Abstract
Background
Group B streptococcus (GBS) is a major cause of invasive disease in young infants. Infants born to women with sufficient pre-existing anti-GBS capsular IgG antibodies are at reduced risk of GBS disease, making maternal immunisation a potential strategy for prevention. We aimed to assess the safety and immunogenicity of a novel hexavalent (serotypes Ia, Ib, II, III, IV, and V) GBS conjugate vaccine (GBS6).
Methods
This phase 1/2, placebo-controlled, observer-blinded, dose-escalation trial, was done at four clinical research centres in the USA (Kentucky, Georgia, and two sites in Utah). Healthy, non-pregnant adults aged 18–49 years were randomly assigned using an interactive, web-based response technology system. Within each dose group (low, medium, or high), participants in sentinel cohorts were randomly assigned 2:2:1 and expanded cohort participants were randomly assigned 4:4:1 to receive GBS6 with aluminium phosphate (AlPO4), GBS6 without AlPO4, or placebo (saline control). One 0·5 mL dose of either saline placebo or 5 μg capsular polysaccharide per serotype in the low-dose group, 10 μg capsular polysaccharide per serotype in the medium-dose group, or 20 μg capsular polysaccharide per serotype in the high-dose group was administered by intramuscular injection into the deltoid muscle on day 1. The primary outcome was safety up to 6 months after vaccination, including the proportion of sentinel cohort participants with clinical laboratory abnormalities at 1 week, the proportion of all participants reporting solicited local reactions, systemic events, or use of antipyretic or pain medication within 14 days, adverse events up to 1 month, and medically attended or serious adverse events up to 6 months. The secondary outcome was GBS immunogenicity (serotype-specific IgG geometric mean concentrations at 1 month). This study is registered with ClinicalTrials.gov, NCT03170609.
Findings
Between June 5, 2017, and June 25, 2018, 365 participants were randomly assigned and 364 (52 in each dose group) were vaccinated and included in the safety analysis. Unsolicited adverse events were reported by 15 (29%) participants in the 5 μg with AlPO4 group, 13 (25%) in the 5 μg without AlPO4 group, 22 (42%) in the 10 μg with AlPO4 group, 12 (23%) in the 10 μg without AlPO4 group, 25 (48%) in the 20 μg with AlPO4 group, 21 (40%) in the 20 μg without AlPO4 group, and 20 (38%) in the placebo group. The most common unsolicited adverse events were in the system organ class of infections and infestations in any dose or formulation of GBS6 (ranging from six [12%] in the 10 μg without AlPO4 group to 15 [29%] in the 20 μg with AlPO4 group and placebo group). Three participants reported at least one serious adverse event during the study, one each in the 5 μg GBS6 with AlPO4 group (diabetic ketoacidosis, two events; resolved), 10 μg GBS6 with AlPO4 group (died by suicide), and 20 μg GBS6 with AlPO4 group (metrorrhagia; resolved). None of these serious adverse events were considered related to the vaccine. 11 of the 365 participants were excluded from the evaluable immunogenicity population, including one participant who did not receive the vaccine, and ten who at 1 month after vaccination were withdrawn for various reasons. GBS serotype-specific IgG geometric mean concentrations increased by 1 week after vaccination for all GBS6 groups, peaked at 2 weeks, stabilised by 1 month, and declined gradually but remained higher than placebo at 6 months.
Interpretation
GBS6 was well tolerated in healthy adults and elicited robust immune responses for all dose levels and formulations that persisted 6 months after vaccination. This study supports further evaluation of GBS6 in pregnant women.
Funding
Pfizer.
Introduction
Streptococcus agalactiae, also known as group B streptococcus (GBS), is a leading cause of invasive bacterial infection in young infants and causes substantial infant morbidity and mortality worldwide, including in the USA.1, 2, 3, 4 GBS can cause serious disease, such as sepsis, meningitis, and pneumonia, and the incidence of invasive disease in infants ranges from 0·55 cases per 1000 livebirths in the USA to 2·59 cases per 1000 livebirths in South Africa; mortality from GBS infection is 6–14% in high-income countries and 10–60% in low-income and middle-income countries.1, 3, 5, 6, 7, 8, 9 In pregnant women, GBS can be associated with ascending infections ranging from urinary tract infections to chorioamnionitis, which can result in stillbirth, preterm delivery, and puerperal sepsis.10
Research in context.
Evidence before this study
We searched PubMed using the keywords “group B streptococcus vaccine”, “clinical trial”, and “human”, for studies published in English up to Aug 25, 2020. Published data are available for monovalent, bivalent, and trivalent group B streptococcus (GBS) vaccines evaluated in non-pregnant and pregnant women. Investigational monovalent and bivalent GBS vaccines containing capsular polysaccharides of serotypes Ia, Ib, II, III, or V conjugated to tetanus toxoid using various doses have been evaluated and studied in non-pregnant, healthy volunteers. The GBS–tetanus toxoid conjugate vaccine candidates were generally safe and well tolerated, characterised primarily by mild reactogenicity, and elicited IgG and opsonophagocytic activity responses that peaked 2–8 weeks after vaccination, and although they declined over time, remained above baseline 2 years after immunisation. A GBS serotype III capsular polysaccharide–tetanus toxoid conjugate vaccine was evaluated in a study of 30 pregnant women and elicited IgG antibodies in the cord blood of infants born to vaccine recipients. Serotype III IgG and opsonophagocytic activity responses were shown at 2 months of age in infants born to vaccinated pregnant women. The vaccine was well tolerated, and no safety concerns were identified in the pregnant women or their infants. A trivalent (Ia, Ib, and III) GBS capsular polysaccharide–cross-reactive material 197 (CRM197) conjugate vaccine has also been evaluated in clinical trials of non-pregnant and pregnant women in Belgium, Canada, South Africa, and Malawi. These studies showed an acceptable safety profile of GBS polysaccharide conjugate vaccines administered to pregnant women, as well as the induction of immune responses to the GBS vaccine serotypes that resulted in transplacental transfer of antibodies to their infants.
Added value of this study
The data from this study suggest that three doses (5 μg, 10 μg, and 20 μg) of the first hexavalent GBS vaccine (GBS6) formulated with or without aluminium phosphate (AlPO4) are safe and well tolerated, and induce immune responses to six GBS serotypes lasting at least 6 months after vaccination in healthy men and non-pregnant women aged 18–49 years. This is the first study to assess a hexavalent CRM197-conjugated GBS vaccine with and without AlPO4 that was designed to maximise global GBS serotype coverage. This study expands the amount of safety and immunogenicity data to support the development of a global GBS vaccine that can eventually be administered to pregnant women for protection of infants from birth throughout the period of greatest risk (approximately 90 days of life).
Implications of all the available evidence
Streptococcus agalactiae, also known as GBS, is a leading cause of invasive bacterial infection in young infants and a substantial cause of infant morbidity and mortality worldwide. The epidemiology of invasive GBS disease in infants and the limitations of prevention and treatment support the need for a prophylactic vaccine, particularly to address the substantial unmet medical need in low-resource countries. Findings from this study will support development of GBS6 for maternal immunisation.
Because young infants are particularly vulnerable to the devastating consequences of GBS disease, even if appropriate treatment is available, preventing infection would be the most effective approach to reduce disease and its associated sequelae. Studies done in the 1980s found that antibiotics administered during labour and before delivery reduced transmission of GBS from colonised mothers to their infants and reduced rates of early onset disease (ie, disease onset at <7 days of life).11, 12 As a result, in the late 1990s, US guidelines were broadened to include recommendations for screening of pregnant women for GBS carriage and the use of intrapartum antibiotic prophylaxis for those who were colonised.13, 14 Although largely effective in the prevention of early onset disease, intrapartum antibiotic prophylaxis is not optimal because some cases occur even when adequate screening is available (eg, in the setting of preterm births). Additionally, screening failures or incomplete intrapartum antibiotic prophylaxis coverage can occur even in high-income countries that have resources for broad implementation. Intrapartum antibiotic prophylaxis has no effect on late-onset disease (ie, disease onset at 7–90 days of life) and prevents neither GBS-associated stillbirth nor GBS infection of the mother in the peripartum period.13, 14 Finally, intrapartum antibiotic prophylaxis might be too late to affect disease in the setting of preterm deliveries, might not be available to women with little access to routine antenatal care, might contribute to antimicrobial resistance, and might affect the neonatal microbiome.15, 16 Maternal immunisation could address these shortcomings of intrapartum antibiotic prophylaxis, and reduce the global burden of invasive GBS infection in infants and pregnant women. Monovalent, bivalent, and trivalent GBS vaccines against serotypes Ia, Ib, II, III, and V have been previously studied in non-pregnant and pregnant women. These vaccines were shown to be safe and elicited postvaccination increases in GBS antibodies in infants and in vaccinees.17, 18 There are no licensed vaccines against GBS disease, and no vaccine has targeted immunity against serotype IV, which is becoming an important contributor to invasive GBS disease.19
This study aimed to evaluate the safety and immunogenicity of a GBS-containing vaccine that includes the six most prevalent capsular polysaccharide serotypes (Ia, Ib, II, III, IV, and V; GBS6), accounting for approximately 98% of disease-causing isolates worldwide.20
Methods
Study design and participants
This phase 1/2, randomised, placebo-controlled, observer-blinded, dose-escalation trial was done at four clinical research centres in the USA (in Kentucky, Georgia, and two sites in Utah). This study used a sentinel cohort design with progression and dose escalation taking place after review of the sentinel cohort at each dose. The sentinel cohorts functioned as a first stage in the study and an expanded cohort served as a second stage to provide an increased number of participants for immunogenicity and further safety assessment.
Healthy, non-pregnant adults aged 18–49 years were eligible for enrolment. Participants were excluded if they were of childbearing potential and were unwilling or unable to use a highly effective method of contraception for at least 3 months after study vaccination; if they had a history of microbiologically proven invasive disease caused by GBS; or if they had any previous vaccination with any licensed or investigational GBS vaccine or vaccination with diphtheria-containing or cross-reactive material 197 (CRM197)-containing vaccines from 6 months before investigational product administration (or planned receipt of these vaccines up to 6 months after vaccination with the study product).
The final protocol and informed consent document were approved by institutional review boards for each of the investigational centres participating in this study, and this study was done in compliance with International Council for Harmonisation Good Clinical Practice guidelines and the ethical principles of the Declaration of Helsinki. Signed and dated written informed consent was required from each participant before any study-specific activity was done. The protocol for this study is included in the appendix (p 23).
Randomisation and masking
Study participants were randomly assigned using an interactive, web-based response technology system following an allocation schedule that was created before the study start. Randomisation was done at each dose level. Participants were enrolled into the lowest dose group, within which participants in a sentinel cohort were randomly assigned 2:2:1 to receive GBS6 with aluminium phosphate (AlPO4), GBS6 without AlPO4, or placebo (saline control) at a rate limited to a maximum of four participants per day. Expanded cohort participants within the lowest dose group were then randomly assigned in a 4:4:1 ratio to receive GBS6 with AlPO4, GBS6 without AlPO4, or placebo, with no limit on the rate of enrolment. The same randomisation process was then repeated with participants being enrolled into the medium dose group after the review of safety data from the low dose group; enrolment started into the high dose group after review of safety data from the medium dose group. The appearance of GBS6 and placebo was not matched, which required an unmasked vaccine administrator at each study site. The unmasked administrators were not involved in other aspects of the study. Internal review committee and external data monitoring committee members reviewed unmasked safety data throughout the study. After a prespecified interim analysis at 1-month postvaccination, the study was unmasked to the sponsor's study team. Sponsor laboratory personnel responsible for doing immunogenicity testing remained masked until study completion. Participants and all other study staff were masked to the allocation throughout the study. The staff who observed the patients were masked.
Procedures
Three doses (5 μg capsular polysaccharide per serotype in the low-dose group, 10 μg capsular polysaccharide per serotype in the medium-dose group, or 20 μg capsular polysaccharide per serotype in the high-dose group) of the GBS6 vaccine candidate were formulated with or without AlPO4 and participants received one 0·5 mL dose of either GBS6 or placebo, administered by intramuscular injection into the deltoid muscle. GBS6 is composed of equal proportions of serotypes Ia, Ib, II, III, IV, and V capsular polysaccharide individually conjugated to the CRM197 carrier protein. The placebo was a sterile saline solution for injection (0·9% sodium chloride injection, in a 0·5 mL dose). Participants received a single vaccine or placebo dose on day 1.
Adverse events were recorded in the following categories: any event (all categories combined); serious adverse event (untoward medical occurrence at any dose that results in death, is life threatening, requires inpatient hospitalisation, results in persistent or clinically significant disability or incapacity, results in congenital anomaly or birth defect, or is considered to be an important medical event, or any combination of these results); immediate adverse events (occurs within the first 30 min after study vaccination); severe adverse events (interferes substantially with the participant's usual function); related adverse events (deemed related to study vaccination by the study investigator); medically attended adverse events (non-serious adverse events that result in an evaluation at a medical facility); and adverse events leading to withdrawal (the primary reason for a participant's study discontinuation). Safety assessments for all participants included 30 min observation after vaccination for immediate adverse events and self-reporting of solicited local reactions (pain at injection site, erythema or redness, and induration or swelling), systemic events (fever, nausea or vomiting, diarrhoea, headache, fatigue, muscle pain, or joint pain), and the use of antipyretic or pain medication by the patient in an electronic diary for 14 days after vaccination (appendix p 9). Unsolicited adverse events were recorded for up to 1 month after vaccination and serious adverse events and medically attended adverse events were recorded until 6 months after vaccination. Adverse events related to study procedures were recorded within 48 h after the 3-month and 6-month blood draws. For the sentinel cohort participants only, blood was collected at screening and 1 week after vaccination for haematology and chemistry assessments (details are included in the protocol; appendix p 23).
The protocol specified safety stopping rules (appendix p 10) for GBS6-vaccinated participants. An internal review committee reviewed the 14-day safety data from each sentinel cohort to determine if expanded cohort enrolment should begin at that dose and if sentinel cohort enrolment for the next higher dose should begin. This study used an external data monitoring committee that provided regular review of cumulative safety data and ad-hoc review if a stopping rule was met.
For immunogenicity assessments, approximately 30 mL of blood was collected from each participant before study vaccination and at 1 and 2 weeks and 1, 3, and 6 months after vaccination. Immunogenicity was measured by IgG antibody concentrations using a direct-binding Luminex (Luminex Corporation, Austin, TX, USA) assay platform. Each of the six GBS capsular polysaccharides included in GBS6 was individually conjugated to poly-L-lysine and the conjugates were chemically coupled to spectrally distinct magnetic Luminex microspheres. The six-plex pool of microspheres was incubated with diluted serum samples to allow serotype-specific primary antibodies to bind to the immobilised polysaccharide antigens. Antibodies that bound to the polysaccharide-coated beads were detected with R-phycoerythrin-conjugated goat antihuman IgG secondary antibodies. Data were captured as median fluorescent intensities using a Luminex reader and converted to μg/mL antibody concentrations using a reference standard curve and accounting for the serum dilution factor. Assay results are reported in weight-based μg/mL units of IgG antibodies. Pfizer's six-plex direct-binding Luminex assay was qualified to measure serotype-specific serum IgG antibodies against GBS6 serotypes Ia, Ib, II, III, IV, and V with appropriate accuracy, precision, and specificity.
Outcomes
The primary outcomes were the proportion of sentinel cohort participants with clinical laboratory abnormalities 1 week after vaccination; the proportion of sentinel and expanded cohort participants reporting solicited local reactions, systemic events, and use of antipyretic or pain medication within 14 days after vaccination; adverse events up to 1 month after vaccination; and medically attended adverse events and serious adverse events up to 6 months after vaccination. The secondary outcome was GBS serotype-specific IgG geometric mean concentrations 1 month after vaccination. Exploratory outcomes were GBS serotype-specific antibody (IgG) geometric mean concentrations measured at 1 and 2 weeks and 1, 3, and 6 months; geometric mean fold rise from baseline to 1 week, 2 weeks, 1 month, 3 months, and 6 months; and the proportion of participants with serotype-specific IgG concentrations ≥1·0 μg/mL at baseline, 1 week, 2 weeks, and 1 month. Additional exploratory endpoints will be reported elsewhere.
Statistical analysis
The sample size of this study was not based on any formal statistical hypothesis. Safety and immunogenicity analyses were descriptive. The safety endpoints (primary outcomes) were analysed in the safety population, which included all participants receiving a dose of GBS6 or placebo. Point estimates and the exact two-sided 95% CIs using the Clopper and Pearson method were calculated for each vaccine group for the primary endpoints. The planned sample size for this study was about 363 (about 52 participants per group, including 52 participants in total assigned to placebo from each of the three dose groups).
The immunogenicity endpoints (secondary and exploratory outcomes) were analysed in the evaluable immunogenicity population, which included participants who were eligible, received vaccination as randomly assigned, had at least one valid and determinate assay result for the 1-month-after-vaccination visit within a prespecified window (within 27–45 days, inclusive, after vaccination), and had no potentially important protocol violations.
Descriptive summary statistics were calculated for all immunogenicity endpoints. Anticapsular IgG antibody concentrations for the six serotypes at all immunogenicity assessment timepoints were reported as GBS serotype-specific concentrations and were logarithmically transformed for analysis. For each serotype, geometric mean concentrations and the associated two-sided 95% CI from each vaccine group were calculated by back transformation of the mean and CI of the logarithmically transformed assay results computed using Student's t distribution. In addition, mixed-effects models with repeated measurements were used to analyse the GBS serotype-specific IgG antibody concentrations and assess the effect of specific covariates. The proportions of participants achieving serotype-specific IgG concentrations of 1·0 μg/mL or more were also descriptively summarised with two-sided 95% exact CIs. Post-hoc analyses were done for proportions of participants achieving serotype-specific IgG concentrations of 0·5 μg/mL or more in a similar manner. This study is registered with ClinicalTrials.gov, NCT03170609.
Role of the funding source
Pfizer was responsible for the study design, data collection, data analysis, data interpretation, and writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. All study data were available to all authors.
Results
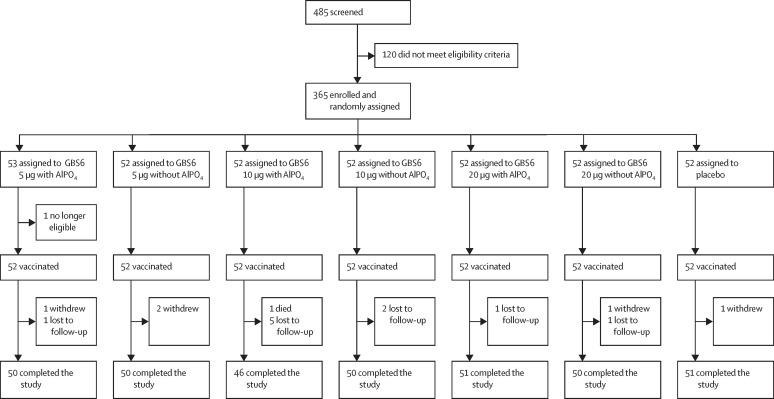
Between June 5, 2017, and June 25, 2018, 485 individuals were screened, 365 of whom were enrolled into the study. 53 participants were randomly assigned to the GBS6 5 μg with AlPO4 group, and 52 participants were randomly assigned to each of the six other study groups (five GBS6 groups and one placebo group; figure 1 ). One patient in the GBS6 5 μg with AlPO4 group was subsequently found to be ineligible and did not receive the study vaccination. 348 (95%) of 365 participants completed the study. The most common reasons for not completing the study included being lost to follow-up (ten [3%] of 365 participants) or withdrawal (four [1%]). No participant was withdrawn because of an adverse event. A specific target for participant enrolment by sex was not prespecified; however, the intention was for each site to enrol at least 50% women within each dose group. The majority of participants were women (256 [70%]), White (307 [84%]), and non-Hispanic (341 [94%]), with a mean age of 32·7 years (SD 8·57), and within each of the study groups, demographic characteristics were fairly similar (table 1 ).
Figure 1.
Trial profile
In the sentinel cohort, 16 patients in each dose group were assigned to receive GBS6 with AlPO4, and 16 patients in each dose group were assigned to receive GBS6 without AlPO4. 24 patients were assigned to receive placebo in the sentinel cohort. AlPO4=aluminium phosphate. GBS6=group B streptococcus-containing vaccine with serotypes Ia, Ib, II, III, IV, and V.
Table 1.
Baseline characteristics in the safety population
| GBS6 5 μg with AlPO4(n=52) | GBS6 5 μg without AlPO4(n=52) | GBS6 10 μg with AlPO4(n=52) | GBS6 10 μg without AlPO4(n=52) | GBS6 20 μg with AlPO4(n=52) | GBS6 20 μg without AlPO4(n=52) | Placebo (n=52) | Total (n=364) | |
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Female | 35 (67%) | 41 (79%) | 32 (62%) | 33 (64%) | 35 (67%) | 37 (71%) | 43 (83%) | 256 (70%) |
| Male | 17 (33%) | 11 (21%) | 20 (39%) | 19 (37%) | 17 (33%) | 15 (29%) | 9 (17%) | 108 (30%) |
| Race | ||||||||
| White | 44 (85%) | 41 (79%) | 46 (89%) | 41 (79%) | 45 (87%) | 46 (89%) | 44 (85%) | 307 (84%) |
| Black or African American | 5 (10%) | 9 (17%) | 5 (10%) | 8 (15%) | 4 (8%) | 4 (8%) | 7 (14%) | 42 (12%) |
| Other | 2 (4%) | 1 (2%) | 0 | 2 (4%) | 2 (4%) | 2 (4%) | 0 | 9 (3%) |
| Asian | 1 (2%) | 0 | 1 (2%) | 1 (2%) | 1 (2%) | 0 | 1 (2%) | 5 (1%) |
| Native Hawaiian or other Pacific Islander | 0 | 1 (2%) | 0 | 0 | 0 | 0 | 0 | 1 (<1%) |
| Ethnicity | ||||||||
| Non-Hispanic or non-Latino | 50 (96%) | 47 (90%) | 50 (96%) | 49 (94%) | 47 (90%) | 51 (98%) | 47 (90%) | 341 (94%) |
| Hispanic or Latino | 2 (4%) | 4 (8%) | 2 (4%) | 2 (4%) | 5 (10%) | 1 (2%) | 5 (10%) | 21 (6%) |
| Not reported | 0 | 1 (2%) | 0 | 0 | 0 | 0 | 0 | 1 (<1%) |
| Unknown | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 0 | 1 (<1%) |
| Age at vaccination, years | ||||||||
| Mean (SD) | 32·9 (8·43) | 34·7 (9·35) | 32·8 (8·31) | 31·9 (8·10) | 33·2 (8·67) | 30·5 (8·25) | 32·8 (8·74) | 32·7 (8·57) |
| Median | 31·0 | 36·5 | 31·0 | 30·0 | 32·0 | 30·0 | 32·0 | 32·0 |
| Range | 20–49 | 18–49 | 18–48 | 19–45 | 18–49 | 18–49 | 18–48 | 18–49 |
Data are n (%) unless otherwise stated. Due to rounding, percentages might not add up to 100%. Race and ethnic group were reported by participants. AlPO4=aluminium phosphate. GBS6=group B streptococcus-containing vaccine with serotypes Ia, Ib, II, III, IV, and V.
Data from the 364 participants who received study vaccine or placebo are included in the safety analyses. Among participants in the sentinel cohorts, two (8%) of 24 placebo recipients and 20 (21%) of 96 GBS6 recipients had grade 1 or higher abnormal laboratory values at the 1-week follow-up visit. One (6%) of 16 participants in the 5 μg GBS6 without AlPO4 group had a grade 3 aspartate aminotransferase abnormality (193 U/L; reference range 11–36 U/L); all other abnormalities were classified as grade 1 or 2, with the majority being grade 1 (appendix p 15). Of the participants whose testing was repeated 1 week after the abnormal result, all laboratory abnormalities were found to have normalised after retesting. None of the changes in laboratory values after vaccination were associated with clinical findings.
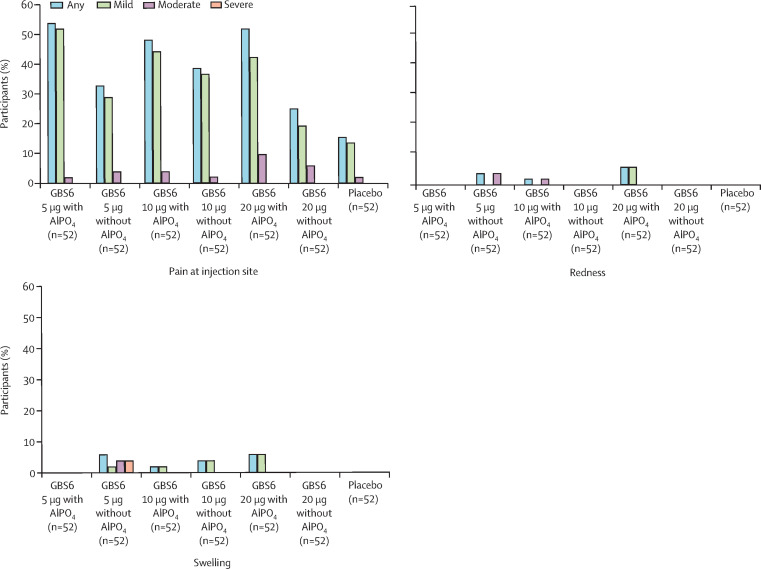
In the 14 days after vaccination, pain at the injection site was the most frequently reported solicited local reaction, ranging from 13 (25%) of 52 participants in the GBS6 20 μg without AlPO4 group to 28 (54%) of 52 participants in the GBS6 5 μg with AlPO4 group. Injection-site pain was reported slightly more frequently among participants in the AlPO4-containing GBS6 groups (figure 2 ). Most local reactions were mild and no severe or grade 4 local reactions were reported (figure 2). The median duration of any local reaction ranged from 1·0 to 3·5 days among GBS6 recipients and was generally similar across groups.
Figure 2.
Participants reporting local reactions, by maximum severity, within 14 days after study vaccination
Solicited injection-site (local) reactions were: pain at injection site (mild: does not interfere with activity; moderate: interferes with activity; severe: prevents daily activity; grade 4: emergency department visit or hospital admission) and redness and swelling (mild: 2·5–5·0 cm in diameter; moderate: 5·5–10·0 cm in diameter; severe: more than 10·0 cm in diameter; grade 4: necrosis or exfoliative dermatitis for redness, and necrosis for swelling). Data were collected with the use of electronic diaries for 14 days after each vaccination. AlPO4=aluminium phosphate. GBS6=group B streptococcus-containing vaccine with serotypes Ia, Ib, II, III, IV, and V.
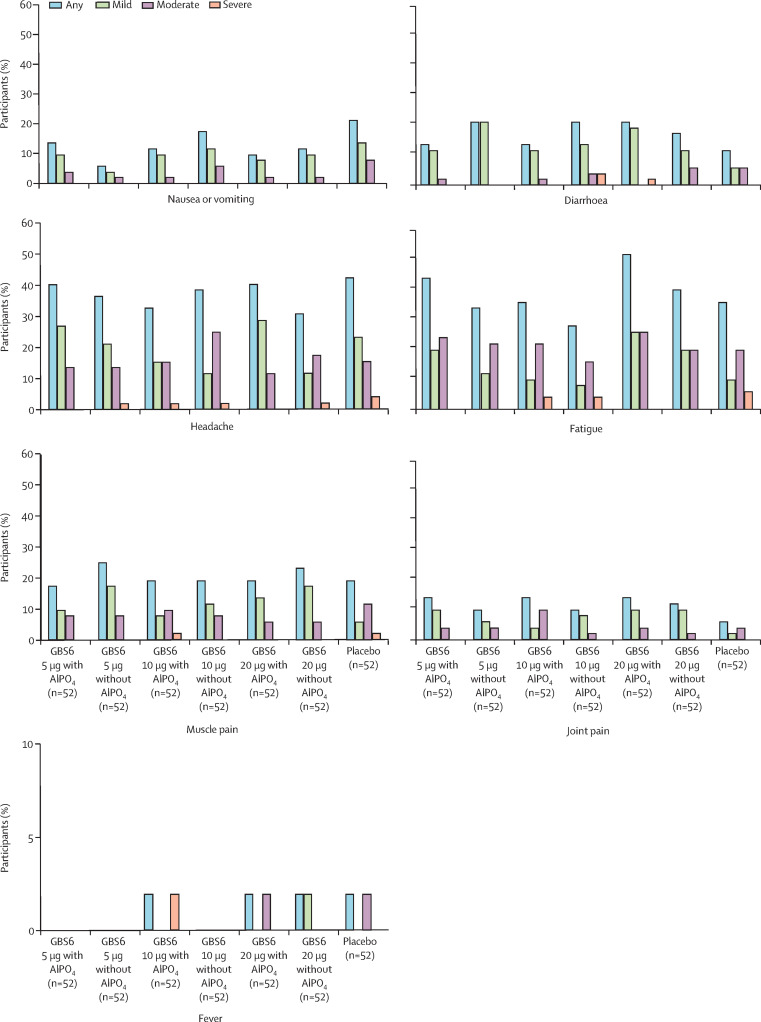
Solicited systemic events reported within 14 days after vaccination for GBS6 and placebo groups are shown in figure 3 . Most systemic events reported among GBS6 recipients were mild or moderate in nature, and similar frequencies and severities were observed across all doses and formulations (ranging from 26 [50%] participants in the GBS6 10 μg with AlPO4 group to 36 [69%] in the GBS6 5 μg with AlPO4 group). No grade 4 systemic events were reported. The most frequently reported systemic events among GBS6 recipients and placebo recipients were fatigue or tiredness and headache. No more than one (2%) of 52 participants at any dose reported a temperature of 100·4°F (38·0°C) or more; no participants in any group reported a fever greater than 102·1°F (38·9°C). The median duration of any reported systemic event ranged from 1·0 to 5·0 days for recipients of GBS6 and 1·0 to 7·0 days in the placebo group. The proportion of patients reporting use of antipyretic or pain medications within 14 days after vaccination ranged from 15% (eight of 52) in the GBS6 5 μg with AlPO4 group to 27% (14 of 52) in the GBS6 10 μg without AlPO4.
Figure 3.
Participants reporting systemic events, by maximum severity, within 14 days after study vaccination
Grading of solicited systemic events is described in the appendix (p 9). AlPO4=aluminium phosphate. GBS6=group B streptococcus-containing vaccine with serotypes Ia, Ib, II, III, IV, and V.
The number of participants reporting unsolicited adverse events in the GBS6 groups ranged from 12 (23%) in the GBS6 10 μg without AlPO4 group to 25 (48%) in the GBS6 20 μg with AlPO4 group (table 2 ). The most common unsolicited adverse events were in the system organ class of infections and infestations in any dose or formulation of GBS6 (ranging from six [12%] in the 10 μg without AlPO4 group to 15 [29%] in the 20 μg with AlPO4 group and placebo group); of these, the most commonly reported adverse events were upper respiratory tract infection and sinusitis (appendix p 20–22).
Table 2.
Unsolicited adverse events in participants in the safety population
| GBS6 5 μg with AlPO4(n=52) | GBS6 5 μg without AlPO4(n=52) | GBS6 10 μg with AlPO4(n=52) | GBS6 10 μg without AlPO4(n=52) | GBS6 20 μg with AlPO4(n=52) | GBS6 20 μg without AlPO4(n=52) | Placebo (n=52) | |
|---|---|---|---|---|---|---|---|
| Any adverse event | 15 (29%; 17–43) | 13 (25%; 14–39) | 22 (42%; 29–57) | 12 (23%; 13–37) | 25 (48%; 34–62) | 21 (40%; 27–55) | 20 (38%; 25–53) |
| Serious adverse event | 1 (2%; 0–10) | 0 (0%; 0–7) | 1 (2%; 0–10) | 0 (0%; 0–7) | 1 (2%; 0–10) | 0 (0%; 0–7) | 0 (0%; 0–7) |
| Immediate adverse event | 0 (0%; 0–7) | 0 (0%; 0–7) | 0 (0%; 0–7) | 0 (0%; 0–7) | 0 (0%; 0–7) | 0 (0%; 0–7) | 0 (0%; 0–7) |
| Severe adverse event | 2 (4%; 1–13) | 2 (4%; 1–13) | 6 (12%; 4–23) | 3 (6%; 1–16) | 2 (4%; 1–13) | 5 (10%; 3–21) | 4 (8%; 2–19) |
| Related adverse event | 2 (4%; 1–13) | 0 (0%; 0–7) | 1 (2%; 0–10) | 1 (2%; 0–10) | 2 (4%; 1–13) | 1 (2%; 0–10) | 0 (0%; 0–7) |
| Medically attended adverse event | 11 (21%; 11–35) | 12 (23·1%; 13–37) | 15 (29%; 17–43) | 10 (19%; 10–33) | 18 (35%; 22–49) | 14 (27%; 16–41) | 18 (35%; 22–49) |
| Adverse event leading to withdrawal | 0 (0%; 0–7) | 0 (0%; 0–7) | 0 (0%; 0–7) | 0 (0%; 0–7) | 0 (0%; 0–7) | 0 (0%; 0–7) | 0 (0%; 0–7) |
Data are n (%; 95% CI). All adverse events reported outside of the electronic diary were recorded in the participant's case report form. Adverse events were reported up to 1 month after vaccination except for serious adverse events and medically attended adverse events, which were reported up to 6 months after vaccination. Participants are counted only once for a specified category. One participant in the 5 μg GBS6 group with AlPO4 had two serious adverse events and is counted only once in this table. AlPO4=aluminium phosphate. GBS6=group B streptococcus-containing vaccine with serotypes Ia, Ib, II, III, IV, and V.
Four serious adverse events in three GBS6 recipients were reported during the study (up to 6 months after vaccination or receipt of placebo). One (2%) participant who received 5 μg of GBS6 with AlPO4, who had known insulin-dependent diabetes, reported two episodes of diabetic ketoacidosis requiring hospital admission (75 and 85 days after vaccination). The participant recovered from both episodes. One (2%) participant who received 10 μg GBS6 with AlPO4 died by suicide 106 days after vaccination, and one (2%) participant who received 20 μg GBS6 with AlPO4, who had a history of metrorrhagia and endometrial ablation, had worsening of metrorrhagia 107 days after vaccination, which required surgical intervention. None of the serious adverse events were considered by the investigator to be related to the study vaccine. No serious adverse events were reported among placebo recipients. The number of participants reporting medically attended adverse events ranged from ten (19%) participants in the GBS6 10 μg without AlPO4 group to 18 (35%) participants in the GBS6 20 μg with AlPO4 group (table 2). The number of participants who reported severe adverse events ranged from two (4%) in the GBS6 5 μg with AlPO4 group, the GBS6 5 μg without AlPO4 group, and the GBS6 20 μg with AlPO4 group to six (12%) in the GBS6 10 μg with AlPO4 group (table 2). Up to two (4%) of 52 GBS6 recipients in each vaccine group reported adverse events that were deemed to be related to the vaccination, the most common of which were injection-site reactions and headache (one participant in the GBS6 20 μg with AlPO4 group and one participant in the GBS6 20 μg without AlPO4 group had injection site discolouration; one participant in the GBS6 10 μg with AlPO4 group and one participant in the GBS6 20 μg with AlPO4 group had headache). No related adverse events were reported in the placebo group. No immediate adverse events were reported during the study (table 2).
Of the 365 participants enrolled into the study, 11 were excluded from the evaluable immunogenicity population, including one participant who did not receive the vaccine and ten who at 1 month after vaccination were withdrawn for one or more of the following reasons: invalid or indeterminate serological results (five participants), blood samples drawn outside of the blood draw window (three), or a potentially important protocol deviation (three).
Overall, few study participants had prevaccination antibody concentrations below the lower limit of quantitation of the assays; however, up to 31% of participants for serotype Ib and 67% of participants for serotype V (depending on the vaccine group) had prevaccination IgG concentrations below the lower limit of quantitation (data not shown). IgG geometric mean concentrations after vaccination were generally lower in these participants than in those with pre-existing antibodies. However, geometric mean fold rises stratified by baseline IgG concentration were similar between groups irrespective of baseline concentration (data not shown).
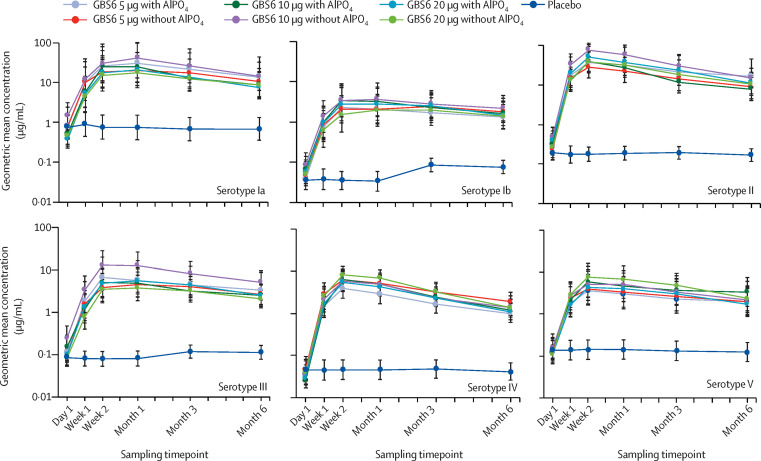
GBS serotype-specific IgG geometric mean concentrations increased rapidly from baseline to 1 week after vaccination across all doses and formulations of GBS6 compared with placebo, peaking by approximately 2 weeks after vaccination for most serotypes and remaining stable up to 1 month after vaccination (figure 4 ; appendix p 11). Although IgG geometric mean concentrations waned by 6 months after vaccination, they remained markedly higher than baseline concentrations and compared with placebo for all doses and formulations of GBS6. No difference in the GBS serotype-specific IgG geometric mean concentration immune response was observed between the GBS6 doses and formulations (figure 4). GBS serotype-specific IgG geometric mean fold rises ranged from approximately 25 to more than 200 for each serotype 1 month after vaccination (appendix p 8). Additionally, GBS serotype-specific IgG geometric mean fold rises remained substantially high for all doses and formulations of GBS6 at 6 months after vaccination (approximately 10-fold to 56-fold rise at 6 months after vaccination) compared with placebo (appendix p 8).
Figure 4.
Group B streptococcus serotype-specific IgG geometric mean concentrations
The IgG assay lower limit of quantitation for each serotype was: Ia 0·008 μg/mL, Ib 0·011 μg/mL, II 0·009 μg/mL, III 0·010 μg/mL, IV 0·005 μg/mL, and V 0·075 μg/mL. GBS6 IgG antibody concentrations more than the lower limit of quantitation and their quantitated values were reported. Values below the lower limit of quantitation were set to 0·5 times the lower limit of quantitation for all analyses. Error bars represent 95% CIs. AlPO4=aluminium phosphate. GBS6=group B streptococcus-containing vaccine with serotypes Ia, Ib, II, III, IV, and V.
The proportion of participants with an IgG antibody concentration of 1·0 μg/mL or more 1 month after vaccination varied by serotype and by dose and formulation. However, at least 75% of participants for at least one dose and formulation group had an antibody concentration of 1·0 μg/mL or more for five of the six vaccine serotypes (Ia, II, III, IV, and V). For serotype Ib, 40–57% of GBS6 participants had reached this threshold at 1 month after vaccination. Geometric mean fold rises from baseline to 1 month after vaccination for serotype Ib were similar to the other serotypes (a 40-fold to 56-fold rise; appendix pp 2, 8). Similar results were observed in post-hoc analyses, using GBS IgG concentration threshold of 0·5 μg/mL for serotypes Ia, II, III, IV, and V (appendix p 5).
Effects of blood sampling timepoint by vaccination group, sex, age, serotype-specific baseline colonisation status, and baseline IgG concentration (on a log scale) were assessed using a mixed-effects model with repeated measurement for serotype-specific IgG concentrations (data not shown). The model-based GBS serotype-specific IgG geometric mean concentrations show similar patterns as the unadjusted GBS serotype-specific IgG geometric mean concentrations. Of the terms evaluated in the model, baseline IgG concentration was strongly correlated with immune response for all six serotypes. Sex, age at vaccination, and baseline colonisation did not considerably or consistently affect the IgG immune response.
Discussion
Overall, GBS6 was safe and well tolerated across all doses and formulations. The vaccine's safety profile was characterised primarily by mild injection-site pain of short duration. There was no evidence of a worsening of reactogenicity with higher doses. The occurrence of local reactions and systemic events with GBS6 was similar to those reported for vaccines that are licensed for use in adults, as reported by Bryant and colleagues,21 and for a different investigational GBS–CRM197 conjugate vaccine, as reported by Madhi and colleagues,17 as well as other vaccines that might be recommended in pregnancy.22, 23, 24, 25
GBS6 induced robust immune responses (as measured by serotype-specific IgG) among vaccine recipients, regardless of dose or formulation. Although we did not observe differences in this non-pregnant population, it is possible that in pregnant women, dose or formulation differences could be observed that directly affect antibody concentrations in the infant. Multiple doses as well as adjuvanted and non-adjuvanted formulations will need to be evaluated in initial studies of GBS6 in pregnant women. For all GBS6-vaccinated groups, a rapid rise in IgG antibody concentration was observed at 1 week after vaccination. Peak responses occurred by 2 weeks and were maintained up to 1 month after vaccination. The rapid responses observed within the first 2 weeks after vaccination might offer an important advantage for pregnant women who might deliver sooner than expected in their third trimester. Geometric mean fold rises at 1 month after vaccination ranged from approximately 25 to more than 200 compared with baseline concentrations. There was a slow decline in IgG concentrations starting at 3 months; however, IgG antibody concentrations and geometric mean fold rises remained substantially elevated from baseline at 6 months after vaccination. This persistent immune response might be important for protection from disease caused by GBS in the mother (eg, post-partum caesarean wound infections)26 and might be an indicator that protection in the infant persists up to at least 3 months of age, with the potential to affect not only early onset disease, but also late-onset disease, for which current prevention approaches are not effective.
A general correlation between greater maternal GBS antibody concentrations and reduced likelihood of GBS disease in newborn babies has been observed in several seroepidemiological studies. The goal of these studies was to identify a maternal serological correlate of protection against GBS disease in the infant to help facilitate vaccine development.27, 28, 29 Baker and colleagues27 did a Bayesian evaluation of absolute disease risk that provided predictions of serotype III early onset disease risk corresponding to a 100% reduction relative to the risk in the general population of colonised women for serotype III-specific IgG concentrations of more than 0·5 μg/mL. For serotypes Ia and V, higher serotype-specific concentrations (>1 to 2 μg/mL) predicted reductions in early onset disease risk of approximately 50–60%. Results reported by Fabbrini and colleagues28 for serotypes Ia and III in European infants were generally consistent with these findings, predicting risk reductions of approximately 75–80% for antibody concentrations of more than 1 to 1·5 μg/mL. Dangor and colleagues29 noted a 90% risk reduction (in early onset disease and late-onset disease combined) in South African infants when using antibody concentration thresholds of 3–6 μg/mL, depending on serotype. Thus, a sufficient concentration of maternal capsular-specific antibody at delivery might provide protection to infants against invasive GBS disease.
Results from these seroepidemiological studies are limited by geographic variability, small samples sizes, and use of different assays, which make direct comparison of proposed protective antibody titres difficult. However, all the studies showed an association between greater maternal antibody concentration and decreased infant disease risk, suggesting that some sufficient concentration of maternal capsular-specific antibody at delivery might provide protection to infants against invasive GBS disease. This association was also shown in a preclinical model of GBS6 before the initiation of this study.20
We selected a threshold IgG concentration of at least 1 μg/mL at 1 month as an early assessment of the immune response after vaccination with GBS6 on the basis of the findings of Baker and colleagues.27 Our results show a robust response to the vaccine. Because there is no definitive protective threshold confirmed for any serotype, and until other seroepidemiological studies have been completed, it is unclear at this time whether IgG concentrations of at least 1 μg/mL are a conservative estimation of a protective threshold. Further studies might also elucidate whether one or multiple antibody protective thresholds (depending on serotype) apply to multivalent GBS vaccines.30
A limitation of this study is that it was done in the USA among quite a homogeneous non-pregnant population, which might reduce the generalisability of our results; however, clinical assessment of GBS6 is already ongoing in another geographic region in a study of pregnant women (NCT03765073). Data from this study will provide the opportunity to assess GBS6 in diverse populations.
This first-in-human trial showed that GBS6 was safe, tolerable, and elicited an immune response among healthy, non-pregnant adults at all doses and formulations tested. The findings from this trial support further evaluation of GBS6 in pregnant women and the amount of passive antibody transfer in newborn babies.
Data sharing
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices: (1) for indications that have been approved in the USA, the EU, or both; or (2) in programmes that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Acknowledgments
Acknowledgments
We thank Kristen Friedman (Pfizer) for writing support, Meichun Ding (Pfizer) and Vani Sundaraiyer (Syneos Health) for statistical support, and Scott Vuocolo (Pfizer) for editorial support.
Contributors
JA, KJC, WJW, SM, and DAS designed the overall study. WCG, KUJ, and ASA provided feedback on the study design. ILS, PCG, DP, and ASA did the immunological analyses. NS, SLB, and JP collected data as study investigators. YP developed the statistical design and oversaw the data analysis. JA drafted the original manuscript. All authors reviewed and edited multiple versions of the manuscript and approved the final version.
Declaration of interests
JA, KJC, ILS, PCG, WJW, WCG, KUJ, YP, SM, DP, DAS, and ASA are current employees of Pfizer and hold stock options. KUJ and ASA have US patent 10226525 issued. ILS has patent WO2016178123A1 issued. NS, SLB, and JP received compensation from Pfizer in their role as study investigators as part of the submitted work.
Supplementary Material
References
- 1.Centers for Disease Control and Prevention Active bacterial core surveillance (ABCs) report. Emerging infections program network, group B streptococcus. 2017. https://www.cdc.gov/abcs/reports-findings/survreports/gbs17.html Updated April 5, 2019.
- 2.Le Doare K, Heath PT. An overview of global GBS epidemiology. Vaccine. 2013;31(suppl 4):D7–12. doi: 10.1016/j.vaccine.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Johri AK, Lata H, Yadav P, et al. Epidemiology of group B streptococcus in developing countries. Vaccine. 2013;31(suppl 4):D43–D45. doi: 10.1016/j.vaccine.2013.05.094. [DOI] [PubMed] [Google Scholar]
- 4.Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59:1–36. [PubMed] [Google Scholar]
- 5.Seale AC, Koech AC, Sheppard AE, et al. Maternal colonization with Streptococcus agalactiae and associated stillbirth and neonatal disease in coastal Kenya. Nat Microbiol. 2016;1 doi: 10.1038/nmicrobiol.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dangor Z, Cutland CL, Izu A, et al. Temporal changes in invasive group B streptococcus serotypes: implications for vaccine development. PLoS One. 2016;11 doi: 10.1371/journal.pone.0169101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dangor Z, Lala SG, Cutland CL, et al. Burden of invasive group B streptococcus disease and early neurological sequelae in South African infants. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards MS, Gonik B. Preventing the broad spectrum of perinatal morbidity and mortality through group B streptococcal vaccination. Vaccine. 2013;31(suppl 4):D66–D71. doi: 10.1016/j.vaccine.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 9.Rivera L, Sáez-Llorens X, Feris-Iglesias J, et al. Incidence and serotype distribution of invasive group B streptococcal disease in young infants: a multi-country observational study. BMC Pediatr. 2015;15:143. doi: 10.1186/s12887-015-0460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker CJ. The spectrum of perinatal group B streptococcal disease. Vaccine. 2013;31(suppl 4):D3–D6. doi: 10.1016/j.vaccine.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 11.Boyer KM, Gotoff SP. Prevention of early-onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis. N Engl J Med. 1986;314:1665–1669. doi: 10.1056/NEJM198606263142603. [DOI] [PubMed] [Google Scholar]
- 12.Lim DV, Morales WJ, Walsh AF, Kazanis D. Reduction of morbidity and mortality rates for neonatal group B streptococcal disease through early diagnosis and chemoprophylaxis. J Clin Microbiol. 1986;23:489–492. doi: 10.1128/jcm.23.3.489-492.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Prevention of perinatal group B streptococcal disease: a public health perspective. MMWR Recomm Rep. 1996;45:1–24. [PubMed] [Google Scholar]
- 14.American College of Obstetricians and Gynecologists Prevention of group B streptococcal early-onset disease in newborns: ACOG Committee opinion, number 782. Obstet Gynecol. 2019;134:e19–e40. doi: 10.1097/AOG.0000000000003334. [DOI] [PubMed] [Google Scholar]
- 15.Van Dyke MK, Phares CR, Lynfield R, et al. Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med. 2009;360:2626–2636. doi: 10.1056/NEJMoa0806820. [DOI] [PubMed] [Google Scholar]
- 16.Seedat F, Stinton C, Patterson J, et al. Adverse events in women and children who have received intrapartum antibiotic prophylaxis treatment: a systematic review. BMC Pregnancy Childbirth. 2017;17:247. doi: 10.1186/s12884-017-1432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madhi SA, Cutland CL, Jose L, et al. Safety and immunogenicity of an investigational maternal trivalent group B streptococcus vaccine in healthy women and their infants: a randomised phase 1b/2 trial. Lancet Infect Dis. 2016;16:923–934. doi: 10.1016/S1473-3099(16)00152-3. [DOI] [PubMed] [Google Scholar]
- 18.Baker CJ, Rench MA, McInnes P. Immunization of pregnant women with group B streptococcal type III capsular polysaccharide-tetanus toxoid conjugate vaccine. Vaccine. 2003;21:3468–3472. doi: 10.1016/s0264-410x(03)00353-0. [DOI] [PubMed] [Google Scholar]
- 19.Francois Watkins LK, McGee L, Schrag SJ, et al. Epidemiology of invasive group B streptococcal infections among nonpregnant adults in the United States, 2008–2016. JAMA Intern Med. 2019;179:479–488. doi: 10.1001/jamainternmed.2018.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buurman ET, Timofeyeva Y, Gu J, et al. A novel hexavalent capsular polysaccharide conjugate vaccine (GBS6) for the prevention of neonatal group B streptococcal infections by maternal immunization. J Infect Dis. 2019;220:105–115. doi: 10.1093/infdis/jiz062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryant KA, Frenck R, Gurtman A, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 18–49 years of age, naive to 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2015;33:5854–5860. doi: 10.1016/j.vaccine.2015.08.080. [DOI] [PubMed] [Google Scholar]
- 22.GlaxoSmithKline Fluarix prescribing information. https://www.fda.gov/media/84804/download
- 23.Seqirus Afluria quadrivalent prescribing information. https://www.fda.gov/media/117022/download
- 24.Sanofi Pasteur Adacel prescribing information. https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Package-Insert—Adacel.pdf
- 25.Seqirus Afluria prescribing information. https://www.immunizationinfo.com/wp-content/uploads/afluria-trivalent-package-insert.pdf
- 26.Lamagni TL, Keshishian C, Efstratiou A, et al. Emerging trends in the epidemiology of invasive group B streptococcal disease in England and Wales, 1991–2010. Clin Infect Dis. 2013;57:682–688. doi: 10.1093/cid/cit337. [DOI] [PubMed] [Google Scholar]
- 27.Baker CJ, Carey VJ, Rench MA, et al. Maternal antibody at delivery protects neonates from early onset group B streptococcal disease. J Infect Dis. 2014;209:781–788. doi: 10.1093/infdis/jit549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabbrini M, Rigat F, Rinaudo CD, et al. The protective value of maternal group B streptococcus antibodies: quantitative and functional analysis of naturally acquired responses to capsular polysaccharides and pilus proteins in European maternal sera. Clin Infect Dis. 2016;63:746–753. doi: 10.1093/cid/ciw377. [DOI] [PubMed] [Google Scholar]
- 29.Dangor Z, Kwatra G, Izu A, et al. Correlates of protection of serotype-specific capsular antibody and invasive group B streptococcus disease in South African infants. Vaccine. 2015;33:6793–6799. doi: 10.1016/j.vaccine.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 30.Andrews NJ, Waight PA, Burbidge P, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014;14:839–846. doi: 10.1016/S1473-3099(14)70822-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices: (1) for indications that have been approved in the USA, the EU, or both; or (2) in programmes that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.