Abstract
Introduction
Traditionally, medical care and research in Parkinson's disease (PD) have been conducted with in-person encounters. The recent COVID-19 pandemic has profoundly impacted the delivery of in-person clinical care and clinical research. We conducted an online survey of active clinician members of the Parkinson Study Group (PSG) to evaluate the adoption of various non-face-to-face methods in clinical practice and research in PD during the COVID-19 pandemic.
Methods
We conducted a survey using the open-access online SurveyMonkey tool (http://www.surveymonkey.com). The survey had 27 items and was designed to elucidate clinical/research care before and during the COVID-19 pandemic. The survey was sent to 414 active PSG members with weekly reminders and it remained accessible for 30 days from May 2020.
Results
We received 142 responses, of which 133 (93.7%) provided demographic data. The clinical use of virtual visits via synchronous video conferencing increased from 39.5% pre-COVID-19 to 94.6% during the COVID-19 pandemic. Lack of access for patients (68.2%) and patient resistance (51.4%) were the top barriers for its use. Approximately 70% respondents stated that 75–100% of their research activities were suspended during the COVID-19 pandemic. Many sites had to fill out protocol deviations (38.2%), protocol exceptions (25.5%) or change their research profile due to layoffs (16.8%). The overall use of video conferencing increased from 30.3% to 64.1%.
Conclusion
The current results suggest a need for flexibility in conducting office visits and clinical trials in PD patients. Technology has the potential to enhance patient care and convenience, when in-person visits can be challenging.
Keywords: Telemedicine, Telehealth, Coronavirus, COVID-19, Barrier, Technology
1. Introduction
The World Health Organization (WHO) declared COVID-19 as a pandemic in March 2020. The vast majority of people with Parkinson's disease (PD) belong to older age groups that are at a higher risk of more severe forms of COVID-19 infection [1]. In response to the pandemic, many hospitals canceled elective surgeries, and clinical and research appointments were modified to limit the risk of COVID-19 infection to patients and clinical staff. While COVID-19 infection might have a direct influence on the health of people with PD, the stress and limited physical activity due to social distancing might have contributed to worsening of motor and non-motor symptoms [2]. In such times, it is paramount that people with PD continue to receive care.
Medical care and research in PD have been conducted traditionally with in-person encounters between patients and care providers, what is termed face-to-face (FTF) clinical care/research [3]. Alternatives to FTF care such as limited in-home data collection (e.g., blood draw by a trained local coordinator) are termed non-face-to-face (NFTF) care [3]. NFTF methods have been used in some centers before the COVID-19 pandemic but were not widespread. The COVID-19 pandemic and the resultant social distancing/stay-at-home guidance issued in many countries profoundly impacted the delivery of care and clinical research in a rapidly transformative way. Across North America, there was an unprecedented surge in NFTF methods of communication to provide continuity of care and maintain clinical research when possible.
We conducted an online survey of active clinician members of the largest North-American network of PD centers of excellence, the Parkinson Study Group (PSG), to evaluate the adoption of various NFTF methods in clinical practice and research activities in PD during the initial months of the COVID-19 pandemic.
2. Methods
We conducted a survey with 27 items divided into a clinical care module and a clinical research module. Both modules included questions designed to elucidate clinical/research care before and during the COVID-19 pandemic. We also collected information on age and professional experience of the clinician members. The open-access online SurveyMonkey tool (http://www.surveymonkey.com) was used to create and administer the survey. The survey was available for 30 days, from May 14, 2020 with weekly reminders e-mailed to 414 active PSG members.
Data analysis: We included only data of those respondents that ended the survey. We present the results using descriptive statistics. The percentages reported are based on the number of respondents for each question, since some questions were skipped by some respondents.
3. Results
A total of 142/414 responses (34.3%) were collected, of which 133 (93.7%) provided demographic data. The majority of the respondents (32.3%, n = 43) were in the 41–50 years age group. 60.1% (n = 80) had more than 15 years of clinical experience, while 57.1% (n = 76) had more than 15 years of research experience (Table 1 ).
Table 1.
Demographic and professional experience of the clinician members of the Parkinson Study Group who responded to the online survey.
| [n = 133] n (%) | |
|---|---|
| Age (years) | |
| <31 | 1 (0.7) |
| 31–40 | 19 (14.3) |
| 41–50 | 43 (32.3) |
| 51–60 | 35 (26.3) |
| >60 | 35 (26.3) |
| Years in clinical practice | |
| <6 | 13 (9.8) |
| 6–10 | 16 (12.0) |
| 11–15 | 24 (18.0) |
| >15 | 80 (60.1) |
| Years in clinical research | |
| <6 | 16 (12.0) |
| 6–10 | 15 (11.3) |
| 11–15 | 26 (19.5) |
| >15 | 76 (57.1) |
| % of dedicated time (mean value) | |
| Clinical | 51.1 |
| Research | 31.6 |
| Administrative | 12.3 |
| Teaching | 7.7 |
3.1. Change in clinical care for PD during the COVID-19 pandemic
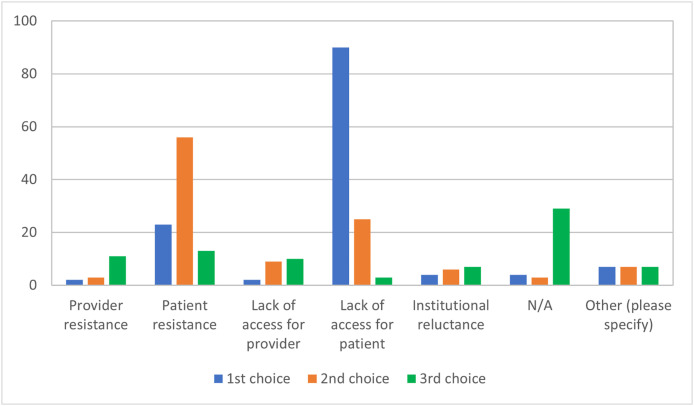
The overall use of synchronous video conferencing increased from 39.5% (n = 49/124) to 94.6% (n = 122/129), with 65.1% (n = 84/129) of respondents reporting that it was the most preferred method of communication. Use of other NFTF modalities, such as asynchronous communication (example: e-mails and messaging) was limited. Institutional preference was more frequently selected as the top reason (75.2%, n = 100/133) for selection of synchronous video conferencing platforms, and HIPAA compliance was more frequently selected as the second most important reason (36.8%, n = 49/133). Among the different video conferencing providers, Zoom (61.6%, n = 82/133) and Doximity (30.8%, n = 41/133) had the greatest uptake. Lack of access by patients (68.2%, n = 90/132) was more frequently selected as the top barrier for adoption of NFTF methods and patient resistance was more frequently selected as the second top barrier (51.4%, n = 56/109) (Fig. 1 ).
Fig. 1.
Barriers for adoption of non-face-to-face methods for clinical care during the COVID-19 pandemic (n = 132 respondents). Participants were asked to rank the top three barriers.
For clinical procedures (botulinum toxin injections, device-aided therapies manipulation) that require FTF interactions, 45.4% (n = 60/132) of the respondents maintained their procedural activities as planned, and 43.9% (n = 58/132) performed remote assessments and deferred a procedure or device manipulation.
3.2. Change in PD clinical research during the COVID-19 pandemic
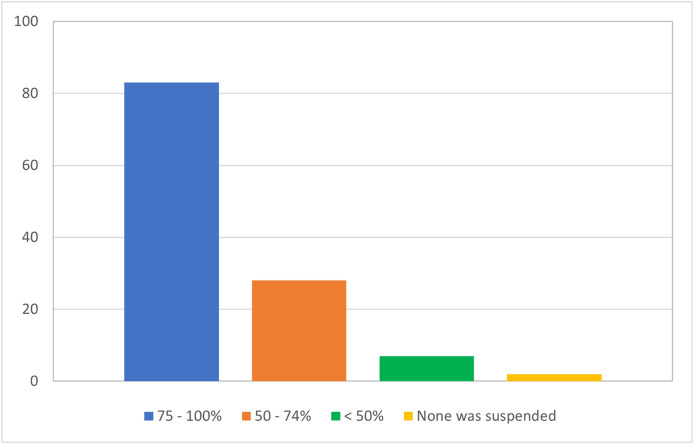
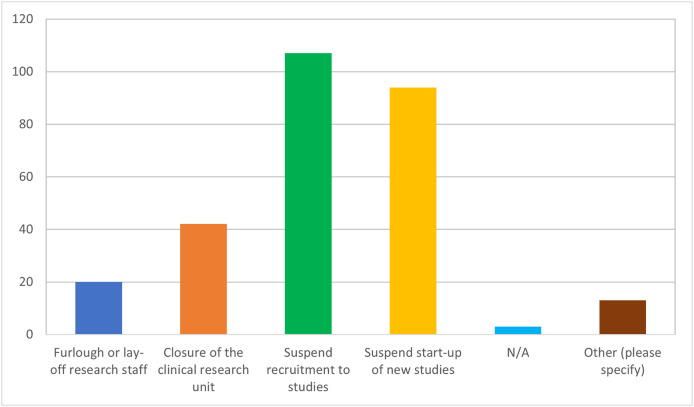
Most of the respondents (69.2%, n = 83/120) stated that 75–100% of their research activities were suspended during the COVID-19 pandemic (Fig. 2 ). 89.9% (n = 107/119) reported suspension of recruitment to studies and 78.9% (n = 94/119) suspended start-up of new studies Fig. 3 . In addition, 35.3% (n = 42/119) reported closure of the research unit, or had a change to the research structure, namely, layoffs (16.8%, n = 20/119). Changes related to converting research visits to a NFTF format included filling out protocol deviations (38.2%, n = 42/110) or protocol exceptions (25.5%, n = 28/110). The overall use of videoconferencing increased from 30.3% to 64.1%, while its use as the most preferred method increased from 10.7% (n = 10/93) to 34.9% (n = 37/106). Most respondents agreed that future studies will use NFTF methods to a larger extent.
Fig. 2.
Proportion of research activities suspended during the COVID-19 pandemic in PSG centers (n = 120 respondents).
Fig. 3.
Implications of the suspension of research activities during COVID-19 pandemic (n = 119 respondents).
4. Discussion
The results of the current survey to members of a consortium of PD tertiary centers dedicated to PD care and research suggest a great ability to adapt clinical and research activities during the COVID-19 pandemic. Overall, the uptake of synchronous video conferencing systems was significant. We found that institutional preference and HIPAA compliance were the major determinants for choosing a telemedicine platform, despite that, in USA, there was a HIPAA flexibility guidance issued by the U.S. Department of Health and Human Services. In response to the COVID-19 pandemic, in the USA and Canada, reimbursement regulations were frequently loosened and telemedicine visits became on par with regular in-office visits for physician reimbursement (facility fees were not reimbursed), which promoted the use of telemedicine for the patient visits by the majority of the survey respondents. In fact, the major barriers for the adoption of NFTF methods were lack of access for patients and patient resistance. Adequate education might overcome patient resistance to use NFTF methods in the future. Patients and caregivers can be trained during their in-office visits to enable compliance with future telemedicine visits. Our results were consistent with the recent Movement Disorder Society (MDS) telemedicine survey showing a global increase in use of telemedicine and similar barriers for its use [4].
The Food and Drug Administration (FDA) issued a guidance in March 2020 with regards to the conduct of clinical trials during the COVID-19 pandemic [5]. Clinical trial sites had to revise existing policies and formulate new policies to continue clinical trials. Studies have shown telemedicine was equally efficacious as in-office visits, and PD patients may prefer them over in-office visits [6,7]. Telemedicine visits help patients and caregivers overcome transportation challenges and save travel time and money [6]. There are current limitations to complete virtually scales of parkinsonism such as the MDS-Unified Parkinson's Disease Rating Scale (MDS-UPDRS), since rigidity and postural instability cannot be rated. However, the feasibility of conducting a modified MDS-UPDRS has been shown previously [8,9]. Imputation methods have also been demonstrated to handle missing values adequately [10]. Even other assessments such as the Montreal Cognitive Assessment can be administered remotely [11]. There can be other challenges in performing assessments over video. Poor lighting and audio, video lag, and smaller spaces can affect certain ratings. Participants have to sit 6–8 feet away from the camera to allow for visualization of the entire body throughout the visit, which, in turn, may impede evaluation of hypomimia, toe tapping or subtle tremors [12]. In order to overcome some of the limitations, wearable devices may be used to complement remote MDS-UPDRS scores. It has been proposed that digital phenotyping of PD can complement in-person care [13]. Web-based portals can be used for patient-reported questionnaires and diaries. Studies comparing the effectiveness of FTF and NFTF visits are needed.
A significant proportion of respondents reported disruption of research activities with research unit closures, suspension of recruitment and staff layoffs. Incorporating a greater routine use of technology into research protocols, at least as a contingency, might minimize disruptions to studies in the future. Electronic signatures have been commonly used in the business world and can be used for obtaining informed consent as well. Training of staff and patients to use these technologies in advance would ensure a smoother transition in the wake of emergencies. Research visit costs include the use of the research facility/research space among others. If FTF visits were to be converted to remote visits, innovative cost structure and budgeting to assure fiscal health will be needed. Pragmatic diligence to avoid missing key research data such as EKG, vital signs and biospecimen collection due to inappropriate use of NFTF methods is essential to ensure the quality of the study is not compromised. Other techniques for remote recording of vital clinical data such as remote EKG, remote monitoring devices, remote blood draws and home weighing machines connected to Wi-Fi may find its utility [14].
The current study has some limitations. The survey response rate of 34.2% can be considered sub-optimal. We minimized the non-response bias by keeping it available for 30 days and having weekly reminders. Finally, while most respondents felt that NFTF visits might be more prevalent in the future, their effectiveness and the quality of clinical care/research care in the context of NFTF was not evaluated in our study.
5. Conclusions
The survey results suggest a need for flexibility in conducting office visits and clinical trials in PD. The COVID-19 pandemic and the subsequent response by the medical community has shown that the use of technology is feasible with the potential to enhance patient care and comfort, namely, for those living in remote areas or with greater disability. We share the concerns of others, that the efficiencies in time and cost for patients and healthcare organizations need to be carefully weighed against less often in-person interactions and potential risks for diagnosis, and patient communication [15]. The validation of these technologies in clinical practice and research is critical, and should not be forgotten, as they have the potential to become an integral part of clinician's lives in a post-COVID 19 era.
Declaration of competing interest
None.
Acknowledgement
Online survey was supported by the Parkinson Study Group.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.parkreldis.2021.03.032.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Petretto D.R., Pili R. Ageing and COVID-19: what is the role for elderly people? Geriatrics. 2020;5(2) doi: 10.3390/geriatrics5020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helmich R.C., Bloem B.R. The impact of the COVID-19 pandemic on Parkinson's disease: hidden sorrows and emerging opportunities. J. Parkinsons Dis. 2020;10(2):351–354. doi: 10.3233/JPD-202038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen B.H., Busis N.A., Ciccarelli L. Coding in the world of COVID-19: non-face-to-face evaluation and management care. Continuum. 2020;26(3):785–798. doi: 10.1212/CON.0000000000000874. [DOI] [PubMed] [Google Scholar]
- 4.Hassan A., Mari Z., Gatto E.M., Cardozo A., Youn J., Okubadejo N., Bajwa J.A., Shalash A., Fujioka S., Aldaajani Z., Cubo E., International Telemedicine Study G. Global survey on telemedicine utilization for movement disorders during the COVID-19 pandemic. Mov. Disord. 2020;35(10):1701–1711. doi: 10.1002/mds.28284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S.F.a.D. Administration . 2020. FDA Guidance on Conduct of Clinical Trials of Medical Products during COVID-19 Public Health Emergency: Guidance for Industry, Investigators, and Institutional Review Boards. Docket Number: FDA-2020-D-1106. [Google Scholar]
- 6.Beck C.A., Beran D.B., Biglan K.M., Boyd C.M., Dorsey E.R., Schmidt P.N., Simone R., Willis A.W., Galifianakis N.B., Katz M., Tanner C.M., Dodenhoff K., Aldred J., Carter J., Fraser A., Jimenez-Shahed J., Hunter C., Spindler M., Reichwein S., Mari Z., Dunlop B., Morgan J.C., McLane D., Hickey P., Gauger L., Richard I.H., Mejia N.I., Bwala G., Nance M., Shih L.C., Singer C., Vargas-Parra S., Zadikoff C., Okon N., Feigin A., Ayan J., Vaughan C., Pahwa R., Dhall R., Hassan A., DeMello S., Riggare S.S., Wicks P., Achey M.A., Elson M.J., Goldenthal S., Keenan H.T., Korn R., Schwarz H., Sharma S., Stevenson E.A., Zhu W., Parkinson I. Connect. National randomized controlled trial of virtual house calls for Parkinson disease. Neurology. 2017;89(11):1152–1161. doi: 10.1212/WNL.0000000000004357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorsey E.R., Venkataraman V., Grana M.J., Bull M.T., George B.P., Boyd C.M., Beck C.A., Rajan B., Seidmann A., Biglan K.M. Randomized controlled clinical trial of "virtual house calls" for Parkinson disease. JAMA Neurol. 2013;70(5):565–570. doi: 10.1001/jamaneurol.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdolahi A., Scoglio N., Killoran A., Dorsey E.R., Biglan K.M. Potential reliability and validity of a modified version of the Unified Parkinson's Disease Rating Scale that could be administered remotely. Park. Relat. Disord. 2013;19(2):218–221. doi: 10.1016/j.parkreldis.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cubo E., Gabriel-Galan J.M., Martinez J.S., Alcubilla C.R., Yang C., Arconada O.F., Perez N.M. Comparison of office-based versus home Web-based clinical assessments for Parkinson's disease. Mov. Disord. 2012;27(2):308–311. doi: 10.1002/mds.24028. [DOI] [PubMed] [Google Scholar]
- 10.Goetz C.G., Luo S., Wang L., Tilley B.C., LaPelle N.R., Stebbins G.T. Handling missing values in the MDS-UPDRS. Mov. Disord. 2015;30(12):1632–1638. doi: 10.1002/mds.26153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdolahi A., Bull M.T., Darwin K.C., Venkataraman V., Grana M.J., Dorsey E.R., Biglan K.M. A feasibility study of conducting the Montreal Cognitive Assessment remotely in individuals with movement disorders. Health Inf. J. 2016;22(2):304–311. doi: 10.1177/1460458214556373. [DOI] [PubMed] [Google Scholar]
- 12.Schneider R.B., Myers T.L., Tarolli C.G., Amodeo K., Adams J.L., Jensen-Roberts S., Dorsey E.R. Remote administration of the MDS-UPDRS in the time of COVID-19 and beyond. J. Parkinsons Dis. 2020;10(4):1379–1382. doi: 10.3233/JPD-202121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhidayasiri R., Mari Z. Digital phenotyping in Parkinson's disease: empowering neurologists for measurement-based care. Park. Relat. Disord. 2020;80:35–40. doi: 10.1016/j.parkreldis.2020.08.038. [DOI] [PubMed] [Google Scholar]
- 14.Inan O.T., Tenaerts P., Prindiville S.A., Reynolds H.R., Dizon D.S., Cooper-Arnold K., Turakhia M., Pletcher M.J., Preston K.L., Krumholz H.M., Marlin B.M., Mandl K.D., Klasnja P., Spring B., Iturriaga E., Campo R., Desvigne-Nickens P., Rosenberg Y., Steinhubl S.R., Califf R.M. Digitizing clinical trials. NPJ Digit Med. 2020;3:101. doi: 10.1038/s41746-020-0302-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulroy E., Menozzi E., Lees A.J., Lynch T., Lang A.E., Bhatia K.P. Telemedicine in Movement Disorders: lecons du COVID-19. Mov. Disord. 2020;35(11):1893–1896. doi: 10.1002/mds.28297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.