Abstract
Many countries had to suspend their colorectal cancer (CRC) screening programme as a result of the COVID-19 pandemic. This eventually may lead to postponed diagnoses of premalignant lesions and CRC, resulting in increased incidence or more advanced CRCs rates. This study aimed to assess the impact of the COVID-19 pandemic on incidence and stage distribution of CRCs in the Netherlands, by monitoring CRC diagnoses and stage distribution in the months before, during and after the first COVID-19 wave. Data on incidence and stage distribution of CRCs of individuals aged 55–75 years in 25 hospitals in the Netherlands were extracted from the Netherlands Cancer Registry. The observed incidence after the suspension (March 2020–December 2020) was compared to the expected incidence in the same period. In the period April to June 2020, we observed the largest decrease in the total incidence of CRC. We found that 48% of the decrease was due to stage I, 23% due to stage II, 23% due to stage III and 5% due to stage IV. After gradually resuming screening mid May 2020, we observed an increase in CRC diagnoses from July 2020 onwards. As of October 2020, the observed number of diagnoses was higher than the expected number. As the decrease was mainly limited to stage I CRCs, it seems that the temporary suspension of the CRC screening programme due to the COVID-19 pandemic will have a minimal long-term impact on stage distribution and CRC mortality.
Keywords: Colorectal cancer screening, Covid-19, Suspension, Stage distribution, Incidence
1. Introduction
In an endeavour to reduce the burden of colorectal cancer (CRC), many countries implemented a CRC screening programme [1,2]. The ultimate goal of CRC screening programmes is to reduce the CRC morbidity and mortality [3,4]. Countries with a long history of successful screening programmes (i.e. high coverage and participation) have indeed been effective in reducing CRC-related incidence and mortality by detecting premalignant lesions and CRC in an early stage [5]. Achieving these CRC screening goals have become challenging in a pandemic-stricken world. Many countries, had to suspend their CRC screening programmes, which eventually may lead to postponed diagnoses of the premalignant lesions and CRCs, compromising the incidence and mortality reduction in the long-term [6,7].
During the first COVID-19 wave, several countries observed a decrease in cancer detection, as a result of suspension of screening, as well as individuals with symptoms avoided or delayed seeking medical care [6,[8], [9], [10], [11]]. The nationwide faecal immunochemical testing (FIT)-based CRC screening programme in the Netherlands was suspended from March till May 2020. Informed by a modelling study, the Dutch government decided to first invite those in the backlog by temporarily increasing the screening interval and expand the screening colonoscopy capacity up to 120%.
A previous Dutch study showed that the COVID-19 pandemic had the largest impact on incidence of CRC among those eligible for screening [12]. But long-term impact on the CRC incidence of the chosen restart CRC screening strategy in the Netherlands is unknown. This study aimed to assess the impact of the COVID-19 pandemic on incidence and stage distribution of CRC in the Netherlands.
2. Materials
2.1. Study period
In the Netherlands, the first COVID-19 case was confirmed in the last week of February. The National Institute of Public Health and Environment (RIVM) decided to stop inviting individuals for CRC screening from March 16, 2020. In the following two weeks individuals were still able to return their FIT and/or undergo a colonoscopy in case of a positive FIT. By the end of March, individuals that already received an invitation were informed to not return their FIT, but wait until further notice. Two months later, in mid-May 2020, the screening invitations were gradually resumed, starting with re-inviting the backlog, prioritising those eligible for subsequent screening round (i.e. second, third, and fourth rounds). June 1, 2020, new invitations were sent to the target population.
2.2. Dataset and study population
Data on incidence and stage distribution were extracted from the Netherlands Cancer Registry (NCR). The NCR registers all newly diagnosed malignancies in the Netherlands. TNM classification 8th edition was used for staging of the tumours, and topography and morphology was classified according to the International Classification of Disease for Oncology [13,14]. For this study, we included patients who were diagnosed with histologically confirmed CRC in the period 2018–2020. To assess the impact of the suspension of CRC screening, we only selected individuals eligible for screening, that is, individuals aged 55–75 years. This was regardless of the diagnostic path, because method of detection (screening or symptoms) was not registered in the NCR. Data on number of CRC diagnoses and stage distribution were available from 25 out of 70 hospitals in the Netherlands: 10 general hospitals, 11 top clinical hospitals and 4 academic hospitals. These 25 hospitals were a representative sample of all hospitals and evenly distributed over the Netherlands; good generalisability of the study is to be expected. Data on stage was available for CRCs diagnosed up to October 2020.
2.3. Outcomes and statistical analysis
In this study we evaluated the monthly number of overall and stage-specific CRC diagnoses. We compared the observed incidence after the suspension (March 2020–December 2020) to the expected incidence in the same period. The expected incidence was based on a trend line extrapolating the incidence prior to the suspension (January 2018–February 2020). The trend line was generated using a regression line, which was fitted to the natural logarithm of the rates. Total incidence as well as stage-specific incidences were compared. Data management and analysis were performed using STATA version 16.1.
3. Results
3.1. CRC incidence
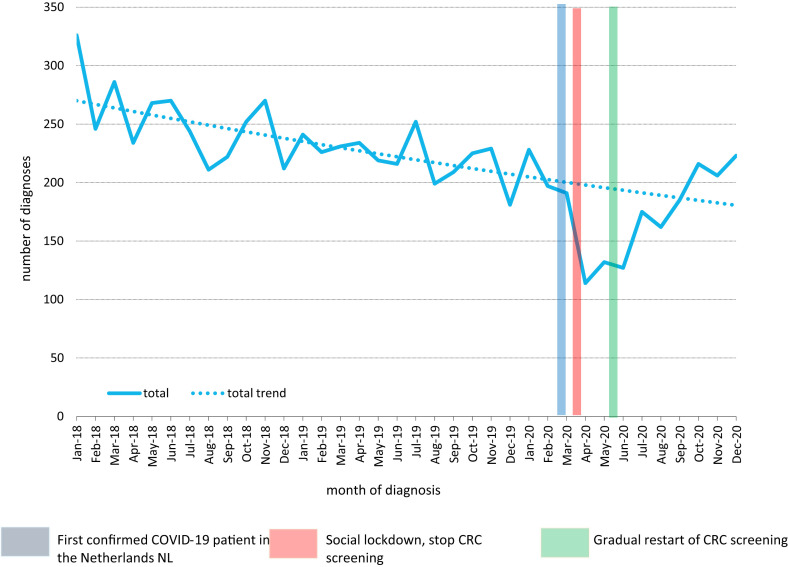
Between 2018 and 2020, 7859 CRCs were detected in the 25 hospitals. Before the pandemic a decrease in CRC incidence was observed; from 326 CRC diagnoses in January 2018 to 191 CRC diagnoses in March 2020. In the period from April to June 2020, we observed the largest decrease in the total incidence of CRC, with the largest drop in incidence in the month after suspending the CRC screening programme. Comparing the number of observed CRC diagnoses to what was expected, we observed a decrease of diagnoses of 42% in April 2020, 33% in May 2020 and 34% in June 2020 (Fig. 1 ). After gradually resuming screening mid-May 2020, we observed a catch-up in CRC diagnoses from July 2020 onwards. As of October 2020, the observed number of diagnoses was higher than the expected number, with the largest increase of 24% beyond the expected incidence rate in December 2020.
Fig. 1.
Observed number of colorectal cancer diagnoses compared to the expected number of colorectal cancer diagnoses (trend line) in patients aged 55–75 years from 2018 to 2020.
3.2. CRC staging
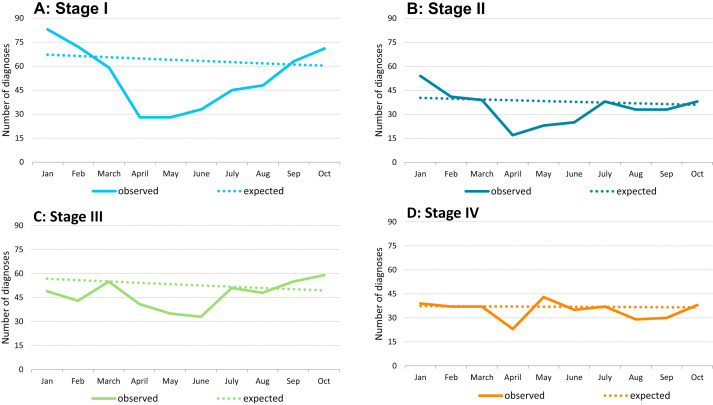
Examining the contribution of each of the stages to the total drop in incidence in the period from April to June 2020, we found that 48% of the decrease was due to stage I, 23% due to stage II, 23% due to stage III and 5% due to stage IV. Fig. 2A-D show the observed number of diagnoses versus the expected number of diagnoses by stage. The largest decrease in incidence was observed for stage I CRCs; compared to the expected there were 57% fewer diagnoses in April 2020 and 56% fewer diagnoses in May 2020 (Fig. 2A). From July 2020 onwards, the incidence rates for stage I CRCs increased. By October 2020, we observed 18% more diagnoses than expected. For stage II CRCs 56% fewer diagnoses were observed in April 2020, and an upward trend was observed from May 2020 onwards (Fig. 2B). From July 2020 onwards, the incidence rates for stage II CRCs was similar to the expected incidence rate. For stage III CRCs, a smaller decrease in incidence was observed with 24% and 34% fewer diagnoses compared to the expected, in April and May 2020, respectively (Fig. 2C). By October 2020, we observed 19% more diagnoses than expected. The number of stage IV diagnoses only dropped in April 2020, with a decrease of 38%, followed by a small increase of 16% to what was expected in May 2020 (Fig. 2D). From June onwards the observed number was quite similar to the expected number. When considering the full period during and after the suspension of the CRC screening programme (March until October 2020), we observed a large difference in the relative decrease of CRC diagnoses compared to what was expected: for stage I, the observed number of diagnoses in this period was 25% lower than expected. These percentages were 18%, 10% and 8% for respectively stage II, III and IV.
Fig. 2.
A–D: Observed number of colorectal cancer diagnoses compared to the expected number of colorectal cancer diagnoses by stage in patients aged 55–75 years.
4. Discussion
As a result of the first COVID-19 wave in the Netherlands, we observed a decrease up to 42% in monthly CRC diagnoses compared to what was expected. However, this was partially compensated in the second half of 2020. In the months during and after the first COVID-19 wave, the largest decrease was observed in stage I CRCs, in contrast to stage IV CRCs which remained mostly stable.
We observed the largest reduction in incidence of CRC the month after the suspension of the Dutch CRC screening programme. The most likely explanation for this delayed drop in CRC incidence, is that the already scheduled colonoscopies for individuals with a positive FIT were not cancelled. The drop in CRC incidence in the Netherlands was similar to the observed drop in CRC incidence in Catalonia (Spain) and in a teaching hospital in Paris (France) where a reduction of 38% and 30%, respectively, in the period March till June 2020 were reported [11,15]. The relative reduction in diagnoses of CRC observed in our study was smaller than the reduction in CRC diagnoses in the UK [8]. The reason for this difference is unknown, but might partially be explained by the chosen specified period of time to calculate the incidence of CRC; monthly versus weekly rates. Apart from the calculation method, the observed difference can most likely be explained by several factors; the chosen strategy to stop and restart screening, the willingness of individuals to participate in CRC screening during the COVID-19 pandemic, accessibility of health care in general, and CRC background risk.
In this study, we not only observed a decrease, but also a catch-up in the number of diagnoses already in the second part of 2020. To our knowledge, a catch-up in CRC diagnoses shortly after the restart of the screening programme has not previously been described. Other countries were unable to observe a stabilisation or catch-up effect; in the Paris teaching hospital in France, the number of new cancer cases were still 4% lower than expected in September 2021 [15]. Noteworthy, the catch -up effect in the Netherlands became visible in October 2020. The explanation for this catch-up effect might be associated with the chosen restart strategy of the Dutch CRC screening programme. The Dutch government decided to first invite those in the backlog, predominantly individuals eligible for the subsequent screening round, by increasing the screening interval and expanding the screening colonoscopy capacity up to 120%. This was only possible because the IT system allows for real-time monitoring of colonoscopy capacity and continuously matches the number of invitations to this capacity. A catch-up effect in incidence in the second part of 2020 was not observed in the breast cancer screening programme in the Netherlands, as only a maximum of 80% of the usual capacity was available in 2020 [10]. Another explanation for the substantial number of CRC diagnoses in the second part of 2020, is that the COVID-19 pandemic had no impact on the willingness to participate in FIT screening in the Netherlands [16]. Another potential explanation is the publication by the cancer registries in the Netherlands shortly after Covid-19 impacted the Netherlands, revealing a significant drop in cancer diagnoses. This was accompanied by a call of clinicians and politicians to continue seeking medical care for symptoms. This may have led to a sense of urgency among people with symptoms to visit a physician or for people invited for screening to participate. Rapid publication of significant changes in cancer incidence is possible as the Dutch cancer screening registry is up-to-date and uses national data (i.e. sufficient numbers for reliable estimates).
The impact of suspension of CRC screening differed by CRC stage; reduction in CRC incidence became smaller with increased CRC stage. The largest reduction in stage I CRCs, is in line with our expectations as they are generally asymptomatic and detected through screening [17]. In contrast, individuals with stage IV CRCs, the stage when in generally symptoms are the most apparent, continued to seek medical care. Limited impact of suspending screening on late stage CRCs (III and IV), was shown by a modelling study that predicted that a delay in diagnoses up to 6 months has limited impact on early detection of CRC [18]. Another modelling study predicted a slightly worse scenario; the diagnostic delay in cancer diagnoses, as a result of the fist COVID-19 wave, will result in significant economic losses [19]. Based on our data, the modest reduction in late stage CRCs, we expect these economic losses to be limited. Although the overall reduction in incidence of stage III CRCs was smaller compared to stage I and II CRCs, the incidence was higher than expected in October 2020. This might be due to a catch up in stage III CRC diagnoses as a result of the extra screening capacity. Another explanation might be that stage I or II CRCs have progressed to stage III CRCs, even despite the fact that screening was not cancelled but postponed with a slight diagnostic delay of two and half months. As the total incidence of stage III CRCs was not higher than expected, the first explanation seems most plausible, but this need to be confirmed with long-term data.
This study is the first that showed the impact of COVID-19 and the associated suspension and restart of the CRC screening programme on the incidence and stage distribution. A limitation of our study is data availability of selected number (25) of hospitals in the Netherlands. Still, the selected hospitals are to be expected to be representative for all hospitals in the Netherlands. Another limitation is that we could not identify the method of detection (i.e. screen-detected or symptom-detected) of the CRC patients. Furthermore, it will be interesting to assess whether the extended screening interval may lead to an increase in interval CRCs. These questions can be addressed when more data and additional information of all CRC diagnoses becomes available.
5. Conclusions
In conclusion, a decrease as well as catch-up in CRC diagnoses was observed in the months during and after the suspension of the Dutch CRC screening programme. The reduction was mainly limited to stage I CRCs. Taken together, we hope that the temporary suspension of the CRC screening programme due to the COVID-19 pandemic will have a minimal long-term impact on stage distribution and CRC mortality.
Funding
This study was supported and funded by the Netherland Organisation for Health Research and Development (ZonMw; grant number 10430022010014). We would like to thank the Netherlands Cancer Registry for provision of the data.
Disclaimer
The funder had no role in the data collection, statistical analyses, interpretation of the results and writing the manuscript.
Data availability
The data that support the findings of this study are available on request from the last author.
Ethics approval
The data of this study originated from the Netherlands Cancer Registry (NCR). The NCR supplied the data anonymously, eliminating the requirement for ethical approval.
Contributors
ME was responsible for study coordination and data collection. ET-Z and ME were responsible for writing the first version of the manuscript. ET-Z, IL-V, ME drafted the final manuscript. All authors gave critical revisions on the intellectual content of the manuscript and approved the final manuscript.
COVID and Cancer-NL consortium members
Dr. J.C. van Hoeve, Prof.dr. M.A.W. Merkx, Prof.dr. N.J. de Wit, MSc. I. Dingemans, Drs. R. Saathof, Prof.dr. C.H. van Gils, Prof.dr. H.C.P.M. van Weert, and Prof.dr. M. Verheij.
Conflict of interest statement
All authors disclose no conflicts of interest.
References
- 1.Schreuders E.H., Ruco A., Rabeneck L., Schoen R.E., Sung J.J.Y., Young G.P., et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64(10):1637–1649. doi: 10.1136/gutjnl-2014-309086. [DOI] [PubMed] [Google Scholar]
- 2.Basu P., Ponti A., Anttila A., Ronco G., Senore C., Vale D.B., et al. Status of implementation and organization of cancer screening in the European Union Member States—summary results from the second European screening report. Int J Cancer. 2018;142(1):44–56. doi: 10.1002/ijc.31043. [DOI] [PubMed] [Google Scholar]
- 3.Lauby-Secretan B., Vilahur N., Bianchini F., Guha N., Straif K. The IARC perspective on colorectal cancer screening. N Engl J Med. 2018;378(18):1734–1740. doi: 10.1056/NEJMsr1714643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gini A., Jansen E.E.L., Zielonke N., Meester R.G.S., Senore C., Anttila A., et al. Impact of colorectal cancer screening on cancer-specific mortality in Europe: a systematic review. Eur J Cancer. 2020;127:224–235. doi: 10.1016/j.ejca.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso R., Guo F., Heisser T., Hackl M., Ihle P., De Schutter H., et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: an international population-based study. Lancet Oncol. 2021;22(7):1002–1013. doi: 10.1016/S1470-2045(21)00199-6. [DOI] [PubMed] [Google Scholar]
- 6.Del Vecchio Blanco G., Calabrese E., Biancone L., Monteleone G., Paoluzi O.A. The impact of COVID-19 pandemic in the colorectal cancer prevention. Int J Colorectal Dis. 2020;35(10):1951–1954. doi: 10.1007/s00384-020-03635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jonge L., Worthington J., van Wifferen F., Iragorri N., Peterse E.F.P., Lew J Bin, et al. Impact of the COVID-19 pandemic on faecal immunochemical test-based colorectal cancer screening programmes in Australia, Canada, and The Netherlands: a comparative modelling study. Lancet Gastroenterol Hepatol. 2021;6(4):304–314. doi: 10.1016/S2468-1253(21)00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutter M.D., Brookes M., Lee T.J., Rogers P., Sharp L. Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: a National Endoscopy Database Analysis. Gut. 2020;70(3):537–543. doi: 10.1136/gutjnl-2020-322179. [DOI] [PubMed] [Google Scholar]
- 9.Aitken M., Kleinrock M. IQVIA Institute for Human Data Science: Parsippany; NJ: 2020. IQVIA Institute for Human Data Science. Shifts in healthcare demand, delivery and care during the COVID-19 era: tracking the impact in the United States. [Google Scholar]
- 10.Eijkelboom A.H., de Munck L., Lobbes M.B.I., van Gils C.H., Wesseling J., Westenend P.J., et al. Impact of the suspension and restart of the Dutch breast cancer screening program on breast cancer incidence and stage during the COVID-19 pandemic. Prev Med. 2021;151:106602. doi: 10.1016/j.ypmed.2021.106602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coma E., Guiriguet C., Mora N., Marzo-Castillejo M., Benítez M., Méndez-Boo L., et al. Impact of the COVID-19 pandemic and related control measures on cancer diagnosis in Catalonia: a time-series analysis of primary care electronic health records covering about five million people. BMJ Open. 2021;11(5) doi: 10.1136/bmjopen-2020-047567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinmohamed A.G., Visser O., Verhoeven R.H.A., Louwman M.W.J., van Nederveen F.H., Willems S.M., et al. Fewer cancer diagnoses during the COVID-19 epidemic in The Netherlands. Lancet Oncol. 2020;21(6):750–751. doi: 10.1016/S1470-2045(20)30265-5. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brierley J., Gospodarowicz M.D., Wittekind C.T. 8th. Wiley; Oxford, England: 2017. TNM classification of malignant tumors international union against cancer. [Google Scholar]
- 14.International classification of diseases for Oncology. 3rd ed. WHO; 2000. [Google Scholar]
- 15.Kempf E., Lamé G., Layese R., Priou S., Chatellier G., Chaieb H., et al. New cancer cases at the time of SARS-Cov2 pandemic and related public health policies: a persistent and concerning decrease long after the end of the national lockdown. Eur J Cancer. 2021;150:260–267. doi: 10.1016/j.ejca.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kortlever T.L., de Jonge L., Wisse P.H.A., Seriese I., Otto-Terlouw P., van Leerdam M.E., et al. The national FIT-based colorectal cancer screening program in The Netherlands during the COVID-19 pandemic. Prev Med. 2021;151:106643. doi: 10.1016/j.ypmed.2021.106643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toes-Zoutendijk E., Kooyker A.I., Elferink M.A., Spaander M.C.W., Dekker E., Koning H.J.D., et al. Stage distribution of screen-detected colorectal cancers in The Netherlands. Gut. 2018;67(9):1745–1746. doi: 10.1136/gutjnl-2017-315111. [DOI] [PubMed] [Google Scholar]
- 18.Ricciardiello L., Ferrari C., Cameletti M., Gaianill F., Buttitta F., Bazzoli F., et al. Impact of SARS-CoV-2 pandemic on colorectal cancer screening delay: effect on stage shift and increased mortality. Clin Gastroenterol Hepatol. 2021;19(7):1410–1417.e9. doi: 10.1016/j.cgh.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gheorghe A., Maringe C., Spice J., Purushotham A., Chalkidou K., Rachet B., et al. Economic impact of avoidable cancer deaths caused by diagnostic delay during the COVID-19 pandemic: a national population-based modelling study in England, UK. Eur J Cancer. 2021;152:233–242. doi: 10.1016/j.ejca.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the last author.