Abstract
Background:
COVID-19 infection caused by SARS-COV-2 can result in multi-organ injuries and significant mortality in severe and critical patients, particularly those with type 2 diabetes as a comorbidity. Metformin and insulin are the main diabetes medications that affect the outcome of patients with COVID-19.
Objective:
The purpose of our study was to find out the features of the hematological indicators of patients with COVID-19 patients and type 2 diabetes.
Methods:
This is a retrospective study of the hospital confirmed COVID-19 patients between January to March 2022, who were admitted to Transcarpathian Regional Clinical Infectious Diseases Hospital (Uzhhorod, Ukraine).
Results:
The effect of type 2 diabetes, metformin, and insulin on COVID-19 were analyzed, respectively. Demographics and blood laboratory indices were collected. In patients who took metformin, the level of CRP was significantly lower than in patients who did not take metformin (24 mg/L [IQR 15 - 58] vs 52 mg/L, [IQR 22–121], P = 0.046).
Conclusion:
Our findings suggest that pre-admission metformin use may benefit COVID-19 patients with type 2 diabetes.
Keywords: COVID-19, diabetes, metformin, insulin
1. BACKGROUND
Coronavirus disease 2019 (COVID‐19), caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2), was first reported in December 2019 (1, 2). The severity of COVID-19 ranges from asymptomatic to severe infection leading to death (1).
Diabetes is a chronic disease that affects over 463 million people around the world (3). Studies reported that 20–50% of COVID-19 patients have diabetes depending on different areas (4). Also diabetic patients with COVID-19 had poorer prognosis compared to non-diabetic patients (5-7).
The diabetes medication regimen may have influenced the progression (8, 9). The most commonly used diabetes medications are metformin and insulin. Theoretical evidence suggests that metformin may be beneficial for reducing proinflammatory and profibrotic states (2, 10, 11) and improving immune response (12). Insulin treatment may increase the risk of poor prognosis by increasing renal Angiotensin Converting Enzyme 2 (ACE2) expression in diabetic mice (13).
2. OBJECTIVE
In this study, we sought to perform a retrospective analysis of metformin and insulin effects in COVID-19 patients with Type 2 Diabetes Mellitus (T2DM).
3. MATERIAL AND METHODS
Ethical Approval of the Study Protocol
This was a retrospective study conducted at the Transcarpathian Regional Clinical Infectious Diseases Hospital (Uzhhorod, Ukraine). The study protocol was approved by the Ethics Committees of Transcarpathian Regional Clinical Infectious Hospital.
Study Design and Participants
We used retrospective patient data collected from medical records available from the e-health information system, which included all adult patients (≥18 years old) admitted from January 2021 to March 2022 with a Polymerase Chain Reaction (PCR) confirming diagnosis of SARS-CoV-2 infection using nasal swabs.
In-hospital confirmed COVID-19 patients were divided into T2DM and non-diabetes. T2DM was defined by fasting venous or capillary glucose ≥ 7.0 mmol/L; two-hour postprandial venous plasma glucose or random plasma glucose ≥ 11.1 mmol/L according to the World Health Organization diagnostic criteria (14). A study flow diagram is shown in Figure 1.
Figure 1. Study flow diagram.
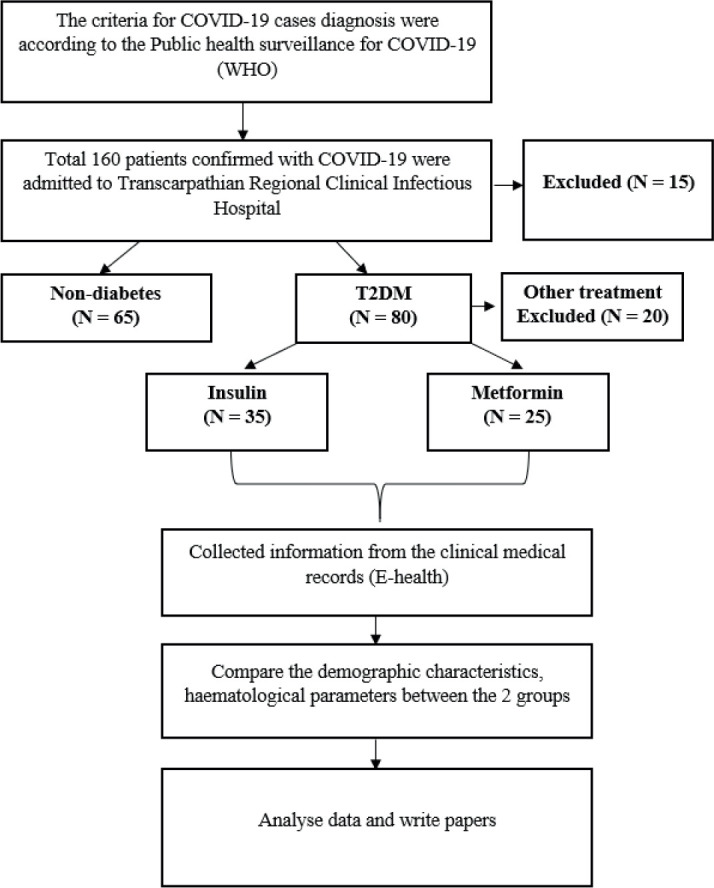
Data Collection
Demographics, comorbidities, laboratory tests, treatments of COVID-19 patients with and without T2DM were collected from the Electronic Medical Records (EMR).
Statistical Analysis
The results are reported as the median (interquartile range [IQR]) for non-normal continuous variables and number [percentage] for categorical variables. The classification variable was represented as a count (%). Differences in parameters among groups were analysed using the Student’s t-test for variables with a normal distribution. The Mann–Whitney U-test was employed for continuous variables with a non-normal distribution, and the χ2 test was used for categorical variables. A predictive model based on logistic regression was developed to predict the presence of metformin treatment based on significant laboratory parameters. All statistical analyses were performed using SPSS. P value ≤0.05 was considered to indicate statistical significance
Table 1. Characteristics of baseline laboratory indices of patients with or without T2DM in COVID-19 cases.
| Characteristics | Normal range | Non-diabetes (N = 65) | T2DM (N = 80) | P-value |
|---|---|---|---|---|
| Age (years) | - | 53.66 ± 13.37 | 67.00 ± 7.70 | <0.001 |
| Male (n, %) | - | 46.10 % | 45.00 % | 0.459 |
| Comorbidities (n, %) | ||||
| Hypertension | - | 8 (12.3 %) | 36 (45 %) | <0.001 |
| Cardiovascular disease | - | 27 (41.5 %) | 66 (82.5 %) | 0.015 |
| Chronic kidney disease | - | 8 (12.3 %) | 28 (35 %) | 0.017 |
| Severity categories (n, %) | ||||
| Mild | - | - | - | - |
| Moderate | - | 60 (92.3 %) | 44 (55 %) | - |
| Severe | - | 4 (6.2 %) | 12 (15 %) | - |
| Critical | - | 1 (1.5 %) | 24 (30 %) | - |
| Baseline laboratory indices | ||||
| Blood glucose | 3.90–5.6 mmol/L | 5 (4-6) | 12 (-15) | < 0.001 |
| White blood cells | 3.50–9.50 × 109/L | 7 (5-10) | 9 (6-12) | 0.139 |
| Lymphocytes | 1.10–3.20 × 109/L | 1 (1-1) | 1 (1-2) | 0.647 |
| Monocytes | 0.12–1.20 × 109/L | 0 (0-0) | 0 (0-0) | 0.651 |
| Neutrophils | 1.80–6.30 × 109/L | 6 (4-8) | 8 (5-10) | 0.100 |
| Creatinine | 57.00–97.00 μmol/L | 106 (87-119) | 102 (84-124) | 0.647 |
| CRP | 0–8.00 mg/L | 50 (13-120) | 28 (20-72) | 0.553 |
| D-dimer | 0–0.55 μg/mL | 1 (0-2) | 2 (1-3) | 0.013 |
| PCT | <0.10 ng/mL | 0 (0-0) | 0 (0-1) | 0.129 |
| COVID-19 treatment protocols | ||||
| Antibiotics (n, %) | - | 29 (44.6 %) | 52 (65 %) | 0.328 |
4. RESULTS
Demographic characteristics of the study population
Among the 145 confirmed patients, the median age was 62.66 ± 12.96, 66 patients (45.5%) were male.
Patients with T2DM were older than patients without T2DM 53.66 ± 13.37 vs 67.00 ± 7.70 (P = <0.001). Among these 80 patients with diabetes, 100% are T2DM. At hospital admission, the distribution of severity category was: 92.3% vs 55% moderate, 6.2% vs 15% severe, and 1.5% vs 30% critical COVID-19 patients without or with TD2M, respectively.
Most of the hematological indicators, such as the level of white blood cells (P=0.139), lymphocytes (P=0.647), monocytes (P=0.651), neutrophils (P=0.100), creatinine (P=0.647), CRP (P=0.553) and procalcitonin (P=0.129) did not differ significantly in the 2 groups of patients (with or without T2DM). The level of d-dimer was higher in patients who had comorbid pathology - TD2M (P=0.013). Hematological parameters of COVID-19 patients with type 2 diabetes depending on insulin or metformin treatment.
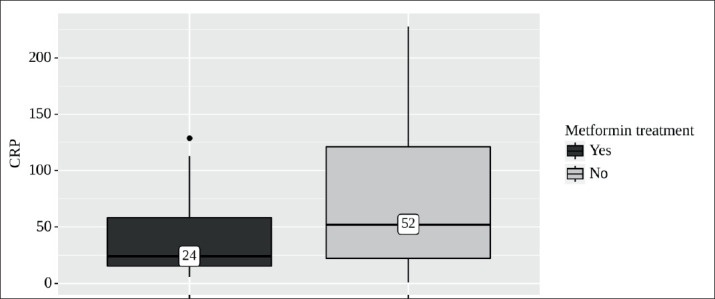
During hospitalization, 25 cases with T2DM had metformin. Patients had in-hospital metformin with a median of 1.0 g per day [IQR 0.55–1.52]. In patients who took metformin, the level of CRP was significantly lower than in patients who did not take metformin (24 mg/L [IQR 15 - 58] vs 52 mg/L, [IQR 22–121], P = 0.046) (Table 2, Figure 2).
Table 2. Characteristics of baseline laboratory indices in metformin or insulin users.
| Characteristics | Non-metformin (N =16) | Metformin (N =25) | P-value | Non-insulin (N = 10) | Insulin (N = 35) | P-value |
|---|---|---|---|---|---|---|
| Concentrations (per day) | - | 1 (0.55 – 1.52) | NA | - | 35 (28.2 – 50.3) | NA |
| Age (years) | 58.67 ± 10.81 | 60.80 ± 12.56 | 0.518 | 52.67 ± 8.81 | 67.8 ± 9.26 | 0.038 |
| Gender (M, %) | 7 (43.7 %) | 12 (48 %) | 0.657 | 6 (60 %) | 17 (48.5 %) | |
| Comorbidities (n, %) | ||||||
| Hypertension | 7 (43.7%) | 7 (28 %) | 0.257 | 3 (30 %) | 9 (25.7%) | 0.436 |
| Cardiovascular disease | 2 (12.5%) | 4 (16 %) | 0.368 | 1 (10%) | 5 (14.2 %) | 0.813 |
| Chronic kidney disease | 7 (43.7 %) | 7 (28 %) | 0.537 | 4 (40 %) | 10 (28.5%) | 0.251 |
| Baseline laboratory indices | ||||||
| Blood glucose | 7 (5-14) | 10 (8-14) | 0.014 | 5 (4-8) | 14 (9-15) | < 0.001 |
| White blood cells | 8 (6-12) | 9 (6-10) | 0.463 | 7 (5-11) | 9 (7-12) | 0.082 |
| Lymphocytes | 1 (1-1) | 1 (1-1) | 0.666 | 1 (1-2) | 1 (1-1) | 0.430 |
| Monocytes | 0 (0-0) | 0 (0-0) | 0.040 | 0 (0-0) | 0 (0-0) | 0.368 |
| Neutrophils | 7 (5-11) | 7 (4-9) | 0.315 | 6 (4-8) | 9 (6—11) | 0.022 |
| Creatinine | 102 (86-119) | 105 (90-124) | 0.644 | 102 (86-118) | 102 (87-127) | 0.647 |
| CRP | 52 (22-121) | 24 (15-58) | 0.046 | 40 (16-104) | 43 (18-100) | 0.846 |
| D-dimer | 1 (0-3) | 1 (0-3) | 0.544 | 1 (0-3) | 2 (1-3) | 0.181 |
| PCT | 0 (0-1) | 0 (0-1) | 0.660 | 0 (0-1) | 0 (0-1) | 0.340 |
| COVID-19 treatment protocols | ||||||
| Antibiotics (n, %) | 13 (81.2 %) | 14 (56 %) | 0.486 | 8 (80%) | 25 (71.4%) | 0.587 |
Figure 2. Analysis of CRP conditioning on Metformin treatment.
35 patients had insulin after admission. In-hospital insulin users with a median of 35.0 unit per day [IQR 28.2 - 50.3]. Higher blood glucose levels (14 mmol/L, [IQR 9 –15] vs. 5 mmol/L [IQR 4 –8], P = < 0.001) were seen in insulin users. Patients in the insulin group had higher WBC (9 × 109/L, [IQR 7 –12] vs. 7 × 109/L [IQR 5 –11], P = 0.082) and neutrophils levels than those of the non-insulin group (9 × 109/L, [IQR 6 –11] vs. 6 × 109/L [IQR 4–8], P = 0.022) with no difference in general characteristics and other laboratory indices.
5. DISCUSSION
This retrospective study is aiming to explore the effects of metformin and insulin on hematological parameters in patients with COVID-19 and T2DM. We validated the risk of T2DM in COVID-19 patients. Patients with T2DM had poorer prognosis (ICU admission and more patients were severe and critically) compared to non-diabetic patients. In terms of mortality from COVID-19 in patients with diabetes, several lines of evidence suggest that diabetes is one of the major causes of death in COVID-19 patients (7, 15). The negative effects of COVID-19 are thought to be caused by microvascular disease, endothelial dysfunction, severe pneumonia, and inflammatory storm (16). Older age and high CRP were thought to be factors contributing to the high mortality rate from COVID-19 in diabetic patients (17).
Metformin is the marketplace leader among glucose-lowering agents for the management of T2DM. In recent years, metformin has attracted attention again for its antiviral effect and anti-inflammatory activity that may help in reducing the risk of severe COVID-19 beyond the effects of glucose control (11, 12, 18).
Additionally, it has been discovered that metformin is linked to a significantly lower mortality rate in people with respiratory pathologies like a chronic obstructive pulmonary disease (19). In our study, a decrease in CRP was observed when using metformin. These findings suggest that metformin use prior to admission may have protective effects for COVID-19 patients with T2DM.
Regarding the COVID-19 in diabetic patients who used insulin, a lot of studies implied an increased risk of mortality in confirmed COVID-19 cases with pre-admission insulin treatment (17, 20-22). We found in-hospital insulin usage was closely associated with higher levels of WBC and neutrophils which indicates deteriorated inflammation.
6. CONCLUSION
In this study, we found that COVID-19 patients with T2DM experienced negative outcomes, which may have been caused by worsening inflammation. Metformin use prior to admission was linked to declining CRP levels among COVID-19 patients with T2DM. In the long run, metformin may be more advantageous than insulin for COVID-19 patients with T2DM. To confirm the current findings, more research is required.
Ethical Approval:
This experiment was approved by the ethics committee of Transcarpathian Regional Clinical Infectious Hospital. The data are de-identified; therefore, the requirement of written informed consent was waived. The study was conducted in compliance with the Declaration of Helsinki principles for ethical research.
Author’s contribution:
All authors were involved in all steps of preparation of this article. Final proofreading was made by the first author.
Conflicts of interest:
There are no conflicts of interest.
Financial support and sponsorship:
None.
REFERENCES
- 1.Stawicki SP, Jeanmonod R, Miller AC, Paladino L, Gaieski DF, Yaffee AQ, et al. The 2019–2020 novel coronavirus (severe acute respiratory syndrome coronavirus 2) pandemic: A joint american college of academic international medicine-world academic council of emergency medicine multidisciplinary COVID-19 working group consensus paper. J Journal of global infectious diseases. 2020;12(2):47. doi: 10.4103/jgid.jgid_86_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petakh P, Kamyshna I, Nykyforuk A, Yao R, Imbery JF, Oksenych V, et al. Immunoregulatory Intestinal Microbiota and COVID-19 in Patients with Type Two Diabetes: A Double-Edged Sword. J Viruses. 2022;14(3):477. doi: 10.3390/v14030477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. J Nature reviews endocrinology. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 4.Bornstein SR, Rubino F, Khunti K, Mingrone G, Hopkins D, Birkenfeld AL, et al. Practical recommendations for the management of diabetes in patients with COVID-19. J The lancet Diabetes endocrinology. 2020;8(6):546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. J The lancet Diabetes endocrinology. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu L, Chen S, Fu Y, Gao Z, Long H, Ren H-w, et al. Risk factors associated with clinical outcomes in 323 coronavirus disease 2019 (COVID-19) hospitalized patients in Wuhan, China. J Clinical infectious diseases. 2020;71(16):2089–2098. doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. J Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penlioglou T, Papachristou S, Papanas N. COVID-19 and diabetes mellitus: May old anti-diabetic agents become the new philosopher’s stone? J Diabetes Therapy. 2020;11(6):1195–1197. doi: 10.1007/s13300-020-00830-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ursini F, Ciaffi J, Landini MP, Meliconi R. COVID-19 and diabetes: Is metformin a friend or foe? J Diabetes research clinical practice. 2020;164:108167. doi: 10.1016/j.diabres.2020.108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma S, Ray A, Sadasivam B. Metformin in COVID-19: a possible role beyond diabetes. Diabetes research and clinical practice. 2020;164:108183. doi: 10.1016/j.diabres.2020.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menendez JA. Metformin and SARS-CoV-2: mechanistic lessons on air pollution to weather the cytokine/thrombotic storm in COVID-19. J Aging. 2020;12(10):8760. doi: 10.18632/aging.103347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Wang L, Li J, Fan Z, Yang L, Zhang Z, et al. Metformin-induced reduction of CD39 and CD73 blocks myeloid-derived suppressor cell activity in patients with ovarian cancer. J Cancer research. 2018;78(7):1779–1791. doi: 10.1158/0008-5472.CAN-17-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salem ES, Grobe N, Elased KM. Insulin treatment attenuates renal ADAM17 and ACE2 shedding in diabetic Akita mice. American Journal of Physiology-Renal Physiology. 2014;306(6):F629–F639. doi: 10.1152/ajprenal.00516.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alberti KGMM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. J Diabetic medicine. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 15.Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? J The Lancet Neurology. 2020;395(10231):1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, et al. Diabetes is a risk factor for the progression and prognosis of COVID‐19. J Diabetes/metabolism research reviews. 2020;36(7):e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Yang D, Cheng B, Chen J, Peng A, Yang C, et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. J Diabetes care. 2020;43(7):1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 18.Scheen A. Metformin and COVID-19: from cellular mechanisms to reduced mortality. J Diabetes metabolism. 2020;46(6):423–426. doi: 10.1016/j.diabet.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendy A, Gopal R, Alcorn JF, Forno E. Reduced mortality from lower respiratory tract disease in adult diabetic patients treated with metformin. J Respirology. 2019;24(7):646–651. doi: 10.1111/resp.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal S, Schechter C, Southern W, Crandall JP, Tomer Y. Preadmission diabetes-specific risk factors for mortality in hospitalized patients with diabetes and coronavirus disease 2019. J Diabetes Care. 2020;43(10):2339–2344. doi: 10.2337/dc20-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu B, Li C, Sun Y, Wang DW. Insulin treatment is associated with increased mortality in patients with COVID-19 and type 2 diabetes. J Cell metabolism. 2021;33(1):65–77. doi: 10.1016/j.cmet.2020.11.014. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riahi S, Sombra LRS, Lo KB, Chacko SR, Neto AGM, Azmaiparashvili Z, et al. Insulin use, diabetes control, and outcomes in patients with COVID-19. J Endocrine Research. 2021;46(2):45–50. doi: 10.1080/07435800.2020.1856865. [DOI] [PubMed] [Google Scholar]


