Abstract
Background
Streptococcus pneumoniae isolated from patients with invasive pneumococcal disease has been subjected to laboratory-based surveillance in Latin American and Caribbean countries since 1993. Invasive pneumococcal diseases remain a major cause of death and disability worldwide, particularly in children. We therefore aimed to assess the direct effect of pneumococcal conjugate vaccines (PCVs) on the distribution of pneumococcal serotypes causing invasive pneumococcal disease in children younger than 5 years before and after PCV introduction.
Methods
We did a multicentre, retrospective observational study in eight countries that had introduced PCV (ie, PCV countries) in the Latin American and Caribbean region: Argentina, Brazil, Chile, Colombia, Dominican Republic, Mexico, Paraguay, and Uruguay. Cuba and Venezuela were also included as non-PCV countries. Isolate data for Streptococcus pneumoniae were obtained between 2006 and 2017 from children younger than 5 years with an invasive pneumococcal disease from local laboratories or hospitals. Species' confirmation and capsular serotyping were done by the respective national reference laboratories. Databases from the Sistema Regional de Vacunas (SIREVA) participating countries were managed and cleaned in a unified database using Microsoft Excel 2016 and the program R (version 3.6.1). Analysis involved percentage change in vaccine serotypes between pre-PCV and post-PCV periods and the annual reporting rate of invasive pneumococcal diseases per 100 000 children younger than 5 years, which was used as a population reference to calculate percentage vaccine type reduction.
Findings
Between 2006 and 2017, 12 269 isolates of invasive pneumococcal disease were collected from children younger than 5 years in the ten Latin American and Caribbean countries. The ten serotypes included in ten-valent pneumococcal conjugate vaccine (PCV10) decreased significantly (p<0·0001) after any PCV introduction, except for the Dominican Republic. The percentage change for the ten vaccine serotypes in PCV10 countries was −91·6% in Brazil (530 [72·9%] of 727 before, 27 [6·1%] of 441 after); −85·0% in Chile (613 [72·6%] of 844 before, 44 [10·9%] of 404] after); −84·7% in Colombia (231 [63·1%] of 366 before, 34 [9·7%] of 352 after); and −73·8% in Paraguay (127 [77·0%] of 165 before, 22 [20·2%] of 109 after). In the 13-valent pneumococcal conjugate vaccine (PCV13) countries, the percentage change for the 13 vaccine serotypes was −59·6% in Argentina (853 [85·0%] of 1003 before, 149 [34·3%] of 434 after); −16·5% in the Dominican Republic (95 [80·5%] of 118 before, 39 [67·2%] of 58 after); −43·7% in Mexico (202 [73·2%] of 276 before, 63 [41·2%] of 153 after); and −45·9% in Uruguay (138 [80·7%] of 171 before, 38 [43·7%] of 87 after). Annual reporting rates showed a reduction from −82·5% (6·21 before vs 1·09 after per 100 000, 95% CI −61·6 to −92·0) to −94·7% (1·15 vs 0·06 per 100 000, −89·7 to −97·3) for PCV10 countries, and −58·8% (2·98 vs 1·23 per 100 000, −21·4 to −78·4) to −82·9% (7·80 vs 1·33 per 100 000, −76·9 to −87·4) for PCV13 countries. An increase in the amount of non-vaccine types was observed in the eight countries after PCV introduction together with an increase in their percentage in relation to total invasive strains in the post-PCV period.
Interpretation
SIREVA laboratory surveillance was able to confirm the effect of PCV vaccine on serotypes causing invasive pneumococcal disease in the eight PCV countries. Improved monitoring of the effect and trends in vaccine type as well as in non-vaccine type isolates is needed, as this information will be relevant for future decisions associated with new PCVs.
Funding
None.
Translations
For the Portuguese and Spanish translations of the abstract see Supplementary Materials section.
Research in context.
Evidence before this study
The Sistema Regional de Vacunas (SIREVA) was instituted in the Latin American region in 1993 by the Pan American Health Organization and WHO for the development of an epidemiological and laboratory surveillance system aimed at determining the relative prevalence of Streptococcus pneumoniae capsular types and antimicrobial susceptibility causing invasive disease, especially pneumonia, in children younger than 6 years. Argentina, Brazil, Chile, Colombia, Mexico, and Uruguay were initially involved in the project and were joined later by Cuba, Paraguay, the Dominican Republic, and Venezuela. Data generated from the national reference laboratories showed that 13 capsular types accounted for more than 85% of the isolates (serotypes 14, 6A/6B, 5, 1, 23F, 19F, 18C, 19A, 9V, 7F, 3, and 9N). Most importantly such data highlighted the need for including serotypes 1 and 5 in the pneumococcal conjugate vaccine (PCV) formulations. The potential benefit of PCV developed or in development at the time against invasive pneumococcal diseases in children younger than 2 years was estimated as 64·2% coverage for the seven-valent pneumococcal conjugate vaccine (PCV7), 80·4% for the ten-valent pneumococcal conjugate vaccine (PCV10), and 89·5% for the 13-valent pneumococcal conjugate vaccine (PCV13). With this information, countries were able to make decisions regarding the introduction of PCV vaccines in their expanded programme on immunisation and by 2012 most of the countries in the region of the Americas had introduced either PCV10 or PCV13. Ongoing surveillance is now able to provide data for describing the changes resulting from PCV vaccination.
We searched Scopus using the search terms “streptococcus pneumoniae” OR “pneumococc*” AND “serotype”, and further selected for “laboratory” AND “surveillance” for all the Latin American countries for the period Jan 1, 2009, to Aug 31, 2019. We only selected those papers published in English, Spanish, or Portuguese. Selection was continued using the keywords “pneumococcus vaccine”, “pneumococcal vaccines”, “10-valent pneumococcal conjugate vaccine”, “heptavalent pneumococcal conjugate vaccine”, OR “13-valent pneumococcal vaccine”. Our search gave 38 publications that were analysed and 20 were selected related to observational studies and population-based studies that compared trends in pneumococcal vaccine types and non-vaccine types before and after the introduction of PCV; they were from Brazil (n=11), Venezuela (n=2), Peru (n=2), Uruguay (n=2), Argentina (n=1), Mexico (n=1), and the Latin American and Caribbean region (n=1).
Added value of this study
Several countries in the Latin American and Caribbean region have documented the effect of PCV following its introduction showing a reduction in vaccine type serotypes and an increase in non-vaccine types. We show the impact of the introduction of two PCVs in selected countries in Latin America using SIREVA's laboratory-based surveillance data. Although new vaccines incorporating other serotypes are being developed and are undergoing clinical trials, the pertinent regional data being produced will be useful in determining whether new formulations might be suitable for the region. So far, the results from this study have indicated that there is no common geographical pattern and that other serotypes not included in the new vaccines might be of regional importance.
Implications of all the available evidence
This study has shown that data from passive, laboratory-based surveillance can provide pertinent results for decision makers if basic quality criteria are followed and that there is a supporting network of intra-country and inter-country laboratories working with similar standards, protocols, and criteria.
Introduction
Invasive pneumococcal diseases are a major cause of death and disability worldwide, mainly in children and older people.1 The ten-valent pneumococcal conjugate vaccine (PCV10) and 13-valent pneumococcal conjugate vaccine (PCV13) are currently the most important public health tools for preventing invasive pneumococcal diseases.2, 3 Ascertaining local epidemiology of invasive pneumococcal diseases before and after the introduction of the pneumococcal conjugate vaccines (PCVs) is therefore crucial in understanding the epidemiological relevance and impact of PCV, since there are geographical and age-related differences, as well as temporal variations in the prevalence of disease-causing serotypes.4 Such understanding has been provided by Streptococcus pneumoniae surveillance studies. The Sistema Regional de Vacunas (SIREVA) has been one of the most successful laboratory-based surveillance programmes in Latin American and Caribbean countries; it has been encouraged by the Pan American Health Organization (PAHO) and supported by the ministries of health of participating countries since 1993. SIREVA has been described as a multicentre, multicountry, passive laboratory surveillance network for monitoring the prevalence of relative pneumococcal serotypes causing invasive pneumococcal diseases in children younger than 5 years. The surveillance programme was aimed at providing reliable data for PCV formulation for the region, thereby facilitating decisions for introducing the vaccine and monitoring changes after the intervention.5
National reference laboratories in Argentina, Brazil, Chile, Colombia, Mexico, and Uruguay published data from 1993 to 1999 showing that 13 capsular types (14, 6A/6B, 5, 1, 23F, 19F, 18C, 19A, 9V, 7F, 3, and 9N) accounted for 86·1% of the isolates causing invasive pneumococcal diseases in children younger than 6 years.6 Ten national reference laboratories (the initial six laboratories, plus Cuba, Paraguay, the Dominican Republic, and Venezuela) updated data from 2000 to 2005, which identified the same 13 serotypes accounting for 86·0% of all isolates.7 The potential benefit of PCV against invasive pneumococcal diseases in children younger than 2 years was estimated at 64·2% for the seven-valent pneumococcal conjugate vaccine (PCV7), 80·4% for PCV10, and 89·5% for PCV13 serotypes. SIREVA data also highlighted the importance of including serotypes 1 and 5 in PCV formulations.6, 7
PCV started to be introduced as early as 2008 and the eight countries considered in this study were using these vaccines in their expanded programme on immunisation by 2013 (appendix 3 pp 4–8).8 In this study, we aimed to establish the direct effect of PCV in these countries by analysing the data regarding the serotypes causing invasive pneumococcal diseases in children younger than 5 years before and after introducing the vaccine.
Methods
Participating countries and national reference laboratories
We did a multicentre, retrospective observational study. The description of participating centres has already been reported.6, 7 Countries that had introduced PCV,8 having better surveillance systems, and a convenience value of invasive pneumococcal isolates (400) collected between 2006 and 2017 were included in the analysis. These countries were Argentina, Brazil, Chile, Colombia, the Dominican Republic, Mexico, Paraguay, and Uruguay. As Cuba and Venezuela had not introduced any PCV during the study period they were included as non-PCV countries.9
Procedures
Isolates were obtained from normally sterile body sites from children younger than 5 years with invasive pneumococcal disease (pneumonia, meningitis, bacteraemia, or another invasive pneumococcal disease), following the WHO clinical criteria.9 S pneumoniae isolates identified in local laboratories or hospitals were voluntarily referred to the national reference laboratories in each country.6, 7 Only one isolate per patient was included in the study. In cases where S pneumoniae was recovered simultaneously from cerebrospinal fluid (CSF) and blood, or pleural fluid and blood, the isolates from CSF in the first case and blood in the second case were considered.
The national reference laboratories did species' confirmation and capsular serotyping using a common protocol that had already been validated by an external quality assurance programme.6, 7, 9 The Quellung reaction for serotyping involved using anti-sera from the Statens Serum Institute (Copenhagen, Denmark). Non-typable strains from Argentina and Brazil were confirmed by PCR.10 Demographic data and clinical diagnoses concerning each patient were collected. PCV10 contains serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F while PCV13 includes three additional serotypes (3, 6A, and 19A). All of them are named vaccine types (eg, PCV10 is named vaccine type ten [VT10], and PCV13 is named vaccine type 13 [VT13]); serotypes not included in PCV10 and PCV13 are named non-vaccine types.
The participating countries' ministries of health were aware of SIREVA's work. Collection of the isolates and information was considered as surveillance activity and was obtained with the approval of each participating hospital. The data and isolates could not be traced back to the source.
Statistical analysis
Databases from SIREVA participating countries were managed and cleaned in a unified database using Microsoft Excel 2016 and the program R (version 3.6.1). Variables were described using percentages, depending on the variable type (discrete or continuous). The CI for evaluating statistical significance was set at 95%. Annual serotype percentages were compared using the χ2 test for percentage trend with 95% CI or Fisher's exact test when small samples precluded using the χ2 test.11 Serotype temporal trend p values could be interpreted as trend variations regarding percentages and absolute numbers during the 2006–17 study period.
For the impact analysis, two periods were used, a pre-PCV period (0, −1, −2), and a post-PCV period (the last 3 years, 2015–17) considering year zero as the year when PCV was introduced into each country. For countries that changed PCV during the study years (Mexico and Uruguay), year zero was considered as the introduction of PCV13 (appendix 3 pp 4–8).
Two impact analyses were done, one involving percentage change, and the other, annual reporting rate of invasive pneumococcal diseases. Percentage change comprised changes in serotype distribution before and after PCV introduction, calculated according to the following formula:12
A positive change indicated an increase in the percentage of the serotype, whereas a negative change indicated a reduction, comparing the post-PCV period with the pre-PCV period.
The annual reporting rate of invasive pneumococcal diseases was per 100 000 children younger than 5 years, and was estimated using the UN's population prospects as the denominator to have a population reference for S pneumoniae isolates in each country.13 Annual reporting rates were estimated for vaccine type, non-typable strains, and non-vaccine types, and used for calculating percentage reductions in serotypes included in PCV10 (VT10) or serotypes included in PCV13 (VT13) in all study countries.
A trend analysis with join-point regression was made in each country to estimate the average annual percentage of change in the annual reporting rates per 100 000 children younger than 5 years, by serotype, associated with year of PCV introduction.14
Role of the funding source
There was no funding source for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
12 269 isolates of invasive pneumococcal disease were collected from children younger than 5 years in ten Latin American and Caribbean countries between 2006 and 2017. Most isolates were collected from children younger than 12 months (4745 [38·7%]), from patients diagnosed with pneumonia (4763 [38·8%]), and from blood (7555 [61·6%]; appendix 3 pp 10–12). A 45% reduction was observed concerning the amount of isolates collected in PCV countries between 2006 and 2017 (table 1 ).
Table 1.
Distribution of Streptococcus pneumoniae isolates causing invasive pneumococcal diseases from children younger than 5 years by country and year of isolation
|
Year |
Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | ||
| Argentina | 195 | 156 | 249 | 239 | 355 | 376 | 272 | 200 | 171 | 157 | 146 | 131 | 2647 (21·6%) |
| Brazil | 318 | 310 | 309 | 185 | 233 | 211 | 173 | 155 | 143 | 144 | 148 | 149 | 2478 (20·2%) |
| Chile | 281 | 343 | 322 | 321 | 282 | 241 | 171 | 181 | 153 | 132 | 137 | 135 | 2699 (22·0%) |
| Colombia | 141 | 160 | 141 | 107 | 137 | 122 | 85 | 78 | 91 | 92 | 114 | 146 | 1414 (11·5%) |
| Dominican Republic | 47 | 36 | 57 | 46 | 28 | 39 | 35 | 44 | 33 | 25 | 22 | 11 | 423 (3·4%) |
| Mexico | 49 | 50 | 62 | 77 | 102 | 97 | 88 | 73 | 64 | 49 | 61 | 43 | 815 (6·6%) |
| Paraguay | 107 | 81 | 74 | 50 | 67 | 57 | 41 | 37 | 22 | 29 | 35 | 45 | 645 (5·3%) |
| Uruguay | 100 | 63 | 75 | 55 | 41 | 29 | 25 | 29 | 15 | 25 | 33 | 29 | 519 (4·2%) |
| Subtotal | 1238 (10·6%) | 1199 (10·3%) | 1289 (11·1%) | 1080 (9·3%) | 1245 (10·7%) | 1172 (10·1%) | 890 (7·6%) | 797 (6·8%) | 692 (5·9%) | 653 (5·6%) | 696 (6·0%) | 689 (5·9%) | 11 640 |
| Cuba | 0 | 2 | 0 | 19 | 27 | 25 | 24 | 15 | 38 | 59 | 48 | 63 | 320 (2·6%) |
| Venezuela | 36 | 40 | 37 | 22 | 28 | 27 | 21 | 42 | 28 | 17 | 7 | 4 | 309 (2·5%) |
| Total | 1274 | 1241 | 1326 | 1121 | 1300 | 1224 | 935 | 854 | 758 | 729 | 751 | 756 | 12 269 |
Data are n or n (%).
Appendix 3 (pp 13–22) summarises the S pneumoniae isolate distribution by country, serotype, and surveillance year. A significant decreasing trend was observed in PCV10 countries for serotype 1 (Colombia and Paraguay p<0·0001; Brazil p=0·003; Chile p=0·006), serotype 5 (Chile, Colombia and Paraguay p<0·0001), serotype 6B (Brazil, Chile, and Colombia p<0·0001), and serotype 14 (all four countries p<0·0001). By contrast, a significantly increased trend (p<0·0001) was observed for two of the three additional serotypes (3 and 19A) in all four countries. A significant increase (p<0·0001) was observed for non-vaccine types in all PCV10 countries. Regarding PCV13 countries, a significant decreasing trend (p<0·0001) was observed for serotypes 14 in Argentina, Mexico, and Uruguay, serotype 1 in Uruguay, and serotype 5 in Argentina and Uruguay. A significant increasing trend (p<0·0001) was also observed for serotype 3 in Uruguay and serotype 19A in Mexico. An increasing trend was observed for non-vaccine types in Argentina (p=0·0004), Mexico (p<0·0001), and Uruguay (p<0·0001). The Dominican Republic was excluded from analysis to avoid bias because of the few isolates reported. Remarkably, serotype 6B was not reported in 2017 in any country regardless of the type of PCV used; serotype 5 has not been isolated since 2015 in the PCV10 countries and has not been reported in Uruguay since 2011 (appendix 3 pp 13–22). The most frequent pneumococcal serotypes causing invasive pneumococcal disease in non-PCV countries during the surveillance period were serotypes 14, 19A, 6B, 6A, and 19F for Cuba (2007–17) and serotypes 19A, 14, 6B, 19F, and 5 for Venezuela (2006–17). The data showed an increase for serotype 14 in Cuba and serotype 19A in Venezuela. Figure 1 shows the yearly serotype percentage by country and highlights the effect of PCVs on vaccine type and non-vaccine type distribution.
Figure 1.
Number of Streptococcus pneumoniae isolates and percentage of serotypes by vaccine type, non-vaccine type, and non-typable isolates per year and by country
(A–D) PCV10 countries. (E–H) PCV13 countries. PCV7=seven-valent pneumococcal conjugate vaccine. PCV10=ten-valent pneumococcal conjugate vaccine. PCV13=13-valent pneumococcal conjugate vaccine.
Table 2, Table 3 show changes in vaccine type and non-vaccine type frequencies observed in PCV10 and PCV13 countries after the introduction of PCV. Percentage change for VT10 in PCV10 countries was −91·6% in Brazil (530 [72·9%] of 727 before, 27 [6·1%] of 441 after); −85·0% in Chile (613 [72·6%] of 844 before, 44 [10·9%] of 404 after); −84·7% in Colombia (231 [63·1%] of 366 before, 34 [9·7%] of 352 after); and −73·8% in Paraguay (127 [77·0%] of 165 before, 22 [20·2%] of 109 after). A greater than −80% change was observed for serotype 5 in all countries, as well as serotypes 6B and 14 in all countries except Paraguay. The three PCV13 additional serotypes had a positive percentage change, driven by changes in serotypes 19A and 3. Concerning PCV13 countries, the percentage change for VT13 was −59·6% in Argentina (853 [85·0%] of 1003 before, 149 [34·3%] of 434 after); −16·5% in the Dominican Republic (95 [80·5%] of 118 before, 39 [67·2%] of 58 after); −43·7% in Mexico (202 [73·2%] of 276 before, 63 [41·2%] of 153 after); and −45·9% in Uruguay (138 [80·7%] of 171 before, 38 [43·7%] of 87 after). A greater than −80% change was observed for serotypes 5 and 18C in Argentina, Mexico, and Uruguay, and for serotype 14 in Argentina and Mexico. Only Argentina had a negative percentage change for serotype 19A. An increase in the total number of non-vaccine types, as well as in their percentage compared with the total isolates was observed in all PCV10 and PCV13 countries. The percentage change for non-vaccine types ranged from 76·3% (69 [18·9%] of 366 before, 117 [33·2%] of 352 after) in Colombia to 337·1% (147 [14·7%] of 1003 before, 278 [64·1%] of 434 after) in Argentina.
Table 2.
Percentage change of Streptococcus pneumoniae serotypes by VT, NVT, and NT for the PCV10 countries
|
Brazil |
Chile |
Colombia |
Paraguay |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before 2008–10 | After 2015–17 | Percentage change | Before 2009–11 | After 2015–17 | Percentage change | Before 2009–11 | After 2015–17 | Percentage change | Before 2010–12 | After 2015–17 | Percentage change | ||
| Shared serotypes | |||||||||||||
| 1 | 6 (0·8%) | 2 (0·5%) | −45·0% | 64 (7·6%) | 11 (2·7%) | −64·1% | 34 (9·3%) | 4 (1·1%) | −87·8% | 5 (3·0%) | 0 | −100% | |
| 4 | 11 (1·5%) | 0 | −100% | 13 (1·5%) | 0 | −100% | 5 (1·4%) | 2 (0·6%) | −58·4% | 3 (1·8%) | 0 | −100% | |
| 5 | 16 (2·2%) | 0 | −100% | 29 (3·4%) | 0 | −100% | 4 (1·1%) | 0 | −100% | 3 (1·8%) | 0 | −100% | |
| 6B | 91 (12·5%) | 1 (0·2%) | −98·2% | 70 (8·3%) | 2 (0·5%) | −94·0% | 32 (8·7%) | 3 (0·9%) | −90·3% | 15 (9·1%) | 2 (1·8%) | −79·8% | |
| 7F | 13 (1·8%) | 4 (0·9%) | −49·3% | 30 (3·6%) | 5 (1·2%) | −65·2% | 4 (1·1%) | 0 | −100% | 5 (3·0%) | 2 (1·8%) | −39·4% | |
| 9V | 11 (1·5%) | 4 (0·9%) | −40·1% | 11 (1·3%) | 3 (0·7%) | −43·0% | 8 (2·2%) | 4 (1·1%) | −48·0% | 3 (1·8%) | 3 (2·8%) | +51·4% | |
| 14 | 264 (36·3%) | 7 (1·6%) | −95·6% | 276 (32·7%) | 13 (3·2%) | −90·2% | 100 (27·3%) | 12 (3·4%) | −87·5% | 75 (45·5%) | 11 (10·1%) | −77·8% | |
| 18C | 44 (6·1%) | 1 (0·2%) | −96·3% | 38 (4·5%) | 3 (0·7%) | −83·5% | 16 (4·4%) | 1 (0·3%) | −93·5% | 5 (3·0%) | 1 (0·9%) | −69·7% | |
| 19F | 35 (4·8%) | 2 (0·5%) | −90·6% | 49 (5·8%) | 4 (1·0%) | −82·9% | 10 (2·7%) | 5 (1·4%) | −48·0% | 8 (4·8%) | 1 (0·9%) | −81·1% | |
| 23F | 39 (5·4%) | 6 (1·4%) | −74·6% | 33 (3·9%) | 3 (0·7%) | −81·0% | 18 (4·9%) | 3 (0·9%) | −82·7% | 5 (3·0%) | 2 (1·8%) | −39·4% | |
| VT10 | 530 (72·9%) | 27 (6·1%) | −91·6% | 613 (72·6%) | 44 (10·9%) | −85·0% | 231 (63·1%) | 34 (9·7%) | −84·7% | 127 (77·0%) | 22 (20·2%) | −73·8% | |
| Three additional serotypes | |||||||||||||
| 3 | 26 (3·6%) | 39 (8·8%) | +147·3% | 15 (1·8%) | 38 (9·4%) | +429·2% | 21 (5·7%) | 39 (11·1%) | +93·1% | 1 (0·6%) | 23 (21·1%) | +3381·7% | |
| 6A | 38 (5·2%) | 9 (2·0%) | −61·0% | 69 (8·2%) | 13 (3·2%) | −60·6% | 12 (3·3%) | 22 (6·3%) | +90·6% | 11 (6·7%) | 7 (6·4%) | −3·7% | |
| 19A | 35 (4·8%) | 131 (29·7%) | +517·0% | 44 (5·2%) | 118 (29·2%) | +460·3% | 32 (8·7%) | 140 (39·8%) | +354·9% | 4 (2·4%) | 22 (20·2%) | +732·6% | |
| VT13 | 629 (86·5%) | 206 (46·7%) | −46·0% | 741 (87·8%) | 213 (52·7%) | −39·9% | 296 (80·9%) | 235 (66·8%) | −17·5% | 143 (86·7%) | 74 (67·9%) | −21·7% | |
| NVT | 93 (12·8%) | 234 (53·1%) | +314·8% | 96 (11·4%) | 187 (46·3%) | +306·9% | 69 (18·9%) | 117 (33·2%) | +76·3% | 17 (10·3%) | 32 (29·4%) | +184·9% | |
| NT | 5 (0·7%) | 1 (0·2%) | −67·0% | 7 (0·8%) | 4 (1·0%) | +19·4% | 1 (0·3%) | 0 | −100% | 5 (3·0%) | 3 (2·8%) | −9·2% | |
| Total* | 727 | 441 | .. | 844 | 404 | .. | 366 | 352 | .. | 165 | 109 | .. | |
Data are n (%), unless otherwise specified (appendix 3 pp 13–16). NT=non-typable. NVT=non-vaccine type. PCV10=ten-valent pneumococcal conjugate vaccine. VT=vaccine type. VT10=vaccine type 10. VT13=vaccine type 13.
The total comprises VT13, NVT, and NT.
Table 3.
Percentage change of Streptococcus pneumoniae serotypes by VT, NVT, and NT for the PCV13 countries
|
Argentina |
Dominican Republic |
Mexico |
Uruguay |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before 2010–12 | After 2015–17 | Percentage change | Before 2010–13 | After 2015–17 | Percentage change | Before 2009–11 | After 2015–17 | Percentage change | Before 2008–10 | After 2015–17 | Percentage change | ||
| Shared serotypes | |||||||||||||
| 1 | 128 (12·8%) | 35 (8·1%) | −36·8% | 3 (2·5%) | 4 (6·9%) | +171·3% | 3 (1·1%) | 0 | −100% | 24 (14·0%) | 1 (1·1%) | −91·8% | |
| 4 | 12 (1·2%) | 1 (0·2%) | −80·7% | 1 (0·8%) | 0 | −100% | 0 | 0 | NA | 1 (0·6%) | 0 | −100% | |
| 5 | 132 (13·2%) | 3 (0·7%) | −94·7% | 3 (2·5%) | 4 (6·9%) | +171·3% | 3 (1·1%) | 0 | −100% | 14 (8·2%) | 0 | −100% | |
| 6B | 42 (4·2%) | 2 (0·5%) | −89·0% | 17 (14·4%) | 6 (10·3%) | −28·2% | 13 (4·7%) | 0 | −100% | 6 (3·5%) | 1 (1·1%) | −67·2% | |
| 7F | 65 (6·5%) | 22 (5·1%) | −21·8% | 0 | 0 | NA | 2 (0·7%) | 0 | −100% | 17 (9·9%) | 0 | −100% | |
| 9V | 24 (2·4%) | 10 (2·3%) | −3·7% | 0 | 0 | NA | 9 (3·3%) | 2 (1·3%) | −59·9% | 1 (0·6%) | 0 | −100% | |
| 14 | 212 (21·1%) | 16 (3·7%) | −82·6% | 29 (24·6%) | 5 (8·6%) | −64·9% | 8 (2·9%) | 0 | −100% | 43 (25·1%) | 9 (10·3%) | −58·9% | |
| 18C | 37 (3·7%) | 2 (0·5%) | −87·5% | 5 (4·2%) | 3 (5·2%) | +22·1% | 7 (2·5%) | 0 | −100% | 2 (1·2%) | 0 | −100% | |
| 19F | 22 (2·2%) | 5 (1·2%) | −47·5% | 4 (3·4%) | 1 (1·7%) | −49·1% | 33 (12·0%) | 5 (3·3%) | −72·7% | 1 (0·6%) | 3 (3·4%) | +489·7% | |
| 23F | 30 (3·0%) | 3 (0·7%) | −76·9% | 19 (16·1%) | 3 (5·2%) | −67·9% | 18 (6·5%) | 0 | −100% | 3 (1·8%) | 1 (1·1%) | −34·5% | |
| VT10 | 704 (70·2%) | 99 (22·8%) | −67·5% | 81 (68·6%) | 26 (44·8%) | −34·7% | 96 (34·8%) | 7 (4·6%) | −86·8% | 112 (65·5%) | 15 (17·2%) | −73·7% | |
| Three additional serotypes | |||||||||||||
| 3 | 35 (3·5%) | 25 (5·8%) | +65·1% | 5 (4·2%) | 4 (6·9%) | +62·8% | 9 (3·3%) | 7 (4·6%) | +40·3% | 14 (8·2%) | 18 (20·7%) | +152·4% | |
| 6A | 55 (5·5%) | 6 (1·4%) | −74·8% | 6 (5·1%) | 2 (3·4%) | −32·2% | 18 (6·5%) | 4 (2·6%) | −59·9% | 3 (1·8%) | 0 | −100% | |
| 19A | 59 (5·9%) | 19 (4·4%) | −25·6% | 3 (2·5%) | 7 (12·1%) | +374·7% | 79 (28·6%) | 45 (29·4%) | +2·8% | 9 (5·3%) | 5 (5·7%) | +9·2% | |
| VT13 | 853 (85·0%) | 149 (34·3%) | −59·6% | 95 (80·5%) | 39 (67·2%) | −16·5% | 202 (73·2%) | 63 (41·2%) | −43·7% | 138 (80·7%) | 38 (43·7%) | −45·9% | |
| NVT | 147 (14·7%) | 278 (64·1%) | +337·1% | 16 (13·6%) | 18 (31·0%) | +128·9% | 65 (23·6%) | 82 (53·6%) | +127·6% | 33 (19·3%) | 49 (56·3%) | +191·8% | |
| NT | 3 (0·3%) | 7 (1·6%) | +439·2% | 7 (5·9%) | 1 (1·7%) | −70·9% | 9 (3·3%) | 8 (5·2%) | +60·3% | 0 | 0 | NA | |
| Total* | 1003 | 434 | .. | 118 | 58 | .. | 276 | 153 | .. | 171 | 87 | .. | |
Data are n (%), unless otherwise specified. NA=not applicable. NT=non-typable. NVT=non-vaccine type. PCV13=thirteen-valent pneumococcal conjugate vaccine. VT=vaccine type. VT10=vaccine type 10. VT13=vaccine type 13.
The total comprises VT13, NVT, and NT.
High non-vaccine type diversity was observed after introducing PCVs (appendix 3 p 23). A list of the 15 predominant non-vaccine types was defined using the cumulative information collected from the eight PCV countries, selecting those that had percentages higher than 1% of total isolates. Table 4 shows the distribution of the three additional vaccine types and the 15 most frequent non-vaccine types and non-typable strains in the post-PCV period. Considering a percentage greater than 4% for non-vaccine type, the non-vaccine types in PCV10 countries, besides the three additional serotypes, were 6C and 24/24F for Brazil and Chile, 6C and 23A for Colombia, and 12F, and 24/24F for Paraguay. Concerning PCV13 countries, the non-vaccine types were serotypes 12F, 23B, and 24/24F in Argentina; 15A, 15C, 23B, and 35B in Mexico; and 12F, 15A, 23B, and 24/24F in Uruguay.
Table 4.
Distribution of the most frequent NVT, NT, and three additional Streptococcus pneumoniae serotypes by PCV10 and PCV13 countries in the post-PCV period (2015–17)
|
PCV10 countries |
PCV13 countries |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Brazil (n=441) | Chile (n=404) | Colombia (n=352) | Paraguay (n=109) | Argentina (n=434) | Dominican Republic (n=58) | Mexico (n=153) | Uruguay (n=87) | ||
| NVT shared | |||||||||
| 6C | 35 (7·9%) | 19 (4·7%) | 19 (5·4%) | 4 (3·7%) | 5 (1·2%) | 2 (3·4%) | 4 (2·6%) | 0 | |
| 8 | 10 (2·3%) | 4 (1·0%) | 2 (0·6%) | 2 (1·8%) | 6 (1·4%) | 1 (1·7%) | 2 (1·3%) | 2 (2·3%) | |
| 9N | 7 (1·6%) | 7 (1·7%) | 5 (1·4%) | 0 | 7 (1·6%) | 1 (1·7%) | 0 | 2 (2·3%) | |
| 10A | 15 (3·4%) | 7 (1·7%) | 2 (0·6%) | 0 | 7 (1·6%) | 0 | 4 (2·6%) | 0 | |
| 11A | 10 (2·3%) | 1 (0·2%) | 3 (0·9%) | 2 (1·8%) | 9 (2·1%) | 0 | 3 (2·0%) | 0 | |
| 12F | 14 (3·2%) | 9 (2·2%) | 0 | 5 (4·6%) | 45 (10·4%) | 0 | 1 (0·7%) | 11 (12·6%) | |
| 15A | 10 (2·3%) | 10 (2·5%) | 8 (2·3%) | 2 (1·8%) | 12 (2·8%) | 1 (1·7%) | 7 (4·6%) | 4 (4·6%) | |
| 15B | 10 (2·3%) | 8 (2·0%) | 3 (0·9%) | 2 (1·8%) | 11 (2·5%) | 1 (1·7%) | 6 (3·9%) | 3 (3·4%) | |
| 15C | 13 (2·9%) | 4 (1·0%) | 6 (1·7%) | 0 | 1 (0·2%) | 0 | 11 (7·2%) | 0 | |
| 16F | 11 (2·5%) | 3 (0·7%) | 3 (0·9%) | 1 (0·9%) | 6 (1·4%) | 0 | 2 (1·3%) | 0 | |
| 22F | 8 (1·8%) | 15 (3·7%) | 1 (0·3%) | 0 | 6 (1·4%) | 0 | 1 (0·7%) | 1 (1·1%) | |
| 23A | 7 (1·6%) | 4 (1·0%) | 14 (4·0%) | 0 | 12 (2·8%) | 0 | 4 (2·6%) | 1 (1·1%) | |
| 23B | 12 (2·7%) | 7 (1·7%) | 8 (2·3%) | 1 (0·9%) | 18 (4·1%) | 0 | 8 (5·2%) | 4 (4·6%) | |
| 24/24F | 19 (4·3%) | 30 (7·4%) | 10 (2·8%) | 5 (4·6%) | 33 (7·6%) | 1 (1·7%) | 7 (4·6%) | 6 (6·9%) | |
| 35B | 11 (2·5%) | 4 (1·0%) | 6 (1·7%) | 0 | 0 | 0 | 7 (4·6%) | 0 | |
| Others | 42 (9·5%) | 55 (13·6%) | 27 (7·7%) | 8 (7·3%) | 100 (23·0%) | 11 (19·0%) | 15 (9·8%) | 15 (17·2%) | |
| NT | 1 (0·2%) | 4 (1·0%) | 0 | 3 (2·8%) | 7 (1·6%) | 1 (1·7%) | 8 (5·2%) | 0 | |
| Subtotal | 235 (53·3%) | 191 (47·3%) | 117 (33·2%) | 35 (32·1%) | 285 (65·7%) | 19 (32·8%) | 90 (58·8%) | 49 (56·3%) | |
| Three additional serotypes | |||||||||
| 3 | 39 (8·8%) | 38 (9·4%) | 39 (11·1%) | 23 (21·1%) | 25 (5·8%) | 4 (6·9%) | 7 (4·6%) | 18 (20·7%) | |
| 6A | 9 (2·0%) | 13 (3·2%) | 22 (6·3%) | 7 (6·4%) | 6 (1·4%) | 2 (3·4%) | 4 (2·6%) | 0 | |
| 19A | 131 (29·7%)v | 118 (29·2%) | 140 (39·8%) | 22 (20·2%) | 19 (4·4%) | 7 (12·1%) | 45 (29·4%) | 5 (5·7%) | |
| Subtotal | 179 (40·6%) | 169 (41·8%) | 201 (57·1%) | 52 (47·7%) | 50 (11·5%) | 13 (22·4%) | 56 (36·6%) | 23 (26·4%) | |
| Total | 414 (93·9%) | 360 (89·1%) | 318 (90·3%) | 87 (79·8%) | 335 (77·2%) | 32 (55·2%) | 146 (95·4%) | 72 (82·8%) | |
Data are n (%). NT=non-typable. NVT=non-vaccine types. PCV=pneumococcal conjugate vaccine. PCV10=ten-valent pneumococcal conjugate vaccine. PCV13=13-valent pneumococcal conjugate vaccine.
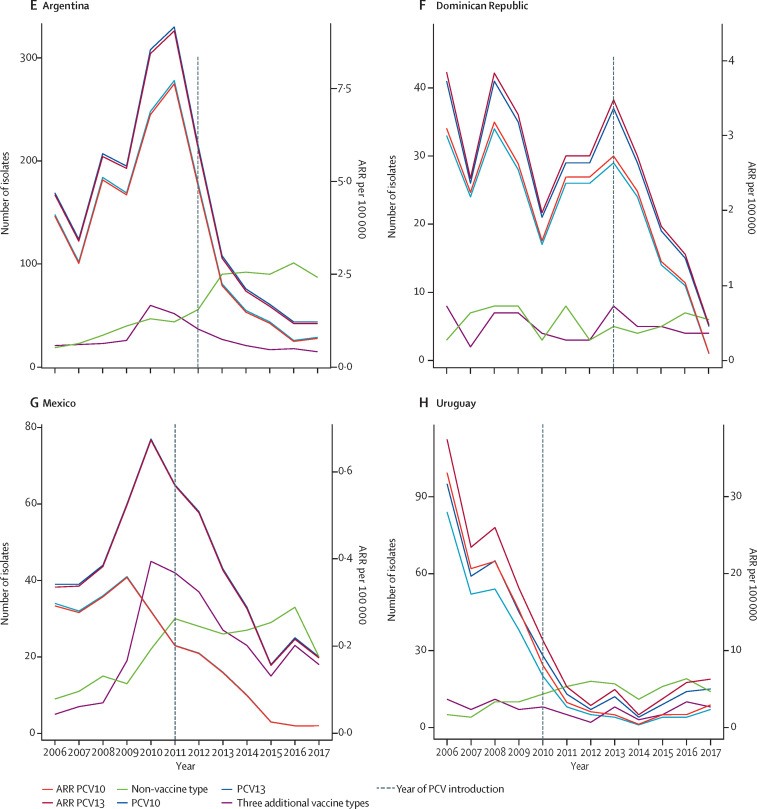
Figure 2 shows the amount of isolates and annual reporting rate per 100 000 children younger than 5 years by vaccine type and non-vaccine type according to PCV, country, and year of surveillance. Annual reporting rates per 100 000 children younger than 5 years regarding total S pneumoniae serotypes by country, VT10, VT13, non-vaccine type, serotypes 3, 6A, 19A, and year of vaccine introduction are shown in appendix 3 (pp 31–35). Annual reporting rates for VT10 in PCV10 countries decreased between −82·5% (6·21 before vs 1·09 after per 100 000, 95% CI −61·6 to −92·0) and −94·7% (1·15 vs 0·06 per 100 000, −89·7 to −97·3), whereas annual reporting rates for VT13 in PCV13 countries decreased between −58·8% (2·98 vs 1·23 per 100 000, −21·4 to −78·4) and −82·9% (7·80 vs 1·33 per 100 000, −76·9 to −87·4; table 5 ). Serotypes of non-vaccine type had a significant increase in Brazil, Chile, Colombia, and Argentina. Brazil and Chile had a decrease in serotype 6A, whereas serotype 19A increased in Brazil, Chile, and Colombia. According to the year of vaccine introduction, PCV10 countries reported an increase in annual reporting rate trends for serotype 19A. Remarkably, the annual reporting rate for serotypes 4, 5, 6A, 7F, 9V, and 18C has been zero in Uruguay since 2013. Non-vaccine type isolates had similar increasing annual reporting rate patterns among countries after any PCV introduction (appendix 3 pp 36–49). It is worth reiterating that Mexico and Uruguay had introduced the PCV7 vaccine before the introduction of PCV13. For our analysis, we have taken year zero as the year of PCV13 introduction and, as such, PCV7 might have affected specific serotypes during the pre-PCV13 period and thus the extent of percentage changes or reduction of annual reporting rates, or both, for PCV13. Data considering earlier pre-vaccine period is presented in appendix 3 (pp 19–20, 42–43).
Figure 2.
Number of isolates and ARR per 100 000 children younger than 5 years by vaccine type and non-vaccine type, country, and year of surveillance
(A–D) PCV10 countries. (E–H) PCV13 countries. Dotted line indicates the year of PCV introduction. ARR=annual reported rate. PCV10=ten-valent pneumococcal conjugate vaccine. PCV13=13-valent pneumococcal conjugate vaccine.
Table 5.
Difference between average ARR by 100 000 children younger than 5 years before and after PCV and percentage of change of ARR by country
| Pre-ARR average | Post-ARR average | Post-ARR–Pre-ARR (95% CI) | Percentage change (95% CI) | ||
|---|---|---|---|---|---|
| PCV10 countries | |||||
| Brazil* | |||||
| PCV10 | 1·15 | 0·06 | 0·05 (0·03 to 0·10) | −94·7% (−89·7 to −97·3) | |
| PCV13 | 1·38 | 0·46 | 0·34 (0·26 to 0·44) | −66·2% (−55·7 to −74·3) | |
| 3 | 0·06 | 0·09 | 1·55 (0·66 to 3·65) | +54·6% (−34·5 to 265·2) | |
| 6A | 0·08 | 0·02 | 0·24 (0·07 to 0·86) | −75·6% (−14·1 to −93·1) | |
| 19A | 0·08 | 0·29 | 3·86 (2·02 to 7·36) | +285·9% (102·3 to 636·1) | |
| NVT | 0·20 | 0·52 | 2·59 (1·71 to 3·93) | +159·4% (71·1 to 293·3) | |
| Total | 1·58 | 0·99 | 0·62 (0·51 to 0·77) | −37·5% (−23·2 to −49·1) | |
| Chile† | |||||
| PCV10 | 16·44 | 1·24 | 0·08 (0·048 to 0·13) | −92·5% (−87·2 to −95·6) | |
| PCV13 | 19·87 | 5·99 | 0·30 (0·23 to 0·39) | −69·9% (−60·8 to −76·9) | |
| 3 | 0·40 | 1·07 | 2·65 (0·94 to 7·47) | +165·4% (−5·7 to 647·3) | |
| 6A | 1·85 | 0·36 | 0·20 (0·07 to 0·55) | −80·3% (−44·9 to −92·9) | |
| 19A | 1·18 | 3·32 | 2·81 (1·54 to 5·12) | +181·0% (54·3 to 411·8) | |
| NVT | 2·57 | 5·25 | 2·04 (1·33 to 3·13) | +104·1% (33·3 to 212·6) | |
| Total | 22·63 | 11·35 | 0·50 (0·41 to 0·62) | −49·8% (−38·4 to −59·2) | |
| Colombia‡ | |||||
| PCV10 | 1·96 | 0·30 | 0·16 (0·08 to 0·29) | −84·4% (−71·0 to −91·7) | |
| PCV13 | 2·51 | 2·11 | 0·84 (0·62 to 1·13) | −16·0% (−37·6 to 13·0) | |
| 3 | 0·18 | 0·35 | 1·96 (0·78 to 4·92) | +96·5% (−21·6 to 392·4) | |
| 6A | 0·10 | 0·20 | 1·94 (0·57 to 6·56) | +94·0% (−42·6 to 555·8) | |
| 19A | 0·27 | 1·26 | 4·63 (2·38 to 9·00) | +362·8% (138·0 to 800·1) | |
| NVT | 0·59 | 1·05 | 1·79 (1·07 to 3·00) | +79·4% (7·2 to 200·3) | |
| Total | 3·11 | 3·16 | 1·02 (0·79 to 1·31) | +1·7% (−21 to 31·1) | |
| Paraguay§ | |||||
| PCV10 | 6·21 | 1·09 | 0·18 (0·08 to 0·38) | −82·5% (−61·6 to −92·0) | |
| PCV13 | 7·00 | 3·66 | 0·52 (0·32 to 0·85) | −47·7% (−14·9 to −67·8) | |
| 3 | 0·05 | 1·14 | 23·37 (0·73 to 749·37) | +2237·0% (−27·1 to 74837·4) | |
| 6A | 0·54 | 0·35 | 0·64 (0·12 to 3·32) | −35·6% (−87·5 to 232·3) | |
| 19A | 0·20 | 1·09 | 5·52 (0·87 to 34·92) | +451·7% (−12·8 to 3392·0) | |
| NVT | 0·83 | 1·58 | 1·90 (0·69 to 5·27) | +90·4% (−31·3 to 427·4) | |
| Total | 8·37 | 5·79 | 0·69 (0·46 to 1·04) | −33·2% (−56·1 to 1·6) | |
| PCV13 countries | |||||
| Argentina¶ | |||||
| PCV10 | 6·43 | 0·88 | 0·14 (0·10 to 0·20) | −86·3% (−80·2 to −90·4) | |
| PCV13 | 7·80 | 1·33 | 0·17 (0·13 to 0·23) | −82·9% (−76·9 to −87·4) | |
| 3 | 0·32 | 0·22 | 0·70 (0·29 to 1·70) | −30·2% (−71·3 to 69·9) | |
| 6A | 0·50 | 0·05 | 0·11 (0·02 to 0·46) | −89·3% (−54·1 to −97·5) | |
| 19A | 0·54 | 0·17 | 0·32 (0·13 to 0·77) | −68·5% (−22·9 to −87·1) | |
| NVT | 1·34 | 2·48 | 1·85 (1·31 to 2·61) | +84·9% (30·8 to 161·4) | |
| Total | 9·17 | 3·88 | 0·42 (0·35 to 0·51) | −57·7% (−48·6 to −65·2) | |
| Dominican Republic‖ | |||||
| PCV10 | 2·54 | 0·82 | 0·32 (0·15 to 0·69) | −67·8% (−30·8 to −85·0) | |
| PCV13 | 2·98 | 1·23 | 0·41 (0·22 to 0·79) | −58·8% (−21·4 to −78·4) | |
| 3 | 0·16 | 0·13 | 0·80 (0·08 to 7·83) | −19·7% (−91·8 to 682·8) | |
| 6A | 0·19 | 0·06 | 0·34 (0·02 to 5·37) | −66·5% (−97·9 to 435·6) | |
| 19A | 0·09 | 0·22 | 2·34 (0·22 to 24·37) | +134·2% (−77·5 to 2337·3) | |
| NVT | 0·50 | 0·57 | 1·13 (0·32 to 3·62) | +12·9% (−64·8 to 262·5) | |
| Total | 3·70 | 1·83 | 0·49 (0·29 to 0·85) | −50·7% (−15·0 to −71·4) | |
| Mexico** | |||||
| PCV10 | 0·28 | 0·02 | 0·07 (0·02 to 0·27) | −92·8% (−72·7 to −98·1) | |
| PCV13 | 0·59 | 0·18 | 0·31 (0·19 to 0·50) | −69·1% (−49·6 to −81·1) | |
| 3 | 0·03 | 0·02 | 0·77 (0·14 to 4·26) | −22·9% (−86·1 to 326·4) | |
| 6A | 0·05 | 0·01 | 0·22 (0·03 to 1·44) | −78·0% (−96·6 to 43·8) | |
| 19A | 0·23 | 0·13 | 0·56 (0·30 to 1·06) | −43·6% (−70·1 to 6·4) | |
| NVT | 0·19 | 0·24 | 1·25 (0·71 to 2·20) | +25·0% (−28·9 to 119·6) | |
| Total | 0·80 | 0·44 | 0·55 (0·39 to 0·77) | −45·1% (−22·7 to −61·0) | |
| Uruguay†† | |||||
| PCV10 | 15·02 | 2·08 | 0·14 (0·06 to 0·35) | −86·1% (−64·7 to −94·5) | |
| PCV13 | 18·51 | 5·28 | 0·28 (0·15 to 0·53) | −71·5% (−46·8 to −84·7) | |
| 3 | 1·88 | 2·50 | 1·33 (0·40 to 4·47) | +33·3% (−60·2 to 346·9) | |
| 6A | 0·40 | 0 | NA | −100% (NA) | |
| 19A | 1·21 | 0·70 | 0·58 (0·09 to 3·82) | −42·4% (−91·3 to 282·5) | |
| NVT | 4·43 | 6·81 | 1·54 (0·72 to 3·31) | +53·9% (−28·3 to 230·6) | |
| Total | 22·93 | 12·10 | 0·53 (0·34 to 0·82) | −47·3% (−17·5 to −66·3) | |
ARR=annual reported rates. NA=not applicable. NVT=non-vaccine type. PCV=pneumococcal conjugate vaccine. PCV10=ten-valent pneumococcal conjugate vaccine. PCV13=thirteen-valent pneumococcal conjugate vaccine.
Pre-ARR average included the years 2008–10, and post-ARR average included the years 2015–17.
Pre-ARR average included the years 2009–11, and post-ARR average included the years 2015–17.
Pre-ARR average included the years 2009–11, and post-ARR average included the years 2015–17.
Pre-ARR average included the years 2010–12, and post-ARR average included the years 2015–17.
Pre-ARR average included the years 2010–12, and post-ARR average included the years 2015–17.
Pre-ARR average included the years 2011–13, and post-ARR average included the years 2015–17.
Pre-ARR average included the years 2009–11, and post-ARR average included the years 2015–17.
Pre-ARR average included the years 2008–10, and post-ARR average included the years 2015–17.
The join-point regression analysis provided very few significant trends. Only Chile and Colombia showed variation in serotype groups of vaccine type after PCV introduction: Chile decreased the VT10 annual reporting rate trend (annual percentage change −65·57%, 95% CI −87·00 to −8·84) and Colombia increased the three additional VT13 annual reporting rate trend (54·71%, 45·55 to 65·58). appendix 3 (p 50) summarises these results.
Discussion
Serotype surveillance of S pneumoniae causing invasive pneumococcal disease represents an important tool for evaluating the effect of PCV introduction. SIREVA enabled Latin American and Caribbean countries to introduce regional laboratory surveillance programmes with the help of PAHO from 1993 onwards;5, 6, 7 PCV began to be introduced as early as 2008. The eight countries considered in this study were using PCVs in their expanded programme on immunisation by 2013.8 This study was thus designed to ascertain the distribution of S pneumoniae serotypes obtained during the 12 years of surveillance (2006–17), after PCV, ranging from 4 years in the Dominican Republic to 9 years in Uruguay. Data from Cuba and Venezuela, non-PCV countries having similar surveillance programmes, were included for comparison. As expected, the main findings of this analysis highlighted the significant reduction or even elimination of PCV serotypes and the emergence of non-vaccine types, in line with data reported elsewhere about PCV impact.15, 16, 17 Brazil,12 Uruguay,18, 19 Mexico,20 and Argentina21 have already published results regarding the effect of PCV introduction, showing a significant decrease in cases and a reduced amount of vaccine type seven, VT10, or VT13 isolates. An important decrease could already be observed 1–2 years after PCV introduction in serotypes 1, 5, 6B, and 14 that had been prevalent in the region in the pre-PCV period.
Regarding the three additional vaccine types, serotype 19A increased significantly in PCV10 countries and decreased only significantly in Argentina. Serotype 3 increased in absolute numbers for all eight countries but because of the low numbers the increase was not significant. This finding has been attributed to capsular 3 polysaccharide's poor immunogenicity.15, 22, 23 Serotype 6A frequency decreased in both PCV10 and PCV13 countries certainly by cross protection with serotype 6B.15, 24 Serotype 6C was observed in PCV10 countries among the most predominant non-vaccine types, not so in PCV13 countries. This observation has already been described in Brazil,12 and in Sweden in PCV10 counties, not so in PCV13 counties,15 suggesting that cross protection occurred with serotype 6A.
Similar results were obtained when the reduction in annual reporting rates was taken into consideration. Estimation of annual reporting rates enabled year-by-year comparisons for each country, especially for evaluating differences between pre-PCV and post-PCV introduction years. It is understood that annual reporting rates represent an underestimation of population incidence because of underreporting of S pneumoniae isolates to the national reference laboratories as invasive pneumococcal disease reporting is not compulsory in Latin American and Caribbean countries, except for meningitis cases. Analysis of temporal trends based on a population proxy of invasive pneumococcal disease incidence with annual reporting rate reinforces a reduction in vaccine type and increased non-vaccine type trends after year zero of vaccine introduction in PCV10 and most PCV13 countries. An increasing serotype 19A trend was also evident in PCV10 countries. Statistical significance for these temporal trends in association with PCV introduction by year were evaluated with join-point regression, giving few significant results.
It is worth stressing that the differences in the results when measured as percentage change or annual reporting rates were due to the different ways both were calculated. Percentage change was measured as percentage vaccine type, group of vaccine types, or non-vaccine types in relation to the amount of isolates at two different times. Concerning annual reporting rates, the change in absolute amount of a vaccine type, group of vaccine types, or non-vaccine types was measured at two different timepoints regarding a population denominator.12
A remarkable finding concerned the significant increase in high non-vaccine type diversity during the most recent surveillance years. The main non-vaccine types in PCV10 countries were 6C and 24/24F, besides the three additional vaccine types, whereas in PCV13 countries non-vaccine types were 12F, 24/24F, and 23B (appendix 3 pp 23–30). The increased amount of serotype 19A isolates in countries after PCV10 vaccination is perturbing because of the increased penicillin non-susceptibility (minimum inhibitory concentration ≥0·12 μg/mL) and multi-drug resistance in isolates. S pneumoniae 19A strains have been molecularly characterised in Colombia,25 Argentina,26 and Brazil.27 Serotype 19A has increased in Colombia and Brazil related to the expansion of CC320, by contrast with Argentina where it was mainly associated with clonal complex CC172.
Serotype replacement after PCV immunisation has been reported worldwide because of replacement by non-vaccine type in the nasopharynx, the site where invasive disease arises. Serotype replacement has been reported in Brazil,12 Uruguay,19 Mexico,20 and Argentina;21 similar reports have been published in the USA,28, 29 and in England and Wales.24
Molecular studies such as multilocus sequencing typing and whole-genome sequencing for characterising ancestries have revealed high serotype genetic diversity that has been used for understanding serotype replacement. The Global Pneumococcal Sequencing Consortium has proposed a cluster concept. Some clusters include vaccine type and non-vaccine type, which could explain serotype replacement in these lineages.30
Information from SIREVA passive laboratory surveillance can guide the formulation of new PCVs to be introduced into the Latin American and Caribbean region. However, the situation with the prevalent serotypes is very different in the pre-vaccination period compared with the post-vaccination period.6, 7 A group of few serotypes was prevalent in causing invasive pneumococcal disease in children during the pre-vaccination period; it was thus possible to define a PCV formulation for this age group. It is difficult to predict a group of common serotypes for the region from the information available to date as there is clear country diversity.
A 20-valent pneumococcal conjugate vaccine (PCV20) is undergoing clinical evaluation, in principle for the adult population, but it could become available for children younger than 2 years in the future. This vaccine incorporates serotypes 8, 10A, 11A, 12F, 15B, 22F, and 33F in the formulation, besides those present in PCV13.31 The percentage of these extra serotypes in the eight countries involved in the surveillance was calculated for 2015–17: Brazil 15·2% (67 of 441), Chile 13·6% (55 of 404), Colombia 3·1% (11 of 352), Paraguay 10·1% (11 of 109), Argentina 20·7% (90 of 434), the Dominican Republic 3·4% (two of 58), Mexico 11·1% (17 of 153), and Uruguay 23·0% (20 of 87). However, one must also consider that during 2015–17, serotype 24F (non-PCV20) alone represented 7·4% (30 of 404) in Chile, 7·6% (33 of 434) in Argentina, and 6·9% (six of 87) in Uruguay.
Adding a few serotypes to new conjugated vaccines should be carefully thought out, as there is no clear pattern concerning which non-vaccine type serotypes should be considered in a new vaccine formulation; surveillance continuity is thus of the utmost importance because of the high variety of non-vaccine types between countries. Effective strategies for developing innovative vaccines including non-capsular pneumococcal antigens, proving immunogenic in children, must be prioritised.32
Our analysis regarding interpreting invasive pneumococcal disease surveillance data for serotype replacement relies on countries that have had a surveillance duration of 4–7 years before the intervention and after PCV introduction, have serotyped all isolates, have an invasive pneumococcal disease case definition, and have collected clinical and epidemiological data.28 Laboratory practices have remained stable during the study period and although some countries have shown an enhancement of their surveillance after vaccine introduction, interestingly, the results still show a decrease in the total amount and percentage in relation to total isolates for vaccine types, whereas there is an increase in the number and percentage for the non-vaccine types, as is the case in Colombia and Argentina. This study relies on a laboratory-based surveillance with its inherent limitations and differences within and among countries. Identification, reporting, and laboratory diagnosis of case is not necessarily uniform, differing among health-care providers, hospitals, and countries. One of the study's limitations concerns the lack of information about the vaccination status of the cases reported. These limitations require better and stronger communication between clinicians, epidemiologists, and laboratories. It is strongly recommended that regional and local health authorities improve surveillance data by making it compulsory to report invasive pneumococcal diseases, thereby facilitating future analysis; at present, only pneumococcal meningitis is considered compulsory.
All Latin American and Caribbean countries' investment in introducing PCVs by the expanded programme on immunisation in the region justify better monitoring of the impact and trends in vaccine type as well as in non-vaccine type isolates. This information will be relevant for future decisions regarding whether new vaccines are needed.
Acknowledgments
Acknowledgments
We thank the staff of the national laboratory networks, and the clinicians and epidemiologists of the ten participating countries. We also thank Jean Marc Gabastou (PAHO, Mexico); Gabriela Andrade Pereira, Camile de Moraes, and Camila de Oliveira Portela (SVS-Ministério da Saúde, Brasília, Brazil); Félix Dickinson Meneses (Departamento de Epidemiología, La Habana, Cuba); Zacarías Garib (Director de Programa Ampliado de Inmunizaciones, Santo Domingo, Dominican Republic), Gilda Tolari and Juana Baez (Laboratorio Hospital Plaza de la Salud, Santo Domingo, Dominican Republic), Genara Santana (Hospital Pediátrico Dr Arturo Grullón, Santiago, Dominican Republic), and Josefina Fernández, Hilma Coradín, Chabela Peña, Milagro Peña, and Pablo Mancebo (Hospital Infantil Dr Roberto Reid Cabral, Santo Domingo, Dominican Republic); Gloria Gómez (Hospital Nacional de Itaugua, Paraguay), Juana Ortellado (Hospital de Clínicas, Paraguay), Myrian Leguizamon (Instituto de Previsión Social, Paraguay), Raquel Blasco (Hospital Regional de Ciudad del Este, Paraguay), and Juan Irala (Instituto de Medicina Tropical, Paraguay); and Evelys Villarroel, Carmen Moreno, Carmen Ugarte, Faviola González, Yuraima Escalona, Lisette Sandrea, Alberto Calvo, Ninoska Montilla, and Celina Elster (Maria C Martinez Instituto Nacional de Higiene Rafael Rangel, Caracas, Venezuela). We finally thank Jason Christopher Garry for reviewing the English version of this manuscript.
Contributors
All authors were responsible for the literature search. CIA, MCdCB, GE-A, and EC proposed the study idea. CIA, CC-O, MCdCB, and EC did the study design and designed the overall study protocol. CIA, CC-O, ALA, and EC were responsible for data collection and data management. CIA, CC-O, and EC developed the analysis plan and did the analysis. CC-O and ALA did the statistical analyses of the pooled data. MCdCB, GE-A, SCGA, MNC-B, CD, MR, SF, PAl, PAr, JS, MN, GT-P, MR-O, GC-C, AK, GG-G, TC, ES, and DP were the microbiological leads for pneumococcal surveillance activities. CIA, CC-O, MCdCB, GE-A, SCGA, JLDF, and EC wrote the different versions of the draft. The SIREVA working group read and approved the final version. All authors read, commented on, and approved the final version of the manuscript.
SIREVA Working Group
Argentina A Corso, P Gaguetti, O Veliz, C S Pereira, D Napoli, M A Moscoloni (INEI-ANLIS Dr Carlos G Malbrán, Buenos Aires). Brazil M L L Silva e Guerra, S Bokermann, L S do Prado, U J Días (Centro de Bacteriología, Instituto Adolfo Lutz, São Paulo). Chile J C Hormazabal, B Rojas, S Castro, D Ibáñez, J Fernandez (Instituto de Salud Pública, Santiago de Chile). Colombia O Sanabria, J Moreno, Z Alarcón, M K Rodriguez, A Bautista (Instituto Nacional de Salud, Bogotá). Cuba L P Solis, D S Apaza, M A Capote (IPK, Laboratorio Referencia Nacional). Dominican Republic J M Feris (Hospital Robert Reid Cabral, Santo Domingo), D Cedano (Laboratorio de Microbiología del Hospital Robert Reid Cabral, Santo Domingo). Mexico A Soto-Noguerón, M E Velázquez-Meza, M Hernández-Salgado (Instituto Nacional de Salud Pública, Cuernavaca, Morelos). Paraguay M E León, M Nagai, L Rojas (Laboratorio Central de Salud Pública, Asunción). Uruguay M L Vega, G P Giffoni, V Félix (Departamento de Laboratorios, Ministerio de Salud, Montevideo).
Declaration of interests
CIA and EC have contributed to a systematic literature review for GlaxoSmithKline. MCdCB has received a lecture travel grant and support for research from Pfizer. SCGA has received a travel grant and support for research from Pfizer. ALA has received a grant for a carrier study from Pfizer and belongs to the Merck Sharp & Dohme's advisory board. All other authors declare no competing interests.
Contributor Information
SIREVA Working Group:
A Corso, Paula Gaguetti, Omar Veliz, C S Pereira, D Napoli, M A Moscoloni, M L L Silva e Guerra, S Bokermann, Lincoln S do Prado, U J Días, J C Hormazabal, B Rojas, S Castro, D Ibáñez, J Fernandez, O Sanabria, J Moreno, Z Alarcón, M K Rodriguez, A Bautista, L P Solis, D S Apaza, M A Capote, J M Feris, D Cedano, Araceli Soto-Noguerón, Maria E Velázquez-Meza, Margarita Hernández-Salgado, M E León, Minako Nagai, Liliana Rojas, M L Vega, G P Giffoni, and V Félix
Supplementary Materials
References
- 1.McAllister DA, Liu L, Shi T, et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob Health. 2019;7:e47–e57. doi: 10.1016/S2214-109X(18)30408-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wahl B, O'Brien KL, Greenbaum A, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health. 2018;6:e744–e757. doi: 10.1016/S2214-109X(18)30247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . World Health Organization; Geneva: 2017. Pneumococcal conjugate vaccine (PCV) review of impact evidence (PRIME). Summary of findings from systematic review.https://www.who.int/immunization/sage/meetings/2017/october/presentations_background_docs/en/ [Google Scholar]
- 4.Izurieta P, Bahety P, Adegbola R, Clarke C, Hoet B. Public health impact of pneumococcal conjugate vaccine infant immunization programs: assessment of invasive pneumococcal disease burden and serotype distribution. Expert Rev Vaccines. 2018;17:479–493. doi: 10.1080/14760584.2018.1413354. [DOI] [PubMed] [Google Scholar]
- 5.Di Fabio JL, Homma A, de Quadros C. Pan American Health Organization epidemiological surveillance network for Streptococcus pneumoniae. Microb Drug Resist. 1997;3:131–133. doi: 10.1089/mdr.1997.3.131. [DOI] [PubMed] [Google Scholar]
- 6.Di Fabio JL, Castañeda E, Agudelo CI, et al. Evolution of Streptococcus pneumoniae serotypes and penicillin susceptibility in Latin America, Sireva-Vigía Group. 1993–1999. Pediatr Infect Dis J. 2001;20:959–967. doi: 10.1097/00006454-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Castañeda E, Agudelo CI, Regueira M, et al. Laboratory-based surveillance of Streptococcus pneumoniae invasive disease in children in 10 Latin American countries: a SIREVA II project, 2000–2005. Pediatr Infect Dis J. 2009;28:e265–e270. doi: 10.1097/INF.0b013e3181a74b22. [DOI] [PubMed] [Google Scholar]
- 8.de Oliveira LH, Trumbo SP, Matus CR, Sanwogou NJ, Toscano CM. Pneumococcal conjugate vaccine introduction in Latin America and the Caribbean: progress and lessons learned. Expert Rev Vaccines. 2016;15:1295–1304. doi: 10.1586/14760584.2016.1166961. [DOI] [PubMed] [Google Scholar]
- 9.Pan American Health Organization/WHO . Pan American Health Organization/World Health Organization; Washington DC: 2016. SIREVA II.www.paho.org/hq/index.php?option=com_content&view=article&id=5536:2011-sireva-ii&Itemid=3966&lang=frS [Google Scholar]
- 10.da Gloria Carvalho M, Pimenta FC, Jackson D, et al. Revisiting pneumococcal carriage by use of broth enrichment and PCR techniques for enhanced detection of carriage and serotypes. J Clin Microbiol. 2010;48:1611–1618. doi: 10.1128/JCM.02243-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalgaard P. Introductory statistics with R statistics and computing. 2002. http://www.academia.dk/BiologiskAntropologi/Epidemiologi/PDF/Introductory_Statistics_with_R__2nd_ed.pdf
- 12.Brandileone MC, Almeida SCG, Minamisava R, Andrade AL. Distribution of invasive Streptococcus pneumoniae serotypes before and 5 years after the introduction of 10-valent pneumococcal conjugate vaccine in Brazil. Vaccine. 2018;36:2559–2566. doi: 10.1016/j.vaccine.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 13.UN Department of Economic and Social Affairs Population Dynamics World population prospects: the 2017 revision. 2017. https://esa.un.org/unpd/wpp/
- 14.Muggeo VMR. Interval estimation for the breakpoint in segmented regression: a smoothed score-based approach. Aust N Z J Stat. 2017;59:311–322. [Google Scholar]
- 15.Naucler P, Galanis I, Morfeldt E, Darenberg J, Örtqvist Å, Henriques-Normark B. Comparison of the impact of pneumococcal conjugate vaccine 10 or pneumococcal conjugate vaccine 13 on invasive pneumococcal disease in equivalent populations. Clin Infect Dis. 2017;65:1780–1789. doi: 10.1093/cid/cix685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Wals P, Lefebvre B, Deceuninck G, Longtin J. Incidence of invasive pneumococcal disease before and during an era of use of three different pneumococcal conjugate vaccines in Quebec. Vaccine. 2018;36:421–426. doi: 10.1016/j.vaccine.2017.11.054. [DOI] [PubMed] [Google Scholar]
- 17.Waight PA, Andrews NJ, Ladhani NJ, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15:535–543. doi: 10.1016/S1473-3099(15)70044-7. [DOI] [PubMed] [Google Scholar]
- 18.Pírez MC, Algorta G, Cedrés A, et al. Impact of universal pneumococcal vaccination on hospitalizations for pneumonia and meningitis in children in Montevideo, Uruguay. Pediatr Infect Dis J. 2011;30:669–674. doi: 10.1097/INF.0b013e3182152bf1. [DOI] [PubMed] [Google Scholar]
- 19.García Gabarrot G, López Vega M, Pérez Giffoni G, et al. Effect of pneumococcal conjugate vaccination in Uruguay, a middle-income country. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Echániz-Aviles G, Soto-Noguerón A, Miranda-Novales G, et al. Streptococcus pneumoniae serotypes identified in Mexican children with invasive disease before and after introduction of PCV7 (1993–2012) Arch Med Res. 2015;46:149–153. doi: 10.1016/j.arcmed.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Gentile A, Bakir J, Firpo V, et al. A multicenter study of pneumonia in 10 pediatric hospitals in Argentina. PLoS One. 2018;13 doi: 10.1371/journal.pone.0199989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva-Costa C, Brito MJ, Pinho MD, et al. Pediatric complicated pneumonia caused by Streptococcus pneumoniae serotype 3 in 13-valent pneumococcal conjugate vaccinees, Portugal, 2010–2015. Emerg Infect Dis. 2018;24:1307–1314. doi: 10.3201/eid2407.180029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hausdorff WP, Hoet B, Adegbola RA. Predicting the impact of new pneumococcal conjugate vaccines: serotype composition is not enough. Expert Rev Vaccines. 2015;14:413–428. doi: 10.1586/14760584.2015.965160. [DOI] [PubMed] [Google Scholar]
- 24.Domingues CM, Verani JR, Montenegro Renoiner EI, et al. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study. Lancet Respir Med. 2014;2:464–471. doi: 10.1016/S2213-2600(14)70060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramos V, Parra EL, Duarte C, Moreno J. Characterization of Streptococcus pneumoniae invasive serotype 19A isolates recovered in Colombia. Vaccine. 2014;32:755–758. doi: 10.1016/j.vaccine.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 26.Gagetti P, Faccone D, Reijtman V, et al. Characterization of Streptococcus pneumoniae invasive serotype 19A isolates from Argentina (1993–2014) Vaccine. 2017;35:4548–4553. doi: 10.1016/j.vaccine.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 27.Cassiolato AP, Almeida SCG, Andrade AL, Minamisava R, Brandileone MCdC. Expansion of the multidrug-resistant clonal complex 320 among invasive Streptococcus pneumoniae serotype 19A after the introduction of a ten-valent pneumococcal conjugate vaccine in Brazil. PLoS One. 2018;13 doi: 10.1371/journal.pone.0208211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feikin DR, Kagucia EW, Loo JD, et al. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olarte L, Kaplan SL, Barson WJ, et al. Emergence of multidrug-resistant pneumococcal serotype 35B among children in the United States. J Clin Microbiol. 2017;55:724–734. doi: 10.1128/JCM.01778-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo SW, Gladstone RA, van Tonder AJ, et al. Pneumococcal lineages associated with serotype replacement and antibiotic resistance in childhood invasive pneumococcal disease in the post-PCV13 era: an international whole-genome sequencing study. Lancet Infect Dis. 2019;19:759–769. doi: 10.1016/S1473-3099(19)30297-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson A, Lamberth E, Severs J, et al. Phase 1 trial of a 20-valent pneumococcal conjugate vaccine in healthy adults. Vaccine. 2019;37:6201–6207. doi: 10.1016/j.vaccine.2019.08.048. [DOI] [PubMed] [Google Scholar]
- 32.Chan WY, Entwisle C, Ercoli G, et al. A novel, multiple-antigen pneumococcal vaccine protects against lethal Streptococcus pneumoniae challenge. Infect Immun. 2019;87:e00846–e00918. doi: 10.1128/IAI.00846-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.