Background:
Dilated cardiomyopathy (DCM) is a life-threatening disease, resulting in refractory heart failure. An immune disorder underlies the pathophysiology associated with heart failure progression. Invariant natural killer T (iNKT) cell activation is a prospective therapeutic strategy for ischemic heart disease. However, its efficacy in nonischemic cardiomyopathy, such as DCM, remains to be elucidated, and the feasible modality for iNKT cell activation in humans is yet to be validated.
Methods:
Dendritic cells isolated from human volunteers were pulsed with α-galactosylceramide ex vivo, which were used as α-galactosylceramide-pulsed dendritic cells (αGCDCs). We treated DCM mice harboring mutated troponin TΔK210/ΔK210 with αGCDCs and evaluated the efficacy of iNKT cell activation on heart failure in DCM mice. Furthermore, we investigated the molecular basis underlying its therapeutic effects in these mice and analyzed primary cardiac cells under iNKT cell-secreted cytokines.
Results:
The number of iNKT cells in the spleens of DCM mice was reduced compared with that in wild-type mice, whereas αGCDC treatment activated iNKT cells, prolonged survival of DCM mice, and prevented decline in the left ventricular ejection fraction for 4 weeks, accompanied by suppressed interstitial fibrosis. Mechanistically, αGCDC treatment suppressed TGF (transforming growth factor)-β signaling and expression of fibrotic genes and restored vasculature that was impaired in DCM hearts by upregulating angiopoietin 1 (Angpt1) expression. Consistently, IFNγ (interferon gamma) suppressed TGF-β-induced Smad2/3 signaling and the expression of fibrotic genes in cardiac fibroblasts and upregulated Angpt1 expression in cardiomyocytes via Stat1.
Conclusions:
Immunomodulatory cell therapy with αGCDCs is a novel therapeutic strategy for heart failure in DCM.
Keywords: α-galactosylceramide-pulsed dendritic cell, chronic heart failure, dilated cardiomyopathy, immune system, natural killer T cell
What is New?
Dilated cardiomyopathy is a fatal cardiomyopathy, resulting in refractory heart failure. The immune system has been recognized as a prospective therapeutic target for severe heart failure, and invariant natural killer T cell activation may be a potential strategy for treating dilated cardiomyopathy.
In this study, we showed that the α-galactosylceramide-pulsed dendritic cell is a potent modality for activating invariant natural killer T cells in the heart, and invariant natural killer T cell activation through alpha-galactosylceramide-pulsed dendritic cell treatment prevents the decline of cardiac contractility, suppresses fibrosis, and improves survival in dilated cardiomyopathy mice.
Mechanistically, invariant natural killer T cell activation promotes vasculogenesis and suppresses TGF-β (transforming growth factor-beta)-Smad signaling, and IFNγ (interferon-gamma) plays a key role in these processes.
What are the Clinical Implications?
Despite the advances in medical and device therapies, prognosis of patients with dilated cardiomyopathy remains poor, and the number of patients receiving heart transplantation or destination therapy with a left ventricular assist device is increasing.
Here, we demonstrate cell therapy with α-galactosylceramide-pulsed dendritic cells as a novel therapeutic modality through immune-modulation via iNKT cell activation.
Cell therapy with alpha-galactosylceramide-pulsed dendritic cell may be a new therapeutic strategy for patients with heart failure who have dilated cardiomyopathy, and clinical studies investigating its efficacy and safety are currently underway.
Dilated cardiomyopathy (DCM) is characterized by left ventricular dilatation and contractile dysfunction. It is a heterogeneous syndrome caused by numerous etiologies, including genetic mutations and acquired myocardial damage.1,2 Although its prognosis is based on an underlying etiology, the overall outcomes remain poor with high morbidity and mortality despite the advances in standard medical therapy with β-blockers and renin-angiotensin-aldosterone system inhibitors.2 Hence, the development of new therapeutics is imperative to improve outcomes for patients with DCM-heart failure (HF).
The immune system is one of the most promising therapeutic targets in chronic HF.3 In the early 1990s, it was reported that circulating TNF-α (tumor necrosis factor-α) is elevated in patients with severe chronic HF.4 Furthermore, animal studies showed that overexpressing TNF-α or administering it aggravates cardiac remodeling and HF in mice,5,6 demonstrating that immune system plays the key role in HF progression.7,8 However, blocking TNF-α using infliximab (ATTACH Trial) or etanercept (RENEWAL) failed to demonstrate clinical benefits in patients with chronic HF.9,10 In addition, a nonspecific immunomodulatory therapy using celecade was not effective for patients with severe HF, categorized as New York Heart Association functional class III-IV chronic HF (ACCLAIM study).11 The efficacy of several therapeutic approaches targeting the immune system in HF was also evaluated.12–15 However, these studies were small and inconclusive, and thus immunomodulatory therapy for HF remains unestablished.
Natural killer T (NKT) cells, characterized by the expression of a specific (invariant) antigen receptor (Vα14Jα18 in mice and Vα24Jα18 in humans) and discovered in the 1980s,16,17 are CD1d-restricted innate-like immune cells that express both an natural killer cell marker and a unique antigen receptor, of which gene fragments are located in the T cell receptor (TCR) loci, but not used by conventional T cells. Although the endogenous physiological regulation of NKT cell activation remains unknown, α-galactosylceramide (αGalCer), identified as an exogenous NKT cell ligand, activates invariant NKT (iNKT) cells.18 Since the first 4 amino acids of Jα18 of iNKT cell receptors, which are important for the recognition of αGalCer presented on human and mouse CD1d, are conserved between humans and mice,19 both human and mouse iNKT cells can be activated by αGalCer-pulsed antigen-presenting cells (APCs) of either mouse or human origin, demonstrating their cross-species reactivity.20 Notably, iNKT cells, a major subset of NKT cells in mammals, secrete multiple cytokines, such as IFNγ (interferon gamma), IL-4 (interleukin-4), and IL-10 (interleukin-10), activate various immune cells, and thereby induce anti-tumor responses.21–23
Previously, we demonstrated that iNKT cell activation induced by αGalCer ameliorates cardiac remodeling, improves survival following myocardial infarction, and reduces ischemia/reperfusion injury in mice.24,25 Moreover, we showed that anti-IL-10 receptor antibodies abolish the cardioprotection induced by iNKT cell activation in these models, suggesting that the anti-inflammatory effect of IL-10 is a key mediator of the cardioprotection through iNKT cell activation against myocardial ischemic insults. However, the post–myocardial infarction HF model, referred to as ischemic cardiomyopathy, is intimately associated with necrosis-driven inflammation of the heart,26 and the efficacy of iNKT cell activation pertaining to HF in nonischemic cardiomyopathy, such as DCM, remains unknown.
In addition, the long-term administration of αGalCer potentially causes lethal liver injuries and anergy, thereby limiting the possibility of directly administering αGalCer to humans.23,27 To overcome these limitations, APCs expressing CD1d, such as dendritic cells (DCs), can be pulsed ex vivo with αGalCer.23 The safety of using prepared αGalCer-pulsed DCs (αGCDCs) in humans has been confirmed.28 Therefore, the use of αGCDCs might be considered a potential modality for clinically treating patients with HF. However, the efficacy of this approach needs to be fully elucidated in an animal HF model.
In this study, we investigated the efficacy of αGCDCs in activating iNKT cells and the mechanism underlying this process in a murine DCM model harboring mutated troponin TΔK210/ΔK210.
Methods
A full description of the materials and methods can be found in the Supplemental Material. The authors declare that all supporting data are available within the article and its Supplemental Material.
Animal Study
All procedures involving animals and animal care protocols were approved by the Committee on Ethics of Animal Experiments of the Kyushu University Graduate School of Medical and Pharmaceutical Sciences (A29-348, A19-029, and A21-032) and were performed in accordance with the Guideline for Animal Experiments of Kyushu University and the Guideline for the Care and Use of Laboratory Animals published by the US National Institutes of Health (revised in 2011). BALB/c mice were purchased from Kyudo Co. Ltd. (Saga, Japan), and all mice, including DCM mice,29,30 were housed in a temperature- and humidity-controlled room and fed a commercial diet (CRF-1LID; Oriental Yeast Co. Ltd, Tokyo, Japan) with free access to water. The study design is described in the Supplemental Material.
Preparation of αGCDCs From Human Peripheral Blood
All procedures involving the preparation of αGCDCs were approved by the Kyushu University Hospital Ethics Committee (Permission numbers are 29-62, 30-571, 2019-498, 2020-516, and 2021-381). The αGCDCs were prepared by performing apheresis on volunteers as described previously.31 Detailed methods are described in the Supplemental Material.
Statistical Analysis
Statistical analyses were performed using JMP16 software (SAS Institute, Cary, NC) and GraphPad Prism version 9.3.1 (GraphPad Software, La Jolla, CA). Data are presented as mean±SD. Paired or unpaired Student t test, Dunnett test, and 1 way-ANOVA followed by Tukey’s post hoc test were used. The ratiometric data were presented on a log-scale axis, and log-transformed data, obtained with log2 (x+1) data transformation, were analyzed using these statistical tests. The log-rank test was used for comparison among the 3 groups (PBS, CTRL-DC, and αGCDC groups), followed by log-rank test for calculating the statistical significance, hazard ratio, and CI between 2 groups. Statistical significance of the results was set at P<0.05.
Results
αGCDC Treatment Induces iNKT Cell Activation and Subsequent Immune Responses
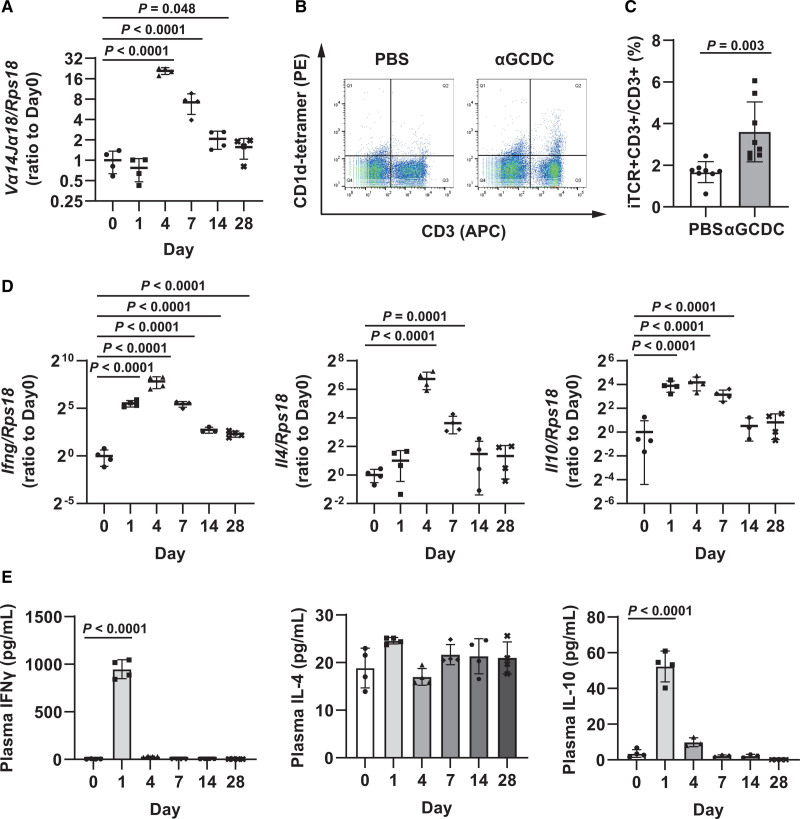
The αGCDCs were produced by loading αGalCer on human APCs, including DCs, collected from the peripheral blood of volunteers via leukapheresis as previously described.31 We examined the dose-dependent effects of αGCDCs in the murine myocardium in vivo and found that the gene expression of invariant TCR (Vα14Jα18) and iNKT cell-secreted cytokines such as interferon-γ (Ifng), interleukin-4 (Il4), and interleukin-10 (Il10) was upregulated (Figure S1A). Consistent with the upregulation of these genes, the corresponding transcription factors, Stat1 and Stat6, were phosphorylated in a dose-dependent manner (Figure S1B and S1C). We used a higher dose (3×106 αGCDCs per DCM mouse) to achieve maximum effects on the myocardium in this study. In comparison with αGCDCs, the effect of IFNγ on Stat1 phosphorylation peaked and plateaued at a relatively lower dose of αGalCer (Figure S2A). Additionally, the phosphorylation of Stat1 with αGCDC treatment was stronger than that with αGalCer treatment, whereas the phosphorylation of Stat6 in αGCDCs was equivalent to that under higher dose of αGalCer (Figure S2A). The transcriptional upregulation of invariant TCR (Vα14Jα18) and Il10 was not observed in mice treated with isolated APCs and control-DCs without αGalCer (CTRL-DCs), although the gene expression of Ifng and Il4 was slightly upregulated in mice treated with isolated APCs and CTRL-DCs, respectively (Figure S2B).
To examine the sustained effect of a single treatment with αGCDCs (3×106 cells in 50 μL PBS per mouse) on iNKT cell activation in mice, we analyzed BALB/c mice treated with αGCDCs on days 1, 4, 7, 14, and 28. The expression of invariant TCR in the myocardium peaked on day 4 (Figure 1A). Concomitantly, the number of iNKT cells in the spleen that were labeled with anti-CD3 antibodies as well as αGalCer-loaded CD1d tetramers also increased on day 4 following αGCDC administration (Figure 1B and 1C). Consistent with the upregulation of invariant TCR expression in the myocardium, iNKT cell-secreted cytokines in the myocardium peaked on day 4 and reverted to nearly normal levels on day 14 (Figure 1D). However, a single administration of αGCDCs maintained the slight upregulation of Ifng, Il4, and Il10 (≈ 5-, 2.5-, and 2-fold, respectively) even on day 28 following treatment (Figure 1D). Stat6 phosphorylation, representing the effect of IL-4, peaked on day 4, whereas Stat1 phosphorylation, representing the effect of IFNγ, peaked on day 7 and continued until day 14 following the treatment (Figure S3A and S3B). In contrast, plasma IFNγ and IL-10 levels peaked on day 1 after the treatment, whereas no significant response was observed for plasma IL-4 (Figure 1E).
Figure 1.
Immune responses to α-galactosylceramide (αGalCer)-pulsed dendritic cell (αGCDC) treatment in the myocardium of BALB/c mice. A, Gene expression of invariant T cell receptor (TCR; Vα14Jα18) in the myocardium of BALB/c mice following a single αGCDC treatment until day 28, measured using reverse transcription-quantitative polymerase chain reaction (RT-qPCR; n=3–4). B, Flow cytometry data of the spleens of BALB/c mice, following a single administration of PBS and αGCDCs, on day 4. CD1d-tetramer binding (PE)- and CD3 (allophycocyanin [APC])-positive cells were defined as invariant natural killer T (iNKT) cells. C, Quantification of the percentage of iNKT cells to CD3-positive cells following a single αGCDC treatment (n=8). D, Gene expression of iNKT cell-secreted cytokines, such as IFNγ (interferon gamma; Ifng), IL-4 (interleukin-4; Il4), and IL-10 (interleukin-10; Il10), in the myocardium of BALB/c mice, after a single αGCDC treatment until day 28, measured using RT-qPCR (n=3–4). E, Plasma concentrations (pg/mL) of iNKT cell-secreted cytokines such as IFNγ, IL-4, and IL-10 in BALB/c mice after a single αGCDC treatment (n=3–4). Data are presented as mean±SD. Statistical significance was determined using Student t test (C) or Dunnett test (other panels).
The Number of iNKT Cells Is Reduced in DCM Mice
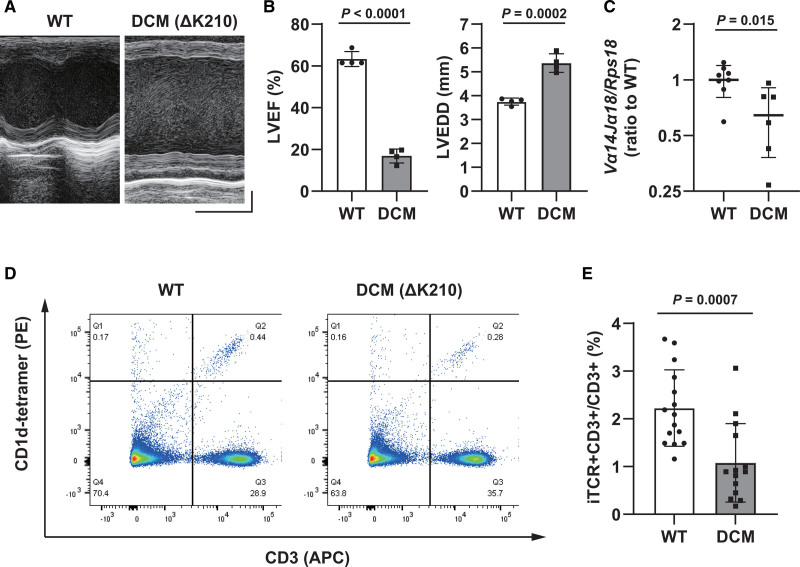
Echocardiograms showed severely impaired contractility and left ventricular dilatation in 5-week-old DCM mice (Figure 2A and 2B). Gene expression of invariant TCR in the spleens of DCM mice was downregulated compared with that in WT mice (Figure 2C). Analysis using flow cytometry also showed a significant decrease in the number of invariant TCR+CD3+ cells, representing iNKT cells, in the spleens of DCM mice (Figure 2D and 2E).
Figure 2.
Characterization of dilated cardiomyopathy (DCM) mice (5 weeks old). A, Representative echocardiographic images of wild-type (WT) and DCM mice. Horizontal scale indicates 100 ms‚ and vertical scale indicates 1 mm. B, Left ventricular ejection fraction (LVEF, left) and left ventricular end-diastolic diameter (LVEDD, right) in WT and DCM mice (n=4). C, Gene expression of invariant T cell receptor (TCR; Vα14Jα18) in the myocardium of WT and DCM mice, measured using reverse transcription-quantitative polymerase chain reaction (n=8 and 6, respectively). D, Flow cytometry data of the spleens of WT and DCM mice. CD1d-tetramer binding (PE)- and CD3 (allophycocyanin [APC])-positive cells were defined as invariant natural killer T (iNKT) cells. E, Quantification of the percentage of iNKT cells to CD3-positive cells (n=15 and 14, respectively). Data are presented as mean±SD. Statistical significance was determined using Student t test.
αGCDC Treatment Maintains Systolic Function, Suppresses Myocardial Fibrosis, and Prolongs the Survival of DCM Mice
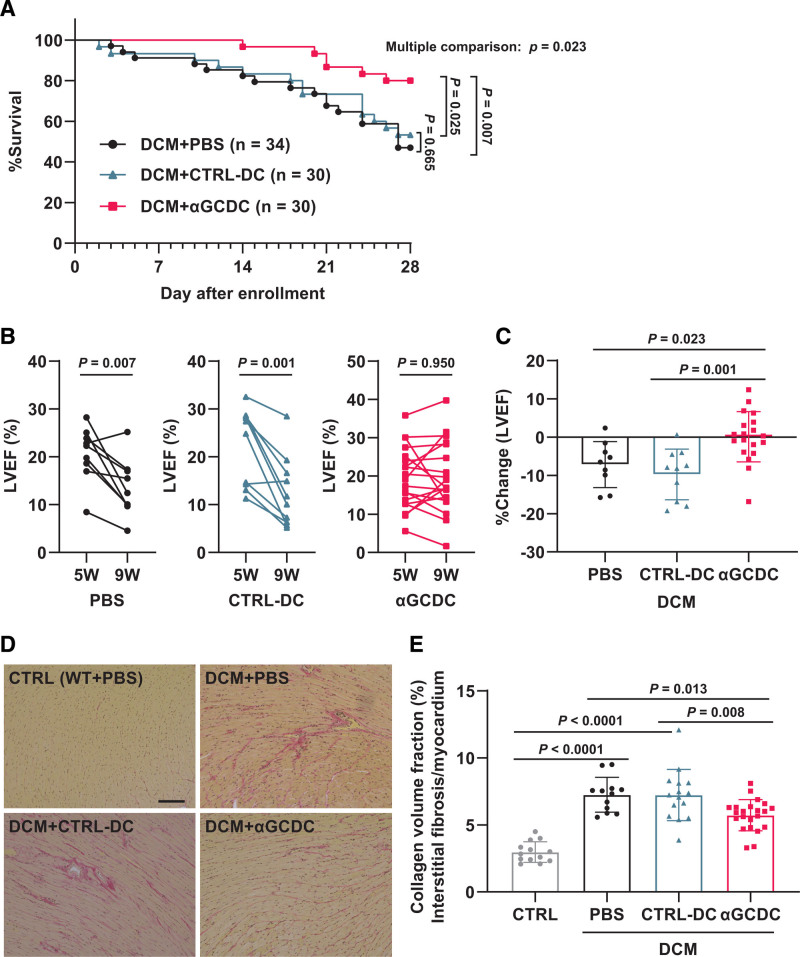
DCM mice were assigned to the PBS, CTRL-DC, and αGCDC groups; among them, echocardiographic parameters did not differ significantly at baseline (5 weeks of age; Table S1). Overall survival was significantly improved in DCM mice treated with αGCDCs, but not in the CTRL-DC group, compared with that in mice in the PBS group (survival rate: 47%, 53%, and 80% in the PBS, CTRL-DC, and αGCDC groups, respectively; hazard ratio, confidence interval, and P: 0.86, 0.43–1.72, and P=0.665 for CTRL-DC versus PBS groups, 0.31, 0.14–0.68, and P=0.007 for αGCDC versus PBS groups, and 0.36, 0.15–0.86, and P=0.025 for αGCDC versus CTRL-DC groups; Figure 3A). In surviving DCM mice, the mice treated with PBS or CTRL-DCs showed a decline in the left ventricular ejection fraction (LVEF) at 4 weeks after each treatment, whereas αGCDC treatment significantly prevented this decline (%Change in LVEF: −7%, −10%, and 0% in the PBS, CTRL-DC, and αGCDC groups, respectively; Figure 3B and 3C), although there was no significant difference in the average of the echocardiographic parameters in the surviving mice among all groups (Table S2). The histological analysis showed that interstitial fibrosis in DCM mice was significantly suppressed in the αGCDC group compared with that in the PBS and CTRL-DC groups (Figure 3D and 3E).
Figure 3.
Chronic effect of α-galactosylceramide (αGalCer)-pulsed dendritic cells (αGCDCs) on dilated cardiomyopathy (DCM) in mice. A, Survival of DCM mice, treated with PBS (n=34, enrollment; n=18, death), control (CTRL)-DCs (n=30, enrollment; n=14, death), and αGCDCs (n=30, enrollment; n=6, death) until day 28 following treatments. B, Individual changes in left ventricular ejection fraction (LVEF); DCM+PBS, n=9; DCM+CTRL-DC, n=10; DCM+αGCDC, n=19. C, Percent change in the LVEF from the beginning (5 weeks of age) to the end of the study (4 weeks after each treatment) in mice treated with PBS (n=9), CTRL-DCs (n=10), and αGCDCs (n=19). Echocardiographic data of some surviving mice (PBS, n=7; CTRL-DC, n=6; αGCDC, n=5) could not be obtained because of their death before or during echocardiography. D, Representative images of Picro-Sirius Red staining in wild-type (WT) and DCM mice treated with PBS, CTRL-DCs, and αGCDCs. The PBS+WT group was used as a control (CTRL). Scale bar‚ 100 µm. E, Quantification of fibrotic area per myocardium in WT mice treated with PBS (Control [CTRL], n=13) and DCM mice treated with PBS (n=12), CTRL-DCs (n=15), and αGCDCs (n=22). Data are presented as mean±SD. Statistical significance was determined using a log-rank test for A, a paired Student t test for B, and 1-way ANOVA with a post hoc test (Tukey) for C and E.
αGCDC Treatment Improves Contractile Function in DCM Mice 4 Days After Treatment
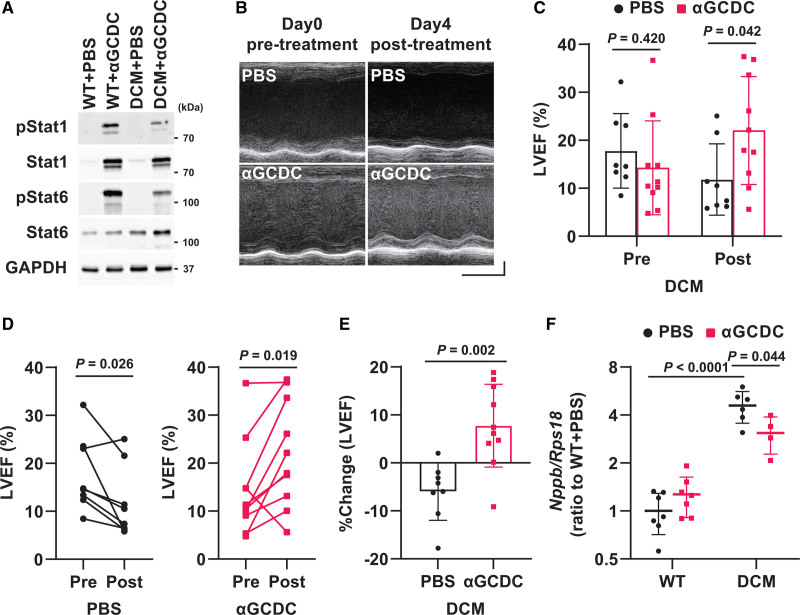
To further investigate the mechanistic basis underlying the effect of αGCDC treatment on DCM mice, we analyzed DCM mice treated with αGCDCs on day 4 and found that the expression of invariant TCR, Ifng, Il4, and Il10 was markedly upregulated (Figure S4A). The phosphorylation of Stat1 and Stat6 in the myocardium of DCM mice was also enhanced by αGCDC treatment (Figure 4A). Notably, the LVEF of DCM mice treated with αGCDCs was significantly higher than that of mice treated with PBS on day 4 following αGCDC treatment (Figure 4B and 4C). A detailed analysis showed that the LVEF of DCM mice significantly improved in response to αGCDC treatment, whereas that of mice in the PBS group was slightly reduced on day 4 after PBS treatment (%Change in LVEF: −6% and 8% in the PBS and αGCDC groups, respectively; Figure 4D and 4E), although there was no significant difference in the average of the echocardiographic parameters, excluding the LVEF and FS, between mice in these groups (Figure 4C‚ Table S3). Furthermore, whereas Nppb expression was upregulated in the PBS-treated DCM mice, it was attenuated in the αGCDC-treated DCM mice (Figure 4F). On day 4 after αGCDC treatment, no difference in interstitial fibrosis was observed between the PBS-treated and αGCDC-treated DCM mice (Figure S4B and S4C). In contrast, the upregulation of iNKT cell-secreted cytokines and the cardioprotective phenotypes of LVEF and Nppb gene expression were not observed in CTRL-DC-treated mice, although Ifng gene expression was slightly upregulated in CTRL-DC-treated mice (Figure S5A through S5E‚ Table S4).
Figure 4.
Acute effect of α-galactosylceramide (αGalCer)-pulsed dendritic cells (αGCDCs) on the cardiac function of dilated cardiomyopathy (DCM) in mice. A, Phosphorylation of Stat1 and Stat6 in the myocardium on day 4 following αGCDC treatment. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. B, Representative echocardiographic images of DCM mice at pretreatment and posttreatment (day 4) with PBS and αGCDCs. Horizontal scale indicates 100 ms, and vertical scale indicates 1 mm. C, Left ventricular ejection fraction (LVEF) in DCM mice at pretreatment (Pre) and posttreatment (Post; day 4) with αGCDCs (n=8–10). D, Individual changes in LVEF; DCM+PBS, n=8; DCM+αGCDC, n=10. E, Percent change in the LVEF from the beginning (pretreatment) to the end of the study (day 4 following each treatment, posttreatment) in mice treated with PBS and αGCDCs (n=8–10). F, Gene expression of BNP (brain natriuretic peptide, Nppb) in the myocardium of wild-type (WT) and DCM mice on day 4 after αGCDC treatment, measured using reverse transcription-quantitative polymerase chain reaction (n=4–7). Data are presented as mean±SD. Statistical significance was determined using Student t test for C and E, paired Student t test for D, and 1-way ANOVA with a post hoc test (Tukey) for F.
αGCDC Treatment Suppresses TGF-β Signaling and Improves the Vasculature Density in the Myocardium of DCM Mice
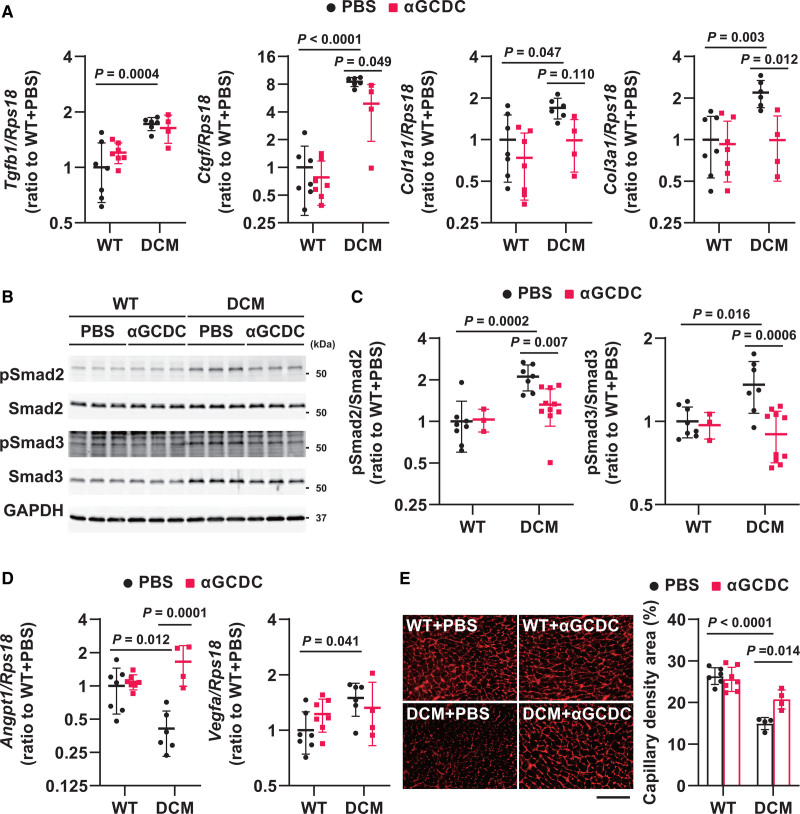
To comprehensively investigate the effects of αGCDCs on cardiac function and fibrosis in DCM mice, we performed microarray analysis on the myocardium on day 4 following treatment. We selected genes according to an algorithm (Figure S6A) and identified 277 genes (defined as cluster 2) whose expression was upregulated in DCM+PBS mice but suppressed in DCM+αGCDC mice. Moreover, we identified 117 genes (defined as cluster 9) whose expression was downregulated in DCM+PBS mice but restored in DCM+αGCDC mice (Figure S6B). Gene ontology (GO) analysis of cluster 2 and 9 revealed that αGCDCs promoted vasculogenesis and suppressed the fibrotic response, particularly TGF-β (transforming growth factor-beta) signaling, in DCM mice (Figure S6C). Reverse transcription-quantitative polymerase chain reaction analysis revealed that αGCDC treatment suppressed the upregulation of fibrotic genes, such as connective tissue growth factor (Ctgf), Col1a, and Col3a, in the myocardium of DCM mice (Figure 5A). Although αGCDC treatment did not suppress the upregulation of Tgfb1 expression (Figure 5A), the phosphorylation of Smad2 and Smad3 was suppressed in the myocardium of αGCDC-treated DCM mice (Figure 5B and 5C). Taken together, these findings indicate that αGCDC treatment suppresses fibrosis by inhibiting Smad2 and Smad3 phosphorylation but not TGF-β expression. Further detailed GO analysis revealed that αGCDC treatment restored the downregulation of angiopoietin 1 (Angpt1) in the myocardium of DCM mice (Figure 5D) but did not alter vascular endothelial growth factor A (Vegfa) expression. Consistent with the restoration of Angpt1 expression, αGCDC treatment also restored the myocardial capillary density, which was impaired in the hearts of DCM mice (Figure 5E). In contrast, these cardioprotective responses, such as fibrotic gene downregulation and Angpt1 upregulation, were not observed in CTRL-DC-treated DCM mice (Figure S7A and S7B).
Figure 5.
Fibrotic gene expression and vasculogenesis in dilated cardiomyopathy (DCM) mice on day 4 after α-galactosylceramide (αGalCer)-pulsed dendritic cell (αGCDC) treatment. A, Expression of fibrotic genes such as Tgfb1, Ctgf, Col1a1, and Col3a1 in the myocardium of wild-type (WT) and DCM mice on day 4 following αGCDC treatment (n=4–7). B, Western blot analysis of phosphorylated Smad2 and Smad3 in the myocardium of WT and DCM mice following treatment with αGCDCs on day 4 (n=3–10). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. C, Quantification of the Western blotting results shown in B. D, Gene expression of angiopoietin 1 (Angpt1) and vascular endothelial growth factor (Vegfa) in the myocardium of WT and DCM mice following treatment with αGCDCs on day 4 (n=4–7). E, Capillary density in the myocardium of WT and DCM mice (n=4–7). Scale bar‚ 100 µm. Data are presented as mean±SD. Statistical significance was determined using 1-way ANOVA with a post hoc test (Tukey).
IFNγ Suppresses TGF-β–Smad2/3 Signaling in Primary Fibroblasts
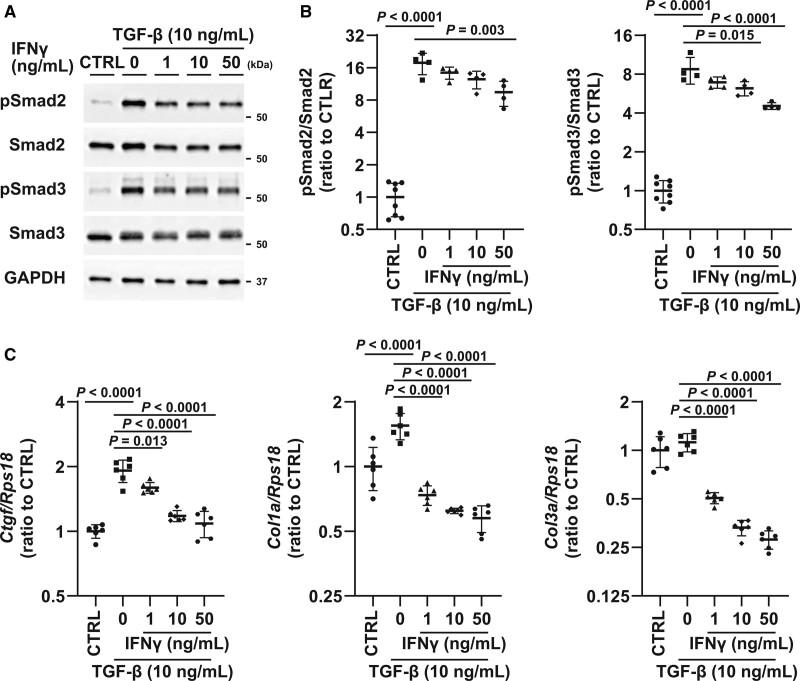
To further investigate the mechanisms underlying the suppression of the TGF-β–Smad signaling axis mediated by αGCDC treatment, we treated primary fibroblasts with major cytokines, specifically IFNγ, IL-4, and IL-10, which are secreted from iNKT cells. IFNγ and IL-4 induced Stat1 and Stat6 phosphorylation, respectively; however, IL-10 did not alter Stat3 phosphorylation (Figure S8A and S8B). IFNγ suppressed the expression of fibrotic genes, such as Ctgf, Col1a1, and Col3a1, whereas IL-4 and IL-10 did not, suggesting that IFNγ is the candidate suppressor of TGF-β signaling (Figure S8C). Consistent with the findings in vivo, the phosphorylation of Smad2 and Smad3 was significantly attenuated by IFNγ pretreatment (Figure 6A and 6B). Furthermore, the upregulation of fibrotic genes by TGF-β was suppressed by IFNγ pretreatment in a dose-dependent manner (Figure 6C).
Figure 6.
Role of IFNγ (interferon gamma) in TGF (transforming growth factor)-β/Smad signaling during α-galactosylceramide (αGalCer)-pulsed dendritic cell (αGCDC) treatment. A, Phosphorylation of Smad2 and Smad3 with TGF-β (10 ng/mL) and IFNγ pretreatment (1, 10, and 50 ng/mL). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. B, Quantification of Western blotting results shown in B (n=4–8). C, Expression of fibrotic genes such as Ctgf, Col1a, and Col3a in response to TGF-β (10 ng/mL) with IFNγ pretreatment (1, 10, and 50 ng/mL) in primary cultured cardiac fibroblasts (n=6). Data are presented as mean±SD. Statistical significance was determined using the 1-way ANOVA with a post hoc test (Tukey).
IFNγ Upregulates Angpt1 in Primary Cultured Cardiomyocytes
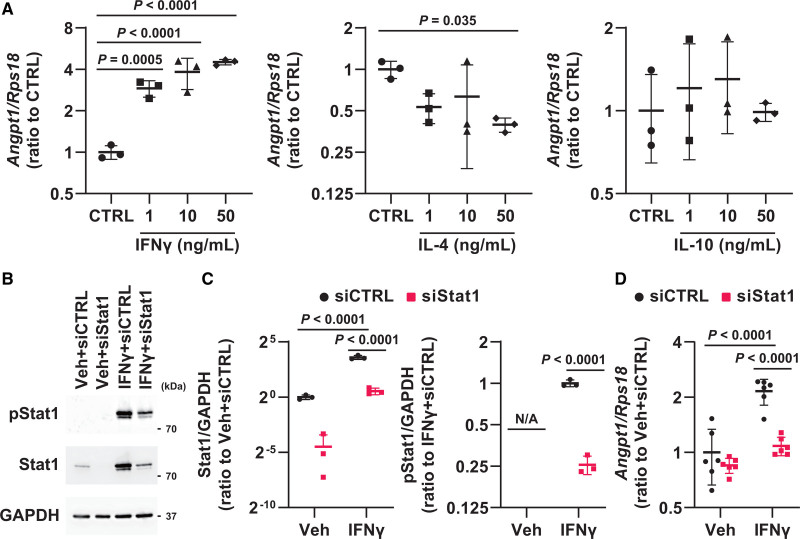
To identify the mechanisms by which αGCDC treatment upregulated Angpt1 expression, we treated cultured cardiomyocytes with IFNγ, IL-4, and IL-10 and found that treatment with IFNγ and IL-4 led to the phosphorylation of Stat1 and Stat6, respectively, whereas treatment with IL-10 did not alter Stat3 phosphorylation (Figure S9A and S9B). Notably, treatment with IFNγ, but not IL-4 or IL-10, upregulated Angpt1 expression (Figure 7A), and the silencing of Stat1 abolished this upregulation (Figure 7B through 7D), indicating that αGCDC treatment promotes vasculogenesis via the IFNγ-Stat1-Angpt1 axis. Based on the present findings, we have summarized the mechanistic basis of αGCDC treatment for chronic HF in DCM mice in Figure 8.
Figure 7.
Role of IFNγ (interferon gamma) in vasculogenesis during α-galactosylceramide (αGalCer)-pulsed dendritic cell (αGCDC) treatment. A, Gene expression of Angpt1 in response to IFNγ, IL-4 (interleukin-4), or IL-10 (interleukin-10) treatment in primary cultured cardiomyocytes (n=3). B, Phosphorylated Stat1 and total Stat1 in response to IFNγ (50 ng/mL) in primary cultured cardiomyocytes transfected with siRNA targeting Stat1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. C, Quantification of Western blotting results shown in B (n=3). D, Gene expression of Angpt1 in response to IFNγ in primary cultured cardiomyocytes transfected with siRNA targeting Stat1 (n=6). Data are presented as mean±SD. Statistical significance was determined using Dunnett test for A and 1-way ANOVA with a post hoc test (Tukey) for the other panels.
Figure 8.
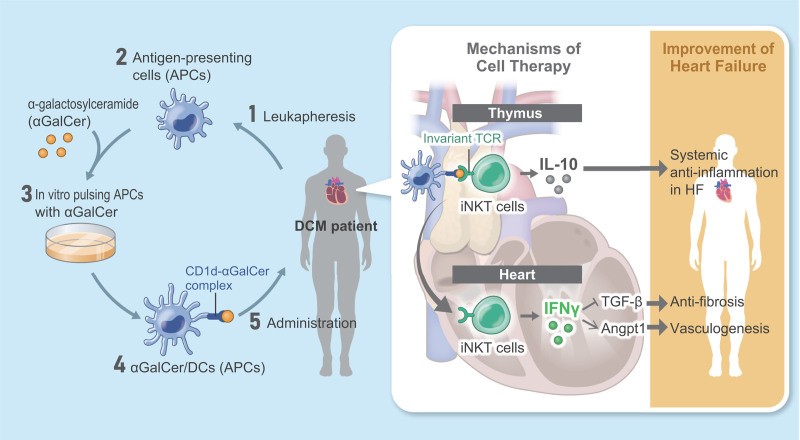
Mechanistic scheme of cell therapy with α-galactosylceramide (αGalCer)-pulsed dendritic cells (αGCDCs) for invariant natural killer T (NKT) cell activation in heart failure (HF). 1‚ Leukapheresis from patients with dilated cardiomyopathy (DCM). 2‚ Isolation of antigen-presenting cells (APCs). 3‚ In vitro pulsing of APCs with αGalCer. 4‚ Preparation of αGCDCs. 5‚ Administration of αGCDCs to patients with DCM. IFNγ indicates interferon-gamma; IL-10, interleukin-10; TCR, T cell receptor; and TGF-β, transforming growth factor.
Discussion
Our study on αGCDC treatment of DCM mice harboring mutated troponin TΔK210/ΔK210 revealed 3 major findings as follows: (1) the immune system, particularly iNKT cells, is impaired in DCM mice; (2) αGCDCs activate iNKT cells, ameliorate contractile dysfunction and interstitial fibrosis in the heart, and prolong the survival of DCM mice; and (3) IFNγ plays a key role in cardioprotection induced by αGCDCs by suppressing the TGF-β–Smad2/3 signaling pathway and upregulating Angpt1 expression.
Previously, we demonstrated that iNKT cell activation with αGalCer ameliorates cardiac remodeling and improves survival following myocardial infarction.24 However, little is known regarding alterations to the immune system in severe HF observed in DCM,26 and the efficacy of immunomodulatory therapeutics has not been established. Here, we found that the iNKT cell number decreased in the spleens of DCM mice (Figure 2D and 2E). Given that αGCDC treatment activated iNKT cells and prolonged the survival of DCM mice, impairment of the immune system in terms of iNKT cells might be responsible for the progression of cardiomyopathy and HF in DCM. Interestingly, Stat1 and Stat6 phosphorylation in the heart in response to αGCDC treatment correlated with the gene expression of invariant TCR in the myocardium and not with the increase in cytokine levels in the plasma (Figure 1‚ Figure S3), indicating that Stat1 and Stat6 phosphorylation in the myocardium depends on iNKT cells localized in the heart.
To identify the potential mechanisms by which αGCDC treatment ameliorates HF in DCM mice, we performed GO analysis using microarray data and investigated the 2 phenotypes produced following treatment, namely antifibrosis and vasculogenesis. Fibrosis is a detrimental feature observed in failing myocardia, and thus, anti-fibrotic mechanisms are the mainstay of cardioprotective effects mediated by αGCDC treatment. Based on microarray analysis, we found that αGCDC treatment suppressed Smad2/3 phosphorylation and the expression of fibrotic genes, such as Ctgf, Col1a, and Col3a, which were upregulated in DCM mice (Figure 5). Several studies have shown that the TGF-β–Smad axis plays a key role in the progression of HF via fibrosis of the heart. Kuwahara et al32 demonstrated that the inhibition of TGF-β using a neutralizing antibody prevents myocardial fibrosis in pressure overload-induced cardiac hypertrophy. In addition, Sakata et al33 demonstrated that a TGF-β antagonist suppresses myocardial fibrosis in mice with the cardiac-restricted overexpression of TNF-α. Recently, Khalil et al34 reported that TGF-β–Smad3 signaling in fibroblasts is a major regulator of myocardial fibrosis. Collectively, these findings indicate that the anti-fibrotic effects of αGCDC treatment result from the suppression of TGF-β–Smad2/3 signaling.
Furthermore, we showed that IFNγ suppressed the TGF-β–Smad2/3 axis. IFNγ has an established role in inducing anti-fibrotic effects, with particular reference to the TGF-β–Smad2/3 axis. Ulloa et al35 first reported that IFNγ/Stat inhibits TGF-β–Smad signaling by upregulating Smad7, an inhibitory Smad. In addition, Kimura et al36 demonstrated that αGalCer attenuates the development of bleomycin-induced pulmonary fibrosis and improves survival, with IFNγ playing a pivotal role in the antifibrotic effects induced by αGalCer. In the present study, we demonstrated that fibrosis was suppressed in DCM mice treated with αGCDCs and that IFNγ suppressed the phosphorylation of Smad2/3 and fibrotic genes induced by TGF-β stimulation in primary cardiac fibroblasts. Nevertheless, Smad7 was not upregulated in response to αGCDC treatment in vivo and IFNγ in primary cardiac fibroblasts (Figure S10A and S10B). These results suggest that an inhibitory interaction between TGF-β and IFNγ, which is not mediated via Smad7, plays a key role in the anti-fibrotic effects of αGCDCs or IFNγ in the heart. Thus, further investigations are needed to clarify these interactions.
Vasculogenesis and angiogenesis are dysregulated in the hypertrophied myocardium,37,38 and the insufficient vasculature of coronary artery circulation is a major cause of cardiac dysfunction.39,40 In this study, transcriptome analysis indicated that αGCDC treatment ameliorated impaired vasculogenesis in DCM mice. Indeed, αGCDC treatment restored the capillary density in the hearts of DCM mice, which was less dense than that in WT mice (Figure 5). GO analysis of genes involved in vasculogenesis revealed that Angpt1 expression, which was markedly downregulated in DCM mice, was completely restored by αGCDC treatment (Figure 5). Angpt1 is essential for vasculogenesis in the developing heart41 as it promotes vascular maturity and activates the endothelial-specific tyrosine kinase Tie2, strengthening reciprocal interactions between the endothelium and the surrounding matrix as well as the mesenchyme. The overexpression of Angpt1 using viral vectors increases capillary density and improves cardiac function in a murine model of HF,39,42 suggesting that Angpt1 is essential not only for heart development but also for the preservation of cardiac function. Given that αGCDCs restored Angpt1 expression and vasculature density in the DCM heart, upregulated Angpt1 expression, at least in part, contributes to the improvement in the survival of DCM mice with preserved left ventricular contractility. Furthermore, other mechanisms might contribute to the improvement in LVEF on day 4 after αGCDC treatment. As the improvement in LVEF following αGCDC treatment was not evident in wild-type BALB/c mice (Figure S11), we deduced that iNKT cell activation would revert some impairments associated with cardiac contractility observed in severe HF. Indeed, a microarray analysis also revealed other potential mechanisms as shown in Figure S6. However, further investigation might be needed to fully confirm the mechanism by which αGCDC treatment improves contractility in the HF model.
We demonstrated that IFNγ, but not IL-4 and IL-10, mediated upregulated Angpt1 expression induced by αGCDC treatment via Stat1 activation (Figure 7). Some studies have demonstrated that IFNγ exerts a proangiogenetic effect,43 whereas others have suggested that it exhibits an inhibitory effect on angiogenesis, especially in malignant tumors.44 These observations suggest that the role of IFNγ in angiogenesis or vasculogenesis depends on the cell type, organ, and specific conditions.
In this study, we demonstrated the cardioprotective effects of IFNγ during αGCDC treatment on contractile function and fibrosis. Although IFNγ is widely recognized as a pro-inflammatory cytokine, previous studies have also shown its significance in cardioprotection considering that its deletion aggravates HF in an aldosterone-induced hypertrophy model and a pressure overload model.45,46 This evidence indicates that IFNγ is necessary for maintaining cardiac function during HF and that it plays a key role in cardioprotection induced by iNKT cell activation. Nevertheless, we deduced that multiple effectors, including IFNγ induced by iNKT cell activation, contribute to cardioprotection in the HF model. Indeed, we and others have demonstrated that IL-10, an anti-inflammatory cytokine, plays a pivotal role in cardioprotection mediated by αGalCer in post–myocardial infarction HF and cardiac hypertrophy induced by angiotensin II.24,47
This study has 2 major limitations. First, we transplanted human αGCDCs into DCM mice (thereby producing a xenograft model), mainly because this study was designed to develop and validate a cell product for humans. Human APCs can present αGalCer to murine iNKT cells and activate them,20 and iNKT cells in mice were evidently activated in response to human αGCDCs in our study; however, xenografting, which can induce immune rejection, could be considered a limitation. Second, we utilized a DCM model that harbors a troponin T mutant. Although this mutation is also observed in human DCM, DCM is a heterogeneous cardiomyopathy induced by numerous etiologies, and therefore, the present findings cannot be broadly generalized to all clinical DCMs. Thus, detailed investigations are needed to validate the application of this concept to clinical DCMs.
In conclusion, our findings revealed the novel and beneficial effects of αGCDC treatment on chronic HF in a DCM model. This supports the clinical application of αGCDCs as a therapeutic modality for DCM patients with chronic HF. A clinical trial investigating the efficacy and safety of αGCDC treatment for chronic HF is currently underway (Japan Registry of Clinical Trials; jRCT2073210116).
Article Information
Acknowledgments
The authors appreciate the technical assistance provided by the Research Support Center, Research Center for Human Disease Modeling, Kyushu University Graduate School of Medical Sciences. Drs M. Ikeda‚ S. Ikeda, Okabe, Ishikita, Tadokoro, Sada, and Abe and M. Sato, and A. Hanada performed the experiments. Drs M. Ikeda, Ide, Matsushima, Arai, Ohtani, and Tsutsui interpreted and analyzed data obtained from the experiments. Drs M. Ikeda, Ide, Matsushima, and Tsutsui designed the experimental protocols. Drs M. Ikeda and Ide wrote the article and prepared the figures. Dr Tsutsui conceived the project. Drs Nonami, Mizuno, Morimoto, Motohashi, Akashi, and Taniguchi provided critical advice and commented on the project. Dr Tsutsui approved and supervised the project. All authors have read and approved the final draft.
Sources of Funding
This work was supported by the Japan Intractable Diseases (Nanbyo) Research Foundation (2021A04; Dr M. Ikeda) and Japan Agency for Medical Research and Development under grants 20ek0109339h0003 and 21ek0109476s1202 (Dr Tsutsui).
Disclosures
This was a collaborative research project with MEDINET Co Ltd (Tokyo, Japan). Dr Ide received research funding from SBI Pharmaceuticals and Pfizer Japan Co Ltd. Dr Tsutsui received remunerations from Kowa, Teijin Pharma, Nippon Boehringer Ingelheim, Mitsubishi Tanabe Pharma, Pfizer Japan, Ono Pharmaceutical, Daiichi Sankyo, Novartis Pharma, Bayer Yakuhin, Otsuka Pharmaceutical, and AstraZeneca; manuscript fees from Nippon Rinsho; research funding from Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, IQVIA Services Japan, MEDINET, Medical Innovation Kyushu, Kowa, Daiichi Sankyo, Johnson & Johnson, and NEC Corporation; and scholarship funds or donations from Abbott Medical Japan, Otsuka Pharmaceutical, Boston Scientific Japan, Ono Pharmaceutical, Bayer Yakuhin, Nippon Boehringer Ingelheim, St. Mary’s Hospital, Teijin Pharma, Daiichi Sankyo, and Mitsubishi Tanabe Pharma. The Department of Immunoregulatory Cardiovascular Medicine is an endowment department supported by an unrestricted grant from MEDINET Co Ltd. A patent pertaining to the results presented in this article is pending.
Supplemental Material
Supplemental Methods
Figures S1–S11
Tables S1–S4
Supplementary Material
Nonstandard Abbreviations and Acronyms
- αGalCer
- alpha-galactosylceramide
- αGCDC
- alpha-galactosylceramide-pulsed dendritic cell
- APC
- antigen-presenting cell
- DCM
- dilated cardiomyopathy
- HF
- heart failure
- IFNγ
- interferon gamma
- IL-4
- interleukin-4
- IL-10
- interleukin-10
- iNKT
- invariant natural killer T
- LV
- left ventricle
- LVEF
- left ventricular ejection fraction
- NKT
- natural killer T
- TCR
- T cell receptor
- TGF-β
- transforming growth factor-beta
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCHEARTFAILURE.122.009366.
For Sources of Funding and Disclosures, see page 1137.
Contributor Information
Shouji Matsushima, Email: shouji-m@cardiol.med.kyushu-u.ac.jp.
Soichiro Ikeda, Email: ikedasoi0215@gmail.com.
Kosuke Okabe, Email: bbknumber31@yahoo.co.jp.
Akihito Ishikita, Email: akihito@junnai.org.
Tomonori Tadokoro, Email: tadokoro.tomonori.0526@gmail.com.
Masashi Sada, Email: sadamasa@junnai.org.
Ko Abe, Email: akou@cardiol.med.kyushu-u.ac.jp.
Midori Sato, Email: msatou@cardiol.med.kyushu-u.ac.jp.
Akiko Hanada, Email: hana@molcar.med.kyushu-u.ac.jp.
Shinobu Arai, Email: arai_s@nakamura-u.ac.jp.
Kisho Ohtani, Email: ohtani@cardiol.med.kyushu-u.ac.jp.
Atsushi Nonami, Email: anonami@camiku.kyushu-u.ac.jp.
Shinichi Mizuno, Email: mizuno_s@med.kyushu-u.ac.jp.
Sachio Morimoto, Email: morimoto@iuhw.ac.jp.
Shinichiro Motohashi, Email: motohashi@faculty.chiba-u.jp.
Koichi Akashi, Email: akashi.koichi.357@m.kyushu-u.ac.jp.
Masaru Taniguchi, Email: masaru.taniguchi@riken.jp.
Hiroyuki Tsutsui, Email: htsutsui@med.kyushu-u.ac.jp.
References
- 1.Hershberger RE, Siegfried JD. Update 2011: clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol. 2011;57:1641–1649. doi: 10.1016/j.jacc.2011.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502 [DOI] [PubMed] [Google Scholar]
- 3.Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116:1254–1268. doi: 10.1161/CIRCRESAHA.116.302317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–241. doi: 10.1056/NEJM199007263230405 [DOI] [PubMed] [Google Scholar]
- 5.Bozkurt B, Kribbs SB, Clubb FJ, Jr, Michael LH, Didenko VV, Hornsby PJ, Seta Y, Oral H, Spinale FG, Mann DL. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 1998;97:1382–1391. doi: 10.1161/01.cir.97.14.1382 [DOI] [PubMed] [Google Scholar]
- 6.Bryant D, Becker L, Richardson J, Shelton J, Franco F, Peshock R, Thompson M, Giroir B. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1998;97:1375–1381. doi: 10.1161/01.cir.97.14.1375 [DOI] [PubMed] [Google Scholar]
- 7.Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002;91:988–998. doi: 10.1161/01.res.0000043825.01705.1b [DOI] [PubMed] [Google Scholar]
- 8.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055 [DOI] [PubMed] [Google Scholar]
- 9.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT; Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2 [DOI] [PubMed] [Google Scholar]
- 10.Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2 [DOI] [PubMed] [Google Scholar]
- 11.Torre-Amione G, Anker SD, Bourge RC, Colucci WS, Greenberg BH, Hildebrandt P, Keren A, Motro M, Moyé LA, Otterstad JE, et al. ; Advanced Chronic Heart Failure CLinical Assessment of Immune Modulation Therapy Investigators. Results of a non-specific immunomodulation therapy in chronic heart failure (ACCLAIM trial): a placebo-controlled randomised trial. Lancet. 2008;371:228–236. doi: 10.1016/S0140-6736(08)60134-8 [DOI] [PubMed] [Google Scholar]
- 12.Gullestad L, Aass H, Fjeld JG, Wikeby L, Andreassen AK, Ihlen H, Simonsen S, Kjekshus J, Nitter-Hauge S, Ueland T, et al. Immunomodulating therapy with intravenous immunoglobulin in patients with chronic heart failure. Circulation. 2001;103:220–225. doi: 10.1161/01.cir.103.2.220 [DOI] [PubMed] [Google Scholar]
- 13.McNamara DM, Holubkov R, Starling RC, Dec GW, Loh E, Torre-Amione G, Gass A, Janosko K, Tokarczyk T, Kessler P, et al. Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy. Circulation. 2001;103:2254–2259. doi: 10.1161/01.cir.103.18.2254 [DOI] [PubMed] [Google Scholar]
- 14.Champion S, Lapidus N, Cherié G, Spagnoli V, Oliary J, Solal AC. Pentoxifylline in heart failure: a meta-analysis of clinical trials. Cardiovasc Ther. 2014;32:159–162. doi: 10.1111/1755-5922.12076 [DOI] [PubMed] [Google Scholar]
- 15.Deftereos S, Giannopoulos G, Panagopoulou V, Bouras G, Raisakis K, Kossyvakis C, Karageorgiou S, Papadimitriou C, Vastaki M, Kaoukis A, et al. Anti-inflammatory treatment with colchicine in stable chronic heart failure: a prospective, randomized study. JACC Heart Fail. 2014;2:131–137. doi: 10.1016/j.jchf.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 16.Imai K, Kanno M, Kimoto H, Shigemoto K, Yamamoto S, Taniguchi M. Sequence and expression of transcripts of the T-cell antigen receptor alpha-chain gene in a functional, antigen-specific suppressor-T-cell hybridoma. Proc Natl Acad Sci U S A. 1986;83:8708–8712. doi: 10.1073/pnas.83.22.8708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koseki H, Imai K, Nakayama F, Sado T, Moriwaki K, Taniguchi M. Homogenous junctional sequence of the V14+ T cell antigen receptor α-chain expanded in unprimed mice. Proc Natl Acad Sci USA. 1990;87:5248–5252. doi: 10.1073/pnas.87.14.5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626 [DOI] [PubMed] [Google Scholar]
- 19.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907 [DOI] [PubMed] [Google Scholar]
- 20.Shimizu K, Hidaka M, Bickham K, Moriwaki M, Fujimoto K, Kawano F, Fujii S. Human leukemic cells loaded with alpha-galactosylceramide (alpha-GalCer) activate murine NKT cells in situ. Int J Hematol. 2010;92:152–160. doi: 10.1007/s12185-010-0616-7 [DOI] [PubMed] [Google Scholar]
- 21.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623 [DOI] [PubMed] [Google Scholar]
- 22.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, Kondo E, Harada M, Koseki H, Nakayama T, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc Natl Acad Sci U S A. 1998;95:5690–5693. doi: 10.1073/pnas.95.10.5690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–874. doi: 10.1038/ni827 [DOI] [PubMed] [Google Scholar]
- 24.Sobirin MA, Kinugawa S, Takahashi M, Fukushima A, Homma T, Ono T, Hirabayashi K, Suga T, Azalia P, Takada S, et al. Activation of natural killer T cells ameliorates postinfarct cardiac remodeling and failure in mice. Circ Res. 2012;111:1037–1047. doi: 10.1161/CIRCRESAHA.112.270132 [DOI] [PubMed] [Google Scholar]
- 25.Homma T, Kinugawa S, Takahashi M, Sobirin MA, Saito A, Fukushima A, Suga T, Takada S, Kadoguchi T, Masaki Y, et al. Activation of invariant natural killer T cells by α-galactosylceramide ameliorates myocardial ischemia/reperfusion injury in mice. J Mol Cell Cardiol. 2013;62:179–188. doi: 10.1016/j.yjmcc.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 26.Dick SA, Epelman S. Chronic heart failure and inflammation: what do we really know? Circ Res. 2016;119:159–176. doi: 10.1161/CIRCRESAHA.116.308030 [DOI] [PubMed] [Google Scholar]
- 27.Hasegawa H, Yamashita K, Otsubo D, Kakeji Y. Liver injury after invariant NKT cell activation by free alpha-galactosylceramide and alpha-galactosylceramide-loaded dendritic cells. Anticancer Res. 2016;36:3667–3672. [PubMed] [Google Scholar]
- 28.Motohashi S, Ishikawa A, Ishikawa E, Otsuji M, Iizasa T, Hanaoka H, Shimizu N, Horiguchi S, Okamoto Y, Fujii S, et al. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2006;12(20 Pt 1):6079–6086. doi: 10.1158/1078-0432.CCR-06-0114 [DOI] [PubMed] [Google Scholar]
- 29.Du CK, Morimoto S, Nishii K, Minakami R, Ohta M, Tadano N, Lu QW, Wang YY, Zhan DY, Mochizuki M, et al. Knock-in mouse model of dilated cardiomyopathy caused by troponin mutation. Circ Res. 2007;101:185–194. doi: 10.1161/CIRCRESAHA.106.146670 [DOI] [PubMed] [Google Scholar]
- 30.Li L, Morimoto S, Take S, Zhan DY, Du CK, Wang YY, Fan XL, Yoshihara T, Takahashi-Yanaga F, Katafuchi T, et al. Role of brain serotonin dysfunction in the pathophysiology of congestive heart failure. J Mol Cell Cardiol. 2012;53:760–767. doi: 10.1016/j.yjmcc.2012.08.006 [DOI] [PubMed] [Google Scholar]
- 31.Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M, Iizasa T, Nakayama T, Taniguchi M, Fujisawa T. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005;11:1910–1917. doi: 10.1158/1078-0432.CCR-04-1453 [DOI] [PubMed] [Google Scholar]
- 32.Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, Imaizumi T. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation. 2002;106:130–135. doi: 10.1161/01.cir.0000020689.12472.e0 [DOI] [PubMed] [Google Scholar]
- 33.Sakata Y, Chancey AL, Divakaran VG, Sekiguchi K, Sivasubramanian N, Mann DL. Transforming growth factor-beta receptor antagonism attenuates myocardial fibrosis in mice with cardiac-restricted overexpression of tumor necrosis factor. Basic Res Cardiol. 2008;103:60–68. doi: 10.1007/s00395-007-0689-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khalil H, Kanisicak O, Prasad V, Correll RN, Fu X, Schips T, Vagnozzi RJ, Liu R, Huynh T, Lee SJ, et al. Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac fibrosis. J Clin Invest. 2017;127:3770–3783. doi: 10.1172/JCI94753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulloa L, Doody J, Massagué J. Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway. Nature. 1999;397:710–713. doi: 10.1038/17826 [DOI] [PubMed] [Google Scholar]
- 36.Kimura T, Ishii Y, Morishima Y, Shibuya A, Shibuya K, Taniguchi M, Mochizuki M, Hegab AE, Sakamoto T, Nomura A, et al. Treatment with alpha-galactosylceramide attenuates the development of bleomycin-induced pulmonary fibrosis. J Immunol. 2004;172:5782–5789. doi: 10.4049/jimmunol.172.9.5782 [DOI] [PubMed] [Google Scholar]
- 37.Marcus ML, Koyanagi S, Harrison DG, Doty DB, Hiratzka LF, Eastham CL. Abnormalities in the coronary circulation that occur as a consequence of cardiac hypertrophy. Am J Med. 1983;75(3A):62–66. doi: 10.1016/0002-9343(83)90120-1 [DOI] [PubMed] [Google Scholar]
- 38.Tomanek RJ. Response of the coronary vasculature to myocardial hypertrophy. J Am Coll Cardiol. 1990;15:528–533. doi: 10.1016/0735-1097(90)90620-5 [DOI] [PubMed] [Google Scholar]
- 39.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602 [DOI] [PubMed] [Google Scholar]
- 40.Giordano FJ, Gerber HP, Williams SP, VanBruggen N, Bunting S, Ruiz-Lozano P, Gu Y, Nath AK, Huang Y, Hickey R, et al. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc Natl Acad Sci U S A. 2001;98:5780–5785. doi: 10.1073/pnas.091415198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9 [DOI] [PubMed] [Google Scholar]
- 42.Tao Z, Chen B, Tan X, Zhao Y, Wang L, Zhu T, Cao K, Yang Z, Kan YW, Su H. Coexpression of VEGF and angiopoietin-1 promotes angiogenesis and cardiomyocyte proliferation reduces apoptosis in porcine myocardial infarction (MI) heart. Proc Natl Acad Sci U S A. 2011;108:2064–2069. doi: 10.1073/pnas.1018925108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee H, Schlereth SL, Park EY, Emami-Naeini P, Chauhan SK, Dana R. A novel pro-angiogenic function for interferon-γ-secreting natural killer cells. Invest Ophthalmol Vis Sci. 2014;55:2885–2892. doi: 10.1167/iovs.14-14093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayakawa Y, Takeda K, Yagita H, Smyth MJ, Van Kaer L, Okumura K, Saiki I. IFN-gamma-mediated inhibition of tumor angiogenesis by natural killer T-cell ligand, alpha-galactosylceramide. Blood. 2002;100:1728–1733. [PubMed] [Google Scholar]
- 45.Garcia AG, Wilson RM, Heo J, Murthy NR, Baid S, Ouchi N, Sam F. Interferon-γ ablation exacerbates myocardial hypertrophy in diastolic heart failure. Am J Physiol Heart Circ Physiol. 2012;303:H587–H596. doi: 10.1152/ajpheart.00298.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kimura A, Ishida Y, Furuta M, Nosaka M, Kuninaka Y, Taruya A, Mukaida N, Kondo T. Protective roles of interferon-γ in cardiac hypertrophy induced by sustained pressure overload. J Am Heart Assoc. 2018;7:e008145. doi: 10.1161/JAHA.117.008145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang HX, Li WJ, Hou CL, Lai S, Zhang YL, Tian C, Yang H, Du J, Li HH. CD1d-dependent natural killer T cells attenuate angiotensin II-induced cardiac remodelling via IL-10 signalling in mice. Cardiovasc Res. 2019;115:83–93. doi: 10.1093/cvr/cvy164 [DOI] [PubMed] [Google Scholar]
- 48.Ikeda M, Ide T, Fujino T, Arai S, Saku K, Kakino T, Tyynismaa H, Yamasaki T, Yamada K, Kang D, et al. Overexpression of TFAM or twinkle increases mtDNA copy number and facilitates cardioprotection associated with limited mitochondrial oxidative stress. PLoS One. 2015;10:e0119687. doi: 10.1371/journal.pone.0119687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ikeda M, Ide T, Fujino T, Matsuo Y, Arai S, Saku K, Kakino T, Oga Y, Nishizaki A, Sunagawa K. The Akt-mTOR axis is a pivotal regulator of eccentric hypertrophy during volume overload. Sci Rep. 2015;5:15881. doi: 10.1038/srep15881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikeda M, Ide T, Tadokoro T, Miyamoto HD, Ikeda S, Okabe K, Ishikita A, Sato M, Abe K, Furusawa S, et al. Excessive hypoxia-inducible factor-1α expression induces cardiac rupture via p53-dependent apoptosis after myocardial infarction. J Am Heart Assoc. 2021;10:e020895. doi: 10.1161/JAHA.121.020895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikeda M, Ide T, Furusawa S, Ishimaru K, Tadokoro T, Miyamoto HD, Ikeda S, Okabe K, Ishikita A, Abe K, et al. Heart rate reduction with ivabradine prevents cardiac rupture after myocardial infarction in mice. Cardiovasc Drugs Ther. 2022;36:257–262. doi: 10.1007/s10557-020-07123-5 [DOI] [PubMed] [Google Scholar]
- 52.Arai S, Ikeda M, Ide T, Matsuo Y, Fujino T, Hirano K, Sunagawa K, Tsutsui H. Functional loss of DHRS7C induces intracellular Ca2+ overload and myotube enlargement in C2C12 cells via calpain activation. Am J Physiol Cell Physiol. 2017;312:C29–C39. doi: 10.1152/ajpcell.00090.2016 [DOI] [PubMed] [Google Scholar]
- 53.Inoue T, Ikeda M, Ide T, Fujino T, Matsuo Y, Arai S, Saku K, Sunagawa K. Twinkle overexpression prevents cardiac rupture after myocardial infarction by alleviating impaired mitochondrial biogenesis. Am J Physiol Heart Circ Physiol. 2016;311:H509–H519. doi: 10.1152/ajpheart.00044.2016 [DOI] [PubMed] [Google Scholar]
- 54.Namba T, Tsutsui H, Tagawa H, Takahashi M, Saito K, Kozai T, Usui M, Imanaka-Yoshida K, Imaizumi T, Takeshita A. Regulation of fibrillar collagen gene expression and protein accumulation in volume-overloaded cardiac hypertrophy. Circulation. 1997;95:2448–2454. doi: 10.1161/01.cir.95.10.2448 [DOI] [PubMed] [Google Scholar]
- 55.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185 [DOI] [PubMed] [Google Scholar]
- 56.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quackenbush J. Microarray data normalization and transformation. Nat Genet. 2002;32(Suppl):496–501. doi: 10.1038/ng1032 [DOI] [PubMed] [Google Scholar]
- 58.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, et al. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01 [DOI] [PubMed] [Google Scholar]
- 59.Tadokoro T, Ikeda M, Ide T, Deguchi H, Ikeda S, Okabe K, Ishikita A, Matsushima S, Koumura T, Yamada KI, et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 2020;5:132747. doi: 10.1172/jci.insight.132747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tadokoro T, Ikeda M, Abe K, Ide T, Miyamoto DH, Furusawa S, Ishimaru K, Watanabe M, Ishikita A, Matsushima S, et al. Ethoxyquin is a competent radical-trapping antioxidant for preventing ferroptosis in doxorubicin cardiotoxicity. J Cardiovasc Pharmacol. In press. doi: 10.1097/FJC.0000000000001328 [DOI] [PubMed] [Google Scholar]
- 61.Miyamoto DH, Ikeda M, Ide T, Tadokoro T, Furusawa S, Abe K, Ishimaru K, Enzan N, Sada M, Yamamoto T, et al. Iron overload via heme degradation in the endoplasmic reticulum triggers ferroptosis in myocardial ischemia-reperfusion injury. J Am Coll Cardiol Basic Trans Sci. 2022;7:800–819. doi: 10.1016/j.jacbts.2022.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deguchi H, Ikeda M, Ide T, Tadokoro T, Ikeda S, Okabe K, Ishikita A, Saku K, Matsushima S, Tsutsui H. Roxadustat markedly reduces myocardial ischemia reperfusion injury in mice. Circ J. 2020;84:1028–1033. doi: 10.1253/circj.CJ-19-1039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.