Abstract
Objectives
During the ongoing pandemic of COVID-19, wearing face masks was recommended, including patients with epilepsy doing the hyperventilation (HV) test during electroencephalogram (EEG) examination somewhere. However, evidence was still limited about the effect of HV with face mask on cortical excitability of patients with epilepsy. The motivation of this work is to make use of the graph theory of EEG to characterize the cortical excitability of patients with epilepsy when they did HV under the condition wearing a surgical face mask.
Methods
We recruited 19 patients with epilepsy and 17 normal controls. All of participants completed two HV experiments, including HV with face mask (HV+) and HV without a mask (HV). The interval was 30 min and the sequence was random. Each experiment consisted of three segments: resting EEG, EEG of HV, and EEG of post-HV. EEG were recorded successively during each experiment. Participants were asked to evaluate the discomfort degree using a questionnaire when every HV is completed.
Results
All of the participants felt more uncomfortable after HV + . Moreover, not only HV decreased small-worldness index in patients with epilepsy, but also HV + significantly increased the clustering coefficient in patients with epilepsy. Importantly, the three-way of Mask*HV*Epilepsy showed interaction in the clustering coefficient in the delta band, as well as in the path length and the small-worldness index in the theta band.
Conclusions
The results of this study indicated that patients with epilepsy showed the increased excitability of brain network during HV + . We should pay more attention to the adverse effect on brain network excitability caused by HV + in patients with epilepsy. In the clinical practice under the COVID-19 pandemic, it is important that the wearing face mask remain cautious for the individuals with epilepsy when they carried out HV behavior such as exercise (e.g., running, etc.).
Keywords: COVID-19, Hyperventilation, Electroencephalogram, Face mask, Cortical excitability
1. Introduction
The outbreak of coronavirus disease 2019 (COVID-19) has rapidly evolved into a global pandemic. COVID-19 was caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Sun et al., 2020) and was transmitted by breathing of infected droplets or contact with infected droplets (Madabhavi et al., 2020; Berger, 2020). As of March 31, 2021, the virus has been responsible for 100 million confirmed cases worldwide and the number is still increasing (Ren et al., 2021). There is some evidence to demonstrate that wearing face masks in public can reduce the risks of SARS-CoV-2 transmission (Sunjaya and Jenkins, 2020; Esposito et al., 2020; Liu and Zhang, 2020). Judging from the current situation of the epidemic, wearing face masks in public places will be the norm. However, breathing wearing a face mask may simulate HV, which might be disadvantageous to the patients with epilepsy (Asadi Pooya and Cross, 2020).
Epilepsy is characterized by a predisposition to having recurrent seizures, which is caused by abnormal excessive or hyper-synchronized neuronal activity in the brain (Alexander et al., 2016). Therefore, epilepsy is also explained as the brain network disruption (Kalitzin et al., 2019). Electroencephalography (EEG) is a common examination in patients with epilepsy (Chen and Koubeissi, 2019). Hyperventilation (HV) is always the EEG “activation procedure”, which can strengthen preexisting EEG abnormalities (Baldin et al., 2017). Previous studies demonstrated that HV significantly increased abnormal discharge and functional connectivity in the delta and theta bands in the epilepsy patients (Mazzucchi et al., 2017). Under the situation of COVID-19, patients with epilepsy were often required to wear face mask during EEG examinations, including HV procedure (Assenza et al., 2020). Of note, Lazzarino et al. found in the healthy individuals that the breathing frequency and depth were increased and more CO2 was inhaled when they breathed with face mask (Lazzarino et al., 2020). However, whether unfavorable impact of HV with face mask on cortical excitability and brain network awaits to be investigated, which has strong implications for clinical practice especially for patients with epilepsy.
Graph theory has been recently used to characterize complex brain networks. Epileptic networks of EEG data is typically suitable within the graph theory framework. In the present study, we employed the analysis method of graph theory of EEG data to characterize brain network profile of patients with epilepsy during HV with face mask, including network density, small-world properties, path length, clustering coefficient. Network density is ratio of the number of edges present in the network to total possible number of edges (Watson et al., 2018). Small-world properties are associated with low wiring cost and rapid information propagation in the brain (Buzsáki et al., 2004). Characteristic path length reflects the average minimum distance between two nodes and efficiency of the network (Huang et al., 2020). The clustering coefficient reflects the density metric for whole networks (Wang et al., 2018). These metrics can provide valuable information about brain excitability.
Our primary goal is to identify the effects of HV with face mask on cortical excitability in patients with epilepsy. We therefore set out to conduct two experiments to examine stimulation induced response of the brain and the interaction of Mask*HV*Epilepsy. This will provide a novel insight for patients with epilepsy at great risk for seizures.
2. Material and methods
2.1. Participants
Epilepsy participants were recruited between April 2020 and June 2020 from the epileptic center at Xuanwu Hospital of Capital Medical University. The inclusion criteria were as follows: (1) ages 18∼65 years old; (2) diagnosis of epilepsy was accomplished by two neurologists; (3) history of epilepsy ≥1month; (4) did not take medication or had taken antiepileptic drugs regularly for more than 1 month. Normal controls (NC) were recruited in the community through advertising and met the following criteria: (1) no history of head trauma or neuropsychiatric disorders; (2) ages 18∼65 years old. The exclusion criteria for patients and NC were as follows: (1) psychogenic non-epileptic seizure; (2) evidence of progressive brain disorders or systemic diseases other than epilepsy; (3) drug addiction; (4) breastfeeding or pregnancy. All participants provided written informed consent for this research and publication. The study was approved by the Xuanwu Hospital Ethics Committee and was in accordance with the Declaration of Helsinki. All research materials were available to any qualified investigator. Requests for study materials and data should be submitted to Dr. Hua Lin.
2.2. Experimental procedures
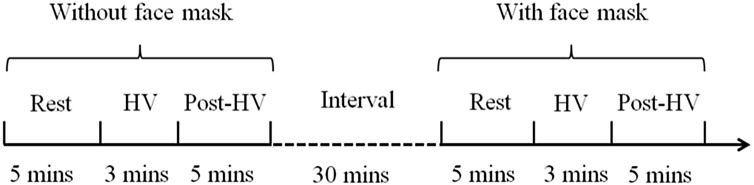
All individuals underwent two HV experiment, including HV with face mask (HV+) and HV without a mask (HV). The interval was 30 min and the sequence was random. Each experiment consisted of three segments: resting EEG, EEG of HV, and EEG of post-HV (Fig. 1 ). EEG were recorded successively during each experiment. Participants were asked to evaluate discomfort when every HV is completed, using a 10 point questionnaire which ‘0 point: without any discomfort’ and ‘10 point: maximal discomfort that I can imagine’.
Fig. 1.
Schematic representation of experimental design. HV, hyperventilation.
2.3. EEG data recording and preprocessing
EEG signals were recorded from 32 scalp electrodes (21 recording channels: Fp1, Fpz, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, Oz and O2) using an EEG amplifier (Yunshen, Beijing, China) and EasyCap (Greentek, Wuhan, China). Electrodes were placed in accordance with the international 10–20 system. EEG signals were continuously recorded at a sampling frequency of 1,024 Hz and band-pass filtered from 0.05 to 100 Hz. Interelectrode impedance was kept below 5 kΩ. EEG was referenced to the average of the two earlobe-electrodes. One electrode was put on the nose as ground. Electrooculogram electrodes were positioned above and below the left eye as well as the outer canthi of each eye. None of the patients with epilepsy was taking medication at the time of the recordings.
Data preprocessing was completed with EEGLAB version 14.0.0.0b (Delorme and Makeig, 2004), which was an open source toolbox for Matlab (Mathworks, MA, USA). Data were re-referenced using the Reference Electrode Standardization Technique (REST) that were proved to be efficient in recovering the waveform and the spectral properties of the potential referenced at infinity (Yao, 2001). Data were low-pass filtered at 30 Hz to exclude high-frequency noise including muscle activities. EEG was inspected visually for the presence of artifacts. Eye blink artifacts and muscle artifacts were removed using an independent component analysis approach. For all participants, we selected 120 s of artifact-free EEG data and segmented them into 2 s epochs (2048 sample points per epoch). EEG data from the patients with epilepsy were extracted from interictal EEG recordings, regardless of abnormal discharges. EEG epochs with artifacts (>±100 μV) were excluded. Besides, artifacts that exceeded 5 standard deviations of all channels were also excluded. Finally, we selected the maximum number of artifact-free epochs for each participants for the following analysis. Functional connectivity analysis and computation of graph theory parameters were in the Supplement Material.
2.4. Statistical analysis
To compare the group differences in the topological properties of brain functional network, repeated-measures analysis of variance (ANOVA) was performed to test the significant difference of the mean Phase locking value (PLV) and the graph parameters (C, L and σ), between two factors of Mask (without mask vs with mask) and two conditions (resting vs HV), as well as two group (controls vs patients) for each frequency band. The analysis of the graph parameters were performed at each value of K. Repeated-measures ANOVA was performed for the discomfort ratings between two factors of Mask (without mask vs with mask) as well as between two groups (controls vs patients)
For all the analyses in this study, p values less than 0.05 (corrected for multiple comparisons) were considered significant. The simple-effects analysis was performed if any interaction between factors was found. All statistical analyses were conducted using SPSS 22.0.
3. Results
3.1. Demographic information and behavioral results
We recruited 19 patients with epilepsy and 17 NC in this study, including 8 females in the patients and 9 females in the controls. Detailed information is summarized in Table 1 . No significant difference was found in age and gender between the patients and the controls using independent t-tests (all p > .05).
Table 1.
Demographic information of the patients with epilepsy and the healthy controls as well as the discomfort ratings (mean ± std.) by the two groups.
| Controls (n = 17) | Patients (19) | t/F, p | |
|---|---|---|---|
| Female/Male | 9/8 | 8/11 | t(34)=-0.636, p = .529 |
| age (years) | 36.5 ± 9.3 | 32.7 ± 11.9 | t(34) = 1.072, p = .291 |
| discomfort ratings under the HV without the masks | 3.1 ± 2.0 | 3.8 ± 2.0 | F(1,34) = 68.463, p < .001, η2 = .668 |
| discomfort ratings under the HV with the masks | 4.7 ± 2.3 | 5.8 ± 1.7 |
All participants wearing the face masks felt more discomfort. There were significantly higher scores in the discomfort ratings under the condition with the face masks (5.4 ± 2.1) than condition without the masks (3.6 ± 2.1) (F(1,34) = 68.463, p < .001, η2 = .668). The discomfort symptoms included dizziness, numbness of the head or limb, and difficulty of expiration.
There was no difference in the routine EEG findings in individuals with mask and without mask.
3.2. Overview of the graphical parameters in different network densities
We firstly examined the clustering coefficient, path length and small-world network in different network densities in resting functional networks (Supplementary Figure). For epilepsy and NCs group, the clustering coefficient within the delta and theta bands were increased as the network density upgraded The path length within both bands were increased in the spare network (from K = 0.05 to K = 0.1) and then were gradually declined in the dense networks. The characteristics of small-world networks were evident for both groups, specifically when the network density was sparse (K = 0.05, 0.1).
Before we performed between-group comparisons, the interaction analysis of Mask*Group, HV*Group and Mask*HV*Group in different network densities (K) were necessary (Supplementary Table). Significant interactive effects of HV and epilepsy were found for the small-worldness index in the delta band (K = 0.05, F(2,68) = 5.593, p = .006,η2 = .141) and in the theta band(K = 0.05, F(2,68) = 4.195, p = .019, η2 = .110; K = 0.15, F(2,68) = 3.832, p = .026, η2 = .101). Moreover, the three-way interactions of Mask*HV*Group were also significant for the clustering coefficient in the delta band (K = 0.15, F(2,68) = 6.250, p = .003,η2 = .155), for the path length (K = 0.10, F(2,68) = 4.329,p = .017,η2 = .113) and for the small-worldness index (K = 0.25, F(2,68) = 3.571,p = .034,η2 = .095) in the theta band. The significant interaction results indicated that the effect of HV and HV + was greater in patients with epilepsy.
3.3. Difference on in the resting functional networks between the two groups
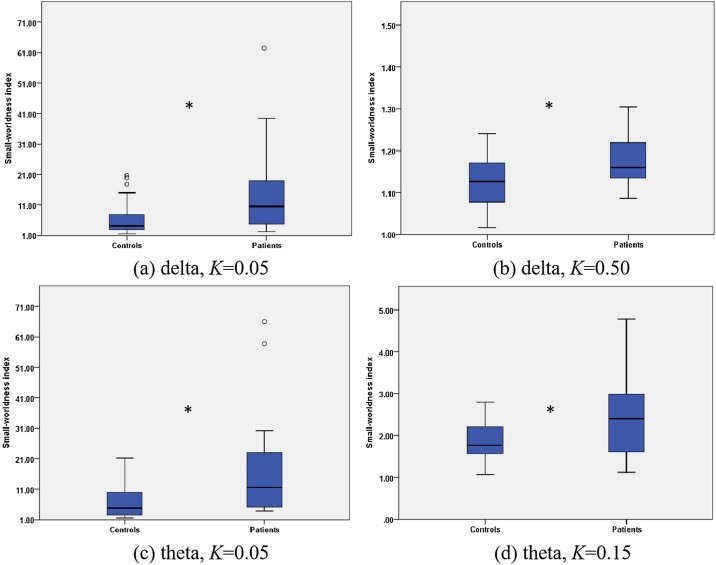
We also observed the resting functional networks of the patients and the controls. The small-worldness index in the controls was smaller than that in the patients in the delta band as shown in Fig. 2 (a) and (b) (K = 0.05: t(34)=-2.144, p = .042; K = 0.5: t(34)=-2.596, p = .014). The same results were also obtained in the theta band as shown in Fig. 2(c) and (d) (K = 0.05: t(34)=-2.367, p = .027; K = 0.15: t(34)=-2.312, p = .029).
Fig. 2.
The difference of the brain network under the resting state between the patients and the controls. (a) The small-worldness index in the controls was smaller than that in the patients in the delta band when the network density (K) was 0.05 as well as when K was 0.50 (b). (c) The same results were also obtained in the theta band as K was 0.05 as well as K was 0.15 (d). * denotes P < 0.05.
3.4. Effect of HV on the functional networks
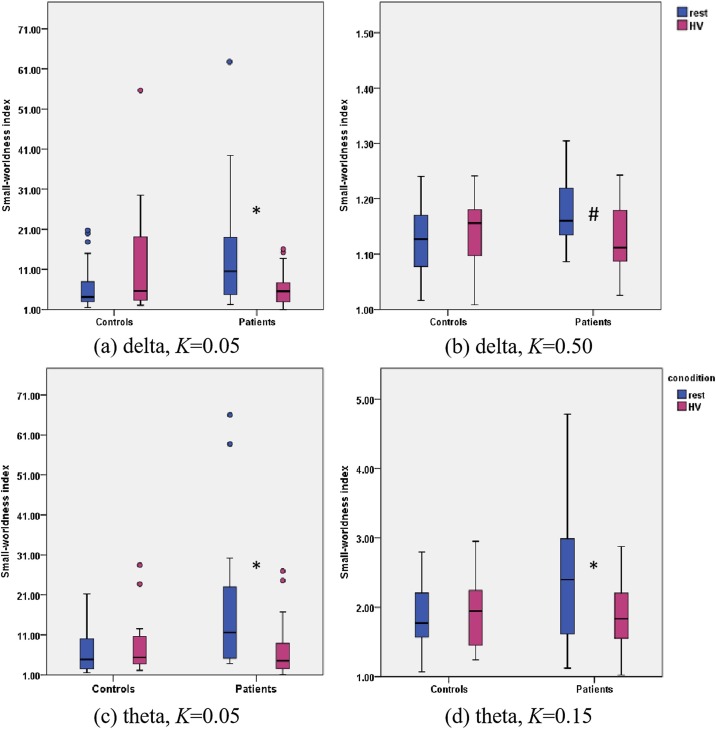
We then further detected the effect of HV on the small-worldness index within the delta and theta bands (Fig. 3 ). In the patient group, the small-worldness index decreased significantly under HV in the delta band in K = 0.05 (Fig. 3(a)) and in K = 0.50 (Fig. 3(b)). Decreased small-worldness index by HV were also found in the theta band in K = 0.05 (Fig. 3(c)) and in K = 0.15 (Fig. 3(d)). Nevertheless, no difference was found in the control group.
Fig. 3.
The HV condition affected the brain networks of the patients within the delta and theta bands. (a) In the patient group, the HV condition significantly impaired the small-worldness index of the brain network relative to the resting state within the delta band as K was 0.05. The HV condition did not affect the brain networks of the controls. (b) As K was 0.50, the HV condition impaired the small-worldness index of the brain networks in the patient group relative to the resting state at trend level. No influence was found for the controls. (c) and (d) In the theta band, the HV condition also impaired the small-worldness index of the brain networks of the patients relative to the resting state as K was 0.05 and 0.15, respectively. * denotes P < 0.05. # denotes P < 0.10. HV, hyperventilation.
3.5. Interaction of mask by HV on the functional networks
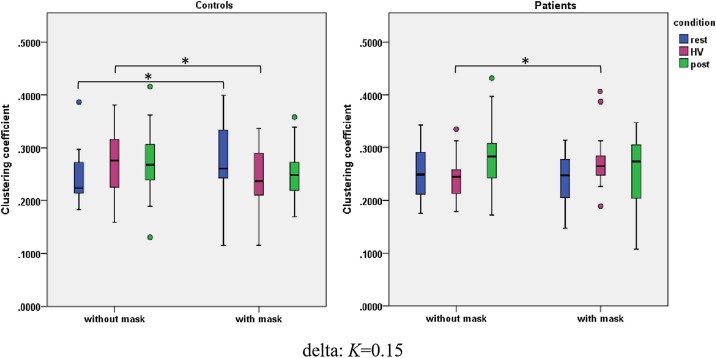
The interaction of Mask*HV were found both in the patient group and in the NC group (Fig. 4 ). HV + increased the clustering coefficient in the patients (F(1,18) = 7.664, p = .013, η2 = .299). In contrast, HV + decreased the clustering coefficient in the NCs (F(1,16) = 5.058, p = .039, η2 = .240).
Fig. 4.
Wearing the face masks affected the clustering coefficient of the brain networks within the delta band (K = 0.15) for the control and patient groups. The controls wearing the face masks increased the clustering coefficient relative to the case without the face masks under the resting state. Moreover, the controls wearing the face masks impaired the clustering coefficient relative to the case without the face masks under the HV condition. The patients wearing the face masks increased the clustering coefficient relative to the case without the face masks under the HV condition. * denotes P < 0.05. HV, hyperventilation.
4. Discussion
Under the COVID-19pandemic, it is important to clarify the effects of HV + on cortical excitability in patients with epilepsy. We found that HV + significantly increased the clustering coefficient in the patients with epilepsy. Importantly, the three-way of Mask*HV*Epilepsy significantly interact in the clustering coefficient in the delta band, as well as in the path length and the small-worldness index in the theta band (Table 2 ). These findings have been observed for the first time in the patients of epilepsy and indicated the abnormal high excitability of brain network under HV+, which suggested the increased risk of seizures in the patients of epilepsy. It is suggested in the clinical practice the behaviour of HV + remain cautious for the individuals with epilepsy.
Table 2.
The clustering coefficient of K = 0.15 within th delta band for the patient and the controls during the resting, HV and post-HV with or without the mask.
| clustering coefficient | without mask | with mask |
|---|---|---|
| NC | ||
| rest * | 0.224 ± 0.048 | 0.278 ± 0.068 |
| HV * | 0.274 ± 0.060 | 0.244 ± 0.059 |
| post-HV | 0.275 ± 0.065 | 0.254 ± 0.054 |
| Patient | ||
| rest | 0.255 ± 0.050 | 0.245 ± 0.046 |
| HV * | 0.241 ± 0.040 | 0.275 ± 0.049 |
| post-HV | 0.283 ± 0.068 | 0.256 ± 0.064 |
Note: * means significant effect of Mask on the resting and the HV conditions.
NC, normal controls; HV, hyperventilation.
Previous studies reported the discomfort symptoms of participants during HV such as dizziness, paraesthesias and headaches (Kane et al., 2014). We observed that the participants felt more serious dizziness and paraesthesias under HV + . Person et al. found that dyspnea variation was significantly higher when the individuals wearing surgical face mask executed the pulmonary function test (Person et al., 2018). HV enhances the air exchange, which leads to decrease the partial pressure of carbon dioxide and increase the partial pressure of oxygen (Keir et al., 2018). Granados et al. provided preliminary evidence that some subjects exhibited severe hypoxia under HV+ (Granados et al., 2016). As such, serious dizziness and paraesthesias under HV + maybe related with hypoxia.
The brain networks features of epileptic discharges were strongly relevant to small-world properties (Ni et al., 2014). Indeed, our data confirmed previous results that the patients with epilepsy showed higher small-worldness index compared with NC in the rest state. We further demonstrated that HV + significantly increased the clustering coefficient of the patients with epilepsy. Bernhardt et al. observed that patients with temporal lobe epilepsy showed increased clustering coefficient, altered distributions of hubs, and vulnerability (Bernhardt et al., 2011). Recently, Goodale et al. also demonstrated in patient of epilepsy that the higher clustering coefficient was, the greater decay of functional connectivity with distance was (Goodale et al., 2020). Increased clustering coefficient is thought to represent abnormal excitable brain network. It should be noted for epilepologists and patients that HV + can excite the brain network in patients with epilepsy and it is likely also the driver of seizures.
The excitable effect of HV on brain network was related to its physiological mechanism. Physiological research has demonstrated HV induced respiratory alkalosis with a drop of the PaCO2 by18 mmHg and an increase of pH (Sakamoto et al., 2018, 2015). HV + aggravated hypocapnia, which could further worsen cerebral ischemic anoxia. The research of hypercapnic challenge (5% CO2) confirmed that hypoxia worsened vasoconstriction and reduced cerebral blood flow (Lawley et al., 2017). The hemodynamic response during hypercapnia might be relative to the higher excitability of brain network (Savelov et al., 2013). For the patients with epilepsy, a decrease in the efficiency of brain network connection was significant under the condition of hypoxia (Brenner, 2005). Besides, the modification of connectivity throughout the brain due to hypoxia was more likely related to the effect of blood gas changes on the NMDA receptor (Wang et al., 2000).Previous study had been demonstrated the NMDA receptors activity related to H+ (Cechetto, 2014). The activation of these receptors may be involved in the node degree of cortical origin and the alteration of brain connectivity (Wakhloo et al., 2020). This could mean that the excitable effects of HV + on brain networks can be further magnified especially in patients with epilepsy.
There are several limitations in this research, some of which may merit future investigation. The first limitation of our study is the small sample size. Although our study tried to provide a new insight for the effects of HV + on brain network in patients with epilepsy, the small sample sizes still require future study. We look forward to increasing the sample size to examine the reproducibility of the results. Secondly, we didn’t monitor the levels of PaO2 and PaCO2 in this work due to limited time and funding, which were sensitive indexs for detecting hypoxia. We will add those examination in the subsequent study. Thirdly, we did not rule out the effect of antiepileptic drugs on cortical excitability and brain connectivity in patients. Notwithstanding, the purpose of the present study was to investigate the brain network influenced by HV + and not by the disease. In this sense, the effect of antiepileptic drugs on cortical excitability is somehow unimportant. Last but not least, there is no EEG recording in the interval of 30 min between HV and HV + . Although we did not observe how long routine EEG regained normal after HV+, the EEG was similar to baseline level after the interval of 30 min. It is better in the future to record the EEG during the whole process.
5. Conclusion
The present results provide evidence that the excitable effects of HV + on brain network can be further magnified in patients with epilepsy. Furthermore, this work revealed that the three-way of Mask*HV*Epilepsy significantly interact. This finding is particularly significant during the COVID-19 pandemic in order to minimize the adverse effects of HV + behavior for patients with epilepsy.
Author contributions
HL contributed to the conception of the study; PS and SL performed the experiments; PS and DC performed the data analyses and wrote the manuscript; HL, RW and YW helped perform the analysis with constructive discussions.
Funding
The work was supported in part by the National Natural Science Foundation of China (No. 82001388, 81501119, 81771398, 81771909, 81871438), the National Key Research and Development Program of China (No. 2019YFC0121200, 2019YFC0121202, 2019YFC0121203, 2018YFC1314500), the China postdoctoral Science Foundation (No. 2019M660720) and the Beijing Postdoctoral Research Foundation (No. 2020-ZZ-014).
Availability of data and material
De-identified data will be shared on reasonable request with any qualified investigator.
Ethics approval
The study has been approved by the local ethics review board of Xuanwu Hospital and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
All patients gave their written informed consent before entering the study.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
The authors wish to thank all participants and their caregivers for supporting this study. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eplepsyres.2021.106741.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Alexander A., Maroso M., Soltesz I. Organization and control of epileptic circuits in temporal lobe epilepsy. Prog. Brain Res. 2016;226:127–154. doi: 10.1016/bs.pbr.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi Pooya A.A., Cross J.H. Is wearing a face mask safe for people with epilepsy? Acta Neurol. Scand. 2020;142(4):314–316. doi: 10.1111/ane.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assenza G., Lanzone J., Ricci L., Boscarino M., Tombini M., Galimberti C.A., Alvisi L., Tassi L., Broglia L., Di Lazzaro V., Mecarelli O. Electroencephalography at the time of Covid-19 pandemic in Italy. Neurol. Sci. 2020;41:1999–2004. doi: 10.1007/s10072-020-04546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldin E., Hauser W.A., Buchhalter J.R., Hesdorffer D.C., Ottman R. Utility of EEG activation procedures in epilepsy: a population-based study. J. Clin. Neurophysiol. 2017;34:512–519. doi: 10.1097/WNP.0000000000000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J.R. COVID-19 and the nervous system. J. Neurovirol. 2020;26:143–148. doi: 10.1007/s13365-020-00840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt B.C., Chen Z., He Y., Evans A.C., Bernasconi N. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb. Cortex. 2011;21:2147–2157. doi: 10.1093/cercor/bhq291. [DOI] [PubMed] [Google Scholar]
- Brenner R.P. The interpretation of the EEG in stupor and coma. Neurologist. 2005;11:271–284. doi: 10.1097/01.nrl.0000178756.44055.f6. [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Geisler C., Henze D.A., Wang X.J. Interneuron Diversity series: circuit complexity and axon wiring economy of cortical interneurons. Trends Neurosci. 2004;27:186–193. doi: 10.1016/j.tins.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Cechetto D.F. Cortical control of the autonomic nervous system. Exp. Physiol. 2014;99:326–331. doi: 10.1113/expphysiol.2013.075192. [DOI] [PubMed] [Google Scholar]
- Chen H., Koubeissi M.Z. Electroencephalography in epilepsy evaluation. Continuum (Minneap Minn) 2019;25:431–453. doi: 10.1212/CON.0000000000000705. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Esposito S., Principi N., Leung C.C., Migliori G.B. Universal use of face masks for success against COVID-19: evidence and implications for prevention policies. Eur. Respir. J. 2020:55. doi: 10.1183/13993003.01260-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale S.E., González H.F.J., Johnson G.W., Gupta K., Rodriguez W.J., Shults R., Rogers B.P., Rolston J.D., Dawant B.M., Morgan V.L., Englot D.J. Resting-state SEEG may help localize epileptogenic brain regions. Neurosurgery. 2020;86:792–801. doi: 10.1093/neuros/nyz351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados J., Gillum T.L., Castillo W., Christmas K.M., Kuennen M.R. “Functional” respiratory muscle training during endurance exercise causes modest hypoxemia but overall is well tolerated. J. Strength Cond. Res. 2016;30:755–762. doi: 10.1519/JSC.0000000000001151. [DOI] [PubMed] [Google Scholar]
- Huang Y.A., Dupont P., Van de Vliet L., Jastorff J., Peeters R., Theys T., Loon J., Van Paesschen W., Van den Stock J., Vandenbulcke M. Network level characteristics in the emotion recognition network after unilateral temporal lobe surgery. Eur. J. Neurosci. 2020;52:3470–3484. doi: 10.1111/ejn.14849. [DOI] [PubMed] [Google Scholar]
- Kalitzin S., Petkov G., Suffczynski P., Grigorovsky V., Bardakjian B.L., Lopes D.S.F., Carlen P.L. Epilepsy as a manifestation of a multistate network of oscillatory systems. Neurobiol. Dis. 2019;130:104488. doi: 10.1016/j.nbd.2019.104488. [DOI] [PubMed] [Google Scholar]
- Kane N., Grocott L., Kandler R., Lawrence S., Pang C. Hyperventilation during electroencephalography: safety and efficacy. Seizure. 2014;23:129–134. doi: 10.1016/j.seizure.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Keir D.A., Pollock M., Thuraisingam P., Paterson D.H., Heigenhauser G., Rossiter H.B., Kowalchuk J.M. Slow V˙O(2) kinetics in acute hypoxia are not related to a hyperventilation-induced hypocapnia. Respir. Physiol. Neurobiol. 2018;251:41–49. doi: 10.1016/j.resp.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Lawley J.S., Macdonald J.H., Oliver S.J., Mullins P.G. Unexpected reductions in regional cerebral perfusion during prolonged hypoxia. J. Physiol. 2017;595:935–947. doi: 10.1113/JP272557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarino A.I., Steptoe A., Hamer M., Michie S. Covid-19: important potential side effects of wearing face masks that we should bear in mind. BMJ. 2020:m2003. doi: 10.1136/bmj.m2003. [DOI] [PubMed] [Google Scholar]
- Liu X., Zhang S. COVID-19: face masks and human-to-human transmission. Influenza Respir. Viruses. 2020;14:472–473. doi: 10.1111/irv.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madabhavi I., Sarkar M., Kadakol N. COVID-19: a review. Monaldi Arch. Chest Dis. 2020:90. doi: 10.4081/monaldi.2020.1298. [DOI] [PubMed] [Google Scholar]
- Mazzucchi E., Vollono C., Losurdo A., Testani E., Gnoni V., Di Blasi C., Giannantoni N.M., Lapenta L., Brunetti V., Della Marca G. Hyperventilation in patients with focal epilepsy. J. Clin. Neurophysiol. 2017;34:92–99. doi: 10.1097/WNP.0000000000000329. [DOI] [PubMed] [Google Scholar]
- Ni Y., Wang Y., Yu T., Li X. Vol. 2014. 2014. pp. 1–6. (Analysis of Epileptic Seizures with Complex Network: Computational and Mathematical Methods in Medicine). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person E., Lemercier C., Royer A., Reychler G. Effect of a surgical mask on six minute walking distance. Rev. Mal. Respir. 2018;35:264–268. doi: 10.1016/j.rmr.2017.01.010. [DOI] [PubMed] [Google Scholar]
- Ren Z., Wang H., Cui G., Lu H., Wang L., Luo H., Chen X., Ren H., Sun R., Liu W., Liu X., Liu C., Li A., Wang X., Rao B., Yuan C., Zhang H., Sun J., Chen X., Li B., Hu C., Wu Z., Yu Z., Kan Q., Li L. Alterations in the human oral and gut microbiomes and lipidomics in COVID-19. Gut. 2021;70(7):1253–1265. doi: 10.1136/gutjnl-2020-323826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto A., Naito H., Chow C.M. Hyperventilation-induced respiratory alkalosis falls short of countering fatigue during repeated maximal isokinetic contractions. Eur. J. Appl. Physiol. 2015;115:1453–1465. doi: 10.1007/s00421-015-3134-8. [DOI] [PubMed] [Google Scholar]
- Sakamoto A., Naito H., Chow C.M. Effects of hyperventilation on repeated pedaling sprint performance: short vs. long intervention duration. J. Strength Cond. Res. 2018;32:170–180. doi: 10.1519/JSC.0000000000001789. [DOI] [PubMed] [Google Scholar]
- Savelov A.A., Petrovskii E.D., Karamamed-Ogly E.S., Shtark M.B. Functions of the hemodynamic response during hypercapnia. Functional MRI study. Bull. Exp. Biol. Med. 2013;155:1–5. doi: 10.1007/s10517-013-2065-9. [DOI] [PubMed] [Google Scholar]
- Sun J., He W., Wang L., Lai A., Ji X., Zhai X., Li G., Suchard M.A., Tian J., Zhou J., Veit M., Su S. COVID-19: epidemiology, evolution, and cross-disciplinary perspectives. Trends Mol. Med. 2020;26:483–495. doi: 10.1016/j.molmed.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunjaya A.P., Jenkins C. Rationale for universal face masks in public against COVID ‐19. Respirology. 2020;25:678–679. doi: 10.1111/resp.13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakhloo D., Scharkowski F., Curto Y., Javed B.U., Bansal V., Steixner-Kumar A.A., Wüstefeld L., Rajput A., Arinrad S., Zillmann M.R., Seelbach A., Hassouna I., Schneider K., Qadir I.A., Werner H.B., Martens H., Miskowiak K., Wojcik S.M., Bonn S., Nacher J., Nave K.A., Ehrenreich H. Functional hypoxia drives neuroplasticity and neurogenesis via brain erythropoietin. Nat. Commun. 2020;11:1313. doi: 10.1038/s41467-020-15041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chiou A.L., Jeng C.H., Yang S.T., Lin J.C. Ethanol potentiates dopamine release during acute hypoxia in rat striatum. Pharmacol. Biochem. Behav. 2000;66:679–685. doi: 10.1016/s0091-3057(00)00224-0. [DOI] [PubMed] [Google Scholar]
- Wang M.Y., Lu F.M., Hu Z., Zhang J., Yuan Z. Optical mapping of prefrontal brain connectivity and activation during emotion anticipation. Behav. Brain Res. 2018;350:122–128. doi: 10.1016/j.bbr.2018.04.051. [DOI] [PubMed] [Google Scholar]
- Watson C.G., Stopp C., Newburger J.W., Rivkin M.J. Graph theory analysis of cortical thickness networks in adolescents with d-transposition of the great arteries. Brain Behav. 2018;8:e00834. doi: 10.1002/brb3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D. A method to standardize a reference of scalp EEG recordings to a point at infinity. Physiol. Meas. 2001;22:693–711. doi: 10.1088/0967-3334/22/4/305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data will be shared on reasonable request with any qualified investigator.