Abstract
A difficulty that has emerged in the development and preclinical evaluation of adjuvant therapies for gram-negative sepsis is the lack of easily studied animal models that closely mimic human infection. An objective of this study was to adapt a previously described model of infection in burned mice to rats with a defined bacterial strain of Escherichia coli. Challenge with two colonies of live E. coli O18:K1:H7 bacteria into an 8% full-thickness burn of the dorsal skin surface of rats produced predictable bacteremia at 24 to 48 h and 80 to 100% mortality at 3 to 4 days. E. coli O18:K1:H7 was approximately 10-million-fold more virulent than several other gram-negative bacterial strains. The model should be a useful tool in studying the pathogenicity of burn wound infections and in evaluating the efficacy of novel adjuvant therapies for gram-negative sepsis.
Secondary infections with gram-negative bacteria cause considerable morbidity and occasional mortality in burn patients. Sometimes the infection rapidly leads to overwhelming bacteremia with shock and subsequent death.
Although numerous candidate therapies have been proposed in the last decade for the adjuvant treatment of gram-negative sepsis, it has become clear that many of the animal models that are currently available for studying gram-negative sepsis may inadequately mimic human disease. Problems with current models include the following: purified lipopolysaccharide is sometimes utilized, large amounts of bacterial inoculum are needed that result in endotoxin intoxication rather than infection, the bacteria are injected or infused as a bolus, and the organism utilized either is not virulent or is not a human pathogen. These issues have been recently summarized (1). There are few models in which a small amount of bacterial inoculum of a clinically relevant gram-negative human pathogen is utilized that subsequently multiplies in vivo, leading to predictable bacteremia and eventual mortality. Simpler models using purified lipopolysaccharide or large amounts of bacterial inoculum may not reflect the complex interplay between host defense and pathogenicity of the organism.
Several burn models have previously been reported (6, 8–11). In many models, Pseudomonas species and/or a relatively large amount of bacterial inoculum has been utilized. In order to develop a more relevant model for the evaluation of novel therapies and to study the roles of capsule and certain outer membrane proteins common to Enterobacteriaceae in protective immunity (2), we sought to adapt one of these models that had utilized mice infected with a strain of Pseudomonas aeruginosa (8) for use in rats infected with a highly defined strain of Escherichia coli. This report describes these experiments.
MATERIALS AND METHODS
E. coli strains O16:K1:H6, O18:K1:H7 (and the K− mutant of this strain), and O25:K5:H1 and P. aeruginosa strain 12.4.4 were the kind gifts of Alan Cross (University of Maryland Cancer Center, Baltimore, Md.). E. coli O4:K54:H5 was the kind gift of Thomas Russo (State University of New York at Buffalo, Buffalo, N.Y.) (7). The outer membrane protein A (OmpA)-negative mutant (OmpA−) of E. coli O18:K1:H7 was the kind gift of Kwang Sik Kim, (Los Angeles Children's Hospital, Los Angeles, Calif.) and has been previously described (5). P. aeruginosa ATCC 19660 was obtained from the American Type Culture Collection, Rockville, Md.
A single colony of bacteria was inoculated into 10 ml of Trypticase soy broth (Difco Labs, Detroit, Mich.) and incubated overnight at 20°C. A 20-μl aliquot was then added to 10 ml of Trypticase soy broth, incubated on a shaker at 37°C, and grown to mid-log phase. The bacteria were centrifuged at 2,800 × g for 10 min, and the cell pellet was washed three times with 0.9% saline solution and resuspended in 5 ml of 0.9% saline solution. Bacterial concentrations were determined by generating standard curves of bacterial concentration versus absorbance at 550 nm. The final concentration was adjusted as required by serial dilutions with 0.9% saline solution. Exact numbers of CFU injected were determined by plating serial 10-fold dilutions of the bacteria on Trypticase soy agar plates, incubating them overnight at 37°C, and counting the colonies the next day.
The model represents an adaption of a previously reported murine model (8). Permission for animal studies was obtained from the Subcommittee on Animal Studies of Massachusetts General Hospital, Boston, Mass., and development of the burn and infection protocol was closely supervised by a veterinarian from the Office for Laboratory Animal Research of Massachusetts General Hospital. Six-week-old male Sprague-Dawley rats (Charles River Animal Farms, Cambridge, Mass.) weighing 250 to 275 g were anesthetized with ether (Sigma Chemical Co., St. Louis, Mo.). The hair on the rats' backs was shaved with clippers, and the skin was washed with 95% ethanol. Brass blocks (2.54 by 2.54 by 15.24 cm; Small Parts, Inc., Miami, Fla.) were preheated by immersion in boiling water. For each rat, a dorsal skin fold was elevated, and two blocks of 1-in2 surface area (each) were applied to opposing sides of the skin fold as required to make a 2-, 4-, or 8-in2 total burn area that would represent an approximately 4, 8, or 16% total body surface area burn, calculated with Meeh's formula (4). The skin fold was compressed for 15 s to deliver a full-thickness skin burn. Immediately following the burn, 500 μl of saline containing 2 × 100 to 2 × 108 CFU of bacteria was inoculated into the midline crease of the burn eschar. The rats were allowed access to food and water ad libitum after recovery from anesthesia. No wound dressings or topical or systemic antimicrobial agents were used. Blood for cultures was obtained at 24-h intervals from the tail vein and serially diluted by factors of 10 and 1,000. A 100-μl aliquot of undiluted solution or 1:10 or 1:1,000 dilution solution was applied to different Trypticase soy agar plates. After 24 h of incubation time at 37°C, the colonies were counted, and the undiluted concentration of bacteria in the blood of the animals was calculated. Organisms present in cultures of blood were confirmed to be E. coli O18 by blotting colonies onto nitrocellulose paper and immunostaining with a rabbit immunoglobulin G specific for the O polysaccharide antigen of E. coli O18. The animals were observed twice a day for 10 days.
To measure colony counts in tissues, rats were humanely sacrificed at intervals up to 72 h following burn and infection. The burn area and a remote, previously denuded area were immediately washed with 95% ethanol and allowed to dry in a sterile area. The skin was removed, by scissoring, under sterile conditions, and one triangle of tissue was cut from each of the extreme corners of two diagonally opposed quadrants. The underlying eschar and musculature were each removed in a similar fashion. The tissue specimens were weighed and homogenized in 3 ml of 0.9% saline solution by 5 min of grinding in a sterile Ten Broeck tissue grinder (Wheaton Science Products, Millville, N.J.). Samples were then diluted and cultured on agar plates. Quantitative colony counts were performed for each serial dilution, and the concentration of bacteria was calculated per gram of wet tissue.
Statistics of survival data were determined by the log rank test with GraphPad Prism, version 2.01 (GraphPad Software, Inc., San Diego, Calif.).
RESULTS
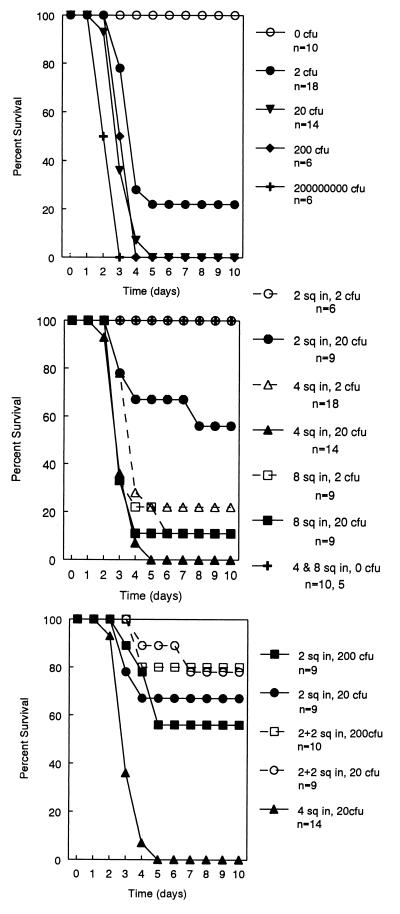
We initially assumed that a relatively large amount of bacterial inoculum might be necessary for the induction of bacteremia. However, because of high mortality, we progressively reduced the number of E. coli O18:K1:H7 injected into the 4-in2 burn. We found that the injection of only 2 CFU resulted in predictable bacteremia and death (Fig. 1, top panel). There was 100% survival in the absence of a bacterial inoculum. To confirm that a one-to-one correspondence existed between the number of viable bacteria and the number of colonies formed on the agar plates, E. coli O18:K1:H7 bacteria were grown as described above and serially diluted. One 100-μl aliquot of each 10-fold dilution was plated onto agar plates as above, and another was inoculated into 10 ml of broth; all aliquots were then held for 7 days. At dilutions in which there were no CFU on the plates, the broth remained sterile.
FIG. 1.
Effects of the amount of inoculum (top) and burn size (middle and bottom) on survival in the model with E. coli O18:K1:H7. In the experiment shown in the top panel, all burns were 4 in2 in area. Rats under all inoculation conditions had significantly greater mortality (P ≤ 0.0001) than uninfected burned rats. As shown in the middle panel, rats with the 2-in2 burn had significantly less mortality (P ≤ 0.002) than those with either the 8- or the 4-in2 burn receiving either amount of inoculum. As shown in the bottom panel, survival of rats with one infected burn of 2 in2 and an additional burn of 2 in2 was significantly greater than survival of rats with a single infected 4-in2 burn (P < 0.0001). However, there was no significant difference in survival between rats with one infected burn of 2 in2 and an additional uninfected burn of 2 in2 and rats with a single 2-in2 infected burn receiving either 20 (P = 0.50) or 200 (P = 0.25) CFU.
The relationship of burn size to survival in the model is shown in Fig. 1, middle panel. Survival was significantly higher in rats with a 2-in2 burn than in rats with larger burns (P ≤ 0.002). To assess whether the configuration of the burn was important for survival, we compared two burns of 2 in2 each (one of which was infected) with a single infected burn of 4 in2. Animals with the same total burn size (4 in2) composed of two burns (one of which was infected) had a significantly higher rate of survival than animals with a single infected burn (P < 0.0001) (Fig. 1, bottom panel).
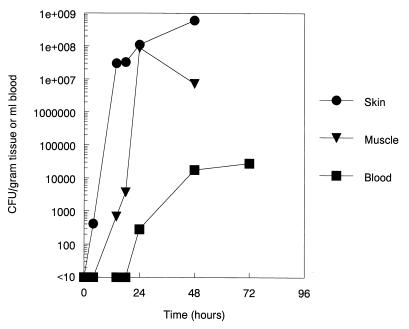
The small amount of bacterial inoculation permitted us to determine the kinetics of bacterial multiplication in the tissues and blood. These data, shown in Fig. 2, suggest that local bacterial replication begins immediately in the burn wound and is detectable in blood after 18 h.
FIG. 2.
Bacterial CFU per gram of excised skin or muscle tissue in area of the burn or per milliliter of blood at different time points in rats with 4-in2 burns infected with E. coli O18:K1:H7. The limit of detection for bacteremia was 10 CFU/ml. The limit of detection for burn tissue cultures was 100 CFU/gram of tissue.
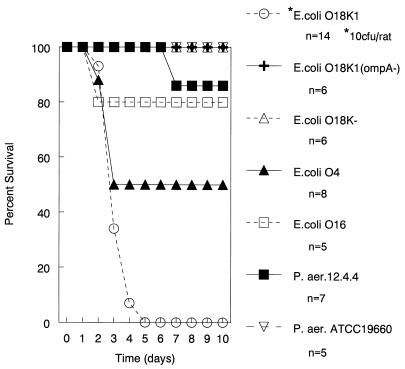
The striking pathogenicity of E. coli O18:K1:H7 prompted us to evaluate several other gram-negative bacteria with the same model. These data are shown in Fig. 3. For all other bacteria studied, there was 50% or greater survival rate with a bacterial inoculum of 107 CFU. These strains included the E. coli O18 K− and O18:K1:H7 OmpA− mutants (5).
FIG. 3.
Survival of rats with 4-in2 burns infected with different strains of gram-negative bacteria. A total of 107 CFU of each strain shown was inoculated into the burned tissue, except for E. coli O18:K1:H7, which was inoculated at 10 CFU. E. coli O18:K1:H7 at a 10-CFU inoculation resulted in significantly greater mortality (P ≤ 0.04) than all other strains inoculated with 107 CFU.
DISCUSSION
We have adapted a previously described model of burn wound infection in mice for use in rats. An unexpected finding was that inoculation of as few as two colonies of live E. coli O18:K1:H7 bacteria into the burn wound resulted in predictable bacteremia at 24 to 48 h and subsequent death at 3 to 4 days. Mortality with this organism was dependent upon burn size. Rats given a 10-million-fold-higher inoculation with two other encapsulated E. coli strains, two previously studied strains of P. aeruginosa, and E. coli O18 organisms that were deficient in either the K1 capsule or OmpA had higher survival rates under identical conditions. The contributions of the capsule and OmpA to pathogenicity have been previously described for models of meningitis (3, 5).
At this time, we do not have an explanation for the striking pathogenicity of E. coli O18:K1:H7. While some caution is needed because the virulence of E. coli O18:K1:H7 is so much higher than the other organisms studied, an advantage of the model for studies of efficacy and pathogenicity compared to most previously described models of gram-negative infection is that it is a model in which gram-negative bacteria replicate in vivo prior to bacteremia.
ACKNOWLEDGMENTS
This work was supported by the Shriners Hospital for Crippled Children (grant 15855); the Office of the Chief of Naval Research, Department of the Navy (grant N0014-94-C-0021); and the National Institutes of Health (grant AI28943).
REFERENCES
- 1.Cross A S, Opal S M, Sadoff J C, Gemski P. Choice of bacteria in animal models of sepsis. Infect Immun. 1993;61:2741–2747. doi: 10.1128/iai.61.7.2741-2747.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hellman J, Zanzot E M, Loiselle P M, Amato S F, Black K M, Ge Y, Kurnick J T, Warren H S. Antiserum against Escherichia coli J5 contains antibodies reactive with outer membrane proteins of heterologous Gram-negative bacteria. J Infect Dis. 1997;176:1260–1268. doi: 10.1086/514121. [DOI] [PubMed] [Google Scholar]
- 3.Kim K S, Itabashi H, Gemski P, Sadoff J, Warren R L, Cross A S. The K1 capsule is the critical determinant in the development of Escherichia coli meningitis in the rat. J Clin Investig. 1992;90:897–905. doi: 10.1172/JCI115965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meeh K. Oberflachen Messungen des Menschilchen Korpers. Z Biol. 1879;15:425–430. [Google Scholar]
- 5.Prasadarao N V, Wass C A, Weiser J N, Stins M F, Huang S-H, Kim K S. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect Immun. 1996;64:146–153. doi: 10.1128/iai.64.1.146-153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rigal C, Pieraggi M T, Serre G. Optimization of a model of full-thickness epidermal burns in the pig and immunohistochemical study of epidermodermal junction regeneration during burn healing. Dermatology. 1992;184:103–108. doi: 10.1159/000247514. [DOI] [PubMed] [Google Scholar]
- 7.Russo T A, Liang Y, Cross A S. The presence of K54 capsular polysaccharide increases the pathogenicity of Escherichia coli in vivo. J Infect Dis. 1994;169:112–118. doi: 10.1093/infdis/169.1.112. [DOI] [PubMed] [Google Scholar]
- 8.Stevens E J, Ryan C M, Friedberg J S, Barnhill R L, Yarmush M L, Tompkins R G. A quantitative model of invasive Pseudomonas infection in burn injury. J Burn Care Rehabil. 1994;15:232–235. doi: 10.1097/00004630-199405000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Stieritz D D, Holder I A. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J Infect Dis. 1968;131:688–691. doi: 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- 10.Teplitz C, Davis D, Mason A D, Moncrief J A. Pseudomonas burn wound sepsis. I. Pathogenesis of experimental Pseudomonas aeruginosa burn wound sepsis. J Surg Res. 1964;4:200–222. doi: 10.1016/s0022-4804(64)80026-3. [DOI] [PubMed] [Google Scholar]
- 11.Yurt R W, McManus A T, Mason A D, Pruitt B A. Increased susceptibility to infection related to extent of burn injury. Arch Surg. 1984;119:183–188. doi: 10.1001/archsurg.1984.01390140047008. [DOI] [PubMed] [Google Scholar]