Abstract
Background
Control of human hookworm infection would be greatly aided by the development of an effective vaccine. We aimed to develop a live attenuated human hookworm vaccine.
Methods
This was a two-part clinical trial done at Q-Pharm in Brisbane (QLD, Australia) using a live ultraviolet C (UVC)-attenuated Necator americanus larvae vaccine. Part one was an open-label, dose-finding study using 50 L3 larvae suspended in water to a volume of 200 μL, attenuated with UVC exposure of 700 μJ (L3–700) or 1000 μJ (L3–1000). Part two was a randomised, double-blind, placebo-controlled, challenge study, in which participants were randomly assigned 2:1 to the vaccine group or placebo group. Healthy hookworm-naive adults aged 18–65 years with body-mass index 18–35 kg/m2 received two doses of either placebo (Tabasco sauce) or vaccine (50 L3–700) on day 1 and day 42, followed by challenge with 30 unattenuated L3 larvae to both groups. All participants received a single oral dose of 400 mg albendazole 4 weeks after each inoculation and a 3-day course (400 mg orally daily) initiated on day 161 after the challenge phase, to eliminate any remaining infection. The primary outcome of part 1 was the level of larval attenuation the resulted in a grade 2 or 3 dermal adverse event. The primary outcome of part 2 was safety and tolerability, assessed by frequency and severity of adverse events in all randomly assigned participants. Prespecified exploratory outcomes in the challenge study were faecal N americanus DNA concentration, the number of N americanus larvae recovered per g of faeces cultured, hookworm antigen-specific serum IgG antibody responses, and hookworm antigen-specific peripheral blood cytokine responses. The trial is registered with the Australian New Zealand Clinical Trials Registry (ACTRN12617001007325).
Findings
Between Sept 19, 2017, and Oct 24, 2018, seven participants were enrolled into three cohorts in part one (two participants in cohort 1, who received L3-700; two participants in cohort 2, who received L3-700; and three participants in cohort 3, who received L3-1000) and a further 15 were enrolled into part two. There were no serious adverse events in part one or part two. In part one, a greater number of skin penetration sites were observed after administration of L3-700 than L3-1000 (mean 15·75 [95% CI 11·18 to 20·32] with L3-700 vs 4·33 [–1·40 to 10·07] with L3-1000). Similarly, greater erythema (median 225 mm2 [IQR 150 to 325] vs 25 mm2 [12·5 to 80]) and a longer duration of the dermal reaction (median 8·0 days [IQR 3·5 to 11·5] vs 2·0 days [2·0 to 4·5]) were observed after L3-700 than L3-1000. The mean number of adverse events per participant did not differ between the groups (3·25 [95% CI 1·48 to 5·02] vs 3·00 [1·04 to 4·96]). Thus, L3-700 was used for vaccination in part two. In part two, ten participants were randomly assigned to receive L3-700 and five to placebo. Significantly more adverse events occurred after vaccination with attenuated larvae than with placebo (incident rate ratio [IRR] 2·13 [95% CI 2·09 to 5·51]; p=0·0030). There was no difference between groups in the frequency of adverse events after challenge (IRR 1·25 [0·78 to 2·01]; p=0·36). Most adverse events were mild in severity, with only one severe adverse event reported (erythematous and indurated pruritic rash >100 mm in a vaccine group participant after challenge). The eosinophil count increased in all participants after challenge, with a significantly greater increase among vaccinated participants than placebo participants (1·55 × 109 cells per L [IQR 0·92 to 1·81] in the vaccine group vs 0·49 × 109 cells per L [0·43 to 0·63] in the placebo group; p=0·014). Vaccinated participants had an IgG response to larval extract after challenge that was higher than that in placebo participants (increase in IgG titre 0·22 [IQR 0·10 to 0·41] vs 0·03 [–0·40 to 0·06]; p=0·020). Significantly fewer larvae per g of faeces were recovered in the vaccine group than in the placebo group after challenge (median larvae per g 0·8 [IQR 0·00 to 3·91] vs 10·2 [5·1 to 18·1]; p=0·014). The concentration of N americanus DNA in faeces was not significantly different between the vaccinated group and the placebo group (log10 DNA intensity 4·28 [95% CI 3·92 to 4·63] vs 4·88 [4·31 to 5·46]; p=0·14). Peripheral blood mononuclear cells from vaccinated participants exhibited significantly greater cytokine production at day 112 than placebo participants for IFNγ, TNFα, IL-2, IL-4, and IL-5 (p<0·05), but not IL-10.
Interpretation
Vaccination with UVC-attenuated N americanus larvae is well tolerated, induces humoral and cellular responses to hookworm antigens, and reduces larval output after challenge with unattenuated larvae. Larger studies are required to confirm protective efficacy.
Funding
National Health and Medical Research Council of Australia.
Introduction
Ancylostoma duodenale, Ancylostoma ceylanicum, and Necator americanus are soil-transmitted helminths that reside in the small intestines of their human hosts where they feed on blood. Globally, 450 million people have chronic hookworm infection, which results in an estimated 2·1 million disability-adjusted life-years lost and accounts for more than US$100 billion in global economic losses.1 Mass drug administration using either albendazole or mebendazole to school-aged children is recommended for control of intestinal nematodes in endemic populations. However, in high-prevalence populations, reinfection within 6 months results in infection intensities that equal or even exceed pretreatment levels,2 and modelling indicates that such programmes will not result in elimination without high and frequent coverage over a long period of time.3
Alternative measures for control of human hookworm disease are therefore required, such as an effective vaccine. After initially promising phase 1 trial results, development of the N americanus ancylostoma-secreted protein 2 (Na-ASP-2) subunit vaccine was terminated because of urticarial hypersensitivity reactions that occurred when the vaccine was administered to participants in an area endemic for hookworm.4 N americanus recombinant subunit vaccine candidates glutathione-S-transferase-1 (Na-GST-1) and aspartic protease-1 (Na-APR-1) have been tested as monovalent formulations in phase 1 trials in Brazil, Gabon, and the USA where they have been shown to be safe and immunogenic.5 However, the efficacy of these subunit vaccines remains to be established,6 and an eventual hookworm subunit vaccine is likely to require multiple distinct components targeting different developmental stages and parasitism pathways.7
A highly effective live attenuated vaccine against the canine hookworm Ancylostoma caninum was marketed in the USA in the 1970s. The vaccine comprised γ-irradiated third-stage infective A caninum larvae (L3) to be administered by subcutaneous injection to young dogs.8 This work has been replicated by others9 and importantly has shown that inoculation with live attenuated larvae elicits greater protective immunity than inoculation with sonicated (dead) larvae.10 Similar results have been demonstrated in hamsters and mice using N americanus L3 attenuated by exposure to ultraviolet C light (UVC).11, 12 These animal studies provide proof of concept for an attenuated human hookworm vaccine.
This study describes the development and investigation of a live attenuated human hookworm vaccine. We developed a method for attenuating N americanus L3 with UVC exposure and conducted a two-part clinical trial to investigate the safety, tolerability, immunogenicity, and protective efficacy of the vaccine in healthy volunteers. Although the safety and tolerability of dermally applied unattenuated N americanus larvae in humans has been established in clinical trials,6, 13 the effect of UVC attenuation on the ability of larvae to penetrate human skin and any resulting clinical effects are unknown. Thus, we first did a dose-finding study (part one) to inform dose selection for a subsequent randomised placebo-controlled study (part two).
Research in context.
Evidence before this study
We searched PubMed for clinical trials and animal studies assessing hookworm vaccines from database inception up to July 30, 2021, with the search terms “Necator” OR “Ancylostoma” OR “hookworm” AND “vaccin*”. No language restriction or inclusion and exclusion criteria were applied. Of the 422 results, 61 described hookworm vaccination experiments. Four phase 1 clinical trials were identified investigating recombinant protein vaccines in hookworm-naive and exposed populations. The remaining 57 studies detailed vaccination in animals with a variety of vaccine candidates comprising recombinant proteins, attenuated larvae, abbreviated infection, and infection in non-permissive hosts. Seven of these studies describe controlled challenge trials of a radiation-attenuated Ancylostoma caninum larvae vaccine in dogs, which reliably produced immunity to hookworm disease. This product was then commercialised and is the only marketed hookworm vaccine to date. The success of live attenuated hookworm vaccines was subsequently confirmed in seven additional animal studies with attenuation using ionising radiation, ultraviolet C (UVC) light, and 5-fluorouracil. The fact that there are no publications detailing negative results associated with live attenuated hookworm vaccines might be indicative of significant publication bias.
Added value of this study
To our knowledge, this is the first attenuated helminth vaccine trialled in humans, and indeed is the first helminth vaccine trial in humans that incorporates a challenge phase. We have shown that dermal administration of UVC-attenuated Nector americanus larvae is safe and tolerable in healthy volunteers and elicits specific humoral and cellular immune responses. After challenge with unattenuated larvae, modest reductions in hookworm DNA in faeces and significant reductions in larval numbers recovered by coproculture were observed in vaccinated individuals compared with those receiving a placebo.
Implications of all the available evidence
The results demonstrate that experimental human infection models are appropriate for trialling potential hookworm interventions and that a measurable immune response can be generated in humans by vaccination with live attenuated N americanus larvae. This pilot trial will inform the design of larger trials powered to test efficacy outcomes, and to benchmark other vaccine approaches.
Methods
Study design and participants
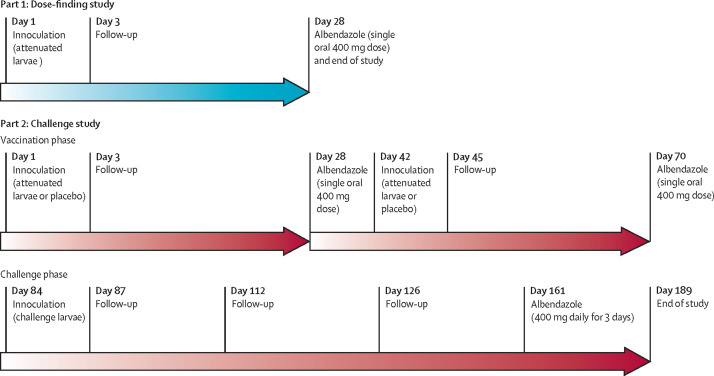
This study was composed of two parts done at Q-Pharm (Brisbane, QLD, Australia). Part one was an open-label dose-finding study to assess the attenuation of UVC-treated N americanus L3 administered by dermal inoculation. Part two was a randomised, double-blind, placebo-controlled challenge study in which participants received two doses of attenuated larvae or placebo followed by challenge with unattenuated N americanus larvae (figure 1 ).
Figure 1.
Study design
Non-pregnant, non-lactating adults aged 18–65 years with body-mass index 18–35 kg/m2 were eligible to participate in this study. Exclusion criteria were a history of immunosuppression, possible past exposure to hookworms assessed by travel history, allergy to any of the study drugs, history of atopy or severe allergic reaction following any vaccination or infusion, treatment in the past 6 months with immune-modulating or cytotoxic medications, anaemia (haemoglobin <130 g/L in men and <120 g/L in women), vaccination in past 30 days, heavily tattooed forearms, and current participation in any other clinical trial (appendix p 2). Individuals screened for this study were interviewed by the principal investigator and educated about natural and experimentally induced hookworm infection, the common adverse events (dermal reactions, gastrointestinal symptoms, respiratory symptoms, and anaemia), the risks of pharmacotherapy (albendazole), and the hypothetical risks of receiving attenuated hookworms. Written information was provided in the consent documentation.
The study was approved by the QIMR Berghofer Medical Research Institute Human Research Ethics Committee. Written informed consent was obtained from all participants. The trial was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines.
Randomisation and masking
Participants in the challenge study (part two) were randomly assigned to either vaccination (attenuated larvae) or placebo (Tabasco sauce) in a 2:1 ratio. The randomisation schedules were generated electronically by an independent, unmasked statistician using a random number generation sequence in Stata, version 15. Participants were masked to treatment allocation. Staff assessing adverse events were also masked to treatment allocation.
Procedures
In-vitro studies demonstrated that UVC exposure produced dose-dependent larval attenuation, as assessed in a thermally induced motility assay (appendix p 5), with reliably detectable attenuation developing after exposure to 700 μJ. It was unknown what effect UVC exposure would have on larval skin penetration, an essential property for dermal delivery of this attenuated vaccine, so a dose-finding study (part one) was first done.
Full details of larval production, attenuation, and administration are in the appendix (pp 3–6). Briefly, larvae of N americanus were cultured by a modified Harada-Mori culture method from the faeces of an experimentally infected donor, as previously described.14 7–10-day-old larvae were harvested and viability was estimated using a thermally induced motility assay.15 For each batch, viability was assessed on the day of harvest and after storage for 14 days (appendix p 5). All larvae were administered to participants within 48 h of harvest and within 24 h of preparation (washing and decontamination in antiseptic). Attenuation was done at the clinical trial site immediately before inoculation by placing 96-well plates loaded with 25±3 L3 in 100 μL of sterile water per well in a UVC cross linker (Hoefer, San Francisco, CA, USA) set to deliver the desired UVC exposure.
Dermal application of 50 unattenuated L3 has previously resulted in tolerable dermal reactions.16 Thus, cohort 1 received 50 L3 attenuated with 700 μJ UVC exposure in water to a volume of 200 μL, administered by dermal application to the forearm of participants on day 1. Participants were observed for 1 h and then returned to the clinic for follow-up visits on day 3 and day 28 (end of study), when a single oral dose of albendazole (400 mg) was administered to eliminate any patent infection from incompletely attenuated larvae (figure 1). A staggered sentinel approach was used for cohort 1 whereby a single participant was initially inoculated and the dermal reaction was reviewed on day 3 before inoculation of the second participant. In successive cohorts, different doses of larval inoculum and UVC exposure were planned to be assessed until the primary outcome had been reached.
For part one, adverse event and skin penetration data were reviewed by the safety review team after each dose cohort to establish the dose of larvae and degree of attenuation to be used in the following cohort. The safety review team comprised the principal investigators and an independent medical monitor. At the conclusion of part one, the safety review team established the dose of larvae and degree of attenuation to be used for vaccination in part two.
In the challenge study (part two), participants received either attenuated larvae or placebo in a volume of 200 μL by dermal application to the forearm on day 1, with a second inoculation performed 6 weeks later on day 42. Tabasco sauce was used as the placebo to replicate the pruritic, erythematous skin reaction caused by dermal hookworm inoculation.17 Albendazole (400 mg orally, single dose) was administered 4 weeks after each inoculation (figure 1). 6 weeks after the second vaccination (day 84), all participants received a challenge dose of 30 non-attenuated L3 by dermal application of 15 L3 to each forearm. Participants returned to the clinic for follow-up visits on days 87, 112, and 126 before a 3-day course of oral albendazole (400 mg daily) was initiated on day 161. The end-of-study visit occurred on day 189.
Inoculation sites were examined 1 h and 2 days after each inoculation and the length and width of the dermal reaction (erythema and induration) were recorded (mm) and a photograph of the area was taken. The inoculation site was also examined under × 4 magnification with a hand lens, and the number of penetration sites was recorded. Adverse events were recorded throughout the study and an assessment of their severity and cause was performed by the investigator. The severity of adverse events was classified according to criteria adapted from US Food and Drug Administration guidance.18 An adverse event was classified as a serious adverse event if it resulted in death, was life threatening, required hospital admission, resulted in a birth defect, resulted in a significant disability or incapacity, or was another important medical event. Participants were given a diary card to record symptoms at home and were asked to bring the card to every clinic visit for review.
Faecal samples were collected on day 70 (before challenge), for 3 days before review on day 161, and after administration of albendazole. Faecal N americanus DNA content was assessed using qPCR as previously described,19 and coproculture was done as described in the appendix (p 3). Formal quantitative egg counts by microscopy were not performed because the required immediate processing of fresh samples was not possible.20 Safety blood samples (complete blood counts, electrolytes, and liver function tests) were collected at screening and days 1, 28, 70, 112, 161, and 189. Additional blood samples were collected for immunological analyses at days 1, 3, 28, 42, 45, 70, 84, 87, 112, 126, and 161. Total IgE was estimated from samples from day 1 and day 112 by the clinical pathology provider. N americanus L3 antigen-specific IgG production was assessed by ELISA and cytokine production by peripheral blood mononuclear cell stimulation as described in the appendix (pp 7–8).
Outcomes
The primary outcome of the dose-ranging study (part one) was the level of larval attenuation that resulted in a mild-to-moderate (grade 1–2) dermal reaction. This outcome determined the level of larval attenuation to be used in the challenge study (part two). The primary outcome of the challenge study was the frequency and severity of adverse events in the vaccinated group compared with the placebo group after vaccination with attenuated larvae and challenge with unattenuated larvae. Prespecified exploratory outcomes in the challenge study were faecal N americanus DNA concentration, the number of N americanus larvae recovered per g of faeces cultured, hookworm antigen-specific serum IgG antibody responses, and hookworm antigen-specific peripheral blood cytokine responses. Eosinophil counts were analysed as an exploratory immunological outcome (cell-mediated immunity).
Statistical analysis
The sample size planned for this phase 1 study was not based on formal statistical calculations but was designed to assess the primary objective of the study: to establish the safety and tolerability of the attenuated hookworm vaccine. The mean and 95% CI of the number and incident rate ratio (IRR) of adverse events per participant were derived and compared between treatment groups using a Poisson regression model. Associations between treatment groups and an adverse event severity index calculated per participant (1 for each mild event, 2 for moderate, and 3 for severe) were assessed using a Mann-Whitney U test. Categorical clinical measures were summarised by frequency and percentage and continuous clinical measures were summarised by mean and SD or median and IQR for non-normally distributed data. Continuous measures such as dermal reactions and hookworm larvae characteristics were compared between treatment groups using a two-sample t test for normally distributed data, or a Mann-Whitney U test for non-normally distributed data. Paired comparisons of continuous measures between timepoints within vaccine or challenge trial stages were performed using a paired t test for normally distributed data or an Exact sign test for non-normal and non-symmetrical data. Associations between continuous clinical measures were assessed using a Spearman's rank order correlation for non-normally distributed data. Associations between faecal hookworm DNA content (continuous) and treatment groups were assessed using a generalised linear model (identity link) with a randomised block design. Treatment group, daily samples as blocks, and technical qPCR replicates were used to estimate intra-assay variability.
The primary outcome (safety) was assessed in all randomly assigned participants. Statistical analyses were done in Stata, version 15 and SPSS, version 22.0. The trial is registered with the Australian New Zealand Clinical Trials Registry (ACTRN12617001007325).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
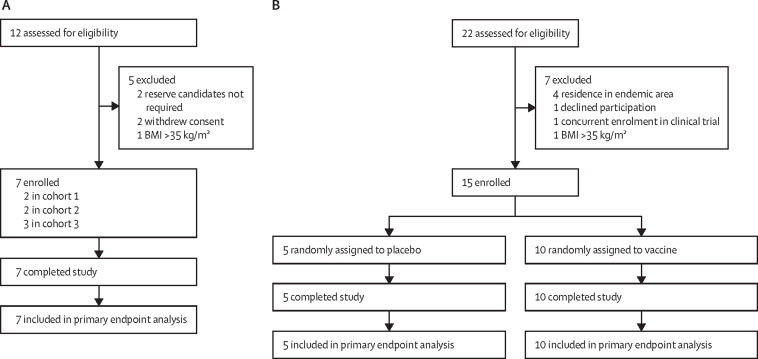
In part one, seven participants were enrolled across three cohorts between Sept 19, 2017, and Jan 11, 2018 (figure 2A ). Most participants were women and White (table 1 ). Participants in cohorts 1 and 2 received 50 L3 attenuated with 700 μJ of UVC (L3-700), whereas those in cohort 3 received 50 L3 attenuated with 1000 μJ of UVC (L3-1000). All participants completed the study per protocol. A greater number of skin penetration sites were evident among participants receiving L3-700 than participants receiving L3-1000 (mean 15·75 [95% CI 11·18 to 20·32] with L3-700 vs 4·33 [–1·40 to 10·07] with L3-1000; appendix p 9). Additionally, the area of erythema was larger (median 225 mm2 [IQR 150 to 325] vs 25 mm2 [12·5 to 80]) and the duration of the dermal reaction was longer (median 8·0 days [IQR 3·5 to 11·5] vs 2·0 days [2·0 to 4·5]) in participants administered L3-700 than in those administered L3-1000 (appendix p 9).
Figure 2.
Trial profile
(A) Part one dose-finding study. (B) Part two challenge study. BMI=body-mass index.
Table 1.
Baseline patient characteristics
| Dose-finding study (n=7) |
Challenge study |
|||
|---|---|---|---|---|
| Vaccine group (n=10) | Placebo group (n=5) | |||
| Sex | ||||
| Female | 6 (86%) | 2 (20%) | 2 (40%) | |
| Male | 1 (14%) | 8 (80%) | 3 (60%) | |
| Age, years | 34·0 (22·0–47·0) | 39·0 (29·0–56·0) | 48·0 (48·0–54·0) | |
| Race | ||||
| White | 6 (86%) | 9 (90%) | 5 (100%) | |
| Asian | 1 (14%) | 0 | 0 | |
| Pacific Islander | 0 | 1 (10%) | 0 | |
| Height, cm | 171·0 (160·0–175·0) | 180·0 (175·0–188·0) | 163·0 (161·0–172·0) | |
| Weight, kg | 62·3 (58·3–79·8) | 84·4 (74·1–101·7) | 87·3 (76·4–87·4) | |
| Body-mass index, kg/m2 | 24·3 (21·0–25·8) | 26·5 (24·2–28·8) | 29·8 (29·5–33·7) | |
Data are n (%) or median (IQR).
Adverse events were reported by all participants in part one and were mild (grade 1) in severity; dermal reactions were observed universally and accounted for most adverse events. The mean number of adverse events per participant did not differ substantially between participants inoculated with larvae attenuated with 700 μJ or 1000 μJ UVC (3·25 [95% CI 1·48–5·02] vs 3·00 [1·04–4·96]; appendix p 10). No serious adverse events occurred and no participant reported respiratory symptoms. Increased eosinophil counts were not detected. One participant who received L3-700 reported transient diarrhoea lasting less than 24 h that commenced 27 days after the administration of larvae; the diarrhoea resolved before albendazole administration. Another participant who received L3-700 developed normocytic anaemia (haemoglobin 10·6 g/dL) at day 28. This participant had a history of hypothyroidism requiring thyroxine replacement therapy. It was discovered that the participant had been non-compliant with this therapy and had a thyroid-stimulating hormone level of more than 100 mIU/L. The participant's haemoglobin returned to normal upon resumption of thyroxine replacement. Because the L3-700 dose produced a grade 1 dermal reaction, which met the prespecified criteria for the vaccine inoculum, the safety review team approved progression to the challenge study (part two) using L3-700 as the dose for vaccination.
In the challenge study, 15 participants were enrolled between March 8 and Oct 24, 2018, with ten participants randomly assigned to receive UVC-attenuated hookworm larvae (L3-700) and five participants randomly assigned to receive placebo (figure 2B). Most participants were men and White (table 1). All participants completed the study per protocol.
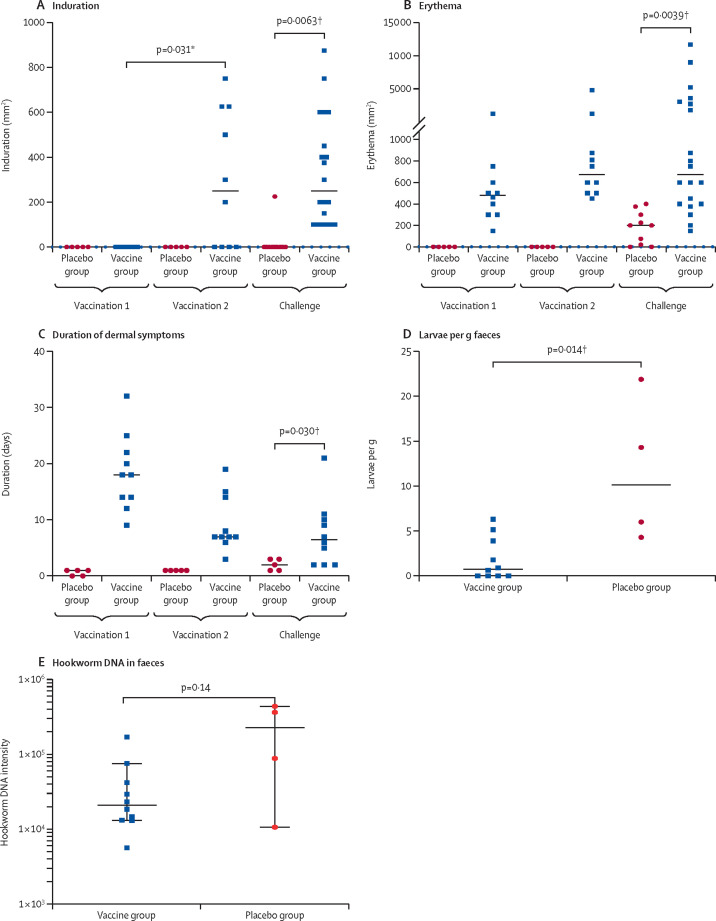
Dermal reaction was universal among participants administered the L3-700 vaccine, and in the placebo group was observed in four (80%) of five participants after the first inoculation and five (100%) of five after the second inoculation. Among participants who received L3-700, induration was not observed after the first vaccination but was observed in six (60%) of ten participants after the second vaccination (figure 3A ). After challenge with unattenuated L3, dermal reaction and pruritus occurred in all participants. 3 days after challenge, the median areas of induration and erythema were significantly greater in the vaccinated group than the placebo group (induration 375 mm2 [IQR 100–475] in the vaccine group vs 0 mm2 [0–0] in the placebo group, p=0·0063; and erythema 594 mm2 [500–2675] vs 226 mm2 [110–263], p=0·0039; figure 3A, B). Similarly, the median duration of dermal symptoms was longer in the vaccinated group than the placebo group (6·5 days [2·0–10·0] vs 2·0 days [1·0–3·0], p=0·030; figure 3C). Blistering and exudation were observed exclusively in the vaccinated group (blistering in six [60%] of ten and exudation in four [40%] of ten participants). Representative photographs of a dermal reaction after vaccination and challenge are in the appendix (p 12).
Figure 3.
Dermal reactions and faecal larval content in the challenge study
The area of induration (A), area of erythema (B), and duration of dermal symptoms (C) at the inoculation site after each vaccination and challenge with non-attenuated larvae. Induration and erythema were measured 2 days after each inoculation (both groups were measured for the challenge inoculation). The number of Necator americanus larvae recovered per g of faeces cultured (D) and the N americanus DNA intensity in faecal culture (E) from samples collected on day 161. Datapoints are for individual participants, horizontal lines show the group median, and error bars show the 95% CI. *Calculated using exact sign test. †Calculated using Mann-Whitney U test.
No serious adverse events were observed during the challenge study. A significantly greater number of adverse events were observed following vaccination with L3-700 than with placebo, with participants having a mean of 8·10 (95% CI 6·3–9·9) adverse events in the L3-700 group compared with 3·8 (2·1–5·5) in the placebo group (IRR 2·13 [95% CI 2·09–5·51]; p=0·0030). Following challenge, there was no difference in the frequency of adverse events between the two groups (mean 6·0 [95% CI 4·5–7·5] for the vaccine group and 4·8 [2·9–6·7] for the placebo group; IRR 1·25 [0·78–2·01]; p=0·36). Most adverse events were mild (table 2 ). One adverse event was classified as severe: a participant from the vaccine group developed an erythematous and indurated pruritic rash of greater than 100 mm in length at both challenge inoculation sites. The rash self-resolved after 6 days. Respiratory and gastrointestinal symptoms were infrequently reported and were predominantly of mild severity. There were no notable changes in haemoglobin or haematocrit.
Table 2.
Severity of treatment-related adverse events in the challenge study
|
Vaccine group (n=10) |
Placebo group (n=5) |
p value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Adverse event index | Mild | Moderate | Severe | Adverse event index | |||
| Vaccine stage 1 and 2 | ||||||||||
| All treatment-related adverse events | 63 | 11 | 0 | 4·5 (3·0–5·0) | 13 | 3 | 0 | 1·0 (1·0–2·0) | 0·0064 | |
| Dermal reactions | 53 | 11 | 0 | 3·0 (2·5–5·0) | 8 | 1 | 0 | 1·0 (1·0–2·0) | 0·00084 | |
| Induration or erythema | 19 | 1 | 0 | 1·0 (1·0–2·0) | 5 | 1 | 0 | 1·0 (1·0–1·0) | 0·32 | |
| Other dermal | 34 | 10 | 0 | 2·0 (2·0–3·0) | 3 | 0 | 0 | 1·0 (1·0–1·0) | 0·010 | |
| Gastrointestinal symptoms | 7 | 0 | 0 | 1·0 (1·0–2·0) | 5 | 2 | 0 | 2·0 (1·0–3·5) | 0·42 | |
| Respiratory reactions | 0 | 0 | 0 | .. | 0 | 0 | 0 | .. | .. | |
| Other | 3 | 0 | 0 | 1·0 (1·0–1·0) | 0 | 0 | 0 | .. | .. | |
| Challenge stage | ||||||||||
| All treatment-related adverse events | 47 | 8 | 1 | 5·5 (5·0–7·0) | 19 | 2 | 0 | 4·0 (3·0–5·0) | 0·090 | |
| Dermal reactions | 30 | 6 | 1 | 4·0 (4·0–5·0) | 6 | 2 | 0 | 2·0 (2·0–2·0) | 0·0026 | |
| Induration or erythema | 13 | 3 | 1 | 2·0 (2·0–3·0) | 2 | 0 | 0 | 1·0 (1·0–1·0) | 0·37 | |
| Other dermal | 17 | 3 | 0 | 2·0 (1·0–3·0) | 4 | 2 | 0 | 2·0 (1·0–2·0) | 0·27 | |
| Gastrointestinal symptoms | 7 | 2 | 0 | 1·5 (1·0–2·0) | 6 | 0 | 0 | 3·0 (1·0–5·0) | 0·59 | |
| Respiratory reactions | 0 | 0 | 0 | .. | 2 | 0 | 0 | 2·0 (NA) | .. | |
| Other | 10 | 0 | 0 | 1·0 (1·0–1·0) | 5 | 0 | 0 | 1·0 (1·0–1·0) | .. | |
Data are number of events and median (IQR). NA=not applicable.
Faecal samples were collected from all participants on day 161 (43 samples; 13 participants produced three samples and two participants produced two samples over the 72-h collection period) for coproculture and to quantify the faecal concentration of N americanus DNA by qPCR. One participant in the placebo group submitted insufficient sample for coproculture (6 g), allowing only qPCR. Additionally, the qPCR assay on the sample from another participant in the placebo group was unsuccessful (did not pass quality control amplification of the control gene target). Thus, coproculture and qPCR data were only available for four participants in the placebo group. Significantly fewer larvae per g of faeces were recovered in the vaccine group than in the placebo group (median larvae per g 0·8 [IQR 0·00–3·91] in the vaccine group vs 10·2 [5·1–18·1] in the placebo group; p=0·014; figure 3D). Although the median concentration of N americanus DNA in the faeces of placebo participants was slightly higher than that of vaccinated participants, the difference was not significant (log10 DNA intensity 4·28 [95% CI 3·92–4·63] vs 4·88 [95% CI 4·31–5·46]; p=0·14; figure 3E).
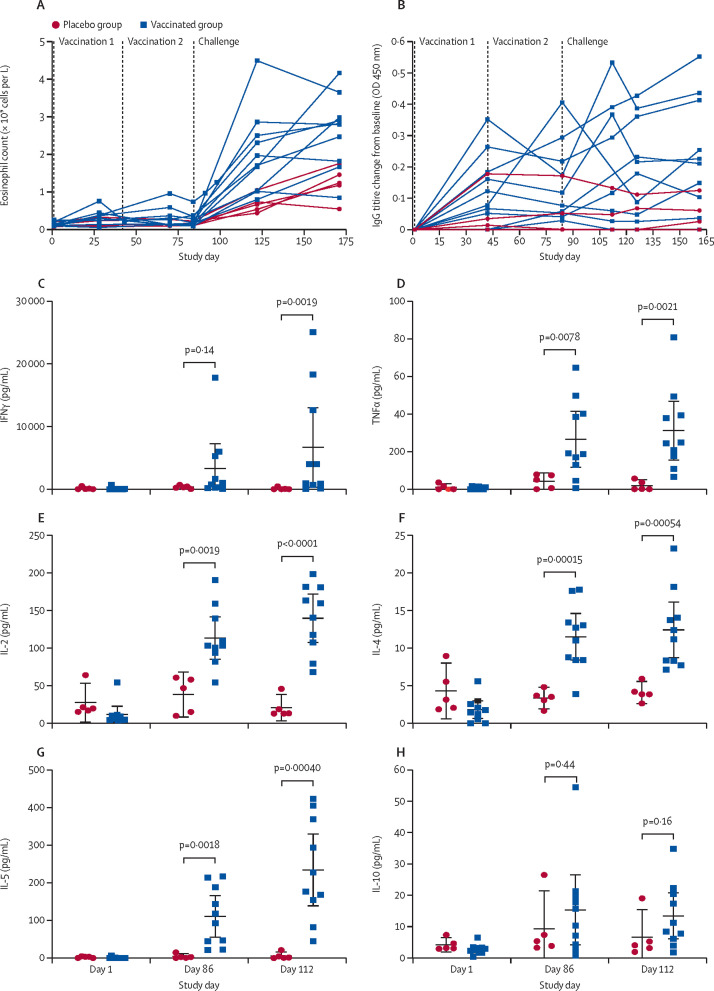
In an exploratory analysis, there was no significant increase in eosinophil counts after vaccination, with a median increase in peripheral eosinophil count at day 70 compared with baseline (day 1) of 0·04 × 109 cells per L (IQR 0·04 to 0·11; p=0·11) among the vaccine group participants (figure 4A ). After challenge, an increase in eosinophil count occurred in all participants (figure 4A), with a significantly greater median increase among vaccine group participants than placebo group participants at day 161 (1·55 × 109 cells per L [IQR 0·92 to 1·81] in the vaccine group vs 0·49 × 109 cells per L [0·43 to 0·63] in the placebo group; p=0·014). Total IgE increased in the vaccinated group but not in the placebo group (appendix p 11). The median increase in total IgE from day 1 to day 112 was 1·0 kIU/L (−15·0 to 1·0) in the placebo group compared with 12·5 kIU/L (2·0 to 21·0) in the vaccinated group (p=0·0195). An increase in N americanus L3 soluble antigen-specific IgG titre was also observed in the vaccinated group, particularly after challenge with unattenuated larvae (figure 4B). The median increase in IgG titre on day 161 compared with baseline (day 1) was 0·22 (0·10 to 0·41) for the vaccine group compared with 0·03 (–0·40 to 0·06) for the placebo group (p=0·020). However, individual IgG responses were heterogeneous, with two (20%) of ten vaccine recipients not having a measurable IgG response. Two individuals in the placebo group had unexplained high baseline IgG on day 1. In both individuals, the level decreased by day 28 and remained low after challenge. Analysis of hookworm antigen-specific cytokine responses by peripheral blood mononuclear cells stimulated with N americanus L3 soluble antigen showed that vaccine group participants exhibited significantly greater production of IFNγ, TNFα, IL-2, IL-4, and IL-5, but not IL-10, than participants who received placebo (figure 4C–H), when assessed at day 112 (28 days after challenge).
Figure 4.
Immune response over the course of the challenge study
Peripheral blood eosinophil count (A) and N americanus L3 soluble antigen-specific IgG titre change from baseline (B). Cytokine production by stimulated peripheral blood mononuclear cells: IFNγ (C), TNFα (D), IL-2 (E), IL-4 (F), IL-5 (G), and IL-10 (H). Datapoints are for individual participants, horizontal lines show the group mean, and error bars show the 95% CI. p values were calculated using a two-sample t test.
Discussion
To the best of our knowledge, this study describes the first investigation of an attenuated helminth vaccine in humans and is the first time that an experimental helminth vaccine has been tested for efficacy with a controlled parasite challenge. Overall, the results of the study indicated that this approach is safe, with no serious adverse events observed. Both the attenuated larvae and challenge larvae were well tolerated, with no rescue medication required. As expected, participants who received vaccination with attenuated hookworm larvae reported more adverse events (principally dermal reactions) than those who received placebo. After challenge with unattenuated larvae in all participants, adverse events occurred at similar frequencies in both groups. Dermal reactions became more prominent with repeat exposure to larvae, with larger areas of erythema, the development of induration, blistering, and exudation at the site. This phenomenon has been noted previously, most compellingly in a series of nine patients who received repeated inoculation with 400 A duodenale larvae as treatment for polycythaemia and hypertension.21
Relatively little is known about the immunological correlates of natural or acquired immune protection against hookworm infection in humans. Although parasite-specific antibody responses (IgE and IgG) and eosinophilia are features of natural hookworm infections in endemic areas,7 and were increased in the vaccine group participants in our study, it is unclear whether these immune responses are protective in the context of natural infection or vaccination. Animal models of hookworm infection suggest that eosinophils might be associated with protection against helminth reinfection, particularly while the parasites transit the lungs.22, 23 In our study, two participants from the placebo group had unexplained high antigen-specific IgG titres at baseline. One of these participants also had an increased total IgE (which did not change after challenge). We have no explanation for the increased baseline IgG readings in these two participants. However, given the absence of a compatible exposure history, pre-existing infection is an unlikely explanation. The likelihood that this is a spurious result is supported by the normalisation of parasite-specific IgG levels by day 28, and by the absence of cell-mediated immunity correlates. Similarly, the significance of an increased total IgE level in the absence of allergy, atopy, or exposure to parasites is questionable.
Vaccinated participants in the present study also displayed a mixed cytokine response to hookworm challenge, involving type 2 (IL-4 and IL-5), type 1 (IFNγ, TNF, and IL-2), and regulatory cytokines, which is consistent with that seen in natural infections24 and in experimental infection studies.25 Notably, increased IL-5 has been correlated with the development of natural immunity to reinfection in endemic areas.24 Geiger and colleagues described similar production of TNFα, INFγ, IL-5, and IL-13 following stimulation with hookworm antigens to those observed in the control group of this study after challenge.26 However, the magnitude of response to stimulation in the vaccine group was much greater than in the placebo group, consistent with the development of an immune response. The positive association between increased antiparasitic cellular, antibody, and cytokine responses identified in our study warrants further investigation, such as detailed analysis to identify memory T cell subsets and antibody targets to guide future vaccine design strategies.
Parasitological outcomes were an exploratory component of part two of this study. Part one was not designed to assess for patency after inoculation with attenuated larvae, and all participants were treated with albendazole at 4 weeks. As it is possible that attenuation is incomplete, in future studies it will be important to assess for patency after a longer interval. Faecal microscopy was not done in this study. Traditionally, faecal egg counts have been used to assess efficacy of anthelmintic chemotherapy, although the usefulness of the so-called faecal egg count reduction test is modest at best,27 and a gold standard for diagnosis and quantification of hookworm burden does not exist.28 Considerable day-to-day variation in faecal egg count is observed, particularly in controlled human hookworm infection where parasite burden is low.13 Furthermore, without prompt preservation or refrigeration of faecal samples, the sensitivity of microscopy for detection of N americanus eggs diminishes significantly within hours of faecal sample production20, 29 and preservation or refrigeration would prevent coproculture. In this trial, because parasite burden was an exploratory outcome, it was established that requiring the participants to manipulate their faecal samples (for preservation or refrigeration) or attend the trial site within hours of defecation was not feasible. Molecular estimation of faecal hookworm DNA content was therefore used, and has previously been described to have greater sensitivity than parasitological methods.19 However, at low faecal egg density, estimation of parasite burden might be confounded by other issues such as density-dependent worm fecundity, and the maturation of embryonated eggs with variable numbers of nuclei.30 Coproculture has been demonstrated to be more sensitive than microscopy for diagnosis of Strongyloides stercoralis and hookworm infection31 and has been used to confirm patency of infection with N americanus in microscopy-negative participants in clinical trials.32, 33 In the current study, there was a significant reduction in the number of hookworm larvae recovered upon faecal culture between the vaccinated and placebo groups, despite there being no significant difference in N americanus faecal DNA content. In future trials, assessment of the protective effect of vaccination using a larger challenge dose would be informative, although there is a risk of increased dermal and gastrointestinal adverse events.16 Similarly, as patency has been shown to take up to 100 days after inoculation to develop,6 increasing the duration of observation after challenge and the number of faecal samples analysed might improve the reliability of parasitological endpoints.
Interpretation of the exploratory outcomes in this trial is limited by its small sample size. As a phase 1 study, with little previous safety and efficacy data to guide study design, this study could not be powered for an efficacy outcome. Using the observed difference in faecal hookworm DNA content by qPCR, we estimate that 35 participants (26 vaccine group and nine placebo group) would be required to establish efficacy with 80% power. An additional limitation of this study is the absence of an active comparator group. It is possible that serial inoculation with hookworm larvae followed by administration of albendazole might produce similar results to the use of attenuation, although this effect has not been observed in field studies where participants are constantly exposed to infective L3 and receive regular anthelmintic treatment.2 Furthermore, there are practical limitations to the scalability of an attenuated hookworm larvae vaccine. Larval production remains dependent on culture of faeces from infected donors, is labour intensive, and the product has a limited shelf life with specific storage requirements.
Despite the limitations of our study, it provides robust proof of concept that controlled human hookworm infection can be used to assess the efficacy of hookworm therapeutics. Additionally, the observed development of sensitisation to larvae and parasite-specific immune response, trend toward lower faecal hookworm DNA, and significant reduction in culturable larvae per g of faeces are important findings that support the hypothesis that protective immunity to hookworm infection can be induced in humans by vaccination. Further work is needed to optimise the protocols for vaccination and challenge, including increasing the challenge dose on the basis of the favourable profile of adverse events observed in a recent dose-escalation safety study.13
Data sharing
All data obtained in this study will be made available upon reasonable request by contacting the corresponding author.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This study was funded by a National Health and Medical Research Council (NHMRC) Program Grant (1132975). JSM was supported by a Practitioner Fellowship (1135955). AL was supported by an NHMRC Senior Principal Research Fellowship (1117504). We would like to thank the volunteers for their participation in the study, and Ihab Caraguli from Icon Cancer Centre (Cairns, QLD, Australia) for help with preparatory preclinical work.
Contributors
PRC, PG, AL, and JSM designed the study and prepared the manuscript. PRC and SL did preclinical studies and N americanus larval production. PRC performed larval culture, production, and attenuation and performed clinical reviews at the trial site. RW was the trial co-ordinator. PG, LB, MSP, and AL performed the ELISA experiments. FDLR and CRE performed the cell-mediated assays. SL and PO'R performed the statistical analysis. PRC drafted the manuscript and all authors edited and approved the manuscript. JSM and PRC accessed and verified the data. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Bartsch SM, Hotez PJ, Asti L, et al. The global economic and health burden of human hookworm infection. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0004922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunn JC, Bettis AA, Wyine NY, et al. Soil-transmitted helminth reinfection four and six months after mass drug administration: results from the delta region of Myanmar. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0006591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrell SH, Coffeng LE, Truscott JE, et al. Investigating the effectiveness of current and modified World Health Organization guidelines for the control of soil-transmitted helminth infections. Clin Infect Dis. 2018;66(suppl 4):S253–S259. doi: 10.1093/cid/ciy002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diemert DJ, Pinto AG, Freire J, et al. Generalized urticaria induced by the Na-ASP-2 hookworm vaccine: implications for the development of vaccines against helminths. J Allergy Clin Immunol. 2012;130:169. doi: 10.1016/j.jaci.2012.04.027. 76.e6. [DOI] [PubMed] [Google Scholar]
- 5.Diemert DJ, Freire J, Valente V, et al. Safety and immunogenicity of the Na-GST-1 hookworm vaccine in Brazilian and American adults. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diemert D, Campbell D, Brelsford J, et al. Controlled human hookworm infection: accelerating human hookworm vaccine development. Open Forum Infect Dis. 2018;5 doi: 10.1093/ofid/ofy083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loukas A, Hotez PJ, Diemert D, et al. Hookworm infection. Nat Rev Dis Primers. 2016;2 doi: 10.1038/nrdp.2016.88. [DOI] [PubMed] [Google Scholar]
- 8.Miller TA. Industrial development and field use of the canine hookworm vaccine. Adv Parasitol. 1978;16:333–342. doi: 10.1016/s0065-308x(08)60577-1. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara RT, Loukas A, Mendez S, et al. Vaccination with irradiated Ancylostoma caninum third stage larvae induces a Th2 protective response in dogs. Vaccine. 2006;24:501–509. doi: 10.1016/j.vaccine.2005.07.091. [DOI] [PubMed] [Google Scholar]
- 10.Vinayak VK, Gupta NK, Chopra AK, Sharma GL, Kumar A. Efficacies of vaccines against canine hookworm disease. Parasitology. 1981;82:375–382. doi: 10.1017/s0031182000066907. [DOI] [PubMed] [Google Scholar]
- 11.Jian X, Jun-Min Y, Hai-Chou X, et al. Protective immunity elicited by ultraviolet-irradiated third-stage infective hookworm (Necator americanus and Ancylostoma caninum) larvae in mice and hamsters. Southeast Asian J Trop Med Public Health. 2006;37:885–895. [PubMed] [Google Scholar]
- 12.Menon S, Bhopale MK. Efficacy of UV-irradiated larval vaccine of Ancylostoma ceylanicum (Looss, 1911) in golden hamsters (Mesocricetus auratus) J Helminthol. 1985;59:287–293. doi: 10.1017/s0022149x00025815. [DOI] [PubMed] [Google Scholar]
- 13.Hoogerwerf MA, Coffeng LE, Brienen EAT, et al. New insights into the kinetics and variability of egg excretion in controlled human hookworm infections. J Infect Dis. 2019;220:1044–1048. doi: 10.1093/infdis/jiz218. [DOI] [PubMed] [Google Scholar]
- 14.Croese J, Giacomin P, Navarro S, et al. Experimental hookworm infection and gluten microchallenge promote tolerance in celiac disease. J Allergy Clin Immunol. 2015;135:508–516. doi: 10.1016/j.jaci.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Kotze AC, Clifford S, O'Grady J, Behnke JM, McCarthy JS. An in vitro larval motility assay to determine anthelmintic sensitivity for human hookworm and Strongyloides species. Am J Trop Med Hyg. 2004;71:608–616. [PubMed] [Google Scholar]
- 16.Mortimer K, Brown A, Feary J, et al. Dose-ranging study for trials of therapeutic infection with Necator americanus in humans. Am J Trop Med Hyg. 2006;75:914–920. [PubMed] [Google Scholar]
- 17.Daveson AJ, Jones DM, Gaze S, et al. Effect of hookworm infection on wheat challenge in celiac disease—a randomised double-blinded placebo controlled trial. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Department of Health and Human Services Guidance for industry. Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. September, 2007. https://www.fda.gov/media/73679/download [DOI] [PubMed]
- 19.Llewellyn S, Inpankaew T, Nery SV, et al. Application of a multiplex quantitative PCR to assess prevalence and intensity of intestinal parasite infections in a controlled clinical trial. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0004380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosch F, Palmeirim MS, Ali SM, Ame SM, Hattendorf J, Keiser J. Diagnosis of soil-transmitted helminths using the Kato-Katz technique: what is the influence of stirring, storage time and storage temperature on stool sample egg counts? PLoS Negl Trop Dis. 2021;15 doi: 10.1371/journal.pntd.0009032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brumpt LC. Deductions cliniques tirées de cinquante cas d'ankylostomose provoquée. Ann Parasitol Hum Comp. 1952;27:237–249. [PubMed] [Google Scholar]
- 22.Obata-Ninomiya K, Ishiwata K, Nakano H, et al. CXCR6+ST2+ memory T helper 2 cells induced the expression of major basic protein in eosinophils to reduce the fecundity of helminth. Proc Natl Acad Sci USA. 2018;115:E9849–E9858. doi: 10.1073/pnas.1714731115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knott ML, Matthaei KI, Giacomin PR, Wang H, Foster PS, Dent LA. Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int J Parasitol. 2007;37:1367–1378. doi: 10.1016/j.ijpara.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Quinnell RJ, Pritchard DI, Raiko A, Brown AP, Shaw MA. Immune responses in human necatoriasis: association between interleukin-5 responses and resistance to reinfection. J Infect Dis. 2004;190:430–438. doi: 10.1086/422256. [DOI] [PubMed] [Google Scholar]
- 25.Gaze S, McSorley HJ, Daveson J, et al. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geiger SM, Fujiwara RT, Santiago H, Corrêa-Oliveira R, Bethony JM. Early stage-specific immune responses in primary experimental human hookworm infection. Microbes Infect. 2008;10:1524–1535. doi: 10.1016/j.micinf.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Levecke B, Speybroeck N, Dobson RJ, Vercruysse J, Charlier J. Novel insights in the fecal egg count reduction test for monitoring drug efficacy against soil-transmitted helminths in large-scale treatment programs. PLoS Negl Trop Dis. 2011;5 doi: 10.1371/journal.pntd.0001427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikolay B, Brooker SJ, Pullan RL. Sensitivity of diagnostic tests for human soil-transmitted helminth infections: a meta-analysis in the absence of a true gold standard. Int J Parasitol. 2014;44:765–774. doi: 10.1016/j.ijpara.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dacombe RJ, Crampin AC, Floyd S, et al. Time delays between patient and laboratory selectively affect accuracy of helminth diagnosis. Trans R Soc Trop Med Hyg. 2007;101:140–145. doi: 10.1016/j.trstmh.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Kotze AC, Kopp SR. The potential impact of density dependent fecundity on the use of the faecal egg count reduction test for detecting drug resistance in human hookworms. PLoS Negl Trop Dis. 2008;2:e297. doi: 10.1371/journal.pntd.0000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inês EJ, Souza JN, Santos RC, et al. Efficacy of parasitological methods for the diagnosis of Strongyloides stercoralis and hookworm in faecal specimens. Acta Trop. 2011;120:206–210. doi: 10.1016/j.actatropica.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Feary J, Venn A, Brown A, et al. Safety of hookworm infection in individuals with measurable airway responsiveness: a randomized placebo-controlled feasibility study. Clin Exp Allergy. 2009;39:1060–1068. doi: 10.1111/j.1365-2222.2009.03187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feary JR, Venn AJ, Mortimer K, et al. Experimental hookworm infection: a randomized placebo-controlled trial in asthma. Clin Exp Allergy. 2010;40:299–306. doi: 10.1111/j.1365-2222.2009.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data obtained in this study will be made available upon reasonable request by contacting the corresponding author.