Abstract
Clustered regularly interspaced short palindromic repeats‐associated protein (CRISPR/Cas9), an adaptive microbial immune system, has been exploited as a robust, accurate, efficient and programmable method for genome targeting and editing. This innovative and revolutionary technique can play a significant role in animal modeling, in vivo genome therapy, engineered cell therapy, cancer diagnosis and treatment. The CRISPR/Cas9 endonuclease system targets a specific genomic locus by single guide RNA (sgRNA), forming a heteroduplex with target DNA. The Streptococcus pyogenes Cas9/sgRNA:DNA complex reveals a bilobed architecture with target recognition and nuclease lobes. CRISPR/Cas9 assembly can be hijacked, and its nanoformulation can be engineered as a delivery system for different clinical utilizations. However, the efficient and safe delivery of the CRISPR/Cas9 system to target tissues and cancer cells is very challenging, limiting its clinical utilization. Viral delivery strategies of this system may have many advantages, but disadvantages such as immune system stimulation, tumor promotion risk and small insertion size outweigh these advantages. Thus, there is a desperate need to develop an efficient non‐viral physical delivery system based on simple nanoformulations. The delivery strategies of CRISPR/Cas9 by a nanoparticle‐based system have shown tremendous potential, such as easy and large‐scale production, combination therapy, large insertion size and efficient in vivo applications. This review aims to provide in‐depth updates on Streptococcus pyogenic CRISPR/Cas9 structure and its mechanistic understanding. In addition, the advances in its nanoformulation‐based delivery systems, including lipid‐based, polymeric structures and rigid NPs coupled to special ligands such as aptamers, TAT peptides and cell‐penetrating peptides, are discussed. Furthermore, the clinical applications in different cancers, clinical trials and future prospects of CRISPR/Cas9 delivery and genome targeting are also discussed.
Keywords: cancer, clinical trials, CRISPR/Cas9, genome editing, mechanism, nanoparticles, structure
Abbreviations
- Cas
CRISPR‐associated system
- CPP
Cell‐penetrating peptide
- CRISPR/Cas9
Clustered regularly interspaced short palindromic repeats‐associated protein
- crRNAs
CRISPR RNAs
- CTD
Carboxy‐terminal domain
- DSB
Double‐stranded break
- EGFR
Epidermal growth factor receptor
- gRNA
Guide RNA
- HDR
Homology‐directed repair
- Indels
Insertions and/or deletions
- LNPs
Lipid NPs
- NHEJ
Non‐homologous end joining
- NLS
Nuclear localization sequence
- NPs
Nanoparticles
- NUC lobe
Nuclease lobe
- PAM
Protospacer adjacent motif
- PDB
Protein data bank
- PEG
Polyethylene glycol
- REC lobe
Recognition lobe
- RNP
Ribonucleoprotein
- sgRNA
Single guide RNA
- TALENs
Transcription activator‐like effector nucleases
- tracrRNA
Trans‐activating crRNA
- VEGF
Vascular endothelial growth factor
- ZFNs
Zinc finger nucleases
1. BACKGROUND
Genetic engineering has gained robust momentum by the emergence of clustered regularly interspaced short palindromic repeats (CRISPR)‐associated system (Cas) (CRISPR/Cas) genome editing strategy [1]. Among different classes and categories of the CRISPR/Cas system based on DNA and RNA targeting, CRISPR/Cas9 represents the most simple and best‐studied system and earned the Nobel Prize in 2020. The recent advances in the CRISPR/Cas9 system have gained tremendous attention for its precise genome targeting and editing in different model systems, including human cells. Basically, it is a bacterial defense system against mobile genetic elements, plasmid transfer and phage infections. This system has been repurposed as a robust tool of RNA‐guided DNA targeting for genome editing. In addition to genome editing, this system has been applied for transcriptional regulation, epigenetic modeling and genome imaging. With the help of the CRISPR/Cas9 system, precise manipulation of any DNA sequence is possible, defined by a short stretch of guide RNA (gRNA) [2]. This technique allows us to elucidate the proper role of some genes in the development and progression of different diseases.
The crystal structure of Streptococcus pyogenes (Sp)‐Cas9 in complex with single guide RNA (sgRNA) and target DNA at a resolution of 2.5 Å has been previously reviewed [3]. The Cas9 reveals a bilobed architecture with nuclease (NUC) and target recognition (REC) lobes, having a positively charged groove at the interface for accommodating sgRNA:DNA heteroduplex. The NUC lobe contains RuvC and HNH nuclease domains, whereas the REC lobe binds to sgRNA and DNA. The target DNA is positioned properly for its cleavage at complementary and non‐complementary strands. Furthermore, the carboxy‐terminal domain (CTD) present in the NUC lobe has been reported to be essential for the interaction with the protospacer‐adjacent motif (PAM) sequence [3]. The domain organization and three‐dimensional structure of Streptococcus pyogenes Cas9‐sgRNA‐DNA ternary complex is shown, obtained from the protein data bank (PDB) (https://www.rcsb.org) with PDB code 4OO8 (Figure 1).
FIGURE 1.
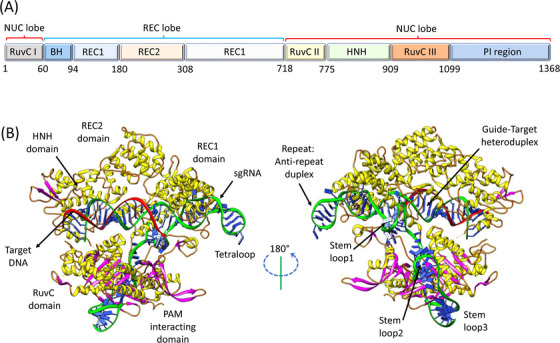
Overall three‐dimensional structure of Streptococcus pyogenes Cas9‐sgRNA‐DNA ternary complex. (A) Domain organization. (B) Ribbon representation of Cas9‐sgRNA‐DNA complex at different angles, obtained from the protein data bank (https://www.rcsb.org, PDB ID: 4OO8), edited using software developed by Resource for Biocomputing, Visualization, and Informatics at University of California, San Francisco Chimera. Abbreviations: NUC, nuclease; REC, recognition; PI, PAM interacting; PAM, protospacer adjacent motif; sgRNA, single‐guide RNA
CRISPR/Cas9 has emerged as a versatile tool for the study and treatment of diverse cancers. In the last few years, this genome editing technology has been widely applied in cancer research to understand the mechanism of cancer progression and the advancements in various cell‐based therapies. This strategy can also be used to activate the deactivated tumor suppressor genes and inactivate oncogenes, besides amendment of the disease‐causing mutations [4]. The CRISPR/Cas9 system utilizes a single programmable endonuclease strategy or modulation of several gene functions by instantly targeting multiple genomic loci designed in a single experiment [5]. This strategy has broadened our views about the pathological phenomena involving several mutations or genes. The CRISPR/Cas9 system can help identify disease‐resistant genes and rapidly assess drug targets [6]. Thus, genome engineering using the CRISPR/Cas9 strategy seems promising in curing genetic disorders, immunological disorders, viral infections, neurodegeneration, cardiovascular diseases, and cancers [7]. The effective use of the CRISPR/Cas9 methodology for genome engineering involves escaping and minimizing potential undesirable off‐target mutations for suitable clinical applications [8]. A complete genome editing strategy of using the CRISPR/Cas9 methodology is still a great challenge, as some obstacles remain to be resolved [9].
The precise delivery of the CRISPR/Cas9 system as ribonucleoproteins (RNPs) within specific cells or tissues via viral vectors and non‐viral carriers is the most challenging. Some reasons include degradation or denaturation of RNPs during formulation and delivery processes, large size of Cas9, and excessive negative charge of sgRNA. Recently, several non‐viral nanoformulations have been used for in vitro RNP delivery within target cells. These formulations include cationic lipid nanoparticles (NPs) and lipoplexes [10], DNA clews [11], zeolitic imidazole frameworks [12], and gold NPs (AuNPs) [13]. However, it is challenging to control the stability, size, and uniformity of the resulting nanoformulations [14].
Even though the translational potential of the CRISPR/Cas9 system has been increasingly explored, translation of this system in clinical trials remains a challenge. Some major concerns include safety, vast immunological effects, uncontrolled off‐target complications, Cas nuclease immunogenicity, and induction of carcinogenic effects by CRISPR components. The ultimate precision and efficiency are highly recommended for the future use of the CRISPR/Cas9 system to minimize off‐target effects. Another concern of Cas9 is its immunogenicity which needs to be properly considered during its clinical translation. Some donors contain naturally occurring Cas9 antibodies in their serum, with 65% exhibiting anti‐SpCas9 and 79% exhibiting anti‐saCas9 [15]. In one study, 96% of the donors were evaluated, reporting a pre‐existing T‐cell immune memory against SpCas9 [16]. Furthermore, low editing efficiency is caused by human anti‐Cas9 immune response, while some patients undergoing CRISPR/Cas9 treatment may suffer from severe immune responses.
This review aimed to summarize recent updates of nanoformulation‐based CRISPR/Cas9‐mediated genome editing within diverse tumor cells. In this review, we elaborated on the conventional cancer treatment strategies, history and current updates about CRISPR/Cas9 biology, structural and mechanistic perceptions into RNA‐guided DNA targeting and cleavage by Cas9 enzyme. In addition, CRISPR/Cas9 nanoformulation‐based delivery within cancer cells and clinical applications in different cancers are discussed. At last, challenges, future prospects, and clinical trials are also discussed.
2. DIFFERENT CANCER TREATMENT STRATEGIES AND THEIR LIMITATIONS
Even though significant progress has been achieved in medicine, cancer still remains the reason of death for millions of people. Tremendous efforts are put forward by oncological researchers to search for more novel therapeutics to alleviate the critical complications caused by conventional methods. Additional strategies have been introduced in the recent past to treat cancer in clinical practice or are under evaluation in clinical trials. Some well‐known cancer treatment strategies include nanomedicine, extracellular vesicles (EVs), natural antioxidants, targeted therapy, gene therapy, thermal ablation, radiomics, and pathomics [17].
Conventional chemotherapeutic drugs are now administered as nanomedicine in the form of NPs, as a versatile platform of biodegradable and biocompatible systems, increasing their concentration and bioavailability near the tumor mass, thus improving their therapeutic profile. The use of such NPs is exploited for diverse applications, ranging from diagnosis to therapy [18]. The proper designing of anticancer drug‐loaded NPs specific to each cancer type is a big challenge in such a treatment plan.
Circulating EVs are clinically significant for the early identification of biomarkers for cancer diagnosis, prognosis prediction, and follow‐up, which can be isolated and exploited as anti‐tumor vaccines or nanosized drug cargoes for cancer therapy [18]. However, some challenges related to the use of EVs for the clinical translation include its isolation, quantification, storage and standard protocols for drug loading.
The magnetic hyperthermia and thermal ablation of different tumors are opening new prospects for precision medicine, with localized treatment in very narrow and precise areas. These approaches could be a novel substitute for more invasive practices such as surgery [19]. However, these approaches also have some limitations, such as low penetration power, efficiency only for localized areas, and the need for a highly skilled operator for treatment performance.
Furthermore, some new fields of cancer therapeutics, such as pathomics and radiomics, contribute in data collection for the utilization of other treatment strategies with predicted response, better clinical outcome, and minimum cancer recurrence [20, 21]. However, these approaches are laborious, as it requires to follow univocal data acquisition guidelines, description of parameters and statistical/computational methods to set, and standardization of procedures to facilitate clinical translation. All together, all these strategies provide only limited personalized anticancer therapies, highlighting the importance of combining multiple disciplines to get the best outcome.
Another promising opportunity relies on gene therapy and expression of genes triggering apoptosis and wild‐type cancer suppressors or the targeted silencing mediated through siRNA [22]. This cancer treatment approach is under evaluation in different clinical trials globally [23]. However, this approach has several challenges that make it less practicable. These challenges include genome integration, off‐target effects, limited efficacy in a specific subset of patients, need of an ad hoc delivery system for RNA interference, high chance of neutralization by the immune system, set up of suitable conditions, and controlled RNA interference.
Zinc‐finger nucleases (ZFNs) and transcription activator‐like effector nucleases (TALENs) comprise a powerful class of tools that are redefining the boundaries of biological research. However, target‐based designing of ZFNs and TALENs limits theri broader applications. These gene‐editing systems have been used to change some important genes such as oncogenes, thus rendering them non‐functional [24].
Compared to conventional genome editing systems using ZFNs and TALENs, the CRISPR/Cas9 system offers several advantages. These advantages mainly include high efficiency, target design simplicity (sgRNA synthesis and its modification to direct Cas9), and multiplexed mutations. CRISPR/Cas9 has presented a great promise in identifying the essential genes, important to regulate various biological activities, in addition to assistance in drug targeting and developing innovative therapies against a wide range of diseases [25]. Moreover, CRISPR/Cas9 can be used to induce permanent as well as non‐permanent modifications in DNA by CRISPR interference (CRISPRi) or CRISPR activation (CRISPRa) [26]. However, all these gene‐editing technologies have some limitations and complications, such as off‐target effects, mosaicism, and the formation of multiple alleles.
3. HISTORY OF CRISPR
CRISPR was first reported in 1987 in E. coli DNA as a curious set of repeats interspaced by non‐repetitive sequences [27]. The importance of this discovery was not seriously recognized at that time, but 13 years later, in 2000, these sequences were proposed to exist in most prokaryotes [27]. In 2002, these repetitive DNA sequences, present in both prokaryotes and archaea, but absent in viruses and eukaryotes, caught the attention of researchers. This family of repetitive DNA sequences, referred to as CRISPR, is characterized by 21 to 37 bp direct repeats interspaced by non‐repetitive sequences of a similar size [28]. Since then, CRISPR has opened its legendary path, and 5 years later, the role of CRISPR in acquired immunity in prokaryotes was confirmed for the first time [29]. In 2010, based on the structure and sequence of the Cas protein, the CRISPR/Cas system was classified into types I‐III with further subtypes [30]. Later, it was observed that transactivating CRISPR RNA (tracrRNA) forms a duplex structure with CRISPR RNA (crRNA) in association with Cas9. This discovery led to a rapid momentum of CRISPR/Cas9 research and the Cas9/crRNA/tracrRNA complex was widely used to cut double‐stranded DNA (dsDNA) targets complementary to the 20 nt guide sequence in crRNA [31].
In 2013, CRISPR/Cas9 research moved from prokaryotes to eukaryotes, as the CRISPR/Cas9 system was used for mammalian cell genome editing [32]. CRISPR/Cas9 was efficiently used to generate knockout mice [33, 34]. This discovery stamped the CRISPR/Cas9 as a powerful genetic engineering tool. Later, in a human liver cancer cell line Huh 7.5 OC, the genome‐wide screening of almost 700 genes associated with cancer and other diseases with long non‐coding RNA (lncRNA) was performed [34]. In October 2016, the application of CRISPR was used for the first time to knockout the programmed cell death protein‐1 (PD‐1) gene in T cells isolated from cancer patients [35]. In 2017, Cox et al. [36] discovered a new method while working on human cells by fusing RNA‐editing enzymes with target RNA‐targeted Cas protein, which can edit specific nucleotides, and this technique was named RNA editing for programmable A‐to‐I replacement (REPAIR). This discovery led to some controversy as the risk of cancer was believed to result from CRISPR/Cas9 treatment [37]. However, in 2018, further development of single‐base editing by CRISPR gene‐editing technology disrupted this panic [38]. In brief, CRISPR/Cas9 has emerged as a cutting‐edge gene‐editing technology, which plays a significant role in genetic engineering and has gained the Nobel Prize in 2020.
4. CRISPR/CAS9 BIOLOGY AND MECHANISM OF ACTION
The CRISPR/Cas9 system is encoded by CRISPR loci accompanied by CRISPR‐associated (cas) genes and forms an RNA‐guided adaptive immune system in many bacteria and archaea [39] (Figure 2). The exposure to foreign plasmids, mobile genetic elements, and phage DNA leads to its integration into the CRISPR repeat spacer array within the bacterial chromosome as a new spacer [40]. So this integration provides a new genetic record of past infections, enabling the bacteria to counteract future invasion by the same attack [29]. The CRISPR array transcription is followed by endonucleolytic cleavage of these transcripts and yields short mature CRISPR RNAs (crRNAs) [41]. The 5′‐end of crRNA includes a spacer, a small segment of RNA that matches with foreign genetic element sequence, and a 3′‐end having a sequence of CRISPR repeat (Figure 2).
FIGURE 2.
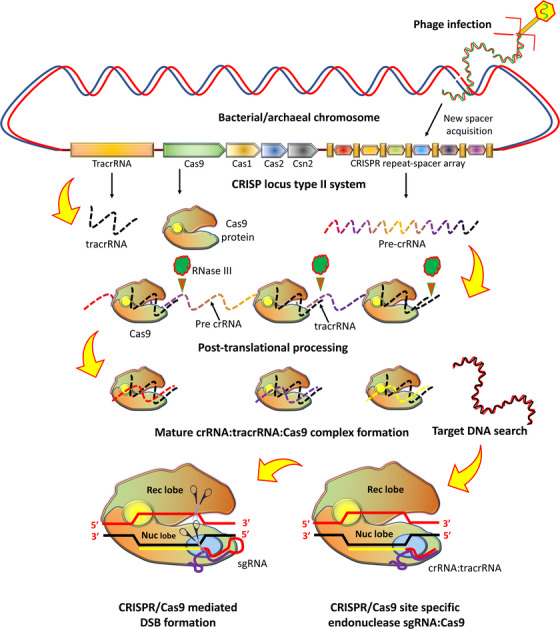
Biology of the CRISPR/Cas9 system and relevant transcription/translation products. Engineered CRISPR/Cas9 system can be devised for site‐specific genome editing as sgRNA:Cas9. Abbreviations: Cas9, CRISPR associated protein 9; CRISPR, clustered regularly interspaced short palindromic repeats; tracrRNA, trans‐activating CRISPR RNA; Rec, recognition; Nuc, nuclease; sgRNA, single‐guide RNA
Any complementary sequence matching between foreign target (proto‐spacer) and the crRNA spacer initiates sequence‐specific damage of assaulting RNA or DNA by Cas nucleases during a second infection [42]. The association of Cas proteins with mature crRNAs to form the CRISPR/Cas system can catechize foreign nucleic acid targets and extinguish any matching sequences [43]. Notably, in most CRISPR/Cas systems, PAM, a short conserved sequence (2‐5 bp), is positioned in close vicinity to the crRNA‐targeted sequence on the foreign assaulting DNA and plays a significant role of double check in selection and degradation of target DNA [44]. According to the present classification of CRISPR‐cas loci, the CRISPR system is categorized into types I‐VI [30], each employing a distinct set of Cas proteins accompanied by crRNA for CRISPR interference [45] and type III systems employ large multi‐Cas protein complexes that bind with crRNA and degrade the target sequence. In contrast, the type II CRISPR system utilizes a single protein as DNA endonuclease, Cas9, with distinct NUC domains (RuvC or HNH) that cleave the target DNA [46]. In addition, tracrRNA, a small non‐coding RNA, is required, which forms a dual‐RNA hybrid during base pairing with crRNA. This dual RNA guides Cas9 to initiate a DNA cleavage with a complementary 20 nucleotide (nt) target sequence and an adjoining PAM [31, 33]. For the maturation of crRNA, the requirement of tracrRNA is important in type II systems [47]. The combination of tracrRNA and crRNA as chimeric sgRNA (Figure 3) simplifies the system and retains fully Cas9‐mediated sequence‐specific DNA cleavage function [46].
FIGURE 3.
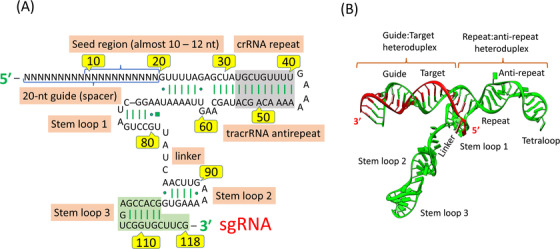
The secondary structure of sgRNA complexed with the target DNA. (A) sgRNA showing extra repeat‐antirepeat regions usually truncated in designing sgRNAs for genomic engineering. (B) Ribbon representation of sgRNA‐DNA complex. Abbreviations: crRNA, CRISPR RNA; tracrRNA, trans‐activating CRISPR RNA
The CRISPR/Cas9 system can be easily programmed to target effectively any DNA sequence within a genome by changing the guide RNA (gRNA) sequence. On the recognition of target DNA, the two domains of Cas9 (RuvC and HNH) sunder the double strands of DNA. A blunt‐ended double‐stranded break (DSB) is produced by Cas9, which is repaired by non‐homologous end joining (NHEJ) or homology‐directed repair (HDR) [48]. This results in randomly small insertions and/or deletions (indels) at the cleavage location. Furthermore, at the site of DSB, a defined genome amendment occurs with high fidelity by a homologous repair template. In addition, the introduction of premature stop codons and frame shift mutations caused by NHEJ can lead to premature inhibition of gene expression and gene knockout, respectively (Figure 4). A 20‐nt gRNA sequence determines the DNA recognition and its editing by CRISPR/Cas9 system, which is not specified by protein [49].
FIGURE 4.
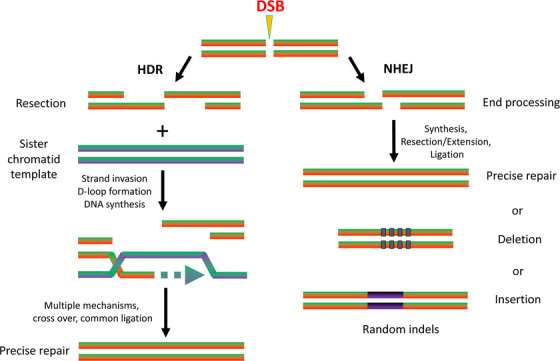
Formation of a DSB and repair by NHEJ and HDR. Repair by NHEJ results in the formation of random indels. Repair by HDR requires a template DNA strand for precise repair. Abbreviations: DSB, double‐stranded break; HDR, homology‐directed repair; NHEJ, non‐homologous end joining
In contrast, conventional DNA‐editing techniques such as transcription activator‐like effector nuclease (TALENs) and zinc finger nucleases (ZFNs) are specified fully by proteins [50]. Thus, the CRISPR/Cas9 system eliminates the need for DNA‐recognition protein engineering for site‐specific DNA modification [51]. The limitations of such tedious tasks have profoundly boosted CRISPR/Cas9 applicability for wide‐ranging genomic screening and manipulation. The high efficiency, ease of design, and simplicity in the use of the CRISPR/Cas9 system are the reasons for its robust implementation as a powerful tool for genome editing in a wide range of organisms [4].
4.1. Cas9 enzyme structure and functions
Cas9 from Streptococcus pyogenes consists of a multidomain structure with 1368 amino acids possessing multifunctional DNA endonuclease activity. It consists of two distinct nuclease domains: the HNH‐like nuclease domain and the RuvC‐like nuclease domain. The HNH‐like nuclease domain snips the target strand complementary to the gRNA sequence. The RuvC‐like nuclease domain nicks the non‐target strand, which is opposite to the complementary strand [52, 53]. Furthermore, Cas9 is involved in crRNA maturation and spacer acquisition [54]. The apo state of Cas9 possesses two lobes: the REC lobe and the NUC lobe with HNH and RuvC nuclease domains and CTD [55]. The REC lobe consists of three regions: a bridge helix (residue 60‐93), the REC1 domain (residue 94‐79 and 308‐713), and the REC2 domain (residue 180‐307).
The PAM‐interacting sites (residue 1099‐1368) are present in elongated CTD, which possesses a Cas9‐specific fold. Apo‐Cas9 comprises the PAM‐recognition sites as largely disordered, indicating that inactive configuration is maintained in the apo‐Cas9 enzyme, which cannot recognize the target DNA before the binding of gRNA. The Cas9 RuvC nuclease domains (residue 1‐59, 718‐769 and 909‐1098) resemble retroviral integrase in structure and are characterized by RNase H fold. For the cleavage of non‐target DNA strands, RuvC uses a two‐metal‐ion catalytic mechanism [3]. The HNH nuclease domain (residue 775‐908) of SpyCas9, like other HNH endonucleases, adopts the ββα‐metal signature fold for the target DNA cleavage and mostly employs a one‐metal‐ion mechanism. The trademarks of one‐ or two‐metal‐ion‐dependent DNA cleavage are conserved in histidine and aspartic acid residue and are consistent with Cas9 mutagenesis [56]. The mutation of either HNH (H840A) or RuvC domain (D10A) transforms Cas9 into nickase, whereas the mutation of both the Cas9 nuclease domains leaves its RNA‐guided DNA binding capacity unchanged but eliminates its endonuclease activity, resulting in dead Cas9 or dCas9 enzyme [46] (Figure 1).
4.2. Assembly of the CRISPR/Cas9 complex
For the recognition of site‐specific DNA cleavage, Cas9 assembles with gRNA, a native crRNA‐tracrRNA complex, and forms an active DNA surveillance complex [54]. The tracrRNA performs a vital role in Cas9 recruitment, while the DNA target specificity is achieved by the 20‐nt spacer sequence of crRNA [49]. For the target specificity, the sequence of nucleotides within the spacer RNA of crRNA is principally important [57]. Any mismatch in this region affects severely the abrogation of target DNA binding and cleavage and close homology in the seed region and leads to off‐target binding incidents [58].
Comparing sgRNA‐bound structures to apo‐Cas9 illustrates how gRNA binding initiates Cas9 structural rearrangements. Low‐resolution electron microscopic studies have revealed a significant structural reorganization between inactive conformations and DNA recognition conformation. The majority of structural reorganization of Cas9 occurs before the target DNA binding, thus emphasizing that gRNA loading may act as a key regulator of Cas9 activity. In the REC lobe, the binding of sgRNA pushes Hel‐III almost 65A° toward the HNH domain, that represent the most prominent conformational changes [55].
4.3. Target search, recognition and cleavage
After the complex formation between Cas9 and gRNA, the target DNA complementary sites were searched [59]. A complementary base pairing is crucial between the protospacer of target DNA and the 20‐nt spacer sequence. In addition, the presence of a conserved PAM sequence adjoining the target site is crucial [31]. The sequence of PAM is significant for distinguishing self and non‐self sequences [60]. Any single mutation in this region disables Cas9 cleavage, and bacteriophage can dodge the immune response of host [61]. A constant three‐dimensional collision between Cas9 and DNA occurs, and once the target site locates its appropriate PAM sequence, DNA melting is triggered at the PAM‐adjacent nucleation site. This step is followed by RNA strand invasion and forms a DNA‐RNA hybrid, in addition to displaced DNA strand (termed as R‐loop) [62].
The entire 20‐nt spacer sequence of sgRNA hybridizes with target DNA through 20 Watson‐Crick base pairs and forms DNA‐RNA heteroduplex with a distorted conformation having a predominantly A‐form structure. The negatively charged DNA‐RNA hybrid occupies the positively charged central channel between NUC and REC lobes, acknowledged by Cas9 in a sequence‐independent manner. This indicates that, rather than the nucleobases, the geometry of guide‐target heteroduplex is recognized by the Cas9. Proper PAM‐Cas9 interface triggers the structural changes to destabilize the adjacent DNA and assist Watson‐Crick base pairing between gRNA and target DNA [59]. The adjacent DNA helix is noticeably bent by Cas9 and alters its trajectory, creating a twist from 180° to ∼150° in the bound DNA segment.
The Cas9 enzyme is activated upon PAM recognition and subsequent DNA‐RNA duplex creation [62]. The RuvC domain with three split RuvC motifs and the HNH domain lies in the middle of the protein Cas9. The target dsDNA is cleaved by each domain at a specific site 3 bp from the PAM sequence, producing a blunt‐ended DSB [63]. A single‐stranded break (SSB) is produced by Cas9 nickase, resulting in a cut in only one DNA duplex. Within the target DNA, Cas9 nickase can make staggered cuts, creating a double nick‐induced DSB for enhanced genome‐editing specificity [64]. The proposed mechanism of target recognition and its cleavage by the CRISPR/Cas9 system is illustrated in Figure 5.
FIGURE 5.

A proposed mechanism of target DNA recognition and its cleavage by the CRISPR/Cas9 system. (A) Large conformational rearrangement occurs in Cas9 upon sgRNA loading to achieve a target‐recognition mode. Apo‐Cas9 consists of a PAM‐interacting cleft, largely disordered, that becomes prestructured for PAM sampling. (B) The guide RNA seed is preorganized for interrogation of adjacent DNA for guide RNA complementarity. (C) A coordinated multiple steps further activate Cas9, starting with PAM recognistion. (D) Local DNA melting, RNA strand invasion and (E) subsequent R‐loop formation. (F) A conformational change of the HNH domain allosterically regulates the RuC domain, ensuring concerted DNA cleavage. Abbreviations: CRISPR, clustered regularly interspaced short palindromic repeats; Rec, recognition; Nuc, nuclease; sgRNA, single‐guide RNA; PAM, protospacer adjacent motif
5. DELIVERY STRATEGIES OF THE CRISPR/CAS9 COMPLEX WITHIN CANCER CELLS
The ability of CRISPR/Cas9 to influence target cells for its therapeutic efficacy with minimal biodegradation depends on its proper delivery strategy. The delivery approaches can be mainly classified as physical, viral and non‐viral. Each delivery approach has its limitations, advantages, and complications. The sgRNA and Cas9 can be delivered within target cells as plasmids, RNPs or a combination of sgRNA and Cas9 mRNA. The delivery of plasmids (∼4.2 kbp SpCas9 gene) with a strong negative charge is mostly hindered [65]. The large size of sgRNA (∼31 kDa, 130 bases) and Cas9 (160 kDa, 4300 bases) is an obstacle for conventional viral and non‐viral delivery systems [66]. Moreover, plasmids also require transcription and translation phases after transfection, which usually leads to delayed editing [67]. Conversely, the delivered RNPs employ faster action with a relatively short expression time before protease degradation. However, the cellular uptake of these RNPs may be challenging due to its large protein size and net negative charge [68].
5.1. CRISPR/Cas9 delivery by physical methods
The physical methods of transporting and targeting a Cas9/sgRNA complex within a particular cell include hydrodynamic injection, microinjection, electroporation, and laser irradiation. These strategies are usually difficult to practice and mainly damage target cells [69].
Physical methods are usually restricted to both in vitro and ex vivo systems. However, this approach avoids immunogenic complications, inherent to viral vectors. Physical methods of gene delivery system directly within the cytoplasm or nucleus can bypass the complications associated with targeting and internalization through endocytotic pathways [70]. The physical methods of targeting the CRISPR/Cas9 system are schematically illustrated in Figure 6, and some characteristics, advantages and limitations of physical and virus‐mediated methods [71, 72, 73, 74, 75, 76] are described in Table 1.
FIGURE 6.
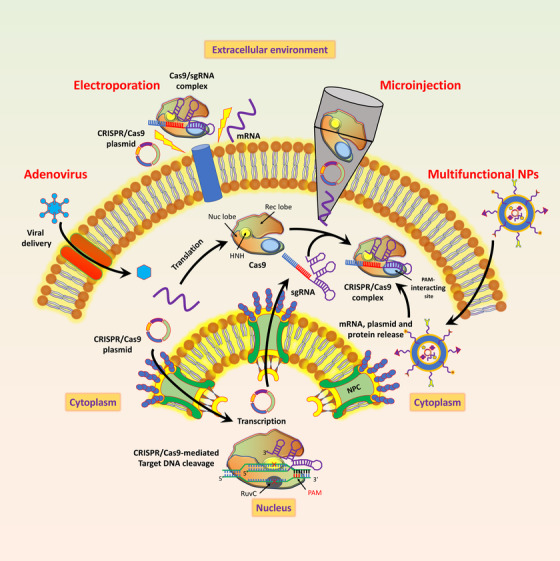
Different strategies of CRISPR/Cas9 delivery as mRNA, DNA, or protein. These methods include adenovirus transport, electroporation, microinjection, and the use of multifunctional NPs. Abbreviations: Cas9/sgRNA, CRISPR associated protein 9/single‐guide RNA; CRISPR, clustered regularly interspaced short palindromic sequence; NPs, nanoparticles; NPC, nuclear pore complex
TABLE 1.
Different approaches of CRISPR/Cas9 delivery by physical and viral methods elucidating their advantages and limitations
| Delivery vehicle and method | Most common cargo | Capacity | Advantages | Limitations | Reference |
|---|---|---|---|---|---|
| Microinjection by needle | DNA plasmid; mRNA (Cas9 + sgRNA); protein (RNP) | nmol/L levels of Cas9 and sgRNA | Guaranteed delivery into cells of interest | Time‐consuming, difficult and generally in vitro only | [71] |
| Electroporation and nucleofection by electric current | DNA plasmid; mRNA (Cas9 + sgRNA) | nmol/L levels of Cas9 and sgRNA | Delivery to cell population; well‐known technique | Generally in vitro only and some cells are not amenable | [72] |
| Hydrodynamic delivery by high‐pressure injection | DNA plasmid; protein (RNP) | nmol/L levels of Cas9 and sgRNA | Virus‐free; low cost; easy | Non‐specific and traumatic to tissues | [73] |
| AAV by non‐enveloped ssDNA | DNA plasmid | <5 kb nucleic acid | Minimal immunogenicity | Low capacity | [74] |
| Adenovirus by non‐enveloped dsDNA | DNA plasmid | 8 kb nucleic acid | High‐efficiency delivery | Inflammatory response and difficult scaled production | [75] |
| Lentivirus by enveloped RNA | DNA plasmid | Almost 10 kb, up to 18 kb nucleic acid | Persistent gene transfer | Prone to gene rearrangement and transgene silencing | [76] |
Abbreviations: Cas9, CRISPR associated protein 9; sgRNA, single guide RNA; RNP, ribonucleoprotein; AAV, adeno‐associated virus; kb, kilobase.
5.2. CRISPR/Cas9 delivery by viral particles
Viruses are naturally occurring transduction agents, which can transfect their own genes in host cells, and some viruses can be exploited to transfer some additional genes of interest for therapeutic purposes [77]. The viral vectors such as adenovirus, adeno‐associated virus (AAV), and herpes virus can persist within the nucleus as extrachromosomal episomes or integrate within the host genome like lentiviruses or oncoretroviruses [78]. Because of the ability to accommodate large DNA payloads to support a strong expression in non‐dividing and dividing cells, lentiviral vectors have become progressively popular in clinical applications [79]. The lentiviral vectors can translocate across the nuclear pore of an intact nuclear membrane. However, the constitutive expression of Cas9 and sgRNA by lentivirus vectors can lead to non‐specific RNA‐DNA interactions and undesirable off‐target effects. In addition, the high integration capacity of retroviral vectors can lead to undesirable off‐target insertional mutagenesis for CRISPR/Cas9 [80]. Furthermore, the use of retroviral vectors can lead to recombination events during large‐scale vector manufacturing, resulting in replication‐competent vectors [81].
Some attractive features of using AAV vectors for clinical applications include the lack of integration machinery, low immunogenicity, and ease of reproduction. However, the concerns of limited packaging capacity and limited transduction efficiency complicate the design of these systems [82]. However, the discovery of a smaller Cas9 variant from Staphylococcus aureus (SaCas9) with comparable editing efficiency to SpCas9 has led to the formation of SaCas9/sgRNA systems which can be packaged in AAV vectors [65].
Adenovirus has received a remarkable attention as an effective gene delivery vector because of its well‐defined biology, genetic stability, ease of large production and high gene transduction efficiency [83]. Compared to AAV vectors, adenoviral vectors have a large packaging capacity of almost 35 kb and offer significant advantages [84]. However, adenoviral vectors with high dosage are highly immunogenic and can produce inflammatory cytokines, initiating the differentiation of antigen‐presenting cells from dendritic cells [85, 86]. In spite of this, adenoviral CRISPR/Cas9 genome editing tools have been used to deliver Cas9 and DNA template in somatic cells of some animals to create models for a particular cancer [86].
Some integrating viruses and mobile genetic elements have incorporated domesticated genes within the eukaryotic genome throughout evolution. Several mammalian Gag homologs (capsid protein homologs) of a long terminal repeat (LTR) have been identified, which form virus‐like particles. PEG10, as one LTR retrotransposon homolog, selectively attaches and facilitates the vesicular secretion of its own mRNA. It has been reported that mRNA cargo of PEG10 can be reprogrammed by flanking genes of interest with PEG10's untranslated regions [87]. By using this reprogramming, Selective Endogenous eNcapsidation for cellular Delivery (SEND) has been developed by engineering both human and mouse PEG10 to package, secrete and deliver specific RNAs. SEND is a modular platform which can be used as an efficient therapeutic delivery modality [87]. SEND technology enables the delivery of exogenous mRNA cargos, such as Cas9 and Cre, within the cells in vitro without using non‐human components. Although this transportation approach is still in its infancy, it is considered a safer alternative to other methods [88].
Although possessing a high transfection efficiency, viral vectors suffer from some limitations, such as large‐scale processing, complexity of synthesis, limited packaging size, and carcinogenic and immunogenic possibilities. These limitations shifted the targeting approach of interested genetic elements to non‐viral vectors and in particular to nanoparticle (NP) delivery strategy [89, 90].
5.3. CRISPR/Cas9 delivery by NPs
The delivery strategy by the NP system for CRISPR/Cas9 has opened a new window of therapeutic purpose for disease management [91]. The NPs (1‐100 nm) have been commonly used as drug and gene delivery vehicles, showing tunable synthesis, high loading capacity and low immunogenicity [92, 93]. These NPs can be coated with various polymers that enhance their facility to bind and protect CRISPR/Cas9. In addition, NP functionalization with various compounds can enhance its circulation time and specific targeting [94].
Some shortcomings of the delivery approach by this synthetic system include low delivery efficiency in comparison with viral delivery [95]. However, the development of organelle‐specific advanced nanocarriers has overcome such limitations. The innovative strategies of surface functionalization of NPs with different agents such as polyethylene glycol (PEG), aptamers, cell‐penetrating peptides (CPP), and nuclear localization signals (NLS) have revolutionized the art of NP‐mediated CRISPR/Cas9 delivery within host cells [96]. Different inorganic NPs such as silica, selenium and AuNPs have been widely explored as vehicles for cancer therapeutic drug delivery and CRISPR/Cas9‐mediated genome editing.
5.3.1. CRISPR/Cas9 delivery by inorganic NPs
Some rigid inorganic nanocarriers have been used recently with noticeable interest as these NPs possess some controllable features. These features include a high surface area to volume ratio, easy surface functionalization, tunable size, immunologically inert, and high stability [97]. These inorganic NPs include carbon nanotubes, calcium carbonate NPs, AuNPs, graphene NPs, iron oxide NPs, and silica NPs. These NPs possess a strong potential to deliver the CRISPR/Cas9 system within specific cells [98]. Out of these NPs, black phosphorus NPs, calcium carbonate NPs, and notably AuNPs have attracted the attention to develop more advanced carrier structures (Table 2) [11, 12, 53, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124].
TABLE 2.
Different delivery vehicles for the CRISPR/Cas9 system and important observations
| Delivery vehicle | Cargo | Target | Route | Observations | Reference |
|---|---|---|---|---|---|
| Lipid‐based delivery | |||||
| LHNPs | ‐ | Plk1 | ‐ | LHNPs as a versatile CRISPR/Cas9‐delivery tool to study cancer biology and gene therapy. | [99] |
| LNP (lipoid, cholesterol, DOPE, DSPE‐PEG2k) | ‐ | GFP | ‐ | NP formulation used in diseased models and study of therapeutics. | [100] |
| LNP‐7C3 | mRNA | ICAM‐2 | IV/IM | A system to quantify how more than 100 nm LNPs deliver mRNA, translated into a functional protein. | [101] |
| LNP‐MK571 | mRNA | TSC2 | IV | Enhances intracellular mRNA delivery both in vivo and in vitro, acts as leukotriene‐antagonists, and is approved for asthma treatment and some other lung diseases. | [102] |
| Cationic lipid‐assisted NPs | mRNA | NLRP3 | IV | A promising strategy for treating NIRP3‐dependent inflammatory diseases and an affordable carrier for delivery of CRISPR/Cas9 into a macrophage. Effective genome editing efficiency (≈53% in the Raw264.7 cell line). | [103] |
| Exosome‐liposome hybrid NPs | Plasmid | mRunx2, hCTNNB1 | Can be used to deliver the CRISPR/Cas9 system in MSCs and study of in vivo gene manipulation. | [104] | |
| LNP‐INT01 | mRNA | Ttr | IV | Enabled ≈ 97% knockout of the mouse Ttr gene in the liver. | [105] |
| PEG‐b‐PLGA‐based cationic lipid‐assisted NPs | Plasmid | NE | IV | CLANpCas9 gene disrupted the NE gene and eased the insulin resistance of T2D mice by decreasing the epididymal white adipose tissue inflammation in the rat liver. | [106] |
| Chalcogen‐containing lipidoids | ‐ | GFP | IV | Combinatorial library of chalcogen (O, S, Se) comprising lipidoid NPs for intracellular delivery of anionic Cas9:single‐guide RNA for genome editing. | [107] |
| ZALNPs | mRNA | Luciferase | IV | ZALNPs guide the design of long RNA carriers and a promising safety and utility of genome editing. The in vitro knockout was reported as 95% | [108] |
| Cationic lipid‐assisted PEG‐PLGA NPs | Plasmid | Ntnl | IV | To express Cas9 in macrophages and precursor monocytes, leading to 20% gene knockout in vivo and 30% in vitro. | [109] |
| Polymeric‐based delivery | |||||
| PBAE NPs | Plasmid | E7 | ‐ | These NPs (with PBAE and CRISPR/shRNA) could be potentially developed as PV‐targeting drugs and used in studies on HPV‐related cervical malignancies. | [110] |
| PEG‐PLGA‐based CLANs | Plasmid | BCR‐ABL | IV | A strategy for targeted treatment of CML with an in vitro indel frequency of almost 46%. | [106] |
| PEGylated chitosan | Plasmid | CFTR | ‐ | Delivery of gene‐editing system by PEGylated chitosan nano complexes. | [111] |
| Cationic polymer PC | Plasmid | β‐subunit of Hb, rhomboid 5 homolog 1 (RHBDFI) | ‐ | A strategy for the large plasmid delivery encoding Cas9/sgRNA for efficient genome editing. | [112] |
| Rigid nanoparticle‐based delivery | |||||
| Black phosphorus nanosheets | ‐ | ‐ | IT | 2D delivery biodegradable platform for CRISPR/Cas9 RNP delivery and some bioactive compounds for biomedical applications. Induction of indel frequency in MCF‐7 cells ≈ 32%. | [113] |
| Arg functionalized gold NPs | ‐ | ‐ | ‐ | For the study of fabrication of Cas9En‐RNP/ArgNPs nano‐assembly. | [114], [115] |
| Polymer/inorganic hybrid NPs (protamine sulfate, CaCO3 and CaPO4) | Plasmid | CDKII | ‐ | Effective genome editing and in situ detection of protein expression. | [116] |
| Gold nanocluster, lipid core‐shell nanocarrier | Plasmid | Plkl | Delivery of protein‐nucleic acid hybrids for gene therapy. | [117] | |
| Gold NPs | ‐ | mG1uR5 | IT | Brain‐targeted therapeutics and development of focal brain‐knockout animal models. The protein and mRNA of mGluR5 reduced ≈ 50%. | [118] |
| Nanoparticle coupled to specific ligand structures | |||||
| pVLPs | Plasmid | ‐ | ‐ | Penetration through the cellular membrane to deliver genetic cargos within the nucleus through the viral entry route. | [119] |
| Arg NPs | ‐ | AAVSI, PTEN | ‐ | Cytoplasmic delivery of Cas9/sgRNA RNP through the co‐engineering of Cas9 protein and Arg NPs. | [114], [115] |
| Cas9 protein and sgRNA‐coated endoporter | Plasmid | CDKII | ‐ | Effective Cas9 RNP delivery initiating targeted gene products in cultured cells and in vivo. | [120] |
| Amphiphilic penetrating peptide NPs | ‐ | EGFP | ‐ | The amphiphilic vectors can deliver Cas9 with low toxicity and good efficiency. | [121] |
| Arg NPs | ‐ | SIRP‐a | ‐ | Can be used as weaponized macrophages for cancer immunotherapy. | [122] |
| TAT peptide‐modified Au NPs | Plasmid | Plkl | IV | CRISPR/Cas9 delivery and targeted genome editing for different diseases. In A375 cells, the irradiation of LACP led to ≈ 65% down‐regulation of the Plk‐l. An intra‐tumoral injection in xenograft models of human melanoma showed tumor volume of the LACP group (no irradiation) ≈ 42% of the volume of the control group. | [53] |
| CPP‐nanoscale ZIFs | mRNA | EGFP | ‐ | CPP‐ZIFs work as easily scaled‐up with excellent loading capacity for co‐delivery of intact Cas9 protein and sgRNA. Possessing ≈ 30% gene knockout | [12] |
| Amino‐ester (MPA‐A, MPA‐Ab) NPs | mRNA | EGFP | ‐ | Biodegradable lipid‐like NPs used as genome‐editing delivery tools for biological and therapeutic applications. | [123] |
| Aptamer AS 1411/ACMC/KALA NPs | Plasmid | CDKII | ‐ | Multi‐functional delivery system NPs for the delivery of plasmid into cancer cell nuclei. | [124] |
| Self‐assembled DNA nanoclews | RNP | EGFP | IT | Delivery of functional nucleic acids and DNA‐binding proteins | [11] |
“‐” refers to: not reported.
Abbreviations: LHNPs, Liposome‐templated hydrogel nanoparticles; PlKI, polo‐like kinase 1; LNP, lipid nanoparticle; DOPE, dioleoyl‐phosphatidyl ethanol‐amine; DSPE‐PEG2k, 1,2‐distearoyl‐sn‐glycero‐3‐phosphoethanolamine‐N‐[amino(polyethylene glycol)‐2000] (ammonium salt); GFP, green fluorescent protein; LNP‐7C3, lipid nanoparticle with specifc lipid composition; ICAM‐2, intracellular adhesion molecule‐2; IV/IM. Intravenous/intramuscular; nm, nanomolar; LNP‐MK571, lipid nanoparticle containing leukotriene antagonsit; TSC2, tuberous sclerosis complex 2; NLRP3, pyrin‐like protein containing a pyrin domain; mRunx2, mouse Runt‐related transcription factor 2; hCTNNB1, human catenin β1; MSCs, mesenchymal stem cells; LNP‐INT01, a biodegradable and ionizable lipid; Ttr, trans‐thyretin; PEG‐b‐PLGA, polyethylene glycol‐β‐poly(lactic‐co‐glycolic acid); CLANpCas9, cationic lipid‐assisted polymeric NPs containing Cas9; T2D, type 2 diabetes; ZALNPs, zwitterionic amino lipid nanoparticles; PBAE NPs, poly(β‐amino ester) nanoparticles; CLANs, cationic lipid‐assisted polymeric nanoparticles; BCR‐ABL, breakpoint cluster region‐Abelson murine leukemia; CML, chronic myeloid leukemia; PEGylated, poly ethylene glycated; CFTR, cystic fibrosis transmembrane conductance regulator; PC, polyethyleneimine‐β‐cyclodextrin; RHBDFI, Rhomboid 5 Homolog 1; MCF‐7, Michigan Cancer Foundation‐7; CaCO3, calcium carbonate; CaPO4, calcium phosphate; CDKII, cyclin dependent kinase II; Plkl, polo‐like kinase 1; pVLPs, peptidyl virus‐like particles; AAVSI, adeno‐associated virus integration site 1; PTEN, phosphate and tensin homolog; EGFP, enhanced green fluorescent protein; SIRP‐a, signal regulatory protein‐alpha; TAT, trans‐activator of transcription; CPP, cell penetrating peptide; ZIFs, zeolitic imidazole Frameworks; MPA‐A, N‐methyl‐1,3‐propanediamine‐A; AS 1411, guanosine rich oligonucleotide aptamer; NE, neutrophil elastase.
5.3.2. CRISPR/Cas9 delivery by AuNPs
AuNPs have been used for both in vitro and in vivo environments to deliver CRISPR components within various tumor cells. Some RNPs have been delivered by Arg‐coated AuNPs, facilitating phosphatase and tensin homolog (PTEN) and adeno‐associated virus integration site 1 (AASV1) knockdown during in vitro conditions [125]. AuNPs enter the cells by membrane fusion process, avoiding lysosomal degradation, leading to an editing efficiency of almost 20%‐30%. The thiol‐modified crRNA has been used to bind with AuNPs, and further binding has been achieved by using Cas9 to form RNPs [126].
The targeting of polo‐like kinase 1 (Plk1) gene under in vitro and in vivo conditions of melanoma tumors was achieved by delivering large CRISPR/Cas9 plasmid by using lipid‐encapsulated TAT‐coated AuNPs [53]. The plasmid release was achieved by light irradiation at 514 nm followed by transfection without promoting cell death, getting a synergistic effect in cancer management. Due to the large loading capacity of AuNPs, they have been used to deliver HDR templates simultaneously with sgRNA and Cas9. The polyethyleneimine (PEI) layer was used to complex with ssDNA, achieving a layer‐by‐layer approach of binding with AuNP‐RNP [126].
In another study, AuNPs were complexed with thiol‐linked ssDNA which bound to donor DNA and RNP and were coated with polymer (poly{N‐[N‐(2‐aminoethyl)‐2‐aminoethyl]aspartamide}) (PAsp(DET) [13]. These AuNPs attained an HDR frequency of 3%‐4%, which is appreciably more than lipofectamine transfection. This approach was used to facilitate in vivo dystrophin gene correction. The lipid‐encapsulated TAT‐coated AuNPs have also been used to carry Plk1 sgRNA encoded as a plasmid and Cas9 protein. This complex produced an editing efficiency of more than 26% in vitro and noticeably inhibited the in vivo melanoma tumor growth [125]. At a physiologic pH, glutathione capped gold (GSH‐Au) nanocrystals were found to self‐assemble with Cas9, forming SpCas9‐Au nanocrystals which can dissociate in an acidic pH environment [127]. In addition, HeLa cells had been used to check the editing efficiency of these complexes and sgRNA by transfection targeting the viral E6 oncogene. It resulted in significant protein expression reduction and showed an editing efficiency of 34% [128] (Table 2).
5.3.3. CRISPR/Cas9 delivery by calcium carbonate and black phosphorus NPs
The remarkable biocompatibility and biodegradability have led to a rapid rise in the synthesis of nanomaterials synthesized from calcium, such as calcium carbonate (CaCO3), calcium phosphate [Ca(H2PO2)2], hydroxoyapatite [Ca5(PO4)3OH] and tricalcium phosphate [Ca3(PO4)2] [129]. Some novel NPs from CaCO3 were synthesized as gene carriers for the delivery of Cas9‐single guide cyclin dependent kinase 11 (Cas9‐SgCDK11) plasmids [129].
Some novel biodegradable nanosheets from black phosphorus have been used with increased efficiency for the delivery of Cas9N3, a new form of Cas9 having three NLS repeats at C‐terminus. This form possesses an increased efficiency for nuclear targeting. An efficient indel frequency of ∼32% was reported in MCF‐7 cells [12]. Furthermore, it has been observed that in comparison to other nanocarrier systems, low dose of Cas9N3‐black phosphorus nanosheets enhanced the efficiency of genome editing and gene silencing in A549/EGFP cancer‐bearing mice [130].
5.4. CRISPR/Cas9 delivery by lipid NPs (LNPs)
LNPs are considered novel carriers of the CRISPR/Cas9 system as they possess low toxicity and protect CRISPR/Cas9 vectors or sgRNAs from nuclease digestion. In addition, LNPs show a significant reduction in immune response stimulation and possess good renal clearance [131]. Lipids, especially phospholipids, have emerged as versatile units for the synthesis of LNPs to improve the biocompatibility and stability of NPs synthesized from inorganic materials, which can be cytotoxic and are not stable in aqueous suspension [132].
Liposomes are common lipid‐based formulations used to encapsulate both hydrophobic and hydrophilic molecules and are widely investigated carriers for the delivery of gene‐editing tools. The vesicular structure, adjustable surface properties and biocompatibility allow effective delivery of proteins or nucleic acids into target cells. Lipoplexes (cationic liposomes) are recommended for the delivery of CRISPR/Cas9 components as gRNA is highly anionic in nature. For the encapsulation of Cas9 protein within LNPs, the fusion of Cas9 to cationic protein is essential to decrease its positive charge [133]. To preserve the RNP integrity of the CRISPR/Cas9 complex, ionizable LNP formulation has been used to target and edit DNA. Lipoplexes are now commpercially available and have been widely used in in vitro gene delivery studies.
Being entirely biocompatible and biodegradable, native liposomes have poor stability, short life, low encapsulation efficiency, and rapid clearance rate [134]. To overcome some of these limitations, nanostructured lipid nanocarriers (NLCs) and solid lipid NPs (SLNPs) have been engineered with superior physical stability, targeted drug delivery, and sustained release profile [135]. Cationic SLNPs form a strong electrostatic interaction with nucleic acids and thus facilitate their transport, protecting them from enzymatic degradation. NLCs are distinctive in their capability to co‐deliver lipophilic drugs and nucleic acids [136].
Several non‐viral transfection reagents have been used to transport nucleic acids, and lipofectamine‐based reagents are the most commonly used in LNP system. Lipofectamine, a cationic liposome formulation, can be complexed with negatively charged nucleic acids through electrostatic interactions.
PEGylation is a novel strategy to reduce non‐specific interactions of LNPs with serum proteins and avoid aggregation and subsequent clearance by the immune system [137]. Polyethylene glycol (PEG) phospholipid‐modified cationic LNPs have been constructed to maintain an electrostatic interaction between negatively charged Cas9/sgRNA‐fused plasmid DNA/chondroitin sulfate complex and positively charged protamine solution with an optimized ratio to form a tightly packed core [138]. This core is subsequently encapsulated with cationic lipids composed of dioleoyl‐phosphatidyl ethanol‐amine (DOPE), 1,2‐dioleoyl‐3‐trimethylammonium propane (DOTAP) and cholesterol, post‐modified with PEG phospholipid to produce NPs with low toxicity, enhanced stability, less immunogenicity and decreased clearance. The dense core helps to reduce the size of the CRISPR system, and cationic shell helps to facilitate NP‐cell interaction and proper internalization of these particles.
Ionizable lipids are another class of lipids suitable for the delivery of nucleic acids. These lipids carry a pH‐dependent charge with a positive charge in an acidic medium to enable the encapsulation of nucleic acids and bear a neutral charge at physiological pH. These LNPs also have additional standard components, for example, helper phospholipid such as dioleoylphosphatidylethanolamine (DOPE) and cholesterol, for structural stability. In addition, it also contains PEG derivatives for physiological stability [139]. These features provide the ability to target specific tissues and to survive in circulation for a longer duration.
Compared to the delivery of short RNAs and plasmids by LNPs, the delivery of longer RNAs characteristic of the CRISPR/Cas9 system, such as sgRNA or Cas9 mRNA, is challenging as an ideal formulation and composition have yet to be efficiently determined. In this regard, zwitterionic amino lipids have been synthesized to uniquely co‐deliver long RNAs such as sgRNA and Cas9 mRNA, minimizing the protein expression by >90% in cells [108]. The tissue‐targeting specificity of NPs can be further enhanced by adding ligands such as peptides, proteins, antibodies, or aptamers that interact with target cell receptors.
For the delivery of cationic LNPs, super negatively charged Cre protein (to maintain electrostatic self‐assembly with cationic lipids) and Cas9:sgRNA were performed in HeLa cells that enabled genome editing and recombination efficiency of more than 70% [140]. It is hypothesized that this lipid/protein nanocarrier facilitates efficient endosomal escape, supporting the cargo to enter the nucleus efficiently.
5.5. Polymer‐based delivery of CRISPR/Cas9
Different types of oligonucleotides, RNA and DNA plasmids, have been widely delivered by polymer‐based NPs [141]. Such NPs possess efficient encapsulation capability, stabilize plasmid DNA encoding sgRNA and Cas9, and aim at specific targets [142]. The in vitro delivery of CRISPR/Cas9 by polyethyleneimine‐β‐cyclodextrin (PC) led to the editing of rhomboid 5 homolog 1 (RHBDF1) and β‐subunit of hemoglobin in HeLa cells [112]. PC encapsulation of plasmid encoding sgRNA and Cas9 was highly efficient and showed lower cytotoxicity. These features led PC to be used for repeated phases with a high dose transfection level.
A novel nanostructure has been constructed by using poly(β‐amino ester) (PBAE) with low toxicity, high biocompatibility, fast drug delivery, and high transfection efficiency [110]. It has been used to transfer CRISPR and short hairpin RNA (shRNA) into human papillovirus 16 (HPV16) transgenic mice, showing reduced tumor progression. To reduce the opsonization and clearance by the reticuloendothelial system (RES), PEGylation of NPs is a good alternative. PEGylation has also been applied for CRISPR/Cas9 NPs to avoid immune recognition [143]. Compared to non‐PEGylated NPs, chitosan and methoxy polyethylene glycol (mPEG) have been added to transfer CRISPR/Cas9 in mucus model,which has helped reduce the nuclease digestion [119] (Table 2).
Recently, DNA has been used as innovative nanostructures which are programmable with a small and uniform size. These nanostructures are biodegradable and possess spatial addressability. These DNA nanostructures include various forms such as nanosuitcase, tetrahedron, nanorobot, origami, and nanoclew, and are used to deliver different cargos [144]. A cationic polymer‐coated DNA nanoclew was engineered to deliver Cas9/sgRNA within the nuclei of some human cells. It has been reported that a partial complementarity between nanoclew sequence and sgRNA guide sequence could improve the genome editing [11]. These new nanocarrier systems could be modified with different ligands to deliver CRISPR/Cas9 and could be noteworthy in tumor management.
5.6. CRISPR/Cas9 delivery by ligand‐mediated carriage system
Some specific ligands such as anisamide, antibodies, aptamers, arginyl‐glycylaspartic acid (RGD), CPP, follicle‐stimulating hormone, folic acid, hyaluronic acid, and lutinizing hormone‐releasing hormone have been used for gene and drug delivery system [145]. Among these aptamers, CPP‐ and RGD‐based delivery strategies have been used for the CRISPR/Cas9 complex.
5.6.1. CRISPR/Cas9 delivery by CPP
CPPs are short peptides (≤30 amino acids) predominantly capable of translocating through cell membranes and can facilitate the attached drugs or cargo complex to achieve intracellular transport. CPP can be efficiently used to edit the genome design by targeting Cas9‐sgRNA ribonucleoprotein [146]. Compared to the plasmid strategy, which may lead to concerns such as immune response stimulation and insertion into the host genome, the delivery of CPP‐mediated Cas9‐sgRNA could be a promising strategy in the future [147]. CPPs are covalently bonded directly to Cas9 proteins and then complexed with sgRNA to deliver to cells. A separate delivery of CPP‐sgRNA and CPP‐Cas9 was performed on different human cell lines [148]. The cellular and sub‐cellular localization of CPP‐delivered CRISPR/Cas9 RNP had been demonstrated by confocal microscopy [146]. In fact, different cells show that CPPs have lower efficiency of desired targeted mutations as ∼10%‐20%. However, compared to transfection from bare plasmids, CPPs are ∼40‐fold more efficient. Thus, CPPs have been proven to be acceptable methods as a delivery strategy for the CRISPR/Cas9 system within different cells (Table 2).
5.6.2. CRISPR/Cas9 delivery by RGD
RGDs play a significant role in cell adhesion for extracellular matrix [149]. These peptides can recognize integrin receptors, which are highly expressed in angiogenic endothelial cells. The RGD peptides are good candidates for tumor‐specific drug delivery systems [150]. Liposome‐template hydrogel NPs (LHNPs) were used for encapsulating Plk1 sgRNA and Cas9 protein for the delivery to specific cells [99]. The core of LHNPs was also layered by PEI hydrogel to encapsulate the Cas9 protein. DOTAP was used to deliver the genetic materials. These procedures prolonged animal model survival and were reported with tumor shrinkage. The conjugation of internalizing RGD (iRGD) on DOTAP improves the NP delivery within tumor cells. The clinical translation of cancer gene therapy had been performed using LHNPs, as these NPs deliver CRISPR/Cas9 with higher delivery efficiency within targeted cancer cells [99].
5.6.3. CRISPR/Cas9 delivery by aptamers
Aptamers represent single‐stranded oligonucleotides with multiple applications. Aptamers can bind to specific receptors on the cell surface and thus help to internalize the delivery system with which they are liganded. Aptamers are emerging as novel ligands to deliver cargo molecules, including genes, into specific target cells [151].
Multifunctional and self‐assembled NPs have been designed from aptamer AS1411, chitosan and KALA, an endosomolytic peptide to deliver Cas9‐sgCDK11 plasmid within MCF‐7 tumor cells [152]. An autonomous DNA nanorobot modified with AS1411 aptamer has been designed to deliver thrombin within tumor cells [110]. A remarkable tumor shrinkage with efficient delivery was reported by this method. Thus, DNA nanorobot‐aptamer‐based delivery system can be a novel CRISPR/Cas9 transfer strategy within target cells. The examples of different delivery vehicles for CRISPR/Cas9 within in vivo and in vitro systems with some important observations are elaborated in Table 2.
5.7. Co‐delivery of CRISPR/Cas9 complex and anticancer drugs by NPs
The development and delivery strategies of CRISPR/Cas9 within cancer cells have offered a robust therapeutic potential to overcome multidrug resistance (MDR) [153]. The anticancer strategy has gained its momentum further by co‐delivery of antitumor drugs and CRISPR/Cas9 using different NPs [154]. This dual antitumor approach works as the CRISPR/Cas9 system selectively knocks down MDR‐related genes while the antitumor drugs simply kill the cells using different approaches. The synergistic approach of using antitumor drugs and CRISPR/Cas9 can reduce the drug dosage, further minimizing adverse effects [155].
PEGylated NPs comprising cationic alpha‐poly‐glutamate‐based polypeptides (P‐HNPs) were used to deliver sgRNA and Cas9 plasmid in both in vivo and in vitro conditions. It resulted in more than 47% of genome editing and 35% delivery of Cas9 plasmid/sgRNA and ∼66% decrease in PLK1protein level in HeLa cancer cells [156]. The co‐delivery of non‐efflux pump‐resistant sgRNA/Cas9 and antitumor drugs is a strong strategy for cancer management. The co‐delivery strategy of chemotherapy drugs and efflux/non‐efflux pump‐resistant sgRNA/Cas9 may be a new advance in cancer chemotherapy.
6. ROLE OF CRISPR/CAS9 IN CANCER RESEARCH AND THERAPY
Cancer is a leading cause of death worldwide, originating from different genetic and epigenetic aberrations. The current treatment strategies face certain limitations for this complex disease, emphasizing the application of highly efficient alternative approaches. The complexity of cancer is recorded in Cancer Genome Atlas, which describes more than 15,000 tumors [157]. Cancer patients usually present different genetic alterations/aberrations, which can significantly change the tumor progression and susceptibility to treatment procedures. In the recent past, a significant thrust has been stressed to identify different genetic and epigenetic mutations responsible for the cancer progression or its therapy [158]. The discovery of ZFNs and TALENs offers a vast opportunity to analyze the role of different genes in cancer.
Due to its gene‐editing capabilities, CRISPR/Cas9 has proven to be a powerful tool in the identification of novel targets in cancer. It is now considered a tool of choice to study the regulation and functions of genes of interest. This strategy is used to generate genetic knockouts in cell populations to monitor their phenotypic effects. In addition, the CRISPR/Cas9 strategy is used to study gene‐drug interactions in conjunction with small molecules [158].
The advancements in DNA sequencing and identification of thousands of single nucleotide polymorphisms (SNPs) have attributed to different diseases. Some genomic repositories such as Cancer Cell Line Encyclopedia, Encyclopedia of DNA elements, and The Cancer Genome Atlas have emerged, pointing out the genomic level catalog of disease‐specific variation [159]. This has also led to the encouragement of personalized medicine based on patient data and genetic information. The introduction of CRISPR technology has helped generate isogenic human knockout and genetically modified cells to tackle this problem [64].
Different cell types such as primary cells, induced pluripotent stem cells, cancer cell lines, and organoids have been investigated using CRISPR technology [160]. To test the hypothesis of synthetic lethality in a particular cell line, CRISPR technology is used if the cell line possesses genetic lesions and is supposed to be sensitized with a specific therapeutic drugs [161]. Furthermore, newly engineered Cas enzymes enable researchers to change specific bases, alter the genome, and modify primary cells. For example, Cas9 and cytidine deaminase fusion enabled the researchers to modify RNA‐guided base editing, a promising technique for editing different cell types [162].
The human genome consists of non‐protein‐coding regions with regulatory elements such as enhancers, insulators, and silencers. Any dysregulation of these regions could contribute to tumorigenesis [163]. The utilization of CRISPR/Cas9 technology in studying these regions has also helped, especially cis‐regulatory elements such as enhancers and trans‐acting factors. Thus, comprehensive know‐how about these regulatory elements in association with CRISPR/Cas9 technology will be helpful in understanding the genomic landscape of cancer cells [164]. Furthermore, CRISPR interference has also been used to screen different cell lines to study the role of lncRNAs on cell viability [133]. The recent updates about the role of the CRISPR/Cas9 system in different cancers are listed below.
6.1. The CRISPR/Cas9 system for the treatment of lung cancer
The CRISPR/Cas9 system is now being used as a ground‐breaking technology for treating lung cancer by using different delivery strategies like AAV as gene transfection agents within the lung tissues [165]. This approach works in two ways. The first method works by designing sgRNA, which contains complementary sequences with the mutated epidermal growth factor receptor (EGFR). This method is accompanied by Cas9 for further process. This CRISPR/Cas9 system is introduced in the concerned patients, having a complementary sequence with mutated EGFR. As this complementary sequence binds to the mutated EGFR, the endonuclease activity of Cas9 protein creates an SSB or DSB depending on the enzyme type. The DNA repair mechanism such as homologous or non‐homologous DNA repair comes into play after then [166]. The EGFR inhibition activates major histocompatibility complex (MHC) class I, which provokes cytotoxic lymphocyte recognition and cancer cell breakdown [167].
The second strategy of lung cancer treatment includes searching for immune cells such as lymphocytes. In China, T cells were extracted from the blood of lung cancer patients undergoing clinical trial, and the gene encoding PD‐1 protein was knocked down by CRISPR/Cas9 system [168]. These cells with edited genes would be propagated and injected back into the bloodstream of the same patients [168]. Thus lymphocytes with no expression of PD‐1 will have less contact between the tumor ligand and the receptor, making the T cell receptor able to identify the problematic cells and perform its function [165]. The knockdown of PD‐1 in immune cells is compulsory for caspase activation, required for programmed death in cancer cells [167].
6.2. The CRISPR/Cas9 system for the treatment of liver cancer
Hepatocellular carcinoma (HCC) is reported as the fifth most common cancer and the third leading cause of death globally [169]. In HCC, an up‐regulation of lysine methyl transferase (G9a) implies a poor prognosis of the disease [170]. The primary function of G9a is to di‐methylate histone H3 at lysine 9 (H3K9me2). The gene‐silencing activity is promoted by H3K9me2‐binding proteins, which are recruited by G9a‐dependent H3K9me2 and prevent transcription activation [171]. The proliferation and migration of HCC cells can be surpassed by the G9a‐KO strategy by using CRISPR/Cas9, transfected through lentivirus, as an in vivo or in vitro system [172].
It has been reported that eukaryotic elongation factor 2 (eEF2) and phosphorylated eEF2 were prognostic markers for the survival of HCC patients [173]. The regulation of eEF2 may be a potential drug target for cancer therapy. In HCC cell lines, eEF2 KO has been performed by using the CRISPR/Cas9 system transferred through electroporation [173]. The results indicated that the growth and proliferation rate of HCC declined by CRISPR/Cas9‐mediated elimination of eEF2 kinase. In addition, nuclear receptor coactivator 5 (NCOA5) promoted different malignancies, and CRISPR/Cas9‐mediated NCOA5‐KO HCC cellular genome has been produced. The epithelial‐mesenchymal transition (EMT) was suppressed by NCOA5‐KO, leading to diminished cell proliferation and migration. Furthermore, a marked association was reported between invasiveness and metastasis of HCC and CXC chemokine receptor 4 (CXCR4) expression [174]. The CRISPR/Cas9 transfection by lipofectamine 2000 as LNPs mediates the targeting of chemokine receptor 4 (CXCR4), which could inhibit the proliferation, migration and invasion of HepG2 cells and decrease the HCC malignancy [14].
6.3. The CRISPR/Cas9 system for the treatment of pancreatic cancer
CRISPR/Cas9 has been proven to be a powerful gene‐editing tool for exploring pancreatic cancer. In the human pancreatic ductal adenocarcinoma (PDAC) cell line, CRISPR/Cas9 was used to knockout the lysine‐specific demethylase 6A (KDM6A) gene [175]. This experiment was performed to demonstrate the aggressive phenotype of KDM6A‐deficient cells. It has been reported that annexin A1 (ANXA1) knockout in Mia PaCa2 cells by CRISPR/Cas9 led to a weaker motile phenotype and less extracellular vesicle secretion [176]. The Capan1 cell line has been found to lose the ability of self‐renewal and migration and formed fewer tumor spheres after the knockout of the polypeptide N‐Acetylgalactosaminyltransferase 3 (GALNT3) gene by using lipofectamine 2000 [177]. In addition, it has been demonstrated that the knockout of sphingosine kinase 1 (SPHK1) in PAN02 cells by lipofectamine LTX transfection reagent led to increased proliferation and migration [178]. Furthermore, the knockout of core 1 synthase, glycoprotein‐N‐acetylgalactosamine‐3‐beta‐galactosyltransferase 1 (C1GALT1) in PDAC cells by using CRISPR/Cas9, transferred by lipofectamine 2000 led to the expression of truncated glycan (Tn) and sialyl Tn (sTn) (carbohydrate tumor antigens) in addition to increased growth, migration, and tumorigenicity [179]. The antitumor strategy of the CRISPR/Cas9 system for pancreatic cancer eradication is still in its infancy but is believed to be revolutionary in future time [179].
6.4. The CRISPR/Cas9 system for the treatment of ovarian cancer
DNA methyltransferase 1 (DNMT1), a key enzyme of DNA methylation, is a significant target for epigenetic therapies in different cancers [180]. Tumor suppressor genes, such as BReast CAncer gene 1 (BRCA1) and RAS association domain family 1 isoform A (RASSF1A), are inactivated by the abnormal overexpression of DNMT1, which can be indispensable for cancer stem cell maintenance [181]. Ovarian cancer is closely associated with a high expression of DNMT1 [182]. The inhibition of this gene suppressed ovarian tumor growth and cisplatin resistance [183]. Thus, targeting DNMT1 in ovarian cancers may be a promising strategy and treatment method [184]. Editing the DNMT1 gene in ovarian cancer cells by using the CRISPR/Cas9 system may be a potential approach to tumor therapy [185].
6.5. The CRISPR/Cas9 system for the treatment of prostate cancer
An emerging evidence showed that the pathogenesis of prostate cancer was related to the increased expression of G protein‐coupled receptor family C group 6 member A (GPRC6A) [186]. In human prostate cancer cells, the GPRC6A activation led to enhanced proliferation and chemotaxis, which further promoted EMT [187]. The prostate cancer cell migration and invasion was reduced by the knockdown of GPRC6A. In the human prostate cancer cell line PC‐3, the CRISPR/Cas9 system, transfected through lentiCRISPR‐v2 plasmid vector, has been used to disrupt the expression of the GPRC6A gene [188]. The editing of this gene resulted in the inhibition of osteocalcin activation of extracellular signal‐regulated kinse (ERK), Ak strain transforming (AKT) and mammalian target of rapamycin (mTOR) signaling. These signaling cascades led to cell proliferation and migration. Thus, to suppress prostate cancer progression, CRISPR/Cas9‐mediated deletion of GPRC6A may be a novel strategy for tumor management.
6.6. The CRISPR/Cas9 system for the treatment of breast cancer
CRISPR/Cas9 technology plays a significant role in ongoing researches associated with breast cancer, and this treatment approach can positively tackle the drug resistance and may improve the immunotherapy on CRISPR cancer in near future [189]. Activation of protein degradation in cancer cells is considered an effective strategy to enhance tumor cell proliferation. A multi‐catalytic enzyme known as 26S proteasome is a protein‐degrading enzyme which regulates polyubiquitylated protein degradation, cell cycle and apoptosis‐related proteins such as caspases [190]. Proteasome inhibitors in cancer cells possess antiapoptotic and antitumor activities. In addition, these inhibitors sensitize cancer cells to intrinsic and extrinsic pro‐apoptotic signals. Thus, these proteasomes have become novel targets for anticancer treatments. The proliferation of breast cancer is also regulated by site‐specific proteasome phosphorylation [46]. Thus, the disruption of this activity may help control the disease.
CRISPR/Cas9 dual‐specificity tyrosine‐regulated kinase 2 (DYRK2) knockout was established to interrupt the tumorigenesis of the proteasome‐addicted human breast carcinoma cells in mice [191]. Estrogen receptor (ER)‐positive breast cancer cells could be treated via the inhibition of estrogen synthesis or by using fulvestrant and tamoxifen, that competes estrogen on ERα. The mutations in ERα such as ERαY537S and ERαD538G adapts to superior metastatic breast cancer that is insensitive to tamoxifen and aromatase inhibitors [192]. For the validation of these mutations, CRISPR/Cas9 had been used to create ERα‐positive breast cancer model having wild‐type ERα replaced with ERαY537S or ERαD538G. In addition, the progression and metastasis of breast cancer were related to migration and invasion enhancer 1 (MIEN1), and its enhanced expression might accelerate tumor migration and metastasis [192]. A targeted deletion of MIEN1 gene by CRISPR/Cas9, transfected through cloning vectors such as [pSpCas9(BB)‐2A‐GFP (PX458)], effectively silenced its expression and thus controlled the disease spreading [193].
6.7. The CRISPR/Cas9 system for the treatment of colorectal cancer
In the murine colorectal tumor cell line MC38, the transcriptional expression of fucosyltransferase 4 (Fut4) and Fut9 genes was performed by using the CRISPR/dCas9‐VPR plasmid as an expression vector [194]. The introduction of these genes led to the neo‐expression of Lewis‐antigen, affecting the expression of core‐fucosylation and sialylation. In addition, HPV16, an anal cancer‐derived gene, has been expressed in immunodeficient mice to check the suppression of cancer growth by CRISPR/Cas9 system. The delivery of Cas9/sgRNA by AAV vectors encoding Cas9 in mice reduced the tumor mass by targeting HPV16. These observations suggest that the CRISPR/Cas9 system may be a potential option for the treatment of HPV‐induced tumors in humans [195].
In the colorectal tumoral cell line CaCO‐2, the lentiviral delivery of Cas9/sgRNA by lipofectamine 3000, containing lentivirus with pSpCas9(BB)‐2A‐GFP plasmid, led to the knockout of Par3L proteins by inhibiting cell proliferation, inducing apoptosis, and activating the expression of signaling cascade‐3 [196]. It induced cell apoptosis and activated cascade‐3 expression. Anticancer chemotherapy is more effective on Par3L‐knockout cells, and their inactivation by the CRISPR/Cas9 system by the suppression of AMP‐activating protein kinase (AMPK) signaling may be a potential target of cancer treatment. Furthermore, it has been revealed that the delivery of hyaluronic acid‐associated CP/Ad‐SS‐GD/RNP nanocomplex efficiently inhibited colorectal tumor growth and metastasis by targeting KRAS mutations [197]. The novel applications of CRISPR/Cas9 in the management of different cancers, including liver, lung, breast, prostate, colorectal and anal cancers, are described in Table 3 [94, 188, 194, 195, 196, 197, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215, 216, 217].
TABLE 3.
The in vitro and in vivo roles of CRISPR/Cas9 in the management of different cancer types
| Cancer type | Cell line | Gene | Target choice | CRISPR effect and mechanism | Reference |
|---|---|---|---|---|---|
| Liver cancer | H2.35 | PTEN, p53 | C57‐HBV | Loss‐of‐function alterations of p53 and PTEN genes in H235 cells, Akt phosphorylation that resulted in corresponding somatic dysfunction and lipid accumulation | [198] |
| ‐ | PTEN and p53 (TP53 and Trp53) | β‐catenin | Knockout of both alleles results in Akt phosphorylation | [199] | |
| CD44‐C3A‐iCSCs DO clone and CD44‐C3A‐iCSCs C10 clone | Oct4, Sox2, NANOG | CD44 | Knockout CD44 to reduce cell proliferation | [200] | |
| Embryonic liver progenitor cells | p53 | HMGA2 (Nf1, Plxnb1, Flrt2, and B9d1) | Knockout both alleles to reduce the mitogen‐activated protein kinase | [201] | |
| AAV9‐HS‐CRM8‐TTRmin‐Cas9, AAV9‐U6‐mF9‐Exon1‐gRNA | F9 | Factor IX (F9) | Loss of FIX activity that induces a dsDNA break in a DNA sequence‐specific manner | [202] | |
| Lung cancer | T cells | Immune checkpoint genes | PD‐1 | Knockout the PD‐1 gene to effectively target exon 2 of the PD‐1 gene and reduce lung cancer size | [203] |
| HEK293T | Trp53, Rb1, Rosa26 | p107 and p130 | Loss of tumor suppressor genes (p107 and p130) to induce metastasis and cell proliferation | [204] | |
| NCI‐H1975, NCI‐H1650 | EGFR | EGFR | Knockout EGFR to inhibit cell proliferation and EGFR expression in lung cancer | [205] | |
| A549, NCI‐H460 | PTEM | PTEN | Knockout of both alleles of PTEN by CRISPR/Cas9 KO PTEN in slug/snail lung tumor which contributes to EMT by nuclear translocation of β‐catenin | [206] | |
| Breast cancer | Cal‐51 | MASTL | PP2A‐B55 | Knockout both alleles to inhibit cell proliferation and tumor suppression | [207] |
| MCF‐7 cells | miR‐23b | miR‐23b, miR27‐b | Knockout niR‐23b and miR27‐b genes to inhibit proliferation, and migration, of miR27‐b‐depleted cells | [208] | |
| MDA‐MB‐231, MDA‐MB‐436 | BRCA1 | BRCAI wild‐type, BRCA1m | Knockout PARPI that results in apoptosis | [209] | |
| MDA‐MB‐231, MCF‐7/TamR‐1 | Sox reductase | Sox2, Sox9 | Knockout of Sox9 reductase promote Wnt signaling and reduce tumor cell invasion | [210] | |
| ‐ | P53, PTEN, RB1, NF1 | Nutlin‐3a | Knockout PTEN, NH, P53, and RBI genes to induce cellular senescence and cell proliferation | [211] | |
| MDA‐MB‐231 | CXCR4, CXCR7 | TNBC | Knockout of the CXCR4 or CXCR7 gene that results in delay in conversion of the G1/S cycle, inhibits cell proliferation, invasion, and mitigation | [212] | |
| MCF‐I0A, HCC1806 | APOBEC3G | APOBEC3G | Knockout both alleles of APOBEC3 to inhibit cell proliferation | [94] | |
| Prostate cancer | ΔPTEN, 2924 V, PSA | ΔPTEN, 2924 V, PSA | PTEN | Knockout PTEN by CRISPR/Ca9 in ΔPTEN cell line to check the role of PTEN in prostate cancer | [213] |
| PC‐3, LNCap, DU145, | GPRC6A | GPRC6A | Activation of GPRC6A that block ERK, mTOR, and AkT phosphorylation and inhibit cell proliferation | [188] | |
| LNCaP | AR | AR | Inhibition of AR that induce apoptosis and inhibit cell proliferation | [214] | |
| PC3, DU145, 293FT | PGC1α | ERRa, PGC‐1α | Knockout ERRa and inhibit MYC levels to suppress metastasis and invasion of lung cancer growth | [215] | |
| DU 145 | NANOG, NANOGP8 | NANOG, NANOGP8 | Knockout NANOG and NANOGP8 to inhibit cell proliferation and migration, and promote drug sensitivity | [216] | |
| PC‐3 (CRL‐1435 and ATCC) | PC‐3 | TP53 | Knockout of both alleles of TP53 to induce apoptosis and inhibit cell proliferation | [217] | |
| Colorectal cancer | SW‐480 CRC cells | Mutant KRAS | KRAS | Knockout both alleles to induce apoptosis, suppress cell proliferation, and promote tumor cell death | [197] |
| CaCO‐2 | Par3L | Par3L | Knockout both alleles to induce apoptosis and inhibit cell proliferation | [196] | |
| MC‐38 | FUT | Fut4 and Fut9 | Inhibit glycosylation by the CRISPR‐dCas9‐VPR system, and activate the Fut4 and Fut9 genes to induce the neo‐expression | [194] | |
| 293T | HPV16 E6 and E7 | HPV16 | Knockout oncogenes (E6 and E7) and inhibit the expression of HPV16 E6 and E7 genes to reduce anal tumor | [195] |
“‐” refers to: not reported.
Abbreviations: PTEN, Phosphatase and tensin homolog; p53, tumor suppressor protein; C57‐HBV, hepatitis B virus transgenic mice line C57; Akt, serine threonine protein kinase; TP53, tumor protein 53; Trp53, tryptophan 53; CD, cluster of differentiation; iCSCs, induced cancer stem‐like cells; HMGA2, high mobility group A2; Plxnb1, plexin b1; Flrt2, fibronectin leucine rich transmembrane protein 2; B9d1, B9 domain containing 1; AAV, adeno‐associated virus; gRNA, guide RNA; F9, factor 9, FIX, human factor IX gene; PD‐1, programmed cell death ligand‐1; Rb1, retinoblastoma1; Rosa26, reverse orientation splice acceptor; NCI‐H1975, non‐small cell lung cancer cell line; EGFR, epidermal growth factor receptor; NCI‐H460, non‐small‐cell lung cancer cell line; PTEN, phosphatase and tensin homolog; EMT, epithelial‐mesenchymal transition; PP2A‐B55, protein phosphatase 2A having B55 substrate; MCF7, Michigan Cancer Foundation‐7 (epithelial cell line); MDA‐MB‐231, epithelial breast cancer cell line type 231; MDA‐MB‐436, epithelial breast cancer cell line type 436; BRCAI, breast cancer gene‐1; PARPI, poly(ADP‐ribose)polymerase inhibitor; MCF‐7/TamR‐1, tamoxifen‐resistant cell line derived from the MCF‐7/S0.5 human breast cancer cell line; Sox2, (sex determining region Y)‐box 2; Wnt, wingless and Int‐1 (group of signal transduction pathways); CXCR4, chemokine receptor type 4; G1/S, growth 1/synthesis phase; MCF‐I0A, noncancer human mammary cell line; HCC1806, epithelial cancer cell line; APOBEC3G, apolipoprotein B mRNA editing enzyme, catalytic subunit 3G; LNCap, lymph node carcinoma of the prostate; DU145, human prostate cancer cell line; GPRC6A, G protein‐coupled receptor class C; ERK, extracellular signal‐regulated kinase; mTOR, mammalian target of rapamycin; AR, androgen receptor; PC3, human prostate cancer cell line; 293FT, human embroyoic kidney cell line; ERRa, estrogen related receptor α; PGC1α, peroxisome‐proliferator‐activated receptor gamma co‐activator 1α; MYC, proto‐oncogene; NANOG, (Nanog homeobox) protein coding gene; ATCC, American type culture collection; TP53, tumor protein 53 gene; SW‐480 CRC cells, colorectal cancer cell line; KRAS, Kirsten rat sarcoma virus gene; CaCO‐2, human colorecta adenocarcinoma cell line; Par3L, partitioning defective 3‐like protein; MC‐38, murine colon adenocarcinoma cell line; Fut4, fucosyl transferase 4 protein coding gene; HPV16, human papilloma virus 16.
7. CLINICAL TRIALS INVOLVING CRISPR/CAS9 GENOME EDITING
The first clinical trial involving CRISPR/Cas9 was based on non‐small cell lung cancer (NSCLC) patient T cells to knockout the PD‐1 (NCT02793856) [35]. Different cancer cells express programmed cell death‐ligand 1 (PD‐L1) that bind with PD‐1 receptors present on activated T cells and inhibit the cytotoxic T cell proliferation and cytokine function. This pathway represents an immune checkpoint mechanism in response to endogenous anticancer activity [218]. Different cancers have also been treated recently by using neutralizing antibodies of PD‐1 or PD‐L1 [219]. This gene therapy approach involved the collection of peripheral blood from patients and ex vivo knockout of PD‐1 by the CRISPR/Cas9 system. These PD‐1‐knockout cells were introduced back to patients. However, the production of engineered T cells is a laborious and costly process compared to therapeutic antibodies against PD‐1 or PD‐L1 [35].
Similarly, another phase I/II clinical trial for Epstein‐Barr virus‐positive cancers, such as gastric carcinoma, lymphoma, and nasopharyngeal carcinoma, involved gene knockout in Epstein‐Barr virus‐specific autologous T cells (NCT03044743). Chimeric antigen receptor (CAR)‐T cells with CRISPR/Cas9 have been generated to fight cancer [220, 221]. In another clinical trial, these CAR cells were delivered to T‐cell receptor α‐chain loci using CRISPR/Cas9 to enhance the tumor rejection capacity [222]. Some more phase II clinical trials have been registered with other cancers, such as bladder, esophageal and renal cancers (clinical‐trials.gov) (Table 4).
TABLE 4.
Different ongoing clinical trials involving the CRISPR/Cas9 system
| Cancer type | Treatment strategy | Phase | Clinical trial identifier |
|---|---|---|---|
| Bladder cancer | PD‐1 knockout T cells | I | NCT02863913 |
| B‐cell lymphoma/leukemia | CRISPR‐Cas9‐edited CAR‐T cells targeting CD19 and CD20 or CD22 | I/II | NCT03166878 |
| B‐cell lymphoma/leukemia | CRISPR‐Cas9‐edited CAR‐T cells targeting CD19 | I/II | NCT03398967 |
| Esophageal cancer | PD‐1 knockout T cells | II | NCT03081715 |
| EBV‐positive advanced stage malignancies | PD‐1 knockout EBV‐CTL | I/II | NCT03044743 |
| Hormone‐refractory prostate cancer | PD‐1 knockout T cells | I | NCT02867345 |
| HPV‐related cervical intraepithelial neoplasia | CRISPR‐Cas9‐sg HPV E6/E7 gel to disrupt HPV DNA | I | NCT03057912 |
| Non‐small cell lung cancer | PD‐1 knockout T cells | I | NCT02793856 |
| Renal cell carcinoma | PD‐1 knockout T cells | I | NCT02867332 |
Abbreviations: PD‐1, programmed cell death protein 1; NCT, national clinical trial; CAR‐T, chimeric antigen receptor‐T cell; CD, cluster of differentiation; HPV, human papillomavirus; EBV, Epstein‐bar virus; EBV‐CTL, Epstein‐bar virus‐specific cytotoxic T cell.
Even though many clinical trials use ex vivo genome editing, only two trials have been implemented for in vivo clinical trials. One trial (NCT03057912) involves the recruitment of the patients and delivery of constructs targeting HPV16 and HPV18 using CRISPR/Cas9 through a gel locally applied on infected cervix. However, more advanced research is obligatory to improve the specific delivery of 2011the CRISPR/Cas9 system to target the desired tissues.
8. CHALLENGES AND FUTURE PROSPECTS
To achieve efficient CRISPR/Cas9 delivery by different NPs, some challenges must be solved to get the best outcome. Some challenges of NP‐based CRISPR/Cas9 delivery within cancer cells include CRISPR/Cas9 packaging (as it is highly anionic); NP size, shape, design, surface properties, and stability during circulation; in vivo toxicity, immunogenicity, and overall efficiency of genome‐editing delivery system by different NPs. The clearance system of systemic circulation hinders the active targeting, as NPs are easily recognized by this system and are removed before the delivery of gene‐editing material is achieved.
Cargo size plays a significant role in determining vascular flow, diffusion profile, adhesion properties, and velocity distribution, all of which contribute to cellular uptake efficiency of CRISPR/Cas9‐NPs. NPs with a size longer than 200 nm accumulate in the liver and spleen and are subsequently cleared by RES. Thus, an ideal CRISPR/Cas9 nanoformulation should have key features such as prolonged blood circulation time, efficient penetration into tumor tissues, high cellular uptake, and efficient endosomal escape to allow CRISPR/Cas9 to release from the endosomes, resulting in high cytoplasm delivery [223, 224].
Despite the distinctive reputation of CRISPR/Cas9 genome editing technology, its efficiency and safety are challenging concerns that require comprehensive research in the future. The off‐target alterations often impede the clinical translation of the CRISPR/Cas9 system [125]. The Cas9 protein commonly cleaves off‐target sites as gRNA tends to have comparatively higher mismatch tolerance and sequence similarity with target sites [225]. The off‐target alterations by CRISPR/Cas9 genome editing are one form of genotoxicity. The CRISPR/Cas9‐edited cells can undergo unexpected large deletions and unusual rearrangements [226]. In human and mouse cell lines, major deletions and complex genomic rearrangements such as insertions and inversions have been reported at adjacent and distal regions of target cut sites [227].
Thus, new versions of Cas9 nuclease with upgraded gRNA design, recognizing a broad range of PAM sequences and targeting it with improved delivery vehicles within specific cells, are often tried to increase the specificity of CRISPR/Cas9 [228]. The newly designed HypaCas9 and xCas9 variants are believed to have efficient target activity with precise genome editing capability [229, 230]. Some new CRISPR/Cas9 inhibitors have been identified to maintain an effective regulation of genome editing, and more related function compounds are expected to be discovered in the future [230]. In addition, some new tools such as BLESS, Digenome‐seq, GUIDE‐seq and HTGTS have been designed to predict potential off‐target sites and gene‐editing outcomes [231].
The efficiency of CRISPR/Cas9 gene‐editing technology and its generalizability are also serious concerns. For example, the PAM;NGG sequence is indispensable at target sites for perfect CRISP/Cas9 genome editing. The applications of CRISPR/Cas9 were limited as only a few PAM sequences were recognized in the recent past. However, recently engineered xCas9 can acknowledge a broader range of PAM sequences (GAA, GAT and NG), multiplying many folds of the scope of this genome‐editing technology.
The future perspectives of CRISPR/Cas9‐based technology will be significant to workout new cancer biomarkers and novel genes which may be highly significant to target for cancer management. The research based on CRISPR/Cas9 technology will be helpful in identifying novel drug targets and their lethal interactions to eradicate cancer. The manipulation of non‐coding regions by this system might boost the exploration of these genes and enhance our understanding on their relationship with different cancers. A deeper insight into various biological changes by the CRISPR/Cas9 system, related to their precise engineering of the driver and pathogenic mutations, will boost the understanding of cancer provocation by these genes. Furthermore, novel advancements in the delivery of the CRISPR/Cas9 system will accelerate their applications for different diseases, including cancer.
9. CONCLUSIONS
The CRISPR/Cas9 system has revolutionized the art of genome editing due to its simplicity and versatility, leading to its widespread use in cancer research. A simple redesign of the RNA sequence can make this system targetable to any oncogenic mutation. The clinical applications of CRISPR/Cas9‐mediated gene therapy face many challenges related to its proper delivery system, fast degradation, short half‐life, reduced uptake by target cells, non‐specific uptake, inability to escape the endosomes, and complications arising due to some immune responses. Due to its high cost and adverse effects, using viral‐based vectors is not a fully proficient delivery strategy. Because of some novel properties of synthetic nanoformulations, the CRISPR/Cas9 delivery within target cells and tissues has shown its potential applications in clinical gene therapy. Overall, the CRISPR/Cas9 system can be applied to treat a wide range of cancers and to develop effective precision medicines. With further optimization, the CRISPR/Cas9 system can produce effective anticancer treatments that may overcome the common limitations encountered by current therapies. Thus, the survival of cancer patients will be significantly improved in the near future.
AUTHOR CONTRIBUTIONS
KSA and AAK planned and designed the diagrams and wrote the final draft of the review. MAA, AA, FA, FKA, and AHR collected information, participated in individual sections, and designed the tables with cited articles. All the authors read and approved the final manuscript.
CONFLICT OF INTERESTS
The authors declare no conflict of interest
FUNDING INFORMATION
This research has no external funding.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
Not applicable
CONSENT FOR PUBLICATION
Not applicable
ACKNOWLEDGMENT
The researchers would like to thank the Deanship of Scientific Research, Qassim University for funding the publication of this project.
Allemailem KS, Alsahli MA, Almatroudi A, Alrumaihi F, Alkhaleefah FK, Rahmani AH, et al. Current updates of CRISPR/Cas9‐mediated genome editing and targeting within tumor cells: an innovative strategy of cancer management. Cancer Commun. 2022;42:1257–1287. 10.1002/cac2.12366
Contributor Information
Khaled S. Allemailem, K.allemailem@qu.edu.sa
Mohammed A Alsahli, Email: shly@qu.edu.sa.
Ahmad Almatroudi, Email: aamtrody@qu.edu.sa.
Faris Alrumaihi, Email: f_alrumaihi@qu.edu.sa.
Fahd Khaleefah Alkhaleefah, Email: fkalkhaleefah@moh.gov.sa.
Arshad Husain Rahmani, Email: Ah.rahmani@qu.edu.sa.
Amjad Ali Khan, Email: akhan@qu.edu.sa.
DATA AVAILABILITY STATEMENT
Not applicable
REFERENCES
- 1. Zhou W, Deiters A. Conditional control of CRISPR/Cas9 function. Angew Chem Int Ed Engl. 2016;55(18):5394–9. [DOI] [PubMed] [Google Scholar]
- 2. Charpentier E, Doudna JA. Biotechnology: Rewriting a genome. Nature. 2013;495(7439):50–1. [DOI] [PubMed] [Google Scholar]
- 3. Nishimasu H, Ran FA, Hsu PD, Konermann S, Shehata SI, Dohmae N, et al. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell. 2014;156(5):935–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR‐Cas9 for genome engineering. Cell. 2014;157(6):1262–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dominguez AA, Lim WA, Qi LS. Beyond editing: repurposing CRISPR‐Cas9 for precision genome regulation and interrogation. Nat Rev Mol Cell Biol. 2016;17(1):5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strong A, Musunuru K. Genome editing in cardiovascular diseases. Nat Rev Cardiol. 2017;14(1):11–20. [DOI] [PubMed] [Google Scholar]
- 8. Shmakov S, Abudayyeh OO, Makarova KS, Wolf YI, Gootenberg JS, Semenova E, et al. Discovery and functional characterization of diverse class 2 CRISPR‐Cas systems. Mol Cell. 2015;60(3):385–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cox DB, Platt RJ, Zhang F. Therapeutic genome editing: prospects and challenges. Nat Med. 2015;21(2):121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao X, Tao Y, Lamas V, Huang M, Yeh WH, Pan B, et al. Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature. 2018;553(7687):217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun W, Ji W, Hall JM, Hu Q, Wang C, Beisel CL, et al. Self‐assembled DNA nanoclews for the efficient delivery of CRISPR‐Cas9 for genome editing. Angew Chem Int Ed Engl. 2015;54(41):12029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alsaiari SK, Patil S, Alyami M, Alamoudi KO, Aleisa FA, Merzaban JS, et al. Endosomal escape and delivery of CRISPR/Cas9 genome editing machinery enabled by nanoscale zeolitic imidazolate framework. J Am Chem Soc. 2018;140(1):143–6. [DOI] [PubMed] [Google Scholar]
- 13. Lee K, Conboy M, Park HM, Jiang F, Kim HJ, Dewitt MA, et al. Nanoparticle delivery of Cas9 ribonucleoprotein and donor DNA in vivo induces homology‐directed DNA repair. Nat Biomed Eng. 2017;1:889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang X, Zhang W, Ding Y, Guo X, Yuan Y, Li D. CRISPR/Cas9‐mediated genome engineering of CXCR4 decreases the malignancy of hepatocellular carcinoma cells in vitro and in vivo. Oncol Rep. 2017;37(6):3565–71. [DOI] [PubMed] [Google Scholar]
- 15. Charlesworth CT, Deshpande PS, Dever DP, Camarena J, Lemgart VT, Cromer MK, et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat Med. 2019;25(2):249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wagner DL, Amini L, Wendering DJ, Burkhardt LM, Akyüz L, Reinke P, et al. High prevalence of streptococcus pyogenes Cas9‐reactive T cells within the adult human population. Nat Med. 2019;25(2):242–8. [DOI] [PubMed] [Google Scholar]
- 17. Pucci C, Martinelli C, Ciofani G. Innovative approaches for cancer treatment: current perspectives and new challenges. Ecancermedicalscience. 2019;13:961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martinelli C. Exosomes: new biomarkers for targeted cancer therapy. Molecular Oncology: Underlying Mechanisms and Translational Advancements: Springer; 2017. p. 129–57. [Google Scholar]
- 19. CJIp Brace. Thermal tumor ablation in clinical use. 2011;2(5):28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu KH, Zhang C, Berry GJ, Altman RB, Ré C, Rubin DL, et al. Predicting non‐small cell lung cancer prognosis by fully automated microscopic pathology image features. Nat Commun. 2016;7:12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aerts HJ. The potential of radiomic‐based phenotyping in precision medicine: a review. JAMA Oncol. 2016;2(12):1636–42. [DOI] [PubMed] [Google Scholar]
- 22. Shanker M, Jin J, Branch CD, Miyamoto S, Grimm EA, Roth JA, et al. Tumor suppressor gene‐based nanotherapy: from test tube to the clinic. J Drug Deliv. 2011;2011:465845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vaishnaw AK, Gollob J, Gamba‐Vitalo C, Hutabarat R, Sah D, Meyers R, et al. A status report on RNAi therapeutics. Silence. 2010;1(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shankar S, Sreekumar A, Prasad D, Das AV, Pillai MR. Genome editing of oncogenes with ZFNs and TALENs: caveats in nuclease design. Cancer Cell Int. 2018;18:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lino CA, Harper JC, Carney JP, Timlin JA. Delivering CRISPR: a review of the challenges and approaches. Drug Deliv. 2018;25(1):1234–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kampmann M. CRISPRi and CRISPRa screens in mammalian cells for precision biology and medicine. ACS Chem Biol. 2018;13(2):406–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol. 1987;169(12):5429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jansen R, Embden JD, Gaastra W, Schouls LM. Identification of genes that are associated with DNA repeats in prokaryotes. Mol Microbiol. 2002;43(6):1565–75. [DOI] [PubMed] [Google Scholar]
- 29. Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315(5819):1709–12. [DOI] [PubMed] [Google Scholar]
- 30. Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, Saunders SJ, et al. An updated evolutionary classification of CRISPR‐Cas systems. Nat Rev Microbiol. 2015;13(11):722–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gasiunas G, Barrangou R, Horvath P, Siksnys V. Cas9‐crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A. 2012;109(39):E2579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, et al. RNA‐guided human genome engineering via Cas9. Science. 2013;339(6121):823–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Higashijima Y, Hirano S, Nangaku M, Nureki O. Applications of the CRISPR‐Cas9 system in kidney research. Kidney Int. 2017;92(2):324–35. [DOI] [PubMed] [Google Scholar]
- 34. Zhu S, Li W, Liu J, Chen CH, Liao Q, Xu P, et al. Genome‐scale deletion screening of human long non‐coding RNAs using a paired‐guide RNA CRISPR‐Cas9 library. Nat Biotechnol. 2016;34(12):1279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cyranoski D. CRISPR gene‐editing tested in a person for the first time. Nature. 2016;539(7630):479. [DOI] [PubMed] [Google Scholar]
- 36. Cox DBT, Gootenberg JS, Abudayyeh OO, Franklin B, Kellner MJ, Joung J, et al. RNA editing with CRISPR‐Cas13. Science. 2017;358(6366):1019–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haapaniemi E, Botla S, Persson J, Schmierer B, Taipale J. CRISPR‐Cas9 genome editing induces a p53‐mediated DNA damage response. Nat Med. 2018;24(7):927–30. [DOI] [PubMed] [Google Scholar]
- 38. Koblan LW, Doman JL, Wilson C, Levy JM, Tay T, Newby GA, et al. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat Biotechnol. 2018;36(9):843–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marraffini LA. CRISPR‐Cas immunity in prokaryotes. Nature. 2015;526(7571):55–61. [DOI] [PubMed] [Google Scholar]
- 40. Amitai G, Sorek R. CRISPR‐Cas adaptation: insights into the mechanism of action. Nat Rev Microbiol. 2016;14(2):67–76. [DOI] [PubMed] [Google Scholar]
- 41. Brouns SJ, Jore MM, Lundgren M, Westra ER, Slijkhuis RJ, Snijders AP, et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008;321(5891):960–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garneau JE, Dupuis M, Villion M, Romero DA, Barrangou R, Boyaval P, et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 2010;468(7320):67–71. [DOI] [PubMed] [Google Scholar]
- 43. Wiedenheft B, Sternberg SH, Doudna JA. RNA‐guided genetic silencing systems in bacteria and archaea. Nature. 2012;482(7385):331–8. [DOI] [PubMed] [Google Scholar]
- 44. Jiang F, Zhou K, Ma L, Gressel S, Doudna JA. STRUCTURAL BIOLOGY. A Cas9‐guide RNA complex preorganized for target DNA recognition. Science. 2015;348(6242):1477–81. [DOI] [PubMed] [Google Scholar]
- 45. Wright AV, Nuñez JK, Doudna JA. Biology and applications of CRISPR systems: harnessing nature's toolbox for genome engineering. Cell. 2016;164(1‐2):29–44. [DOI] [PubMed] [Google Scholar]
- 46. Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual‐RNA‐guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, et al. CRISPR RNA maturation by trans‐encoded small RNA and host factor RNase III. Nature. 2011;471(7340):602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Perez‐Pinera P, Kocak DD, Vockley CM, Adler AF, Kabadi AM, Polstein LR, et al. RNA‐guided gene activation by CRISPR‐Cas9‐based transcription factors. Nat Methods. 2013;10(10):973–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR‐Cas9. Science. 2014;346(6213):1258096. [DOI] [PubMed] [Google Scholar]
- 50. Chandrasegaran S, Carroll D. Origins of programmable nucleases for genome engineering. J Mol Biol. 2016;428(5 Pt B):963–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hu JH, Davis KM, Liu DR. Chemical biology approaches to genome editing: understanding, controlling, and delivering programmable nucleases. Cell Chem Biol. 2016;23(1):57–73. [DOI] [PubMed] [Google Scholar]
- 52. Chen H, Choi J, Bailey S. Cut site selection by the two nuclease domains of the Cas9 RNA‐guided endonuclease. J Biol Chem. 2014;289(19):13284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang P, Zhang L, Zheng W, Cong L, Guo Z, Xie Y, et al. Thermo‐triggered release of CRISPR‐Cas9 system by lipid‐encapsulated gold nanoparticles for tumor therapy. Angew Chem Int Ed Engl. 2018;57(6):1491–6. [DOI] [PubMed] [Google Scholar]
- 54. Heler R, Samai P, Modell JW, Weiner C, Goldberg GW, Bikard D, et al. Cas9 specifies functional viral targets during CRISPR‐Cas adaptation. Nature. 2015;519(7542):199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jinek M, Jiang F, Taylor DW, Sternberg SH, Kaya E, Ma E, et al. Structures of Cas9 endonucleases reveal RNA‐mediated conformational activation. Science. 2014;343(6176):1247997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang W. An equivalent metal ion in one‐ and two‐metal‐ion catalysis. Nat Struct Mol Biol. 2008;15(11):1228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Semenova E, Jore MM, Datsenko KA, Semenova A, Westra ER, Wanner B, et al. Interference by clustered regularly interspaced short palindromic repeat (CRISPR) RNA is governed by a seed sequence. Proc Natl Acad Sci U S A. 2011;108(25):10098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pattanayak V, Lin S, Guilinger JP, Ma E, Doudna JA, Liu DR. High‐throughput profiling of off‐target DNA cleavage reveals RNA‐programmed Cas9 nuclease specificity. Nat Biotechnol. 2013;31(9):839–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jiang F, Doudna JA. The structural biology of CRISPR‐Cas systems. Curr Opin Struct Biol. 2015;30:100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Marraffini LA, Sontheimer EJ. Self versus non‐self discrimination during CRISPR RNA‐directed immunity. Nature. 2010;463(7280):568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jiang W, Bikard D, Cox D, Zhang F, Marraffini LA. RNA‐guided editing of bacterial genomes using CRISPR‐Cas systems. Nat Biotechnol. 2013;31(3):233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA. DNA interrogation by the CRISPR RNA‐guided endonuclease Cas9. Nature. 2014;507(7490):62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hale CR, Zhao P, Olson S, Duff MO, Graveley BR, Wells L, et al. RNA‐guided RNA cleavage by a CRISPR RNA‐Cas protein complex. Cell. 2009;139(5):945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR‐Cas9 system. Nat Protoc. 2013;8(11):2281–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520(7546):186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rosenblum D, Gutkin A, Kedmi R, Ramishetti S, Veiga N, Jacobi AM, et al. CRISPR‐Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci Adv. 2020;6(47). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim S, Kim D, Cho SW, Kim J, Kim JS. Highly efficient RNA‐guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 2014;24(6):1012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zuris JA, Thompson DB, Shu Y, Guilinger JP, Bessen JL, Hu JH, et al. Cationic lipid‐mediated delivery of proteins enables efficient protein‐based genome editing in vitro and in vivo. Nat Biotechnol. 2015;33(1):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Glass Z, Lee M, Li Y, Xu Q. Engineering the delivery system for CRISPR‐based genome editing. Trends Biotechnol. 2018;36(2):173–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mellott AJ, Forrest ML, Detamore MS. Physical non‐viral gene delivery methods for tissue engineering. Ann Biomed Eng. 2013;41(3):446–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chuang CK, Chen CH, Huang CL, Su YH, Peng SH, Lin TY, et al. Generation of GGTA1 mutant pigs by direct pronuclear microinjection of CRISPR/Cas9 plasmid vectors. Anim Biotechnol. 2017;28(3):174–81. [DOI] [PubMed] [Google Scholar]
- 72. Chen S, Lee B, Lee AY, Modzelewski AJ, He L. Highly efficient mouse genome editing by CRISPR ribonucleoprotein electroporation of zygotes. J Biol Chem. 2016;291(28):14457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Guan Y, Ma Y, Li Q, Sun Z, Ma L, Wu L, et al. CRISPR/Cas9‐mediated somatic correction of a novel coagulator factor IX gene mutation ameliorates hemophilia in mouse. EMBO Mol Med. 2016;8(5):477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Long C, Amoasii L, Mireault AA, McAnally JR, Li H, Sanchez‐Ortiz E, et al. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science. 2016;351(6271):400–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Voets O, Tielen F, Elstak E, Benschop J, Grimbergen M, Stallen J, et al. Highly efficient gene inactivation by adenoviral CRISPR/Cas9 in human primary cells. PLoS One. 2017;12(8):e0182974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Roehm PC, Shekarabi M, Wollebo HS, Bellizzi A, He L, Salkind J, et al. Inhibition of HSV‐1 replication by gene editing strategy. Sci Rep. 2016;6:23146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chew WL, Tabebordbar M, Cheng JK, Mali P, Wu EY, Ng AH, et al. A multifunctional AAV‐CRISPR‐Cas9 and its host response. Nat Methods. 2016;13(10):868–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4(5):346–58. [DOI] [PubMed] [Google Scholar]
- 79. Ortinski PI, O'Donovan B, Dong X, Kantor B. Integrase‐deficient lentiviral vector as an all‐in‐one platform for highly efficient CRISPR/Cas9‐mediated gene editing. Mol Ther Methods Clin Dev. 2017;5:153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chen X, Gonçalves MA. Engineered viruses as genome editing devices. Mol Ther. 2016;24(3):447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bear AS, Morgan RA, Cornetta K, June CH, Binder‐Scholl G, Dudley ME, et al. Replication‐competent retroviruses in gene‐modified T cells used in clinical trials: is it time to revise the testing requirements? Mol Ther. 2012;20(2):246–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Li C, Samulski RJ. Engineering adeno‐associated virus vectors for gene therapy. Nat Rev Genet. 2020;21(4):255–72. [DOI] [PubMed] [Google Scholar]
- 83. Crystal RG. Adenovirus: the first effective in vivo gene delivery vector. Hum Gene Ther. 2014;25(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Squire LR, Dronkers N, Baldo J. Encyclopedia of neuroscience: Elsevier; Amsterdam, The Netherlands; 2009. [Google Scholar]
- 85. Ghosh SS, Gopinath P, Ramesh A. Adenoviral vectors: a promising tool for gene therapy. Appl Biochem Biotechnol. 2006;133(1):9–29. [DOI] [PubMed] [Google Scholar]
- 86. Maddalo D, Manchado E, Concepcion CP, Bonetti C, Vidigal JA, Han YC, et al. In vivo engineering of oncogenic chromosomal rearrangements with the CRISPR/Cas9 system. Nature. 2014;516(7531):423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Segel M, Lash B, Song J, Ladha A, Liu CC, Jin X, et al. Mammalian retrovirus‐like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science. 2021;373(6557):882–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gutkin A, Rosenblum D, Peer D. RNA delivery with a human virus‐like particle. Nat Biotechnol. 2021;39(12):1514–5. [DOI] [PubMed] [Google Scholar]
- 89. Li L, Hu S, Chen X. Non‐viral delivery systems for CRISPR/Cas9‐based genome editing: challenges and opportunities. Biomaterials. 2018;171:207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rui Y, Wilson DR, Green JJ. Non‐viral delivery to enable genome editing. Trends Biotechnol. 2019;37(3):281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cunningham FJ, Goh NS, Demirer GS, Matos JL, Landry MP. Nanoparticle‐mediated delivery towards advancing plant genetic engineering. Trends Biotechnol. 2018;36(9):882–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Allemailem KS, Almatroudi A, Alrumaihi F, Almatroodi SA, Alkurbi MO, Basfar GT, et al. Novel approaches of dysregulating lysosome functions in cancer cells by specific drugs and its nanoformulations: a smart approach of modern therapeutics. Int J Nanomedicine. 2021;16:5065–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. SA K, Almatroudi A, Alsahli MA, Aljaghwani A, ME‐K A, Rahmani AH, et al. Novel strategies for disrupting cancer‐cell functions with mitochondria‐targeted antitumor drug‐loaded nanoformulations. Int J Nanomedicine. 2021;16:3907–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mendes R, Fernandes AR, Baptista PV. Gold nanoparticle approach to the selective delivery of gene silencing in cancer‐the case for combined delivery? Genes (Basel). 2017;8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nelson CE, Gersbach CA. Engineering delivery vehicles for genome editing. Annu Rev Chem Biomol Eng. 2016;7:637–62. [DOI] [PubMed] [Google Scholar]
- 96. Khan AA, Allemailem KS, Almatroodi SA, Almatroudi A, Rahmani AH. Recent strategies towards the surface modification of liposomes: an innovative approach for different clinical applications. 3 Biotech. 2020;10(4):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kim YD, Park TE, Singh B, Maharjan S, Choi YJ, Choung PH, et al. Nanoparticle‐mediated delivery of siRNA for effective lung cancer therapy. Nanomedicine (Lond). 2015;10(7):1165–88. [DOI] [PubMed] [Google Scholar]
- 98. Bharti C, Nagaich U, Pal AK, Gulati N. Mesoporous silica nanoparticles in target drug delivery system: a review. Int J Pharm Investig. 2015;5(3):124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chen Z, Liu F, Chen Y, Liu J, Wang X, Chen AT, et al. Targeted delivery of CRISPR/Cas9‐mediated cancer gene therapy via liposome‐templated hydrogel nanoparticles. Adv Funct Mater. 2017;27(46):1703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li Y, Bolinger J, Yu Y, Glass Z, Shi N, Yang L, et al. Intracellular delivery and biodistribution study of CRISPR/Cas9 ribonucleoprotein loaded bioreducible lipidoid nanoparticles. Biomater Sci. 2019;7(2):596–606. [DOI] [PubMed] [Google Scholar]
- 101. Sago CD, Lokugamage MP, Paunovska K, Vanover DA, Monaco CM, Shah NN, et al. High‐throughput in vivo screen of functional mRNA delivery identifies nanoparticles for endothelial cell gene editing. Proc Natl Acad Sci U S A. 2018;115(42):E9944–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Patel S, Ashwanikumar N, Robinson E, DuRoss A, Sun C, Murphy‐Benenato KE, et al. Boosting intracellular delivery of lipid nanoparticle‐encapsulated mRNA. Nano Lett. 2017;17(9):5711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Xu C, Lu Z, Luo Y, Liu Y, Cao Z, Shen S, et al. Targeting of NLRP3 inflammasome with gene editing for the amelioration of inflammatory diseases. Nat Commun. 2018;9(1):4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lin Y, Wu J, Gu W, Huang Y, Tong Z, Huang L, et al. Exosome‐liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv Sci (Weinh). 2018;5(4):1700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Finn JD, Smith AR, Patel MC, Shaw L, Youniss MR, van Heteren J, et al. A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 2018;22(9):2227–35. [DOI] [PubMed] [Google Scholar]
- 106. Liu Y, Zhao G, Xu CF, Luo YL, Lu ZD, Wang J. Systemic delivery of CRISPR/Cas9 with PEG‐PLGA nanoparticles for chronic myeloid leukemia targeted therapy. Biomater Sci. 2018;6(6):1592–603. [DOI] [PubMed] [Google Scholar]
- 107. Li Y, Yang T, Yu Y, Shi N, Yang L, Glass Z, et al. Combinatorial library of chalcogen‐containing lipidoids for intracellular delivery of genome‐editing proteins. Biomaterials. 2018;178:652–62. [DOI] [PubMed] [Google Scholar]
- 108. Miller JB, Zhang S, Kos P, Xiong H, Zhou K, Perelman SS, et al. Non‐viral CRISPR/Cas gene editing in vitro and in vivo enabled by synthetic nanoparticle co‐delivery of Cas9 mRNA and sgRNA. Angew Chem Int Ed Engl. 2017;56(4):1059–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Luo YL, Xu CF, Li HJ, Cao ZT, Liu J, Wang JL, et al. Macrophage‐specific in vivo gene editing using cationic lipid‐assisted polymeric nanoparticles. ACS Nano. 2018;12(2):994–1005. [DOI] [PubMed] [Google Scholar]
- 110. Zhu D, Shen H, Tan S, Hu Z, Wang L, Yu L, et al. Nanoparticles based on poly (β‐amino ester) and HPV16‐targeting CRISPR/shRNA as potential drugs for HPV16‐related cervical malignancy. Mol Ther. 2018;26(10):2443–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhang H, Bahamondez‐Canas TF, Zhang Y, Leal J, Smyth HDC. PEGylated chitosan for nonviral aerosol and mucosal delivery of the CRISPR/Cas9 system in vitro. Mol Pharm. 2018;15(11):4814–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhang Z, Wan T, Chen Y, Chen Y, Sun H, Cao T, et al. Cationic polymer‐mediated CRISPR/Cas9 plasmid delivery for genome editing. Macromol Rapid Commun. 2019;40(5):e1800068. [DOI] [PubMed] [Google Scholar]
- 113. Zhou W, Cui H, Ying L, Yu XF. Enhanced cytosolic delivery and release of CRISPR/Cas9 by black phosphorus nanosheets for genome editing. Angew Chem Int Ed Engl. 2018;57(32):10268–72. [DOI] [PubMed] [Google Scholar]
- 114. Mout R, Ray M, Yesilbag Tonga G, Lee YW, Tay T, Sasaki K, et al. Direct cytosolic delivery of CRISPR/Cas9‐ribonucleoprotein for efficient gene editing. ACS Nano. 2017;11(3):2452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mout R, Rotello VM. Cytosolic and nuclear delivery of CRISPR/Cas9‐ribonucleoprotein for gene editing using arginine functionalized gold nanoparticles. Bio Protoc. 2017;7(20):e2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Liu BY, He XY, Xu C, Xu L, Ai SL, Cheng SX, et al. A dual‐targeting delivery system for effective genome editing and in situ detecting related protein expression in edited cells. Biomacromolecules. 2018;19(7):2957–68. [DOI] [PubMed] [Google Scholar]
- 117. Liang Z, Chen K, Li T, Zhang Y, Wang Y, Zhao Q, et al. Efficient DNA‐free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat Commun. 2017;8:14261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lee B, Lee K, Panda S, Gonzales‐Rojas R, Chong A, Bugay V, et al. Nanoparticle delivery of CRISPR into the brain rescues a mouse model of fragile X syndrome from exaggerated repetitive behaviours. Nat Biomed Eng. 2018;2(7):497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kong J, Wang Y, Zhang J, Qi W, Su R, He Z. Rationally designed peptidyl virus‐like particles enable targeted delivery of genetic cargo. Angew Chem Int Ed Engl. 2018;57(43):14032–6. [DOI] [PubMed] [Google Scholar]
- 120. Shen Y, Cohen JL, Nicoloro SM, Kelly M, Yenilmez B, Henriques F, et al. CRISPR‐delivery particles targeting nuclear receptor‐interacting protein 1 (Nrip1) in adipose cells to enhance energy expenditure. J Biol Chem. 2018;293(44):17291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lostalé‐Seijo I, Louzao I, Juanes M, Montenegro J. Peptide/Cas9 nanostructures for ribonucleoprotein cell membrane transport and gene edition. Chem Sci. 2017;8(12):7923–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ray M, Lee YW, Hardie J, Mout R, Yeşilbag Tonga G, Farkas ME, et al. CRISPRed macrophages for cell‐based cancer immunotherapy. Bioconjug Chem. 2018;29(2):445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhang X, Li B, Luo X, Zhao W, Jiang J, Zhang C, et al. Biodegradable amino‐ester nanomaterials for Cas9 mRNA Delivery in vitro and in vivo. ACS Appl Mater Interfaces. 2017;9(30):25481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Liu BY, He XY, Zhuo RX, Cheng SX. Tumor targeted genome editing mediated by a multi‐functional gene vector for regulating cell behaviors. J Control Release. 2018;291:90–8. [DOI] [PubMed] [Google Scholar]
- 125. Wang P, Zhang L, Xie Y, Wang N, Tang R, Zheng W, et al. Genome editing for cancer therapy: delivery of Cas9 protein/sgRNA plasmid via a gold nanocluster/lipid core‐shell nanocarrier. Adv Sci (Weinh). 2017;4(11):1700175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Shahbazi R, Sghia‐Hughes G, Reid JL, Kubek S, Haworth KG, Humbert O, et al. Targeted homology‐directed repair in blood stem and progenitor cells with CRISPR nanoformulations. Nat Mater. 2019;18(10):1124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ju E, Li T, Ramos da Silva S, Gao SJ. Gold nanocluster‐mediated efficient delivery of Cas9 protein through pH‐induced assembly‐disassembly for inactivation of virus oncogenes. ACS Appl Mater Interfaces. 2019;11(38):34717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Padayachee J, Singh M. Therapeutic applications of CRISPR/Cas9 in breast cancer and delivery potential of gold nanomaterials. Nanobiomedicine (Rij). 2020;7:1849543520983196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Dong Z, Feng L, Zhu W, Sun X, Gao M, Zhao H, et al. CaCO(3) nanoparticles as an ultra‐sensitive tumor‐pH‐responsive nanoplatform enabling real‐time drug release monitoring and cancer combination therapy. Biomaterials. 2016;110:60–70. [DOI] [PubMed] [Google Scholar]
- 130. Liu W, Dong A, Wang B, Zhang H. Current advances in black phosphorus‐based drug delivery systems for cancer therapy. Adv Sci (Weinh). 2021;8(5):2003033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wan T, Niu D, Wu C, Xu F‐J, Church G, Ping YJMT. Material solutions for delivery of CRISPR/Cas‐based genome editing tools: current status and future outlook. 2019;26:40–66. [Google Scholar]
- 132. Luchini A, Vitiello G. Understanding the nano‐bio interfaces: lipid‐coatings for inorganic nanoparticles as promising strategy for biomedical applications. Front Chem. 2019;7:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Liu SJ, Horlbeck MA, Cho SW, Birk HS, Malatesta M, He D, et al. CRISPRi‐based genome‐scale identification of functional long noncoding RNA loci in human cells. Science. 2017;355(6320). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Naseri N, Valizadeh H, Zakeri‐Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull. 2015;5(3):305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Rehman Z, Zuhorn IS, Hoekstra D. How cationic lipids transfer nucleic acids into cells and across cellular membranes: recent advances. J Control Release. 2013;166(1):46–56. [DOI] [PubMed] [Google Scholar]
- 136. Han Y, Zhang Y, Li D, Chen Y, Sun J, Kong F. Transferrin‐modified nanostructured lipid carriers as multifunctional nanomedicine for codelivery of DNA and doxorubicin. Int J Nanomedicine. 2014;9:4107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non‐viral gene delivery particles. Eur J Cell Biol. 2004;83(3):97–111. [DOI] [PubMed] [Google Scholar]
- 138. Zhang L, Wang P, Feng Q, Wang N, Chen Z, Huang Y, et al. Lipid nanoparticle‐mediated efficient delivery of CRISPR/Cas9 for tumor therapy. NPG Asia Mater. 2017;9(10):e441–e. [Google Scholar]
- 139. Granot Y, Peer D. Delivering the right message: Challenges and opportunities in lipid nanoparticles‐mediated modified mRNA therapeutics‐An innate immune system standpoint. Semin Immunol. 2017;34:68–77. [DOI] [PubMed] [Google Scholar]
- 140. Wang M, Zuris JA, Meng F, Rees H, Sun S, Deng P, et al. Efficient delivery of genome‐editing proteins using bioreducible lipid nanoparticles. Proc Natl Acad Sci U S A. 2016;113(11):2868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Aghamiri S, Jafarpour A, Shoja M. Effects of silver nanoparticles coated with anti‐HER2 on irradiation efficiency of SKBR3 breast cancer cells. IET Nanobiotechnol. 2019;13(8):808–15. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 142. Liu B, Saber A, Haisma HJ. CRISPR/Cas9: a powerful tool for identification of new targets for cancer treatment. Drug Discov Today. 2019;24(4):955–70. [DOI] [PubMed] [Google Scholar]
- 143. Turecek PL, Bossard MJ, Schoetens F, Ivens IA. PEGylation of biopharmaceuticals: a review of chemistry and nonclinical safety information of approved drugs. J Pharm Sci. 2016;105(2):460–75. [DOI] [PubMed] [Google Scholar]
- 144. Ruan W, Zheng M, An Y, Liu Y, Lovejoy DB, Hao M, et al. DNA nanoclew templated spherical nucleic acids for siRNA delivery. Chem Commun (Camb). 2018;54(29):3609–12. [DOI] [PubMed] [Google Scholar]
- 145. Aghamiri S, Mehrjardi KF, Shabani S, Keshavarz‐Fathi M, Kargar S, Rezaei N. Nanoparticle‐siRNA: a potential strategy for ovarian cancer therapy? Nanomedicine (Lond). 2019;14(15):2083–100. [DOI] [PubMed] [Google Scholar]
- 146. Axford DS, Morris DP, McMurry JL. Cell penetrating peptide‐mediated nuclear delivery of Cas9 to enhance the utility of CRISPR/Cas genome editing. FASEB J. 2017;31:909.4–.4. [Google Scholar]
- 147. Huang Y‐W, Lee H‐J. Cell‐penetrating peptides for medical theranostics and targeted drug delivery. Peptide applications in biomedicine, biotechnology and bioengineering: Elsevier; 2018. p. 359–70. [Google Scholar]
- 148. Ramakrishna S, Kwaku Dad AB, Beloor J, Gopalappa R, Lee SK, Kim H. Gene disruption by cell‐penetrating peptide‐mediated delivery of Cas9 protein and guide RNA. Genome Res. 2014;24(6):1020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Huettner N, Dargaville TR, Forget A. Discovering cell‐adhesion peptides in tissue engineering: beyond RGD. Trends Biotechnol. 2018;36(4):372–83. [DOI] [PubMed] [Google Scholar]
- 150. Fu S, Xu X, Ma Y, Zhang S, Zhang S. RGD peptide‐based non‐viral gene delivery vectors targeting integrin α(v)β(3) for cancer therapy. J Drug Target. 2019;27(1):1–11. [DOI] [PubMed] [Google Scholar]
- 151. Allemailem KS, Almatroudi A, Alsahli MA, Basfar GT, Alrumaihi F, Rahmani AH, et al. Recent advances in understanding oligonucleotide aptamers and their applications as therapeutic agents. 3 Biotech. 2020;10(12):551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Liu Y, Cao ZT, Xu CF, Lu ZD, Luo YL, Wang J. Optimization of lipid‐assisted nanoparticle for disturbing neutrophils‐related inflammation. Biomaterials. 2018;172:92–104. [DOI] [PubMed] [Google Scholar]
- 153. Yang Y, Qiu JG, Li Y, Di JM, Zhang WJ, Jiang QW, et al. Targeting ABCB1‐mediated tumor multidrug resistance by CRISPR/Cas9‐based genome editing. Am J Transl Res. 2016;8(9):3986–94. [PMC free article] [PubMed] [Google Scholar]
- 154. Ratan ZA, Son YJ, Haidere MF, Uddin BMM, Yusuf MA, Zaman SB, et al. CRISPR‐Cas9: a promising genetic engineering approach in cancer research. Ther Adv Med Oncol. 2018;10:1758834018755089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lage H. Gene therapeutic approaches to overcome ABCB1‐mediated drug resistance. Recent Results Cancer Res. 2016;209:87–94. [DOI] [PubMed] [Google Scholar]
- 156. Aghamiri S, Talaei S, Ghavidel AA, Zandsalimi F, Masoumi S, Hafshejani NH, et al. Nanoparticles‐mediated CRISPR/Cas9 delivery: recent advances in cancer treatment. J Drug Deliv Sci Technol. 2020;56:101533. [Google Scholar]
- 157. Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hudson TJ, Anderson W, Artez A, Barker AD, Bell C, Bernabé RR, et al. International network of cancer genome projects. Nature. 2010;464(7291):993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Drost J, van Jaarsveld RH, Ponsioen B, Zimberlin C, van Boxtel R, Buijs A, et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature. 2015;521(7550):43–7. [DOI] [PubMed] [Google Scholar]
- 161. Shi J, Wang E, Milazzo JP, Wang Z, Kinney JB, Vakoc CR. Discovery of cancer drug targets by CRISPR‐Cas9 screening of protein domains. Nat Biotechnol. 2015;33(6):661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Nishida K, Arazoe T, Yachie N, Banno S, Kakimoto M, Tabata M, et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science. 2016;353(6305). [DOI] [PubMed] [Google Scholar]
- 163. Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21(11):1253–61. [DOI] [PubMed] [Google Scholar]
- 164. Sanjana NE, Wright J, Zheng K, Shalem O, Fontanillas P, Joung J, et al. High‐resolution interrogation of functional elements in the noncoding genome. Science. 2016;353(6307):1545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Castillo A. Gene editing using CRISPR‐Cas9 for the treatment of lung cancer. Colomb Med (Cali). 2016;47(4):178–80. [PMC free article] [PubMed] [Google Scholar]
- 166. Tang H, Shrager JB. CRISPR/Cas‐mediated genome editing to treat EGFR‐mutant lung cancer: a personalized molecular surgical therapy. EMBO Mol Med. 2016;8(2):83–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Zhao Z, Shi L, Zhang W, Han J, Zhang S, Fu Z, et al. CRISPR knock out of programmed cell death protein 1 enhances anti‐tumor activity of cytotoxic T lymphocytes. Oncotarget. 2018;9(4):5208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Doudna JA. The promise and challenge of therapeutic genome editing. Nature. 2020;578(7794):229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Iwagami Y, Huang CK, Olsen MJ, Thomas JM, Jang G, Kim M, et al. Aspartate β‐hydroxylase modulates cellular senescence through glycogen synthase kinase 3β in hepatocellular carcinoma. Hepatology. 2016;63(4):1213–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Qin J, Li Q, Zeng Z, Wu P, Jiang Y, Luo T, et al. Increased expression of G9A contributes to carcinogenesis and indicates poor prognosis in hepatocellular carcinoma. Oncol Lett. 2018;15(6):9757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Scheer S, Zaph C. The lysine methyltransferase G9a in immune cell differentiation and function. Front Immunol. 2017;8:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Wei L, Chiu DK, Tsang FH, Law CT, Cheng CL, Au SL, et al. Histone methyltransferase G9a promotes liver cancer development by epigenetic silencing of tumor suppressor gene RARRES3. J Hepatol. 2017;67(4):758–69. [DOI] [PubMed] [Google Scholar]
- 173. Pott LL, Hagemann S, Reis H, Lorenz K, Bracht T, Herold T, et al. Eukaryotic elongation factor 2 is a prognostic marker and its kinase a potential therapeutic target in HCC. Oncotarget. 2017;8(7):11950–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Jeng KS, Jeng CJ, Jeng WJ, Chang CF, Sheen IS. Role of C‐X‐C chemokine ligand 12/C‐X‐C chemokine receptor 4 in the progression of hepatocellular carcinoma. Oncol Lett. 2017;14(2):1905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Watanabe S, Shimada S, Akiyama Y, Ishikawa Y, Ogura T, Ogawa K, et al. Loss of KDM6A characterizes a poor prognostic subtype of human pancreatic cancer and potentiates HDAC inhibitor lethality. Int J Cancer. 2019;145(1):192–205. [DOI] [PubMed] [Google Scholar]
- 176. Pessolano E, Belvedere R, Bizzarro V, Franco P, Marco I, Porta A, et al. Annexin A1 may induce pancreatic cancer progression as a key player of extracellular vesicles effects as evidenced in the in vitro MIA PaCa‐2 model system. Int J Mol Sci. 2018;19(12):3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Barkeer S, Chugh S, Karmakar S, Kaushik G, Rauth S, Rachagani S, et al. Novel role of O‐glycosyltransferases GALNT3 and B3GNT3 in the self‐renewal of pancreatic cancer stem cells. BMC Cancer. 2018;18(1):1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Yuza K, Nakajima M, Nagahashi M, Tsuchida J, Hirose Y, Miura K, et al. Different roles of sphingosine kinase 1 and 2 in pancreatic cancer progression. J Surg Res. 2018;232:186–94. [DOI] [PubMed] [Google Scholar]
- 179. Yang H, Bailey P, Pilarsky C. CRISPR Cas9 in pancreatic cancer research. Front Cell Dev Biol. 2019;7:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Cai Y, Tsai HC, Yen RC, Zhang YW, Kong X, Wang W, et al. Critical threshold levels of DNA methyltransferase 1 are required to maintain DNA methylation across the genome in human cancer cells. Genome Res. 2017;27(4):533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Zagorac S, Alcala S, Fernandez Bayon G, Bou Kheir T, Schoenhals M, González‐Neira A, et al. DNMT1 inhibition reprograms pancreatic cancer stem cells via upregulation of the miR‐17‐92 cluster. Cancer Res. 2016;76(15):4546–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Hao X, Luo H, Krawczyk M, Wei W, Wang W, Wang J, et al. DNA methylation markers for diagnosis and prognosis of common cancers. Proc Natl Acad Sci U S A. 2017;114(28):7414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Flanagan JM, Wilson A, Koo C, Masrour N, Gallon J, Loomis E, et al. Platinum‐based chemotherapy induces methylation changes in blood DNA associated with overall survival in patients with ovarian cancer. Clin Cancer Res. 2017;23(9):2213–22. [DOI] [PubMed] [Google Scholar]
- 184. Sander JD, Joung JK. CRISPR‐Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32(4):347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. He ZY, Zhang YG, Yang YH, Ma CC, Wang P, Du W, et al. In vivo ovarian cancer gene therapy using CRISPR‐Cas9. Hum Gene Ther. 2018;29(2):223–33. [DOI] [PubMed] [Google Scholar]
- 186. Pi M, Quarles LD. GPRC6A regulates prostate cancer progression. Prostate. 2012;72(4):399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Liu M, Zhao YY, Yang F, Wang JY, Shi XH, Zhu XQ, et al. Evidence for a role of GPRC6A in prostate cancer metastasis based on case‐control and in vitro analyses. Eur Rev Med Pharmacol Sci. 2016;20(11):2235–48. [PubMed] [Google Scholar]
- 188. Ye R, Pi M, Cox JV, Nishimoto SK, Quarles LD. CRISPR/Cas9 targeting of GPRC6A suppresses prostate cancer tumorigenesis in a human xenograft model. J Exp Clin Cancer Res. 2017;36(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Banerjee B, Sherwood RI. A CRISPR view of gene regulation. Curr Opin Syst Biol. 2017;1:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Banerjee S, Ji C, Mayfield JE, Goel A, Xiao J, Dixon JE, et al. Ancient drug curcumin impedes 26S proteasome activity by direct inhibition of dual‐specificity tyrosine‐regulated kinase 2. Proc Natl Acad Sci U S A. 2018;115(32):8155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Guo X, Wang X, Wang Z, Banerjee S, Yang J, Huang L, et al. Site‐specific proteasome phosphorylation controls cell proliferation and tumorigenesis. Nat Cell Biol. 2016;18(2):202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Zhao HB, Zhang XF, Wang HB, Zhang MZ. Migration and invasion enhancer 1 (MIEN1) is overexpressed in breast cancer and is a potential new therapeutic molecular target. Genet Mol Res. 2017;16(1). [DOI] [PubMed] [Google Scholar]
- 193. Van Treuren T, Vishwanatha JK. CRISPR deletion of MIEN1 in breast cancer cells. PLoS One. 2018;13(10):e0204976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Blanas A, Cornelissen LAM, Kotsias M, van der Horst JC, van de Vrugt HJ, Kalay H, et al. Transcriptional activation of fucosyltransferase (FUT) genes using the CRISPR‐dCas9‐VPR technology reveals potent N‐glycome alterations in colorectal cancer cells. Glycobiology. 2019;29(2):137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Hsu DS, Kornepati AV, Glover W, Kennedy EM, Cullen BR. Targeting HPV16 DNA using CRISPR/Cas inhibits anal cancer growth in vivo. Future Virol. 2018;13(7):475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Li T, Liu D, Lei X, Jiang Q. Par3L enhances colorectal cancer cell survival by inhibiting Lkb1/AMPK signaling pathway. Biochem Biophys Res Commun. 2017;482(4):1037–41. [DOI] [PubMed] [Google Scholar]
- 197. Wan T, Chen Y, Pan Q, Xu X, Kang Y, Gao X, et al. Genome editing of mutant KRAS through supramolecular polymer‐mediated delivery of Cas9 ribonucleoprotein for colorectal cancer therapy. J Control Release. 2020;322:236–47. [DOI] [PubMed] [Google Scholar]
- 198. Liu Y, Qi X, Zeng Z, Wang L, Wang J, Zhang T, et al. CRISPR/Cas9‐mediated p53 and Pten dual mutation accelerates hepatocarcinogenesis in adult hepatitis B virus transgenic mice. Sci Rep. 2017;7(1):2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Xue W, Chen S, Yin H, Tammela T, Papagiannakopoulos T, Joshi NS, et al. CRISPR‐mediated direct mutation of cancer genes in the mouse liver. Nature. 2014;514(7522):380–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Han S, Guo J, Liu Y, Zhang Z, He Q, Li P, et al. Knock out CD44 in reprogrammed liver cancer cell C3A increases CSCs stemness and promotes differentiation. Oncotarget. 2015;6(42):44452–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Song CQ, Li Y, Mou H, Moore J, Park A, Pomyen Y, et al. Genome‐wide CRISPR screen identifies regulators of mitogen‐activated protein kinase as suppressors of liver tumors in mice. Gastroenterology. 2017;152(5):1161–73.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Singh K, Evens H, Nair N, Rincón MY, Sarcar S, Samara‐Kuko E, et al. Efficient in vivo liver‐directed gene editing using CRISPR/Cas9. Mol Ther. 2018;26(5):1241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Lu Y, Xue J, Deng T, Zhou X, Yu K, Deng L, et al. Safety and feasibility of CRISPR‐edited T cells in patients with refractory non‐small‐cell lung cancer. Nat Med. 2020;26(5):732–40. [DOI] [PubMed] [Google Scholar]
- 204. Ng SR, Rideout WM, 3rd , Akama‐Garren EH, Bhutkar A, Mercer KL, Schenkel JM, et al. CRISPR‐mediated modeling and functional validation of candidate tumor suppressor genes in small cell lung cancer. Proc Natl Acad Sci U S A. 2020;117(1):513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Cheung AH, Chow C, Zhang J, Zhou Y, Huang T, Ng KC, et al. Specific targeting of point mutations in EGFR L858R‐positive lung cancer by CRISPR/Cas9. Lab Invest. 2018;98(7):968–76. [DOI] [PubMed] [Google Scholar]
- 206. Perumal E, So Youn K, Sun S, Seung‐Hyun J, Suji M, Jieying L, et al. PTEN inactivation induces epithelial‐mesenchymal transition and metastasis by intranuclear translocation of β‐catenin and snail/slug in non‐small cell lung carcinoma cells. Lung Cancer. 2019;130:25–34. [DOI] [PubMed] [Google Scholar]
- 207. Álvarez‐Fernández M, Sanz‐Flores M, Sanz‐Castillo B, Salazar‐Roa M, Partida D, Zapatero‐Solana E, et al. Therapeutic relevance of the PP2A‐B55 inhibitory kinase MASTL/Greatwall in breast cancer. Cell Death Differ. 2018;25(5):828–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Hannafon BN, Cai A, Calloway CL, Xu YF, Zhang R, Fung KM, et al. miR‐23b and miR‐27b are oncogenic microRNAs in breast cancer: evidence from a CRISPR/Cas9 deletion study. BMC Cancer. 2019;19(1):642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Mintz RL, Lao YH, Chi CW, He S, Li M, Quek CH, et al. CRISPR/Cas9‐mediated mutagenesis to validate the synergy between PARP1 inhibition and chemotherapy in BRCA1‐mutated breast cancer cells. Bioeng Transl Med. 2020;5(1):e10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Domenici G, Aurrekoetxea‐Rodríguez I, Simões BM, Rábano M, Lee SY, Millán JS, et al. A Sox2‐Sox9 signalling axis maintains human breast luminal progenitor and breast cancer stem cells. Oncogene. 2019;38(17):3151–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Dekkers JF, Whittle JR, Vaillant F, Chen HR, Dawson C, Liu K, et al. Modeling breast cancer using CRISPR‐Cas9‐mediated engineering of human breast organoids. J Natl Cancer Inst. 2020;112(5):540–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Yang M, Zeng C, Li P, Qian L, Ding B, Huang L, et al. Impact of CXCR4 and CXCR7 knockout by CRISPR/Cas9 on the function of triple‐negative breast cancer cells. Onco Targets Ther. 2019;12:3849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Takao A, Yoshikawa K, Karnan S, Ota A, Uemura H, De Velasco MA, et al. Generation of PTEN‑knockout (−/−) murine prostate cancer cells using the CRISPR/Cas9 system and comprehensive gene expression profiling. Oncol Rep. 2018;40(5):2455–66. [DOI] [PubMed] [Google Scholar]
- 214. Wei C, Wang F, Liu W, Zhao W, Yang Y, Li K, et al. CRISPR/Cas9 targeting of the androgen receptor suppresses the growth of LNCaP human prostate cancer cells. Mol Med Rep. 2018;17(2):2901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Valcarcel‐Jimenez L, Macchia A, Crosas‐Molist E, Schaub‐Clerigué A, Camacho L, Martín‐Martín N, et al. PGC1α suppresses prostate cancer cell invasion through ERRα transcriptional control. 2019;79(24):6153–65. [DOI] [PubMed] [Google Scholar]
- 216. Kawamura N, Nimura K, Nagano H, Yamaguchi S, Nonomura N, Kaneda Y. CRISPR/Cas9‐mediated gene knockout of NANOG and NANOGP8 decreases the malignant potential of prostate cancer cells. Oncotarget. 2015;6(26):22361–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Batır MB, Şahin E, Çam FS. Evaluation of the CRISPR/Cas9 directed mutant TP53 gene repairing effect in human prostate cancer cell line PC‐3. Mol Biol Rep. 2019;46(6):6471–84. [DOI] [PubMed] [Google Scholar]
- 218. Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD‐L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD‐L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Reck M, Rodríguez‐Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375(19):1823–33. [DOI] [PubMed] [Google Scholar]
- 220. Forsberg EMV, Lindberg MF, Jespersen H, Alsén S, Bagge RO, Donia M, et al. HER2 CAR‐T cells eradicate uveal melanoma and T‐cell therapy‐resistant human melanoma in IL2 transgenic NOD/SCID IL2 receptor knockout mice. Cancer Res. 2019;79(5):899–904. [DOI] [PubMed] [Google Scholar]
- 221. Upadhyay R, Boiarsky JA, Pantsulaia G, Svensson‐Arvelund J, Lin MJ, Wroblewska A, et al. A critical role for fas‐mediated off‐target tumor killing in T‐cell immunotherapy. Cancer Discov. 2021;11(3):599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Eyquem J, Mansilla‐Soto J, Giavridis T, van der Stegen SJ, Hamieh M, Cunanan KM, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543(7643):113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Zhen S, Li X. Liposomal delivery of CRISPR/Cas9. Cancer Gene Ther. 2020;27(7‐8):515–27. [DOI] [PubMed] [Google Scholar]
- 224. Ashok B, Peppas NA, Wechsler ME. Lipid‐ and polymer‐based nanoparticle systems for the delivery of CRISPR/Cas9. J Drug Deliv Sci Technol. 2021;65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Tsai SQ, Joung JK. Defining and improving the genome‐wide specificities of CRISPR‐Cas9 nucleases. Nat Rev Genet. 2016;17(5):300–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Shin HY, Wang C, Lee HK, Yoo KH, Zeng X, Kuhns T, et al. CRISPR/Cas9 targeting events cause complex deletions and insertions at 17 sites in the mouse genome. Nat Commun. 2017;8:15464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Kosicki M, Tomberg K, Bradley A. Repair of double‐strand breaks induced by CRISPR‐Cas9 leads to large deletions and complex rearrangements. Nat Biotechnol. 2018;36(8):765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Hu JH, Miller SM, Geurts MH, Tang W, Chen L, Sun N, et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature. 2018;556(7699):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Chen JS, Dagdas YS, Kleinstiver BP, Welch MM, Sousa AA, Harrington LB, et al. Enhanced proofreading governs CRISPR‐Cas9 targeting accuracy. Nature. 2017;550(7676):407–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Koonin EV, Makarova KS. Anti‐CRISPRs on the march. Science. 2018;362(6411):156–7. [DOI] [PubMed] [Google Scholar]
- 231. Zhang XH, Tee LY, Wang XG, Huang QS, Yang SH. Off‐target effects in CRISPR/Cas9‐mediated genome engineering. Mol Ther Nucleic Acids. 2015;4(11):e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable


