Abstract
Background
To assess the prevalence, severity, and mortality of COVID-19 in people with epilepsy (PWE) and evaluate seizure control in PWE during and after COVID-19.
Methods
Retrospective, observational, multicenter study conducted in 14 hospitals. Medical records of randomly selected PWE followed at neurology outpatient clinics were reviewed. Proportion of PWE with a positive test for SARS-CoV-2 during 2020 was calculated. Risk factors associated with COVID-19 and its morbimortality were evaluated.
Results
2751 PWE were included, mean age 48.8 years (18–99), 72.4% had focal epilepsy, and 35% were drug-refractory. COVID-19 prevalence in PWE was 5.53%, while in the Spanish population was 4.26%. Proportion of admissions to hospital, ICU, and deaths in PWE were 17.1%, 2%, and 4.61% of COVID-19 cases, while in Spanish population were 10.81%, 0.95%, and 2.57%, respectively. A severe form of COVID-19 occurred in 11.8%; dyslipidemia, institutionalization at long-term care facilities, intellectual disability, and older age were associated risk factors. Older age, hypertension, dyslipidemia, cardiac disease, and institutionalization were associated with mortality from COVID-19. Seizure control was stable in 90.1% of PWE during acute COVID-19, while 8.6% reported an increase in seizure frequency. During post-COVID-19 follow-up, 4.6% reported seizure control worsening.
Conclusions
COVID-19 was moderately prevalent in PWE. One out of 5 patients required medical attention and 4.6% died due to COVID-19. Older age, dyslipidemia, institutionalization, and intellectual disability were significant risk factors associated with severe COVID-19. Seizure control remained stable during COVID-19 and throughout long-term follow-up in most PWE who contracted the infection.
Keywords: COVID-19; Coronavirus, SARS-CoV-2; Prevalence; Epilepsy; Seizures
1. Introduction
The current coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has challenged the world. Spain is one of the most affected countries since the pandemic outbreak, with one of the highest prevalence and mortality rates worldwide [1]. The scientific community has made a huge effort to identify the population at higher risk of suffering COVID-19 or a severe form of the disease. It is well known that age is the main factor associated with death due to COVID-19, but other risk factors have been proposed in different studies, sometimes with contradictory results. Whether some chronic conditions, such as epilepsy, are related in some manner to SARS-CoV-2 infection remains unclear.
The influence of the COVID-19 pandemic on people with epilepsy (PWE) is beyond question. Many studies have shown that a significant proportion of PWE (ranging from 8 to 31% depending on the series) have suffered a worsening in their epilepsy control, having an increase of seizure frequency during the pandemic [2], [3], [4], [5], [6], [7], [8], [9], [10]. Whether seizures, anti-seizure treatments, or epilepsy itself may be associated with any risk of immunosuppression, and thereby increase the risk to be infected by SARS-CoV-2 or having severe COVID-19 is under discussion [11], [12]. Investigating this and assessing the specific morbidity and mortality of COVID-19 in PWE is of crucial importance to identify effective strategies to prevent infection and to reduce its impact. However, thus far, only a couple of studies have systematically evaluated the incidence, prevalence, and death rates of COVID-19 in patients with epilepsy [13], [14]. The evidence suggests that the risk of having COVID-19 was higher in PWE and that the case-fatality rate was higher in those patients with active epilepsy than in the rest of the hospitalized patients with severe COVID-19. Though of high relevance, these results studied relatively small samples and require further scientific support.
This study aimed to assess the prevalence of confirmed cases of SARS-CoV-2 infection in PWE. The severity of COVID-19, the associated morbidity and mortality in PWE, as well as the factors associated with the infection or its severity were also analyzed. Likewise, seizure control in PWE during and after COVID-19 infection was evaluated. The results were contrasted with the official data of people infected by SARS-CoV-2 in Spain during the study period, given by the Health Ministry's national records [15], [16].
2. Methods
2.1. Design and settings
A retrospective, observational, multicenter study was conducted in 14 hospitals distributed throughout the country. We included data between January 1, 2020, and December 31, 2020.
2.2. Study population
The study population were patients over 18 years old diagnosed with epilepsy according to the current criteria [17]. Patients were excluded if the diagnosis of epilepsy could not be confirmed and if there were any doubts between epilepsy or other nonepileptic paroxysmal disorders. Also, patients who met the criteria of resolved epilepsy [17] were excluded. In addition, patients who had died before March 2020 (when the COVID-19 pandemic reached Spain) were excluded. Subjects were selected randomly among the study population previously described. To obtain the randomized sample, each center collected all PWE followed up in their neurology outpatient clinics (N) and performed a random selection of N by creating a table of random numbers from 1 to N and selecting the first 200 numbers generated. Demographic and clinical characteristics of PWE were described: age, sex, intellectual disability, type of epilepsy (according to the current ILAE classifications of seizures and epilepsies [18], [19], etiology of epilepsy, active epilepsy [20], drug-resistant epilepsy, and history of epilepsy surgery.
2.3. Variables collected
The primary outcome of this study was the prevalence of SARS-CoV-2 infection in PWE in 2020, defined as the proportion of PWE with at least one positive test demonstrating the presence of SARS-CoV-2 in the nasopharynx (by real-time reverse transcription-polymerase chain reaction [rt-PCR] and/or antigenic detection test [AT]) or the presence of SARS-CoV-2 specific antibodies in serum (by positive Enzyme-Linked ImmunoSorbent Assay [ELISA] or rapid antibody test [RAbT]), among the total sample of PWE. A secondary outcome was the severity of COVID-19, classified as asymptomatic, mild, or severe infection according to the 2007 Infectious Diseases Society of America/American Thoracic Society criteria [21]. Admissions to the emergency department, hospital, or intensive care unit (ICU) and fatality rate (proportion of deaths related to COVID-19 among those infected) were also recorded. The risk of progression to severe COVID-19 was evaluated using the CALL score [22]. We evaluated several comorbidities of COVID-19 patients and their relationship with infection severity and death were explored. Finally, seizure control during and after COVID-19 infection was divided into worsening (increase of seizure frequency > 50% compared with the pre-COVID-19 period [mean per month during previous three months]), stability, or improvement (decrease of seizure frequency > 50% compared with the pre-COVID-19 period), considering the COVID-19 period as the first 14 days of the infection. All variables were collected by reviewing the hospital and primary care medical records and/or face-to-face or telephone consultation with the patients by neurologists and neurology residents.
The results were compared with the proportion of positive rt-PCR, AT, ELISA, and RAbT for SARS-CoV-2 within the national general population, published by the Spanish Health Ministry during the same period [15], [16]. Also, the proportion of admissions to hospital, to ICU, and proportion of deaths due to COVID-19 reported by the Ministry were compared with those found in our series during the same period [15].
2.4. Estimation of the sample size
The size of the sample was calculated based on a prior epidemiologic study in our country [13] that reported a cumulated incidence of COVID-19 of 0.0119 and 0.005 in PWE and general population, respectively. Assuming an alpha risk of 0.05, a power (beta) of 80%, and a percentage of data loss of 20%, the sample should be at least 2466 PWE. Assuming the possibility that the references used do not represent our population of PWE, we decided to increase the sample up to 2750 patients.
2.5. Statistical analysis
The statistical analysis was performed using Stata/IC 14.2 (StataCorp LLC). A descriptive analysis of the variables collected was performed: the qualitative variables were expressed as a percentage and the quantitative variables using mean ± standard deviation (SD) or median-interquartile range (IQR) whether they had a normal distribution. Prevalence of COVID-19 and mortality due to COVID-19 were calculated as the proportion of positive tests for SARS-CoV-2 and deaths due to COVID-19 among the total sample of PWE and were compared with the national prevalence and mortality during the same period. Similarly, admissions to hospital and ICU due to COVID-19 were calculated as the proportion of these events among the total sample of PWE and compared with the national data. An analysis of the main factors associated with contracting and dying from COVID-19 was performed: the ratios were compared using the X2 test or Fisher exact test, quantitative variables were compared using Student’s t test or the U-Mann–Whitney test when appropriate. Finally, univariate and multivariate analyses using logistic regression were performed following biological plausibility, based on current scientific knowledge regarding the etiology and mechanisms of COVID-19. The confidence intervals (CI) and odds ratios (OR) were calculated. In all cases, p-values less than 0.05 were considered statistically significant. To assess the fit of the logistic regression model we used the Hosmer-Lemeshow goodness-of-fit test.
2.6. Ethics
This study received approval from the ethical standards committee of the Complejo Hospitalario Universitario de Albacete (identifier: 2021/01/026). A waiver of written informed consent was obtained since all data were collected retrospectively and anonymously.
3. Results
3.1. Demographic characteristics of PWE
We included 2751 PWE from 14 hospitals across the country. The mean age was 48.8 years, range 18–99 (Fig. 1 ), 50.8% were men. According to epilepsy type, 71.5% had focal epilepsy, 23.1% generalized epilepsy (68.4% of whom had idiopathic generalized epilepsy) and 5.4% had unknown epilepsy. The most frequent known etiologies were vascular (11.2%), malformation of cortical development (7.2%), and tumoral (6.1%). The 66.8% had active epilepsy, 29.4% were drug-refractory, and 6.4% had a history of epilepsy surgery. More epidemiological details are shown in Table 1 .
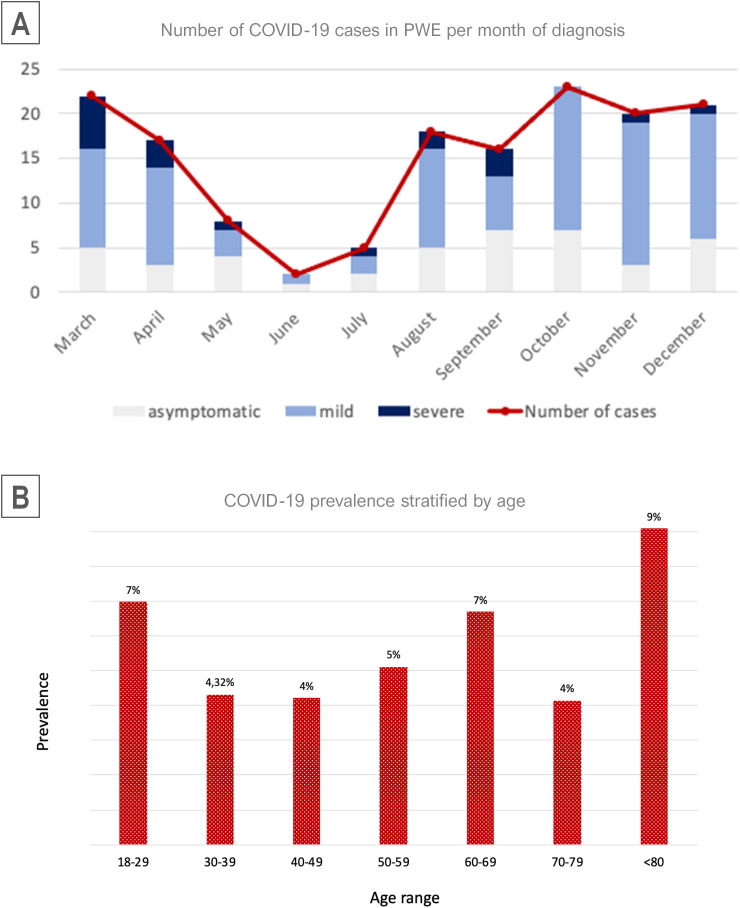
Fig. 1.
Cases of COVID-19 diagnosed per month and distribution per ages Legend: (A) Number of COVID-19 cases in PWE per month, divided per severity. The first wave (March to May) comprised most of the severe cases, but most cases were diagnosed during the second wave (October-December). (B) Distribution of cases per age. The proportion of COVID-19 cases over the total cases were divided per age.
Table 1.
Demographic characteristics of population of people with epilepsy.
| COVID-19 – n (%) | COVID-19 + n (%) | p value | ||
|---|---|---|---|---|
| Total | 2599 (94.47%) | 152 (5.53%) | ||
| Sex | Man | 1397 (50.8%) | 73 (48%) | 0.505 |
| Woman | 1354 (49.2%) | 79 (52%) | ||
| Age | Mean | 48.8 | 49.9 | 0.456 |
| SD | 18.8 | 20.7 | ||
| Range | 18–99 | 18–96 | ||
| Epilepsy type | Generalized | 636 (23.1%) | 35 (23%) | >0.999 |
| IGE with GTCS only | 259 (43.4%) | 10 (33.3%) | ||
| JME | 106 (18.7%) | 8 (26.7%) | ||
| Juvenile absence | 42 (7.4%) | 3 (10%) | ||
| Childhood absence | 19 (3.3%) | - | ||
| Epileptic encephalopathy | 30 (5.0%) | 3 (10%) | ||
| Other genetic generalized epilepsies# | 80 (13.4%) | 4 (13.3%) | ||
| Others | 50 (8.4%) | 2 (6.7%) | ||
| Focal | 1966 (71.5%) | 110 (72.4%) | 0.854 | |
| Frontal | 395 (20%) | 22 (19.8%) | ||
| Temporal | 734 (37.2%) | 32 (28.8%) | ||
| Posterior quadrant | 226 (11.5%) | 11 (9.9%) | ||
| Unknown | 617 (31.3%) | 46 (41.4%) | ||
| Unknown | 149 (5.5%) | 7 (4.6%) | 0.853 | |
| Etiology | Unknown | 950 (34.5%) | 62 (40.8%) | 0.096 |
| Genetic | 144 (5.2%) | 6 (3.9%) | 0.576 | |
| MCD | 198 (7.2%) | 7 (4.6%) | 0.257 | |
| Perinatal | 114 (4.1%) | 10 (6.6%) | 0.138 | |
| Vascular | 307 (11.2%) | 15 (9.9%) | 0.692 | |
| Infectious | 70 (2.5%) | 6 (3.9%) | 0.280 | |
| TBI | 125 (4.5%) | 3 (2%) | 0.157 | |
| Autoimmune | 35 (1.3%) | 3 (2%) | 0.440 | |
| Tumoral | 169 (6.1%) | 5 (3.3%) | 0.163 | |
| Others | 203 (7.4%) | 14 (9.2%) | 0.341 | |
| IGE | 435 (15.8%) | 21 (13.8%) | 0.568 | |
| Intellectual disability | No | 2354 (85.6%) | 121 (79.6%) | 0.042* |
| Yes | 397 (14.4%) | 31 (20.4%) | ||
| Active epilepsy | No | 871 (33.5%) | 42 (27.6%) | 0.156 |
| Yes | 1728 (66.5%) | 110 (72.4%) | ||
| Drug-refractory epilepsy | No | 1844 (70.9%) | 99 (65.1%) | 0.142 |
| Yes | 755 (29.1%) | 53 (34.9%) | ||
| Epilepsy surgery | No | 2433 (93.6%) | 142 (93.4%) | 0.865 |
| Yes | 166 (6.4%) | 10 (6.6%) |
Significant values were marked with *
COVID-19: coronavirus disease 2019. GTCS: generalized tonic-clonic seizure; IGE: idiopathic generalized epilepsy; JME: juvenile myoclonic epilepsy; MCD: malformation of cortical development; PWE: people with epilepsy; TBI: traumatic brain injury.
Including generalized epilepsies with genetic confirmation different than IGE and epileptic encephalopathies
3.2. COVID-19 prevalence and risk factors of infection
COVID-19 prevalence in our sample was 5.53% (95% CI 4.73–6.44), 152 out of 2751 PWE tested positive for SARS-CoV-2, with no reinfection cases (Fig. 2 ). Almost half of the patients (42.1%) tested positive during the second wave in Spain (October-December), compared to 30.9% during the first wave (March-May) (Fig. 1, Table 2 ). Seventy-two percent of COVID-19 patients had active epilepsy, almost half of them (48%) were taking 2 or more anti-seizure medications (ASMs) and 20.4% of patients infected had an intellectual disability. Patients with intellectual disability were more likely to contract COVID-19 (OR 1.56, 95% CI 1.04–2.35, p = 0.042). Sex, age, active epilepsy, drug-refractoriness, and etiology were not associated with a higher risk of being infected by the virus (Table 1). Comorbidities of COVID-19 patients are presented in Table 2. Globally, 62.5% of patients who contracted COVID-19 had some comorbidity other than epilepsy, being the neurological ones the most frequent (32.2%), followed by hypertension (25.7%) and dyslipidemia (19.7%).
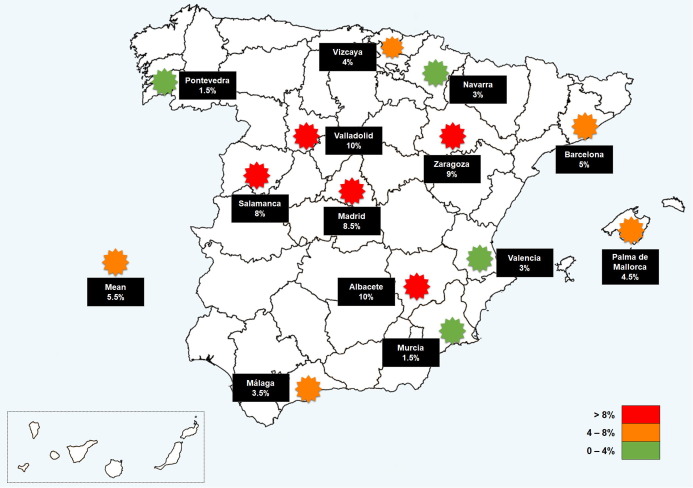
Fig. 2.
Prevalence of COVID-19 in PWE distributed per localities in Spain. Legend: Prevalence differences across localities ranged from 1.5% to 10.5%. The regions with higher prevalence were those located around the center of the country, similar to that observed in the general population of the country.
Table 2.
Characteristics of people with epilepsy with COVID-19 and infection course.
| Variables | COVID-19 + = 152 (%) | |
|---|---|---|
| Date infection | 1st wave (Mar-May) | 47 (30.9%) |
| Summer (Jun-Sep) | 41 (27.0%) | |
| 2nd wave (Oct-Dec) | 64 (42.1%) | |
| COVID-19 severity | Asymptomatic | 43 (28.3%) |
| Mild | 91 (59.9%) | |
| Severe | 18 (11.8%) | |
| Call score | Class A (4–6) | 28 (18.4%) |
| Class B (7–9) | 19 (12.5%) | |
| Class C (10–13) | 10 (6.6%) | |
| Not classifiable | 95 (62.5%) | |
| Health care required | Emergency department | 38 (25%) |
| Admission to hospital | 26 (17.1%) | |
| Intensive Care Unit | 3 (2%) | |
| Death due to COVID-19 | No | 145 (95.4%) |
| Yes | 7 (4.6%) | |
| N° ASM | 0–1 | 78 (51.3%) |
| 2–3 | 63 (41.4%) | |
| >3 | 11 (7.2%) | |
| Comorbidities | Hypertension | 39 (25.7%) |
| Diabetes mellitus | 15 (9.9%) | |
| Dyslipidaemia | 30 (19.7%) | |
| Cardiac | 16 (10.5%) | |
| Respiratory | 14 (9.2%) | |
| Autoimmune | 5 (3.3%) | |
| Haematological (non-neoplastic) | 6 (3.9%) | |
| Haematological (neoplasia) | 5 (3.3%) | |
| Active neoplasia | 2 (1.3%) | |
| Cured neoplasia | 5 (3.3%) | |
| Neurological (different from epilepsy) | 49 (32.2%) | |
| Institutionalized | No | 124 (81.6%) |
| Yes | 28 (18.4%) | |
| Seizure baseline | No seizures > 1 year | 76 (50%) |
| Yearly | 37 (24.3%) | |
| Monthly | 25 (16.4%) | |
| Weekly | 12 (7.9%) | |
| Daily | 2 (1.3%) | |
| Seizure control during COVID-19 | Better than baseline | 2 (1.3%) |
| Same as baseline | 137 (90.1%) | |
| Worse than baseline | 13 (8.6%) | |
| Seizure control posterior to COVID-19 | Better than baseline | 4 (2.6%) |
| Same as baseline | 141 (92.8%) | |
| Worse than baseline | 7 (4.6%) | |
ASM: anti-seizure medication; COVID-19: coronavirus disease 2019.
3.3. COVID-19 severity and mortality. Risk factors in PWE
According to COVID-19 severity, 11.8% had a severe disease, 59.9% had mild disease, and 28.3% were asymptomatic. Having a severe form of COVID-19 was more likely to occur with older age (OR 1.09, 95% CI 1.03–1.17, p = 0.005), intellectual disability (OR 10.11, 95% CI 1.24–82.14, p = 0.030), institutionalization at long-term care facilities (OR 19.49, 95% CI 2.95–128.92, p = 0.002), and dyslipidemia (OR 7.45, 95% CI 1.46–38.34, p = 0.016). Hypertension (4.53, 95% CI 1.65–12.51, p = 0.004), cardiac disorders (8.84, 95% CI 2.76–28.31, p < 0.0001), respiratory disorders (7.88, 95% CI 2.34–26.50, p = 0.001), and other neurological diseases (3.97, 95% CI 1.43–11, p = 0.008) were statistically significant in the univariate analysis but not in the multivariate one, as shown in Table 3 .
Table 3.
Univariate and multivariate analyses of risk factors for severe COVID-19 infection.
| Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|
| OR | CI | p | OR | CI | p | ||
| Age | 1.07 | 1.04 – 1.11 | <0.0001* | 1.09 | 1.03–1.17 | 0.005* | |
| Sex (male) | 0.91 | 0.34–2.44 | 0.858 | - | - | - | |
| Intellectual disability | 2.92 | 1.03–8.30 | 0.045* | 10.11 | 1.24–82.14 | 0.030* | |
| Epilepsy type | 2.16 | 0.92–5.08 | 0.076 | - | - | - | |
| Generalized | 0.93 | 0.29–3.09 | 0.931 | - | - | - | |
| Focal | 0.99 | 0.33–2.98 | 0.988 | - | - | - | |
| Unknown | 1.25 | 0.14–11.06 | 0.838 | - | - | - | |
| Etiology | Unknown | Reference | - | - | - | ||
| Genetic | 11.40 | 1.8–71.9 | 0.010* | 7.69 | 0.58–102.1 | 0.122 | |
| MCD | - | - | - | - | - | - | |
| Perinatal | 1.26 | 0.13–12.10 | 0.838 | - | - | - | |
| Vascular | 7.6 | 1.91–30.19 | 0.004* | 1.14 | 0.11–12.4 | 0.911 | |
| Infectious | 2.28 | 0.22–23.50 | 0.489 | - | - | - | |
| TBI | - | - | - | - | - | - | |
| Autoimmune | - | - | - | - | - | - | |
| Tumoral | - | - | - | - | - | - | |
| Others | 1.90 | 0.33–10.98 | 0.98 | - | - | - | |
| IGE | - | - | - | - | - | - | |
| Active epilepsy | 0.55 | 0.20–1.54 | 0.260 | - | - | - | |
| Drug-refractory epilepsy | 0.49 | 0.15–1.59 | 0.238 | - | - | - | |
| Institutionalization | 3.42 | 1.19–9.84 | 0.022* | 19.49 | 2.95–128.92 | 0.002* | |
| Hypertension | 4.53 | 1.65–12.51 | 0.004* | 0.29 | 0.05–1.86 | 0.194 | |
| Diabetes mellitus | 2.03 | 0.51–8.03 | 0.311 | - | - | - | |
| Dyslipidemia | 9.51 | 3.28–27.58 | <0.0001* | 7.45 | 1.46,38.34 | 0.016* | |
| Cardiac disorder | 8.84 | 2.76–28.31 | <0.0001* | 2.41 | 0.48–12.16 | 0.287 | |
| Respiratory disorder | 7.88 | 2.34–26.50 | 0.001* | 4.56 | 0.71–29.13 | 0.109 | |
| Autoimmune disorder | 1.91 | 0.20–18.11 | 0.572 | - | - | - | |
| Neurological comorbidity | 3.97 | 1.43–11.00 | 0.008* | 1.28 | 0.30–5.37 | 0.738 | |
Significant values were marked with *.
IGE: idiopathic generalized epilepsy; MCD: malformation of cortical development; TBI: traumatic brain injury.
Overall, 25% of PWE with COVID-19 consulted in an Emergency Department (ED), 17.1% were admitted to hospital, and only 2% were transferred to an ICU (Table 2). The case-fatality rate was 4.61% (95% CI 2.19–9.42): seven patients died from COVID-19. Older age (OR 1.21, 95% CI 1.06–1.38, p = 0.004), hypertension (OR 8.16, 95% CI 1.51–43.98, p = 0.015), dyslipidemia (OR 12, 95% CI 2.20–65.39, p = 0.004), cardiac disease (OR 7.61, 95% CI 1.53–37.78, p = 0.013), and institutionalization at long-term care facilities (OR 33, 95% CI 3.85–292.36, p = 0.001) were all associated with mortality attributable to COVID-19 in the univariate analysis (Table 4 ). No association was found between intellectual disability and mortality (p = 0.684). Multivariate analysis was not appropriate due to the low sample size for the number of variables to test. The mean age of deceased patients was 85.6 (SD 10.8) years, 6 of 7 patients were institutionalized in residencies. Noteworthy, 6 out of 7 patients were admitted to the hospital but none of them was transferred to the ICU. Mortality was higher among those patients who contracted COVID-19 during the first wave: 5 out of 7 deceased patients got infected from March to May 2020.
Table 4.
Univariate analysis of risk factors for death due to COVID-19.
| Univariate analysis |
|||
|---|---|---|---|
| OR | CI | p | |
| Age | 1.21 | 1.06–1.38 | 0.004* |
| Sex (male) | 0.35 | 0.07–1.88 | 0.223 |
| Intellectual disability | 0.64 | 0.07–5.51 | 0.684 |
| Epilepsy type | 1.04 | 0.25–5.73 | 0.82 |
| Etiology | 1.04 | 0.86–1.26 | 0.66 |
| Active epilepsy | 2.36 | 0.28–20.26 | 0.432 |
| Institutionalized | 33.54 | 3.84–292.36 | 0.001* |
| Hypertension | 8.16 | 1.51–43.98) | 0.015* |
| Diabetes mellitus | - | - | - |
| Dyslipidemia | 12 | 2.20–65.39 | 0.004* |
| Cardiac disorder | 7.61 | 1.53–37.78 | 0.013* |
| Respiratory disorder | 4.43 | 0.77–25.33 | 0.094 |
| Autoimmune disorder | - | - | - |
| Neurological comorbidity | 2.96 | 0.64–13.79 | 0.166 |
3.4. Seizure control during COVID-19 infection
According to control of epilepsy in patients with COVID-19, 47% were taking one ASM, 35% were drug-refractory. The most frequent ASMs used were levetiracetam (36.2%), valproate (26.3%), lacosamide (19.7%), and lamotrigine (19.1%). Basal seizure control in COVID-19 patients is described in Table 2. During the course of the infection, nearly all (90.1%) remained with the same seizure frequency, 13 patients (8.6%) had a worsening of their seizure frequency, 6 of whom returned to their baseline during the convalescence period (Table 2). Patients had a mean post-COVID-19 follow-up time of 4.6 months (SD 3.19 months) and no relevant change of seizure frequency was found.
3.5. Spanish demographics and COVID-19 epidemiological results
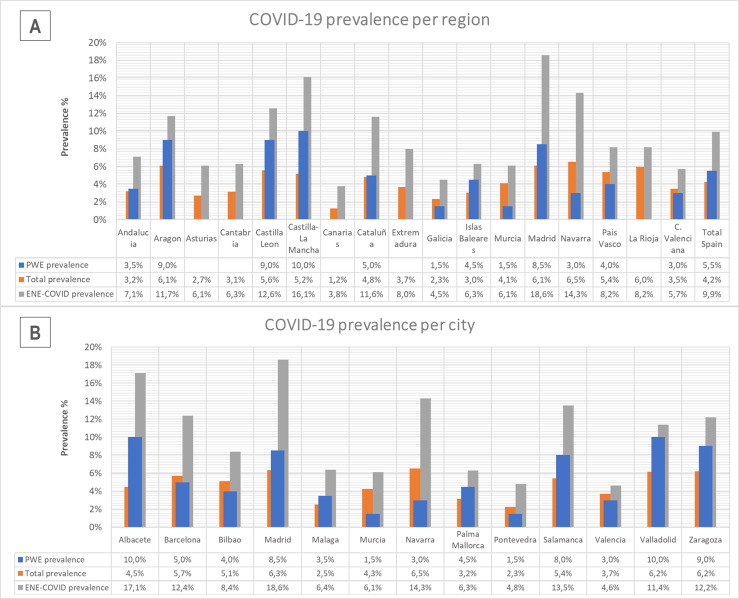
The total population in Spain in 2020 was 47.332.614 inhabitants [23]; the Spanish Health Ministry reported 2.028.472 cases of SARS-CoV-2 infection (almost all through rt-PCR and RAbT) during 2020 [15], with a prevalence of 4.26%. When considering only the population older than 19 years, in Spain there were 38.055.467 inhabitants in 2020, with a mean age of 51.2 years [23], and the Spanish Health Ministry reported 1.699.649 cases of SARS-CoV-2 infection during 2020 [15]. Therefore, 4.47% of the Spanish population above 19 years of age tested positive for SARS-CoV-2 during 2020. The comparison between the prevalence of each participating center and that of their respective city and region is represented in Fig. 3 . The proportion of admissions to hospital, ICU, and deaths because of COVID-19 in 2020 in Spain were 10.81%, 0.95%, and 2.57% of COVID-19 cases, respectively. Excluding people under 19 years of age, the proportion of hospitalizations due to COVID-19 increased to 12.76% of patients, 1.11% cases were admitted to ICU, and 3.06% of cases died during 2020 [15]. On the other side, the nationwide seroepidemiological study reported a prevalence of SARS-CoV-2 infection in the Spanish population of 9.9% of the sample studied as of December 15, 2020 [16]. Fig. 3 represents the reported prevalence per city and region.
Fig. 3.
Comparison of the prevalence of COVID-19 in PWE of the participant centers with that officially reported for their respective city and region. Legend: Prevalence of COVID-19 in PWE compared with that officially reported by their respective region (A) and city (B) [15]. Prevalence reported by the national seroprevalence study (ENE-COVID) [16] is also represented for comparison.* The prevalence for PWE of the current study is calculated as the number of cases in PWE over 18 years during 2020/total PWE over 18 years analyzed. The prevalence for each city (B) is calculated as the number of cases in population over 19 years during 2020/total population of the locality over 19 years on January 1st, 2020 [15], [23]. Prevalence for each region (A) is calculated as the total number of cases during 2020/total population of the region on January 1st, 2020 [15], [23] since the Spanish Health Ministry has not divided the regional data by age. For the study ENE-COVID, data are also for the total population studied, with no age division [16].
4. Discussion
4.1. Risk of COVID-19 in PWE
Controversy about whether PWE are at higher risk of suffering COVID-19 or a severe form of the infection has arisen since the COVID-19 outbreak [24], [25]. To date, only a few studies aimed to solve this issue [13], [14], [26], with contradictory results and some of them important biases. Our study presents data collected by accessing each patient's medical records, from a large, nation-wide, multicenter, randomly selected sample of PWE. COVID-19 prevalence in PWE was 5.53%, while in the Spanish population over 19 years old it was 4.47% (Fig. 3). As illustrated in Fig. 3, the prevalence of COVID-19 revealed by each participating center was rather similar to that officially reported for their respective local population. Also, the impact of the pandemic in our country varied markedly from one region to another, explaining the important prevalence differences across regions seen in our study, ranging from 1.5% to 10% (Fig. 2, Fig. 3). However, it should be noted that COVID-19 prevalence both in our study and in the Spanish population is far below the given by the national seroprevalence survey conducted by the Spanish government, where 9.9% of the population analyzed tested positive for SARS-CoV-2 antibodies on December 15, 2020 [16]. Therefore, in our population there are probably more cases of COVID-19 than diagnosed. Previous studies highlighted that PWE had limited access to health services or medication during pandemic period and that no testing was performed in most PWE who reported symptoms compatible with COVID-19 [2], [3], [4], [5], [10], [14], [27], [28], [29], making unlikely that the higher prevalence of COVID-19 in PWE was due to an easier access to health care resources of these patients during the pandemic because of their condition.
4.2. Immune response in PWE
In our cohort of PWE, SARS-CoV-2 infection was more likely for patients with active epilepsy (72.4% vs 66.5%) and intellectual disability (20.4% vs 14.4%), being significantly associated only with the latter one. So far, neurological conditions have not been independently associated with a predisposition to suffer COVID-19 [30]; however, there is evidence that the immune response can be impaired in people with neurological disorders. It is known that a direct immune modulation is exerted by the central nervous system (CNS) through the vagus-splenic reflex, referred to as the cholinergic anti-inflammatory pathway [31], [32]. Experimental studies demonstrated that brain lesions in some autonomic regulatory regions prevent immune activation depending on T-cell response [33], [34], supporting that parasympathetic and sympathetic drives mediate immune adaptative responses. Ictal and interictal autonomic disturbances can occur in PWE with a possible impact on immune response [35], [36], [37], [38]. Moreover, some data suggest that ASM can directly affect both humoral and cellular immunity, modifying the synthesis and expression of cytokines [12], but the mechanisms are not fully understood and other intercurrent factors may act as confounders.
4.3. Risk factors for severe COVID-19 and mortality in PWE
In our cohort of PWE, the proportion of patients who needed medical assistance because of COVID-19 was high. A large population-based study reported that epilepsy was a risk factor for hospital admission and mortality due to COVID-19 in adults [25]. Other studies reported a high proportion of severe COVID-19 courses and deaths caused by the infection in PWE [4], [13], [39], [40]. However, these studies were limited to institutionalized PWE or hospitalized patients during the first wave of the pandemic, so selection and severity biases may exist. The 11.9% of severe infections in PWE outlined in our study is close to the 10.2% found in a literature review [30], and clearly below the reported for cerebrovascular diseases (19.3%), dementias (22.2%), or multiple sclerosis (39.4%) [30]. It should be considered that epilepsy can have many causes and associations that can act as risk factors for developing severe respiratory or systemic infections. Some comorbidities such as older age, hypertension, diabetes, dyslipidemia, cardiac, and respiratory pathologies are demonstrated risk factors for severe COVID-19 [25], [41], although most of them are also risk factors for epilepsy [42]. It should be noted that the prevalence of these comorbidities was not known in the national population, hindering the comparison. On the other hand, intellectual disability acted as a risk factor for contracting COVID-19 and for having a severe course of the disease, though it was not associated with higher mortality. Epidemiological evaluations of long-term care facilities for PWE revealed a COVID-19 prevalence of 10% to 63% of their residents, the majority of whom had intellectual disabilities [39], [40]. However, this higher prevalence of COVID-19 is probably related to the facility of the virus to spread among the institution residents rather than with their cognitive comorbidities. Mortality due to COVID-19 in our study affected mainly elderly patients, institutionalized in nursing homes, and with other comorbidities, so that epilepsy did not appear to be the main risk factor for death.
4.4. Influence of COVID-19 infection in epilepsy control
Neurological symptoms are usual during the COVID-19 course [43], [44]. However, although electroencephalographic abnormalities have been reported in high percentages of patients with SARS-CoV-2 infection [45], [46], seizures are very infrequent in these cases [43], [44], [47], [48], [49]. Besides that, during the first months of the pandemic, seizure control has worsened in a significant proportion of PWE [2], [3], [4], [5], [6], [7], [8], [9], [10]. In most cases, this decompensation was not directly related to SARS-CoV-2 infection, but attributable to different pandemic-related factors such as psychological distress, sleep disorders, or difficulties obtaining ASM. Our study evaluated the seizure control of a broad cohort of PWE during the acute phase of COVID-19, and the results point in the same direction, with a few (8.6%) of our COVID-19 patients reporting a significant worsening of their seizures during the infection. Therefore, SARS-CoV-2 infection has little impact on seizure control in PWE, which is striking, since fever and systemic infections are well-known seizure triggers [50]. Also valuable, we conducted a long-term post-infection follow-up in a large number of patients. Indeed, half of the patients in whom seizure control worsened during COVID-19 returned to baseline at the convalescence phase, only 4.6% maintained an increase in seizure frequency after the infection, and when considering a long-term follow-up, the vast majority of our cohort were stable in their epilepsy control.
4.5. Study limitations
Despite the high number of subjects included in the study and the adequate potency in order to calculate COVID-19 prevalence in PWE, this is a retrospective study and the association of the infection and its course with the analyzed risk factors must be treated with caution, since direct causality cannot be properly inferred. In addition, other clinical or socio-demographic factors not assessed in the study may have influenced the risk of contracting COVID-19 or determining its morbimortality. The circumstances of the infection could not be investigated, and seizure count was based in many cases on patients’ subjective recall of the infection period. Finally, socio-demographic characteristics of the reference group could not be evaluated, limiting the comparison of these data with our results.
5. Conclusion
COVID-19 was moderately prevalent in PWE. One out of 5 patients required medical attention and 4.6% died due to COVID-19. Older age, dyslipidemia, intellectual disability, and institutionalization at long-term care facilities, were significant risk factors associated with severe COVID-19, and for mortality, older age, hypertension, dyslipidemia, cardiac disease, and institutionalization at long-term care facilities were significantly associated. Seizure control remained stable on most of the PWE who contracted COVID-19 both during the acute phase and throughout long-term follow-up after the infection, suggesting that SARS-CoV-2 infection has little impact on seizure control in PWE.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
In memory of all the people in our country who have suffered the consequences of COVID-19.
References
- 1.Johns Hopkins University and Medicine. COVID-19 Map. Johns Hopkins Coronavirus Resource Centre, https://coronavirus.jhu.edu/map.html [accessed 20 February 2021].
- 2.Aledo‐Serrano Á., Mingorance A., Jiménez‐Huete A., Toledano R., García‐Morales I., Anciones C., et al. Genetic epilepsies and COVID-19 pandemic: lessons from the caregiver perspective. Epilepsia. 2020;61(6):1312–1314. doi: 10.1111/epi.v61.610.1111/epi.16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conde Blanco E., Manzanares I., Centeno M., Khawaja M., Betrán O., Donaire A., et al. Epilepsy and lockdown: a survey of patients normally attending a Spanish centre. Acta Neurol Scand. 2021;143(2):206–209. doi: 10.1111/ane.v143.210.1111/ane.13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonseca E., Quintana M., Lallana S., Luis Restrepo J., Abraira L., Santamarina E., et al. Epilepsy in time of COVID-19: a survey-based study. Acta Neurol Scand. 2020;142(6):545–554. doi: 10.1111/ane.v142.610.1111/ane.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez-Larsen A., Gonzalez-Villar E., Díaz-Maroto I., Layos-Romero A., Martínez-Martín Á., Alcahut-Rodriguez C., et al. Influence of the COVID-19 outbreak in people with epilepsy: analysis of a Spanish population (EPICOVID registry) Epilepsy Behav. 2020;112:107396. doi: 10.1016/j.yebeh.2020.107396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alkhotani A., Siddiqui M.I., Almuntashri F., Baothman R. The effect of COVID-19 pandemic on seizure control and self-reported stress on patient with epilepsy. Epilepsy Behav. 2020;112:107323. doi: 10.1016/j.yebeh.2020.107323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assenza G., Lanzone J., Brigo F., Coppola A., Di Gennaro G., Di Lazzaro V., et al. Epilepsy Care in the Time of COVID-19 Pandemic in Italy: Risk Factors for Seizure Worsening. Front Neurol. 2020;11 doi: 10.3389/fneur.2020.0073710.3389/fneur.2020.00737.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang S., Wu C., Jia Y., Li G., Zhu Z., Lu K., et al. COVID-19 outbreak: The impact of stress on seizures in patients with epilepsy. Epilepsia. 2020;61(9):1884–1893. doi: 10.1111/epi.v61.910.1111/epi.16635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puteikis K., Jasionis A., Mameniškienė R. Recalling the COVID-19 lockdown: Insights from patients with epilepsy. Epilepsy Behav. 2021;115:107573. doi: 10.1016/j.yebeh.2020.107573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosengard J.L., Donato J., Ferastraoaru V., Zhao D., Molinero I., Boro A., et al. Seizure control, stress, and access to care during the COVID-19 pandemic in New York City: the patient perspective. Epilepsia. 2021;62(1):41–50. doi: 10.1111/epi.v62.110.1111/epi.16779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aronica E., Crino P.B. Inflammation in epilepsy: clinical observations. Epilepsia. 2011;52(Suppl 3):26–32. doi: 10.1111/j.1528-1167.2011.03033.x. [DOI] [PubMed] [Google Scholar]
- 12.Beghi E., Shorvon S. Antiepileptic drugs and the immune system. Epilepsia. 2011;52(Suppl 3):40–44. doi: 10.1111/j.1528-1167.2011.03035.x. [DOI] [PubMed] [Google Scholar]
- 13.Cabezudo-García P., Ciano-Petersen N.L., Mena-Vázquez N., Pons-Pons G., Castro-Sánchez M.V., Serrano-Castro P.J. Incidence and case fatality rate of COVID-19 in patients with active epilepsy. Neurology. 2020;95(10):e1417–e1425. doi: 10.1212/WNL.0000000000010033. [DOI] [PubMed] [Google Scholar]
- 14.Bosak M., Mazurkiewicz I., Wężyk K., Słowik A., Turaj W. COVID-19 among patients with epilepsy: risk factors and course of the disease. Epilepsy Behav. 2021;120:107996. doi: 10.1016/j.yebeh.2021.107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Documentación y datos Centro Nacional de Epidemiología de España, https://cnecovid.isciii.es/covid19/#documentaci%C3%B3n-y-datos. [accessed 20 February 2021].
- 16.Estudio Nacional de sero-Epidemiología de la Infección por SARS-CoV-2 en España (ENE-Covid), https://www.mscbs.gob.es/gabinetePrensa/notaPrensa/pdf/15.12151220163348113.pdf [accessed 15 February 2021].
- 17.Fisher R.S., Acevedo C., Arzimanoglou A., Bogacz A., Cross J.H., Elger C.E., et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 18.Scheffer I.E., Berkovic S., Capovilla G., Connolly M.B., French J., Guilhoto L., et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher R.S., Cross J.H., French J.A., Higurashi N., Hirsch E., Jansen F.E., et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522–530. doi: 10.1111/epi.2017.58.issue-410.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- 20.Forsgren L., Beghi E., Oun A., Sillanpaa M. The epidemiology of epilepsy in Europe - a systematic review. Eur J Neurol. 2005;12(4):245–253. doi: 10.1111/ene.2005.12.issue-410.1111/j.1468-1331.2004.00992.x. [DOI] [PubMed] [Google Scholar]
- 21.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al., Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27-72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed]
- 22.Ji D, Zhang D, Xu J, Chen Z, Yang T, Zhao P, et al., Prediction for Progression Risk in Patients With COVID-19 Pneumonia: The CALL Score. Clin Infect Dis. 2020;71:1393-9. doi: 10.1093/cid/ciaa414. [DOI] [PMC free article] [PubMed]
- 23.National Statistics Institute. Spanish Statistical Office: population in Spain as of January 1, 2020, https://www.ine.es/dyngs/INEbase/en/operacion.htm?c=Estadistica_C&cid=1254736176951&menu=ultiDatos&idp=1254735572981 [accessed 20 February 2021].
- 24.Kuroda N. Epilepsy and COVID-19: updated evidence and narrative review. Epilepsy Behav. 2021;116:107785. doi: 10.1016/j.yebeh.2021.107785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clift AK, Coupland CAC, Keogh RH, et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371:m3731. doi: 10.1136/bmj.m3731. [DOI] [PMC free article] [PubMed]
- 26.Asadi-Pooya A.A., Shahisavandi M., Sadeghian S., Nezafat A., Nabavizadeh S.A., Barzegar Z. Is the risk of COVID-19 contraction increased in patients with epilepsy? Epilepsy Behav. 2021;115:107734. doi: 10.1016/j.yebeh.2020.107734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guilhoto L.M., Mosini A.C., Susemihl M.A., Pinto L.F. COVID-19 and epilepsy: how are people with epilepsy in Brazil? Epilepsy Behav. 2021;122:108115. doi: 10.1016/j.yebeh.2021.108115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asadi‐Pooya A.A., Farazdaghi M., Bazrafshan M. Impacts of the COVID-19 pandemic on Iranian patients with epilepsy. Acta Neurol Scand. 2020;142(4):392–395. doi: 10.1111/ane.v142.410.1111/ane.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorpe J., Ashby S., Hallab A., Ding D., Andraus M., Dugan P., et al. Evaluating risk to people with epilepsy during the COVID-19 pandemic: Preliminary findings from the COV-E study. Epilepsy Behav. 2021;115:107658. doi: 10.1016/j.yebeh.2020.107658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubota T., Kuroda N. Exacerbation of neurological symptoms and COVID-19 severity in patients with preexisting neurological disorders and COVID-19: a systematic review. Clin Neurol Neurosurg. 2021;200:106349. doi: 10.1016/j.clineuro.2020.106349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borovikova L.V., Ivanova S., Zhang M., Yang H., Botchkina G.I., Watkins L.R., et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 32.Carnevale D., Lembo G. Heart, Spleen, Brain. Circulation. 2018;138(18):1917–1919. doi: 10.1161/CIRCULATIONAHA.118.035628. [DOI] [PubMed] [Google Scholar]
- 33.Harrison DG. The immune system in hypertension. Trans Am Clin Climatol Assoc. 2014;125:130-38; discussion 138-40. [PMC free article] [PubMed]
- 34.Zubcevic J., Santisteban M.M., Perez P.D., Arocha R., Hiller H., Malphurs W.L., et al. A single angiotensin II hypertensive stimulus is associated with prolonged neuronal and immune system activation in Wistar-Kyoto rats. Front Physiol. 2017;8 doi: 10.3389/fphys.2017.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazzola L, Rheims S. Ictal and Interictal Cardiac Manifestations in Epilepsy. A Review of Their Relation With an Altered Central Control of Autonomic Functions and With the Risk of SUDEP. Front Neurol. 2021;12:642645. doi: 10.3389/fneur.2021.642645. [DOI] [PMC free article] [PubMed]
- 36.Benarroch E.E. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68(10):988–1001. doi: 10.1016/S0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- 37.Cechetto D.F. Cortical control of the autonomic nervous system. Exp Physiol. 2014;99(2):326–331. doi: 10.1113/eph.2014.99.issue-210.1113/expphysiol.2013.075192. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez‐Larsen A., Principe A., Ley M., Navarro‐Cuartero J., Rocamora R. Characterization of the insular role in cardiac function through intracranial electrical stimulation of the human insula. Ann Neurol. 2021;89(6):1172–1180. doi: 10.1002/ana.v89.610.1002/ana.26074. [DOI] [PubMed] [Google Scholar]
- 39.Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R., et al. Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. N Engl J Med. 2020;382(22):2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balestrini S., Koepp M.J., Gandhi S., Rickman H.M., Shin G.Y., Houlihan C.F., et al. Clinical outcomes of COVID-19 in long-term care facilities for people with epilepsy. Epilepsy Behav. 2021;115:107602. doi: 10.1016/j.yebeh.2020.107602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson E.L., Krauss G.L., Lee A.K., Schneider A.L.C., Dearborn J.L., Kucharska-Newton A.M., et al. Association between midlife risk factors and late-onset epilepsy: results from the atherosclerosis risk in communities study. JAMA Neurol. 2018;75(11):1375. doi: 10.1001/jamaneurol.2018.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á., Layos-Romero A., García-García J., et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95(8):e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mao L., Jin H., Wang M., Hu Y.u., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antony A.R., Haneef Z. Systematic review of EEG findings in 617 patients diagnosed with COVID-19. Seizure. 2020;83:234–241. doi: 10.1016/j.seizure.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asadi-Pooya A.A., Simani L., Shahisavandi M., Barzegar Z. COVID-19, de novo seizures, and epilepsy: a systematic review. Neurol Sci. 2021;42(2):415–431. doi: 10.1007/s10072-020-04932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu L.u., Xiong W., Liu D., Liu J., Yang D., Li N., et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia. 2020;61(6) doi: 10.1111/epi.v61.610.1111/epi.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cagnazzo F., Arquizan C., Derraz I., Dargazanli C., Lefevre P.-H., Riquelme C., et al. Neurological manifestations of patients infected with the SARS-CoV-2: a systematic review of the literature. J Neurol. 2021;268(8):2656–2665. doi: 10.1007/s00415-020-10285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nalleballe K., Reddy Onteddu S., Sharma R., Dandu V., Brown A., Jasti M., et al. Spectrum of neuropsychiatric manifestations in COVID-19. Brain Behav Immun. 2020;88:71–74. doi: 10.1016/j.bbi.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frucht M.M., Quigg M., Schwaner C., Fountain N.B. Distribution of seizure precipitants among epilepsy syndromes. Epilepsia. 2000;41:1534–1539. doi: 10.1111/j.1499-1654.2000.001534.x. [DOI] [PubMed] [Google Scholar]