Abstract
Haemophilus influenzae, especially the nontypeable strains, are among the most common pathogens encountered in patients with chronic lung disease and otitis media. We and others have demonstrated that respiratory isolates of nontypeable H. influenzae bind to human mucins, but the mechanism of binding is not entirely clear. We have therefore examined the role of pili in the adherence of both type b and nontypeable H. influenzae to human respiratory mucins. We used isogenic H. influenzae strains with a mutation in the structural gene for pilin (hifA), a laboratory H. influenzae strain transformed with a type b pilus gene cluster (from strain C54), antibodies raised against H. influenzae HifA, and Escherichia coli strains carrying a cloned type b pilus gene cluster (from strain AM30) in these studies. All bacteria lacking HifA or the pilus gene cluster had decreased adherence of piliated H. influenzae to mucins, and Fab fragments of anti-HifA antibodies inhibited the adherence. E. coli strains carrying the cloned type b pilus gene cluster were six to seven times more adhesive than strains carrying the vector. The role of other putative adhesins was not examined and thus cannot be excluded, but these studies support a role for pili in the binding of H. influenzae to human respiratory mucins.
Haemophilus influenzae continues to be an important pathogen encountered in lung diseases such as chronic bronchitis and cystic fibrosis (25) and in otitis media (37). This organism is able to colonize the respiratory tract for prolonged periods in patients with chronic bronchitis and cystic fibrosis, undergoing antigenic drift in certain outer membrane proteins (15). It has the potential to attach to both airway mucus (3, 22, 24, 29) and airway cells (11, 20, 34, 35, 40) or both (43), but there appears to be a preference for mucus during the early encounter with the respiratory epithelium (30). This ability to attach to mucus may in part explain the potential of this organism to persist in the respiratory tract, since mucociliary clearance is abnormal in these diseases.
A variety of surface structures on H. influenzae can mediate adherence to eukaryotic cells. Scott and Old (32) first reported that H. influenzae possessed mannose-resistant hemagglutinating (MRHA) fimbriae. In 1982, two groups separately reported that type b H. influenzae possessed MRHA surface structures, which they called pili, that mediated adherence to human buccal epithelial cells or oropharyngeal cells (16, 27). Others have found that type b H. influenzae with peritrichous pili had greater binding to buccal epithelial cells than to HEp-2 cells (31), while a different laboratory, surveying nontypeable H. influenzae (ntHi) isolated from sputum or conjunctiva, found that 3 of 15 isolates possessed fimbriae, primarily polar in location (1).
Pili are present on both typeable H. influenzae and ntHi and mediate MRHA through the erythrocyte (RBC) antigen AnWj. These structures also mediate binding to buccal epithelial cells (39), with the receptors on those cells being sialyl gangliosides (38). The pilins from all strains examined up to this time, whether on typeable H. influenzae or ntHi, are 68% identical and 77% similar at the amino acid level (2, 6, 7, 12).
Other surface structures which mediate the adhesion of ntHi to eukaryotic cells have also been characterized. High-molecular-weight surface proteins identified as HMW-1 and HMW-2 facilitate adherence to Chang epithelial cells (33). HMW-1 and HMW-2 are unique to ntHi and are not found on type b H. influenzae (33). The ntHi isolates which lack HMW-1 and HMW-2 genes usually possess another adhesin gene, identified as hia, and occasionally a portion of the pilus gene cluster (20). Some ntHi isolates also produce a protein identified as Hap with sequence similarity to H. influenzae immunoglobulin A (IgA) protease, which also facilitates binding to Chang cells (34). Surface fimbrils (Hsf), proteins closely related to Hia, are also present on the surfaces of certain H. influenzae isolates (35). We chose not to study these other adhesins.
Recently, the affinity of H. influenzae for mucus, particularly that of the nontypeable strains, has been demonstrated in vitro by quantitative studies of adhesion to highly purified mucins (8, 30), native mucins (22), and crude mucus (3), but the surface structures that mediate the adherence of H. influenzae to these targets have not been clearly defined. Pili, which are found on both type b (11) and nontypeable strains (1), have been implicated in adherence to oropharyngeal cells, and we suspected that this structure might be involved in the binding to mucin. However, there are conflicting reports on the role of surface appendages in binding to mucus. Read and colleagues reported that a piliated ntHi strain (strain R890 from our collection) was bound to mucus after inoculation of human nasal turbinates in organ culture (29); but a different laboratory reported that a clone from the same transformation (R881) did not bind better to crude mucus in vitro (8). However, Davies et al. (8) used an agglutination assay, while Read and colleagues (29) assessed adherence with the scanning electron microscope. Others have reported that certain outer membrane proteins mediate the interaction of ntHi with highly purified mucins (30). Thus, the issue appears to be unsettled. Since pili appear to be important for colonization of host tissue by other bacterial species and because hifA mutants have decreased ability to colonize primate respiratory mucosa (42), we sought to reexamine their role in binding to mucins harvested from the human respiratory tract. In this manuscript, we report studies on the role of pili in the adherence of H. influenzae to human respiratory mucins.
MATERIALS AND METHODS
Bacteria.
Table 1 depicts the bacterial strains used in this study. The parental strain (Eagan) is phenotypically a nonpiliated H. influenzae type b isolate, while strain R1369 is its fimbriated derivative which mediates MRHA (36). Strain RKAW5 is a nonpiliated derivative of R1369 (42) in which a kanamycin cassette is inserted in hifA, the gene encoding pilin. R906 is a laboratory derivative of H. influenzae Rd (5) lacking the pilus gene cluster, and R881 is the piliated derivative of R906 produced by transformation with a DNA fragment containing the pilus gene cluster from strain C54 (27). A library of chromosomal DNA from H. influenzae strain C54 was partially digested with SauA1 and ligated into BamHI-digested pHC79 (18). Clones in Escherichia coli DH5α were screened by colony hybridization with a nick-translated PstI fragment (pCD1) (42) containing the 5′ end of the pilus gene cluster (hifA, hifB, and a portion of hifC) from strain R1369. One cosmid, containing a 28-kb insert, which had homology to pCD1 was partially digested with ClaI and was used to transform strain R906 made competent by the M-IV technique (17). Transformants adhering to human RBCs were subcultured and banded on Percoll (13), and the band was aspirated and subcultured. Several colonies which were hemagglutination positive (R881 through R890) were frozen in skim milk at −70°C. The presence or absence of the type b capsule on H. influenzae was tested by slide agglutination with anti-type antisera (Difco, Detroit, Mich.).
TABLE 1.
Bacteria used in this study
| Strain | Origin | HAa | Reference or source |
|---|---|---|---|
| H. influenzae | |||
| 3-15 | Nontypeable respiratory strain | + | 22 |
| E1a | Streptomycin-resistant derivative of Eagan; type b | − | 5 |
| R1369 | Piliated, strain E1a | + | 36 |
| RKAW5 | hifA mutant of R1369 | − | 42 |
| R906 | Nontypeable laboratory derivative of Rd | − | 4 |
| C54 | Piliated type b | + | 27 |
| R881 | R906 transformed with the pilus gene cluster; from strain C54; nontypeable | + | This study |
| E. coli | |||
| R3180 | DH5α/pEMBL8 | − | 10 |
| R2746 | DH5α/pMH140; expressing type b pili from strain AM30 | + | 40 |
| R2747 | ORN103/pEMBL8 | − | 23 |
| R2745 | ORN103/pMH140; expressing type b pili from strain AM30 | ±b | This study |
HA, hemagglutination.
±, microscopic hemagglutination.
E. coli DH5α has been described before and was obtained from Bethesda Research Laboratories; E. coli strain ORN103, obtained from Paul Orndorff (23), has genotype thr-l leu-6 thi-l Δ(argF-lac) U169 xyl-7 ara-13 mtl-2 gal-6 rpsL fhuA2 minA minB recA13 Δ(pilABCDFE hyp). E. coli strains were transformed with plasmids by the calcium heat shock method, which we have performed previously (5). Plasmid pEMBL8 (10) was obtained from Riccardo Cortese, Heidelberg, Germany, and plasmid pMH140, which contains the pilus gene cluster from a type b strain (AM30; also called 770235 f+b0), was obtained from Marieke van Ham, Amsterdam, The Netherlands (40).
H. influenzae strains were grown on chocolate agar plates, which were made with tryptic soy agar enriched with 5% horse RBCs, 2% Fildes enrichment (Difco), and bacitracin (100 μg/ml; Sigma, St. Louis, Mo.) and then stored at 4°C in sealed bags. The same medium was used to grow strain RKAW5, but it also contained 20 μg of kanamycin/ml. All strains were kept frozen at −70°C in aliquots in 25% (vol/vol) glycerol and taken from the frozen stocks for each adhesion experiment. All E. coli strains were grown on L agar containing ampicillin at 100 μg/ml.
Respiratory mucin.
Human tracheobronchial mucin (HTBM) was prepared from the tracheobronchial secretions of a single patient with chronic obstructive pulmonary disease. The mucin was purified from the secretions by a method previously described (22, 44). Extensive delipidation was not carried out; therefore, these mucins represent native mucins.
Assay of bacterial adherence to mucin.
Adherence assays were performed by using 96-well microtiter plates (catalog no. 76-242-05; ICN Biomedicals, Costa Mesa, Calif.). One hundred microliters of HTBM solution at a concentration of 100 μg/ml was added to the wells and left at 37°C overnight to allow the mucin to coat the wells. Prior to addition of the bacteria, the wells were washed five times with 0.2 ml of sterile phosphate-buffered saline (PBS) by using a hand-held microtiter plate washer. Three coated wells and a “negative,” uncoated control well were used for each assay.
Bacteria were grown for 8 to 12 h on chocolate agar plates containing antibiotics as appropriate in a 5% CO2 incubator at 37°C. The organisms were harvested from the plates, suspended in sterile PBS, and adjusted to a density of 108 to 5 × 108 CFU/ml (A580 of 0.2); this density was used for all experiments. One hundred microliters of the bacterial suspension was added to the mucin-coated wells and to the negative-control wells and allowed to incubate for 60 min at 37°C. The wells were then washed 15 times with sterile PBS to remove unbound bacteria. At the density used, nonspecific binding of H. influenzae to the polystyrene microtiter plates was minimal. The inoculum at the beginning of the experiment was determined by performing serial dilutions of the bacterial suspension in sterile PBS and then plating 100 μl of each dilution in duplicate on chocolate agar plates. For studies examining the effect of antipilin antibodies the procedure was modified as follows. The initial bacterial concentration was doubled, 100 μl of the bacterial suspension was added to 100 μl of the antipilin or control (not agglutinating piliated cells) antibody, and the mixture was mixed and allowed to stand at room temperature for 10 min. Next, 100 μl of this mixture was placed in the experimental and control wells and incubated for 60 min. Each organism was tested in triplicate on each microtiter plate.
In the original method for assessing mucin adherence, 0.5% Triton X-100 (Sigma) was used as a desorbing agent to remove bacteria from the wells. We have recently reported that 0.5% Tween 20 (Sigma) provided increased recovery of H. influenzae over Triton X-100 (22). On the basis of these observations, 250 μl of 0.5% Tween 20 was added to each well and allowed to desorb bacteria for 15 min at room temperature. The wells were then mixed 20 times with a micropipette, and 100 μl of the solution was removed and diluted in sterile PBS. The diluted solution was plated in duplicate on chocolate agar plates. The plates were incubated for 24 h in a 5% CO2 incubator at 37°C. The colonies were identified as H. influenzae by morphological characteristics, Gram staining, and their dependence on heme and β-NAD+ for aerobic growth. Randomly selected colonies from each experiment were tested to confirm that the colonies were H. influenzae. Each experiment was replicated at least three times. Adherent E. coli cells were enumerated by the same technique except that the bacteria were quantitated by overnight incubation on L agar containing ampicillin at 100 μg/ml.
Hemagglutination.
To test whether a correlation between bacterial adherence to HTBM and hemagglutination exists, the relevant strains were tested as follows. H. influenzae cells were grown for 12 h on chocolate agar plates in a 5% CO2 incubator at 37°C. The organisms were suspended in sterile PBS and adjusted to an A580 of 1.0. Serial dilutions of the bacterial suspension were made, and 100 μl of each concentration was placed in a glass agglutination plate. One hundred microliters of a 2% suspension of multiply washed human RBCs (type O+) was placed in each well, and the contents of the wells were mixed thoroughly. The plates were gently agitated for 1 h at 37°C in a humidified environment and examined for hemagglutination at each dilution. Control wells contained and equal amount of sterile PBS and RBCs. E. coli strains were processed in the same manner except that they were grown on L agar containing ampicillin. A positive control was run with each set of experiments by using a hemagglutination-positive piliated strain of Pseudomonas aeruginosa (28).
ELISA.
Costar (Corning Science Products, Acton, Mass.) 96-well enzyme immunoassay plates were coated with gel-purified HifA at 1.0 and 4.0 μg/ml in the 20 mM bicarbonate buffer by being incubated for 18 h at 4°C. To reduce nonspecific binding, 100 μl of 3% bovine serum albumin in PBS (blocking buffer) was added to the rinsed plates and an additional overnight incubation was performed. The antibody source (serum or ascitic fluid) was then geometrically diluted in blocking buffer and incubated for 2 h at room temperature. Each well was then washed three times with blocking buffer before addition of the secondary antibody (goat anti-mouse IgG or goat anti-rabbit IgG; Bio-Rad, Richmond, Calif.) conjugated to horseradish peroxidase (HRP). The plates were then rinsed three times with blocking buffer and twice with PBS; 100 μl of HRP substrate (Kirkegaard-Perry Inc., Gaithersburg, Md.) was added, and the plates were incubated for 30 min at room temperature; 50 μl of 2 M H2SO4 was added, and A450 was read with an MR5000 enzyme-linked immunosorbent assay (ELISA) reader (Dynatech, Chantilly, Va.).
Antipilin antibody production.
AF100 is ascitic fluid from mice elicited by sarcoma 180/TG cells after the mice have been immunized with HifA purified from strain R1369 (26). HifA, the structural subunit of the MRHA pili, was purified as described previously (36), except that the final product from that purification scheme was electrophoresed in sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis gel. After localization of the band with the appropriate mobility by immersion of the gel in ice-cold 0.1 M KCl, the band was excised and the protein was eluted with a Hoefer Transphor model TE50X into 0.05 M ammonium bicarbonate, pH 10.5. To “renature” the pilin, this fraction was lyophilized and the pellet was dissolved in 1 ml of PBS. The protein content was estimated from its absorbance at 280 mm. Female Swiss Webster mice, 6 to 8 weeks old, were inoculated subcutaneously with 100 μg of purified HifA emulsified with 0.2 ml of Ribi adjuvant. Thirty and 45 days later each mouse was inoculated intraperitoneally with an additional 100 μg of HifA dissolved in 0.1 ml of PBS. Two weeks later ascites was induced in the mice by the intraperitoneal injection of 106 sarcoma 180/TG cells (26). Ascitic fluid was harvested daily from each mouse beginning 1 week later and centrifuged at 12,000 × g for 15 min, and the supernatant was used as a source of antibody. AF100 is the ascitic fluid from a single mouse.
Mouse IgG was purified from AF100 using the Pierce (Rockford, Ill.) ImmunoPure G kit. Ascites fluid (2 ml) was centrifuged at 10,000 × g for 10 min at 4°C to remove the particulate material. It was then applied to a protein G column, which was washed extensively, and the IgG was eluted. The fractions with an A280 >0.1 were pooled and stored in 20 mM sodium phosphate buffer (pH 7.0) containing 10 mM EDTA. Two IgG purification procedures yielded 800 μg of protein. This material was digested overnight with papain, and the Fab fragments were isolated with an ImmunoPure Fab preparation kit (Pierce). The mixture was applied to a protein A column, and the early A280 peak was pooled according to the manufacturer's instructions. This material was dialyzed against PBS and used as an anti-HifA Fab fragment.
Monoclonal antibodies were developed against HifA by the method of Davis (9). Six-week-old female BALB/C mice were immunized by the intraperitoneal administration of 50 μg of purified HifA (gel purified as described above) emulsified in Ribi adjuvant. Three weeks later the animals were boosted with the same amount and by the same route, again after emulsification in Ribi adjuvant. Seven to 10 days after the second immunization, the animals were bled by retro-orbital puncture and the antibody titer was determined by ELISA. ELISA was performed using polyclonal goat anti-mouse IgG antisera conjugated to alkaline phosphatase. Crude HifA fractions prepared by ammonium sulfate precipitation of sheared-cell (R1369) supernatant were used as the antigen, coating the ELISA plates with 5 μg of protein. If the titer (defined as the highest dilution yielding an A405 exceeding that seen with nonimmune sera by 0.1) was >1:2,000, the spleen was harvested. If the titer was <1:2,000, then an additional 50 μg of HifA in PBS was administered intraperitoneally. Five to 7.5 Sp2/O cells, on average, were fused to each spleen cell. Thymocyte feeder cells were used during cloning by limiting dilution. Culture supernatants were screened by ELISA (as described above) when the growth in the wells was 30 to 60% confluent. One reactive IgG3 monoclonal antibody was developed and identified as 37-D.
Western blot analysis.
To verify the specificity of the anti-HifA antisera, we performed Western blot analysis, essentially as previously described (5). Fifty micrograms of protein derived from whole-cell lysates (in running buffer) was electrophoresed on a sodium dodecyl sulfate–12.5% polyacrylamide gel electrophoresis gel. The separated proteins were electrophoretically transferred to Immobilon, along with prestained molecular size standards loaded concurrently. After transfer, the membranes were stained with Ponceau S to determine the locations of the relevant bands and to determine that transfer had occurred. The membrane was then immersed in a blocking solution consisting of 1% skim milk in PBS for 60 min at room temperature. Following this, they were rinsed and immersed for 60 min at room temperature in solution containing anti-HifA antibody (mouse ascites fluid AF100 diluted 1:500). The membranes were subsequently washed in PBS containing 0.1% sodium dodecyl sulfate at room temperature for 10 min and then were washed five times for 5 min each in PBS. They were then reacted with goat antibody directed to mouse IgG purchased from Bio-Rad. The secondary antibody was conjugated to HRP, and the membranes were developed using 4-chloro-1-naphthol and hydrogen peroxide in Tris-buffered saline at pH 7.5.
RESULTS
All H. influenzae isolates which were previously found to agglutinate O+ human RBCs had this property, as indicated in Table 1, before they were tested for adherence to mucin. Only E. coli strains R2745 and R2746 hemagglutinated human RBCs.
Effect of piliation on adherence of H. influenzae to mucin.
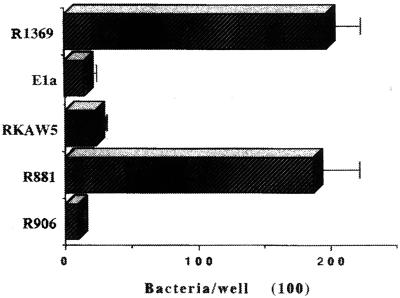
Strain R1369 is a phase variant selected for pilus expression by RBC adsorption, while the other type b derivative lacking pili was produced by the insertion of an antibiotic resistance cassette into hifA (RKAW5). Strain R1369 bound to mucin extremely well (Fig. 1), as extensively as the previously reported nontypeable sputum strains (22); the isogenic, nonpiliated hifA mutant was markedly reduced in its ability to adhere to mucin. Similarly, the laboratory ntHi strain R906 lacking the pilus gene cluster showed about 5% of the adherence of the same strain expressing a type b pilus gene cluster (R881) (Fig. 1).
FIG. 1.
Adherence of piliated and nonpiliated isogenic mutants of H. influenzae to native respiratory mucins. Strains R1369 and R881 are piliated, type b and nontypeable respectively. All other strains lack detectable pili (Table 1). Values are means. Error bars, standard deviations.
Studies with the pilus gene cluster.
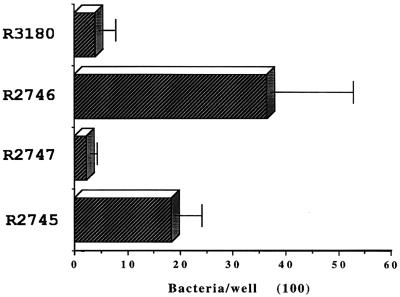
E. coli strains DH5α and ORN103 were both transformed with the pilus gene cluster in the vector pEMBL8. Both E. coli strains carrying and expressing the cloned pilus gene cluster from a type b H. influenzae isolate had six- to sevenfold increased binding to mucin compared to E. coli carrying the vector alone (Fig. 2). However the number of bacteria adhering was considerably lower than that seen with the H. influenzae strains.
FIG. 2.
Adherence of E. coli strains carrying the pilus gene cluster to mucins. Plasmid pEMBL8 is the vector into which the pilus gene cluster from strain AM30 was cloned, yielding pMH140. Values are means. Error bars, standard deviations.
Specificity of the anti-HifA antibody.
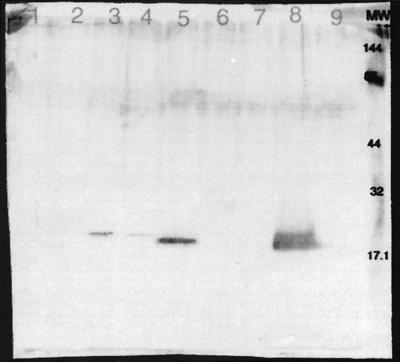
Figure 3 depicts the reactivity of AF100 antisera with the test E. coli and H. influenzae. The reactivity was greatest with H. influenzae strain R1369, the source of the HifA used for immunization. Although the HifA expressed by strain R881 is from a different type b strain (C54 versus R1369), cross-reactivity is seen (lane 5 versus lane 8). This antibody preparation also reacted with a protein of similar mobility encoded by pMH140, but the reactivity was considerably diminished (lanes 3 and 4). The E. coli expressing the pilus gene cluster (pMH140) was derived from H. influenzae strain AM30. AM30 is a type b H. influenzae strain in which 148 of the 196 amino acid residues in HifA are identical to those in strain R1369 HifA (2, 37). Thus the cross-reactivity is not unexpected.
FIG. 3.
Western blot analysis of H. influenzae and E. coli strains with AF100 antibody preparation. Lane 1, R3180; lane 2, R2747; lane 3, R2746; lane 4, R2745; lane 5, R881; lane 6, RKAW5; lane 7, 3-15; lane 8, R1369; lane 9, R906. The mobility of the molecular weight standards is indicated to the right.
Effect of antifimbrial antibodies on adherence.
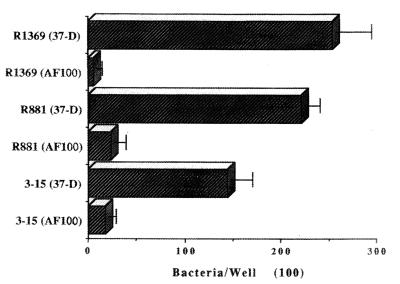
Antibodies raised against renatured pilin from a type b strain (R1369) were used to test for inhibition of adhesion. The adherence of the type b strain R1369 was reduced about 95%, and those of the hemagglutinating ntHi strains R881 and 3-15 were reduced by about 90%, in the presence of AF100 compared with adherence in the presence of control antibody 37-D (Fig. 4). Monoclonal antibody 37-D, which does not agglutinate alpha-fimbriated strains, had no detectable effect on mucin adherence.
FIG. 4.
Effect of mouse anti-HifA antibodies on adhesion of type b H. influenzae and ntHi strains to mucins. 37-D, a mouse monoclonal antibody that reacts with pilin from strain R1369 in ELISA but not on Western blots or in agglutination assays with any of the H. influenzae strains used in these studies and that was used as a control. AF100 is a mouse polyclonal antibody against pilin raised in ascitic fluid; it agglutinates piliated H. influenzae strains at a 1:4 dilution. Error bars, standard deviations.
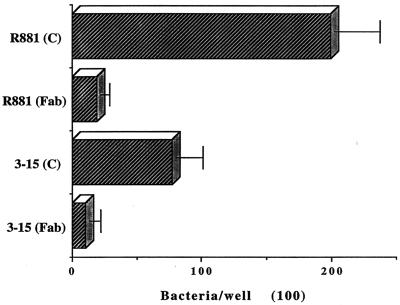
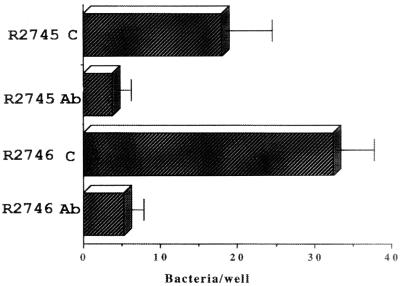
Although we could not observe bacterial agglutination in the antibody inhibition experiments with an inverted microscope, we tested Fab fragments of AF100 IgG prepared as described above. A commercially available heterologous mouse Fab preparation was used as a control reagent (Accurate Chemicals, Westbury, N.Y.). Both the anti-HifA Fab and the control Fab preparations were used at a protein concentration of 0.86 μg/ml, which was the maximum concentration of the anti-HifA Fab available. Neither preparation (undiluted) caused microscopic agglutination of the strains tested. The levels of adhesion of strains R881 and 3-15 were reduced by about 95 and 90%, respectively, by the anti-HifA Fab fragment, compared to the effect of the control Fab reagent (Fig. 5). Furthermore, this anti-HifA Fab preparation also inhibited the adherence of the E. coli strains carrying the gene encoding the pilus gene cluster (Fig. 6).
FIG. 5.
Effect of mouse anti-HifA Fab fragments (Fab) on the adherence of ntHi to respiratory mucins. C, treatment with control mouse Fab fragments. R881 expresses type b pili, while strain 3-15 is a piliated nontypeable isolate. Error bars, standard deviation.
FIG. 6.
Effect of mouse anti-HifA Fab fragments (Ab) on adhesion of E. coli strains carrying the pilus gene cluster. C, control (treatment with nonimmune mouse Fab fragments). Error bars, standard deviations.
DISCUSSION
We investigated the role of hemagglutinating pili in the binding of H. influenzae to human tracheobronchial mucin. We show that strains bearing pili of both type b and ntHi bound to mucin better than their nonpiliated derivatives. Furthermore, the binding of both encapsulated and nonencapsulated but piliated H. influenzae to mucin was blocked by pretreatment with antibodies directed to its structural subunit, HifA. To further support the observation that fimbriae are involved in adhesion to mucins, we examined the adherence of two E. coli strains carrying and expressing the cloned pilus gene cluster (hifABCDE) from H. influenzae strain AM30 (41). The E. coli strains which contained the cloned pilus gene cluster had a six- to sevenfold increased binding compared to the E. coli strains carrying the vector alone.
A polyclonal mouse antibody raised against highly purified HifA inhibited binding to mucin. Monoclonal antibody 37-D, also directed against HifA of strain R1369 but only recognizing a three-dimensional epitope, did not inhibit binding. This is not surprising as Gilsdorf et al. (14) found that polyclonal antibodies raised against hydrophilic peptides comprising various portions of pilin did not bind well to the native structure on the surface of type b H. influenzae. Others have found a similar dependence of the immunogenicity of E. coli WF96 fimbriae on the three-dimensional conformation (19). We found that monoclonal antibody 37-D reacts with HifA or with piliated H. influenzae only under ELISA conditions. There is no detectable reactivity with HifA on Western blot analysis, nor does this monoclonal antibody mediate agglutination of piliated H. influenzae (data not shown). Monoclonal antibody 37-D can be viewed as a control for mouse polyclonal antibody AF100, which agglutinates piliated organisms.
To verify that the inhibition of mucin binding by the antibody preparations was not due to bacterial agglutination, we prepared IgG and then monovalent Fab fragments from the AF100 antibody preparation. We found that this preparation also inhibited mucin binding of H. influenzae and E. coli expressing H. influenzae pili on their surfaces.
There are multiple surface adhesins which could have mediated the adhesion of H. influenzae to mucin. These studies have only examined the role of hemagglutinating pili and find that pili play a major role. However, these data do not rule out a role for other morphologically distinct H. influenzae adhesins (high-molecular-weight proteins, Hia, and Hsf-Hap, for example) which may be present on naturally occurring clinical isolates which lack pili. The binding of several nonpiliated H. influenzae strains to highly purified mucins (in an agglutination assay) was demonstrated by Davies et al. (8), indicating that there are other adhesins present on some ntHi strains. These studies allow us to conclude that pili are involved in the adherence of H. influenzae to mucus but do not rule out the existence of other adhesins for mucin epitopes.
This study differs significantly in its conclusions from one of the previous reports on the role of pili (8), wherein this structure was found not to be involved in adherence to mucins. In fact two of the same strains used in the present study, the nontypeable Rd derivative strain R906 and strain R881, a transformant with a type b pilus gene cluster, were used by Davies et al.; neither strain bound to mucins in that study (8). It is our belief that these differences may reflect the method of mucin preparation and/or the sensitivities of the assay systems used. Mucins for our assay were prepared by ultracentrifugation in Cs-Br (44), whereas the mucins used in the previous report were prepared by solubilization in 6 M guanidinium chloride and then two cycles of Cs-Cl guanidinium chloride ultracentrifugation. The latter method is reported to remove more of the noncovalently attached lipids and nucleic acids from the native mucins compared to what is seen in situ. However, the assay system used in the previous study was not quantitative, and the lower limit of detection of bacterial binding by aggregation and slot blot was not reported. Theoretically, our assay can detect 100 CFU bound to the microtiter plates. One additional factor is the choice of mucins for the assays. We used mucins from the same patient throughout our studies, but it is not clear whether the same mucin preparations were used in the previous studies (8). Aside from technical differences, the differing conclusions raise the question of what is the most appropriate target to study in order to mimic as close as one can the in vivo environment. Is it more appropriate to study crude mucus, native mucins, or highly purified mucins? Each may have its advantages for a given purpose. If one is interested in a specific chemical group of mucins interacting with a defined microbial product, then highly purified mucin may be the substance of choice. On the other hand, native mucins or crude mucus may be more appropriate to detect interactions that occur in vivo. The results of this study also contrast with those investigating the role of pili in another organism, P. aeruginosa, which resides in the mucus of patients with cystic fibrosis. Pili are not needed for the binding of P. aeruginosa to preparations of native mucins (28), even though H. influenzae and P. aeruginosa are reported to bind to the same cellular glycolipids (21).
The presence of pili may allow ntHi a survival advantage in that these surface appendages allow binding to human mucus, which may permit progression to other stages of the infectious process such as tissue injury and invasion. Alternately, in conditions where the clearance of tracheobronchial secretions is impaired (i.e., cystic fibrosis and chronic bronchitis) this mucus adherence may mediate a state of chronic colonization.
ACKNOWLEDGMENTS
This work was supported by grants from the American Lung Association of Florida (M.K.) and Public Health Service grants NHLBI HL 33622 (R.R.) and NIAID AI 20625 (A.L.S.) from the National Institute of Heart, Lung and Blood Diseases and the National Institute of Allergy and Infectious Diseases.
We are indebted to Joseph St. Geme, Jr., for his careful reading of the manuscript and helpful comments.
REFERENCES
- 1.Apicella M A, Shero M, Dudas K C, Stack R R, Klohs W, LaScolea L J, Murphy T F, Mylotte J M. Fimbriation of Haemophilus species isolated from the respiratory tract of adults. J Infect Dis. 1984;150:40–43. doi: 10.1093/infdis/150.1.40. [DOI] [PubMed] [Google Scholar]
- 2.Armes L G, Forney L J. The complete primary structure of pilin from Haemophilus influenzae type b strain Eagan. J Protein Chem. 1990;9:45–52. doi: 10.1007/BF01024983. [DOI] [PubMed] [Google Scholar]
- 3.Barsum W, Wilson R, Read R C, Rutman A, Todd H C, Houdret N, Roussel P, Cole P J. Interaction of fimbriated and nonfimbriated strains of unencapsulated Haemophilus influenzae with human respiratory tract mucus in vitro. Eur Respir J. 1995;8:709–714. [PubMed] [Google Scholar]
- 4.Caitlin B W, Bendler III J W, Goodgal S H. The type b capsulation locus of Haemophilus influenzae: map location and size. J Gen Microbiol. 1972;70:411–422. doi: 10.1099/00221287-70-3-411. [DOI] [PubMed] [Google Scholar]
- 5.Chanyangam M, Smith A L, Moseley S L, Kuehn M, Jenny P. Contribution of a 28 kilodalton membrane protein to the virulence of Haemophilus influenzae. Infect Immun. 1991;59:600–608. doi: 10.1128/iai.59.2.600-608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemans D L, Marrs C F, Patel M, Duncan M, Gilsdorf J R. Comparative analysis of Haemophilus influenzae hifA (pilin) genes. Infect Immun. 1998;66:656–663. doi: 10.1128/iai.66.2.656-663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman T, Grass S, Munson R., Jr Molecular cloning, expression, and sequence of the pilin gene from nontypeable Haemophilus influenzae M37. Infect Immun. 1991;59:1716–1722. doi: 10.1128/iai.59.5.1716-1722.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies J, Carlstedt I, Nilsson A-K, Hakansson A, Sabharwal H, van Alphen L, van Ham M, Svanborg C. Binding of Haemophilus influenzae to purified mucins from the human respiratory tract. Infect Immun. 1995;63:2485–2492. doi: 10.1128/iai.63.7.2485-2492.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis J M. A single-step technique for selecting and cloning hybridomas for monoclonal antibody production. Methods Enzymol. 1986;121:307–322. doi: 10.1016/0076-6879(86)21029-0. [DOI] [PubMed] [Google Scholar]
- 10.Dente L, Cesareni G, Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983;11:1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farley M M, Stephens D S, Kaplan S L, Mason E O. Pilus- and non-pilus mediated interactions of Haemophilus influenzae type b with human erythrocytes and human nasopharyngeal mucosa. J Infect Dis. 1990;161:274–280. doi: 10.1093/infdis/161.2.274. [DOI] [PubMed] [Google Scholar]
- 12.Forney L J, Marrs C F, Bektesh S L, Gilsdorf J R. Comparison and analysis of the nucleotide sequences of pilin genes from Haemophilus influenzae type b strains Eagan and M43. Infect Immun. 1991;59:1991–1996. doi: 10.1128/iai.59.6.1991-1996.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giesa F R, Zajack I, Bartus H F, Actor P. Isopycnic separation of Escherichia coli cultures possessing colonization factor antigen I. J Clin Microbiol. 1982;15:1074–1076. doi: 10.1128/jcm.15.6.1074-1076.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilsdorf J R, Forney L J, McCrea K W. Reactivity of antibodies against conserved regions of pilin of Haemophilus influenzae type b. J Infect Dis. 1993;163:962–965. doi: 10.1093/infdis/167.4.962. [DOI] [PubMed] [Google Scholar]
- 15.Groeneveld K, van Alphen L, Voorter C, Eijk P P, Jansen H M, Zanen H C. Antigenic drift of Haemophilus influenzae in patients with chronic obstructive pulmonary disease. Infect Immun. 1989;57:3038–3044. doi: 10.1128/iai.57.10.3038-3044.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerina N G, Langermann S, Clegg H W, Kessler T W, Goldmann D A, Gilsdorf J R. Adherence of piliated Haemophilus influenzae type b to human oropharyngeal cells. J Infect Dis. 1982;146:564. doi: 10.1093/infdis/146.4.564. [DOI] [PubMed] [Google Scholar]
- 17.Herriott R M, Meyer E M, Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970;101:517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hohn B, Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980;11:291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- 19.Karch H, Leying H, Goroncy-Bermes P, Kroll H-P, Opferkuch W. Three-dimensional structure of fimbriae determines specificity of immune response. Infect Immun. 1985;50:517–522. doi: 10.1128/iai.50.2.517-522.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krasan G P, Cutter D, Block S L, St. Geme J W., III Adhesin expression in matched nasopharyngeal and middle ear isolates of nontypeable Haemophilus influenzae from children with acute otitis media. Infect Immun. 1999;67:449–454. doi: 10.1128/iai.67.1.449-454.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krivan H C, Roberts D D, Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAcβ1-4Gal found in some glycolipids. Proc Natl Acad Sci USA. 1988;85:6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubiet M, Ramphal R. Adhesion of nontypeable Haemophilus influenzae from blood and sputum to human tracheobronchial mucins and lactoferrin. Infect Immun. 1995;63:899–902. doi: 10.1128/iai.63.3.899-902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maurer L, Orndorff P E. Identification and characterization of genes determining receptor binding and pilus length of Escherichia coli type 1 pili. J Bacteriol. 1987;169:640–645. doi: 10.1128/jb.169.2.640-645.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyamoto N, Bakaletz L O. Selective adherence of non-typeable Haemophilus influenzae (NTHi) to mucus or epithelial cells in the chinchilla eustachian tube and middle ear. Microb Pathog. 1996;21:343–356. doi: 10.1006/mpat.1996.0067. [DOI] [PubMed] [Google Scholar]
- 25.Moller L V, Regelink A G, Grasselier H, Dankert-Roelse J E, Dankert J, van Alphen L. Multiple Haemophilus influenzae strains and strain variants coexist in the respiratory tract of patients with cystic fibrosis. J Infect Dis. 1995;172:1388–1392. doi: 10.1093/infdis/172.5.1388. [DOI] [PubMed] [Google Scholar]
- 26.Ou S K, Hwang J M, Patterson P H. A modified method for obtaining large amounts of high titer polyclonal ascites fluid. J Immunol Methods. 1993;165:75–80. doi: 10.1016/0022-1759(93)90108-j. [DOI] [PubMed] [Google Scholar]
- 27.Pichichero M E, Loeb M, Anderson P, Smith D H. Do pili play a role in pathogenicity of Haemophilus influenzae type b? Lancet. 1982;ii:960–962. doi: 10.1016/s0140-6736(82)90161-1. [DOI] [PubMed] [Google Scholar]
- 28.Ramphal R, Koo L, Ishimoto K S, Totten P A, Lara J C, Lory S. Adhesion of Pseudomonas aeruginosa pilin deficient mutants to mucin. Infect Immun. 1991;59:1307–1311. doi: 10.1128/iai.59.4.1307-1311.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Read R C, Wilson R, Rutman A, Lund V, Todd H C, Brain A P R, Jeffery P, Cole P J. Interaction of nontypeable Haemophilus influenzae with human respiratory mucosa in vitro. J Infect Dis. 1991;163:549–558. doi: 10.1093/infdis/163.3.549. [DOI] [PubMed] [Google Scholar]
- 30.Reddy M S, Bernstein J M, Murphy T F, Faden H S. Binding between outer membrane proteins of nontypeable Haemophilus influenzae and human nasopharyngeal mucin. Infect Immun. 1996;64:1477–1479. doi: 10.1128/iai.64.4.1477-1479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sable N S, Connor E M, Hall C B, Loeb M R. Variable adherence of fimbriated Haemophilus influenzae type b to human cells. Infect Immun. 1985;48:119–123. doi: 10.1128/iai.48.1.119-123.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott S S, Old D C. Mannose-resistant and eluting (MRE) haemagglutinins, fimbriae and surface structure in strains of Haemophilus. FEMS Microbiol Lett. 1981;10:235–240. [Google Scholar]
- 33.St. Geme J W, III, Falkow S, Barenkamp S J. High-molecular-weight proteins of nontypeable Haemophilus influenzae mediate attachment to human epithelial cells. Proc Natl Acad Sci USA. 1993;90:2875–2879. doi: 10.1073/pnas.90.7.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.St. Geme J W, III, de la Morena M L, Falkow S A. Haemophilus influenzae IgA protease-like protein promotes intimate interaction with human epithelial cells. Mol Microbiol. 1994;14:217–233. doi: 10.1111/j.1365-2958.1994.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 35.St. Geme J W, III, Cutter D, Barenkamp S J. Characterization of the genetic locus encoding Haemophilus influenzae type b surface fibrils. J Bacteriol. 1996;178:6281–6287. doi: 10.1128/jb.178.21.6281-6287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stull T L, Mendelman P M, Haas J E, Schoenborn M A, Mack K D, Smith A L. Characterization of Haemophilus influenzae type b fimbriae. Infect Immun. 1984;46:787–796. doi: 10.1128/iai.46.3.787-796.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trujillo H, Callejas R, Mejia G I, Castrillon L. Bacteriology of middle ear fluid specimens obtained by tympanocentesis from 111 Colombian children with acute otitis media. Pediatr Infect Dis J. 1989;8:361–363. doi: 10.1097/00006454-198906000-00007. [DOI] [PubMed] [Google Scholar]
- 38.van Alphen L, Geelen-van Den Broek L, Blass L, van Ham M, Dankert J. Blocking of fimbria-mediated adherence of Haemophilus influenzae by sialyl gangliosides. Infect Immun. 1991;59:4473–4477. doi: 10.1128/iai.59.12.4473-4477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Alphen L, van den Berghe N, Geelen-van den Broek L. Interaction of Haemophilus influenzae with human erythrocytes and oropharyngeal epithelial cells is mediated by a common fimbrial epitope. Infect Immun. 1988;56:1800–1806. doi: 10.1128/iai.56.7.1800-1806.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Ham S M, Mooi F R, Sindhunata M G, Maris W R, van Alphen L. Cloning and surface expression in Escherichia coli of fimbriae of Haemophilus influenzae establishes adherence to human cells. EMBO J. 1989;11:3535–3540. doi: 10.1002/j.1460-2075.1989.tb08519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Ham S M, van Alphen L, Mooi F R, van Putten J P. The fimbrial gene cluster of Haemophilus influenzae type b. Mol Microbiol. 1994;13:673–684. doi: 10.1111/j.1365-2958.1994.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 42.Weber A, Harris K, Lohrke S, Forney L, Smith A L. Inability to express fimbriae results in impaired ability of Haemophilus influenzae b to colonize the nasopharynx. Infect Immun. 1991;59:4724–4728. doi: 10.1128/iai.59.12.4724-4728.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson R, Read R, Cole P. Interaction of Haemophilus influenzae with mucus, cilia, and respiratory epithelium. J Infect Dis. 1992;165(Suppl. 1):S100–S102. doi: 10.1093/infdis/165-supplement_1-s100. [DOI] [PubMed] [Google Scholar]
- 44.Woodward H, Horsey B, Bhavarnandan V P, Davidson E A. Isolation, purification, and properties of respiratory mucus glycoproteins. Biochemistry. 1982;21:694–701. doi: 10.1021/bi00533a017. [DOI] [PubMed] [Google Scholar]