Abstract
Background
The tetravalent dengue vaccine (CYD-TDV) has been shown to provide protection against dengue disease over 5-year follow-up in participants with previous dengue infection, but increased the risk of dengue hospitalisation and severe dengue during long-term follow-up in those without previous dengue infection. WHO recommended pre-vaccination screening to identify those with previous dengue infection (ie, dengue seropositive) who would benefit from vaccination. We re-evaluated CYD-TDV efficacy in those identified as dengue seropositive using five commercially available immunoassays, and assessed immunoassay performance.
Methods
We included participants in the immunogenicity subsets of the phase 3 CYD14 (NCT01373281) and CYD15 (NCT01374516) CYD-TDV efficacy trials, which enrolled children aged 2–16 years in 2011–12 in five countries in the Asia-Pacific region (CYD14) and five Latin American countries (CYD15). Participants assessed had received at least one injection of study drug (CYD-TDV or placebo) and had baseline samples available. We tested baseline samples by IgG-based immunoassays to classify baseline dengue serostatus, using two ELISAs (EUROIMMUN and Panbio) and three rapid diagnostic tests (RDTs; TELL ME FAST, SD BIOLINE, and OnSite). Vaccine efficacy in preventing symptomatic, hospitalised, and severe virologically confirmed dengue was determined for participants who tested positive by each immunoassay. The specificity and sensitivity of each immunoassay was determined as percentage negative and positive agreement compared with the reference algorithm, which used dengue plaque reduction neutralisation test with 50% and 90% cutoffs and non-structural protein 1 IgG ELISA results to assign baseline serostatus.
Findings
Samples were available for 3967 participants, 2735 (69·0%) of whom were classified as seropositive by the reference algorithm. Vaccine efficacy against symptomatic virologically confirmed dengue in immunoassay-positive participants was high across all five immunoassays (EUROIMMUN ELISA 88·2% [95% CI 77·3 to 93·9], Panbio ELISA 87·6% [76·7 to 93·4], TELL ME FAST RDT 88·8% [67·0 to 96·2], SD BIOLINE RDT 82·8% [66·9 to 91·1], and OnSite RDT 89·7% [64·6 to 97·0]), as was vaccine efficacy against hospitalised virologically confirmed dengue (EUROIMMUN-ELISA 72·8% [38·9 to 87·9], Panbio ELISA 77·5% [52·8 to 89·3], TELL ME FAST RDT 92·4% [37·8 to 99·1], SD BIOLINE RDT 87·2% [54·5 to 96·4], and OnSite RDT 73·7% [–5·1 to 93·4]) and severe virologically confirmed dengue (EUROIMMUN ELISA 86·9% [–16·8 to 98·5], Panbio ELISA 91·3% [27·6 to 99·0], TELL ME FAST RDT 100·0% [not estimable to 100·0%], SD BIOLINE RDT 89·4% [9·6 to 98·8], and OnSite RDT 73·4% [–193·7 to 97·6]). The immunoassays exhibited high specificity (≥98·8% for all immunoassays apart from SD BIOLINE RDT) but variable sensitivities, with higher sensitivities observed for the ELISAs (EUROIMMUN 89·2% [87·9 to 90·3] and Panbio 92·5 [91·4 to 93·5]) than the RDTs (TELL ME FAST 52·5% [50·6 to 54·4], SD BIOLINE 71·1% [69·3 to 72·8], and OnSite 47·6% [45·7 to 49·5]).
Interpretation
Our findings suggest that these immunoassays could be used for pre-vaccination screening for CYD-TDV as tools to assist risk stratification until more sensitive and convenient tests become available.
Funding
Sanofi Pasteur.
Introduction
There is a continued and urgent need for vaccination against dengue. The global incidence of dengue has continued to increase over recent years, with a reported doubling of symptomatic infections every 10 years between 1990 and 2013.1 More than 3·3 million cases globally were reported to WHO in 2016,2 and a further increase in cases was seen in 2019,3, 4 with more than 3 million cases reported in the Americas alone.5 In addition to the increase in cases in dengue-endemic regions, an increase in dengue cases among travellers has also been seen over recent years.6, 7 The tetravalent dengue vaccine (CYD-TDV) has been shown to reduce the incidence of symptomatic virologically confirmed dengue, hospitalised virologically confirmed dengue, and severe virologically confirmed dengue in two phase 3 efficacy studies in dengue-endemic areas.8, 9 A case-cohort analysis of the results from three CYD-TDV efficacy studies showed that the vaccine provided durable protection against hospitalised and severe virologically confirmed dengue over 5 years in participants who had a previous dengue infection (classified as dengue seropositive in the study), whereas those without previous natural exposure to the dengue virus (classified as dengue seronegative) had an increased risk of being admitted to hospital for dengue and of severe dengue over the 5-year follow-up.10
Research in context.
Evidence before this study
The case-cohort analysis of the tetravalent dengue vaccine (CYD-TDV) efficacy studies showed an increased risk of hospitalised and severe dengue over 5 years of follow-up for those without evidence of previous dengue infection before vaccination (ie, dengue seronegative by measured or inferred dengue serostatus). In those who had previous dengue infection (dengue seropositive), CYD-TDV conferred robust efficacy against subsequent virologically confirmed dengue over 5 years. As a consequence of these findings, WHO recommended pre-vaccination screening to determine dengue serostatus in dengue vaccination programmes to minimise the risk of inadvertently vaccinating those who are seronegative.
We searched PubMed using the terms “dengue”, “rapid diagnostic test”, “enzyme-linked immunosorbent assay”, and “IgG” for English-language papers published between Jan 1, 2015, and March 31, 2020. ELISAs and rapid diagnostic tests (RDTs) might be suitable for identifying those with previous dengue infection in clinical practice. While various tests have been assessed in endemic and non-endemic regions in people with suspected current dengue infection or in the convalescent period, there is little evidence of their use in detecting previous dengue infection. Previous assessment of these assays using samples from dengue-endemic and non-endemic regions to determine previous dengue infection have indicated their utility in classifying individual serostatus, with moderate–high sensitivity and high specificity.
Added value of this study
To our knowledge, this is the first study in which currently available dengue immunoassays were applied in a setting that simulated pre-vaccination screening in populations considered for dengue vaccination programmes. As such, the favourable assessment of vaccine efficacy against symptomatic dengue and hospitalised dengue in participants of the CYD-TDV phase 3 efficacy studies who tested positive before vaccination predicts the benefits to test-positive individuals if these tests were prospectively applied in actual vaccination programmes.
Implications of all the available evidence
The five immunoassays assessed in this study could be suitable temporising tools for pre-vaccination screening in populations in dengue-endemic areas as part of a vaccination programme. The RDTs showed lower sensitivity compared with the ELISAs, but ELISAs are usually less convenient in terms of costs, practical use, and need for a laboratory, and might be more difficult to implement. This finding highlights the need for a test that is more sensitive than the currently available RDTs but more convenient than ELISAs.
WHO has subsequently provided guidance for the use of CYD-TDV in vaccination programmes, recommending the pre-vaccination screening strategy in which only individuals with evidence of previous dengue infection are vaccinated. Individuals with no documented previous laboratory-confirmed dengue infection (serological or virological confirmation) need to undergo dengue serostatus determination, to ensure that only those who are dengue seropositive are vaccinated.11 This guidance requires the availability of suitable serological assays to assess dengue serostatus with high specificity to minimise the risk of severe outcomes from inadvertently vaccinating those who are seronegative. While risk minimisation stands as a top priority, high sensitivity in an immunoassay is also desirable to identify those who would benefit from vaccination to maximise individual and public health impact.
Two methods could potentially be useful for the determination of dengue serostatus in practice: ELISAs and rapid diagnostic tests (RDTs). Both methods can detect IgG antibodies against dengue virus in blood or serum samples, which are useful serological markers as they can persist over a person's lifetime12 and have been shown to be sufficient to determine previous dengue infection.13 A systematic review of studies assessing commercially available RDTs found them to have a specificity of 75–80% when compared with laboratory ELISA testing; however, these studies evaluated the detection of IgG in those with suspected dengue infection or on convalescent samples after recent infection, and therefore did not assess their accuracy for identifying remote previous dengue infection.14 Assessment of dengue IgG ELISAs and RDTs to detect previous dengue infection using samples from dengue-endemic and non-endemic regions have indicated their utility in serostatus classification, with very high specificity and moderate–high sensitivity, with the highest sensitivity seen with ELISAs.13, 15 These initial studies were limited by using a sample population that was not fully reflective of the vaccine target population and by not having a single comparator for all groups.
In this current study, we use data and samples from two phase 3 CYD-TDV studies to evaluate the efficacy of CYD-TDV for the prevention of symptomatic virologically confirmed dengue, hospitalised virologically confirmed dengue, and severe virologically confirmed dengue in those classified as dengue seropositive at baseline by five currently available dengue immunoassays. Additionally, we assessed the performance characteristics of these immunoassays in a cohort representative of the vaccine target population in dengue-endemic areas.
Methods
Study design and participants
The two phase 3 CYD-TDV studies (CYD14 and CYD15) were randomised, placebo-controlled trials, and have been described in detail elsewhere.8, 9 CYD14 was done in five countries in the Asia-Pacific region in children aged 2–14 years who were enrolled from June 3 to Dec 1, 2011 (NCT01373281), and CYD15 was done in five Latin American countries in children aged 9–16 years who were enrolled from June 8, 2011, to March 16, 2012 (NCT01374516). The children were randomly assigned (2:1) to receive three doses of CYD-TDV or placebo 6 months apart; follow-up data were available up to 6 years. A random subset of participants from each of these studies enrolled in the first 2–4 months were selected for the immunogenicity subsets: 19% (1983/10 275) of participants enrolled in CYD14 and 10% (2000/20 869) of participants enrolled in CYD15. These participants provided pre-vaccination and post-vaccination blood samples and were included in the current study. This study adhered to the principles of Good Clinical Laboratory Practice.
Procedures
Five commercially available immunoassays were used to classify participants' baseline dengue serostatus: two ELISAs and three RDTs. These assays were chosen from those that were commercially available and had shown high specificity and low cross-reactivity in our previous studies,13, 15 with the inclusion of one additional ELISA that was available in countries where the vaccine was licensed at the time. The two ELISAs used in this study were the EUROIMMUN anti-dengue virus IgG ELISA (EUROIMMUN, Luebeck, Germany) and the Panbio Dengue IgG Indirect ELISA (Abbott, Chicago, IL, USA). The three RDTs used were TELL ME FAST Dengue IgG/IgM Combo Test Device (Biocan Diagnostics, Vancouver, Canada), SD BIOLINE Dengue IgG/IgM WB (Abbott, Chicago, IL, USA), and the OnSite Dengue IgG/IgM Combo Rapid Test CE (CTK Biotech, San Diego, CA, USA). A summary of the characteristics of these assays is presented in the appendix (pp 2–3). Testing was done according to each manufacturer's instructions; EUROIMMUN ELISA, TELL ME FAST RDT, and OnSite RDT testing was done by Sanofi Pasteur's Global Clinical Immunology Laboratory (Swiftwater, PA, USA) and Panbio ELISA and SD BIOLINE RDT testing was done externally by the Central Virology Laboratory (Chaim Sheba Medical Center, Ramat Gan, Israel). The laboratory testing personnel were blinded to study treatment group, dengue clinical outcomes, dengue serostatus, or previous dengue exposure of study participants who provided the source samples.
Each immunoassay was done on baseline samples from the immunogenicity subset where possible. The immunoassays were initiated in sequence according to the availability of testing kits and testing slots (OnSite RDT first, TELL ME FAST RDT second, Panbio ELISA and SD BIOLINE RDT third, and EUROIMMUN ELISA last); therefore, for each successive immunoassay tested, the number of available samples decreased due to insufficient sample volumes.
For the reference baseline dengue serostatus, we used results from previous assessments of each sample by plaque reduction neutralisation test with 50% cutoff (PRNT50) or 90% cutoff (PRNT90) and anti-dengue non-structural protein 1 (NS1) IgG ELISA.13, 15, 16, 17 These methods are considered the most reliable for determining dengue serostatus, and the results of both of these assays were used to classify samples as reference seropositive or seronegative through assignment to one of six groups (appendix p 4). These six groups represent a gradient of certainty for identifying previous dengue infection, with group 1 representing the closest possible reference to true dengue seronegative (ie, negative results for all three reference tests) and group 6 the closest reference to true dengue seropositive (ie, positive results for both PRNT50 and PRNT90). Participants categorised as belonging to groups 1–3 were classified as reference dengue seronegative whereas those in groups 4–6 were classified as reference dengue seropositive.
Outcomes
The prespecified outcomes of this study were the efficacy of CYD-TDV in preventing symptomatic virologically confirmed dengue and hospitalised virologically confirmed dengue for participants in the immunogenicity subsets who tested positive by each of the immunoassays. Symptomatic virologically confirmed dengue cases during the active phase of the studies (months 0–25) were defined as acute febrile illness (temperature ≥38°C on at least 2 consecutive days) that were virologically confirmed by dengue RT-PCR or dengue NS1 antigen ELISA, as described in the original studies.8, 9 Hospitalised virologically confirmed dengue cases during the entire 6-year study period (months 0–72) were defined as cases with hospital admission associated with virological confirmation by dengue RT-PCR or dengue NS1 antigen ELISA.10, 18 An additional outcome was the efficacy of CYD-TDV against severe virologically confirmed dengue cases in those who were immunoassay positive; severe virologically confirmed dengue cases over 6 years of follow-up (months 0–72) were those adjudicated to be severe by the independent data monitoring committee, as described in the original studies.8, 9, 10, 18
Vaccine efficacy was not evaluated in test-negative individuals as CYD-TDV is not intended for use in seronegative individuals. Furthermore, the immunoassays exhibited a wide range of sensitivities in identifying previous dengue infections in our preliminary assessments,13, 15 so a proportion of seropositive individuals would be erroneously classified as seronegative. We therefore elected not to include immunoassay-negative individuals in our analyses to avoid generating misleadingly favourable vaccine efficacy estimates in these subsets.
Statistical analysis
Outcomes were assessed in the pooled population of participants of the immunogenicity subsets of the CYD14 and CYD15 studies who received at least one study injection and had available baseline samples.
The sensitivity and specificity of the five immunoassays were derived using the reference algorithm as comparator. For each immunoassay, true seropositive samples were those determined as seropositive by both the immunoassay and reference algorithm and true seronegative samples were those determined as seronegative by both the immunoassay and reference algorithm. The sensitivity of the immunoassay was determined as the proportion of samples correctly classified as seropositive by the immunoassay, and specificity was calculated as the proportion of samples correctly classified as seronegative by the immunoassay. The positive predictive value (PPV) was calculated as the number of true seropositive samples divided by all seropositive samples identified by the immunoassay, and the negative predictive value (NPV) was calculated as the number of true seronegative samples divided by all seronegative samples identified by the immunoassay.
Incidence of symptomatic, hospitalised, or severe virologically confirmed dengue was calculated as the number of cases per 100 person-years, with the person-years at risk being the sum of the individual years that participants contributed to the analysis, with corresponding 95% CIs calculated by the exact binomial method (Clopper-Pearson method). For the efficacy assessment, a Cox regression model using the vaccine group and study as fixed effects was used and vaccine efficacy was calculated as (1 – hazard ratio) × 100. 95% CIs for sensitivity, specificity, PPV, and NPV were estimated using the Clopper-Pearson method. Main analyses were done on the prespecified primary population of those aged 2–16 years to maximise power for the evaluation of vaccine efficacy, while additional sensitivity analyses included analysis by age group (≥9 years, <9 years, and ≥6 years).
Role of the funding source
This study was sponsored by Sanofi Pasteur. The study sponsor participated in the trial design and managed all operational aspects of the study, including monitoring data collection, statistical analyses, and writing of the report. Assays were done either at Sanofi Pasteur's Global Clinical Immunology Laboratory or at the Central Virology Laboratory, with the latter funded by Sanofi Pasteur under a research agreement. All authors had full access to all of the data and had final responsibility for the decision to submit for publication.
Results
Samples were available from 3967 participants in the immunogenicity subsets of both studies, with determination of serostatus by one or more of the immunoassays for 3962 participants. 2735 (69·0%) of 3962 participants were classified as dengue seropositive at baseline by the reference algorithm (appendix p 5). 2369 (61·7%) of 3841 participants tested positive by EUROIMMUN ELISA, 2466 (63·9%) of 3862 by Panbio ELISA, 1426 (36·4%) of 3913 by TELL ME FAST RDT, 1935 (50·1%) of 3862 by SD BIOLINE RDT, and 1307 (33·0%) of 3962 by OnSite RDT. The baseline characteristics of those who tested positive by each immunoassay were similar between those in the CYD-TDV group and those in the placebo group (appendix pp 6–10).
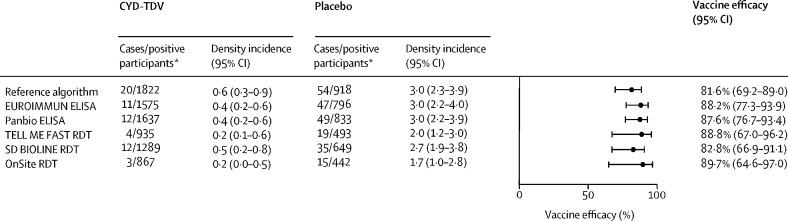
Vaccine efficacy against symptomatic virologically confirmed dengue (months 0–25) in all test-positive participants ranged from 82·8% (95% CI 66·9–91·1) by SD BIOLINE RDT to 89·7% (64·6–97·0) by OnSite RDT, which compared favourably to the estimate among participants categorised as dengue seropositive by the reference algorithm (figure 1 ). Similar results were observed for each of the age strata (appendix p 11); for participants younger than 9 years, efficacy estimates generated with the TELL ME FAST and OnSite RDTs had consistent point estimates but the corresponding 95% CIs crossed the null due to lower number of dengue-positive participants by these assays.
Figure 1.
CYD-TDV efficacy against symptomatic virologically confirmed dengue up to month 25 in immunoassay-positive participants
Data are pooled from CYD14 and CYD15 studies from participants aged 2–16 years. Density incidence is per 100 person-years, with the person-years at risk being the sum of the individual years for which the participants contributed to the analysis. RDT=rapid diagnostic test. *Immunoassay-positive participants.
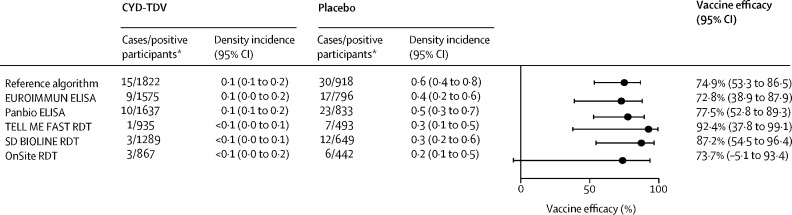
Vaccine efficacy against hospitalised virologically confirmed dengue over the entire follow-up period (months 0–72) ranged from 72·8% (95% CI 38·9–87·9) by EUROIMMUN ELISA to 92·4% (37·8–99·1) by TELL ME FAST RDT in test-positive participants, and efficacy among participants categorised as dengue seropositive by the reference algorithm was in the same range (figure 2 ). The lower bound of the 95% CI was above the null for all but the OnSite RDT. The vaccine efficacy estimates against hospitalised virologically confirmed dengue were more variable and less precise when examined by the different age strata due to the low number of events per age group (appendix p 12).
Figure 2.
CYD-TDV efficacy against hospitalised virologically confirmed dengue up to month 72 in immunoassay-positive participants
Density incidence is per 100 person-years, with the person-years at risk being the sum of the individual years for which the participants contributed to the analysis. RDT=rapid diagnostic test. *Immunoassay-positive participants.
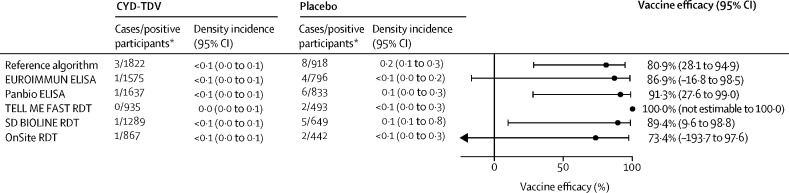
There were very few severe virologically confirmed dengue cases over the entire follow-up (months 0–72) in test-positive participants and, as such, vaccine efficacy estimates lacked precision (figure 3 ); nevertheless, point estimates were generally consistent with those observed for hospitalised virologically confirmed dengue, and were similar to the vaccine efficacy determined in those found to be dengue seropositive by the reference algorithm.
Figure 3.
CYD-TDV vaccine efficacy against severe virologically confirmed dengue up to month 72 in immunoassay-positive participants
Density incidence is per 100 person-years, with the person-years at risk being the sum of the individual years for which the participants contributed to the analysis. RDT=rapid diagnostic test. *Immunoassay-positive participants.
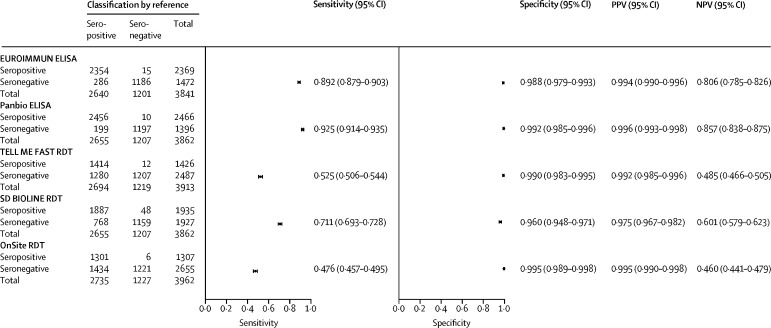
All immunoassays, except SD BIOLINE RDT, exhibited very high specificity (≥98·8%) for identifying previous dengue infection (figure 4 ). The two ELISAs (EUROIMMUN and Panbio) exhibited higher sensitivities than the three RDTs (figure 4). For the dengue seroprevalence observed in these studies (2735 [69·0%] of 3967 participants), all the immunoassays exhibited high PPVs (≥97·5%). The NPVs were higher for the two ELISAs than for the RDTs (figure 4).
Figure 4.
Immunoassay performance for dengue serostatus classification
Values presented here are specific for the observed dengue seropositivity by the reference algorithm in this study (69·1%); PPVs and NPVs for other seroprevalences would differ from the estimates presented here. NPV=negative predictive value. PPV=positive predictive value. RDT=rapid diagnostic test.
Discussion
To our knowledge, this study is the first evaluation of CYD-TDV efficacy in those with previous dengue infection identified using currently available dengue IgG immunoassays that could be used for pre-vaccination screening. For each of the five immunoassays, in test-positive participants, there is evidence of robust CYD-TDV efficacy against symptomatic virologically confirmed dengue over 2 years of follow-up and against hospitalised virologically confirmed dengue over 6 years of follow-up. Vaccine efficacy was consistent across the different immunoassays, indicating the benefit of the pre-vaccination screening strategy in populations generally representative of those targeted for dengue vaccination. The results for vaccine efficacy against symptomatic virologically confirmed dengue in test-positive participants aged 2–16 years in this study are consistent with estimates for those classified as seropositive (by PRNT50 only) in the immunogenicity subsets of CYD14 (74·3% [95% CI 53·2–86·3] in children aged 2–14 years)9 and CYD15 (83·7% [62·2–93·7] in children aged 9–16 years),8 or seropositive in the case-cohort study (73% [59–82] in children aged 2–16 years)10 over 25 months. Vaccine efficacy estimates against hospitalised virologically confirmed dengue are also consistent with interim data in seropositive participants from these studies.10
The ideal immunoassay for dengue pre-vaccination screening should have very high specificity to minimise the safety risk of inadvertently vaccinating individuals who are seronegative but have tested as positive.19, 20 The very high specificity across the EUROIMMUN ELISA, Panbio ELISA, TELL ME FAST RDT, and OnSite RDT suggests that these tests would be of value in minimising inadvertent vaccination of dengue-seronegative individuals. While the observed specificity for the SD BIOLINE RDT was lower than for the other assays, this assay was still associated with high PPV given the dengue endemicity in the study populations. However, the application of this test rather than any of the others evaluated would result in more inadvertent vaccination of seronegative individuals in settings with lower dengue endemicity. High sensitivity will maximise the benefits of vaccination through fewer false negatives; with higher sensitivity, more individuals who are eligible for vaccination would be identified and could be vaccinated, leading to a higher coverage. The test sensitivity was higher for the two ELISAs compared with the three RDTs. Consequently, ELISAs would identify greater numbers of people who would benefit from vaccination with CYD-TDV. However, compared with RDTs, in clinical practice ELISAs are less convenient, logistically more difficult to implement, and likely to have lower compliance due to the extra visit required for screening. The OnSite and TELL ME FAST RDTs had notably lower sensitivity than the ELISAs, at approximately 50% compared with approximately 90% for the ELISAs. As a result, these tests would identify far fewer eligible individuals and lead to lower vaccine coverage, thus reducing the associated public health benefits. These data highlight a gap for a test that is more sensitive than the currently available RDTs, but more convenient than ELISAs, to ensure the greatest public health benefit of a dengue vaccination programme incorporating pre-vaccination screening.
An assessment of commercially available dengue IgG RDTs and ELISAs has previously been done by Sanofi Pasteur's Global Clinical Immunology Laboratory in samples from dengue-endemic and non-endemic areas.13, 15 This current study supports the finding of very high specificity and high sensitivity with the Panbio ELISA, as found in the previous assessment. The previous finding of very high specificity for the TELL ME FAST and OnSite RDTs was also reflected in the current study, whereas our sensitivity estimates are lower than those of the previous study (52·5% vs 61·0% for TELL ME FAST RDT, and 47·6% vs 67·0% for OnSite RDT). This is likely to be explained by the difference in study populations; the previous studies had more participants with documented symptomatic dengue infection and were therefore more likely to have higher IgG levels that would be more readily detectable by the RDTs, while in the current study the immunosubsets from CYD14 and CYD15 were more representative of the overall population of dengue-endemic regions; therefore, the current data are more informative. Finally, the SD BIOLINE RDT shows a different performance profile between previous experience (specificity 99·6%, sensitivity 53·7%) and the current study (specificity 96·0%, sensitivity 71·1%).
Modelling studies of the potential impact of pre-vaccination screening at a population level have shown such a strategy improves efficiency compared with no screening, with a larger number of hospital admissions prevented per vaccination.21 Also, the public health impact of pre-vaccination screening was maximised in low dengue transmission regions when test specificity was high (which minimised individual harm), whereas in high dengue transmission areas, high sensitivity was important for maximising public health benefits.22 School-based screening in Vietnam was estimated to cost US$9·25 per 9-year-old child screened, where ELISA kits constituted the main costs and 22% of the costs related to sample transfers to a laboratory.23 The use of RDTs would eliminate the need to transfer samples to a laboratory, as well as the costs associated with laboratory staff, indicating a potential cost advantage over ELISAs. Pre-vaccination screening in combination with CYD-TDV vaccination was projected to be highly cost-effective (cost-effectiveness ratio 1 × per-capita gross domestic product [GDP]) from a health system perspective over a 10-year period using an RDT with 70% sensitivity and 99% specificity for most countries where at least 50% of 9-year-olds were seropositive.21 Another study predicted that pre-vaccination screening and subsequent vaccination would only be cost-effective (cost-effectiveness ratio 3 × per-capita GDP) from a public payer perspective (assuming similar RDT performance, transmission rates, and duration assessed) in countries with a relatively high per-capita GDP (such as Mexico [$8201] or Thailand [$5807]),22 highlighting the need for further assessment at a local level before implementation of pre-vaccination strategies.
Cross-reactivity of these immunoassays with other flaviviruses that might be circulating in dengue-endemic areas is another important consideration. Low cross-reactivity is important because individuals from dengue-endemic areas might also be exposed to other flaviviruses or flavivirus vaccines. Exposure to these might result in false positives in cross-reactive tests and the inadvertent vaccination of people who are dengue seronegative. In previous studies, we observed low-to-no cross-reactivity with other flaviviruses (Zika virus, Japanese encephalitis, yellow fever, and West Nile virus) for the immunoassays evaluated here, with the exception of the Panbio ELISA, which has exhibited high cross-reactivity with Zika virus and West Nile virus.13, 15 Of note, no cross-reactivity was identified with the OnSite RDT immunoassay. Evaluation of the EUROIMMUN ELISA showed no (yellow fever, Japanese encephalitis) or low (Zika virus, West Nile virus) flavivirus cross-reactivity, distinguishing it from the Panbio ELISA. Importantly, the CYD-TDV efficacy trials were initiated before the emergence of Zika virus in Latin America, and so the impact of Zika virus exposure on test performance could not be directly evaluated in the present study. However, this study is likely to be representative of test performance and pre-vaccination screening outcomes in the context of non-Zika flaviviruses, particularly yellow fever in Latin America and Japanese encephalitis in Asia, given that endemicity to these viruses or availability of their corresponding vaccines preceded the execution of the CYD14 and CYD15 studies.
One advantage of this study was the use of an algorithm to determine the reference baseline serostatus from each participant's sample. We believe that this algorithm is the most accurate method to determine serostatus for pre-vaccination screening as it used a combination of dengue PRNT50,PRNT90, and NS1 IgG ELISA results. In addition, given that we used samples and data from two double-blinded, randomised controlled trials, the results generated here are expected to be generally valid with a low risk for bias. However, there are several limitations to this study. Given the use of archived serum samples from phase 3 clinical trials, this study might not be reflective of epidemiological conditions that are expected to change over time to some extent. Also, the RDT assays were done in a controlled laboratory environment by trained staff, and thus might differ from a practical, real-world, implementation of these assays.
Our study supports the use of these immunoassays for pre-vaccination screening for CYD-TDV; those who were identified as dengue seropositive by the immunoassays were protected against virologically confirmed dengue following vaccination with CYD-TDV. The immunoassays assessed would be suitable tools until more sensitive or convenient tests become available. The selection of an appropriate immunoassay should take into consideration local epidemiological context, testing infrastructure, and test availability.
Data sharing
Qualified researchers can request access from Sanofi Pasteur to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymised and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and the process for requesting access can be found online.
Acknowledgments
Acknowledgments
Editorial assistance with the preparation of the manuscript was provided by Nicola Truss (inScience Communications, Springer Healthcare, London, UK). Funding for this assistance was provided by Sanofi Pasteur. The authors would also like to thank Jean-Sébastien Persico (Sanofi Pasteur, Lyon, France) for editorial assistance and manuscript coordination on behalf of Sanofi Pasteur.
Contributors
CAD, MB, MZ, YA-Ö, and SJS were involved in the study concept and design. MB, YL, ES, RF, GHD, and SH were involved in data acquisition. All authors had access to and can verify the study data. CAD, MB, HW, YL, ES, YA-Ö, and SJS were involved in data analysis and interpretation. All authors were involved in critical revision of the manuscript and reviewed the final version and provided approval for submission.
Declaration of interests
CAD, MB, HW, MZ, RF, GHD, SH, YA-Ö, and SJS are employees of Sanofi Pasteur. YL and ES declare no competing interests.
Supplementary Material
References
- 1.Stanaway JD, Shepard DS, Undurraga EA, et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16:712–723. doi: 10.1016/S1473-3099(16)00026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Dengue and severe dengue. Nov 4, 2019. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue
- 3.WHO Dengue situation update number 584. December, 2019. https://iris.wpro.who.int/bitstream/handle/10665.1/14329/Dengue-20191219.pdf
- 4.European Centre for Disease Prevention and Control Dengue worldwide overview. 2019. https://www.ecdc.europa.eu/en/dengue-monthly
- 5.Pan American Health Organization Dengue and severe dengue. 2019. http://www.paho.org/data/index.php/en/mnu-topics/indicadores-dengue-en/dengue-regional-en/261-dengue-reg-ano-en.html
- 6.Halstead S, Wilder-Smith A. Severe dengue in travellers: pathogenesis, risk and clinical management. J Travel Med. 2019;26 doi: 10.1093/jtm/taz062. [DOI] [PubMed] [Google Scholar]
- 7.Wilder-Smith A. Risk of dengue in travelers: implications for dengue vaccination. Curr Infect Dis Rep. 2018;20:50. doi: 10.1007/s11908-018-0656-3. [DOI] [PubMed] [Google Scholar]
- 8.Villar L, Dayan GH, Arredondo-García JL, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med. 2014;372:113–123. doi: 10.1056/NEJMoa1411037. [DOI] [PubMed] [Google Scholar]
- 9.Capeding MR, Tran NH, Hadinegoro SR, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014;384:1358–1365. doi: 10.1016/S0140-6736(14)61060-6. [DOI] [PubMed] [Google Scholar]
- 10.Sridhar S, Luedtke A, Langevin E, et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med. 2018;379:327–340. doi: 10.1056/NEJMoa1800820. [DOI] [PubMed] [Google Scholar]
- 11.WHO Dengue vaccine: WHO position paper—September 2018. Weekly Epidemiol Rec. 2018;36:457–476. [Google Scholar]
- 12.Imrie A, Meeks J, Gurary A, et al. Antibody to dengue 1 detected more than 60 years after infection. Viral Immunol. 2007;20:672–675. doi: 10.1089/vim.2007.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonaparte M, Zheng L, Garg S, et al. Evaluation of rapid diagnostic tests and conventional enzyme-linked immunosorbent assays to determine prior dengue infection. J Travel Med. 2019;26 doi: 10.1093/jtm/taz078. [DOI] [PubMed] [Google Scholar]
- 14.Luo R, Fongwen N, Kelly-Cirino C, Harris E, Wilder-Smith A, Peeling RW. Rapid diagnostic tests for determining dengue serostatus: a systematic review and key informant interviews. Clin Microbiol Infect. 2019;25:659–666. doi: 10.1016/j.cmi.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonaparte M, Huleatt J, Hodge S, et al. Evaluation of dengue serological tests available in Puerto Rico for identification of prior dengue infection for prevaccination screening. Diagn Microbiol Infect Dis. 2020;96 doi: 10.1016/j.diagmicrobio.2019.114918. [DOI] [PubMed] [Google Scholar]
- 16.Nascimento EJM, George JK, Velasco M, et al. Development of an anti-dengue NS1 IgG ELISA to evaluate exposure to dengue virus. J Virol Methods. 2018;257:48–57. doi: 10.1016/j.jviromet.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Timiryasova TM, Bonaparte MI, Luo P, Zedar R, Hu BT, Hildreth SW. Optimization and validation of a plaque reduction neutralization test for the detection of neutralizing antibodies to four serotypes of dengue virus used in support of dengue vaccine development. Am J Trop Med Hyg. 2013;88:962–970. doi: 10.4269/ajtmh.12-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadinegoro SR, Arredondo-García JL, Capeding MR, et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med. 2015;373:1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 19.Wilder-Smith A, Hombach J, Ferguson N, et al. Deliberations of the Strategic Advisory Group of Experts on Immunization on the use of CYD-TDV dengue vaccine. Lancet Infect Dis. 2019;19:e31–e38. doi: 10.1016/S1473-3099(18)30494-8. [DOI] [PubMed] [Google Scholar]
- 20.Wilder-Smith A, Smith PG, Luo R, et al. Pre-vaccination screening strategies for the use of the CYD-TDV dengue vaccine: a meeting report. Vaccine. 2019;37:5137–5146. doi: 10.1016/j.vaccine.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Coudeville L, Baurin N, Shepard DS. The potential impact of dengue vaccination with, and without, pre-vaccination screening. Vaccine. 2020;38:1363–1369. doi: 10.1016/j.vaccine.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 22.España G, Yao Y, Anderson KB, et al. Model-based assessment of public health impact and cost-effectiveness of dengue vaccination following screening for prior exposure. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner HC, Wills BA, Rahman M, et al. Projected costs associated with school-based screening to inform deployment of Dengvaxia: Vietnam as a case study. Trans R Soc Tropl Med Hyg. 2018;112:369–377. doi: 10.1093/trstmh/try057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers can request access from Sanofi Pasteur to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymised and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and the process for requesting access can be found online.