Abstract
Background
Treatment options for Chinese patients with locally advanced or metastatic squamous‐cell non‐small‐cell lung cancer (sqNSCLC) after failure of first‐line chemotherapy are limited. This study (ORIENT‐3) aimed to evaluate the efficacy and safety of sintilimab versus docetaxel as second‐line treatment in patients with locally advanced or metastatic sqNSCLC.
Methods
ORIENT‐3 was an open‐label, multicenter, randomized controlled phase 3 trial that recruited patients with stage IIIB/IIIC/IV sqNSCLC after failure with first‐line platinum‐based chemotherapy. Patients were randomized in a 1:1 ratio to receive either 200 mg of sintilimab or 75 mg/m2 of docetaxel intravenously every 3 weeks, stratified by the Eastern Cooperative Oncology Group performance status. The primary endpoint was overall survival (OS) in the full analysis set (FAS). Secondary endpoints included progression‐free survival (PFS), objective response rate (ORR), disease control rate (DCR), duration of response (DoR) and safety.
Results
Between August 25, 2017, and November 7, 2018, 290 patients were randomized. For FAS, 10 patients from the docetaxel arm were excluded. The median OS was 11.79 (n = 145; 95% confidence interval [CI], 10.28‐15.57) months with sintilimab versus 8.25 (n = 135; 95% CI, 6.47‐9.82) months with docetaxel (hazard ratio [HR]: 0.74; 95% CI, 0.56‐0.96; P = 0.025). Sintilimab treatment significantly prolonged PFS (median 4.30 vs. 2.79 months; HR: 0.52; 95% CI, 0.39‐0.68; P < 0.001) and showed higher ORR (25.50% vs. 2.20%, P < 0.001) and DCR (65.50% vs. 37.80%, P < 0.001) than the docetaxel arm. The median DoR was 12.45 (95% CI, 4.86‐25.33) months in the sintilimab arm and 4.14 (95% CI, 1.41‐7.23) months in the docetaxel arm (P = 0.045). Treatment‐related adverse events of grade ≥ 3 were reported in 26 (18.1%) patients in the sintilimab arm and 47 (36.2%) patients in the docetaxel arm. Exploratory biomarker analysis showed potential predictive values of expression levels of two transcription factors, including OVOL2 (HR: 0.35; P < 0.001) and CTCF (HR: 3.50; P < 0.001),for sintilimab treatment.
Conclusions
Compared with docetaxel, sintilimab significantly improved the OS, PFS, and ORR of Chinese patients with previously treated locally advanced or metastatic sqNSCLC.
Keywords: Non‐small cell lung cancer; Carcinoma, squamous cell; Sintilimab; Immunotherapy; Survival; Randomized controlled trial
Abbreviations
- sqNSCLC
squamous‐cell non–small‐cell lung cancer
- ECOG
Eastern Cooperative Oncology Group
- PS
performance status
- OS
overall survival
- FAS
full analysis set
- PFS
progression‐free survival
- ORR
objective response rate
- CI
confidence interval
- HR
hazard ratio
- NSCLC
non–small‐cell lung cancer
- PD‐1
Programmed cell death‐1
- PD‐L1
programmed cell death‐ligand 1
- UICC
Union for International Cancer Control
- AJCC
American Joint Committee on Cancer
- RECIST v1.1
Response Evaluation Criteria in Solid Tumors version 1.1
- CTLA‐4
cytotoxic lymphocyte antigen‐4
- DCR
disease control rate
- DoR
duration of response
- CR
complete response
- PR
partial response
- SD
stable disease
- PD
progressive disease
- QoL
Quality of life
- FFPE
formalin‐fixed paraffin‐embedded
- cfDNA
cell‐free DNA
- BEP
biomarker evaluable population
- mTBI
molecular tumor burden index
- DEGs
differentially expressed genes
- SAEs
serious adverse events
- TRAEs
treatment‐related adverse events
- irAEs
immune‐related adverse events
- GHS
Global Health Status
- VAS
visual analog scale
- OVOL2
ovo‐like zinc finger 2
- CTCF
CCTC‐binding factor
- EMT
epithelial to mesenchymal transition
1. BACKGROUND
Lung cancer remains the leading cause of cancer‐related mortality worldwide [1, 2]. In China, estimations showed that there were 815,563 newly diagnosed lung cancer cases and approximately 714,699 lung cancer‐related deaths in 2020 [1]. Squamous‐cell non‐small‐cell lung cancer (sqNSCLC) represents approximately 30% of all non‐small‐cell lung cancer (NSCLC) cases [3]. Most targeted agents are not indicated for sqNSCLC owing to a lack of actionable genetic alterations. Platinum‐based chemotherapy is the standard first‐line treatment for advanced sqNSCLC, while therapeutic options for sqNSCLC patients who failed first‐line chemotherapy are limited. Docetaxel has been the standard of care for second‐line treatment with a median overall survival (OS) of 6.0 (95% confidence interval [CI], 5.1‐7.3) months [4].
Programmed cell death‐1 (PD‐1) or programmed cell death‐ligand 1 (PD‐L1) inhibitors benefited second‐line patients with advanced NSCLC and improved their OS compared with docetaxel [4, 5, 6]. In the CheckMate 017 [4] and CheckMate 078 [7] studies, nivolumab showed a significantly prolonged OS compared to docetaxel (hazard ratio [HR], 0.59 and 0.68, respectively). Until now, no randomized clinical studies have focused on Chinese sqNSCLC patients with second‐line anti‐PD‐1/PD‐L1 treatment.
Sintilimab is a fully human IgG4 monoclonal PD‐1 antibody [8]. For both sqNSCLC and non‐sqNSCLC patients in the first‐line setting, sintilimab combined with platinum‐based chemotherapy has shown better survival benefits over chemotherapy alone [9, 10]. The ORIENT‐3 study aimed to evaluate the efficacy and safety of sintilimab versus docetaxel in the second‐line treatment of Chinese patients with locally advanced or metastatic sqNSCLC.
2. METHODS
2.1. Study design and patients
The ORIENT‐3 was an open‐label, randomized controlled phase 3 trial conducted in 39 centers across China. This study was approved by the Ethics Committee of each study center. Written informed consent was obtained from all patients. The study was performed in full accordance with the guidelines for Good Clinical Practice and the Declaration of Helsinki. The study was registered at ClinicalTrials.gov (identifier: NCT03150875).
Key inclusion criteria were: 1) histologically or cytologically diagnosed with sqNSCLC; 2) locally advanced, metastatic, or recurrent sqNSCLC of stage IIIB, IIIC, or IV (8th edition of Union for International Cancer Control [UICC]/American Joint Committee on Cancer [AJCC] tumor stage classification) who had progressed or recurred after or intolerable to first‐line platinum‐based chemotherapy; 3) 18‐75 years of age; 4) had at least one measurable lesion per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1); 5) Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1; 6) before signing the informed consent form, life expectancy of at least 12 weeks, and; 7) adequate major organ functions. Key exclusion criteria were: EGFR‐sensitive mutation or ALK rearrangement; adenosquamous carcinoma of the lung; prior therapy of anti‐PD‐1, anti‐PD‐L1, anti‐cytotoxic lymphocyte antigen‐4 (CTLA‐4) antibodies or docetaxel; symptomatic central nervous system metastasis and/or carcinomatous meningitis; active autoimmune disease (inherited or acquired); interstitial lung disease.
2.2. Randomization
Eligible patients were randomly assigned (ratio, 1:1) to receive sintilimab or docetaxel via the Medidata‐rtsm system. The random list was generated by a blinded statistician independently from the study sponsor. The minimization method was done centrally (block size = 2) with ECOG PS (0 or 1) as a stratification factor to minimize imbalances between the groups, with a probability of 0.9. The randomization table of the patients (random code of participants) was generated and submitted to the randomization system as an electronic file. The allocated treatments were not marked by the participants or investigators.
2.3. Procedures
Intravenous infusion of sintilimab injection (sintilimab, IBI308, Innovent Biologics, Inc., Suzhou, China) at a dose of 200 mg once every 3 weeks or docetaxel (Qilu Pharmaceutical Co., Ltd., Haikou, China) at a dose of 75 mg/m2 once every 3 weeks was administered. The treatment was continued until disease progression, death, intolerable toxicity, withdrawal of consent, initiation of a new anti‐cancer therapy, or other protocol‐specified reasons. Sintilimab treatment beyond initial radiographic disease progression was allowed if the investigators deemed the patient was clinically stable and might benefit from the treatment.
2.4. Outcomes
The primary endpoint was OS, defined as the time from randomization to death from any cause in the full analysis set (FAS). Patients in the docetaxel arm who received anti‐PD‐1/PD‐L1 therapy before documented disease progression after randomization were excluded from the FAS. Secondary endpoints included progression‐free survival (PFS), objective response rate (ORR), disease control rate (DCR), and duration of response (DoR). PFS was defined as the time from randomization to the first disease progression. ORR refers to the proportion of patients with a confirmed complete response (CR) or partial response (PR). DCR refers to the proportion of patients with CR, PR, or stable disease (SD). DoR refers to the time from the initial response to progressive disease (PD) or death from any cause. Patients without PD or death were censored at the date of their last imaging evaluation.
Investigators assessed tumor responses according to the RECIST v1.1. Radiographic imaging examinations were performed at baseline and were repeated every 6 weeks for 24 weeks and every 9 weeks thereafter until PD, initiation of a new anti‐tumor therapy, death, or withdrawal of consent. Survival follow‐up was performed every 60 days after discontinuing the study treatment.
For the safety evaluation, the Medical Dictionary for Regulatory Activities (MedDRA) version 23.0 was used to code and classify all adverse events. The severity of adverse events was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
The PD‐L1 tumor proportion score (TPS), which is defined as the percentage of viable tumor cells with partial or complete membrane staining at any intensity, was evaluated using the 22C3 pharmDx assay (Agilent, Santa Clara, USA) at a central lab (Covance, Shanghai, China), as previously described [10].
Quality of life (QoL) was evaluated using the EQ 5D‐5L, EORTC QLQ‐C30, and EORTC QLQ‐LC13 questionnaires. The QoL was evaluated on the first dosing day, at every radiographic imaging examination, and on the first safety follow‐up.
RNA was extracted from formalin‐fixed paraffin‐embedded (FFPE) baseline tumor samples using the RNeasy FFPE Kit (Qiagen, Hilden, Germany). Peripheral blood was collected in Streck tubes and processed within 72 h to isolate cell‐free DNA (cfDNA) using a QIAamp Circulating Nucleic Acid Kit (Qiagen, Hilden, Germany). Sequencing was performed using the Illumina HiSeq 3000 platform (Illumina, San Diego, USA) at a central laboratory (Geneplus, Beijing, China).
2.5. Statistical analysis
The FAS was defined as all patients randomly assigned to a treatment group having at least one efficacy assessment after randomization. Notably, the FAS excluded patients in the docetaxel arm who had received anti‐PD‐1/PD‐L1 therapy before documented disease progression after randomization. The primary endpoint was OS in the FAS population. Overall, 266 patients were required to provide a 90% power to test OS. Considering a certain dropout rate, a total of 290 patients would be required, with 145 patients in each arm. The final analysis would occur when events (deaths) were observed in 75% of patients. The sponsor and principal investigator reviewed whether the patient had protocol deviation that seriously affected the efficacy evaluation. The safety set included all patients who received at least one dose of the study drugs.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NY, USA) and R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). Continuous data with a normal distribution were presented as mean ± standard deviation or median (min, max). Categorical data were described as numbers and percentages. For the analysis of OS, PFS, and DoR, the Kaplan‐Meier method was used to estimate the median and 95% CI and plot the survival curve. The stratified log‐rank test was used for the comparisons between groups. The stratified Cox proportional hazard regression model was used to estimate the HR, with the stratification factors in randomization included in the model. Fisher's exact test was used to compare ORR and DCR between groups. All statistical analyses were two‐sided, and P values < 0.05 were considered statistically significant.
2.6. Exploratory biomarker analysis based on tissue/blood sequencing data
Among 157 sequenced patients (86 in the sintilimab arm and 71 in the docetaxel arm) with archival tumor tissue samples available, 110 samples (61 in the sintilimab arm and 49 in the docetaxel arm) with qualified RNA sequencing data were defined as biomarker evaluable population (BEP) to conduct downstream analysis. To screen for survival‐related genes, based on the Transcripts Per Million (TPM) normalized gene expression level of each protein‐coding gene, the BEP were split into high or low expression groups by the median value of the whole cohort of the respective gene. Then, the “survival” package was used for Cox regression analysis between high and low BEP groups in each treatment arm to derive respective HR and unadjusted P values. All genes were ranked by unadjusted P value, whereas those with P < 0.05 were considered as potentially survival‐related genes. To screen for survival‐related gene signatures, gene set signature scores were first calculated using the GSVA algorithm [11], then the same screening procedure described above was conducted. Survival curves were plotted using the R survminer package.
Baseline cfDNA from 135 patients in the sintilimab arm was sequenced, and the molecular tumor burden index (mTBI) was calculated [12] to explore peripheral indicators of clinical benefit. Differentially expressed genes (DEGs) were calculated using the R DESeq2 package [13].
3. RESULTS
3.1. Patient baseline characteristics
Between August 25, 2017, and November 7, 2018, 368 patients were screened, of whom 290 were randomized (Figure 1 and Supplementary Table S1). The median follow‐up duration was 23.56 (range, 0.03‐34.30) months. Efficacy results were based on FAS, which included 280 patients, with 145 in the sintilimab arm and 135 in the docetaxel arm. Ten patients in the docetaxel arm were excluded from FAS due to receiving anti‐PD‐1/PD‐L1 therapy before documented disease progression after randomization, of whom 2 did not receive docetaxel treatment after randomization. The median docetaxel treatment duration of those 10 patients was 3.5 (range, 0‐11) cycles. Among the 10 patients, 8 had at least one post‐baseline tumor assessment, and the overall assessment per RECIST v1.1 prior to anti‐PD‐1 therapy was PR for 2 patients and SD for the rest 6 patients (Supplementary Table S2).
FIGURE 1.
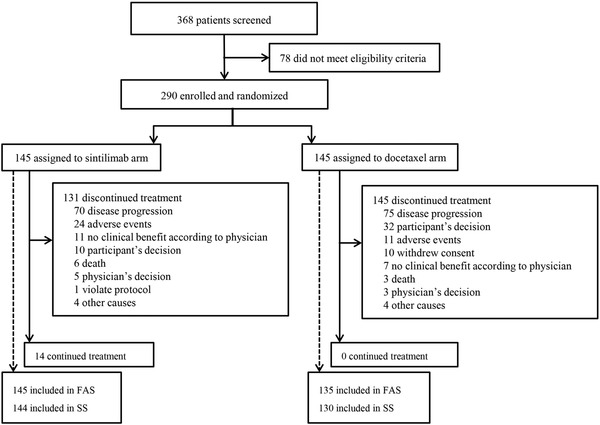
Patient Disposition Consort Figure. Between August 25, 2017, and November 7, 2018, 368 patients were screened, of whom 290 were randomized. FAS included 280 patients, with 145 in the sintilimab arm and 135 in the docetaxel arm.
Abbreviations: FAS, full analysis set; SS, safety set.
Among all randomized patients, 144 in the sintilimab arm and 130 in the docetaxel arm who received at least one dose of study treatment were included in the safety set. The median treatment duration of sintilimab and docetaxel was 8 (range, 1‐45) and 2 (range, 1‐15) cycles, respectively. In total, 40.3% (58/144) of patients in the sintilimab arm and 3.1% (4/130) of patients in the docetaxel arm completed 10 or more treatment cycles.
Fourteen patients in the sintilimab arm remained on study treatment at the cut‐off date on July 31, 2020, while no patient continued treatment in the docetaxel arm. As shown in Figure 1, the most common reason for treatment discontinuation was disease progression (48.3% [70/145] in the sintilimab arm and 51.7% [75/145] in the docetaxel arm), followed by adverse events (16.6% [24/145] in the sintilimab arm and 7.6% [11/145] in the docetaxel arm).
In the FAS, the median age of the patients in the sintilimab and docetaxel arms was 61.0 (range, 38.0‐74.0) and 60.0 (range, 34.0‐75.0) years, respectively. Most patients were male (sintilimab vs docetaxel arms, 93.8% vs 90.4%), had ECOG PS of 1 (75.9% vs 77.0%), and had stage IV disease (80.7% vs 80.7%). The baseline demographic and disease characteristics of the patients were comparable between the two arms (Table 1).
TABLE 1.
Patients baseline characteristics in FAS
| Characteristics | Sintilimab arm(n = 145) | Docetaxel arm(n = 135) |
|---|---|---|
| Sex, n (%) | ||
| Male | 136 (93.8) | 122 (90.4) |
| Female | 9 (6.2) | 13 (9.6) |
| Age (years) | ||
| Mean ± SD | 60.6 ± 8.2 | 59.5 ± 8.4 |
| Median (min, max) | 61.0 (38.0, 74.0) | 60.0 (34.0, 75.0) |
| > 60, n (%) | 84 (57.9) | 65 (48.1) |
| ≤ 60, n (%) | 61 (42.1) | 70 (51.9) |
| Ethnicity, n (%) | ||
| Han | 141 (97.2) | 129 (95.6) |
| Others | 4 (2.8) | 6 (4.4) |
| BMI (kg/m2) | ||
| Mean ± SD | 23.1 ± 3.1 | 23.6 ± 3.4 |
| Median (min, max) | 22.9 (15.0, 30.8) | 23.5 (17.4, 34.1) |
| ECOG PS, n (%) | ||
| 0 | 35 (24.1) | 31 (23.0) |
| 1 | 110 (75.9) | 104 (77.0) |
| PD‐L1 expression, n (%) | ||
| TPS < 1% | 27 (42.9) | 27 (52.9) |
| TPS ≥ 1% | 36 (57.1) | 24 (47.1) |
| Missing data | 82 | 84 |
| Smoking status, n (%) | ||
| Current smoker/quitted | 130 (89.7) | 108 (80.0) |
| Non‐smoker | 15 (10.3) | 27 (20.0) |
| Disease conditions, n (%) | ||
| Locally advanced | 28 (19.3) | 26 (19.3) |
| Metastatic | 117 (80.7) | 109 (80.7) |
| Brain metastases, n (%) | ||
| Yes | 12 (12.0) | 10 (10.8) |
| No | 88 (88.0) | 83 (89.2) |
| Missing data | 45 | 42 |
| Liver metastases, n (%) | ||
| Yes | 17 (11.7) | 15 (11.2) |
| No | 128 (88.3) | 119 (88.8) |
| Missing data | 0 | 1 |
| Staging, n (%) | ||
| Stage IIIB | 21 (14.5) | 17 (12.6) |
| Stage IIIC | 7 (4.8) | 9 (6.7) |
| Stage IVA | 69 (47.6) | 64 (47.4) |
| Stage IVB | 48 (33.1) | 45 (33.3) |
| Prior systemic chemotherapy | 145 (100.0) | 135 (100.0) |
| Paclitaxel‐based chemotherapy | 54 (37.2) | 44 (32.6) |
| Response to prior chemotherapy * | ||
| Complete or partial response | 37 (25.5) | 39 (28.9) |
| Stable disease | 59 (40.7) | 50 (37.0) |
| Progressive disease | 33 (22.8) | 37 (27.4) |
| Not evaluable or unknown | 37 (25.5) | 45 (33.3) |
| Time since prior treatment (months) # , median (95% CI) | 2.7 (1.4‐5.5) | 2.0 (1.2‐6.2) |
Patients’ response to any chemotherapy, including prior neoadjuvant therapy, adjuvant therapy or first‐line chemotherapy were recorded. Therefore, the data was not equal to 145 (sintilimab arm) or 135 (docetaxel arm).
Defined as the time from the last dose of prior chemotherapy to randomization.
Abbreviations: FAS: full analysis set; SD: standard deviation; BMI: body mass index; ECOG: Eastern Cooperative Oncology Group; PS: performance status; PD‐L1: programmed cell death‐ligand 1; TPS: tumor proportional score; CI: confidence interval.
In total, 45.5% (66/145) and 53.8% (78/145) of patients in the sintilimab and docetaxel arms received at least one subsequent anti‐tumor treatment, respectively (Supplementary Table S3). Specifically, 21.4% (31/145) of patients in the docetaxel arm received subsequent immunotherapy, including pembrolizumab, nivolumab and sintilimab. Only 6.2% (9/145) of patients in the sintilimab arm received subsequent immunotherapy. Subsequent targeted therapies, including afatinib, icotinib and anlotinib, were given to 30.3% (44/145) of patients in the sintilimab arm and 31.0% (45/145) in the docetaxel arm. Subsequent chemotherapy was given to 31.7% (46/145) of patients in the sintilimab arm and 28.3% (41/145) in the docetaxel arm (Supplementary Table S3).
3.2. Efficacy
In the FAS, 75.2% (109/145) and 77.8% (105/135) of patients in the sintilimab and docetaxel arms died. The median OS was 11.79 (95% CI, 10.28‐15.57) months in the sintilimab arm and 8.25 (95% CI, 6.47‐9.82) months in the docetaxel arm. Sintilimab was associated with significant improvement in OS compared with docetaxel, with an HR of 0.74 (95% CI, 0.56‐ 0.96; P = 0.025) (Figure 2). The 12‐month OS rate was 49% (95% CI, 41‐57) and 38% (95% CI, 30‐47), and the 24‐month OS rate was 27% (95% CI, 20‐34) and 16% (95% CI, 10‐24) in the sintilimab and docetaxel arms, respectively. Subgroup analysis also showed survival benefits in the sintilimab arm compared with the docetaxel arm in several subgroups, such as those of age >60 years (Figure 3).
FIGURE 2.
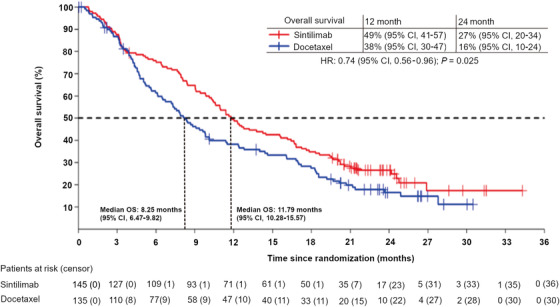
Kaplan‐Meier survival curve of overall survival in the full analysis set. The median OS was 11.79 (95% CI, 10.28‐15.57) months in the sintilimab arm and 8.25 (95% CI, 6.47‐9.82) months in the docetaxel arm. Sintilimab significantly improved OS compared with docetaxel with a HR of 0.74 (95% CI, 0.56‐0.96; P = 0.025).
Abbreviations: OS, overall survival; CI, confidence interval; HR, hazard ratio
FIGURE 3.
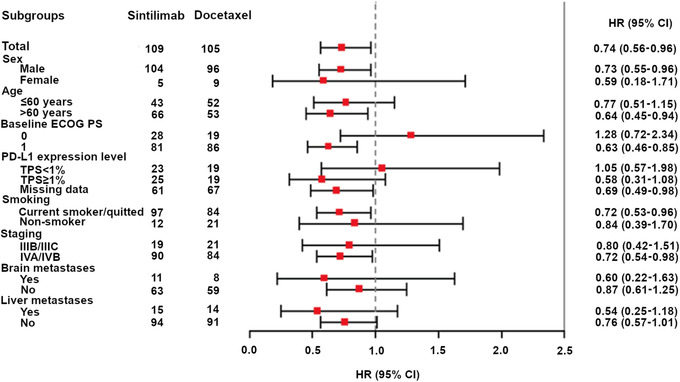
Subgroup analysis of overall survival, showing the overall survival benefits in the overall population and across subgroups. Note: For brain metastases, as there were patients with missing data of brain metastases at baseline in the sintilimab arm and docetaxel arm, respectively, the total number of events plus non‐events did not equal to 109 and 105, respectively.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status; PD‐L1, programmed cell death ligand‐1; TPS, tumor proportion score; HR, hazard ratio; CI, confidence interval.
As assessed by investigators in the FAS, the median PFS was 4.30 (95% CI, 4.04‐5.78) months and 2.79 (95% CI, 1.91‐3.19) months in the sintilimab and docetaxel arms, respectively. Sintilimab demonstrated significant improvement in PFS over docetaxel (HR: 0.52; 95% CI, 0.39‐0.68; P < 0.001) (Figure 4). The 6‐month PFS rate was 42% (95% CI, 33‐ 50) in the sintilimab arm and 16% (95% CI, 10‐24) in the docetaxel arm. The 24‐month PFS rate was 14% (95% CI, 8‐21) in the sintilimab arm and 0% in the docetaxel arm.
FIGURE 4.
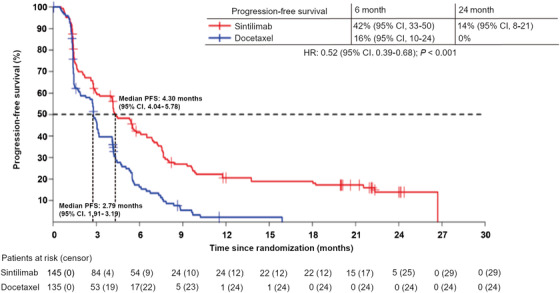
Kaplan‐Meier survival curve of progression‐free survival in the full‐analysis set. The median PFS was 4.30 (95% CI, 4.04‐5.78) and 2.79 (95% CI, 1.91‐3.19) months in the sintilimab and docetaxel arms, respectively. Sintilimab demonstrated significant improvement of PFS over docetaxel (HR: 0.52; 95% CI, 0.39‐0.68; P < 0.001).
Abbreviations: PFS, progression‐free survival; CI, confidence interval; HR, hazard ratio
Table 2 illustrates the best overall response of patients from the two arms. The confirmed ORR was significantly higher in the sintilimab arm (25.50%, 95% CI, 18.60‐33.40) than that in the docetaxel arm (2.20%, 95% CI, 0.50‐6.40) (P < 0.001). The median DoR was 12.45 (95% CI, 4.86‐25.33) months in the sintilimab arm and 4.14 (95% CI, 1.41‐7.23) months in the docetaxel arm (P = 0.045). The DCR was 65.50% (95% CI, 57.20‐73.20) and 37.80% (95% CI, 29.60‐46.50) in the sintilimab and docetaxel arms (P < 0.001), respectively.
TABLE 2.
Patient's response according to RECIST v1.1 in FAS
| Best overall response, n (%) | Sintilimab arm (n = 145) | Docetaxel arm (n = 135) |
|---|---|---|
| CR | 1 (0.7) | 0 |
| PR | 36 (24.8) | 3 (2.2) |
| SD | 58 (40.0) | 48 (35.6) |
| PD | 39 (26.9) | 48 (35.6) |
| NE | 11 (7.6) | 36 (26.7) |
RECIST: Response Evaluation Criteria in Solid Tumors; v1.1: version 1.1; FAS, full analysis set; CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; NE: not evaluable according to RECIST v1.1 criteria; CI: confidence interval.
3.3. Safety
By the data cut‐off date on July 31, 2020, the safety set included 274 patients, with 144 in the sintilimab arm and 130 in the docetaxel arm. Among 145 patients allocated to the docetaxel arm, 15 (10.3%) did not receive docetaxel at all, reflecting the open‐label nature of the study. A total of 97.2% (140/144) and 96.2% (125/130) patients in the sintilimab and docetaxel arms experienced at least one treatment‐emergent adverse event (TEAEs), respectively (data not shown). The incidence of grade 3 or above TEAEs was 43.1% (62/144) and 46.9% (61/130). Serious adverse events (SAEs) occurred in 38.9% (56/144) and 26.2% (34/130) of patients in the sintilimab and docetaxel arms, respectively. Fatal TEAEs occurred in 6.3% (9/144) and 3.8% (5/130) of patients in the sintilimab and docetaxel arms (data not shown).
In the safety set, 84.7% (122/144) and 83.1% (108/130) of patients had at least one treatment‐related adverse event (TRAE) of any grade in the sintilimab and docetaxel arms, respectively (Table 3). The incidence of grade 3 or above TRAEs was 18.1% (26/144) in the sintilimab arm and 36.2% (47/130) in the docetaxel arm. Among SAEs, 20.1% (29/144) were related to sintilimab, and 13.8% (18/130) were related to docetaxel. In addition, 6.3% (9/144) and 7.7% (10/130) of patients in the sintilimab and docetaxel arms had TRAEs that interrupted study treatment, respectively, and 12.5% (18/144) and 5.4% (7/130) of patients in the sintilimab and docetaxel arms had TRAEs that led to permanent discontinuation of treatment, respectively.
TABLE 3.
Any grade TRAEs and all grade ≥3 TRAEs in the safety set
| Events | Sintilimab arm (n = 144)n (%) | Docetaxel arm (n = 130)n (%) | ||
|---|---|---|---|---|
| All grades | Grade 3‐4 | All grades | Grade 3‐4 | |
| At least one time | 122 (84.7) | 26 (18.1) | 108 (83.1) | 47 (36.2) |
| Laboratory test | 68 (47.2) | 4 (2.8) | 66 (50.8) | 32 (24.6) |
| Increased ALT | 24 (16.7) | 2 (1.4) | 5 (3.8) | 0 (0) |
| Increased AST | 24 (16.7) | 2 (1.4) | 4 (3.1) | 0 (0) |
| Increased γ‐GT | 8 (5.6) | 1 (0.7) | 3 (2.3) | 2 (1.5) |
| Increased blood bilirubin | 8 (5.6) | 1 (0.7) | 1 (0.8) | 0 (0) |
| Lymphopenia | 4 (2.8) | 0 (0) | 7 (5.4) | 2 (1.5) |
| Leukopenia | 3 (2.1) | 0 (0) | 40 (30.8) | 25 (19.2) |
| Hemoglobin reduction | 3 (2.1) | 0 (0) | 8 (6.2) | 0 (0) |
| Neutropenia | 2 (1.4) | 0 (0) | 38 (29.2) | 31 (23.8) |
| Diseases of the skin and subcutaneous tissues | 42 (29.2) | 4 (2.8) | 46 (35.4) | 0 (0) |
| Skin rash | 21 (14.6) | 2 (1.4) | 5 (3.8) | 0 (0) |
| Hair loss | 0 (0) | 0 (0) | 45 (34.6) | 0 (0) |
| Dermatitis | 4 (2.8) | 2 (1.4) | 0 (0) | 0 (0) |
| Maculopapule | 3 (2.1) | 0 (0) | 0 (0) | 0 (0) |
| Pruritus | 6 (4.2) | 0 (0) | 0 (0) | 0 (0) |
| Others | 11 (7.6) | 0 (0) | 4 (3.1) | 0 (0) |
| Systemic diseases and reactions of drug administration sites | 39 (27.1) | 3 (2.1) | 39 (30.0) | 2 (1.5) |
| Weakness | 18 (12.5) | 0 (0) | 30 (23.1) | 0 (0) |
| Fever | 17 (11.8) | 0 (0) | 8 (6.2) | 1 (0.8) |
| Fatigue | 3 (2.1) | 1 (0.7) | 4 (3.1) | 1 (0.8) |
| Others | 10 (6.9) | 2 (1.4) | 13 (10.0) | 0 (0) |
| Diseases of the endocrine system | 35 (24.3) | 0 (0) | 0 (0) | 0 (0) |
| Hypothyroidism | 26 (18.1) | 0 (0) | 0 (0) | 0 (0) |
| Hyperthyroidism | 12 (8.3) | 0 (0) | 0 (0) | 0 (0) |
| Metabolic and nutritional diseases | 39 (21.5) | 7 (4.9) | 41 (31.5) | 3 (2.3) |
| Loss of appetite | 13 (9.0) | 0 (0) | 20 (15.4) | 2 (1.5) |
| Hyponatremia | 6 (4.2) | 2 (1.4) | 2 (1.5) | 0 (0) |
| Hypokalemia | 2 (1.4) | 2 (1.4) | 3 (2.3) | 0 (0) |
| Weight loss | 8 (5.6) | 0 (0) | 8 (6.2) | 0 (0) |
| Others | 10 (6.9) | 3 (2.1) | 8 (6.2) | 1 (0.8) |
| Hematological and lymphatic diseases | 23 (16.0) | 1 (0.7) | 40 (30.8) | 7 (5.4) |
| Anemia | 22 (15.3) | 1 (0.7) | 39 (30.0) | 3 (2.3) |
| Bone marrow failure | 0 (0) | 0 (0) | 3 (2.3) | 3 (2.3) |
| Blood coagulation disorder | 1 (0.7) | 0 (0) | 0 (0) | 0 (0) |
| Neutropenia | 0 (0) | 0 (0) | 1 (0.8) | 1 (0.8) |
| Gastrointestinal diseases | 20 (13.9) | 0 (0) | 28 (21.5) | 2 (1.5) |
| Diarrhea | 6 (4.2%) | 0 (0) | 8 (6.2%) | 0 (0) |
| Nausea | 5 (3.5) | 0 (0) | 10 (7.7%) | 1 (0.8) |
| Constipation | 5 (3.5%) | 0 (0) | 7 (5.4%) | 0 (0) |
| Emesis | 4 (2.8) | 0 (0) | 4 (3.1) | 1 (0.8) |
| Diseases of kidney and urinary system | 12 (8.3%) | 0 (0) | 5 (3.8%) | 0 (0) |
| Proteinuria | 10 (6.9%) | 0 (0) | 4 (3.1%) | 0 (0) |
| Others | 3 (2.1) | 0 (0) | 1 (0.8) | 0 (0) |
| Infectious diseases | 9 (6.3%) | 4 (2.8%) | 17 (13.1%) | 7 (5.4%) |
| Infectious pneumonia | 3 (2.1%) | 2 (1.4%) | 9 (6.9%) | 4 (3.1%) |
| Anal abscess | 0 (0) | 0 (0) | 2 (1.5%) | 2 (1.5%) |
| Urinary tract infection | 3 (2.1) | 0 (0) | 1 (0.8) | 0 (0) |
| Upper respiratory infection | 1 (0.7) | 1 (0.7) | 2 (1.5) | 0 (0) |
| Others | 3 (2.1) | 2 (1.4) | 5 (3.8) | 1 (0.8) |
Note: The number of cases for the row title refers to the number of patients with the corresponding main condition, while the number of cases for the corresponding row subtitle refers to the number of cases with the specified event. Since one patient may have several specified events (including hypo/hyperthyroidism but at different time points during the study period), therefore the number of cases of specified events may surpass the number of cases of main condition events.
“Others” in the table refer to cases that were not mentioned as their occurrence was ≤ 1%.
Abbreviations: TRAEs: treatment‐related adverse events; ALT: alanine aminotransferase; AST: aspartate aminotransferase; γ‐GT: γ‐glutamyltransferase.
A total of 58.3% (84/144) of patients in the sintilimab arm had immune‐related adverse events (irAEs) based on the investigators’ assessment; the most common irAEs included hypothyroidism (14.6% [21/144]), rash (11.8% [17/144]), and aspartate aminotransferase increased (10.4% [15/144]) (Table 4). The incidence of infusion reactions assessed by investigators was 2.8% (4/144) and 3.8% (5/130) in the sintilimab and docetaxel arms, respectively. Most of them were in grade 1‐2.
TABLE 4.
irAEs with an incidence of ≥ 2% assessed by investigators in the safety set
| Sintilimab (n = 144) | ||
|---|---|---|
| irAEs, n (%) | All grades | Grade 3‐4 |
| At least one time | 84 (58.3) | 14 (9.7) |
| Laboratory test | 35 (24.3) | 3 (2.1) |
| Increased ALT | 14 (9.7) | 1 (0.7) |
| Increased AST | 15 (10.4) | 1 (0.7%) |
| Diseases of the skin and subcutaneous tissues | 35 (24.3) | 4 (2.8) |
| Skin rash | 17 (11.8) | 2 (1.4) |
| Dermatitis | 4 (2.8) | 2 (1.4) |
| Pruritus | 4 (2.8) | 0 (0) |
| Others | 11 (7.6) | 0 (0) |
| Diseases of the endocrine system | 30 (20.8) | 0 (0) |
| Hypothyroidism | 21 (14.6) | 0 (0) |
| Hyperthyroidism | 11 (7.6) | 0 (0) |
| Systemic diseases and reactions of drug administration sites | 19 (13.2) | 1 (0.7) |
| Fatigue | 9 (6.3) | 0 (0) |
| Others | 12 (8.3) | 1 (0.7) |
| Metabolic and nutritional diseases | 12 (8.3) | 1 (0.7) |
| Loss of appetite | 8 (5.6) | 0 (0) |
| Others | 9 (6.3) | 1 (0.7) |
Note: The number of cases for the row title refers to the number of patients with the corresponding main condition, while the number of cases for the corresponding row subtitle refers to the number of cases with the specified event. Since one patient may have several specified events (including hypo/hyperthyroidism but at different time points during the study period), therefore the number of cases of specified events may surpass the number of cases of main condition events.
“Others” in the table refer to cases that were not mentioned as their occurrence were ≤ 2%.
Abbreviations: irAEs: immune‐related adverse events; ALT: alanine aminotransferase; AST: aspartate aminotransferase.
3.4. Quality of life
Patients in the sintilimab arm reported better QLQ‐C30 Global Health Status (GHS)/QoL score, as well as EQ‐5D visual analog scale (VAS) from baseline to Cycle 15 compared to those in the docetaxel arm (Figure 5A, 5B). Patients who received sintilimab had improvement from baseline in EORTC QLQ‐C30 fatigue, pain, dyspnea and appetite loss than those who received docetaxel. Also, in EORTC QLQ‐LC13, patients in the sintilimab arm showed improvement in dyspnea, coughing, hemoptysis, alopecia, pain in the arm or shoulder and pain in other parts (Figure 5C, 5D).
FIGURE 5.
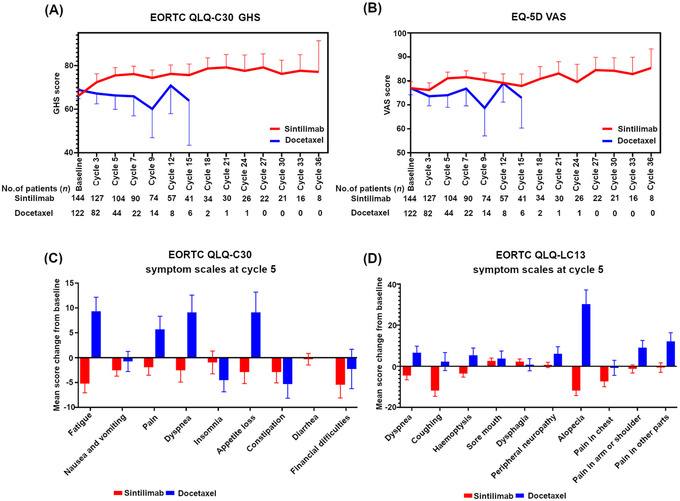
Quality of life analysis in ORIENT‐3. (A) Patients in the sintilimab arm reported better QLQ‐C30 Global Health Status scores from baseline to Cycle 15 than those in the docetaxel arm. (B) Patients in the sintilimab arm reported better EQ‐5D Visual Analog Scale from baseline to cycle 15 than those in the docetaxel arm. (C) Patients in the sintilimab arm had improvement in EORTC QLQ‐C30 fatigue, pain, dyspnea and appetite loss than those in the docetaxel arm. (D) Patients in the sintilimab arm showed improvement in EORTC QLQ‐LC13 dyspnea, coughing, hemoptysis, alopecia, pain in the arm or shoulder and pain in other parts than those in the docetaxel arm.
Abbreviations: EORTC, European Organization for Research and Treatment of Cancer; QLQ‐C30, quality of life questionnaire score 30; GHS, Global Health Status; EQ‐5D, EuroQol five‐dimensional questionnaire; VAS, visual analog scale; QLQ‐LC13, Quality of life questionnaire‐lung cancer 13.
3.5. Biomarker analysis
Tumor RNA sequencing was performed for patients with tumor tissue samples available, and 110 patients (61 in the sintilimab arm and 49 in the docetaxel arm) had qualified data for downstream analysis. The 28 immune cell populations [14] were used to conduct association analyses with PFS. Only a low expression level of central memory CD4 T cell (red dot in figure; HR: 1.76; P = 0.042) was significantly associated with longer PFS in the sintilimab arm (Supplementary Figure S1). When analyzing survival‐related individual genes using the cox proportional hazards regression model, two transcription factors, ovo‐like zinc finger 2 (OVOL2) and CCCTC‐binding factor (CTCF), were significantly associated with PFS in the sintilimab arm. In the sintilimab arm, patients with high OVOL2 expression showed significantly superior PFS compared with patients with low OVOL2 expression (median PFS: 6.87 [95% CI, 5.45‐13.80] months versus 2.79 [95% CI, 2.04‐4.40] months, HR: 0.35, 95% CI, 0.19‐0.62; P < 0.001) (Figure 6A); patients with high CTCF expression had remarkably inferior PFS compared to patients with low CTCF expression (median PFS: 2.69 [95% CI, 1.74‐4.21] months versus 6.90 [95% CI, 5.52‐11.80] months, HR: 3.50, 95% CI, 1.90‐6.50; P < 0.001) (Figure 6B). Among patients with high OVOL2 expression in the sintilimab arm, patients with high CTCF expression levels had significantly inferior PFS compared with those with low CTCF expression levels, with a median PFS of 5.39 (95% CI, 2.96‐not available [NA]) months versus 11.80 (95% CI, 6.51‐NA) months (HR: 4.30, 95% CI, 1.50‐12.0; P = 0.007) (Figure 6C). Among patients with low OVOL2 expression levels, patients with high CTCF expression levels showed worse PFS than those with low CTCF expression levels (median PFS: 2.07 [95% CI, 1.41‐4.17] months versus 4.98 [95% CI, 2.79‐7.95] months, HR: 2.80, 95% CI, 1.30‐6.00; P = 0.008)] (Figure 6D). The BEP was further split into two sub‐cohorts by the median OVOL2 or CTCF expression to identify differentially expressed genes (DEGs). As a result, 30 and 2497 DEGs (adjusted P < 0.05) might be regulated by OVOL2 or CTCF, respectively. OVOL2 was reported to suppress the Epithelial‐to‐Mesenchymal Transition (EMT) [15, 16]. Thus, we defined DEGs that were highly expressed in patients with low OVOL2 expression levels as the EMT‐related signature and that were highly expressed in patients with high OVOL2 expression as the non‐EMT‐related signature (Supplementary Figure S2A). In the sintilimab arm, patients with a high level of EMT‐related signature showed inferior PFS than patients with a low level of EMT‐related signature (median PFS: 3.68 [95% CI, 2.07‐6.90] months versus 5.39 [95% CI, 4.17‐11.80] months, HR: 1.80, 95% CI, 1.00‐3.10; P = 0.036) (Supplementary Figure S2B), while patients with a high level of non‐EMT related signature had significantly superior PFS compared with those with a low level of non‐EMT related signature (median PFS: 6.87 [95% CI, 5.39‐13.80] months versus 4.11 [95% CI, 2.63‐5.45] months, HR: 0.45, 95% CI, 0.26‐0.80; P = 0.006) (Supplementary Figure S2C), which indicated that patients without EMT could benefit much more from sintilimab treatment. Lastly, baseline cfDNA from 135 patients in the sintilimab arm was sequenced to explore peripheral indicators of clinical benefit. The mTBI based on cfDNA was analyzed, which indicated that patients with low mTBI had significantly longer PFS (median PFS: 6.87 [95% CI, 4.17‐9.30] versus 4.14 [95% CI, 2.20‐5.55], HR: 0.64, 95% CI, 0.44‐0.95; P = 0.025) (Supplementary Figure S3A) and OS (median OS: 16.70 [95% CI, 10.90‐21.10] versus 10.40 [95% CI, 8.41‐12.80], HR: 0.66, 95% CI, 0.45‐0.98; P = 0.038) compared to those with high mTBI (Supplementary Figure S3B).
FIGURE 6.
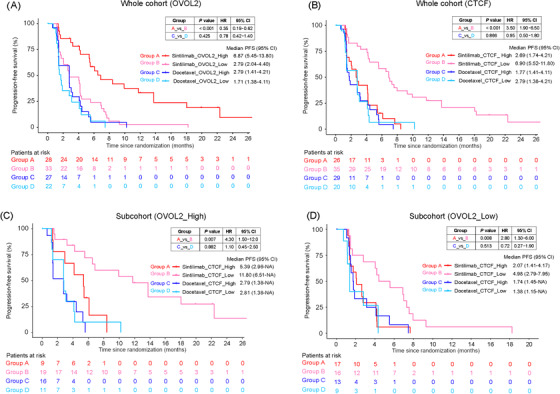
Correlation between PFS and OVOL2 or CTCF. (A) Correlation between PFS and OVOL2 expression level in the BEP. (B) Correlation between PFS and CTCF expression level in the BEP. (C) Correlation between PFS and CTCF expression level in the sub‐cohort of BEP with high OVOL2 expression level. (D) Correlation between PFS and CTCF expression level in the sub‐cohort of BEP with low OVOL2 expression level. Samples were split into high (‘_High’) or low (‘_Low’) expression groups by the median value of the BEP (whole cohort) of the respective gene.
Abbreviation: PFS, Progression‐free survival; HR, Hazard ratio; CI, Confidential interval; OVOL2, ovo‐like zinc finger 2; CTCF, CCCTC‐binding factor; BEP, biomarker evaluable population; NA, not available.
4. DISCUSSION
ORIENT‐3 is the first randomized controlled trial investigating the efficacy and safety of sintilimab in Chinese patients with locally advanced, metastatic, or recurrent sqNSCLC who failed first‐line platinum‐based chemotherapy. This study found that second‐line therapy with sintilimab significantly improved OS compared with docetaxel. Sintilimab generated a superior and more durable response compared with docetaxel.
Primary endpoint analysis of the FAS showed that compared with docetaxel, sintilimab significantly prolonged the median OS by 3.54 months (11.79 months vs. 8.25 months, HR: 0.74; P = 0.025) (Figure 2). Nivolumab as second‐line therapy achieved a median OS of 12.3 months in the sqNSCLC subgroup compared to 7.9 months with docetaxel (HR: 0.61; 95% CI, 0.42‐0.89) [7]. For previously treated and PD‐L1‐positive patients with stage IIIB/IV NSCLC, pembrolizumab showed longer median OS than docetaxel (10.4 months vs 8.5 months, HR: 0.71; 95% CI, 0.58‐0.88; P < 0.001) [5].
The OS, PFS, ORR and DoR benefits of sintilimab over docetaxel were demonstrated in the FAS. In ORIENT‐3 study, 21.4% (31/145) of patients in the docetaxel arm received subsequent immunotherapy. In comparison, the rate was only 2% (3/137) in the CheckMate 017 [4] and 13% (45/343) in the KEYNOTE‐010 clinical trials [5]. A previous study showed that for second‐line NSCLC patients who received chemotherapy, OS was greatly impacted by subsequent anti‐neoplastic therapies [17].
Further, our study found that sintilimab prolonged the median PFS by 1.51 months (4.30 months vs. 2.79 months) and reduced the risk of disease progression by 48% (HR: 0.52; P < 0.001) (Figure 4). The median PFS of the sqNSCLC patients in the CheckMate 017 study who received nivolumab and docetaxel treatment was 3.5 months versus 2.8 months, respectively (HR: 0.62; 95% CI, 0.47‐0.81; P < 0.001) [4]. The survival curve of the sintilimab arm and the docetaxel arm separated shortly after the initiation of therapy, and the PFS rate was 26% higher in the sintilimab arm than that in the docetaxel arm after 6 months of treatment (42%, 95% CI, 33‐50 versus 16%, 95% CI, 10‐24) (Figure 4). These findings suggest that treatment benefits appeared rapidly in patients treated with sintilimab.
The confirmed ORR of sintilimab as second‐line therapy for patients with sqNSCLC was 25.50% (37/145) as assessed by investigators, which was significantly higher than that of docetaxel (2.20%, 3/135). The ORR of nivolumab in the CheckMate 017 study was 20% (27/135) [4], and the ORR of nivolumab for the subgroup of sqNSCLC in the CheckMate 078 trial was 21.1% (28/133) [7]. The median DoR in the sintilimab arm was 12.45 months. These results suggest that the clinical benefits of sintilimab persisted in respondents. In addition, various biomarkers for predicting long‐term responses to immunotherapy were suggested [18].
The median number of cycles of the docetaxel arm was 2 (range: 1‐15), which was relatively lower than the 4 cycles of the REVEL study [19]. In the docetaxel arm, 32 patients discontinued treatment due to their own decision, and 10 withdrew consent, while the number was 10 and 0 in the sintilimab arm, respectively (Figure 1). Many of the patients allocated to the docetaxel arm who discontinued treatment probably did so to seek anti‐PD‐1 treatment because some might have suspected known that the efficacy of anti‐PD‐1 therapy was better than docetaxel. A similar phenomenon was also found in the CheckMate 057 trial [20], where 8% of patients assigned to docetaxel did not receive it and in the KEYNOTE‐010 trial [5], where 45 out of 343 patients assigned to docetaxel withdrew consent.
The findings of the subgroup analyses on OS showed that patients with higher PD‐L1 expression tended to benefit from sintilimab, as observed in previous studies in solid tumors [5, 21, 22]. Still, it is suggested that PD‐L1 expression as a predictive marker of response should be taken with caution due to the variability in positivity thresholds [23]. Prior clinical evidence has indicated that both PD‐L1 positive and negative sqNSCLC patients could benefit from PD‐1/PD‐L1 inhibitors in second‐line settings [4, 24, 25]. In the CheckMate 017 [4], a study with similar settings as ORIENT‐3, the HRs for OS benefit were 0.69 (95% CI, 0.45‐1.05) in PD‐L1 expression level ≥ 1% population and 0.58 (95% CI, 0.37‐0.92) in PD‐L1 < 1% population. In addition, PD‐L1 expression varies among different tumor areas and in time [26] and is influenced by prior therapies [27]. In this ORIENT‐3 study, although there was no significant difference regarding the status of PD‐L1 expression between the sintilimab and docetaxel arms, it was limited by relatively fewer PD‐L1 quantifiable patients and not employing PD‐L1 status as a stratification factor. There were only 54 patients in the TPS < 1% subgroup, and PD‐L1 expression was assessed in archival tumor tissues. Those facts might have limited subgroup analysis and resulted in relatively fewer OS benefits observed in the TPS < 1% population.
In addition, ECOG PS 1 and stage IV appeared to favor benefits from sintilimab. Previous studies reported worse immunotherapy outcomes with ECOG PS > 2 [28, 29], but such patients were not included in this study. In addition, current/previous smokers also benefited more from sintilimab, consistent with findings in a recent meta‐analysis [30]. The response rate for docetaxel in the ORIENT‐3 study was relatively lower. Lower response rates of docetaxel in the East‐Asian population were found in several trials [7, 31, 32]. The underlying mechanisms remain unknown and worthy of future exploration.
Based on the OS benefits in the overall population and subgroups, as well as PFS, ORR and DoR benefits of sintilimab over docetaxel, the OS benefit brought by sintilimab could be considered robust and meaningful.
Generally, the frequency of TEAEs and grade 3 or higher TEAEs were comparable between the sintilimab and docetaxel arms. The TRAEs in the sintilimab arm were mostly of grade 1 or 2, and the incidence of grade ≥ 3 TRAEs was much lower in the sintilimab arm than in the docetaxel arm (18.1% vs. 36.2%) despite longer exposure. No new safety signal was observed from the safety analysis of TEAE. The incidence, types and severity of irAEs were similar to previously reported results of sintilimab [8, 10, 33, 34]. A recent meta‐analysis that analyzed 16 studies involving 8278 NSCLC patients suggested that sintilimab had comparable safety profiles with other approved PD‐1/L1 inhibitors [35]. The relatively higher fatal TEAEs in the sintilimab arm might be due to longer treatment exposure (median, 8 vs 2 cycles). In the sintilimab arm, 5 out of 9 fatal TEAEs were treatment‐related; 2 (one case each of exacerbation of myocardial injury and lung infection) were evaluated by investigators as “possibly related” to sintilimab, and the other 3 (2 cases of unknown cause of death and one of lung infection aggravated) were considered by investigators as “not determinable” to sintilimab.
In this study, tissue and peripheral blood biomarkers, including tumor tissue transcriptomes and cfDNA, were simultaneously studied for their potential to identify patient populations which derived selective benefits from sintilimab treatment in this indication. Two transcription factors, namely OVOL2 and CTCF, were found to be the topmost genes related to the survival of patients treated with sintilimab. Biologically, OVOL2 suppresses the EMT process [15, 16], and CTCF mediates cancer‐specific gene expression by regulating chromosome architecture [36, 37]. CTCF was recently reported to be a significant suppressor of PD‐L1 [38]. The combination of OVOL2 and CTCF could further enhance the predictive value of the sintilimab treatment response. Particularly, patients with high OVOL2 and low CTCF expression levels had significantly superior PFS (median PFS: 11.8 months), whereas patients with low OVOL2 and high CTCF expression levels had significantly inferior PFS (median PFS: 2.07 months), which were comparable with those receiving docetaxel treatment (Figure 6C and 6D). Moreover, the mTBI, which reflected the percentage of circulating tumor DNA (ctDNA) detected in blood cfDNA [12], was found to predict benefits from sintilimab treatment. The association of low baseline ctDNA levels with good clinical outcomes was also supported by previous studies [39, 40]. In this study, we also found that a low central memory CD4 T cell expression level was associated with longer PFS in the sintilimab arm. The function of central memory CD4 T cells in the tumor microenvironment remains unclear [41, 42]. However, it was reported that transforming growth factor‐β could induce CD4+CD62L+ central memory T cells to differentiate into Foxp3+ T cells [43], which might then suppress immune reaction and lead to worse survival.
This study has some limitations. First, all patients were Chinese, potentially limiting the generalizability of the results to other populations. Second, exploratory biomarker analysis was performed post‐hoc on a limited sample size without adjustment for multiplicity.
5. CONCLUSIONS
Sintilimab showed a statistically significant survival benefit over docetaxel as a second‐line treatment in Chinese patients with advanced or metastatic sqNSCLC, with a manageable safety profile. Sintilimab could be a new treatment option for patients with locally advanced or metastatic sqNSCLC who had disease progression or recurrence after or were intolerable to first‐line platinum‐based chemotherapy.
DECLARATIONS
AUTHOR CONTRIBUTIONS
YK Shi contributed to study conception and design, manuscript revision and editing; JF Feng contributed to study conception and design; LY Sun contributed to data analysis and interpretation, manuscript writing; YQ Wang contributed to data analysis and interpretation; B Peng contributed to data analysis and interpretation, manuscript writing; All authors contributed to administrative support, provision of study materials or patients, collection and assembly of data. All authors read and approved the final version of the manuscript. The corresponding authors take full responsibility for the credibility of the descriptions and data presented in this work.
CONFLICT OF INTEREST
Disclosure: Bo Peng, Jiya Sun, Christoph Mancao, Yanqi Wang and Luyao Sun are full‐time employees of Innovent Biologics,Inc. The other authors declare that they have no competing interests.
FUNDING
This study was funded by Innovent biologics, Inc. and Eli Lilly and Company, and partly supported by China National Major Project for New Drug Innovation (2017ZX09304015).
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethics Committee of each study center. Written informed consent was obtained from all patients. Trial registration: NSCLC, NCT03150875. Registered May 12, 2017, https://clinicaltrials.gov/ct2/show/NCT03150875
CONSENT FOR PUBLICATION
Not applicable.
Supporting information
Supporting information
ACKNOWLEDGMENTS
The study was funded by Innovent Biologics, Inc. and Eli Lilly and Company. The authors thank the patients and their families who made this study possible and the clinical study teams who participated in the study. The authors would also like to thank Dr. Haizhu Chen (National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, China) for providing medical editing assistance with this article.
Shi Y, Wu L, Yu X, Xing P, Wang Y, Zhou J, et al. Sintilimab versus docetaxel as second‐line treatment in advanced or metastatic squamous non‐small‐cell lung cancer: an open‐label, randomized controlled phase 3 trial (ORIENT‐3). Cancer Commun. 2022;42:1314–1330. 10.1002/cac2.12385
Trial registration: NSCLC, NCT03150875. Registered May 12, 2017, https://clinicaltrials.gov/ct2/show/NCT03150875
Contributor Information
Yuankai Shi, Email: syuankai@cicams.ac.cn.
Jifeng Feng, Email: fjif@vip.sina.com.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209‐49. [DOI] [PubMed] [Google Scholar]
- 2. Cao M, Li H, Sun D, Chen W. Cancer burden of major cancers in China: A need for sustainable actions. Cancer Commun (Lond). 2020;40(5):205‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Travis WD. Pathology of lung cancer. Clin Chest Med. 2011;32(4):669‐92. [DOI] [PubMed] [Google Scholar]
- 4. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced Squamous‐Cell Non‐Small‐Cell Lung Cancer. N Engl J Med. 2015;373(2):123‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herbst RS, Baas P, Kim DW, Felip E, Pérez‐Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet. 2016;387(10027):1540‐50. [DOI] [PubMed] [Google Scholar]
- 6. Kang J, Zhang C, Zhong WZ. Neoadjuvant immunotherapy for non‐small cell lung cancer: State of the art. Cancer Commun. 2021;41(4):287‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok T, et al. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J Thorac Oncol. 2019;14(5):867‐75. [DOI] [PubMed] [Google Scholar]
- 8. Liu X, Yi Y. Recent updates on Sintilimab in solid tumor immunotherapy. Biomark Res. 2020;8(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou C, Wu L, Fan Y, Wang Z, Liu L, Chen G, et al. Sintilimab Plus Platinum and Gemcitabine as First‐Line Treatment for Advanced or Metastatic Squamous NSCLC: Results From a Randomized, Double‐Blind, Phase 3 Trial (ORIENT‐12). J Thorac Oncol. 2021;16(9):1501‐11. [DOI] [PubMed] [Google Scholar]
- 10. Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Efficacy and Safety of Sintilimab Plus Pemetrexed and Platinum as First‐Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC: a Randomized, Double‐Blind, Phase 3 Study (Oncology pRogram by InnovENT anti‐PD‐1‐11). J Thorac Oncol. 2020;15(10):1636‐46. [DOI] [PubMed] [Google Scholar]
- 11. Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA‐seq data. BMC Bioinformatics. 2013;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Zhao C, Chang L, Jia R, Liu R, Zhang Y, et al. Circulating tumor DNA analyses predict progressive disease and indicate trastuzumab‐resistant mechanism in advanced gastric cancer. EBioMedicine. 2019;43:261‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA‐seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Charoentong P, Finotello F, Angelova M, Mayer C, Efremova M, Rieder D, et al. Pan‐cancer Immunogenomic Analyses Reveal Genotype‐Immunophenotype Relationships and Predictors of Response to Checkpoint Blockade. Cell Rep. 2017;18(1):248‐62. [DOI] [PubMed] [Google Scholar]
- 15. Wang ZH, Li Z, Hu M, Yang QJ, Yan S, Wu RS, et al. Ovol2 gene inhibits the Epithelial‐to‐Mesenchymal Transition in lung adenocarcinoma by transcriptionally repressing Twist1. Gene. 2017;600:1‐8. [DOI] [PubMed] [Google Scholar]
- 16. Kitazawa K, Hikichi T, Nakamura T, Mitsunaga K, Tanaka A, Nakamura M, et al. OVOL2 Maintains the Transcriptional Program of Human Corneal Epithelium by Suppressing Epithelial‐to‐Mesenchymal Transition. Cell Rep. 2016;15(6):1359‐68. [DOI] [PubMed] [Google Scholar]
- 17. Kotake M, Miura Y, Imai H, Mori K, Sakurai R, Kaira K, et al. Post‐Progression Survival Associated with Overall Survival in Patients with Advanced Non‐Small‐Cell Lung Cancer Receiving Docetaxel Monotherapy as Second‐Line Chemotherapy. Chemotherapy. 2017;62(4):205‐13. [DOI] [PubMed] [Google Scholar]
- 18. Bai R, Lv Z, Xu D, Cui J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res. 2020;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garon EB, Ciuleanu T‐E, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second‐line treatment of stage IV non‐small‐cell lung cancer after disease progression on platinum‐based therapy (REVEL): a multicentre, double‐blind, randomised phase 3 trial. Lancet (London, England). 2014;384(9944):665‐73. [DOI] [PubMed] [Google Scholar]
- 20. Borghaei H, Paz‐Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non‐Small‐Cell Lung Cancer. N Engl J Med. 2015;373(17):1627‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kowanetz M, Zou W, Gettinger SN, Koeppen H, Kockx M, Schmid P, et al. Differential regulation of PD‐L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti‐PD‐L1). Proc Natl Acad Sci. 2018;115(43):E10119‐e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reck M, Rodríguez‐Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus Chemotherapy for PD‐L1‐Positive Non‐Small‐Cell Lung Cancer. N Engl J Med. 2016;375(19):1823‐33. [DOI] [PubMed] [Google Scholar]
- 23. Davis AA, Patel VG. The role of PD‐L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen R‐L, Zhou J‐X, Cao Y, Li S‐H, Li Y‐H, Jiang M, et al. The efficacy of PD‐1/PD‐L1 inhibitors in advanced squamous‐cell lung cancer: a meta‐analysis of 3112 patients. Immunotherapy. 2019;11(17):1481‐90. [DOI] [PubMed] [Google Scholar]
- 25. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): a phase 3, open‐label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mansfield AS, Aubry MC, Moser JC, Harrington SM, Dronca RS, Park SS, et al. Temporal and spatial discordance of programmed cell death‐ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol. 2016;27(10):1953‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang J, Dang F, Ren J, Wei W. Biochemical Aspects of PD‐L1 Regulation in Cancer Immunotherapy. Trends Biochem Sci. 2018;43(12):1014‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sehgal K, Gill RR, Widick P, Bindal P, McDonald DC, Shea M, et al. Association of Performance Status With Survival in Patients With Advanced Non‐Small Cell Lung Cancer Treated With Pembrolizumab Monotherapy. JAMA Netw Open. 2021;4(2):e2037120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dall'Olio FG, Maggio I, Massucci M, Mollica V, Fragomeno B, Ardizzoni A. ECOG performance status ≥2 as a prognostic factor in patients with advanced non small cell lung cancer treated with immune checkpoint inhibitors‐A systematic review and meta‐analysis of real world data. Lung Cancer. 2020;145:95‐104. [DOI] [PubMed] [Google Scholar]
- 30. Mo J, Hu X, Gu L, Chen B, Khadaroo PA, Shen Z, et al. Smokers or non‐smokers: who benefits more from immune checkpoint inhibitors in treatment of malignancies? An up‐to‐date meta‐analysis. World J Surg Oncol. 2020;18(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park K, Kim J‐H, Cho EK, Kang J‐H, Shih J‐Y, Zimmermann AH, et al. East Asian Subgroup Analysis of a Randomized, Double‐Blind, Phase 3 Study of Docetaxel and Ramucirumab Versus Docetaxel and Placebo in the Treatment of Stage IV Non‐small Cell Lung Cancer Following Disease Progression after One Prior Platinum‐Based Therapy (REVEL). Cancer Res Treat. 2016;48(4):1177‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun Y, Wu YL, Zhou CC, Zhang L, Zhang L, Liu XY, et al. Second‐line pemetrexed versus docetaxel in Chinese patients with locally advanced or metastatic non‐small cell lung cancer: a randomized, open‐label study. Lung cancer. 2013;79(2):143‐50. [DOI] [PubMed] [Google Scholar]
- 33. Zhang L, Mai W, Jiang W, Geng Q. Sintilimab: A Promising Anti‐Tumor PD‐1 Antibody. Front Oncol. 2020;10:594558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jiang H, Zheng Y, Qian J, Mao C, Xu X, Li N, et al. Efficacy and safety of sintilimab in combination with chemotherapy in previously untreated advanced or metastatic nonsquamous or squamous NSCLC: two cohorts of an open‐label, phase 1b study. Cancer Immunol Immunother. 2021;70(3):857‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu L, Bai H, Wang C, Seery S, Wang Z, Duan J, et al. Efficacy and Safety of First‐Line Immunotherapy Combinations for Advanced NSCLC: A Systematic Review and Network Meta‐Analysis. J Thorac Oncol. 2021;16(7):1099‐117. [DOI] [PubMed] [Google Scholar]
- 36. Ong CT, Corces VG. CTCF: an architectural protein bridging genome topology and function. Nat Rev Genet. 2014;15(4):234‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fang C, Wang Z, Han C, Safgren SL, Helmin KA, Adelman ER, et al. Cancer‐specific CTCF binding facilitates oncogenic transcriptional dysregulation. Genome Biol. 2020;21(1):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oreskovic E, Wheeler EC, Mengwasser KE, Fujimura E, Martin TD, Tothova Z, et al. Genetic analysis of cancer drivers reveals cohesin and CTCF as suppressors of PD‐L1. Proc Natl Acad Sci. 2022;119(7):e2120540119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Snyder A, Morrissey MP, Hellmann MD. Use of Circulating Tumor DNA for Cancer Immunotherapy. Clin Cancer Res. 2019;25(23):6909‐15. [DOI] [PubMed] [Google Scholar]
- 40. Zhang J, Dai D, Tian J, Li L, Bai J, Xu Y, et al. Circulating Tumor DNA Analyses Predict Disease Recurrence in Non‐Muscle‐Invasive Bladder Cancer. Front Oncol. 2021;11:657483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu Q, Sun Z, Chen L. Memory T cells: strategies for optimizing tumor immunotherapy. Protein & cell. 2020;11(8):549‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu J, Zhang T, Xiong H, Zeng L, Wang Z, Peng Y, et al. Tumor‐Infiltrating CD4 Central Memory T Cells Correlated with Favorable Prognosis in Oral Squamous Cell Carcinoma. J Inflamm Res. 2022;15:141‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang X, Chang Li X, Xiao X, Sun R, Tian Z, Wei H. CD4(+)CD62L(+) central memory T cells can be converted to Foxp3(+) T cells. PloS one. 2013;8(10):e77322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.


