Abstract
Invasive lung disease caused by Aspergillus species is a potentially fatal infection in immunocompromised patients. The adhesion of Aspergillus fumigatus conidia to proteins in the basal lamina is thought to be an initial step in the development of invasive aspergillosis. The purpose of this study was to determine the mechanism of adhesion of A. fumigatus conidiospores to basal-lamina proteins and to determine whether conidia possess unique adhesins which allow them to colonize the host. We compared conidia from different Aspergillus species for the ability to bind to purified fibronectin and intact basal lamina. Adhesion assays using immobilized fibronectin or type II pneumocyte-derived basal lamina showed that A. fumigatus conidia bound significantly better than those of other Aspergillus species to both fibronectin and intact basal lamina. Neither desialylation nor complete deglycosylation of fibronectin decreased the binding of A. fumigatus conidia to fibronectin, suggesting that oligosaccharides on fibronectin were not involved in conidiospore binding. Further evidence for this hypothesis came from experiments using purified fragments of fibronectin; A. fumigatus conidia preferentially bound to the nonglycosylated 40-kDa fragment which contains the glycosaminoglycan (GAG) binding domain. Negatively charged carbohydrates, including dextran sulfate and heparin, as well as high-ionic-strength buffers, inhibited binding of A. fumigatus conidia to both fibronectin and intact basal lamina, suggesting that negatively charged carbohydrates on the surface of the conidium may bind to the GAG binding domain of fibronectin and other basal-lamina proteins. These data provide evidence for a novel mechanism of conidial attachment whereby adherence to fibronectin and other basal-lamina proteins is mediated via negatively charged carbohydrates on the conidial surface.
Over the past decade, the emergence of new fungal pathogens and the reemergence of previously uncommon fungal diseases has escalated (14). This is primarily due to increases in the numbers of immunocompromised persons, for example, bone marrow and organ transplant recipients (47), cancer patients being treated with cytotoxic chemotherapy (4), and people with AIDS (28). The most common invasive mold infection worldwide is caused by Aspergillus species (34). The genus Aspergillus contains several species that are capable of causing disease (Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, Aspergillus terreus, and Aspergillus nidulans); however, over 90% of all cases are caused by A. fumigatus (12). Clinical manifestations of Aspergillus infection include aspergilloma (colonization of preexisting lung cavities) and invasive aspergillosis (8). Inhalation of infectious particles (conidia) results in conidial adhesion followed by hyphal invasion of the bronchial wall (5). Dissemination through the vasculature can result in colonization of other organs, which is associated with a poor prognosis (5). If not treated, the mortality rate of invasive aspergillosis is nearly 100% (13), and the overall success rate of antifungal therapy is only 34% (12). New strategies for the prevention and treatment of invasive aspergillosis are therefore urgently needed.
Since A. fumigatus conidia account for less than 1% of all airborne conidiospores (2), their virulence is thought to be unrelated to their prevalence in the environment. Thus, A. fumigatus must possess unique virulence factors which allow it to colonize the host. The colonization of a host by a pathogenic microorganism depends on its ability to adhere to and, in some cases, invade host cells and tissues (16). Similarly, the development of an Aspergillus infection is thought to be dependent on the adhesion of Aspergillus conidia to host cells and/or to the extracellular matrix (ECM) (7). Recently, several groups have investigated the adhesion of A. fumigatus conidia to purified ECM proteins, such as fibronectin (9, 31), laminin (9, 20, 45), and type IV collagen (9, 20). In addition, Bromley and Donaldson have demonstrated that A. fumigatus conidia can bind to intact lung cell basal lamina in vitro (9).
Various mechanisms have been proposed by which A. fumigatus conidia adhere to these ECM proteins. The cell binding domain of fibronectin contains a sequence, GRGDS, which is specifically recognized by mammalian integrins (33). The pathogenic yeast Candida albicans can bind the GRGDS sequence in complement fragment iC3b (3) via a receptor which shares some similarities with the integrin family of proteins (17, 24), and it has been suggested that integrinlike proteins may be found in A. fumigatus (24). Support for this hypothesis comes from experiments using GRGDS as a competitive ligand which demonstrated that the peptide inhibited the binding of conidia to immobilized fibronectin by approximately 40% (9, 20). In contrast, the literature suggests that the binding of A. fumigatus conidia to laminin occurs by a different mechanism, because addition of the synthetic peptides RGD, GPRP, and YIGSR did not prevent spore attachment to laminin (6). Adhesion to laminin was found to be inhibited by the negatively charged sugars N-acetylneuraminic acid and sialyllactose (6). The authors proposed that sialic acids on the oligosaccharide moiety of laminin were bound by a fungal lectinlike receptor (6). Interestingly, Penicillium marneffei, another fungal pathogen, has been shown to interact with laminin and fibronectin via a sialic acid-dependent mechanism (21, 22); N-acetylneuraminic acid inhibited binding of P. marneffei conidia to both laminin (22) and fibronectin (21).
Preliminary results in our laboratory suggested that A. fumigatus and other Aspergillus species differed in their extents of binding to fibronectin. Furthermore, binding was not inhibited by GRGDS, indicating that the cell binding domain may not be involved in conidiospore adhesion to fibronectin. Therefore, the aims of the present study were twofold: (i) to determine whether A. fumigatus conidia bound to purified ECM proteins and basal lamina to a greater extent than the conidia of other Aspergillus species and (ii) to establish the mechanism by which A. fumigatus conidia adhere to fibronectin and basal lamina. The results of these studies provide strong evidence for a novel binding mechanism involving negatively charged carbohydrates present on the conidiospore cell wall.
MATERIALS AND METHODS
Aspergillus strains and growth conditions.
The following Aspergillus species were used in this study. Three strains of A. fumigatus, ATCC 13073, ATCC 42202, and CHUV (a gift from M. Monod, Laboratoire de Mycologie, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland); three strains of A. flavus, hospital isolate number 1 (HI 1) (isolated from a patient with chronic granulomatous disease at British Columbia Children's Hospital, Vancouver), ATCC 11495, and ATCC 64841; two strains of Aspergillus ornatus, ATCC 16921 and ATCC 66492; and two strains of Aspergillus wentii, ATCC 10584 and ATCC 1023. The fungi were grown on YM agar (0.3% yeast extract, 0.3% malt extract, 0.5% peptone, and 0.5% dextrose) for 5 to 14 days at 28°C (except A. ornatus ATCC 66492, which was grown at 24°C) until the conidia were fully mature. The conidia were harvested by flooding the plate with phosphate-buffered saline, pH 7.4 (PBS)–0.05% Tween 20 and gently scraping it with a sterile cotton swab. The suspension was then vortexed for 1 min to break up the chains of conidia, and the hyphal fragments were removed by filtration through sterile glass wool. The conidia were pelleted by centrifugation (1,500 × g; 2 min), resuspended in PBS, and counted with a hemacytometer.
Preparation of basal lamina from cultured lung cells.
The type II pneumocyte cell line A549 was obtained from the American Type Culture Collection (Manassas, Va.) (CCL-185). The cells were seeded overnight in RPMI 1640 medium (Life Technologies, Burlington, Ontario, Canada) containing 10% fetal bovine serum (ICN Pharmaceuticals, Montreal, Canada), 100 mg of streptomycin/liter, and 16 mg of penicillin/liter (both from Sigma-Aldrich Canada, Oakville, Ontario, Canada) at 1.5 × 105 cells/well (for chamber slide experiments) or at 1.0 × 105 cells/well (for microtiter plate experiments). The following day, the cells were stripped off by treating them with 0.1 M NH4OH for 30 min at 37°C, and the wells were then washed two times with PBS. This procedure removes the cells but leaves the basal lamina intact (27). The presence of basal lamina was confirmed by immunodetection with a mouse monoclonal anti-type IV collagen antibody (M3F-7) (Developmental Studies Hybridoma Bank, University of Iowa).
Peroxidase labeling of A. fumigatus conidia.
The biotinylation and peroxidase labeling of conidia were carried out essentially as described by Peñalver et al. (31) with minor modifications. Briefly, 2 × 108 A. fumigatus ATCC 13073 conidia/ml were resuspended in 100 mM carbonate buffer, pH 10.0, containing 2 mg of n-hydroxysuccinimidobiotin (Sigma)/ml (previously dissolved in dimethyl sulfoxide) and incubated at 28°C for 75 min on a rotator. The biotinylated conidia were then washed twice with 50 mM sodium phosphate (pH 6.0) and once with 10 mM Tris-Cl (pH 7.4)–0.9% NaCl (TBS) and finally resuspended in TBS containing 0.05% Tween 20 and 1% bovine serum albumin (BSA) (ICN). ExtrAvidin peroxidase (Sigma) was added at 1:100, and the solution was incubated for 1 h at room temperature. The labeled conidia were then washed three times with PBS and counted with a hemacytometer. Peroxidase labeling of conidia does not interfere with adhesion of the conidia to fibronectin, laminin, or type IV collagen (20).
Adherence assays on glass slides.
Fibronectin at 0 or 50 μg/ml (dissolved in 300 μl of PBS) was added to eight-well chamber slides (Becton-Dickinson Canada, Inc., Mississauga, Ontario, Canada) and incubated for 1 h at 37°C and then overnight at 4°C. The basal lamina was prepared in chamber slides as described above. The slides were blocked with PBS–0.1% BSA (500 μl per well) for 1 h at 37°C. This blocking solution was then aspirated, and 200 μl of conidium suspension at 108/ml was added to protein-coated wells or control wells without protein. The slides were incubated for 1 h at 37°C, after which nonadherent conidia were removed by washing the slides three times with 500 μl of PBS–0.05% Tween 20/well. The bound conidia were then fixed for 30 min with 300 μl of 2.5% glutaraldehyde in PBS and counted with an Olympus Vanox microscope at ×1,000 magnification. Five fields were captured for each well (by a Sony 950 video camera connected to the microscope), and the number of conidia per field was determined by the computer program Eclipse (Empix Imaging, Mississauga, Ontario, Canada).
Adherence assay on microtiter plates.
Adherence assays were performed essentially as described by Peñalver et al. (31). Immulon 2 microtiter plates (VWR Canlab, Edmonton, Canada) were coated with 200 μl of a 50-μg/ml fibronectin solution (dissolved in PBS) for 1 h at 37°C and then overnight at 4°C. Microtiter plates were coated with the basal lamina as described above. For the experiments using fibronectin fragments, each well was coated with 200 μl of a 2.5 μM solution of each fragment (or 0.12 μM intact fibronectin). The plates were blocked with 200 μl of PBS–0.1% BSA per well for 1 h at 37°C. The wells were aspirated, and then 200 μl of peroxidase-labeled A. fumigatus ATCC 13073 conidia at 108/ml was added to each well. Nonadherent conidia were removed by washing the plates three times with 200 μl of PBS–0.05% Tween 20 per well. Bound conidia were detected by the addition of 100 μl of substrate solution (phosphate-citrate buffer [pH 5.0] containing 0.4 mg of o-phenylenediamine/ml and 0.4 μl of H2O2/ml) per well for 2 to 8 min at room temperature. The reaction was stopped with the addition of 25 μl of 3 M H2SO4 per well. The plates were read on a microplate reader (Bio-tek Instruments) at 490 nm. For fibronectin fragment inhibitor experiments, the conidia were preincubated with a 3.75 μM solution of one of the fragments for 1 h at 37°C before being added to fibronectin-coated wells. For carbohydrate inhibitor experiments, the conidia were coincubated with either dextran sulfate (Sigma), porcine heparin (Calbiochem, La Jolla, Calif.), keratan sulfate, or chondroitin sulfate at the concentrations noted in the figure legends. All carbohydrate solutions were used at physiological pH. For the peptide inhibitor experiments, the conidia were coincubated with GRGDS peptide (Sigma) or scrambled peptide SGGDR (University of Victoria Microsequencing Center, Victoria, British Columbia, Canada). For the experiments examining the effects of ionic strength on conidial adhesion, conidia were suspended in PBS containing differing concentrations of NaCl (1.6, 4.0, 8.0, 16.0, 40.0, or 80.0 g/liter).
Desialylation of fibronectin.
Fibronectin (50 μg) was incubated with 0 or 0.05 U of Vibrio cholerae α 2-3,6,8 neuraminidase (Calbiochem) for 16 h at 37°C in 50 mM sodium acetate (pH 5.5) containing 1 mM CaCl2 and 1× protease inhibitor cocktail (Sigma). The following day, the samples were diluted in PBS and 10 μg of protein was added to the wells of an Immulon 2 plate. Another sample (1.5 μg) was tested for desialylation by lectin blotting as described below. The plate was incubated at 37°C for 1 h and then at 4°C overnight. The next day a standard microtiter plate adherence assay was performed.
Lectin blotting of desialylated fibronectin.
Desialylated and native fibronectin were run on a 7.5% sodium dodecyl sulfate (SDS) polyacrylamide gel (Bio-Rad Mini Protean II system) (1.5 μg/lane) and transferred to nitrocellulose membrane using the Pharmacia semidry transfer apparatus according to the manufacturer's directions. The membrane was blocked for 1 h in PBS–0.05% Tween 20–1% skim milk and then probed with 10 μg of biotinylated Sambucus nigra agglutinin (SNA) (Sigma)/ml diluted in PBS–0.05% Tween 20 for 2 h. To biotinylate SNA, 0.06 mg of N-hydroxysuccinimido-long chain Biotin (Pierce, Rockford, Ill.) was incubated with 1 mg of SNA in 0.1 M sodium phosphate–0.15 M NaCl (pH 7.2) for 2 h at room temperature. Unreacted biotin was removed by filtration through an Ultrafree 10,000 molecular weight cutoff filter (Millipore) according to the manufacturer's instructions. SNA recognizes sialic acid α (2→6) galactose (39), which is the predominant sialic acid found on bovine fibronectin (25). The blot was rinsed three times for 10 min each time with PBS–0.05% Tween and then incubated with streptavidin-horseradish peroxidase (Life Technologies) diluted 1:2,000 in PBS–0.05% Tween 20 for 1 h. The membrane was washed again as described above (four times) and then developed with the DAB substrate (6 mg of diaminobenzidine tetrahydrochloride [Sigma] in 10 mM Tris-Cl [pH 7.6] containing 0.3% NiCl2 and 1 μl of H2O2/ml).
Deglycosylation of fibronectin.
Fibronectin was deglycosylated according to the manufacturer's instructions with the ProLink deglycosylation kit (Prozyme Inc., San Leandro, Calif.). Briefly, 40 μg of protein was deglycosylated under denaturing or nondenaturing conditions. Fibronectin was denatured by heating it at 100°C in denaturation buffer prior to the addition of glycosidase enzymes. The protease inhibitors aprotinin, leupeptin (at 10 μg/ml), and pepstatin (at 0.7 μg/ml) (all from Sigma) were included to prevent degradation of fibronectin by any contaminating proteases. Deglycosylation reactions were carried out for 3 h (for denatured samples) or 6 h (for nondenatured samples) at 37°C. Deglycosylation was confirmed by running deglycosylated and native proteins on SDS-polyacrylamide gel electrophoresis and observing the decrease in molecular mass; native fibronectin had a molecular mass of 206 kDa, and deglycosylated fibronectin was 201 kDa. Microtiter plates were then coated with the samples for use in spore adherence assays.
Miscellaneous chemicals and reagents.
Bovine fibronectin and the 40-kDa fibronectin fragment were obtained from Sigma Chemical Co., and the 45- and 120-kDa fibronectin fragments were from Canadian Life Technologies. Microbial culture supplies were from Difco Laboratories (Detroit, Michigan). Miscellaneous chemicals were from Sigma. Keratan sulfate and chondroitin sulfate were obtained from C. R. Roberts, Department of Oral Biological and Medical Sciences, University of British Columbia, Vancouver, British Columbia, Canada.
Statistics.
Differences in binding between Aspergillus species were analyzed by Proc Mixed analysis of the difference as a randomized complete block design in SAS (version 6.12; SAS Institute). Variations were reported as the 95% confidence interval of the mean and therefore are equivalent for all data sets. For all other experiments, the results are expressed as the mean ± standard deviation of three replicates, and each experiment was done three times unless otherwise noted. The Student t test was used for statistical analysis of data.
RESULTS
A. fumigatus conidia bind significantly better to basal lamina and fibronectin than those of other Aspergillus species.
To determine whether A. fumigatus conidia preferentially adhere to extracellular matrix proteins compared to those of other Aspergillus species, the relative levels of binding to intact lung cell basal lamina and to fibronectin in four species (two to three strains/species) within the Aspergillus genus were compared. In addition to the three A. fumigatus strains, A. flavus (three strains), A. wentii (two strains), and A. ornatus (two strains) were also studied. A. flavus and A. wentii can cause aspergillosis, although they are much less frequently observed as causative agents of invasive aspergillosis (especially A. wentii) than A. fumigatus (12). A. ornatus is a nonpathogenic Aspergillus species (34).
We first investigated the adhesion of Aspergillus species to intact basal lamina from cultured lung cells. Conidia from each species were added to basal-lamina-coated plates, and the bound conidia were counted by computer-aided microscopy. The number of A. fumigatus conidia bound to the basal lamina was dependent on the amount of basal lamina present (data not shown). All A. fumigatus strains bound significantly better to the basal lamina than both A. ornatus strains and A. wentii strains (P < 0.05) (up to 50-fold greater) (Table 1).
TABLE 1.
Adhesion of Aspergillus species to A549-derived basal laminaa
| Species and strain | No. of conidia bound per field (magnification, ×1000) |
|---|---|
| A. fumigatus ATCC 13073 | 242 ± 36 |
| A. fumigatus ATCC 42202 | 234 ± 36 |
| A. fumigatus CHUV | 225 ± 36 |
| A. wentii ATCC 10584 | 18 ± 36b |
| A. wentii ATCC 1023 | 27 ± 36b |
| A. ornatus ATCC 16921 | 5 ± 36b |
| A. ornatus ATCC 66492 | 7 ± 36b |
A549 cells (1.5 × 105/well) were seeded into chamber slides and incubated overnight. The following day, the basal lamina was prepared (see Materials and Methods) and added to each well. Bound conidiospores were counted as described in Materials and Methods.
Statistically different from A. fumigatus strains (P < 0.05) as determined by Proc Mixed analysis of the difference as a randomized complete block design in SAS. The values are the least-squares means (background subtracted) ± 2 standard errors of triplicate wells of two independent experiments performed on separate days.
We next investigated the adhesion of Aspergillus species to purified fibronectin, a component of the basal lamina (37). A. fumigatus conidia bound significantly better (P < 0.05) to fibronectin (up to 90-fold) than those of the nonpathogenic A. ornatus strains and A. wentii ATCC 10584; A. wentii ATCC 1023, a rare pathogen, showed intermediate levels of binding (Table 2). Interestingly, A. flavus strains bound to fibronectin at levels comparable to (or less than) those of A. wentii strains; adhesion was 10- to 50-fold less than the adhesion of A. fumigatus.
TABLE 2.
Adhesion of Aspergillus species to fibronectina
| Species and strain | No. of conidia bound per field (magnification, ×1000) |
|---|---|
| A. fumigatus ATCC 13073 | 273 ± 52 |
| A. fumigatus ATCC 42202 | 263 ± 52 |
| A. fumigatus CHUV | 151 ± 52 |
| A. flavus HI no. 1 | 30 ± 52b |
| A. flavus ATCC 11495 | 4 ± 52b |
| A. flavus ATCC 64841 | 30 ± 52b |
| A. wentii ATCC 10584 | 13 ± 52b |
| A. wentii ATCC 1023 | 91 ± 52 |
| A. ornatus ATCC 16921 | 3 ± 52b |
| A. ornatus ATCC 66492 | 4 ± 52b |
Chamber slides were coated with fibronectin at the indicated concentrations. Conidia were allowed to adhere, and then unbound conidia were washed off with PBS-Tween. Bound conidia were counted as described in Materials and Methods.
Statistically different from A. fumigatus strains (P < 0.05) as determined by Proc Mixed analysis of the difference as a randomized complete block design in SAS. The values are the least-squares means (background subtracted) ± 2 standard errors of triplicate wells of three independent experiments performed on separate days.
Taken together, these results demonstrate that A. fumigatus conidia adhere to both fibronectin and basal lamina significantly better than those of other, less pathogenic Aspergillus species. Furthermore, the results with fibronectin paralleled those obtained using intact basal lamina, suggesting that fibronectin may be a good model ECM protein for the study of A. fumigatus spore adhesion to lung basal lamina. All subsequent experiments employed A. fumigatus strain ATCC 13073.
Adhesion of A. fumigatus conidia to fibronectin is not mediated via the RGD peptide or oligosaccharides on the fibronectin glycoprotein.
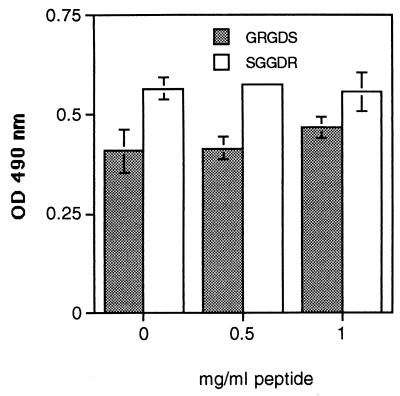
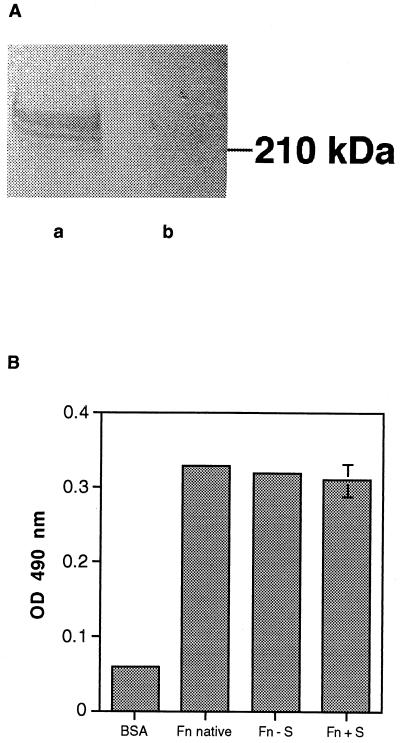
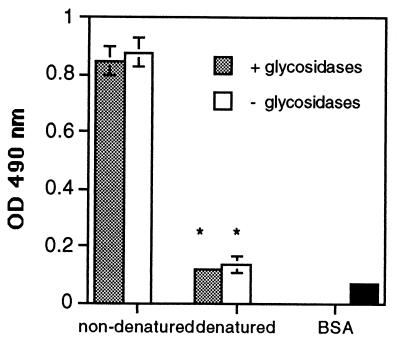
Previous studies have indicated that A. fumigatus conidia may bind to fibronectin at the cell binding domain via the RGD sequence, because addition of GRGDS peptide reduced binding by 40% (9, 20). However, we have found that neither GRGDS nor a scrambled peptide control, SGGDR, significantly inhibited the adhesion of A. fumigatus conidia to fibronectin (Fig. 1). From their studies of spore adhesion to laminin, Bouchara et al. concluded that sialic acids present in the oligosaccharide moieties of the laminin glycoprotein bind to a receptor on the spore surface (6). Because fibronectin is also glycosylated, we postulated that a similar mechanism may mediate spore binding to fibronectin. Fibronectin was desialylated by incubating it for 16 h in the presence or absence of V. cholerae α 2-3,6,8 neuraminidase. The removal of sialic acid was confirmed by immunoblotting both native and desialylated fibronectin with a sialic acid-specific lectin, SNA. SNA detected a 220-kDa band only in the native, sialylated form (Fig. 2A). A microtiter plate was then coated with the samples and used in an adherence assay. A. fumigatus conidia bound equally well to the sialidase-treated and untreated fibronectin (Fig. 2B). To determine whether conidia were binding to other sugars on the remaining portions of the oligosaccharide chains, fibronectin was completely deglycosylated under denaturing and nondenaturing conditions and conidial attachment was measured using a spore binding assay in microtiter plates. Deglycosylation was monitored by SDS-polyacrylamide gel electrophoresis (data not shown). A. fumigatus conidia bound equally well to glycosylated and deglycosylated fibronectin (Fig. 3); however, denaturation of fibronectin significantly decreased conidiospore binding (P < 0.05) (Fig. 3). Taken together, these results suggest that the oligosaccharide chains of fibronectin are not required for conidiospore binding and that fibronectin must have an intact tertiary protein structure for A. fumigatus conidia to adhere.
FIG. 1.
Effect of GRGDS peptide on the adhesion of A. fumigatus conidia to fibronectin. Microtiter plates were coated with fibronectin at 50 μg/ml for 1 h at 37°C, and the next day a standard conidium binding assay was performed. Background wells contained BSA only. Peroxidase-labeled conidia were diluted in 0, 0.5, or 1.0 mg of GRGDS or SGGDR peptide/ml and added to the fibronectin-coated wells for 1 h at 37°C. Unbound conidia were washed with PBS-Tween, and the number of bound conidia was determined by measuring the optical density (OD) at 490 nm after the addition of substrate. The values are the means ± standard deviations of triplicate wells, and the figures are representative of three independent experiments.
FIG. 2.
Adhesion of A. fumigatus conidia to desialylated fibronectin. (A) Fibronectin was incubated with or without V. cholerae neuraminidase for 16 h at 37°C. Samples were run on a 7.5% acrylamide gel, transferred to nitrocellulose, and then probed with a biotinylated sialic acid-binding lectin, SNA. The lectin bound only to the untreated fibronectin (a) and not to the sialidase-treated sample (b). The location of the molecular mass standard is shown on the right. (B) Microtiter plates were coated with fibronectin (Fn) (untreated and desialylated as described above) from panel A for 1 h at 37°C, and the next day a standard conidium binding assay was performed. A sample of native fibronectin (not pretreated with acetate buffer overnight) was included as a control (native). Background wells contained BSA only. Peroxidase-labeled conidia were added to the wells, and the number of bound conidia was determined by measuring the optical density (OD) at 490 nm after the addition of substrate. The values are the means ± standard deviations of triplicate wells, and the figures are representative of three independent experiments. Fn + S, sialidase treated fibronectin; Fn − S, sham treated fibronectin (no enzyme).
FIG. 3.
Adhesion of A. fumigatus conidia to native and deglycosylated fibronectin. Fibronectin was treated with glycosidases (shaded bars) or no enzymes (open bars) under denaturing (3 h at 37°C following heating to 100°C for 5 min in denaturation buffer) or nondenaturing (6 h at 37°C) conditions, and then microtiter plates were coated with it. The background wells contained BSA only (solid bars). Peroxidase-labeled conidia were added to the wells, and the number of bound conidia was determined by measuring the optical density (OD) at 490 nm after the addition of substrate. The values are the means ± standard deviations of triplicate wells, and the figures are representative of three independent experiments. ∗, P < 0.05 versus nondenatured.
A. fumigatus conidia bind to the GAG binding region of fibronectin.
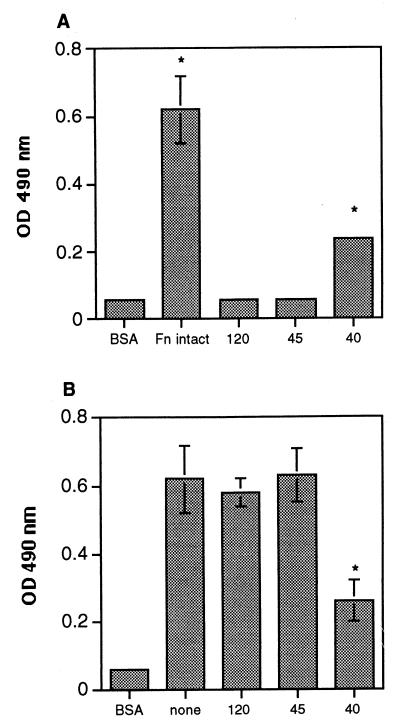
To establish whether the conidia were attaching to a specific region of the fibronectin protein, microtiter plates were coated with individual fragments of fibronectin containing either the gelatin-binding domain (45 kDa) (26, 42), the cell binding domain (120 kDa) (32), or the glycosaminoglycan (GAG) binding domain (40 kDa) (18, 26), and the fragments were tested for the ability to promote conidial binding. Of the three fragments, only the 40-kDa fragment (containing the GAG binding domain) bound significant amounts of conidia (P < 0.05) (Fig. 4A). However, binding of the conidia to the 40-kDa fragment was only observed when at least a 2.5 μM concentration of this fragment was bound to the plate, whereas 0.12 μM intact fibronectin could promote adhesion; i.e., 20 times more fragment than intact protein was required to bind comparable numbers of conidia. These data provide additional evidence that the intact three-dimensional structure of fibronectin may be necessary for conidia to adhere. To confirm that conidiospores bound selectively to the 40-kDa fragment, we tested the ability of the three fibronectin fragments to act as competitive inhibitors in a fibronectin-binding assay. Conidia were preincubated with one of the three fragments and then added to microtiter plates coated with intact fibronectin. Preincubation of the conidia with the 40-kDa fragment inhibited their binding to immobilized fibronectin by 64% (versus background), and this was the only fragment that was able to block adhesion (P < 0.05) (Fig. 4B). These data further support our hypothesis that the cell binding domain of fibronectin is not involved in spore binding. In addition, they also confirm that conidia do not adhere to fibronectin via oligosaccharide chains on this protein; oligosaccharide chains are found on both the 45- and the 120-kDa fragments, but not the 40-kDa fragment (42).
FIG. 4.
Adhesion of A. fumigatus conidia to fibronectin protein fragments. Microtiter plates were coated with intact fibronectin (Fn intact) or fibronectin fragments containing the gelatin-binding domain (45 kDa [45]), the cell binding domain (120 kDa [120]), or the GAG binding domain (40 kDa [40]) and tested for the ability to promote spore adhesion. Background wells contained BSA only. Peroxidase-labeled conidia were added to wells containing fragments alone (A) or were preincubated with the fragments before conidia were added to wells coated with fibronectin (B). Unbound conidia were removed by washing the plates with PBS–0.05% Tween 20, and the number of bound conidia was determined by measuring the optical density (OD) at 490 nm. The values are the means ± standard deviations of triplicate wells, and the figures are representative of two independent experiments. ∗, P < 0.05 versus BSA (for panel A) or versus none (for panel B).
Negatively charged carbohydrates block adhesion of A. fumigatus conidia to fibronectin.
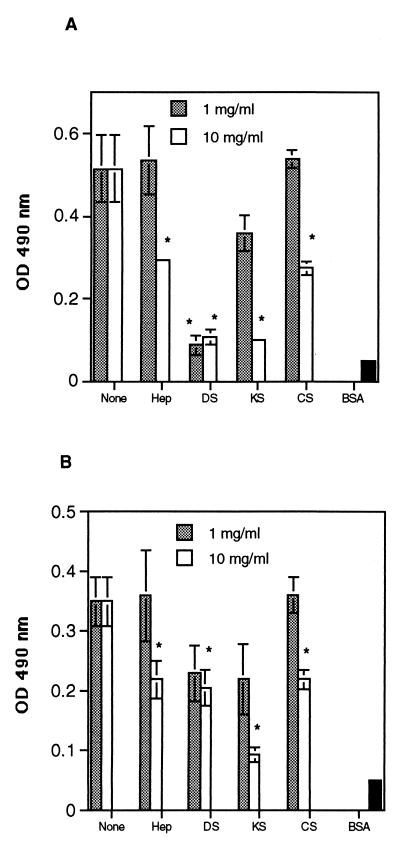
Like many other ECM proteins, fibronectin is made up of repeating modules: each module is 40 to 90 amino acids in length and is classified as either a type I, II, or III repeat (35). The 40-kDa GAG binding fragment contains three type III repeats (12 to 14), and GAG binding occurs at modules 13 and 14 (1, 10, 41). Previous work has demonstrated that a threshold level of sulfate content in GAGs is essential for fibronectin affinity (29). Other sulfated polysaccharides, such as dextran sulfate, bind to fibronectin if they are sufficiently negatively charged (29). To determine whether negatively charged carbohydrates on the conidial cell wall were responsible for binding to the GAG binding region of fibronectin, different GAGs and negatively charged carbohydrates were used as competitive inhibitors in a fibronectin binding assay. Binding of A. fumigatus conidia to immobilized fibronectin was inhibited by 48% with 10 mg of heparin/ml and was inhibited by 88% with 10 mg of dextran sulfate/ml (P < 0.05) (Fig. 4A). Interestingly, keratan sulfate and chondroitin sulfate (at 10 mg/ml), which have not been reported as known ligands of the GAG binding domain of fibronectin, also decreased binding of conidia to fibronectin by 100 and 52%, respectively (P < 0.05) (Fig. 5A). In contrast, the neutral sugars glucose and galactose did not inhibit spore binding at concentrations up to 30 mg/ml (data not shown).
FIG. 5.
Effects of negatively charged carbohydrates on the adhesion of A. fumigatus conidia to fibronectin and basal lamina. Peroxidase-labeled conidia were added to fibronectin-coated microtiter plates (A) or to basal-lamina-coated microtiter plates (B) in the presence of heparin (Hep), dextran sulfate (DS), keratan sulfate (KS), or chondroitin sulfate (CS). Unbound conidia were removed by washing the plates with PBS–0.05% Tween 20, and the number of bound conidia was determined by measuring the optical density (OD) at 490 nm. Background wells contained BSA only. The values are the means ± standard deviations of triplicate wells. These figures are representative of two independent experiments. ∗, P < 0.05 versus none.
We next investigated whether negatively charged sugars could inhibit conidial binding to intact basal lamina. Binding of conidia to basal lamina was inhibited by 44% with heparin, 49% with dextran sulfate, 87% with keratan sulfate, and 44% with chondroitin sulfate (all at 10 mg/ml) (P < 0.05) (Fig. 5B). Thus, negatively charged carbohydrates inhibited binding of A. fumigatus conidia to both fibronectin and intact basal lamina.
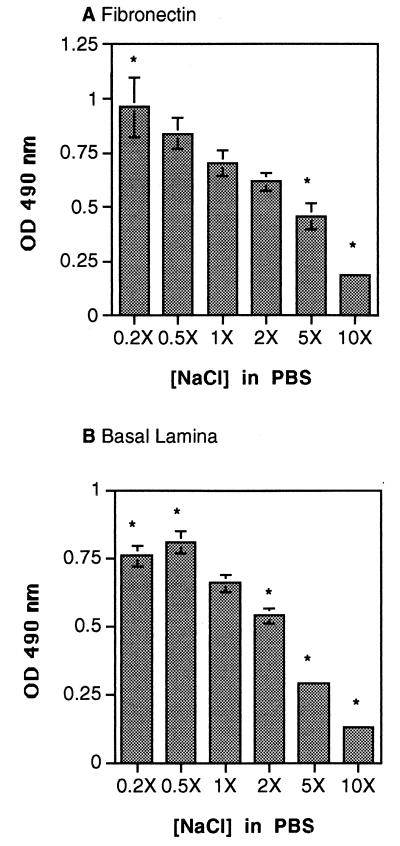
Adhesion of A. fumigatus conidia to fibronectin and basal lamina is dependent on ionic strength.
Binding of anionic heparin to the high-affinity GAG binding domain is known to occur through a cluster of positively charged amino acids in modules 13 and 14 of fibronectin (10, 41). Consequently, binding strength is dependent on the ionic strength of the buffer, and the dissociation constant (Kd) of heparin for fibronectin increases as the ionic strength increases (26). To establish whether spore binding to fibronectin and to basal lamina was mediated by ionic bonds, we monitored the extent of spore adhesion to fibronectin and to intact basal lamina in buffers of various ionic strengths. We found that conidial binding to both fibronectin and basal lamina increased by 15 to 25% in low-ionic-strength buffer (1.6 to 4.0 g of NaCl/liter) (Fig. 6), whereas high-ionic-strength buffer (40 to 80 g of NaCl/liter) inhibited the binding of conidia to both fibronectin and basal lamina by 70 to 80% (Fig. 6). Thus, spore adhesion to fibronectin occurs via ionic bonds, probably at the high-affinity GAG binding site. Ionic bonds also appear to mediate conidial binding to intact basal lamina.
FIG. 6.
Effect of ionic strength on the adhesion of A. fumigatus conidia to fibronectin and basal lamina. Peroxidase-labeled conidia were added to fibronectin-coated (A) or basal-lamina-coated (B) microtiter plates in the presence of increasing amounts of NaCl in the assay buffer (PBS): 0.2×, 1.6 g/liter; 0.5×, 4.0 g/liter; 1×, 8.0 g/liter (standard PBS amount); 2×, 16.0 g/liter; 5×, 40.0 g/liter; and 10×, 80.0 g/liter. Unbound conidia were removed by washing the plates with PBS–0.05% Tween 20, and the number of bound conidia was determined by measuring the optical density (OD) at 490 nm. Background wells contained BSA only. The values are the means ± standard deviations of triplicate wells. These figures are representative of two independent experiments. ∗, P < 0.05 versus 1× NaCl in PBS.
DISCUSSION
This study presents evidence that negatively charged carbohydrates on the surface of A. fumigatus conidia may mediate their adhesion to purified fibronectin. Furthermore, results from parallel experiments using intact basal lamina suggest that conidia may bind to fibronectin and possibly other proteins present in the basal lamina via a similar mechanism. Support for these conclusions comes from the finding that A. fumigatus conidia bound to the 40-kDa fragment containing the GAG binding domain of fibronectin but not to the 45-kDa gelatin-binding domain or the 120-kDa cell binding domain. Moreover, adhesion of conidia to fibronectin and intact basal lamina was inhibited by negatively charged carbohydrates and by high-ionic-strength buffers, implicating ionic bonds as the major binding mechanism.
Alveolar basal lamina is a specialized ECM composed of laminin, type IV and V collagen, entactin, chondroitin sulfate proteoglycan, heparan sulfate proteoglycan, and fibronectin (15). The origin of the fibronectin is not clear; however, some studies suggest that circulating plasma fibronectin may become deposited in the alveolar basal lamina (19, 30). Along with neutropenia, lung tissue damage is a known risk factor for developing invasive aspergillosis (5). Lung injury is accompanied by interstitial edema, rupture of the basal lamina, and accumulation of migrating inflammatory cells (38). During lung injury and inflammation, the distribution and deposition of ECM components are altered (37). In comparison to healthy lungs, the basal lamina of diseased lungs have increased amounts of fibronectin and other ECM proteins (38, 44), and it has been suggested that deposition of fibronectin upon denuded basal lamina may assist in reepithelialization by providing an anchor for cell attachment (11). In the damaged lung, inhaled conidia may have increased access to fibronectin and other ECM proteins, and therefore adhesion to these proteins may be important in the development of invasive aspergillosis. The finding that A. fumigatus binds to fibronectin and intact basal lamina to a greater extent than other Aspergillus species supports this hypothesis, as A. fumigatus infections account for over 90% of all cases of invasive aspergillosis. Nevertheless, adhesion is only one factor of many that promote the development of invasive aspergillosis.
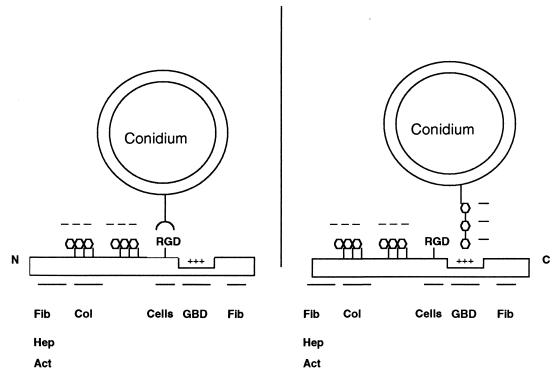
Previous studies have suggested that A. fumigatus conidia possess a lectinlike receptor which may bind to sialic acids on the oligosaccharide chains of both laminin and fibrinogen (6). This conclusion was based on the findings that sialic acid and sialylated glycoproteins such as mucin inhibited binding of A. fumigatus conidia to laminin and fibrinogen (6). In contrast, our results demonstrated that conidia bound equally well to native, desialylated, and fully deglycosylated fibronectin. We have also found that conidia bound equally well to the sialidase-treated and native laminin (J. A. Wasylnka and M. M. Moore, unpublished observations), a result not consistent with the model proposed by Bouchara et al. (6). These data strongly suggest that conidia do not bind to fibronectin or laminin via its oligosaccharides. Furthermore, we have shown that negatively charged carbohydrates, such as dextran sulfate and heparin, decreased spore binding to both fibronectin and basal lamina. Therefore, negatively charged carbohydrates on the spore cell wall may be ligands for fibronectin and other ECM proteins. Two current models of conidial binding to fibronectin are shown in Fig. 7.
FIG. 7.
Two models of A. fumigatus conidium adherence to fibronectin. Fibronectin is depicted as a linear structure with the location of the domains noted, as well as the major ligands for these domains, abbreviated as noted below. (Left model) An integrinlike protein on the conidium binds to the RGD peptide in the cell binding domain. (Right model) Proposed mechanism of adherence based on the data presented here. Negatively charged carbohydrates on the conidial cell wall bind to a cluster of positively charged amino acids in the GAG binding domain of fibronectin. Note: fibronectin is a dimer linked by two disulfide bonds at the C terminal; these are not shown for clarity. Fib, fibrin; Hep, heparin; Act, actin; Col, collagen; Cells, cell binding domain; GBD, GAG binding domain.
We have established that adhesion of A. fumigatus conidia to fibronectin and basal lamina was dependent on ionic strength and was inhibited by negatively charged carbohydrates. This could be explained in two ways: fibronectin may be the only binding site for conidia in the basal lamina, or more plausibly, binding of conidia to other ECM proteins is also mediated by ionic interactions. Glycoproteins, such as laminin and collagen, are also known to contain GAG binding domains (46, 49); therefore, we postulate that A. fumigatus conidia may bind to basal lamina via the GAG binding domains present in fibronectin and other ECM proteins. Further research is necessary to confirm this hypothesis.
The crystal structure of the GAG binding domain of human fibronectin has been determined (41). The major GAG binding site (HBS-1) is found in fibronectin type III repeat 13 and consists of four basic amino acids which are arranged in a continuous cluster (41). A minor GAG binding site is also found in fibronectin type III repeat 14 (HBS-2); the distance between HBS-1 and HBS-2 is 60 Å (41). Other studies have determined that 12 to 16 oligosaccharides (∼51 to 69 Å) are required for optimal binding of heparin to fibronectin (48). Consequently, Sharma et al. have proposed that GAG binding spans from HBS-1 to HBS-2 (41). Our study suggests that negatively charged molecules on the spore surface bind to the positively charged cleft of the GAG binding domain of fibronectin, but at present, the identity of the ligand on the conidial surface is not known.
The cell wall of A. fumigatus contains over 70% carbohydrate, specifically β (1→3) glucan and chitin (23). In addition, many of the proteins present on the conidium surface are heavily glycosylated (23). Since negatively charged polysaccharides inhibited conidium binding to both fibronectin and basal lamina, negatively charged carbohydrates are good candidates for ligands to ECM proteins. Negatively charged carbohydrates can include GAG-like polysaccharides, glucuronic or iduronic acid polysaccharides, or sialic acids. Sialic acids are negatively charged carbohydrates and could represent potential conidiospore ligands for ECM proteins. Other pathogenic fungi, such as Paracoccidioides brasilensis (43) and Cryptococcus neoformans (36), have been shown to contain sialic acids on the surface of the cell wall. Lectin binding experiments in our laboratory have shown that A. fumigatus conidia also possess sialic acids (J. A. Wasylnka and M. M. Moore, unpublished observations).
If conidia cell wall carbohydrates mediate adhesion to components of the ECM, then oxidation of these carbohydrates should decrease adhesion to fibronectin and intact basal lamina. Preliminary results in our laboratory suggest that A. fumigatus sialic acids cannot be oxidized by sodium periodate (data not shown). Sialic acids that are acetylated at carbons 7 to 9 are resistant to periodate oxidation (40). Such acetylated sialic acids have been shown to be present in the cell wall of C. neoformans (36). If A. fumigatus possesses acetylated sialic acids, this may explain its resistance to periodate oxidation.
We have presented evidence that suggests that negatively charged carbohydrates present on the A. fumigatus conidium surface interact with the GAG binding domain of fibronectin. Further experiments are under way to identify the A. fumigatus ligand that interacts with fibronectin and intact basal lamina.
ACKNOWLEDGMENTS
This work was supported by grants from the British Columbia Health Care Research Foundation and the Natural Sciences and Engineering Research Council of Canada (NSERC) to M.M.M. J.A.W. was supported by an NSERC predoctoral fellowship. The M3F-7 antibody developed by Heinz Furthmayr was obtained from the Developmental Studies Hybridoma Bank maintained by the Department of Biological Sciences, University of Iowa, Iowa City, IA 52242, under contract N01-HD-7-3263 from the NICHD.
We thank Bryan Crawford for assistance with Eclipse and Ian Bercowitz, Department of Mathematics and Statistics, SFU, for performing the SAS analysis. We are grateful to C. R. Roberts, Department of Oral Biological and Medical Sciences, University of British Columbia, for valuable discussions and critical reading of the manuscript. We also thank Linda J. S. Pinto and Anna H. T. Gifford for critical reading of the manuscript and many helpful discussions. Anat Reicher Feldman is gratefully acknowledged for help with the lectin blots, critical reading of the manuscript, and valuable discussions.
REFERENCES
- 1.Barkalow F J, Schwarzbauer J E. Localization of the major heparin-binding site in fibronectin. J Biol Chem. 1991;266:7812–7818. [PubMed] [Google Scholar]
- 2.Beguin H, Nolard-Tintigner N, Claus B. Fluctuations saisonnières des spores d'Aspergillus dans l'air à Bruxelles en 1982. Bull Soc Fr Mycol Méd. 1985;14:195–200. [Google Scholar]
- 3.Bendel C M, Hostetter M K. Distinct mechanisms of epithelial adhesion for Candida albicans and Candida tropicalis. Identification of the participating ligands and development of inhibitory peptides. J Clin Investig. 1993;92:1840–1849. doi: 10.1172/JCI116775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodey G, Bueltmann B, Duguid W, Gibbs D, Hanak H, Hotchi M, Mall G, Martino P, Meunier F, Milliken S, et al. Fungal infections in cancer patients: an international autopsy survey. Eur J Clin Microbiol Infect Dis. 1992;11:99–109. doi: 10.1007/BF01967060. [DOI] [PubMed] [Google Scholar]
- 5.Bodey G P, Vartivarian S. Aspergillosis. Eur J Clin Microbiol Infect Dis. 1989;8:413–437. doi: 10.1007/BF01964057. [DOI] [PubMed] [Google Scholar]
- 6.Bouchara J P, Sanchez M, Chevailler A, Marot-Leblond A, Lissitzky J C, Tronchin G, Chabasse D. Sialic acid-dependent recognition of laminin and fibrinogen by Aspergillus fumigatus conidia. Infect Immun. 1997;65:2717–2724. doi: 10.1128/iai.65.7.2717-2724.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouchara J P, Tronchin G, Chabasse D. Mechanisms and implications of the adhesion phenomenon in Aspergillus fumigatus. Pathol Biol (Paris) 1994;42:640–646. [PubMed] [Google Scholar]
- 8.Broderick L S, Conces D J, Jr, Tarver R D, Bergmann C A, Bisesi M A. Pulmonary aspergillosis: a spectrum of disease. Crit Rev Diagn Imaging. 1996;37:491–531. [PubMed] [Google Scholar]
- 9.Bromley I M, Donaldson K. Binding of Aspergillus fumigatus spores to lung epithelial cells and basement membrane proteins: relevance to the asthmatic lung. Thorax. 1996;51:1203–1209. doi: 10.1136/thx.51.12.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busby T F, Argraves W S, Brew S A, Pechik I, Gilliland G L, Ingham K C. Heparin binding by fibronectin module III-13 involves six discontinuous basic residues brought together to form a cationic cradle. J Biol Chem. 1995;270:18558–18562. doi: 10.1074/jbc.270.31.18558. [DOI] [PubMed] [Google Scholar]
- 11.Clark R A F, Lanigan J M, DellaPelle P, Manseau E, Dvorak H F, Colvin R B. Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J Investig Dermatol. 1982;79:264–269. doi: 10.1111/1523-1747.ep12500075. [DOI] [PubMed] [Google Scholar]
- 12.Denning D W. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–803. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 13.Denning D W. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996;23:608–615. doi: 10.1093/clinids/23.3.608. [DOI] [PubMed] [Google Scholar]
- 14.Dixon D M, McNeil M M, Cohen M L, Gellin B G, La Montagne J R. Fungal infections: a growing threat. Public Health Rep. 1996;111:226–235. [PMC free article] [PubMed] [Google Scholar]
- 15.Dunsmore S E, Rannels D E. Extracellular matrix biology in the lung. Am J Physiol. 1996;270:L3–L27. doi: 10.1152/ajplung.1996.270.1.L3. [DOI] [PubMed] [Google Scholar]
- 16.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gale C, Finkel D, Tao N, Meinke M, McClellan M, Olson J, Kendrick K, Hostetter M. Cloning and expression of a gene encoding an integrin-like protein in Candida albicans. Proc Natl Acad Sci USA. 1996;93:357–361. doi: 10.1073/pnas.93.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Pardo A, Rostagno A, Frangione B. Primary structure of human plasma fibronectin. Characterization of a 38 kDa domain containing the C-terminal heparin-binding site (Hep III site) and a region of molecular heterogeneity. Biochem J. 1987;241:923–928. doi: 10.1042/bj2410923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gil J, Martinez-Hernandez A. The connective tissue of the rat lung. J Histochem Cytochem. 1984;32:230–238. doi: 10.1177/32.2.6363520. [DOI] [PubMed] [Google Scholar]
- 20.Gil M L, Peñalver M C, Lopez-Ribot J L, O'Connor J E, Martinez J P. Binding of extracellular matrix proteins to Aspergillus fumigatus conidia. Infect Immun. 1996;64:5239–5247. doi: 10.1128/iai.64.12.5239-5247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton A J, Jeavons L, Youngchim S, Vanittanakom N. Recognition of fibronectin by Penicillium marneffei conidia via a sialic acid-dependent process and its relationship to the interaction between conidia and laminin. Infect Immun. 1999;67:5200–5205. doi: 10.1128/iai.67.10.5200-5205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton A J, Jeavons L, Youngchim S, Vanittanakom N, Hay R J. Sialic acid-dependent recognition of laminin by Penicillium marneffei conidia. Infect Immun. 1998;66:6024–6026. doi: 10.1128/iai.66.12.6024-6026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hearn V M, Sietsma J H. Chemical and immunological analysis of the Aspergillus fumigatus cell wall. Microbiology. 1994;140:789–795. doi: 10.1099/00221287-140-4-789. [DOI] [PubMed] [Google Scholar]
- 24.Hostetter M K. An integrin-like protein in Candida albicans: implications for pathogenesis. Trends Microbiol. 1996;4:242–246. doi: 10.1016/0966-842X(96)10036-6. [DOI] [PubMed] [Google Scholar]
- 25.Hynes R. Structure of fibronectins. In: Rich A, editor. Fibronectins. New York, N.Y: Springer-Verlag; 1990. pp. 113–175. [Google Scholar]
- 26.Ingham K C, Brew S A, Atha D H. Interaction of heparin with fibronectin and isolated fibronectin domains. Biochem J. 1990;272:605–611. doi: 10.1042/bj2720605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLaughlin M E, Kao R, Liener I E, Hoidal J R. A quantitative in vitro assay of polymorphonuclear leukocyte migration through human amnion membrane utilizing 111in-oxine. J Immunol Methods. 1971;95:89–98. doi: 10.1016/0022-1759(86)90321-2. [DOI] [PubMed] [Google Scholar]
- 28.Minamoto G Y, Barlam T F, Els N J V. Invasive aspergillosis in patients with AIDS. Clin Infect Dis. 1992;16:66–74. doi: 10.1093/clinids/14.1.66. [DOI] [PubMed] [Google Scholar]
- 29.Ogamo A, Nagai A, Nagasawa K. Binding of heparin fractions and other polysulfated polysaccharides to plasma fibronectin: effects of molecular size and degree of sulfation of polysaccharides. Biochim Biophys Acta. 1985;841:30–41. [PubMed] [Google Scholar]
- 30.Oh E, Pierschbacher M, Ruoslahti E. Deposition of plasma fibronectin in tissues. Proc Natl Acad Sci USA. 1981;78:3218–3221. doi: 10.1073/pnas.78.5.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peñalver M C, O'Connor J E, Martinez J P, Gil M L. Binding of human fibronectin to Aspergillus fumigatus conidia. Infect Immun. 1996;64:1146–1153. doi: 10.1128/iai.64.4.1146-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierschbacher M D, Hayman E G, Ruoslahti E. Location of the cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell. 1981;26:259–267. doi: 10.1016/0092-8674(81)90308-1. [DOI] [PubMed] [Google Scholar]
- 33.Pierschbacher M D, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 34.Pitt J I. The current role of Aspergillus and Penicillium in human and animal health. J Med Vet Mycol. 1994;32:17–32. [PubMed] [Google Scholar]
- 35.Potts J R, Campbell I D. Fibronectin structure and assembly. Curr Opin Cell Biol. 1994;6:648–655. doi: 10.1016/0955-0674(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues M L, Rozental S, Couceiro J N S S, Angluster J, Alviano C S, Travassos L R. Identification of N-acetylneuraminic acid and its 9-0 acetylated derivative on the cell surface of Cryptococcus neoformans: influence of fungal phagocytosis. Infect Immun. 1997;65:4937–4942. doi: 10.1128/iai.65.12.4937-4942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roman J. Extracellular matrix and lung inflammation. Immunol Res. 1996;15:163–178. doi: 10.1007/BF02918505. [DOI] [PubMed] [Google Scholar]
- 38.Roman J, McDonald J A. Fibronectins and fibronectin receptors in lung development, injury and repair. In: Crystal R G, West J B, editors. The lung: scientific foundations. Philadelphia, Pa: Lippincott-Raven; 1997. pp. 737–755. [Google Scholar]
- 39.Schauer R, Kelm S, Reuter G, Roggentin P, Shaw L. Biochemistry and role of sialic acids. In: Rosenberg A, editor. The biology of the sialic acids. New York, N.Y: Plenum Press; 1995. pp. 7–67. [Google Scholar]
- 40.Schulte B A, Spicer S S, Miller R L. Histochemical localization of sialoglycoconjugates with a sialic acid-specific lectin from the slug Limax flavus. Histochem J. 1984;16:1125–1132. doi: 10.1007/BF01002899. [DOI] [PubMed] [Google Scholar]
- 41.Sharma A, Askari J A, Humphries M J, Jones E Y, Stuart D I. Crystal structure of a heparin- and integrin-binding segment of human fibronectin. EMBO J. 1999;18:1468–1479. doi: 10.1093/emboj/18.6.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skorstengaard K, Jensen M S, Petersen T E, Magnusson S. Purification and complete primary structures of the heparin-, cell-, and DNA-binding domains of bovine plasma fibronectin. Eur J Biochem. 1986;154:15–29. doi: 10.1111/j.1432-1033.1986.tb09353.x. [DOI] [PubMed] [Google Scholar]
- 43.Soares R M, Costa e Silva-Filho F, Rozental S, Angluster J, de Souza W, Alviano C S, Travassos L R. Anionogenic groups and surface sialoglycoconjugate structures of yeast forms of the human pathogen Paracoccidioides brasiliensis. Microbiology. 1998;144:309–314. doi: 10.1099/00221287-144-2-309. [DOI] [PubMed] [Google Scholar]
- 44.Torikata C, Villiger B, Kuhn III C, McDonald J A. Ultrastructural distribution of fibronectin in normal and fibrotic human lung. Lab Investig. 1985;52:399–408. [PubMed] [Google Scholar]
- 45.Tronchin G, Bouchara J P, Larcher G, Lissitzky J C, Chabasse D. Interaction between Aspergillus fumigatus and basement membrane laminin: binding and substrate degradation. Biol Cell. 1993;77:201–208. doi: 10.1016/s0248-4900(05)80189-3. [DOI] [PubMed] [Google Scholar]
- 46.Tsilibary E C, Koliakos G G, Charonis A S, Vogel A M, Reger L A, Furcht L T. Heparin type IV collagen interactions: equilibrium binding and inhibition of type IV collagen self-assembly. J Biol Chem. 1988;263:19112–19118. [PubMed] [Google Scholar]
- 47.Wald A, Leisenring W, van Burik J A, Bowden R A. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis. 1997;175:1459–1466. doi: 10.1086/516480. [DOI] [PubMed] [Google Scholar]
- 48.Walker A, Gallagher J T. Structural domains of heparan sulfate for recognition of the C-terminal domain of human plasma fibronectin (HEPII) Biochem J. 1996;317:871–877. doi: 10.1042/bj3170871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamada K M. Fibronectin and other adhesive glycoproteins. In: Hay E D, editor. Cell biology of extracellular matrix. 2nd ed. New York, N.Y: Plenum Press; 1991. pp. 111–146. [Google Scholar]