Abstract
Articular cartilage has a low self-repair capacity due to the lack of vessels and nerves. In recent times, nanofiber scaffolds have been widely used for this purpose. The optimum nanofiber scaffold should stimulate new tissue's growth and mimic the articular cartilage nature. Furthermore, the characteristics of the scaffold should match those of the cellular matrix components of the native tissue to best merge with the target tissue. Therefore, selective modification of prefabricated scaffolds based on the structure of the repaired tissues is commonly conducted to promote restoring the tissue. A thorough analysis is required to find out the architectural features of scaffolds that are essential to make the treatment successful. The current review aims to target this challenge. The article highlights different optimization approaches of nanofibrous scaffolds for improved cartilage tissue engineering. In this context, the influence of the architecture of nanoscaffolds on performance is discussed in detail. Finally, based on the gathered information, a future outlook is provided to catalyze development in this promising field.
Keywords: Articular cartilage, Scaffolds, Articular cartilage defect, Collagen type II
Introduction
There are three forms of cartilage in the body; hyaline or articular cartilage, fibroelastic cartilage (meniscus), and elastic cartilage 1. Articular cartilage (AC) covers the end of the long bones at the joint place. The main function of AC is to create friction-free movement, reduce load, increase traction, and resist pressure distribution at the joint surface 2. At the same time, it must have high tensile strength, resist lateral tension, and ensure tissue integrity. Also known as hyaline cartilage, it is a unique and durable connective tissue that provides nearly frictionless articulation for mechanical load transmission between joints. Thus, it plays a crucial role in the physiological mobility of joints 3,4.
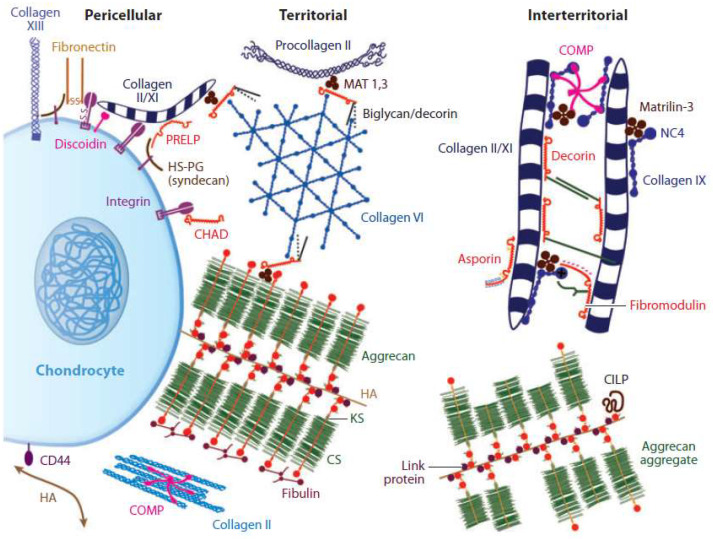
Cartilage architecture predominantly comprises collagen type II, VI, IX, X, chondrocytes, and glycosaminoglycans. Collagen type II mainly forms 90-95% of the fiber network of the extracellular matrix of cartilage and provides its cartilaginous framework and tensile strength 5-8. It exhibits the stress-shielding of the solid matrix components due to its high water content, incompressible under these conditions, and the structural organization of the proteoglycan and collagen molecules 9,10. Furthermore, carbohydrate groups in collagen type II allow more interaction with water than other collagens. This sort of collagen, together with classes IX and XI, forms a fibrous network that leads to the elastic strength of the cartilage 11. Besides these materials, proteoglycans, hyaluronic acid, and heparan sulfate are some of the glycosaminoglycans found in the AC 12. The most prominent proteoglycan is aggrecan, which generates tensile strength, porosity, permeability, and reinforcement together with collagen fibrils in AC (Figure 1) 13.
Figure 1.
Extracellular matrix of articular cartilage. Two major load-bearing macromolecules are present in articular cartilage: Collagens (mainly type II) and proteoglycans (notably, aggrecan). Smaller classes of molecules, such as non-collagenous proteins and smaller proteoglycans, are present in lower amounts. The interaction between the highly negatively charged cartilage proteoglycans and type II collagen provides the compressive and tensile strength of the tissue. Reproduced with permission 13. Copyrighted by the Authors (2019).
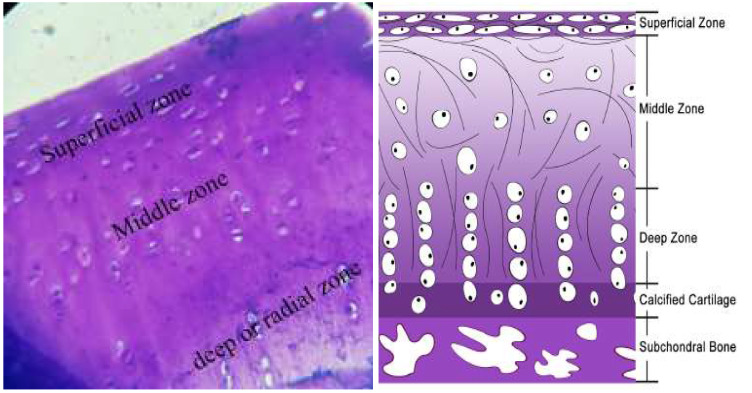
AC is divided into three areas: superficial, middle, and deep, each of which has its distinct properties; the number of cells, shape, size, and direction of collagen fibers and proteoglycans (Figure 2) 14-16. The superficial layer of AC is the thinnest, and the elastic properties of cartilage belong to this layer. Chondrocytes and collagen fibers (mainly type II and type IX collagen fibers with an ultra-small diameter (20 nm)) in this layer are aligned parallel to the surface to protect the deeper layers against tensile, compressive, and shear forces 17-19. Given the hierarchical nature of the AC, its repair is non-trivial. This perspective article aims to summarize recent progress in solving this issue using nanoscaffolds and provides directions how to facilitate further development in this field.
Figure 2.
Structure of the articular cartilage layer. Reproduced with permission 20. Copyrighted by the Authors (2021).
Articular Cartilage damages
AC has a low post-traumatic injury or wear self-repair capacity due to a lack of vessels and nerves. Furthermore, its cell proliferation capacity is not satisfactory 21. Therefore, AC-related defects are especially problematic. In general, people with joint injuries and meniscal or ligament tears are prone to cartilage joint damage 22,23. Consequently, damaged cartilage can lead to arthritis in the joint. Also, AC can be damaged by injury or physiological wear and tear during aging. Initially, in osteoarthritis, chondrocytes release many anabolic factors to repair the lesions. However, the factors alter the phenotype of chondrocytes by forming non-functional cartilage (fibrocartilage), which is thinner than regular AC and degenerates under mechanical pressure 24.
Treatment procedures for articular cartilage defects
Treatment of AC injuries is one of the most challenging issues of musculoskeletal medicine due to the poor intrinsic ability of this tissue to repair itself. Nevertheless, restoring AC is essential as it can relieve pain and improve function. Most importantly, it can delay or prevent the onset of arthritis. The most common procedures for cartilage restoration are as follows.
Microfracture
Microfracture is commonly used for cartilage restoration by marrow stimulation. This method increases the migration of mesenchymal stem cells (MSCs), the influx of growth factors, and platelets from bone marrow to the defect site. However, this method results in the formation of fibrocartilage rather than normal hyaline AC 25, which is unwanted.
Drilling
This therapeutic approach creates holes in the subchondral bone and supplies blood flow into defects to induce the repair of cartilage. The holes are made with a surgical drill or wire. Although the method is similar to microfracture, in this technique, the heat of the drill damages the AC tissues. It again results in the formation of fibrocartilage 26,27, so it is also not recommended.
Abrasion Arthroplasty
Abrasion Arthroplasty removes some areas of the AC to create new capacities for joint surfaces. Hence, it can eliminate the injured cartilage tissue 28. But unfortunately, this is only a palliative approach as it does not promote AC regeneration. Consequently, it is employed as a last resort treatment.
Autologous chondrocyte implantation (ACI)
Autologous Chondrocyte Implantation (ACI) is used to repair AC defects in a surgical approach. ACI is divided into a two-step procedure. In the first surgical procedure, healthy cartilage tissue is taken from a non-weight-bearing area of the joint during an arthroscopic procedure. The cartilage tissue is then sent to the laboratory, and chondrocytes are isolated, which are subsequently cultured and proliferated. The second surgical procedure (arthrotomy) is an open procedure in which the newly grown cells are injected into the articular cartilage defects 29. ACI is the most useful technique for treating isolated cartilage defects in younger patients with larger defects in AC who would like to return to activities of daily living. Unfortunately, despite its merits, this approach does not restore the possibility of competing in high-level sports 30-32.
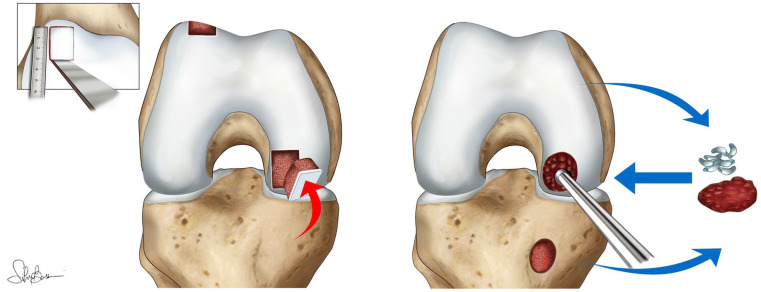
Osteochondral Autograft Transplantation (OCA)
OCA has demonstrated consistent clinical results and can be used to treat a variety of articular defects of the knee using size-matched cadaveric donor plugs that permit immediate structural restoration of the joint articular surface 33,34 (Figure 3).
Figure 3.
Schematic representation of osteochondral autograft transplantation. Reproduced with permission 35. Copyrighted by the Authors (2021).
All described treatment strategies have been applied to regenerate cartilage lesions to restitute articular function and relieve the associated pain. Although they decrease patient discomfort and enhance joint mobility, the repaired tissue is often fibrocartilage with less clinical action 36.
Engineering articular cartilage tissue
Tissue engineering is used to reconstruct and regenerate damaged tissues. It is a medical technology that has attracted considerable attention in recent times. The main purpose of this approach is not only to repair tissue but primarily to improve organ function. Tissue engineering also has diagnostic applications made in vitro and is used to test the biocompatibility of the materials 37. Three components are required to achieve an ideal tissue engineering procedure: appropriate scaffold, cell, and induction factors. In recent decades, success in tissue engineering has mainly influenced the therapy of defects and tissue regeneration. A stable increase in life expectancy and a related improvement in life quality are linked with this development, including the understandable request of the population to accept no damage in increasing age during the whole life. It is evident that modern medicine seeks solutions to avoid the loss of cell or tissue functions 3,38, which is more beneficial than treating advanced state diseases.
Nanofiber scaffolds
Since the current surgical procedures for regenerating a cartilage defect have considerable drawbacks, 3D scaffolds might offer promising results to facilitate the restoration of target tissues 39. Also, the advances in nanotechnology have offered groundbreaking progress in the field of tissue engineering by providing a microenvironment for the induction of cell expansion and differentiation into the desired lineage 40. The development in this area is particularly dynamic as nanomaterials exhibit good physicochemical and biomimetic properties that can stimulate the growth of chondrocytes and the regeneration of AC 41. Nanofibers have been considered one of the most extensively studied nanomaterials for cartilage regeneration and are constructed via different techniques, including electrospinning, self-assembly, phase separation, and drawing 42-44. The polymer nanofibers used for the regeneration of AC are both synthetic and natural scaffolds. Natural nanofiber scaffolds include alginate 45, gelatin 46, agarose 47, hyaluronic acid 48, fibrin, and collagen 49. Synthetic scaffolds are very diverse and mostly contain polycaprolactone 50, polyethylene glycol, polyurethane 51, poly(p-dioxanone) 52, poly(lactic acid) 53. It is of utmost importance to ensure that the selected scaffold exhibits tissue compatibility and biodegradability.
On the one hand, synthetic polymers are reproducible, and their properties can be easily controlled 54. Many polymer nanofibers well maintain cartilage stem cell differentiation and proliferation in vitro 55,56. Nevertheless, Earth-abundant natural polymers have more applications in clinical studies, and collagen is the most prevalently used type 48. Collagen polymers provide an essential network 57 for cartilage formation, both with and without cells 58. In general, natural scaffolds are used as gels in which the polymer network retains a large amount of water. This environment supports the proliferation, differentiation, and adhesion of cells. However, unfortunately, these polymers have poor mechanical properties and weak resistance to pressure and tension 59.
Regardless of the origin, the nanofiber scaffolds should stimulate the growth of new tissue in all aspects and mimic the characteristics of AC and the area below the articular cartilage. In addition, the scaffold should exhibit the potential to merge with the target and withstand physical activity tissue 60.
The effect of architectural features of scaffolds on cartilage repair
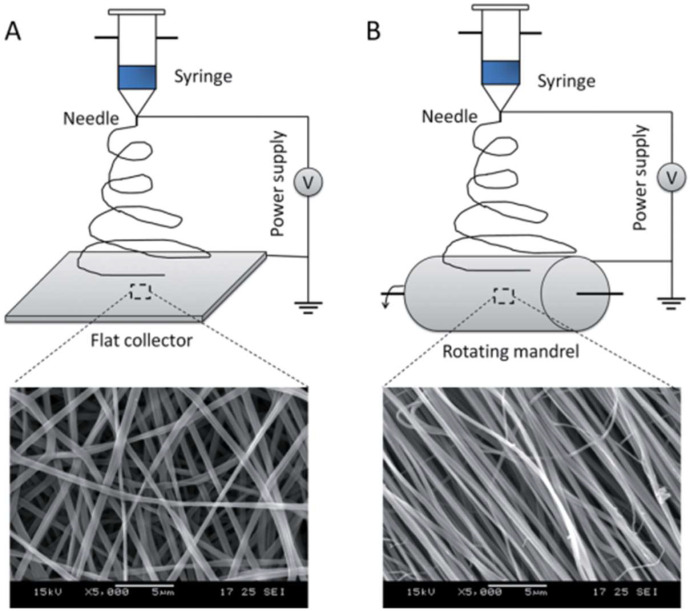
Scaffolds have been applied to improve tissue regeneration by recapitulating a difference in the physical structure of the tissue. Different strategies have been used, including fibrous self-synthesis, phase segregation, and electrospinning of nanofibers to produce the fibrous collagen network of the extracellular matrix via the aid of nanotechnology. Today, in reconstructive medicine, electrospinning scaffolds are used to repair and regenerate the tissues, such as heart muscle, cartilage, bone, nerve, and others. Interestingly, optimization of the nanoscaffold synthesis conditions may even lead to fiber alignment to obtain anisotropic materials for cell growth (Figure 4) 61.
Figure 4.
Electrospinning of fibrous (A) isotropic and (B) anisotropic nanoscaffolds. Reproduced with permission from 61. Copyrighted by Wiley (2012).
Some research has shown the potential of electrospinning of fiber scaffolds for cartilage tissue restoration 62. Different 2D electrospun nanofibrous matrices containing a single polymer have been implemented in the chondrogenic differentiation of BM-derived SCs 63. It has been shown that soluble factors minimally affect a cellular orientation, and mainly physical cues are connected with this issue. However, chondrogenic factors influence cell shape, which should be taken into account 64. The expression of collagen type II and S GAG content has shown a substantial increment in cells cultured on the nanofibrous scaffold in the presence of chondrogenic media.
In another study, the culture of MSCs on electrospun PCL nanofiber stimulated the fibroblast-like morphology. In contrast, the dynamic condition led to a round-shaped morphology with elevated collagen type I, II, and sGAG 65. Dahl et al. reported the effects of PLGA/PCL electrospun nanofibers on chondrogenic differentiation of human Umbilical Cord MSCs 66, resulting in high levels of proteoglycans and sGAG.
Other hybrid nanofiber scaffolds have also been applied in this context. For instance, PLGA/collagen scaffolds have shown optimal chondrogenesis potential 52. Li et al. have developed a PLLA/silk fibroin (PLLA/SF) composite that provided a suitable platform for the adhesion and growth of chondrocytes for cartilage tissue engineering purposes 67. Aligned nanofibrous scaffolds have been shown to mimic the naturally-occurring extracellular matrix and result in fibrochondrogenesis 68. Moreover, Shafiee et al. have shown that aligned PLLA/PCL nanofibrous scaffolds and compared them with randomly oriented scaffolds. The results showed that aligned scaffolds led to a bipolar extension along the fiber and a significantly higher expression of the chondrogenesis markers 69.
Although nano-sized structures simulate well the ECM components, they can also promote cell spreading and restrict infiltration 70,71. Thus, the construction of micro-nanofibers can overcome these limitations and assist in yielding larger pore sizes, improved cellular differentiation, and formation of ECM 72. Leverson et al. have fabricated electrospun PCL microfibers (Pμ), PCL microfibers with PCL nanofibers (PμPn), and PCL microfibers and fibrin nanofibers (PμFn) scaffolds. Accordingly, similar porosity was observed in both PμFn and PμPn scaffolds. However, larger pore sizes were obtained in Pμ scaffolds. Also, higher density was observed in PμPn scaffolds. More elongated spindle-like cells of human umbilical MSCs appeared on Pμ and PμFn scaffolds, whereas flattened, broad polygonal morphology was observed for PμPn scaffolds 73.
The synthesis of 3D nanofibrous scaffolds has also highly enhanced the chondrogenesis of different SCs 74. Li et al. have shown that a 3D nanofibrous scaffold produced a higher level of cartilaginous ECM from chondrocyte-like cells 75. Moreover, it has been revealed that biomolecules influence the chondrogenic differentiation of SCs seeded on nanofibrous structures. Schagemann et al. have reported that augmentation of the nanofibrous scaffold with or without TGF-β1 and/or hyaluronan could positively affect the chondrogenic differentiation of MSCs. Also, they showed that microfibrous scaffolds release higher amounts of TGF-β1. However, these scaffolds show a lower level of chondrogenic markers compared to nanofibrous scaffolds 76.
Research has also shown that scaffolds with parallel fibers and random Poly(l-lactide) (PLLA)/Polycaprolactone (PCL) 69 and Polycaprolactone (PCL)/Poly(lactic-co-glycolic acid) (PLGA) can support progenitor cells and human bone marrow-derived stem cells, adhesion, proliferation, and regeneration of cartilage 77. However, the proliferation of stem cells was higher on the random scaffolds than on the aligned scaffolds. In contrast, the differentiation of stem cells was higher on the scaffolds with parallel fibers. Thus, such a scaffold with paralleled fibers structure is suitable for the superficial area of AC wherein its fibers are arranged parallel to the surface.
Although nano- and microfibers electrospun scaffolds maintain and support the growth and proliferation of bone marrow-derived stem cells, nano-fibrous PCL scaffolds play a greater cartilaginous activity. That was shown by produced sGAG and collagen type II expression, and this scaffold may be suitable for the repair of the superficial area of the scaffold. Other studies have reported similar results with nanofiber scaffolds, as opposed to microfiber scaffolds or smooth (film) Poly(L-lactide) (PLLA) 78 or poly (L-co-D,L-lactide) (PLDLA) 79 scaffolds. The nano-fibrous structures preserve the morphology of chondrocytes and increase the expression of cartilage markers and the formation of the extracellular matrix. Nevertheless, it should be noted that not only the fiber size and porosity but also the pore size acts as a significant factor in carcinogenesis 80. Electrospun nanofiber scaffolds with low porosity/high density often cause negligible cell permeability and limit nutrient infiltration to the deep zone of the cartilage tissue 81. Thus, various modifications in the fiber size and density have been applied to strengthen and increase the permeability and transport potential of biomaterials through scaffold fibers 82,83. Paralleled PCL strands combined with synovial-derived stem cells have shown a significant repair of meniscal hoop structure injuries in rabbits 84 (Figure 5).
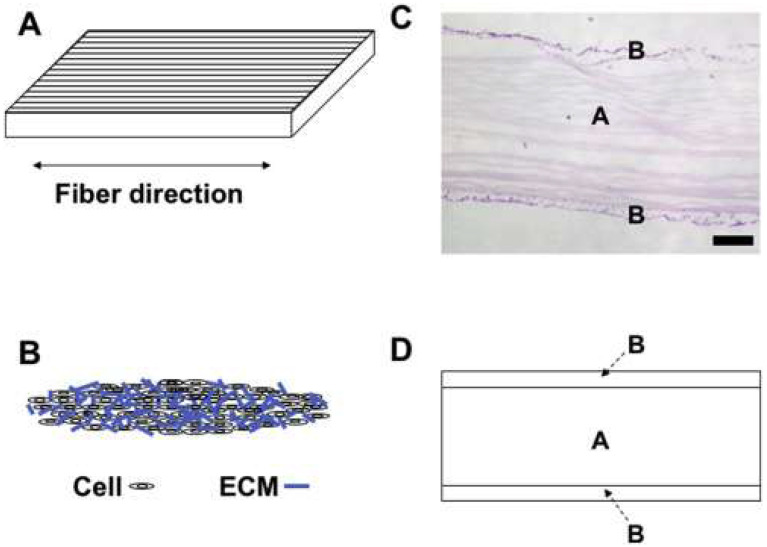
Figure 5.
Schematic illustration of a mixed material of a nanofiber scaffold along with scaffold-free tissue-engineered construct (TEC) consisting of cells and extracellular matrix (ECM). A: The parallel structure of the scaffold. B: TEC structure. C: H&E staining of the combined material consisting of an electrospun nanofibrous scaffold (A) and a TEC (B). D: Schematic depiction of combined material comprising an electrospun nanofibrous scaffold (A) and a TEC (B). Reproduced with permission 84. Copyrighted by the Authors.
In swine, as a large animal, the implantation of PCL/BMSCs in full-thickness cartilage defect of AC led to a complete repair and formation of hyaline cartilage-like tissue 6 months after seeding 85. Seeding collagen/PLCL scaffolds with chondrocytes in layer-by-layer sandwich constructs of collagen/PLCL in mice harvested 83% of native cartilage after 12 weeks of transplantation 86. PVA/chondroitin sulfate electrospun fibers seeded in a rat articular cartilage defect showed increased chondrogenesis compared to the control group of the empty defect 87. Resveratrol-PLA-gelatin scaffolds, compared with PLA-gelatin scaffolds, were found to treat faster the rat articular cartilage defect 12 weeks post-transplantation 88. Repairing the cartilage defects in the rabbit model by aligned PLLA-polydopamine chondroitin sulfate fibers was facilitated, and cartilage defects were regenerated by hyaline cartilage-like tissue 89. In light of the foregoing, the application of nanoscaffolds for cartilage restoration is an auspicious approach with a high application potential, which can gain from recent breakthroughs in biomedical engineering 90-93.
Conclusion and Future Outlooks
This review discussed the application of nanofiber scaffolds that can result in the complete synthesis of native hyaline cartilage tissue in optimum situations. Tissue replacement through non-biological and regenerative systems in vivo and the formation and design of nanofiber scaffolds are currently hot topics in the tissue engineering field because of its merits. Still, the examined literature reveals increasing demand for qualified tissue regeneration strategies to increase the number of successful transplantations. Thus, it is vital to develop strategies for creating cells, tissues, and organs with good compatibility to reduce the future rejection rate of clinical programs 94. In light of the presented research, tissue engineering appears to be a promising approach to reach these goals. Furthermore, judging by the maturity of the results, the production of appropriate scaffolds for tissue engineering may soon materialize at a sufficiently large scale 95. Nevertheless, the multidisciplinary nature of this challenge hampers progress. Thus, it is necessary to promote interactions between engineers and physicians to provide possibilities for their collaboration for the sake of tissue engineering.
Importantly, there is much room to optimize the performance of nanoscaffolds. For instance, future scaffold constructs will only be successful if improved compatibility regarding the ECM is realized 96. In addition, a broader spectrum of nanofibers, stem cell sources, and differentiation-inducing growth factors should be evaluated to reach the most suitable combination of materials for cartilage repair (Figure 6).
Figure 6.
Most important factors, which should be the matter of further research to increase the technology readiness level of nanofiber-bases scaffolds for cartilage engineering.
In this context, the fabrication and implantation of appropriate bioreactors that eventually aid in more ECM formation and the achievement of artificial constructs mimicking native cartilage tissues should be considered. Finally, although several investigations produced promising results regarding the efficacy of nanofibers for repairing cartilage defects in vivo, more effort is still necessary for shifting the information from in vitro to in vivo phase. Only then this promising concept will serve society and improve the quality of our life.
Acknowledgments
D.J. would like to thank the National Science Centre, Poland (under the SONATA program, Grant agreement UMO-2020/39/D/ST5/00285).
References
- 1.Decker RS, Koyama E, Pacifici M. Articular Cartilage: Structural and Developmental Intricacies and Questions. Curr Osteoporos Rep. 2015;13:407–14. doi: 10.1007/s11914-015-0290-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W, Chen S, Morsi Y, El-Hamshary H, El-Newhy M, Fan C. et al. Superabsorbent 3D Scaffold Based on Electrospun Nanofibers for Cartilage Tissue Engineering. ACS Appl Mater Interfaces. 2016;8:24415–25. doi: 10.1021/acsami.6b06825. [DOI] [PubMed] [Google Scholar]
- 3.Weigel T, Schinkel G, Lendlein A. Design and preparation of polymeric scaffolds for tissue engineering. Expert Review of Medical Devices. 2006;3:835–51. doi: 10.1586/17434440.3.6.835. [DOI] [PubMed] [Google Scholar]
- 4.Lim E-H, Sardinha JP, Myers S. Nanotechnology Biomimetic Cartilage Regenerative Scaffolds. Arch Plast Surg. 2014;41:231–40. doi: 10.5999/aps.2014.41.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calejo I, Costa-Almeida R, Reis RL, Gomes ME. A Physiology-Inspired Multifactorial Toolbox in Soft-to-Hard Musculoskeletal Interface Tissue Engineering. Trends in Biotechnology. 2020;38:83–98. doi: 10.1016/j.tibtech.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Casanellas I, García-Lizarribar A, Lagunas A, Samitier J. Producing 3D Biomimetic Nanomaterials for Musculoskeletal System Regeneration. Frontiers in Bioengineering and Biotechnology. 2018. 6. [DOI] [PMC free article] [PubMed]
- 7.Li Y, Xu L. Advances in understanding cartilage remodeling. F1000Res. 2015;4:642. doi: 10.12688/f1000research.6514.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilusz RE, Sanchez-Adams J, Guilak F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biology. 2014;39:25–32. doi: 10.1016/j.matbio.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vonk LA, de Windt TS, Kragten AHM, Beekhuizen M, Mastbergen SC, Dhert WJA. et al. Enhanced cell-induced articular cartilage regeneration by chondrons; the influence of joint damage and harvest site. Osteoarthritis and Cartilage. 2014;22:1910–7. doi: 10.1016/j.joca.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Casanova MR, Reis RL, Martins A, Neves NM. The Use of Electrospinning Technique on Osteochondral Tissue Engineering. In: Oliveira JM, Pina S, Reis RL, San Roman J, editors. Osteochondral Tissue Engineering: Nanotechnology, Scaffolding-Related Developments and Translation. 2018. pp. 247–63. [DOI] [PubMed]
- 11.Cheng A, Schwartz Z, Kahn A, Li X, Shao Z, Sun M. et al. Advances in Porous Scaffold Design for Bone and Cartilage Tissue Engineering and Regeneration. Tissue Engineering Part B: Reviews. 2019;25:14–29. doi: 10.1089/ten.teb.2018.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J. 2008;17:467–79. doi: 10.1007/s00586-008-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinegård D. Fell-Muir Lecture: Proteoglycans and more - from molecules to biology. International Journal of Experimental Pathology. 2009;90:575–86. doi: 10.1111/j.1365-2613.2009.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao X, Hu DA, Wu D, He F, Wang H, Huang L, Applications of Biocompatible Scaffold Materials in Stem Cell-Based Cartilage Tissue Engineering. Frontiers in Bioengineering and Biotechnology. 2021. 9. [DOI] [PMC free article] [PubMed]
- 15.Correia CR, Reis RL, Mano JF. Multiphasic, Multistructured and Hierarchical Strategies for Cartilage Regeneration. In: Bertassoni LE, Coelho PG, editors. Engineering Mineralized and Load Bearing Tissues. 2015. pp. 143–60. [DOI] [PubMed]
- 16.Vyas C, Poologasundarampillai G, Hoyland J, Bartolo P. 12 - 3D printing of biocomposites for osteochondral tissue engineering. In: Ambrosio L, editor. Biomedical Composites (Second Edition) 2017. pp. 261–302.
- 17.Nickien M, Thambyah A, Broom ND. How a decreased fibrillar interconnectivity influences stiffness and swelling properties during early cartilage degeneration. Journal of the Mechanical Behavior of Biomedical Materials. 2017;75:390–8. doi: 10.1016/j.jmbbm.2017.07.042. [DOI] [PubMed] [Google Scholar]
- 18.Nazempour A, Van Wie BJ. Chondrocytes, Mesenchymal Stem Cells, and Their Combination in Articular Cartilage Regenerative Medicine. Ann Biomed Eng. 2016;44:1325–54. doi: 10.1007/s10439-016-1575-9. [DOI] [PubMed] [Google Scholar]
- 19.Bhosale AM, Richardson JB. Articular cartilage: structure, injuries and review of management. British Medical Bulletin. 2008;87:77–95. doi: 10.1093/bmb/ldn025. [DOI] [PubMed] [Google Scholar]
- 20.Zhao X, Hu DA, Wu D, He F, Wang H, Huang L, Applications of Biocompatible Scaffold Materials in Stem Cell-Based Cartilage Tissue Engineering. Frontiers in Bioengineering and Biotechnology. 2021. 9. [DOI] [PMC free article] [PubMed]
- 21.Dahlin RL, Kasper FK, Mikos AG. Polymeric Nanofibers in Tissue Engineering. Tissue Engineering Part B: Reviews. 2011;17:349–64. doi: 10.1089/ten.teb.2011.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vahedi P, Jarolmasjed SH, Soleimani A. Transplantation of ASCs-Poly (ε-Caprolactone) Nanofiber Scaffold and Evaluate the Effect of Mechanical Loading of Walking on Articular Cartilage Repair in Sheep Model. Cell Tiss Biol. 2021;15:199–207. [Google Scholar]
- 23.Cole BJ, Pascual-Garrido C, Grumet RC. Surgical Management of Articular Cartilage Defects in the Knee. JBJS. 2009;91:1778–90. [PubMed] [Google Scholar]
- 24.Lamplot JD, Schafer KA, Matava MJ. Treatment of Failed Articular Cartilage Reconstructive Procedures of the Knee: A Systematic Review. Orthopaedic Journal of Sports Medicine. 2018;6:2325967118761871. doi: 10.1177/2325967118761871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang KC, Frank RM, Cotter EJ, Christian DR, Cole BJ. Arthroscopic Management of Isolated Tibial Plateau Defect With Microfracture and Micronized Allogeneic Cartilage-Platelet-Rich Plasma Adjunct. Arthroscopy Techniques. 2017;6:e1613–8. doi: 10.1016/j.eats.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mistry H, Connock M, Pink J, Shyangdan D, Clar C, Royle P. et al. Autologous chondrocyte implantation in the knee: systematic review and economic evaluation. Health Technology Assessment. 2017;21:1–294. doi: 10.3310/hta21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huisman SM, Brunner D. Cell polarity in fission yeast: A matter of confining, positioning, and switching growth zones. Seminars in Cell & Developmental Biology. 2011;22:799–805. doi: 10.1016/j.semcdb.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Ye K, Di Bella C, Myers DE, Choong PFM. The osteochondral dilemma: review of current management and future trends. ANZ Journal of Surgery. 2014;84:211–7. doi: 10.1111/ans.12108. [DOI] [PubMed] [Google Scholar]
- 29.Ma H-L, Hung S-C, Wang S-T, Chang M-C, Chen T-H. Osteochondral autografts transfer for post-traumatic osteochondral defect of the knee—2 to 5 years follow-up. Injury. 2004;35:1286–92. doi: 10.1016/j.injury.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Ludvigsen TC, Løken S. et al. A Randomized Multicenter Trial Comparing Autologous Chondrocyte Implantation with Microfracture: Long-Term Follow-up at 14 to 15 Years. JBJS. 2016;98:1332–9. doi: 10.2106/JBJS.15.01208. [DOI] [PubMed] [Google Scholar]
- 31.Vahedi P, Jarolmasjed S, Shafaei H, Roshangar L, Soleimani Rad J, Ahmadian E. In vivo articular cartilage regeneration through infrapatellar adipose tissue derived stem cell in nanofiber polycaprolactone scaffold. Tissue and Cell. 2019;57:49–56. doi: 10.1016/j.tice.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Huang BJ, Hu JC, Athanasiou KA. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials. 2016;98:1–22. doi: 10.1016/j.biomaterials.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vahedi P, Moghaddamshahabi R, Webster TJ, Calikoglu Koyuncu AC, Ahmadian E, Khan WS. et al. The Use of Infrapatellar Fat Pad-Derived Mesenchymal Stem Cells in Articular Cartilage Regeneration: A Review. International Journal of Molecular Sciences. 2021;22:9215. doi: 10.3390/ijms22179215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stone AV, Christian DR, Redondo ML, Yanke AB, Southworth TM, Tauro TM. et al. Osteochondral Allograft Transplantation and Osteochondral Autograft Transfer. Operative Techniques in Sports Medicine. 2018;26:183–8. [Google Scholar]
- 35.Di Martino A, Silva S, Andriolo L, Merli G, Reale D, Zaffagnini S. et al. Osteochondral autograft transplantation versus autologous bone-cartilage paste grafting for the treatment of knee osteochondritis dissecans. International Orthopaedics (SICOT) 2021;45:453–61. doi: 10.1007/s00264-020-04804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camp CL, Stuart MJ, Krych AJ. Current Concepts of Articular Cartilage Restoration Techniques in the Knee. Sports Health. SAGE Publications. 2014;6:265–73. doi: 10.1177/1941738113508917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campos F, Bonhome-Espinosa AB, Carmona R, Durán JDG, Kuzhir P, Alaminos M. et al. In vivo time-course biocompatibility assessment of biomagnetic nanoparticles-based biomaterials for tissue engineering applications. Materials Science and Engineering: C. 2021;118:111476. doi: 10.1016/j.msec.2020.111476. [DOI] [PubMed] [Google Scholar]
- 38.Vahedi P, Roshangar L, Jarolmasjed S, Shafaei H, Samadi N, Soleimanirad J. Effect of low-intensity pulsed ultrasound on regenerative potential of transplanted ASCs –PCL construct in articular cartilage defects in sheep. The Indian Journal of Animal Sciences. 2016. 86.
- 39.Khadijeh K, Dizaj SM, Saadat YR, Sharifi S, Shahi S, Ahmadian E, Eftekhari A, Abdolahinia ED, Farzaneh Lotfipour. Osteogenic differentiation of mesenchymal stem cells via curcumin-containing nanoscaffolds. Stem Cells International. 2021. 1520052. [DOI] [PMC free article] [PubMed]
- 40.Yang Y, Chawla A, Zhang J, Esa A, Jang HL, Khademhosseini A. Chapter 29 - Applications of Nanotechnology for Regenerative Medicine; Healing Tissues at the Nanoscale. In: Atala A, Lanza R, Mikos AG, Nerem R, editors. Principles of Regenerative Medicine (Third Edition). Boston: Academic Press. 2019. p. 485-504.
- 41.Eftekhari A, Maleki Dizaj S, Sharifi S, Salatin S, Rahbar Saadat Y, Zununi Vahed S. et al. The Use of Nanomaterials in Tissue Engineering for Cartilage Regeneration; Current Approaches and Future Perspectives. International Journal of Molecular Sciences. 2020;21:536. doi: 10.3390/ijms21020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie X, Chen Y, Wang X, Xu X, Shen Y, Khan A ur R. et al. Electrospinning nanofiber scaffolds for soft and hard tissue regeneration. Journal of Materials Science & Technology. 2020;59:243–61. [Google Scholar]
- 43.Cao L, Zhang F, Wang Q, Wu X. Fabrication of chitosan/graphene oxide polymer nanofiber and its biocompatibility for cartilage tissue engineering. Materials Science and Engineering: C. 2017;79:697–701. doi: 10.1016/j.msec.2017.05.056. [DOI] [PubMed] [Google Scholar]
- 44.Venugopal J, Low S, Choon AT, Ramakrishna S. Interaction of cells and nanofiber scaffolds in tissue engineering. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2008;84B:34–48. doi: 10.1002/jbm.b.30841. [DOI] [PubMed] [Google Scholar]
- 45.Müller M, Öztürk E, Arlov Ø, Gatenholm P, Zenobi-Wong M. Alginate Sulfate-Nanocellulose Bioinks for Cartilage Bioprinting Applications. Ann Biomed Eng. 2017;45:210–23. doi: 10.1007/s10439-016-1704-5. [DOI] [PubMed] [Google Scholar]
- 46.Visser J, Melchels FPW, Jeon JE, van Bussel EM, Kimpton LS, Byrne HM. et al. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat Commun. 2015;6:6933. doi: 10.1038/ncomms7933. [DOI] [PubMed] [Google Scholar]
- 47.Selmi T a. S, Verdonk P, Chambat P, Dubrana F, Potel J-F, Barnouin L, et al. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel. The Journal of Bone and Joint Surgery British volume. 2008. 90-B:597-604. [DOI] [PubMed]
- 48.Schagemann J, Behrens P, Paech A, Riepenhof H, Kienast B, Mittelstädt H. et al. Mid-term outcome of arthroscopic AMIC for the treatment of articular cartilage defects in the knee joint is equivalent to mini-open procedures. Arch Orthop Trauma Surg. 2018;138:819–25. doi: 10.1007/s00402-018-2887-z. [DOI] [PubMed] [Google Scholar]
- 49.Xu T, Binder KW, Albanna MZ, Dice D, Zhao W, Yoo JJ. et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2012;5:015001. doi: 10.1088/1758-5082/5/1/015001. [DOI] [PubMed] [Google Scholar]
- 50.Visser J, Peters B, Burger TJ, Boomstra J, Dhert WJA, Melchels FPW. et al. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication. 2013;5:035007. doi: 10.1088/1758-5082/5/3/035007. [DOI] [PubMed] [Google Scholar]
- 51.Hung K-C, Tseng C-S, Hsu S. Synthesis and 3D Printing of Biodegradable Polyurethane Elastomer by a Water-Based Process for Cartilage Tissue Engineering Applications. Advanced Healthcare Materials. 2014;3:1578–87. doi: 10.1002/adhm.201400018. [DOI] [PubMed] [Google Scholar]
- 52.Ahmed M, Ramos TA da S, Damanik F, Quang Le B, Wieringa P, Bennink M. et al. A combinatorial approach towards the design of nanofibrous scaffolds for chondrogenesis. Sci Rep. 2015;5:14804. doi: 10.1038/srep14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Müller M, Becher J, Schnabelrauch M, Zenobi-Wong M. Nanostructured Pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication. 2015;7:035006. doi: 10.1088/1758-5090/7/3/035006. [DOI] [PubMed] [Google Scholar]
- 54.Stoop R. Smart biomaterials for tissue engineering of cartilage. Injury. 2008;39:77–87. doi: 10.1016/j.injury.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 55.Wagner ER, Parry J, Dadsetan M, Bravo D, Riester SM, van Wijnen AJ. et al. Chondrocyte Attachment, Proliferation, and Differentiation on Three-Dimensional Polycaprolactone Fumarate Scaffolds. Tissue Engineering Part A. 2017;23:622–9. doi: 10.1089/ten.tea.2016.0341. [DOI] [PubMed] [Google Scholar]
- 56.Recha-Sancho L, Moutos FT, Abellà J, Guilak F, Semino CE. Dedifferentiated Human Articular Chondrocytes Redifferentiate to a Cartilage-Like Tissue Phenotype in a Poly(ε-Caprolactone)/Self-Assembling Peptide Composite Scaffold. Materials. 2016;9:472. doi: 10.3390/ma9060472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pascarella A, Ciatti R, Pascarella F, Latte C, Di Salvatore MG, Liguori L. et al. Treatment of articular cartilage lesions of the knee joint using a modified AMIC technique. Knee Surg Sports Traumatol Arthrosc. 2010;18:509–13. doi: 10.1007/s00167-009-1007-6. [DOI] [PubMed] [Google Scholar]
- 58.Kon E, Filardo G, Berruto M, Benazzo F, Zanon G, Della Villa S. et al. Articular Cartilage Treatment in High-Level Male Soccer Players: A Prospective Comparative Study of Arthroscopic Second-Generation Autologous Chondrocyte Implantation Versus Microfracture. Am J Sports Med. 2011;39:2549–57. doi: 10.1177/0363546511420688. [DOI] [PubMed] [Google Scholar]
- 59.Sánchez-Téllez DA, Téllez-Jurado L, Rodríguez-Lorenzo LM. Hydrogels for Cartilage Regeneration, from Polysaccharides to Hybrids. Polymers. 2017;9:671. doi: 10.3390/polym9120671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koons GL, Diba M, Mikos AG. Materials design for bone-tissue engineering. Nat Rev Mater. 2020;5:584–603. [Google Scholar]
- 61.Kai D, Jin G, Prabhakaran MP, Ramakrishna S. Electrospun synthetic and natural nanofibers for regenerative medicine and stem cells. Biotechnology Journal. 2013;8:59–72. doi: 10.1002/biot.201200249. [DOI] [PubMed] [Google Scholar]
- 62.Zheng X, Wang W, Liu S, Wu J, Li F, Cao L. et al. Enhancement of chondrogenic differentiation of rabbit mesenchymal stem cells by oriented nanofiber yarn-collagen type I/hyaluronate hybrid. Materials Science and Engineering: C. 2016;58:1071–6. doi: 10.1016/j.msec.2015.07.066. [DOI] [PubMed] [Google Scholar]
- 63.Kazemnejad S, Khanmohammadi M, Baheiraei N, Arasteh S. Current State of Cartilage Tissue Engineering using Nanofibrous Scaffolds and Stem Cells. Avicenna Journal of Medical Biotechnology. Avicenna Research Institute. 2017;9:50–65. [PMC free article] [PubMed] [Google Scholar]
- 64.Wise JK, Yarin AL, Megaridis CM, Cho M. Chondrogenic Differentiation of Human Mesenchymal Stem Cells on Oriented Nanofibrous Scaffolds: Engineering the Superficial Zone of Articular Cartilage. Tissue Engineering Part A. 2009;15:913–21. doi: 10.1089/ten.tea.2008.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alves da Silva ML, Martins A, Costa-Pinto AR, Costa P, Faria S, Gomes M. et al. Cartilage Tissue Engineering Using Electrospun PCL Nanofiber Meshes and MSCs. Biomacromolecules. 2010;11:3228–36. doi: 10.1021/bm100476r. [DOI] [PubMed] [Google Scholar]
- 66.Dahl JP, Caballero M, Pappa AK, Madan G, Shockley WW, van Aalst JA. Analysis of Human Auricular Cartilage to Guide Tissue-Engineered Nanofiber-Based Chondrogenesis: Implications for Microtia Reconstruction. Otolaryngol Head Neck Surg. 2011;145:915–23. doi: 10.1177/0194599811419092. [DOI] [PubMed] [Google Scholar]
- 67.Li Z, Liu P, Yang T, Sun Y, You Q, Li J. et al. Composite poly(l-lactic-acid)/silk fibroin scaffold prepared by electrospinning promotes chondrogenesis for cartilage tissue engineering. J Biomater Appl. 2016;30:1552–65. doi: 10.1177/0885328216638587. [DOI] [PubMed] [Google Scholar]
- 68.Baker BM, Nathan AS, Gee AO, Mauck RL. The influence of an aligned nanofibrous topography on human mesenchymal stem cell fibrochondrogenesis. Biomaterials. 2010;31:6190–200. doi: 10.1016/j.biomaterials.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shafiee A, Seyedjafari E, Sadat Taherzadeh E, Dinarvand P, Soleimani M, Ai J. Enhanced chondrogenesis of human nasal septum derived progenitors on nanofibrous scaffolds. Materials Science and Engineering: C. 2014;40:445–54. doi: 10.1016/j.msec.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 70.Pham QP, Sharma U, Mikos AG. Electrospun Poly(ε-caprolactone) Microfiber and Multilayer Nanofiber/Microfiber Scaffolds: Characterization of Scaffolds and Measurement of Cellular Infiltration. Biomacromolecules. 2006;7:2796–805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 71.Shalumon KT, Binulal NS, Deepthy M, Jayakumar R, Manzoor K, Nair SV. Preparation, Characterization and Cell Attachment Studies of Electrospun Multi-scale Poly(caprolactone) Fibrous Scaffolds for Tissue Engineering. Journal of Macromolecular Science, Part A. 2010;48:21–30. [Google Scholar]
- 72.Srinivasan S, Jayakumar R, Chennazhi KP, Levorson EJ, Mikos AG, Nair SV. Multiscale Fibrous Scaffolds in Regenerative Medicine. In: Jayakumar R, Nair S, editors. Biomedical Applications of Polymeric Nanofibers. Berlin, Heidelberg: Springer. 2012. pp. 1–20.
- 73.Levorson EJ, Sreerekha PR, Chennazhi KP, Kasper FK, Nair SV, Mikos AG. Fabrication and characterization of multiscale electrospun scaffolds for cartilage regeneration. Biomed Mater. 2013;8:014103. doi: 10.1088/1748-6041/8/1/014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu J, Feng K, Liu X, Ma PX. Chondrogenic and osteogenic differentiations of human bone marrow-derived mesenchymal stem cells on a nanofibrous scaffold with designed pore network. Biomaterials. 2009;30:5061–7. doi: 10.1016/j.biomaterials.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li W-J, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ. et al. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 76.Schagemann JC, Paul S, Casper ME, Rohwedel J, Kramer J, Kaps C. et al. Chondrogenic differentiation of bone marrow-derived mesenchymal stromal cells via biomimetic and bioactive poly-ε-caprolactone scaffolds. Journal of Biomedical Materials Research Part A. 2013;101A:1620–8. doi: 10.1002/jbm.a.34457. [DOI] [PubMed] [Google Scholar]
- 77.Zamanlui S, Mahmoudifard M, Soleimani M, Bakhshandeh B, Vasei M, Faghihi S. Enhanced chondrogenic differentiation of human bone marrow mesenchymal stem cells on PCL/PLGA electrospun with different alignments and compositions. International Journal of Polymeric Materials and Polymeric Biomaterials. 2018;67:50–60. [Google Scholar]
- 78.Markway BD, Tan G-K, Brooke G, Hudson JE, Cooper-White JJ, Doran MR. Enhanced Chondrogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells in Low Oxygen Environment Micropellet Cultures. Cell Transplant. 2010;19:29–42. doi: 10.3727/096368909X478560. [DOI] [PubMed] [Google Scholar]
- 79.Wimpenny I, Ashammakhi N, Yang Y. Chondrogenic potential of electrospun nanofibres for cartilage tissue engineering. Journal of Tissue Engineering and Regenerative Medicine. 2012;6:536–49. doi: 10.1002/term.459. [DOI] [PubMed] [Google Scholar]
- 80.Shanmugasundaram S, Chaudhry H, Arinzeh TL. Microscale Versus Nanoscale Scaffold Architecture for Mesenchymal Stem Cell Chondrogenesis. Tissue Engineering Part A. 2011;17:831–40. doi: 10.1089/ten.TEA.2010.0409. [DOI] [PubMed] [Google Scholar]
- 81.Skotak M, Ragusa J, Gonzalez D, Subramanian A. Improved cellular infiltration into nanofibrous electrospun cross-linked gelatin scaffolds templated with micrometer-sized polyethylene glycol fibers. Biomed Mater. 2011;6:055012. doi: 10.1088/1748-6041/6/5/055012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wright L, McKeon-Fischer K, Cui Z, Nair L, Freeman J. PDLA/PLLA and PDLA/PCL nanofibers with a chitosan-based hydrogel in composite scaffolds for tissue engineered cartilage. Journal of Tissue Engineering and Regenerative Medicine. 2014;8:946–54. doi: 10.1002/term.1591. [DOI] [PubMed] [Google Scholar]
- 83.Fiejdasz S, Szczubiałka K, Lewandowska-Łańcucka J, Osyczka AM, Nowakowska M. Biopolymer-based hydrogels as injectable materials for tissue repair scaffolds. Biomed Mater. 2013;8:035013. doi: 10.1088/1748-6041/8/3/035013. [DOI] [PubMed] [Google Scholar]
- 84.Shimomura K, Rothrauff BB, Hart DA, Hamamoto S, Kobayashi M, Yoshikawa H. et al. Enhanced repair of meniscal hoop structure injuries using an aligned electrospun nanofibrous scaffold combined with a mesenchymal stem cell-derived tissue engineered construct. Biomaterials. 2019;192:346–54. doi: 10.1016/j.biomaterials.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 85.Li J, Chen G, Xu X, Abdou P, Jiang Q, Shi D. et al. Advances of injectable hydrogel-based scaffolds for cartilage regeneration. Regenerative Biomaterials. 2019;6:129–40. doi: 10.1093/rb/rbz022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He X, Feng B, Huang C, Wang H, Ge Y, Hu R. et al. Electrospun gelatin/polycaprolactone nanofibrous membranes combined with a coculture of bone marrow stromal cells and chondrocytes for cartilage engineering. IJN. 2015;10:2089–99. doi: 10.2147/IJN.S79461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coburn JM, Gibson M, Monagle S, Patterson Z, Elisseeff JH. Bioinspired nanofibers support chondrogenesis for articular cartilage repair. Proceedings of the National Academy of Sciences. Proceedings of the National Academy of Sciences. 2012;109:10012–7. doi: 10.1073/pnas.1121605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu F, Li M, Yuan Z, Rao F, Fang X, Jiang B. et al. Mechanism research on a bioactive resveratrol–PLA–gelatin porous nano-scaffold in promoting the repair of cartilage defect. IJN. 2018;13:7845–58. doi: 10.2147/IJN.S181855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ren X, Li J, Li J, Jiang Y, Li L, Yao Q. et al. Aligned porous fibrous membrane with a biomimetic surface to accelerate cartilage regeneration. Chemical Engineering Journal. 2019;370:1027–38. [Google Scholar]
- 90.Banitaba SN, Ebadi SV, Salimi P, Bagheri A, Gupta A, Arifeen WU, Biopolymer-based electrospun fibers in electrochemical devices: versatile platform for energy, environment, and health monitoring. Mater Horiz. 2022. https://pubs.rsc.org/en/content/articlelanding/2022/mh/d2mh00879c. [DOI] [PubMed]
- 91.Kumar A, Sood A, Singhmar R, Mishra YK, Thakur VK, Han SS. Manufacturing functional hydrogels for inducing angiogenic-osteogenic coupled progressions in hard tissue repairs: prospects and challenges. Biomater Sci. The Royal Society of Chemistry. 2022;10:5472–97. doi: 10.1039/d2bm00894g. [DOI] [PubMed] [Google Scholar]
- 92.Panda S, Hajra S, Mistewicz K, Nowacki B, In-na P, Krushynska A. et al. A focused review on three-dimensional bioprinting technology for artificial organ fabrication. Biomater Sci. The Royal Society of Chemistry. 2022;10:5054–80. doi: 10.1039/d2bm00797e. [DOI] [PubMed] [Google Scholar]
- 93.Serrano-Aroca Á, Cano-Vicent A, Sabater i Serra R, El-Tanani M, Aljabali AlaaAA, Tambuwala MM. et al. Scaffolds in the microbial resistant era: Fabrication, materials, properties and tissue engineering applications. Materials Today Bio. 2022;16:100412. doi: 10.1016/j.mtbio.2022.100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muncie JM, Weaver VM. Chapter One - The Physical and Biochemical Properties of the Extracellular Matrix Regulate Cell Fate. In: Litscher ES, Wassarman PM, editors. Current Topics in Developmental Biology [Internet]. Academic Press; 2018 [cited. 2022. Nov 2]. p. 1-37. Available from: https://www.sciencedirect.com/science/article/pii/S0070215318300346. [DOI] [PMC free article] [PubMed]
- 95.Sato M, Yamato M, Mitani G, Takagaki T, Hamahashi K, Nakamura Y. et al. Combined surgery and chondrocyte cell-sheet transplantation improves clinical and structural outcomes in knee osteoarthritis. npj Regen Med. 2019;4:1–11. doi: 10.1038/s41536-019-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peroglio M, Gaspar D, Zeugolis DI, Alini M. Relevance of bioreactors and whole tissue cultures for the translation of new therapies to humans. Journal of Orthopaedic Research. 2018;36:10–21. doi: 10.1002/jor.23655. [DOI] [PubMed] [Google Scholar]