Supplemental Digital Content is Available in the Text.
BACKGROUND
Aging is a multifactorial response to genetic preprogramming nuances, sun exposure, and ultraviolet radiation. Recently, there has been a paradigm shift toward minimally invasive rejuvenation.
OBJECTIVE
This prospective multicenter study aims to evaluate the efficacy and safety of a novel hands-free bipolar bulk radiofrequency (RF) device in terms of improvement in skin appearance.
PATIENTS AND METHODS
This multicenter prospective study enrolled subjects aged 35 to 75 years with visible signs of aging. The primary objective was to evaluate skin appearance pretreatment and at 1, 3, and 6 months after the final treatment. Each patient received 3 total treatments to the chin and cheeks using the hands-free RF device spaced 2 weeks apart.
RESULTS
In total, data from 87 patients were assessed from 6 treatment sites. The average age was 54 years (range 35–75 years). Most patients were female (97%), and Fitzpatrick skin types I to V were represented. Overall, patients found the procedures to be relatively pain-free, and both patients and investigators felt they noted some improvement in their skin appearance. Histological sections demonstrated an increase in collagen or elastic fibers within the papillary dermis.
CONCLUSION
This study supports the use of this novel noninvasive hands-free bipolar facial remodeling device for the improvement of skin appearance.
The aging face is characterized by thinning dermis, extracellular matrix atrophy, bone loss, and decreased collagen synthesis.1 This process is multifactorial; in addition to genetic nuances, sun exposure and ultraviolet radiation play a major role in not only causing but also accelerating the undesirable skin changes of the aging process. These changes include fine and coarse wrinkles, roughness, laxity, dyspigmentation, lentigines, actinic keratosis, xerosis, textural changes, and telangiectasia.2 In the recent past, there has been a paradigm shift toward minimally invasive rejuvenation techniques to address these changes. Specifically, patients seek to achieve skin tightening with no or minimal downtime. As a result, nonsurgical skin tightening treatments have grown rapidly over the last 10 years. In particular, radiofrequency (RF) devices have been shown to achieve modest clinical efficacy in skin tightening with limited or no downtime or excessive pain and have therefore become the main treatment choice for noninvasive skin tightening among dermatologists, plastic surgeons, and aesthetic physicians.3–7
Most commercially available RF devices are administrated by an operator, whose required credentials vary by state. Regardless of the operator, the treatments are time-consuming, can be monotonous, leading to operator fatigue, reducing the effectiveness of the treatment. To circumvent this issue, investigation has turned toward the safety and efficacy of a noninvasive hands-free bipolar facial remodeling device for the improvement of skin appearance.8 The novel hands-free RF platform discussed herein is designed such that the cheek and chin applicators are secured comfortably on the patient's face, enabling an automatic, hands-free, treatment that is based on a predefined protocol. This protocol incorporates a skin temperature control to ensure a consistent, accurate, and safe treatment.8 In addition, the system can be individually adjusted to achieve maximum safety, efficacy, and comfort to each patient. This prospective multicenter study aims to evaluate the efficacy and safety of this novel hands-free RF device in terms of improvement in skin appearance.
Methods
The data from this multicenter prospective study were obtained from the combined data from 2 separate Institutional Review Board (IRB)-approved studies and complied with the Declaration of Helsinki (IRB-8470 Sterling IRB; ClinicalTrials.gov: NCT05398159 and IRB-8267 Sterling IRB; ClinicalTrials.gov: NCT04721600). Of note, this study was originally designated to be a single-site trial but was upgraded to a multicenter trial due to slow subject recruitment during the coronavirus 2019 pandemic, thus the need for 2 separate IRB protocols.
Subjects were healthy adults between the ages of 35 and 75 years with visible signs of facial aging, seeking skin tightening treatments with the aim to evaluate a novel noninvasive hands-free bipolar facial remodeling device (InMode, Lake Forest, CA) for the improvement of skin appearance. Six centers conducted this study including: Laser & Skin Surgery Center of Northern California (Sacramento, CA; primary investigator [PI]: S.K.), AboutSkin Dermatology and DermSurgery, PC (Greenwood Village, CO; PI: J.C.), Refresh Dermatology (Houston, TX; PI: S.C.), Lupo Center for Aesthetic and General Dermatology (New Orleans, LA; PI: M.L.), Dallas Plastic Surgery Institute (Dallas, TX; PI: R.R.), and SkinCare Physicians (Chestnut Hill, MA; PI: J.D.) with the goal of enrolling at least 15 subjects per site. Exclusion criteria included any type of electrical implant (e.g., pacemaker, defibrillator, and brain stimulator) or any permanent implant near the treatment area (cochlear implant), a current or history of skin cancer, immune compromise, history of wound healing skin disorders, any facial skin treatment within 6 months (e.g., filler, microneedling, or laser), or use of isotretinoin.
The primary objective of this study was to evaluate overall skin appearance pretreatment and at 3 and 6 months after the final treatment. This was assessed using blinded evaluators, which included cosmetic dermatologists and plastic surgeons not familiar with the device or study. Three blinded evaluators per site were trained and judged to be competent before being able to undertake assessment of images for the trial. They received instructions to evaluate overall changes/improvement in skin appearance, and carried out their assessments separately, without knowledge of treatment allocation. Each evaluator received 2 files: Powerpoint presentation with before (B) and after (A) images (marked with either letter A or B) randomized in a different order and an excel file with subjects' codes to document their assessment. The aim was to identify the before image (A or B). It was hypothesized that 2 of the 3 blind evaluators would agree on overall improvement in skin appearance by picking the correct before images based on photographs for at least 70% of patients. The secondary objectives included investigator assessment of the skin appearance comparing pretreatment and posttreatments at 1, 3, and 6 months, as well as subject assessment of improvement and satisfaction. Finally, biopsies from the treated areas of the first 4 consented subjects were collected at baseline and at 3-month follow-up and stained for hematoxylin and eosin (H&E), elastin, and Masson trichrome to assess for changes consistent with collagen remodeling.
Treatment Details
After informed consent was obtained, the skin was thoroughly cleansed. The patient was seated in an upright sitting position and clear ultrasound gel was applied to the treatment area (2–4 mm thick). The hands-free RF device was applied and secured in place to the treatment areas on the chin and cheeks, respectively (Figure 1). The hands-free RF system used in this study consists of an alternating current/direct current power supply unit, 2 RF generators, a controller, and a user interface including an liquid crystal display touch screen. The noninvasive applicators are connected to the console via a cable, and the delivery of the RF Energy is controlled by a Start/Stop button on the liquid crystal display screen. Importantly, the system also incorporates a treatment deactivation button. Pressing the deactivation button by either the patient or the caregiver would immediately halt the treatment and switch the device into a Pause mode until the operator re-enables it.
Figure 1.
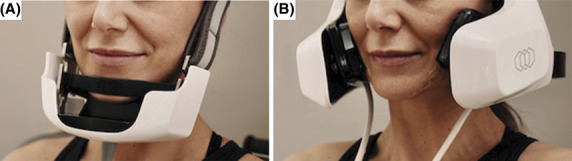
Representative patient wearing the hands-free RF device with the chin (A) and cheek (B) applicators in place. RF, radiofrequency.
The patient was told to keep their head in the specific position in which the device was set up (and not tilt down to view a phone or book, for example). The patient was provided the call button to alert the staff if any immediate attention was required for any reason. The energy level was titrated according to the patient's comfort level. No anesthesia of any kind was used, including topical anesthetic, and no pain medications were provided or taken. If the patient complained of pain or discomfort, the RF energy level was decreased. All patients were treated in both the cheek and the chin areas, and on average, each combined treatment lasted approximately 75 minutes. Each patient received 3 total treatments spaced 2 weeks apart and then presented for follow-up appointments at 1, 3, and 6 months after the final treatment.
Photography
Standard photographic images were obtained at each follow-up and treatment session. Five of the 6 sites used Canfield technology and 1 site used Quantificare.
Statistics
Investigator and subject assessments were obtained using a 0- to 4-point Likert scale at all treatment and follow-up visits. Investigators used a scale anchored by 0 (no difference) and 4 (significantly marked improvement). Scores were compared with the hypothesized lowest value (0, no difference) using 1-sample Wilcoxon signed rank tests. Adverse events (AEs) and treatment discomfort were closely monitored after each treatment.
Treatment discomfort metrics were measured on an 11-point scale, where 0 = most comfortable and 10 = most uncomfortable. Subjects were asked to assess their pain for both the cheek and the chin areas. The midpoint of 5 (moderate pain) was selected as the hypothesized value by which to compare the participants' mean on the pain scale.
Finally, patients assessed their satisfaction with the procedure at 1, 3, and 6 months posttreatment and used a scale anchored by −2 (very disappointed) and 2 (very satisfied). Scores were compared with the hypothesized middle value (0, indifferent) using 1-sample t-tests. All outcome data were measured at 1 or 3 time points; for outcomes with only 1 time point, 1-sample t-tests or Wilcoxon signed rank tests were used against a hypothesized mean or median (the midpoint or anchor of the scale) to determine if the mean values were considered improved. For outcomes with 3 measurements, repeated-measure analysis of variances (ANOVAs) were used.
Results
In all, 87 patients completed the study through to the 6-month follow-up, though at various points; some of those 87 patients had missing photographs due to technology malfunction. Thus, for the primary outcome, only subjects who had before and after photographs at all time points were used for analysis (n = 64). For secondary evaluations, data from all 87 patients were used for analysis. The average age of the patients was 54 years (range 35–75 years), most patients were female (97%), and Fitzpatrick skin types I to V were represented. Subject demographics are outlined in Table 1.
TABLE 1.
Subject Demographics
| Age (yrs) | Average: 54 | Range: 35–75 | |
| Gender | |||
| Female | 84 | ||
| Male | 3 | ||
| Fitzpatrick skin type | Average: III | Range: I–V |
For the primary outcome blinded evaluations at 3 months, the evaluators correctly classified 85.9% of patients. A 1-tailed, 1-sample z-test, to compare the observed proportion (85.9%) against a hypothesized proportion (70%), was statistically significant (z = 2.78, p < .01). After treatment, the blinded evaluators determined that the treatment was significantly more effective than the 70% benchmark, indicating a greater success above the baseline threshold.
For the primary outcome blinded evaluations at 6 months, the evaluators correctly classified 80.3% of patients. Similar to the 3-month data, this observed proportion (80.3%) was statistically significant compared with the 70% hypothesis (z = 1.83, p = .03). Figure 2 displays before and after photographs of a representative subject demonstrating modest skin tightening after 3 RF treatments spaced 2 weeks apart.
Figure 2.
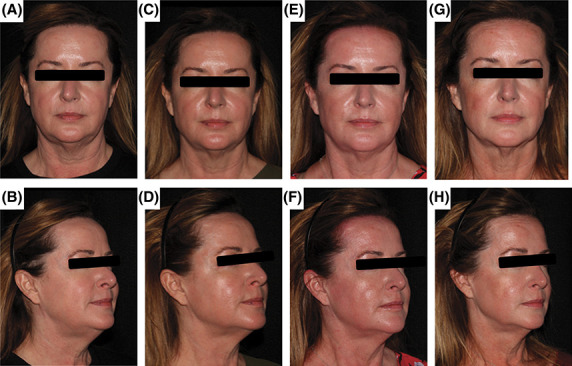
Before and after photographs of a representative subject demonstrating modest skin tightening after 3 RF treatments spaced 2 weeks apart. Photographs at baseline (A and B), 1-month follow-up (C and D), 3-month follow-up (E and F), and 6-month follow-up (G and H) are shown. RF, radiofrequency.
Pain Scores
The average cheek pain value for each treatment (Treatment 1: mean = 1.64, Treatment 2: mean = 1.42, Treatment 3: mean = 0.97) was significantly lower than 5, indicating participants believed the cheek pain caused by each treatment was significantly lower than moderate pain (Treatment 1: t[94] = −17.10, p < .001; Treatment 2: t[94] = −19.07, p < .001; Treatment 3: t[89] = −28.41, p < .001). In addition, subject cheek pain evaluation significantly differed across the range of treatments, with Treatment 3 being considered less painful than Treatment 1, as demonstrated by the within-subjects ANOVA test pairwise comparison results (F [2, 178] = 6.27, p = .01).
The average chin pain value for each treatment (Treatment 1: mean = 1.26, Treatment 2: mean = 1.07, Treatment 3: mean = 0.70) was also significantly lower than 5, indicating participants believed the chin pain caused by each treatment was significantly lower than moderate pain (Treatment 1: t[93] = −21.56, p < .001; Treatment 2: t[93] = −22.85, p < .001; Treatment 3: t[88] = −30.27, p < .001). Similar to the cheek, chin pain evaluation significantly differed across the range of treatments, with Treatment 3 being considered less painful than the first 2 treatments, as demonstrated by the within-subject ANOVA (F [2, 176] = 4.95, p = .03). Supplemental Digital Content, Figure S1, http://links.lww.com/DSS/B183 displays the mean values of both pain scales at each test administration.
Investigator Assessment
Investigators assessed the level of the patients' improvement at 1, 3, and 6 months posttreatment. The median values at all 3 administrations were significantly higher than 0 (1 month: median = 1, standardized test statistic = 6.59, p < .001; 3 months: median = 1, standardized test statistic = 7.30, p < .001; 6 months: median = 2, standardized test statistic = 7.39, p < .001). In other words, investigators believed that the improvement of the patients at 1, 3, and 6 months were significantly higher than “no difference.” In addition, at 1 month, investigators believed 77% of patients had any improvement; at 3 months, investigators believed 75% of patients had any improvement; and at 6 months, investigators believed 83% of patients had any improvement. Supplemental Digital Content, Figure S2, http://links.lww.com/DSS/B183 displays the distributions of investigator improvement scores at both administrations.
Subject Assessment
Patients assessed the level of their skin appearance improvement at 1, 3, and 6 months posttreatment. Patients used a scale anchored by 0 (no difference) and 4 (significantly marked improvement), identical to the Investigator Assessment scale. Scores were compared with the hypothesized lowest value (0, no difference) using 1-sample Wilcoxon signed rank tests. The median values at all 3 administrations were significantly higher than 0 (1 month: median = 1, standardized test statistic = 6.59, p < .001; 3 months: median = 1, standardized test statistic = 7.30, p < .001; 6 months: median = 2, standardized test statistic = 7.39, p < .001). In other words, patients believed they improved at all measurement periods significantly higher than “no difference.” In addition, at 1 month, 69% of patients believed they had any improvement; at 3 months, 70% of patients believed they had any improvement; and at 6 months, 74% of patients believed they had any improvement. Supplemental Digital Content, Figure S3, http://links.lww.com/DSS/B183 displays the distributions of subject improvement scores at both administrations.
Subject Satisfaction
Finally, patients assessed their satisfaction with the procedure at 1, 3, and 6 months posttreatment. The average values at all administrations were significantly higher than 0 (1 month: mean = 0.76, t[66] = 7.45, p < .001; 3 months: mean = 0.73, t[90] = 7.16, p < .001; 6 months: mean = 0.57, t[84] = 4.66, p < .001). In other words, patients were satisfied at a higher level compared with being indifferent. In addition, at 1 month, 57% of patients were satisfied or very satisfied with the procedure; at 3 months, 61% of patients were satisfied or very satisfied with the procedure; and at 6 months, 59% of patients were satisfied or very satisfied with the procedure. Supplemental Digital Content, Figure S4, http://links.lww.com/DSS/B183 displays the distributions of patient satisfaction scores at all 3 administrations.
Histology
The first 4 consented subjects underwent biopsies of the treated areas at baseline and at the 3-month follow-up visit. Histological sections were stained using H&E, elastin, and Masson trichrome, and all samples demonstrated an increase in either collagen or elastic fibers within the papillary and upper reticular dermis, and loss of rete pegs within superficial dermis. No dermal scar formation was noted. All changes were consistent with collagen remodeling.
Figure 3 is a representative histology image.
Figure 3.
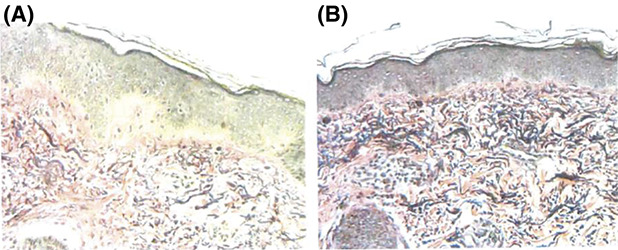
Representative histology slide at baseline (A) and at 3 months posttreatment (B) showing increased network of collagen fibers and dermal elastic fibers compared with baseline biopsy.
Adverse Events
Only 6 of the 87 patients reported AEs, all of which were mild and short lived. One patient reported persistent erythema that resolved 2 days after treatment. Another patient reported mild tenderness that resolved the day after treatment. The more significant AEs included hypopigmentation (seen in 1 patient) and epidermal skin changes consistent with a superficial burn (seen in 3 patients). All patients had resolution of AEs by the end of the study date.
Discussion
Over the past 2 decades, RF has emerged as a safe and effective method for delivering energy to the skin to induce neocollagenesis, resulting in modest skin tightening.6–8 Current RF devices are tightly regulated to deliver electrical currents, heating the skin and its contents to a precise temperature, resulting in a thermal effect. Following Ohm law, as RF is applied to the skin, the inherent resistance of varying tissue types within the skin leads to the generation of thermal energy. As a result, tissues with higher amounts of resistance, like fat, receive more energy.6–8
Soft tissue remodeling via bulk RF is achieved by 2 main mechanisms: (1) cleavage of hydrogen bonds within the collagen resulting in fibril denaturation and collagen contraction and (2) initiation of a wound healing cascade triggering neocollagenesis, angiogenesis, and elastogenesis.6–8 Both mechanisms of action result in modest long-term tissue tightening.6–8 Clinical and basic science studies have shown that the amount of collagen in the skin increases approximately 15% to 20% by 3-month follow-up after only 10 minutes of exposures to higher temperatures (39–43°C).9
Since the inception of the first commercially available RF device in 2004 (ThermaCool Thermage, Inc., Hayward, CA), RF devices have only continued to improve in efficacy, consistency, and safety.10 Consistent results coupled with little to no pain, no downtime, and few side effects have catapulted RF devices to the forefront of the noninvasive skin tightening options. The main drawback of this original device, however, is the operator-dependent nature of the treatment and the fact that treatments are time-intensive, leading to operator fatigue. The introduction of a noninvasive hands-free bipolar facial remodeling device addresses these issues by allowing the provider to place the device on the patient, set the target temperature, and automate the treatment without the need for regular manual intervention. The device automatically reaches the target temperature within 1 to 2 minutes and delivers consistent thermal energy throughout the treatment time, ultimately eliminating user variability and operator fatigue. Others have reported the efficacy and safety of this hands-free thermoregulatory device for face and neck contouring.8 In their recent prospective trial, Dayan and colleagues8 showed that this hands-free device is able to concentrate thermal energy consistently to a depth (4 mm) that allows for fibroseptal network tightening and ultimately submental soft tissue contraction.
The present study complements previously published work and, to the authors' knowledge, is the largest prospective trial to date evaluating the safety and efficacy of the device. The primary outcome data confirmed that at least 2 of 3 blind evaluators agreed on skin appearance based on photographs for at least 70% of patients. Secondary outcomes including investigator assessment of skin appearance, as well as subject assessment of improvement and satisfaction further supported these data. Patients believed that the procedures were relatively pain free and AEs were relatively rare and transient. In addition, investigators and patients believed they saw some improvement compared with no improvement in their skin condition, and patient satisfaction was higher than neutral. Finally, biopsies stained for H&E, elastin, and Masson trichrome confirmed changes consistent with collagen remodeling.
There were, however, a number of limitations to this study. Even though this study was a multicenter trial with 87 patients enrolled, a larger sample size would have been preferred to obtain more information on treatment efficacy for various age brackets and Fitzpatrick skin types. In addition, 23 of the 87 enrolled patients had incomplete data due to missing images (either lack of before or follow-up photographs or technology malfunction resulting in loss of data storage). Thus, in the end, only 64 subjects were included in the final primary end point analysis. However, for secondary evaluations, data from all 87 participants were included in the analysis. In terms of treatment length, it is important to note that while treatment time took on average 75 minutes, this was for the combined chin and cheek treatment. It is not mandatory to treat both zones in 1 visit, and each zone can take at least 20 minutes with typically the chin taking longer than the cheeks. In addition, regarding histology data, only 4 patients underwent biopsies of the treated sites. Although all samples demonstrated an increase in either collagen or elastic fibers within the papillary and upper reticular dermis, the fact that there was loss of rete pegs within superficial dermis was unexpected. Future studies with a comprehensive histological component are needed to determine the meaning and significance of these minute but important histological changes. Finally, the statistical analysis could have been improved using a more objective scale, such as the Fitzpatrick wrinkle scale or Alexiades skin laxity scale, or surface area measurements. The present study assessed global changes/improvement in skin appearance of the subjects based on before and after photographs. It is our understanding that studies are underway using more objective measurements for improved statistical analysis.
Conclusion
In conclusion, the data collected from the study supported that the use of the noninvasive hands-free bulk bipolar facial remodeling device for the improvement of skin appearance was safe and effective. Patients believed the procedures were relatively pain free. Investigators and patients also believed they noted some improvement compared with no improvement in their skin condition. Finally, patient satisfaction was higher than neutral. Given the favorable primary and secondary outcome data, it is logical to conclude that the hands-free bipolar facial remodeling device 3-treatment regimen has a positive effect on chin and cheek skin appearance.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.dermatologicsurgery.org).
The authors have indicated no significant interest with commercial supporters.
S. Chilukuri, J. Cohen, S. Kilmer, M. Lupo, and J. S. Dover loaned equipment for the completion of the study.
Contributor Information
Suneel Chilukuri, Email: dermsurg@gmail.com.
Joel Cohen, Email: jcohenderm@yahoo.com.
Suzanne Kilmer, Email: skilmer@skinlasers.com.
Mary Lupo, Email: drlupo@drmarylupo.com.
Rod Rohrich, Email: rod.rohrich@dpsi.org.
Jeffrey S. Dover, Email: jdover@skincarephysicians.net.
References
- 1.Rohrich RJ. The “science of aging” supplement. Plast Reconstr Surg 2021;147:1S–2S. [DOI] [PubMed] [Google Scholar]
- 2.Gloau RG. Aesthetic and anatomic analysis of the aging skin. Semin Cutan Med Surg. 1996;15:134–8 [DOI] [PubMed] [Google Scholar]
- 3.Gold MH. Tissue tightening: a hot topic utilizing deep dermal heating. J Drugs Dermatol 2007;6:1238–42. [PubMed] [Google Scholar]
- 4.Mulholland RS. Radiofrequency energy for noninvasive and minimally invasive skin tightening. Clin Plast Surg 2011;38:437–48, vi. [DOI] [PubMed] [Google Scholar]
- 5.Alster TS, Lupton JR. Nonablative cutaneous remodeling using radiofrequency devices. Clin Dermatol 2007;25:487–91. [DOI] [PubMed] [Google Scholar]
- 6.Dayan E, Burns AJ, Rohrich RJ, Theodorou S. The use of radiofrequency in aesthetic surgery. Plast Reconstr Surg Glob Open 2020;8:e2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dayan E, Rovatti P, Aston S, Chia CT, et al. Multimodal radiofrequency application for lower face and neck laxity. Plast Reconstr Surg Glob Open 2020;8:e2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dayan E, Chapas A, Marte J, Chia C, Theodorou S. A prospective trial: handsfree thermoregulated bipolar radiofrequency for face and neck contouring. Plast Reconstr Surg Glob Open 2022;10:e4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritz K, Salavastru C. Ways of noninvasive facial skin tightening and fat reduction. Facial Plast Surg 2016;32:276–82. [DOI] [PubMed] [Google Scholar]
- 10.Hodgkinson DJ. Clinical applications of radiofrequency: nonsurgical skin tightening (thermage). Clin Plast Surg 2009;36:261–8, viii. [DOI] [PubMed] [Google Scholar]


