Background:
Pharmacologic termination of paroxysmal supraventricular tachycardia (PSVT) often requires medically supervised intervention. Intranasal etripamil, is an investigational fast-acting, nondihydropyridine, L-type calcium channel blocker, designed for unsupervised self-administration to terminate atrioventricular nodal–dependent PSVT. Phase 2 results showed potential safety and efficacy of etripamil in 104 patients with PSVT.
Methods:
NODE-301, a phase 3, multicenter, double-blind, placebo-controlled study evaluated the efficacy and safety of etripamil nasal spray administered, unsupervised in patients with symptomatic sustained PSVT. After a medically supervised etripamil test dose while in sinus rhythm, patients were randomized 2:1 to receive etripamil 70 mg or placebo. When PSVT symptoms developed, patients applied a cardiac monitor and attempted a vagal maneuver; if symptoms persisted, they self-administered blinded treatment. An independent Adjudication Committee reviewed continuous electrocardiogram recordings. The primary efficacy endpoint was termination of adjudicated PSVT within 5 hours after study drug administration.
Results:
NODE-301 accrued 156 positively adjudicated PSVT events treated with etripamil (n=107) or placebo (n=49). The hazard ratio for the primary endpoint, time-to-conversion to sinus rhythm during the 5-hour observation period, was 1.086 (95% CI, 0.726–1.623; P=0.12). In predefined sensitivity analyses, etripamil effects (compared with placebo) occurred at 3, 5, 10, 20, and 30 minutes (P<0.05). For example, at 30 minutes, there was a 53.7% of SVT conversion in the treatment arm compared to 34.7% in the placebo arm (hazard ratio, 1.87 [95% CI, 1.09–3.22]; P=0.02). Etripamil was well tolerated; adverse events were mainly related to transient nasal discomfort and congestion (19.6% and 8.0%, respectively, of randomized treatment-emergent adverse events.
Conclusions:
Although the primary 5-hour efficacy endpoint was not met, analyses at earlier time points indicated an etripamil treatment effect in terminating PSVT. Etripamil self-administration during PSVT was safe and well tolerated. These results support continued clinical development of etripamil nasal spray for self-administration during PSVT in a medically unsupervised setting.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03464019.
Keywords: atrioventricular node, blood pressure, calcium, etripamil, tachycardia, verapamil
What is Known?
Symptomatic sustained atrioventricular nodal–dependent paroxysmal supraventricular tachycardia (PSVT) unresponsive to a vagal maneuver frequently requires intervention in an emergency or acute care setting.
No currently approved self-administered pharmacological agents for termination of PSVT are available, and only etripamil is under investigation for this use.
What the Study Adds
Etripamil was designed to terminate atrioventricular nodal–dependent PSVT in a medically unsupervised setting, given its parenteral administration via an intranasal route and its pharmacokinetic characteristics.
A statistically significant difference in PSVT termination rates between etripamil treatment and placebo was achieved at 3, 5, 10, and 30 minutes.
Etripamil treatment effect during the first 30 minutes is consistent with its expected rapid onset.
Etripamil treatment was observed to be well tolerated, with a majority of adverse events being limited to the administration site and being mild and transient.
More patients in the placebo group than the etripamil group sought additional medical interventions—mainly in an emergency department setting—to terminate PSVT during the 5-hour monitoring period.
Paroxysmal supraventricular tachycardia (PSVT) is characterized by episodes of regular tachyarrhythmia.1–3 Because the atrioventricular node is a necessary component of the arrhythmia circuit for atrioventricular nodal reentrant tachycardia and atrioventricular reentrant tachycardia, the 2 most common types of PSVT, a pharmaceutical agent capable of transiently prolonging atrioventricular nodal refractoriness could terminate the arrhythmia and restore sinus rhythm.4–6
Intravenous verapamil, diltiazem, and adenosine are effective agents for the rapid termination of acute PSVT.4 However, these medications require the establishment of intravenous access and, therefore, are not appropriate for self-administration outside a healthcare setting. Patients may self-administer oral beta-blockers or calcium channel blockers at home to acutely manage their PSVT episodes. However, there is limited medical evidence to support the efficacy and safety of this so-called pill-in-the-pocket approach in the context of PSVT.7−10 European SVT guidelines do not suggest the acute use of oral medications for terminating PSVT, and US guidelines only recommend that this approach be considered for patients with infrequent, well-tolerated atrioventricular nodal reentrant tachycardia.1,2 Furthermore, these oral medications are slower-acting than medications with intravenous, sublingual, or intranasal routes of administration, and there may be associated risks of hypotension during PSVT and bradycardia following conversion. As such, there currently is no rapidly acting and safe drug available for patient self-administration for the acute termination of PSVT.
Etripamil, an investigational new drug, was formulated to be a fast-acting, L-type calcium channel blocker for administration with a nasal spray delivery device. Like other nondihydropyridine calcium channel blockers, etripamil appears to slow atrioventricular node conduction and increase its refractory period.5,6 Etripamil is being studied as a potential patient-administered treatment for the rapid termination of episodes of atrioventricular nodal–dependent SVT.
In the first-in-human, single ascending-dose phase 1 study in healthy volunteers, etripamil, when administered intranasally at doses between 3.5 and 140 mg during sinus rhythm, was rapidly absorbed, with a time after drug administration to peak plasma concentration of 5 minutes, and a dose-dependent maximum peak concentration and greater prolongation of the PR interval at higher doses.9 Following a dose of 70 mg, peak plasma concentrations of etripamil occurred at ≈8 minutes and fell by 60% at 25 minutes and ≈80% within 50 minutes.
In a phase 2, parallel-design, double-blind study, 104 randomized patients received etripamil at doses from 35 to 140 mg or placebo during atrioventricular nodal–dependent SVT episodes induced in an electrophysiology laboratory.10 The SVT conversion rates at 15 minutes (primary end point) were significantly higher with the 3 highest etripamil doses, including etripamil 70 mg, which demonstrated a conversion rate of 87%, compared with 35% with placebo.8 Etripamil was well tolerated, and the majority of adverse events (eg, nasal discomfort, nasal congestion, and oropharyngeal pain, each of which occurred in ≥10% of patients in the etripamil and placebo treatment groups) were related to nasal administration of the drug.10 Only the 140-mg and 105-mg doses, but not the 70-mg dose, transiently reduced blood pressure.10 Based on its efficacy and safety in phase 2, etripamil 70 mg was selected as the dose for the current study, which was conducted outside the healthcare environment in medically unsupervised settings.
The primary objective of this phase 3 study was to determine whether etripamil 70-mg nasal spray self-administered by patients is superior to placebo at terminating PSVT, measured as both the proportion of patients converted to sinus rhythm and as the time to sinus rhythm conversion, over a 5-hour observation period.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Overview
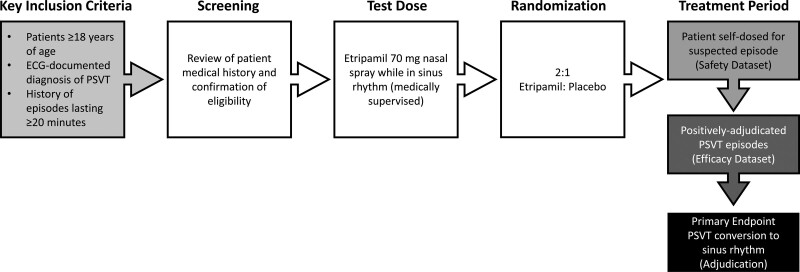
NODE-301 was a phase 3, randomized, double-blind, placebo-controlled, event-driven multicenter study conducted at 65 sites in the United States and Canada to evaluate the efficacy and safety of etripamil 70-mg nasal spray when self-administered for termination of spontaneous, symptomatic PSVT (Figure 1; please see the Supplemental Material S1 for a full list of study sites and principal investigators). The primary efficacy endpoint was adjudicated termination of positively adjudicated PSVT episodes and conversion to sinus rhythm for at least 30 seconds. The independent adjudication committee was blinded to study assignments. The primary efficacy variable was time to conversion of PSVT to sinus rhythm after study drug administration. The observation period for study endpoints was 5 hours.
Figure 1.
Study design. The treatment period occurred from randomization until the patient had paroxysmal supraventricular tachycardia (PSVT) treated with study drug.
Following screening, patients self-administered a 70-mg test dose of etripamil nasal spray (200 μL total; 100 μL in each nostril) in a seated position with the head upright, under medical supervision while in sinus rhythm to evaluate safety and tolerability. Vital signs (heart rate and blood pressure) and ECG monitoring were carried out within 10 minutes before the test dose and for 30 minutes following test dose. In addition to a standard ECG, a cardiac monitoring system (CMS; Preventice BodyGuardian Heart, Eagan, MN) was applied to the chest to record the entire test, and the central analysis report was immediately sent to the investigator. Failure of the test dose occurred if any of the following criteria were met: symptoms consistent with clinically severe hypotension (eg, presyncope, syncope, nausea, or vomiting), marked decrease in systolic blood pressure (SBP; for patients with a pre–test dose SBP >100 mm Hg, a decrease in SBP ≥40 mm Hg or post–test dose SBP <80 mm Hg; for patients with a pre–test dose SBP between 90 mm Hg and 100 mm Hg [inclusive], a post–test dose SBP <75 mm Hg), second- or third-degree atrioventricular block, new marked sinus bradycardia (heart rate ≤40 beats per minute or sinus pauses ≥3 seconds), ventricular arrhythmias, or atrial fibrillation or flutter.
Patients with test dose failure who had an identifiable and modifiable cause (eg, beta-blocker therapy with potential for withdrawal) were eligible to repeat the test dose within 14 days of the initial test dose once the modifiable cause was eliminated. Patients could be randomized if they passed the second test dose, and the cause of the initial test dose failure was eliminated for the duration of the study. Patients without an identifiable and modifiable cause for test dose failure, or who failed a repeat test dose, were not randomized. Patients who passed either test dose and did not present with any criteria (see Study Population section) were randomized to receive either double-blind etripamil or placebo in a 2:1 ratio by an investigator using interactive response technology. Randomized patients were given a study kit, which included the blinded study drug, a CMS kit, and a diary, and were trained on steps to undertake following suspected spontaneous PSVT. The CMS kit included the wireless CMS that is applied directly to the skin of the chest by the patient or caregiver via a medical-grade adhesive strip and a kit-specific, paired smartphone to transmit the data for adjudication review following an event. The placebo control patients received a formulation consisting of water, sodium acetate, disodium EDTA, and sulfuric acid that matched the pH of the etripamil formulation (pH of 4.0–4.8).
Study Population
Patients aged 18 years and older with ECG documentation of previous PSVT and a history of episodes typically lasting ≈20 minutes or longer were eligible to participate in the study. If the patient had previously undergone an ablation procedure for PSVT, documented ECG evidence of postablation PSVT was required. Important exclusion criteria included SBP <90 mm Hg after a 5-minute rest in a sitting position at the screening visit or before the test dose, previous allergic reaction to verapamil, severe ventricular arrhythmia (eg, torsades de pointes, ventricular fibrillation, or sustained ventricular tachycardia), ventricular preexcitation (eg, Wolff–Parkinson–White syndrome), second- or third-degree atrioventricular block, an atrial arrhythmia not dependent on the atrioventricular node for perpetuation (ie, atrial fibrillation, atrial tachycardia), history of severe hypotension or syncope during tachycardia, class I or III antiarrhythmic drug or digoxin use (unless stopped >5 half-lives before test dose randomization), current New York Heart Association class II–IV heart failure, a history of stroke within 6 months of screening, and hepatic or renal dysfunction.
This report focuses on the primary efficacy and safety end points of the NODE-301 study, which include the outcomes of the efficacy population (n=156; all randomized patients who used study drug at the time of positively adjudicated atrioventricular nodal–dependent PSVT), the safety population (n=198; all randomized patients who took study drug for a suspected PSVT episode), and the overall safety population, which includes all patients who took any study drug (n=431; 233 of whom only received the etripamil test dose and comprised the test dose–only population).
Adjudication Committee
An independent Adjudication Committee comprising at least 5 cardiac electrophysiologists blinded to study assignments determined whether recorded episodes were consistent with true atrioventricular nodal–dependent PSVT, the elapsed time between study drug administration and PSVT termination and whether PSVT was terminated following a vagal maneuver. The Adjudication Committee also confirmed that a positively adjudicated episode converted to sinus rhythm for at least 30 seconds following study drug administration.
Postrandomization Treatment
At the postrandomization study follow-up visit, patients were retrained on study procedures, including how to perform a vagal maneuver. Consistent with the real-world environment, the type of vagal maneuver was left to the discretion of the investigators. Only self-administered, patient-initiated vagal maneuvers were allowed. Patients randomized to study drug who experienced symptoms consistent with PSVT contacted a telephone coach to guide them through study procedures or proceeded with using the provided study guides (printed or electronic). Patients were instructed to apply the CMS and perform a vagal maneuver. If symptoms of PSVT were successfully relieved following the vagal maneuver, patients were instructed to refrain from self-administering study drug but to keep the CMS device on for 5 hours. Patients with PSVT that resolved with a vagal maneuver remained in the study until a subsequent episode occurred.
If symptoms persisted after a vagal maneuver, patients were instructed to press the CMS event marker button to mark the time of study drug administration on the recording, then immediately self-administer the randomly allocated study drug nasal spray (ie, 100 μL in each nostril via the Aptar Pharma Nasal Spray Bidose System, Crystal Lake, IL) in a seated position with the head upright and remain in that position for at least 10 minutes following study drug administration. If symptoms did not resolve within 20 minutes, patients could seek appropriate alternative medical care.
Statistical Methods
Sample Size
Assuming a type 1 error of alpha=0.01 and a 2:1 ratio in the number of perceived PSVT episodes adjudicated to be verified PSVT, etripamil: placebo, a minimum of 46 positive conversion events without censoring (ie, technical issue of recording, conversion not observed, or due to other medical intervention over the 5 hours) were required to attain at least 90% power on the primary efficacy variable of time to conversion using a 2-sided log-rank test. Because this was an event-driven trial and patients were randomized before they had a qualifying PSVT episode, it was unlikely that 2:1 etripamil: placebo verified PSVT episodes ratio would match the 2:1 randomization ratio. Anticipating such a case, it was preferable to target a higher sample size to cover the worst-case scenarios in terms of imbalance. A total of 150 PSVT episodes that are adjudicated to be verified PSVT would ensure at least 90% power for cases of marked imbalance in the etripamil: placebo PSVT episodes ratio. It was anticipated that 500 randomized patients might be required to accrue a sufficient number of events within 18 months of enrollment. The study continued to enroll patients until at least 150 patients experienced positively adjudicated PSVT.
Data Analyses
The primary efficacy endpoint was analyzed in the efficacy population. First, the proportional hazards assumption was tested. Because the proportional hazards assumption was not met, a Peto-Peto test was used, as specified, to calculate a P value. The hazard ratio and 95% CI were calculated using the Cox proportional hazards model.
The P values at each time point for the predefined sensitivity analysis of efficacy were obtained from χ2 tests using patients converted and patients not converted (censored and at risk) by each time point. The probability of conversion to sinus rhythm was calculated and reported as a Kaplan–Meier plot.
If the CMS recording accidentally stopped before conversion of PSVT, the episode was censored at the time of last recording for lack of data. If PSVT was terminated by a medical intervention (eg, intravenous or oral administration of medication to terminate PSVT, electrical cardioversion, physician-initiated vagal maneuver), the episode was censored at the time of PSVT termination following the intervention or at 5 hours if the PSVT did not terminate by the end of the 5-hour recording.
Safety data were summarized with descriptive statistics and classified using the Medical Dictionary Regulatory Activities version 21.0. Safety variables included clinical adverse events, vital signs, laboratory tests, arrhythmias, and conduction disorders detected on surface ECG or CMS recordings. An adverse event was defined as any untoward medical occurrence associated with the use of study drug, and included observed or volunteered problems, complaints, or symptoms that were recorded on an appropriate electronic case report form. An adverse event was considered serious if it resulted in death, was life-threatening, or required hospitalization or prolonged an existing hospitalization. Treatment-emergent adverse events were defined as those occurring within 24 hours after receiving the test dose or within 24 hours after receiving a randomized study drug dose.
The exploratory objectives of the study were to evaluate (1) the safety, hemodynamic (ie, blood pressure), and cardiac conduction effects of a test dose of etripamil 70 mg; and (2) the safety and efficacy of etripamil 70 mg in subgroups of interest (eg, concomitant medications). Data for exploratory efficacy endpoints were summarized using descriptive statistics.
Ethics
This clinical study complied with the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice Guidelines, and applicable local regulatory requirements. Institutional review board approval was obtained at each participating center, and all patients underwent an informed consent process that included signing an informed consent form before any study-specific procedures were conducted.
Results
Disposition
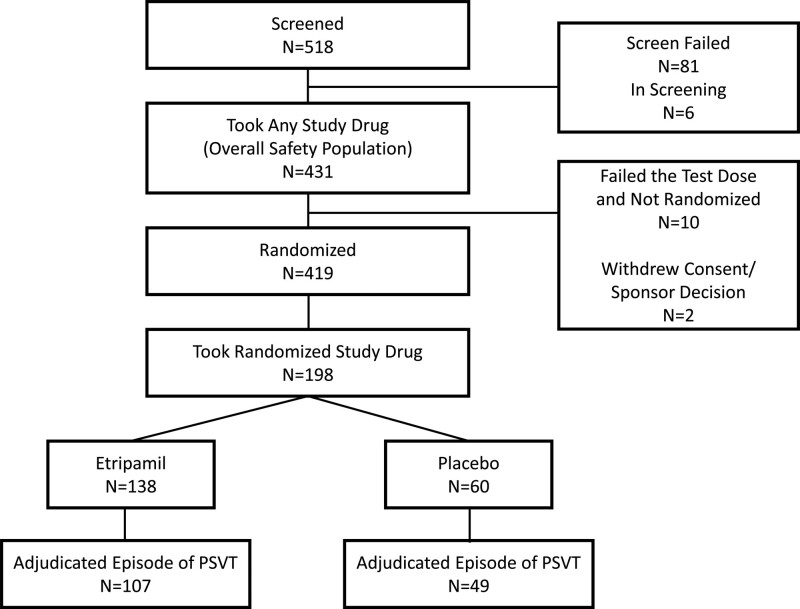
Of the 431 patients who received the etripamil test dose and who comprised the overall safety population, 419 (97%) were randomized to etripamil or placebo after passing on either their first or second test dose (total includes 1 patient who failed test dose but was randomized). A total of 10 patients (2.3%) had test dose failure and were not randomized (refer to the Safety section for reasons for failure), and 2 patients withdrew consent after the test dose but before randomization. A total of 3 patients in the etripamil group had CMS failure before 30 minutes and were censored at the time of CMS failure.
During the treatment period, 198 patients developed symptoms perceived by the patient to be due to PSVT that did not terminate with a vagal maneuver, and they self-administered study drug. Of the 198 patients, 156 (79%) had an episode of atrioventricular nodal–dependent PSVT that was positively adjudicated by the adjudication committee, of whom 107 received etripamil and 49 placebo, and who comprised the efficacy population (Figure 2). A total of 233 patients received the etripamil test dose only and did not self-administer study drug during an episode of perceived PSVT following randomization (test dose–only population). The overall safety population was composed of the 198 patients who passed the etripamil test dose in sinus rhythm and also administered study drug during perceived PSVT, along with the 233 patients who only administered the etripamil test dose while in sinus rhythm.
Figure 2.
Patient disposition−all screened patients. PSVT indicates paroxysmal supraventricular tachycardia.
Patient Characteristics
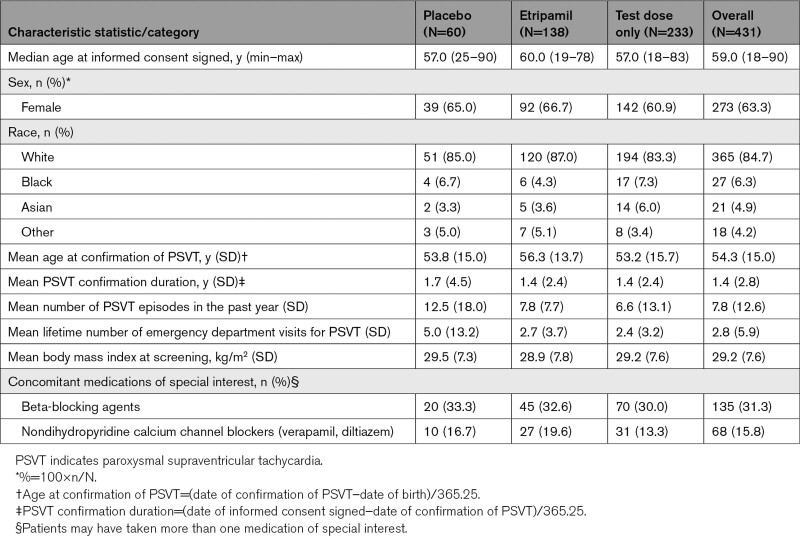
Overall, the randomized etripamil and placebo groups were balanced with respect to baseline characteristics (Table 1). Median age was 57 years and 60 years in the placebo and etripamil groups, respectively (minimum–maximum, 19–90 years), and almost two-thirds of patients were women. Although patients in the placebo group had a higher mean frequency of PSVT in the past year (mean±SD; placebo, 12.5±17.9 versus etripamil, 7.8±7.7) and a higher mean number of emergency department visits over their lifetime (placebo, 5.0±13.2 versus etripamil, 2.7±3.7), the median values for these 2 parameters were similar or identical between groups (6.0 versus 5.0 for PSVT in the past year and 2.0 versus 2.0 for lifetime emergency department visits in the placebo and etripamil groups, respectively). Use of beta-blockers and nondihydropyridine calcium channel blockers was similar in the placebo and etripamil groups, with beta-blockers used in 33.3% and 32.6% of patients, and calcium channel blockers used in 16.7% and 19.6% of patients, respectively.
Table 1.
Demographic and Patient Characteristics−Overall Safety Population
Outcomes
Efficacy
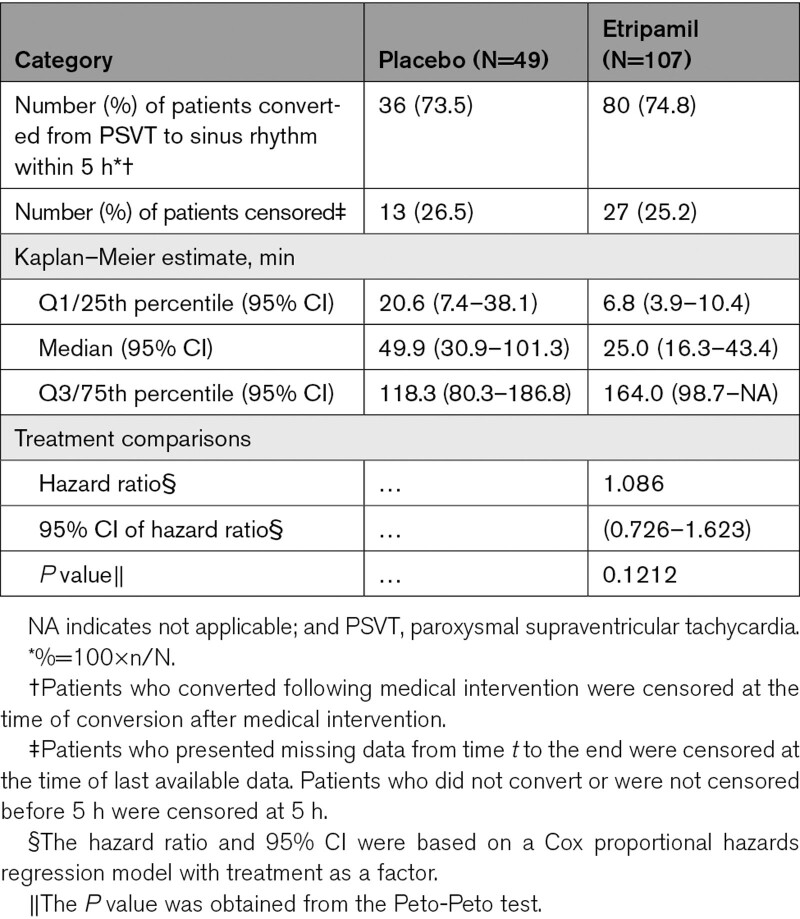
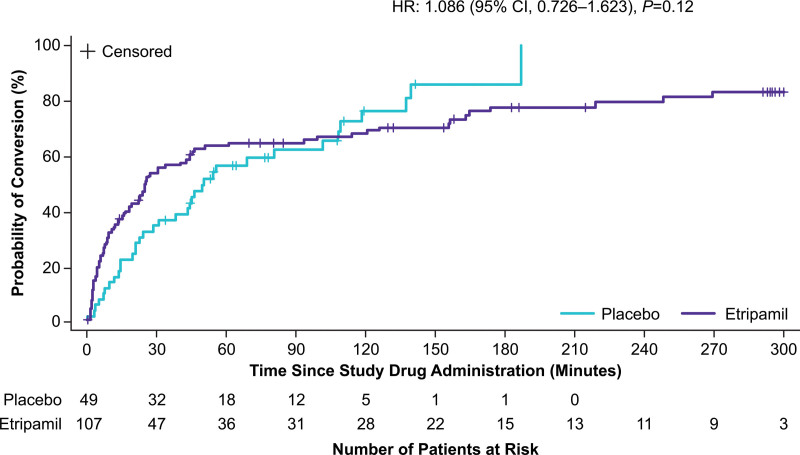
The hazard ratio of time to conversion over the 5-hour monitoring period was 1.086 (95% CI, 0.726–1.623; P=0.1212; Peto-Peto analysis; Table 2). A Kaplan–Meier plot of cumulative conversions over time is presented in Figure 3. Patients who took etripamil had a numerically shorter estimated median time to conversion than those who took placebo (25 versus 50 minutes), with a shorter first quartile conversion time (6.8 versus 20.6 minutes).
Table 2.
Analysis of Primary End Point in the Efficacy Population: Conversion of Adjudicated PSVT to Sinus Rhythm
Figure 3.
Kaplan–Meier plot of conversion up to 5 hours. HR indicates hazard ratio.
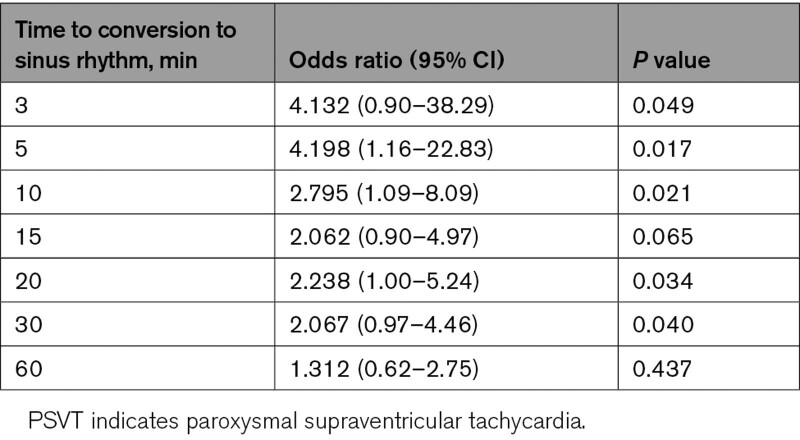
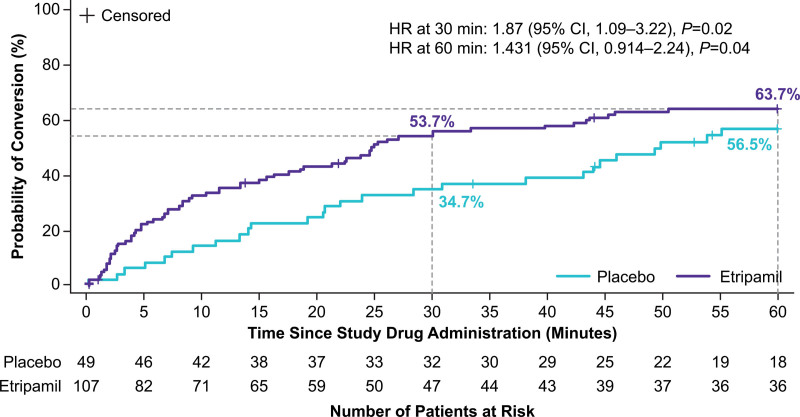
Although the study was not powered for specific time point analyses, in a predefined sensitivity analysis, etripamil treatment effects (compared with placebo) were observed at 3, 5, 10, 20, and 30 minutes (Table 3). Odds ratio was 4.132 (95% CI, 0.90–38.29; P=0.049) at 3 minutes and 2.067 (95% CI, 0.97–4.46; P=0.04) at 30 minutes. The etripamil treatment group had a maximum odds ratio of 4.198 (95% CI, 1.16–22.83; P=0.017) at 5 minutes versus placebo. Following study drug administration, the probability of conversion within the first 30 minutes was 54% for etripamil versus 35% for placebo (Figure 4). A total of 9 patients (8.4%) in the etripamil group and none in the placebo group were censored at 5 hours due to persistence of PSVT throughout the entire recording period.
Table 3.
Odds Ratio and P Value of Conversion of PSVT to Sinus Rhythm at Predefined Time Points (Efficacy Population)
Figure 4.
Kaplan–Meier plot of conversion up to 60 minutes. HR indicates hazard ratio.
Subgroup analyses showed no difference in treatment effect in any subgroup category, including age, sex, weight, or body mass index; concomitant treatment with beta-blockers or calcium channel blockers; or preexisting first-degree atrioventricular block. The heart rate during SVT before study drug administration was similar in the etripamil and placebo groups (respectively, mean±SD: 183±29 and 178±28 beats per minute; median, range, 183, 106–250 and 175, 125–250). For patients who converted at 5 minutes, the mean±SD heart rate during PSVT before study drug administration was lower than for those who were nonconverters at 5 minutes (167.2±26.2 versus 182.3±28.0 beats per minute, respectively; P=0.032).
Safety
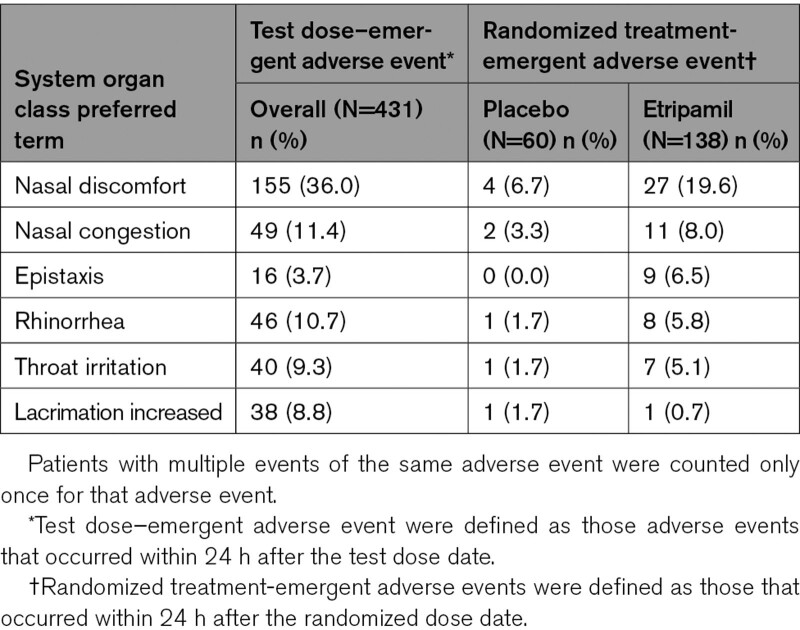
Overall, etripamil was well tolerated when self-administered as a test dose during sinus rhythm or as a postrandomization dose during symptomatic PSVT. The most common (≥5%) treatment-emergent adverse events (those occurring within 24 hours of a test dose or those occurring more frequently with etripamil within 24 hours of a randomized dose) were nasal discomfort (test dose, 36.0%; randomized dose, 19.6%), nasal congestion (test dose, 11.4%, randomized dose, 8.0%), epistaxis (test dose, 3.7%; randomized dose, 6.5%), rhinorrhea (test dose, 10.7%; randomized dose, 5.8%), throat irritation (test dose, 9.3%; randomized dose, 5.1%), and increased lacrimation (test dose, 8.8%; randomized dose, 0.1%), all of which were related to the nasal route of administration (Table 4). The incidence of all other adverse events, including those related to abnormal vital signs, laboratory results, and ECG findings, was balanced between the 2 groups. No serious adverse events were observed within 24 hours of taking study drug.
Table 4.
Test Dose or Treatment-Emergent Adverse Events Occurring in ≥5% of Patients Overall or in the Etripamil Group
CMS monitoring data showed new first-degree atrioventricular block lasting >30 seconds in 8 (5.8%) patients in the etripamil 70-mg group (median duration, 137 minutes; range, 0.7–359), 1 (1.7%) patient in the placebo group (duration, 30 minutes) in the overall safety population, and no (0.0%) patients in the test dose–only population. Two etripamil-treated patients receiving concomitant nondihydropyridine calcium channel blockers had first-degree atrioventricular block lasting >30 seconds, and 4 patients in the etripamil group and 1 patient in the placebo group receiving concomitant beta-blockers had first-degree atrioventricular block lasting >30 seconds. There were no episodes of second- or third-degree atrioventricular block. No cases of preexcitation were revealed following etripamil dosing. A total of 15 of 138 (11.2%) patients in the etripamil group and 7 of 60 (11.7%) patients receiving placebo developed nonsustained ventricular tachycardia (defined as ≥3 consecutive ventricular beats at a rate of ≥100 beats per minute with a duration of <30 seconds) that did not require medical treatment; no patient in either group experienced sustained ventricular tachycardia. PSVT recurred in 3 of 138 (2.2%) patients in the etripamil group and 4 of 60 (6.7%) patients in the placebo group during the post-administration monitoring period following initial conversion to sinus rhythm.
There were no reported episodes of syncope in patients randomized and treated with study drug nasal spray during PSVT or the 5-hour observation period in either group. One patient who experienced a transient episode of syncope that occurred 168 days after etripamil test dose administration was withdrawn from the study, and the event was considered not related to study drug.
The reasons for test dose failure in 10 (2.3%) patients who were not randomized were premature ventricular contractions (3 patients, 2 with previously documented premature ventricular contractions), hypotension (2 patients), adverse events of burning nose (1 patient) and lacrimation/sneezing (1 patient), dizziness (1 patient), PSVT occurring immediately before test dose administration (1 patient), and other (1 patient; following successful test dose administration, the site determined that this patient did not meet study eligibility criteria but documented this as a test dose failure rather than a screen failure). One additional patient with a documented history of premature ventricular contractions had test dose failure due to premature ventricular contractions but was randomized into the study. No patients developed clinically significant sinus bradycardia or second- or third-degree atrioventricular block during the test dose.
Discussion
The NODE-301 study demonstrated the safety of etripamil 70-mg nasal spray when self-administered by patients in a medically unsupervised setting and monitored for 5 hours with a CMS during symptomatic PSVT and after conversion. No severe adverse events related to study drug were reported. The NODE-301 study did not meet the prespecified primary efficacy endpoint during the 5-hour observation period after study drug administration. It is possible that the study design, which incorporated a prolonged assessment time of 5 hours after etripamil or placebo administration, contributed to this outcome. When designing the study, the 5-hour observation period was selected based primarily on an intention to fully capture any potential safety events during this first study of etripamil conducted outside a clinical setting, monitored with a CMS, and without any direct patient observation during PSVT. A primary effectiveness time point of 30 minutes, more commensurate with the pharmacokinetic properties of etripamil, may have been a more appropriate time to accurately assess the efficacy of etripamil. Indeed, PSVT is usually limited in time; hence, the Kaplan–Meier curves were expected to converge with the extended monitoring duration.11 Additionally, given the short duration of action of etripamil, conversion to sinus rhythm would be expected to occur during the period of direct pharmacologic effect, that is, within the first 30 to 40 minutes. Etripamil treatment effect was observed as early as 3 minutes, and over the first 30 minutes after study drug administration the Kaplan–Meier probability of conversion was 54% with etripamil compared with 35% with placebo (P=0.04). This early treatment effect is consistent with the pharmacokinetic properties of etripamil.
Although the earlier studies of etripamil were limited to assessing the effects of etripamil on PSVT conversion in a medically supervised environment, the primary endpoint in this study of nasal drug self-treatment of an arrhythmia outside a healthcare setting was influenced by patient behaviors, such as whether patients sought other medical intervention, which represents an important potential confounder in the study beyond the pharmacologic effects of etripamil. Patients with additional medical interventions unrelated to study drug treatment were censored at the time of conversion, which in some cases occurred temporally near the crossover of the Kaplan–Meier curves. Patients in the placebo arm were generally censored earlier because of medical interventions; all 13 (26.5%) censored patients in the placebo arm were censored up to 141 minutes of monitoring, whereas 13 (12.1%) patients receiving etripamil (ie, less than half the rate compared with placebo) were censored before that time point and 14 (13.1%) beyond that point.
The Kaplan–Meier curves separated during the first 60 minutes before converging. The effect of evaluating the primary efficacy endpoint of time to conversion over the full 5-hour monitoring period in the etripamil group may have been further confounded by the study design, which used a 2:1 ratio with a smaller placebo group.
The sample size calculations for NODE-301 were based on the phase 2 NODE-1 study, which demonstrated a 52% absolute higher conversion rate (87% versus 35%) with etripamil 70 mg than with placebo at 15 minutes after dosing to treat induced SVT.12 Reasons for the reduced efficacy of etripamil in this study may include the medically unsupervised environment and the potential for alterations in autonomic tone with the seated patient position in NODE-301 versus the supine position in NODE-1. The upright position was chosen to ensure optimal delivery of the nasal spray while also minimizing the potential for local adverse events due to accidental oral ingestion of the study drug.10 Heightened adrenergic response in alert patients who experienced PSVT in NODE-301 compared with patients who received intravenous sedation in NODE-1 may also be a contributing factor. Additionally, there may have been differing responsiveness to pharmacologic conversion in spontaneously occurring PSVT that is nonresponsive to vagal maneuvers compared with SVT induced and sustained for only 5 minutes in the electrophysiology laboratory. Patients enrolled in the current study had a long history of PSVT with a high burden and frequency of episodes that might have been more recalcitrant to conversion. Accordingly, these assumptions may have resulted in a significant underestimation of the sample size required to demonstrate statistical significance over a 5-hour time period.
Clinical Significance and Implications
This is the first study to show the potential of a self-administered intranasal treatment for spontaneous symptomatic PSVT in a medically unsupervised setting. The current study’s results along with prior studies of etripamil nasal spray support the potential for its use to allow patients to rapidly treat their PSVT outside a healthcare setting, possibly precluding the need for emergency intervention. Further investigation is required to support this indication.
Limitations
Although the range of patient ages in the study was broad (18−90 years), the median and mean ages of the patient population were >55 years, likely reflecting the known difficulties of recruiting the 18- to 30-year age group.12 Future studies are required to define efficacy, safety, and optimal etripamil dosing in pediatric and lower-body-weight patients with PSVT. The lack of a prespecified time when study drug must be taken after PSVT initiation could influence the conversion rate and the time to conversion in each group; however, this design is more reflective of how the drug will be used by patients on their own in a real-world setting. The decision to include only a single dose, rather than several doses or sequential doses of etripamil 70 mg, may have limited safety and efficacy data that could ultimately factor into determining the optimal etripamil dose for unsupervised use. The Adjudication Committee was limited to CMS single-channel recordings of PSVT, and their mechanisms (eg, atrioventricular reentrant tachycardia or atrioventricular nodal reentrant tachycardia) were not definitively identified via an electrophysiology study.
Future Directions
There is an unmet need for an acute, rapid treatment for PSVT that allows patients to safely self-treat their PSVT to reduce symptom burden and reduce urgent care encounters. Etripamil appears to be a promising self-administered treatment for patients with PSVT. The planned “RAPID” study (part 2 of the NODE-301 study) will evaluate the efficacy of etripamil at early time points (≤30 minutes) and will include assessment of both efficacy and safety of a different dosing regimen of etripamil nasal spray. Specifically, patients will have the option of administering a second 70-mg dose of etripamil if symptoms persist for 10 minutes after the first dose.
Conclusions
In the current study, self-administration of etripamil 70-mg nasal spray in adults during symptomatic‚ sustained PSVT was well tolerated when administered outside the healthcare environment in a medically unsupervised setting. Despite not meeting its primary efficacy endpoint, there is evidence of an early treatment effect that persisted through 30 to approximately 60 minutes compared with placebo. For example, at 30 minutes, there was a 53.7% of SVT conversion in the treatment arm compared to 34.7% in the placebo arm (hazard ratio, 1.87 [95% CI, 1.09–3.22; P=0.02]). These findings support the ongoing clinical development of etripamil nasal spray for patient-actuated, on-demand, acute treatment of PSVT outside a healthcare environment.
Article Information
Acknowledgments
Milestone would like to acknowledge the contributions of the Adjudication Committee members (José Dizon, MD, Chair; Angelo Biviano, MD; Ioanna Kosmidou, MD, PhD; John Morrow, MD [Columbia University Medical Center, New York, NY]; James Peacock, MD [White Plains Hospital, White Plains, NY]); the Data Safety Monitoring Committee (Daniel Beyerbach, MD, Chair [The Christ Hospital, Cincinnati, OH]; and Hussein-Al-Khalidi, PhD, and Sean Pokorney, MD [Duke University, Durham, NC]) to this article. The corresponding author, Francis Plat, MD, had full access to all the data in the study and takes responsibility for data integrity and data analysis.
Sources of Funding
This study was funded by Milestone Pharmaceuticals. Medical writing assistance was provided by Mayur Kapadia, MD, of Cadent Medical Communications, LLC, a Syneos Health group company, and was supported by Milestone Pharmaceuticals.
Disclosures
Drs Camm, Stambler, Potvin, Mondesert, Sager, and Haberman are consultants for Milestone Pharmaceuticals, Inc. Dr Sager has equity in Milestone Pharmaceuticals. Francis Plat‚ Silvia Shardonofsky‚ and Douglas Wight are or were employees of Milestone Pharmaceuticals at the time of this study. The other authors report no conflicts.
Supplemental Material
Supplemental Methods
S1 List of Study Sites and Primary Investigators
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CMS
- cardiac monitoring system
- PSVT
- paroxysmal supraventricular tachycardia
- SBP
- systolic blood pressure
- SVT
- supraventricular tachycardia
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCEP.122.010915.
For Sources of Funding and Disclosures, see page 822.
Contributor Information
Bruce S. Stambler, Email: bss4@cwru.edu.
Philip T. Sager, Email: psager@sagerconsulting.com.
Silvia Shardonofsky, Email: sshardonofsky@milestonepharma.com.
Douglas Wight, Email: dwight@milestonepharma.com.
Diane Potvin, Email: dpotvin@Excelsus-stat.com.
A. Shekhar Pandey, Email: pandey@cambridgecardiaccare.com.
James E. Ip, Email: jei9008@med.cornell.edu.
Benoit Coutu, Email: benoit.coutu@umontreal.ca.
Blandine Mondésert, Email: blandine.mondesert@icm-mhi.org.
Laurence D. Sterns, Email: ldsterns@gmail.com.
Matthew Bennett, Email: Matthew.Bennett@vch.ca.
Jeffrey L. Anderson, Email: jeffreyl.anderson@imail.org.
Roger Damle, Email: RogerD@southdenver.com.
Ronald Haberman, Email: rhaberman@milestonepharma.com.
A. John Camm, Email: jcamm@sgul.ac.uk.
References
- 1.Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomström-Lundqvist C, Calkins H, Corrado D, Deftereos SG, Diller G-P, et al. ; ESC Scientific Document Group. 2019 ESC guidelines for the management of patients with supraventricular tachycardia. The Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC) [published correction appears in Eur Heart J. 2020;41:4258]. Eur Heart J. 2020;41:655–720. doi: 10.1093/eurheartj/ehz467 [DOI] [PubMed] [Google Scholar]
- 2.Page RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, Deal BJ, Estes MI, III, Field ME, Goldberger ZD, Hammill SC, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society [published correction appears in Circulation. 2016;134:e232–233]. Circulation. 2016;133:e471–e505. doi: 10.1161/cir.0000000000000310 [DOI] [PubMed] [Google Scholar]
- 3.Issa ZF, Miller JM, Zipes DP. Clinical Arrhythmology and Electrophysiology: A Companion to Braunwald’s Heart Disease. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2012. [Google Scholar]
- 4.Ferguson JD, DiMarco JP. Contemporary management of paroxysmal supraventricular tachycardia. Circulation. 2003;107:1096–1099. doi: 10.1161/01.cir.0000059743.36226.e8 [DOI] [PubMed] [Google Scholar]
- 5.Hamer A, Peter T, Mandel W. Atrioventricular node reentry: intravenous verapamil as a method of defining multiple electrophysiologic types. Am Heart J. 1983;105:629–642. doi: 10.1016/0002-8703(83)90488-x [DOI] [PubMed] [Google Scholar]
- 6.Hamer A, Peter T, Platt M, Mandel W. Effects of verapamil on supraventricular tachycardia in patients with overt and concealed Wolff-Parkinson-White syndrome. Am Heart J. 1981;101:600–612. doi: 10.1016/0002-8703(81)90227-1 [DOI] [PubMed] [Google Scholar]
- 7.Alboni P, Tomasi C, Menozzi C, Bottoni N, Paparella N, Fucà G, Brignole M, Cappato R. Efficacy and safety of out-of-hospital self-administered single-dose oral drug treatment in the management of infrequent, well-tolerated paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 2001;37:548–553. doi: 10.1016/s0735-1097(00)01128-1 [DOI] [PubMed] [Google Scholar]
- 8.Yeh SJ, Lin FC, Chou YY, Hung JS, Wu D. Termination of paroxysmal supraventricular tachycardia with a single oral dose of diltiazem and propranolol. Circulation. 1985;71:104–109. doi: 10.1161/01.cir.71.1.104 [DOI] [PubMed] [Google Scholar]
- 9.Plat F, Broughton A, Douville P, Sager PT, Soh B, Wight D. Abstract 19713: The electrocardiographic effects of intranasal formulations of a new calcium channel blocker, MSP-2017, are consistent with a potential treatment of paroxysmal supraventricular tachycardia: results of a phase 1 dose escalation study. Circulation. 2015;132:A19713. doi: 10.1161/circ.132.suppl_3.19713 [DOI] [Google Scholar]
- 10.Stambler BS, Dorian P, Sager PT, Wight D, Douville P, Potvin D, Shamszad P, Haberman RJ, Kuk RS, Lakkireddy DR, et al. Etripamil nasal spray for rapid conversion of supraventricular tachycardia to sinus rhythm. J Am Coll Cardiol. 2018;72:489–497. doi: 10.1016/j.jacc.2018.04.082 [DOI] [PubMed] [Google Scholar]
- 11.Medi C, Kalman JM, Freedman SB. Supraventricular tachycardia. Med J Aust. 2009;190:255–260. doi: 10.5694/j.1326-5377.2009.tb02388.x [DOI] [PubMed] [Google Scholar]
- 12.Hays L, McSweeney J, Mitchell A, Bricker C, Green A, Reid D, Landes RD. Recruitment issues in emerging adult populations: focus on adult congenital heart disease. Nurs Rep. 2020;10:135–145. doi: 10.3390/nursrep10020017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.