Abstract
Purpose
The purpose of this systematic review was to assess potential gender differences in prevalence and clinical relevance of insulin-related lipohypertrophy (LH).
Patients and Methods
Five electronic databases (PubMed, Embase, CNKI, Wanfang and VIP) were systematically searched for studies, from inception to 1st Sep 2022, on the prevalence of insulin-related LH. The eligibility of articles was independently screened, and the included studies were evaluated using standardized quality assessment tools.
Results
A total of 22 studies mentioned the LH prevalence in different genders, of which two are about gestational diabetes; therefore, 20 studies were eventually included, providing data on 6238 patients. The prevalence of LH varied from 30.26% to 72.54%. Ten studies (4392 patients) were conducted with the adult diabetes patients of different genders over the age of 18, the total prevalence rate of LH was 51.73%, the LH prevalence in male gender was from 41.94% to 68.57% and the rate of the total population was 54.89% (2046 patients); The LH prevalence in female gender was from 33.18% to 70% and the rate of the total population was 48.98% (2346 patients), and the prevalence of LH was significantly different between male and female gender (P<0.001). Interestingly, only one study (n=1227) showed that there were dramatic differences between different genders (P<0.001), the subjects were T2DM patients, the LH prevalence rate of male vs female was 70.52% (299/424) VS 52.18% (419/803), while the other studies either only include T1DM or both T1DM and T2DM.
Conclusion
The evidence shows that the results of gender differences in the LH prevalence are inconsistent with different types of DM. Probably, there is no gender differences in the LH prevalence in adult patients with T1DM, but it has a gender difference between male and female in T2DM. More strictly designed clinical studies are needed to further verify and reveal the underlying mechanisms.
Keywords: insulin injection, lipohypertrophy, gender differences, hypertrophy, diabetes mellitus, prevalence
Introduction
Diabetes mellitus (DM) is an increasingly serious public health problem that is affecting the whole world. The global prevalence of DM was estimated to be 9.3% in 2019, which will rise to 10.2% by 2030 and 10.9% by 20451. According to a recent investigation,2 It is estimated that the total prevalence of DM is 12.4%, and the prevalence of pre-diabetes is 38.1% in China, which is higher than the global diabetes prevalence (8.3%, 2019), and the number of patients with DM has exceeded 140 million.
All T1DM patients and around one-third of T2DM patients need extrinsic insulin therapy for controlling hyperglycemia.3 Despite the technical progress of insulin injection and pump equipment, the inaccuracy of basic injection technology is still an obstacle to diabetes control. If the clinical medical staff cannot guide the patients to master the correct insulin injection method, including the selection of appropriate injection equipment, the selection of injection site, the angle and length of injection, the replacement of needles, and the dynamic monitoring of Lipohypertrophy, the prevalence of Lipohypertrophy would increase significantly.4
Lipohypertrophy (LH) is characterized by nodular swelling caused by fat accumulation around the sites of subcutaneous insulin injection. The insulin-related LH occurs in subcutaneous tissue associated with insulin injection or infusion therapy, which are very common, the average lipohypertrophy rate is 41.8%,5 with a wide rage as 1.9% to 77.1%.6,7 Primarily, the trauma of the repeated injection-related and the lack of rotation and replacement of injection sites would play a key role in the formation of LH; however, the pathophysiological mechanism is unclear. The occurrence of LH may be related to the growth promoting properties and immune process of insulin, as well as the excessive inflammatory cytokines like the tumor necrosis factor-a (TNF-α) produced at the injection site of insulin.8,9 The biggest impact of LH on patients is to affect the insulin absorption at the site of injection, which may cause the blood sugar to rise and fall conspicuously, insulin injection at the LH sites can reduce drug absorption by 25%10 and the variability of insulin uptake would increase by 3 to 5 times.11
LH prevalence has been reported worldwide with wide variations among individual studies, indicating that there are incomparable obstacles against proper lipohypertrophy recognition.12 Some recent studies5,13 suggest that frequency of LH would depend on the type of DM, different age, different gender, duration of insulin use, duration of DM, needle reuse, times of injection, etc. As everyone knows, numerous diseases, including type 2 diabetes, have gender differences between male and female gender. However, there was no separate research report on gender differences in LH prevalence of T1DM or T2DM, nor other studies on the mechanism of LH gender differences.
Although the overall LH prevalence of T1DM or T2DM has been fully clarified, the gender differences in the prevalence of LH of T1DM or T2DM have historically received little attention. Hence, we conducted gender differences in the LH prevalence of different types of DM by integrating data from existing research literatures (N=6238). The purpose of this systematic review was to assess potential gender differences in prevalence and clinical relevance of insulin-related lipohypertrophy (LH).
Methods
Search Strategy
Two reviewers independently conducted a comprehensive literature search. Databases of PubMed, Embase, CNKI, Wanfang and VIP were screened from inception to 1st Sep 2022. Both Medical Subject Headings (MeSH) and free-text keywords were used for retrieving related studies, as the terms “lipohypertrophy”, “insulin”, “diabetes”, “diabetes mellitus”, “lipodystrophy”, “subcutaneous nodules”, “prevalence”, “gender differences”, “Insulin injection”, “insulin hypertrophy” and “sex differences” were utilized in various kinds of combinations for the electronic search.
Inclusion Criteria
This system review was conducted according to PRISMA statement, except for the protocol registration.14
The Following Inclusion Criteria
All cross-sectional or longitudinal study data
Researches associated the LH prevalence in T1DM or T2DM or Pancreatic DM subjects on insulin injection or infusion treatment
Researches that detection for LH with palpation, ultrasonography, or observation
The Following Studies Were Excluded
Researches that not disclosing relevant data
Researches not performed on subjects of DM patients with insulin treatment, such as subjects on long-term hemodialysis
Researches that not disclosing the number of different gender subjects on insulin treatment
Researches on gestational DM patients
Review studies, non-English and non-Chinese language literatures and researches that lack the corresponding data
Intervention study, case report and irrelevant information
Data Extraction
The data were independently extracted from the included articles by two reviewers. The main results are the prevalence of LH caused by insulin injection in patients of different genders, and the prevalence of gender differences in different types of diabetes. Additional data collected included study design, publishing time, age, population, sample size, duration of DM, time of insulin treatment, patient characteristics, type of insulin and LH location.
Data Synthesis
The data were synthesized applying a narrative method handling the displayed review subjects. Due to the diversity and high heterogeneity of the included studies, meta-analysis cannot be provided, but all the included articles were performing a comprehensive assessment of the research conclusions.
Quality Assessment
Relevant studies confirmed to be included in the review reported the prevalence of LH in different genders. We used a variety of assessment tools to evaluate the scientific rigor and study quality, including the Cross-Sectional Studies devised by the National Institutes of Health.15 The quality of the included researches was independently evaluated by two reviewers. The quality of the estimations of the studies was performed by the Recommended Rating Assessment, Development and Evaluation (GRADE) methods, when considering the above inclusion criteria, and the risk of bias, heterogeneity, imprecision, and other information that is provided in the included studies. We would use quality assessments to grade the quality of evidence from high to low.16
Ethical Considerations
This review does not include any data from other new research conducted by any authors of human subjects; therefore, no ethical approval is needed.
Results
The Summary of Included Studies
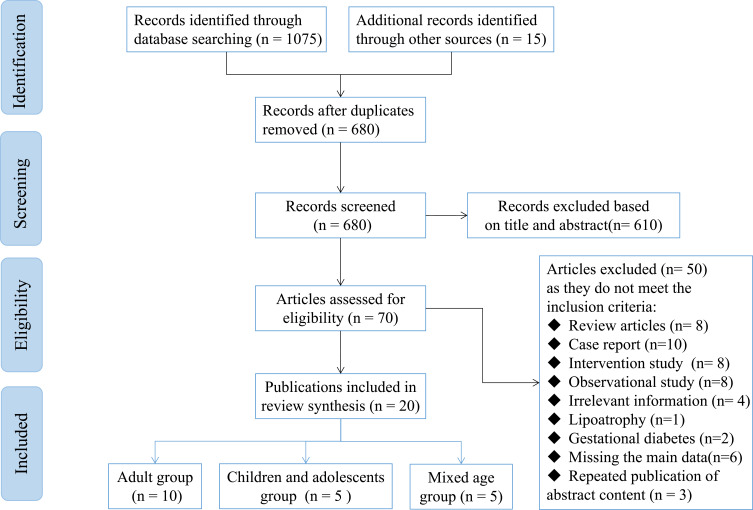
As depicted in Table 1 and Figure 1, a total of 22 studies mention the LH prevalence in different genders, of which two are gestational diabetes; therefore, 20 studies were finally included, containing 6238 patients. Distinguished by age, there are 10 studies involving adult diabetes, four studies are on juvenile diabetes, and five studies are on mixed juvenile and adult diabetes. In terms of different types of DM, only one study is on T2DM, 12 studies are on T1DM, five studies are on T1DM and T2DM or Pancreatic DM, and two studies do not mention type of DM. The mean of the diabetes duration was over 4 years, most studies were performed to confirm LH through observation and palpation, and four studies combined ultrasound examination. As the BMI and HbA1c (%) data are shown in Table 1, the heterogeneity of data of the included studies is very large, but a trend could be seen, that is, the larger the BMI, or the higher the average HbA1c (%), and the higher the incidence of LH.
Table 1.
Summary of Included Studies
| Author (Year), Country | Study Population | Sample Population | Mean Diabetes Duration (Years, SD) | Mean Duration of Insulin use (Years, SD) | Detection Method(s), | BMI | HbA1c (%) | |
|---|---|---|---|---|---|---|---|---|
| Sample | Type of Diabetes | |||||||
| Lin et al (2021)19 | Adult diabetes | Size: n=120 Mean age (years, SD): 59.21 ± 11.44 Gender (M/F): 50/70 |
T1DM: 120 T2DM: 0 |
ND | 6.56 ± 4.26 | Detection Observation, palpation, ultrasound examination | 24.93 ± 3.18 | 9.45 ± 1.85% |
| Arora et al (2021)20 | Adult diabetes | Size: n=500 Mean age (years, SD): 45.2 ± 16.5 Gender (M/F): 272/228 |
T1DM: 113 T2DM: 356 Pancreatic DM: 31 |
26.8 ± 6.9 | 3 (2.5–5.0) | Palpation, ultrasound examination | <18.5: 88 (17.6) 18.5–23.5: 192 (38.4) ≥23.5220 (44.0) |
Mean: 9.9 ± 1.9 LH: 10.53 ± 1.73 No LH: 8.953 ± 0.5281 |
| Gentile et al (2021)21 | Adult diabetes | Size: n=780 Mean age (years, SD): 62 ± 15 Gender (M/F): 387/393 |
T1DM: 224 T2DM: 556 |
18 ± 11 | 10.1 ± 2.11 | Palpation | 29 ± 6 | 7.8 ± 1.3 |
| Thewjitcharoen et al (2020)22 | Adult diabetes | Size: n=400 Mean age (years, SD): 65.6 ± 15.4 Gender (M/F): 186/214 |
T1DM: 56 T2DM: 344 |
Mean: 23.0 ± 10.2 LH: 20.1 ± 11.6 No LH: 23.5 ± 9.8 |
Mean: 11.4 ± 8.7 LH: 18.9 ± 11.0 No LH: 10.2 ± 7.6 |
Palpation | Mean: 26.2 ± 4.8 LH: 23.8 ± 3.6 No LH: 26.5 ± 4.8 |
Mean: 7.9 ± 1.6 LH: 7.8 ± 1.5 No LH: 7.9 ± 1.6 |
| Gentile et al (2020)12 | Adult diabetes | Size: n=1227 Mean age (years, SD): 50.1 ± 10.5 Gender (M/F): ND |
T1DM: 0 T2DM: 1227 |
26.8 ± 6.9 | Age≤65: 8 ± 4 Age >65: 12 ± 5 |
Palpation, ultrasound examination | Age≤65: 8 ± 4 Age >65: 12 ± 5 |
Age≤65: 8.1 ± 1.2 Age >65: 8.9 ± 1.4 |
| Lian et al (2018)23 | Adult diabetes | Size: n=300 Mean age (years, SD): Male: 48.00 ± 3.0 Femal: 47.0 ± 4.0 Gender (M/F): 148/152 |
T1DM: ND T2DM: ND |
LH: 10.98 ± 3.12 No LH: 7.24 ± 3.53 |
LH: 4.47 ± 3.12 No LH: 7.24 ± 3.53 |
Palpation, ultrasound examination | ND | ND |
| Tsadik et al (2018)24 | Adult diabetes | Size: n=176 Mean age (years, SD): 51.36 ± 3.96 Gender (M/F): 86/90 |
T1DM: 176 T2DM: 0 |
ND | 1–5: 65/46 >5: 38/27 |
Observation and the palpation | Underweight: 14/14 Healthy weight: 78/52 Obese: 6/4 |
ND |
| Hernar et al (2017)17 | Adult diabetes | Size: n=215 Mean age (years, SD): 36.0 (18–82) Gender (M/F): 111/104 |
T1DM: 215 T2DM: 0 |
17.0 (1–57) | ND | Observation and the palpation | ND | 8.0 (5.5–14.0) |
| Al Hayek et al (2016)25 | Adult diabetes | Size: n=174 Mean age (years, SD): 55.43 ± 1.97 Gender (M/F): 90/84 |
T1DM: 174 T2DM: 0 |
6.1 ± 4.5 | ND | Observation and the palpation | ND | ND |
| Sawatkar et al (2014)26 | Adult diabetes | Size: n=500 Mean age (years, SD): 26.9 ± 6.9 Gender (M/F): 272/228 |
T1DM: 500 T2DM: 0 |
4.43 ± 4.4 | 12.51 ± 6.68 | Observation and the palpation | 19.2 ± 5.4 | 9.72 ± 3.0 |
| Singha et al (2021)27 | Juvenile diabetes | Size: n=91 Mean age (years, SD): 13.3 ± 4.1 Gender (M/F): 34/57 |
T1DM: 91 T2DM: 0 |
LH: 5.3 ± 2.7 No LH: 5.5 ± 2.8 |
ND | Observation and the palpation | ND | LH: 9.0 ± 2.6 No LH: 7.7 ± 1.1 |
| McCann et al (2019)28 | Juvenile diabetes | Size: n=76 Mean age (years, SD): 4–18 Gender (M/F): ND |
T1DM: 76 T2DM: 0 |
<4: 43%LH >4: 57%LH |
ND | Observation and the palpation | ND | ND |
| Magdy et al (2011)33 | Juvenile diabetes | Size: n=119 Mean age (years, SD): 2 months to 21 Years Gender (M/F): 64/55 |
T1DM: 119 T2DM: 0 |
4.13 ± 3.67 | ND | Observation and the palpation | ND | Normal: 76.1% Overweight: 15.0% Obese: 3.5% under weight: 5.3% |
| Conwell et al (2008)29 | Juvenile diabetes | Size: n=50 Mean age (years, SD): 13.3 ± 3.5 Gender (M/F): 24/26 |
T1DM: 50 T2DM: 0 |
6.5 ± 3.7 | 2.8 ± 1.7 | Observation and the palpation | 21.6 ± 4.8 | 7.7 ± 1.1 |
| Lombardo et al (2022)37 | Juvenile diabetes | Size: n=212 Mean age (years, SD): 11.9 ± 4.7 Gender (M/F): 123/89 |
T1DM: 212 T2DM: 0 |
4.8 ± 3.4 | ND | Inspection and palpation | ND | 6.8 ± 1.6 |
| Yan et al (2021)30 | Mixed juvenile and Adult diabetes | Size: n=142 Mean age (years, SD): ≤18: 43/8 >18: 60/31 Gender (M/F): 82/60 |
T1DM: ND T2DM: ND |
ND | LH: 4 (3, 7) No LH: 5 (3,7) |
Observation and the palpation | LH: 20.56 (17.89, 23.0) No LH: 21.48 (20.06,22.6) |
ND |
| Barola et al (2018)31 | Mixed juvenile and Adult diabetes | Size: n=372 Mean age (years, SD): 17.1 (5.0–50.6) Gender (M/F): 204/168 |
T1DM: 372 T2DM: 0 |
5.6 ± 5.3 | Mean: 7.9 ± 1.6 LH: 7.8 ± 1.5 No LH: 7.9 ± 1.6 |
Observation and the palpation | ND | Mean: 9.7 ± 2.6 LH: 10.0 ± 2.7 No LH: 9.2 ± 2.4 |
| Gupta et al (2018)32 | Mixed juvenile and Adult diabetes | Size: n=139 Mean age (years, SD): 21.71 ± 11.78 <18: 11.73 ± 3.96 ≥18: 25.51 ± 9.79 Gender (M/F): 75/64 |
T1DM: 139 T2DM: 0 |
8.70 ± 7.52 | ND | Observation and the palpation | 19.90 ± 4.68 | 9.71 ± 2.29 |
| Blanco et al (2013)9 | Mixed juvenile and Adult diabetes | Size: n=430 Mean age (years, SD): 49.0 ± 22.8 Gender (M/F): 221/202 |
T1DM: 177 T2DM: 253 |
ND | ND | Observation and the palpation | ND | ND |
| Vardar et al (2007)34 | Mixed juvenile and Adult diabetes | Size: n=215 Mean age (years, SD): ND Gender (M/F): 137/78 |
T1DM: 31 T2DM: 184 |
26.8 ± 6.9 | 0–5 years: 66 (30.7%) 6–10 years: 59 (27.4%) 11–15 years: 57 (26.5%) 16–20 years: 33 (15.4%) |
Observation and the palpation | Normal: 72 (33.5%) Overweight: 86 (40%) Obese: 57 (26.5%) |
|
Abbreviation: ND, not detected.
Figure 1.
PRISMA flowchart for inclusion of selected articles in this systematic review.
Note: PRISMA figure adapted from Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. Creative Commons.14
Gender Differences in the Demographic, Prevalence, and Clinical Features of LH in Diabetes Mellitus Patients
The LH prevalence varied from 30.26% to 72.54%. And LH prevalence in males is mildly higher than in females in most included studies. As shown in Table 2, the total 10 articles (4392 patients) were studies about the adult diabetes patients of different genders over the age of 18, the total prevalence rate of LH was 51.73%, the LH prevalence rate of male adult ranged from 41.94% to 68.57%, while in female adult ranged from 33.18% to 70.0%, and the overall population ranged from 37.25% to 69.17%. Most studies showed that the LH prevalence in male adults is mildly higher than that in female genders, as shown in Table 2, but most of them have no significant statistical difference between different genders (P>0.05). Only one study12 suggested that the LH prevalence in males is dramatically significantly higher than that in female (P<0.0001). However, the gender differences in the LH prevalence in juvenile diabetes were diametrically opposite in different studies, such as shown in the studies of Singha et al27 (31.25% vs 55.32%, P=0.0110<0.05) and Lombardo et al37 (52.03% vs 35.96%, P=0.0203<0.05), this also could be seen in the other five mixed juvenile and adult DM studies (Tables 3 and 4).
Table 2.
Gender Differences in the Prevalence, Demographic and Clinical Features of LH in DM Patients (Adult DM)
| Author (Year) | Male | Female | All | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Present No (% Among Patients with LH) | Absent No (% Among Patients Without LH) | LH Prevalence (%) | Present No (% Among Patients with LH) | Absent No (% Among Patients Without LH) | LH Prevalence (%) | Present No (% Among Patients with LH) | Absent No (% Among Patients Without LH) | Overall | LH Prevalence (%) | ||
| Lin et al (2021)19 | 48(57.83%) | 22(59.46%) | 68.57% | 35(42.17%) | 15(40.54%) | 70.00% | 83(69.17%) | 37(30.83%) | 120 | 69.17% | 0.8673 |
| Arora et l. (2021)20 | 151(52.07%) | 272(54.40%) | 55.51% | 139(47.93%) | 228(45.60%) | 60.96% | 290(58.00%) | 210(42.00%) | 500 | 58.00% | 0.2188 |
| Gentile et al (2021)21 | 183(50.83%) | 204(48.57%) | 47.29% | 177(49.17%) | 216(51.43%) | 45.04% | 360(46.15%) | 420(53.85%) | 780 | 46.15% | 0.5288 |
| Thewjitcharoen et al (2020)22 | 78(52.35%) | 108(43.03%) | 41.94% | 71(47.65%) | 143(56.97%) | 33.18% | 149(37.25%) | 251(62.75%) | 400 | 37.25% | 0.0708 |
| Gentile et al (2020)12 | 299(41.64%) | 125(24.56%) | 70.52% | 419(58.36%) | 384(75.44%) | 52.18% | 718(58.52%) | 509(41.48%) | 1227 | 58.52% | <0.0001 |
| Lian et al (2018)23 | 85(50.00%) | 63(48.46%) | 57.43% | 85(50.00%) | 67(51.54%) | 55.92% | 170(56.67%) | 130(43.33%) | 300 | 56.67% | 0.7917 |
| Tsadik et al (2018)24 | 53(51.46%) | 33(45.21%) | 61.63% | 50(48.54%) | 40(54.79%) | 55.56% | 103(58.52%) | 73(41.48%) | 176 | 58.52% | 0.4137 |
| Hernar et al (2017)17 | 64(57.66%) | 47(45.19%) | 57.66% | 47(42.34%) | 57(54.81%) | 45.19% | 111(51.63%) | 104(48.37%) | 215 | 51.63% | 0.0767 |
| Al Hayek et al (2016)25 | 43(51.81%) | 47(51.65%) | 47.78% | 40(48.19%) | 44(48.35%) | 47.62% | 83(47.70%) | 91(52.30%) | 174 | 47.70% | 0.9833 |
| Sawatkar et. (2014)26 | 119(58.05%) | 153(51.86%) | 43.75% | 86(41.95%) | 142(48.14%) | 37.72% | 205(41.00%) | 295(59.00%) | 500 | 41.00% | 0.1721 |
| Total | 1123(49.43%) | 923(43.54%) | 54.89% | 1149(50.57%) | 1197(56.46%) | 48.98% | 2272(51.73%) | 2120(48.27%) | 4392 | 51.73% | <0.0001 |
Table 3.
Gender Differences in the Prevalence, Demographic and Clinical Features of LH in DM Patients (Juvenile DM)
| Author (Year) | Male | Female | All | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Present No (% Among Patients with LH) | Absent No (% Among Patients Without LH) | LH Prevalence (%) | Present No (% Among Patients with LH) | Absent No (% Among Patients without LH) | LH Prevalence (%) | Present No (% Among Patients with LH) | Absent No (% Among Patients Without LH) | Overall | LH Prevalence (%) | ||
| Singha et al (2021)27 | 20(43.48%) | 44(67.69%) | 31.25% | 26(56.52%) | 21(32.31%) | 55.32% | 46(50.55%) | 45(49.45%) | 91 | 50.55% | 0.0110 |
| McCann et al (2019)28 | 13(56.52%) | 28(50.00%) | 31.71% | 10(43.48%) | 28(50.00%) | 26.32% | 23(30.26%) | 53(69.74%) | 76 | 30.26% | 0.6290 |
| Blanco et al (2013)9 | 34(52.31%) | 21(38.89%) | 61.82% | 31(47.69%) | 33(61.11%) | 48.44% | 65(54.62%) | 54(45.38%) | 119 | 54.62% | 0.1438 |
| Conwell et al (2008)29 | 11(52.38%) | 13(44.83%) | 45.83% | 10(47.62%) | 16(55.17%) | 38.46% | 21(42.00%) | 29(58.00%) | 50 | 42.00% | 0.5977 |
| Lombardo et al (2022)37 | 64(66.67%) | 59(50.86%) | 52.03% | 32(33.33%) | 57(49.14%) | 35.96% | 96(45.28%) | 116(54.72%) | 212 | 45.28% | 0.0203 |
| Total | 142(56.57%) | 162(51.10%) | 46.71% | 109(43.43%) | 155(48.90%) | 41.29% | 251(45.80%) | 297(54.20%) | 548 | 45.80% | 0.1943 |
Table 4.
Gender Differences in the Prevalence, Demographic and Clinical Features of LH in DM Patients (Mixed Juvenile and Adult DM)
| Author (Year) | Male | Female | All | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Present No (% Among Patients with LH) | Absent No (% Among Patients Without LH) | LH Prevalence (%) | Present No (% Among Patients with LH) | Absent No (% Among Patients Without LH) | LH Prevalence (%) | Present No (% Among Patients with LH) | Absent No (% Among Patients Without LH) | Overall | LH Prevalence (%) | ||
| Yan et al (2021)30 | 57(55.34%) | 25(64.10%) | 69.51% | 46(44.66%) | 14(35.90%) | 76.67% | 103(72.54%) | 39(27.46%) | 142 | 72.54% | 0.3454 |
| Barola et al (2018)31 | 139(60.17%) | 65(46.10%) | 68.14% | 92(39.83%) | 76(53.90%) | 54.76% | 231(62.10%) | 141(37.90%) | 372 | 62.10% | 0.0081 |
| Gupta et al (2018)32 | 50(51.55%) | 25(59.52%) | 66.67% | 47(48.45%) | 17(40.48%) | 73.44% | 97(69.78%) | 42(30.22%) | 139 | 69.78% | 0.3862 |
| Blanco et al (2013)9 | 142(52.21%) | 79(52.32%) | 64.25% | 130(47.79%) | 72(47.68%) | 64.36% | 277(64.42%) | 153(35.58%) | 430 | 64.42% | 0.9824 |
| Vardar et al (2007)34 | 35(33.65%) | 33(32.67%) | 51.47% | 69(66.35%) | 68(67.33%) | 50.36% | 104(48.37%) | 111(51.63%) | 215 | 48.37% | >0.9999 |
| Total | 423(52.42%) | 227(47.89%) | 65.08% | 384(47.58%) | 247(52.11%) | 60.86% | 812(62.56%) | 486(37.44%) | 1298 | 62.56% | 0.1187 |
LH Prevalence in Patients with Different Types of Diabetes Mellitus
The prevalence of LH is different in different genders of subjects with different types of DM, as Tables 5 and 6 reveal, in the research of Lin et al,19 Lian et al,23 Tsadik et al,24 Hernar et al,17 Al Hayek et al,25 Sawatkar et al,26 the study population is T1DM patients, the average prevalence rate of LH in male (51.99%) is higher than that in female (46.40%) in adult T1DM patients (≥18 years). However, there is no significant difference between them in general (P=0.055>0.05).
Table 5.
LH Prevalence in Patients with Different Types of Diabetes (Adult DM)
| Author (year) | TIDM | T2DM | Pancreatic DM | Overall | |||
|---|---|---|---|---|---|---|---|
| Present | Absent | Present | Absent | Present | Absent | ||
| Lin et al (2021)19 | 83 (69.17%) | 37 (30.83%) | 120 | ||||
| Arora et al (2021)20 | 58 | 55 | 216 | 140 | 16 | 15 | 500 |
| Gentile et al (2021)21 | 118 | 116 | 326 | 230 | 780 | ||
| Thewjitcharoen et al (2020)22 | 26 | 30 | 87 | 57 | 400 | ||
| Gentile et al (2020)12 | 718(58.52%) | 509(41.48%) | 1227 | ||||
| Lian et al (2018)23 | ND | ND | 300 | ||||
| Tsadik et al (2018)24 | 103(58.52%) | 73(41.48%) | 176 | ||||
| Hernar et al (2017)17 | 111(51.63%) | 104(48.37%) | 215 | ||||
| Al Hayek et al (2016)25 | 83(47.70%) | 91(52.30%) | 174 | ||||
| Sawatkar et al (2014)26 | 205(41.00%) | 295(59.00%) | 500 | ||||
Abbreviation: ND, not detected.
Table 6.
LH Prevalence in Patients with T1DM
| Author (Year) | Male | Female | All | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Present No (% Among Patients with LH) | Absent No (% Among Patients Without LH) | LH Prevalence (%) | Present No (% Among Patients with LH) | Absent No (% Among Patients Without LH) | LH Prevalence (%) | Present No (% Among Patients with LH) | Absent No (% Among Patients Without LH) | Overall | LH Prevalence (%) | ||
| Lin et al (2021)19 | 48(57.83%) | 22(59.46%) | 68.57% | 35(42.17%) | 15(40.54%) | 70.00% | 83(69.17%) | 37(30.83%) | 120 | 69.17% | 0.8673 |
| Tsadik et al (2018)24 | 53(51.46%) | 33(45.21%) | 61.63% | 50(48.54%) | 40(54.79%) | 55.56% | 103(58.52%) | 73(41.48%) | 176 | 58.52% | 0.4137 |
| Hernar et al (2017)17 | 64(57.66%) | 47(45.19%) | 57.66% | 47(42.34%) | 57(54.81%) | 45.19% | 111(51.63%) | 104(48.37%) | 215 | 51.63% | 0.0767 |
| Al Hayek et al (2016)25 | 43(51.81%) | 47(51.65%) | 47.78% | 40(48.19%) | 44(48.35%) | 47.62% | 83(47.70%) | 91(52.30%) | 174 | 47.70% | 0.9833 |
| Sawatkar et al (2014)26 | 119(58.05%) | 153(51.86%) | 43.75% | 86(41.95%) | 142(48.14%) | 37.72% | 205(41.00%) | 295(59.00%) | 500 | 41.00% | 0.1721 |
| Total | 327(55.90%) | 302(50.33%) | 51.99% | 258(44.10%) | 298(49.67%) | 46.40% | 103(58.53%) | 73(41.49%) | 1185 | 49.37% | 0.055 |
As Table 5 shown, in the study of Arora et al,20 Gentile et al21 and Thewjitcharoen et al,22 they were associated with T1DM and T2DM, there are no significant gender differences in the LH prevalence rate (P>0.05). Because the male-to-female ratio in these three studies has not been revealed, it is impossible to compare gender differences in patients with different types of DM. As Tables 1 and 2 depict, only one study (N=1227)12 involved simple T2DM population, interestingly, it suggested that there was a dramatic difference between different genders (P<0.001), the prevalence rate of LH in male vs female was 70.52% (299/424) vs 52.18% (419/803).
To sum up, in the study of adult diabetes, although the LH prevalence in males is mildly higher than that in females in the population of T1DM patients, it is probably that there are no significant gender differences (P>0.05). However, in the study12 of the population of T2DM, result of the large sample data shows that the LH prevalence in male gender is remarkably higher than that in female gender (P<0.0001).
Additionally, for the gestational diabetes mellitus, the LH prevalence was 37.50% in the study of Yi et al,36 and it was 35.86% in the study of Tang et al.35
LH Prevalence in Patients of Different Age Grades
With the increasing age, the incidence of LH in diabetic patients over 60 years old is generally higher than that in people under 60 years old, as the study20 (Table 7) shown, the LH prevalence rate in the age 31–60 years (61.54%) was higher than that of age 18–30 years (61.54%) (P=0.0142<0.05), and in the age >60 years was remarkably higher than that the age 31–60 years (P<0.0001)12 (Table 7).
Table 7.
LH Prevalence in Patients of Different Age Grades
| Author (Year) | Age | LH Prevalence (%) | Overall | P-value | ||
|---|---|---|---|---|---|---|
| >18, ≤30 | 31–60 | >60 | ||||
| Lin et al (2021)19 | 40/23 (63.49%) | 43/14 (75.44%) | 69.17% | 120 | 0.1570 | |
| Arora et al (2021)20 | 54/58 (48.21%) | 192/120 (61.54%) | 44/32 (57.89%) | 58.00% | 500 | 0.0142 |
| Gentile et al (2021)21 | 62.0 ± 15.0 | 46.15% | 780 | |||
| Thewjitcharoen et al (2020)22 | 64.8 ± 14.2 | 37.25% | 400 | |||
| Gentile et al (2020)12 | 396/817 (32.65%) | 322/410 (43.99%) | 58.52% | 1227 | <0.0001 | |
| Lian et al (2018)23 | 48.0± 3.0 | 56.67% | 300 | |||
| Tsadik et al (2018)24 | 58.52% | 176 | ||||
| Hernar et al (2017)17 | 36.0 (18–82) | 51.63% | 215 | |||
| Al Hayek et al (2016)25 | 47.70% | 174 | ||||
| Sawatkar et al (2014)26 | 41.00% | 239 | ||||
LH Prevalence in Patients with Different DM Duration and Time of Insulin Use
As Table 8 shown, the patients, with the DM duration over 5 years, were more likely to develop LH (P<0.0001); And the patients with the duration of insulin use over 5 years, had a significantly higher incidence of LH than those with insulin use of less than 5 years (P<0.0001).
Table 8.
LH Prevalence in Patients with Different Diabetes Duration and Time of Insulin Use
| Author (Year) | Duration of Diabetes | P-value | Duration of Insulin Use | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| ≤5 | 6–10 | >10 | ≤5 | 6–10 | >10 | |||
| Lin et al (2021)19 | 26/28 (48.15%) | 29/8 (78.38%) | 28/1 (96.55%) | <0.0001 | ||||
| Arora et al (2021)20 | 83/99 (45.6%) | 115/63 (64.61%) | 92/48 (65.71%) | <0.0001 | 224/179 (55.58%) | 46/21 (68.66%) | 20/10 (66.67%) | <0.0001 |
| Gentile et al (2021)21 | 20 ± 11 | 221/311 (41.54%) | 58/65 (47.15%) | 81/44 (64.8%) | <0.0001 | |||
| Thewjitcharoen et al (2020)22 | 24.1 ± 8.9 | 13.4 ± 9.2 | ||||||
| Gentile et al (2020)12 | 12 ± 9 | 12 ± 5 | ||||||
| Lian et al (2018)23 | 10.98 ± 3.12 | 4.47±1.93 | ||||||
| Tsadik et al (2018)24 | 65/46 (58.56%) | 38/27 (58.46%) | >0.05 | |||||
| Hernar et al (2017)17 | 17.0 (1–57) | |||||||
| Al Hayek et al (2016)25 | 38/68 (35.85%) | 45/23 (66.18%) | <0.0001 | |||||
| Sawatkar et al (2014)26 | 4.43 ± 4.4 | |||||||
LH Prevalence in Different Body Sites
As Table 9 depicts, data extracted from the included studies show that the incidence of LH in different body sites was closely related to age level. There are significant differences between different age levels. LH prevalence of the older group (>60 years) was 52.9% in the abdomen, while the younger group (31–60 years) had a lower rate (38.3%, P<0.0001). However, the younger has a higher incidence rate of LH in the arms (35.8% vs 25.4%, P<0.05), in the thighs (33.4% vs 26.7%, P<0.05) and in the buttocks (26.2% vs 4.9%, P<0.01).
Table 9.
LH Prevalence in Different Body Sites
| Author (Year) | Abdomen | Thighs | Arms | Other Unusual Areas | LH Prevalence(%) | Overall | P-value |
|---|---|---|---|---|---|---|---|
| Lin et al (2021)19 | - | - | - | - | 69.17% | 120 | 0.1570 |
| Arora et al (2021)20 | - | - | - | - | 58.00% | 500 | 0.0142 |
| Gentile et al (2021)21 | 52.4% | 23.3% | 19.9% | 4.55% | 46.15% | 780 | |
| Thewjitcharoen et al (2020)22 | - | - | - | - | 37.25% | 400 | |
| Gentile et al (2020)12 | 52.9%/38.3% (O/Y) | 26.7%/33.4%(O/Y) | 25.4%/35.8%(O/Y) | 4.9%/26.2%(O/Y) | 58.52% | 1227 | <0.0001 |
| Lian et al (2018)23 | - | - | - | - | 56.67% | 300 | |
| Tsadik et al (2018)24 | 11.4% | 28.4% | 15.9% | 44.3% | 58.52% | 176 | |
| Hernar et al (2017)17 | 110/97 (53.14%) | 82/49 (62.6%) | 9/5 (64.29%) | 8/13 (38.10%) | 51.63% | 215 | |
| Al Hayek et al (2016)25 | 21.6% | 33.7% | 27.7% | 17% | 47.70% | 174 | |
| Sawatkar et al (2014)26 | 52.26% | 11.91% | 11.58% | 23.26% | 41.00% | 239 |
Notes: Y, younger patients (31–60 years); O, older patients (>60 years).
Discussion
However, there are numerous studies on the overall prevalence of LH in patients with different types of DM and no relevant studies and exact conclusions on the LH prevalence between different genders. In the same type of diabetes (T1DM or T2DM), there is a certain gender difference in the prevalence of LH among people of different age periods. There is a trend that the prevalence rate of male gender is higher than that of female gender, and this difference might be more significant with the increasing age. In the T1DM population studies, the prevalence of LH in male (31.25%–61.82%) was slightly higher than that in female (26.32%–55.32%), but it was no remarkable difference (P>0.05), as shown in the studies Tsadik et al,24 Hernar et al,17 Al Hayek et al25 and Sawatkar et al.26 However, the average prevalence rate of LH in male (51.99%) is little higher than that in female (46.40%) in adult T1DM patients. Although there is no remarkable difference between them overall (P=0.055>0.05), there is a trend that males are larger than females, and this difference also makes gender differences increasingly obvious with increasing age.
Unfortunately, in the included studies, only one study12 purely selected T2DM as the study population. However, interestingly, this 1227 sample size study found that, the average age was 50.1±10.5, the LH prevalence was 70.52% in male gender versus 52.18% in female, with a markedly significant statistical difference (P<0.0001). In other studies, either the number of T1DM or T2DM and the corresponding prevalence rate of LH were not determined or they included a mixture of T1DM and T2DM. Although there was no significant difference between the male and female groups (P>0.05), it was mildly higher in the male group than that in the female group. We also believed that there was no significance in comparing gender differences among patients with mixed type of diabetes or mixed age-level subjects. In the meta-analysis5 study of data from 26,865 participants, forty-five studies were included, the average lipohypertrophy rate was 41.8%, the pooled LH prevalence in studies on T1DM or T2DM subjects was 39.9% and 45.9% separately. However, in our study, we found that the LH prevalence in juvenile diabetes was 46.13% (<18 years), and in adult diabetes (mix TIDM andT2DM) was 49.37% (≥18 years), and in T2DM patients was 58.52% (≥18 years), which was higher than that of the meta-analysis research. Considering that the authors may not take age into account age level and other confounding factors in the analysis.
Additionally, there are also some differences in the LH prevalence in different regions, pooled the LH prevalence in Europe was 44.6%, and it was mildly lower in Africa (34.8%) and Asia (41.3%).2 This result may be caused by local medical level or other research-related factors, such as diagnostic technology, injection technology, multiple use of syringes by patients and different ethnic characteristics.
In age-wise analysis, with increasing age, the incidence of LH in diabetic patients over 60 years old is generally higher than that under 60 years old, as shown in the study.20 LH prevalence rate in age 31–60 years (61.54%) was higher than that in age 18–30 years (61.54%) (P=0.0142<0.05) and in age >60 years was remarkably higher than the age 31–60 years (P<0.0001).12
However, numerous studies have suggested that LH prevalence is related to many factors, including diabetes duration and time of insulin use, reuse of needles, non-rotation of the injection site, dose of insulin use, HbA1c (%) level, needle length, injection times/day, etc.8,9,18,19,25,30,34–36 However, these influencing factors were not consistent, and as the meta-analysis study5 found, only the time of insulin treatment was found to be the major reason that significantly affected the LH prevalence rate.
The great heterogeneity of the LH prevalence rate could be owed to lack of defects in LH’s unified official definition and the standardized inspection procedure.17 The checked experience and skills of the examiner in different classes could not be defined, resulting in the huge heterogeneity of the LH prevalence rate. Of the included studies, only four studies12,19,20,23 defined LH by observation and palpation and combined with ultrasound examination, the other studies diagnosed LH only through observation and palpation. Certainly, in the two studies,12,18 they depicted standardized examination methods involving adequate lighting, such as patient’s correct posture and warming hands of examiner. It is very important for LH detection to use jelly for auxiliary palpation and detect the skin with less elasticity.
Analysis of body sites occurring in LH because of these studies come from different regions, different medical institutions, different patient populations and different medical schemes, the results of LH prevalence in different body sites are inconsistent, and even the opposite results may happen. For convenience and confidentiality, most people still choose to inject insulin into the abdomen for treatment, so the prevalence of LH is high, followed by multiple sites for alternate injection. In addition, there are few data on the effect of different types of insulin on the incidence of LH in the included studies, compared with analogue (47.9%), the human insulin (61.15%) causes a higher incidence of LH.20 As mentioned above,5 the main factor in the incidence of LH is the time of insulin use, which is also similar to the times or days of insulin injection. Unfortunately, there is very weak evidence in the included studies that is not enough to explain.
Certainly, our research also has certain limitations. First, this is a systematic analysis at the study-level, which is not like a patient group study that collects patient-level data to provide better evidence. Secondly, due to the different diagnostic techniques and standards of different studies, there is a high heterogeneity in the LH prevalence rate and lack of sufficient data for analysis, which makes it impossible to perform a meta-regression analysis for all studies. Moreover, in virtue of the uneven quality of the included studies, it may be difficult to obtain the most reliable data analysis results. However, most importantly, we preliminarily found some undiscovered phenomena, that is, both T1DM and T2DM population have a higher incidence of LH with increasing age and the extension of insulin use. While there may not be gender differences in all age T1DM populations, there are probably significant differences in T2DM population, and the most important reason may be closely associated with the pathogenesis of both. Briefly, the pathogenesis of T1DM is the absolute lack of insulin due to the destruction of islet cells, while T2DM is the relative deficiency of insulin due to insulin resistance. Insulin resistance is mostly related to abdominal obesity in males, while women pay more attention to the maintenance of body shape. The accumulation of abdominal fat is also the main reason why the incidence of abdominal LH in males is markedly higher than in female gender, while gender difference data in other body parts has not been disclosed.
Furthermore, because most of the studies did not list the detailed data of BMI and HbA1c (%), the association analysis could not be carried out, and the impact of BMI and HbA1c (%) on gender differences in the LH prevalence could not be calculated, which is also a limitation of this study. It requires more standardized and strictly designed clinical research to verify and more in-depth research to find the mechanism of gender differences.
Conclusion
The evidence shows that the results of gender differences in the LH prevalence are inconsistent in different types of DM. Probably, there is no gender difference in the LH prevalence in adult patients with T1DM, but there is a gender difference between male and female in T2DM. And more strictly designed clinical studies are needed to further verify and reveal the underlying mechanisms.
Acknowledgments
We are very grateful to all the participants of this study for their contributions to this research. Siping Peng, Mingming Xu, and Hengxia Zhao contributed equally to this manuscript.
Funding Statement
This study was supported by the Research Project of Traditional Chinese Medicine Bureau of Guangdong Province, China (No.20211324) and the “3030 PLAN” project of TCM clinical research of Shenzhen Traditional Chinese Medicine Hospital (G3030202123).
Disclosure
The authors state that they have no conflicts of interest in this work.
References
- 1.Saeedi P, Petersohn I, Salpea P., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Peng W, Zhao Z, et al. Prevalence and treatment of diabetes in China, 2013-2018. JAMA. 2021;326(24):2498–2506. doi: 10.1001/jama.2021.22208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gentile S, Strollo F, Ceriello A. Lipodystrophy in insulin-treated subjects and other injection-site skin reactions: are we sure everything is clear? Diabetes Therapy. 2016;7(3):401–409. doi: 10.1007/s13300-016-0187-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Coninck C, Frid A, Gaspar R, et al. Results and analysis of the 2008–2009 insulin injection technique questionnaire survey. J Diabetes. 2010;2(3):168–179. doi: 10.1111/j.1753-0407.2010.00077.x [DOI] [PubMed] [Google Scholar]
- 5.Wang K, Zhang S, Liu C, et al. A meta-analysis and meta-regression on the prevalence of lipohypertrophy in diabetic patients on insulin therapy. Therapies. 2021;76(6):617–628. doi: 10.1016/j.therap.2021.04.002 [DOI] [PubMed] [Google Scholar]
- 6.Strollo F, Guarino G, Armentano V. Unexplained hypoglycaemia and large glycaemic variability: skin lipohypertrophy as a predictive sign. Diabetes Res Open J. 2016;2(1):24–32. doi: 10.17140/DROJ-2-126 [DOI] [Google Scholar]
- 7.Gentile S, Strollo F, Guarino G, et al. Why are so huge differences reported in the occurrence rate of skin lipohypertrophy? Does it depend on method defects or on lack of interest? Diabetes Metabolic Syndrome. 2019;13(1):682–686. doi: 10.1016/j.dsx.2018.11.042 [DOI] [PubMed] [Google Scholar]
- 8.Pahuja V, Punjot P, Fernandes G, et al. Exploring the factors associated with lipohypertrophy in insulin-treated type 2 diabetes patients in a tertiary care hospital in Mumbai, India. Int J Diabetes Dev Ctries. 2019;39(3):426–431. doi: 10.1007/s13410-019-00735-0 [DOI] [Google Scholar]
- 9.Blanco M, Hernández MT, Strauss KW, et al. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39(5):445–453. doi: 10.1016/j.diabet.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 10.Spollett G, Edelman SV, Mehner P, et al. Improvement of insulin injection technique: examination of current issues and recommendations. Diabetes Educ. 2016;42(4):379–394. doi: 10.1177/0145721716648017 [DOI] [PubMed] [Google Scholar]
- 11.Famulla S, Hövelmann U, Fischer A, et al. Insulin injection into lipohypertrophic tissue: blunted and more variable insulin absorption and action and impaired postprandial glucose control. Diabetes Care. 2016;39(9):1486–1492. doi: 10.2337/dc16-0610 [DOI] [PubMed] [Google Scholar]
- 12.Gentile S, Guarino G, Corte TD, et al. Insulin-induced skin lipohypertrophy in type 2 diabetes: a multicenter regional survey in Southern Italy. Diabetes Therapy. 2020;11(9):2001–2017. doi: 10.1007/s13300-020-00876-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng N, Zhang X, Zhao F, et al. Prevalence of lipohypertrophy in insulin‐treated diabetes patients: a systematic review and meta‐analysis. J Diabetes Investig. 2018;9(3):536–543. doi: 10.1111/jdi.12742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372():n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institutes of Health. Quality assessment tool for observational cohort and cross-sectional studies; 2014. Available from: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort. Accessed December 4, 2017.
- 16.Ryan R, Hill S How to GRADE the quality of the evidence. Version 3.0; 2016. Available from: http://cccrg.cochrane.org/author-resources. Accessed Dec 4, 2017.
- 17.Hernar I, Haltbakk J, Broström A. Differences in depression, treatment satisfaction and injection behaviour in adults with type 1 diabetes and different degrees of lipohypertrophy. J Clin Nurs. 2017;26:4583–4596. doi: 10.1111/jocn.13801 [DOI] [PubMed] [Google Scholar]
- 18.Ji L, Sun Z, Li Q, et al. Lipohypertrophy in China: prevalence, risk factors, insulin consumption, and clinical impact. Diabetes Technol Ther. 2017;19(1):61–67. doi: 10.1089/dia.2016.0334 [DOI] [PubMed] [Google Scholar]
- 19.Lin Y, Lin L, Wang W, et al. Insulin-related lipohypertrophy: ultrasound characteristics, risk factors, and impact of glucose fluctuations. Endocrine. 2022;75(3):768–775. doi: 10.1007/s12020-021-02904-w [DOI] [PubMed] [Google Scholar]
- 20.Arora S, Agrawal NK, Shanthaiah DM, et al. Early detection of cutaneous complications of insulin therapy in type 1 and type 2 diabetes mellitus. Prim Care Diabetes. 2021;15(5):859–864. doi: 10.1016/j.pcd.2021.06.004 [DOI] [PubMed] [Google Scholar]
- 21.Gentile S, Guarino G, Della Corte T, et al. Bruising: a neglected, though patient-relevant complication of insulin injections coming to light from a real-life nationwide survey. Diabetes Therapy. 2021;12(4):1143–1157. doi: 10.1007/s13300-021-01026-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thewjitcharoen Y, Prasartkaew H, Tongsumrit P, et al. Prevalence, risk factors, and clinical characteristics of lipodystrophy in insulin-treated patients with diabetes: an old problem in a new era of modern insulin. Diabetes Metab Syndrome Obesity. 2020;13:4609. doi: 10.2147/DMSO.S282926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lian L, Fu Jingling, F Longyan. Investigation on the current situation and related factors of subcutaneous fat hyperplasia in diabetic patients with insulin injection. Nursing Practice Res. 2018;15(7):18–20. [Google Scholar]
- 24.Tsadik AG, Atey TM, Nedi T, et al. Effect of insulin-induced lipodystrophy on glycemic control among children and adolescents with diabetes in Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. J Diabetes Res. 2018;2018:1–7. doi: 10.1155/2018/4910962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al Hayek AA, Robert AA, Braham RB, et al. Frequency of lipohypertrophy and associated risk factors in young patients with type 1 diabetes: a cross-sectional study. Diabetes Therapy. 2016;7(2):259–267. doi: 10.1007/s13300-016-0161-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawatkar GU, Kanwar AJ, Dogra S, et al. Spectrum of cutaneous manifestations of type 1 diabetes mellitus in 500 South Asian patients. Br J Dermatol. 2014;171(6):1402–1406. doi: 10.1111/bjd.13077 [DOI] [PubMed] [Google Scholar]
- 27.Singha A, Bhattacharjee R, Dalal BS, et al. Associations of insulin-induced lipodystrophy in children, adolescents, and young adults with type 1 diabetes mellitus using recombinant human insulin: a cross-sectional study. J Pediatric Endocrinol Metabo. 2021;34(4):503–508. doi: 10.1515/jpem-2020-0556 [DOI] [PubMed] [Google Scholar]
- 28.McCann A. Frequency of injection site assessment for lipohypertrophy in children and young people with type 1 diabetes. Nurs Child Young People. 2022;34(4):548. [DOI] [PubMed] [Google Scholar]
- 29.Conwell LS, Pope E, Artiles AM, et al. Dermatological complications of continuous subcutaneous insulin infusion in children and adolescents. J Pediatr. 2008;152(5):622–628. doi: 10.1016/j.jpeds.2007.10.006 [DOI] [PubMed] [Google Scholar]
- 30.Dan Y, Min Z, Yue W, et al. Analysis of influencing factors of subcutaneous fat hyperplasia caused by insulin injection based on accurate ultrasonic detection. Chine J Diabetes. 2021;29(10):738–741. [Google Scholar]
- 31.Barola A, Tiwari P, Bhansali A, et al. Insulin-related lipohypertrophy: lipogenic action or tissue trauma? Front Endocrinol (Lausanne). 2018;9:638. doi: 10.3389/fendo.2018.00638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta SS, Gupta KS, Gathe SS, et al. Clinical implications of lipohypertrophy among people with type 1 diabetes in India. Diabetes Technol Ther. 2018;20(7):483–491. doi: 10.1089/dia.2018.0074 [DOI] [PubMed] [Google Scholar]
- 33.Omar MA, El-Kafoury AA, El-Araby RI. Lipohypertrophy in children and adolescents with type 1 diabetes and the associated factors. BMC Res Notes. 2011;4(1):290. doi: 10.1186/1756-0500-4-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vardar B, Kızılcı S. Incidence of lipohypertrophy in diabetic patients and a study of influencing factors. Diabetes Res Clin Pract. 2007;77(2):231–236. doi: 10.1016/j.diabres.2006.12.023 [DOI] [PubMed] [Google Scholar]
- 35.Chundan T. Study on the related factors of subcutaneous fat hyperplasia caused by insulin treatment of gestational diabetes. Shanghai Nursing. 2019;1:548. [Google Scholar]
- 36.Yi Y, Yihua J, Xiaowen C. Study on the related factors of subcutaneous fat hyperplasia caused by insulin injection in the treatment of gestational diabetes. J Clin Exp Med. 2021;20(12):4. [Google Scholar]
- 37.Lombardo F, Bombaci B, Alibrandi A, et al. The impact of insulin-induced lipodystrophy on glycemic variability in pediatric patients with type 1 diabetes. Children. 2022;9(7):1087. doi: 10.3390/children9071087 [DOI] [PMC free article] [PubMed] [Google Scholar]