Abstract
Background
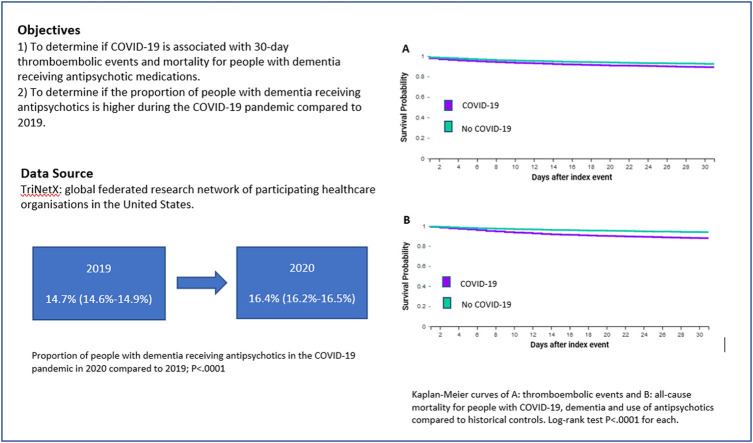
Antipsychotic medications are frequently prescribed to people with dementia to manage behavioural and psychological symptoms. Using a global federated research network, the objectives were to determine: 1) if COVID-19 is associated with 30-day thromboembolic events and mortality for people with dementia receiving antipsychotic medications; and 2) if the proportion of people with dementia receiving antipsychotics is higher during the COVID-19 pandemic compared to 2019.
Methods
A retrospective cohort study was conducted using TriNetX, a global federated health research network. The network was searched for people aged ≥ 65 years with dementia, COVID-19 and use of antipsychotics in the 30-days prior to COVID-19 recorded in electronic medical records between 20/01/2020 and 05/12/2020. These individuals were compared to historical controls from 2019 with dementia and use of antipsychotics in the 30-days before a visit to a participating healthcare organisation. Propensity score matching for age, sex, race, co-morbidities and use of antidepressants and anticonvulsants was used to balance cohorts with and without COVID-19.
Results
Within the TriNetX network, 8414 individuals with COVID-19, dementia and use of antipsychotics and 31,963 historical controls were identified. After propensity score matching there were 8396 individuals with COVID-19 and 8396 historical controls. The cohorts were well balanced for age, sex, race, co-morbidities and use of antidepressants and anticonvulsants. The odds of 30-day thromboembolic events and all-cause mortality were significantly higher in adults with COVID-19 (Odds Ratios: 1.36 (95% confidence interval (CI): 1.21–1.52) and 1.93 (1.71–2.17), respectively). The number of people with dementia with a visit to a participating healthcare organisation was lower between 20/01/2020 and 05/12/2020 (n = 165,447) compared to the same period in 2019 (n = 217,391), but the proportion receiving antipsychotics increased from 14.7% (95%CI: 14.6–14.9%) to 16.4% (95%CI: 16.2–16.5%), P < .0001.
Conclusions
These findings add to the evidence base that during the COVID-19 pandemic there was an increase in the proportion of people with dementia receiving antipsychotics. The negative effects of antipsychotics in patients with dementia may be compounded by concomitant COVID-19.
Keywords: Dementia, Alzheimer’s disease, Antipsychotics, COVID-19, Mortality
Graphical Abstract
1. Introduction
Antipsychotic medications are frequently prescribed to older adults living with dementia in response to behavioural and psychological symptoms, and concomitant use with other psychotropic medications is high [1]. The high use of antipsychotic medications for people with dementia occurs even though many studies and national guidelines for dementia have highlighted their small-moderate effect size for reducing behavioural and psychological symptoms, high-risk of adverse events (including falls, thromboembolic events and mortality), and availability of safer alternatives [2], [3], [4], [5]. Since 2005, the Food and Drug Administration (FDA) in the United States (US) have produced black-box warnings against prescription of antipsychotic medications for people with dementia, due to increased mortality [6]. Risperidone is the only second-generation antipsychotic approved for the treatment of behavioural and psychological symptoms of dementia in some countries such as the United Kingdom (UK), while other antipsychotics are often used off-label. Risperidone and other antipsychotic medications including haloperidol, quetiapine and olanzapine are not approved for treatment of behavioural and psychological symptoms of dementia by the FDA in the US.
Non-pharmacological approaches are recommended for behavioural and psychological symptoms for people with dementia due to their similar effect size for reducing the symptoms without adverse events. Antipsychotic medications should only be utilised for behavioural and psychological symptoms for people with dementia when all other approaches have failed, the symptoms are severe, or the person is at an immediate risk of harm to themselves or others [2], [3], [4].
Concerns have been raised that the use of antipsychotic medications for people living with dementia may increase during the coronavirus disease 2019 (COVID-19) pandemic. A study of the National Health Service in England showed that although the absolute number of antipsychotic prescriptions for people with dementia decreased between March-July 2020, the proportion of patients who were prescribed antipsychotics substantially increased [7]. Although the underlying reasons for an increase in the proportion of people with dementia prescribed antipsychotic medications remains unclear, it has been proposed that this may be due to increases in behavioural and psychological symptoms for people with dementia in response to restrictions imposed, such as reduction or cessation of visitors and group activities [7]. International organisations such as Alzheimer’s Disease International have drawn attention to the increased need for psychological support for people living with dementia during the COVID-19 pandemic [8].
It has been hypothesized that people with COVID-19 may encounter a dysregulation of coagulation and a hyperinflammatory response which may result in a “hypercoagulable state” and subsequent increased risk of thrombosis [9]. A review of evidence published in July 2020 concluded that the use of antipsychotic medications may worsen respiratory function and increase the risk of thromboembolism for people with COVID-19 [10].
The primary objective of the current study was to determine if COVID-19 is associated with 30-day thromboembolic events and all-cause mortality for people with dementia receiving antipsychotics compared to historical controls. A secondary objective was to determine if the proportion of people with dementia receiving antipsychotic medications had increased during the COVID-19 pandemic compared to people with dementia during the same time-period in 2019.
2. Materials and methods
2.1. Study participants
A retrospective cohort study was conducted using TriNetX, a global federated health research network. TriNetX provided access to statistics on electronic medical records from participating healthcare organisations predominately in the United States. Available data include demographics, diagnoses using International Classification of Diseases, Ninth Revision and Tenth Revision, Clinical Modification (ICD-10-CM) codes, procedures, medications, and measurements (e.g., laboratory test results). The healthcare organisations include a mixture of hospitals, primary care, and specialist providers and contribute data from insured and uninsured patients alike. The data are continuously updated; healthcare organisations update their data at various times, refreshing every 1, 2, or 4 weeks. To comply with legal frameworks and ethical guidelines guarding against data re-identification, the identity of participating healthcare organisations and their individual contribution to each dataset are not disclosed. The TriNetX platform only uses aggregated counts and statistical summaries of de-identified information. No protected health information or personal data are made available to the users of the platform.
For the first objective, the TriNetX online research platform was searched on 5th February 2021 for patients aged ≥ 65 years with dementia and COVID-19 recorded in their electronic medical records between 20th January 2020 and 5th December 2020. Participants were included if they had antipsychotic medication use recorded in electronic medical records in the 30-days prior to COVID-19. The start date was chosen as January 20th 2020 because COVID-19 was first confirmed in the US on this date, and the TriNetX network is predominately US-based [11]. The end date was set at 5th December 2020 to allow for at least 30-days follow-up for all participants and to ensure up to four weeks additional time to allow the update of data from all participating healthcare organisations. Identification of COVID-19 was determined using codes in electronic medical records following criteria provided by TriNetX based on Centers for Disease Control and Prevention (CDC) coding guidelines, or a positive test result identified with COVID-19 specific laboratory codes. Specifically, COVID-19 was identified by one or more of the following ICD-10-CM codes in the electronic medical records of the patients: U07.1 COVID-19, virus identified; U07.2 COVID-19, virus not identified; B97.29 Other coronavirus as the cause of diseases classified elsewhere; B34.2 Coronavirus infection, unspecified; or a positive test result identified with COVID-19 specific laboratory Logical Observation Identifiers Names and Codes (LOINCs). Patients with ICD-9 code 079.89 were excluded because this code may still be used code for > 50 viral infections. Dementia was identified using the following ICD-10-CM codes: G30 Alzheimer’s disease; F01 vascular dementia; F02 dementia in other diseases classified elsewhere; F03 unspecified dementia. Antipsychotic medications were identified using the World Health Organisation Anatomical Therapeutic Chemical (ATC) codes N05A*. A second cohort of historical controls included patients aged ≥ 65 years with dementia and a visit to a participating healthcare organisation recorded between 20th January 2019 and 5th December 2019. Participants were included if they had antipsychotic medication use recorded in electronic medical records in the 30-days prior to or on a visit to a healthcare organisation. Individuals with any history of schizophrenia (ICD-10-CM code F20) or bipolar disorder (ICD-10-CM code F31) were excluded from all analyses.
For the second objective, the proportion of participants receiving antipsychotic medications 30 days prior to or on a visit to a healthcare organisation were compared in two cohorts: 1) participants aged ≥ 65 years with dementia and a visit to a participating healthcare organisation recorded in their electronic medical records between 20th January 2020 and 5th December 2020 and 2) a historical control cohort of participants aged ≥ 65 years with dementia and a visit to a participating healthcare organisation recorded in their electronic medical records between 20th January 2019 and 5th December 2019.
2.2. Statistical analysis
Statistical analyses were completed on the TriNetX online research platform. Baseline characteristics were compared with chi-squared tests for categorical variables and independent-sample t-tests for continuous variables. For the first objective, 1:1 propensity score matching was used to balance the cohort with dementia and COVID-19 receiving antipsychotics with the historical controls. The TriNetX platform calculates propensity scores using logistic regression through implementation of the scikit-learn package in Python. ‘Greedy nearest neighbour matching’ is used with a caliper of 0.1 pooled standard deviations. The following variables were included in propensity score matching: age, sex, race, other use of psychotropic medications (antidepressants and anticonvulsants), and any of the following health conditions recorded in the previous 30 days identified from ICD-10-CM codes in electronic medical records: hypertension, ischaemic heart diseases, cerebrovascular diseases, diabetes mellitus, chronic obstructive pulmonary disease (COPD), diseases of the genitourinary system, neoplasms, mood [affective] disorders and anxiety, dissociative, stress-related, somatoform and other nonpsychotic mental disorders. The health conditions were chosen because they are known to be associated with antipsychotic use and/or thromboembolic events or mortality. Kaplan-Meier analysis was used to estimate the probability of thromboembolic events and all-cause mortality up to 30-days following recording of COVID-19 in electronic medical records. Thromboembolic events included were cerebral infarction (ICD-10-CM code: I63), transient cerebral ischaemic attacks and related syndromes (G45), pulmonary embolism (I26), arterial embolism and thrombosis (I74) and other venous embolism and thrombosis (I82). Logistic regression was used to calculate odds ratios with 95% confidence intervals (CIs). For the second objective, the proportion of individuals with dementia receiving antipsychotics in 2020 compared to 2019 were compared with a chi-squared test. Statistical significance was pre-specified at P < .05.
2.3. Ethical approval
As a federated network, research studies using the TriNetX research network do not require ethical approvals as no patient identifiable identification is received.
3. Results
3.1. Participant characteristics for people with dementia and COVID-19 receiving antipsychotics compared to historical controls with dementia receiving antipsychotics
Within the TriNetX network, 8414 individuals were identified with COVID-19, dementia and use of antipsychotics recorded in their electronic medical records in the 30-day period before COVID-19 was noted. The most common antipsychotics received were quetiapine (42.1%, n = 3539), haloperidol (38.4%, n = 3232), olanzapine (21.1%, n = 1776) and risperidone (12.5%, n = 1050); the remaining antipsychotics were received by < 3% of the participants. Analyses were completed for all individuals with dementia, and no further analyses were completed by type of dementia because the majority of individuals (75.0%) had more than one type of dementia recorded in their electronic medical records.
Compared to 31,963 historical controls with dementia and receiving antipsychotic medications without COVID-19, individuals with COVID-19, dementia and antipsychotic use were significantly older (mean age (standard deviation) 80.7 (7.3) vs. 80.0 (7.5), P < .0001), had a significantly lower proportion of females (55.5% vs. 57.8%, P = .0002), a significantly lower proportion of individuals identified as white (74.3% vs. 76.4%, P < .0001) and a higher proportion of individuals with hypertensive diseases, diseases of the genitourinary system, cerebrovascular disease, ischaemic heart disease, COPD, diabetes mellitus and neoplasms. Individuals with COVID-19 also had a significantly higher proportion of individuals with a history of antidepressant and anticonvulsant use. After 1:1 propensity score matching, there were 8396 individuals in each cohort, and the cohorts were well balanced on characteristics including age, sex, race, co-morbidities and antidepressant and anticonvulsant use ( Table 1). However, ischaemic heart disease and the proportion of individuals with race unknown remained significantly higher in the COVID-19 cohort.
Table 1.
Characteristics for people with dementia and COVID-19 receiving antipsychotics compared to historical controls in 2019 receiving antipsychotics, before and after propensity score matching.
| Initial populations |
Propensity score matched populations |
|||||
|---|---|---|---|---|---|---|
| Characteristic | COVID-19 cohort (n = 8414) | 2019 cohort (n = 31,963) | P-value | COVID-19 cohort (n = 8396) | 2019 cohort (n = 8396) | P-value |
| Age (years), mean (SD) | 80.7 (7.3) | 80.0 (7.5) | < 0.0001 | 80.7 (7.3) | 80.8 (7.3) | 0.14 |
| Female | 55.5 (4667) | 57.8 (18,462) | 0.0002 | 55.6 (4667) | 55.6 (4665) | 0.98 |
| Race | ||||||
| White | 74.3 (6247) | 76.4 (24,421) | < 0.0001 | 74.4 (6247) | 74.9 (6286) | 0.49 |
| Black or African American | 15.1 (1266) | 12.1 (3854) | < 0.0001 | 15.1 (1266) | 16.0 (1343) | 0.56 |
| Asian | 1.1 (95) | 1.3 (408) | 0.28 | 1.1 (95) | 1.1 (95) | 0.10 |
| Native Hawaiian or other Pacific Islander | 0.1 (10) | 0.1 (26) | 0.31 | 0.1 (10) | 0.1 (10) | 1.00 |
| American Indian or Alaska Native | 0.2 (16) | 0.2 (56) | 0.77 | 0.1 (10) | 0.1 (10) | 0.68 |
| Unknown | 9.4 (787) | 10.0 (3198) | 0.07 | 9.2 (769) | 7.9 (664) | 0.003 |
| Hypertensive Diseases | 59.5 (5002) | 52.9 (16,895) | < 0.0001 | 59.4 (4996) | 59.1 (4961) | 0.58 |
| Diseases of the genitourinary system | 53.8 (4528) | 40.1 (12,812) | < 0.0001 | 53.9 (4523) | 54.0 (4532) | 0.89 |
| Cerebrovascular Disease | 18.5 (1555) | 13.5 (4306) | < 0.0001 | 18.5 (1552) | 17.4 (1458) | 0.06 |
| Ischaemic Heart Disease | 27.8 (2336) | 21.1 (6730) | < 0.0001 | 27.8 (2332) | 26.1 (2193) | 0.02 |
| Chronic Obstructive Pulmonary Disease | 12.0 (1008) | 10.7 (3427) | 0.001 | 12.0 (1008) | 11.2 (938) | 0.09 |
| Diabetes mellitus | 25.6 (2152) | 21.7 (6929) | < 0.0001 | 25.6 (2151) | 24.6 (2066) | 0.13 |
| Neoplasms | 12.8 (1076) | 11.2 (3587) | < 0.0001 | 12.8 (1076) | 12.2 (1025) | 0.23 |
| Mood [affective] disorders | 21.7 (1825) | 21.5 (6869) | 0.69 | 21.7 (1823) | 21.2 (1780) | 0.42 |
| Anxiety, dissociative, stress-related, somatoform and other nonpsychotic mental disorders | 18.5 (1555) | 17.9 (5733) | 0.25 | 18.5 (1554) | 18.1 (1519) | 0.48 |
| Antidepressant use | 46.6 (3921) | 40.7 (13,004) | < 0.0001 | 46.6 (3909) | 46.4 (3898) | 0.92 |
| Anticonvulsant use | 25.5 (2143) | 20.4 (6524) | < 0.0001 | 25.5 (2139) | 24.6 (2069) | 0.21 |
Baseline characteristics were compared using a chi-squared test for categorical variables and an independent-sample t-test for continuous variables. Data are % (n), unless otherwise stated. Presence of a condition or use of medication is based on recording with electronic medical records 30-days up to COVID-19 recording or healthcare organisation visit. COVID-19: coronavirus disease 2019, SD: standard deviation.
3.2. Outcomes for people with dementia and COVID-19 receiving antipsychotics compared to historical controls with dementia receiving antipsychotics
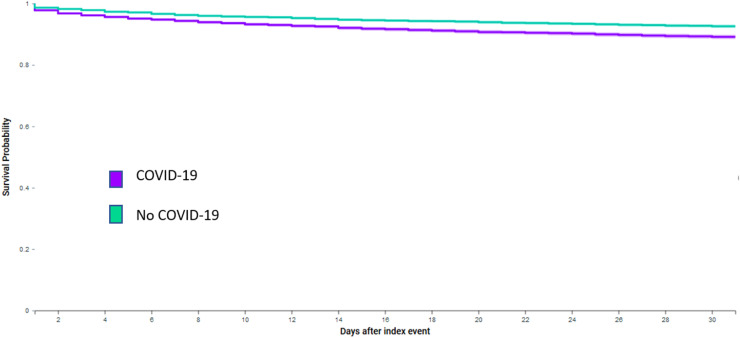
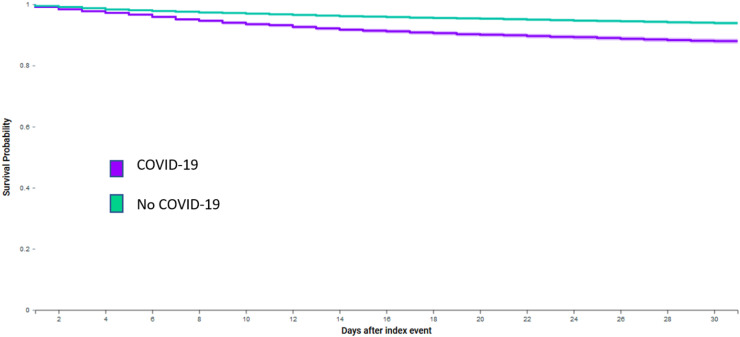
After propensity-score matching, compared to historical controls, people with dementia and COVID-19 who received antipsychotics had higher odds of 30-day thromboembolic events (9.1% vs. 6.9%, Log-Rank test P < .0001, Fig. 1; Odds Ratio 1.36 (95% CI: 1.21–1.52)), and all-cause mortality (10.1% vs. 5.5%, Log-Rank test P < .0001, Fig. 2; Odds Ratio 1.93 (95% CI: 1.71–2.17)). Table 2 also shows the results stratified by the four most common types of antipsychotics prescribed. COVID-19 was associated with significantly higher odds of 30-day thromboembolic events and mortality for people receiving quetiapine, haloperidol or risperidone compared to historical controls. No significant association was observed between COVID-19 and 30-day thromboembolic events for people receiving olanzapine, but a significant association was observed with mortality.
Fig. 1.
Kaplan-Meier curve of thromboembolic events for people with dementia, COVID-19 and use of antipsychotics compared to historical controls.
Fig. 2.
Kaplan-Meier curve of all-cause mortality for people with dementia, COVID-19 and use of antipsychotics compared to historical controls.
Table 2.
Outcomes for people with dementia and COVID-19 receiving antipsychotics compared to historical controls in 2019 with dementia receiving antipsychotics.
| Exposure | Propensity score matched populations (n) | 30-day thromboembolic events | 30-day thromboembolic events OR (95% CI) | 30-day mortality | 30-day mortality OR (95% CI) |
|---|---|---|---|---|---|
| Any antipsychotic | |||||
| 2019 cohort | 8396 | 6.9% | Reference | 5.5% | Reference |
| COVID-19 cohort | 8396 | 9.1% | 1.36 (1.21–1.52) | 10.1% | 1.93 (1.71–2.17) |
| Quetiapine | |||||
| 2019 cohort | 3534 | 6.0% | Reference | 4.3% | Reference |
| COVID-19 cohort | 3534 | 8.3% | 1.42 (1.18–1.70) | 8.9% | 2.18 (1.78–2.66) |
| Haloperidol | |||||
| 2019 cohort | 3216 | 6.9% | Reference | 10.3% | Reference |
| COVID-19 cohort | 3216 | 8.3% | 1.23 (1.02–1.48) | 13.8% | 1.39 (1.20–1.62) |
| Olanzapine | |||||
| 2019 cohort | 1774 | 8.3% | Reference | 7.0% | Reference |
| COVID-19 cohort | 1774 | 7.8% | 0.94 (0.74–1.20) | 9.0% | 1.32 (1.03–1.68) |
| Risperidone | |||||
| 2019 cohort | 1047 | 4.3% | Reference | 3.2% | Reference |
| COVID-19 cohort | 1047 | 6.7% | 1.60 (1.09–2.34) | 7.9% | 2.57 (1.71–3.86) |
COVID-19: coronavirus disease 2019; OR: odds ratio; 95% CI: 95% confidence interval.
3.3. Antipsychotic use for people with dementia during the COVID-19 pandemic compared to 2019
Between 20th January 2019 and 5th December 2019, 217,391 people living with dementia (with no previous history of schizophrenia or bipolar disorder) had a recorded visit at one of 44 healthcare organisations. Between 20th January 2020 and 5th December 2020, 165,447 people living with dementia (with no previous history of schizophrenia or bipolar disorder) had a visit recorded at 43 healthcare organisations. Compared to the period examined in 2019, in 2020 there was a statistically significant higher proportion of people with dementia with a record of receiving antipsychotic medications 30 days before or on the date of their visit (16.4% (95%CI: 16.2%−16.5%, n = 27,050) vs. 14.7% (95%CI: 14.6%−14.9%, n = 31,963) (P < .0001)).
4. Discussion
4.1. Principal findings
By utilising a global health research network, this study suggests that for people with dementia and no recorded history of schizophrenia or bipolar disorder and receiving antipsychotics, COVID-19 associated with higher odds of 30-day thromboembolic events and all-cause mortality. This study also suggests that the proportion of people with dementia receiving antipsychotic medications increased during the COVID-19 pandemic compared to the proportion of people with dementia receiving antipsychotic medications in 2019.
High rates of death have been reported for people living with dementia during the COVID-19 pandemic, which may be in part due to a high prevalence of dementia amongst older people living in care homes [12], [13]. Increasing evidence from large-scale observational studies suggests people living with dementia are at increased risk of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and mortality with COVID-19 [14], [15]. A previous study which utilised the TriNetX global health research network also showed dementia to be a risk factor for mortality for older adults with COVID-19 [16]. Furthermore, within the TriNetX network, COVID-19 was associated with a 2–3-fold increase in the incidence of dementia compared to other health events, and a psychiatric diagnosis, including dementia, in the previous year was associated with a higher incidence of COVID-19 [17]. The findings of the current study suggest the use of antipsychotic medications for people with dementia and COVID-19 may be a contributing factor to high rates of mortality reported for these individuals. Furthermore, stratified by type of antipsychotic, the results indicated that the highest rates of 30-day all-cause mortality were seen in patients receiving haloperidol. However, the largest difference in all-cause mortality between patients with and without COVID-19 was for patients receiving risperidone (3.2% vs. 7.9%, OR 2.57, 95% CI: 1.71–3.86). Therefore, the potentially negative impact of COVID-19 for patients with dementia receiving antipsychotics may be more pronounced for patients receiving risperidone, although the mortality rate remained lower than for patients receiving haloperidol, quetiapine or olanzapine.
Previous evidence has suggested that the use of antipsychotic medications may worsen respiratory function and increase the risk of thromboembolism for people with COVID-19 [10]. To our knowledge, no previous study has examined associations between COVID-19 and outcomes specifically for people with dementia receiving antipsychotic medications. The findings of this study add to the evidence base to suggest further caution of the use of antipsychotic medications during the COVID-19 pandemic for people with dementia.
A previous study suggested there has been a reduction in the absolute number of patients registered with dementia in England during the pandemic, but an increase in the proportion of individuals with dementia receiving antipsychotic medications [7]. The findings of the current study are in line with the results of the previous study [7]. A reduction in the absolute number of people with dementia within the TriNetX global health research network was observed compared to the same time-period in 2019, and there was a significant increase in the proportion of individuals with dementia who received antipsychotic medications, from 14.7% to 16.4%. The underlying reasons for this could not be explored in the current study but may include an increase in behavioural and psychological symptoms for people with dementia in response to restrictions imposed by the COVID-19 pandemic. It has been estimated that prior to the COVID-19 pandemic, approximately one in five older adults living in care homes were prescribed antipsychotic medications in countries including the United States, the United Kingdom and Australia [18], [19], [20]. COVID-19 restrictions for older people living in the community and care homes have varied between countries and settings, but have included recommendations of shielding, cessation or reduction in family visits, changes in routine and activities, changes in availability of non-pharmacological approaches/therapies, and some people living in care homes have been asked to isolate in their bedrooms. Furthermore, a possible reason for prescribing antipsychotics during the COVID-19 pandemic is that patients may be at higher risk of developing delirium potentially due to infection with COVID-19 or environmental factors [21]. However, this could not be explored in the current study.
Although often referred to clinically as behavioural and psychological symptoms of dementia, behaviours such as agitation, depression and aggression experienced by people with dementia are often not triggered by underlying dementia pathology. Such behaviours are often in response to unmet needs which have resulted in discomfort, pain or distress for the individual [22]. Consensus of an expert panel identified caregiver training, environmental adaptations, person-centred care, and tailored activities as first-line approaches for behavioural and psychological symptoms of dementia overall and for agitation, prior to the use of pharmacological approaches. If pharmacological strategies are needed, citalopram and analgesic approaches were prioritised ahead of the use of antipsychotic medications [23]. Non-pharmacological approaches for people with dementia experiencing changes in behaviours should be considered after conducting a formal assessment to explore possible reasons for their distress and checking for clinical or environmental causes [4]. The Describe, Investigate, Create, Evaluate (DICE) approach can help with assessment and to create and implement a treatment plan [24]. Non-pharmacological approaches should be person-centred and a range of activities should be offered [4]. There is no formal evidence to show the extent to which such non-pharmacological approaches have been suspended or ceased during the COVID-19 pandemic. However, the restrictions imposed during the pandemic would suggest it would not be possible in many settings to carry out such activities to the same extent as before the COVID-19 pandemic.
These findings should encourage further research into the underlying reasons of an increase in the use of antipsychotic medications during the COVID-19 pandemic. Furthermore, strategies should be considered to improve implementation of non-pharmacological approaches both during the COVID-19 pandemic and more generally, for people with behavioural and psychological symptoms with dementia.
4.2. Limitations
The main limitation is that the data used in the study were from a research network which can only capture electronic medical records from participating healthcare organisations, and some health conditions may be underreported. Furthermore, the majority of people with dementia in this study had a history of more than one classification of dementia recorded. It is unclear if this is due to variation in the use of ICD-10-CM codes amongst different healthcare staff or if people were living with more than one type of underlying dementia pathology. Therefore, we did not further stratify analyses by type of dementia. The research network does not have information on the indication for medications, the dose or how long the person has received the medication, so this could not be explored. There was also no information collected on whether the participants were living in a care home so this could also not be considered in this study. Although the cohorts were propensity score matched for factors including age, sex, race, co-morbidities and antidepressant and anticonvulsant use, residual confounding may include socioeconomic factors, cognitive measures and behavioural measures which were not available, including global behaviour measures such as the Neuropsychiatric Inventory (NPI) or specific behaviour measures e.g. the Cohen Mansfield Agitation Inventory (CMAI). Within TriNetX, it is also not possible to determine the impact of attending different healthcare organisations due to data privacy restrictions. All deaths captured within the TriNetX network were included; however, deaths outside of the participating health care organisations are not well captured.
5. Conclusions
It is well-known that antipsychotic medications are associated with increased risk of severe adverse events for people living with dementia, but little research has previously focused on the use of antipsychotic medications for people with dementia and COVID-19. The results of this study indicate an association between COVID-19 and higher odds of thromboembolic events and all-cause mortality for people with dementia receiving antipsychotics. Therefore, the negative effects of antipsychotics in patients with dementia may be increased by concomitant COVID-19. In addition, this study also demonstrates there has been an increase in the proportion of people living with dementia receiving antipsychotic medications during the COVID-19 pandemic.
Availability of data and materials
To gain access to the data in the TriNetX research network, a request can be made to TriNetX (https://live.trinetx.com), but costs may be incurred, a data sharing agreement would be necessary, and no patient identifiable information can be obtained.
Funding
There was no specific funding received for the study. TriNetX LLC, US funded the acquisition of the data used.
CRediT authorship contribution statement
SLH and GYHL made a substantial contribution to the design of the work. PU made substantial contributions to the acquisition of the data. SLH analysed the data. SLH, BJRB, DAL and GYHL made a substantial contribution to the interpretation of the data. SLH drafted the work and all other co-authors revised it critically for important intellectual content. All authors read and approved the final manuscript.
Declaration of Competing Interest
Stephanie Harrison has received funding from Bristol Myers Squibb (BMS). Benjamin JR Buckley has received funding from BMS/Pfizer. Deirdre A Lane has received investigator-initiated educational grants from BMS, has been a speaker for Boehringer Ingeheim, and BMS/Pfizer and has consulted for BMS, Boehringer Ingelheim, and Daiichi-Sankyo. Paula Underhill is an employee of TriNetX LLC. Gregory Lip: consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon and Daiichi-Sankyo and speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo. No fees are directly received to Gregory Lip personally.
References
- 1.Nørgaard A., Jensen-Dahm C., Gasse C., Hansen E.S., Waldemar G. Psychotropic polypharmacy in patients with dementia: prevalence and predictors. J. Alzheimers Dis. 2017;56:707–716. doi: 10.3233/JAD-160828. [DOI] [PubMed] [Google Scholar]
- 2.Laver K., Cumming R.G., Dyer S.M., Agar M.R., Anstey K.J., Beattie E., Brodaty H., Broe T., Clemson L., Crotty M., Dietz M., Draper B.M., Flicker L., Friel M., Heuzenroeder L.M., Koch S., Kurrle S., Nay R., Pond C.D., Thompson J., Santalucia Y., Whitehead C., Yates M.W. Clinical practice guidelines for dementia in Australia. Med. J. Aust. 2016;204:191–193. doi: 10.5694/mja15.01339. [DOI] [PubMed] [Google Scholar]
- 3.Reus V.I., Fochtmann L.J., Eyler A.E., Hilty D.M., Horvitz-Lennon M., Jibson M.D., Lopez O.L., Mahoney J., Pasic J., Tan Z.S., Wills C.D., Rhoads R., Yager J. The American Psychiatric Association Practice Guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am. J. Psychiatry. 2016;173:543–546. doi: 10.1176/appi.ajp.2015.173501. [DOI] [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Excellence, Dementia: assessment, management and support for people living with dementia and their carers, (2018). [PubMed]
- 5.Papola D., Ostuzzi G., Gastaldon C., Morgano G.P., Dragioti E., Carvalho A.F., Fusar‐Poli P., Correll C.U., Solmi M., Barbui C. Antipsychotic use and risk of life-threatening medical events: umbrella review of observational studies. Acta Psychiatr. Scand. 2019;140:227–243. doi: 10.1111/acps.13066. [DOI] [PubMed] [Google Scholar]
- 6.Dorsey E.R., Rabbani A., Gallagher S.A., Conti R.M., Alexander G.C. Impact of FDA black box advisory on antipsychotic medication use. Arch. Intern. Med. 2010;170:96–103. doi: 10.1001/archinternmed.2009.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard R., Burns A., Schneider L. Antipsychotic prescribing to people with dementia during COVID-19. Lancet Neurol. 2020;19:892. doi: 10.1016/S1474-4422(20)30370-7. 892-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alzheimer's Disease, International. Alzheimer's Disease International, From plan to impact III maintaining dementia as a priority in unprecedented times, (2020).
- 9.Colling M.E., Kanthi Y. COVID-19-associated coagulopathy: an exploration of mechanisms. Vasc. Med. 2020;25:471–478. doi: 10.1177/1358863X20932640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostuzzi G., Papola D., Gastaldon C., Schoretsanitis G., Bertolini F., Amaddeo F., Cuomo A., Emsley R., Fagiolini A., Imperadore G., Kishimoto T., Michencigh G., Nosé M., Purgato M., Dursun S., Stubbs B., Taylor D., Thornicroft G., Ward P.B., Hiemke C., Correll C.U., Barbui C. Safety of psychotropic medications in people with COVID-19: evidence review and practical recommendations. BMC Med. 2020;18:215. doi: 10.1186/s12916-020-01685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alzheimer's Society, Dementia UK update, (2014).
- 13.Burki T. England and Wales see 20,000 excess deaths in care homes. Lancet. 2020;395 doi: 10.1016/S0140-6736(20)31199-5. (1602-1602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., Holden K.A., Read J.M., Dondelinger F., Carson G., Merson L., Lee J., Plotkin D., Sigfrid L., Halpin S., Jackson C., Gamble C., Horby P.W., Nguyen-Van-Tam J.S., Ho A., Russell C.D., Dunning J., Openshaw P.J., Baillie J.K., Semple M.G. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atkins J.L., Masoli J.A.H., Delgado J., Pilling L.C., Kuo C.L., Kuchel G.A., Melzer D. Preexisting comorbidities predicting COVID-19 and mortality in the UK biobank community cohort. J. Gerontol. Ser. A. 2020;75:2224–2230. doi: 10.1093/gerona/glaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison S.L., Fazio-Eynullayeva E., Lane D.A., Underhill P., Lip G.Y.H. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLOS Med. 2020;17 doi: 10.1371/journal.pmed.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2020 doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Department of Health and Human Services, Department of Health and Human Services, National partnership to improve dementia care in nursing homes, Centers for Medicare and Medicaid Services, (2016).
- 19.Szczepura A., Wild D., Khan A.J., Owen D.W., Palmer T., Muhammad T., Clark M.D., Bowman C. Antipsychotic prescribing in care homes before and after launch of a national dementia strategy: an observational study in English institutions over a 4-year period. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-009882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison S.L., Sluggett J.K., Lang C., Whitehead C., Crotty M., Corlis M., Wesselingh S.L., Inacio M.C. The dispensing of psychotropic medicines to older people before and after they enter residential aged care. Med. J. Aust. 2020;212:309–313. doi: 10.5694/mja2.50501. [DOI] [PubMed] [Google Scholar]
- 21.LaHue S.C., Douglas V.C., Miller B.L. The one-two punch of delirium and dementia during the COVID-19 pandemic and beyond. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.596218. 596218-596218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Algase D.L., Beck C., Kolanowski A., Whall A., Berent S., Richards K., Beattie E. Need-driven dementia-compromised behavior: an alternative view of disruptive behavior. Am. J. Alzheimer's Dis. 1996;11:10–19. [Google Scholar]
- 23.Kales H.C., Lyketsos C.G., Miller E.M., Ballard C. Management of behavioral and psychological symptoms in people with Alzheimer's disease: an international Delphi consensus. Int. Psychogeriatr. 2019;31:83–90. doi: 10.1017/S1041610218000534. [DOI] [PubMed] [Google Scholar]
- 24.Kales H.C., Gitlin L.N., Lyketsos C.G. Detroit expert panel on A, management of neuropsychiatric symptoms of D. management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J. Am. Geriatr. Soc. 2014;62:762–769. doi: 10.1111/jgs.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
To gain access to the data in the TriNetX research network, a request can be made to TriNetX (https://live.trinetx.com), but costs may be incurred, a data sharing agreement would be necessary, and no patient identifiable information can be obtained.