.H., Figueiredo J. de A., Ferraz V., Yoshida M.I., Fernandes R.V.D.B., Botrel D.A., et al. Hygroscopic thermal and chemical properties of cinnamon essential oil microparticles obtainded by spray drying. Emirates Journal of Food and Agriculture. 2017;29(11):884. doi: 10.9755/ejfa.2017.v29.i11.1499. [DOI] [Google Scholar]
- Carmo E. L. do, Teodoro R.A.R., Félix P.H.C., Fernandes R.V. de B., Oliveira É. R. de, Veiga T.R.L.A., et al. Stability of spray-dried beetroot extract using oligosaccharides and whey proteins. Food Chemistry. 2018;249(October 2017):51–59. doi: 10.1016/j.foodchem.2017.12.076. [DOI] [PubMed] [Google Scholar]
- Carvalho J.D. dos S., Oriani V.B., de Oliveira G.M., Hubinger M.D. Solid lipid microparticles loaded with ascorbic acid: Release kinetic profile during thermal stability. Journal of Food Processing and Preservation. 2021;45(6):1–9. doi: 10.1111/jfpp.15557. [DOI] [Google Scholar]
- Carvalho J.D.S., Oriani V.B., Oliveira G.M., Hubinger M.D. Characterization of ascorbic acid microencapsulated by the spray chilling technique using palm oil and fully hydrogenated palm oil. Lebensmittel-Wissenschaft & Technologie. 2019;101(March 2018):306–314. doi: 10.1016/j.lwt.2018.11.043. [DOI] [Google Scholar]
- Chalella Mazzocato M., Thomazini M., Favaro-Trindade C.S. Improving stability of vitamin B12 (Cyanocobalamin) using microencapsulation by spray chilling technique. Food Research International. 2019;126(May):108663. doi: 10.1016/j.foodres.2019.108663. [DOI] [PubMed] [Google Scholar]
- Chambi H.N.M., Alvim I.D., Barrera-Arellano D., Grosso C.R.F. Solid lipid microparticles containing water-soluble compounds of different molecular mass: Production, characterisation and release profiles. Food Research International. 2008;41(3):229–236. doi: 10.1016/j.foodres.2007.11.012. [DOI] [Google Scholar]
- Chawda P.J., Shi J., Xue S., Young Quek S. Co-encapsulation of bioactives for food applications. Food Quality and Safety. 2017;1(4):302–309. doi: 10.1093/fqsafe/fyx028. [DOI] [Google Scholar]
- Chenarides L., Grebitus C., Lusk J.L., Printezis I. Food consumption behavior during the COVID‐19 pandemic. Agribusiness. 2021;37(1):44–81. doi: 10.1002/agr.21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho T.J., Kim S.A., Kim H.W., Park S.M., Rhee M.S. Changes in consumers' food purchase and transport behaviors over a decade (2010 to 2019) following health and convenience food trends. International Journal of Environmental Research and Public Health. 2020;17(15):5448. doi: 10.3390/ijerph17155448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consoli L., Grimaldi R., Sartori T., Menegalli F.C., Hubinger M.D. Gallic acid microparticles produced by spray chilling technique: Production and characterization. Lebensmittel-Wissenschaft und -Technologie- Food Science and Technology. 2016;65:79–87. doi: 10.1016/j.lwt.2015.07.052. [DOI] [Google Scholar]
- Cutrim C.S., Alvim I.D., Cortez M.A.S. Microencapsulation of green tea polyphenols by ionic gelation and spray chilling methods. Journal of Food Science & Technology. 2019;56(8):3561–3570. doi: 10.1007/s13197-019-03908-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delshadi R., Bahrami A., Tafti A.G., Barba F.J., Williams L.L. Micro and nano-encapsulation of vegetable and essential oils to develop functional food products with improved nutritional profiles. Trends in Food Science & Technology. 2020;104(July):72–83. doi: 10.1016/j.tifs.2020.07.004. [DOI] [Google Scholar]
- Eftimov T., Popovski G., Petković M., Seljak B.K., Kocev D. COVID-19 pandemic changes the food consumption patterns. Trends in Food Science & Technology. 2020;104(August):268–272. doi: 10.1016/j.tifs.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadini A.L., Alvim I.D., Ribeiro I.P., Ruzene L.G., Silva L. B. da, Queiroz M.B., et al. Innovative strategy based on combined microencapsulation technologies for food application and the influence of wall material composition. Lebensmittel-Wissenschaft und -Technologie- Food Science and Technology. 2018;91(January):345–352. doi: 10.1016/j.lwt.2018.01.071. [DOI] [Google Scholar]
- Favaro-Trindade C.S., de Matos Junior F.E., Okuro P.K., Dias-ferreira J., Cano A., Severino P., et al. 2021. Encapsulation of active pharmaceutical ingredients in lipid micro/nanoparticles for oral administration by spray-cooling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro-trindade C.S., Okuro P.K., Matos-Junior F.E. Handbook of Encapsulation and Controlled Release; Janeiro: 2016. Encapsulation via spray chilling/cooling/congealing; pp. 71–87. [Google Scholar]
- Fernandes R.V. de B., Borges S.V., Botrel D.A. Gum Arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carbohydrate Polymers. 2014;101:524–532. doi: 10.1016/j.carbpol.2013.09.083. [DOI] [PubMed] [Google Scholar]
- Fernandes R.V.D.B., Borges S.V., Botrel D.A., Oliveira C. R. De. Physical and chemical properties of encapsulated rosemary essential oil by spray drying using whey protein-inulin blends as carriers. International Journal of Food Science and Technology. 2014;49(6):1522–1529. doi: 10.1111/ijfs.12449. [DOI] [Google Scholar]
- Figueiredo J. de A., Mt Lago A., M Mar J., S Silva L., A Sanches E., P Souza T., et al. Stability of camu-camu encapsulated with different prebiotic biopolymers. Journal of the Science of Food and Agriculture. 2020;100(8):3471–3480. doi: 10.1002/jsfa.10384. [DOI] [PubMed] [Google Scholar]
- Figueiredo J. de A., Teixeira M.A., Campelo P.H., Lago A.M.T., Souza T. P. de, Yoshida M.I., et al. Encapsulation of camu-camu extracts using prebiotic biopolymers: Controlled release of bioactive compounds and effect on their physicochemical and thermal properties. Food Research International. 2020;137(July):109563. doi: 10.1016/j.foodres.2020.109563. [DOI] [PubMed] [Google Scholar]
- Fredes C., Osorio M.J., Parada J., Robert P. Stability and bioaccessibility of anthocyanins from maqui (Aristotelia chilensis [Mol.] Stuntz) juice microparticles. Lebensmittel-Wissenschaft und -Technologie- Food Science and Technology. 2018;91:549–556. doi: 10.1016/j.lwt.2018.01.090. 2017. [DOI] [Google Scholar]
- Furuta T., Neoh T.L. Microencapsulation of food bioactive components by spray drying: A review. Drying Technology. 2020:1–32. doi: 10.1080/07373937.2020.1862181. 0(0) [DOI] [Google Scholar]
- Galanakis C.M., Aldawoud T.M.S., Rizou M., Rowan N.J., Ibrahim S.A. Food ingredients and active compounds against the coronavirus disease (COVID-19) pandemic: A comprehensive review. Foods. 2020;9(11):1701. doi: 10.3390/foods9111701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamboa O.D., Gonçalves L.G., Grosso C.F. Microencapsulation of tocopherols in lipid matrix by spray chilling method. Procedia Food Science. 2011;1:1732–1739. doi: 10.1016/j.profoo.2011.09.255. [DOI] [Google Scholar]
- Gharibzahedi S.M.T., Smith B. Legume proteins are smart carriers to encapsulate hydrophilic and hydrophobic bioactive compounds and probiotic bacteria: A review. Comprehensive Reviews in Food Science and Food Safety. 2021;20(2):1250–1279. doi: 10.1111/1541-4337.12699. [DOI] [PubMed] [Google Scholar]
- Gomes G.V.L., Sola M.R., Rochetti A.L., Fukumasu H., Vicente A.A., Pinho S.C. β-Carotene and α-tocopherol coencapsulated in nanostructured lipid carriers of murumuru (Astrocaryum murumuru) butter produced by phase inversion temperature method: Characterisation, dynamic in vitro digestion and cell viability study. Journal of Microencapsulation. 2019;36(1):43–52. doi: 10.1080/02652048.2019.1585982. [DOI] [PubMed] [Google Scholar]
- Gu C., Hu C., Ma C., Fang Q., Xing T., Xia Q. Development and characterization of solid lipid microparticles containing vitamin C for topical and cosmetic use. European Journal of Lipid Science and Technology. 2016;118(7):1093–1103. doi: 10.1002/ejlt.201500373. [DOI] [Google Scholar]
- Gunel Zehra., Varhan Emine., Koç Mehmet., Topuz Ayhan., Hilal Sahin-Nadeem. Production of pungency-suppressed capsaicin microcapsules by spray chilling. Food Bioscience. 2021;40(100918) doi: 10.1016/j.fbio.2021.100918. [DOI] [Google Scholar]
- Huang J., Qian A., Sun R., Xia Q. Preparation and characterization of coenzyme Q10 loaded solid lipid-based formulations for enhancement of gastrointestinal solubilization. Journal of Dispersion Science and Technology. 2019;40(10):1385–1395. doi: 10.1080/01932691.2018.1515023. [DOI] [Google Scholar]
- Jafari S.M., Assadpoor E., He Y., Bhandari B.R. Encapsulation efficiency of food flavours and oils during spray drying. Drying Technology. 2008;26(7):816–835. doi: 10.1080/07373930802135972. [DOI] [Google Scholar]
- Jaskulski M., Kharaghani A., Tsotsas E. In: Thermal and nonthermal encapsulation methods. Krokida M.K., editor. CRC Press; 2018. Chapter 3- encapsulation methods: Spray drying, spray chilling and spray cooling; pp. 67–114. [Google Scholar]
- Landim Neves M.I., de Souza Queirós M., Soares Viriato R.L., Badan Ribeiro A.P., Gigante M.L. Anhydrous milk fat blended with fully hydrogenated soybean oil as lipid microparticles: Characterization, stability, and trends for application. Lebensmittel-Wissenschaft & Technologie. 2021;152(August):112276. doi: 10.1016/j.lwt.2021.112276. [DOI] [Google Scholar]
- Loh H.C., Seah Y.K., Looi I. The COVID-19 pandemic and diet change. Progress In Microbes & Molecular Biology. 2021;4(1):1–14. doi: 10.36877/pmmb.a0000203. [DOI] [Google Scholar]
- Maschke A., Becker C., Eyrich D., Kiermaier J., Blunk T., Göpferich A. Development of a spray congealing process for the preparation of insulin-loaded lipid microparticles and characterization thereof. European Journal of Pharmaceutics and Biopharmaceutics. 2007;65(2):175–187. doi: 10.1016/j.ejpb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Matos F.E., Jr., Comunian T.A., Thomazini M., Favaro-Trindade C.S. Effect of feed preparation on the properties and stability of ascorbic acid microparticles produced by spray chilling. Lebensmittel-Wissenschaft & Technologie. 2017;75:251–260. doi: 10.1016/j.lwt.2016.09.006. [DOI] [Google Scholar]
- Matos F.E., Jr., Di Sabatino M., Passerini N., Favaro-Trindade C.S., Albertini B. Development and characterization of solid lipid microparticles loaded with ascorbic acid and produced by spray congealing. Food Research International. 2015;67:52–59. doi: 10.1016/j.foodres.2014.11.002. [DOI] [Google Scholar]
- Matos-Junior F.E., Thomazini M., Fávaro-trindade C.S. Aplicação de vitamina C livre e encapsulada por spray chilling em salsicha de carne de frango : Características físico-químicas , estabilidade e aceitação sensorial application of free or encapsulated vitamin C to chicken frankfurter sausage by spray. Brazilian Journal of Food Technology. 2016:322–331. [Google Scholar]
- Maurya V.K., Bashir K., Aggarwal M. Vitamin D microencapsulation and fortification: Trends and technologies. The Journal of Steroid Biochemistry and Molecular Biology. 2020;196(March 2019) doi: 10.1016/j.jsbmb.2019.105489. [DOI] [PubMed] [Google Scholar]
- McClements D.J. Advances in nanoparticle and microparticle delivery systems for increasing the dispersibility, stability, and bioactivity of phytochemicals. Biotechnology Advances. 2020;38(August 2018):107287. doi: 10.1016/j.biotechadv.2018.08.004. [DOI] [PubMed] [Google Scholar]
- Müller R.H., Petersen R.D., Hommoss A., Pardeike J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Advanced Drug Delivery Reviews. 2007;59(6):522–530. doi: 10.1016/j.addr.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Müller R., Radtke M., Wissing S. Nanostructured lipid matrices for improved microencapsulation of drugs. International Journal of Pharmaceutics. 2002;242(1–2):121–128. doi: 10.1016/S0378-5173(02)00180-1. [DOI] [PubMed] [Google Scholar]
- Müller R.H., Radtke M., Wissing S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Advanced Drug Delivery Reviews. 2002;54(SUPPL):131–155. doi: 10.1016/S0169-409X(02)00118-7. [DOI] [PubMed] [Google Scholar]
- Nahum V., Domb A.J. Recent developments in solid lipid microparticles for food ingredients delivery. Foods. 2021;10(2):1–25. doi: 10.3390/foods10020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri-Numa I.A., DellaTorre A., Oriani V.B., Franch G.C., Angolini C.F.F., Dupas Hubinger M., et al. In vitro bioactivity approach of unripe genipap (Genipa americana L., Rubiaceae) fruit extract and its solid lipid microparticle. Food Research International. 2020;127(April 2019):108720. doi: 10.1016/j.foodres.2019.108720. [DOI] [PubMed] [Google Scholar]
- Oh C.M., Guo Q., Wan Sia Heng P., Chan L.W. Spray-congealed microparticles for drug delivery-an overview of factors influencing their production and characteristics. Expert Opinion on Drug Delivery. 2014;11(7):1047–1060. doi: 10.1517/17425247.2014.915805. [DOI] [PubMed] [Google Scholar]
- Okuro P.K., de Matos F.E., Jr., Favaro-Trindade C.S. Technological challenges for spray chilling encapsulation of functional food ingredients. Food Technology and Biotechnology. 2013;51(2):171–182. [Google Scholar]
- Oriani V.B., Alvim I.D., Consoli L., Molina G., Pastore G.M., Hubinger M.D. Solid lipid microparticles produced by spray chilling technique to deliver ginger oleoresin: Structure and compound retention. Food Research International. 2016;80:41–49. doi: 10.1016/j.foodres.2015.12.015. [DOI] [Google Scholar]
- Oriani V.B., Alvim I.D., Paulino B.N., Procópio F.R., Pastore G.M., Hubinger M.D. The influence of the storage temperature on the stability of lipid microparticles containing ginger oleoresin. Food Research International. 2018;109(February):472–480. doi: 10.1016/j.foodres.2018.04.066. [DOI] [PubMed] [Google Scholar]
- Oxley J.D. Encapsulation technologies and delivery systems for food ingredients and nutraceuticals. Elsevier; 2012. Spray cooling and spray chilling for food ingredient and nutraceutical encapsulation; pp. 110–130. [DOI] [Google Scholar]
- Paucar O.C., Tulini F.L., Thomazini M., Balieiro J.C.C., Pallone E.M.J.A., Favaro-Trindade C.S. Production by spray chilling and characterization of solid lipid microparticles loaded with vitamin D 3. Food and Bioproducts Processing. 2016;100:344–350. doi: 10.1016/j.fbp.2016.08.006. [DOI] [Google Scholar]
- Pedroso D.L., Dogenski M., Thomazini M., Heinemann R.J.B., Favaro-Trindade C.S. Microencapsulation of Bifidobacterium animalis subsp. lactis and Lactobacillus acidophilus in cocoa butter using spray chilling technology. Brazilian Journal of Microbiology. 2013;44(3):777–783. doi: 10.1590/S1517-83822013000300017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelissari J.R., Souza V.B., Pigoso A.A., Tulini F.L., Thomazini M., Rodrigues C.E.C., et al. Production of solid lipid microparticles loaded with lycopene by spray chilling: Structural characteristics of particles and lycopene stability. Food and Bioproducts Processing. 2016;98:86–94. doi: 10.1016/j.fbp.2015.12.006. [DOI] [Google Scholar]
- Pezeshki A., Hamishehkar H., Ghanbarzadeh B., Fathollahy I., Keivani Nahr F., Khakbaz Heshmati M., et al. Nanostructured lipid carriers as a favorable delivery system for β-carotene. Food Bioscience. 2019;27(January 2018):11–17. doi: 10.1016/j.fbio.2018.11.004. [DOI] [Google Scholar]
- Procopio F.R., Oriani V.B., Paulino B.N., do Prado-Silva L., Pastore G.M., Sant'Ana A.S., et al. Solid lipid microparticles loaded with cinnamon oleoresin: Characterization, stability and antimicrobial activity. Food Research International. 2018;113(March):351–361. doi: 10.1016/j.foodres.2018.07.026. [DOI] [PubMed] [Google Scholar]
- Queirós M. de S., Viriato R.L.S., Ribeiro A.P.B., Gigante M.L. Dairy-based solid lipid microparticles: A novel approach. Food Research International. 2020;131(November 2019):109009. doi: 10.1016/j.foodres.2020.109009. [DOI] [PubMed] [Google Scholar]
- Ray S., Raychaudhuri U., Chakraborty R. Food Bioscience an overview of encapsulation of active compounds used in food products by drying technology. Food Bioscience. 2016;13:76–83. doi: 10.1016/j.fbio.2015.12.009. [DOI] [Google Scholar]
- Ribeiro M.D.M.M., Arellano D.B., Grosso C.R.F. The effect of adding oleic acid in the production of stearic acid lipid microparticles with a hydrophilic core by a spray-cooling process. Food Research International. 2012;47(1):38–44. doi: 10.1016/j.foodres.2012.01.007. [DOI] [Google Scholar]
- Richard D.O. 2009. Fats & oils: Formulating and processing for applications. [Google Scholar]
- Rodríguez-Roque M.J., Rojas-Graü M.A., Elez-Martínez P., Martín-Belloso O. Changes in vitamin C, phenolic, and carotenoid pro fi les Throughout in vitro gastrointestinal digestion of a blended fruit juice. Journal of Agricultural and Food Chemistry. 2013;61:1859–1867. doi: 10.1021/jf3044204. [DOI] [PubMed] [Google Scholar]
- Santos V. da S., Braz B.B., Silva A.Á., Cardoso L.P., Ribeiro A.P.B., Santana M.H.A. Nanostructured lipid carriers loaded with free phytosterols for food applications. Food Chemistry. 2019;298(February):125053. doi: 10.1016/j.foodchem.2019.125053. [DOI] [PubMed] [Google Scholar]
- Salvim, et al. Production and structural characterization of solid lipid microparticles loaded with soybean protein hydrolysate. Food Research International. 2015;76:689–696. doi: 10.1016/j.foodres.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Santos C.A., Carpenter C.S., Arid J.D., da Silva Á.Á., Cardoso L.P., Ribeiro A.P.B., et al. Production and characterization of promising β-stable seed crystals to modulate the crystallization of fat-based industrial products. Food Research International. 2020;130(November 2019):108900. doi: 10.1016/j.foodres.2019.108900. [DOI] [PubMed] [Google Scholar]
- Sartori T., Consoli L., Hubinger M.D., Menegalli F.C. Ascorbic acid microencapsulation by spray chilling: Production and characterization. Lebensmittel-Wissenschaft und -Technologie- Food Science and Technology. 2015;63(1):353–360. doi: 10.1016/j.lwt.2015.03.112. [DOI] [Google Scholar]
- Sato K. Crystallization behaviour of fats and lipids. Chemical Engineering Science. 2001;56:2256–2265. doi: 10.15446/dyna.v85n204.66626. [DOI] [Google Scholar]
- Sato K., Ueno S. Crystallization, transformation and microstructures of polymorphic fats in colloidal dispersion states. Current Opinion in Colloid & Interface Science. 2011;16(5):384–390. doi: 10.1016/j.cocis.2011.06.004. [DOI] [Google Scholar]
- Shishir M.R.I., Chen W. Trends of spray drying: A critical review on drying of fruit and vegetable juices. Trends in Food Science & Technology. 2017;65:49–67. doi: 10.1016/j.tifs.2017.05.006. [DOI] [Google Scholar]
- Silva M.P., Thomazini M., Holkem A.T., Pinho L.S., Genovese M.I., Fávaro-Trindade C.S. Production and characterization of solid lipid microparticles loaded with guaraná (Paullinia cupana) seed extract. Food Research International. 2019;123(December 2018):144–152. doi: 10.1016/j.foodres.2019.04.055. [DOI] [PubMed] [Google Scholar]
- Sorita G., Santamaria-Echart A., Gozzo A., Gonçalves O., Leimann F., Bona E., et al. Lipid composition optimization in spray congealing technique and testing with curcumin-loaded microparticles. Advanced Powder Technology. 2021;32(5):1710–1722. doi: 10.1016/j.apt.2021.03.028. [DOI] [Google Scholar]
- Tavares L., Santos L., Zapata Noreña C.P. Bioactive compounds of garlic: A comprehensive review of encapsulation technologies, characterization of the encapsulated garlic compounds and their industrial applicability. Trends in Food Science & Technology. 2021;114(September 2020):232–244. doi: 10.1016/j.tifs.2021.05.019. [DOI] [Google Scholar]
- Timms R.E. Physical properties of oils and mixtures of oils. Journal of the American Oil Chemists’ Society. 1985;62(2):241–249. doi: 10.1007/BF02541385. [DOI] [Google Scholar]
- Tomšik A., Šarić L., Bertoni S., Protti M., Albertini B., Mercolini L., et al. Encapsulations of wild garlic (Allium ursinum L.) extract using spray congealing technology. Food Research International. 2019;119(August 2018):941–950. doi: 10.1016/j.foodres.2018.10.081. [DOI] [PubMed] [Google Scholar]
- Trojanowska A., Nogalska A., Valls R.G., Giamberini M., Tylkowski B. Technological solutions for encapsulation. Physical Sciences Reviews. 2017;2(9):1–20. doi: 10.1515/psr-2017-0020. [DOI] [Google Scholar]
- Tulini F.L., Souza V.B., Thomazini M., Silva M.P., Massarioli A.P., Alencar S.M., et al. Evaluation of the release profile, stability and antioxidant activity of a proanthocyanidin-rich cinnamon (Cinnamomum zeylanicum) extract co-encapsulated with α-tocopherol by spray chilling. Food Research International. 2017;95:117–124. doi: 10.1016/j.foodres.2017.03.010. [DOI] [PubMed] [Google Scholar]
- Van Duong T., Lüdeker D., Van Bockstal P.J., De Beer T., Van Humbeeck J., Van Den Mooter G. Polymorphism of indomethacin in semicrystalline dispersions: Formation, transformation, and segregation. Molecular Pharmaceutics. 2018;15(3):1037–1051. doi: 10.1021/acs.molpharmaceut.7b00930. [DOI] [PubMed] [Google Scholar]
- Van Duong T., Van den Mooter G. The role of the carrier in the formulation of pharmaceutical solid dispersions. Part II: Amorphous carriers. Expert Opinion on Drug Delivery. 2016;13(12):1681–1694. doi: 10.1080/17425247.2016.1198769. [DOI] [PubMed] [Google Scholar]
- Wegmuller Rita., et al. Development, stability, and sensory testing of microcapsules containing iron, iodine, and vitamin a for use in food fortification. Journal of Food Science. 2006;71(2):181–187. doi: 10.1111/j.1365-2621.2006.tb08923.x. [DOI] [Google Scholar]
- Yang R., Zhou Z., Sun G., Gao Y., Xu J. Ferritin, a novel vehicle for iron supplementation and food nutritional factors encapsulation. Trends in Food Science & Technology. 2015;44(2):189–200. doi: 10.1016/j.tifs.2015.04.005. [DOI] [Google Scholar]
- Yin Y., Cadwallader K.R. Spray-chilling encapsulation of 2-acetyl-1-pyrroline zinc chloride using hydrophobic materials: Storage stability and flavor application in food. Food Chemistry. 2019;278(August 2018):738–743. doi: 10.1016/j.foodchem.2018.11.122. [DOI] [PubMed] [Google Scholar]
- Zafimahova-Ratisbonne A., Wardhono E.Y., Lanoisellé J.-L., Saleh K., Clausse D. Stability of W/O emulsions encapsulating polysaccharides. Journal of Dispersion Science and Technology. 2014;35(1):38–47. doi: 10.1080/01932691.2013.773444. [DOI] [Google Scholar]
- Zanetti M., Carniel T.K., Dalcanton F., dos Anjos R.S., Gracher Riella H., de Araújo P.H.H., et al. Use of encapsulated natural compounds as antimicrobial additives in food packaging: A brief review. Trends in Food Science & Technology. 2018;81:51–60. doi: 10.1016/j.tifs.2018.09.003. [DOI] [Google Scholar]
- Zhang R., Zhou L., Li J., Oliveira H., Yang N., Jin W., et al. Microencapsulation of anthocyanins extracted from grape skin by emulsification/internal gelation followed by spray/freeze-drying techniques: Characterization, stability and bioaccessibility. Lebensmittel-Wissenschaft & Technologie. 2020;123(January):109097. doi: 10.1016/j.lwt.2020.109097. [DOI] [Google Scholar]