Abstract
Background
Patients with refractory or relapsed acute myeloid leukemia (AML) have poor survival, necessitating the exploration of optimized therapeutic strategy. Here, we aimed to investigate clinical outcomes and health‐related quality of life (HR‐QoL) after total therapy, which included allogeneic hematopoietic stem cell transplantation (allo‐HSCT), and prophylactic donor lymphocyte infusion (DLI) in the early phase after transplantation, followed by multiple measurable residual disease (MRD) and graft‐versus‐host disease (GvHD)‐guided DLIs.
Methods
Consecutive patients who had refractory or relapsed AML and had received non‐T‐cell‐depleted allo‐HSCT at Peking University Institute of Hematology were included in the study. If the patients achieved complete remission at 30 days after transplantation and had no evidence of relapse, severe infection, organ failure, and active GvHD at the time of planned DLI, prophylactic DLI was administered at 30 days after transplantation for human leukocyte antigen (HLA)‐matched related HSCT or at 45‐60 days after transplantation for haploidentical or unrelated HSCT. Subsequently, multiple DLIs were administered based on MRD results and whether they developed GvHD after transplantation.
Results
A total of 105 patients were eligible. Eighty‐seven patients received prophylactic DLI (group B), while 18 did not receive prophylactic DLI (group A). Among 105 patients, the cumulative incidence of grade 2‐4 acute GvHD and chronic GvHD was 40.6% (95% confidence interval [CI] = 30.6%‐50.6%) and 73.3% (95% CI = 67.4%‐79.2%), respectively. The cumulative incidence of relapse (CIR), transplant‐related mortality (TRM), and leukemia‐free survival (LFS) at 5 years after transplantation were 31.5% (95% CI = 21.9%‐41.1%), 22.1% (95% CI = 11.3%‐32.9%), and 46.4% (95% CI = 36.8%‐56.0%), respectively. In group B, the CIR, TRM, and LFS at 5 years after transplantation were 27.6% (95% CI = 17.6%‐37.6%), 21.6% (95% CI = 11.2%‐32.0%), and 50.8% (95% CI = 40.0%‐61.6%), respectively. At the end of follow‐up, 48 patients survived, and more than 90% of survivors had satisfactory recoveries of HR‐QoL.
Conclusions
Our study indicated that total therapy is not only associated with decreased CIR, comparable TRM, and better long‐term LFS, but also with satisfactory HR‐QoL for refractory or relapsed AML, compared with those of standard of care therapy reported previously. Therefore, total therapy may be an optimized therapeutic strategy for refractory or relapsed AML.
Keywords: acute myeloid leukemia, allogeneic hematopoietic stem cell transplantation, refractory, relapsed, total therapy
List of abbreviations
- AML
acute myeloid leukemia
- allo‐HSCT
allogeneic hematopoietic stem cell transplantation
- CR
complete remission
- CIR
cumulative incidence of relapse
- LFS
leukemia‐free survival
- DLI
donor lymphocyte infusion
- GvL
graft‐versus‐leukemia
- GvHD
graft‐versus‐host disease
- G‐CSF
granulocyte colony stimulating factor
- GPBSCs
G‐CSF mobilized peripheral blood stem cells
- HLA
human leukocyte antigen
- TRM
transplant‐related mortality
- MRD
measurable residual disease
- HR‐QoL
health‐related quality of life
- Bu
busulfan
- Cy
cyclophosphamide
- TBI
total body irradiation
- ATG
anti‐human thymocyte immunoglobulin
- CSA
cyclosporine
- MNC
mononuclear cells
- LAIPs
leukemia‐associated immune phenotypes
- FCM
flow cytometry
- BM
bone marrow
- PWB
physical well‐being
- SWB
social/family well‐being
- EWB
emotional well‐being
- FWB
functional well‐being
- BMTS
bone Marrow Transplant Subscale
- CR1
first complete remission
- CR2
second complete remission
- CI
confidence interval
- N/A
Not applicable
1. BACKGROUND
Refractory or relapsed acute myeloid leukemia (AML) is associated with a dismal prognosis [1, 2, 3]. Currently, allogeneic hematopoietic stem cell transplantation (allo‐HSCT) remains a potentially curative approach for refractory or relapsed AML. Although allo‐HSCT provides a 70%‐90% complete remission (CR) rate in patients with refractory or relapsed AML [4, 5], cumulative incidence of relapse (CIR) at 2 years after transplantation is still as high as 40%‐60% [4, 5, 6, 7, 8], while leukemia‐free survival (LFS) remains at 20%‐40% [4, 5, 6, 7, 8]. Notably, the therapeutic strategy for refractory or relapsed AML needs to be optimized.
Donor lymphocyte infusion (DLI) exhibits graft‐versus‐leukemia (GvL) effects and is an effective method for the treatment and prevention of relapse after allo‐HSCT. However, the success of DLI is usually impeded by the morbidity and mortality associated with severe graft‐versus‐host disease (GvHD) after DLI, particularly in patients receiving haploidentical DLI [9, 10]. Modified DLI includes the infusion of granulocyte colony stimulating factor (G‐CSF)‐mobilized peripheral blood stem cells (GPBSCs) instead of unstimulated donor lymphocytes and application of immunosuppressive agents after infusion to prevent GvHD. Our previous studies suggested that modified DLI could not only reduce the incidence acute GvHD, but could also preserve GvL effect [11, 12]. Our previous retrospective studies found that in patients with refractory or relapsed acute leukemia, those who received prophylactic modified DLI in the early phase after transplantation had lower CIR than patients who did not receive prophylactic DLI (3‐year CIR: 46% vs. 66%, P = 0.020 for human leukocyte antigen [HLA]‐matched related; 3‐year CIR: 36% vs. 55%, P = 0.017 for haploidentical) [13]; moreover, transplant‐related mortality (TRM) of patients who received prophylactic modified DLI did not increase (3‐year TRM: 20% vs. 20%, P = 0.83 for HLA‐matched related; 3‐year TRM: 38% vs. 33%, P = 0.95 for haploidentical) [14]. Additionally, according to the results of measurable residual disease (MRD) after transplantation, pre‐emptive modified DLI could also prevent relapse compared to interleukin‐2 (3‐year CIR: 27.8% vs. 64.4%, P = 0.001) and did not increase TRM (3‐year TRM: 14.4% vs. 11.4%, P = 0.897) [15]. These results supported the safety and efficacy of modified DLI. Furthermore, our prospective study revealed that prophylactic modified DLI followed by multiple MRD and GvHD‐guided modified DLIs could prevent relapse (3‐year CIR: 32.4%), did not increase TRM (3‐year TRM: 17.3%), and improved LFS (3‐year LFS: 50.3%) in patients who had refractory or relapsed acute leukemia and received allo‐HSCT [16]. These results suggested that total therapy, which includes allo‐HSCT, and prophylactic DLI in the early phase after transplantation, followed by multiple MRD and GvHD‐guided DLIs, likely improves the LFS and can serve as an optimized therapeutic strategy for refractory or relapsed AML. However, the study has some limitations. In particular, the follow‐up period was relatively short, different eligible diseases such as AML and acute lymphoblastic leukemia were evaluated, and health‐related quality of life (HR‐QoL) was not assessed. It is difficult to demonstrate the safety and efficacy of total therapy for refractory or relapsed AML based on current results. Here, we extended this prospective study to investigate whether total therapy was associated with a better long‐term LFS and a better recovery of HR‐QoL in patients with refractory or relapsed AML. We aimed to explore the optimized therapeutic strategy for refractory or relapsed AML.
2. MATERIALS AND METHODS
2.1. Eligibility criteria
Consecutive patients with refractory or relapsed AML who received non‐T‐cell‐depleted allo‐HSCT at Peking University Institute of Hematology (Beijing, China) between October 12, 2011 and December 31, 2018, were eligible in this study. Refractory or relapsed AML included primary refractory disease and refractory relapse. Primary refractory disease was defined as AML unable to achieve CR after ≥2 cycles of chemotherapy. Refractory relapse was defined as relapsed AML unable to achieve CR after 1‐2 courses of salvage chemotherapy. This study was an extension of the prospective study, which was registered at www.ClinicalTrials.gov (NCT01455272). The study was approved by the Ethics Committee of Peking University Peoples Hospital and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
2.2. Study protocol of total therapy
2.2.1. Transplants
Details of transplants including the conditioning regimen, GvHD prophylaxis, and supportive care have been described previously [17]. For patients receiving HLA‐matched related HSCT, the conditioning regimen included busulfan (Bu; 3.2 mg/kg/day iv for 3 days; Otsuka Pharmaceutical Co., Ltd, Hangzhou, Zhejiang, China) and cyclophosphamide (Cy; 1.8 g/m2/day for 2 days; Jiangsu Hengrui Pharmaceutical Co., Ltd, Lianyungang, Jiangsu, China); or total body irradiation (TBI; 7.7 Gy in one dose) and Cy. For patients receiving haploidentical or unrelated HSCT, the conditioning regimen included Bu, Cy, and anti‐human thymocyte immunoglobulin (ATG; 2.5 mg/kg/day iv for 4 days; Genzyme Corp, Boston, MA, USA) or TBI, Cy, and ATG. Cyclosporine (CSA), mycophenolate mofetil, and short‐term methotrexate was administered to prevent GvHD.
2.2.2. Protocol of DLI
2.2.2.1. Prophylactic DLI
Prophylactic DLI was administered if patients met the following criteria: (1) achievement of CR at 30 days after transplantation and (2) no evidence of relapse, severe infection, serious organ failure, or active GvHD at the time of the planned prophylactic DLI. Prophylactic DLI was administered at 30 days after transplantation in patients receiving HLA‐matched related HSCT or at 45‐60 days after transplantation in patients receiving haploidentical or unrelated HSCT. The infusion of GPBSCs was used for DLI. The dose of mononuclear cells (MNC) was 1.0×108/kg.
2.2.2.2. MRD and GvHD‐guided DLIs
After prophylactic DLI, discontinuation of CSA and application of DLIs were performed based on the results of MRD and whether the patients developed GvHD. In patients with persistent negative MRD results, if patients had no GvHD, CSA was stopped at 90 days after HLA‐matched related HSCT, or at 100 days after haploidentical or unrelated HSCT. If patients had GvHD, CSA was reduced by 50% when GvHD was controlled and then stopped after 2 weeks. Subsequently, if patients had no GvHD, DLI was repeated at 6 months after transplantation; if patients had persistent GvHD, DLI was not repeated.
In patients with positive MRD results, CSA was stopped immediately. If patients had no GvHD, chemotherapy and DLI were administered promptly. If patients had GvHD, chemotherapy without DLI was initiated immediately. Subsequently, (1) if MRD results became negative and GvHD was controlled, chemotherapy and DLI were repeated after 6 months; (2) if MRD results became negative and GvHD was not controlled, chemotherapy and DLI were not repeated; (3) if MRD results remained positive and GvHD was controlled, chemotherapy and DLI were repeated after 3 months; and (4) if MRD results remained positive but GvHD was not controlled, chemotherapy and DLI were repeated after 6 months.
The infusion of GPBSCs was used for DLI. The dose of MNC for each DLI was 1.0×108/kg. After DLI, CSA was administered to prevent DLI‐associated GvHD. For patients receiving HLA‐matched related HSCT, CSA was administered for 2‐4 weeks after each infusion [11]; for patients receiving haploidentical or unrelated HSCT, CSA was administered for 6‐8 weeks after each infusion [12].
2.2.2.3. Chemotherapy before DLI
Chemotherapy regimens included aclacinomycin (10 mg/m2/day for 5 days) and cytarabine (100 mg/m2/day for 5 days); or homoharringtonine (2 mg/m2/day for 5 days), aclacinomycin (10 mg/m2/day for 5 days), and cytarabine (100 mg/m2/day for 5 days).
2.2.3. MRD monitoring and definition
The monitoring of MRD using leukemia‐associated immune phenotypes (LAIPs) and WT1 was performed at 1, 2, 3, 4.5, 6, 9, and 12 months, and at 6‐month intervals thereafter after transplantation. LAIPs were detected by eight‐color flow cytometry (FCM) as previously described [18]. A total of 250,000‐1,000,000 cells were collected for routine analysis. If a cluster of more than 25 cells with LAIPs, results was considered positive. A lower limit of detection of 0.01% was targeted. Any measurable level of FCM was considered positive. Therefore, FCM‐positivity was defined as > 0.01% of cells with LAIPs in bone marrow (BM) samples. WT1 mRNA level was detected using TaqMan‐based real‐time quantitative PCR technology as previously described [19]. The PCR primer pair of WT1 was as follows: 5’‐GATAACCACACAACGCCCATC and 3’‐CACACGTCGCACATCCTGAAT. The sequencing probe was 5’‐ACACCGTGCGTGTGTATTCTGTATTGC‐3’ [19]. WT1‐positivity was defined as a transcript level > 0.60% in BM samples. When FCM‐positivity or WT1‐positivity was detected, the test was repeated after 2 weeks. Patients’ MRD results were considered positive if they had two consecutive positive results for FCM or WT1 or exhibited both FCM‐positivity and WT1‐positivity in a single BM sample [15].
2.3. Aassessment of HR‐QoL
The Functional Assessment of Cancer Therapy‐Bone Marrow Transplant scale (FACT‐BMT) was administered to assess the HR‐QoL of the patient. The FACT‐BMT Version 4.0 is a 39‐item self‐reported questionnaire, which includes physical well‐being (PWB, seven items), social/family well‐being (SWB, seven items), emotional well‐being (EWB, six items), functional well‐being (FWB, seven items), and Bone Marrow Transplant Subscale (BMTS, 12 items). Every item in each subscale consists of a Likert‐type scale ranging from 0 to 4 (0 = not at all, 1 = a little bit, 2 = somewhat, 3 = quite a bit, and 4 = very much). The FACT‐BMT is scored by adding the scores of all subscales. The FACT‐G is scored by adding the scores of PWB, SWB, EWB, and FWB. The FACT trial outcome index (FACT‐TOI) is scored by adding the scores of PWB, FWB, and BMTS [20]. If the score is higher than 60%, it is defined as recovery of HR‐QoL. Higher scores indicate better HR‐QoL.
2.4. Definitions
Since thrombolytic engraftment could be postponed by factors other than leukemia and cytotoxic therapy (i.e., GvHD, virus infection, and drugs), CR was defined as less than 5% BM blasts without evidence of dysplasia in BM, no myeloblasts with Auer rods, no extra‐medullary leukemia, and an absolute neutrophil count ≥ 1×109/L. Leukemia relapse was defined as recurrence of ≥ 5% BM blasts or at least one extra‐medullary leukemia site. Grading of acute GvHD and chronic GvHD was conducted using published criteria [21, 22]. Overall survival was defined as the interval from transplant to death from any cause. LFS was defined as the interval from transplantation to leukemia relapse or death, whichever occurred first.
2.5. Statistical analysis
The CIR, TRM, and GvHD were calculated using a competing risk model. LFS and overall survival were calculated using the Kaplan–Meier method and compared using the log‐rank test. A univariate analysis was performed using the X 2 test for categorical variables and the Mann–Whitney test for continuous variables. A multivariate analysis was performed using Cox proportional hazards model. The endpoint of follow‐up for the surviving patients was September 30, 2021. Unless specified, all P‐values were 2‐sided, and a P‐value < 0.05 was considered significant. The SPSS 20.0 (IBM, Armonk, NY, USA) and R 2.6.1 software packages (R Foundation for Statistical Computing; 2018, Vienna, Austria) were used for the statistical analyses.
3. RESULTS
3.1. Patient characteristics
A total of 105 consecutive patients were eligible for this study. All patients achieved neutrophil engraftment. Overall, 103 (98.1%) patients (95% confidence interval [CI] = 93.3%‐99.5%) achieved CR at 30 days after transplantation. Eighteen (17.1%) patients did not receive prophylactic DLI (group A) and the reasons for this are described in Figure 1. Eighty‐seven (82.9%) patients received prophylactic DLI (group B) (Figure 1). The characteristics of the patients are displayed in Table 1. After prophylactic DLI, 54 (54/87, 62.1%) patients (95% CI = 51.6%‐71.6%) had persistent negative MRD results, and 33 (33/87, 37.9%) patients (95% CI = 28.5%‐48.4%) exhibited positive MRD results. Of the 33 patients, 7 (21.2%), 14 (42.4%), and 12 (36.4%) patients exhibited positive MRD results because of two consecutive positive results of FCM, two consecutive positive results of WT1, and both FCM‐positivity and WT1‐positivity in a single BM sample, respectively. In 54 patients with persistent negative MRD results, 35 (64.8%) patients did not receive MRD and GvHD‐guided DLI because of active GvHD. In 33 patients with positive MRD results, 5 (15.2%) patients did not receive MRD and GvHD‐guided DLI because of relapse within 2 weeks after prophylactic DLI. At the end of the follow‐up, 47 patients received MRD and GvHD‐guided DLIs, among whom 21 (44.7%) received one course of DLI, 17 (36.2%) received two courses, 5 (10.6%) received three courses, and 4 (8.5%) received four courses after prophylactic DLI. After MRD and GvHD‐guided DLI, 69 (69/87, 79.3%) patients (95% CI = 69.7%‐86.5%) had persistent negative MRD results, and the remaining 18 (18/87, 20.7%) patients (95% CI = 13.5%‐30.4%) had persistent positive MRD results (Figure 1).
FIGURE 1.
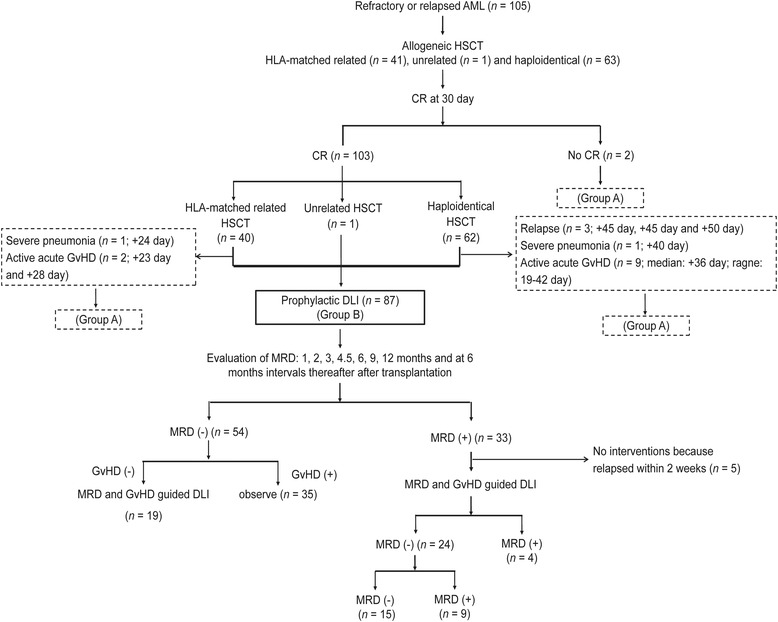
The diagram of patient subgrouping (n = 105). Abbreviations: AML, acute myeloid leukemia; HSCT, hematopoietic stem cell transplantation; HLA, human leucocyte antigen; CR, complete remission; GvHD, graft‐versus‐host disease; DLI, donor lymphocyte infusion; MRD, measurable residual disease
TABLE 1.
Characteristics of 105 patients with refractory or relapsed acute myeloid leukemia
| Characteristic | Total cases | Group A (no DLI) | Group B (DLI) | P value | |
|---|---|---|---|---|---|
| Case number | 105 | 18 | 87 | ||
| Age (years, median [range]) | 35 (6‐66) | 39 (17‐66) | 35 (6‐66) | 0.792 | |
| Sex (cases [%]) | 0.415 | ||||
| Male | 67 (63.8) | 13 (72.2) | 54 (62.1) | ||
| Female | 38 (36.2) | 5 (27.8) | 33 (37.9) | ||
| Risk stratification by genetics † (cases [%]) | 0.116 | ||||
| Favorable | 11 (10.5) | 2 (11.1) | 9 (10.3) | ||
| Intermediate | 57 (54.3) | 6 (33.3) | 51 (58.6) | ||
| Poor | 37 (35.2) | 10 (55.6) | 27 (31.0) | ||
| Count of bone marrow blasts at transplant (%, median [range]) | 24.0 (7.0‐73.0) | 23.5 (7.0‐70.0) | 24.0 (7.0‐73.0) | 0.496 | |
| Type of donor (cases [%]) | 0.197 | ||||
| HLA‐matched related | 41 (39.0) | 4 (22.2) | 37 (42.5) | ||
| Haploidentical | 63 (60.0) | 14 (77.8) | 49 (56.3) | ||
| HLA‐matched unrelated | 1 (1.0) | 0 (0) | 1 (1.1) | ||
| HLA‐mismatch (cases [%]) | 0.251 | ||||
| 0 locus mismatch | 43 (41.0) | 4 (22.2) | 39 (44.8) | ||
| 1 locus mismatch | 1 (1.0) | 0 (0.0) | 1 (1.1) | ||
| 2 loci mismatch | 16 (15.2) | 3 (16.7) | 13 (14.9) | ||
| 3 loci mismatch | 45 (42.9) | 11 (61.1) | 34 (39.1) | ||
| Donor‐patient sex match (cases [%]) | 0.203 | ||||
| Female‐female | 12 (11.4) | 0 (0.0) | 12 (13.8) | ||
| Female‐male | 30 (28.6) | 8 (44.4) | 22 (25.3) | ||
| Male‐male | 35 (33.3) | 5 (27.8) | 30 (34.5) | ||
| Male‐female | 28 (26.7) | 5 (27.8) | 23 (26.4) | ||
| ABO match (cases [%]) | 0.292 | ||||
| Match | 62 (59.0) | 10 (55.6) | 52 (59.8) | ||
| Major mismatch | 25 (23.8) | 6 (33.3) | 19 (21.8) | ||
| Minor mismatch | 16 (15.2) | 1 (5.6) | 15 (17.2) | ||
| Major and minor mismatch | 2 (1.9) | 1 (5.6) | 1 (1.1) | ||
| Conditioning regimen (cases [%]) | 0.344 | ||||
| TBI‐based | 7 (6.7) | 2 (11.1) | 5 (5.7) | ||
| Bu‐based | 98 (93.3) | 16 (88.9) | 82 (94.3) | ||
| Stem cell (cases [%]) | 0.182 | ||||
| Bone marrow and peripheral blood stem cells | 96 (91.4) | 15 (83.3) | 81 (93.1) | ||
| Peripheral blood stem cells | 9 (8.6) | 3 (16.7) | 6 (6.9) | ||
| Dose of MNCs for transplant (×108/kg, median [ range]) | 7.69 (5.08‐13.02) | 8.05 (5.09‐11.99) | 7.63 (5.08‐13.02) | 0.364 | |
| Dose of CD34+ cells for transplant (×106/kg, median [ range]) | 2.52 (0.60‐9.80) | 2.46 (0.63‐9.46) | 2.65 (0.60‐9.80) | 0.752 | |
| Neutrophil engraftment (cases [%]) | 1.000 | ||||
| Yes | 105 (100.0) | 18 (100.0) | 87 (100.0) | ||
| No | 0 (0) | 0 (0) | 0 (0) | ||
| Time of neutrophil engraftment (days, median [ range]) | 14 (10‐26) | 14 (10‐21) | 14 (10‐26) | 0.277 | |
| Platelet engraftment (cases [%]) | 0.003 | ||||
| Yes | 100 (95.2) | 14 (77.8) | 86 (98.9) | ||
| No | 5 (4.8) | 4 (22.2) | 1 (1.1) | ||
| Time of platelet engraftment (days, median [ range]) | 16.0 (8.0‐74.0) | 17.0 (9.0‐34.0) | 15.5 (8.0‐74.0) | 0.701 | |
| Dose of CD3+ cells for each DLI (×108/kg, median [ range]) | N/A | N/A | 0.35 (0.12‐0.64) | N/A | |
| Dose of CD4+ cells for each DLI (×108/kg, median [ range]) | N/A | N/A | 0.20 (0.11‐0.42) | N/A | |
| Dose of CD8+ cells for each DLI (×108/kg, median [ range]) | N/A | N/A | 0.12 (0.05‐0.28) | N/A | |
| Dose of CD34+ cells for each DLI (×106/kg, median [ range]) | N/A | N/A | 0.38 (0.32‐1.22) | N/A | |
Risk stratification by genetics are according to 2017 ELN recommendation.
Abbreviations: DLI, donor lymphocyte infusion; HLA, human leukocyte antigen; TBI, total body irradiation; Bu, busulfan; MNC, mononuclear cell; N/A, not applicable.
3.2. GvHD
Among a total of 105 patients, the cumulative incidence of grade 2‐4 acute GvHD and chronic GvHD was 40.6% (95% CI = 30.6%‐50.6%) and 73.3% (95% CI = 67.4%‐79.2%), respectively. In group B, 38 (38/87, 43.7%) patients developed grade 1‐4 acute GvHD, and 71 (71/87, 81.6%) patients developed chronic GvHD. The cumulative incidences of grade 2‐4 acute GvHD and chronic GvHD was 36.5% (95% CI = 26.1%‐46.9%) and 81.6% (95% CI = 75.7%‐87.5%), respectively (Table 2). The outcomes of group A are also described in Table 2.
TABLE 2.
Clinical outcomes after transplant in 105 patients with refractory or relapsed acute myeloid leukemia
| Characteristic | Total cases | Group A (no DLI) | Group B (DLI) | Type of donor | |||
|---|---|---|---|---|---|---|---|
| HLA‐matched | Haploidentical | P value † | |||||
| Case number | 105 | 18 | 87 | 42 | 63 | ||
| Onset time of Acute GvHD (day, median [range]) | 46.0 (19.0‐100.0) | 34.0 (19.0‐42.0) | 72.5 (42.0‐100.0) | 56.0 (19.0‐75.0) | 44.5 (19.0‐100.0) | 0.905 | |
| Severity of Acute GvHD (n) | |||||||
| Grade 1 | 8 | 0 | 8 | 3 | 5 | ||
| Grade 2 | 30 | 5 | 25 | 8 | 22 | ||
| Grade 3 | 6 | 3 | 3 | 2 | 4 | ||
| Grade 4 | 5 | 3 | 2 | 2 | 3 | ||
| Cumulative incidence of acute GvHD (%, [95% CI]) | |||||||
| Grade 1‐4 | 46.7 (36.9‐56.5) | 61.1 (46.4‐75.8) | 43.7 (33.3‐54.1) | 35.7 (25.3‐46.1) | 54.0 (41.5‐66.5) | 0.071 | |
| Grade 2‐4 | 40.6 (30.6‐50.6) | 61.1 (46.4‐75.8) | 36.5 (26.1‐46.9) | 29.7 (19.3‐40.1) | 48.5 (35.4‐61.6) | 0.066 | |
| Grade 3‐4 | 12.2 (5.1‐19.3) | 34.3 (19.6‐49.0) | 7.5 (1.0‐14.0) | 11.2 (1.7‐20.7) | 14.0 (5.6‐22.4) | 0.628 | |
| Onset time of chronic GvHD (day, median [range]) | 138.0 (100.0‐200.0) | 114.5 (110.0‐150.0) | 140.0 (100.0‐200.0) | 145.0 (100.0‐200.0) | 130.0 (109.0‐200.0) | 0.322 | |
| Severity of chronic GvHD (n) | |||||||
| Mild | 31 | 2 | 29 | 12 | 19 | ||
| Moderate | 38 | 4 | 34 | 14 | 24 | ||
| Severe | 8 | 0 | 8 | 4 | 4 | ||
| Cumulative incidence of chronic GvHD (%, [95% CI]) | |||||||
| ≥ mild | 73.3 (67.4‐79.2) | 33.3 (17.4‐49.2) | 81.6 (75.7‐87.5) | 71.4 (61.4‐81.4) | 74.6 (67.3‐81.9) | 0.510 | |
| ≥ moderate | 60.1 (51.1‐69.1) | 26.2 (10.3‐42.1) | 67.2 (55.8‐78.6) | 56.8 (43.7‐69.9) | 62.4 (53.8‐71.0) | 0.571 | |
| ≥ severe | 19.5 (8.3‐30.7) | 0.0 (0.0‐0.0) | 23.0 (9.9‐36.1) | 15.6 (3.8‐27.4) | 24.1 (16.8‐31.4) | 0.469 | |
| CIR at 5 years (%, [95% CI]) | 31.5 (21.9‐41.1) | 50.0 (34.1‐65.9) | 27.6 (17.6‐37.6) | 35.7 (23.4‐48.0) | 28.5 (18.5‐38.5) | 0.448 | |
| Onset time of relapse (day, median [range]) | 128.0 (34.0‐1173.0) | 68.0 (34.0‐480.0) | 147.0 (60.0‐1173.0) | 120.0 (50.0‐1173.0) | 130.5 (34.0‐624.0) | 0.929 | |
| TRM at 5 years (%, [95% CI]) | 22.1 (11.3‐32.9) | 33.3 (16.6‐50.0) | 21.6 (11.2‐32.0) | 28.9 (16.2‐30.6) | 19.7 (10.9‐28.5) | 0.285 | |
| Onset time of TRM (day, median [range]) | 280.0 (60.0‐1963.0) | 88.0 (60.0‐176.0) | 329.0 (108.0‐1963.0) | 329.0 (88.0‐1353.0) | 220.0 (60.0‐1963.0) | 0.413 | |
| LFS at 5 years (%, [95% CI]) | 46.4 (36.8‐56.0) | 16.7 (3.4‐30.0) | 50.8 (40.0‐61.6) | 35.4 (20.9‐49.9) | 51.7 (43.1‐60.3) | 0.148 | |
| Median LFS (months, median [95% CI]) | 51.5 (4.6‐82.6) | 3.5 (1.1‐5.9) | 69.2 (6.2‐84.3) | 41.4 (3.8‐78.7) | 60.8 (6.0‐86.5) | 0.148 | |
| Survival at 5 years (%, [95% CI]) | 46.4 (36.8‐56.0) | 16.7 (3.4‐30.0) | 50.8 (40.0‐61.6) | 35.4 (20.9‐49.9) | 51.7 (43.1‐60.3) | 0.132 | |
| Median survival (months, median [95% CI]) | 52.2 (4.6‐83.2) | 4.7 (3.3‐6.1) | 70.0 (6.5‐91.9) | 43.2 (4.1‐79.6) | 60.8 (6.7‐86.5) | 0.132 | |
| Cause of mortality (n) | |||||||
| Relapse | 32 | 9 | 23 | 15 | 17 | ||
| Infection | 19 | 4 | 15 | 10 | 9 | ||
| Grade 4 acute GvHD | 3 | 2 | 1 | 1 | 2 | ||
| Severe chronic GvHD (lung) | 3 | 0 | 3 | 0 | 3 | ||
P value represents comparison between patients receiving HLA‐matched HSCT and patients receiving haploidentical HSCT.
Abbreviations: DLI, donor lymphocyte infusion; HLA, human leucocyte antigen; GvHD, graft‐versus‐host disease; CIR, cumulative incidence of relapse; TRM, treatment‐related mortality; LFS, leukemia‐free survival.
In group B, with increasing courses of DLIs, the incidence of ≥ moderate chronic GvHD gradually increased (0 course vs. 1 course vs. 2 courses vs. 3‐4 courses = 40.2% vs. 47.7% vs. 75.9% vs. 100.0%, P = 0.001). However, the courses of DLIs were not associated with the incidence of grade 1‐4 acute GvHD (P = 0.500), grade 2‐4 acute GvHD (P = 0.471), grade 3‐4 acute GvHD (P = 0.398), ≥ mild chronic GvHD (P = 0.533), and ≥ severe chronic GvHD (P = 0.321).
3.3. Relapse
During a median follow‐up period of 86.0 months (range = 34.6‐124.2 months), 33 patients experienced relapse after transplantation in a total of 105 patients. The CIR was 29.5% (95% CI = 20.1%‐38.9%) at 2 years and 31.5% (95% CI = 21.9%‐41.1%) at 5 years after transplantation (Table 2 and Figure 2A). In group B, 24 patients experienced relapse. The CIR was 25.3% (95% CI = 15.5%‐35.1%) at 2 years and 27.6% (95% CI = 17.6%‐37.6%) at 5 years (Table 2). The CIR of group A are displayed in Table 2.
FIGURE 2.
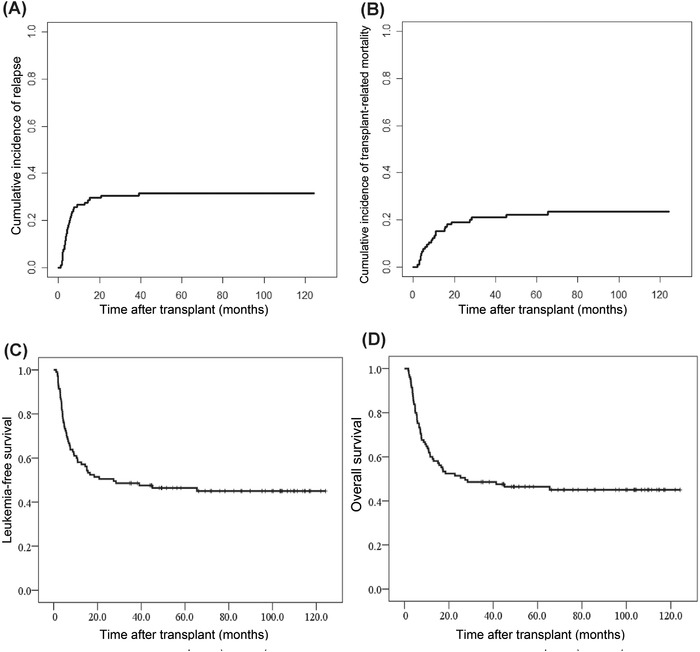
Cumulative incidence of relapse, transplant‐related mortality, leukemia‐free survival and overall survival after transplantation (n = 105). (A) Cumulative incidence of relapse after transplantation. (B) Cumulative incidence of transplant‐related mortality after transplantation. (C) Leukemia‐free survival after transplantation. (D) Overall survival after transplantation
In group B, based on the results of univariate analysis (Supplementary Table S1), multivariate analysis showed that persistent negative MRD results (hazard ratio [HR] = 0.028, 95% CI = 0.009‐0.091, P < 0.001) and ≥ mild chronic GvHD (HR = 5.876, 95% CI = 2.859‐9.582, P = 0.001) were associated with a lower risk of relapse after transplantation (Table 3). In 33 patients with positive MRD results after transplantation, with the increasing course of DLIs, the frequency of conversion to negative MRD results gradually increased (0 course vs. 1 course vs. 2 courses vs. 3‐4 courses = 0.0% vs. 42.9% vs. 50.0% vs. 75.0%, P = 0.014), and the CIR gradually decreased (0 course vs. 1 course vs. 2 courses vs. 3‐4 courses = 100.0% vs. 57.1% vs. 50.0% vs. 12.5%, P = 0.005). Moreover, the development and severity of chronic GvHD were also associated with a lower relapse risk in patients with positive MRD results (P = 0.003) (Figure 3A), or even in patients with persistent negative MRD results (P = 0.005) (Figure 3B).
TABLE 3.
Multivariate analysis for outcomes after transplant in 87 patients who had refractory or relapsed acute myeloid leukemia and received prophylactic donor lymphocyte infusion
| Characteristics | Hazard ratio (95% CI) | P value | |
|---|---|---|---|
| Relapse | |||
| ≥ mild chronic GvHD after prophylactic DLI: no vs. yes | 5.876 (2.859‐9.582) | 0.001 | |
| ≥ moderate chronic GvHD after prophylactic DLI: no vs. yes | 2.867 (0.866‐7.493) | 0.103 | |
| MRD results after DLI: negative vs. positive | 0.028 (0.009‐0.091) | <0.001 | |
| Leukemia‐free survival | |||
| Age: < 35 vs. ≥ 35 | 1.848 (0.759‐3.875) | 0.147 | |
| Type of donor: HLA‐matched related and unrelated vs. haploidentical | 0.712 (0.377‐1.345) | 0.234 | |
| ABO match (match vs. mismatch) | 0.790 (0.387‐1.616) | 0.585 | |
| ≥ mild chronic GvHD after prophylactic DLI: no vs. yes | 0.025 (0.009‐0.067) | <0.001 | |
| ≥ moderate chronic GvHD after prophylactic DLI: no vs. yes | 0.640 (0.272‐1.506) | 0.307 | |
| MRD results after DLI: negative vs. positive | 4.463 (2.517‐8.826) | <0.001 | |
Abbreviations: GvHD, graft‐versus‐host disease; DLI, donor lymphocyte infusion; MRD, measurable residual disease; HLA, human leukocyte antigen.
FIGURE 3.
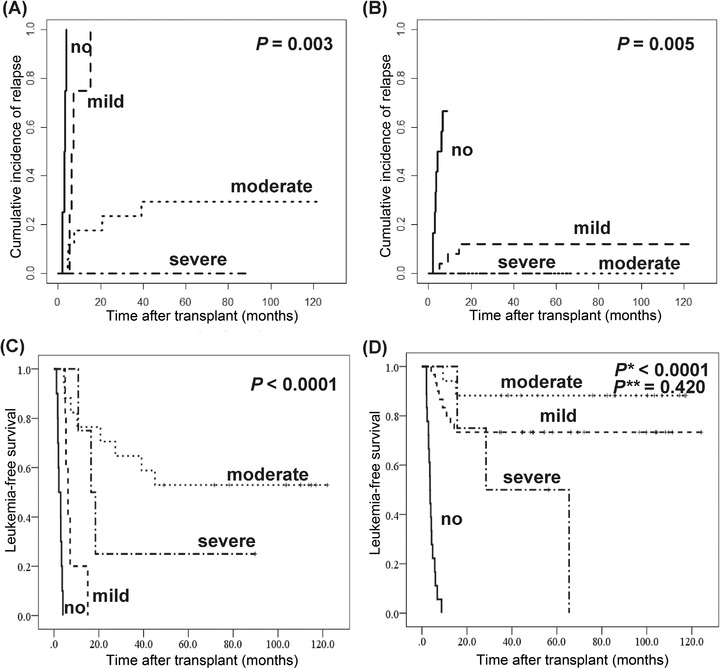
Cumulative incidence of relapse (CIR) and leukemia‐free survival (LFS) after transplantation in group B, according to MRD results and GvHD (n = 87). (A) CIR after transplantation in patients with positive MRD results. (B) CIR after transplantation in patients with persistent negative MRD results. (C) LFS after transplantation in patients with positive MRD results. (D) LFS after transplantation in patients with persistent negative MRD results. Eighty‐seven patients received prophylactic donor lymphocyte infusion and divided into groups B. According to the severity of chronic GvHD, patients were divided into four groups, such as no chronic GvHD, mild chronic GvHD, moderate chronic GvHD, and severe chronic GvHD. P* represents the comparison among four groups. P** represents the comparison between patients with mild chronic GvHD and patients with moderated chronic GvHD. Abbreviations: CIR, cumulative incidence of relapse; MRD, measurable residual disease; LFS, leukemia‐free survival; GvHD, graft‐versus‐host disease
3.4. TRM
Among a total of 105 patients, 25 patients experienced TRM. The TRM was 19.0% (95% CI = 9.6%‐28.4%) at 2 years and 22.1% (95% CI = 11.3%‐32.9%) at 5 years after transplantation (Table 2 and Figure 2B). In group B, 19 patients experienced TRM. The TRM was 16.1% (95% CI = 6.7%‐25.5%) at 2 years and 21.6% (95% CI = 11.2%‐32.0%) at 5 years (Table 2). The TRM of group A and the causes of mortality are displayed in Table 2.
3.5. LFS and overall survival
Among a total of 105 patients, the LFS was 51.4% (95% CI = 41.8%‐61.0%) at 2 years and 46.4% (95% CI = 36.8%‐56.0%) at 5 years after transplantation. The overall survival was 52.4% (95% CI = 42.8%‐62.0%) at 2 years and 46.4% (95% CI = 36.8%‐56.0%) at 5 years (Table 2 and Figure 2C‐2D). In group B, the LFS was 58.5% (95% CI = 48.1%‐68.8%) at 2 years and 50.8% (95% CI = 40.0%‐61.6%) at 5 years. The overall survival was 59.8% (95% CI = 49.4%‐70.2%) at 2 years and 50.8% (95% CI = 40.0%‐61.6%) at 5 years (Table 2). The LFS and overall survival of group A are also described in Table 2.
In group B, multivariate analysis revealed that persistent negative MRD‐test results (HR = 4.463, 95% CI = 2.517‐8.826, P < 0.001) and ≥ mild chronic GvHD (HR = 0.025, 95% CI = 0.009‐0.067, P < 0.001) were associated with a better LFS (Table 3). In patients with positive MRD results, patients with moderate chronic GvHD had the best LFS (P < 0.001) (Figure 3C). However, in patients with persistent negative MRD results, those with mild or moderate chronic GvHD had better LFS than patients with severe chronic GvHD or without chronic GvHD (P < 0.001). Nevertheless, there was no significant difference in LFS between patients with mild and moderate chronic GvHD (P = 0.420) (Figure 3D).
3.6. HR‐QoL after transplantation
At the end of the follow‐up, 48 patients survived, and their HR‐QoL was assessed. Three survivors were in group A, and the remaining 45 survivors were in group B. Of the 48 survivors, more than 90% of the survivors exhibited recoveries in all subscales (Table 4). Compared with that of patients with mild or moderate chronic GvHD, patients with severe chronic GvHD had the poorest recovery of HR‐QoL in all subscales (Table 4). However, there was no significant difference in recovery of HR‐QoL between patients with mild and moderate chronic GvHD, except for PWB (26.5 vs. 24.1, P = 0.003) and EWB (22.3 vs. 20.4, P = 0.010) (Table 4). The HR‐QoL scores of the survivors in group A and group B are described in Table 4. Additionally, the 48 survivors did not develop second malignancies.
TABLE 4.
Health‐related quality of life after transplant in 48 survivors with refractory or relapsed acute myeloid leukemia
| Characteristics | Recovery rate (n = 48) | Total (n = 48) | Group A (no DLI) (n = 3) | Group B (DLI) (n = 45) | Chronic GvHD | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mild (n = 22) | Moderate (n = 24) | Severe (n = 2) | P* | P** | P*** | |||||
| PWB (mean ± SD) | 97.9% | 24.9 ± 3.6 | 28.0 ± 0.0 | 24.6 ± 3.6 | 26.5 ± 2.0 | 24.1 ± 2.9 | 16.0 ± 9.9 | 0.003 | 0.001 | 0.037 |
| SWB (mean ± SD) | 97.9% | 23.3 ± 3.2 | 26.3 ± 0.6 | 23.1 ± 3.2 | 23.3 ± 3.2 | 23.8 ± 2.6 | 17.5 ± 5.0 | 0.521 | 0.028 | 0.047 |
| EWB (mean ± SD) | 97.9% | 21.1 ± 3.0 | 23.0 ± 1.0 | 20.9 ± 3.0 | 22.3 ± 1.8 | 20.4 ± 2.8 | 15.0 ± 7.1 | 0.010 | 0.001 | 0.026 |
| FWB (mean ± SD) | 93.8% | 23.3 ± 4.7 | 26.7 ± 0.6 | 23.0 ± 4.7 | 23.9 ± 3.4 | 23.5 ± 4.0 | 12.5 ± 13.5 | 0.739 | 0.002 | 0.004 |
| BMTS (mean ± SD) | 97.9% | 40.3 ± 0.8 | 45.0 ± 1.0 | 39.9 ± 5.6 | 41.0 ± 5.2 | 40.8 ± 3.5 | 26.5 ± 14.8 | 0.875 | 0.003 | 0.001 |
| FACT‐G (mean ± SD) | 97.9% | 92.5 ± 12.2 | 104.0 ± 1.0 | 91.7 ± 12.2 | 96.0 ± 8.8 | 91.9 ± 8.9 | 61.0 ± 35.4 | 0.133 | 0.001 | 0.001 |
| FACT‐TOI (mean ± SD) | 97.9% | 88.4± 12.4 | 99.7 ± 0.6 | 87.6 ± 12.4 | 91.3 ± 9.6 | 88.4 ± 7.8 | 55.0 ± 38.2 | 0.266 | 0.001 | 0.001 |
| FACT‐BMTS (mean ± SD) | 97.9% | 132.7 ± 17.3 | 149.0 ± 0.0 | 131.6 ± 17.4 | 136.9 ± 13.9 | 132.7 ± 11.7 | 87.5 ± 50.2 | 0.267 | 0.001 | 0.001 |
P * represents the comparison between mild and moderate chronic GvHD; P ** represents the comparison between mild and severe chronic GvHD; P *** represents the comparison between moderate and severe chronic GvHD.
Abbreviations: DLI, donor lymphocyte infusion; GvHD, graft‐versus‐host disease; PWB, physical well‐being; SWB, social/family well‐being; EWB, emotional well‐being; FWB, functional well‐being; BMTS, bone marrow transplant subscale; FACT, functional assessment of cancer therapy; FACT‐TOI, FACT trial outcome index.
4. DISCUSSION
The present study suggested that in patients with refractory or relapsed AML, total therapy is associated with a CIR of 29.5% at 2 years and 31.5% at 5 years after transplantation and an LFS of 51.4% at 2 years and 46.4% at 5 years after transplantation. This result is significantly better than that reported in previous studies involving patients with refractory or relapsed AML (2‐year CIR: 29.5% vs. 40.0%‐60.0% and 2‐ year LFS: 51.4% vs. 20.0%‐40.0%) [4, 5, 6, 7, 8]. Moreover, this result is also similar to that in patients with AML in second complete remission (CR2) (2‐year CIR: 29.5% vs. 23.0%‐30.0%; 5‐year CIR: 31.5% vs. 25.0%‐32.0%; 2‐year LFS: 51.4% vs. 50.8%‐54.0%; and 5‐year LFS: 46.4% vs. 28.0%‐42.0%) [23, 24, 25, 26]. Although the cross‐trial comparison has a potential bias for conclusion, these results suggest that total therapy is most likely effective for patients with refractory or relapsed AML.
The efficacy of total therapy can be attributed to its capacity in making more patients achieve and maintain negative MRD results. Several studies have reported that a positive MRD result after transplantation correlates with an increased relapse risk [18, 19]. In the present study, 62.1% of patients achieved negative MRD results after prophylactic DLI. After MRD and GvHD‐guided DLI, 79.3% of patients achieved negative MRD results. The frequency of negative MRD results after transplantation was similar to those of patients with standard‐risk acute leukemia (79.3% vs. 87.1%) [15]. With the increasing courses of DLIs, the frequency of conversion to negative MRD results in patients with positive MRD results gradually increased (P = 0.014) while the relapse rate gradually decreased (P = 0.005). It is notable that among 69 patients with negative MRD results, 7 (10.1%) patients relapsed. This result suggests that the MRD‐test platform has some limitations. In the present study, MRD‐positivity was defined more strictly than in most prior reports to avoid excessive TRM associated with the application of DLI. This resulted in the MRD‐test exhibiting imperfect sensitivity but increased specificity. Our previous study suggested that the combined use of WT1 and FCM for the monitoring of MRD after transplantation was associated with a sensitivity of 50.0% and a specificity of 97.2% in AML patients [19]. Although compared with single MRD parameter (two consecutive positive results of WT1 or FCM), the combined use of WT1 and FCM was associated with higher sensitivity (50.0% vs. 33.3% vs. 25.9%) and relatively similar specificity (97.2% vs. 97.2% vs. 98.8%) [19], new techniques with higher sensitivity may be used to monitor the state of MRD after transplantation, such as quantitative‐digital PCR or next generation sequencing. However, the use of these techniques in the post‐transplant setting should be investigated in future studies.
Another probable reason to explain the efficacy of total therapy is a higher incidence of chronic GvHD in the present study. Researchers have demonstrated that the development of chronic GvHD usually induces a stronger GvL effect. After pre‐emptive DLI, the development of chronic GvHD was typically associated with a higher frequency of conversion to negative MRD results (85.7% vs. 16.7%) and a lower CIR (1‐year CIR: 3.2% vs. 11.9%, P = 0.002) [27]. The present study also suggested that the incidence and severity chronic GvHD were associated with lower CIR (P = 0.003 and P = 0.005) in patients with both positive and negative MRD results. Moreover, with the increasing courses of DLIs, the incidence of moderate to severe chronic GvHD (P = 0.001) and the frequency of conversion to negative MRD results gradually increased (P = 0.014); concurrently, the relapse rate gradually decreased (P = 0.005). Additionally, 18 patients in group B exhibited persistent positive MRD results, and 17 patients relapsed. These results suggest that these patients may need a more intensive therapy to achieve negative MRD results. Several novel drugs, such as hypomethylating agents [28] and Bcl‐2 inhibitors [29] have been used in combination with DLI in some pilot studies, and an enhanced GvL effect was observed [30]. However, this warrants further investigation.
Severe GvHD is a major risk of DLI and is always correlated with higher TRM [10]. However, compared with the previous study, the incidence of grade 2‐4 and grade 3‐4 acute GvHD in the present study was not significantly higher (grade 2‐4 acute GvHD: 40.6% vs. 31.0%‐49.0% and grade 3‐4 acute GvHD: 12.2% vs. 13.2%‐24.0%) [14, 31, 32]. Moreover, although the incidence of chronic GvHD was higher than that described in the previous study (73.3% vs. 41.2%‐45.7%) [14, 31, 32], the incidence of severe chronic GvHD was only 19.5%. The present study also suggested that total therapy was associated with a TRM of 19.0% at 2 years and 22.1% at 5 years after transplantation. This result was comparable with those of previous studies conducted on patients with refractory or relapsed AML (2‐year TRM: 19% vs. 16%‐26%) [4, 5, 6, 7, 8] and on patients with AML in first complete remission (CR1) or CR2 (2‐year TRM: 19.0% vs. 13.0%‐20.0%) [15, 33]. These results demonstrate the safety of total therapy. The safety of total therapy most likely benefits from the application of modified DLI. Several studies found that the application of G‐CSF could induce T cell hypo‐responsiveness [34, 35], and augment NKT cell‐dependent CD8+ cytotoxicity [36]. Additionally, our previous studies suggested that the use of short‐term immunosuppressive agents after HLA‐matched related DLI [11] or haploidentical DLI [12] could reduce the incidence of acute GvHD, but did not influence GvL effect. This resulted in that the duration of immunosuppressive agents used for the treatment of severe GvHD after DLI was significantly shorter [12]. This probably led to a better immune reconstitution, thus resulted in a lower incidence of infection, and a lower TRM.
In the present study, after total therapy, more than 90% of long‐term survivors were reported with satisfactory recovery of HR‐QoL. The mean HR‐QoL scores were good and comparable with those reported in a previous study conducted on patients with AML in CR1 [37]. Pidala et al. [20] reported that, compared with that of patients with mild or moderate chronic GvHD, patients with severe chronic GvHD had the poorest recovery of HR‐QoL. The present study also revealed similar results. In particular, there was no significant difference in the recovery of HR‐QoL between patients with mild and moderate chronic GvHD. These results suggest that although the incidence of moderate chronic GvHD gradually increases with increasing courses of DLIs, the patients’ HR‐QoL scores do not decrease. Thus, most patients exhibited satisfactory recovery of HR‐QoL.
The interpretation of our results entails some limitations. This was not a randomized control study. Instead, this was a single center prospective study, with a larger number of cases with refractory or relapsed AML (n = 105), longer follow‐up periods (86 months [range = 34.6‐124.2]), and with the assessment of HR‐QoL. Herein, total therapy resulted in better long‐term LFS and more satisfactory HR‐QoL scores in patients with refractory or relapsed AML than it did in patients who received the standard of care therapy reported in previous studies. Additionally, these results were comparable with those obtained from patients with AML in CR2. Therefore, total therapy could be an optimized therapeutic strategy for refractory or relapsed AML. In the future, a multicenter randomized control study may be needed to further confirm our results.
5. CONCLUSIONS
These data demonstrated that total therapy is associated with decreased CIR, comparable TRM, better long‐term LFS, and satisfactory HR‐QoL for patients with refractory or relapsed AML, compared with those of standard of care therapy reported previously. Therefore, this method may be an optimized therapeutic strategy for refractory or relapsed AML.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethics Committee of Peking University Peoples Hospital (Permit number: PUPH IRB [2010] (78)) and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
This manuscript has no shared data and materials.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Xiao‐jun Huang designed the study. Chen‐hua Yan analyzed the data and draft this manuscript. The other authors provided subject data and analyzed the data. All authors read and approved the final manuscript.
Supporting information
Supporting information
ACKNOWLEDGEMENTS
Not applicable.
Yan C‐H, Wang Y, Sun Y‐Q, Cheng Y‐F, Mo X‐D, Wang F‐R, et al. Optimized therapeutic strategy for patients with refractory or relapsed acute myeloid leukemia: long‐term clinical outcomes and health‐related quality of life assessment. Cancer Commun. 2022;42:1387–1402. 10.1002/cac2.12376
Trial registration: This study was registered at www.ClinicalTrials.gov with the accession number NCT01455272.
REFERENCES
- 1. Breems DA, Van Putten WLJ, Huijgens PC, Ossenkoppele GJ, Verhoef GEJ, Verdonck LF, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23(9):1969–78. [DOI] [PubMed] [Google Scholar]
- 2. Schlenk RF, Döhner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008; 358(18):1909–18. [DOI] [PubMed] [Google Scholar]
- 3. Ou Z, Yu D, Liang Y, He W, Li Y, Zhang M, et al. Analysis of the Global Burden of Disease study highlights the trends in death and disability‐adjusted life years of leukemia from 1990 to 2017. Cancer Commun (Lond). 2020;40(11):598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Poiani M, Labopin M, Battipaglia G, Beelen DW, Tischer J, Finke J, et al. The impact of cytogenetic risk on the outcomes of allogeneic hematopoietic cell transplantation in patients with relapsed/refractory acute myeloid leukemia: On behalf of the acute leukemia working party (ALWP) of the European group for blood and marrow transplantation (EBMT). Am J Hematol. 2021;96(1):40–50. [DOI] [PubMed] [Google Scholar]
- 5. Saraceni F, Labopin M, Brecht A, Kröger N, Eder M, Tischer J, et al. Fludarabine‐treosulfan compared to thiotepa‐busulfan‐fludarabine or FLAMSA as conditioning regimen for patients with primary refractory or relapsed acute myeloid leukemia: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT). J Hematol Oncol. 2019;12(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodríguez‐Arbolí E, Labopin M, Tischer J, Brecht A, Ganser A, Finke J, et al. FLAMSA‐Based Reduced‐Intensity Conditioning versus Myeloablative Conditioning in Younger Patients with Relapsed/Refractory Acute Myeloid Leukemia with Active Disease at the Time of Allogeneic Stem Cell Transplantation: An Analysis from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2020;26(11):2165–73. [DOI] [PubMed] [Google Scholar]
- 7. Brissot E, Labopin M, Ehninger G, Stelljes M, Brecht A, Ganser A, et al. Haploidentical versus unrelated allogeneic stem cell transplantation for relapsed/refractory acute myeloid leukemia: a report on 1578 patients from the Acute Leukemia Working Party of the EBMT. Haematologica. 2019;104(3):524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brissot E, Labopin M, Stelljes M, Ehninger G, Schwerdtfeger R, Finke J, et al. Comparison of matched sibling donors versus unrelated donors in allogeneic stem cell transplantation for primary refractory acute myeloid leukemia: a study on behalf of the Acute Leukemia Working Party of the EBMT. J Hematol Oncol. 2017;10(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Or R, Hadar E, Bitan M, Resnick IB, Aker M, Ackerstein A, et al. Safety and efficacy of donor lymphocyte infusions following mismatched stem cell transplantation. Biol Blood Marrow Transplant. 2006;12(2):1295–1301. [DOI] [PubMed] [Google Scholar]
- 10. Yan CH, Liu DH, Xu LP, Liu KY, Zhao T, Wang Y, et al. Modified donor lymphocyte infusion‐associated acute graft‐versus‐host disease after haploidentical T‐cell‐replete hematopoietic stem cell transplantation: incidence and risk factors. Clin Transplant. 2012;26(6):868–76. [DOI] [PubMed] [Google Scholar]
- 11. Huang XJ, Wang Y, Liu DH, Xu LP, Liu KY, Chen H, et al. Administration of short‐term immunosuppressive agents after DLI reduces the incidence of DLI‐associated acute GVHD without influencing the GVL effect. Bone Marrow Transplant. 2009;44(5):309–16. [DOI] [PubMed] [Google Scholar]
- 12. Yan CH, Xu LP, Liu DH, Chen H, Wang Y, Liu KY, et al. Immunosuppression for 6‐8 weeks after modified donor lymphocyte infusion reduced acute graft‐versus‐host disease without influencing graft‐versus‐leukemia effect in haploidentical transplant. Chin Med J (Engl). 2014;127(20):3602–9. [PubMed] [Google Scholar]
- 13. Wang Y, Liu DH, Fan ZP, Sun J, Wu XJ, Ma X, et al. Prevention of relapse using DLI can increase survival following HLA‐identical transplantation in patients with advanced‐stage acute leukemia: a multi‐center study. Clin Transplant. 2012;26(4):635–43. [DOI] [PubMed] [Google Scholar]
- 14. Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Zhang XH, et al. Prevention of relapse using granulocyte CSF‐primed PBPCs following HLA‐mismatched/haploidentical, T‐cell‐replete hematopoietic SCT in patients with advanced‐stage acute leukemia: a retrospective risk‐factor analysis. Bone Marrow Transplant. 2012;47(8):1099–1104. [DOI] [PubMed] [Google Scholar]
- 15. Yan CH, Liu DH, Liu KY, Xu LP, Liu YR, Chen H, et al. Risk stratification‐directed donor lymphocyte infusion could reduce relapse of standard‐risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood. 2012;119 (14):3256–62. [DOI] [PubMed] [Google Scholar]
- 16. Yan CH, Liu QF, Wu DP, Zhang X, Xu LP, Zhang XH, et al. Prophylactic Donor Lymphocyte Infusion (DLI) Followed by Minimal Residual Disease and Graft‐versus‐Host Disease‐Guided Multiple DLIs Could Improve Outcomes after Allogeneic Hematopoietic Stem Cell Transplantation in Patients with Refractory/Relapsed Acute Leukemia. Biol Blood Marrow Transplant. 2017;23(8):1311–9. [DOI] [PubMed] [Google Scholar]
- 17. Lu DP, Dong LJ, Wu T, Huang XJ, Zhang MJ, Han W, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA‐mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA‐identical sibling transplantation. Blood. 2006;107(8):3065–73. [DOI] [PubMed] [Google Scholar]
- 18. Chang YJ, Wang Y, Liu YR, Xu LP, Zhang XH, Chen H, et al. Haploidentical allograft is superior to matched sibling donor allograft in eradicating pre‐transplantation minimal residual disease of AML patients as determined by multiparameter flow cytometry: a retrospective and prospective analysis. J Hematol Oncol. 2017;10(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao XS, Yan CH, Liu DH, Xu LP, Liu YR, Liu KY, et al. Combined use of WT1 and flow cytometry monitoring can promote sensitivity of predicting relapse after allogeneic HSCT without affecting specificity. Ann Hematol. 2013; 92(8):1111‐9. [DOI] [PubMed] [Google Scholar]
- 20. Pidala J, Kurland B, Chai XY, Majhail N, Weisdorf DJ, Pavletic S, e al. Patient‐reported quality of life is associated with severity of chronic graft‐versus‐host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117(17):4651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tomas ED, Storb R, Clift RA, Fefer A, Johnson L, Neiman PE, et al. Bone marrow transplantation. N Engl J Med. 1975;92(17):895–902. [DOI] [PubMed] [Google Scholar]
- 22. Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft‐versus‐host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–56. [DOI] [PubMed] [Google Scholar]
- 23. Wang Y, Liu DH, Liu KY, Xu LP, Zhang XH, Han W, et al. Impact of pretransplantation risk factors on post transplantation outcome of patients with acute myeloid leukemia in remission after haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(2):283–90. [DOI] [PubMed] [Google Scholar]
- 24. Malard F, Labopin M, Stuhler G, Bittenbring J, Ganser A, Tischer J, et al. Sequential Intensified Conditioning Regimen Allogeneic Hematopoietic Stem Cell Transplantation in Adult Patients with Intermediate‐ or High‐Risk Acute Myeloid Leukemia in Complete Remission: A Study from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2017;23(2):278–84. [DOI] [PubMed] [Google Scholar]
- 25. Weisdorf DJ, Millard HR, Horowitz MM, Hyare PS, Champlin R, Ho V, et al. Allogeneic transplantation for advanced acute myeloid leukemia: The value of complete remission. Cancer. 2017;123(11):2025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sierra J, Storer B, Hansen JA, Martin PJ, Petersdorf EW, Woolfrey A, et al. Unrelated donor marrow transplantation for acute myeloid leukemia: an update of the Seattle experience. Bone Marrow Transplant. 2000;26(4):397–404. [DOI] [PubMed] [Google Scholar]
- 27. Mo XD, Xu LP, Zhang XH, Liu DH, Wang Y, Chen H, et al. Chronic GVHD induced GVL effect after unmanipulated haploidentical hematopoietic SCT for AML and myelodysplastic syndrome. Bone Marrow Transplant. 2015;50(1):127–33. [DOI] [PubMed] [Google Scholar]
- 28. Guillaume T, Thépot S, Peterlin P, Ceballos P, Le Bourgeois A, Garnier A, et al. Prophylactic or Preemptive Low‐Dose Azacitidine and Donor Lymphocyte Infusion to Prevent Disease Relapse following Allogeneic Transplantation in Patients with High‐Risk Acute Myelogenous Leukemia or Myelodysplastic Syndrome. Transplant Cell Ther. 2021;27(10):839.e1‐839.e6. [DOI] [PubMed] [Google Scholar]
- 29. Amit O, On YB, Perez G, Shargian‐Alon L, Yeshurun M, Ram R. Venetoclax and donor lymphocyte infusion for early relapsed acute myeloid leukemia after allogeneic hematopoietic cell transplantation. A retrospective multicenter trial. Ann Hemato. 2021;100(3):817–24. [DOI] [PubMed] [Google Scholar]
- 30. Kwon YR, Kim HJ, Sohn MJ, Lim JY, Park KS, Lee S et al. Effects of decitabine on allogeneic immune reactions of donor lymphocyte infusion via activation of dendritic cells. Exp Hematol Oncol. 2020;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jaiswal SR, Zaman S, Chakrabarti A, Sen S, Mukherjee S, Bhargava S, et al. Improved Outcome of Refractory/Relapsed Acute Myeloid Leukemia after Post‐Transplantation Cyclophosphamide‐Based Haploidentical Transplantation with Myeloablative Conditioning and Early Prophylactic Granulocyte Colony‐Stimulating Factor‐Mobilized Donor Lymphocyte Infusions. Biol Blood Marrow Transplant. 2016;22(10):1867–73. [DOI] [PubMed] [Google Scholar]
- 32. Schmid C, Schleuning M, Ledderose G, Tischer J, Kolb HJ. Sequential regimen of chemotherapy, reduced‐intensity conditioning for allogeneic stem‐cell transplantation, and prophylactic donor lymphocyte transfusion in high‐risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23(24):5675–87. [DOI] [PubMed] [Google Scholar]
- 33. Baron F, Labopin M, Ruggeri A, Cornelissen JJ, Meijer E, Sengeloev H, et al. Impact of Donor Type in Patients with AML Given Allogeneic Hematopoietic Cell Transplantation After Low‐Dose TBI‐Based Regimen. Clin Cancer Res. 2018;24(12):2794–2803. [DOI] [PubMed] [Google Scholar]
- 34. Huang XJ, Chang YJ, Zhao XY. In vivo induction of T‐cell hyporesponsiveness and alteration of immunological cells of bone marrow grafts using granulocyte colony‐stimulating factor. Haematologica. 2004;89(12):1517–24. [PubMed] [Google Scholar]
- 35. Huang XJ, Chang YJ, Zhao XY. Maintaining hyporesponsiveness and polarization potential of T cells after in vitro mixture of G‐CSF mobilized peripheral blood grafts and G‐CSF primed bone marrow grafts in different proportions. Transpl Immunol. 2007; 17(3):193–7. [DOI] [PubMed] [Google Scholar]
- 36. Morris ES, MacDonald KP, Rowe V, Banovic T, Kuns RD, Don ALJ, et al. NKT cell‐ dependent leukemia eradication following stem cell mobilization with potent G‐CSF analogs. J Clin Invest. 2005;115(11):3093–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wright R, Oremek M, Davies D, Kewley C, Singh A, Taitt N, et al. Quality of Life following Allogeneic Stem Cell Transplantation for Patients Age >60 Years with Acute Myelogenous Leukemia. Biol Blood Marrow Transplant. 2020;26(8):1527–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
This manuscript has no shared data and materials.


